Abstract
Yarrowia lipolytica A-101-1.22 produces high citric acid (112 g l−1) with a yield of 0.6 g g−1 and a productivity of 0.71 g l−1 h−1 during batch cultivation in the medium with glycerol-containing waste of biodiesel industry. However, it was observed that the specific citric acid production rate, which was maximal at the beginning of the biosynthesis, gradually decreases in the late production phase and it makes continuation of the process over 100 h pointless. The cell recycle and the repeated batch regimes were performed as ways for prolongation of citric acid synthesis by yeast. Using cell recycle, the active citric acid biosynthesis (96–107 g l−1) with a yield of 0.64 g g−1 and a productivity of 1.42 g l−1 h−1 was prolongated up to 300 h. Repeated batch culture remained stable for over 1000 h; the RB variant of 30% feed every 3 days showed the best results: 124.2 g l-1 citric acid with a yield of 0.77 g g-1 and a productivity of 0.85 g l-1 h-1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the twentieth century, oil hydrocarbons were considered to be the main source of energy and the cheapest and readily available raw material for biotechnology. However, the ever-increasing rise in the cost of oil starting with the oil crisis of the seventies and the deterioration of the global ecological situation in the last years made us turn to alternative energy sources, such as biodiesel. Biodiesel production worldwide has been on an exponential growth curve over the past several years. Under Directive 2003/30/EC Europe established the goal of reaching a 5.75% share of renewable energy in the transport sector by 2010.
Biodiesel can be produced from various vegetable oils and animal fats. The technology consists in that oil triglycerides are hydrolyzed and then methylated with the formation of methylated fatty acids, which are just used as biodiesel. One of the major wastes from this technological process is glycerol, which is formed in an amount of more than 1 kg per 10 kg of the biodiesel produced. In 2007, the amount of glycerol-containing waste in Europe reached 600,000 tons (Papanikolaou et al. 2008) that poses the problem of its utilization.
Biodiesel waste, which contains glycerol (up to 80%), oil residue, free fatty acids (about 1%), sodium and potassium salts, and water, may serve as raw material for various processes of biotechnological bulk metabolites, in particular citric acid (CA). Global CA production has reached 1.4 million tons, increasing annually at 3.5–4.0% in demand and consumption (Anastassiadis et al. 2008).
Traditionally, different strains of filamentous fungus Aspergillus niger have been used in the commercial production of CA from molasses, sucrose, or glucose. Moreover, there is a great interest in various yeasts belonging to Candida (Yarrowia) lipolytica, which is capable of CA production from various carbon sources, such as n-alkanes (Akiyama et al. 1973; Gledhill et al. 1973; Lozinov et al. 1974; Stottmeister et al. 1982), glucose (Anastassiadis et al. 2002; Anastassiadis et al. 2008; Behrens et al. 1987; Briffaud and Engasser 1979; Förster et al. 2007a; Lozinov et al. 1974; Moresi 1994; Stottmeister et al. 1982; Wojtatowicz et al. 1991), ethanol (Arzumanov et al. 2000; Finogenova et al. 2005), and plant oils (Fickers et al. 2005; Förster et al. 2007b; Holz et al. 2009; Kamzolova et al. 2005). The necessary condition for the synthesis of CA and its excretion into the surrounding medium is the retardation of growth of Y. lipolytica under the conditions of carbon excess and nitrogen deficiency in the cultivation medium (Akiyama et al. 1973; Behrens et al. 1987; Fickers et al. 2005; Finogenova et al. 2005; Gaden 1959; Lozinov et al. 1974; Stottmeister et al. 1982). Most strains of Y. lipolytica produced CA and threo-Ds-isocitric acid (ICA) simultaneously in the proportion that depended on the strain, carbon source, and composition of the growth medium (Anastassiadis et al. 2008; Fickers et al. 2005; Holz et al. 2009; Lozinov et al. 1974; Stottmeister et al. 1982).
The relevant literature data on attempts to use glycerol as carbon source for CA production are rare (Förster et al. 2007a, b; Holz et al. 2009; Imandi et al. 2007; Levinson et al. 2007; Papanikolaou et al. 2002; Papanikolaou et al. 2008; Rymowicz et al. 2006; Rywińska et al. 2009). The available reports are mainly focused on cultivation in flasks and batch process. Recently, we reported CA production by Y. lipolytica on glycerol in repeated batch bioreactor (Rywińska and Rymowicz 2010). Yet, implementation of a continuous process for CA production from crude glycerol has not been developed.
The aim of the present work was to study the possibility of CA production by Y. lipolytica in a cell recycle system and repeated batch (RB) on the medium containing glycerol-containing waste of biodiesel manufacture (crude glycerol), and to conduct a comparative analysis of biochemical peculiarities (protein content; C, H, N content, lipid content and fatty acid composition) of the producer in batch mode and continuous cultivation.
Materials and methods
Strain
The acetate-negative mutant strain Y. lipolytica A-101-1.22 used in this study was from the yeast culture collection belonging to the Department of Biotechnology and Food Microbiology, Wroclaw University of Environmental and Life Sciences in Poland. It was obtained from a parental wild-type, asporogenous strain A-101 of Y. lipolytica by using NTG (N-methyl-N'-nitro-N-nitrosoguanidine) treatment. The parental strain of Y. lipolytica A-101 was isolated from an oil-field in southeastern Poland. In comparison to the A-101 strain, the A-101-1.22 mutant is able to produce lower amount of by-product such as threo-Ds-isocitric acid (Wojtatowicz et al. 1991; Rymowicz et al. 1993). The yeast strain was maintained on YM slants under a layer of paraffin oil at 4 °C.
Chemicals
The carbon source used was raw glycerol from biodiesel (fatty acid methyl esters) production unit [Rafineria Trzebinia S.A—glycerol content—76% (w/w)]. The impurities in the industrial raw glycerol solution were sodium salts [4% (w/w)], methanol [0.1% (w/w], metals [Cu 0.3, Mg 100, Fe 13.7, Zn 2.9, and Ca 46 (ppm)], heavy metals [Cd, Cr, Hg not detected], other organic materials [0.8% (w/w)] and water [19.5% (w/w)].
Culture conditions
The growth medium for a seed culture contained: 50 g glycerol, 2 g yeast extract, 3 g bacto peptone (Difco, USA) in 1 l of tap water. A seed culture was grown in a 300-ml flask (containing 100 ml of growth medium) on a shaker at 30 °C for 2 days. An inoculum of 200 ml was introduced into the fermenter containing 1.8 l of the production medium.
Batch and RB regimes were performed in a 5-l jar fermenter (Biostat B Plus, Sartorius, Germany) with a working volume of 2 l at 30 °C. The aeration rate was fixed at 0.6 l min−1. The stirrer speed was adjusted to 800 rpm, and the dissolved oxygen concentration was maintained at 25 ± 5% saturation. The pH was maintained automatically at 5.5 by the addition of NaOH (40% w/v).
Batch mode was conducted in a medium consisting of 125 g l−1 crude glycerol, 3 g l−1 NH4Cl, 1 g l−1 MgSO4 × 7H2O, 0.2 g l−1 KH2PO4, and 1 g l−1 yeast extract. After 24 and 60 h of cultivation, crude glycerol (62.5 g l−1) was added until a total concentration of 250 g l−1.
Production medium for RB cultivation contained: 250 g l−1 crude glycerol, 3 g l−1 NH4Cl, 1 g l−1 MgSO4 × 7H2O, 0.2 g l−1 KH2PO4, and 1 g l−1 yeast extract. For the first 75 h, the cultivation was conducted in batch mode, then a portion of the culture liquid (0.8, 0.6, or 0.4 l) was withdrawn, and the same volume of the replaced production medium was added. This procedure was repeated four times for each volume. The replaced medium contained: 4 g l−1 NH4Cl, 1 g l−1 MgSO4 × 7H2O, 0.2 g l−1 KH2PO4, and 1 g l−1 yeast extract. The volume of culture broth at the start of each cycle of RB culture was 2 l, and the concentration of crude glycerol was 125 g l-1. The end of each RB cycle was determined when the concentration of residual glycerol was below 0.5 g l−1 and an appropriate volume of replaced medium was added.
The cell recycle was carried in a 5-l tank reactor with working volume of 1.4 l. Bioreactor was coupled with the inside spiral membrane module (Bioengineering AG), which contained a flat polyethersulfone membrane (Sartorius), diameter of membrane was 47 mm, pore size was 0.45 µm. The membrane module was equipped with a peristaltic pomp (Ismatec). The spiral module with membrane was sterilized in autoclave at 121 °C for 20 min. This operating membrane system reduces membrane fouling, therefore the membrane was changed after 2 weeks of the continuous culture. Production medium contained: 187.5 g l−1 of crude glycerol, 4 g l−1 of NH4Cl, 0.2 g l−1 of KH2PO4, 1.0 g l−1 of MSO4 × 7H2O, and 1 g l−1 of yeast extract (Difco, USA) in tap water. Medium for continuous process contained: 212.5 g l−1 of crude glycerol and 0.7 g l−1 of bacto peptone in tap water.
Analytical methods
The biomass was determined gravimetrically after drying in a drier at 105 °C. Concentration of total CA and ICA, glycerol, mannitol, and erythritol were determined by HPLC (Beckman Gold System, USA) on an Aminex HPX87H organic acid column coupled to a UV (λ = 210 nm) and refractive index (RI) detector. The column was eluted with 20 mM H2SO4 at room temperature and a flow rate of 0.6 ml min−1. The retention times for citric acids, mannitol, erythritol, and glycerol were 7.9, 9.8, 11.3, and 12.9 min, respectively.
ICA was identified using enzymatic methods as described by Goldberg and Ellis (1983). Protein was determined by the Lowry method.
The intracellular content of carbon, hydrogen, and nitrogen was measured in a C, H, N analyzer (Carlo Erba Strumentazione); the ash content was determined by burning in a muffle furnace. The oxygen content (O) was calculated from: \( {\hbox{O}} = {1}00 - \left( {{\hbox{C}} + {\hbox{H}} + {\hbox{N}} + {\hbox{H}} + {\hbox{Ash}}} \right) \), where C, H, N—values of carbon content, hydrogen content, and nitrogen content.
Methyl esters of fatty acids were obtained by the method of Sultanovich et al. (1982) and analyzed by gas-liquid chromatography on a Chrom-5 chromatograph with a flame-ionization detector. The column (2 m × 3 mm) was packed with 15% Reoplex-400 applied to Chromaton N-AW (0.16–0.20 mm). The temperature of the column was 200 °C. The lipid content in the biomass was determined from the total fatty acid content with docosane (C22H46) as internal standard. The energy content in the biomass was calculated on the basis of the theory of the material-energy balance (Erickson et al. 2000).
Calculation of fermentation parameters
To take into account the medium dilution due to the addition of NaOH solution for maintaining the constant pH value, the total amounts of citric acid in the culture broth were used for calculations of the mass yield of CA (YCA), the volumetric citric acid productivity (QCA), and the specific citric acid production rate (q CA) at batch and repeated batch regimes.
Mass yield of CA (YCA), expressed in g g−1 from crude glycerol was calculated from:
the volumetric citric acid productivity (QCA), expressed in g l−1 h−1 was calculated from:
the specific citric acid production rate (q CA), expressed in g (g cells h)−1 was calculated from:
where P, total amount of CA in the culture liquid at the end of a regime/cultivation (g); S, total amount of crude glycerol consumed (g); V, the initial volume of culture liquid (l); t, the fermentation duration (h); and X, working biomass in the fermentor (g).
For the cell recycle cultivation the following equations were used to calculate mass yield of CA (YCA), the volumetric citric acid productivity (QCA) and the specific citric acid production rate (q CA):
where C, average CA concentration in cell recycle (g l−1); Sr, crude glycerol consumed in the recycle fermentor (g l−1); Si, inlet concentration of crude glycerol in the recycle fermentor g l−1; So, average outlet concentration of crude glycerol in the recycle fermentor g l−1; D—dilution rate in the recycle fermentor (h−1).
Results
Biosynthesis of CA in batch regime
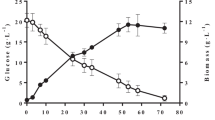
The strain Y. lipolytica A-101-1.22 was cultivated under nitrogen deficiency in a 5-l fermentor with working volume of 2 l with crude glycerol as the carbon source; pH = 5.5 was adjusted automatically with 40% NaOH. The initial concentration of crude glycerol in the medium was 125 g l−1; after 24 and 60 h of cultivation, crude glycerol (62.5 g l−1) was added until a total concentration of 250 g l−1.
Figure 1 shows the results of a typical experiment. During the first 24 h of cultivation, the cell biomass increased to 25.3 g l−1 and then the culture passed to the stationary growth phase because of nitrogen exhaustion in the medium. Most of CA was accumulated in the medium during the stationary growth phase; by the end of the cultivation period (158 h), the culture produced 112 g l−1 CA. The mass yield of CA (YCA) and the volumetric citric acid productivity (QCA) reached 0.6 g g−1 and 0.71 g l−1 h−1, respectively. The concentrations of by-products ICA, erythritol, and mannitol consisted of 7.1, 8.9, and 7.9 g l−1, respectively.
The biomass composition of CA-producing strain Y. lipolytica A-101-1.22 during the cultivation on crude glycerol is presented in Table 1. In the growth phase the crude glycerol was essentially converted into protein (26.9% of the dry biomass), while the lipid content in yeast cells was low (7.4% of the dry biomass), indicating predominance of functional lipids in their compositions. The protein content of Y. lipolytica A-101-1.22 grown on crude glycerol was lower than that obtained in Y. lipolytica, grown on pure substrates, such as glucose (43% of the dry biomass; Kozlova et al. 1981) or ethanol (45% of the dry biomass; Kamzolova et al. 1996). It seems the impurities in crude glycerol inhibited of protein synthesis in CA-producing strain Y. lipolytica A-101-1.22.
The transition of the culture to the stationary phase initiated by nitrogen limitation of cell growth was accompanied by a decrease in the protein amount (in 1.7 times) and by an increase in the lipid content (in 2.41 times); the intracellular contents of carbon (46.6–48.6% of the dry biomass), hydrogen (6.7–7.0% of the dry biomass), and oxygen (36.8–39.9% of the dry biomass) changed insignificantly during the cultivation period (Table 1).
It should be noted that the intracellular amount of nitrogen decreased from 4.7% of dry biomass in the growth phase to 2.5% of dry biomass in the acid production phase showing the importance of both nitrogen limitation and a balance between nitrogen concentration and other nutrients for the optimum citrate excretion by yeast. Similar data on a decrease in nitrogen content of Saccharomycopsis lipolytica D1805 biomass during yeast transition to the stationary phase (from 8.5% in the trophophase to 4% at the end of exponential phase) have been reported by Briffaud and Engasser (1979). Moresi (1994) revealed a reduction in intracellular nitrogen content from 7–8% to 2.3–4.4% in Y. lipolytica ATCC 20346. There are also data on the importance of nitrogen limitation of Candida oleophila ATCC 20177 growth for citric acid production; the optimum concentration of NH +4 was found to be 1.2 mg g−1 (Anastassiadis et al. 2002).
Under cell growth limitation by nitrogen the energy content of biomass increased from 18.9 to 19.7 kJ g−1 (Table 1) that can be explained by a correlation between the energy content of biomass and the amount of lipids, the most energy-rich component of the cells, which essentially increased in this case.
As seen from Table 1, during the cultivation oleic acid (Δ9C18:1), palmitic (C16:0), palmitoleic (Δ9C16:1), and linoleic (Δ9,12C18:2) acids prevailed among the fatty acids. In the course of cultivation, the significant alterations were observed in the fatty acid ratio: the content of linoleic acid (Δ9,12C18:2) decreased from 36.5% to 12.3% and that of oleic acid (Δ9C18:1) increased from 28.1% to 45.6%; the contents of palmitic acid (C16:0) and palmitoleic acid (Δ9C16:1) did not markedly alterate during the cultivation. Fatty acid desaturase activity during cultivation has been estimated by calculating the ratios of desaturase product to substrate (C16:1/C16:0; C18:1/C18:0; C18:2/C18:1). The high C18:1/C18:0 ratios calculated for all variants suggest an important Δ9 activity in the yeast cells, especially in the growth phase (12 h).
As can be seen from the data presented in Table 2, the specific citric acid production rate (q CA) of Y. lipolytica A-101-1.22, which is maximal at the beginning of CA biosynthesis (0.076–0.06 g (g cell)−1 h−1, gradually decreases during cultivation, which makes continuation of the process over 100 h pointless. The disadvantages of batch cultivation mentioned determine the necessity to use other cultivation methods, allowing extended active biosynthesis of CA.
Cell recycle culture
One of the promising ways to prolong microbiological processes during the metabolites production, which are excreted into culture liquid, is the cultivation in a membrane bioreactor (Gledhill et al. 1973; Rane and Sims 1995). Using a membrane bioreactor allows to increase the duration of fermentation by maintaining the CA concentration level, which is not inhibiting the cell metabolism, also the mass yield from substrate consumed increases by decreasing its consumption for cell growth, therefore, biomass accumulated of producer functions longer than in batch culture.
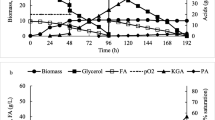
Membrane bioreactor with the inside spiral membrane module was used in the present work. Figure 2 illustrates the results obtained in the cell recycle system. After growing Y. lipolytica A-101.1.22 in the batch mode during 72 h, the yeast was concentrated prior to starting continuous operation of the recycle fermentor. The dilution rate in the recycle (D) was 0.014 h−1 and the fermentation was operated for 500 h. The active CA synthesis (96–107 g l−1) was observed between 100 and 300 h. ICA concentration varied from 6.1 to 7.0 g l−1 and did not exceed 6% from total CA plus ICA, which was similar to the batch cultivation data, while the concentrations of erythritol (0.29 ± 0.19 g l−1) and mannitol (1.97 ± 0.75 g l−1) were significantly lower than those obtained in batch regime; the residual glycerol maintained at trace level. In this period of cultivation the average the volumetric citric acid productivity (QCA) and the specific citric acid production rate (q CA).remained high and consisted of 1.42 g l−1 h−1 and 0.064 g (g cell)−1 h−1, respectively; average mass yield of CA (YCA) of 0.64 g g−1 was slightly higher to the batch cultivation data. After 300 h of cultivation, CA synthesis decreased indicating that the continuous withdrawal of CA produced is not enough for extending active CA synthesis.
RB cultivation
RB cultivation represents a cultivation process, where culture liquid is withdrawn with cells and fresh cultivation medium added to the fermentor at fixed periods of time. As compared with traditional batch operation, RB mode often makes a fermentation process more efficient. It has been successfully employed for the production of CA from ethanol (Arzumanov et al. 2000; 2002), glucose (Anastassiadis and Rehm 2006; Moresi 1994), and crude glycerol (Rywińska and Rymowicz 2010).
In present experiments, the amount of the medium added and the duration of a cycle were varied. The following variants of RB cultivation were studied: 40% feed every 3 days, 30% feed every 3 days, and 20% feed every 5 days. Each RB culture was repeated four times. In Fig. 3, four cycles of each variant of RB cultivation are shown. RB culture remains stable for a long period of time (1,129 h), even at 886 h CA concentration consisted of 119.9 g l−1.
Table 3 lists the biomass level, CA concentration, the concentrations of by-products ICA, erythritol, mannitol, and calculated values of the mass yield of CA (YCA), the volumetric citric acid productivity (QCA), and the specific citric acid production rate (q CA) at different modes of RB cultivation.
RB cultivation mode of 30% feed every 3 days showed the best results: CA concentration (124.2 g l−1), the volumetric citric acid productivity (QCA; 0.85 g l−1 h−1), and the mass yield of CA (YCA; 0.77 g g−1) were 11%, 20%, and 28%, respectively, greater than those obtained in batch fermentor operating under similar conditions; the specific citric acid production rate (q CA) (0.05 g (g cell)−1 h−1) of Y. lipolytica A-101-1.22 was comparable to rate obtained at 39–61 h of batch cultivation (the period of maximal CA biosynthesis). An increase in the amount of replaced medium from 30% to 40% resulted in reduced CA concentration (108.4 g l−1), which was probably due to the high degree of culture renewal; while the volumetric citric acid productivity (QCA; 0.77 g l−1 h−1) and the mass yield of CA (YCA; 0.64 g g−1) were higher than those obtained in batch mode. A decrease in the amount of replaced medium from 30% to 20% and an increase in the duration of a cycle from 3 to 5 days resulted in a decrease in CA concentration (112.6 g l−1), the volumetric citric acid productivity (QCA; 0.51 g l−1 h−1) and the mass yield of CA (YCA; 0.55 g g−1), and an significant increase in erythritol production (up to 45.2 g l−1). In all RB variants used, ICA concentration did not exceed 7% from total CA plus ICA, which was similar to the batch cultivation data.
The biomass composition of CA-producer, cultivated on crude glycerol during the long RB cultivation (1,129 h) is presented in Table 4. The intracellular contents of carbon (46.3–48.5% of the dry biomass), hydrogen (6.7–7.0% of the dry biomass), and oxygen (35.0–39.4% of the dry biomass) changed insignificantly. This is why RB culture allows maintenance of more stable activity of the culture for a long period of time and achieves better results, as compared to batch mode.
Discussion
In this study, CA production from glycerol-containing waste of biodiesel production by Y. lipolytica A-101-1.22 in batch, cell recycle, and RB bioreactors is reported. Table 5 lists CA, citric–isocitric acid ratios, the yields and productivities obtained in all type of bioreactor at similar biomass concentrations, and operating conditions.
Batch culture Y. lipolytica A-101-1.22 produced 112 g l−1 CA with yield of 0.6 g g−1; the citric acid to isocitric acid ratio in the strain studied was high (15.8). In our previous study (Rywińska et al. 2009), we showed that other acetate-negative mutant of Y. lipolytica Wratislavia AWG7 produced 131.5 g l−1 CA with yield of 0.66 g g−1 and the citric acid to isocitric acid ratio equal 28. For comparison, Y. lipolytica NRRL YB-423 produced 21.6 g l−1 of CA with yield of 0.54 g g−1 (Levinson et al. 2007) and Y. lipolytica LGAM S(7)1 produced 62.5 g l−1 of CA with yield of 0.56 g g−1 (Papanikolaou et al. 2002; 2008) with ratio between 2 and 6. According to Anastassiadis et al. (2002) the highest ratio, 49.6, was obtained using C. lipolytica DSM 3286 on a glucose medium after 196 h. These authors showed that in all strains used, the citrate to isocitrate ratio, increased with the fermentation time and that this ratio depended on the particular strain used. Other authors showed that Y. lipolytica strains produced CA and ICA simultaneously in a proportion that depended on the carbon source and the composition medium (Finogenova et al. 2005; Levinson et al. 2007; Rymowicz et al. 2006; Wojtatowicz et al. 1991).
As can be seen from the data presented in Table 2, the specific citric acid production rate (q CA), which is maximal at the beginning of the citrate biosynthesis, gradually decreases during the cultivation. Our data is in agreement with the findings of Arzumanov et al. (2000) and Wojtatowicz et al. (1991) who reported two distinctly different CA production phases using Y. lipolytica.
The cell recycle and RB regimes were performed as the ways for prolongation of CA synthesis by yeast. It was found that an acetate-negative mutant Y. lipolytica A-101-1.22 shows the stability for 500 h (Fig. 2) in the cell recycle bioreactor and over 1,000 h for RB cultivation (Fig. 3). Several researchers have investigated the long-term production of CA by yeast. Enzminger and Asenjo (1986) studied CA production by Saccharomycopsis lipolytica Y 7576 from glucose in a cell reactor; the yield of 0.86 g g−1, productivity of 1.16 g l−1 h−1, and a constant rate of acid production were maintained for over 200 h. C. lipolytica Y 1095, grown on glucose, indicated the stability for nearly 600 h in the cell recycle (Rane and Sims 1995). Arzumanov et al. (2000) described RB process with Y. lipolytica grown on ethanol, which maintained the high acid-producing activity for 700 h. About 20 RB experiments were sequentially carried out without any technical and microbiological stability problems at 100% conversion for Candida oleophila grown on glucose (Anastassiadis and Rehm 2006). Recently, we reported long RB process (more than 1,650 h) with an acetate-negative mutant of Y. lipolytica Wratislavia AWG7 (Rywińska and Rymowicz 2010).
CA concentrations and the volumetric productivities (QCA) in cell recycle and RB regimes were comparable to the best values reported previously for long-term cultivation on various substrates (Anastassiadis and Rehm 2006; Arzumanov et al. 2000; Enzminger and Asenjo 1986; Klasson et al. 1989; Rane and Sims 1995; Rywińska and Rymowicz 2010). Moreover the data obtained on the use of Y. lipolytica A-101-1.22 RB culture show that this cultivation method achieves better results than batch cultivation and that the element composition of producer remains stable for a long period of time (Table 4).
The maximum yield (0.77 g g−1) obtained in this study using RB mode was comparable with the values obtained with glucose-grown Y. lipolytica (Rane and Sims 1995) and lower than those reported for ethanol-grown Y. lipolytica 187/1 yeast (Arzumanov et al. 2000). The citrate to isocitrate ratio in cell recycle and RB regimes were similar to batch fermentation.
It should be noted that Y. lipolytica A-101-1.22 grown on crude glycerol produced a high amount of erythritol and mannitol, especially at RB cultivation. As noted, sugar alcohols, including mannitol and erythritol, protect plants, fungi, yeasts, and bacteria during stress conditions, e.g., osmotic stress (Aoki et al. 1993). It is quite likely that the production of polyols in the present study resulted from the exposure of the strain to high concentration of CA and glycerol. Moreover, our recent investigation has shown that a high initial concentration of glycerol up to 150 g l−1 and the total glycerol concentration of 250 g l−1 favors erythritol production by other strain Y. lipolytica Wratislavia K1 (Rymowicz et al. 2006). Probably in response to a high external osmotic environment, Y. lipolytica accumulates a high amount of erythritol and mannitol, which compensates for the difference between the extracellular and intracellular water potential. To date, biochemical pathways involved in the regulation of the erythritol and mannitol production from crude glycerol by Y. lipolytica have not been studied in depth.
In conclusion, based on the data presented in this study, it appears that the production of CA by Y. lipolytica A-101-1.22 using proposed cultivation methods could potentially compete with the more traditional A. niger process. Moreover, the cell recycle and RB regimes have the advantages offered by continuous processes, e.g., the decrease in the expenditure of sterilization and preparation of a fermentor and inoculum preparation, and hence, increases the economical efficiency of CA biosynthesis.
References
Akiyama S-I, Suzuki T, Sumino Y, Nakao Y, Fukuda H (1973) Induction and citric acid productivity of fluoroacetate-sensitive mutant strains of Candida lipolytica. Agric Biol Chem 37:879–884
Anastassiadis S, Rehm HJ (2006) Citric acid production from glucose by yeast Candida oleophila ATCC 20177 under batch, continuous and repeated batch cultivation. Electron J Biotechnol 9:26–39
Anastassiadis S, Aivasidis A, Wandrey C (2002) Citric acid production by Candida strains under intracellular nitrogen limitation. Appl Microbiol Biotechnol 60(1–2):81–87
Anastassiadis S, Morgunov IG, Kamzolova SV, Finogenova TV (2008) Citric acid production patent review. Recent Pat Biotechnol 2(2):107–123
Aoki M, Pastore G, Park Y (1993) Microbial transformation of sucrose and glucose to erythritol. Biotechnol Lett 15:383–388
Arzumanov TE, Shishkanova NV, Finogenova TV (2000) Biosynthesis of citric acid by Yarrowia lipolytica repeated-batch culture on ethanol. Appl Microbiol Biotechnol 53(5):525–529
Arzumanov TE, Sidorov IA, Shishkanova NV, Finogenova TV (2002) Mathematic modeling of citric acid production by repeated-batch culture. Enzyme Microb Technol 26(9–10):826–833
Behrens U, Thierrsch A, Weissbrodt E, Stottmeister U (1987) Particularities in the kinetics of growth and citric acid accumulation by Saccharomycopsis lipolytica. Acta Biotechnol 7(2):179–183
Briffaud J, Engasser J (1979) Citric acid production from glucose. I. Growth and excretion kinetics in a stirred fermentor. Biotechnol Bioeng 21(11):2083–2092
Enzminger JD, Asenjo JA (1986) Use of cell recycle in the aerobic fermentative production of citric acid by yeast. Biotechnol Lett 8:7–12
Erickson LE, Minkevich IG, Eroshin VK (2000) Application of mass and energy balance regularities in fermentation. Biotechnol Bioeng 67(6):748–774
Fickers P, Benetti PH, Waché Y, Marty A, Mauersberger S, Smit MS, Nicaud JM (2005) Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res 5(6–7):527–543
Finogenova TV, Morgunov IG, Kamzolova SV, Cherniavskaia OG (2005) Organic acid production by the yeast Yarrowia lipolytica (a review). Prikl Biokhim Mikrobiol 41(5):478–486, Review. Russian
Förster A, Aurich A, Mauersberger S, Barth G (2007a) Citric acid production from sucrose using a recombinant strain of the yeast Yarrowia lipolytica. Appl Microbiol Biotechnol 75(6):1409–1417
Förster A, Jacobs K, Juretzek J, Mauersberger S, Barth G (2007b) Overexpression of the ICL1 gene changes the product ration of citric acid production by Yarrowia lipolytica. Appl Microbiol Biotechnol 77(4):681–689
Gaden EL Jr (1959) Fermentation process kinetics. J Biochem Microbiol Technol Eng 1(4):413–429
Gledhill WE, Hill ID, Hodson PH (1973) Citrate production from hydrocarbons by use of a nonsterile, semicontinuous cell recycle system. Biotechnol Bioeng 15:963–972
Goldberg D, Ellis G (1983) Isocitrate dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie, Weinheim
Holz M, Förster A, Mauersberger S, Barth G (2009) Aconitase overexpression changes the product ratio of citric acid production by Yarrowia lipolytica. Appl Microbiol Biotechnol 81(6):1087–1096
Imandi SB, Bandaru VVR, Somalanka SR, Garapati HR (2007) Optimization of medium constituents for the production of citric acid from byproduct glycerol using Doehlert experimental design. Enzyme Microb Technol 40(5):1367–1372
Kamzolova SV, Chistiakova TI, Dediukhina EG, Shishkanova NV, Finogenova TV (1996) Effect of ethanol concentration on the maximum specific growth rate and composition of biomass in a mutant strain of Yarrowia lipolytica No. 1. Biull Eksp Biol Med 121(1):71–73, Russian
Kamzolova SV, Morgunov IG, Aurich A, Perevoznikova OA, Shishkanova NV, Stottmeister U, Finogenova TV (2005) Lipase secretion and citric acid production in Yarrowia lipolytica yeast grown on animal and vegetable fat. Food Technol Biotechnol 43(2):113–122
Klasson TK, Clausen EC, Gaddy JL (1989) Continuous Fermentation for the Production of Citric Acid from Glucose. Appl Biochem Biotechnol 20/21:491–509
Kozlova TM, Medvedeva GA, Glazunova LM, Finogenova TV (1981) Structural changes in Candida lipolytica cells during citric acid biosynthesis. Mikrobiologiia 50(3):508–514, Russian
Levinson WE, Kurtzman CP, Kuo TM (2007) Characterization of Yarrowia lipolytica and related species for citric acid production from glycerol. Enzyme Microb Technol 41:292–295
Lozinov AB, Finogenova TV, Glazunova LM, Illarionova VI (1974) Growth limitation in Candida lipolytica cultures and supersynthesis of metabolites. Mikrobiologiia 43(5):786–790, Russian
Moresi M (1994) Effect of glucose concentration on citric acid production by Yarrowia lipolytica. J Chem Technol Biotechnol 60(4):387–395
Papanikolaou S, Muniglia L, Chevalot I, Aggelis A, Marc I (2002) Yarrowia lipolytica as a potential producer of citric acid from raw glycerol. J Appl Microbiol 92(4):737–744
Papanikolaou S, Fakas S, Fick M, Chevalot I, Galiotou-Panayotou M, Komaitis M, Marc I, Aggelis G (2008) Biotechnological valorisation of raw glycerol discharged after bio-diesel (fatty acid methyl esters) manufacturing process: Production of 1, 3-propanediol, citric acid and single cell oil. Biomass Bioenerg 32:60–71
Rane KD, Sims KA (1995) Citric acid production by Candida lipolytica Y 1095 in cell recycle and fed-batch fermentors. Biotechnol Bioeng 46:325–332
Rymowicz W, Kautola H, Yu-Yen L, Linko P (1993) Studies on citric acid production with immobilized Yarrowia lipolytica in repeated batch and continuous air-lift bioreactors. Appl Microbiol Biotechnol 39(1):1–4
Rymowicz W, Rywińska A, Żarowska B, Juszczyk P (2006) Citric acid production from raw glycerol by acetate mutants of Yarrowia lipolytica. Chem Pap 60:391–395
Rywińska A, Rymowicz W (2010) High-yield production of citric acid by Yarrowia lipolytica on glycerol in repeated-batch bioreactors. J Ind Microbiol Biotechnol. doi:10.1007/s10295-009-0687-8
Rywińska A, Rymowicz W, Żarowska B, Wojtatowicz M (2009) Biosynthesis of citric acid from glycerol by acetate mutants of Yarrowia lipolytica in fed-batch fermentation. Food Technol Biotechnol 47(1):1–6
Stottmeister U, Behrens U, Weissbrodt E, Barth G, Franke-Rinker D, Schulze E (1982) Utilization of paraffins and other noncarbohydrate carbon sources for microbial citric acid synthesis. Z Allg Mikrobiol 22(6):399–424, Review. German
Sultanovich YA, Nechaev AP, Barsukova IA (1982) Method for quantitatively detecting fatty acid composition of lipids of microorganisms. SU 968072 (A1), C12Q1/00
Wojtatowicz M, Rymowicz W, Kautola H (1991) Comparison of different strains of the yeast Yarrowia lipolytica for citric acid production from glucose hydrol. Appl Biochem Biotechnol 31:165–174
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rymowicz, W., Fatykhova, A.R., Kamzolova, S.V. et al. Citric acid production from glycerol-containing waste of biodiesel industry by Yarrowia lipolytica in batch, repeated batch, and cell recycle regimes. Appl Microbiol Biotechnol 87, 971–979 (2010). https://doi.org/10.1007/s00253-010-2561-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2561-z