Abstract
Key message
Molecular breeding of Cucumis sativus L. is based on traditional breeding techniques and modern biological breeding in China. There are opportunities for further breeding improvement by molecular design breeding and the automation of phenotyping technology using untapped sources of genetic diversity.
Abstract
Cucumber (Cucumis sativus L.) is an important vegetable cultivated worldwide. It bears fruits of light fragrance, and crisp texture with high nutrition. China is the largest producer and consumer of cucumber, accounting for 70% of the world’s total production. With increasing consumption demand, the production of Cucurbitaceae crops has been increasing yearly. Thus, new cultivars that can produce high-quality cucumber with high yield and easy cultivation are in need. Conventional genetic breeding has played an essential role in cucumber cultivar innovation over the past decades. However, its progress is slow due to the long breeding period, and difficulty in selecting stable genetic characters or genotypes, prompting researchers to apply molecular biotechnologies in cucumber breeding. Here, we first summarize the achievements of conventional cucumber breeding such as crossing and mutagenesis, and then focus on the current status of molecular breeding of cucumber in China, including the progress and achievements on cucumber genomics, molecular mechanism underlying important agronomic traits, and also on the creation of high-quality multi-resistant germplasm resources, new variety breeding and ecological breeding. Future development trends and prospects of cucumber molecular breeding in China are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cucumber, (Cucumis sativus L.) (2n = 2x = 14) which belongs to the gourd family (Cucurbitaceae) and originate from the south of the Himalayas (Naegele and Wehner 2017), is a major vegetable crop with important economic and biological value. It produces fruits with rich nutrients including protein, multi-vitamins, cucurbitacin, calcium, iron, propanol diacid and other essential substances for human diet (Li et al. 2015). The polysaccharides and flavonoids in the fruit have antioxidant activity to scavenge free radicals, hence to enhance human immunity and delay aging (Shi et al. 2010; He et al. 2011). Cucurbitacin C in the fruit has an effect to inhibit proliferation of various tumors (Zhang et al. 2012), and also played a role in preventing liver from inflammation (Yang et al. 2005). In addition, the wild cucumber fruit has sometimes been used as an herb medicine in a number of health-related products (Zhang et al. 2012).
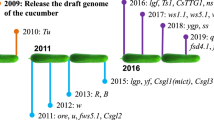
China is the lead producer of cucumber with cultivation area accounts for at present 54% of the world total cultivation area (FAOSTAT3). This rapid increase in cucumber production in China has created not only high demand on the continuous improvement of field cultivation and management, but most urgent demand on breeding new varieties with high quality, high yield and strong bio-/abio-resistance (Fig. 1). Therefore, Chinese cucumber breeders made remarkable achievements in these last decades, especially in cucumber molecular breeding by using high-quality worldwide cucumber germplasm resources and employing innovative molecular approaches and bio-engineering technologies combined with field test and verification in a complex environment. In this article, we review the recent progress on the breeding of new cucumber varieties by these approaches include cross-breeding, mutagenesis breeding, marker-assisted breeding, genetic engineering breeding and genome-wide design breeding (Fig. 2).
Illustration of the agronomic traits-related genes in cucumber molecular breeding. Genes regulating leaf development, flower differentiation, optimal fruit production and stress resistance were identified by either molecular marker-assisted breeding or genome-wide design breeding and/or genetic engineering breeding in cucumber
Cross-breeding
Hybridization makes use of the principle of gene recombination to transfer the good traits of parents to the hybrid offspring to obtain the required performance type. As the genetic source of hybrid offspring can be known, trait performance can be predicted, making it one of the main methods of cucumber breeding (Lv et al. 1994). For example, the early cucumber variety “JinChun 4” generated by cross-breeding from its parents that derived from a high-generation inbred line with strong disease resistances, therefore owns its parental traits with high resistance to downy mildew, powdery mildew and fusarium wilt. This is also the case for “JiNing 736” which was obtained by crossing between pure inbred lines 159 and 131. This variety combined the advantages of early maturity, high yield, low temperature resistance and good taste from the two parent lines (Yang 2012). In another work, “TangJiao 8” was highlighted from 45 hybrid combinations strains, comparative analysis of five advantage combination strains, the gynoecious inbred line “09-17” with strong parthenocarpy ability; cold resistance was chosen as the female parent of “TangJiao 8,” while the subgynoecious inbred line with moderate parthenocarpy ability and strong cold resistance was served as male parent. This variety integrated the advantages of higher rate of female flower formation at each node, higher parthenocarpy ability and stronger cold resistance from the two parent lines (Li et al. 2017). “NingJia 1” was a distant hybridization line which was also successfully achieved by crossing a wild cucumber as the female parent with a cultivated cucumber as the male (Chen et al. 2005). After multiple generations of selfing and backcrossing, an excellent germplasm line 7012A which has strong abio/bio-stress resistance was selected. Then, the cucumber line was further crossbred with an American processed cucumber 7011A to obtain “NingJia 1,” a strong growth potential, good quality and high-yield F1 hybrid (Chen et al. 2005). In recent years, many popular cucumber varieties, such as “Jinyou 10,” “Jinyou 12,” “Jinyou 20,” “Jinyou 21,” “Jinyou 30,” “Jinyou 32” and “Jinyou 35,” have been cultivated by Tianjin Kerun cucumber research institute in China. The cucumber variety “Jinyou 10” was generated by cross-breeding from its parents that derived from holland cucumber, after multiple generations of systematic breeding, conferring its traits with high resistance to disease, low temperature in juvenile stage and high temperature in adult phase. This is also the case for “Jinyou 12” which was obtained by crossing between pure female parent lines “Q12” and male parent lines “131.” This variety combined the advantages of high yield, low temperature resistance and high resistance to Fusarium wilt, downy mildew, powdery mildew and Cucumber mosaic virus. “Jinyou 20” is another widely cultivated Chinese variety, which combined the advantages of early maturity, strong growth potential, high yield, cold/hot tolerance and strong disease resistance. With the same traits, “Jinyou 21,” “Jinyou 30,” “Jinyou 32” and “Jinyou 35” have strong tolerance to low temperature and weak light; furthermore, “Jinyou 21,” “Jinyou 32” and “Jinyou 35” both possess high disease resistant and high yield. It is worth noting that among these varieties, “Jinyou 35” is the first Chinese facility cucumber variety integrating high quality, high yield, disease resistance, wide adaptability, low light tolerance and low temperature tolerance. It was successfully achieved by crossing a inbred line, which was with high yield and resistance, as the female parent with another inbred line G3515.
Mutagenesis breeding
Mutation breeding artificially uses physical or chemical factors and/or space radiation to make changes in the structure of genetic material to induce gene mutation and screen the target traits from mutant groups. It can directly use germplasm resources, and the required mutants can be obtained in a short time by increasing mutation frequency. A large population of chemical mutagenesis on North China cucumber cultivar “406” was generated and showed that the half lethal dose was 1.2% EMS treatment of 12 h (Shang et al. 2014); the mutation frequency in M2 generation was 12.49%, and mutations mainly manifested in the color and shape of leaf, flower and fruit (Table 1). Moreover, the plant architecture was affected (Table 1). It was interesting to find that mutagenesis effect of nitrogen accumulation on cucumber seeds showed that plants with variations in M1 generation showed obvious separation on the fruit shape traits in M2 generation, but the variations in fruit shape and prickles in M3 generation can be stably inherited (Cui et al. 2012). This study has created indeed a plenty of germplasm materials for cucumber breeding in the future. A space mutation breeding using dragon fruit cucumber was carried out and obtained an excellent variation of white cucumber strain “055-33-6-1-2-49” after five generations of mutated line selection (Li et al. 2016). The cucumber varieties space “96-1”(Zhang et al. 2005), “HangYan 1”(Li et al. 2009) and “HangYu 1”(Wang et al. 2016) were also obtained by space mutation breeding.
Molecular marker-assisted breeding in cucumber
With the development of molecular biotechnology, molecular markers have emerged as a powerful tool in breeding. Because molecular markers can appear directly in the form of DNA, no epistatic effect or other forms of gene interaction are observed between non-allelic markers, and molecular markers are not affected by the environment (Juwattanasomran et al. 2011). In addition, because polymorphism occurs almost throughout the entire genome, the molecular marker is stable and reliable and has good repeatability. Molecular markers are widely applied in genetic breeding, gene mapping, gene cloning, genetic map construction, systematic taxonomy, genetic germplasm resource research and assisted selective breeding (Yan et al. 2006; Xu and Zhao 2009; Hu and Zhao 2010). There are many types of genetic markers, each with particular limitations and strengths. Within genetic markers, there are four common categories: markers based on hybridization, such as RFLP (restriction fragment length polymorphism); markers based on PCR included SSR (simple sequence repeat), RAPD (random amplified polymorphic DNA), SRAP (sequence-related amplified polymorphism); markers based on restriction enzyme and PCR, such as AFLP (amplified fragment length polymorphism); and new-generation markers, for instance, SNP (single nucleotide polymorphism) (Maheswaran 2004).
SSR-related breeding in cucumber
Owing to their high polymorphism, reliability and rapid and ready detection, SSR has proven to be powerful tool in plant molecular breeding and molecular biological studies (Ohbayashi et al. 2019).
The number of fruit-bearing branches was positively correlated with the yield of processed cucumber. Taking advantage of multiple lateral branching in MAS (marker-assisted selection) will likely become an effective tool in cucumber breeding (Fazio et al. 2003). Polymorphism of the SSR markers was analyzed between non-branched and branched gene pools. A non-branched linkage marker SSR10018 was mapped on chromosome 1 of cucumber. After further verification, a closer marker SSR19673 with a genetic distance of 15.9 cM was obtained (Table 1; Ren 2013). Leaf size is an important trait that control photosynthesis and yield. Weng et al. (2010) found that the leaf size-related gene LITTLE LEAF (LL) is located on chromosome 6 in between simple sequence repeat (SSR) markers SSR02355 and SSR03940. Further research found six new SSR markers with polymorphisms by plotting 145 recombinant inbred lines (RILs) and showed that SSR21758 and UW083795 are the closest to LL with distances of 1.0 and 0.6 cM, respectively. These two markers were used for identifying 29 recombinants from 1423 individual F2 population derived from lobular strain H19 crossing normal leaf strain G421. After sequencing, 960 SNPS were identified, and four SNPS flanked with the LL locus were converted to dCAPS markers (ll-dcaps1–ll-dcaps4), which was used to identify 29 recombinants. These results revealed that the LL gene-related SSR markers is an effective molecular tool to bred cucumber leaf-associated varieties.
Fruit shape is one of the important characters of cucumber appearance. The curving degree, fruit thickness, fruit length and fruit neck length are closely related to fruit shape. In cucumber breeding, fruit curving degree seriously affects cucumber ornamental traits, flavor, taste and sales, decreasing commercial performance (Liang and Li 2001). Therefore, studying the curvature of cucumber fruit in breeding is important. Zhang (2009) conducted genetic analysis on the curving properties of cucumber fruits by using curving and straightening varieties as parents. Molecular markers and quantitative trait locus (QTL) assay were used to identify an F2 population. Seven SSR markers were detected, and one QTL related to the curvature of cucumber fruit was identified. The nearest marker distance of the curved correlated QTL was 2.5 cM (Table 1). Sun et al. (2010) used a molecular marker to study the cross-diameter of cucumber fruits. A total of 116 pairs of SSR primers were used to identify the QTLs in the cswct25-cswct29-cswta03 linkage group (Table 1).
The peel is one of the most intuitive traits that reflect the advantages or disadvantages of cucumber appearance. Skin color and gloss are key factors in determining market acceptance. Green fruit peel is the main type of cucumber in the market; thus, a great deal of attention has been paid to the green peel gene. A previous study has shown that a dominant SRAP marker ME9EM1-369, which has 6-cM genetic distance, is linked to the green peel gene (Table 1; Li 2008). The gloss character of cucumber inbred line “1101” was controlled by dominant gene G and was located between SSR markers Cs28 and UW013295 on chromosome 5, with genetic distances of 1.5 and 4.8 cM, respectively (Table 1; Dong 2013).
Fruit wart is an exterior character of cucumber fruit; it is a tuberculate base bulged from fruit surface that is generally topped by a fruit spine. The fruit spines and warts are collectively referred to as the warty trait in cucumber (Fig. 1). Appearance of fruit-free tumor is related to consumers’ choice of cucumber and its market value. SSR analysis showed that the fruit wart-related gene Tu is located between CSWGATT01C and CSCT335, flanking the gene at 20.0 and 14.1 cM, respectively (Table 1; Wang et al. 2007). Zhang et al. (2010) identified 15 markers associated with Tu/tu loci by combining SRAP and SSR markers. Another work developed 20 SNP markers and 332 SSR markers in the final interval of Tu gene and found two dominant markers TY-1 and TY-2 that can be used in molecular MAS breeding for cucumber warts (Yang 2014). Map-based cloning identified CsTS1, which encodes an oleosin protein, as the gene controlling the size of fruit warts (Yang et al. 2019). Mutation in the promoter region resulted in low expression of CsTS1 in 22 small or wartless cucumber lines. On the contrary, it was highly expressed in 44 different cucumber lines with enlarged fruit wart (Table 1 and Fig. 1; Yang et al. 2019).
Fruit spine is another important fruit appearance character that can be used to judge the quality of cucumber. Guan (2008) took European greenhouse-type inbred line S06 and cucumber hairless (fruiting spine) mutant gl (Glabrous) required as parents to map the gene of fruiting spine formation. Combined with BSA and F2 mapping population, 18 SSR markers related to the gl-2 gene of cucumber were screened. The gene was located on chromosome 2, and the closest linkage markers flanking the gene were SSR10522 and SSR13275, with genetic distances of 0.6 and 3.8 cM, respectively (Table 1; Yang et al. 2011). Li et al. (2013) investigated the hybridization between two inbred lines WI7200 and WI7201 and confirmed that a single dominant gene B controlled the color of black spines and spines of ripe fruits. Fine mapping with SSR markers concluded that a R2R3-MYB gene might be the best candidate gene for the B locus (Table 1 and Fig. 1).
Cucumber flesh thickness is another important trait for cucumber fruit quality and commercial value. A 0.19-Mb-long quantitative trait locus (QTL fft2.1) was identified by specific length amplified fragment sequencing (SLAF-seq), SSR and marker-based classical QTL mapping in 138 F2 individuals. Twenty genes were predicted in this 0.19-Mb region. Q-RT-PCR revealed that the SET domain protein lysine methyltransferase (Csa2M058670.1) was high expression in the paternal cultivated variety (thick fruit flesh) D8; oppositely, the thin fruit flesh parent XUE1 has the low expression of the gene. Sequence alignment analysis showed that a 4-bp deletion mutation in the promoter region of Csa2M058670.1, which may result in the low expression of Csa2M058670.1 in thin fruit flesh lines (Xu et al. 2015). This work provides a genetic resource for flesh thickness breeding.
Parthenocarpy ability is directly related to the yield of cucumber, and many relevant markers have been found. A previous work had identified that the SSR marker SSR22338 may be linked to the main effect QTL of parthenocarpy (Table 1; Wang 2011). The major-effect QTL Parth2.1 and six minor-effect QTLs have been demonstrated mainly to contribute to the genetic architecture of parthenocarpy in cucumber. Indel-T-39 and SSR16226 can be directly used in MAS of cucumber breeding. Among this region, 57 genes with nonsynonymous SNPs/InDels were found in the coding sequence. Based on further combined analysis using RNA-Seq data, CsWD40, CsEIN1, CsARF19, CsPPR, CsMDL, CsHEXO3, CsSMAX1 and CsDJC77 were predicted as the possible candidate genes regulating parthenocarpy (Table 1 and Fig. 1; Wu et al. 2016).
The hypocotyl-derived adventitious root (AR) is an important morphological response to flooding stress. A genetic linkage map consisting of 149 SSR markers and spanning 550.8 cM was constructed. Three QTLs (ARN3.1, ARN5.1 and ARN6.1) located on chromosomes 3, 5 and 6 were identified by composite interval mapping. The major-effect QTL ARN6.1 was narrowed down to a 0.79-Mb interval flanked by SNP25558853 and SSR12898. Transcriptome data generated from hypocotyls under flooding stress indicated that 15 genes in the 0.79 Mb had differential expression, including a salicylic acid methyl transferase-like protein (Csa6G503880), a cytochrome P450 monooxygenase (Csa6G504590) and a heavy metal-associated protein (Csa6G505230) (Table 1; Xu et al. 2017). Subsequent work showed that CsARN6.1 encoding AAA ATPase is the major candidate gene for ARN6.1. Transgenic plants over-expressing the CsARN6.1Asp allele from Zaoer-N line increased the number of ARs compared with the WT expressing the allele from pepino line under flooding conditions (Table 1 and Fig. 1; Xu et al. 2018a, b). This finding sheds light on the genetic and molecular mechanism underlying adventitious root development during flooding stress in cucumber and provides potential gene resources for further illuminating flooding tolerance in plants.
AFLP marker-related breeding in cucumber
AFLP marker technology is widely used due to its strong stability, high polymorphism and no radiation pollution. Cucumber fruit bitterness is one of the important characters that affect the quality of fruit. Previous studies developed a number of molecular markers for bitter genes. The biosynthesis of cucurbitacin C (CuC) in cucumber is well-studied, and several genes involved in CuC biosynthesis have been identified. A pivotal gene Bi (bitterness) encoded a cucurbitadienol synthase conferring bitterness throughout the plant (Table 1; Shang et al. 2014). Bi is regulated specifically by two transcription factors, Bl (bitter leaf) and Bt (bitter fruit); these two genes are expressed specifically in cucumber leaves and fruits, respectively (Shang et al. 2014). In addition, Bt’s expression is positively correlated with increased fruit bitterness in wild cucumber lines (Shang et al. 2014). An AFLP marker E4M6 is linked with Bi, and the linkage distance is 15.0 cM (Guo 2003). Similarly, two dominant AFLP markers, E23M66-101 and E25M65-213, are mapped closely to Bt (Table 1; Gu et al. 2006). Furthermore, the AFLP markers linked to parthenocarpy were screened by BSA method. The specific band with a molecular weight of approximately 325 bp was amplified by the primer E41/M47, which is linked to the parthenocarpy gene with a genetic distance of 9.7 cM (Table 1; Yan 2009).
SRAP marker-related breeding in cucumber
Based on the difference in base content of exons, intron and promoter, SRAP primers have the advantages of high yield, medium yield, high codominance and easy separation. The fruit neck length of cucumber influences its quality and commercial value. Most consumers prefer cucumbers with short or no fruit neck. Therefore, to meet the needs of the market, more studies focused on fruit neck length. Zhao (2011) used bulked segregant analysis SRAP and BSA technology to perform QTL analysis of cucumber neck length. Polymorphic bands were detected in nine markers, and two QTLs might affect the length of fruit stalk (Table 1).
The quality characteristics of cucumber mainly include internal and nutritional quality, internal quality texture and flavor. Texture includes hardness, toughness, compactness and bitterness. Flavor generally refers to the unique smell and taste of cucumbers. Studies have shown that the aroma of plants is mainly due to the presence of volatile compounds, such as 2-acetyl-1-pyrrolidine (2AP). In rice (Oryza sativa) and soybean (Glycine max), the deletion mutation of BADH2 gene had been identified as the cause of fragrance (Table 1 and Fig. 1; Bradbury et al. 2005; Juwattanasomran et al. 2011). In cucumbers, analysis with F2 and backcross population also indicated that the aroma is recessive and controlled by a single gene, fgr (Table 1 and Fig. 1; Pramnoi et al. 2013). And the linkage SRAP marker Cs-BADH-AG showed a distance of 1.1 cM (Yundaeng et al. 2015).
SCAR marker-related breeding in cucumber
SCAR marker is used to clone the target RAPD (random amplified polymorphic DNA) fragment. Specific primers are designed according to the end sequences of RAPD fragment, and PCR amplification is performed on the gene DNA fragment to identify the single locus corresponding to the original RAPD fragment. SCAR marker is convenient, fast and reliable, and can quickly detect a large number of individuals with good stability and reproducibility. Yield is one of the most important agronomic characters in cucumber production. A great deal of studies have focused on the development of yield markers. A total of 58 QTLs of nine yield-related traits were identified by QTL analysis. Among them, the expressions of ffa2a and ffa2b are stable (Table 1; Chen et al. 2010). Female flower ratio of cucumber is also another important factors affecting yield. The sex phenotype of cucumber is determined by three types of genes, namely, F, M and A, and their linkage markers have been widely reported. For example, the ACC synthase gene (CsACS1 gene) markers are closely linked to F-locus (Ye et al. 2000). SSR markers SSR19914 (3.2 cM), SSR23487 (0.28 cM), SCAR markers SCAR123 (0.94 cM), SRAP markers ME23SA4 (17.8 cM) and SCAP markers SCAP123 (0.94 cM) are linked to the M gene (Table 1; Shi et al. 2009). The application of SSR markers between subgynoecious S-2-98 and monoecious M95 bulks constructed from BC1 plants has identified three QTLs: sg3.1, sg6.1 and sg6.2. The major QTL sg3.1 accounts for 54.6% of the phenotypic variation. PCR-based-markers from the SNP profile was developed to indicated that sg3.1 was delimited to a 799-kb genomic region (Table 1; Bu et al. 2016). The flowering time of cucumber is an important factor affecting yield. Ef1.1 is a candidate gene for early flowering QTL. Combining QTL-seq and traditional QTL analyses in F2 and BC1 populations, which derived from a cross between the late flowering line “9930” and the early flowering line “Muromskij,” confirmed Ef1.1 to an 890-kb genomic region. The homolog gene Csa1G651710 of main flowering switch gene FLOWERINGLOCUS T (FT) was identified in this region. Gene expression study showed that Csa1G651710 is higher expression in “Muromskij.” In brief, these data supported that Csa1G651710 is a potential candidate gene for early flowering in “Muromskij” and provides theoretical basis for molecular MAS of cucumber yield (Table 1 and Fig. 1; Lu et al. 2014).
QTLs-related breeding in cucumber
Quantitative trait locus (QTL) is a locus (section of DNA) which correlates with variation of a quantitative trait in the phenotype of a population of organisms. QTLs are mapped by identifying which molecular markers correlate with an observed trait. Fruit length is a pivotal agronomic and domesticated trait controlled by QTLs. Additionally, the potential genetic and molecular mechanisms that determine fruit length of cucumber remain unclear. QTL mapping has identified eight QTLs for fruit length, while the major-effect QTL fl3.2, which explained a maximum of 38.87% of the phenotypic variation, had been detected. A genome-wide comparison of SNP profiles between two DNA bulks had identified six QTLs for fruit length. QTLs ovl3.1 and ovl3.2 both had major effects on ovary length with a △ (SNP-index) of 0.80 (P < 0.01) and 0.74 (P < 0.01), respectively (Table 1; Wei et al. 2016).
Fruit size is another important trait in the diversifying selection and domestication in cucumber, but the genetic mechanism is poorly understood. QTL analysis revealed that 11 QTLs underlying fruit size variation and flowering time in the semi-wild Xishuangbanna cucumber, FS5.2 played the major roles in determining the round fruit shape (characteristic of the WI7167 XIS cucumber). (Table 1; Pan et al. 2017a). Future QTL analysis revealed two interacting loci, FS1.2 and FS2.1, which were also conferred the round fruit shape in WI7239 line. CsSUN, a homolog of the tomato fruit-shaped gene SUN, was identified as a candidate for FS1.2 (Table 1 and Fig. 1). Moreover, the round-fruited WI7239 had a 161-bp deletion in the first exon of CsSUN. A marker derived from this deletion was also mapped at the peak location of FS1.2 in QTL analysis (Pan et al. 2017b).
The common cucumber diseases in production include DM (downy mildew), PM (powdery mildew), anthracnose (Colletotrichum orbiculare), target leaf spot (TLS) and blight. They seriously affect the quality of cucumber, reducing its yield and damaging its commodity value. Therefore, the development of gene linkage markers related to resistance to various diseases has become a research hotspot (Sakata et al. 2006). A population of F7 RILs has been developed from the cultivar “Santou” and PI197088-1 and multiple QTLs for PM tolerance have been identified. Among these QTLs, only one locus was effective under both high and low temperatures. Nevertheless, the other loci were only effective under high or low temperatures. This result indicated the temperature dependence of PM resistance gene expression/function, and their combination plays important roles for higher resistance to the PM pathogen (Table 1; Sakata et al. 2006). In a recent study, a dominantly inherited major-effect QTL for PM resistance was fine mapped in cucumber. QTL mapping and linkage analysis in a subset of F2 plants delimited the Pm1.1 locus into a 41.1-kb region. Comparative gene expression analysis revealed that two genes (Csa1M064780 and Csa1M064790) encoding the same function of a cysteine-rich receptor-like protein kinase were the most possible candidate genes. These results provided a new insight into understanding the phenotypic and genetic molecular mechanisms of PM resistance in cucumber (Table 1 and Fig. 1; Xu et al. 2016). A natural loss-of-function mutation at CsMLO1 locus was found to confer PM resistance in cucumber. CsMLO1 encodes a cell membrane protein, which was induced by PM after host–pathogen interaction. These findings will facilitate PM-resistant varieties breeding in cucumber (Table 1 and Fig. 1; Nie et al. 2015). The Gy14 cucumber has higher resistance to pathogens bacterial angular leaf spot, fungal anthracnose and oomyceteous DM, but the underlying genetic molecular mechanisms are poorly unknown. QTL mapping and further map-based cloning in Gy14 have identified STAYGREEN (CsSGR) gene as a candidate gene for the triple disease-resistant loci (Table 1; Wang et al. 2018). Map-based cloning has also identified CsSGR as a possible gene for resistance to the anthracnose fungal pathogen Colletotrichum orbiculare in cucumber (Table 1 and Fig. 1; Pan et al. 2018). This work indicated a novel function for the highly conserved STAYGREEN family genes for different disease resistance in plants (Pan et al. 2018). TLS is one of the most serious foliar diseases in cucumber. Fine genetic mapping was conducted to identify cca-3, a CC-NB-ARC-type resistance gene analog, against TLS. And one SNP was found in the NB-ARC domain of this gene that may lead to nonsynonymous mutation of amino acid. Furthermore, the expression level of cca-3 was positively correlated with the necrotic spots on leaves after infection. In brief, the cca-3 resistance gene may be able to induce hypersensitive responses to infection by TLS pathogen (Table 1 and Fig. 1; Wen et al. 2015).
Map-based cloning-assisted breeding in cucumber
The dwarf plant architecture is an important agronomic trait in cucumber molecular breeding and has the potential to be used in once-over mechanical harvest of cucumber production. Dwarf plant architecture is controlled by a recessive gene cp (compact plant architecture). Map-based cloning with 1269 “F2” plants delimited the cp locus to a 220-kb genomic region, and the cytokinin oxidase (CKX) gene in cucumber was predicted as the candidate gene. Sequencing results showed that a 3-bp deletion in the first exon of CKX gene in PI308915 line were further selected as a marker-assisted selection (MAS) marker for the dwarf phenotype (Table 1; Li et al. 2011).
MutMap method was used to identify an EMS-induced dwarf mutant si (short internode) in cucumber. Genetic linkage analysis and genome sequencing combination results showed that the occurrence of a premature stop codon in a VIER F-BOX gene of cucumber was strongly associated with the dwarf phenotype in cucumber (Table 1 and Fig. 1; Lin et al. 2016). In plants, a good deal of genes controlling plant height have been identified, which are responsible for biosynthesis or signal transduction of plant hormones, such as brassinosteroids (BRs). Screening from EMS-induced mutagenesis population, a super compact (SCP) mutant C257, which was extremely dwarf due to the absence of internode elongation, was identified. Map-based cloning combined with a modified MutMap identified a plant cytochrome P450 monooxygenase gene CsCYP85A1 as the possible candidate gene for scp-1 (Table 1 and Fig. 1; Wang et al. 2017). In another study, a spontaneous dwarf mutant, super compact-2 (scp-2), that has wrinkled and dark green leaves was identified. Map-based cloning displayed that the dwarf phenotype was due to a missense mutation and a truncated protein lacking in the conserved catalytic domains of the CsDET2 gene (Table 1 and Fig. 1). Measurement of endogenous BR levels revealed a reduced level of brassinolide in scp-2. Moreover, the mutant phenotype could be partially rescued by the application of epibrassinolide. These data support that scp-2 is a BR biosynthesis-deficient mutant, and the CsDET2 gene plays a key role in BR-related regulation of plant architecture in cucumber (Hou et al. 2017). GA3 application could partially rescue EMS-induced dwarf mutant Csdw. Endogenous GA3 levels from the stem of Csdw decreased significantly. Kompetitive Allele Specific PCR genotyping and MutMap results revealed that Csa3G872760 (CsCLAVATA1), which encodes a CLAVATA1-type receptor-like kinase, is a possible candidate gene for dwarf phenotype of Csdw (Table 1 and Fig. 1; Xu et al. 2018a, b).
Leaf color mutants in higher plants are ideal materials for studying chloroplast development and the structure and function of photosynthetic system. Map-based cloning experiment showed that the cucumber vyl (virescent-yellow leaf) mutant, which exhibited delayed chloroplast development process and reduced pigment contents, is controlled CsVYL, which encodes a DnaJ-like zinc finger protein (Table 1 and Fig. 1; Song et al. 2018). In an EMS mutagenesis population, the chlorophyll-deficient mutant C528 with golden leaf color was identified. Map-based cloning showed CsChlI as the potentiality possible gene for this mutation, which encoded the CHLI of cucumber Mg-chelatase. This mutant has potential in cucumber breeding and provides a useful tool in understanding the CHLI function, especially in the chloroplast development and chlorophyll biosynthesis pathway (Table 1 and Fig. 1; Gao et al. 2016). In virescent leaf mutants, juvenile leaves are yellow in color. Phenotypic characterization and genetic mapping of the cucumber virescent leaf mutant showed that the v-1 locus was the hypothetic region. Multiple lines of evidence-supported CsaCNGCs, which encodes a cyclic-nucleotide-gated ion channel protein, was the only candidate gene for the v-1 locus. RT-PCR revealed a significantly lower expression of CsaCNGCs in the true leaves of 9110Gt than in 9110G (Table 1 and Fig. 1; Miao et al. 2016).
Cucumber genome-wide design breeding
Given the narrow genetic background, developing cucumber markers through conventional means is difficult and results in a backward cucumber genetic research system, which seriously restricts the development of cucumber biological research and molecular design breeding. In 2007, a Chinese group initiated and organized the international cucumber genome project, which was the first attempt to draw the cucumber genome sequence map by using new-generation DNA sequencing technology (Huang et al. 2009). The sequencing cucumber variety, East Asian line “Chinese Long” (9930), bears long fruit with an elongated stalk. Deep resequencing of 115 cucumber lines has identified 112 putative domestication sweeps, which contain genes related to leaf size, fruit length and bitterness (Qi et al. 2013). On the basis of the deep sequencing of 115 cucumber strains, a genome-wide genetic variation map containing more than 360 loci was built, providing a new way of thinking to understand cucumber evolution and diversity and laying the foundation for genome-wide design breeding.
Investigating the genomic basis of divergence among different cultivated populations led to the discovery of a natural heritable variation in a β-carotene hydroxylase gene. As we know, the most obvious difference between the Xishuangbanna group and the other three groups is the orange endocarp of the fruit, which is due to the different accumulation of β-carotene caused by the single recessive gene ore. Searching for nonsynonymous SNPs fixed between the Xishuangbanna group and other groups has identified only one SNP resided within the physical interval of ore gene (Qi et al. 2013). It causes amino acid change at residue 257 in Csa3G183920, which encoded a putative β-carotene hydroxylase, and designated as CsaBCH1. Future biochemical experiments showed that functional CsaBCH1 confers the biosynthesis of β-carotene (Table 1 and Fig. 1; Qi et al. 2013). The identification of the ore gene demonstrates that the genomic landscape difference of population divergence should be further used to clone genes related to important agronomic traits.
Wild cucumber plants currently bear extremely bitter fruits. Obviously, an essential step in fruit domestication must have involved the silence of fruit bitterness. Bi and Bt genetic loci are known to be the major genetic loci for bitterness in cucumber. The recessive bi allele confers bitter-free leaf; on the contrary, the dominant Bt allele renders the fruit extremely bitter. A selective sweep at the genomic region spanning the Bi gene has no signature. Otherwise, there is a strong signature of a selective sweep during domestication in the Bt region, but no diversity was observed in the cultivated groups (Shang et al. 2014). In future, a high-resolution genetic map consisting of 1822 F2 individuals from a cross between the line 9110Gt and 9930 of the Bt locus was generated. Based on this analysis, the Bt locus was located to a 442-kb region on chromosome 5 that includes 67 predicted genes (Table 1 and Fig. 1; Shang et al. 2014).
Structural variations (SVs) represent a major source of genetic diversity. Another work of the sequencing group showed a nucleotide-resolution SV map of cucumber that contains 26,788 SVs based on deep resequencing results of 115 diverse accessions. These SVs affect 1676 coding genes, some of which are associated with cucumber domestication. On the basis of the map, a copy number variation (CNV) involving four genes defines the female (F) flowers locus, which produce only female flowers and bear fruit at almost all nodes. The SV set provides a snapshot of SVs in plants and will serve as an important characteristic for exploring functional genes with underlying important agronomic traits and for promoting practical breeding in cucumber (Table 1; Zhang et al. 2015). Moreover, cucumber genome-wide design breeding system has been widely used in genome research of other vegetable crops, such as cabbage, watermelon and tomato. Under the concerted efforts of many research groups in China, more than ten research papers have been published in the top international journals, such as Nature, Science and Cell, which has promoted Chinese vegetable genome research.
Genetic engineering breeding of cucumber
Genetic engineering is the act of modifying the genetic makeup of an organism. Modifications can be generated by nuclear transplantation, gene targeting, transfection of synthetic chromosomes or viral insertion methods. Selective breeding is not considered as a form of genetic engineering. Genetic engineering breeding uses plant biotechnology to obtain gene fragments needed for encoding, cloning and transferring target traits into recipient cells for replication and expression to obtain new varieties with the target traits. Genetic transformation system and genetic engineering improvement in cucumber provide abundant germplasm materials and gene resources for cucumber breeding. In addition, the widely used plant gene editing methods, CRISPR/Cas9, was also used in cucumber. The first report about CRISPR/Cas9 gene editing in cucumber was focused on the development of broad virus resistance in non-transgenic cucumber (Chandrasekaran et al. 2016). In a recent work, Chinese cucumber breeders optimized the CRISPR/Cas9 system by using stronger CsU6 promoter and a GFP tag to facilitate selection both the transformants and transgene-free mutants among the progeny. With the optimized procedures, they generated transgene-free cucumber plants with edited CsWIP1, CsVFB1, CsMLO8 and CsGAD1 genes. This was the first study that knocked out the CsWIP1 to increase female flowers development in cucumber (Hu et al. 2017).
Fruit length is an important agricultural trait during cucumber domestication and breeding. A recent study showed two alleles of the FRUITFULL-like MADS-box gene CsFUL1 with a C-A SNP variation among 150 cucumber lines (Zhao et al. 2019). Reduce CsFUL1A by RNA interference (RNAi) result in further elongated fruit, whereas elevated expression of CsFUL1A resulted in greatly reduced fruit length in cucumber. Further molecular studies indicated that CsFUL1A acts as a repressor for fruit elongation through SUPERMAN-mediated cell division/expansion and PINFORMED-mediated auxin transport in cucumber (Zhao et al. 2019). This work suggests a strategy for manipulating fruit length in cucumber breeding via modulation of CsFUL1A expression (Table 1 and Fig. 1; Zhao et al. 2019).
Cucumber is an important vegetable crop with indeterminant growth habit. Its leaves are derived from the shoot apical meristem (SAM), and unisexual flowers are generated from the leaf axils. LEAFY (LFY) and its homologs have been shown to promote flower development and branching (Weigel et al. 1992; Blázquez et al. 1997; Ratcliffe et al. 1999). The LFY homolog gene CsLFY was cloned and functionally characterized in cucumber. Knockdown of CsLFY led to defective shoot development and termination of leaf initiation in cucumber. Further, biochemical analyses indicated that CsLFY physically interacts with WUSCHEL (CsWUS) in regulating meristem maintenance in cucumber (Table 1 and Fig. 1; Zhao et al. 2018). HANABA TARANU (HAN) is a GATA3-type transcription factor that functions in floral organ development and SAM organization in Arabidopsis (Ding et al. 2015). CsHAN1 is predominantly expressed at the junction of the SAM and the stem. The overexpression or knockdown (RNAi) of CsHAN1 resulted in retarded growth and highly lobed leaves in cucumber (Table 1 and Fig. 1; Ding et al. 2015). Further studies indicated that CsHAN1 may regulate SAM development through mediating the CsWUS and SHOOTMERISTEMLESS (STM) pathways in cucumber. Future studies using the CRISPR system to obtain knockout transgenic lines of these genes may be helpful in obtaining germplasm resources of shoot meristem maintenance in cucumber.
Anther and ovule genesis preconditions crop fertilization and fruit production. To dissect the molecular regulation of anther and ovule development in cucumber, SPOROCYTELESS (SPL)/NOZZLE (NZZ) homolog CsSPL was detected and its expression was specifically in the developing anthers or ovules. Knockdown of CsSPL with RNAi reduced male and female fertility with malformed pollen and suppressed ovule development (Table 1 and Fig. 1). Furthermore, CsSPL acted as a linker between CsWUS and CsPHB (PHABULOSA) functioning to orchestrate sex organ development in cucumber (Liu et al. 2018).
Shoot branching is an important agronomic trait that directly determines plant architecture and affects crop productivity. To promote crop yield and quality, axillary branches need to be manually removed during cucumber production for fresh market and thus are undesirable. In a recent research, the homologous gene of TEOSINTE BRANCHED1/CYCLOIDEA/PCF(TCP) family gene BRANCHED1 (BRC1) in cucumber is expressed in axillary buds and displays a higher expression level in cultivated cucumber than in wild varieties. Transgenic experiments showed that knockdown of CsBRC1 leads to increased bud outgrowth and reduced auxin accumulation in buds. Further molecular experiments showed that cucumber CsBRC1 directly binds to the promoter of auxin efflux transporter CsPIN3 and repress its expression. Tissue-specific expression of CsPIN3 driven by the CsBRC1 promoter leads to highly branched and decreased auxin levels in lateral buds. This study provides a new strategy to breed for cucumber cultivars with varying degrees of shoot branching grown in different cucumber production systems (Shen et al. 2019).
Perspective
Cucumber is beneficial for researchers to apply molecular breeding technology to improve cultivars, owing to its relatively small genome size (367 Mbp) and short growing life cycle (50–90 days) (Che and Zhang 2019). The development of high-throughput sequencing technology and whole genome sequencing has already provided fundamental data for growing elite cucumber varieties to improve quality and enhance stress resistance. Investigating these sequencing data associated with commercial traits is essential to the success of cucumber molecular breeding. To shorten breeding life and speed up cucumber breeding, we propose following activities: (1) To identify new marker genes associated with commercial and agronomic traits which include precocity, high quality, disease resistance and fertility for gene integration breeding thus to combine molecular markers of good traits into one cultivar. (2) Explore the study on functional genes and optimize transgenic system. Focus on the relationship between gene function and important agronomic traits of cucumber and to establish high-efficiency genetic transformation methods and gene editing technology in cucumber, which is indispensable for functional research in this important Cucurbitaceae crop. (3) Expand germplasm resources and increase the utilization of genetic map. Introduce and make full use of wild germplasm resources and bank of mutants from mutagenesis or natural crossing. (4) Use artificial intelligence, genome sequencing, gene editing, and other technologies to realize the rapid accumulation of cucumber omics genotype and phenomics. Integrate genetic variation data to realize the rapid mining of regulatory genes for crop traits and accurate prediction of phenotypes. Engineered and synthetic genes should be used to confer crops with new biological traits such as stress tolerance and high yield. The machine learning prediction model will be established at the whole genome level, and the breeding design scheme of intelligent combination of natural variation, artificial variation and quantitative trait locus of excellent alleles will also be created. Finally, realize the automation of molecular phenotyping technology, and achieve intelligent, efficient and directional cultivation of new cucumber varieties.
References
Blázquez MA, Soowal LN, Lee L, Weigel D (1997) LEAFY expression and flower initiation in Arabidopsis. Development 124:3835–3844
Bradbury LMT, Fitzgerald TL, Henry RJ, Jin Q, Waters DLE (2005) The gene for fragrance in rice. Plant Biotechnol J 3:363–370
Bu F, Chen H, Shi Q, Zhou Q, Gao D (2016) A major quantitative trait locus conferring subgynoecy in cucumber. Theor Appl Genet 129:97–104
Chandrasekaran J, Brumin M, Wolf D, Leibman D, Klap C, Pearlsman M, Sherman A, Arazi T, Gal-On A (2016) Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol Plant Pathol 17(7):1140–1153
Che G, Zhang X (2019) Molecular basis of cucumber fruit domestication. Curr Opin Plant Biol 47:38–46
Chen C (2015) Screening and identification of cucumber “406” mutant by EMS mutagenesis (dissertation). Hunan Agricultural University, Changsha (in Chinese with English abstract)
Chen QJ, Zhang HY, Wang YJ, Li WY, Zhang F, Mao AJ, Chen JH et al (2010) Mapping and analyzing QTLs of yield-associated agronomic traits of greenhouse cucumbers. Sci Agric Sin 43:112–122 (in Chinese with English abstract)
Chen LZ, Chen JF, Jack S, Qian CT (2005) A new pickling cucumber F1 hybrid bred from inter specific hybridization. China Veg (3):4–6 (in Chinese with English abstract)
Cui XH, Liu N, Han YK, Wei AM, Zhang GH, Du SL (2012) Mutation and inheritance of nitrogen ion injection induce mutant in cucumber. China Cucurbits Veg 25:17–19 (in Chinese with English abstract)
Ding L, Yan S, Jiang L, Liu M, Zhang J, Zhao J, Zhao W, Han Y, Wang Q, Zhang X (2015) HANABA TARANU regulates the shoot apical meristem and leaf development in cucumber (Cucumis sativus L.). J Exp Bot 66:7075–7087
Dong S (2013) Genetic mechanism and gene mapping of glossy fruit skin in cucumber (dissertation). Chinese Academy of Agricultural Sciences, Beijing (in Chinese with English abstract)
Fazio G, Chung SM, Staub JE (2003) Comparative analysis of response to phenotypic and marker-assisted selection for multiple lateral branching in cucumber (Cucumis sativus L.). Theor Appl Genet 107:875–883
Gao M, Hu L, Li Y, Weng Y (2016) The chlorophyll-deficient golden leaf mutation in cucumber is due to a single nucleotide substitution in CsChlI for magnesium chelatase I subunit. Theor Appl Genet 129:1961–1973
Gu X, Zhang S, Zhang S (2006) The AFLP makers linkedwith the bitter fruit gene (Bt) in cucumber. Acta Hortic Sin 33:140–142 (in Chinese with English abstract)
Guan Y (2008) Mapping and cloning of related gene for fruitspines formation in cucumber (dissertation). Shanghai Jiao Tong University, Shanghai (in Chinese with Englishabstract)
Guo Y (2003) Studies on the inheritance of the cucumber bitterness and AFLP molecular marker (dissertation). Northeast Agricultural University, Harbin (in Chinese with English abstract)
He NW, Yang XB, Tian LM, Zhao Y (2011) In vitro antioxidant activity of cucumber polysaccharides. Food Sci 32:70–74 (in Chinese with English abstract)
Hou S, Niu H, Tao Q, Wang S, Gong Z (2017) A mutant in the CsDET2 gene leads to a systemic brassinosteriod deficiency and super compact phenotype in cucumber (Cucumis sativus L.). Theor Appl Genet 130:1693–1703
Hu Y, Zhao S (2010) RAPD technology and its application on plant research. Biotechnol Bull 5:74–77 (in Chinese with English abstract)
Hu BW, Li DW, Liu X, Qi JJ, Gao DL, Zhao SQ, Huang SW, Sun JJ, Yang L (2017) Engineering non-transgenic gynoecious cucumber using an improved transformation protocol and optimized CRISPR/Cas9 system. Mol Plant 10:1575–1578
Huang SW, Li RQ, Zhang ZH, Li L, Gu XF, Fang W, Lucas WJ et al (2009) The genome of the cucumber, Cucumis sativus L. Nat Genet 41:1275–1281
Juwattanasomran R, Somta P, Chankaew S, Shimizu T, Wongpornchai S (2011) A SNP in GmBADH2 gene associates with fragrance in vegetable soybean variety “Kaori” and SNAP marker development for the fragrance. Theor Appl Genet 122:533–541
Li Y (2008) SRAP markers linked to the green skin trait of cucumber (dissertation). Northwest A&F University, Yangling (in Chinese with English abstract)
Li Y, Yang L, Pathak M, Li D, He X, Weng Y (2011) Fine genetic mapping of cp: a recessive gene for compact (dwarf) plant architecture in cucumber, Cucumis sativus L. Theor Appl Genet 123:973–983
Li Y, Wen C, Weng Y (2013) Fine mapping of the pleiotropic locus B for black spine and orange mature fruit color in cucumber identifies a 50 kb region containing a R2R3-MYB transcription factor. Theor Appl Genet 126:2187–2196
Li YP, Liang KJ, Li MT, Zhang Y, Zhou SK, Chen X, Lang JB (2015) Nutritional content and function of cucumber and grafting technique. Shanghai Agric Sci Technol 1:89–131 (in Chinese)
Li PK, Wang P, Wang FQ, Guo ZF, Yin HP, Zhang ZP (2016) Report on white cucumber strain 05-33-6-1-2-49 by space mutation. Gansu Agric Sci Technol 8:27–29 (in Chinese with English abstract)
Li CX, Li YH, Su SY, Liu G, Han JL (2017) Breeding and cultivation techniques of a new cucumber variety Tangza No.8. J Hebei Agric Sci 21:89–92 (in Chinese with English abstract)
Li HB, Wang H, Fang BF (2009) New variety Hang Yan 1 of cucumber. J Changjiang Veg 23:10 (in Chinese)
Liang C, Li G (2001) Factors affecting the appearance quality of cucumber and preventive measures. Inner Mong Agric Sci Technol S1:18 (in Chinese)
Lin T, Wang S, Zhong Y, Gao D, Cui Q, Chen H, Zhang Z, Shen H, Weng Y, Huang S (2016) A truncated f-box protein confers the dwarfism in cucumber. J Genet Genom 43:223–226
Liu X, Ning K, Che G, Yan S, Han L (2018) CsSPL functions as an adaptor between HD-ZIP III and CsWUS transcription factors regulating anther and ovule development in cucumber. Plant J 94:535–547
Lu H, Lin T, Klein J, Wang S, Qi J, Zhou Q, Sun J, Zhang Z, Weng Y, Huang S (2014) QTL-seq identifies an early flowering QTL located near Flowering Locus T in cucumber. Theor Appl Genet 127(7):1491–1499
Lv SZ, Ma DH, Huo ZR, Shen WY, Li SJ, Chen ZW (1994) A new variety Jinchun NO.4 of cucumber with high yield and disease resistance. China Veg 2:1–3 (in Chinese)
Maheswaran M (2004) Molecular markers: history, features and applications. Department of Plant Molecular Biology and Biotechnology
Miao H, Zhang S, Wang M, Wang Y, Weng Y, Gu X (2016) Fine mapping of virescent leaf gene v-1 in cucumber (Cucumis sativus L.). Int J Mol Sci 17:1602
Naegele RP, Wehner TC (2017) Genetic resources of cucumber. In: Grumet R, Katzir N, Garcia-Mas J (eds) Genetics and genomics of Cucurbitaceae. Springer, Berlin, pp 61–86
Nie J, Wang Y, He H, Guo C, Zhu W, Pan J, Li D, Lian H, Pan J, Cai R (2015) Loss-of-function mutations in CsMLO1 confer durable powdery mildew resistance in cucumber (Cucumis sativus L.). Front Plant Sci 6:1155
Ohbayashi K, Ishikawa N, Hodoki Y, Okada Y, Shimada M (2019) Rapid development and characterization of EST-SSR markers for the honey locust seedbeetle, Megabruchidius dorsalis (Coleoptera: Bruchidae), using de novo transcriptome analysis based on next-generation sequencing. Appl Entomol Zool 54:141–145
Pan Y, Qu S, Bo K, Gao M (2017a) QTL mapping of domestication and diversifying selection related traits in round-fruited semi-wild Xishuangbanna cucumber (Cucumis sativus L. var. xishuangbannanesis). Theor Appl Genet 130:1531–1548
Pan YP, Liang XJ, Gao ML, Liu HQ, Meng HW, Weng YQ, Chen ZH (2017b) Round fruit shape in WI7239 cucumber is controlled by two interacting quantitative trait loci with one putatively encoding a tomato SUN homolog. Theor Appl Genet 130:573–586
Pan J, Tan J, Wang Y, Zheng X, Owens K, Li D, Li Y, Weng Y (2018) STAYGREEN (CsSGR) is a candidate for the anthracnose (Colletotrichum orbiculare) resistance locus cla in Gy14 cucumber. Theor Appl Genet 131:1577–1587
Pramnoi P, Somta P, Chankaew S, Juwattanasomran R, Srinives P (2013) A single recessive gene controls fragrance in cucumber (Cucumis sativus L.). J Genet 92:147–149
Qi J, Xin L, Shen D, Han M, Xie B, Li XX, Zeng P et al (2013) A genomic variation map provides insights into the genetic basis of cucumber domestication and diversity. Nat Genet 45:1510–1515
Ratcliffe OJ, Bradley DJ, Coen ES (1999) Separation of shoot and floral identity in Arabidopsis. Development 126:1109–1120
Ren G (2013) The research on genetic transformation of tuberculate fruit gene Tu in cucumber (Cucumis sativus L.) and gene mapping of non-lateral-branch gene nlb in cucumber (Cucumis sativus L.) (dissertation). Shanghai Jiao Tong University, Shanghai (in Chinese with English abstract)
Sakata Y, Kubo N, Morishita M, Kitadani E, Sugiyama M, Hirai M (2006) QTL analysis of powdery mildew resistance in cucumber (Cucumis sativus L.). Theor Appl Genet 112:243–250
Shang Y, Ma Y, Zhou Y, Zhang H, Duan L, Chen H, Zeng J et al (2014) Plant science. Biosynthesis, regulation, and domestication of bitterness in cucumber. Science 346:1084–1088
Shen JJ, Zhang YQ, Ge DF, Wang ZY, Song WY, Gua R, Che G, Cheng ZH, Liu RY, Zhang XL (2019) CsBRC1 inhibits axillary bud outgrowth by directly repressing the auxin efflux carrier CsPIN3 in cucumber. Proc Natl Acad Sci USA 116:17105–17114
Shi QX, Liu SQ, Li Z, Cao CX, Li Y, Huang SW (2009) Three co-dominant markers linked to M gene in Cucumis sativus. Acta Hortic Sin 36:737–742 (in Chinese with English abstract)
Shi XF, Li Q, Li XH, Xiao C, Wang SN (2010) The anti-oxidation effect of Cucumis sativus Linn. flavonoids. Food Res Dev 31:85–86 (in Chinese with English abstract)
Song M, Wei Q, Wang J, Fu W, Qin X, Lu X, Cheng F et al (2018) Fine mapping of CsVYL, conferring virescent leaf through the regulation of chloroplast development in cucumber. Front Plant Sci 9:432
Sun HT, Qin ZW, Zhou XY, Wu T, Pan DD (2010) Genetic analysis and molecular localization of the fruit diameter in cucumber. Chin Agric Bull 26:38–42 (in Chinese with English abstract)
Wang L (2011) Isolation, expression, SSR marker analysis of genes related to parthenocary of cucumber (dissertation). Nanjing Agricultural University, Nanjing (in Chinese with English abstract)
Wang GL, Qin ZW, Zhou XY, Zhao CY (2007) Genetic analysis and SSR markers of tuberculate trait in Cucumis sativus. Chin Bull Bot 24:168–172 (in Chinese with English abstract)
Wang FQ, Yin HP, Guo ZF, Zhang ZP (2016) Breeding and characteristics of a white cucumber cultivar Hangyu Cucumber No.1. J Changjiang Veg 16:30–33 (in Chinese with English abstract)
Wang H, Wanqing L, Yaguang Q, Yupeng P, Xiaofeng W (2017) The cytochrome P450 gene CsCYP85A1 is a putative candidate for super compact-1 (Scp-1) plant architecture mutation in cucumber (Cucumis sativus L.). FRONT PLANT SCI 8:266
Wang Y, Junyi T, Zhiming WKV, Todd CW, Changlong W, Zheng X, Ken O et al (2018) STAYGREEN, STAY HEALTHY: a loss-of-susceptibility mutation in the STAYGREEN gene provides durable, broad-spectrum dis- ease resistances for over 50 years of US cucumber production. New Phytol 221:415–430
Wei Q, Fu W, Wang Y, Qin X, Wang J, Li J, Lou Q et al (2016) Rapid identification of fruit length loci in cucumber (Cucumis sativus L.) using next-generation sequencing (NGS)-based QTL analysis. SCI Rep UK 6:27496
Weigel D, Alvarez J, Smyth DR, Yanofsky MF, Meyerowitz EM (1992) LEAFY controls floral meristem identity in Arabidopsis. Cell 69:843–859
Wen C, Mao A, Dong C, Liu H, Yu S, Guo Y, Weng Y, Xu Y (2015) Fine genetic mapping of target leaf spot resistance gene cca-3 in cucumber, Cucumis sativus L. Theor Appl Genet 128:2495–2506
Weng Y, Johnson S, Staub JE, Huang S (2010) An extended intervarietal microsatellite linkage map of cucumber, Cucumis sativus L. HortScience 45:882–886
Wu Z, Zhang T, Li L, Xu J, Qin XD, Zhang TL, Cui L et al (2016) Identification of a stable major-effect QTL (Parth 2.1) controlling parthenocarpy in cucumber and associated candidate gene analysis via whole genome re-sequencing. BMC Plant Biol 16:182
Xu C, Zhao BH (2009) The development and application of SRAP molecular markers. Life Sci Instrum 7:24–27 (in Chinese with English abstract)
Xu XW, Lu L, Zhu BY, Xu Q, Qi XH, Chen XH (2015) QTL mapping of cucumber fruit flesh thickness by SLAF-seq. Sci Rep 5:15829
Xu X, Yu T, Xu R, Shi Y, Lin X, Xu Q, Qi X, Weng Y, Chen X (2016) Fine mapping of a dominantly inherited powdery mildew resistance major-effect QTL, Pm1.1, in cucumber identifies a 41.1 kb region containing two tandemly arrayed cysteine-rich receptor-like protein kinase genes. Theor Appl Genet 129:507–516
Xu X, Ji J, Xu Q, Qi X, Chen X (2017) Inheritance and quantitative trail loci mapping of adventitious root numbers in cucumber seedlings under waterlogging conditions. Mol Genet Genomics 292:353–364
Xu L, Wang C, Cao W, Zhou S, Wu T (2018a) CLAVATA1-type receptor-like kinase CsCLAVATA1 is a putative candidate gene for dwarf mutation in cucumber. Mol Genet Genomics 293:1393–1405
Xu X, Ji J, Xu Q, Qi X, Weng Y, Chen X (2018b) The major-effect quantitative trait locus CsARN6.1 encodes an AAA ATPase domain-containing protein that is associated with waterlogging stress tolerance by promoting adventitious root formation. Plant J 93:917–930
Yan L (2009) Studies on physiological and genetic analysis and molecular markers of parthenocarpy in cucumber (Cucumis sativus L.) (dissertation). Nanjing Agricultural University, Nanjing (in Chinese with English abstract)
Yan H, Gao L, Li G (2006) Development and application of molecular marker technology. Bull Biol 41:17–20 (in Chinese)
Yang XH (2012) Breeding and cultivation techniques of new cucumber variety ‘Jining 726’ (dissertation). Shangdong Agricultural University, Tianan (in Chinese with English abstract)
Yang X (2014) Mapping and functional analyses of the tuberculate fruit gene Tu and the dull fruit skin gene D in cucumber (dissertation). Shanghai Jiao Tong University, Shanghai (in Chinese with English abstract)
Yang SJ, Chang YQ, Zheng LH, Wei ZR, Qu HG, Cao SG (2005) Protective effects of cucurbitacin B on the acute liver injury induced by CCL4. Food Sci 26:9 (in Chinese with English abstract)
Yang SJ, Miao H, Zhang SP, Chen ZC, Zhou J, Dong SY, Gu XF (2011) Genetic analysis and mapping of gl-2 gene in cucumber (Cucumis sativus L.). Acta Hortic Sin 38:1685–1692 (in Chinese with English abstract)
Yang L, Liu H, Zhao J, Pan Y, Cheng S, Lietzow CD, Wen C et al (2018) LITTLE LEAF (LL) encodes a WD40 repeat domain-containing protein associated with organ size variation in cucumber. Plant J 95:834–847
Yang S, Wen C, Liu B, Cai Y, Xue S, Bartholomew ES, Dong M et al (2019) A CsTu-TS1 regulatory module promotes fruit tubercule formation in cucumber. Plant Biotechnol J 17:289–301
Ye BP, Bai SN, Cao ZX (2000) ACC synthase gene (ACSG) as a possible molecular marker for female lines in cucumber. Acta Bot Sin 42:765–766 (in Chinese with English abstract)
Yundaeng C, Somta P, Tangphatsornruang S, Chankaew S, Srinives P (2015) A single base substitution in BADH/AMADH is responsible for fragrance in cucumber (Cucumis sativus L.), and development of SNAP markers for the fragrance. Theor Appl Genet 128:1881–1892
Zhang P (2009) Mapping quantitative traits loci and proteomics studies on bending of cucumber fruit (dissertation). Northeast Agricultural University, Harbin (in Chinese with English abstract)
Zhang ZY, Ni LF, Rui MF, Ye GR (2005) Space breeding cucumber-space 96-1. Shanghai Veg 5:26 (in Chinese with English abstract)
Zhang W, He H, Guan Y, Du H, Yuan L, Li Z, Yao D, Pan J, Cai R (2010) Identification and mapping of molecular markers linked to the tuberculate fruit gene in the cucumber (Cucumis sativus L.). Theor Appl Genet 120:645–654
Zhang YT, Ou YDY, He XH (2012) Progress in antitumor effect of cucurbitacin B and its mechanism. Chin J Pharmacol Toxicol 26:112–115 (in Chinese with English abstract)
Zhang Z, Mao L, Chen H, Bu F, Li G (2015) Genome-wide mapping of structural variations reveals a copy number variant that determines reproductive morphology in cucumber. Plant Cell 27:1595–1604
Zhao P (2011) Genetic analysis of carpopodium length in cucumber and identification of its quantative trait loci (dissertation). Northeast Agricultural University, Harbin (in Chinese with English abstract)
Zhao W, Chen Z, Liu X, Che G, Gu R, Zhao J, Wang Z, Hou Y, Zhang X (2018) CsLFY is required for shoot meristem maintenance via interaction with WUSCHEL in cucumber (Cucumis sativus). New Phytol 218:344–356
Zhao J, Jiang L, Che G, Pan Y, Li Y, Hou Y, Zhao W, Zhong Y, Ding L, Yan S, Sun C, Liu R, Yan L, Wu T, Li X, Weng Y, Zhang X (2019) A functional allele of CsFUL1 regulates fruit length through repressing CsSUP and inhibiting auxin transport in cucumber. Plant Cell 31:1289–1307
Acknowledgments
This research was supported by the Science Foundation of Zhejiang Province (Grant No. Y19C150016) and National Natural Science Foundation of China (Grant Nos. 31872105, 31801862 and 3180186).
Author information
Authors and Affiliations
Contributions
HSW, SJF and ZL jointly conceived the review, conducted the literature review and wrote the manuscript. JPZ, ZHM and YJW collected the literature. CLW, TW and YC provided critical comments on the manuscript. All authors read and approved the manuscript.
Corresponding authors
Additional information
Communicated by Kai Shi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Feng, S., Zhang, J., Mu, Z. et al. Recent progress on the molecular breeding of Cucumis sativus L. in China. Theor Appl Genet 133, 1777–1790 (2020). https://doi.org/10.1007/s00122-019-03484-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-019-03484-0