Abstract
Transgenic plants are genetically engineered crops having desirable traits, which are stable and often pass to the progeny. The wild varieties grown in the natural environment are prone to attack by various biotic and abiotic factors which result in yield reduction, qualitatively and quantitatively. Genetic engineering provides a plausible way to combat such stresses by reorganizing biochemical photosynthetic pathways. Cucumber (Cucumis sativus L.) is one of the most popular economically important crops which is widely cultivated throughout the world. Apart from its economical uses, cucumber is also used as a model plant to study sex determination and cell trafficking system in plant vascular biology. The availability of assembled draft genome sequences for cucumber provides the treasured basis for the transformation of desired traits by the identification of candidate genes. Various techniques are employed for Agrobacterium-mediated and other gene transfer methods for cucumber. Tissue culture techniques using different explants for complete plantlet regeneration are discussed in this chapter. This chapter also summarizes the current status of cucumber transgenic with respect to phenotypic stability of transformed trait and its inheritance to the progeny. The availability of transgenic plants with respect to new and improved variety is likely to be an important factor influencing the continued development of transgenic technology, its subsequent field trials, and commercial availability. However, various social and ethical factors have been an obstacle in the process of transgenic plant development.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
The cucumber (Cucumis sativus L.) is one kind of major cucurbitaceous fruit, bred all over the world (He et al. 2008). Cucumber (Cucumis sativus L.) is a major fruit utilized as an economically important edible food and is widely cultivated throughout the globe (Huang et al. 2009; Wang et al. 2013). As a fresh market culinary crop, the economic importance of cucumber varies differently along different geographical regions. It is categorized as a popular vegetable in southern Asia, America, and most of the European countries (Nishibayashi et al. 1996b; Esquinas-Alcazar et al. 1983).
13.1.1 Distribution and Importance of Cucumber
Cucumber is a member of the family Cucurbitaceae. Family Cucurbitaceae comprises annual or perennial herbs, creepers, and climbers which are native to temperate and tropical regions. The family houses 98 genera and about 975 species of food and ornamental plants. Important members of the family are cucumbers, gourds, melons, squashes, and pumpkins that make up the family Cucurbitaceae economically important. The Cucumis genus comprises over 30 different species. Cucumis is an important genus of the Cucurbitaceae family comprising mostly creepers which include the cucumber (Cucumis sativus), muskmelons (Cucumis melo), and Indian gherkin (Cucumis anguria) (Sebastian et al. 2010). This crop is mostly produced for the fresh market throughout the world where China, as a market leader in terms of production, being the highest producer among all cucumber-producing countries. Cucumber also holds the position to be among the top ten vegetables produced throughout the world (Sebastian et al. 2010).
13.1.2 Genome Organization and Inheritance
Cucumber possesses a small haploid genome having a size of 367 Mbp with a basic number of x = 7 (Arumuganathan and Earle 1991). Three different modes of transmission of chromosomes are employed by cucumber, which makes it a unique model to study the inheritance traits in filial generations. Chromosomes are transmitted maternally for chloroplasts, paternally for mitochondria, and biparentally for nuclear DNA (Havey 1997; Havey et al. 1998). Indeed, this mitochondrial genome of cucumber happens to be the largest genome among all eukaryotic organisms (Plader et al. 2007).
Due to these properties cucumber has received much attentiveness for being a model crop for vascular biology and inheritance pattern studies. The precise genome sequence for cucumber was decoded by Huang et al. (2009), and further efforts are now made to develop a detailed genetic and cytogenetic map for this crop (Ren et al. 2009). More details for cucumber genome annotation, genetic maps, and QTLs are being constantly appended in the International Cucurbit Genomics Initiative (http://icugi.org) which has now been completely upgraded to (http://cucurbitgenomics.org). This newly appended database has a genome sequence available for 15 members of the Cucurbitaceae family. Furthermore, the database also provides EST and expression analysis data which includes the transcriptomic data for cucumber and other related species.
13.1.3 Importance of Cucumber Transformation
Though its geographical distribution being so varied, still the crop faces significant challenges against several biotic factors, namely diseases caused due to infestation of pests and microorganisms, especially Cucumber mosaic virus, Green mottle mosaic virus, leaf fungal diseases, angular leaf spot, fusarium wilt and cucurbit scab, nematode infestation, anthracnose, leaf blotch which causes a huge loss in the net attainable yield (Bacci et al. 2006; He et al. 2008). Apart from this, the yield is further compromised by other environmental vagaries mostly in the form of abiotic factors that include extreme temperature regime and prolonged water deficit and salinity stress (Chojak-Koźniewska et al. 2018). A lot of efforts are being made for developing an improved variety that shows a better yield and significant tolerance against environmental extremes. However, it is still a challenge to improve yield and tolerance of cucumber by conventional breeding owing to its narrow genetic base, having a genetic variability of only 3–8% (Plader et al. 2007). Improvement via conventional breeding-related strategies is further exacerbated because of long crop breeding time and extreme incompatibility toward related species (Kho et al. 1980). Although several defence and pathogenesis-related genes and their alleles already exist in nature, however, it is still a challenge to breed an improved variety just by conventional breeding. Therefore, it is imperative to make use of recombinant DNA technology for transferring the elite genes directly to the host genome in a relatively short span. Also, transformation in cucumber is not only aimed to provide resistance against biotic or abiotic stresses but it also applies to generate new improved varieties with respect to reduce bitterness, fruit size, sweetness, and to some extent to increase the content of secondary metabolites. The genome sequence of cucumber has also provided the list of important genes that may further help to augment the process (Huang et al. 2009). The transformation efficiency and stabilization of transgene construct in plants are largely governed by the availability of regeneration protocols from diverse explants. A major barrier limiting transformation is the recalcitrant nature of the crop, the requirement of sterile culture for plant regeneration, and the non-efficient transformation process (Niazian et al. 2017). All these factors impede the generation of novel transformants carrying agronomically important traits and limit other reverse genetics-related studies.
The chapter mostly focuses on methods and factors that are used for enhancing the transformation processes and also highlights the current status and future perspective of transgenic cucumbers possessing elite traits for disease resistance and stress tolerance and their commercial utility.
13.2 Methods Used for Cucumber Transformation
Genetic improvement of cucumber using biotechnological tools requires an efficient and reproducible method of plant regeneration which ascertains an efficient and stable basis for its genetic transformation. Several groups have defined a number of diverse protocols, however, every protocol suffers from some of the other lacunae. Moreover, other extraneous factors, namely genotypes, explant source, seedling age, growth regulator, media composition, and environment, also play a key role in influencing the reproducibility of regeneration and transformation (Punja et al. 1990; Sarmento et al. 1992; Nishibayashi et al. 1996b; Vasudevan et al. 2007; Wang et al. 2013). The problem of sterility barrier and crossing incompatibility seems an acute barrier especially in cucumber, (C. sativus L.) where the successful crossing is only reported in C. sativus and closely related C. hardwickii, but very few reports of successful crossing exist in other species (Trulson et al. 1986). To overcome this concern various biotechnological interventions have been exclusively utilized for developing an improved cucumber genotype. Among them, the most utilized are tissue culture-based techniques, namely micropropagation, in vitro regeneration using various explants, Agrobacterium-mediated transformation, and direct gene transfer for incorporating one or more traits of interest.
13.2.1 Tissue Culture-Based Regeneration: Direct and Indirect Regeneration
Two different methodologies are employed for plantlet regeneration through tissue culture, namely indirect regeneration having distinguishable callus phase and direct regeneration that is free from intermittent callus phase. The direct regeneration procedure holds superior standards as having a minimal deviation from the mother explants or having negligible somaclonal variation. Both types can be achieved by using cotyledonary explants (Msikita et al. 1990; Colijn-Hooymans et al. 1994) or leaf-derived callus (Nadolska-Orczyk et al. 1984; Mishra and Bhatnagar 1995) and leaf microexplants (Burza and Malepszy 1995b, Burza et al., 2006) or may also be sometimes protoplast-derived (Burza and Malepszy 1995a, Burza et al., 2006). Various groups have reported cotyledonary explants for direct shoot organogenesis and achieved encouraging results in cucumber (He et al. 2006; Rajagopalan and Perl-Treves 2005; Tabei et al. 1998).
Usually without using any extraneous inducer the time required, from explant inoculation to the time when plants get ready for greenhouse transfer, is around 10 weeks to 6 months. Transformation efficiency obtained by using this regeneration protocol was recorded in the range of 1.5–6.3% for regenerated shoots and varied from 1.4% to 10% in the obtained transgenic plants calculated against the total number of inoculated explants (He et al. 2008). The obtained results can be optimized for other explants like cotyledons, hypocotyls, leaves, and petioles, to further curtail the time spent on cucumber transformation.
13.2.2 Micropropagation
Micropropagation technique entails using synthetic media for in vitro multiplication of cells or organs utilizing a small part as an explant from a superior or elite parent. Several groups have attempted to utilize in vitro micropropagation or mass multiplication of cucumber with variable efficiencies. An efficient procedure for micropropagation of cucumber from seeds is reported by Hisajima et al. (1989). It utilizes a multi-stepped protocol for shoot induction from seeds, followed by shoot multiplication and lastly root induction which displayed an exceptionally high efficiency. Using this protocol approximately 1,600,000 cucumber shoots, with minimal deviation, were regenerated from a single shoot in a span of one year. Another study performed by Burza and Malepszy (1995b) utilizes the use of 2−3 mm2 pieces of leaf micro explants of C. sativus and C. anguria for shoot regeneration in a short span of five weeks. However, in vitro micropropagation also suffers from a major drawback as the plants regenerated from the mature explants are mostly found to have aberrant morphology and are mostly polyploid/mixoploid at the chromosomal level. Unfortunately, tissue culture-based regeneration likely resulted in callus formation and thus a high rate of somaclonal variations is observed in the progeny. Hence more progressive methods are required that are able to precisely transfer the gene of interest with minimal linkage drag which is the major drawback of conventional breeding. Recombinant DNA technology has paved the path for further development of cucumber, as among various techniques employed, the most utilized is Agrobacterium-mediated gene transfer and direct gene transfer methods.
13.2.3 Recombinant DNA Technology for Transgenic Generation
In crop improvement, genetic transformation provides a platform for transferring genes, cloned from prokaryotic or eukaryotic origin to a suitable host, creating new properties in a relatively short span of time without altering the existing traits (Gardner 1993). Yin et al. 2005 reported that mostly all transformation procedures for cucumber improvement programs utilize tissue culture-based methods where gene transformation is mostly performed via Agrobacterium-mediated gene transfer (Miao et al., 2009). Although few researchers also report direct gene transfer, namely the biolistic method, which will be described in the latter part of this chapter.
13.2.3.1 Agrobacterium-Mediated Gene Transfer
Agrobacterium is a type of plant-pathogen also known as a natural genetic engineer that infects plants especially the wounded sites and leads to tumorous outgrowth (Xu et al., 1993). Agrobacterium is distributed into five species based on their disease-causing ability and the choice of their host range in plants (Otten et al. 1996; Gelvin 2003). However, A. tumefaciens and A. rhizogenes are the most utilized species for plant transformation. Both differ in their specific properties as the former induces crown gall disease while the latter is known to induce hairy root disease. This variability among various strains of Agrobacterium is governed by a plasmid inherited inside the bacteria, namely Ti (tumor) and Ri (Root) inducing plasmids (Hwang et al. 2017). However, it becomes too labor-intensive to regenerate plantlets from transgenic hairy root cultures, therefore Ri plasmid-based transformation technique holds a limited role in cucumber transformation (Tang and Samuels 2001).
13.2.3.2 Direct Gene Transfer
Another method used for the successful transfer of ectopic genes into cucumber is a direct gene transfer method. This method makes use of microprojectile bombardment and pollen tube pathway for transgene integration into the cucumber genome. Microprojectile or particle bombardment makes use of DNA coated gold or tungsten particles which are accelerated under an inert environment of Helium gas. These accelerated particles are allowed to bombard in a suitable explant to release the plasmid DNA where it gets integrated stably. However, this technique is not often used to transform cucumber since this is prone to integrate multiple copies of the desired gene resulting in gene silencing of the obtained transgenic line. In a related study, Chee et al. (1992) generated transgenic cucumber by bombarding a reporter gene in embryogenic callus suspension cultures and obtained a transformation frequency of 16%. Shockingly, they concluded that among them only one-fourth of plantlets were successfully able to express the reporter gene. Later, it was concluded that multiple copies of transgene were incorporated into the genome which triggered gene silencing hampering the expression of a reporter gene. Multicopy integration and rearrangement were also reported using uidA (GUS) and nptII (kanamycin/Neomycin) as probes which were confirmed by PCR and Southern blot analysis.
13.2.3.3 Pollen Tube Pathway Method
This method entails directly injecting the exogenous DNA into the reproductive part of cucumber, especially the pollen tube or the ovary. Injecting DNA is directly performed 12 h post-fertilization which results in the successful integration of the exogenous DNA. However, very few reports exist for the successful transformation of cucumber using a pollen tube pathway. According to Li et al. (2000) very high transformation frequency was achieved using cucumber variety “Jingyan” when 100 mg/kg of DNA was directly injected into the ovary 12 h after pollination but contrarily a very low fruit setting ratio and the number of seeds per fruit was obtained, making this technique to be of less utility.
Apart from all these major techniques utilized in gene transfer, several other relatively less utilized techniques are also used. Among them, direct gene transfer using protoplast culture, suspension culture, haploid cell culture, viz., ovary culture, anther culture, and somatic embryo culture have been sparingly utilized for gene transformation. Very few authors have even attempted to generate transgenic cucumber plants via somatic embryogenesis using cotyledonary explants which were mediated through A. tumefaciens (Chee 1990; Tabei et al. 1994). The reason for this is the occurrence of a spontaneous mutation that is seen more in somatic embryogenesis as compared to direct organogenesis; therefore, direct organogenesis is supposed to be more suitable for regeneration studies and for the generation of transgenic plants. However, very scanty reports are available to date for direct gene transfer which succeeds in generating transgenic cucumber plants having single-copy trait integration that is stably inherited over several generations.
13.3 Factors Affecting Transformation Efficiency
Reports indicated that using acetosyringone at 50–200 μM concentration during co-cultivation was found helpful in augmenting Agrobacterium-mediated transformation frequency (Gelvin 2003). In addition, another chemical namely abscisic acid (ABA) was efficiently able to induce the production of adventitious shoots using cotyledonary explants (Tabei et al. 1998). Several research groups have used ABA treatment for cotyledonary explants and reported increased organogenesis in various cucumber cultivars (Gal-On et al. 2005; He et al. 2006; Rajagopalan and Perl-Treves 2005; Vengadesan et al. 2005).
Optimization transformation procedure in plants initially requires a reporter gene, mostly GUS, and one selectable marker gene mostly kanamycin or neomycin to be introduced into the plant cell prior to the integration of gene (s) of interest. A reporter gene encodes an enzyme that displays assayable activity by substrate cleavage which can be used as an indicator to monitor the transcriptional activity of a gene for which the gene product is not known or is not easily identifiable so as to get a fair idea that the plant tissue subjected to transformation has really been transformed or not (Gardner 1993). The original gene sequence to be studied is replaced with the reporter gene sequence and fused downstream to a strong promoter. This modified vector is now introduced into Agrobacterium cells which will be used to infect the plant tissue followed by co-cultivation screening. After the process is optimized the reporter gene is replaced with the gene of interest and the transformation process is repeated for obtaining viable transformants.
13.4 Applications of Biotechnology for Cucumber Improvement
The continued exponential increase of the human population demands the availability of more food products. On the other hand, limited acreages and various stresses slower down the production of edible plants. Traditional breeding programs can be combined with our recent understanding of genome biology to produce quality crops. Genetic engineering and plant biotechnology for crop improvement can broadly be categorized into three main areas: (1) Combating plants against continued and ever-changing biotic and abiotic stress; (2) increasing crop yield and control of plant growth vegetatively and reproductively; and (3) production of biochemicals and pharmaceuticals in plants.
Recently, the available annotated draft genome of cucumber provides valuable insight for the identification of candidate genes in the cucumber for diverse important agronomic traits. These genes often act in a cascade and provide a better understanding of the activation of metabolic pathways. A complete understanding of functional genomics of the cucumber genome is the superior resource for a scientist to impede the new varieties with valuable and economically marketable agricultural traits. Traditional breeding methods are often obstacles to sexual incompatibility between and within the species. Hence, breeding offers a narrow window for the up-gradation of elite germplasm. Recent advances in genome editing and transgenic technologies have provided a new impulse to cucumber transformation. The goal of cucumber transgenic program is to increase productivity and quality of fruit by identifying and pyramiding the genetic potential of different cultivars and thereby providing resistance against multiple biotic and abiotic stresses. Further, the availability and standardization of efficient tissue culture systems from different explant sources also facilitated rapid developments of novel trait transfer in cucumber. This section reviews the progress in cucumber biotechnology.
13.4.1 Biotic Stress Resistance
Various biotic factors, viz., viruses, fungi, nematodes, and bacteria pose a serious threat to the cultivation and production of cucumber throughout the globe. The only solution to combat such biotic threats was either to use pesticides extensively or to rely on conventional breeding programs. The use of pesticides, nowadays, is very limited owing to their health deteriorating effects on living organisms. On the other hand, conventional breeding is very limited in cucumber due to restricted genetic diversity and sexual incompatibility. Hence transgenic approaches by the introduction of exogenous genes are in application to overcome limitations of conventional breeding in cucumber development.
13.4.1.1 Virus Resistance
Compared to other biotic infections, viral infections are difficult or nearly impossible to cure by conventional chemical methods. Viruses often change their epitope profiles or they will show visible symptoms only after successful disease establishments in host plants. So far, an efficient chemical method to cure viral diseases of cucumber is unavailable. On the other hand, no useful genetic resource against virus resistance is available in important pickle type and vegetable type cultivar of cucumber. Potyviruses namely Cucumber Mosaic Virus (CMV), Watermelon Mosaic Virus (WMV), and Zucchini Yellow Mosaic Virus (ZYMV) are among the viruses that infect cucumber. Virus resistance strategies are centered to provide resistance against these viruses. Coat protein genes are important candidate genes to provide virus resistance in transformed plants. Transgenic plants expressing virus coat protein genes show delayed symptom appearance or resistance against viruses. Chee and Slightom 1991) were first to transfer CMV-C coat protein (CP) gene into cucumber by Agrobacterium-mediated transformation. Field trials showed that transgenic plants did not obtain resistance against CMV infection (Chee and Slightom 1991). Successful establishment of virus resistance through coat protein was first reported by Gonsalves et al. (1992) in a vegetable-type cultivar of cucumber by the transformation of CMV-C CP gene. Out of the total, 36% transgenic plants showed no symptoms against CMV infection under greenhouse condition. Furthermore, transgenic cucumbers showed more vegetative and fruit growth than those of non-transformed plants. Transgenic cucumber expressing CMV-O CP gene was reported by Nishibayashi et al. (1996a). Transformed cucumber plants showed a higher degree of resistance to CMV infection and produce milder symptoms on double inoculation of CMV and ZYMV, which individually produce severe symptoms of necrosis on susceptible varieties. Agrobacterium-mediated transfer of WMV CP gene and plant regeneration from cotyledon explant was reported by Wang et al. (2000). Transgenic plants showed a lower incidence of virus disease. A novel approach to tobamovirus resistance by transferring the viral replicase gene was done by Gal-On et al. (2005). Putative 54 KDa Cucumber fruit mottle mosaic tobamovirus (CFMMV) replicase gene was transferred to cotyledonary explant of cucumber by Agrobacterium. Transgenic plants showed higher immunity immune to soil-borne CFMMV infection by mechanical and graft inoculation. Transgenic plants also showed resistance to root infection by CFMMV when planted in soil containing a significant amount of CFMMV titer. Another novel strategy of transformation of the pokeweed antiviral protein (PAP) gene was reported by Cao et al. (2011). PAP inhibits the effect of plant viruses that belong to a group of ribosome-inactivating protein I (RIP I). Transgenic T0 plants harboring the PAP gene from plants of the Phytolacca genus did not show any symptoms of CMV infection in the field condition. Agrobacterium-mediated genetic transformation of cucumber with GFP-tagged WMV genes, under constitutive expression of CaMV 35S promoter was achieved by Khidr et al. (2012). Integration of viral genes into the host plant genome confers broad spectrum resistance against these viruses (Wilson 1993; Gonsalves et al. 1994). Genome editing provides an elucidative way for substantial immunity against a broad spectrum of viruses. Chandrasekaran et al. (2016) reported the use of CRISPER/Cas9 technology to confer virus resistance. T3 progeny, homozygous for Cas9/subgenomic RNA targets N- and C-terminus of eIF4e (eukaryotic translation initiation factor 4E) gene. Edited plants exhibited a higher level of immunity against Cucumber vein yellowing virus (Ipomovirus), Zucchini yellow mosaic virus (Potyvirus), and Papaya ringspot mosaic virus-W (Potyvirus). In contrast, heterozygous and non-edited wild varieties showed susceptibility to these viruses. All these reports clearly highlight the importance of transgenic technology to raise virus-resistant cucumber variety without compromising botanical and fruiting characteristics.
13.4.1.2 Fungal Resistance
Various fungi are reported to infect cucumber plants in the field. Fungal infection on cucumber is characterized by yellow lesions on the leaf and small venation. Opposite to viral infection, fungal infections are self-transferred and seed-borne too. Botrytis, Phytophthora, Fusarium, and Alternaria are among the most common fungal pathogens infecting cucumber. Chitinase are hydrolyzing proteins that degrade chitin. The role of chitinase as an antifungal protein was reported in many plants to confer antifungal properties. Chitinases are pathogenesis-related proteins that show higher-level expression during fungal infection. Cucumber chitinase is acidic in nature. Transformation of cucumber with chitinase genes from tobacco, bean, and petunia was reported by Punja and Raharjo (1996). Expression of transgenes in transgenic plants showed no significant disease resistance when inoculated with Botrytis, Colletotrichum, Alternaria, and Rhizoctonia (Raharjo et al. 1996). Chitinase cDNA under the intrinsic expression of CaMV 35S promoter from the rice was transformed by Agrobacterium. When transgenic plants were infected with gray mold, Botrytis cinerea, 15 out of 20 independent shoots showed resistance compared to non-transgenic plants (Tabei et al. 1998). Enhanced expression and intracellular accumulation of rice chitinase genes have provided resistance against gray mold in transgenic cucumber (Kishimoto et al. 2002). Overexpression of the rice class I chitinase gene also confer resistance to Phytophthora rot (Phytophthora nicotianae var. parasitica) (Kishimoto et al. 2003). Overexpression of class III chitinase gene (CHI2) in cucumber exhibits revocable resistance against infection to gray mold, as transgenic plants did not exhibit disease symptoms initially but later developed serious disease symptoms (Kishimoto et al. 2004). Transgenic expression of a novel antifungal gene from Ginko biloba seed kernels in cucumber showed enhanced resistance against blight disease caused by Fusarium oxysporum (Liu et al. 2010). Recently, miRNA-mediated resistance against target leaf spot (TLS), which is caused by Corynespora cassiicola, was reported by Wang et al. (2019). Agarobacteria-infiltered novel miRNA constructs silence the indigenous expression of miRNA in cucumber cotyledon and thus improve disease resistance in transgenic cucumber plants.
13.4.2 Abiotic Stress Resistance
Abiotic stress is a key environmental factor that leads to significant retarded plant growth and a decrease in yield. Abiotic stress can prominently impede plant growth by declining biomass production. Various abiotic stresses, viz., salt, chilling, temperature, pesticides, etc. were reported to hinder plant growth and development. Chilling stress resistance and pesticide residue stress resistance are the two most important reported in transgenic cucumber. Chilling at or below 4 °C induces oxidative damage and produces irreversible injury in cucumber fruit. Oxidative stress accompanied by lipid peroxidation is an earlier response to chilling stress in cucumber. Such damage to the tissue will evoke the cascade of biotic infection, eventually producing fruit with no edible value.
13.4.2.1 Chilling Stress Tolerance
The deficiency of cold‐tolerant cucumber germplasm is a hindrance in classical breeding. Genetic transformation techniques providing chilling stress resistance mainly target cis-acting elements and inducible genes, thereby providing chilling tolerance in sensitive crop varieties. Upon exposure to cold stress, the plant immediately activates a cascade of the signaling process to combat negative effects induced by cold stress. Various cis-elements activate the expression of functional genes. Among these elements, C-repeat elements/dehydration-responsive elements (CRT/DRE), where C-repeat binding factors (CBFs) bind, are induced positively by an upstream inducer of CBF expression (ICE) gene, with two homologs, ICE1 and ICE2.
Dehydrins (DHN) proteins from late embryogenesis abundant [Lea] D11 family shows upregulation in chilling stress (Close 1997). Dhn10 gene from Solanum sogarandinum fused with 1633 bp promoter region of Solanum sogarandinum glucosyl transferase (GT) gene (pGT::Dhn10). Fusion constructs with all regulatory elements were transferred by Agrobacterium, and transgenic lines of cucumber are produced. The prospective role of the Dhn10 gene in cold tolerance was observed in three T1 transgenic cucumber lines. Out of three, one line showed a significant increase in its chilling tolerance with no apparent chilling injury. The rest two lines showed no or significantly decreased freezing tolerance in experimental conditions (Yin et al. 2004a). Similarly, Mróz et al. (2015) transform pGT::Dhn24 fusion from Solanum sogarandinum. Transgenic cucumber showed no evidence for increased cold tolerance. The transformation of cbf1 gene in cucumber cotyledon was reported by Deng et al. (2004). Transgenic cucumber expressing the Arabidopsis cbf1 gene showed a significantly decreased level of chilling injury symptoms, viz., decrease in membrane injury, increase in the level of SOD and CAT enzymes for scavenging-free radicle oxygen, and increase in proline and relative water content (Gupta et al. 2012). Arabidopsis thaliana nit2 gene encodes enzyme nitrilase which indole-3-acetonitrile (IAN) to indole-3-acetic acid (IAA), an important auxin. Transgenic cucumber expressing exogenous nit gene showed enhanced resistance to various abiotic environmental stresses (Jang et al. 2013).
Fusion of the trehalose-6-phosphate (T-6-P) synthase (TPS) and T-6-P phosphatase (TPP) of Escherichia coli is called TPSP (Trehalose 6-phosphate synthase and phosphatase). This novel bifunctional TPSP possesses both activities of phosphatase and synthetase and is reported to accumulate trehalose upon various abiotic stresses in Escherichia coli and tobacco (Jang et al. 2003). To evaluate the role of TPSP in providing chilling tolerance to cucumber, Kim et al. (2010) transformed the TPSP gene construct under CaMV 35S promoter and nopaline synthase (nos) and bar gene as marker system. Transformed plants were shown to synthesize and accumulate three times more amount of trehalose than non-transformed plants. Conversely, transformed plants show abnormal growth morphology including stunted growth and sterility. These results clearly suggest that the accumulation of trehalose is toxic to growth in cucumber (Kim et al. 2010).
Signal transduction pathways often initiate a cascade of cross-talks where several molecules are involved. These cross-talks are extremely complex to decipher in plants. One such signaling cascade is the mitogen-activated protein kinase (MAPK) cascade, which shows upregulation in various abiotic stresses. The signaling cascade involves a series of phosphorylation of MAPK to MPAK kinase (MAPKK) to MAPKK kinase (MAPKKK). This signaling cascade leads to various cell responses including cell division, production of reactive oxygen species (ROS) scavengers, and other signaling molecules to combat abiotic stress. Wang et al. (2013) use the affecting MAPK signaling pathway to confer abiotic stress resistance in Cucumis sativus L. Genetic construct containing MAPK was transformed to cucumber cotyledons. Successful transformants showed various levels of resistance against abiotic stress (Wang et al. 2013).
G-proteins (guanine nucleotide-binding proteins) are multimeric heterotrimers composed of three subunits: Gα, Gβ, and Gγ. In plants, the Gγ subunit is a trimer composed of three structurally distinct subunits: A, B, and C (Yan et al. 2018). These proteins play important signaling molecules providing cellular response against abiotic stress. Type C subunit of Gγ subunit is transformed in cucumber. Transgenic cucumber constitutively expressing type C subunit of Gγ subunit showed increased accumulation of CBF and anti-oxidative enzymes such as SOD, peroxidase (POD), catalase, and glutathione reductase and glutathione S-transferase (GST). Thus, mediating signal transduction in response to cold stimuli in cucumber without compromising other growth parameters (Bai et al. 2018).
YUC proteins (YUCCA) catalyze the conversion of Indole 3-pyruvic acid to Indole 3-acetic acid (IA), an important phytohormone auxin. Thus, the YUC gene family catalyzes the rate-limiting step in the auxin biosynthesis pathway. Cucumber possesses 10 YUC family genes (CsYUCs), which work in a coordinated manner to maintain auxin concentration in cucumber. Experimental studies showed that CsYUC8 and CsYUC9 are upregulated under high-temperature stress. CsYUC4 shows downregulation in response to the lower temperature. CsYUC10b is upregulated under chilling stress and regulated by CsYUC10b and CsYUC11. CsYUC11 provides tolerance against salinity stress (Yan et al. 2016).
13.4.2.2 Pesticide Resistance
Chemical pesticides are widely used to control pests in cucumber plants. These pesticides are recalcitrant to natural degradation and often absorb and form pesticide residue in cucumber fruit. Consumption of such fruit is toxic to human health and the environment. One such pesticide used for cucumber cultivation is Propamocarb (PM). Some of the cucumber varieties are known to accumulate a lower amount of PM in their tissues. In such cultivars, genes namely CsMAPEG (Cucumis sativus membrane-associated proteins in eicosanoid and glutathione metabolism) are strongly and constitutively expressed. Transfer of CsMAPEG gene constructs in high pesticide residue abundance cultivar of cucumber results in increased concentration of SOD, POD, and GST accompanied by metabolic degradation of PM in transgenic plants. The results showed the CsMAPEG cascade in PM degradation in cucumber (Zhang et al. 2019).
13.4.3 Yield Improvement
The typical plant breeding programs for crop improvement are tedious and time-consuming. The exponential increase in the human population demands a rapid solution for yield improvement in plants without compromising nutrient content. Plant tissue culture and marker-assisted selection that form the basis of plant biotechnology offer unique opportunities to provide sustainable agricultural practices. Modern genomic editing practices should be used as an aid of classical breeding to increase the yield.
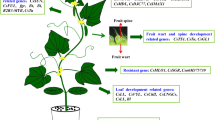
The first successful attempt toward yield increase in cucumber was reported by Salyaev et al. (2002). Uridine diphosphate glucuronosyltransferase (UDT) from Zea mays and acetyl Co-A binding protein gene (ACB) from Arabidopsis thaliana are transformed to cucumber. UDT encodes the enzyme IAA glucose synthase and thereby overexpression of UDT leads to the accumulation of auxin (IAA) in transformed cucumber. ACB acetyl Co-A binding protein gene (ACB) catalyzes the prime step in fatty acid biosynthesis. Transformed cucumber showed a marked increase in yield compared to non-transformed plants (Salyaev et al. 2002a, b). The role of G-proteins in enhanced seed germination and root growth was reported by Yan et al. (2018). G-protein gene, CsGPA1, was transformed to cucumber. Transformed plants showed earlier seed division, root growth, and hypocotyl elongation through cell division and enhanced activity of root apical meristem (RAM) (Yan et al. 2018). Phytohormone, gibberellin (GA) is reported to regulate many functions, especially in fruit development. The role of GA in locule, interior cavities of fruit development is a complex process and is regulated by a series of different genes. (Cong et al. 2008). Overexpression of GA receptor gene CsGID1a is shown to control locule formation in transgenic cucumbers (Liu et al. 2016). Another important epidermal feature of cucumber fruit is tubercle formation. Yang et al. (2019) demonstrated that CsTS1 gene expression positively regulates tubercle formation and increases the economic value of cucumber (Yang et al. 2019). Little Leaf (LL) locus in cucumber is known to control organ size and development. Yang et al. (2018) showed that overexpressing of the LL allele in wild-type cucumber varieties leads to an increase in leaf size, flowers fruits, and seeds (Yang et al. 2018).
13.4.4 Nutrition and Taste Enhancement
Improvement in nutrition and taste enhancement are important traits that are to be addressed by plant biotechnology. There are several reports available for taste development and nutritional enhancement in cucumber. Thaumatin is a plant protein production in the fruit of the West African perennial plant, Thaumatococcus daniellii Bentham. Thaumatin is a natural sweetener and is often used as a taste enhancer in food products. Szwacka et al. (2000) reported transformation of Thaumatin gene is cloned in plant binary vector and express under CaMV 35S constitutive promoter and under neomycin phosphotransferase II (NPT II) marker selection. Transformed cucumber lines show accumulation of a higher level of thaumatin mRNA and protein expression from T1 progeny (Szwacka et al. 2000, 2002; Yin et al. 2004b). Another important trait transfer to cucumber is an enhancement of carotene content. Carotenoids are important precursor producing chromogenic substances which imparts peculiar color to the plant parts. Also, carotenoids are precursors for abscisic acid (ABA), and especially β-carotene is a precursor for vitamin A. Jang et al. (2016) reported successful stable transformation of to introduce phytoene synthase-2a carotene desaturase (PAC genes) into a cucumber. Transformants overexpressing PAC gene showed enhanced beta-carotene content (Jang et al. 2016).
13.4.5 Improvement in Fruit Quality
Improving the fruit quality for taste, size, shelf life, ripening, nutritional enhancement, etc. are demanding traits for transformation by plant biotechnology. An important trait for fruit quality in cucumber is parthenocarpy or seedless fruit. Seedless fruit is a choice over wild-type seed fruits for processing and ease of consumption. As pollinating agents are not necessary for parthenocarpic fruits, plants can be grown under a suitable controlled enclosed condition like a greenhouse when environmental factors are not suitable for plant growth. The first successful event of transgenic cucumber for parthenocarpy was reported by Yin et al. (2006). IaaM gene from Pseudomonas syringae pv savastona, coding for auxin, indole acetic acid (IAA), under the control of a specific promoter, DefH9, from Antirrhinum majus, was transformed to cucumber. IAA is expressed specifically in developing ovule, and increased expression of auxin gene promotes parthenocarpy in developing fruit (Mezzetti et al. 2004, Yin et al. 2006). Total fruit yield produced by the transformed cucumber, 70–90% were parthenocarpic (Yin et al. 2004, 2006). Auxin-binding protein 1 (ABP1) from Arabidopsis thaliana is transformed in cucumber cotyledon by Agrobacterium tumefaciens. Successful transformants showed 31% production of parthenocarpic fruit (Bai et al. 2004). MADS box genes are important genes that regulate inflorescence and fruit development. MADS box genes, CSMADS06, are transformed and overexpressed in cucumber varieties S06 and S52. Positive cucumber transformants show inflorescence blooming and shown to set fruits (Lai et al. 2007).
13.4.6 Nutraceuticals and Biopharming
Biopharming is the use of transgenic plants or animals carrying foreign genes coding for medicinally important therapeutic proteins such as hormones, enzymes, antibodies, vaccines, etc. The term “edible vaccine” is popularly in use when vaccine protein is developed as transgenic in the edible part of the plant and can be consumed directly. Apart from, biopharmaceuticals, nutrition enhancers are also the prime target for transgenic plant development. Anti-oxidative and anti-aging enzyme superoxide dismutase (SOD) coding gene, CuZnSOD cDNA (mSOD1) from Manihot esculenta, under ascorbate oxidase promoter and phosphinothricin (PPT) resistance as the selectable marker are transformed in cucumber. The transformed cucumbers showed a higher level of SOD expression that was three times higher in fruits of transgenic plants compared to fruits of non-transgenic plants (Lee et al. 2003). Cucumis sativus also served as a potential system for the transformation and production of exogenous proteins. Shi et al. (2006) used hairy root culture produced after transformation with Agrobacterium rhizogenes carrying binary vector with the gene sequence of EHRH cardenolide 16′-O-glucohydrolase derived from Digitalis lanata. This enzyme catalyzes the deglycosylation of steroid glycosides. Deglycosylation activities of transformed cucumber were measured by HPLC (Shi et al. 2006). GLP-1 (Glucagon-like peptide-1) is a 30-amino acid residue hormone which regulates the secretion of insulin to control blood glucose levels. A synthetic analog of the GLP-1 gene was constructed and transformed into a cucumber. Oral administration of transgenic fusion protein (GLP-T) to diabetic mice reduces blood glucose levels (Lei et al. 2009). These results provide a new strategy to cure diabetes. Production of oral vaccines in cucumber is useful and economically viable. Sindhu et al. (2010) reported the expression of hepatitis B virus surface antigen (HBsAg) in cucumber. HBsAg gene was cloned in plant binary vector pCAMBIA 3300 under constitutive expression of CaMV 35S promoter and PPT gene as a selection marker. Transformed cucumber plants were shown to express HBsAg, as confirmed by Western blot, ELISA, and MALDI-ToF.
The number of transformed traits produced in transgenic plants is often low due to low transgenic frequency and gene expression level. Future efforts need to be directed toward marker-free selection, tissue and organ-specific expression, and modification of promoters.
13.5 Conclusion and Future Prospects
Cucumber (Cucumis sativus L.) is an important horticultural crop and is consumed worldwide. The most important efforts in transgenic cucumber are directed toward virus resistance and changes in the ripening process. Other important traits such as fungal resistance, abiotic stress tolerance, and biofortification increase the yield and nutritive value of cucumber. Enhanced expression of SOD, carotene, HBsAg, GLP-1, etc. in cucumber provides prime examples of the successful and stable transformation of transgenes in cucumber. Standardization of agrobacterium or biolistic-based transformation methods followed by standard techniques of tissue culture to raise complete plantlets led to an increase in transformation efforts in cucumber. Availability of draft genome sequence of cucumber opened up the door of genome editing and gene silencing by RNAi using dsRNA, siRNA, and artificial miRNA to incorporate desirable traits such as fruit quality, biotic and abiotic stress resistance, and biopharmaceutical production. Construction of chimeric construct provides multiple trait transfer in cucumber. Genetically modified (GM) cucumber has a potential future. The release of GM cucumber as a commercial variety needs a mandatory risk assessment to avoid the risk effects of transgene escape.
References
Arumuganathan K, Earle ED (1991) Nuclear DNA content of some important plant species. Plant Mol Biol Report 9(3):208–218
Bacci L, Picanço MC, Gonring AHR, Guedes RNC, Crespo ALB (2006) Critical yield components and key loss factors of tropical cucumber crops. Crop Prot 25(10):1117–1125
Bai JG, Wang XJ, Yin QX, Tian M (2004) Regeneration and transformation of Cucumis sativus cv Jinyanshihao. Scientia Agriculture Sinica 37:263–267 (in Chinese)
Bai L, Liu Y, Mu Y, Anwar A, He C, Yan Y, Li Y, Yu X (2018) Heterotrimeric G-protein γ subunit CsGG3.2 positively regulates the expression of CBF genes and chilling tolerance in cucumber. Front Plant Sci 9:488
Burza W, Malepszy S (1995a) In vitro culture of Cucumis sativus L. XVIII. Plants from protoplasts through direct somatic embryogenesis. Plant Cell, Tissue Organ Cult 41:259–266
Burza W, Malepszy S (1995b) Direct plant regeneration from leaf explants in cucumber (Cucumis sativus L.) is free of stable genetic variation. Plant Breeding 114(4):341–345
Burza W, Zuzga S, Yin Z, Malepszy S (2006) Cucumber (Cucumis sativus L.). In: Wang K (ed) Agrobacterium protocols, Vol 1, Methods in Molecular Biology, Humana Press, 427–438
Cao B, Lei J, Chen G, Cao P, Liu X, Chen Q, Wei X (2011) Testing of disease-resistance of pokeweed antiviral protein gene (PacPAP) in transgenic cucumber(Cucumis sativus.) African J Biotechnol 10(36):6883–6890
Chandrasekaran J, Brumin M, Wolf D, Liebman D, Klap C, Pearlsman M, Sherman A, Arazi T, Gal-On A (2016) Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol Plant Pathol 17(7):1140–1153
Chee PP (1990) Transformation of Cucumis sativus tissue by Agrobacterium tumefaciens and the regeneration of transformed plants. Plant Cell Rep 9(5):245–248
Chee PP, Slightom JL (1991) Transfer and expression of cucumber mosaic virus coat protein gene in the genome of Cucumis sativus. J Am Soc Hort Sci 116:1098–1102
Chee PP, Slightom JL (1992) Transformation of cucumber tissues by microprojectile bombardment: identification of plants containing functional and non-functional transferred genes. Gene 118(2):255–260
Chojak-Koźniewska J, Kuźniak E, Zimny J (2018) The effects of combined abiotic and pathogen stress in plants: Insights from salinity and Pseudomonas syringae pv. lachrymans interaction in cucumber. Front Plant Sci 9:1691
Close TJ (1997) Dehydrins: A commonalty in the response of plants to dehydration and low temperature. Physiol Plantarum 100:291–296
Colijn-Hooymans CM, Hakkert JC, Jansen J, Custers JBM (1994) Competence for regeneration of cucumber cotyledons is restricted to specific developmental stages. Plant Cell, Tissue Organ Cult 39(3):211–217
Cong B, Barrero L, Tanksley S (2008) Regulatory change in YABBY-like transcription factor led to evolution of extreme fruit size during tomato domestication. Nat Genet 40:800–804
Deng XY, Zhang XG, Jing X, Shao CW, Su CG (2004) Transformation of cold-induced transcription activator CBF3 into cucumber (Cucumis sativus L.). J Southwest Agric Univ (natural Science) 26:603–605 (In Chinese)
Esquinas-Alcazar JT, Gulick PJ (1983) Genetic resources of Cucurbitaceae. A global report. IBPGR Secretariat
Gal-On A et al (2005) Transgenic cucumbers harboring the 54-kDa putative gene of Cucumber fruit mottle mosaic tobamovirus are highly resistant to viral infection and protectnon-transgenic scions from soil infection. Trans Res 14:81–93
Gardner RC (1993) Gene transfer into tropical and subtropical crops. Sci Hortic 55(1–2):65–82
Gelvin SB (2003) Agrobacterium-mediated plant transformation: The biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev 67(1):16–37
Gonsalves C, Xue B, Yepes M, Fuchs M, Ling K, Namba S (1994) Transferring cucumber mosaic virus-white leaf strain coat protein gene into Cucumis melo L. and evaluating transgenic plants for protection against infections. J Am Soc Hort Sci 119:345–355
Gonsalves D, Chee PP, Provvidenti R, Seem R, Slightom JL (1992) Comparison of coat protein-mediated and genetically-derived resistance in cucumbers to infection by cucumber mosaic virus under field conditions with natural challenge inoculations by vectors. Nat Biotechnol 10:1562–1570
Gupta N, Rathore M, Goyary D, Khare N, Anandhan S, Pande V, Ahmed Z (2012) Marker-free transgenic cucumber expressing Arabidopsis cbf1 gene confers chilling stress tolerance. Biol Plant 56:57–63
Havey MJ (1997) Predominant paternal transmission of the cucumber mitochondrial genome. J Hered 88:232–235
Havey MJ, McCreight JD, Rhodes B, Taurick G (1998) Differential transmission of the Cucumis organellar genomes. Theor Appl Genet 97(1–2):122–128
He Z, Chen L, Yao W, Dai J (2008) Recent progress in cucumber (Cucumis sativus L.) transformation. Transgenic Plant J 2(1):39–44
He Z, Duan Z, Liang W, Chen F et al (2006) Mannose selection system used for cucumber transformation. Plant Cell Rep 25(9):953–958
Hisajima S, Arai Y, Namwongprom K, Subhadrabandhu S (1989) Micropropagation of cucumber plant through reproductive organ culture and semi-aquaculture of regenerated plants. Jpn J Tropic Agric 33(1):1–5
Huang S, Li R, Zhang Z et al (2009) The genome of the cucumber, Cucumis sativus L. Nat Genet 41:1275–1281
Hwang HH, Yu M, Lai EM (2017) Agrobacterium-mediated plant transformation: Biology and Applications. The Arabidopsis Book, 15:e0186
Jang HA, Lim KM, Kim HA, Park YI, Kwon SY, Choi PS (2013) Development of transgenic cucumbers expressing Arabidopsis Nit gene. J Plant Biotechnol 40:198–202
Jang HA, Utomo SD, Kwon SY, Ha S-H, Xing-guo Y, Choi PS (2016) Production of transgenic cucumber expressing phytoene synthase-2A carotene desaturase gene. J Plant Biotechnol 43(3):341–346
Jang IC, Oh SJ, Seo JS et al (2003) Expression of a bifunctional fusion of the Escherichia coli genes for trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase in transgenic rice plants increases trehalose accumulation and abiotic stress tolerance without stunting growth. Plant Physiol 131(2):516–524
Khidr YA, Mahmoud A, El-Absawy E, Hemeida AA, Hasan ME (2012) Production of transgenic cucumber plantlets containing sequences from watermelon mosaic virus-ii fused with GFP gene Egypt. J Genet Cytol 41:19–35
Kho YO, Den Nijs APM, Franken J (1980) Interspecific hybridization in Cucumis L. II. The crossability of species, an investigation of in vivo pollen tube growth and seed set. Euphytica 29(3):661–672
Kim HA, Min SR, Choi DW, Choi PS, Hong SG (2010) Development of transgenic cucumber expressing TPSP gene and morphological alterations. J Pl Biotechnol 37:72–76
Kishimoto K, Nakajima M, Nishizawa Y, Tabei Y, Hibi T, Akutsu K (2003) Response of transgenic cucumber expressing a rice class I chitinase gene to two fungal pathogens with different infectivities. J Gen Pl Pathol 69:358–363
Kishimoto K, Nishizawa Y, Tabei Y, Hibi T, Nakajima M, Akutsu K (2002) Detailed analysis of rice chitinase gene expression in transgenic cucumber plants showing different levels of disease resistance to gray mold (Botrytis cinerea). Plant Sci 162:655–662
Kishimoto K, Nishizawa Y, Tabei Y, Nakajima M, Hibi T, Akutsu K (2004) Transgenic cucumber expressing an endogenous class III chitinase gene has reduced symptoms from Botrytis cinerea. J Gen Pl Pathol 70:314–320
Lai L, Pan JS, He HL, Cai R (2007) Agrobacterium-mediated genetic transformation of cucumber (Cucumis sativus L.) with MADS-box like gene. Journal of Shanghai Jiaotong University (agricultural Science) 25:374–382 (in Chinese)
Lee HS, Kwon EJ, Kwon SY, Jeong YJ et al (2003) Transgenic cucumber fruits that produce elevated level of an anti-aging superoxide dismutase. Mol Breeding 11:213–220
Lei Z, Fang L, CuiYan W et al (2009) Generation of transgenic cucumbers with expression of a ten-tandem repeat long-acting GLP-1 analogue and their biological function on diabetic rats. Chinese Sci Bull 54:4658–4663
Li Y, Wang G, Ge X (2000) Studies on gene transformation by introducing exogenous DNA into cucumber after pollination. Acta Agriculturae Boreali-Sinica 15(2):89–94
Liu B, Liu X, Yang S, Chen C et al (2016) Silencing of the gibberellin receptor homolog, CsGID1a, affects locule formation in cucumber (Cucumis sativus) fruit. New Phytol 210:551–563
Liu J, Tian HL, Wang YH, Guo AG (2010) Overexpression of a novel antifungal protein gene GNK2-1 results in elevated resistance of transgenic cucumber to Fusarium oxysporum. Chin Bull Bot 45:411–418 (In Chinese)
Mezzetti BL, Pandolfini Y, Spena A (2004) The defH9-iaaM auxin-synthesizing gene increases plant fecundity and fruit production in strawberry and raspberry. BMC Biotechnol 4:4
Miao MM, Xu R, Zheng LJ, Zhou HL, Zhang ZD, Cheng H (2009) High-efficiency Agrobacterium tumefaciens mediated transformation of cucumber (Cucumis sativus L.) using stem nodes as explants. J Hortic Sci Biotech 84:199–203
Mishra AK, Bhatnagar SP (1995) Direct shoot regeneration from the leaf explant of cucumber (Cucumis sativus L.). Phytomorphology 45:47–55
Mróz TL, Ziółkowska A, Gawroński P, Pióro-Jabrucka E, Kacprzak S, Mazur M, Malepszy S, Bartoszewski G (2015) Transgenic cucumber lines expressing the chimeric pGT::Dhn24 gene do not show enhanced chilling tolerance in phytotron conditions. Plant Breeding 134:468–476
Msikita W, Skirvin RM, Juvik JA, Splittstoesser WE, Ali N (1990) Regeneration and flowering In vitro of ‘Burpless Hybrid’ cucumber cultured from excised seed. Hort Science 25(4):474–477
Nadolska-Orczyk A, Malepszy S (1984) Cucumber plant regeneration from leaf explants-selected characteristics. Bull Polish Acad Sci Biol 32(11–12):425–428
Niazian M, Noori SS, Galuszka P, Mortazavian SMM (2017) Tissue culture-based Agrobacterium mediated and in planta transformation methods. Soil Water Res 53(4):133–143
Nishibayashi S, Hayakawa T, Nakajima T, Suzuki M, Kaneko H (1996a) CMV protection in transgenic cucumber plants with an introduced CMV-O cp gene. Theor Appl Genet 93:672–678
Nishibayashi S, Kaneko H, Hayakawa T (1996b) Transformation of cucumber (Cucumis sativus L.) plants using Agrobacterium tumefaciens and regeneration from hypocotyl explants. Plant Cell Rep 15(11):809–814
Otten L, Ruffray PD, Momol EA, Momol MT, Burr TJ (1996) Phylogenetic relationships between Agrobacterium vitis isolates and their Ti plasmids. Mol Plant-Microbe Interact 9:782–786
Plader W, Burza W, Malepszy S (2007) Cucumber. In: Pua EC, Davey MR (eds) Transgenic Crops IV, 1st edn. Springer, Berlin, Heidelberg, pp 181–199
Punja ZK, Abbas N, Sarmento GG, Tang FA (1990) Regeneration of Cucumis sativus var. sativus and C. sativus var. hardwickii, C. melo, and C. metuliferus from explants through somatic embryogenesis and organogenesis. Plant Cell, Tissue and Organ Culture 21(2):93–102
Punja ZK, Raharjo SHT (1996) Response of transgenic cucumber and carrot plants expressing different chitinase enzyme to inoculation with fungal pathogens. Plant Dis 80(9):999–1005
Raharjo SHT, Hernadez MO, Zhang YY, Punja ZK (1996) Transformation of picking cucumber with chitinase-encoding genes using Agrobacterium tumefaciens. Plant Cell Rep 15:591–596
Rajagopalan PA, Perl-Treves R (2005) Improved cucumber transformation by a modified explant dissection and selection protocol. Hort Sci 40(2):431–435
Ren Y, Zhang ZH, Liu JH et al (2009) Integrated genetic and cytogenetic map of the cucumber genome. PLoS One 4(6):e5795
Salyaev RK, Rekoslavsaya NI, Mapelli S (2002) Increase of productivity in transgenic plants with introduced genes UGT, ACB and ACP.13th Congr. of the Federation of European Societies of Plant Physiology, Crete, Greece. Abstract, Section 8, Biological Applications:49
Sarmento GG, Alpert K, Tang FA, Punja ZK (1992) Factors influencing Agrobacterium tumefaciens mediated transformation and expression of kanamycin resistance in pickling cucumber. Plant Cell, Tissue Organ Cult 31(3):185–193
Sebastian P, Schaefer H, Telford IR, Renner SS (2010) Cucumber (Cucumis sativus) and melon (C. melo) have numerous wild relatives in Asia and Australia, and the sister species of melon is from Australia. Proc Natl Acad Sci USA 107(32):14269–14273
Shi HP, Lindermann P (2006) Expression of recombinant Digitalis lanata EHRH. Cardenolide 16-O-glucohydrolase in Cucumis sativus L. hairy roots. Plant Cell Rep 25:1193–1198
Sindhu C, Unni E, Soniya V (2010) Transgenic Cucumis sativus expressing the hepatitis B surface antigen. Plant Mol Biol Rep 28:627–634
Szwacka M, Krzymowska M, Kowalczyk M E, Osuch A (2000) Transgenic cucumber plants expressing the thaumatin gene. In: Bielecki S, Tramper J, Polak J (eds.) Progress in Biotechnology, 17: 43–48
Szwacka M, Krzymowska M, Osuch A, Kowalczyk ME, Malepszy S (2002) Variable properties of transgenic cucumber plants containing the thaumatin II gene from Thaumatococcus daniellii. Acta Physiol Plant 24:173–185
Tabei Y, Kitade S, Nishizawa Y, Kikuchi N, Kayano T, Hibi T, Akutsu K (1998) Transgenic cucumber plants harboring a rice chitinase gene exhibit enhanced resistance to gray mold (Botrytis cinerea). Plant Cell Rep 17:159–164
Tabei Y, Nishio T, Kurihara K, Kanno T (1994) Selection of transformed callus in a liquid medium and regeneration of transgenic plants in cucumber (Cucumis sativus L.). Jpn J Breed 44(1):47–51
Tang W, Samuels V (2001) Genetic transformation and its practical application in plants. J Dev Reprod Biol 02
Trulson AJ, Simpson RB, Shahin EA (1986) Transformation of cucumber (Cucumis sativus L.) plants with Agrobacterium rhizogenes. Theor Appl Genet 73(1):11–15
Vasudevan A, Selvaraj N, Ganapathi A, Choi CW (2007) Agrobacterium mediated genetic transformation in cucumber (Cucumis Sativus L.). Am J Biotechnol Biochem 3:24–32
Vengadesan G, Anand RP, Selvaraj N, Perl-Treves R, Ganapathi A (2005) Transfer and expression of nptII and bar genes in cucumber (Cucumis Sativus L.). In vitro Cellular and Developmental Biology-Plant 41(1):17–21
Wang HZ, Zhao PJ, Zhou XY (2000) Regeneration of transgenic cucumber plants with a WMV-2 CP gene. Acta Phytophysiologica Sinica 26:267–272
Wang J, Zhang SJ, Wang X, Wang LN, Xu HN, Wang XF, Shi QH, Wei M, Yang FJ (2013) Agrobacterium-mediated transformation of cucumber (Cucumis sativus L.) using a sense mitogen-activated protein kinase gene (CsNMAPK) Plant Cell. Tissue and Organ Culture (PCTOC) 113:269–277
Wang X, Yu G, Zhao J, Cui N, Yu Y, Fan H (2019) Functional identification of Corynespora cassiicola-responsive miRNAs and their targets in cucumber. Front Plant Sci 10:668
Wilson TMA (1993) Strategies to protect crop plants against viruses: pathogen-derived resistance blossoms. Proc Natl Acad Sci USA 90:3134–3141
Xu Y, Bu W, Li B (1993) Metabolic factors capable of inducing Agrobacterium vir gene expression are present in rice (Oryza sativa L.). Plant Cell Reports 12(3):160–164
Yan S, Che G, Ding L et al (2016) Different cucumber CsYUC genes regulate response to abiotic stresses and flower development. Sci Rep 6:20760
Yan Y, Zhang W, Li Y et al (2018) Functions of CsGPA1 on the hypocotyl elongation and root growth of cucumbers. Sci Rep 8:15583
Yang L, Liu H, Zhao J, Pan Y et al (2018) LITTLELEAF (LL) encodes a WD40 repeat domain-containing protein associated with organ size variation in cucumber. Plant J 95:834–847
Yang S, Wen C, Liu B, Cai Y et al (2019) A CsTu-TS1 regulatory module promotes fruit tubercule formation in cucumber. Plant Biotechnol J 17:289–301
Yin Z, Bartoszewski G, Szwacka M, Malepszy S (2005) Cucumber transformation methods—the review. Biotechnologia 1(68):95–113
Yin Z, Pawłowicz I, Bartoszewski G, Malinowski R, Malepszy S, Rorat T (2004a) Transcriptional expression of a Solanum sogarandinum GTDhn10 gene fusion in cucumber and its correlation with chilling tolerance in transgenic seedlings. Cellular Mol. Biol. Lett 9:891–902
Yin Z, Plader W, Malepszy S (2004b) Transgene inheritance in plants. J Appl Genet 45:127–144
Yin ZM, Malinowski R, Ziółkowska A, Sommer H, Plader W, Malepszy S (2006) The defH9-iaaM-containing construct efficiently induces parthenocarpy in cucumber. Cell Mol Biol Lett 11:279–290
Zhang F, Qin Z, Zhou X et al (2019) Expression and functional analysis of the propamocarb-related gene CsMAPEG in cucumber. BMC Plant Biol 19:371
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bhatt, B. et al. (2022). Biotechnological Innovations in Cucumber (Cucumis sativus L.) Development—Current Scenario and Future Perspectives. In: Pandey, S., Weng, Y., Behera, T.K., Bo, K. (eds) The Cucumber Genome. Compendium of Plant Genomes. Springer, Cham. https://doi.org/10.1007/978-3-030-88647-9_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-88647-9_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-88646-2
Online ISBN: 978-3-030-88647-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)