Abstract
The yeast Yarrowia lipolytica is able to secrete high amounts of several organic acids under conditions of growth limitation and carbon source excess. Here we report the production of citric acid (CA) in a fed-batch cultivation process on sucrose using the recombinant Y. lipolytica strain H222-S4(p67ICL1) T5, harbouring the invertase encoding ScSUC2 gene of Saccharomyces cerevisiae under the inducible XPR2 promoter control and multiple ICL1 copies (10–15). The pH-dependent expression of invertase was low at pH 5.0 and was identified as limiting factor of the CA-production bioprocess. The invertase expression was sufficiently enhanced at pH 6.0–6.8 and resulted in production of 127–140 g l−1 CA with a yield Y CA of 0.75–0.82 g g−1, whereas at pH 5.0, 87 g l −1 with a yield Y CA of 0.51 gg−1 were produced. The CA-productivity Q CA increased from 0.40 g l −1 h−1 at pH 5.0 up to 0.73 g l −1 h−1 at pH 6.8. Accumulation of glucose and fructose at high invertase expression level at pH 6.8 indicated a limitation of CA production by sugar uptake. The strain H222-S4(p67ICL1) T5 also exhibited a gene–dose-dependent high isocitrate lyase expression resulting in strong reduction (<5%) of isocitric acid, a by-product during CA production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Considering the finiteness of fossil raw materials, it is a current challenge to develop strategies for producing chemicals from renewable resources. Intermediates from the microbiological metabolism, particularly organic acids, are excellent sources for the production of such chemicals. At the beginning of this decade, the worldwide production of citric acid (CA) was approximately 1.0–1.5 million tonnes (BACAS-Belgian Academy Council of Applied Science 2004; http://www.sriconsulting.com). Because of its chemical features, CA is used in many industrial applications, for instance as flavour, acidifying and preservative additive in the food and pharmaceutical industry, as stabiliser for vegetable oils and fats or as complex-forming and bleaching component in many washing detergents. Large-scale production of CA is carried out mainly with submerged fermentations by the filamentous fungus Aspergillus niger using beet or cane molasses or glucose syrup (Crolla and Kennedy 2001; Karaffa and Kubicek 2003). However, this process is accompanied by some environmental problems such as the accumulation of solid (gypsum) and organically as well as heavy-metal-loaded liquid waste (sewage and ferrocyanide sludge) products (Röhr et al. 1996; Kubicek 2001).
One of the remarkable features of the yeast Yarrowia (Y.) lipolytica is its ability to secrete high amounts (up to 200–240 g l −1) of organic acids, such as pyruvate and the tricarboxylic acid cycle intermediates citrate, isocitrate (ICA) or 2-oxoglutarate, into the medium (Stottmeister et al. 1982; Barth and Gaillardin 1997; Fickers et al. 2005). Wild type strains of this yeast can use a wide spectrum of carbon sources as substrates for overproduction of organic acids, except for sucrose, a cheap and commonly used carbon source for CA production by A. niger. The special cultivation condition necessary for the organic acid production by Y. lipolytica is a carbon source excess with simultaneous growth limitation by nitrogen, mineral salts or thiamine. For production of CA, the nitrogen limitation of growth is favoured.
Because of the above-mentioned ecological problems of the Aspergillus process, it is of interest to develop alternative processes using yeast as producing organisms. A bioprocess for CA production using Y. lipolytica would have several additional advantages compared to the Aspergillus process, including a larger substrate variety (selection of low-priced substrates would be possible), a smaller sensitivity to low dissolved oxygen concentrations and heavy metals, and higher product yields (Stottmeister and Hoppe 1991).
However, the cheap and often-used substrate sucrose cannot be utilised by wild type strains of Y. lipolytica because of the lack of the sucrose-cleaving enzyme invertase. A disadvantage of using wild type strains of Y. lipolytica for CA production is the secretion of ICA as by-product of the process. Contaminations of ICA above 5% of the CA produced disturb the crystallisation of CA during the purification process.
To eliminate these disadvantages, recombinant strains of Y. lipolytica have been constructed (Kruse et al. 2004; Förster 2006). These strains, e.g. H222-S4(p67ICL1) T5, harbour in the genome one copy of the invertase-encoding SUC2 gene of Saccharomyces cerevisiae under control of the XPR2 promoter and multiple copies of their own isocitrate lyase-encoding ICL1 gene. Therefore, these recombinant strains also exhibited high isocitrate lyase activity, resulting in high yield of CA accompanied by a strong reduction in the ICA content, a by-product during CA production by Y. lipolytica. Here, we report on the improvement of the CA production process with the recombinant strain H222-S4(p67ICL1) T5 using sucrose as sole carbon source by changing the pH of the medium. Furthermore, the gene–dose-dependent effect of the multiple ICL1 copies on the isocitrate lyase expression and on the reduced secretion of ICA during CA production on sucrose is shown.
Materials and methods
Strains, media, and growth conditions
The ICL1 multicopy (copy number 10–15) strain Y. lipolytica H222-S4(p67ICL1) T5 (MATA ura3-302::ScSUC2 ura3d4mc ICL1mc; mc-multicopy; short strain name T5) was obtained by integrative transformation of the strain H222-S4 (MATA ura3-302::ScSUC2, Mauersberger et al. 2001), a derivative of the wild type strain H222 (Barth and Weber 1983; Barth and Gaillardin 1996), with the multicopy vector p67ICL1 (ura3d4, LTR zeta, ICL1) as described by Juretzek et al. (2001) and Kruse et al. (2004).
For cultivation, 500-ml shaking flasks were used. The strains were grown at 28°C and 220 rpm in 20–30 ml complete medium yeast peptone dextrose (YPD; Barth and Gaillardin 1996) or in 100 ml minimal medium M (Mauersberger et al. 2003) containing (a) mineral salts: 3 g l −1 (medium Mg for growth) or 1 g l −1 (medium Mp for citrate production) (NH4)2SO4, 1 g l −1 KH2PO4, 0.16 g l −1 K2HPO4 × 3H2O, 0.7 g l −1 MgSO4 × 7H2O, 0.5 g l −1 NaCl and 0.4 g l −1 Ca(NO3)2 × 4H2O; (b) trace elements: 0.5 mg l−1 H3BO3, 0.04 mg l −1 CuSO4 × 5H2O, 0.1 mg l −1 KI, 0.4 mg l −1 MnSO4 × 4H2O, 0.2 mg l −1 Na2MoO4 × 2H2O, 0.4 mg l −1 ZnSO4 × 7H2O and 0.6 mg l −1 FeCl3 × 6H2O; (c) vitamin: 0.3 mg l −1 thiamine hydrochloride; (d) proteose-peptone: 0–5.0 g l −1. The pH of the final medium M was 5.5–6.0. For cultivation using sucrose as carbon source, the pH at the beginning of cultivation was kept at 6.8; in the shaking flasks, it was adjusted by the addition of 50 mM potassium phosphate buffer (KKP) to pH 6.8. The concentration of the carbon sources was between 1–2% (in Mg) and 5–10% (in Mp). During cultivation the pH was regulated using 2.5 or 10 N NaOH.
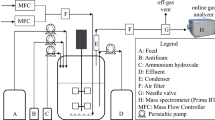
For cultivation in a stirred tank bioreactor (2.0 l Biostat MD 2, Braun Biotech International, Melsungen, Germany), the strain T5 was grown at 30°C in 1.2 l production medium according to Behrens et al. (1978) containing (a) mineral salts: 1.5 g l −1 NH4Cl, 0.7 g l −1 KH2PO4, 0.35 g l −1 MgSO4 × 7H2O, 3.5 mg l −1 FeSO4 × 7H2O, 30 mg L−1 CaCl2; (b) trace elements: 20 mg l −1 CuSO4 × 5H2O, 20 mg l −1 MnSO4 × 5H2O, 10.5 mg l −1 ZnCl2, 2.5 mg l −1 CoSO4 × 7H2O and 28.5 mgl −1 H3BO3; (c) vitamin: 1 mgl −1 thiamine hydrochloride; (d) proteose-peptone: 0.4 g l −1. During cultivation, the pH was regulated with 7.5 N NaOH. The total concentration of sucrose was 170 g l −1. Silicone anti-foam reagent no.85390 (Fluka AG, Taufkirchen, Germany) was added if necessary. All media (except sterile filtrated sucrose and thiamine) were sterilised at 121°C for 20 min.
Invertase activity was determined by a spectrophotometric assay according to Goldstein and Lampen (1975) using whole yeast cells after separation from culture medium. The cells were harvested by centrifugation (5000 × g, 3 min) and re-suspended in mineral salts according to Behrens et al. (1978). This step was necessary to avoid an interference of the assay with high glucose content in the fermentation broth.
Determination of citric and isocitric acid content in cell-free samples of the fermentation broth was carried out using the DX 600 ion chromatography system (Dionex, Sunnyvale, CA, USA) or using the enzymatic test kits no. 139076 and 414433 (R-Biopharm, Darmstadt, Germany).
Determination of glucose, fructose and sucrose was performed using the enzymatic test kit no. 716260 (R-Biopharm, Darmstadt, Germany).
Determination of ammonium was performed using LCK 302 and 303 enzymatic test kits (Dr. Lange GmbH, Düsseldorf, Germany).
Results
Growth on sucrose
Naturally, Y. lipolytica is not able to utilise sucrose as sole carbon source because of the absence of an invertase-encoding gene. To develop a direct selection system for ura3 markers in Y. lipolytica wild type strains, Nicaud et al. (1989) constructed integrative vectors for the heterologous expression of the ScSUC2 gene from S. cerevisiae under the control of the promoter and the secretion sequences of the XPR2 gene (extracellular alkaline protease AEP) of Y. lipolytica. After integrative transformation with the pXPR2-ScSUC2 expression cassette containing a 4.3 kb SalI fragment from pINA302 (Barth and Gaillardin 1996), Ura-Y. lipolytica strains such as H222-S4 (MATA ura3-302) growing on sucrose (Suc+) were selected, which thus contained the ura3-302 allele (Mauersberger et al. 2001). Subsequently the strain H222-S4 was re-transformed with the multicopy vector p67ICL1 (ura3, LTR zeta, ICL1; Juretzek et al. 2001) to increase the ICL1 copy number to at least 10–12, resulting in the recombinant strain H222-S4(p67ICL1) T5 (Kruse et al. 2004; Förster 2006), referred to as T5 hereafter. Because of its high CA yields accompanied by very low content of the by-product ICA (<5%), detected during the CA production on all tested substrates, this strain was selected for the present study to optimise the CA production process from sucrose.
The highest induction of the strong XPR2 promoter takes place in the presence of peptone; derepression starts at pH values >5.5–6.0 in the medium (Ogrydziak et al. 1977; Madzak et al. 1999). In the past, the growth on sucrose as sole carbon source of Suc+-strains of Y. lipolytica, containing the pXPR2-ScSUC2 fusion, has only been described under conditions of induction (1.7 g l −1 peptone) and simultaneous derepression (medium pH 6.8) of pXPR2 (Nicaud et al. 1989; Wojtatowicz et al. 1997).
Preliminary plate tests and shaking flask experiments with the strain T5 showed that the derepression of pXPR2 caused by choosing a pH of 6.0–6.8 in the culture medium is sufficient to support growth on sucrose. Pre-cultures of strain T5 in minimal media with glucose or in YPD were used to inoculate the production medium Mp containing sucrose, 50 mM potassium phosphate buffer (KKP) pH 6.8 and either peptone or not (Fig. 1). In the presence of peptone, the growth of strain T5 occurred without a long lag phase when using a pre-culture in Mg with glucose for the inoculation (Fig. 1). In contrast, a nearly 20 h lag phase was observed in the absence of peptone using medium Mg with glucose as pre-culture before the growth started. After this lag phase, no further significant difference could be observed for the growth rate in the main culture with or without peptone, when using medium Mg with glucose as the pre-culture. The observed long lag phase is probably caused by the time necessary for the derepression of pXPR2 and synthesis of the invertase. Interestingly, it took more than 80 h before a slow growth started using the YPD pre-culture (Fig. 1).
Growth features of the Y. lipolytica strain H222-S4(p67ICL1) T5 on sucrose media with (D) or without (A, B, C) the addition of proteose-peptone. Cultivation in 500 ml shaking flasks with 100 ml medium at 28°C and 220 rpm. Pre-cultures: A, B, D, in minimal medium Mg with glucose; C, in YPD. Main cultures: A, C, Mg with 2% sucrose, 50 mM potassium phosphate buffer (KKP) pH 6.8; D, Mp with 7% sucrose, 50 mM KKP pH 6.8 and 0.8 g l −1 proteose-peptone; B, Mp with 7% sucrose, 50 mM KKP pH 6.8. Growth (biomass) was monitored as optical density at 600 nm (OD600)
Invertase activity
To optimise the bioprocess for CA production on sucrose, the course of the invertase activity during the cultivation under nitrogen exhaustion was studied in more detail. As shown in Fig. 2a, during cultivation of T5 in the bioreactor in presence of 0.4 g l −1 peptone, the maximal invertase expression was observed in the early exponential growth phase (6–12 h). Thereafter the activity of invertase decreased rapidly (Fig. 2a,b). This observation was also made in shaking-flasks experiments using various peptone concentrations (0.1–5.0 g l −1; Förster 2006). These experiments also showed that during the first 17 h of cultivation in peptone-containing media, the invertase activity was 1.7–2.5 times higher than in a medium without peptone. With increasing peptone concentrations (up to 2.0 g l −1), a moderate enhancement of the maximal achieved invertase expression was observed. No further increase was detected at peptone concentrations above 2.0 g l −1 (data not shown). The majority (60–70%) of invertase activity was found under these conditions on the cell surface (cell-bound activity detectable with whole cells), whereas 30–40% of the invertase was detectable in the cell-free culture medium (data not shown).
Course of growth and invertase activity of the Y. lipolytica strain H222-S4(p67ICL1) T5 during cultivation on sucrose in the bioreactor at pH 5.0 (a) and comparison of invertase activities during cultivation at pH 5.0, 6.0, and 6.8 (b). Cultivation conditions: 1.2 l working volume of production medium according to Behrens et al. (1978) with 0.4 g l−1 proteose-peptone (see “Materials and methods”); total 170 g l −1 sucrose (start concentration 100 g l −1, first addition of 45–50 g l −1 at 51 h [pH 6.8] or at 73 h [pH 5.0], second addition of 20–25 g l −1 at 100 h, see Fig. 3); stirrer speed, 1,000 rpm; pO2 content, 50%; aeration, 0 to 1.6 l min−1; T, 30 °C; pH 6.8 (growth phase-g) and 5.0, 6.0, or 6.8 (adjusted in the production phase I, pI). Growth (biomass) was monitored as optical density at 600 nm (OD600). Invertase activity was measured with whole cells, given as U per g dry weight biomass (BM)
Because of acidification during cell growth and later secretion of CA and ICA after N-exhaustion, the pH of the medium decreased from 6.8 at the start of cultivation to pH 3–4. Therefore, it was necessary to adjust the pH of the medium by the addition of NaOH. For growth and CA production using Y. lipolytica, a pH of 5.0–5.5 is usually applied (Stottmeister et al. 1982; Mattey 1992). Preliminary shaking-flask experiments showed a significant increase in the invertase activity at pH 6.0–6.8 compared to pH 5.0 because of the derepression of pXPR2. To confirm these results, cultivation experiments were performed in 2.0-l bioreactors for the production of CA on sucrose in the presence of 0.4 g l −1 peptone to avoid a longer lag phase (Fig. 2). Three different cultivation conditions were tested. From cultivation start in the bioreactor until N-exhaustion (18–23 h, end of growth phase g; Fig. 5), the pH was kept at 6.8. With the beginning of the production phase (p I), the pH was either kept at pH 6.8 or allowed to fall down to 6.0 or 5.0 then maintained at this level for the rest of the fermentation time.
Within a cultivation time of up to 20 h, only moderate differences of the invertase activity were observed. As shown in Fig. 2a, a maximum of invertase activity (10–14 U g−1 dry weight [d.w.] biomass) was usually detected during the initial growth phase (g) at pH 6.8 within 6–12 h. Thereafter, upon changing the pH to 5.0 at the beginning of the production phase (p I), the invertase activity decreased steadily to low levels of 3–4 U g−1 and persisted at this level until the end of cultivation at 215 h. In contrast, a constant increase in invertase activity from 8–10 after 20 h up to 24–26 or even 110 U g−1 was observed after changing the pH 6.8 at the beginning of the production phase (p I) to pH 6.0 or keeping it at pH 6.8 during the entire fermentation time, respectively. This occurred obviously because of permanent derepression of pXPR2 (Fig. 2b). Only a moderate increase in invertase expression was observed at pH 6.0 compared to the 4.4-fold higher invertase activity at pH 6.8.
In conclusion, using the strain T5 harbouring the pXPR2-SUC2 expression cassette, a pH-dependent derepression of the invertase synthesis under control of the XPR2 promoter was demonstrated, allowing an excellent growth of this strain on sucrose and subsequent CA production without further addition of the inducing peptone.
Utilisation of sucrose, glucose and fructose
The decline of the sucrose in the medium showed that during cultivation at pH 6.8, the sucrose was cleaved much faster than at pH 5.0. Almost the complete bulk of sucrose (100 g l −1) was cleaved at pH 6.8 nearly 46 h after start of fermentation (Fig. 3a). In contrast, more than 70 h of cultivation was necessary to split the same bulk of sucrose during cultivation at pH 5.0, commensurate with the lower invertase expression level (Fig. 2). The much faster cleavage of sucrose due to the increased invertase expression at pH 6.8 was confirmed by additional feedings of sucrose (Fig. 3a). The 45 g l −1 sucrose added after 51 h of cultivation was almost completely cleaved into glucose and fructose after 73 h. In contrast, at pH 5.0 of the 50 g l −1 sucrose added after 73 h of cultivation, 15 g l −1 still remained after 100 h (Fig. 3a). Concerning the sucrose cleavage, comparable results were obtained for cultivation at pH 6.0.
Kinetics of sucrose utilisation during cultivation of the Y. lipolytica strain H222-S4(p67ICL1) T5 in the bioreactor at pH 5.0 compared to pH 6.8 (a) and time course of glucose and fructose concentrations at pH 6.8 (b). Cultivation conditions: see Fig. 2. Second additional dosage of 20–25 g l −1 sucrose made at 100 h cultivation time. Arrows indicate additional sucrose feedings after 51, 73 and 100 h cultivation
In all cultivation experiments for CA production from sucrose, a significant accumulation of the monosaccharides glucose (5–8 g l −1) and fructose (maximal accumulation of 56 g l −1 at pH 6.8 vs 15 g l −1 at pH 5.0) was observed, resulting from the cleavage of the disaccharide sucrose (Fig. 3b). According to these results, it appeared that Y. lipolytica first utilised glucose and then fructose, because the glucose concentration was clearly lower than the fructose concentration in the medium at all times. Similar observations have been described by other authors (Mansfeld et al. 1995; Bizukojc and Ledakowicz 2004). For a more precise investigation of this phenomenon, strain T5 was cultivated in shaking flasks under N-exhaustion conditions in medium Mp using 8% glucose only, 8% fructose only or a mixture of 4% glucose and 4% fructose as carbon sources. As shown in Fig. 4a, the consumption rate of glucose and fructose was the same when these sugars were offered separately. In contrast, however, glucose was utilised first when both sugars were offered simultaneously (Fig. 4b). The consumption of fructose did not start until the glucose concentration was reduced to a level of 5–10 g l −1. Thus, during high-level invertase expression, the utilisation of the accumulated fructose could become the rate-limiting step for the CA production process from sucrose.
Course of the glucose and fructose concentrations in the medium during cultivation of the Y. lipolytica strain H222-S4(p67ICL1) T5. Culture conditions: 500 ml shaking flasks with 100 ml minimal medium Mp at 28°C and 220 rpm; a Cultivation with 8% glucose or 8% fructose in separate experiments; b Presence of 4% glucose and 4% fructose simultaneously
CA production
In Fig. 5, a typical time course of CA fermentation is shown for Y. lipolytica strain T5 on sucrose at pH 6.8. After N-exhaustion at 25–30 h, the production of biomass stopped, and the secretion of CA and ICA started. Up to a cultivation time of about 50 h, no larger differences concerning the CA concentration were observed for the tested pH values of 5.0, 6.0 and 6.8. The CA concentration was in the range of 25–35 g l −1. After 73 h cultivation time, the CA concentration at pH 6.0 and 6.8 was already in the range of 60–65 g l −1 compared to 38 g l −1 at pH 5.0 (data not shown). The maximum CA concentration of 140 g l −1 was obtained at pH 6.8 after 191 h (Table 1). The CA concentration at pH 6.0 (127 g l −1) was nearly in the same range as that at pH 6.8. At the end of cultivation at 215 h, the CA concentration had decreased from 140 to 130 g l −1 at pH 6.8 because of the utilisation of CA as carbon source after exhaustion of the carbohydrate substrates (glucose and fructose). In contrast to the cultivations at pH 6.0 or 6.8, the CA concentration at pH 5.0 was much lower. Only 78 g l −1 CA was produced after 191 h of cultivation; the maximum CA concentration of 87 g l −1 was detected after 215 h (Table 1), although in this case the CA product formation was not yet completely finished.
Fed-batch cultivation of the Y. lipolytica strain H222-S4(p67ICL1) T5 on sucrose at pH 6.8. Cultivation conditions: see Fig. 2. Concentration of sucrose at cultivation start was 100 g l −1; first additional sucrose dosage (45 g l −1) after 51 h; second sucrose dosage (25 g l −1) after 100 h cultivation; pH 6.8 maintained throughout the growth phase (g) and the production phases p I and p II; acids, CA or ICA content; OD600, optical density at 600 nm; BM, biomass in g dry weight; NH 4-N, ammonium nitrogen in mg l−1; Arrows indicate additional sucrose feedings
The volumetric productivity (or space-time yield) Q CA, the biomass-specific rate of CA production q CA and the substrate yields Y CA at pH 6.0 and 6.8 were 1.5- to 1.8-fold higher than at pH 5.0 over the total fermentation time, including growth (g) and production phases (p I and II). The productivity Q CA at pH 6.8 was 0.73 g l −1 h−1 compared to 0.40 g l −1 h−1 at pH 5.0. The specific productivity q CA at pH 6.8 was 0.091 g g−1 h−1 vs only 0.054 g g−1 h−1 at pH 5.0 (Table 1).
The highest CA productivity values (Q CA max, q CA max) were observed in the first production phase (p I) lasting from 30 to 100 h of cultivation with a moderate 1.4-fold increase in the volumetric productivity (Q CA max from 1.33 to 1.90 g l −1 h−1) and the biomass-specific productivity (q CA max from 0.14 to 0.19 g g−1 h−1), observed during cultivation at pH 5.0 or pH 6.8, respectively (Table 1). Otherwise, the maximal productivities detected in shaking-flask experiments in the presence of 1.7 or 0.4 g l −1 peptone and with changing pH-conditions from 3 to 5.5 were significantly lower (Q CA max of 0.25 to 0.38 g l −1 h−1, q CA max of 0.019 to 0.044 g g−1 h−1, respectively) than in the bioreactor. This was obviously because of the oxygen limitation, dominating the CA production process in shaking flasks, which was eliminated in the bioreactor experiments.
Especially in the second production phase in bioreactor (p II, 100–215 h, initiated by an additional 20–25 g l −1 sucrose feeding) with decreasing overall production rates (Fig. 5), the pH-mediated increase in CA production was even more striking. The Q CA and q CA values in phase p II were 0.29, 0.53, 0.68 g l −1 h−1 and 0.039, 0.071, 0.085 g g−1 h−1 for cultivation at pH 5.0, 6.0 and 6.8, respectively. Thus, the productivity increase from pH 5.0 to 6.8 was 2.3-fold in the production phase p II.
The increase in the CA productivity correlated with the higher invertase activities detected during cultivation at pH 6.0 and 6.8 compared to pH 5.0 (Table 1, Fig. 2b). Interestingly, the only moderate increase in invertase expression (from 8–10 to 24–26 U g−1) during cultivation at pH 6.0 obviously already resulted in a significant productivity increase. A further strong increase in invertase expression at pH 6.8 to 105–110 U g−1 only led to a moderate productivity increase (Table 1), indicating that the invertase activity was no longer the rate-limiting factor for the CA production process. The high level of invertase expression at pH 6.8 resulted in faster sucrose splitting and subsequent accumulation of glucose and especially fructose in the medium (Fig. 3). The maximal intermediate accumulation of fructose (56 g l −1) and glucose (6–8 g l −1) was highest during cultivation at pH 6.8. For comparison, the maximal values at pH 5.0 were 15 g l −1 fructose and 5–6 g l −1 glucose.
The ICA proportion of the total acid production (CA and ICA), independent of the chosen pH value, was smaller than 5% because of the ICL1 gene–dose-dependent high level expression of the isocitrate lyase in the strain H222-S4(p67ICL1) T5, containing 10–15 ICL1 copies. Normally, in wild type strains of Y. lipolytica, the proportion of ICA in the total produced acids on carbohydrates is approximately 10–12% (Barth and Gaillardin 1997; Kruse et al. 2004; Förster 2006).
Discussion
The recombinant Y. lipolytica strain H222-S4(p67ICL1) T5, containing the ScSUC2 gene of S. cerevisiae under the control of the promoter and secretion signal sequence of the Y. lipolyticaXPR2 gene as well as multiple copies of its own ICL1 gene, was used for CA production from sucrose. Low invertase activity was established during CA production on sucrose at pH 5.0, indicating the limitation of the process by the enzyme. To increase the invertase activity, the pH value during the production phase was raised from 5.0 to 6.0 or 6.8, respectively. This resulted in a strong increase in the invertase activity because of the derepression of pXPR2 (up to 8-fold at pH 6.0, up to 37-fold at pH 6.8). The pXPR2 derepression during cultivation at pH 6.0 or 6.8 had a stronger effect on the invertase expression level than the pXPR2-inducing peptone. This observation has never been described before in the literature for this type of recombinant strains of Y. lipolytica. At pH 6.0, the derepression of pXPR2 obviously functioned to a smaller extent than at pH 6.8, resulting in a lower increase in invertase activity. At pH 5.0, only a minor invertase activity was observed after N-exhaustion. This is because of the loss of the pXPR2-inducing effect of peptone because of its consumption by the yeast cells and also because of the lack of pXPR2 derepression at pH 5.0. Concerning the observed long delay in invertase expression after a YPD pre-culture, one could speculate that the transport mechanism for extracellular proteins in the cell is overloaded because in YPD, beside invertase secretion, the transport of protease takes place simultaneously.
The transfer of the CA production from shaking flasks to bioreactor conditions eliminated the oxygen limitation of the bioprocess and allowed the pH-mediated fine regulation of invertase expression. The increased invertase expression at pH 6.0 and 6.8 in the bioreactor resulted in a further significant improvement of the CA production process from sucrose (Table 1). The characteristics of CA production after optimisation of the bioprocess by pH-mediated increased invertase expression in the bioreactor (product yield Y CA 0.82 g g−1, CA concentration 127–140 g l −1, productivities Q CA 0.66–0.73 g l −1 h−1, q CA 0.088–0.091 g g−1 h−1) are comparable to the data found by other authors using carbohydrates as substrates.
Kautola et al. (1992) described specific CA productivity rates q CA of 0.10 and 0.05 g g−1 h−1, and total yields of Y (CA+ICA) of 0.67 and 0.56 g g−1 for the Y. lipolytica strain A-101 on inverted sucrose or inverted molasses, respectively. For the Y. lipolytica Suc+ strain W29ura3-302, containing the same pXPR2-ScSUC2 fusion as H222-S4(p67ICL1) T5, Zarowska et al. (2001) reported a maximal specific CA productivity rate q CAmax of 0.06 g g−1 h−1, volumetric productivity Q CA of 0.37 g l −1 h−1 and total yield Y (CA+ICA) of 0.61 g g−1 from non-inverted beet molasses. For the Y. lipolytica Suc- strain A-101-1.22, Zarowska et al. (2001) presented values for productivity Q CA of 0.78 g l −1 h−1 and maximal specific CA productivity q CAmax of 0.12 g g−1 h−1 on inverted molasses. Up to now, there are no data available for the CA production on non-inverted sucrose with Y. lipolytica.
For comparison, maximal specific CA productivity rates q CA max of 0.19–0.20 g g−1 h−1 have been reported for Y. lipolytica on glucose (Stottmeister and Hoppe 1991). Rane and Sims (1995, 1996) reported Q CA of 0.6–1.3 g l −1 h−1 and q CA of 0.02–0.03 g g−1 h−1 for Y. lipolytica strain Y-1095 using high biomass content and glucose as substrate. In our experiments using sucrose as the sole carbon source, the maximal specific CA production rate q CA max on sucrose was 0.19 g g−1 h−1, which was in the range of q CAmax described by Stottmeister and Hoppe (1991) for glucose. Thus, the productivity for CA formation with Y. lipolytica is nearly the same when using glucose or sucrose as substrates.
Concluded from the data presented here, it appears that the production of CA from sucrose by the recombinant Y. lipolytica strain H222-S4(p67ICL1) T5 could potentially compete with the more traditional surface or submerged A. niger -processes (CA 120–140 g l −1, Q CA 0.4–0.8 g l −1 h−1, Y CA 0.8–0.9 g g−1; Stottmeister and Hoppe 1991; Rane and Sims 1995; Röhr et al. 1996; Karaffa and Kubicek 2003).
Interestingly, the characteristics of the CA production process in the bioreactor at pH 6.0 given a moderate invertase expression level were almost in the same range as the data for pH 6.8 (Table 1). The strong increase in invertase expression in the production phase at pH 6.8 resulted in a higher intermediate accumulation of glucose and especially fructose but only in a small further enhancement of the CA production characteristics. Obviously, the rate-limiting step of the CA production process from sucrose was the sucrose splitting in the case of low invertase expression, whereas with a sufficiently high level of invertase expression the uptake of fructose and glucose was rate limiting.
In contrast to the situation with Y. lipolytica, more information on the sugar uptake is available for other yeasts. In S. cerevisiae, at least 20 proteins (HXT genes) are known to be involved in the hexose transport through the plasma membrane (Wieczorke et al. 1999). According to DNA sequence comparison, at least five genes of Y. lipolytica showed homology to the S. cerevisiae genes HXT2, HXT3, HXT4 and HXT8. Lee et al. (2002) described the kinetics of glucose and xylose uptake in S. cerevisiae. At high glucose concentrations, glucose was taken up mainly by low-affinity transporters. The uptake of glucose by high-affinity transporters occurred at low glucose concentrations. For the uptake of the pentose xylose, the same transporter systems were used. The xylose uptake was low in case of high glucose concentration in the medium and high in case of low glucose concentration. The yeast Schizosaccharomyces pombe contains at least four hexose transporters (Heiland et al. 2000), and in Pichia stipitis three glucose transporters are known (Weierstall et al. 1999). Most of the known hexose transporters function passively by diffusion processes without energy consumption. However, for Kluyveromyces lactis, an active fructose transporter has been described that also exhibits a small affinity for glucose (Diezemann and Boles 2003).
For further optimisation of the CA production process on sucrose using Y. lipolytica, the improvement of the glucose and fructose uptake into the cell should be the favoured strategy. For this purpose the genes encoding for hexose transporters could be overexpressed in Y. lipolytica. An additional means of process optimisation could be the development of efficient sucrose feeding strategies to avoid possible substrate limitation and inhibition effects.
References
BACAS-Belgian Academy Council of Applied Science (2004) Industrial biotechnology and sustainable chemistry. Brussels, 1-29, http://wbt.dechema.de/img/wbt_/Literatur/BACAS-Studie.pdf
Barth G, Gaillardin C (1996) Yarrowia lipolytica. In: Wolf K (ed) Nonconventional yeasts in biotechnology. Springer, Berlin Heidelberg New York, pp 313–388
Barth G, Gaillardin C (1997) Physiology and genetics of the dimorphic fungus Yarrowia lipolytica. FEMS Microbiol Rev 19:219–237
Barth G, Weber H (1983) Genetic studies in the yeast Saccharomyces lipolytica. Inactivation and mutagenesis. Z Allg Mikrobiol 23:147–157
Behrens U, Weißbrodt E, Lehmann W (1978) Zur Kinetik der Citronensäurebildung bei Candida lipolytica. Z Allg Mikrobiol 18:549–558
Bizukojc M, Ledakowicz S (2004) The kinetics of simultaneous glucose and fructose uptake and product formation by Aspergillus niger in citric acid fermentation. Process Biochem 39:2261–2268
Crolla A, Kennedy KJ (2001) Optimization of citric acid production from Candida lipolytica Y-1095 using n-paraffin. J Biotechnol 89:27–40
Diezemann A, Boles E (2003) Functional characterization of the Frt1 sugar transporter and fructose uptake in Klyveromyces lactis. Curr Genet 43:281–288
Fickers P, Benetti PH, Wache Y, Marty A, Mauersberger S, Smit MS, Nicaud JM (2005) Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res 5:527–543
Förster A (2006) Untersuchungen zur Nutzung der Hefe Yarrowia lipolytica für die Gewinnung von Citronensäure aus nachwachsenden Rohstoffen. Ph.D. thesis, Institut für Mikrobiologie, Technische Universität Dresden
Goldstein A, Lampen JO (1975) Beta-D-fructofuranoside fructohydrolase from yeast. Methods Enzymol 42:504–511
Heiland S, Radovanovic N, Höfer M, Winderickx J, Lichtenberg H (2000) Multiple hexose transporters of Schizosaccharomyces pombe. J Bacteriol 182:2153–2162
Juretzek T, Le Dall MT, Mauersberger S, Gaillardin C, Barth G, Nicaud JM (2001) Vectors for gene expression and amplification in the yeast Yarrowia lipolytica. Yeast 18:97–113
Karaffa L, Kubicek CP (2003) Aspergillus niger citric acid accumulation: do we understand this well working black box? Appl Microbiol Biotechnol 61:189–196
Kautola H, Rymowicz W, Linko YY, Linko P (1992) The utilization of beet molasses in citric acid production with yeast. Sci aliments 12:383–392
Kruse K, Förster A, Mauersberger S, Barth G (2004) Method for the biotechnological production of citric acid by means of a genetically modified yeast Yarrowia lipolytica. Patent WO2004/009828, DE10333144
Kubicek C (2001) Chapter 4.2, Citric acid. In: Ratledge C, Kristiansen B (eds) Basic biotechnology. Cambridge University Press, Cambridge, pp 305–315
Lee WJ, Kim MD, Ryu YW, Bisson LF, Seo JH (2002) Kinetic studies on glucose and xylose transport in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 60:186–191
Madzak C, Tréton B, Blanchin-Roland S, Cordero Otero RR, Gaillardin C (1999) Functional analysis of upstream regulating regions from the Yarrowia lipolyticaXPR2 promoter. Microbiology 145:75–87
Mansfeld J, Förster M, Hoffmann T, Schellenberger A, Dautzenberg H (1995) Coimmobilization of Yarrowia lipolytica cells and invertase in polyelectrolyte complex microcapsules. Enzyme Microb Technol 17:11–17
Mattey M (1992) The production of organic acids. Crit Rev Biotechnol 12:87–132
Mauersberger S, Wang HJ, Gaillardin C, Barth G, Nicaud JM (2001) Insertional mutagenesis in the n-alkane-assimilating yeast Yarrowia lipolytica. Generation of tagged mutants in genes involved in hydrophobic substrates utilization. J Bacteriol 183:5102–5109
Mauersberger S, Kruse K, Barth G (2003) Chapter 63, Induction of citric acid/isocitric acid and α-ketoglutaric acid production in the yeast Yarrowia lipolytica. In: Wolf K, Breunig K, Barth G (eds) Non-conventional yeasts in genetics, biochemistry and biotechnology. Practical protocols. Springer, Berlin Heidelberg New York, pp 393–400
Nicaud JM, Fabre E, Gaillardin C (1989) Expression of invertase activity in Yarrowia lipolytica and its use as a selective marker. Curr Genet 16:253–260
Ogrydziak DM, Demain AL, Tannenbaum SR (1977) Regulation of extracellular protease production in Candida lipolytica. Biochim Biophys Acta 497:525–538
Rane KD, Sims KA (1995) Citric acid production by Candida lipolytica Y-1095 in cell recycle and fed-batch fermentors. Biotechnol Bioeng 46:325–332
Rane KD, Sims KA (1996) Citric acid production by Yarrowia lipolytica: effect of nitrogen and biomass concentration on yield and productivity. Biotechnol Lett 18:1139–1144
Röhr M, Kubicek CP, Kominek J (1996) Citric acid. In: Rehm HJ, Reed G, Delweg D (eds) Biotechnology, 2nd (edn), vol 6. VCH Verlag Chemie, Weinheim, pp 308–345
Stottmeister U, Hoppe K (1991) Organische Genußsäuren. In: Ruttloff H (ed) Lebensmittelbiotechnologie, Entwicklungen und Aspekte. Akademie-Verlag, Berlin, pp 516–547
Stottmeister U, Behrens U, Weissbrodt E, Barth G, Franke-Rinker D, Schulze E (1982) Nutzung von Paraffinen und anderen Nichtkohlenhydrat-Kohlenstoffquellen zur mikrobiellen Citronensäuresynthese. Z Allg Mikrobiol 22:399–424
Weierstall T, Hollenberg CP, Boles E (1999) Cloning and characterization of three genes (SUT1-3) encoding glucose transporters of the yeast Pichia stipitis. Mol Microbiol 31:871–883
Wieczorke R, Krampe S, Weiserstall T, Freidel K, Hollenberg CP, Boles E (1999) Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett 464:123–128
Wojtatowicz M, Rymowics W, Robak M, Zarowska B, Nicaud JM (1997) Kinetics of cell growth and citric acid production by Yarrowia lipolytica Suc+ transformants in sucrose media. Pol J Food Nutr Sci 47:49–54
Zarowska B, Wojtatowicz M, Rymowicz W, Robak M (2001) Production of citric acid on sugar beet molasses by single and mixed cultures of Yarrowia lipolytica. Electron J Pol Agric Univ 4(2)
Acknowledgments
This work was supported by the Sächsisches Staatsministerium für Umwelt und Landwirtschaft (SMUL), Land Saxony, Germany (Grant no. 138811.61/89).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Förster, A., Aurich, A., Mauersberger, S. et al. Citric acid production from sucrose using a recombinant strain of the yeast Yarrowia lipolytica . Appl Microbiol Biotechnol 75, 1409–1417 (2007). https://doi.org/10.1007/s00253-007-0958-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-0958-0