Abstract
In the yeast Yarrowia lipolytica, sugar polyols and organic acids are derived from central metabolism, namely the citrate cycle and the pentose phosphate pathway. Although these metabolites have numerous applications in agro-food, chemical, and pharmaceutical industries, the main challenge is to gain productivity to obtain processes that are economically viable. Y. lipolytica is known for its ability to use industrial wastes or raw materials as feedstock and to grow at high cell density in large-scale bioreactor. Recent advances in metabolic engineering and synthetic biology allowed the development of efficient Y. lipolytica-based cell factories to bioconvert these feedstocks into added-value metabolites. This book chapter will focus on current knowledge on the synthesis of the most important polyols and organic acids in Y. lipolytica.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Several polyols and organic acids have been included in the top 12 platform chemical list of the US Department of Energy (Werpy and Petersen 2004). This highlights their importance in nowadays industries. Historically, most of these compounds have been produced industrially by chemical synthesis in a fossil fuel-dependent fashion. For instance, succinic acid (SA) could be produced by the catalytic hydrogenation of maleic anhydride (Ong et al. 2020) while erythritol, four-carbon sugar alcohol, could be obtained from dialdehyde starch in the presence of nickel as a catalyst at high temperatures (Carly and Fickers 2018).
In microorganisms, these compounds are also intermediates of biochemical pathways, such as SA that is involved in the Krebs cycle, or cell metabolites such as erythritol that is produced by certain osmotolerant yeasts in response to osmotic stress (Carly and Fickers 2018). Actually, there is an increasing demand from global drivers for greener and more sustainable processes to be developed in the context of circular economy. With the advent of molecular technologies such as metabolic engineering and synthetic biology, industrial microorganisms could now be engineered to bioconvert organic and industrial wastes into value-added chemicals using the so-called biorefinery concept. In this book chapter, we will focus on the non-conventional yeast Yarrowia lipolytica as a cell factory and detail recent advances made in strain and process engineering to produce polyols and organic acid of biotechnological interest.
2 Yarrowia lipolytica
Y. lipolytica is an Hemiascomycetes yeast isolated from dairy products, dry sausages, lipid-rich or alkane-containing matrix (polluted soil and sewage) or hypersaline environments such as seawater (Nicaud 2012). This yeast has specific metabolisms for alkanes and triglycerides catabolism (Fickers et al. 2005). Also, it presents other metabolic features such as the ability to sustain high osmotic pressure owing to the synthesis of a specific metabolite, namely erythritol, or the ability to synthesize and secrete enzymes (lipase, protease) with high efficiency (up to several g/L). Interest in Y. lipolytica started in the mid-60s for the production of single-cell proteins (SCP) from alkanes and later the yeast was a research focus for heterologous protein production. It is also considered as a model organism to study dimorphism as Y. lipolytica could grow either as yeast-like cell and pseudo-filamentous forms (Barth and Gaillardin 1996). Y. lipolytica has gained a GRAS status (Generally Recognized As Safe) that is of interest for the development of food-related applications (Groenewald et al. 2014). The complete annotated genome from strain E150 was released in 2004 (Dujon et al. 2004) and the genome of several Yarrowia strains are now publicly available (http://gryc.inra.fr/). Numerous molecular tools have been also developed such as efficient promoters and vectors for fine-tuned gene expression (Shabbir Hussain et al. 2016; Sassi et al. 2016; Trassaert et al. 2017; Park et al. 2019), DNA assembly methods (Wong et al. 2017; Vandermies et al. 2017; Celińska et al. 2017; Larroude et al. 2019), gene disruption systems (Fickers et al. 2003), and genome editing tools including CRISPR/Cas9 (Larroude et al. 2020). All these technologies have been recently reviewed (Abdel-Mawgoud et al. 2018; Larroude et al. 2018; Bilal et al. 2020). Efficient strategies have been also developed to cultivate Y. lipolytica in bioreactors with the aim to maximize recombinant protein or metabolite production titers (Do et al. 2019; Vandermies and Fickers 2019). Also, review papers related to Y. lipolytica physiology have been published (Barth and Gaillardin 1996; Fickers et al. 2005; Nicaud 2012).
3 Organic Acid
Y. lipolytica is considered as a model organism to study the production of organic acids from different carbon sources including glycerol, glucose but also waste carbon sources (waste cooking oil, pineapple waste, olive-mill wastewater, or rapeseed oil, Table 1). Organic acids such as citric acid, iso-citric acid, succinic acid, itaconic acid, and α-ketoglutaric acid which are all intermediates of Krebs cycle will be discussed in this section. The main production data for these organic acids are summarized in Table 1.
3.1 Citric Acid
Citric acid (CA) is a tricarboxylic organic acid that plays a central role in the metabolism of all aerobic organisms. It is an intermediate of the Krebs cycle (Fig. 1). Based on its non-toxic, pH buffering, and chelating properties, CA has become an important industrial product. Applications for CA include the food, cosmetic, and pharmaceutical industries, where it is used as a flavoring agent, antioxidant, preservative, or pH buffering system. About 70% of the global total CA production is used by the food industry, 20% by detergent, and the remaining 10% by the chemical and pharmaceutical industries (Carsanba et al. 2019). The global CA production exceeded 2 million tons in 2018. The market is projected to reach a volume of nearly 3 million tons by 2024, with an expected Compound Annual Growth Rate (CAGR) of 4% between 2019 and 2024 (Fickers et al. 2020; Ciriminna et al. 2017). Historically, CA was isolated from citrus fruits, but this method was displaced by fermentation of sugars using the filamentous fungus Aspergillus niger. In bench-top bioreactors, a production titer of 130 kg/m3 could be obtained after five to eight days of batch cultivation (Ciriminna et al. 2017). Because of its high productivity, A. niger dominated the market for CA production. However, the processes developed for CA production from molasses are multistage, are not environmentally friendly, and are being limited by the raw material sources used (Morgunov et al. 2013).
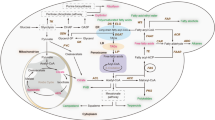
Overview of the principal metabolic pathways for organic acid synthesis in Y. lipolytica. GK: glycerokinase; GDH: Glyceraldehyde-3-phosphate dehydrogenase; PCK: Phosphoenolpyruvate carboxykinase; PYC: pyruvate carboxylase; PDC: pyruvate decarboxylase; ACS: acetyl-coenzyme A synthetase; ACH: acetyl-coenzyme A hydrolase; CIT: citrate synthetase; ACO: aconitase; IDH: isocitrate dehydrogenase; KGDH: αketoglutarate dehydrogenase; SCS: succinyl CoA synthetase; SDH: succinate dehydrogenase; FUM: fumarase; ACL: ATP citrate lyase; ICL: isocitrate lyase; MLS: malate synthase; PEP: phosphoenol pyruvate; CAD: cis-aconitate decarboxylase
In a study by Kamzolova and Morgunov, 43 different wild yeast isolates were evaluated for CA production (Kamzolova and Morgunov 2017). Among these, Y. lipolytica was found as the best CA producer on minimal medium with titer and yield of 85 g/L and 0.70 g/g, respectively (Kamzolova and Morgunov 2017). Based on this special ability and other biochemical characteristics, Y. lipolytica has been recognized as a potential host to produce Krebs cycle intermediates, including CA. In addition, cultivation conditions such as pH 4.5–5.0, temperature of 28 ℃, and glycerol concentration in culture medium from 20 to 80 g/L were found the optimal values for CA production (Morgunov et al. 2013). The main drawback of using Y. lipolytica to produce CA is the co-production of iso-CA (ICA) in high amounts (Cavallo et al. 2017). However, several authors have reported that nitrogen limitation during yeast growth could alleviate this disadvantage. Indeed, nitrogen starvation activates adenosine monophosphate (AMP) deaminase (Beopoulos et al. 2009a; b). This leads to a decrease in the mitochondrial AMP concentration that inhibits the iso-citrate dehydrogenase. This finally blocks the Krebs cycle at the ICA stage allowing accumulation of early metabolites in the mitochondria, such as CA (Fig. 1) (Beopoulos et al. 2009a; b). Compared to other limiting factors, nitrogen starvation yielded to high accumulation of CA. Indeed, upon limitation of nitrogen, phosphorus, or sulfur, CA could accumulate in the culture medium up to 18.0 g/L, 16.0 g/L, and 16.8 g/L, respectively (Morgunov et al. 2013).
Low pH value is considered as a limiting factor for CA production by Y. lipolytica wild-type strains (Tomaszewska et al. 2014). However, the engineered strain AJD pADUTGut1/2 was obtained by overexpression of GUT1 and GUT2 genes encoding, respectively, glycerol kinase (YALI0F00384g) and glycerol-3-phosphate dehydrogenase (YALI0B02948g) were able to produce CA efficiently from glycerol at pH 3 (Mironczuk et al. 2016). Initially, the aim of these modifications was to improve erythritol production (see below), which was indeed achieved when strain AJD pADUTGut1/2 was grown under high osmotic pressure (Mironczuk et al. 2016). However, in the absence of osmotic stress, CA becomes the main metabolite produced during culture. Moreover, it was shown that overexpression GUT1 alone significantly increases glycerol uptake rate, whereas in the strain overexpressing GUT2 gene, higher quantities of CA were obtained. This suggested that GUT2 could be crucial for CA production (Mironczuk et al. 2016). With strain Y. lipolytica AJD pADUTGut1/2, CA titer of 63.9 g/L was obtained at pH 3.0 which corresponds to a 14.5-fold increase as compared to the wild-type strain A101 under the same experimental conditions. On glycerol-based medium, a CA titer of 93 g/L was obtained at pH 6.0 (Mironczuk et al. 2016). The purification of glycerol is an expensive process, leading to CA production process unprofitable. As an economical alternative, crude glycerol, which is a direct byproduct of biodiesel production or saponification could be used. However, the utilization of such substrates could be limited by their high content of impurities such as salts, sodium hydroxide, methanol, or other organic compounds, which could be toxic for yeasts or hinder the production process. Despite these facts, Y. lipolytica can easily grow on such substrates and was found to produce CA with titer of 76 g/L at pH 3 (Rzechonek et al. 2018a). Overexpression of gene ICL1 encoding iso-citrate lyase led to a decrease in ICA production from 10–12 to 3–6% (Forster et al. 2007b). However, the overexpression of ICL1 did not influence the total production of CA and ICA. When ICL1 overexpression was coupled with the disruption of ACL1 gene (YALI0E34793g) encoding ATP citrate lyase and overexpression of the K. marxianus INU1 gene encoding inulinase, CA and ICA titers of 84.0 g/L and 1.8 g/L were obtained, respectively, from inulin (100 g/L) after 214 h of culture in a 2L scale bioreactor (Liu et al. 2013). Besides this, overexpression of genes encoding mitochondrial citrate carrier (YHM2, YALI0B10736g) and adenosine monophosphate deaminase (AMDP, YALI0E11495g) in Y. lipolytica wild-type strain W29, a CA titer with productivity and yield from glucose of 97.1 g/L, 0.93 g/(L h) and 0.5 g/g were obtained, respectively, during culture in bioreactor (Yuzbasheva et al. 2019).
The CA production by different Y. lipolytica wild-type strains has been also reported. With an acetate-negative mutant strain Y. lipolytica A101-1.22 grown on glycerol-containing waste from the biodiesel industry, a CA titer of 124.2 g/L was obtained in repeated batch culture (Rymowicz et al. 2010). From a screening study, strain K57 was found able to produce CA with titer of 72.1 g/L and yield of 0.77 g/g on glucose-based medium during batch culture (Carsanba et al. 2019).
Industrial wastes have been also considered as possible carbon substrates for CA production (Wojtatowicz et al. 1991; Imandi et al. 2008; Papanikolaou et al. 2008; Morgunov et al. 2013; Liu et al. 2014; Morgunov and Kamzolova 2015). Using mutant strain Y. lipolytica NG40/UV7 and glycerol-containing wastes, CA production was found to increase by 40.6% (122.2 g/L) as compared to that obtained with pure glycerol (Morgunov et al. 2013; Morgunov and Kamzolova 2015). Pineapple wastes were also used successfully with a production titer of 202 g/kg of waste in optimized conditions (namely, 0.34% yeast extract, 70.71% moisture content of the substrate, 0.64% KH2PO4, and 0.69% Na2HPO4) (Imandi et al. 2008). Olive-mill wastewater (OMW) blended with glucose was used also for CA production in nitrogen-limited conditions. The titer obtained was 28.9 g/L (Papanikolaou et al. 2008). With Y. lipolytica SWJ-1b grown in the presence of waste cooking oil (80 g/L), a CA titer of 31.7 g/L was obtained within 336 h of culture in a 10 L bioreactor (Liu et al. 2014). The highest CA concentration, 100 g/L, was obtained by the mutant strain A-101–1.14 following the shortest growth (24 h) and production (80 h) phases from potato starch (Wojtatowicz et al. 1991).
3.2 Iso-citric Acid
Iso-citric acid (ICA) exists in the form of four stereoisomers, of which only the threo-Ds-form is an intermediate of Krebs cycle (Kamzolova et al. 2016, 2018). A promising application of ICA is sports medicine. ICA has a marked energetic and anti-hypoxic effect and can be used as a physiological stimulant of sportsmen undergoing intensive long-term physical training (Kamzolova et al. 2016). Recently, ICA has been tested as a natural prophylactic and therapeutic agent. It has been shown efficient for the treatment of iron-deficiency anemia and in the resorption of blood clots (Morgunov et al. 2019).
At present, ICA is produced via chemical synthesis. However, this results in a racemic mixture of stereoisomers that cannot be separated by chemical methods. ICA can be found also in the leaves and stems of some plants from the Crassulaceae family or in fruits such as blackberry and blackcurrant. However, the purification of ICA from plant extracts or fruit juices that contain a wide range of organic acids and other components is a complicated and very expensive technological process. Therefore, the development of biotechnological methods for ICA production is extremely important. At present, the most promising method for ICA production is considered to be microbiological synthesis (Kamzolova et al. 2016; Laptev et al. 2017).
It is known that Y. lipolytica accumulates ICA and CA in the culture medium when cell growth is nutrients limited. Moreover, the ICA/CA ratio greatly depends on the carbon source used for cell growth. For example, wild-type strains of Y. lipolytica secrete mainly CA and about 8–16% ICA on carbohydrates or glycerol while approximately 50–65% CA and 35–50% ICA on substrates like alkanes, triglycerides, ethanol, or acetate. When using ethanol as a carbon source, an ICA proportion of 35–67% was found depending on the cultivation conditions (Kamzolova et al. 2016; Holz et al. 2009). Some authors have conducted several studies to increase the ICA production titer. The recombinant Y. lipolytica H222-S4 strain deleted for ICL1 gene, when grown on glycerol or glucose, showed only a smaller enhancement (by 2–5%) of ICA /CA ratio. With Y. lipolytica H222-S4 T5 that overexpress ICL1 gene, the relative ICA content in the medium was as low as 5–7% of the total acids (CA and ICA) (Forster et al. 2007a, b).
Y. lipolytica VKM Y-2723 and its mutant derivative 704-UV4-A/NG50 were selected from 60 yeast strains for their ability for ICA production. Under optimal culture conditions (i.e., iron concentration of 1.2 mg/L, temperature of 29 °C, pH 6.0, pO2 50–55% of saturation, and 30 mM itaconic acid), Y. lipolytica VKM Y-2373 produced 70.6 g/L ICA and 22.4 g/L CA with an ICA/CA ratio of 1:0.32 when grown on rapeseed oil. In similar conditions, a titer of 86 g/L ICA and 20 g/L CA with an ICA/CA ratio of 1:0.23 was obtained with Y. lipolytica 704-UV4-A/NG50 (Kamzolova et al. 2013). Under the above optimal culture conditions, Y. lipolytica VKM Y-2723 produced 90.5 g/L ICA with a yield of 0.77 g/g (Kamzolova et al. 2018). Also, catalytic activities of enzymes such as alcohol dehydrogenase, citrate synthase, aconitate hydratase, iso-citrate dehydrogenase, and iso-citrate lyase but not aldehyde dehydrogenase were found higher in a repeated-batch cultivation of 748 h than in batch cultivation. Therefore, under optimal repeated-batch culture using ethanol as substrate, Y. lipolytica strain VKM Y-2723 produced 109.6 g/L ICA with a production rate of 1.35 g/L/h (Morgunov et al. 2019). Besides this, overexpression of ACO1 (YALI0D09361g) encoding aconitase allowed to increase ICA titer (Laptev et al. 2017; Holz et al. 2009). For the wild-type strain H222 and H222-S4 grown on sunflower oil, the ICA proportion ranged between 35 and 49%, whereas it increased up to 71% with the ACO1 multi-copy transformant T1 without any modification in the total organic acid titer (both CA and ICA). However, ICA production was only moderately increased from 8–12% up to 13–17% with carbon sources such as glucose, sucrose, and glycerol (Holz et al. 2009). When ACO1 was expressed in multicopy, ICA and CA titers were, respectively, of 72.6 g/L and 29.0 g/L with an ICA/CA ratio of 2.5:1 during culture in 10L bioreactor in a rapeseed oil-based medium (Laptev et al. 2017).
3.3 Succinic Acid
As an almost ubiquitous metabolite in many organisms, succinic acid (SA) is an intermediate of the Krebs cycle. Due to its numerous potential applications, SA has been recognized as one of the most important and high value-added bio-based building block chemicals by the US Department of Energy (DOE) (Beauprez et al. 2010; Ahn et al. 2016). In 2004 and 2010, the US DOE reported SA as one of the five most promising bio-based platform chemicals. SA market worldwide is forecasted to grow at a compound annual growth rate (CAGR) of 15.7% between 2020 and 2026 (Ahn et al. 2016; Tan et al. 2014, 2020a). Among all the platform chemicals, SA is currently used as a surfactant, ion chelator, additive in agricultural and food, and pharmaceutical industries. It can be used as a precursor to synthesize γ-butyrolactone, 1,4-butanedioic acid, tetrahydrofuran, and other value-added chemicals (Ahn et al. 2016; Tan et al. 2014; McKinlay et al. 2007). Currently, succinate is produced mainly from petroleum-derived maleic anhydride. However, due to near future shortages of petroleum resources, severe environmental concerns related to chemical synthesis processes and to reach an economical bio-based production of SA, extensive research works have been focusing on the development of efficient microbial strains by metabolic engineering as well as optimized fermentation and downstream processes (Ahn et al. 2016; Tan et al. 2013). Besides, bio-based SA production technologies can reduce greenhouse gas emissions by 50% and energy demand by 30–40% as compared to the chemical production process (Tan et al. 2014).
By contrast to prokaryotes, some yeasts, such as Y. lipolytica, are highly tolerant to low pH, rendering them attractive as an industrial host for SA synthesis (Cui et al. 2017). A Y. lipolytica evolved strain, named PSA3.0, which can produce SA at low pH using glucose as substrate was selected from a long-lasting culture in in situ fibrous bed bioreactor (isFBB) (Li et al. 2018a). Strain PSA3.0 produced SA with a titer of 18.4 g/L and yield of 0.23 g/g at pH 3.0. These values are, respectively, 4.8 and 4.6-fold higher than those obtained with the parental strain PSA02004 at pH 3.0. Using fed-batch culture in bioreactor, a SA titer of 76.8 g/L was obtained, which is the highest value ever achieved from a glucose-based medium at low pH (Li et al. 2018a). Furthermore, Y. lipolytica PSA02004 produces 33.2 g/L SA with a yield of 0.58 g/g and productivity of 0.33 g/L/h from sugarcane bagasse hydrolysates. Using glucose-rich fruit and vegetable waste (FVW) hydrolysates, SA titer of 140.6 g/L with a productivity of 0.69 g/L/h has been obtained (Li et al. 2018b; Ong et al. 2019). Under optimal conditions of repeated-batch fermentation, SA titer of 55.3 g/L and the maximal productivity 2.6 g/L/h was reached with Y. lipolytica strain VKPM Y3753 (Bondarenko et al. 2016).
An increase in SA titer was also achieved by replacing the native promoter of the succinate dehydrogenase by strong promoters (Cui et al. 2017; Yuzbashev et al. 2010; Jost et al. 2014; Gao et al. 2016; Li et al. 2016, 2017; Yang et al. 2017). Under oxygen limitation, Y. lipolytica strain H222-AZ2 obtained by exchange of the native promoter of the succinate dehydrogenase subunit 2 encoding gene by the inducible promoter POT1, a SA productivity of 0.152 g/L/h and titer of 25 g/L after 165 h of culture in glycerol medium were obtained (Jost et al. 2014). With Y. lipolytica strain PGC01003, deleted for Ylsdh5 genes encoding succinate dehydrogenase subunit 5, SA titer of 43 g/L was obtained during batch culture in 2.5 L bioreactor in medium containing crude glycerol. With a fed-batch strategy, the strain produced 160 g/L SA (Gao et al. 2016). After 21 days of evolution to enable the strain PGC01003 to catabolize glucose, the evolved strain produced 65.7 and 87.9 g/L SA using YPD medium and food waste hydrolysate, respectively (Yang et al. 2017).
Enhanced SA produced was also achieved by genetic modifications of the strains in succinate dehydrogenase or fermentation technology innovation. For example, when the native promoter of SDH2 was replaced by an inducible promoter of POT1, SA titer at 25 g/L with productivity at 0.15 g/L/h was obtained by Y. lipolytica H222-AZ2 from glycerol under oxygen limitation (Jost et al. 2014) After inactivation of SDH5 that encodes succinate dehydrogenase subunit 5, SA titer at 43 g/L was obtained by batch fermentation from crude glycerol By PGC01003, and a much higher titer was achieved at 160 g/L SA via fed-batch fermentation strategy (Gao et al. 2016). Based on this, an in-situ fibrous bed bioreactor (isFBB) which could improve the initial cell density was developed, and SA titer at 198.2 g/L with average productivity of 1.46 g/L/h was achieved by PGC01003 via fed-batch strategy (Li et al. 2016). This value was further improved to 209.7 g/L when the immobilization matrix in isFBB was replaced by the more porous material (e.g., sugarcane bagasse), which is the highest value ever reported (Li et al. 2017). However, it was reported that the accumulation of acetate was a major factor that impeded the fermentation at low pH, resulting in an obvious increase in downstream process cost (Cui et al. 2017). In order to solve this problem, the gene encoding the acetyl-coenzyme A hydrolase (ACH1) was deleted, and PCK from S. cerevisiae encodes phosphoenolpyruvate carboxykinase together with SCS2 encoding endogenous succinyl CoA synthetase were overexpressed in PGC01003 to achieve the strain PGC202. As result, SA titer at 110.7 g/L with a yield of 0.53 g/g and productivity of 0.8 g/L/h was obtained by this strain via fed-batch fermentation with pH without control (final pH at 3.4) (Cui et al. 2017).
Nevertheless, all the metabolic evolution of Y. lipolytica for SA production has led to a partial or total loss of its ability to grow in glucose-based medium, which limits its industrial application (Yang et al. 2017). In this case, a strategy termed metabolic engineering was applied by Yang et al. to obtain a glucose-consuming Y. lipolytica (PSA02004) after a 21-day repeated fermentation of PGC01003 (Yang et al. 2017). As a result, the evolved strain PSA02004 could produce SA at 65.7 g/L and 87.9 g/L from the YPD medium and food waste hydrolysate at pH 6.0, respectively. Interestingly, with the help of isFBB, the pH for the cultivation of PSA02004 could be decreased to a level lower than 3.0 gradually by metabolic evolution, and the evolved strain named Y. lipolytica PSA3.0 that could produce SA with a titer of 19.3 g/L, productivity of 0.52 g/L/h, and yield of 0.29 g/g at pH 3.0 from YPD was achieved (Li et al. 2018a). The enzyme activity analysis demonstrated that the pathway from pyruvate to acetate was partially blocked in Y. lipolytica PSA3.0 after the evolution, which is beneficial to cell growth and SA production at low pH.
However, as for PGC01003, the accumulation of acetate is a limiting factor for further improvement of SA production as SA recovery request a pH adjustment leading to an increase of downstream process cost (Cui et al. 2017). To solve this issue, a new recombinant strain, named PGC202, was deleted for gene ACH1 (YALI0E30965g) encoding the acetyl-coenzyme A hydrolase and overexpressing PCK gene from Saccharomyces cerevisiae encoding phosphoenolpyruvate carboxykinase together with gene SCS2 encoding endogenous succinyl CoA synthetase was constructed. It allowed improving the SA production process through the elimination of acetic acid overflow and by-products formation. Indeed, SA titer of 110.7 g/L with a maximum yield from glycerol of 0.53 g/g and a productivity of 0.8 g/L/h were obtained during fed-batch fermentation of strain PGC202 (Cui et al. 2017). Furthermore, the strain produces 31.7 g/L SA with a yield of 0.52 g/g and productivity of 0.60 g/L/h in isFBB fermentation when using glucose-containing MFWs hydrolysate as the carbon source supplemented with 3% of tryptone (Li et al. 2019).
3.4 Itaconic Acid
Itaconic acid (IA) is naturally produced by several Aspergillus species (Blazeck et al. 2015). The industrially versatile usability of IA and its derivatives are reflected in the wide range of applications such as in plastics, styrene-butadiene rubber, synthetic latex, super-absorbent polymers, unsaturated polyester resins, and detergents. The field of application of these products is widespread and ranges from paint, lacquer, paper industries, hygiene, and medical products as well as in the construction sector (Kuenz and Krull 2018). Thus, IA was recognized as one of 12 value-added chemicals from biomass by the US DOE in 2004 (Blazeck et al. 2015). IA market is estimated at US$28.4 million in the year 2020 in the USA while the world market is expected to reach $129.3 million by 2027 due to increasing demand for bio-based chemicals (2020b). To date, two strategies have been developed to produce IA, a chemical method based on the pyrolysis of citric acid and a biosynthesis process that relies on the decarboxylation of cis-aconitic acid by microorganisms (Kuenz and Krull 2018; Zhao et al. 2019).
Although current industrial production of IA is based on A. terreus fermentation allowing titer higher than 80 g/L, A. terreus suffers from poor growth in optimal media used for IA production and is negatively affected by shear stress in the bioreactor, thus precluding fermentations under conventional conditions (Blazeck et al. 2015). Moreover, a complex and expensive downstream process has been developed at an industrial scale (Kuenz and Krull 2018; Okamoto et al. 2014; Bellasio et al. 2015; Chin et al. 2015; Otten et al. 2015). In order to address these concerns, Y. lipolytica was considered as an alternative IA production host as this organism can natively grow at low pH and sustains a high flux toward the citric acid cycle (Blazeck et al. 2015). There are currently two reports on engineered Y. lipolytica strains used to produce IA. In the study of Blazeck et al., the native cis-aconitate decarboxylase (CAD) gene was overexpressed, and the resulting strain produced IA with a titer of 33 mg/L (Blazeck et al. 2015). Further strain optimizations of the metabolic pathway, enzyme localization, and media optimization strategies enabled IA titer of 4.6 g/L during culture in bioreactors, representing a 140-fold improvement. A recombinant strain which overexpresses genes encoding the mitochondrial cis-aconitate transporter MTT and CAD allowed an IA titer of 22.0 g/L in optimized conditions (Zhao et al. 2019). This represents a 60-fold improvement over the initial titer (0.36 g/L).
3.5 α-ketoglutaric Acid
α-ketoglutaric acid (α-KG), an important dicarboxylic acid, is an intermediate of the Krebs cycle. It is also involved in amino acid metabolism and has an important role in the regulation of the balance between carbon and nitrogen metabolism in many microorganisms. α-KG is widely applied in the industrial scope, e.g., as a building block for the chemical synthesis of heterocycles, dietary supplement, component of infusion solutions, and wound healing compounds (Otto et al. 2011; Yovkova et al. 2013). Currently, α-KG is synthesized by chemical processes or biosynthesis at an industrial scale. The main chemical routes utilized succinic acid and oxalic acid diethyl esters or relied on the oxidation of glyoxylic acid with sodium glutamate using copper as a catalyst. The drawbacks of these chemical routes are a lack of selectivity, a low yield, high risk from manipulation of harsh chemicals, and the generation of environmental hazards. These drawbacks sharply increased the downstream process cost and restricted the utilization of α-KG in food, medicine, and cosmetics applications.
Y. lipolytica is unable to synthesize the pyrimidine structure of the thiamine molecule. Thiamine is the cofactor of α-ketoglutarate dehydrogenase (KGDH) which is a key enzyme in the metabolism of α-KG; thus, the limitation of thiamine availability can reduce the activity of α-ketoglutarate dehydrogenase of the Krebs cycle. Under conditions of thiamine deficiency, the conversion of α-KG in the Krebs cycle is inhibited, which leads to its accumulation in the fermentation broth (Chernyavskayaá et al. 2000; Kamzolova et al. 2012; Yin et al. 2012).
Currently, different strains producing α-KG from different carbon sources have been reported. Y. lipolytica H355 allows α-KG titer of 195 g/L with a productivity of 1.3 g/L/h in the medium containing a mixture of n-alkanes (Guo et al. 2016b). Under optimal conditions, Y. lipolytica VKM Y-2412 produced 172 g/L of α-KG with a yield of 0.70 g/g and 102.5 g/L α-KG with the yield of 0.95 g/g from ethanol and rapeseed oil, respectively (Kamzolova et al. 2012; Kamzolova and Morgunov 2013). However, in order to overcome the disadvantages of low yield and accumulation of byproducts, several approaches including metabolic engineering strategies and different fermentation configurations have been investigated (Guo et al. 2016a). Y. lipolytica H355A that overexpress the iso-citrate dehydrogenase (IDH1) and pyruvate carboxylase (PYC1) genes can produce 186 g/L α-KG from raw glycerol. This represents a 19% increased production as compared to the control strain H355. Y. lipolytica-RoPYC2 which overexpress RoPYC2 gene encoding pyruvate carboxylase produced α-KG with a titer of 62.5 g/L with only 13.5 g/L pyruvate (PA). As compared to the parental strain, α-KG production in strain RoPYC2 increased by 35.3% while PA production is reduced by 69.8% (Yovkova et al. 2013; Yin et al. 2012).
4 Polyols
In some biological systems, polyols are obtained by the reduction of their keto or aldo groups into hydroxyl groups (Rice et al. 2019). As polyols, Y. lipolytica synthesize mainly mannitol (MAN), which is suggested to provide cofactor NADPH for fatty acid synthesis and erythritol (EOL). The latter being produced in response to osmotic stress. Derivatives of erythritol, namely erythrulose (EOSE) and threitol (TOL), are also of industrial interest and will be also discussed below (Table 2).
4.1 Mannitol
MAN is a six-carbon alcohol involved in stress tolerance in microorganisms notably in the scavenging of reactive oxygen species (ROS) (Zhang et al. 2018; Sekova et al. 2019). A possible role of MAN in fatty acid metabolism has been also reported (Dulermo et al. 2015). MAN production has been investigated for several Y. lipolytica strains and different experimental conditions. For cells grown in shake-flasks in the presence of crude glycerol, mannitol was co-produced with EOL with titers ranging from 2.6 to 14.9 g/L. During glycerol fed-batch bioreactor culture, strains A-15 and A UV’1 synthetized MAN with a titer of, respectively, 41.4 and 38.1 g/L, corresponding to the productivity of 0.29 and 0.28 g/(L h) (Tomaszewska et al. 2012). With a derivative of strain AIB overexpressing GUT1 gene encoding glycerol kinase grown in a batch bioreactor on a mixture of molasse and crude glycerol, maximal MAN titer of 11 g/L was obtained (Rakicka et al. 2017a). Similarly, using a mixture of crude glycerol and olive oil mill wastewater, the MAN titer reached 13 g/L within 140 h with a yield from glycerol of 0.21 g/g (Sarris et al. 2019).
4.2 Erythritol
Erythritol (EOL) is a four-carbon polyol. Its industrial interest relies on its sweetening property as it has the texture and taste of table sugar. EOL has also other interesting properties regarding its application. It is not metabolized by the human body, and thus, it is calories-free and does not modify glycemia (Carly and Fickers 2018). It could be synthesized by several osmotolerant microorganisms, including Y. liploytica and Candida magnoliae, as an osmoprotectant. In Y. lipolytica, EOL derives from erythrose-4P, an intermediate of the pentose phosphate pathway (PPP) (Fig. 2). The latter is dephosphorylated by an erythrose-4P phosphatase (E4PP) before being reduced by an erythrose reductase (ER) into erythritol. In this yeast, genes coding for erythrose reductase have been characterized (namely, YALI0F18590g, YALI0D07634g, and YALI0C13508g) (Janek et al. 2017; Cheng et al. 2018). The complete EOL catabolic pathway has been also reported recently (Niang et al. 2020). It is first converted into erythrulose by an erythritol dehydrogenase (EYD1, YALI0F01650g) and subsequently phosphorylated into L-erythrulose-1P by an erythrulose kinase (EYK1, YALI0F01606g). Then, L-erythrulose-1P is isomerized into D-erythrulose-4P by an erythrulose-1P isomerase (EYL1, YALI0F01584g). Finally, erythrose-4P is generated by the activity of an erythrulose-4P isomerase (EYL2, YALI0F01628g).
Overview of the principal metabolic pathways for polyols and derivatives synthesis in Y. lipolytica. DHAP: dihydroxyacetone-P; E4PP: Erythrose 4P phosphatase; ER: erythrose reductase; EDH: erythritol dehydrogenase; EK: erythritol kinase; E1PI: erythrulose-1P isomerase; E4PI: erythrulose-4P isomerase; FBA: fructose-bisphosphate aldolase; F6P-P: Fructose-6P phosphatase; GK: glycerol kinase; G3PDH: glycerol-3P dehydrogenase; GPI: glucose-6P isomerase; G6PDH: glucose-6P dehydrogenase; HK: hexokinase; MDH: mannitol dehydrogenase; PFK: phosphofructokinase; PGDH: phopshogluconate dehydrogenase; RPE: Ribulose-P3 epimerase; R5PI: ribose-5P isomerase; TK: transketolase; TIM: triose isomerase; TAL: transaldolase; 6PGL: 6-phosphonogluconolactonase, Ss-XDH: xylitol dehydrogenase from Scheffersomyces stipites. Pathway in italics is heterologous
Several review papers focusing on EOL have been published, and only the most striking information will be reported below (Carly and Fickers 2018; Regnat et al. 2018; Rzechonek et al. 2018b). An efficient strategy for EOL synthesis consisted of constitutively expressing genes encoding erythrose reductase (ER). Overexpression of ER gene YALI0F18590g in Y. lipolytica strain AMM led to a 20% increase in EOL titer as compared to the parental strain (44.4 g/L) (Janek et al. 2017). This corresponded to the productivity of 0.77 g/(L h) and a yield from glycerol of 0.44 g/g. More recently, two additional ERs have been characterized (YALI0D07634g and YALI0C13508g) (Cheng et al. 2018). Upon overexpression of these three ERs under the control of the strong constitutive promoter hp4d, an EOL titer of 178 g/L was obtained from an initial glucose concentration of 300 g/L within 84 h. This corresponded to productivity and yield of 2.1 g/(L h) and 0.59 g/g, respectively. As ERs are NADPH-dependent enzymes, the authors engineered the co-factor metabolism by overexpressing genes YALI0B15598g and YALI0E22694g encoding 6-phosphogluconate dehydrogenase (GND1) and glucose-6P dehydrogenase (ZWF1), respectively. The corresponding enzymes are known to generate NADPH from NADP+. The resulting strain, HCY108, produced EOL with a titer of 190 g/L within 80 h of culture with productivity and yield from the glucose of 2.4 g/(L h) and 0.63 g/g, respectively (Cheng et al. 2018). Other genes from the PPP pathway have been also overexpressed to increase EOL titer. Overexpression of gene YALI0E06479g encoding transketolase (TKL1) in strains Po1d and MK1 yielded a 19% (43 g/L) and 51% (51 g/L) increased EOL titer, respectively. This corresponded to a productivity of 0.04 and 0.05 g/(gDCW h) (Carly et al. 2017b; Mirończuk et al. 2017). By coexpression of genes YALI0E06479g (TKL1) and YALI0F15587g encoding transaldolase (TAL1), an increase in EOL titer and productivity of 45% and 46% was obtained, respectively (46.7 g/L and 0.5 g/(L h) (Mirończuk et al. 2017). Gene involved in carbon source catabolism were also overexpressed to feed the PPP pathway with precursors (i.e., fructose-6P and glyceraldehyde-3P). Overexpression of genes YALI0F00384g (GUT1) and YALI0B02948g (GUT2) encoding, respectively, glycerol kinase (GK) and glycerol-3-P dehydrogenase (G3P-DH) allowed an EOL titer of 78 g/L from an initial glycerol concentration of 100 g/L within 72 h in a 5-L bioreactor (Mirończuk et al. 2016). Besides this, overexpression of genes GUT1 and TKL1 in a strain disrupted for gene EYK1 allowed an erythritol titer, productivity, and yield from glycerol of 80 g/L, 1.03 g/(L h), and 0.53 g/g, respectively (Carly et al. 2017b). By overexpressing invertase encoding gene SUC2 from Saccharomyces cerevisiae and native GUT1 gene, an EOL titer of 100 g/L with a productivity and yield of 1.1 g/(L h) and 0.67 g/g, respectively, were obtained from a mixture of raw industrial molasses (60 g/L) and crude glycerol (100 g/L) (Rakicka et al. 2017b). Disruption of gene SNF1 (YALI0D02101g) coding for a regulator of lipid accumulation allowed an EOL production of 185.4 mg/g in solid-state fermentation (SSF) using peanut press cake mixed with 40% sesame meal and 10% waste cooking oil as substrate (Li et al. 2019). EOL production in large-scale bioreactor was recently reported. Using raw glycerol feeding, strain Y. lipolytica Wratislavia K1 produced EOL with titer and yield from glycerol of 180 g/L and 0.53 g/g, respectively, after 144 h of cultivation in 500 L bioreactor (Rakicka-Pustułka et al. 2020). With metabolically engineered Y. lipolytica strain HCY118, a production titer of 196 g/L with productivity and yield from glucose of 2.51 g/L h and 0.65 g/g, respectively, were obtained in a 30 m3 bioreactor within 78 h (Wang et al. 2020).
4.3 Erythrulose
As mentioned already, erythrulose (EOSE) is an intermediate of the erythritol catabolic pathway. It has many applications in cosmetics as a sunless tanning agent and in chemistry as a precursor of several drugs such as anticancer (Bengamide E), antifungal (Tanikolide), substitute β-lactam, cytokine modulator (Cytoxazone), or cholesterol-lowering drugs (Crestor, Zetia) (Carly et al. 2018). In a Y. lipolytica Δeyk1 derivative (RIY210), the conversion of EOSE into L-erythrulose-1P is impaired. Therefore, such a mutant strain can convert EOL into EOSE. The conversion rate could be increased by the overexpression of EYD1 encoding erythritol dehydrogenase in a Δeyk1 genetic background. With such a strategy, an efficient fed-batch bioreactor process was developed to convert EOL into EOSE with a conversion rate and yield of 0.116 g/g DCW·h and 0.64 g/g, respectively (Carly et al. 2017a).
4.4 Threitol
Threitol (TOL) is a diastereoisomer of erythritol that is produced naturally as an antifreeze molecule by the fungus Armillaria mellea as well as the Alaskan beetle Upis ceramboides. TOL has application as a precursor for the synthesis of anticancer drugs (treosulfan and threitol ceramide). It is also a constitutive element of oxygen-sensitive pigments incorporated in a smart plastic film used for food packaging (Chi et al. 2019). Recently, xylitol dehydrogenase (Ss-XDH) from Scheffersomyces stipites CBS6054 was found able to oxidize EOL into EOSE irreversibly and then reduce EOSE into threitol (Chi et al. 2019). By overexpression of the corresponding gene in the Y. lipolytica strain CGMCC7326, a good producer of EOL from glucose, a TOL production titer of 112 g/L with a yield from the glucose of 0.37 g/g has been reported (Chi et al. 2019).
5 Conclusion and Prospects
Due to its potential for organic acids and polyols production and good tolerance to low pH, Y. lipolytica has gradually become a cell factory for their production. However, several key steps allowing to increase strain production titer and productivity remain to be identified. Process operations are still not optimal, especially on a large scale. Further investigations must be made to obtain cost-effective processes for most of the metabolites described herein.
References
Abdel-Mawgoud AM, Markham KA, Palmer CM, Liu N, Stephanopoulos G, Alper HS (2018) Metabolic engineering in the host Yarrowia lipolytica. Metab Eng 50:192–208. https://doi.org/10.1016/j.ymben.2018.07.016
Ahn JH, Jang YS, Lee SY (2016) Production of succinic acid by metabolically engineered microorganisms. Curr Opinion Biotechnol 42:54–66. https://doi.org/10.1016/j.copbio.2016.02.034
Barth G, Gaillardin C (1996) Yarrowia lipolytica. In: Wolf K (ed) Nonconventional yeasts in biotechnology: a handbook. Springer, Heidelberg, pp 313–388
Beauprez JJ, De Mey M, Soetaert WK (2010) Microbial succinic acid production: natural versus metabolic engineered producers. Proc Biochem 45:1103–1114. https://doi.org/10.1016/j.procbio.2010.03.035
Bellasio M, Mattanovich D, Sauer M, Marx H (2015) Organic acids from lignocellulose: Candida lignohabitans as a new microbial cell factory. J Ind Microbiol Biotechnol 42:681–691. https://doi.org/10.1007/s10295-015-1590-0
Beopoulos A, Cescut J, Haddouche R, Uribelarrea JL, Molina-Jouve C, Nicaud JM (2009) Yarrowia lipolytica as a model for bio-oil production. Prog Lipid Res 48:375–387. https://doi.org/10.1016/j.plipres.2009.08.005
Beopoulos A, Chardot T, Nicaud JM (2009) Yarrowia lipolytica: a model and a tool to understand the mechanisms implicated in lipid accumulation. Biochimie 91:692–696. https://doi.org/10.1016/j.biochi.2009.02.004
Bilal M, Xu S, Iqbal HMN, Cheng H (2020) Yarrowia lipolytica as an emerging biotechnological chassis for functional sugars biosynthesis. Crit Rev Food Sci Nutr 1–18. https://doi.org/10.1080/10408398.2020.1739000
Bio-Succinic Acid Market Size is Expected to Grow at a CAGR of 15.7% - Valuates Reports (2020a). https://www.prnewswire.com/news-releases/bio-succinic-acid-market-size-is-expected-to-grow-at-a-cagr-of-15-7---valuates-reports-301116323.html
Blazeck J, Hill A, Jamoussi M, Pan A, Miller J, Alper HS (2015) Metabolic engineering of Yarrowia lipolytica for itaconic acid production. Metab Eng 32:66–73. https://doi.org/10.1016/j.ymben.2015.09.005
Bondarenko PY, Fedorov AS, Sineoky SP (2016) Optimization of repeated-batch fermentation of a recombinant strain of the yeast Yarrowia lipolytica for succinic acid production at low pH. Appl Biochem Microbiol 53:882–887. https://doi.org/10.1134/s0003683817090022
Carly F, Fickers P (2018) Erythritol production by yeasts: a snapshot of current knowledge. Yeast 35:455–463. https://doi.org/10.1002/yea.3306
Carly F, Gamboa-Melendez H, Vandermies M, Damblon C, Nicaud JM, Fickers P (2017) Identification and characterization of EYK1, a key gene for erythritol catabolism in Yarrowia lipolytica. Appl Microbiol Biotechnol 101:6587–6596. https://doi.org/10.1007/s00253-017-8361-y
Carly F, Steels S, Telek S, Vandermies M, Nicaud J-M, Fickers P (2018) Identification and characterization of EYD1, encoding an erythritol dehydrogenase in Yarrowia lipolytica and its application to bioconvert erythritol into erythrulose. Bioressour Technol 247:963–969. https://doi.org/10.1016/j.biortech.2017.09.168
Carly F, Vandermies M, Telek S, Steels S, Thomas S, Nicaud J-M, Fickers P (2017) Enhancing erythritol productivity in Yarrowia lipolytica using metabolic engineering. Metab Eng 42:19–24. https://doi.org/10.1016/j.ymben.2017.05.002
Carsanba E, Papanikolaou S, Fickers P, Erten H (2019) Screening various Yarrowia lipolytica strains for citric acid production. Yeast 36:319–327. https://doi.org/10.1002/yea.3389
Cavallo E, Charreau H, Cerrutti P, Foresti ML (2017) Yarrowia lipolytica: a model yeast for citric acid production. FEMS Yeast Res 17:1–16. https://doi.org/10.1093/femsyr/fox084
Celińska E, Ledesma-Amaro R, Larroude M, Rossignol T, Pauthenier C, Nicaud J-M (2017) Golden gate assembly system dedicated to complex pathway manipulation in Yarrowia lipolytica. Microb Biotechnol 10:450–455. https://doi.org/10.1111/1751-7915.12605
Cheng H, Wang S, Bilal M, Ge X, Zhang C, Fickers P, Cheng H (2018) Identification, characterization of two NADPH-dependent erythrose reductases in the yeast Yarrowia lipolytica and improvement of erythritol productivity using metabolic engineering. Microb Cell Fact 17:133. https://doi.org/10.1186/s12934-018-0982-z
Chernyavskayaá OGC, Shishkanova NV, Il’chenko AP, Finogenova TV (2000) Synthesis of alpha-ketoglutaric acid by Yarrowia lipolytica yeast grown on ethanol. Appl Microbiol Biotechnol 53:152–158
Chi P, Wang S, Ge X, Bilal M, Fickers P, Cheng H (2019) Efficient D-threitol production by an engineered strain of Yarrowia lipolytica overexpressing xylitol dehydrogenase gene from Scheffersomyces stipitis. Bioch Eng J 149. https://doi.org/10.1016/j.bej.2019.107259
Chin T, Sano M, Takahashi T, Ohara H, Aso Y (2015) Photosynthetic production of itaconic acid in Synechocystis sp. PCC6803. J Biotechnol 195:43–45, 107259. https://doi.org/10.1016/j.jbiotec.2014.12.016
Ciriminna R, Meneguzzo F, Delisi R, Pagliaro M (2017) Citric acid: emerging applications of key biotechnology industrial product. Chem Cent J 11:22–31. https://doi.org/10.1186/s13065-017-0251-y
Cui Z, Gao C, Li J, Hou J, Lin CSK, Qi Q (2017) Engineering of unconventional yeast Yarrowia lipolytica for efficient succinic acid production from glycerol at low pH. Metab Eng 42:126–133. https://doi.org/10.1016/j.ymben.2017.06.007
Do HD, Vandermies M, Fickers P, Theron CW (2019) Non-conventional yeast species for recombinant protein and metabolite production. Ref Mod Life Sci. Elsevier
Dujon B, Sherman D, Fischer G, Durrens P, Casaregola S, Lafontaine I, De Montigny J, Marck C, Neuvéglise C, Talla E, Goffard N, Frangeul L, Aigle M, Anthouard V, Babour A, Barbe V, Barnay S, Blanchin S, Beckerich JM, Beyne E, Bleykasten C, Boisramé A, Boyer J, Cattolico L, Confanioleri F, De Daruvar A, Despons L, Fabre E, Fairhead C, Ferry-Dumazet H, Groppi A, Hantraye F, Hennequin C, Jauniaux N, Ph, Joyet, Kachouri R, Kerrest A, Koszul R, Lemaire M, Lesur I, Ma L, Muller H, Nicaud JM, Nikolski M, Oztas S, Ozier-Kalogeropoulos O, Pellenz S, Potier S, Richard GF, Straub ML, Suleau A, Swennen D, Tekaia F, Wésolowski-Louvel M, Westhof E, Wirth B, Zeniou-Meyer M, Zivanovic I, Bolotin-Fukuhara M, Thierry A, Ch, Bouchier, Caudron B, Scarpelli C, Gaillardin C, Weissenbach J, Wincker P, Souciet JL (2004) Genome evolution in yeasts. Nature 430:35–44. https://doi.org/10.1038/nature02579
Dulermo T, Lazar Z, Dulermo R, Rakicka M, Haddouche R, Nicaud J-M (2015) Analysis of ATP-citrate lyase and malic enzyme mutants of Yarrowia lipolytica points out the importance of mannitol metabolism in fatty acid synthesis. Biochim Biophys Acta 1851:1107–1117. https://doi.org/10.1016/j.bbalip.2015.04.007
Fickers P, Benetti PH, Waché Y, Marty A, Mauersberger S, Smit MS, Nicaud JM (2005) Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res 5:527–543. https://doi.org/10.1016/j.femsyr.2004.09.004
Fickers P, Cheng H, Sze Ki Lin C (2020) Sugar alcohols and organic acids synthesis in Yarrowia lipolytica: Where are we? Microorganisms 8:574. https://doi.org/10.3390/microorganisms8040574
Fickers P, Le Dall MT, Gaillardin C, Ph, Thonart, Nicaud JM (2003) New disruption cassettes for rapid gene disruption and marker rescue in the yeast Yarrowia lipolytica. J Microbiol Methods 55:727–737
Forster A, Aurich A, Mauersberger S, Barth G (2007) Citric acid production from sucrose using a recombinant strain of the yeast Yarrowia lipolytica. Appl Microbiol Biotechnol 75:1409–1417. https://doi.org/10.1007/s00253-007-0958-0
Forster A, Jacobs K, Juretzek T, Mauersberger S, Barth G (2007) Overexpression of the ICL1 gene changes the product ratio of citric acid production by Yarrowia lipolytica. Appl Microbiol Biotechnol 77:861–869. https://doi.org/10.1007/s00253-007-1205-4
Gao C, Yang X, Wang H, Rivero CP, Li C, Cui Z, Qi Q, Lin CSK (2016) Robust succinic acid production from crude glycerol using engineered Yarrowia lipolytica. Biotechnol Biofuels 9:179–190. https://doi.org/10.1186/s13068-016-0597-8
Global Citric Acid Markets Report, 2011–2018 & 2019–2024 (2019) https://www.prnewswire.com/news-releases/global-citric-acid-markets-report-2011-2018--2019-2024-300814817.html
Global Itaconic Acid (IA) Industry (2020) https://www.reportlinker.com/p03646033/Global-Itaconic-Acid-IA-Industry.html?utm_source=PRN
Groenewald M, Boekhout T, Neuvéglise C, Gaillardin C, van Dijck P, Wyss M (2014) Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit Rev Microbiol 40:187–206. https://doi.org/10.3109/1040841X.2013.770386
Guo H, Madzak C, Du G, Zhou J (2016) Mutagenesis of conserved active site residues of dihydrolipoamide succinyltransferase enhances the accumulation of alpha-ketoglutarate in Yarrowia lipolytica. Appl Microbiol Biotechnol 100:649–659. https://doi.org/10.1007/s00253-015-6995-1
Guo H, Su S, Madzak C, Zhou J, Chen H, Chen G (2016) Applying pathway engineering to enhance production of alpha-ketoglutarate in Yarrowia lipolytica. Appl Microbiol Biotechnol 100:9875–9884. https://doi.org/10.1007/s00253-016-7913-x
Holz M, Forster A, Mauersberger S, Barth G (2009) Aconitase overexpression changes the product ratio of citric acid production by Yarrowia lipolytica. Appl Microbiol Biotechnol 81:1087–1096. https://doi.org/10.1007/s00253-008-1725-6
Imandi SB, Bandaru VV, Somalanka SR, Bandaru SR, Garapati HR (2008) Application of statistical experimental designs for the optimization of medium constituents for the production of citric acid from pineapple waste. Bioresour Technol 99:4445–4450. https://doi.org/10.1016/j.biortech.2007.08.071
Janek T, Dobrowolski A, Biegalska A, Mirończuk AM (2017) Characterization of erythrose reductase from Yarrowia lipolytica and its influence on erythritol synthesis. Microb Cell Fact 16:118. https://doi.org/10.1186/s12934-017-0733-6
Jost B, Holz M, Aurich A, Barth G, Bley T, Muller RA (2014) The influence of oxygen limitation for the production of succinic acid with recombinant strains of Yarrowia lipolytica. Appl Microbiol Biotechnol 99:1675–1686. https://doi.org/10.1007/s00253-014-6252-z
Kamzolova SV, Allayarov RK, Lunina JN, Morgunov IG (2016) The effect of oxalic and itaconic acids on threo-Ds-isocitric acid production from rapeseed oil by Yarrowia lipolytica. Bioresour Technol 206:128–133. https://doi.org/10.1016/j.biortech.2016.01.092
Kamzolova SV, Chiglintseva MN, Lunina JN, Morgunov IG (2012) alpha-Ketoglutaric acid production by Yarrowia lipolytica and its regulation. Appl Microbiol Biotechnol 96:783–791. https://doi.org/10.1007/s00253-012-4222-x
Kamzolova SV, Dedyukhina EG, Samoilenko VA, Lunina JN, Puntus IF, Allayarov RL, Chiglintseva MN, Mironov AA, Morgunov IG (2013) Isocitric acid production from rapeseed oil by Yarrowia lipolytica yeast. Appl Microbiol Biotechnol 97:9133–9144. https://doi.org/10.1007/s00253-013-5182-5
Kamzolova SV, Morgunov IG (2017) Metabolic peculiarities of the citric acid overproduction from glucose in yeasts Yarrowia lipolytica. Bioresour Technol 243:433–440. https://doi.org/10.1016/j.biortech.2017.06.146
Kamzolova SV, Morgunov IG (2013) alpha-Ketoglutaric acid production from rapeseed oil by Yarrowia lipolytica yeast. Appl Microbiol Biotechnol 97:5517–5525. https://doi.org/10.1007/s00253-013-4772-6
Kamzolova SV, Shamin RV, Stepanova NN, Morgunov GI, Lunina JN, Allayarov RK, Samoilenko VA, Morgunov IG (2018) Fermentation conditions and media optimization for isocitric acid production from ethanol by Yarrowia lipolytica. BioMed Res Int 2018:2543210. https://doi.org/10.1155/2018/2543210
Kuenz A, Krull S (2018) Biotechnological production of itaconic acid-things you have to know. Appl Microbiol Biotechnol 102:3901–3914. https://doi.org/10.1007/s00253-018-8895-7
Laptev IA, Filimonova NA, Allayarov RK, Kamzolova SV, Samoilenko VA, Sineoky SP, Morgunov IG (2017) New recombinant strains of the yeast Yarrowia lipolytica with overexpression of the aconitate hydratase gene for the obtainment of isocitric acid from rapeseed oil. Appl Biochem Microbiol 52:699–704. https://doi.org/10.1134/s000368381607005x
Larroude M, Park Y-K, Soudier P, Kubiak M, Nicaud J-M, Rossignol T (2019) A modular golden gate toolkit for Yarrowia lipolytica synthetic biology. Microb Biotechnol 12:1249–1259. https://doi.org/10.1111/1751-7915.13427
Larroude M, Rossignol T, Nicaud J-M, Ledesma-Amaro R (2018) Synthetic biology tools for engineering Yarrowia lipolytica. Biotechnol Adv 36:2150–2164. https://doi.org/10.1016/j.biotechadv.2018.10.004
Larroude M, Trabelsi H, Nicaud J-M, Rossignol T (2020) A set of Yarrowia lipolytica CRISPR/Cas9 vectors for exploiting wild-type strain diversity. Biotechnol Lett 42:773–785. https://doi.org/10.1007/s10529-020-02805-4
Li C, Gao S, Li X, Yang X, Lin CSK (2018) Efficient metabolic evolution of engineered Yarrowia lipolytica for succinic acid production using a glucose-based medium in an in situ fibrous bioreactor under low-pH condition. Biotechnol Biofuels 11:236–248. https://doi.org/10.1186/s13068-018-1233-6
Li C, Gao S, Yang X, Lin CSK (2017) Green and sustainable succinic acid production from crude glycerol by engineered Yarrowia lipolytica via agricultural residue based in situ fibrous bed bioreactor. Bioresour Technol 249:612–619. https://doi.org/10.1016/j.biortech.2017.10.011
Li C, Ong KL, Yang X, Lin CSK (2019) Bio-refinery of waste streams for green and efficient succinic acid production by engineered Yarrowia lipolytica without pH control. Chem Eng J 371:804–812. https://doi.org/10.1016/j.cej.2019.04.092
Li C, Yang X, Gao S, Chuh AH, Lin CSK (2018) Hydrolysis of fruit and vegetable waste for efficient succinic acid production with engineered Yarrowia lipolytica. J Clean Prod 179:151–159. https://doi.org/10.1016/j.jclepro.2018.01.081
Li C, Yang X, Gao S, Wang H, Lin CSK (2016) High efficiency succinic acid production from glycerol via in situ fibrous bed bioreactor with an engineered Yarrowia lipolytica. Bioresour Technol 225:9–16. https://doi.org/10.1016/j.biortech.2016.11.016
Liu X, Lv J, Xu J, Zhang T, Deng Y, He J (2014) Citric acid production in Yarrowia lipolytica SWJ-1b yeast when grown on waste cooking oil. Appl Biochem Biotechnol 175:2347–2356. https://doi.org/10.1007/s12010-014-1430-0
Liu XY, Chi Z, Liu GL, Madzak C, Chi ZM (2013) Both decrease in ACL1 gene expression and increase in ICL1 gene expression in marine-derived yeast Yarrowia lipolytica expressing INU1 gene enhance citric acid production from inulin. Mar Biotechnol (NY) 15:26–36. https://doi.org/10.1007/s10126-012-9452-5
McKinlay JB, Vieille C, Zeikus JG (2007) Prospects for a bio-based succinate industry. Appl Microbiol Biotechnol 76:727–740. https://doi.org/10.1007/s00253-007-1057-y
Mirończuk AM, Biegalska A, Dobrowolski A (2017) Functional overexpression of genes involved in erythritol synthesis in the yeast Yarrowia lipolytica. Biotechnol Biofuels 10:77. https://doi.org/10.1186/s13068-017-0772-6
Mironczuk AM, Rzechonek DA, Biegalska A, Rakicka M, Dobrowolski A (2016) A novel strain of Yarrowia lipolytica as a platform for value-added product synthesis from glycerol. Biotechnol Biofuels 9:180–192. https://doi.org/10.1186/s13068-016-0593-z
Mirończuk AM, Rzechonek DA, Biegalska A, Rakicka M, Dobrowolski A (2016) A novel strain of Yarrowia lipolytica as a platform for value-added product synthesis from glycerol. Biotechnol Biofuels 9. https://doi.org/10.1186/s13068-016-0593-z
Morgunov IG, Kamzolova SV (2015) Physiologo-biochemical characteristics of citrate-producing yeast Yarrowia lipolytica grown on glycerol-containing waste of biodiesel industry. Appl Microbiol Biotechnol 99:6443–6450. https://doi.org/10.1007/s00253-015-6558-5
Morgunov IG, Kamzolova SV, Karpukhina OV, Bokieva SB, Inozemtsev AN (2019) Biosynthesis of isocitric acid in repeated-batch culture and testing of its stress-protective activity. Appl Microbiol Biotechnol 103:3549–3558. https://doi.org/10.1007/s00253-019-09729-8
Morgunov IG, Kamzolova SV, Lunina JN (2013) The citric acid production from raw glycerol by Yarrowia lipolytica yeast and its regulation. Appl Microbiol Biotechnol 97:7387–7397. https://doi.org/10.1007/s00253-013-5054-z
Niang PM, Arguelles-Arias A, Steels S, Denies O, Nicaud J-M, Fickers P (2020) In Yarrowia lipolytica erythritol catabolism ends with erythrose phosphate. Cell Biol Int 44:651–660. https://doi.org/10.1002/cbin.11265
Nicaud J-M (2012) Yarrowia lipolytica. Yeast 29:409–418. https://doi.org/10.1002/yea.2921
Okamoto S, Chin T, Hiratsuka K, Aso Y, Tanaka Y, Takahashi T, Ohara H (2014) Production of itaconic acid using metabolically engineered Escherichia coli. J Gen Appl Microbiol 60:191–197. https://doi.org/10.2323/jgam.60.191
Ong KL, Fickers P, Pang KP, Raffel Dharma P, Luk HS, Uisan K, Lin CSK (2020) Fermentation of fruit and vegetable wastes for biobased products. In: Food industry waste: assessment and recuperation of commodities, Kisseva M.R. and Webb C. Academic Press, pp 255–274
Ong KL, Li C, Li X, Zhang Y, Xu J, Lin CSK (2019) Co-fermentation of glucose and xylose from sugarcane bagasse into succinic acid by Yarrowia lipolytica. Bioch Eng J 148:108–115. https://doi.org/10.1016/j.bej.2019.05.004
Otten A, Brocker M, Bott M (2015) Metabolic engineering of Corynebacterium glutamicum for the production of itaconate. Metab Eng 30:156–165. https://doi.org/10.1016/j.ymben.2015.06.003
Otto C, Yovkova V, Barth G (2011) Overproduction and secretion of alpha-ketoglutaric acid by microorganisms. Appl Microbiol Biotechnol 92:689–695. https://doi.org/10.1007/s00253-011-3597-4
Papanikolaou S, Galiotou-Panayotou M, Fakas S, Komaitis M, Aggelis G (2008) Citric acid production by Yarrowia lipolytica cultivated on olive-mill wastewater-based media. Bioresour Technol 99:2419–2428. https://doi.org/10.1016/j.biortech.2007.05.005
Park Y-K, Vandermies M, Soudier P, Telek S, Thomas S, Nicaud J-M, Fickers P (2019) Efficient expression vectors and host strain for the production of recombinant proteins by Yarrowia lipolytica in process conditions. Microb Cell Fact 18:167. https://doi.org/10.1186/s12934-019-1218-6
Rakicka M, Biegalska A, Rymowicz W, Dobrowolski A, Mirończuk AM (2017) Polyol production from waste materials by genetically modified Yarrowia lipolytica. Bioresour Technol 243:393–399. https://doi.org/10.1016/j.biortech.2017.06.137
Rakicka M, Mirończuk AM, Tomaszewska-Hetman L, Rywińska A, Rymowicz W (2017b) An effective method of continuous production of erythritol from glycerol by Yarrowia lipolytica MK1. Food Technol Biotechnol 55:125–130. https://doi.org/10.17113/ftb.55.01.17.4812
Rakicka-Pustułka M, Mirończuk AM, Celińska E, Białas W, Rymowicz W (2020) Scale-up of the erythritol production technology—process simulation and techno-economic analysis. J Clean Prod 257:120533. https://doi.org/10.1016/j.jclepro.2020.120533
Regnat K, Mach RL, Mach-Aigner AR (2018) Erythritol as sweetener—wherefrom and whereto? Appl Microbiol Biotechnol 102:587–595. https://doi.org/10.1007/s00253-017-8654-1
Rice T, Zannini E, Arendt EK, Coffey A (2019) A review of polyols—biotechnological production, food applications, regulation, labeling and health effects. Crit Rev Food Sci Nutr 1–18. https://doi.org/10.1080/10408398.2019.1625859
Rymowicz W, Fatykhova AR, Kamzolova SV, Rywinska A, Morgunov IG (2010) Citric acid production from glycerol-containing waste of biodiesel industry by Yarrowia lipolytica in batch, repeated batch, and cell recycle regimes. Appl Microbiol Biotechnol 87:971–979. https://doi.org/10.1007/s00253-010-2561-z
Rzechonek DA, Dobrowolski A, Rymowicz W, Mironczuk AM (2018) Aseptic production of citric and isocitric acid from crude glycerol by genetically modified Yarrowia lipolytica. Bioresour Technol 271:340–344. https://doi.org/10.1016/j.biortech.2018.09.118
Rzechonek DA, Dobrowolski A, Rymowicz W, Mirończuk AM (2018) Recent advances in biological production of erythritol. Crit Rev Biotechnol 38:620–633. https://doi.org/10.1080/07388551.2017.1380598
Sarris D, Rapti A, Papafotis N, Koutinas AA, Papanikolaou S (2019) Production of added-value chemical compounds through bioconversions of olive-mill wastewaters blended with crude glycerol by a Yarrowia lipolytica strain. Molecules 24:222. https://doi.org/10.3390/molecules24020222
Sassi H, Delvigne F, Kar T, Nicaud J-M, Coq A-MC-L, Steels S, Fickers P (2016) Deciphering how LIP2 and POX2 promoters can optimally regulate recombinant protein production in the yeast Yarrowia lipolytica. Microb Cell Fact 15:159. https://doi.org/10.1186/s12934-016-0558-8
Sekova VY, Dergacheva DI, Isakova EP, Gessler NN, Tereshina VM, Deryabina YI (2019) Soluble sugar and lipid readjustments in the Yarrowia lipolytica yeast at various temperatures and pH. Metabolites 9:307. https://doi.org/10.3390/metabo9120307
Shabbir Hussain M, Gambill L, Smith S, Blenner MA (2016) Engineering promoter architecture in oleaginous yeast Yarrowia lipolytica. ACS Synth Biol 5:213–223. https://doi.org/10.1021/acssynbio.5b00100
Tan JP, Md. Jahim J, Wu TY, Harun S, Kim BH, Mohammad AW (2014) Insight into biomass as a renewable carbon source for the production of succinic acid and the factors affecting the metabolic flux toward higher succinate yield. Ind Eng Chem Res 53:16123–16134. https://doi.org/10.1021/ie502178j
Tan Z, Zhu X, Chen J, Li Q, Zhang X (2013) Activating phosphoenolpyruvate carboxylase and phosphoenolpyruvate carboxykinase in combination for improvement of succinate production. App Env Microbiol 79:4838–4844. https://doi.org/10.1128/AEM.00826-13
Tomaszewska L, Rakicka M, Rymowicz W, Rywińska A (2014) A comparative study on glycerol metabolism to erythritol and citric acid in Yarrowia lipolytica yeast cells. FEMS Yeast Res 14:966–976. https://doi.org/10.1111/1567-1364.12184
Tomaszewska L, Rywińska A, Gładkowski W (2012) Production of erythritol and mannitol by Yarrowia lipolytica yeast in media containing glycerol. J Ind Microbiol Biotechnol 39:1333–1343. https://doi.org/10.1007/s10295-012-1145-6
Trassaert M, Vandermies M, Carly F, Denies O, Thomas S, Fickers P, Nicaud J-M (2017) New inducible promoter for gene expression and synthetic biology in Yarrowia lipolytica. Microb Cell Fact 16:141. https://doi.org/10.1186/s12934-017-0755-0
Vandermies M, Denies O, Nicaud JM, celin P (2017) EYK1 encoding erythrulose kinase as a catabolic selectable marker for genome editing in the non-conventional yeast Yarrowia lipolytica. J Microbiol Methods 139
Vandermies M, Fickers P (2019) Bioreactor-scale strategies for the production of recombinant protein in the yeast Yarrowia lipolytica. Microorganisms 7:40. https://doi.org/10.3390/microorganisms7020040
Wang N, Chi P, Zou Y, Xu Y, Xu S, Bilal M, Fickers P, Cheng H (2020) Metabolic engineering of Yarrowia lipolytica for thermoresistance and enhanced erythritol productivity. Biotechnol Biofuels 13:176. https://doi.org/10.1186/s13068-020-01815-8
Werpy T, Petersen G (2004) Top value-added chemicals from biomass: volume I—results of screening for potential candidates from sugars and synthesis gas United States: N. p., 2004. Web. https://doi.org/10.2172/15008859.
Wojtatowicz M, Rymowicz W, Kautola H (1991) Comparison of different strains of the yeast Yarrowia lipolytica for citric acid production from glucose hydrolysate. Appl Biochem Biotechnol 31:165–174
Wong L, Engel J, Jin E, Holdridge B, Xu P (2017) YaliBricks, a versatile genetic toolkit for streamlined and rapid pathway engineering in Yarrowia lipolytica. Metab Eng Commun 5:68–77. https://doi.org/10.1016/j.meteno.2017.09.001
Yang X, Wang H, Li C, Lin CSK (2017) Restoring of glucose metabolism of engineered Yarrowia lipolytica for succinic acid production via a simple and efficient adaptive evolution strategy. Agri Food Chem 65:4133–4139
Yin X, Madzak C, Du G, Zhou J, Chen J (2012) Enhanced alpha-ketoglutaric acid production in Yarrowia lipolytica WSH-Z06 by regulation of the pyruvate carboxylation pathway. Appl Microbiol Biotechnol 96:1527–1537. https://doi.org/10.1007/s00253-012-4192-z
Yovkova V, Otto C, Aurich A, Mauersberger S, Barth G (2013) Engineering the alpha-ketoglutarate overproduction from raw glycerol by overexpression of the genes encoding NADP+-dependent isocitrate dehydrogenase and pyruvate carboxylase in Yarrowia lipolytica. Appl Microbiol Biotechnol 98:2003–2013. https://doi.org/10.1007/s00253-013-5369-9
Yuzbashev TV, Yuzbasheva EY, Sobolevskaya TI, Laptev IA, Vybornaya TV, Larina AS, Matsui K, Fukui K, Sineoky SP (2010) Production of succinic acid at low pH by a recombinant strain of the aerobic yeast Yarrowia lipolytica. Biotechnol Bioeng 107:673–682. https://doi.org/10.1002/bit.22859
Yuzbasheva EY, Agrimi G, Yuzbashev TV, Scarcia P, Vinogradova EB, Palmieri L, Shutov AV, Kosikhina IM, Palmieri F, Sineoky SP (2019) The mitochondrial citrate carrier in Yarrowia lipolytica: Its identification, characterization and functional significance for the production of citric acid. Metab Eng 54:264–274. https://doi.org/10.1016/j.ymben.2019.05.002
Zhang M, Gu L, Cheng C, Ma J, Xin F, Liu J, Wu H, Jiang M (2018) Recent advances in microbial production of mannitol: utilization of low-cost substrates, strain development and regulation strategies. World J Microbiol Biotechnol 34:41. https://doi.org/10.1007/s11274-018-2425-8
Zhao C, Cui Z, Zhao X, Zhang J, Zhang L, Tian Y, Qi Q, Liu J (2019) Enhanced itaconic acid production in Yarrowia lipolytica via heterologous expression of a mitochondrial transporter MTT. Appl Microbiol Biotechnol 103:2181–2192. https://doi.org/10.1007/s00253-019-09627-z
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Li, C., Lin, W., Ong, K.L., Mou, J., Lin, C.S.K., Fickers, P. (2022). Synthesis of Polyols and Organic Acids by Wild-Type and Metabolically Engineered Yarrowia lipolytica Strains. In: Darvishi Harzevili, F. (eds) Synthetic Biology of Yeasts. Springer, Cham. https://doi.org/10.1007/978-3-030-89680-5_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-89680-5_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-89679-9
Online ISBN: 978-3-030-89680-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)