Abstract
RUNX2 is a multifunctional transcription factor that controls skeletal development by regulating the differentiation of chondrocytes and osteoblasts and the expression of many extracellular matrix protein genes during chondrocyte and osteoblast differentiation. This transcription factor plays a major role at the late stage of chondrocyte differentiation: it is required for chondrocyte maturation and regulates Col10a1 expression in hypertrophic chondrocytes and the expression of Spp1, Ibsp, and Mmp13 in terminal hypertrophic chondrocytes. It is essential for the commitment of pluripotent mesenchymal cells to the osteoblast lineage. During osteoblast differentiation, RUNX2 upregulates the expression of bone matrix protein genes including Col1a1, Spp1, Ibsp, Bglap, and Fn1 in vitro and activates many promoters including those of Col1a1, Col1a2, Spp1, Bglap, and Mmp13. However, overexpression of Runx2 inhibits osteoblast maturation and reduces Col1a1 and Bglap expression. The inhibition of RUNX2 in mature osteoblasts does not reduce the expression of Col1a1 and Bglap in mice. Thus, RUNX2 directs pluripotent mesenchymal cells to the osteoblast lineage, triggers the expression of major bone matrix protein genes, and keeps the osteoblasts in an immature stage, but does not play a major role in the maintenance of the expression of Col1a1 or Bglap in mature osteoblasts. During bone development, RUNX2 induces osteoblast differentiation and increases the number of immature osteoblasts, which form immature bone, whereas Runx2 expression has to be downregulated for differentiation into mature osteoblasts, which form mature bone. During dentinogenesis, Runx2 expression is downregulated, and RUNX2 inhibits the terminal differentiation of odontoblasts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vertebrate skeleton is composed of cartilage and bone. Bone is formed through either intramembranous or endochondral ossification. Osteoblasts directly form intramembranous bones, whereas chondrocytes first form a cartilaginous skeleton that is then replaced with bone by osteoblasts and osteoclasts through the process of endochondral ossification. In chrondocyte differentiation, after mesenchymal condensation, pluripotent mesenchymal cells differentiate into immature chondrocytes, which produce type II collagen and proteoglycan. The immature chondrocytes further differentiate into hypertrophic chondrocytes, which express Col10a1, and finally become terminal hypertrophic chondrocytes, which express Spp1 (secreted phophoprotein 1/osteopontin), Ibsp (integrin-binding sialoprotein/bone sialoprotein II), and Mmp13 (matrix metallopeptidase 13) (Marks and Odgren 2002; Inada et al. 1999).
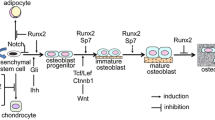
These processes in chondrocyte differentation are regulated by many factors, and specific transcription factors play essential roles in the differentiation of chondrocytes. The transcription factor SOX9 plays a crucial role in mesenchymal condensation, leading to the formation of the cartilaginous template, whereas SOX9, SOX5, and SOX6 are required for the production of cartilaginous matrix, and runt-related transcription factor 2 (RUNX2)/core-binding factor α1 (CBFA1)/polyoma-enhancer-binding protein 2αA (PEBP2αA) has an important function in the terminal diffrentiation of chondrocytes, a function that is a prerequisite for endochondral ossification. RUNX3, which is another Runx family transcription factor, is also involved in the terminal differentiation of chondrocytes (Komori 2005) (Fig. 1).
Regulation of extracellular matrix protein genes by RUNX2 during the differentiation of osteoblasts and chondrocytes. RUNX2 is essential for the commitment of pluripotent mesenchymal cells to the osteoblast lineage. During the process of endochondral ossification, RUNX2 and RUNX3 are crucial for chondrocyte maturation and inhibit chondrocytes from acquiring the phenotype of permanent cartilage. RUNX2 regulates the expression of Col10a1 in hypertrophic chondrocytes and the expression of Spp1, Ibsp, and Mmp13 in terminal hypertrophic chondrocytes. During the process of osteoblast differentiation, RUNX2 triggers the expression of Col1a1, Col1a2, Spp1, Ibsp, and Bglap and maintains the expression of Spp1 and Ibsp in immature osteoblasts. However, Runx2 expression has to be downregulated for bone maturation (Col10a1 collagen 10a1, Spp1 secreted phophoprotein 1/osteopontin, Ibsp integrin-binding sialoprotein/bone sialoprotein II, Mmp13 matrix metallopeptidase 13, Bglap bone gamma-carboxyglutamate [gla] protein/osteocalcin)
During osteoblast differentiation, RUNX2, SP7, and canonical Wnt signaling play essential roles in the commitment of pluripotent mesenchymal cells to the osteoblastic lineage (Komori 2006). After commitment into the osteoblastic lineage, the osteoblasts express bone matrix protein genes at various expression levels depending on the maturation level of the cells. Immature mesenchymal cells and preosteoblasts weakly express Col1a1, and its expression is upregulated in immature osteoblasts (Aubin and Triffitt 2002; Inada et al. 1999). Immature osteoblasts express Spp1 and then Ibsp, and maturated osteoblasts strongly express Bglap (bone gamma-carboxyglutamate [gla] protein/osteocalcin) (Aubin and Triffitt 2002; Maruyama et al. 2007). Mature osteoblasts are embedded into the bone matrix and finally become osteocytes, which express Dmp1 (dentin matrix protein 1) (Toyosawa et al. 2001) (Fig. 2).
Regulation of osteoblast and odontoblast differentiation by RUNX2. RUNX2 directs pluripotent mesenchymal cells to the osteoblast lineage, increases the number of immature osteoblasts, but inhibits osteoblast maturation. Preosteoblasts express Runx2. Immature osteoblasts express Runx2 and Spp1 and, subsequently, Bglap. Mature osteoblasts express Bglap, but Runx2 expression is downregulated. Osteocytes express Dmp1. The transition of immature osteoblasts to osteocytes occurs at an early stage of bone development. The common precursors of osteoblasts and odontoblasts are restricted to neural-crest-derived mesenchymal cells, but the basal process of osteoblast differentiation is similar in the neural-crest-derived and non-neural-crest-derived pluripotent mesenchymal cells. Preodontoblasts differentiate from neural-crest-derived pluripotent mesenchymal cells. RUNX2 is essential for differentiation of pluripotent mesenchymal cells into preodontoblasts. RUNX2 also probably induces the differentiation of preodontoblasts into immature odontoblasts at an early stage but is inhibitory at a late stage. Preodontoblasts express Runx2, immature odontoblasts express Dspp and Nes but Runx2 weakly, and mature odontoblasts express Dspp and Nes but not Runx2. Runx2 expression is downregulated during odontoblast differentiation, and RUNX2 inhibits terminal differentiation of odontoblasts. Overexpression of Runx2 induced transdifferentiation of odontoblasts to osteoblasts (Spp1 secreted phophoprotein 1/osteopontin, Bglap bone gamma-carboxyglutamate [gla] protein/osteocalcin, Dspp dentin sialophosphoprotein, Nes nestin, Dmp1 dentin matrix protein 1)
Expression of Runx2 during skeletal development
RUNX2 is expressed as two isoforms that possess different N-termini (type I RUNX2 starting with the sequence MRIPV and type II RUNX2 starting with the sequence MASNS) and that are expressed under different promoters (Komori and Kishimoto 1998). Both type I and type II Runx2 mRNAs are expressed in chondrocytes and osteoblasts, although type II Runx2 mRNA is predominantly expressed in osteoblasts (Enomoto et al. 2000; Banerjee et al. 2001; Choi et al. 2002). The two isoforms have similar functions but differ in their dependency on CBFB, which is an essential co-transcription factor of RUNX2 (Kundu et al. 2002; Miller et al. 2002; Yoshida et al. 2002; Kanatani et al. 2006).
During skeletal development, both type I and type II Runx2 mRNAs are weakly expressed in proliferating chondrocytes, their expression is upregulated as chondrocytes differentiate, and both type I and type II Runx2 mRNAs are highly expressed in chondrocytes with maturational stages ranging from prehypertrophic to hypertrophic chondrocytes (Simeone et al. 1995; Kim et al. 1999; Enomoto et al. 2000; Inada et al. 1999; Stricker et al. 2002). During the progression of osteoblast differentiation, the expression of Runx2 mRNA and RUNX2 protein dynamically change, and the expression of Spp1 (SPP1) and Bglap (BGLAP) can be used to highlight this dynamic process. During the development of intramembranous bones, RUNX2 is strongly detected in preosteoblasts, immature osteoblasts, and early mature osteoblasts. At 1 week of age in wild-type mice, preosteoblasts in the periosteum of the mandible express RUNX2 but not SPP1 or BGLAP, whereas inside the mandible, both SPP1-positive immature osteoblasts and BGLAP-positive early mature osteoblasts express RUNX2 (Maruyama et al. 2007). During the development of endochondral bones, RUNX2 is first detected in mesenchymal cells in the perichondrial region: in the femur at 1 week of age, the preosteoblasts in the perichondrial region surrounding proliferating and prehypertrophic chondrocytes express RUNX2 but not SPP1 or BGLAP; immature osteoblasts surrounding the hypertrophic chondrocyte layer express RUNX2 and SPP1 but not BGLAP; and BGLAP-positive early mature osteoblasts, which express RUNX2, appear in the metaphyseal cortical bone (Maruyama et al. 2007). During long bone development, osteoblasts at the metaphysis are less mature than those at the diaphysis: in the femur at 4 weeks of age, SPP1-positive immature osteoblasts strongly express RUNX2 and BGLAP-positive mature osteoblasts weakly express RUNX2 in the metaphysis, whereas RUNX2 and SPP1 are undetectable by immunohistochemistry in most of the BGLAP-positive late mature osteoblasts in the diaphysis even though the mRNAs of Runx2 and Spp1 are detectable by in situ hybridization (Maruyama et al. 2007). Thus, RUNX2 is expressed in preosteoblasts, which do not express SPP1 or BGLAP, is strongly expressed in SPP1-positive immature osteoblasts, and then is expressed in BGLAP-positive early mature osteoblasts, but RUNX2 expression is finally downregulated in BGLAP-positive late mature osteoblasts (Maruyama et al. 2007) (Fig. 2).
RUNX2 regulates the expression of extracellular matrix protein genes in chondrocytes
During chondrocyte maturation, immature chondrocytes express Col2a1, mature chondrocytes (hypertrophic chondrocytes) express Col10a1, and terminally differentiated chondrocytes (terminal hypertrophic chondrocytes) express Spp1 and Ibsp. Runx2-deficient (Runx2 −/−) mice completely lack bone formation because of the absence of osteoblasts (Komori et al. 1997; Otto et al. 1997). The skeleton of Runx2 −/− mice is composed of cartilage, chondrocyte maturation is inhibited in Runx2 −/− mice, and the expression of Col10a1 in hypertrophic chondrocytes is drastically reduced (Inada et al. 1999; Kim et al. 1999). In restricted skeletons, including the tibia, fibula, radius, and ulna, however, chondrocytes maturate to terminal hypertrophic chondrocytes. In these skeletons, Col10a1 is detected, whereas Spp1, Ibsp, and Mmp13, which are expressed in terminal hypertrophic chondrocytes, are undetectable. RUNX2 directly regulates the expression of Spp1 in a synergistic manner with ETS1 (Sato et al. 1998). Runx2 also directly regulates Mmp13 expression, and RUNX2, which is activated by protein kinase A, and AP1 physically interact and are required for parathyroid-hormone-dependent Mmp13 expression (Jiménez et al. 1999; Porte et al. 1999; Selvamurugan et al. 2000; Hess et al. 2001) (Fig. 1). MMP13, which efficiently degrades the native helix of fibrillar collagen with preferential activity on type II collagen and is able to degrade aggrecan, plays an important role in the degradation of cartilage matrix at the chondro-osseous junction in the process of endochondral ossification (Fosang et al. 1996; Knäuper et al. 1996; Inada et al. 2004).
Overexpression of Runx2 in chondrocytes under the control of the Col2a1 promoter accelerates chondrocyte maturation and Col10a1 expression in mice, whereas the expression of dominant negative (dn)-Runx2 in chondrocytes under the control of the Col2a1 promoter decelerates chondrocyte maturation and reduces Col10a1 expression in mice (Ueta et al. 2001). RUNX2 and RUNX3 have redundant functions in chondrocyte maturation, and chondrocyte maturation is completely inhibited in whole skeletons of Runx2 −/− Runx3 −/− mice (Yoshida et al. 2004). Hypertrophic chondrocytes expressing Col10a1 are absent in Runx2 −/− Runx3 −/− mice. In vitro analyses have shown that RUNX2 induces Col10a1 expression, and that RUNX2 directly regulates the Col10a1 promoter by using core responsive elements located at −2.4 kb in mouse and chicken and between −89 and −60 bp in humans (Enomoto et al. 2000; Zheng et al. 2003; Drissi et al. 2003; Higashikawa et al. 2009) (Fig. 1).
TNC (tenascin-C) is expressed in chondrocytes once cartilage tissue appears, but its expression becomes limited to the articular chondrocytes as cartilage development progresses (Pacifici 1995). In Runx2 transgenic mice under the control of the Col2a1 promoter, permanent cartilage enters the endochondral pathway, and TNC expression in the presumptive joint region is lost, whereas most chondrocytes in dn-Runx2 transgenic mice under the control of the Col2a1 promoter retain the expression of TNC. Therefore, suppression of Runx2 expression is required for the formation and maintenance of permanent cartilage (Ueta et al. 2001) (Fig. 1). In osteoarthritis, however, RUNX2 is detected in the articular cartilage, and RUNX2 is colocalized with COL10A1 or MMP13 (Wang et al. 2004; Kamekura et al. 2006).
RUNX2 regulates the expression of bone matrix protein genes in osteoblasts
As Runx2 −/− mice lack osteoblasts, the expression of bone matrix protein genes including Spp1, Ibsp, and Bglap is virtually absent in these mice (Komori et al. 1997; Inada et al. 1999). In type II Runx2-specific knockout mice, the expression of Col1a1, Spp1, and Bglap is reduced (Xiao et al. 2005). In accordance with the results of the in vivo studies, in vitro studies have demonstrated that RUNX2 is a positive regulator that can upregulate the expression of bone matrix protein genes including Col1a1, Spp1, Ibsp, Bglap, and Fn1 (fibronectin 1) (Ducy et al. 1997; Sato et al. 1998; Harada et al. 1999; Lee et al. 2000). RUNX2 is involved in the transcriptional activation of many promoters including those of Col1a1, Col1a2, Spp1, and Bglap (Banerjee et al. 1997; Kern et al. 2001; Harada et al. 1999; Jiménez et al. 1999; Sato et al. 1998). However, Ibsp is an exception, because Ibsp expression is reduced by RUNX2 and HDAC3 in vitro, and RUNX2 represses Ibsp promoter activity (Lamour et al. 2007; Javed et al. 2001). Further, expression of dn-Runx2 under the control of the Bglap promoter, which directs reporter gene expression to mature osteoblasts, results in osteopenia because of drastic reductions in the expression of genes encoding the main bone matrix proteins including COL1A1, COL1A2, SPP1, IBSP, and BGLAP (Ducy et al. 1999).
However, transgenic mice that overexpress Runx2 under the control of a 2.3-kb mouse Col1a1 promoter, which directs reporter gene expression to immature and mature osteoblasts, show osteopenia with multiple fractures (Liu et al. 2001; Geoffroy et al. 2002; Kanatani et al. 2006). Most of the osteoblasts of these mice exhibit less mature phenotypes, and the numbers of terminally differentiated osteoblasts, which strongly express Bglap, and of osteocytes are greatly diminished. As a result, in the osteoblasts of these mice, the expression of Col1a1, Alpl (alkaline phosphatase, liver/bone/kidney), Bglap, and Mmp13, all of which normally increase during osteoblast maturation, are reduced (Liu et al. 2001; Geoffroy et al. 2002; Kanatani et al. 2006), although the changes in the expression of the bone matrix protein genes could be, in part, attributable to the abnormal osteoblast differentiation in Runx2 transgenic mice. In dn-Runx2 transgenic mice under the control of the same 2.3-kb mouse Col1a1 promoter, the volume of the trabecular bone is increased, and the expression of major bone matrix protein genes including Col1a1, Spp1, and Bglap, is not significantly affected compared with those in wild-type mice, although dn-RUNX2 rescues the reduction of Bglap expression in the Runx2 transgenic mice (Maruyama et al. 2007). These findings, together with the in vitro data, indicate that RUNX2 induces the expression of major bone matrix protein genes in osteoblast progenitors, allowing the cells to acquire the osteoblastic phenotype while keeping the osteoblastic cells in an immature stage. As the expression patterns of Runx2 and Spp1 are similar during bone development, and as Spp1 expression is increased in Runx2 transgenic mice, RUNX2 is likely to maintain Spp1 expression in immature osteoblasts (Maruyama et al. 2007; Liu et al. 2001; Geoffroy et al. 2002; Kanatani et al. 2006). In mature osteoblasts, a low level of Runx2 expression might nevertheless be required for the maintenance of the expression of Col1a1 and Bglap (Fig. 1).
Osteoblast differentiation and bone maturation
In transgenic mice overexpressing Runx2 under the control of the 2.3-kb Col1a1 promoter, cortical bone has a woven bone-like structure, the cortical bone mass but not the trabecular bone mass is severely reduced, and the reduction in cortical bone mass is attributable to accelerated resorption caused by the increase in recruitment and activity of osteoclasts (Liu et al. 2001; Geoffroy et al. 2002; Kanatani et al. 2006). This seems to be caused by the immature composition of cortical bone, which contains abundant SPP1 with the small cell attachment motif (Arg-Gly-Asp [RGD]); the RGD is recognized by integrins and promotes the attachment of osteoclasts to the extracellular matrix (Young et al. 1993). The expression of IBSP, which also has the RGD motif, is increased in Runx2 transgenic mice under the control of the 2.3-kb Col1a1 promoter (Liu et al. 2001; Geoffroy et al. 2002) and may also contribute to the accelerated resorption of cortical bone.
In contrast, the trabecular bone increases in volume without deceleration of osteoclastogenesis in adult dn-Runx2 transgenic mice under the control of the 2.3-kb Col1a1 promoter (Maruyama et al. 2007). The extent of mineralization in the trabecular bone is higher in dn-Runx2 transgenic mice than in wild-type mice. Further, although the collagen fibrils are loosely deposited in a random orientation in the trabecular bone of wild-type mice, they are densely and regularly packed in the trabecular bone of dn-Runx2 transgenic mice. These characteristics of the trabecular bone of dn-Runx2 transgenic mice are similar to those seen in cortical bone, indicating that the trabecular bone in dn-Runx2 transgenic mice has characteristics of compact bone, which represents a more mature bone than trabecular bone and is more resistant to osteolysis. Thus, RUNX2 directs multipotent mesenchymal cells to the osteoblast lineage and triggers the expression of major bone matrix protein genes, leading to an increase in immature osteoblasts, which form immature bone. However, Runx2 expression has to be downregulated for the phenotype of fully mature osteoblasts, which form mature bone, to be acquired (Figs. 1, 2).
Odontoblast differentiation and RUNX2
Endogenous Runx2 is expressed in preodontoblasts and is downregulated during odontoblast differentiation (Bronckers et al. 2001; Yamashiro et al. 2002; Chen et al. 2005; Miyazaki et al. 2008) (Fig. 2). In Runx2 transgenic mice under the control of the 2.3-kb Col1a1 promoter, transgene expression has been detected in odontoblasts and osteoblasts (Miyazaki et al. 2008). The overexpression of Runx2 in odonotoblasts inhibits their terminal differentiation and induces the transdifferentiation of odontoblasts into osteoblasts, forming a bone structure (Miyazaki et al. 2008). The gene expression of DSPP (dentin sialophosphoprotein), which is known to be a tooth-specific extracellular matrix protein (D’Souza et al. 1997; Begue-Kirn et al. 1998), is severely downregulated in odontoblasts of Runx2 transgenic mice. Further, NES (nestin), which is an intermediate filament protein and an odontoblast marker protein that is not expressed in osteoblasts (Terling et al. 1995), is also severely downregulated in the odontoblasts. The levels of SPP1 and DMP1, which are noncollagenous proteins present in both bone and teeth but with higher expression levels in the former (D’Souza et al. 1997; Aguiar and Arana-Chavez 2007), are increased in the dentin of Runx2 transgenic mice. The mRNA of COL1A1, a major organic component of bone and dentin, is similarly expressed in immature odontoblasts of both wild-type and Runx2 transgenic mice; however, it decreases after transdifferentiation from odontoblasts to osteoblasts in Runx2 transgenic mice. The expression of BGLAP, another protein found in both bone and dentin, is upregulated in immature odontoblasts, but is also downregulated after transdifferentiation in Runx2 transgenic mice. Therefore, RUNX2 is able to alter the expression of extracellular matrix protein genes in odontoblasts and to induce the expression of bone matrix protein genes in odontoblasts, leading to their transdifferentiation to osteoblasts (Fig. 2). After transdifferentiation into osteoblasts, however, the expression of Col1a1 and Bglap is downregulated, as has been observed in the osteoblasts of Runx2 transgenic mice (Liu et al. 2001; Geffroy et al. 2002; Kanatani et al. 2006; Miyazaki et al. 2008).
References
Aguiar MC, Arana-Chavez VE (2007) Ultrastructural and immunocytochemical analysis of osteopontin in reactionary and reparative dentine formed after extrusion of upper rat incisors. J Anat 210:418–427
Aubin JE, Triffitt JT (2002) Mesenchymal stem cells and osteoblast differentiation. In: Bilezikian JP, Raisz LG, Rodan GA (eds) Principles of bone biology. Academic Press, New York, pp 59–81
Banerjee C, McCabe LR, Choi J, Hiebert SW, Stein JL, Stein GS, Lian JB (1997) Runt homology domain proteins in osteoblast differentiation: AML3/CBFA1 is a major component of a bone-specific complex. J Cell Biochem 66:1–8
Banerjee C, Javed A, Choi JY, Green J, Rosen V, Wijnen AJ van, Stein JL, Lian JB, Stein GS (2001) Differential regulation of the two principal Runx2/Cbfa1 N-terminal isoforms in response to bone morphogenetic protein-2 during development of the osteoblast phenotype. Endocrinology 142:4026–4039
Begue-Kirn C, Krebsbach PH, Bartlett JD, Butler WT (1998) Dentin sialoprotein, dentin phosphoprotein, enamelysin and ameloblastin: tooth-specific molecules that are distinctively expressed during murine dental differentiation. Eur J Oral Sci 106:963–970
Bronckers AL, Engelse MA, Cavender A, Gaikwad J, D’Souza RN (2001) Cell-specific patterns of Cbfa1 mRNA and protein expression in postnatal murine dental tissues. Mech Dev 101:255–258
Chen S, Rani S, Wu Y, Unterbrink A, Gu TT, Gluhak-Heinrich J, Chuang H-H, MacDougall M (2005) Differential regulation of dentin sialophosphoprotein expression by Runx2 during odontoblasts cytodifferentiation. J Biol Chem 280:29717–29727
Choi KY, Lee SW, Park MH, Bae YC, Shin HI, Nam S, Kim YJ, Kim HJ, Ryoo HM (2002) Spatio-temporal expression patterns of Runx2 isoforms in early skeletogenesis. Exp Mol Med 34:426–433
Drissi MH, Li X, Sheu TJ, Zuscik MJ, Schwarz EM, Puzas JE, Rosier RN, O’Keefe RJ (2003) Runx2/Cbfa1 stimulation by retinoic acid is potentiated by BMP2 signaling through interaction with Smad1 on the collagen X promoter in chondrocytes. J Cell Biochem 90:1287–1298
Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G (1997) Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747–754
Ducy P, Starbuck M, Priemel M, Shen J, Pinero G, Geoffroy V, Amling M, Karsenty G (1999) A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev 13:1025–1036
D’Souza RN, Cavender A, Sunavala G, Alvarez J, Oshima T, Kulkarni AB, MacDougall M (1997) Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J Bone Miner Res 12:2040–2049
Enomoto H, Enomoto-Iwamoto M, Iwamoto M, Nomura S, Himeno M, Kitamura Y, Kishimoto T, Komori T (2000) Cbfa1 is a positive regulatory factor in chondrocyte maturation. J Biol Chem 275:8695–8702
Fosang AJ, Last K, Knäuper V, Murphy G, Neame PJ (1996) Degradation of cartilage aggrecan by collagenase-3 (MMP-13). FEBS Lett 380:17–20
Geoffroy V, Kneissel M, Fournier B, Boyde A, Matthias P (2002) High bone resorption in adult aging transgenic mice overexpressing cbfa1/runx2 in cells of the osteoblastic lineage. Mol Cell Biol 22:6222–6233
Harada H, Tagashira S, Fujiwara M, Ogawa S, Katsumata T, Yamaguchi A, Komori T, Nakatsuka M (1999) Cbfa1 isoforms exert functional differences in osteoblast differentiation. J Biol Chem 274:6972–6978
Hess J, Porte D, Munz C, Angel P (2001) AP-1 and Cbfa/runt physically interact and regulate parathyroid hormone-dependent MMP13 expression in osteoblasts through a new osteoblast-specific element 2/AP-1 composite element. J Biol Chem 276:20029–20038
Higashikawa A, Saito T, Ikeda T, Kamekura S, Kawamura N, Kan A, Oshima Y, Ohba S, Ogata N, Takeshita K, Nakamura K, Chung UI, Kawaguchi H (2009) Identification of the core element responsive to runt-related transcription factor 2 in the promoter of human type X collagen gene. Arthritis Rheum 60:166–178
Inada M, Yasui T, Nomura S, Miyake S, Deguchi K, Himeno M, Sato M, Yamagiwa H, Kimura T, Yasui N, Ochi T, Endo N, Kitamura Y, Kishimoto T, Komori T (1999) Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev Dyn 214:279–290
Inada M, Wang Y, Byrne MH, Rahman MU, Miyaura C, López-Otín C, Krane SM (2004) Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci USA 101:17192–17197
Javed A, Barnes GL, Jasanya BO, Stein JL, Gerstenfeld L, Lian JB, Stein GS (2001) Runt homology domain transcription factors (Runx, Cbfa, and AML) mediate repression of the bone sialoprotein promoter: evidence for promoter context-dependent activity of Cbfa proteins. Mol Cell Biol 21:2891–2905
Jiménez MJ, Balbín M, López JM, Alvarez J, Komori T, López-Otín C (1999) Collagenase 3 is a target of Cbfa1, a transcription factor of the runt gene family involved in bone formation. Mol Cell Biol 19:4431–4442
Kamekura S, Kawasaki Y, Hoshi K, Shimoaka T, Chikuda H, Maruyama Z, Komori T, Sato S, Takeda S, Karsenty G, Nakamura K, Chung UI, Kawaguchi H (2006) Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis Rheum 54:2462–2470
Kanatani N, Fujita T, Fukuyama R, Liu W, Yoshida CA, Moriishi T, Yamana K, Miyazaki T, Toyosawa S, Komori T (2006) Cbfβ regulates Runx2 function isoform-dependently in postnatal bone development. Dev Biol 296:48–61
Kern B, Shen J, Starbuck M, Karsenty G (2001) Cbfa1 contributes to the osteoblast-specific expression of type I collagen genes. J Biol Chem 276:7101–7107
Kim IS, Otto F, Zabel B, Mundlos S (1999) Regulation of chondrocyte differentiation by Cbfa1. Mech Dev 80:159–170
Knäuper V, López-Otin C, Smith B, Knight G, Murphy G (1996) Biochemical characterization of human collagenase-3. J Biol Chem 271:1544–1550
Komori T (2005) Regulation of skeletal development by the Runx family of transcription factors. J Cell Biochem 95:445–453
Komori T (2006) Regulation of osteoblast differentiation by transcription factors. J Cell Biochem 99:1233–1239
Komori T, Kishimoto T (1998) Cbfa1 in bone development. Curr Opin Genet Dev 8:494–499
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755–764
Kundu M, Javed A, Jeon JP, Horner A, Shum L, Eckhaus M, Muenke M, Lian JB, Yang Y, Nuckolls GH, Stein GS, Liu PP (2002) Cbfβ interacts with Runx2 and has a critical role in bone development. Nat Genet 32:547–552
Lamour V, Detry C, Sanchez C, Henrotin Y, Castronovo V, Bellahcène A (2007) Runx2- and histone deacetylase 3-mediated repression is relieved in differentiating human osteoblast cells to allow high bone sialoprotein expression. J Biol Chem 282:36240–36249
Lee KS, Kim HJ, Li QL, Chi XZ, Ueta C, Komori T, Wozney JM, Kim EG, Choi JY, Ryoo HM, Bae SC (2000) Runx2 is a common target of transforming growth factor β1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol 20:8783–8792
Liu W, Toyosawa S, Furuichi T, Kanatani N, Yoshida C, Liu Y, Himeno M, Narai S, Yamaguchi A, Komori T (2001) Overexpression of Cbfa1 in osteoblasts inhibits osteoblast maturation and causes osteopenia with multiple fractures. J Cell Biol 155:157–166
Marks SC Jr, Odgren PR (2002) Structure and development of the skeleton. In: Bilezikian JP, Raisz LG, Rodan GA (eds) Principles of bone biology. Academic Press, pp 3–15
Maruyama Z, Yoshida CA, Furuichi T, Amizuka N, Ito M, Fukuyama R, Miyazaki T, Kitaura H, Nakamura K, Fujita T, Kanatani N, Moriishi T, Yamana K, Liu W, Kawaguchi H, Nakamura K, Komori T (2007) Runx2 determines bone maturity and turnover rate in postnatal bone development and is involved in bone loss in estrogen deficiency. Dev Dyn 236:1876–1890
Miller J, Horner A, Stacy T, Lowrey C, Lian JB, Stein G, Nuckolls GH, Speck NA (2002) The core-binding factor β subunit is required for bone formation and hematopoietic maturation. Nat Genet 32:645–649
Miyazaki T, Kanatani N, Rokutanda S, Yoshida C, Toyosawa S, Nakamura R, Takada S, Komori T (2008) Inhibition of terminal differentiation of odontoblasts and their transdifferentiation into osteoblasts in Runx2 transgenic mice. Arch Histol Cytol 71:131–146
Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ (1997) Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89:765–771
Pacifici M (1995) Tenascin-C and the development of articular cartilage. Matrix Biol 14:689–698
Porte D, Tuckermann J, Becker M, Baumann B, Teurich S, Higgins T, Owen MJ, Schorpp-Kistner M, Angel P (1999) Both AP-1 and Cbfa1-like factors are required for the induction of interstitial collagenase by parathyroid hormone. Oncogene 18:667–678
Sato M, Morii E, Komori T, Kawahata H, Sugimoto M, Terai K, Shimizu H, Yasui T, Ogihara H, Yasui N, Ochi T, Kitamura Y, Ito Y, Nomura S (1998) Transcriptional regulation of osteopontin gene in vivo by PEBP2aA/CBFA1 and ETS1 in the skeletal tissues. Oncogene 17:1517–1525
Selvamurugan N, Pulumati MR, Tyson DR, Partridge NC (2000) Parathyroid hormone regulation of the rat collagenase-3 promoter by protein kinase A-dependent transactivation of core binding factor α1. J Biol Chem 275:5037–5042
Simeone A, Daga A, Calabi F (1995) Expression of runt in the mouse embryo. Dev Dyn 203:61–70
Stricker S, Fundele R, Vortkamp A, Mundlos S (2002) Role of Runx genes in chondrocyte differentiation. Dev Biol 245:95–108
Terling C, Rass A, Mitsiadis TA, Fried K, Lendahl U, Wroblewski J (1995) Expression of the intermediate filament nestin during rodent tooth development. Int J Dev Biol 39:947–956
Toyosawa S, Shintani S, Fujiwara T, Ooshima T, Sato A, Ijuhin N, Komori T (2001) Dentin matrix protein 1 is predominantly expressed in chicken and rat osteocytes but not in osteoblasts. J Bone Miner Res 16:2017–2026
Ueta C, Iwamoto M, Kanatani N, Yoshida C, Liu Y, Enomoto-Iwamoto M, Ohmori T, Enomoto H, Nakata K, Takada K, Kurisu K, Komori T (2001) Skeletal malformations caused by overexpression of Cbfa1 or its dominant negative form in chondrocytes. J Cell Biol 153:87–100
Wang X, Manner PA, Horner A, Shum L, Tuan RS, Nuckolls GH (2004) Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthritis Cartilage 12:963–973
Xiao Z, Awad HA, Liu S, Mahlios J, Zhang S, Guilak F, Mayo MS, Quarles LD (2005) Selective Runx2-II deficiency leads to low-turnover osteopenia in adult mice. Dev Biol 283:345–356
Yamashiro T, Aberg T, Levanon D, Groner Y, Thesleff I (2002) Expression of Runx1, -2 and -3 during tooth, palate and craniofacial bone development. Mech Dev 119S:S107–S110
Yoshida CA, Furuichi T, Fujita T, Fukuyama R, Kanatani N, Kobayashi S, Satake M, Takada K, Komori T (2002) Core-binding factor β interacts with Runx2 and is required for skeletal development. Nat Genet 32:633–638
Yoshida CA, Yamamoto H, Fujita T, Furuichi T, Ito K, Inoue K, Yamana K, Zanma A, Takada K, Ito Y, Komori T (2004) Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev 18:952–963
Young MF, Ibaraki K, Kerr JM, Heegaard AM (1993) Molecular and cellular biology of the major noncollagenous proteins in bone. In: Noda M (ed) Cellular and molecular biology of bone. Academic Press, London, pp 191–234
Zheng Q, Zhou G, Morello R, Chen Y, Garcia-Rojas X, Lee B (2003) Type X collagen gene regulation by Runx2 contributes directly to its hypertrophic chondrocyte-specific expression in vivo. J Cell Biol 162:833–842
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Komori, T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res 339, 189–195 (2010). https://doi.org/10.1007/s00441-009-0832-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-009-0832-8