Abstract
Summary
Vitamin D (VD) plays an important role in bone mineralization. The present study investigates the effect of VD supplementation alone on bone turnover markers in younger postmenopausal women. It has been shown that VD supplementation in postmenopausal women with hypovitaminosis D is associated with a reduction in bone turnover markers.
Purpose
The purpose of this study is to evaluate the effect of VD supplementation alone on bone turnover markers in younger postmenopausal women.
Methods
In this double-blind, placebo-controlled trial, 160 women were randomized into the VD group (supplementation with 1000 IU of vitamin D3/day, orally; n = 80) or placebo group (n = 80). Women aged 50–65 years with amenorrhea ≥ 12 months and normal bone mineral density were included. The intervention lasted 9 months, and the participants were assessed at the beginning and end of treatment. Serum levels of total calcium, parathormone (PTH), alkaline phosphatase (AP), and 24-h urine calcium were determined. Serum C-terminal telopeptide of type I collagen (s-CTX) and procollagen type 1 N-terminal propeptide (P1NP) were measured by immunoassay as markers of bone resorption and formation, respectively. Plasma 25-hydroxyvitamin-D [25(OH)D] concentrations were measured by HPLC. Intention-to-treat analysis was performed using ANOVA, Student’s t test, Tukey’s test, and gamma distribution.
Results
Over the period of 9 months, 25(OH)D concentrations increased from 15.0 ± 7.5 to 27.5 ± 10.4 ng/mL (+ 45.4%) in the VD group and decreased from 16.9 ± 6.7 to 13.8 ± 6.0 ng/mL (− 18.5%) in the placebo group (p < 0.001). There was a decrease (− 21.3%) of PTH levels in the VD group with a significant difference between groups at the end of the study (p < 0.001). No significant differences were observed in the other laboratory parameters (total calcium, AP, and calciuria) in either group (p > 0.05). A comparison of bone turnover markers showed a significant reduction in of s-CTX (− 24.2%, p < .0001) and P1NP (− 13.4%, p = 0.003) levels in the VD group. No significant variations in bone turnover markers were observed in the placebo group (s-CTX, − 6.9%, p = 0.092 and P1NP, − 0.6%, p = 0.918).
Conclusion
In younger postmenopausal women with VD deficiency, isolated supplementation with 1000 IU of vitamin D3 for 9 months is associated with a reduction in bone turnover markers. However, any between-group differences was not observed in bone turnover markers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The bone is a metabolically active tissue that undergoes continuous renewal through the formation and resorption of bone [1]. This dynamic process is called bone remodeling. The rate of bone resorption increases in postmenopausal women, enhancing the impact of the imbalance in bone remodeling [2]. Growing evidence suggests that a rapid bone remodeling, evaluated by biochemical markers of bone resorption and formation, increases bone fragility and the risk of osteoporotic fractures [3]. Biochemical markers of bone remodeling permit the dynamic assessment of bone activity and health [4, 5]. Although they cannot be used for the diagnosis of osteoporosis, high levels of bone turnover markers indicate more rapid loss of bone mineral density (BMD) and are associated with an increased risk of osteoporotic fractures regardless of BMD in postmenopausal women [6, 7]. Markers of bone remodeling respond rapidly to therapeutic intervention and these changes have been associated with the response of bone to therapy and with a reduction in fracture risk [8].
Vitamin D is essential for maintenance of the skeleton, for calcium absorption, and for regulation of the parathyroid [9]. The active form of vitamin D regulates the transcription of an expressive number of genes that encode calcium transport proteins and bone matrix proteins [10]. Measurement of 25-hydroxyvitamin D [25(OH)D] is nowadays the most suitable method to evaluate and monitor vitamin D status in the human organism, since its plasma levels are the main indicator of body reserves [11, 12]. Vitamin D deficiency is defined when the plasma concentrations of 25(OH)D are below the threshold considered to be sufficient to maintain normal secretion of parathormone (PTH) by the parathyroids [13]. In the case of vitamin D deficiency, an elevation of circulating PTH is observed, which results in secondary hyperparathyroidism accompanied by a reduction in the active form of vitamin D to 1,25-dihydroxyvitamin D [1,25(OH)2D] and by an increase in bone resorption [11]. Studies analyzing the relationship between bone health and vitamin D estimate that the desirable concentration of 25(OH)D is at least 30 ng/mL, with lower values being correlated with secondary hyperparathyroidism and a consequent reduction in bone mass [13, 14]. However, current recommendations show no consensus with regard to the optimal vitamin D status [14]. The Endocrine Society suggests serum 25(OH)D concentrations above 30 ng/mL for at risk populations, including the elderly, to support the possible effect on bone metabolism [15]. In contrast, the Institute of Medicine (IOM) defines serum 25(OH)D levels of 20 ng/mL as adequate, reflecting a level that is more than adequate for the needs of the general population [16]. However, there is general agreement that vitamin D supplements are needed to meet the requirements of postmenopausal women and the elderly population.
While vitamin D supplementation is widely used to prevent and to treat musculoskeletal diseases, it has to be acknowledged that data on vitamin D, BMD, and fracture risk are abundant but heterogeneous. This fact might be explained by the type, dose, and duration of vitamin D, as well as by the starting 25(OH)D level as a potential determinant of the effect of supplementation [17, 18]. Furthermore, most randomized and controlled studies that evaluated the effect of vitamin D supplementation on bone mass included the association with calcium, a fact that makes it difficult to identify the effects specifically attributable to vitamin D [9]. A meta-analysis of 29 studies on supplementation with calcium plus vitamin D or calcium alone suggests that a daily supplementation with 1200 mg calcium combined with 800 IU of vitamin D results in a reduction in fracture rates and in a moderate increase in BMD [19]. Another meta-analysis comprised 23 studies that compared interventions differing only in vitamin D content and that included adults (average age > 20 years) without other metabolic bone diseases. A small benefit of vitamin D supplementation on BMD at the femoral neck was observed, which was heterogenous among trials, but no effect at any other site was reported [20].
Since vitamin D is a well-established bone nutrient, markers of bone formation and turnover have been considered indicators of long-term vitamin D status [21]. A high bone turnover in vitamin D-deficient individuals has been reported in observational studies [22]. However, relatively limited and inconsistent data are available regarding the separate effects of vitamin D on bone turnover markers in different populations of both sexes [17, 18, 22,23,24]. Only one intervention study examined the effect of vitamin D supplementation alone on bone turnover in postmenopausal women [17]. Thus, more research is needed to establish the responses of bone markers to changes in vitamin D status [21].
We hypothesized that the improvement of low vitamin D status in postmenopausal women will affect their bone markers. The aim of the present study was to evaluate the effect of vitamin D supplementation alone on bone turnover markers in Brazilian postmenopausal women.
Methods
Study design and sample selection
This study was a randomized, double-blind, placebo-controlled clinical trial. The population studied consisted of patients seen between September 2013 and February 2014 at the Climacteric and Menopause Outpatient Clinic of the Botucatu Medical School—UNESP, who were taking part in a vitamin D intervention study to investigate the risk of falls, postural balance control and muscle function. Details of this study have been reported previously [25, 26]. In the present study, we investigated all 160 postmenopausal women of the previous studies. Women aged 50 to 65 years who have not had a menstrual period for at least 12 months and with BMD higher than − 1.5 SD were included in the study. Criteria for exclusion were BMD T-scores of the total spine and/or femoral neck ≤ − 1.5 SD; musculoskeletal disease; renal failure (creatinine > 1.4 mg/dL); liver disorders; cancer; abusive alcohol consumption; grade III obesity; use of bisphosphonate, estrogen, fluoride, tamoxifen, and calcitonin; primary hyperparathyroidism or hypercalciuria; and use of pharmacological doses of vitamin D or hormone therapy. Informed consent was obtained from all participants, and the study was approved by the Research Ethics Committee of the Botucatu Medical School, UNESP. The study was registered at the Brazilian Clinical Trials Registry under the registration number RBR-222wfk. This trial was conducted in accordance with the Consolidated Standards of Reporting Trials (CONSORT) 2010 statement [27].
Randomization and supplementation protocol
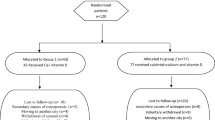
After initial screening, all participants were given a number (1–160) according to their order of inclusion in the study. Central computer randomization was conducted using a specific software (SAS 9.2 for Windows using Procedure Plan). The women were randomly assigned to two groups in a predetermined sequence: supplemented group consisting of patients receiving vitamin D3 supplementation (n = 80) and placebo group consisting of patients receiving placebo (n = 80). All participants started simultaneously in February/March 2014. The investigator and the patients were unaware of the group allocation and different numbers (blinding); only the pharmacist responsible for manipulation of the placebo knew to which group the patients belonged. Thus, 80 patients received five oral drops (each drop containing 200 IU, 20-mL flask) of 1000 IU of vitamin D3 (cholecalciferol, DePura®, Sanofi-Aventis, Brazil) for 9 months. The remaining 80 patients received five oral drops of placebo with the same characteristics and flavor (1% powdered lemon flavor, 0.2% EDTA, liquid flavor qs, and liquid petrolatum qs in 20 mL). The flasks were identical and were packed and coded by the pharmacist so that the participants could not identify the group. The participants were asked to return the flasks during each visit (every 3 months) to determine the amount of unused medication and compliance. The time of follow-up was 9 months, and the patients were submitted to clinical evaluation at baseline and at the end of the 9 months of follow-up. The flowchart shows the recruitment and randomization of the participants (Fig. 1).
Baseline measurements
Individual interviews were held with all participants, and the following data were collected: age, time since menopause, parity, current smoking, osteoarticular diseases, use of medications, and physical activity. Smokers were defined as persons who reported smoking, regardless of the number of cigarettes smoked. Women who performed aerobic exercise of moderate intensity for at least 30 min, five times a week (150/min/week), or resistance exercise three times a week were classified as active. All participants completed a 24-h dietary recall. These data were used to quantify dietary calcium intake. The participants were advised to maintain their usual dietary pattern during the study period.
The following anthropometric measures were obtained: weight, height, body mass index (BMI = weight/height2), and waist circumference. The criteria of the World Health Organization (2002) were used for classification of the participants according to BMI: normal weight, ≤ 24.9 kg/m2; overweight, 25 to 29.9 kg/m2; and obesity, ≥ 30.0 kg/m2. Waist circumference was measured at the midpoint between the lowest rib and the top of the iliac crest, with the subject in the orthostatic position. Measurements were performed at the time of exhalation by a single examiner. Increased waist circumference was defined as a value higher than 88 cm [28]. T-scores (in standard deviations) of the lumbar spine and femoral neck were obtained from the bone densitometry reports of the last 12 months. BMD was measured by dual-energy X-ray absorptiometry at the lumbar spine (L1 to L4) and femoral neck using the Hologic® QDR-2000 system (Waltham, MA, USA).
Laboratory assessment
Serum levels of creatinine, total calcium, alkaline phosphatase (AP), and parathormone (PTH) were determined at baseline and after 9 months. Creatinine, total calcium, and AP were measured by dry chemistry in an automated Vitros 950® analyzer (Johnson-Johnson, Rochester, NY, USA). PTH was measured by chemoluminescence with an automated IMMULITE 2000® immunoassay system (Diagnostic Products Corporation, Los Angeles, CA, USA). The following normal range was considered: creatinine of 0.7 to 1.2 mg/dL, calcium of 8.4 to 10.2 mg/dL, AP of 36 to 126 U/L, and PTH of 11 to 65 pg/mL. Calcium in 24-h urine samples was analyzed by dry chemistry in an Uryxxon 300® apparatus (Düren, Germany).
The plasma concentrations of 25(OH)D were measured at baseline and after 9 months for the evaluation of bioavailability and treatment compliance. The 25(OH)D concentrations were determined by high-performance liquid chromatography (HPLC) using an isocratic HPLC system equipped with a Rheodyne® manual injector (model 7725i), a 20-μL loop, and a Waters UV–vis detector (model M-484). An RP 18 column (4.0 mm × 15 cm, 5-μm particle size; Sigma-Aldrich, St. Louis, MO, USA) was used. The detection limit was 2.5 ng/mL, and the coefficient of variation was < 7%. Serum 25(OH)D levels were classified as normal (≥ 30 ng/mL), insufficiency (20–29 ng/mL), and deficiency (< 20 ng/mL) [29].
The markers of bone turnover were determined at baseline and after 9 months. Serum C-terminal cross-linked telopeptide of type I collagen (s-CTX) was used as the marker of bone resorption and was measured with the Elecsys β-CrossLaps Serum Assay (Roche Diagnostics, Indianapolis, IN, USA) by electrochemiluminescence (ECLIA) in an automated Roche™ Elecsys apparatus. This assay is specific for cross-linked isomers of type I collagen fragments, irrespective of their nature. The specificity of the test is ensured by the use of two monoclonal antibodies that recognize β-8AA octapeptides in a linear manner. Thus, the method quantifies the beta form of aspartic acid of C-terminal telopeptides (β-CTX) [30]. The reference range of s-CTX for premenopausal women is 0.11 to 0.63 ng/mL [31]. Procollagen type 1 amino-terminal propeptide (P1NP) was used as the marker of bone formation and was measured with the Elecsys Total P1NP Kit (Roche Diagnostics, Mannheim, Germany) by ECLIA in an automated Roche™ Elecsys apparatus. Procollagen type 1 contains N-(amino) and C-(carboxy) terminal extensions. These extensions (propeptides) are removed by specific proteases during the conversion of procollagen into collagen and subsequent incorporation into the bone matrix. The extension measured with this assay is the amino-terminal propeptide. P1NP is released as a trimeric structure (derived from the trimeric structure of collagen), but is rapidly broken down to a monomeric form by thermal degradation. The Elecsys P1NP assay detects the two fractions present in blood and is therefore called total P1NP. The reference range of P1NP for premenopausal women is 16.3 to 78.2 ng/mL [31]. The coefficient of variation for the two assays (s-CTX and P1NP) is less than 5%. The analytical sensitivity according to the manufacturer of the kits is 0.01 ng/ml for s-CTX and < 5 ng/mL for P1NP.
Statistical analysis
Sample size calculation was based on the study of Grados et al. [32], which demonstrated a 40% reduction in mean CTX levels after supplementation with vitamin D and calcium. Assuming a 95% confidence interval, a power of the test of 80% and correlation between times of 0.3 to detect a difference between values, a minimum of 77 women per group was estimated. Intention-to-treat analysis was used as the statistical method. The Shapiro–Wilk test was applied to determine whether the variables showed a normal distribution and the Levene test to determine homogeneity. The mean and standard deviation were calculated for quantitative variables and frequency and percentage for qualitative variables. The initial clinical, anthropometric, and biochemical variables were compared between groups using the Student’s t test and gamma distribution (asymmetric). For comparison of the biochemical parameters between time points (baseline and after 9 months) and between groups, a repeated measures design over time (ANOVA) was used, followed by Tukey’s multiple comparisons test adjusted for the group × time interaction. The bone turnover markers were compared between time points and groups using a repeated measures design for the asymmetric variables assuming a gamma distribution, followed by the Wald test for multiple comparisons. The absolute change was determined by subtracting final values from baseline and is expressed as percentage of relative variation. Adherence to the study medication was evaluated based on returned pills. The statistical tests were two tailed/bilateral, and the significance level was set at 5%. Analyses were performed using the Statistical Analysis System (SAS) 9.2 software.
Results
Table 1 shows the comparison of baseline clinical, anthropometric and laboratory variables between women receiving vitamin D supplementation (VD group, n = 80), and placebo (n = 80). The groups were homogenous for all variables analyzed (p > 0.05). The mean age of the participants was 58.8 ± 6.6 years in the VD group and 59.3 ± 6.7 years in the placebo group (p = 0.654), with a time since menopause of 12.0 ± 8.8 and 12.3 ± 8.4 years (p = 0.804), respectively. In both groups, the women were classified on average as overweight (BMI 25 to 29.9 kg/m2) and to have central fat deposition (waist circumference > 88 cm). The estimated mean dietary intake of calcium was higher than 700 mg/day and no difference was observed between groups. The mean BMD at the two sites evaluated was normal in all participants. In both groups, the mean levels of 25(OH)D indicated vitamin D deficiency. Mean creatinine, calcium, AP, and PTH concentrations were within the normal range (Table 1). Smoking was reported by 26.2% of the participants in the supplemented group and by 21.2% in the placebo group, and regular physical activity was reported by 31.2 and 26.5% of the participants, respectively, with no differences between groups (data not shown).
Table 2 shows the comparison of laboratory variables between groups and time points. There was a significant increase (+ 45.4%) in plasma 25(OH)D concentration in the group supplemented with vitamin D, while a decrease (− 18.5%) was observed in the placebo group, with a significant difference between groups (p = 0.049) and time points (p < 0.001). The PTH levels decreased by 21.3% in the supplemented group (p < 0.001) and increased by 8.5% in the placebo group (p = 0.493), with a significant difference between groups (p = 0.002) at the end of the study (Table 2). In both groups, no significant differences were detected in alkaline phosphatase or calciuria (Table 2). Stratification of plasma 25(OH)D concentrations showed that 62.5% of the women in the placebo group had vitamin D insufficiency versus 26.3% in the supplemented group. Vitamin D deficiency was only observed in the placebo group in 21.3% of the participants (data not shown).
Comparison of the bone remodeling markers showed a significant reduction between time points in s-CTX (− 24.2%) (p < 0.0001) and P1NP (− 13.4%) (p < 0.003) only in the group supplemented with vitamin D (Table 3). However, there was not any between-group differences observed in bone turnover markers. And no variations between time points were observed in the placebo group (s-CTX, − 6.9%, p = 0.092, and P1NP, − 0.6%, p = 0.918).
The rate of adherence to the study medications was 92%, with no differences between treatment groups (vitamin D or placebo). Twenty-one of the 160 women analyzed dropped out of the study over the 9 months (Fig. 1). The reported adverse events were mild and equally distributed between the supplementation and placebo groups. Two participants in the VD group and three participants in the placebo group withdrew from the study due to gastrointestinal complaints and epigastric pain. No other adverse effects were reported.
Discussion
In the present study involving younger postmenopausal women with vitamin D deficiency, daily isolated supplementation with 1000 IU of vitamin D alone for 9 months resulted in a reduction of bone remodeling markers in the group supplemented. Biochemical markers of bone remodeling have been useful tools in bone mass assessment for more than two decades [20, 33]. At the end of the 9 months, we observed a reduction of 24.2% in serum s-CTX, of 13.4% in P1NP, and of 21.3% in PTH only in the group supplemented with vitamin D. Only PTH differed from the placebo group at the end of the study. In addition, we found an increase in serum 25(OH)D levels in supplemented women, demonstrating their adherence to treatment.
Vitamin D deficiency impairs bone mineralization due to inefficient absorption of dietary calcium and phosphorus and is associated with an increase in serum PTH concentration [21]. In the present study, supplementation with 1000 IU of vitamin D resulted in an increase of plasma 25(OH)D concentration and in a reduction of serum PTH levels. Our findings agree with the results of previous trials [17, 22, 32, 34, 35]. Garnero et al. [34] investigated associations between serum 25(OH)D, bone turnover markers, and BMD in 669 postmenopausal women (mean age 62.2 years) who belonged to a population-based cohort followed up prospectively for a median of 11.2 years. The authors found no positive correlation between 25(OH)D levels and BMD or bone markers, except for a reduction in PTH [34]. The supplemental forms of vitamin D3 are metabolized in sequential hydroxylations in the liver and kidneys to 1,25(OH)2D, the active form of vitamin D [21]. Together with the intracellular vitamin D receptor (VDR), 1,25(OH)2D exerts important biological functions throughout the body. In the intestine, it binds to VDR to facilitate calcium and phosphorus absorption; in the kidney, 1,25(OH)2D stimulates the PTH-dependent tubular reabsorption of calcium; in bone, PTH and 1,25(OH)2D interact to activate osteoclasts responsible for bone resorption. Furthermore, 1,25(OH)2D suppresses PTH gene expression and inhibits the proliferation of parathyroid cells [21].
In present study, we observed a reduction of 24.2% in serum s-CTX and of 13.4% in P1NP only in the group supplemented with vitamin D. However, we did not observe any between-group differences in circulating bone turnover markers. Some studies have evaluated the effect of isolated supplementation with vitamin D on markers of bone remodeling [18, 22,23,24, 36], and only one study [17] was conducted on postmenopausal women, a fact impairing comparison with our results. MacDonald et al. [17] studied 305 women aged 60 to 70 years who were randomized to receive 400 or 1000 IU of vitamin D or placebo for 12 months. At the end of treatment, PTH was reduced in both treatment groups compared to placebo. Bone loss in the hip was greater in the placebo group (− 0.6%) and in the group receiving 400 IU of vitamin D (− 0.6%) compared to the group receiving 1000 IU of vitamin D (− 0.05%). No differences between groups were found in the lumbar spine or in the markers of bone metabolism [17]. Similar to our result, MacDonald et al. observed no differences in bone remodeling markers (P1NP and s-CTX) between groups. The authors suggested that 25(OH)D may not accurately reflect clinical outcome, nor how much vitamin D is being stored [17]. The type, duration and dose of vitamin D used in the supplementation, and the body stores of vitamin D may explain the discrepancy between the results between studies. Schwetz et al. [18] examined the effects of vitamin D supplementation on bone turnover markers in a double-blind, randomized, placebo-controlled trial. One hundred ninety-seven participants (60.2 ± 11.1 years; 47% women) with 25(OH)D levels < 30 ng/mL were randomized to receive daily doses of 2800 IU of vitamin D or placebo for 8 weeks. Vitamin D had no significant effect on CTX or P1NP. In patients with low 25(OH)D levels, the authors observed no significant effect of vitamin D supplementation for 8 weeks on bone turnover markers [18].
On the other hand, Rossini et al. [36] reported a dose-dependent effect of vitamin D supplementation on bone markers. The authors investigated the impact of high doses of vitamin D in 37 older adults (mean age of 75 years) of both sexes who were randomized to receive a single oral dose of 600,000, 300,000 or 100,000 IU of vitamin D and compared to 34 controls. Blood measurements were obtained over a period of 90 days. The administration of 600,000 IU resulted in a significant increase of s-CTX. Lower s-CTX elevations were obtained with the lower doses, which reached significance with the dose of 300,000 IU. No relevant alterations in bone remodeling were observed in the control group. These results indicate that vitamin D doses higher than 100,000 IU may be associated with acute increases in bone resorption [36].
The vitamin D status of the human organism is evaluated based on blood 25(OH)D concentrations. In the present study, baseline 25(OH)D levels were well below the desirable standards in the women investigated, classifying them as vitamin D insufficiency (< 20 ng/mL). At the end of the 9 months of vitamin D supplementation, mean plasma concentrations of 27.5 ng/mL were achieved. On the other hand, a reduction in plasma concentrations was observed in the unsupplemented group, reaching vitamin D deficiency. The population studied in this clinical trial consisted of postmenopausal women without osteopenia/osteoporosis who received daily doses of 1000 IU of vitamin D according to the recommendation of 800–1000 IU/day for postmenopausal healthy women to maintain sufficient plasma concentrations of 25(OH)D [16]. Grimes et al. [37] evaluated the effect of supplementation with a high dose of vitamin D (6,500 IU/day) compared to the standard dose (800 IU/day) on BMD and bone remodeling (P1NP and s-CTX) in 297 postmenopausal women with a T-score of − 2.0 SD in the lumbar spine and/or femoral neck. All participants received 1000 mg calcium. No changes in BMD were observed after 1 year of follow-up. Although the markers of bone remodeling were reduced in both groups, the decrease in P1NP was more pronounced in the group receiving the standard dose of vitamin D. The authors concluded that the higher dose is not superior to the standard dose in terms of the effect on bone remodeling [37]. However, postmenopausal women with risk factors for hypovitaminosis D should receive adequate and individualized treatment.
The present clinical trial used P1NP and s-CTX as markers of bone turnover to evaluate the effect of isolated supplementation with vitamin D in postmenopausal women with normal bone mass. The markers of bone formation and resorption derived from collagen can be useful for the diagnosis and monitoring of a series of diseases characterized by changes in bone metabolism [8]. Bone formation markers are enzymes or direct or indirect (procollagen) products of active osteoblasts and are generally characterized by moderate biological variability. An important bone formation marker is P1NP, which corresponds to one of the terminal portions of the procollagen molecule that is released into the bloodstream during the synthesis of type I collagen. Its serum concentration reflects bone synthesis [38,39,40]. Cross-linked molecules of type I collagen are currently the best biochemical markers of bone resorption [41]. The carboxy- and amino-terminal telopeptides (ends of the protein chain) of type I collagen, whose proteins chains are cross-linked through pyridinoline compounds, are released during collagen degradation, giving origin to the carboxy-terminal (CTX) and amino-terminal (NTX) telopeptides of type I collagen. Serum CTX shows an important correlation with bone dynamics [39]. The International Osteoporosis Foundation (IOF) and the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) recommend the use of a marker of bone formation (serum procollagen type I N-terminal propeptide, P1NP) and a marker of bone resorption (s-CTX) as reference analytes for bone turnover markers in clinical studies [41].
The present study has some limitations. The first is related to the representativeness of the sample. Since a group of postmenopausal women attending a public health service were studied, it can be assumed that they are periodically seen by medical professionals and have constant access to general healthcare. The second limitation is related to the effective and true control of the correct use of supplements, although instructions on the route of administration and return of empty flasks indicating complete consumption of the product were provided.
On the other hand, some strengths of the study should be highlighted such as the use of HPLC for the measurement of 25(OH)D, which is considered the gold standard in detecting 25(OH)D levels in plasma. While commercial automated immunoassays show variable specificity for 25(OH)D2 and D3, HPLC is more specific and is able to detect 25(OH)D2 and D3 separately [42]. The type of population studied, i.e., postmenopausal women younger than 65 years, is another strength of the study, making important contributions to the elaboration of isolated vitamin D supplementation strategies designed to prevent or at least to delay the loss of bone mass and to reduce the fracture risk in this population at risk of osteoporosis.
In conclusion, in postmenopausal women with vitamin D deficiency, isolated supplementation with 1000 IU of vitamin D3 for 9 months may be is associated with a reduction in bone turnover markers. However, it was not observed any between-group differences in bone turnover markers.
References
Cavalier E, Bergmann P, Bruyère O, Delanaye P, Durnez A, Devogelaer JP, Ferrari SL, Gielen E, Goemaere S, Kaufman JM, Toukap AN, Reginster JY, Rousseau AF, Rozenberg S, Scheen AJ, Body JJ (2016) The role of biochemical of bone turnover markers in osteoporosis and metabolic bone disease: a consensus paper of the Belgian Bone Club. Osteoporos Int 27:2181–2195
Position Statement (2010) Management of osteoporosis in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause 17:25–54
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25:2359–2381
Ivaska KK, Lenora J, Gerdhem P, Akesson K, Väänänen HK, Obrant KJ (2008) Serial assessment of serum bone metabolism markers identifies women with highest rate of bone loss and osteoporosis risk. J Clin Endocrinol Metab 93:2622–2632
Bieglmayer C, Kudlacek S (2009) The bone marker plot: an innovative method to assess bone turnover in women. Eur J Clin Investig 39:230–238
Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, Cormier C, Bréart G, Meunier PJ, Delmas PD (1996) Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res 11:1531–1538
Reginster JY, Collette J, Neuprez A, Zegels B, Deroisy R, Bruyere O (2008) Role of biochemical markers of bone turnover as prognostic indicator of successful osteoporosis therapy. Bone 42:832–836
Vasikaran SD, Glendenning P, Morris HA (2006) The role of biochemical markers of bone turnover in osteoporosis management in clinical practice. Clin Biochem Rev 27:119–121
Rosen CJ (2011) Vitamin D insufficiency. N Engl J Med 364:248–254
Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP (2004) Genetics and biology of vitamin D receptor polymorphisms. Gene 338:143–156
Dawson-Hughes B, Heaney RP, Holick MF, Vieth R, Dawson-Hughes B, Heaney RP (2005) Estimated of optimal vitamin D status. Osteoporos Int 16:713–716
Holick MF (2010) The D-lemma: to screen or not to screen for 25-hydroxyvitamin D concentrations. Clin Chemistry 56:729–731
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357:266–281
Wimalawansa SJ, Razzaque DMS, Al-Daghri NM (2017) Calcium and vitamin D in human health: hype or real? J Steroid Biochem Mol Biol. https://doi.org/10.1016/j.jsbmb.2017.12.009
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 96:1911–1930
Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Manson JE, Mayne ST, Ross AC, Shapses SA, Taylor CL (2012) IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab 97:1146–1152
Macdonald HM, Wood AD, Aucott LS, Black AJ, Fraser WD, Mavroeidi A, Reid DM, Secombes KR, Simpson WG, Thies F (2013) Hip bone loss is attenuated with 1000 IU but not 400 IU daily vitamin D3: a 1-year double-blind RCT in postmenopausal women. J Bone Mineral Res 28:2202–2213
Schwetz V, Trummer C, Pandis M, Grübler MR, Verheyen N, Gaksch M, Zittermann A, März W, Aberer F, Lang A, Treiber G, Friedl C, Obermayer-Pietsch B, Pieber TR, Tomaschitz A, Pilz S (2017) Effects of vitamin D supplementation on bone turnover markers: a randomized controlled trial. Nutrients. https://doi.org/10.3390/nu9050432
Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A (2007) Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet 370:657–666
Reid IR, Bolland MJ, Grey A (2014) Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet 383:146–155
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (2016) Scientific opinion on dietary reference values for vitamin D. EFSA J 14:4547
Madar AA, Knutsen KV, Stene LC, Brekke M, Lagerløv P, Meyer HE, Macdonald HM (2015) Effect of vitamin D3-supplementation on bone markers (serumP1NP and CTX): a randomized, double blinded, placebo controlled trial among healthy immigrants living in Norway. Bone Reports 2:82–88
Andersen R, Molgaard C, Skovgaard LT, Brot C, Cashman KD, Jakobsen J (2008) Effect of vitamin D supplementation on bone and vitamin D status among Pakistani immigrants in Denmark: a randomised double-blinded placebo-controlled intervention study. Br J Nutr 100:197–207
Viljakainen HT, Vaisanen M, Kemi V, Rikkonen T, Kröger H, Laitinen EK, Rita H, Lamberg-Allardt C (2009) Wintertime vitamin D supplementation inhibits seasonal variation of calcitropic hormones and maintains bone turnover in healthy men. J Bone Miner Res 24:346–352
Cangussu LM, Nahas-Neto J, Orsatti CL, Poloni PF, Schmitt EB, Almeida-Filho B, Nahas EAP (2016) Effect of isolated vitamin D supplementation on the rate of falls and postural balance in postmenopausal women fallers: a randomized, double-blind, placebo-controlled trial. Menopause 23:267–274
Cangussu LM, Nahas-Neto J, Orsatti CL, Bueloni-Dias F, Nahas EAP (2015) Effect of vitamin D supplementation alone on muscle function in postmenopausal women: a randomized, double-blind, placebo-controlled clinical trial. Osteoporos Int 26:2413–2421
Schulz KF, Altman DG, Moher D, Group C (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 152(11):726–732
Executive Summary of the Third report of the National Cholesterol Education Program (NCEP) (2002) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106:3143–3421
Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B (2006) Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84:18–28
Paul Chubb SA (2012) Measurement of C-terminal telopeptide of type I collagen (CTX) in serum. Clin Biochem 45:928–935
Glover SJ, Gall M, Schoenborn-Kellenberger O, Wagener M, Garnero P, Boonen S, Cauley JA, Black DM, Delmas PD, Eastell R (2009) Establishing a reference interval for bone turnover markers in 637 healthy, young, premenopausal women from the United Kingdom, France, Belgium, and the United States. J Bone Miner Res 24:389–397
Grados F, Brazier M, Kamel S, Mathieu M, Hurtebize N, Maamer M, Garabédian M, Sebert JL, Fardellone P (2003) Prediction of bone mass density variation by bone remodeling markers in postmenopausal women with vitamin D insufficiency treated with calcium and vitamin D supplementation. J Clin Endocrinol Metab 88:5175–5179
Compstom J (2009) Monitoring osteoporosis treatment. Best Pract Res Clin Rheumatol 23:781–788
Garnero P, Munoz F, Sornay-Rendu E, Delmas PD (2007) Associations of vitamin D status with bone mineral density, bone turnover, bone loss and fracture risk in health postmenopausal women The OFELY study. Bone 40:716–722
Aloia JF, Dhaliwal R, Shieh A, Mikhail M, Islam S, Yeh JK (2013) Calcium and vitamin D supplementation in postmenopausal women. J Clin Endocrinol Metab 98:E1702–E1709
Rossini M, Adami S, Viapiana O, Fracassi E, Idolazzi L, Povino MR, Gatti D (2012) Dose-dependent short-term effects of single high doses of oral vitamin D3 on bone turnover markers. Calcif Tissue Int 91:365–369
Grimnes G, Joakimsen R, Figenschau Y, Torjesen PA, Almås B, Jorde R (2012) The effect of high-dose vitamin D on bone mineral density and bone turnover markers in postmenopausal women with low bone mass-a randomized controlled 1-year trial. Osteoporos Int 23:201–211
Garnero P, Vergnaud P, Hoyle N (2008) Evaluation of a fully automated serum assay for total N-terminal propeptide of type I collagen in postmenopausal osteoporosis. Clin Chem 54:188–196
Funck-Brentano T, Biver E, Chopin F, Bouvard B, Coiffier G, Souberbielle JC, Garnero P, Roux C (2011) Clinical utility of serum bone turnover markers in postmenopausal osteoporosis therapy monitoring: a systematic review. Semin Arthritis Rheum 41:157–169
Koivula MK, Risteli L, Risteli J (2012) Measurement of aminoterminal propeptide of type I procollagen (PINP) in serum. Clin Biochem 45:920–927
Vasikaran S, Eastell R, Bruyère O, Foldes AJ, Garnero P, Griesmacher A, McClung M, Morris HA, Silverman S, Trenti T, Wahl DA, Cooper C, Kanis JA, IOF-IFCC Bone Marker Standards Working Group (2011) Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int 22:391–420
Ford L (2013) Measurement of vitamin D. Methods Mol Biol 1065:245–257
Funding
This work was supported by grants from the São Paulo Research Foundation (FAPESP) process number 2014/00001-0.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent was obtained from all participants, and the study was approved by the Research Ethics Committee of the Botucatu Medical School, UNESP.
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Nahas-Neto, J., Cangussu, L.M., Orsatti, C.L... et al. Effect of isolated vitamin D supplementation on bone turnover markers in younger postmenopausal women: a randomized, double-blind, placebo-controlled trial. Osteoporos Int 29, 1125–1133 (2018). https://doi.org/10.1007/s00198-018-4395-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-018-4395-y