Abstract
Itaconic acid is one of the basic chemicals for the polymer industry, which can be produced on the basis of renewable raw materials. Since the middle of the twentieth century, itaconic acid has been produced industrially using the filamentous fungus Aspergillus terreus. But the demand for the organic acid is low due to the high production costs compared to alternative petrochemical manufactured raw materials. The high production costs are based on a low final titer, low productivities, and the usage of pure sugars, purified molasses, or starch hydrolysates, since the fungus reacts very sensitively to impurities in a culture medium. This review provides a comprehensive overview of the most recent developments, including a spectrum of studied microorganisms and their capabilities for the production of itaconic acid. The technological achievements in the biotechnological production of itaconic acid are presented. Particular attention is paid to current achievements in terms of suitable alternative substrates and their applicability in fermentation processes. Also, the pathway of itaconic acid and especially the influences on the fermentation process, which must be known in order to achieve a high final titer of itaconic acid, a yield close to the theoretical yield, and high productivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1837, itaconic acid was discovered as a thermal decomposition product of citric acid (Baup 1837). Itaconic acid is a single unsaturated dicarbonic acid and also known as methylene succinic acid (Fig. 1). Due to its chemical structure, one unsaturated double bond and two carboxyl groups, itaconic acid can be converted to a number of high-value bio-based chemicals or materials generated from carbohydrates (Werpy et al. 2004). The threefold functional structure allows a variety of reactions and applications and it is able to replace petrochemical-based methacrylic or acrylic acid (Magalhães et al. 2016b; Robert and Friebel 2016). Because of the two carboxyl groups, itaconic acid has two pKa values, pKa1 = 3.84 and pKa2 = 5.55 at 25 °C. In an aqueous solution, the concentration of each dissociated form depends on the present pH value. Mainly the non-dissociated acid occurs at pH values lower than pH 2, wherein at a pH value higher than pH 7, the doubly dissociated itaconate is for the most part present. Between pH 2 and pH 7, a mixture of all dissociation forms occurs (Krull et al. 2017b; Rychtera and Wase 1981). Itaconic acid crystallizes in rhombic double pyramids and has the physical constants given in Table 1 (Willke and Vorlop 2001).
The varieties of functional groups in the itaconic acid molecule make them an effective intermediate in the preparation of complex organic compounds. Different reactions, like salt formation with metals, esterification with alcohols, anhydride formation, addition reactions, and polymerization, are possible.
The industrially versatile usability of itaconic acid and its derivatives, like 3-methylpyrrolidin, 2-methyl-1,4-butanediamine, itaconic acid diamide, 3-methyltetrahydrofuran, or unsaturated esters, are reflected in the wide range of applications, e.g., styrene-butadiene rubber, synthetic latex, superabsorbent polymers, unsaturated polyester resins, and detergents. The field of application of these products is widespread and ranges from the paint, lacquer, and paper industries through to hygiene and medical products up to the construction sector. Usage in coatings, plasticizers, chemical fibers, or other plastics is also known (Delidovich et al. 2016; Klement and Büchs 2013; Kumar et al. 2017; Magalhães et al. 2016b; Okabe et al. 2009; Robert and Friebel 2016; Saha 2017; Willke and Vorlop 2001).
In the case of a further reduction of the itaconic acid production costs, a future application of itaconic acid could be methyl methacrylate production (thermoplastics). Thus, the estimated projected market of itaconic acid in 2020 could reach nearly 407,000 t/a, with a market value of approximately US$500 million (Weastra sro 2013). If the itaconic acid were to be produced on the basis of alternative raw materials, the production costs of polyitaconic acid would have to decline from US$3/kg to US$1.50/kg to replace petrochemical-based polyacrylic acid (Durant 2009).
Chemical production of itaconic acid
A chemical method to produce itaconic acid is the pyrolysis of citric acid and the hydrolysis of these anhydrides (Baup 1837). Itaconic acid was also produced using the decarboxylation of aconitic acid and the name itaconic acid was obtained as an anagram. Other chemical syntheses, such as distillation of citric acid and further treatment of the yielded anhydrides, oxidation of mesityl oxides with the subsequent isomerization of the formed citraconic acid, or oxidation of isoprene, are known (Willke and Vorlop 2001).
Biotechnological production of itaconic acid
The first biotechnological production of itaconic acid was described by Kinoshita (1932) with Aspergillus itaconicus. Calam (1939) achieved higher itaconic acid concentrations with Aspergillus terreus as a surface culture. Charles Pfizer Co. applied the first patent in 1945 for an industrial production process of itaconic acid by a submerged cultivation of A. terreus (Kane et al. 1945) and built the first production plant in Brooklyn, NY, USA, in 1955.
Further production facilities were located in Japan, England, and France. Due to the demands for the lowest possible production costs, the production facilities were increasingly relocated to the Asia-Pacific region (Okabe et al. 2009; Weastra sro 2013).
As a result of continuous optimization of the itaconic acid production with A. terreus since the 1960s, the biotechnological production process is more economical compared to the chemical processes, and the chemical processes cannot compete with the biotechnological process (Tate 1981).
The worldwide estimated itaconic acid production in 2011 was 41,400 t with a market value of US$74.5 million (Weastra sro 2013). The price per kilogram of itaconic acid varies between US$1.80 and US$2.00 depending on the manufacturer and quality of the product.
It was assumed that a phosphate limitation is required for itaconic acid overproduction by A. terreus (Klement and Büchs 2013; Rychtera and Wase 1981; Welter 2000). However, this thesis has been refuted. Neither a phosphate nor a nitrogen limitation is necessary to initiate a production of itaconic acid (Hevekerl et al. 2014b; Krull et al. 2017b). The trigger for the overproduction of itaconic acid in A. terreus is still unknown. However, the biosynthesis including the enzymes and transporters has been very well studied and described.
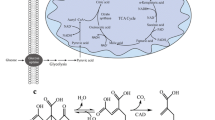
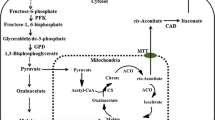
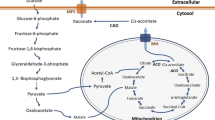
For the formation of itaconic acid (Fig. 2), sugars, such as glucose, xylose, or arabinose, are uptakes from A. terreus and converted to pyruvate in the cytosol via the metabolic pathway glycolysis and the pentose phosphate pathway (Eimhjellen and Larsen 1955; Saha et al. 2017). The pyruvate is transported into the mitochondrion and catalyzed over the citrate cycle to citrate and cis-aconitate. Via a citrate malate antiporter or mitochondrial tricarboxylate transporter, cis-aconitate gets into the cytosol, where it is converted to itaconate by cis-aconitate decarboxylase (CAD) (Bonnarme et al. 1995; Jaklitsch et al. 1991; Steiger et al. 2013). Afterwards, the itaconate enters the medium using a transporter, which presumably belongs to the group of major facilitator superfamily proteins (Li et al. 2011). Thus, in the reaction of 1 mol glucose, xylose, or arabinose, 1 mol of itaconic acid can be formed. This corresponds to a maximum theoretical yield of 72% (Saha et al. 2017).
Simplified illustration of the pathway of itaconic acid in A. terreus (Bonnarme et al. 1995; Jaklitsch et al. 1991; Li et al. 2011) and equilibrium of weak acids in the medium and cytoplasm (Lambert and Stratford 1999). (1) Citrate synthase. (2) Isocitrate dehydrogenase. (3) Citrate malate antiporter, mitochondrial tricarboxylate transporter (mtt). (4) Cis-aconitate decarboxylase (CAD). (5) Major facilitator superfamily protein (mfs). (6) H+-ATPase. EMP Embden-Meyerhof-Parnas, PPP pentose phosphate pathway
In addition to the industrially used A. terreus strain, other organisms, like Ustilaginaceae, are also able to form itaconic acid (Geiser et al. 2014; Guevarra and Tabuchi 1990; Haskins et al. 1955). According to the current literature, the trigger of itaconic acid overproduction in Ustilago maydis is ammonium limitation (Maassen et al. 2014). The metabolic pathway of U. maydis to form itaconic acid is comparable to the metabolic pathway of A. terreus. However, according to Geiser et al. (2016a) U. maydis converts cis-aconitate in the cytosol first to trans-aconitate using a cytosolic aconitate-Δ-isomerase (Adi1). Trans-aconitate is then catalyzed by a novel enzyme, trans-aconitate decarboxylase (Tad1), into itaconic acid. Geiser et al. (2016a) showed that U. maydis synthesized itaconate via the unusual intermediate trans-aconitate. In addition to the genes for the enzymes aconitate-Δ- isomerase and trans-aconitate decarboxylase, also a mitochondrial TCA transporter (mtt1) and an itaconate transporter protein (Itp1), which belong to the major Facilitator superfamily, were found in the same gene cluster of itaconic acid synthesis (Geiser et al. 2016a). Furthermore, Zambanini et al. (2017a) examined two of the native promoters, Ptad1 and Pmtt1 from the itaconate cluster of U. maydis. It is known that the trigger of itaconic acid overproduction in U. maydis is ammonium limitation, and it is postulated that these two promoters are well suited to induce gene expression in response to the nitrogen limitation that is coupled to the itaconic acid production phase. This investigation provides a new set of genetic tools leading to further improvement of the organic acid production using metabolic engineering strategies with Ustilago (Zambanini et al. 2017a).
Wild-type strains
In addition to the industrially used A. terreus strain, other organisms, like Ustilaginaceae, are able to form itaconic acid (Geiser et al. 2014; Guevarra and Tabuchi 1990; Haskins et al. 1955). Also known as itaconic acid producers are yeasts of the species Pseudozyma (Levinson et al. 2006; Specht et al. 2014), Candida (Tabuchi et al. 1981), and the fungi Rhodotorula (Kawamura et al. 1981) and Helicobasidium (Sayama et al. 1994). From these alternative itaconic acid producers, Specht et al. (2014) achieved the highest itaconic acid concentration of 75 g/L with an oscillating dissolved oxygen concentration. The highest currently published titer of itaconic acid produced with the genus Ustilago is 44.5 g/L (Maassen et al. 2014). Both titers are well below achieved concentration of 130–160 g/L itaconic acid with A. terreus (Hevekerl et al. 2014b; Karaffa et al. 2015; Krull et al. 2017b). There are also mammalian macrophages (Strelko et al. 2011) that are known to produce itaconic acid in traces. An overview of various itaconic acid-producing wild-type strains is given in Table 2.
Genetically engineered strains
In addition to the natural itaconic acid-producing strains, genetically modified organisms are increasingly produced. Plasmids with the full itaconic acid biosynthesis cluster are introduced into microorganisms that possess the citrate cycle, to overexpress the production of itaconic acid. That includes enzymes, like the key enzyme cis-aconitate decarboxylase (CAD), which catalyze cis-aconitate to itaconic acid (Kanamasa et al. 2008; Li et al. 2011). Also important transporter systems, including the transport of cis-aconitate from the mitochondrion into the cytosol (mttA) and the transport of itaconic acid out of the cell (mfsA), are transferred to organisms which are not naturally itaconic acid producers (Li et al. 2011). An overview of various itaconic acid-producing genetically modified organisms is given in Table 3. The itaconic acid production by genetically engineered microorganisms is generally based on the following major strategies:
-
Using itaconic acid-producing wild-type strains:
Aspergillus niger, the natural citric acid producer (typical industrial citric acid fermentation up to 200 g/L (Roehr et al. 1996)), is phylogenetically a close relative of A. terreus, but without the key enzyme cis-aconitate decarboxylase and therefore not able to produce itaconic acid. An increased production of citric acid was yielded with a modified pfkA gene in relation to the parental strain. Tevz et al. inserted the modified A. niger pfkA genes into the A. terreus ATCC 20542 strain and increased the average productivity between 48 and 96 h and the final titer of itaconic acid after 168 h to 0.44 g/L/h and 45.5 g/L compared to the parental strain with 0.079 g/L/h and 21.4 g/L itaconic acid (Tevz et al. 2010). According to the literature, the strain A. terreus ATCC 20542 is a wild-type parent strain used for industrial lovastatin-overproducing strains (Alberts et al. 1980; Lai et al. 2007; Monaghan et al. 1981). To study the biosynthesis mechanism and increase the itaconic acid production, Huang et al. provided a gene-targeting system as an efficient platform of sequential and multiple genetic modifications in A. terreus (Huang et al. 2016).
The natural itaconic acid-producing wild-type strain U. maydis also produces 2-hydroxyparaconate (Geiser et al. 2016b). Geiser et al. proposed that the conversion of itaconic acid to 2-hydroxyparaconate is catalyzed by the P450 monooxygenase Cyp3, encoded by cyp3. Due to the deletion of cyp3 and the simultaneous overexpression of the gene cluster regulator ria1, an itaconate hyper producer strain (4.5-fold more itaconate, without the side product 2-hydroxyparaconate) was generated. The combination with a process optimization, led to an increased final itaconate titer, productivity, and yield (Geiser et al. 2016b).
Zambanini et al. (2017b) shifts the organic acid production towards itaconate using metabolic engineering. The transcriptional regulator and mitochondrial transporter, ria1 or mtt1, from the itaconate cluster of U. maydis were overexpressed in Ustilago vetiveriae, which resulted in 2.0-fold (ria1) and 1.5-fold (mtt1) higher itaconic acid titer compared to the wild-type strain. Additionally, the malate production was reduced by 75 and 41%, respectively (Zambanini et al. 2017b).
-
Using non-itaconic acid-producing wild-type strains:
According to the literature, several approaches are published that use metabolic engineering and A. niger as a host to increase the itaconic acid production (Blumhoff et al. 2013; Hossain et al. 2016; Li et al. 2012; van der Straat et al. 2013; van der Straat et al. 2014). So far, the highest achieved titer of itaconic acid with a genetically engineered A. niger is 26.2 g/L (Hossain et al. 2016). Hossein et al. overexpressed a putative cytosolic citrate synthase citB in A. niger strains which contain the full itaconic acid biosynthesis cluster. Using that approach, they achieved a maximum productivity of 0.35 g/L/h without the formation of any side products (Hossain et al. 2016).
Corynebacterium glutamicum is widely used as a host for the industrial production of L-glutamate and L-lysine. It is used as a model organism in industrial biotechnology, but not able to produce itaconic acid because of the absence of the key enzyme cis-aconitate decarboxylase (CAD). Expression of the CAD gene from A. terreus followed by optimizing the CAD activity as well as cultivation conditions, and reduction of the isocitrate dehydrogenase activity, yielding a final titer of 7.8 g/L itaconic acid and a maximum volumetric productivity of 0.27 g/L/h with a genetically engineered C. glutamicum (Otten et al. 2015).
To improve the itaconic acid production, the approach of using faster-growing organisms was conducted. Therefore, genetical engineering of Escherichia coli, as a well-examined and fast-growing organism, was carried out. The generated strain E. coli ita23 (MG1655 ΔaceA ΔsucCD ΔpykA ΔpykF Δpta ΔPicd::cam_P2 pCadCs; plasmid pCadCs: BBa_J23100_cadA_gltA_T, KanR, ColE1) produced 32 g/L itaconic acid after 120 h and a maximum yield of 0.77 mol/molglucose (0.56 g/gglucose). This is the highest titer reported so far using heterologous itaconic acid production (Harder et al. 2016).
Blazeck et al. increased the itaconic acid production in S. cerevisiae using a computationally guided genetic manipulation in order to increase the metabolic capacity. Three genetic knockouts (Δade3 Δbna2 Δtes1), combined with a higher cell density fermentation, improved the itaconic acid final titer to 168 mg/L (Blazeck et al. 2014).
Chin et al. used CO2 as the carbon source for the photosynthetic production of itaconic acid with the engineered cyanobacterium Synechocystis sp. PCC6803 strain, expressing the key enzyme cis-aconitate decarboxylase (pJAK12-cad).
Due to the natural ability to accumulate citric acid intermediates as well as the tolerance against lower pH values, Yarrowia lipolytica was engineered to produce itaconic acid. Blazeck et al. expressed the key enzyme cis-aconitate decarboxylase as well as cytosolic aconitase enzymes and generated the PO1f leucine+uracil+ CAD (2×) ACOnoMLS Epi CAD Epi strain that yielded 4.6 g/L itaconic acid (Blazeck et al. 2015).
Influences on the fermentation process
An economical itaconic acid production requires a high final titer of itaconic acid, a yield close to the theoretical yield, and a high productivity. To achieve these goals, it is essential to know the parameters that influence these factors and to carry out the cultivation under reproducible conditions. In the following section, the most important factors influencing the biotechnological production of itaconic acid are described in more detail.
Morphology
The morphology of filamentous fungi influences the productivity in submersed culture. The reproduction of the filamentous fungus Aspergillus terreus is asexual, either through the formation of conidium spores or via chlamydospores (Deak et al. 2009). The formation of conidium spores is described in surface and submerged cultures (Fuchs and Schlegel 2006; Hevekerl 2016; Hevekerl et al. 2014b).
Chlamydospores are formed in submerged cultures. A pH shift during a cultivation of A. terreus led to a massive formation of submerged conidia. These conidia were derived from the formation of chlamydospores, and also from the formation of submerged conidiophores. The formation of the conidiophores was observed when NaOH and KOH were used as lye. The addition of an equivalent amount of sodium ions to the fermentation broth at the time of the pH shift in the form of NaCl did not result in the formation of any conidium. The use of ammonia solution as lye for the pH shift resulted in the formation of chlamydospores, but no conidiophores were visible (Hevekerl 2016; Hevekerl et al. 2014b). The chlamydospores are twice as large at 4–7 μm as the conidial spores at 2–4 μm (Deak et al. 2009; Hevekerl 2016).
The spores of A. terreus form germ tubes, which become hyphae. The subsequent growth of the hyphae takes place in the form of hyphae growth at the hypha-apexes or about the division of the hypha-apexes (the top of the hyphae). A network-like structure, the mycelium, is formed through the growth and fusion of hyphae (Kück et al. 2009).
Due to chemical and physical influences, A. terreus grows in different morphological forms in submerged cultures. The morphology ranges from branched mycelium filaments (filamentous growth) to densely interwoven mycelium masses (pellets) (Papagianni 2004). According to the literature, loose pellets with a diameter of 0.1–0.5 mm show the highest productivity and yield of itaconic acid (Gyamerah 1995a). The morphology of more compact or larger pellets leads to limitations regarding oxygen supply and the supply with the nutrients. Additionally, the diffusion of the metabolic products will be a problem. Especially a limitation of oxygen supply should be avoided in the production of itaconic acid (Gyamerah 1995a; Kuenz et al. 2012). Moreover, the formation of mycelium causes limitations due to increased viscosity that results in a poorer mixing of the fermentation broth.
In addition to the industrially used A. terreus strain, other organisms, like Ustilaginaceae, are able to form itaconic acid. The best-described species is U. maydis. It serves as a model organism for the study of pathogenesis in plants (Bölker 2001) and was fully sequenced in 2006 (Kamper et al. 2006). The growth of U. maydis is dimorphic. The non-pathogenic haploid cells can reproduce by budding, and show a yeast-like appearance or form pseudohyphae or true hyphae (Begerow et al. 2006; Bölker 2001). Using U. maydis for the production of itaconic acid, a yeast-like unicellular growth has been described throughout the literature (Guevarra and Tabuchi 1990; Klement and Büchs 2013; Maassen et al. 2014).
Medium components
Important for the productivity of the most frequently used commercial itaconic acid producer A. terreus is the shaped morphology which is immensely influenced by several medium components. Iron, zinc, calcium, cobalt, nickel, and manganese ions in the medium can have an especially strong influence on the morphology of A. terreus (Batti and Schweiger 1963; Gyamerah 1995a; Karaffa et al. 2015; Kobayashi 1978; Kuenz et al. 2012; Lockwood and Reeves 1945) For example, too low calcium concentrations lead to very compact pellets, whereas a concentration of 20 g/L CaCl2 × 2 H2O yield an almost pseudo-, yeast-like morphology (Kuenz et al. 2012). With regard to manganese ions, Karaffa et al. (2015) recommend a medium with a concentration of lower than 3 μg/L. Higher manganese concentrations lead to a changed morphology in the form of a loose, highly branched mycelium, resulting in reduced itaconic acid yield and productivity. Thus, the influence of manganese and other heavy metal ions on the morphology, and thus on the production, of itaconic acid with A. terreus is very similar to the production of citric acid with A. niger (Choudhary and Pirt 1966; Clark et al. 1966; Tomlinson et al. 1950). In addition to the morphology, heavy metals also inhibit relevant enzymes, such as the key enzyme CAD, in itaconic acid production (Dwiarti et al. 2002).
pH value
In the literature, very different approaches regarding the pH value and process strategies are described using A. terreus for the cultivation of itaconic acid. For the initial pH, a broad range is used from pH 2 to pH 5.9 (Gyamerah 1995b; Park et al. 1993; Rychtera and Wase 1981; Yahiro et al. 1995). The pH has an influence on the germination speed of the conidia. pH values lower than pH 3 slow the germination and thereby postpone the itaconic acid production (Batti and Schweiger 1963; Hevekerl et al. 2014b). After the germination of the spores, the ammonium ions, which serve as the nitrogen source, are consumed. This process releases protons, which cause a drop of the pH in the unbuffered media (Mattey 1992). Low pH values during the growth of the mycelium are necessary to acquire the ability to produce itaconic acid. Independent of the pH of the production medium, the mycelium grown at pH 2 was able to produce itaconic acid, whereas the mycelium grown at pH 6 did not produce itaconic acid (Larsen and Eimhjellen 1955). Larsen and Eimhjellen suggested that under these low pH values, relevant enzyme systems are activated which are necessary for the itaconic acid production. After the start of itaconic acid production, different pH-related strategies lead to a similar final titer of about 90 g/L itaconic acid. Kuenz et al. (2012) adjusted the pH value to pH 3.1 at the beginning of the cultivation and did not subsequently adjust the pH, so that it dropped below pH 2. On the other hand, Batti and Schweiger (1963) once raised the pH value to pH 3.2 with ammonia water after 5.8 days.
A significant increase in the final titer of itaconic acid, far beyond 90 g/L, was achieved by changing the pH value during the production phase of the cultivation. A rise in the pH value, due to a single pH shift or a pH control, increased the final itaconic acid titer from 87 to 146 g/L (Hevekerl et al. 2014b). Currently, the highest yield of itaconic acid of 160 g/L is achieved under a pH control in the production phase to pH 3.4 (Krull et al. 2017b). The degree of the dissociation of itaconic acid shifts with increasing pH from H2IA to HIA− and finally to IA2− depending on the pH value. Krull et al. showed a correlation between the increased final titer of itaconic acid with the increased itaconic acid form of HIA−, which resulted from the pH control to 3.4 in the production phase. So far, a further increase of the pH value in order to produce more HIA− and thus higher final itaconic acid titer was not successful because of a changed morphology (Krull et al. 2017b).
Using Ustilaginaceae for the production of itaconic acid, the pH dependency of the itaconic acid is described in the literature (Geiser et al. 2014). The well-described U. maydis yielded a titer of 44.5 g/L itaconic acid under pH-controlled cultivations at pH 6 (Maassen et al. 2014).
Oxygen supply
The formation of itaconic acid is strongly dependent on a sufficient oxygen supply. For the itaconic acid production with A. terreus, it is known that a short interruption of the aeration results in the decrease of the productivity or even to a complete stop of the itaconic acid production (Gyamerah 1995b; Kuenz et al. 2012; Larsen and Eimhjellen 1955; Nelson et al. 1952; Pfeifer et al. 1952). The critical duration until a negative effect occurs can be as short as 3 min, but can differ, depending on the used cultivation parameters. According to literature, centrifugation or filtration of the mycelium leads to the loss of itaconic acid production (Larsen and Eimhjellen 1955). Kuenz et al. 2012 showed that independent of the used itaconic acid-producing strain, the sufficient and especially continuous supply of oxygen during the production phase is essential. The fungus is able to regain the ability to produce itaconic acid after an oxygen limitation. After 20–24 h, the itaconic acid production starts again, but with a much lower productivity (Gyamerah 1995b; Pfeifer et al. 1952). This delay is presumably caused by the synthesis of new protein systems in the cells (Gyamerah 1995b).
During the production phase, the dissolved oxygen level only slightly affected the itaconic acid production with A. terreus. Park et al. (1993) varied the dissolved oxygen level between 20 and 60% and found no influence on the reached itaconic acid concentrations, but a slightly better yield at lower dissolved oxygen levels (Park et al. 1993).
Other itaconic acid-producing organisms have not yet been studied for the influence of oxygen levels and limitations. However, it can be assumed that for the cultivation of U. maydis, a high input of oxygen is necessary, due to the high shaking frequencies used in different studies (Carstensen et al. 2013; Geiser et al. 2016b; Guevarra and Tabuchi 1990; Maassen et al. 2014).
Side products
The production of itaconic acid using the fungus A. terreus depends, among other things, on the pH value. During itaconic acid production at pH 2.1, malic acid occurred as a side product, whereas during the cultivation at pH 6, no itaconic acid was formed but malic acid, succinic acid, and fumaric acid were accumulated (Larsen and Eimhjellen 1955). Changing the pH value during the production phase of the cultivation significantly influences the titer of itaconic acid as well as the production of side products. With a start pH of 3.1 and no control during the cultivation, the share of the side products are about 3.3%, consisting of 97% malic acid and α-ketoglutaric acid as the major side products, as well as cis-and trans-aconitate. Carrying out a pH shift after 2.1 days, the share of the side products decreased (Hevekerl et al. 2014b).
Using U. maydis as an itaconic acid-producing strain, the trigger of the overproduction of itaconic acid is ammonium limitation (Maassen et al. 2014). However, nitrogen limitation also causes the formation of intracellular and extracellular lipids (Haskins et al. 1955; Hewald et al. 2005; Maassen et al. 2014). Especially the formation of extracellular glycolipids, such as ustilagic acid in form of oil drops or mannosylerythritol lipid (MEL) in the form of needle-like crystals, has been demonstrated (Bölker et al. 2008).
Guevarra and Tabuchi 1990 provide an overview of Ustilaginaceae, including the product formation. In addition to the main product itaconic acid, U. maydis formed itatartaric acid, l-2-hydroxyparaconic acid, and malic acid.
U. cynodontis and U. rabenhorstiana produced, in addition to itaconic acid, itatartaric acid, l-2-hydroxyparaconic acid, and erythritol. U. spermophora formed the side products malic acid and succinic acid (Guevarra and Tabuchi 1990). Geiser et al. 2014 screened 68 Ustilaginaceae regarding their production of organic acids, polyols, and glycolipids. They identified U. maydis and U. vetiveriae as producers of itaconate, malate, and succinate as well as U. cynodontis and U. xerochloae as producers of itaconate, malate, succinate, and erythritol (Geiser et al. 2014).
Fermentation of alternative substrates
A. terreus is able to utilize not only different substrates like glucose, xylose, sucrose but also glycerol or ethanol for growth and itaconic acid production (Eimhjellen and Larsen 1955). The highest yields are achieved with sucrose and glucose (Hevekerl et al. 2014b; Karaffa et al. 2015; Krull et al. 2017b).
From 100 A. terreus strains, 20 strains showed good itaconic acid production from xylose and arabinose (Saha et al. 2017). Further on, 20 A. terreus strains were evaluated regarding their ability to convert mannose and galactose to itaconic acid. One of these strains, A. terreus NRRL 1971 showed, with 36.4 g/L itaconic acid and a yield of 0.46 g/gmannose, the highest itaconic acid titer reported so far using mannose as the carbon source (Saha and Kennedy 2017a).
To optimize the itaconic acid production, the incubation system was scaled down in microtiter plates (Hevekerl et al. 2014a). To examine the ability of A. terreus to utilize different monosaccharides, like glucose, xylose, arabinose, galactose, and rhamnose, a combination of these monosaccharides, the influence of enzyme formulation, as well as the influence of sugar degradation products, microtiter plates were used successfully (Krull et al. 2017a; Saha and Kennedy 2017b).
As an alternative to the pure sugars, sugar beet and sugarcane molasses (Batti and Schweiger 1963; Kane et al. 1945), as well as starch hydrolysates (Cros and Schneider 1993; Dwiarti et al. 2007; Petruccioli et al. 1999; Reddy and Singh 2002; Yahiro et al. 1997), were successfully used for itaconic acid production. In addition, Reddy and Singh (2002) successfully produced itaconic acid on sugar extract from fruit peel waste.
Cultivation on starch hydrolysates or sugar extracts is significantly more successful because no sugar degradation products can be produced as an inhibitor by harsh pretreatment conditions of the lignocellulosic biomass. On the other hand, these harsh conditions are necessary for lignocellulose in order to break up the significantly more complex structure of lignocellulose compared to starch (Jönsson et al. 2013). The resulting inhibiting sugar degradation products or other impurities must be removed by usually complex purification processes in order to achieve a sufficient production of itaconic acid with A. terreus. Another possibility is to render the strain more resistant to potential inhibitors by mutagenesis. However, it is very difficult to compare different microorganisms cultured on different lignocellulose hydrolysates with different purification methods (Palmqvist and Hahn-Hägerdal 2000).
Li et al. (2016) used an undetoxified enzymatic hydrolysate of steam-exploded corn stover as the sole carbon source. In addition to glucose and xylose, this also contained formic acid and acetic acid. With an A. terreus wild-type strain, only 0.54 g/L itaconic acid is formed, presumably due to the inhibiting effect of the acids. By mutagenesis of the strain through atmospheric and room temperature plasma, the titer increased to 19.3 g/L itaconic acid (Li et al. 2016). By using activated carbon, Wu et al. (2017) reduced the concentration of furfural of the used wheat bran hydrolysate and increased the production of itaconic acid from 8 to 34.2 g/L. A further increase to 49.6 g/L itaconic acid was achieved using an A. terreus mutant (Wu et al. 2017). Krull et al. (2017a) succeeded the cultivation with a wild-type strain of A. terreus and without the use of activated carbon or ion exchanger and achieved 23.3 g/L itaconic acid with a yield of 0.27 (w/w) using a wheat chaff hydrolysate. Due to a deft hydrolysis method, the formation of inhibitors was avoided, and as a result of this, a complex purification was not necessary. Both process variables, final titer, and yield, could additionally increase to 27.7 g/L itaconic acid and 0.41 (w/w) by an optional removal of cations (Krull et al. 2017a). Pedroso et al. (2017) used a phosphoric hydrolysate of rice husks and produced 1.9 g/L itaconic acid with a yield of 49 mg/gsugar using A. terreus ATCC 10020. Jimenez-Quero et al. (2016) carried out different pretreatments of two lignocellulosic biomasses, wheat bran and corn cobs. Depending on the method, different concentrations of sugars, metals, or inhibitors, and different itaconic acid yields were obtained, depending on the Aspergillus strains they tested.
Another attempt is the utilization of the carbon source glycerol for the itaconic acid production. Compared to glucose as a substrate, Kuenz (2008) obtained with glycerol a similar growth of A. terreus DSM 23081 and 69.7 g/L itaconic acid after 15 days of cultivation. Juy et al. (2010) also used glycerol as the sole carbon source and A. terreus MJL05, isolated from vendee’s lands. After varying and optimizing C:N, N:P, and C:P ratios the itaconic acid production was improved to 27.6 g/L itaconic acid with a productivity of 0.192 g/L/h and a yield of 0.44 g/gglycerol. Zambanini et al. (2017b) achieved an itaconic titer of 34.7 g/L combined with 46.2 g/L malate from 196 g/L glycerol with a productivity of 0.09 g/L/h using the strain Ustilago vetiveriae TZ1. Also the itaconic acid production with glycerol, using E. coli scvCadA_No8, has been shown to be successful. Under optimized cultivation conditions, 7.2 g/L itaconic were produced after approximately 85 h (Jeon et al. 2016). A genetically engineered Yarrowia lipolytica produced 2.65 g/L itaconic acid after 168 h in a nitrogen-limited medium containing 100 g/L glycerol (Wang et al. 2012).
Kim et al. (2017) described a whole-cell bioconversion of citrate to itaconate using E. coli, with enhanced aconitase and cis-aconitate decarboxylase activities by controlling the expression of multiple cadA genes. For this reason, the conversion of 500 mM citrate to 319.8 mM (41.6 g/L) itaconate is carried out successfully without using buffer systems or additional cofactors.
An overview of itaconic acid-producing organisms from alternative substrates is given in Table 4.
Product recovery and purification
The quality and the environmental compatibility of product isolation and purification contribute significantly to the evaluation of a biotechnical process. The following methods in the product recovery of itaconic acid have already been described in the literature:
Solids, e.g., biomass, are separated by filtration or centrifugation continuously or at the end of batch cultivations. Subsequently, the itaconic acid can be crystallized using several evaporation and cooling processes, whereby an industrial degree of purity of the itaconic acid is achieved. If activated carbon and filtrations are used in evaporation processes, itaconic acid is obtained at a high quality. Afterwards, the residual solution is treated by liquid/liquid extraction or anion exchanger (Lockwood 1975). Using glucose or sucrose as a substrate, the precipitation of insoluble itaconic acid salts with high purity by crystallization is possible (Kobayashi 1971). To reduce the cost of product recovery and purification, technologies such as ultrafiltration for mycelial separation, bipolar electrodialysis (Kobayashi et al. 1972), reverse osmosis, or ion exchange techniques have been used (Kobayashi et al. 1973; Kobayashi et al. 1980).
Relating to industrial processes, the main steps of the product recovery of itaconic acid are decoloration, clarification, evaporation, and crystallization steps. The purification processes for itaconic acid were recently reviewed by Anjum et al. (2016), López-Garzón and Straathof (2014), and Magalhães et al. (2016b). A schematic diagram of the itaconic acid production and recovery process, including evaporation, crystallization steps, and recrystallization after activated carbon treatment are given by Magalhães et al. (2016b) and Okabe et al. (2009). Magalhães et al. reviewed the current state of the art of crystallization, precipitation, liquid-liquid extraction, membrane separation, and adsorption, including the comparison of the advantages and disadvantages of the separation methods (Magalhães et al. 2016b). According to Okabe et al., a high-purity itaconic acid requires solvent extraction, ion exchange, and decolorization (Okabe et al. 2009).
In addition to the already reviewed and known product recovery and purification processes, the following new findings are published. Holzhäuser et al.(2017) reduced the number of product recovery steps as well as the need of energy-intensive separation steps by using catalytic conversion within the fermentation broth. The potential of a chemo- and electrochemical reduction of itaconic acid to methylsuccinic acid using acidic media or a crude fermentation broth was under investigation. Pure itaconic acid was converted efficiently by means of chemocatalytic hydrogenation over Ru/C or RANEY® nickel. In real fermentation broths, the presence of various salts as well as glucose prevented direct chemocatalytic valorization. However, using electrochemical hydrogenation, the conversion of pure itaconic acid in a dilute sulfuric acid environment decreased only slightly compared to a real fermentation broth. A complete conversion and yield was achieved by simple optimizations of the reaction time and the substrate concentration (Holzhäuser et al. 2017).
Schute et al. (2016) uses the new findings in the field of selective liquid-phase adsorption, in which highly hydrophobic porous materials have been used, as new possibilities for process development and demonstrates the efficiency of selective liquid-phase adsorption for the separation and purification of itaconic acid from aqueous solutions. A wide variety of different adsorbents were under investigation, with surface polarity as well as texture properties as critical parameters for their performance. Depending on the pH value, the itaconic acid occurs as an acid (H2IA), in a single-protonated form (HIA−) as well as in the twice-protonated form (IA2−), which has a great influence on the adsorption performance. In addition, experiments were carried out on a continuously operated fixed bed adsorber and evaluated the desorption behavior (Schute et al. 2016).
Magalhães et al. (2016a) carried out the separation of itaconic acid by adsorption from aqueous solutions using two types of commercial, strongly basic ion exchange resins: Purolite A-500P and PFA-300. Parameters like the pH value, temperature, and itaconic acid concentration were varied in order to evaluate the separation process. After batch experiments, continuous adsorption experiments were performed using a fixed-bed column. A simplified mathematical model was developed; the adsorption parameters were determined and compared with the experimental data (Magalhães et al. 2016a).
Within the framework of the project BioConSept, different process techniques, like membrane, crystallization, electrodialysis, extraction, and adsorption, were evaluated in detail. Regarding the recovery process of itaconic acid, a process of fermentation and product recovery using freeze crystallization were investigated and tested up to bench scale (BioConSept 2016).
Economic aspects
On an industrial scale, itaconic acid is synthesized biotechnologically using the fungus A. terreus and a final concentration of about 80 g/L (Kumar et al. 2017; Willke and Vorlop 2001). Itaconic acid’s global production is estimated at more than 80,000 t/a, and about US$2 kg−1 (Okabe et al. 2009). Reasoned by the good efficiency of this material, it is estimated that the annual production capacity will increase by 5.5% between 2016 and 2023 (Marked Report 2015). Okabe et al. (2009) provide an overview about the supply of itaconic acid including the company, location, production start, and capacity. According to Okabe et al. 2009, the largest capacities of itaconic acid production are located at Eddyville (USA, Cargill) with 30,000 t/a, at Kyogyo (Japan, Iwata Chemicals) with 10,000 t/a, and Melle (France, Rhodia) with 10,000 t/a (Okabe et al. 2009).
Meanwhile, the itaconic acid production in the USA (Cargill), Japan (Iwata Chemicals), and France (Rhodia) have stopped and has been shifted to China (Weastra sro 2013).
Since 2000, China is an important supplier of itaconic acid. Among others, important companies and their capacities are Qingdao Kehai Biochemical Co., Ltd., and Zhejiang Guoguang Biochemistry Co., Ltd. (10,000–15,000 t/a (Tsao et al. 2010; Weastra 2013)) as well as Qingdao Langyatai Group (15,000 t/a, Jiaonan, Shandong), Shandong Kaison Biochemical (5000 t/a, Wulian, Shandong), Shandong Zhongshun Science And Technology Development Co., Ltd. (3000 t/a, Zibo, Shandong), Enjoyrong Biochemical Co.Ltd. (5000 t/a, Wulian, Rizhao, Shandong), Leader Industry Co.,Ltd. (15,000 t/a, Zhangqiu, Jinan, Shandong), and Chengdu Lake Biology Engineering Industry (4000 t/a, Sichuan) (Listofcompanies 2017; Okabe et al. 2009).
Leaf Technologies (LESAFFRE ADVANCED FERMENTATION TECHNOLOGIES, France) and Dutch DNA (The Netherlands), a TNO spin-out, entered into a research and development collaboration to develop a new itaconic acid-producing fungal strain, in order to reduce the itaconic acid production costs and to enable a wider use as a renewable chemical (LeafDutchDNO 2015a; LeafDutchDNO 2015b; McCoy 2015).
Concluding remarks
Itaconic acid is one of the basic chemicals for the polymer industry, which can be produced on the basis of renewable raw materials. Since the middle of the twentieth century, itaconic acid has been produced industrially using the filamentous fungus Aspergillus terreus. Compared to alternative petrochemical manufactured raw materials, the demand for biotechnological produced itaconic acid is low due to the high production costs. The costs of the biotechnological production of itaconic acid are mainly limited by low product concentration, low productivity and low yields. Particularly in recent years, many new findings have been presented in the field of biotechnical production of itaconic acid.
On the one hand, for the well-known production with the filamentous fungus Aspergillus terreus, the parameters influencing the production of itaconic acid, such as media components, pH value during cultivation and morphology, have been clearly pointed out. In this way it was possible to further improve the itaconic acid production with an A. terreus wild-type strain and achieve a similar level of concentration as in the industrial citric acid production.
On the other hand, in the field of pathways, new insights were gained in the enzyme systems involved, but above all in the field of transporter systems. This allows a better understanding of itaconic acid production, of naturally itaconic acid-producing strains, and their metabolic engineering. Furthermore, these findings enable also the metabolic engineering of non-itaconic acid-producing wild-type strains. It remains exciting what will arise in this area and also in the area of the usage of alternative raw materials in the future and their contribution to the price reduction of biotechnologically produced itaconic acid.
References
Alberts AW, Chen J, Kuron G, Hunt V, Huff J, Hoffman C, Rothrock J, Lopez M, Joshua H, Harris E, Patchett A, Monaghan R, Currie S, Stapley E, Albers-Schonberg G, Hensens O, Hirshfield J, Hoogsteen K, Liesch J, Springer J (1980) Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci U S A 77(7):3957–3961
Anjum S, Tripathi S, Singh N, Gupta KS (2016) Reactive extraction a boom for itaconic acid: a review. Int J Recent Sci Res 7(5):11372–11376
Batti M, Schweiger LB (1963) Process for the production of itaconic acid. United States Patent 3 078 217
Baup S (1837) Über eine neue Pyrogen- Citronensäure, und über Benennung der Pyrogen Säure überhaupt. Ann Chim Phys 19:29–38
Begerow D, Stoll M, Bauer R (2006) A phylogenetic hypothesis of Ustilaginomycotina based on multiple gene analyses and morphological data. Mycologia 98(6):906–916
BioConSept (2016) Final report summary—BIOCONSEPT (integration of bio-conversion and separation technology for the production and application of platform chemicals from 2nd generation biomass). Project ID: 289194; funded under: FP7-KBBE
Blazeck J, Hill A, Jamoussi M, Pan A, Miller J, Alper HS (2015) Metabolic engineering of Yarrowia lipolytica for itaconic acid production. Metab Eng 32:66–73. https://doi.org/10.1016/j.ymben.2015.09.005
Blazeck J, Miller J, Pan A, Gengler J, Holden C, Jamoussi M, Alper HS (2014) Metabolic engineering of Saccharomyces cerevisiae for itaconic acid production. Appl Microbiol Biot 98(19):8155–8164. https://doi.org/10.1007/s00253-014-5895-0
Blumhoff ML, Steiger MG, Mattanovich D, Sauer M (2013) Targeting enzymes to the right compartment: metabolic engineering for itaconic acid production by Aspergillus niger. Metab Eng 19:26–32. https://doi.org/10.1016/j.ymben.2013.05.003
Bölker M (2001) Ustilago maydis—a valuable model system for the study of fungal dimorphism and virulence. Microbiol-Sgm 147:1395–1401
Bölker M, Basse CW, Schirawski J (2008) Ustilago maydis secondary metabolism—from genomics to biochemistry. Fungal Genet Biol 45:S88–S93
Bonnarme P, Gillet B, Sepulchre AM, Role C, Beloeil JC, Ducrocq C (1995) Itaconate biosynthesis in Aspergillus-terreus. J Bacteriol 177(12):3573–3578
Calam CT (1939) Studies in the biochemistry of microorganisms. XXIII. Itaconic acid, a metabolic product of Aspergillus terreus Thom. Biochem J 33:1488–1495
Carstensen F, Klement T, Buchs J, Melin T, Wessling M (2013) Continuous production and recovery of itaconic acid in a membrane bioreactor. Bioresour Technol 137:179–187. https://doi.org/10.1016/j.biortech.2013.03.044
Chin T, Sano M, Takahashi T, Ohara H, Aso Y (2015) Photosynthetic production of itaconic acid in Synechocystis sp PCC6803. J Biotechnol 195:43–45. https://doi.org/10.1016/j.jbiotec.2014.12.016
Choudhary AQ, Pirt SJ (1966) The influence of metal-complexing agents on citric acid production by Aspergillus niger. J Gen Microbiol 43(1):71–81. https://doi.org/10.1099/00221287-43-1-71
Clark DS, Ito K, Horitsu H (1966) Effect of manganese and other heavy metals on submerged citric acid fermentation of molasses. Biotechnol Bioeng 8(4):465–471
Cros P, Schneider D (1993) Microbiological production of itaconic acid. United States Patent 5 231 016
Deak E, Wilson SD, White E, Carr JH, Balajee SA (2009) Aspergillus terreus accessory conidia are unique in surface architecture, cell wall composition and germination kinetics. PLoS One 4(10):e7673. https://doi.org/10.1371/journal.pone.0007673
Delidovich I, Hausoul PJ, Deng L, Pfutzenreuter R, Rose M, Palkovits R (2016) Alternative monomers based on lignocellulose and their use for polymer production. Chem Rev 116(3):1540–1599. https://doi.org/10.1021/acs.chemrev.5b00354
Durant Y (2009) Development of integrated production of polyitaconic acid from northeast hardwood biomass—NIFA project 2009–2012. Technical report, Itaconix, LCC
Dwiarti L, Otsuka M, Miura S, Yaguchi M, Okabe M (2007) Itaconic acid production using sago starch hydrolysate by Aspergillus terreus TN484-M1. Bioresour Technol 98(17):3329–3337. https://doi.org/10.1016/j.biortech.2006.03.016
Dwiarti L, Yamane K, Yamatani H, Kahar P, Okabe M (2002) Purification and characterization of cis-aconitic acid decarboxylase from Aspergillus terreus TN484-M1. J Biosci Bioeng 94(1):29–33
Eimhjellen KE, Larsen H (1955) Mechanism of itaconic acid formation by Aspergillus-terreus. 2. Effect of substrates and inhibitors. Biochem J 60(1–4):139–147
Fuchs G, Schlegel HG (2006) Allgemeine Mikrobiologie. Thieme Flexible Taschenbücher, Thieme Georg Verlag
Geiser E, Przybilla SK, Friedrich A, Buckel W, Wierckx N, Blank LM, Bolker M (2016a) Ustilago maydis produces itaconic acid via the unusual intermediate trans-aconitate. Microb Biotechnol 9(1):116–126. https://doi.org/10.1111/1751-7915.12329
Geiser E, Przybilla SK, Engel M, Kleineberg W, Buttner L, Sarikaya E, Hartog TD, Klankermayer J, Leitner W, Bolker M, Blank LM, Wierckx N (2016b) Genetic and biochemical insights into the itaconate pathway of Ustilago maydis enable enhanced production. Metab Eng 38:427–435. https://doi.org/10.1016/j.ymben.2016.10.006
Geiser E, Wiebach V, Wierckx N, Blank LM (2014) Prospecting the biodiversity of the fungal family Ustilaginaceae for the production of value-added chemicals. Fungal Biol Biotechnol 1(1):2. https://doi.org/10.1186/s40694-014-0002-y
Guevara ED, Tabuchi T (1990) Production of 2-hydroxyparaconic and itatartaric acids by Ustilago cynodontis and simple recoyery process of the acids. Agric Biol Chem 54(9):2359–2365
Guevarra ED, Tabuchi T (1990) Accumulation of itaconic, 2-hydroxyparaconic, itatartaric, and malic-acids by strains of the genus Ustilago. Agric Biol Chem 54(9):2353–2358
Gyamerah M (1995a) Factors affecting the growth form of Aspergillus terreus NRRL 1960 in relation to itaconic acid fermentation. Appl Microbiol Biot 44(3–4):356–361
Gyamerah MH (1995b) Oxygen requirement and energy relations of itaconic acid fermentation by Aspergillus terreus NRRL 1960. Appl Microbiol Biot 44(1–2):20–26
Harder BJ, Bettenbrock K, Klamt S (2016) Model-based metabolic engineering enables high yield itaconic acid production by Escherichia coli. Metab Eng 38:29–37. https://doi.org/10.1016/j.ymben.2016.05.008
Haskins RH, Thorn JA, Boothroyd B (1955) Biochemistry of the Ustilaginales. 11. Metabolic products of Ustilago zeae in submerged culture. Can J Microbiol 1(9):749–756
Hevekerl A (2016) Biotechnisch erzeugte Itaconsäure. Cuvillier Verlag
Hevekerl A, Kuenz A, Vorlop K-D (2014a) Filamentous fungi in microtiter plates—an easy way to optimize itaconic acid production with Aspergillus terreus. Appl Microbiol Biot:1–7 doi:https://doi.org/10.1007/s00253-014-5743-2
Hevekerl A, Kuenz A, Vorlop KD (2014b) Influence of the pH on the itaconic acid production with Aspergillus terreus. Appl Microbiol Biot 98(24):10005–10012. https://doi.org/10.1007/s00253-014-6047-2
Hewald S, Josephs K, Bolker M (2005) Genetic analysis of biosurfactant production in Ustilago maydis. Appl Environ Microb 71(6):3033–3040
Holzhäuser FJ, Artz J, Palkovits S, Kreyenschulte D, Büchs J, Palkovits R (2017) Electrocatalytic upgrading of itaconic acid to methylsuccinic acid using fermentation broth as a substrate solution. Green Chem 19(10):2390–2397. https://doi.org/10.1039/C6GC03153F
Hossain AH, Li A, Brickwedde A, Wilms L, Caspers M, Overkamp K, Punt PJ (2016) Rewiring a secondary metabolite pathway towards itaconic acid production in Aspergillus niger. Microb Cell Factories 15(1):130. https://doi.org/10.1186/s12934-016-0527-2
Huang XN, Chen M, Li JJ, Lu XF (2016) Establishing an efficient gene-targeting system in an itaconic-acid producing Aspergillus terreus strain. Biotechnol Lett 38(9):1603–1610. https://doi.org/10.1007/s10529-016-2143-y
Jaklitsch WM, Kubicek CP, Scrutton MC (1991) The subcellular organization of itaconate biosynthesis in Aspergillus terreus. J Gen Microbiol 137:533–539
Jeon HG, Cheong DE, Han Y, Song JJ, Choi JH (2016) Itaconic acid production from glycerol using Escherichia coli harboring a random synonymous codon-substituted 5'-coding region variant of the cadA gene. Biotechnol Bioeng 113(7):1504–1510. https://doi.org/10.1002/bit.25914
Jimenez-Quero A, Pollet E, Zhao M, Marchioni E, Averous L, Phalip V (2016) Itaconic and fumaric acid production from biomass hydrolysates by Aspergillus strains. J Microbiol Biotechnol 26(9):1557–1565. https://doi.org/10.4014/jmb.1603.03073
Jönsson LJ, Alriksson B, Nilvebrant NO (2013) Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels 6:16
Juy MI, Orejas JA, Lucca ME (2010) Study of itaconic acid production by Aspergillus terrus MJL05 strain with different variable. Rev Colom Biotechnol 12:187–193
Kamper J, Kahmann R, Bolker M, Ma LJ, Brefort T, Saville BJ, Banuett F, Kronstad JW, Gold SE, Muller O, Perlin MH, Wosten HAB, de Vries R, Ruiz-Herrera J, Reynaga-Pena CG, Snetselaar K, McCann M, Perez-Martin J, Feldbrugge M, Basse CW, Steinberg G, Ibeas JI, Holloman W, Guzman P, Farman M, Stajich JE, Sentandreu R, Gonzalez-Prieto JM, Kennell JC, Molina L, Schirawski J, Mendoza-Mendoza A, Greilinger D, Munch K, Rossel N, Scherer M, Vranes M, Ladendorf O, Vincon V, Fuchs U, Sandrock B, Meng S, Ho ECH, Cahill MJ, Boyce KJ, Klose J, Klosterman SJ, Deelstra HJ, Ortiz-Castellanos L, Li WX, Sanchez-Alonso P, Schreier PH, Hauser-Hahn I, Vaupel M, Koopmann E, Friedrich G, Voss H, Schluter T, Margolis J, Platt D, Swimmer C, Gnirke A, Chen F, Vysotskaia V, Mannhaupt G, Guldener U, Munsterkotter M, Haase D, Oesterheld M, Mewes HW, Mauceli EW, DeCaprio D, Wade CM, Butler J, Young S, Jaffe DB, Calvo S, Nusbaum C, Galagan J, Birren BW (2006) Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 444(7115):97–101
Kanamasa S, Dwiarti L, Okabe M, Park EY (2008) Cloning and functional characterization of the cis-aconitic acid decarboxylase (CAD) gene from Aspergillus terreus. Appl Microbiol Biot 80(2):223–229. https://doi.org/10.1007/s00253-008-1523-1
Kane J, Finlay A, Amann P (1945) Production of itaconic acid. United States Patent 2 385 283
Karaffa L, Diaz R, Papp B, Fekete E, Sandor E, Kubicek CP (2015) A deficiency of manganese ions in the presence of high sugar concentrations is the critical parameter for achieving high yields of itaconic acid by Aspergillus terreus. Appl Microbiol Biot 99(19):7937–7944. https://doi.org/10.1007/s00253-015-6735-6
Kawamura D, Furuhashi M, Saito O, Matsui H (1981) Japan Patent 56 137 893
Kim J, Seo HM, Bhatia SK, Song HS, Kim JH, Jeon JM, Choi KY, Kim W, Yoon JJ, Kim YG, Yang YH (2017) Production of itaconate by whole-cell bioconversion of citrate mediated by expression of multiple cis-aconitate decarboxylase (cadA) genes in Escherichia coli. Sci Rep 7:39768. https://doi.org/10.1038/srep39768
Kinoshita K (1932) Über die Produktion von Itaconsäure und Mannit durch einen neuen Schimmelpilz, Aspergillus itaconicus. Acta Phytochimica 5:271–287
Klement T, Büchs J (2013) Itaconic acid—a biotechnological process in change. Bioresour Technol 135:422–431. https://doi.org/10.1016/j.biortech.2012.11.141
Kobayashi T (1971) Process for recovering itaconic acid and salts thereof from fermented broth. Japan Patent 3 621 053
Kobayashi T (1978) Production of itaconic acid from wood waste. Process Biochem 13(5):15–22
Kobayashi T, Nakamura I, Nakagawa M (1972) Process design for itaconic acid fermentation. Proc IV IFS: Ferm Technol today. 215–221
Kobayashi T, Nakamura I, Nakagawa M (1973) Itaconic acid production. Japan Patent 48 092 584
Kobayashi T, Nakamura I, Nakagawa M (1980) Itaconic acid production. Japan Patent 51 028 711
Krull S, Eidt L, Hevekerl A, Kuenz A, Prüße U (2017a) Itaconic acid production from wheat chaff by Aspergillus terreus. Process Biochem 63:169–176. https://doi.org/10.1016/j.procbio.2017.08.010
Krull S, Hevekerl A, Kuenz A, Prüße U (2017b) Process development of itaconic acid production by a natural wild type strain of Aspergillus terreus to reach industrially relevant final titers. Appl Microbiol Biot 101:4063–4072. https://doi.org/10.1007/s00253-017-8192-x
Kück U, Nowrousian M, Reiß J, Hoff B, Engh I (2009) Schimmelpilze: Lebensweise, Nutzen, Schaden, Bekämpfung. Springer
Kuenz A (2008) Itaconsäureherstellung aus nachwachsenden Rohstoffen als Ersatz für petrochemisch hergestellte Acrylsäure. PhD thesis, Technical University of Braunschweig
Kuenz A, Gallenmüller Y, Willke T, Vorlop K-D (2012) Microbial production of itaconic acid: developing a stable platform for high product concentrations. Appl Microbiol Biot 96(5):1209–1216. https://doi.org/10.1007/s00253-012-4221-y
Kumar S, Krishnan S, Samal SK, Mohanty S, Nayak SK (2017) Itaconic acid used as a versatile building block for the synthesis of renewable resource-based resins and polyesters for future prospective: a review. Polym Int 66(10):1349–1363. https://doi.org/10.1002/pi.5399
Lai LST, Hung CS, Lo CC (2007) Effects of lactose and glucose on production of itaconic acid and lovastatin by Aspergillus terreus ATCC 20542. J Biosci Bioeng 104(1):9–13
Lambert RJ, Stratford M (1999) Weak-acid preservatives: modelling microbial inhibition and response. J Appl Microbiol 86(1):157–164
Larsen H, Eimhjellen K (1955) The mechanism of itaconic acid formation by Aspergillus terreus. 1. The effect of acidity. Biochem J 60(1):135–139
LeafDutchDNO (2015a) Leaf Technologies and Dutch DNA Biotech enter in R&D collaboration for the development of a value added fermentation solution to produce itaconic acid. http://www.lesaffre.com/wp-content/uploads/2015/10/PR_LeafDutchDNO_EN.pdf. Accessed 22.12.2017
LeafDutchDNO (2015b) LEAF Technologies partners with Dutch DNA to produce itaconic acid. Int Sugar J 117(1403):783–783
Levinson WE, Kurtzman CP, Kuo TM (2006) Production of itaconic acid by Pseudozyma antarctica NRRL Y-7808 under nitrogen-limited growth conditions. Enzyme Microb Tech 39(4):824–827
Li A, Pfelzer N, Zuijderwijk R, Punt P (2012) Enhanced itaconic acid production in Aspergillus niger using genetic modification and medium optimization. BMC Biotechnol 12:57
Li A, van Luijk N, ter Beek M, Caspers M, Punt P, van der Werf M (2011) A clone-based transcriptomics approach for the identification of genes relevant for itaconic acid production in Aspergillus. Fungal Genet Biol 48(6):602–611. https://doi.org/10.1016/j.fgb.2011.01.013
Li X, Zheng K, Lai C, Ouyang J, Yong Q (2016) Improved itaconic acid production from undetoxified enzymatic hydrolysate of steam-exploded corn stover using an Aspergillus terreus mutant generated by atmospheric and room temperature plasma. Bioresources 11(4):9047–9058
Listofcompanies (2017) http://www.listofcompaniesin.com/china/itaconic-acid/. Accessed 22.12.2017
Lockwood LB (1975) Production of organic acids by fermentation. In: Peppler HJ, Perlman D (eds) Microbial technology, vol 2. Academic Press, New York, pp 356–386
Lockwood LB, Reeves MD (1945) Some factors affecting the production of itaconic acid by Aspergillus terreus. Arch Biochem 6(3):455–469
López-Garzón CS, Straathof AJJ (2014) Recovery of carboxylic acids produced by fermentation. Biotechnol Adv 32(5):873–904. https://doi.org/10.1016/j.biotechadv.2014.04.002
Maassen N, Panakova M, Wierckx N, Geiser E, Zimmermann M, Bölker M, Klinner U, Blank LM (2014) Influence of carbon and nitrogen concentration on itaconic acid production by the smut fungus Ustilago maydis. Eng Life Sci 14:129–134. https://doi.org/10.1002/elsc.201300043
Magalhães AI, de Carvalho JC, Ramírez ENM, Medina JDC, Soccol CR (2016a) Separation of itaconic acid from aqueous solution onto ion-exchange resins. J Chem Eng Data 61(1):430–437. https://doi.org/10.1021/acs.jced.5b00620
Magalhães AI Jr, de Carvalho JC, Medina JD, Soccol CR (2016b) Downstream process development in biotechnological itaconic acid manufacturing. Appl Microbiol Biotechnol 101:1–12. https://doi.org/10.1007/s00253-016-7972-z
Marked Report (2015) Transparency market research,Market Report, Itaconic Acid
Mattey M (1992) The production of organic acids. Crit Rev Biotechnol 12(1–2):87–132
McCoy M (2015) European biotechs eye itaconic acid. Chem Eng News 93(41):16–17 Concentrates
Monaghan RL, Alberts AW, Hoffman CH, Albers-Schonberg G (1981) Hypocholesteremic fermentation products and process of preparation. United States Patent 4 294 926
Nelson GEN, Traufler DH, Kelley SE, Lockwood LB (1952) Production of itaconic acid by Aspergillus terreus in 20-Liter fermentors. Ind Eng Chem 44(5):1166–1168. https://doi.org/10.1021/ie50509a062
Okabe M, Lies D, Kanamasa S, Park EY (2009) Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Appl Microbiol Biot 84(4):597–606
Otten A, Brocker M, Bott M (2015) Metabolic engineering of Corynebacterium glutamicum for the production of itaconate. Metab Eng 30:156–165. https://doi.org/10.1016/j.ymben.2015.06.003
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour Technol 74(1):25–33
Papagianni M (2004) Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol Adv 22(3):189–259
Park YS, Ohta N, Okabe M (1993) Effect of dissolved-oxygen concentration and impeller tip speed on itaconic acid production by Aspergillus terreus. Biotechnol Lett 15(6):583–586
Pedroso GB, Montipó S, Mario DAN, Alves SH, Martins AF (2017) Building block itaconic acid from left-over biomass. Biomass Convers Biorefin 7(1):23–35. https://doi.org/10.1007/s13399-016-0210-1
Petruccioli M, Pulci V, Federici F (1999) Itaconic acid production by Aspergillus terreus on raw starchy materials. Lett Appl Microbiol 28(4):309–312
Pfeifer VF, Vojnovich C, Heger EN (1952) Itaconic acid by fermentation with Aspergillus terreus. Ind Eng Chem 44(12):2975–2980. https://doi.org/10.1021/ie50516a055
Reddy CSK, Singh RP (2002) Enhanced production of itaconic acid from corn starch and market refuse fruits by genetically manipulated Aspergillus terreus SKR10. Bioresour Technol 85(1):69–71
Robert T, Friebel S (2016) Itaconic acid - a versatile building block for renewable polyesters with enhanced functionality. Green Chem 18:2922–2934. https://doi.org/10.1039/C6GC00605A
Roehr M, Kubicek CP, Kominek J (1996). Citric acid, in Biotechnology: Products of primary metabolism, Volume 6, Second Edition (eds H.-J. Rehm and G. Reed), pp. 307–345. Wiley-VCH Verlag GmbH
Rychtera M, Wase DAJ (1981) The growth of Aspergillus terreus and the production of itaconic acid in batch and continuous cultures. The influence of pH. J Chem Technol Biotechnol 31:509–521. https://doi.org/10.1002/jctb.503310168
Saha BC (2017) Emerging biotechnologies for production of itaconic acid and its applications as a platform chemical. J Ind Microbiol Biot 44(2):303–315
Saha BC, Kennedy GJ (2017a) Mannose and galactose as substrates for production of itaconic acid by Aspergillus terreus. Lett Appl Microbiol 65(6):527–533. https://doi.org/10.1111/lam.12810
Saha BC, Kennedy GJ (2017b) Ninety six well microtiter plate as microbioreactors for production of itaconic acid by six Aspergillus terreus strains. J Microbiol Methods 144:53–59. https://doi.org/10.1016/j.mimet.2017.11.002
Saha BC, Kennedy GJ, Qureshi N, Bowman MJ (2017) Production of itaconic acid from pentose sugars by Aspergillus terreus. Biotechnol Prog 33(4):1059–1067. https://doi.org/10.1002/btpr.2485
Sayama A, Kobayashi K, Ogoshi A (1994) Morphological and physiological comparisons of Helicobasidium mompa and H. purpureum. Mycoscience 35(1):15–20
Schute K, Detoni C, Kann A, Jung O, Palkovits R, Rose M (2016) Separation in biorefineries by liquid phase adsorption: itaconic acid as case study. ACS Sustain Chem Eng 4(11):5921–5928. https://doi.org/10.1021/acssuschemeng.6b00096
Shin WS, Lee D, Kim S, Jeong YS, Chun GT (2013) Application of scale-up criterion of constant oxygen mass transfer coefficient (kLa) for production of itaconic acid in a 50 L pilot-scale fermentor by fungal cells of Aspergillus terreus. J Microbiol Biotechnol 23(10):1445–1453
Specht R, Aurich A, Kreyß E, Barth G, Bodinus C (2014) Verfahren zur biotechnologischen Herstellung von Itaconsäure. DE 102008011854 B4
Steiger MG, Blumhoff ML, Mattanovich D, Sauer M (2013) Biochemistry of microbial itaconic acid production. Front Microbiol 4: Article 23
Strelko CL, Lu WY, Dufort FJ, Seyfried TN, Chiles TC, Rabinowitz JD, Roberts MF (2011) Itaconic acid is a mammalian metabolite induced during macrophage activation. J Am Chem Soc 133(41):16386–16389
Tabuchi T, Sugisawa T, Ishidori T, Nakahara T, Sugiyama J (1981) Itaconic acid fermentation by a yeast belonging to the genus Candida. Agric Biol Chem 45(2):475–479
Tate BE (1981) Itaconic acid and derivatives. Grayson MEckroth E (eds) Kirk-Othmer Encycl Chem Technol 3:865–873
Tevz G, Bencina M, Legisa M (2010) Enhancing itaconic acid production by Aspergillus terreus. Appl Microbiol Biot 87(5):1657–1664. https://doi.org/10.1007/s00253-010-2642-z
Tippkotter N, Duwe AM, Wiesen S, Sieker T, Ulber R (2014) Enzymatic hydrolysis of beech wood lignocellulose at high solid contents and its utilization as substrate for the production of biobutanol and dicarboxylic acids. Bioresour Technol 167:447–455. https://doi.org/10.1016/j.biortech.2014.06.052
Tomlinson N, Campbell JJR, Trussell PC (1950) The influence of zinc, iron, copper, and manganese on the production of citric acid by Aspergillus niger. J Bacteriol 59(2):217–227
Tsao G, Ouyang P, Chen J (2010 ) Biotechnology in China II: chemicals, energy and environment. Advances in biochemical engineering/biotechnology. Springer Berlin Heidelberg
van der Straat L, Tamayo-Ramos J, Schonewille T, de Graaff L (2013) Overexpression of a modified 6-phosphofructo-1-kinase results in an increased itaconic acid productivity in Aspergillus niger. AMB Express 3(1):57
van der Straat L, Vernooij M, Lammers M, van den Berg W, Schonewille T, Cordewener J, van der Meer I, Koops A, de Graaff LH (2014) Expression of the Aspergillus terreus itaconic acid biosynthesis cluster in Aspergillus niger. Microb Cell Factories 13:11. https://doi.org/10.1186/1475-2859-13-11
Wang JH, Tsai SH, Teng K (2012) Producing itaconic acid in yeast using glycerol as the substrate. United States Patent 8(192):965
Weastra sro (2013) Wp 8.1, determination of market potential for selected platform chemicals: itaconic acid, succinic acid, 2,5-furandicarboxylic acid. Technical report, Bioconsept
Welter K (2000) Biotechnische Produktion von Itaconsäure aus nachwachsenden Rohstoffen mit immobilisierten Zellen. PhD thesis, Technical University of Braunschweig
Werpy T, Petersen G, Aden A, Bozell J, Holladay J, White J, Manheim A, Eliot D, Lasure L, Jones S (2004) Top value added chemicals from biomass: volume I—results of screening for potential candidates from sugars and synthesis gas. Technical report, Department of Energy Washington DC
Willke T, Vorlop KD (2001) Biotechnological production of itaconic acid. Appl Microbiol Biot 56(3–4):289–295
Wu X, Liu Q, Deng Y, Li J, Chen X, Gu Y, Lv X, Zheng Z, Jiang S, Li X (2017) Production of itaconic acid by biotransformation of wheat bran hydrolysate with Aspergillus terreus CICC40205 mutant. Bioresour Technol 241:25–34. https://doi.org/10.1016/j.biortech.2017.05.080
Yahiro K, Shibata S, Jia SR, Park Y, Okabe M (1997) Efficient itaconic acid production from raw corn starch. J Ferment Bioeng 84(4):375–377
Yahiro K, Takahama T, Park YS, Okabe M (1995) Breeding of Aspergillus-terreus mutant TN-484 for itaconic acid production with high-yield. J Ferment Bioeng 79(5):506–508
Zambanini T, Hartmann SK, Schmitz LM, Buttner L, Hosseinpour Tehrani H, Geiser E, Beudels M, Venc D, Wandrey G, Buchs J, Schwarzlander M, Blank LM, Wierckx N (2017a) Promoters from the itaconate cluster of Ustilago maydis are induced by nitrogen depletion. Fungal Biol Biotechnol 4:11. https://doi.org/10.1186/s40694-017-0040-3
Zambanini T, Hosseinpour Tehrani H, Geiser E, Merker D, Schleese S, Krabbe J, Buescher JM, Meurer G, Wierckx N, Blank LM (2017b) Efficient itaconic acid production from glycerol with Ustilago vetiveriae TZ1. Biotechnol Biofuels 10:131. https://doi.org/10.1186/s13068-017-0809-x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kuenz, A., Krull, S. Biotechnological production of itaconic acid—things you have to know. Appl Microbiol Biotechnol 102, 3901–3914 (2018). https://doi.org/10.1007/s00253-018-8895-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-8895-7