Abstract
Normal calcium and bone homeostasis in the adult is virtually fully explained by the interactions of several key regulatory hormones, including parathyroid hormone, 1,25 dihydroxy vitamin D3, fibroblast growth factor-23, calcitonin, and sex steroids (estradiol and testosterone). In utero, bone and mineral metabolism is regulated differently from the adult. During development, it is the placenta and not the fetal kidneys, intestines, or skeleton that is the primary source of minerals for the fetus. The placenta is able to meet the almost inexhaustible needs of the fetus for minerals by actively driving the transport of calcium and phosphorus from the maternal circulation to the growing fetus. These fundamentally important minerals are maintained in the fetal circulation at higher concentrations than those in maternal blood. Maintenance of these inordinately higher fetal levels is necessary for the developing skeleton to accrue sufficient minerals by term. Importantly, in livestock species, prenatal mineralization of the skeleton is crucial for the high levels of offspring activity soon after birth. Calcium is required for mineralization, as well as a plethora of other physiological functions. Placental calcium and phosphate transport are regulated by several mechanisms that are discussed in this review. It is clear that phosphate and calcium metabolism is intimately interrelated and, therefore, placental transport of these minerals cannot be considered in isolation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
The strength and durability of the adult vertebrate skeleton come not from the construction of a monolithic static structure but from building a dynamic and renewable biologically active scaffold (Suva et al. 2005). This process requires an enormous supply of minerals, principally calcium and phosphate, along with the meticulous regulation of mineral storage, availability, and utility by a plethora of hormones acting in concert on kidney, intestine, and bone. Indeed, normal adult calcium and bone homeostasis can be almost fully explained by the interactions of several regulatory hormones, including parathyroid hormone (PTH), PTH-related protein (PTHrP), 1,25 dihydroxyvitamin D3 (1,25(OH)2D3), fibroblast growth factor-23 (FGF23), calcitonin, and sex steroids (estradiol, progesterone, and testosterone).
During normal fetal development minerals such as calcium, phosphate and magnesium are maintained at significantly higher concentrations in utero to achieve adequate bone accretion (Taylor-Miller and Allgrove 2021). The availability of these critical minerals is an integral component of fetal development that facilitates the safe neonatal transition to post-natal life in all mammalian species (Taylor-Miller and Allgrove 2021). It is perhaps not surprising that many of the same mineral transport regulators active throughout adult life are associated with mineral transport across the placenta to the developing fetus; a concept that will be discussed throughout this review.
With regard to mineral ion action, inorganic phosphate (Pi) is critical for a wide variety of metabolic pathways including synthesis of DNA and RNA, formation of phospholipids and membranes, the generation of ATP/GTP/UTP, cellular signaling (phosphorylation and dephosphorylation), the buffering of pH in intracellular fluids and urine, as well as in the extracellular matrix (ECM) of bone and teeth in the forms of hydroxyapatite (Ca10[PO4]6[OH]2) (Voelkl et al. 2021) and glucosamine-phosphate-derived glycoproteins (Wu 2021).
During embryonic development after the early chondrocyte anlage pattern for the endochondral skeleton is laid down, rapid bone formation and mineralization of the developing fetal skeleton creates a significant demand for minerals. In this scenario, kidneys, intestines, and skeleton (active in adults to control the extent of mineral ion metabolism) do not supply minerals to the normal fetus. Rather, the placenta meets the extensive fetal need for minerals by actively transporting calcium, phosphorus, and magnesium from the maternal circulation (Kovacs 2014). The placenta provides these essential minerals even when confronted with their reduced concentrations in the maternal circulation. Indeed, the fetus develops in the face of a significant hypercalcemia yet maintains even higher mineral concentrations than in the mother or a normal adult (Kovacs 2014). These significantly elevated levels are required for the skeleton to form and mineralize normally (Care 1991). In this review, the regulatory mechanisms responsible for the transport of minerals across the placenta to the developing fetus are described in relation to the well-characterized mechanisms in play in the adult.
5.2 Roles of Phosphate, Calcium, and Vitamin D Postnatally in Bone and Kidney
5.2.1 Phosphate
Several factors and endocrine hormones modulate homeostasis of renal calcium and phosphate. Among those, (1,25(OH)2D3), PTH, and FGF23 have gained the most attention as very important regulators of phosphate homeostasis (Suva and Friedman 2020). These three hormones regulate each other, thereby forming a classic regulatory endocrine loop (Kovacs 2014).
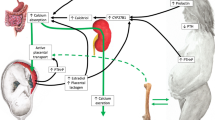
An increase in dietary phosphate intake or plasma phosphate levels stimulates PTH secretion, which enhances bone-derived FGF23 synthesis and release as well as the synthesis of 1,25(OH)2D3 by kidneys (Fig. 5.1). Similarly, modest decreases in serum calcium also produce profound and rapid increases in PTH secretion that enhance bone-derived FGF23 formation and release, as well as the synthesis of 1,25(OH)2D3 by the kidney. The release of FGF23 suppresses PTH and 1,25(OH)2D3 levels (PTH inhibits 1α hydroxylase action in the kidney), whereas 1,25(OH)2D3 stimulates FGF23 release and inhibits PTH synthesis and secretion. In addition to these positive and negative feedback loops, all three hormones are regulated by a plethora of other factors.
PTH, FGF23, and 1,25(OH)2D3 are essential regulators of phosphate homeostasis postnatally. An increase in dietary phosphate intake or plasma phosphate levels stimulates PTH secretion, which enhances bone-derived FGF23 formation and release as well as the synthesis of 1,25(OH)2D3 by kidneys. The release of FGF23 suppresses PTH and 1,25(OH)2D3 levels (PTH inhibits 1α hydroxylase action in the kidney), whereas 1,25(OH)2D3 stimulates FGF23 release and inhibits PTH synthesis and secretion. In addition to these positive and negative feedback loops, all three hormones are regulated by a plethora of other factors.
Blood in the vascular circulation and extracellular fluid are responsible for the transport of phosphate to and from the organs involved in phosphate metabolism. It is the skeleton that provides the largest reservoir of phosphate, and it is also the source of a major hormone regulating phosphate transport, namely FGF23 (Dias et al. 2006; Peacock 2021). Bone mineral phosphate is exchangeable with extracellular fluid phosphate, but this flux does not substantially contribute to extracellular fluid phosphate homeostasis (Dias et al. 2006; Peacock 2021). Fundamentally, two transport pathways for phosphate exist in bone. One involves the transport of extracellular fluid phosphate into and out of bone whereas the second is the transport of phosphate within bone, directly from the bone-forming osteoblast as a component of the mineralizing apatite crystal (Peacock 2021). Daily net transport of phosphate from extracellular fluid to new bone formation and back to the extracellular fluid by bone resorption is less than that managed by the kidney and the gut (Peacock 2021).
It is the kidney that handles the major fraction of daily phosphate transport, as well as being the primary source for the generation of the active form of vitamin D (1,25(OH)2D3), through the action of the PTH-regulated 1α hydroxylase (Li et al. 2020). 1,25(OH)2D3 is a critically important hormone for the regulation of phosphate transport. The process of glomerular filtration transports more than 5,000 mg phosphate every 24 h to the proximal renal tubule that reabsorbs and transfers the majority (>80%) back to extracellular fluid. As such, the kidney is a primary organ responsible for controlling the circulating concentrations of phosphate (Peacock 2018).
Given the fundamental role of the kidney, the impact of the gut on the supply of phosphate to the circulation is also important to consider. The amount of dietary phosphate is inadequate for herbivorous mammals without receiving supplementation (Wu 2018) and humans consuming little or no animal products. It is possible that dietary phosphate is surplus over the daily requirements for phosphate in carnivores. Paracellular diffusion is an important mechanism for phosphate uptake in humans and other animals, but the mechanistic detail(s) and its relative magnitude remain to be fully elucidated. The absorption of phosphate occurs by both paracellular diffusion and active, saturable, transcellular mechanisms that act at low phosphate intakes (Peacock 2021). In addition, the small intestine is an important site for phosphate transport, and it is directly responsible for the bulk of dietary phosphate absorption (Hernando and Wagner 2018). In contrast to animal products, the bioavailability of phosphate in plant-sourced foods is relatively low (Wu 2018). The efficiency of absorption is estimated at around 80% with some 20% of the absorbed phosphate returning to the lumen of the gut from the extracellular fluid as endogenous secretions. With this in mind, it is clear that phosphate transport is both passively and actively performed and regulated by a number of transport mechanisms distributed across many tissues, but with a particularly important focus on the kidney, gut and bone (Peacock 2021).
5.2.2 Calcium
Similar to phosphate, the minute to minute regulation of serum calcium is also intimately and elegantly controlled by the interplay between calcium and phosphate as well as the level of the major calcium regulating hormones in the adult, PTH and 1,25(OH)2D3 (Kovacs 2014). Calcium is involved in many physiological and biochemical processes as it is an essential element for cardiac function, the structural integrity of bone, muscle contraction, and it also acts as a second messenger in cell signaling and a cofactor for many enzymes in a multitude of biochemical pathways. Serum calcium can be measured in venous blood samples, with normal physiologic levels ranging from 8.8 to 10.4 mg/dL for total calcium, and 4.7–5.2 mg/dL for free calcium (Kavsak 2017). Total calcium values should be corrected for current concentrations of albumin as their interaction can affect reported levels.
A consistent finding throughout fetal development is the high demand for calcium. Indeed, concentrations of calcium in fetal serum are significantly greater than the simultaneous concentrations of calcium in maternal serum in many species (Kovacs 2014). In fetal mice, free calcium is 0.25–0.50 mM greater than maternal values (Kovacs et al. 1996), and the values can be even higher in relation to maternal levels in a wide variety of species including pigs (Care et al. 1986), lambs (Delivoria-Papadopoulos et al. 1967), foals (Garel 1972), and perinatal primates (Fleischman et al. 1975). These important measurements indicate that the fetus is indeed hypercalcemic relative to both the maternal and normal adult serum calcium values. Interestingly, an explanation continues to elude us regarding how early in gestation do concentrations of calcium in fetal serum exceed those in maternal serum.
Given the profound hypercalcemia of the fetus, an obvious question is why? What purpose is served by the fetus maintaining a high concentration of calcium in its blood? The conservation of this fetal hypercalcemia across mammals suggests some fundamentally important physiological function. Several rationales exist including the obvious need for high fetal calcium to ensure normal skeletal mineralization (especially in large animals) and the possibility that elevated blood calcium in utero provides a survival advantage after birth. Whatever the physiological reason, the details of why are unclear, especially since moderate fetal hypocalcemia does not appear to impair survival to the end of gestation as shown in murine studies (Kovacs et al. 2001a; Suzuki et al. 2008).
During endochondral bone formation in the fetus, the regulation of fetal mineral homeostasis is of utmost importance. In addition to the requirement for calcium, phosphorus also plays a key role. Since the mineralization of osteoid requires the incorporation of phosphate prior to calcium binding (Zhang et al. 2011), dietary phosphorus is an important determinant of bone mineralization during fetal development. In sum, concentrations of calcium, ionized calcium, and phosphorus in serum are significantly higher in fetuses than in the mother and can generally be maintained despite abnormal concentrations in the maternal circulation (Kovacs 2014).
As discussed previously, and throughout this review, the transport of both phosphate and calcium is regulated by a complex hormonal axis comprised of FGF23, PTH, and 1,25(OH)2D3. Perhaps most importantly, the metabolism of phosphate and calcium does not occur in isolation from each other. They closely interact at transport mechanisms in extracellular fluid, gut, bone, and kidney. Changes in the extracellular fluid concentrations of phosphate and calcium independently regulate secretion of the hormones, FGF23, PTH, and 1,25(OH)2D3 that maintain phosphate and calcium homeostasis. The regulation of phosphate and calcium metabolism is intimately inter-related and neither should be considered in isolation.
5.3 Animal Models for the Study of Placental Mineral Transport (see Enders and Carter 2004; Carter 2007; Barry and Anthony 2008; Grigsby 2016)
The placenta is an underappreciated organ, which is critical for the exchange of minerals, gases, amino acids, sugars, and proteins, while producing regulatory molecules such as cytokines, growth factors, and hormones that are crucial for the development and growth of the conceptus (Enders and Blankenship 1999). Given the ethical concerns and the significant limitations associated with performing human placental research, comparative physiology is essential to improve understanding of the mechanisms governing placental nutrient and mineral transport, and conceptus development and growth. As there are often confounding factors in human pregnancy studies, animal models can provide valuable data from a controlled experimental design to allow further investigation into the importance of mineral transport in pregnancy. Despite the striking similarities that exist between mammalian species during pregnancy, placentation varies substantially, which can lead to differences in mineral and nutrient transport to the fetus (Enders and Blankenship 1999; Enders and Carter 2004; Montiel et al. 2013).
There are many published reports on placental transport of minerals with rodents, sheep, and pigs commonly used as animal models. Rodents are an extensively utilized as an inexpensive animal model for studies of conceptus growth and development. The gestation period for rats, mice, and hamsters is 20–22 days, allowing generation of data from large numbers of animals in a timely manner. Rodents undergo invasive implantation and have hemochorial placentae. While there are several similarities between human and rodent placentae in structure and function, there are several considerations that must be made when interpreting results from studies with rodents (Wu 2022). Considering the significant interspecies differences in bone structure, composition, and density (Aerssens et al. 1998) and physiological maturity at birth, it is unsurprising that there are significant variations in the timings of ossification and skeletal mineralization. Compared with humans, rodents are relatively immature at birth, and their small size makes both surgical and non-surgical procedures challenging (Swanson and David 2015). In contrast to humans, rodents are a litter-bearing species, with individual feto-placental units allowing for direct comparison among samples from different fetuses within the same uterus, without the confounding factors of maternal genotype, nutrition, or husbandry practices. However, as they are a litter bearing species, there are much greater demands being placed upon the mother to ensure that adequate mineral transfer occurs to allow appropriate fetal growth and development. Data suggest that the fetal mineral demand is much greater in rodents than in other commonly utilized animal models, with approximately 80% of the calcium in maternal blood transported to the fetuses in late gestation in rats, compared with 5–10% of calcium in maternal blood of women (Comar 1956; Widdowson 1962). This makes rodents highly susceptible to secondary hyperparathyroidism and hypocalcemia compared with other species. While this allows for unique opportunities to study the effects of these conditions on fetal growth and development, it does raise the question of how comparable placental mineral transport is in rodents compared with other species.
In contrast to rodents, sheep are much larger in size, making them easier to handle and perform experimental manipulations. Importantly, the sheep fetus has a similar physiological maturity at birth to human infants. Furthermore, like humans, sheep usually have singleton or twin pregnancies. Sheep have a much longer gestation length than rodents, which allows investigations to focus on comparable stages of gestation to those for human pregnancies. While the sheep placenta is viewed by some as being anatomically distinct from the human placenta, there are many important similarities. The sheep have a multi-cotyledonary placenta, consisting of placentomes composed of maternal caruncular tissue and fetal cotyledonary tissue. Large variations in cotyledonary numbers exist and it is known that breed, litter size, and parity can influence cotyledonary number and size (Dwyer et al. 2005). Despite the gross anatomical differences, the human placenta also has cotyledonary structures, making them more similar in structure to those for sheep than may be appreciated by some scientists. Additionally, while the fetal vascular trees of sheep and human placentae differ in size, the architecture of the stem, intermediate, and terminal villi is comparable (Leiser et al. 1997).
Similar to rodents, pigs are a litter-bearing species with individual feto-placental units, so comparisons can be made among different fetuses within the same uterus, with minimal confounding effects. Pigs are much larger in size than rodents, making them easier to handle and perform experimental manipulations. Furthermore, piglets have a similar physiological maturity at birth to human infants and a longer gestation length than rodents, which allows investigations to occur at desired, comparable stages of gestation to human pregnancies. Pigs have a true epitheliochorial placenta, wherein the uterine luminal epithelium (LE) remains intact throughout pregnancy and the trophectoderm directly attaches to the LE. This type of conceptus attachment is much less invasive than for other types of placental types; therefore, extensive remodeling must occur to maximize placental surface area available for nutrient, mineral, and gas exchange.
It is important to consider these species-specific differences when designing an experiment to investigate placental transport of minerals and nutrients and when extrapolating the findings from studies of one species to another species. While each animal model has its own merits, it is important to note that no animal model fully recapitulates human pregnancy.
5.4 Maternal Mineral Adaptations
Calcium and phosphorous are two of the most abundant minerals in the body, with approximately 99 and 85% of this calcium and phosphorous, respectively, stored in bone in the form of hydroxyapatite (Ca10[PO4]6[OH]2) (Mitchell et al. 1945; Penido and Alon 2012). In addition to their critical roles in the skeletal and renal systems outlined earlier, calcium and phosphate are regulators of many processes including cellular proliferation, protein synthesis, and cellular metabolism (Chin et al. 1987; Santella 1998; Brostrom and Brostrom 2003; Jeon 2008; Glancy and Balaban 2012; Penido and Alon 2012; Kovacs 2014). During pregnancy, the mother must adapt to meet the nutritional and mineral requirements of the developing fetus, without compromising the maternal mineral reserve. Studies investigating placental transport of minerals have demonstrated that the rate of calcium and phosphate transport significantly increases in the last trimester of pregnancy to allow for fetal skeletal mineralization. In fact, it is estimated that around 80% of the minerals present in a fetus at birth are transported during the last trimester of pregnancy. In humans, this translates to approximately 20 g of phosphate and 30 g of calcium (Givens and Macy 1933; Widdowson and McCance 1965; Ziegler et al. 1976). Unsurprisingly, this is a significant burden on the mother’s mineral reserve and the mechanisms regulating maternal mineral homeostasis must be altered to avoid the mother becoming hypocalcemic or hypophosphatemic.
To accommodate the increasing mineral demands, the maternal intestine must double the absorption of phosphate and calcium (Kent et al. 1991; Ritchie et al. 1998; Kovacs 2016) (Fig. 5.2). Increasing intestinal mineral absorption is a crucial gestational adaptation to provide sufficient minerals for transport across the placenta to meet the demands of the developing fetus. In humans, this begins after approximately 12 weeks of pregnancy and is maintained until parturition (Heaney and Skillman 1971; Kent et al. 1991; Cross et al. 1995). This strategy increases the maternal mineral reserve so that in the latter stages of gestation, during the period of exponential fetal growth and skeletal mineralization, there are sufficient amounts of calcium and phosphate available for the fetus. There are conflicting theories regarding what regulates this change in maternal mineral absorption. Some consider calcitrol to be the central regulator of this adaptation as it is known to increase intestinal expression of molecules involved in calcium transport including calcium-binding proteins, transient receptor potential cation channel family members, and Ca2+ ATPases [reviewed by (Kovacs 2016)]. While this is plausible, the increase in intestinal calcium absorption begins early in gestation, prior to the increase in maternal calcitrol. Additionally, recent evidence from research with mice indicates that calcitrol is not the key regulator of this increase in intestinal calcium absorption during pregnancy (Fudge and Kovacs 2010; Gillies et al. 2018). Evidence from animal models suggests that prolactin and placental lactogen can regulate intestinal calcium absorption; therefore, the potential role of these molecules in the regulation of increased intestinal calcium absorption during the first trimester warrants further investigation. Despite this crucial and significant increase in intestinal calcium absorption, the maternal kidneys do not alter their resorption of minerals, ultimately leading to significant increases in calcium excretion in urine.

source of minerals for the fetus as the fetal intestines can absorb minerals present in amniotic fluid. The fetal kidneys then filter the blood and excrete minerals into urine, which in turn makes up much of the volume of amniotic fluid; thereby forming an intestinal-renal-amniotic fluid loop
Maternal mineral absorption increases during gestation from an increased mineral reserve for fetal skeletal mineralization. During pregnancy, the mother must adapt to meet the nutritional and mineral requirements of the developing fetus, without compromising the maternal mineral reserve. Studies investigating placental mineral transport have demonstrated that the rate of calcium and phosphate transport significantly increases in the last trimester of pregnancy to allow for fetal skeletal mineralization, with the majority of transported calcium and phosphate forming the fetal skeleton. To accommodate the increasing mineral demands, the maternal intestine must double the absorption of phosphate and calcium. Following placental transport of these minerals, they can be stored in amniotic and allantoic fluid. The fetus can drink amniotic fluid, which is an important additional
Considering the extensive transport of calcium that must occur across the placenta to the fetus, it is unsurprising that calcium in maternal serum decreases in late gestation in many species including rats, sheep, goats, and cows [reviewed by Kovacs (2016)]. Results of several studies of women suggest that calcium in maternal serum decreases in pregnancy; however, results of other studies suggest that the abundance of free calcium in maternal serum is not affected by stage of pregnancy [reviewed by Kovacs (2016)].
5.5 Gestational Changes in Phosphate and Calcium in Fetal Fluids
Prior to the establishment of a functional placenta for transplacental exchange of gases, micronutrients (amino acids, glucose), and macromolecules (proteins), the conceptus is entirely reliant upon the secretion and transport of nutrients into the uterine lumen by the maternal uterine glandular epithelium (GE) and LE. A simple and effective manner to assess the nutrient and mineral composition within the uterine luminal environment is to flush the uterus with a buffer solution and compare the nutrient and mineral composition across different days of gestation. Data from pigs and sheep indicate that calcium in uterine flushings increases during the peri-implantation period and is more abundant in uterine flushings from pregnant animals when compared with cyclic animals during the mid-luteal phase of the estrous cycle (Gao et al. 2009; Choi et al. 2019; Stenhouse et al. 2021a). Furthermore, it has been demonstrated in mice that this increase in calcium is essential for the regulation of conceptus development and implantation, with infusion of calcium channel blockers into the mouse uterine lumen causing failure of blastocysts to implant (Banerjee et al. 2011).
As the major components of the skeleton, calcium, and phosphate must be transported across the placenta to the fetus for adequate mineralization of the skeleton (Kovacs 2014, 2016). Studies investigating placental transport of minerals have demonstrated that the rate of calcium and phosphate transport increases significantly during the last trimester of pregnancy to allow mineralization of the fetal skeletal system to occur, as noted previously. In humans, >300 mg of calcium must be transported per day to the fetus between weeks 35 and 38 of gestation. It has been suggested that pregnant ewes in late gestation must transport 1.25 g of phosphate and 2.7 g of calcium to the ovine fetus each day (Grace et al. 1986).
Fetal fluids (allantoic and amniotic fluids) are of maternal origin via active transport of water, as well as other molecules, across the placenta and into the allantoic sac for distribution to other components of the conceptus including the fetus and amniotic sac. The driving force for expansion of the allantois, and in turn the chorioallantois, is the rapid accumulation of water in the allantoic sac. In the pregnant ewe, this volume increases from about 1 ml on Day 18 to 90 ml on Day 40 and then from Day 70 (32 ml) to Day 140 (438 ml) of the 147-day period of gestation. The rapid changes in allantoic fluid volume allow expansion of the chorioallantoic membranes and ensure their intimate apposition across the maximum surface area for attachment to the maternal endometrium. Allantoic fluid is a nutrient reservoir that is rich in electrolytes, sugars, amino acids, minerals, and other nutrients, as well as proteins. Importantly, the allantoic epithelium derives from the hindgut and functions to transport nutrients from allantoic fluid into the fetal-placental vasculature. Amniotic fluid is isosmotic to fetal serum, and its volume increases throughout gestation in sheep from 2 ml on Day 30 to over 700 ml on Day 140 of gestation. Amniotic fluid has several important roles. First, it buoys the fetus to allow it to develop symmetrically in three dimensions. Second, it prevents fetal skin from adhering to the amnion. Third, the fetus may drink up to 1 L of amniotic fluid in the last one-third of gestation to gain water and other nutrients, including proteins secreted by the lungs, salivary glands, and amniotic membranes. This act of drinking amniotic fluid is an important additional source of minerals for the fetus as the fetal intestines can absorb minerals present in amniotic fluid.
Phosphate and calcium are both very abundant in ovine amniotic and allantoic fluids across gestation (Stenhouse et al. 2021a; b), indicative of extensive mineral transport across the placenta. Total phosphate and calcium in ovine allantoic fluid increase significantly with advancing gestational day, highlighting that in late gestation when increased placental mineral transport occurs, these minerals are stored in the allantoic fluid for the fetus to utilize for skeletal mineralization. However, total calcium and phosphate are relatively constant in ovine amniotic fluid across gestation, despite significant changes in fluid volume across pregnancy (Stenhouse et al. 2021a; b). In goats, the concentrations of phosphorous and calcium in allantoic fluid increase with advancing gestational day, whereas in amniotic fluid, the concentrations of both calcium and phosphorous decrease with advancing gestational stage (Tabatabaei 2012). Similarly, it has been suggested that the concentrations of phosphate in human amniotic fluid decrease with advancing gestational day (Fotiou et al. 2015; Correia-Branco et al. 2020). It is important to note that differences in placentation type, stage of gestation, and fetal number may all influence the abundance of these minerals in fetal fluids. Additionally, is known that the volume of fetal fluids fluctuates significantly across gestation (Bazer et al. 2012; Dubil and Magann 2013). Therefore, care should be taken when comparing fetal fluid data presented as concentration as compared with total abundance (concentration X fluid volume).
While it is understood that mineral transport at the maternal–conceptus interface increases in late gestation, it is important to ascertain the spatiotemporal profile of molecules that regulate calcium and phosphate transport and vitamin D metabolism at the maternal–conceptus interface across species. Many studies have investigated calcium, phosphate, and vitamin D signaling in pregnancy. While these are all important signaling pathways individually, we must ascertain how these pathways work in concert at the maternal–conceptus interface to fully understand the importance of these minerals during the course of gestation.
5.6 Impact of Maternal Calcium and Vitamin D Deficiencies on Fetal Development and Pregnancy Outcomes
Hypocalcemia in pregnancy occurs when the fetal demands for calcium exceed the availability of calcium in maternal serum. Deficiency in maternal calcium is a significant problem in pregnancy and has been associated with several adverse pregnancy outcomes including pre-eclampsia, preterm birth, and intrauterine growth restriction (Chhabra and Singh 2017; Wilson et al. 2020). Thus, calcium has an important role in the regulation of conceptus development and growth. In mothers with severe hypocalcemia, the increase in intestinal calcium absorption that occurs in a normal pregnancy is not sufficient to increase the available calcium reserve in the mother. In these instances, calcium must be resorbed from maternal bone, which can decrease maternal bone density and increase the risk of the mother developing osteoporosis during pregnancy (Kovacs and Ralston 2015). Despite these adaptations in mineral status of the mother, it is necessary to monitor mineral status and ensure that there is sufficient mineral transport across the placenta because severe calcium deficiencies impact placental transport of calcium to the fetus which, in severe cases, can lead to demineralization of the fetal skeleton and development of secondary hyperparathyroidism [reviewed by Kovacs (2014)]. Similar findings have been reported for pregnant rats fed a calcium-deficient diet that led to maternal hypocalcemia and secondary hyperparathyroidism in dams, and resorption of minerals from the fetal skeletal system and secondary hyperparathyroidism in the fetuses [reviewed by Kovacs (2014)].
In dairy cows, the periparturient period (4 weeks before to 4 weeks after calving) is often associated with the development of ‘milk fever’ (hypocalcemia). Reports vary substantially on the incidence of this condition (DeGaris and Lean 2009); however, a meta-analysis of 137 controlled trials estimated that the mean incidence of the condition is 21% (range 0–83%) (Lean et al. 2006). In this condition, calcium in maternal serum can reach < 50% of the recommended normal concentrations of calcium in serum (Mayer et al. 1969; Allen and Sansom 1986), which can have severe consequences for both the mother and fetus. Hypocalcemia in dairy cows is associated with an increased risk for many undesirable conditions including parturition recumbency, dystocia, still born caves, ketosis, displaced abomasum, and even death. Uterine involution is less efficient in cows with hypocalcemia, therefore, there is a higher incidence of uterine prolapse and retained fetal membranes in hypocalcemic cows (Risco et al. 1994). Additionally, cows with hypocalcemia are more susceptible to infections, including mastitis and metritis after calving (Ducusin et al. 2003). This condition is not restricted to dairy cows as a similar condition occurs in ewes during the periparturient period that is estimated to account for between 4 and 6% of the mortality rate in sheep (Hindson and Winter 2002). Collectively, these findings indicate that hypocalcemia in large animals during the periparturient period has significant adverse impacts on pregnancy outcomes and maternal and offspring health, which can be costly for the livestock industry.
Feeding low amounts of calcium to pregnant ewes for 2 months from Day 60 of gestation significantly decreased maternal calcium while increasing circulating concentrations of hydroxyproline, parathyroid hormone, and 1,25(OH)2D (Lima et al. 1993). Interestingly, maternal hypocalcemia in ewes does not influence calcium, hydroxyproline, parathyroid hormone, or 1,25(OH)2D in fetal plasma at Day 120 of gestation. Despite the lack of differences in calcium in fetal plasma, higher concentrations of phosphate in fetal plasma result in delayed ossification of the skeletal system, a lower proportion of bone compared with cartilage, lower specific gravity, and lower ash content in fetuses from hypocalcemic mothers (Lima et al. 1993).
Maternal vitamin D deficiency is also a significant problem in pregnancy and has been linked to several adverse pregnancy outcomes including pre-eclampsia, preterm birth, and intrauterine growth restriction (Bodnar et al. 2007; Ma et al. 2012; Gernand et al. 2014; Achkar et al. 2015; Chen et al. 2015; Qin et al. 2016; Zhou et al. 2017; Alimohamadi et al. 2020; Wilson et al. 2020). Thus, vitamin D has an important role in the regulation of conceptus development and growth. In addition to impacts on pregnancy outcome, it has been suggested that maternal hypovitaminosis D (defined as maternal 25(OH)D] levels <20 ng/ml or <50 nmol/l) is a significant risk factor for adverse neonatal outcomes in humans (Karras et al. 2016). There are large variations in the reported incidence of maternal hypovitaminosis D, presumably due to seasonal and locational variations in maternal vitamin D status [reviewed by Karras et al. (2016)], but this problem does affect the global population. Despite the clear associations between maternal calcium status and fetal skeletal development, there are contradicting reports on the association between maternal vitamin D status and fetal skeletal development in humans [reviewed by Karras et al. (2016)]. However, there is some evidence suggesting a link between maternal vitamin D status and fetal bone development, with maternal hypovitaminosis D impacting fetal bone development from as early as 19 weeks of gestation (Mahon et al. 2010; Ioannou et al. 2012; Young et al. 2012).
5.7 Regulators of Placental Calcium and Phosphate Transport
Given the importance of phosphate, calcium, and vitamin D for many critical processes postnatally, it is unsurprising that many regulatory mechanisms exist (Fig. 5.3). In this review, we will briefly summarize some of the available evidence for the roles of several of the regulatory processes present at the maternal–conceptus interface across species affecting phosphate, calcium, and vitamin D.
Many signaling pathways are present at the maternal–conceptus interface across species for the regulation of phosphate, calcium, and vitamin D transport and metabolism. As the major components of the skeleton, calcium and phosphate must be transported across the placenta to the fetus, where they must be present in excess abundance compared with that in the mother to allow adequate mineralization of the skeleton. Considering this, it is perhaps unsurprising that many pathways are expressed at the maternal–conceptus interface for the regulation of the transport of these molecules. These pathways are intimately related to one another and therefore must not be considered in isolation from one another
5.7.1 PTH and PTHrP
Parathyroid hormone (PTH), PTH-related protein (PTHrP), and the cognate G protein-coupled PTH receptor (PTHR) play defining roles in the regulation of extracellular calcium and phosphate metabolism and in controlling skeletal growth and repair. Through a series of complex signaling mechanisms that, in many instances proceed in a tissue-specific manner, precise control of these processes is achieved and maintained.
The shared receptor (PTHR) mediates all known biological actions of PTH and PTHrP (Suva and Friedman 2020). Full length PTH is a peptide of 84 amino acids whereas PTHrP exists as peptide products of 139, 141, or 173 amino acid residues derived from a single alternatively spliced gene distinct from the PTH gene (Suva and Friedman 2020). The fundamental role of PTH in phosphate and calcium metabolism in adults is well defined as it has been the focus of intense investigation for many years (Quarles 2008).
Within the fetal circulation, PTH is less abundant when compared with concentrations in maternal blood of several species such as lambs and rodents (Thomas et al. 1981; Collignon et al. 1996; Simmonds et al. 2010). These low values for PTH in the fetus are from sources within the fetus since intact PTH does not cross the placenta (Garel 1972; Northrop et al. 1977; Günther et al. 2000). During development, fetal parathyroid glands begin to express PTH by mid-gestation (Tucci et al. 1996). The fetal parathyroid gland appears to be the primary source since genetic ablation of the fetal parathyroids using Hoxa3 null mice results in undetectable PTH in serum, measured using a rodent PTH immunoradiometric assay (Kovacs et al. 2001b). An additional source of PTH is likely the placenta since the PTH gene is expressed by placentae of mice. As such, the placenta may contribute a small amount of PTH to fetal circulation (Simmonds and Kovacs 2010). The normally low concentration of PTH in fetal blood is critical for the maintenance of calcium concentrations in fetal blood since fetal mice lacking PTH or the PTHR are hypocalcemic compared with WT littermates (Kovacs et al. 1996).
Only since the discovery and cloning of PTHrP (Suva et al. 1987) have details of the movement of calcium across the placenta begun to be elucidated (Abbas et al. 1989; MacIsaac et al. 1991). Since fetal blood contains high PTH-like bioactivity, but low levels of immunoreactive PTH (Allgrove et al. 1985; Loveridge et al. 1988; Rodda et al. 1988; Bourdeau et al. 1990), the finding of elevated PTHrP in fetal blood (Khosla et al. 1990; Seki et al. 1994) explained earlier reports of PTH-like bioactivity. These results led to the overarching hypothesis that PTHrP is the primary calcium-regulating hormone in fetuses, and that the suppressed PTH levels suggested that PTH is largely unimportant until after birth. Furthermore, studies in chronically cannulated sheep fetuses demonstrated a PTHrP-specific action (Abbas et al. 1989) and investigation of PTHrP null mice fetuses compared with their WT and PTHrP ± littermates demonstrated the importance of PTHrP in regulating mineral and bone metabolism in the fetus (Kovacs 2014).
5.7.2 Calcitrol
Postnatally, vitamin D has a central role in the regulation of mineral homeostasis (Bouillon et al. 2019). Vitamin D is acquired through the diet, in the form of fish liver and fortified dairy products, and vitamin D3 (cholecalciferol) can be synthesized from cholesterol in the skin following exposure to ultraviolet irradiation (Schmid and Walther 2013). Cytochrome P450 mixed-function oxidases (CYPs) are the regulators of vitamin D metabolism (Jones et al. 2014). Vitamin D enters the systemic circulation bound to vitamin D binding protein and is transported to the liver, where it is hydroxylated by CYP2R1 to 25-hydroxyvitamin D (25[OH]D3) (Bikle 2014). 25[OH]D3 is then transported to the kidney, bone, and placenta where it is further hydroxylated to the active hormonal form 1,25-dihydroxyvitamin D (1,25[OH]2D3) by 1α-hydroxylase CYP27B1 (Bikle 2014; Wu 2018). The activity of calcitrol is regulated by the catabolic activity of CYP24A1 (24-hydroxylase) (Bikle 2014), which inactivates 1,25[OH]2D3 via conversion to 1,24,25[OH]3D3. The biologically active form of vitamin D (1,25(OH)2D3 or calcitrol) binds to the vitamin D receptor (VDR). The VDR (also known as NR1I1 (nuclear receptor subfamily 1, group I, member 1) is a member of the nuclear receptor family of transcription factors. 1,25(OH)2D3 binds to the VDR, which then heterodimerizes with the retinoid-X receptor to elicit transcriptional effects. Vitamin D2 from sunlight-dried plants is almost as effective as vitamin D3 in most mammals, including humans, cattle, sheep, and pigs (Wu 2018).
As gestation progresses, the abundance of calcitrol in maternal blood increases significantly in many species including humans, rabbits, rodents, and pigs (Pike et al. 1979; Kubota et al. 1982; Ardawi et al. 1997; Boass et al. 1997; Kovacs et al. 2005; Kirby et al. 2013; Jang et al. 2017). This increase in maternal calcitrol occurs due to increased synthesis of calcitrol and not due to alterations in the metabolic clearance of calcitrol (Ross et al. 1989; Paulson et al. 1990). Species specific differences in the abundance of calcitrol in fetal blood versus maternal blood do exist. In rodents (Lester et al. 1978; Verhaeghe et al. 1988; Kovacs et al. 2005) and pigs (Lachenmaier-Currle et al. 1989; Lachenmaier-Currle and Harmeyer 1989), the abundance of calcitrol in fetal blood is <50% of that in maternal blood. It has been suggested that calcitrol cannot cross the placenta in rats (Noff and Edelstein 1978); therefore, the conceptus itself must be responsible for the generation of calcitrol from 25-hydroxyvitamin D that can be transported across the placenta in rats (Haddad et al. 1971). In contrast, calcitrol abundance is significantly greater in fetal blood than maternal blood in pregnant ewes (Abbas et al. 1987; Paulson et al. 1987) and calcitrol can pass between maternal and fetal blood in pregnant ewes (Devaskar et al. 1984). Furthermore, given the rapid metabolism of calcitrol in sheep fetuses, it is thought that calcitrol production is significantly upregulated in the ovine conceptus when compared with maternal production of calcitrol (Ross et al. 1989).
While it is well established that vitamin D is an important regulator of mineral homeostasis postnatally, contradicting data from animal models exists regarding whether vitamin D is required to maintain normal concentrations of minerals in fetal serum (Kovacs 2014). Loss of 1α-hydroxylase in fetal pigs, vitamin D deficiencies in rats, and some data from VDR null mice suggest that abundances of calcium, phosphorous, and PTH in fetal serum are not regulated by calcitrol, vitamin D, or VDR [reviewed by Kovacs (2014)]. However, other data from studies of other VDR null mice lines are inconsistent with these reports [reviewed by Kovacs (2014)].
In humans, rodents, and pigs, vitamin D metabolizing enzymes and the production of vitamin D at the maternal–conceptus interface have been demonstrated (Shahbazi et al. 2011; Bergada et al. 2014; O’Brien et al. 2014; Jang et al. 2017), suggesting potentially important roles of vitamin D in the establishment and maintenance of pregnancy, and in the regulation of conceptus growth and development. The expression of CYP27B1 mRNA and protein in placentae is positively correlated to the abundance of vitamin D in blood of humans (O’Brien et al. 2014). VDR is more abundant in the endometrium of pregnant mice compared with cyclic mice, and VDR expression increases in both the placenta and decidua with advancing days of gestation (Shahbazi et al. 2011). Additionally, the expression of CYP2R1, CYP27B1, CYP24A1, GC, and VDR varies significantly across gestation at the porcine maternal–conceptus interface (Jang et al. 2017), suggesting that vitamin D metabolism at the maternal–conceptus interface is regulated locally and varies across gestation.
Recently, we demonstrated the expression of vitamin D regulatory molecules at the ovine maternal–conceptus interface across gestation (Stenhouse et al. 2021a). VDR protein is expressed by both the trophectoderm and endoderm of the Day 17 conceptus, with more intense staining in the endoderm. Furthermore, VDR protein is expressed by the myometrium, blood vessels, uterine LE, uterine superficial glandular epithelium (sGE), uterine caruncles, and chorioallantois throughout pregnancy. The expression of VDR protein at the ovine maternal–conceptus interface throughout pregnancy demonstrates that the synthesis of active vitamin D may have broad functional consequences locally throughout pregnancy. Molecules involved in the metabolism of vitamin D (CYP2R1, CYP11A1, CYP24, and CYP27B1, and VDR) are expressed at the mRNA level in both endometria and placentae across gestation in sheep (Stenhouse et al. 2021a). Interestingly, CYP2R1 and CYP27B1 mRNAs have stable expression in ovine endometria and placentae across days of gestation (Stenhouse et al. 2021a). Since CYP2R1 and CYP27B1 are essential for the production of calcitrol, stable expression across gestation suggests an essential role for vitamin D throughout pregnancy across the maternal–conceptus interface. The expression of CYP24, VDR, and CYP11A1 mRNAs at the ovine maternal–conceptus interface varies depending upon the gestational day investigated, with high expression in early pregnancy that decreases with advancing days of gestation (Stenhouse et al. 2021a). The activity of calcitrol is regulated by the catabolic activity of CYP24A1 (24-hydroxylase) that inactivates 1,25[OH]2D3 via conversion to 1,24,25[OH]3D3 (Bikle 2014). The high expression of CYP24 mRNA in ovine endometria and placentae in early pregnancy compared with mid- and late-gestation could be indicative of high requirements for these tissues to catabolize calcitrol during implantation and placentation, which may not be required as extensively in mid- to late-gestation when the placenta is established and readily transporting minerals and nutrients to the fetus. Similar findings have been reported for porcine endometria, with high expression of CYP24 mRNA on Days 12 and 15 of gestation (Jang et al. 2017). Thus, it could be postulated that a localized negative feedback mechanism is present to regulate the abundance of calcitrol at the maternal–conceptus interface. Further investigations into the spatiotemporal expression and enzymatic activity of these enzymes would provide valuable insights into the importance of vitamin D in the establishment and maintenance of pregnancy in mammals.
Using porcine endometrial explant cultures, it has been demonstrated that calcitriol regulates the expression of several genes with essential roles in the establishment and maintenance of pregnancy including fibroblast growth factor 7 (FGF7), and secreted phosphoprotein 1 (SPP1) (Jang et al. 2017). In addition to the essential roles of vitamin D in the regulation of phosphate and calcium homeostasis, there are several ‘non-classical’ extra-skeletal functions of vitamin D. Vitamin D is a known regulator of both the adaptive and innate immune systems (Adams and Hewison 2008), targeting multiple immune cell types including T- and B-lymphocytes, monocytes, macrophages, and dendritic cells [reviewed by Baeke et al. (2010)]. Importantly, vitamin D modulates cytokine production by T-lymphocytes and antigen-presenting cells, and enhances anti-microbial activity of macrophages and monocytes, [reviewed by Baeke et al. (2010)]. During pregnancy, the uterine immune system must be tightly regulated to ensure that required anti-microbial protection occurs while ensuring that the conceptus is not rejected by the maternal immune system (Hunt 2006). Therefore, an immunomodulatory role of vitamin D at the maternal–conceptus interface has been suggested [reviewed by Tamblyn et al. (2015)]. Metabolism of vitamin D stimulates both anti-bacterial and anti-inflammatory responses by human decidual and trophoblast cells (Evans et al. 2006; Díaz et al. 2009; Liu et al. 2009). Additionally, trophoblast-derived vitamin D has been suggested to be a regulator of placental inflammation in mice (Liu et al. 2011). Given the importance of immune modulation during pregnancy, and the described spatiotemporal expression profile of vitamin D regulatory molecules at the maternal–conceptus interface in multiple species, it could be hypothesized that vitamin D not only modulates phosphate and calcium transport but plays an important role in immunomodulation at the maternal–conceptus interface during implantation and placentation, which warrants further investigation.
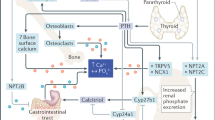
5.7.3 Phosphatonins
Fibroblast growth factor 23 (FGF23), secreted frizzled-related protein 4 (sFRP4), matrix extracellular phosphoglycoprotein, and fibroblast growth factor 7 (FGF7) are members of the phosphatonin family of circulating molecules with suggested roles in the maintenance of phosphate homeostasis (Berndt and Kumar 2007; Shaikh et al. 2008). Of the phosphatonins, FGF23 has been the most extensively investigated and is considered a significant regulator of phosphate transport through its interactions with Klotho (KL). KL acts as a coreceptor with members of the fibroblast growth factor receptor family (FGFR), and FGF23 binds to the FGFR-KL complex. Binding to this complex can regulate WNT (wingless-related integration site), PKC (protein kinase C), cAMP (cyclic adenosine monophosphate), p53 (tumor protein 53), and p21 (cyclin-dependent kinase inhibitor) signaling cascades to maintain phosphate homeostasis (Wang and Sun 2009). The metalloproteinases ADAM10 and ADAM17 can cleave KL from the plasma membrane to form a secreted form of KL (Chen et al. 2007). KL can mediate phosphate absorption by the intestine, phosphate excretion in urine, and phosphate distribution in bone in both an FGF23-dependent and -independent manner. Furthermore, the expression of type II and type III sodium-dependent phosphate transporters is tightly regulated via KL and FGF23 (Bon et al. 2018; Hu et al. 2019). In addition, other FGF family members such as FGF19 and FGF21 can utilize the KL-FGFR complex to enhance their cell signaling (Dolegowska et al. 2019). In addition to the role of KL signaling in the regulation of phosphate homeostasis, KL likely has essential roles in the regulation of calcium (Nabeshima and Imura 2008), acting as a glucuronidase to activate transient receptor potential cation channel 5 (TRPV5) (Chang et al. 2005). Furthermore, KL is a mediator of calcitrol synthesis (Yoshida et al. 2002; Haussler et al. 2013).
In mice, the fetal heart, liver, and somites express FGF23 from embryonic day 12.5 (Sitara et al. 2004). Despite low expression of FGF23 mRNA by the murine placenta, several genes involved in FGF23-KL signaling are expressed including KL, SLC34A1, SLC34A2, SLC34A3, CYP27B1, CYP24A1, FGFR1, FGFR2, FGFR3, and FGFR4 (Ma et al. 2014). Transgenic mouse lines have provided interesting insights into the role of FGF23 in the regulation of placental phosphate transport. While FGF23 null mice are fertile, they have a growth restriction phenotype accompanied by shorter longevity (Sitara et al. 2004). Interestingly, these studies revealed that intact FGF23 cannot cross the murine placenta despite fetal levels of intact FGF23 being comparable to intact FGF23 levels in wild-type pregnant mice (Ma et al. 2014).
A role of KL in the maintenance of calcium and phosphate transport in pigs has also been suggested (Choi et al. 2014a). KL mRNA is expressed in both the porcine endometria and chorioallantois and analysis of porcine endometrial KL mRNA expression demonstrated that KL mRNA decreased between Days 12–15 of the estrous cycle. Interestingly, porcine endometrial KL mRNA has a biphasic expression profile, with the highest expression observed on Days 12 and 90 of gestation. A role of KL-FGF23 signaling in the regulation of phosphate transport during pregnancy in ewes has also been suggested (Stenhouse et al. 2021b). FGF23 and KL proteins are localized to both the endoderm and trophectoderm of Day 17 ovine conceptuses, and both were abundantly expressed in the myometrium, uterine LE, sGE, and GE, and stratum compactum stroma, as well as blood vessels throughout gestation. FGF23 protein is expressed by the mononuclear and binucleate cells at the interface of the chorioallantois and caruncular tissue, and in areas undergoing syncytialization within the placentome. As pregnancy progresses, FGF23 protein is expressed in greater abundance in caruncular tissue than cotyledonary tissue of placentomes. KL protein is expressed in caruncular tissue, the chorioallantois and blood vessels throughout gestation. There is expression of FGF23-KL signaling molecules (FGF23, FGFR1-4, KL, KLB, ADAM10, and ADAM17) at the mRNA level at the ovine maternal–conceptus interface throughout gestation. The spatiotemporal expression profiles suggest potential roles of KL-FGF23 signaling in the regulation of conceptus implantation in sheep, which warrants further investigation.
KL is very abundant in human cord blood (Ohata et al. 2011) and it is expressed by the syncytiotrophoblast (STB) cells of the chorionic and basal plates of the human placenta (Iñiguez et al. 2019). Data from studies with humans suggest that KL regulates placental transport of phosphate and fetal growth, with alterations in the expression of KL, ADAM17, and FGFR1 associated with pre-eclampsia and small for gestational age term placentae (Miranda et al. 2014; Loichinger et al. 2016; Iñiguez et al. 2019).
In addition to the role of KL in the regulation of pathways associated with cellular proliferation and apoptosis, KL can regulate angiogenesis, nitric oxide production by endothelial cells, and protect against endothelial dysfunction, all of which are important in the establishment and maintenance of pregnancy (Saito et al. 1998; Fukino et al. 2002; Yamamoto et al. 2005; Kusaba et al. 2010). It is also known that vascular smooth muscle cells have high expression of KL and that knockdown of KL expression in human vascular cells revealed that they are a KL-dependent target tissue for FGF23 (Lim et al. 2012). Extensive angiogenesis must occur in the uterus and placentae to allow adequate nutrient transport from the mother to the fetus. Future studies to ascertain whether KL-FGF signaling also acts as an essential regulator of angiogenesis at the ovine maternal–conceptus interface should be performed.
Despite extensive research into the role of FGF23 in the regulation of phosphate transport, few studies have investigated the mechanisms whereby the phosphatonin FGF7 regulates phosphate transport. FGF7 inhibits phosphate uptake in tumor cells (Carpenter et al. 2005), and its expression may be regulated by vitamin D in breast cancer cells (Lyakhovich et al. 2000). FGF7 may also have an essential role in the female reproductive tract as it is classified as a progestamedin. FGF7 is expressed by uterine stromal cells in response to progesterone (P4) and acts in a paracrine manner via FGFR2IIIb to regulate the function of uterine epithelial cells and conceptus trophectoderm (Chen et al. 2000). Ovine FGF7 mRNA localizes to the tunica muscularis of blood vessels in the endometrium and myometrium (Chen et al. 2000). Given the essential roles of the progestamedins in the establishment and maintenance of pregnancy, and the limited studies that have implicated FGF7 in phosphate transport, future studies should determine whether progestamedins act as regulators of phosphate and calcium transport during pregnancy.
5.7.4 Calcium-Binding Proteins
The S100 family of proteins are cytosolic calcium-binding proteins that have central roles in the regulation of many cellular processes including cellular proliferation, differentiation, migration, metabolism, apoptosis, protein phosphorylation, and the maintenance of calcium homeostasis (Hermann et al. 2012).
Calbindin-D9K, also known as S100G, is a vitamin D-regulated calcium binding protein expressed by epithelial cells of the intestine for the modulation of calcium transport (Darwish and DeLuca 1992). While S100G is a known regulator of calcium absorption in the kidney and intestine, and multiple studies provide evidence for a critical role of this protein in the establishment of pregnancy and regulation of conceptus growth and development. In the mouse uterus, S100G mRNA is localized to uterine LE and GE when the endometrium is receptive to implantation of the blastocyst (Nie et al. 2000). Furthermore, S100G mRNA expression is down-regulated at the implantation site in mice (Nie et al. 2000). Similarly, S100G protein is localized to the uterine stroma and myometrium in the rat, with decreased uterine expression during the period of blastocyst implantation (An et al. 2003). In contrast to findings from rodents, S100G protein is expressed only by uterine LE and GE of the non-pregnant bovine endometrium, and not by myometrial or uterine stromal cells (Inpanbutr et al. 1994). S100G mRNA and protein expression are greatest during the luteal phase of the estrous cycle, suggesting that S100G may be important in the regulation of uterine gland secretions during this phase of the estrous cycle in cattle. Furthermore, S100G is highly expressed by the porcine uterus on Day 12 of pregnancy, and its expression may be regulated by P4 (Choi et al. 2009, 2012). In sheep, S100G mRNA is expressed in endometria across gestation (Stenhouse et al. 2021a), with high expression at Day 30 of gestation, which could indicate a vital role for S100G in placental development and function. Both the cytotrophoblast (CTB) and STB cells of human term placentae express S100G mRNA, with greater expression by STB than CTB cells (Belkacemi et al. 2004), highlighting a role of S100G in the mediation of calcium flux at the maternal–conceptus interface. S100G is expressed by the yolk sac and placenta in multiple species (reviewed by Choi and Jeung (2008), and it is hypothesized that this protein has a critical role in regulation of the accumulation of excess calcium required in late gestation for mineralization of the fetal skeletal system.
While S100G is expressed at the conceptus–maternal interface in multiple species, with differing spatiotemporal expression profiles across species, the functional significance of S100G in pregnancy remains poorly understood. It is proposed that other calcium regulatory molecules may undergo compensatory increases in the kidney and intestines of S100G null mice, such as TRPV6 and plasma membrane Ca2+-ATPase 1 (PMCA1) (reviewed by (Choi and Jeung 2008). S100G null mice are fertile but it could be hypothesized that in the absence of S100G, other calcium regulatory molecules undergo compensatory increases in expression at the maternal–conceptus interface to ensure that calcium transport is appropriately regulated.
Additional S100 family members, while not as extensively investigated, have been suggested to play critical roles in pregnancy. S100A8 is associated with early recurrent pregnancy loss in humans (Nair et al. 2013) and S100A8 null mice (Passey et al. 1999; Baker et al. 2011). S100A7, S100A8, S100A9, and S100A12 have been suggested to have a role in the establishment of pregnancy in pigs (Zeng et al. 2018, 2019). Similarly, roles for S100A9 and S100A12 in the establishment of pregnancy in sheep have been suggested because of high endometrial expression early in gestation (Days 9, 12, and 17) (Stenhouse et al. 2021a). Interestingly, the spatiotemporal profile of S100A9 protein in the ovine uterus varies significantly across gestation. S100A9 protein is localized to uterine LE, sGE, and GE on Days 9, 12, and 17 of gestation, suggesting a pivotal role of this molecule in the regulation of calcium trafficking at the maternal–conceptus interface during the peri-implantation period of pregnancy. In contrast, S100A9 protein is only detected in uterine GE on Days 30 and 90 of gestation, and uterine LE at Day 125 of gestation. This striking change in the localization of this protein across gestation in uteri of sheep suggests alterations in the functional importance of S100A9 during gestation that warrants further investigation. S100A11 may have a role in the regulation of endometrial receptivity to implantation of blastocysts and immunotolerance through regulation of epidermal growth factor-stimulated adhesion through its roles in regulating intracellular calcium uptake and release (Liu et al. 2012; Poeter et al. 2013). Knockdown of S100A11 in mice reduced the expression of genes associated with uterine receptivity to implantation and reduced rates of implantation of blastocysts (Liu et al. 2012). Additionally, S100A11 has been detected in uterine flushings from ewes on Days 14 and 16 of pregnancy, suggesting a potential role of S100A11 during the peri-implantation period of pregnancy (Romero et al. 2017). The results from studies with these animal models translate directly to those from humans in which S100A11 is a factor associated with pregnancy failure, and low endometrial expression of S100A11 is associated with adverse immune responses (Liu et al. 2012).
While S100 family members are known to regulate calcium flux, many of the S100 proteins are also mediators of inflammation (Donato et al. 2013; Xia et al. 2018). Available data suggest that the S100 family of calcium-binding proteins plays a critical role in early pregnancy when the uterine immune environment must be tightly regulated to ensure that anti-microbial protection occurs and the conceptus is not rejected by the maternal immune system (Hunt 2006). It could be speculated that in addition to ensuring that sufficient calcium is available for the rapidly dividing and differentiating conceptus tissues in early gestation, members of the S100 family play an important role in the establishment of immunotolerance at the maternal–conceptus interface.
5.7.5 Transient Receptor Potential Cation Channel (TRPV) Family
The TRPV family members, TRPV5 and TRPV6, have a critical role in the regulation of calcium transport by epithelial cells in mammals (Islam 2011). The TRPV channels, such as TRPV5 and TRPV6, have important functions in the regulation of calcium transport at the maternal–conceptus interface to regulate calcium transport in a manner similar to that for transport of calcium by intestinal epithelia. It is speculated that TRPV channels are important for the transfer of calcium in maternal blood to cells on the maternal side of the maternal–conceptus interface, and that Ca2+-ATPases are present on the basement membranes of cells on the fetal side of the maternal–conceptus interface that allow that calcium to enter the fetal circulation.
TRPV6 mRNA and protein are expressed by the murine yolk sac (Suzuki et al. 2008) with expression of TRPV6 increasing 14-fold in the placentae of mice during the last 4 days of gestation (Suzuki et al. 2008) to ensure that required calcium is available for mineralization of the fetal skeletal system. TRPV6 null mice have a 40% reduction in placental calcium transport, accompanied by severe hypocalcemia and a 50% decrease in weight of fetal skeletal ash, indicating that placental TRPV6 is critical for the regulation of placental transport of calcium (Suzuki et al. 2008). TRPV6 is highly expressed by the porcine uterus on Day 12 of pregnancy (Choi et al. 2009, 2012), and a role for TRPV channels in early pregnancy has also been suggested for sheep (Stenhouse et al. 2021a). Day 17 ovine conceptus tissue expresses TRPV5 mRNA, and there is also high expression of TRPV6 mRNA in Day 17 endometria and Day 30 placentae. Interestingly, endometria and placentae supplying twin fetuses have greater expression of TRPV6 mRNA compared with endometria from ewes with singleton pregnancies at Day 125 of gestation. Thus, TRPV6 may be critical in regulating the increase in calcium transport required to meet the increased mineral demands in twin pregnancies.
5.7.6 Sodium-Dependent Phosphate Transporters
Solute carrier family members (SLCs) have central roles in the regulation of solute transport across the plasma membrane, which is critical for the regulation of intracellular contents of metabolites and ions, cellular volume, and the removal of waste products from cells (Pizzagalli et al. 2020). Many SLC transporter family members exist, allowing for passive facilitative transport or secondary active transport of molecules such as sugars, amino acids, polyamines, vitamins, nucleotides, metals, and inorganic and organic ions (Pizzagalli et al. 2020). The SLC34 and SLC20 families of type II and type III sodium-dependent phosphate transporters play critical roles in bone, kidney, choroid plexus, and vascular physiology and pathophysiology (Lederer 2014; Segawa et al. 2015).
Interestingly, evidence suggests that members of the SLC34 and SLC20 families of type II and type III sodium-dependent phosphate transporters have a critical role in the regulation of growth and development of mammalian conceptuses. In vitro placental perfusion studies have provided interesting insights into the mechanisms of mineral transport in many species including humans, guinea pigs, rats, mice, and sheep [reviewed by Kovacs (2014)]. Manipulation of Na+ concentrations in these models demonstrated that placental phosphate transport is sodium-dependent (Husain and Mughal 1992; Stulc and Stulcova 1996). Given that sodium-dependent phosphate transporters are regulators of post-natal phosphate homeostasis, it could be hypothesized that they are regulators of phosphate transport at the maternal–fetal interface. SLC20A1 is expressed by STB cells of the human placenta (Yang et al. 2014) and defects in vascular remodeling of the yolk sac in SLC20A1-deficient mice is embryonic lethal (Wallingford and Giachelli 2014). Similarly, knockout of the type II sodium-dependent transporter SLC34A2 is embryonic lethal in mice (Shibasaki et al. 2009). SLC20A2 is localized to intravillous connective tissues in placentae of humans (Yang et al. 2014). A role for sodium-dependent phosphate transporters in the development of preeclampsia is based on evidence that a reduction in placental expression of SLC20A1 and SLC20A2 is associated with development of preeclampsia and, in late gestation, there is a significant increase in expression of SLC20A2 in preeclamptic pregnancies. SLC20A2 deficient mice are fertile, but some 25% of the mice have pregnancy complications including abnormal placental vascular remodeling, fetal growth restriction, and placental calcification and about 50% of the offspring of those mice do not survive until weaning (Wallingford et al. 2016).
A role for sodium-dependent phosphate transporters SLC20A1 and SLC20A2 in the regulation of phosphate transport at the maternal–conceptus interface in sheep has also been suggested (Stenhouse et al. 2021b). Both SLC20A1 and SLC20A2 mRNAs are expressed by ovine placentae and endometria throughout gestation. Interestingly, expression of SLC20A1 mRNA decreased in ovine endometria at Day 17 of gestation, corresponding to the period of conceptus attachment to uterine LE. There was a stable expression of SLC20A2 mRNA across gestation in both ovine endometria and placentae, highlighting a potentially crucial role for this transporter in the regulation of placental phosphate transport across gestation.
5.7.7 Plasma Membrane Ca2+-ATPases
Plasma membrane Ca2+-ATPases (PMCAs) are Ca2+ extrusion pumps that function to maintain intracellular concentrations of calcium within a tightly regulated range. There are four main isoforms of PMCA (PMCA1-4) expressed in adult tissues. PMCA expression doubles during the last week of gestation in rodents (Tuan and Bigioni 1990; Glazier et al. 1992). Similarly, PMCA 1–4 is expressed by the STB of human placentae and ATP-dependent Ca2+ transport increases linearly during the third trimester of pregnancy (Strid and Powell 2000). A critical role for Ca2+-ATPase activity in the regulation of placental transport of calcium was suggested based on results obtained following inhibition of Ca2+-ATPase activity in the rat placentae (Care 1991; Strid and Powell 2000). Homozygous PMCA1 knockout mice have an embryonic lethal phenotype prior to implantation of the blastocyst (Okunade et al. 2004; Prasad et al. 2007). In contrast, PMCA2 and PMCA4 null mice have mild phenotypes although further analyses should be performed to investigate effects on the development of the fetal skeletal system and on placental development and structure (Okunade et al. 2004; Prasad et al. 2007).
The PMCA1-4 mRNAs are expressed by the uterine endometrium of pigs during the estrous cycle and throughout gestation, in a pregnancy status and stage of pregnancy-specific manner (Choi et al. 2014b), suggesting a role of Ca2+-ATPases in the regulation of calcium ion abundance in uterine endometria. A role for plasma membrane Ca2+-ATPases in early pregnancy in sheep has also been suggested (Stenhouse et al. 2021a). Day 17 ovine conceptus tissue expresses PMCA4 mRNA which, in combination with high expression of PMCA3 and PMCA4 mRNA in endometria from Day 17 of pregnancy, and high expression of PMCA4 mRNA in placentae on Day 30 of pregnancy, suggests a role in the regulation of implantation and placental development. Collectively, these findings suggest a role for PMCAs in the regulation of calcium at the maternal–conceptus interface in pregnancy that warrants further investigation, particularly during the critical periods of implantation and placentation.
5.7.8 Stanniocalcin
The homodimeric phosphoglycoprotein stanniocalcin (STC) is a central regulator of calcium levels in fish (Wagner et al. 1986; Lu et al. 1994; Wagner 1994; Wagner and Jaworski 1994). STC decreases the influx of calcium from the aquatic environment through the gills and promotes absorption of phosphate in the kidney, while chelating excess calcium, and inhibiting calcium uptake in the intestine. STC1 and STC2 are mammalian orthologs of STC. STC1 has relatively high amino acid sequence identity (approximately 50%) to fish STC and is expressed in many tissues including brain, lung, and heart. While it is known that mammalian STC1 is a regulator of calcium and phosphate transport in the kidney and intestine, in part through the regulation of sodium-phosphate co-transporters, the role of STC2 remains poorly understood.
In addition to the role of STCs in the kidney and intestine, STC may have an important role in female reproduction. During pregnancy, the abundance of STC1 mRNA in the murine ovary increases substantially and STC1 protein is present in maternal blood during pregnancy despite not normally being found in blood (Deol et al. 2000). The localization of STC1 mRNA in the mouse uterus varies substantially depending upon pregnancy status and stage. In cyclic mice, the uterine LE expresses STC1 mRNA (Stasko et al. 2001). Following implantation, STC1 mRNA localizes to the mesometrial stromal cells bordering the uterine lumen until Days 6.5–8.5 when expression is in the mesometrial lateral sinusoids, after which time expression decreases. STC1 protein, however, is expressed by the uterine epithelia, stromal cells and decidual cells, as well as trophoblast giant cells. Similarly, STC1 and STC2 may have roles in the regulation of implantation and decidualization in the rat (Xiao et al. 2006). STC1 and STC2 mRNA expressions are induced in stromal cells at implantation sites on Day 6 of pregnancy, with additional expression in the uterine LE. Furthermore, STC2 protein is immunolocalized to uterine GE from Day 2 of gestation, with expression peaking on Day 6. STC1 mRNA and protein are expressed by decidualized cells from Days 7–9 of gestation. Interestingly, while STC1 mRNA is expressed by the whole decidua from Days 7–9 of gestation, STC2 mRNA is only expressed in the decidua in close proximity to the implantation site on Days 7 and 8, with weak staining throughout the entire decidua by Day 9.
A role for STC has also been suggested for the female reproductive tract of large animals. In the pig, STC1 mRNA is localized to uterine LE between Days 12 and 15 of the estrous cycle and, in pregnant pigs, it increases between Days 15 and 20 of gestation, suggesting that its expression may be regulated by P4 (Song et al. 2009). After Day 20, expression of STC1 mRNA in the uterus decreases to minimal expression on Day 25 and no expression at Day 30, suggesting that STC1 may be important for conceptus attachment and implantation. Expression of STC1 mRNA by the endometrial glands of the ovine uterus is evident from Day 18 of pregnancy and increased further to Day 80 of gestation (Song et al. 2006). STC1 protein is localized predominantly to the apical surface of uterine GE after Day 16 of pregnancy and in the placental areolae that form opposite the opening of uterine glands to transport histotroph across the placenta and into the fetal-placental blood. Ovine endometrial stroma and glands have low expression of STC2 mRNA in both cyclic and early pregnant uteri (Song et al. 2006). The abundance of STC2 mRNA significantly increases between Days 20 and 40 of gestation, with high expression in both placentomes and endometria until Day 120 of gestation. In late gestation, abundant expression of STC2 mRNA is present in uterine LE and GE, trophectoderm, and uterine caruncules.
In addition to demonstrating that STC1 and STC2 mRNAs and proteins are expressed by ovine uterine and placental tissues, Song et al. established that STC1 protein is present in ovine and porcine uterine luminal fluid (Song et al. 2006, 2009) and ovine allantoic fluid (Song et al. 2006). Thus, STC1 can be secreted by endometrial glands and enter allantoic fluid and the fetal circulation. Further investigations are required to ascertain the role of secreted STC1 in the pregnant uterus, but it may act as a regulator of conceptus growth and development.
Given the spatiotemporal profiles of STC1 expression across gestation, its expression may be hormonally regulated in the female reproductive tract. While interferon tau (IFNT; signal for maternal recognition of pregnancy in ruminants) does not appear to be a regulator of STC1 expression in the ovine uterus, treatment of ewes with P4 does induce expression of STC1 mRNA in the ovine endometria (Song et al. 2006). Intrauterine infusions of ovine placental lactogen increased the abundance of STC1 in progestinized ewes; demonstrating an important role of P4 and placental lactogen in the regulation of calcium transport by STC1 by the ovine uterus (Song et al. 2006). Furthermore, the expression of STC1 mRNA by uterine LE in pigs is stimulated by P4, and this expression is enhanced by estrogen (Song et al. 2009). Given the pattern of expression of STC1 in the uterine LE of pigs, with complete downregulation occurring after conceptus attachment and implantation, the downregulation in expression may be in response to the progestinized uterus being exposed to estrogen, making STC1 a useful marker of implantation in pigs. Furthermore, in that study, treatment of ewes with estradiol (E2) increased the expression of progesterone receptor (PGR) by uterine GE and this was associated with decreased expression of STC1, which could indicate that downregulation of PGR is critical for the expression of STC1 and other genes expressed by uterine GE.
Collectively, the spatiotemporal expression patterns suggest an important role for the calcium and phosphate regulator STC in the female reproductive tract. Despite differences in expression profiles for STC1 among species, it is evident that STC1 plays a role in both the establishment and maintenance of pregnancy in species with differing types of placentation to influence growth and development of the conceptus. The intriguing finding that STC1 and STC2 have opposing expression profiles in the ovine uterus and placentomes indicate that they may work together to regulate calcium and phosphate transport at the ovine maternal–conceptus interface in different ways. The concept warrants further investigation to improve our understanding of these molecules.
5.7.9 Sex Steroids
Sex steroids, specifically estradiol and testosterone, are critical for the regulation of postnatal skeletal development and bone turnover throughout adulthood, and therefore are considered calciotropic hormones. Placental-derived hCG and the fetal pituitary gonadotropins can stimulate sex steroid production by developing fetal ovaries and testes. Additionally, the fetal adrenals synthesize low amounts of sex steroids. During pregnancy, concentrations of both estradiol (Habert and Picon 1984) and progesterone (Ward and Weisz 1984) are similar between male and female fetuses in rats. In contrast, concentrations of testosterone in fetuses are at least four times greater for male than female fetuses in rats (Habert and Picon 1984). In addition, concentrations of testosterone are equal to (Houtsmuller et al. 1995) or higher than those in maternal blood in rats [reviewed in Kovacs (2014)]. Similar findings have been reported for sheep, with higher concentrations of testosterone in plasma of male than female fetuses. Interestingly, there is a dramatic decrease in concentrations of testosterone in fetal plasma immediately prior to parturition, and concentrations of testosterone remain greater than those in maternal plasma. Concentrations of progesterone are less in male and female sheep fetuses compared with much higher concentrations in maternal plasma until just prior to parturition when there is a dramatic decline in progesterone in maternal plasma (Strott et al. 1974). Concentrations of estradiol in fetal plasma are slightly greater than those in maternal plasma throughout most of pregnancy (Strott et al. 1974). Thus, circulating levels of sex steroids in fetal lambs exceed maternal concentrations at all gestational time points investigated [reviewed in Kovacs (2014)]. Finally, the testes in human fetuses produce testosterone in response to gonadotropins and hCG, whereas negligible amounts of testosterone are produced by the ovaries or adrenals in female fetuses (Reyes et al. 1973). Concentrations of estradiol in human fetuses are quite low and similar between males and females, and much lower than those in maternal plasma at term [reviewed in Kovacs (2014)].
The levels of sex steroids in the placenta can be quite high during pregnancy, with concentrations of testosterone, estradiol, or both in umbilical cord blood being equal to or greater than those in maternal plasma [reviewed in Kovacs (2014)]. This appears to result from aromatase enzymatic activity in the placenta converting maternal androgens into estrogens. In the third trimester of pregnancy in humans, the placenta is the source of nearly all of the estradiol, estrone, and estriol present in maternal blood (Menon and Sperling 1998).
Although estrogen and testosterone are considered calciotropic hormones because of their effects on development of the post-natal skeleton, evidence that they play a role in mineral transport and homeostasis during pregnancy is less clear. Studies of calcium and phosphate in serum of murine fetuses that lack sex steroids, or their receptors have not been performed, apart from describing them as being normal at birth. This lack of evidence for mineralization deficiencies suggests that development of fetal bone and mineral homeostasis in fetuses is less dependent upon sex steroids (Kovacs 2015). In the rat, it has been suggested that fluctuations in concentrations of Ca2+ may result from activities of different calcium transporters in uteri that respond differentially to progesterone and estradiol during the estrous cycle (Kim et al. 2006; Yang et al. 2011). Additionally, in the pig, it has been suggested that estrogen increases the release and or transport of calcium into the uterine lumen during the peri-implantation period of gestation (Geisert et al. 1982).
Estrogen is a known regulator of calcium transport in the intestine (Arjmandi et al. 1993) and kidney (Bronner 1989). Estrogen stimulates expression of renal CYP27B1 that increases calcitriol secretion by the kidneys in multiple animal models and humans (Reddy et al. 1983; Kovacs 2016). Estradiol also reduces serum FGF23 and increases PTH in blood, which also may contribute to increase in calcitriol (Saki et al. 2020), although the requirement for estrogen to stimulate the 2–3-fold increase in calcitriol in maternal blood during pregnancy has not been tested directly (Ryan and Kovacs 2021).
In addition to the indirect regulation of calcitriol by estrogen via CYP27B1 expression and decreases in FGF23 and PTH, estrogen may also indirectly regulate calcium transport during pregnancy via regulation of TRPV6, S100G, and vitamin D metabolism. Estrogen is a key regulator of expression of TRPV6 during pregnancy (Lee and Jeung 2007), and TRPV6 is critical for the regulation of placental transport of calcium (Suzuki et al. 2008). TRPV6 is highly expressed in the porcine uterus on Day 12 of pregnancy (Choi et al. 2009, 2012) and in uteri of pregnant sheep (Stenhouse et al. 2021a). Thus, taken together, current evidence supports an indirect role for sex steroids in the regulation of mineral homeostasis during pregnancy, primarily via upregulation of expression of calcitriol.
5.8 Summary and Conclusions
Appropriate transport of nutrients and minerals at the maternal–conceptus interface is essential not only for the establishment and maintenance of pregnancy but also the appropriate regulation of conceptus development and growth. Despite our understanding that phosphate, calcium, and vitamin D are essential for conceptus development and mineralization of the fetal skeletal system, little is known regarding mechanisms of transport and metabolism of these molecules at the maternal–conceptus interface. Current research defining the spatiotemporal expression of the multiple pathways for calcium and phosphate transport and vitamin D metabolism at the maternal–conceptus interface across gestation and species is vital to improving understanding of these processes. Furthermore, understanding how IFNT, E2, P4, and nutrients regulate phosphate and calcium transporters, and enzymes involved in vitamin D metabolism will further understanding of the roles of these molecules in the nutrition and physiology of reproduction under normal and pathological conditions.
Abbreviations
- 1,25(OH)2D3:
-
1,25 Dihydroxyvitamin D3
- ADAM:
-
A disintegrin and metalloprotease domain
- cAMP:
-
Cyclic adenosine monophosphate
- CTB:
-
Cytotrophoblasts
- CYPs:
-
Cytochrome P450 mixed-function oxidases
- E2:
-
Estradiol
- ECM:
-
Extracellular Matrix
- FGF:
-
Fibroblast growth factor
- FGFR:
-
Fibroblast growth factor receptor
- GE:
-
Glandular epithelium
- IFNT:
-
Interferon tau
- KL:
-
Klotho
- LE:
-
Luminal epithelium
- P21:
-
Cyclin-dependent kinase inhibitor
- P4:
-
Progesterone
- P53:
-
Tumor protein 53
- PGR:
-
Progesterone receptor
- PKC:
-
Protein kinase C
- PMCA:
-
Plasma membrane Ca2+ATPase
- PTH:
-
Parathyroid hormone
- PTHR:
-
Parathyroid hormone receptor
- PTHrP:
-
Parathyroid hormone-related protein
- S100:
-
S100 calcium binding proteins
- sFRP4:
-
Secreted frizzled-related protein 4
- sGE:
-
Superficial glandular epithelium
- SLC:
-
Solute carrier
- SLC20:
-
Solute carrier family 20
- SLC34:
-
Solute carrier family 34
- SPP1:
-
Secreted phosphoprotein 1
- STB:
-
Syncytiotrophoblast
- STC:
-
Stanniocalcin
- TRPV:
-
Transient receptor potential cation channel
- VDR:
-
Vitamin D receptor
- WNT:
-
Wingless-related integration site
References
Abbas SK, Care AD, Van Baelen H, Bouillon R (1987) Plasma vitamin D-binding protein and free 1,25-dihydroxyvitamin D3 index in pregnant ewes and their fetuses in the last month of gestation. J Endocrinol 115:7–12
Abbas S, Pickard D, Rodda C, Heath J, Hammonds R, Wood W, Caple I, Martin T, Care A (1989) Stimulation of ovine placental calcium transport by purified natural and recombinant parathryoid hormone-related protein (PTHrP) preparations. Q J Exp Physiol 74:549–552
Achkar M, Dodds L, Giguère Y, Forest JC, Armson BA, Woolcott C, Agellon S, Spencer A, Weiler HA (2015) Vitamin D status in early pregnancy and risk of preeclampsia. Am J Obstet Gynecol 212:e1–e7
Adams JS, Hewison M (2008) Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab 4:80–90
Aerssens J, Boonen S, Lowet G, Dequeker J (1998) Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone reseach. Endocrinology 139:663–670
Alimohamadi S, Esna-Ashari F, Rooy RSB (2020) Relationship of vitamin D serum level with intrauterine growth retardation in pregnant women. Int J Women’s Heal Reprod Sci 8:221–226
Allen WM, Sansom BF (1986) Parturient paresis (milk fever) and hypocalcemia (cows, ewes, and goats). In: Howard JL (ed) Current veterinary therapy: food animal practice, Philadelphia, vol 2, pp 311–320
Allgrove J, Adami S, Manning RM, O’Riordan JL (1985) Cytochemical bioassay of parathyroid hormone in maternal and cord blood. Arch Dis Child 60:110–115
An B, Choi K, Kang SK, Hwang WS, Jeung E (2003) Novel Calbindin-D 9k protein as a useful biomarker for environmental estrogenic compounds in the uterus of immature rats. Reprod Toxicol 17:311–319
Ardawi MSM, Nasrat HAN, Aqueel HSBA (1997) Calcium-regulating hormones and parathyroid hormone-related peptide in normal human pregnancy and postpartum: a longitudinal study. Eur J Endocrinol 137:402–409
Arjmandi BH, Salih MA, Herbert DC, Sims SH, Kalu DN (1993) Evidence for estrogen receptor-linked calcium transport in the intestine. Bone Miner 21:63–74
Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C (2010) Vitamin D: modulator of the immune system. Curr Opin Pharmacol 10:482–496
Baker JR, Jeffery R, May RD, Mathies M, Spencer-Dene B, Poulsom R, Hogg N (2011) Distinct roles for S100a8 in early embryo development and in the maternal deciduum. Dev Dyn 240:2194–2203
Banerjee A, Padh H, Nivsarkar M (2011) Hormonal crosstalk with calcium channel blocker during implantation. Syst Reprod Med 57:186–189
Barry JS, Anthony RV (2008) The pregnant sheep as a model for human pregnancy. Theriogenology 69:55–67
Bazer FW, Spencer TE, Thatcher WW (2012) Growth and development of the ovine conceptus. J Anim Sci 90:159–170
Belkacemi L, Gariépy G, Mounier C, Simoneau L, Lafond J (2004) Calbindin-D9k (CaBP9k) localization and levels of expression in trophoblast cells from human term placenta. Cell Tissue Res 315:107–117
Bergada L, Pallares J, Arcidiacono M, Cardus A, Santacan M, Valls J, Cao G, Fernandez E, Dolcet X, Dusso A, Matias-Guiu X (2014) Role of local bioactivation of vitamin D by CYP27A1 and CYP2R1 in the control of cell growth in normal endometrium and endometrial carcinoma. Lab Invest 94:608–622
Berndt T, Kumar R (2007) Phosphatonins and the regulation of phosphate homeostasis. Annu Rev Physiol 69:341–359
Bikle D (2014) Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 21:319–329
Boass A, Garner SC, Schultz VL, Toverud SU (1997) Regulation of serum calcitriol by serum ionized calcium in rats during pregnancy and lactation. J Bone Miner Res 12:909–914
Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM (2007) Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab 92:3517–3522
Bon N, Frangi G, Sourice S, Guicheux J, Beck-cormier S, Beck L (2018) Phosphate-dependent FGF23 secretion is modulated by PiT2/Slc20a2. Mol Metab 11:197–204
Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-hughes B, Lips P, Munns CF, Lazaretti-castro M, Giustina A, Bilezikian J (2019) Skeletal and extraskeletal actions of Vitamin D: current evidence and outstanding questions. Endocr Rev 40:1109–1151
Bourdeau A, Manganella G, Thil-Trubert CL, Sachs C, Cournot G (1990) Bioactive parathyroid hormone in pregnant rats and fetuses. Am J Physiol 258:E549–E554
Bronner F (1989) Renal calcium transport: mechanisms and regulation-an overview. Am J Physiol 257:F707–F711
Brostrom MA, Brostrom CO (2003) Calcium dynamics and endoplasmic reticular function in the regulation of protein synthesis: implications for cell growth and adaptability. Cell Calcium 34:345–363
Care AD (1991) The placental transfer of calcium. J Dev Physiol 15:253–257
Care AD, Caple IW, Singh R, Peddie M (1986) Studies on calcium homeostasis in the fetal Yucatan miniature pig. Lab Anim Sci 36:389–392
Carpenter TO, Ellis BK, Insogna KL, Philbrick WM, Sterpka J, Shimkets R (2005) Fibroblast growth factor 7: an inhibitor of phosphate transport derived from oncogenic osteomalacia-causing tumors. J Clin Endocrinol Metab 90:1012–1020
Carter AM (2007) Animal models of human placentation—a review. Placenta 28:S41–S47
Chang Q, Hoefs S, Van Der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG (2005) Cell signalling: the β-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310:490–493
Chen C, Spencer TE, Bazer FW (2000) Fibroblast growth factor-10: a stromal mediator of epithelial function in the ovine uterus. Biol Reprod 63:959–966
Chen C, Podvin S, Gillespie E, Leeman SE, Abraham CR (2007) Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA 104:19796–19801
Chen YH, Fu L, Hao JH, Yu Z, Zhu P, Wang H, Xu YY, Zhang C, Tao FB, Xu DX (2015) Maternal vitamin D deficiency during pregnancy elevates the risks of small for gestational age and low birth weight infants in Chinese population. J Clin Endocrinol Metab 100:1912–1919
Chhabra S, Singh A (2017) Role of calcium in hypertensive disorders of pregnancy current status of research a mini review. J Nutr Disord Ther 7:1–5
Chin KV, Cade C, Brostrom CO, Galuska EM, Brostrom MA (1987) Calcium-dependent regulation of protein synthesis at translational initiation in eukaryotic cells. J Biol Chem 262:16509–16514
Choi KC, Jeung EB (2008) Molecular mechanism of regulation of the calcium-binding protein calbindin-D9k, and its physiological role(s) in mammals: a review of current research: molecular medicine. J Cell Mol Med 12:409–420
Choi Y, Seo H, Kim M, Ka H (2009) Dynamic expression of calcium-regulatory molecules, TRPV6 and S100G, in the uterine endometrium during pregnancy in pigs. Biol Reprod 81:1122–1130
Choi Y, Seo H, Shim J, Kim M, Ka H (2012) Regulation of S100G expression in the uterine endometrium during early pregnancy in pigs. Asian-Australasian J Anim Sci 25:44–51
Choi Y, Seo H, Shim J, Hyun S-H, Lee E, Ka H (2014a) Klotho: expression and regulation at the maternal-conceptus interface in pigs. J Anim Reprod Biotechnol 29:375–383
Choi Y, Seo H, Shim J, Yoo I, Ka H (2014b) Calcium extrusion regulatory molecules: differential expression during pregnancy in the porcine uterus. Domest Anim Endocrinol 47:1–10
Choi Y, Jang H, Seo H, Yoo I, Han J, Kim M, Lee S, Ka H (2019) Changes in calcium levels in the endometrium throughout pregnancy and the role of calcium on endometrial gene expression at the time of conceptus implantation in pigs. Mol Reprod Dev 86:883–895
Collignon H, Davicco MJ, Barlet JP (1996) Calcitonin mRNA expression and plasma calciotropic hormones in fetal lambs. Domestric Anim Endocrinol 13:269–276
Comar CL (1956) Radiocalcium studies in pregnancy. Ann NY Acad Sci 64:281–298
Correia-Branco A, Rincon MP, Pereira LM, Wallingford MC (2020) Inorganic phosphate in the pathogenesis of pregnancy-related complications. Int J Mol Sci 21:1–11
Cross N, Hillman L, Allen S, Krause G, Vieira N (1995) Calcium homeostasis pregnancy, lactation, and bone metabolism during and postweaning: a longitudinal study. Am J Clin Nutr 61:514–523
Darwish HM, DeLuca HF (1992) Identification of a 1,25-dihydroxyvitamin D3-response element in the 5′-flanking region of the rat calbindin D-9k gene. Proc Natl Acad Sci USA 89:603–607
DeGaris PJ, Lean IJ (2009) Milk fever in dairy cows: a review of pathophysiology and control principles. Vet J 176:58–69
Delivoria-Papadopoulos M, Battaglia FC, Bruns PD, Meschia G (1967) Total, protein-bound, and ultrafilterable calcium in maternal and fetal plasmas. Am J Physiol 213:363–366
Deol HK, Varghese R, Wagner GF, Dimattia GE (2000) Dynamic regulation of mouse ovarian stanniocalcin expression during gestation and lactation. Endocrinology 141:3412–3421
Devaskar UP, Ho M, Devaskar SU, Tsang RC (1984) 25-hydroxy- and 1 alpha,25-dihydroxyvitamin D. Maternal-fetal relationship and the transfer of 1,25-dihydroxyvitamin D3 across the placenta in an ovine model. Dev Pharmacol Ther 7:213–220
Dias RS, Kebreab E, Vitti DMSS, Roque AP, Bueno ICS, France J (2006) A revised model for studying phosphorus and calcium kinetics in growing sheep. J Anim Sci 84:2787–2794
Díaz L, Noyola-martínez N, Barrera D, Hernández G, Avila E, Halhali A, Larrea F (2009) Calcitriol inhibits TNFa-induced inflammatory cytokines in human trophoblasts. J Reprod Immunol 81:17–24
Dolegowska K, Marchelek-mysliwiec M, Nowosiad-magda M, Slawinski M, Dolegowska B (2019) FGF19 subfamily members: FGF19 and FGF21. J Physiol Biochem 75:229–240
Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, Geczy CL (2013) Functions of S100 Proteins. Curr Mol Med 13:24–57
Dubil EA, Magann EF (2013) Amniotic fluid as a vital sign for fetal wellbeing. Australas J Ultrasound Med 16:62–70
Ducusin RJT, Uzuka Y, Satoh E, Otani M, Nishimura M, Tanabe S, Sarashina T (2003) Effects of extracellular Ca2+ on phagocytosis and intracellular Ca2+ concentrations in polymorphonuclear leukocytes of postpartum dairy cows. Res Vet Sci 75:27–32
Dwyer CM, Calvert SK, Farish M, Donbavand J, Pickup HE (2005) Breed, litter and parity effects on placental weight and placentome number, and consequences for the neonatal behaviour of the lamb. Theriogenology 63:1092–1110
Enders AC, Blankenship TN (1999) Comparative placental structure. Adv Drug Deliv Rev 38:3–15
Enders AC, Carter AM (2004) What can comparative studies of placental structure tell us?—a review. Placenta 25:S3–S9
Evans KN, Nguyen L, Chan J, Innes BA, Bulmer JN, Kilby MD, Hewison M (2006) Effects of 25-hydroxyvitamin D 3 and 1, 25-dihydroxyvitamin D 3 on cytokine production by human decidual cells. Biol Reprod 75:816–822
Fleischman AR, Lerman S, Oakes GK, Epstein MF, Chez RA, Mintz DH (1975) Perinatal primate parathyroid hormone metabolism. Biol Neonate 21:40–49
Fotiou M, Michaelidou AM, Athanasiadis AP, Menexes G, Symeonidou M, Koulourida V, Ganidou M, Theodoridis TD, Tarlatzis BC (2015) Second trimester amniotic fluid glucose, uric acid, phosphate, potassium, and sodium concentrations in relation to maternal pre-pregnancy BMI and birth weight centiles. J Matern Neonatal Med 28:910–915
Fudge NJ, Kovacs CS (2010) Pregnancy up-regulates intestinal calcium absorption and skeletal mineralization independently of the vitamin D receptor. Endocrinology 151:886–895
Fukino K, Suzuki T, Saito Y, Shindo T, Amaki T, Kurabayashi M, Nagai R (2002) Regulation of angiogenesis by the aging suppressor gene klotho. Biochem Biophys Res Commun 293:332–337
Gao H, Wu G, Spencer TE, Johnson GA, Li X, Bazer FW (2009) Select nutrients in the ovine uterine lumen. I. Amino acids, glucose, and ions in uterine lumenal flushings of cyclic and pregnant ewes. Biol Reprod 80:86–93
Garel JM (1972) Distribution of labeled parathyroid hormone in rat fetus. Horm Metab Res 4:131–132
Geisert R, Thatcher W, Roberts R, Bazer F (1982) Establishment of pregnancy in the pig: III. Endometrial secretory response to estradiol administered on day 11 of the estrous cycle. Biol Reprod 27:957–965
Gernand AD, Simhan HN, Caritis S, Bodnar LM (2014) Maternal vitamin D status and small-for-gestational-age offspring in women at high risk for preeclampsia. Obstet Gynecol 123:40–48
Gillies BR, Ryna BA, Tonkin BA, Poulton IJ, Ma Y, Kirby BJ, St-Arnaud R, Sims NA, Kovacs CS (2018) Absence of calcitrol causes increased lactational bone loss and lower milk calcium but does not impair post-lactation bone recovery in Cyp27b1 null mice. J Bone Miner Res 33:16–26
Givens MH, Macy IG (1933) Chemical composition of human fetus. J Biol Chem 102:7–17
Glancy B, Balaban RS (2012) Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry 51:2959–2973
Glazier JD, Sharpe PT, Atkinson DE, Thornburg KL, Edwards D, Boyd RDH, Sibley CP, Boyd RDH (1992) Gestational changes in Ca2+ transport across rat placenta and mRNA for calbinding K and Ca2+-ATPase. Am J Physiol 263:R930–R935
Grace ND, Watkinson JH, Martinson PL (1986) Accumulation of minerals by the foetus(es) and conceptus of single- and twin-bearing ewes. New Zeal J Agric Res 29:207–222
Grigsby PL (2016) Animal models to study placental development and function throughout normal and dysfunctional human pregnancy. Semin Reprod Med 34:11–16
Günther T, Chen ZF, Kim J, Priemel M, Rueger JM, Amling M, Moseley JM, Martin TJ, Anderson DJ, Karsenty G (2000) Genetic ablation of parathyroid glands reveals another source of parathyroid hormone. Nature 406:199–203
Habert R, Picon R (1984) Testosterone, dihydrotestosterone, and estradiol-17b levels in maternal and fetal plasma and in fetal testes in the rat. J Steroid Biochem 21:193–198
Haddad JGJ, Boisseau V, Avioli LV (1971) Placental transfer of vitamin D3 and 25-hydroxycholecalciferol in the rat. J Lab Clin Med 77:908–915
Haussler MR, Whitfield GK, Kaneko I, Forster R, Saini R, Hsieh C, Haussler CA, Jurutka PW (2013) The role of Vitamin D in the FGF23, Klotho, and phosphate bone-kidney endocrine axis. Rev Endocr Metab Disord 13:57–69
Heaney RP, Skillman TG (1971) Calcium metabolism in normal human pregnancy. J Clin Endocrinol 33:661–670
Hermann A, Donato R, Weiger TM, Chazin WJ (2012) S100 calcium binding proteins and ion channels. Front Pharmacol 3:1–10
Hernando N, Wagner CA (2018) Mechanisms and regulation of intestinal phosphate absorption. Compr Physiol 8:1065–1090
Hindson J, Winter A (2002) Appendix 3 nutrition. In: Manual of sheep diseases. Wiley-Blackwell Publisher, Hoboken, New Jersey, 304 pp.
Houtsmuller EJ, De Jong FH, Rowland DL, Slob AK (1995) Plasma testosterone in fetal rats and their mothers on day 19 of gestation. Physiol Behav 57:495–499
Hu MC, Shi M, Moe OW (2019) Role of αKlotho and FGF23 in regulation of type II Na-dependent phosphate co-transporters. Pflugers Arch Eur J Physiol 471:99–108
Hunt JS (2006) Stranger in a strange land. Immunol Rev 213:36–47
Husain S, Mughal M (1992) Mineral transport across the placenta. Arch Dis Child 67:874–878
Iñiguez G, Gallardo P, Castro JJ, Gonzalez R, Garcia M, Kakarieka E, San Martin S, Johnson MC, Mericq V, Cassorla F (2019) Klotho gene and protein in human placentas according to birth weight and gestational age. Front Endocrinol (lausanne) 9:1–8
Inpanbutr N, Miller EK, Petroff BK, Iacopino AM (1994) CaBP(9K) levels during the luteal and follicular phases of the estrous cycle in the bovine uterus. Biol Reprod 50:561–571
Ioannou C, Javaid MK, Mahon P, Yaqub MK, Harvey NC, Godfrey KM, Noble JA, Cooper C, Papageorghiou AT (2012) The effect of maternal vitamin D concentration on fetal bone. J Clin Endocrinol Metab 97:2070–2077
Islam MS (ed) (2011) Transient receptor potential channels. Adv Exp Med Biol 704:1–1095
Jang H, Choi Y, Yoo I, Han J, Hong JS, Kim YY, Ka H (2017) Vitamin D-metabolic enzymes and related molecules: expression at the maternal-conceptus interface and the role of vitamin D in endometrial gene expression in pigs. PLoS ONE 12:1–20
Jeon US (2008) Kidney and calcium homeostasis. Electrolyte Blood Press 6:68–76
Jones G, Prosser DE, Kaufmann M (2014) Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res 55:13–31
Karras SN, Fakhoury H, Muscogiuri G, Grant WB, van den Ouweland JM, Colao AM, Kotsa K (2016) Maternal vitamin D levels during pregnancy and neonatal health: evidence to date and clinical implications. Ther Adv Musculoskelet Dis 8:124–135
Kavsak P (2017) Textbook of clinical chemistry and molecular diagnostics, 6th ed. Elsevier, NewYork
Kent G, Price R, Gutteridge D, Rosman K, Smith M, Allen J, Hickling C, Blakeman S (1991) The efficiency of intestinal calcium absorption is increased in late pregnancy but not in established lactation. Calcif Tissue Int Tissue 48:293–295
Khosla S, Johansen KL, Ory SJ, O’Brien PC, Kao PC (1990) Parathyroid hormone-related peptide in lactation and in umbilical cord blood. Mayo Clin Proc 65:1407–1414
Kim HJ, Lee GS, Ji YK, Choi KC, Jeung EB (2006) Differential expression of uterine calcium transporter 1 and plasma membrane Ca2+ ATPase 1b during rat estrous cycle. Am J Physiol 291:234–241
Kirby BJ, Ma Y, Martin HM, Favaro KLB, Karaplis AC, Kovacs CS (2013) Upregulation of calcitriol during pregnancy and skeletal recovery after lactation do not require parathyroid hormone. J Bone Miner Res 28:1987–2000
Kovacs CS (2014) Bone development and mineral homeostasis in the fetus and neonate: roles of the calciotropic and phosphotropic hormones. Physiol Rev 94:1143–1218
Kovacs CS (2015) Early human development calcium, phosphorus, and bone metabolism in the fetus and newborn. Early Hum Dev 91:623–628
Kovacs CS (2016) Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiol Rev 96:449–547
Kovacs CS, Ralston SH (2015) Presentation and management of osteoporosis presenting in association with pregnancy or lactation. Osteoporos Int 26:2223–2241
Kovacs CS, Lanske B, Hunzelman JL, Guo J, Karaplis AC, Kronenberg HM (1996) Parathyroid hormone-related peptide (PTHrP) regulates fetal-placental calcium transport through a receptor distinct from the PTH/PTHrP receptor. Proc Natl Acad Sci USA 93:15233–15238
Kovacs CS, Chafe LL, Fudge NJ, Friel JK, Manley NR (2001a) PTH regulates fetal blood calcium and skeletal mineralization independently of PTHrP. Endocrinology 142:4983–4993
Kovacs CS, Manley NR, Moseley JM, John Martin T, Kronenberg HM (2001b) Fetal parathyroids are not required to maintain placental calcium transport. J Clin Invest 107:1007–1015
Kovacs CS, Woodland ML, Fudge NJ, Friel JK, Christopher S, Woodland ML, Fudge NJ (2005) The vitamin D receptor is not required for fetal mineral homeostasis or for the regulation of placental calcium transfer in mice. Am J Physiol 289:E133–E144
Kubota M, Ohno J, Shiina Y, Suda T (1982) Vitamin D metabolism in pregnant rabbits: differences between the maternal and fetal response to administration of large amounts of vitamin D3. Endocrinology 110:1950–1956
Kusaba T, Okigaki M, Matui A, Murakami M, Ishikawa K, Kimuraa T, Sonomura K, Adachi Y, Shibuya M, Shirayama T, Tanda S, Hatta T, Sasaki S, Mori Y, Matsubara H (2010) Klotho is associated with VEGF receptor-2 and the transient receptor potential canonical-1 Ca2+ channel to maintain endothelial integrity. Proc Natl Acad Sci USA 107:19308–19313
Lachenmaier-Currle U, Harmeyer J (1989) Placental transport of calcium and phosphorus in pigs. J Perinat Med 17:127–136
Lachenmaier-Currle U, Breves G, Harmeyer J (1989) Role of 1,25-(OH)2D3 during pregnancy: studies with pigs suffering from pseudo-vitamin D-deficiency rickets, type 1. Q J Exp Physiol 74:875–881
Lean IJ, Degaris PJ, Mcneil DM, Block E (2006) Hypocalcemia in dairy cows: meta-analysis and dietary cation anion difference theory revisited. J Dairy Sci 89:669–684
Lederer E (2014) Renal phosphate transporters. Curr Opin Nephrol Hypertens 23:502–506
Lee GS, Jeung EB (2007) Uterine TRPV6 expression during the estrous cycle and pregnancy in a mouse model. Am J Physiol 293:132–138
Leiser R, Krebs C, Ebert B, Dantzer V (1997) Placental vascular corrosion cast studies: a comparison between ruminants and humans. Microsc Res Tech 38:76–87
Lester GE, Gray TK, Lorenc RS (1978) Evidence for maternal and fetal differences in vitamin D metabolism. Proc Soc Exp Biol Med 159:303–307
Li XY, Zheng SX, Wu G (2020) Amino acid metabolism in the kidneys: nutritional and physiological significance. Adv Exp Med Biol 1265:71–95
Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL (2012) Vascular klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation 125:2243–2255
Lima MS, Kallfelz F, Krook L, Nathanielsz PW (1993) Humeral skeletal development and plasma constituent changes in fetuses of ewes maintained on a low calcium diet from 60 days of gestation. Calcif Tissue Int 52:283–290
Liu N, Kaplan AT, Low J, Nguyen L, Liu GY, Equils O, Hewison M (2009) Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biol Reprod 80:398–406
Liu NQ, Kaplan AT, Lagishetty V, Yuxin B, Ouyang Y, Simmons CF, Equils O, Hewison M (2011) Vitamin D and the regulation of placental inflammation. J Immunol 186:5974–5986
Liu XM, Ding GL, Jiang Y, Pan HJ, Zhang D, Wang TT, Zhang RJ, Shu J, Sheng JZ, Huang HF (2012) Down-regulation of S100A11, a calcium-binding protein, in human endometrium may cause reproductive failure. J Clin Endocrinol Metab 97:3672–3683
Loichinger M, Towner D, Thompson K, Jun Ahn H, Bryant-Greenwood G (2016) Systemic and placental α-klotho: effects of preeclampsia in the last trimester of gestation. Placenta 41:53–61
Loveridge N, Caple IW, Rodda C, Martin TJ, Care AD (1988) Further evidence for a parathryoid hormone-related protein in fetal parathyroid glands of sheep. Arthritis Rheum 73:781–784
Lu M, Wagner GF, Renfro JL (1994) Stanniocalcin stimulates phosphate reabsorption by flounder renal proximal tubule in primary culture. Am J Physiol 267:R1356–R1362
Lyakhovich A, Aksenov N, Pennanen P, Miettinen S, Ahonen MH, Syvälä H, Ylikomi T, Tuohimaa P (2000) Vitamin D induced up-regulation of keratinocyte growth factor (FGF-7/KGF) in MCF-7 human breast cancer cells. Biochem Biophys Res Commun 273:675–680
Ma R, Gu Y, Zhao S, Sun J, Groome LJ, Wang Y (2012) Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am J Physiol 303:E928–E935
Ma Y, Samaraweera M, Cooke-hubley S, Kirby BJ, Karaplis AC, Lanske B, Kovacs CS (2014) Neither absence nor excess of FGF23 disturbs murine fetal-placental phosphorus homeostasis or prenatal. Endocrinology 155:1596–1605
MacIsaac RJ, Heath JA, Rodda CP, Moseley JM, Care AD, Martin TJ, Caple IW (1991) Role of the fetal parathyroid glands and parathyroid hormone-related protein in the regulation of placental transport of calcium, magnesium and inorganic phosphate. Reprod Fertil Dev 3:447–457
Mahon P, Harvey N, Crozier S, Inskip H, Robinson S, Arden N, Swaminathan R, Cooper C, Godfrey K (2010) Low maternal vitamin D status and fetal bone development: cohort study. J Bone Miner Res 25:14–19
Mayer GP, Ramberg CFJ, Kronfeld DS, Buckle RM, Sherwood LM, Aurbach GD, Potts JTJ (1969) Plasma parathyroid hormone concentration in hypocalcemic parturient cows. Am J Vet Res 30:1587–1597
Menon RK, Sperling MA (1998) Developmental endocrinology in the fetal-placental unit. In: Cowett RM (ed) Principles of perinatal-neonatal metabolism, 2nd edn. Springer, New York, pp 425–436
Miranda J, Romero R, Korzeniewski SJ, Schwartz AG, Chaemsaithong P, Stampalija T, Yeo L, Dong Z, Hassan SS, Chrousos GP, Gold P, Chaiworapongsa T (2014) The anti-aging factor α-klotho during human pregnancy and its expression in pregnancies complicated by small-for-gestational-age neonates and/or preeclampsia. J Matern Neonatal Med 27:449–457
Mitchell H, Hamilton T, Steggerda F, Bean H (1945) The chemical composition of the adult human body and its bearing on the biochemistry of growth. J Biol Chem 168:625–637
Montiel JF, Kaune H, Maliqueo M (2013) Maternal-fetal unit interactions and eutherian neocortical development and evolution. Front Neuroanat 7:1–14
Nabeshima YI, Imura H (2008) a-Klotho: a regulator that integrates calcium homeostasis. Am J Nephrol 28:455–464
Nair RR, Khanna A, Singh K (2013) Role of inflammatory proteins S100A8 and S100A9 in pathophysiology of recurrent early pregnancy loss. Placenta 34:824–827
Nie GY, Li Y, Wang J, Minoura H, Findlay JK, Salamonsen LA (2000) Complex regulation of calcium-binding protein D9k (calbindin-D(9k)) in the mouse uterus during early pregnancy and at the site of embryo implantation. Biol Reprod 62:27–36
Noff D, Edelstein S (1978) Vitamin D and its hydroxylated metabolites in the rat. Placental and lacteal transport, subsequent metabolic pathways and tissue distribution. Horm Res 9:292–300
Northrop G, Misenhimer HR, Becker FO (1977) Failure of parathyroid hormone to cross the nonhuman primate placenta. Am J Obstet Gynecol 129:449–453
O’Brien KO, Li S, Cao C, Kent T, Young BV, Queenan RA, Pressman EK, Cooper EM (2014) Placental CYP27B1 and CYP24A1 expression in human placental tissue and their association with maternal and neonatal calcitropic hormones. Endocr Res 99:1348–1356
Ohata Y, Arahori H, Namba N, Kitaoka T, Hirai H, Wada K, Nakayama M, Michigami T, Imura A, Nabeshima YI, Yamazaki Y, Ozono K (2011) Circulating levels of soluble α-klotho are markedly elevated in human umbilical cord blood. J Clin Endocrinol Metab 96:943–947
Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O’Connor KT, Neumann JC, Andringa A, Miller DA, Prasad V, Doetschman T, Paul RJ, Shull GE (2004) Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem 279:33742–33750
Passey RJ, Williams E, Lichanska AM, Wells C, Hu S, Geczy CL, Little MH, Hume DA (1999) A null mutation in the inflammation-associated S100 protein S100A8 causes early resorption of the mouse embryo. J Immunol 163:2209–2216
Paulson SK, DeLuca HF, Battaglia F (1987) Plasma levels of vitamin D metabolites in fetal and pregnant ewes. Proc Soc Exp Biol Med 185:267–271
Paulson SK, Ford KK, Langman CB (1990) Pregnancy does not alter the metabolic clearance of 1,25-dihydroxyvitamin D in rats. Am J Physiol 258:E158–E162
Peacock M (2018) Hypoparathyroidism and the kidney. Endocrinol Metab Clin North Am 47:839–853
Peacock M (2021) Phosphate metabolism in health and disease. Calcif Tissue Int 108:3–15
Penido MGMG, Alon US (2012) Phosphate homeostasis and its role in bone health. Pediatr Nephrol 27:2039–2048
Pike JW, Parker JB, Haussler MR, Boass A, Toverud SU (1979) Dynamic changes in circulating 1,25-dihydroxyvitamin D during reproduction in rats. Science 204:1427–1429
Pizzagalli MD, Bensimon A, Superti-Furga G (2020) A guide to plasma membrane solute carrier proteins. FEBS J 1–52
Poeter M, Radke S, Koese M, Hessner F, Hegemann A, Musiol A, Gerke V, Grewal T, Rescher U (2013) Disruption of the annexin A1/S100A11 complex increases the migration and clonogenic growth by dysregulating epithelial growth factor (EGF) signaling. Biochim Biophys Acta 1833:1700–1711
Prasad V, Okunade G, Liu L, Paul RJ, Shull GE (2007) Distinct phenotypes among plasma membrane Ca2+-ATPase knockout mice. Ann NY Acad Sci 1099:276–286
Qin LL, Lu FG, Yang SH, Xu HL, Luo BA (2016) Does maternal Vitamin D deficiency increase the risk of preterm birth: a meta-analysis of observational studies. Nutrients 8:1–10
Quarles LD (2008) Endocrine functions of bone in mineral metabolism regulation. J Clin Invest 118:3820–3828
Reddy GS, Willis DM, Goltzman D, Guyda H, Solomon S, Philips DR, Bishop JE, Mayer E (1983) Regulation of vitamin D metabolism in normal human pregnancy. J Clin Endocrinol Metab 56:363–370
Reyes FI, Winter JSD, Faiman C (1973) Studies on human sexual development. I. Fetal gonadal and adrenal sex steroids. J Clin Endocrinol Metab 37:74–78
Risco C, Drost M, Thatcher WW, Savio J, Thatcher MJ (1994) Effects of calving-related disorders on prostaglandin, calcium, ovarian activity and uterine involution in postpartum dairy cows. Theriogenology 42:183–203
Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Van LMD, Cann CE, King JC (1998) A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr 67:693–701
Rodda CP, Kubota M, Heath JA, Ebeling PR, Moseley JM, Care AD, Caple IW, Martin TJ (1988) Evidence for a novel parathyroid hormone-related protein in fetal lamb parathyroid glands and sheep placenta: comparisons with a similar protein implicated in humoral hypercalcaemia of malignancy. J Endocrinol 117:261–271
Romero JJ, Liebig BE, Broeckling CD, Prenni JE, Hansen TR (2017) Pregnancy-induced changes in metabolome and proteome in ovine uterine flushings. Biol Reprod 97:273–287
Ross R, Halbert K, Tsang RC (1989) Determination of the production and metabolic clearance rates of 1,25-dihydroxyvitamin D3 in the pregnant sheep and its chronically catheterized fetus by primed infusion technique. Pediatr Res 26:633–634
Ryan BA, Kovacs CS (2021) Maternal and fetal vitamin D and their roles in mineral homeostasis and fetal bone development. J Endocrinol Invest 44:643–659
Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, Matsumura Y, Masuda H, Kurabayashi M, Kuro-O M, Nabeshima YI, Nagai R (1998) Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun 248:324–329
Saki F, Sadeghian F, Kasaee SR, Koohpeyma F, Omrani GHR (2020) Effect of prolactin and estrogen on the serum level of 1,25-dihydroxy vitamin D and FGF23 in female rats. Arch Gynecol Obstet 302:265–271
Santella L (1998) The role of calcium in the cell cycle: facts and hypotheses. Biochem Biophys Res Commun 244:317–324
Schmid A, Walther B (2013) Natural vitamin D content in animal products. Adv Nutr 4:453–462
Segawa H, Shiozaki Y, Kaneko I, Miyamoto KI (2015) The role of sodium-dependent phosphate transporter in phosphate homeostasis. J Nutr Sci Vitaminol (tokyo) 61:S119–S121
Seki K, Wada S, Nagata N, Nagata I (1994) Parathyroid hormone-related protein during pregnancy and the perinatal period. Gynecol Obstet Invest 37:83–86
Shahbazi M, Jeddi-tehrani M, Zareie M, Salek-moghaddam A, Akhondi MM, Bahmanpoor M, Sadeghi MR, Zarnani AH (2011) Expression profiling of vitamin D receptor in placenta, decidua and ovary of pregnant mice. Placenta 32:657–664
Shaikh A, Berndt T, Kumar R (2008) Regulation of phosphate homeostasis by the phosphatonins and other novel mediators. Pediatr Nephrol 23:1203–1210
Shibasaki Y, Etoh N, Hayasaka M, Takahashi MO, Kakitani M, Yamashita T, Tomizuka K, Hanaoka K (2009) Targeted deletion of the tybe IIb Na+-dependent Pi-co-transporter, NaPi-IIb, results in early embryonic lethality. Biochem Biophys Res Commun 381:482–486
Simmonds CS, Kovacs CS (2010) Role of parathyroid hormone (PTH) and PTH-related protein (PTHrP) in regulating mineral homeostasis during fetal development. Crit Rev Eukaryot Gene Expr 20:235–273
Simmonds CS, Karsenty G, Karaplis AC, Kovacs CS (2010) Parathyroid hormone regulates fetal-placental mineral homeostasis. J Bone Miner Res 25:594–605
Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, Harald J, Lanske B (2004) Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex- deficient mice. Matrix Biol 421–432
Song G, Bazer FW, Wagner GF, Spencer TE (2006) Stanniocalcin (STC) in the endometrial glands of the ovine uterus: regulation by progesterone and placental hormones 1. Biol Reprod 74:913–922
Song G, Dunlap KA, Kim J, Bailey DW, Spencer TE, Burghardt RC, Wagner GF, Johnson GA, Bazer FW (2009) Stanniocalcin 1. Is a luminal epithelial marker for implantation in pigs regulated by progesterone and estradiol. Endocrinology 150:936–945
Stasko SE, DiMattia GE, Wagner GF (2001) Dynamic changes in stanniocalcin gene expression in the mouse uterus during early implantation. Mol Cell Endocrinol 174:145–149
Stenhouse C, Halloran KM, Newton MG, Gaddy D, Suva LJ, Bazer FW (2021a) Novel mineral regulatory pathways in ovine pregnancy: II. Calcium binding proteins, calcium transporters, and vitamin D signaling. Biol Reprod 105:232–243
Stenhouse C, Halloran KM, Newton MG, Gaddy D, Suva LJ, Bazer FW (2021b) Novel mineral regulatory pathways in ovine pregnancy: 1. Phosphate, klotho signaling, and sodium dependent phosphate transporters. Biol Reprod 104:1084–1096
Strid H, Powell TL (2000) ATP-dependent Ca2+ transport is up-regulated during third trimester in human syncytiotrophoblast basal membranes. Pediatr Res 48:58–63
Strott CA, Sundel H, Stahlman MT (1974) Maternal and fetal plasma progesterone, cortisol, testosterone and 17b-estradiol in preparturient sheep: response to fetal ACTH infusion. Endocrinology 95:1327–1339
Stulc J, Stulcova B (1996) Placental transfer of phosphate in anaesthetized rats. Placenta 17:487–493
Suva LJ, Winslow GA, Wettenhall RE, Hammonds RG, Moseley JM, Diefenbach-Jagger H, Rodda CP, Kemp BE, Rodriguez H, Chen EY (1987) A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. Science 237:893–896
Suva LJ, Gaddy D, Perrien DS, Thomas RL, Findlay DM (2005) Regulation of bone mass by mechanical loading: microarchitecture and genetics. Curr Osteoporos Rep 3:46–51
Suva L, Friedman P (2020) PTH and PTHrP actions on bone. In: Handbook of experimental pharmacology, vol 262. Springer, Berlin, pp 27–45
Suzuki Y, Kovacs CS, Takanaga H, Peng J, Landowski CP, Hediger MA (2008) Calcium channel TRPV6 is involved in murine maternal-fetal calcium transport. J Bone Miner Res 23:1249–1256
Swanson AM, David AL (2015) Animal models of fetal growth restriction: considerations for translational medicine. Placenta 36:623–630
Tabatabaei S (2012) Gestational variations in the biochemical composition of the fetal fluids and maternal blood serum in goat. Comp Clin Path 21:1305–1312
Tamblyn JA, Hewison M, Wagner CL, Bulmer JN, Kilby MD (2015) Immunological role of vitamin D at the maternal—fetal interface. J Endocrinol 224:R107–R121
Taylor-Miller T, Allgrove J (2021) Endocrine diseases of newborn: epidemiology, pathogenesis, therapeutic options, and outcome “Current Insights Into Disorders of Calcium and Phosphate in the Newborn.” Front Pediatr 9:1–8
Thomas ML, Anast CS, Forte LR (1981) Regulation of calcium homeostasis in the fetal and neonatal rat. Am J Physiol 240:E367–E372
Tuan RS, Bigioni N (1990) Ca-activated ATPase of the mouse chorioailantoic placenta: developmental expression, characterization and cytohistochemical localization. Development 110:505–513
Tucci J, Russell A, Senior PV, Fernley R, Ferraro T, Beck F (1996) The expression of parathyroid hormone and parathyroid hormone-related protein in developing rat parathyroid glands. J Mol Endocrinol 17:149–157
Verhaeghe J, Thomasset M, Brehier A, Assche FAV, Bouillon R (1988) 1,25(OH)2D3 and Ca-binding protein in fetal rats: relationship to maternal vitamin D status. Am J Physiol 254:E505–E512
Voelkl J, Egli-Spichtig D, Alesutan I, Wagner CA (2021) Inflammation: a putative link between phosphate metabolism and cardiovascular disease. Clin Sci 135:201–227
Wagner GF (1994) The molecular biology of the corpuscles of stannius and regulation of stanniocalcin gene expression. Fish Physiol 13:273–306
Wagner GF, Jaworski E (1994) Calcium regulates stanniocalcin mRNA levels in primary cultured rainbow trout corpuscles of Stannius. Mol Cell Endocrinol 99:315–322
Wagner GF, Hampong M, Park CM, Copp DH (1986) Purification, characterization, and bioassay of teleocalcin, glycoprotein from salmon corpuscles of stannius. Gen Comp Endocrinol 63:481–491
Wallingford MC, Giachelli CM (2014) Loss of PiT-1 results in abnormal endocytosis in the yolk sac visceral endoderm. Mech Dev 133:189–202
Wallingford M, Gammill H, Giachelli C (2016) SLC20A2 deficiency results in fetal growth restriction and placental calcification associated with thickened basement membranes and novel CD13 and lamininα1 expressing cells. Reprod Biol 16:13–26
Wang Y, Sun Z (2009) Current understanding of klotho. Ageing Res Rev 8:43–51
Ward IL, Weisz J (1984) Differential effects of maternal stress of circulating levels of corticosterone, progesterone, and testosterone in male and female rat fetuses and their mothers. Endocrinology 114:1635–1644
Widdowson EM (1962) Metabolic relationship of calcium, magnesium and phosphorus in the foetus and newly born. Voeding 23:62–71
Widdowson EM, McCance RA (1965) The metabolism of calcium, phosphorus, magnesium and strontium. Pediatr Clin North Am 12:595–614
Wilson RL, Phillips JA, Bianco-Miotto T, McAninch D, Goh Z, Anderson PH, Roberts CT (2020) Reduced dietary calcium and vitamin D results in preterm birth and altered placental morphogenesis in mice during pregnancy. Reprod Sci 27:1330–1339
Wu G (2018) Principles of animal nutrition. CRC Press, Boca Raton, FL
Wu G (2021) Amino acids: biochemistry and nutrition. CRC Press, Boca Raton, FL
Wu G (2022) Nutrition and metabolism: Foundations for animal growth, development, reproduction, and health. Adv Exp Med Biol 1354:1–24
Xia C, Braunstein Z, Toomey AC, Zhong J, Rao X (2018) S100 proteins as an important regulator of macrophage inflammation. Front Immunol 8:1–11
Xiao L, Yuan J, Song X, Li Y, Hu Z, Liu Y (2006) Expression and regulation of stanniocalcin 1 and 2 in rat uterus during embryo implantation and decidualization. Reproduction 131:1137–1149
Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-O M (2005) Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 280:38029–38034
Yang H, Choi KC, Hyun SH, Jeung EB (2011) Coexpression and estrogen-mediated regulation of TRPV6 and PMCA1 in the human endometrium during the menstrual cycle. Mol Reprod Dev 78:274–282
Yang H, Kim TH, Lee GS, Hong EJ, Jeung EB (2014) Comparing the expression patterns of placental magnesium/phosphorus-transporting channels between healthy and preeclamptic pregnancies. Mol Reprod Dev 81:851–860
Yoshida T, Fujimori T, Nabeshima Y (2002) Mediation of unusually high concentrations of 1,25-Dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1a-hydroxylase gene. Endocrinology 143:683–689
Young BE, McNanley TJ, Cooper EM, McIntyre AW, Witter F, Harris ZL, O’Brien KO (2012) Maternal vitamin D status and calcium intake interact to affect fetal skeletal growth in utero in pregnant adolescents. Am J Clin Nutr 95:1103–1112
Zeng S, Bick J, Ulbrich SE, Bauersachs S (2018) Cell type-specific analysis of transcriptome changes in the porcine endometrium on day 12 of pregnancy. BMC Genomics 19:1–19
Zeng S, Ulbrich SE, Bauersachs S (2019) Spatial organization of endometrial gene expression at the onset of embryo attachment in pigs. BMC Genomics 20:1–19
Zhang R, Lu Y, Ye L, Yuan B, Yu S, Qin C, Xie Y, Gao T, Drezner MK, Bonewald LF, Feng JQ (2011) Unique roles of phosphorus in endochondral bone formation and osteocyte maturation. J Bone Miner Res 26:1047–1056
Zhou SS, Tao YH, Huang K, Zhu BB, Tao FB (2017) Vitamin D and risk of preterm birth: up-to-date meta-analysis of randomized controlled trials and observational studies. J Obstet Gynaecol Res 43:247–256
Ziegler E, O’Donnell A, Nelson S, Fomon S (1976) Body composition of the reference fetus. Growth 40:329–341
Acknowledgements
This work was supported, in part, by Texas A&M University Presidential Impact Fellow Funds (to FWB) and funds from Texas A&M AgriLife Research (to FWB and GW), and the National Institutes of Health # 1R21DE028076-01 (to DG).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors have no conflicts of interest to declare.
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Stenhouse, C., Suva, L.J., Gaddy, D., Wu, G., Bazer, F.W. (2022). Phosphate, Calcium, and Vitamin D: Key Regulators of Fetal and Placental Development in Mammals. In: Wu, G. (eds) Recent Advances in Animal Nutrition and Metabolism. Advances in Experimental Medicine and Biology, vol 1354. Springer, Cham. https://doi.org/10.1007/978-3-030-85686-1_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-85686-1_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-85685-4
Online ISBN: 978-3-030-85686-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)