Abstract
A category of new chemicals, phthalic acid esters (PAEs), are used primarily as plastic additives that can be easily leached out of plastics and released into the environment and can create risks when exposed by the humans and other living organisms. The distribution and ecotoxicological risk assessments based on risk quotient (RQ) of six phthalic acid esters (PAEs) including dimethyl phthalate, diethyl phthalate, dibutyl phthalate, benzyl butyl phthalate, bis(2-ethylhexyl) phthalate, and di-n-octyl phthalate in surface water and sediment collected from the Kaveri, Vellar, and Tamiraparani Rivers, Tamil Nadu, India. As far our knowledge is concerned, this is the first comprehensive study that examines phthalate contamination seasonally in these three rivers. This chapter confirms ubiquitous phthalate contamination of fresh water ecosystems in India, and the data contribute for better understanding and managing the pollution from emerging contaminants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Phthalates are a category of chemicals commonly used for softening and improving plastic products’ strength and toughness, mostly polyvinyl chloride (PVC). They are also utilized as dispersants, gelling and emulsifying agents, stabilizers, lubricants, binders, and so forth in numerous user items such as personal care products and cosmetics, plastic and vinyl supplies, vinyl flooring, food packaging and wraps, cleansers, adhesives, plumbing accessories, and medical equipment (NIH, 2019).
Phthalates were initially used in the 1920s, which greatly improved production with the advent of PVC in the 1930s. PVC items may have up to 50% of plasticizers, usually phthalates, by weight. The estimated total worldwide production of phthalates is 11 billion pounds (EUROPA, 2011; Holland, 2018). Asia Pacific region itself uses 52% plasticizers globally, and phthalates make up 87% of the produced plasticizer (Selvaraj et al., 2015). Phthalates are not directly linked to the polymer however by means of secondary molecular interactions. Therefore, they leach out of plastic items over time and might migrate and contaminate the air, water, food, house dust, and soil; particularly under conditions involving heat (Net et al., 2015a). Therefore, monitoring of phthalates in terrestrial and aquatic ecosystems and evaluation of the possible ecological threat is therefore highly significant (Li et al., 2017).
The 2008 US Consumer Product Safety Improvement Act (CPSIA) bans any infant toy or child care items containing amounts of >0.1% by weight of the following phthalates: di-(2-ethylhexyl) phthalate, dibutyl phthalate, benzyl butyl phthalate, diisononyl phthalate, diisobutyl phthalate, di-n-pentyl phthalate, di-n-hexyl phthalate, and dicyclohexyl phthalate (US-CPSC, 2018). Similarly, as set out in revised entry 51 of Annex XVII to the REACH regulation of European Union, the restriction was put in place on things comprising the four phthalates, di-(2-ethylhexyl) phthalate, dibutyl phthalate, benzyl butyl phthalate, and diisobutyl phthalate in an amount equivalent to above 0.1% by weight exclusively or in any blend in plasticized material (EU, 2018).
Long-term exposure to phthalates may lead to health problems, such as altered semen quality, birth defects, hormonal and endocrine disturbances, asthma, breast cancer, and so on (Diamanti-Kandarakis et al., 2009; Mathieu-Denoncourt et al., 2015).
Ceaseless human exposure to phthalates may affect liver-detoxifying enzymes (Praveena et al., 2018). In male rats, chronic exposure of phthalates altered spermatogenesis by altering androgen metabolism even with long-term exposure below the no observed adverse effect level (NOAEL) (Gao et al., 2018). Phthalates have harmful effects on aquatic organisms even at exposure to environmental concentrations (i.e., ng/l to μg/l) (Oehlmann et al., 2009). Chronic exposure to DEHP (20–200 μg/l) has altered fish growth and locomotion in medaka fish Oryzias latipes perhaps by a cumulative impact of neurotoxicity, oxidative stress, and apoptosis pathways (Yang et al., 2018). In addition, chronic DEHP exposure (100–500 μg/l) in juvenile yellow catfish Tachysurus fulvidraco shows disturbance of normal lipid metabolism processes at the enzymatic and molecular levels (Meng et al., 2018). Xu et al. (2013) suggested that DBP and DEP (5–500 μg/l) could induce antioxidant stress and immune response in zebra fish embryos. Further, DBP exposure (0.36–3.6 μM) reported to cause developmental toxicity and adverse effects on the embryo heart (cardiac structure deformities, pericardial edema, and altered cardiac function) in zebrafish (Danio rerio) (Sun & Li, 2019).
Phthalates are shown to affect primary producers in aquatic ecosystems. Acute exposure (0–20 mg/l) of DBP on freshwater algae (Chlorella pyrenoidosa and Scenedesmus obliquus) showed inhibition of photosynthetic activity by disrupting cell membranes and organelles with elevated ROS levels in lipid peroxidation and oxidative stress (Gu et al., 2017). Further, DEHP exposure (1–10 μg/l) on the marine dinoflagellate Alexandrium pacificum showed a noteworthy reduction in cell density and abundance (1.2–50 times lower) with inhibition of photosynthetic activities (M’Rabet et al., 2018).
Rivers are vulnerable to synthetic organic pollutants released into the river as a result of anthropogenic activities. In India, mostly treated/untreated wastewaters are discharged directly to the rivers. Therefore, the water quality is substandard in the majority of the rivers, and they act as major sinks of organic pollutants (Ramaswamy, 2015). Although persistent organic pollutant contamination has been well studied in environmental matrices (Patil et al., 2015), information is scare on phthalates.
Recently, emerging contaminants such as triclosan, triclocarban, benzotriazoles, carbamazepine, nonsteroidal anti-inflammatory drugs, parabens, phenolic compounds, synthetic musks, perfluorinated compounds and phthalates were reported by us in water, sediment, and fish from the rivers such as Kaveri, Vellar, and Tamiraparani (Ramaswamy et al., 2011; Selvaraj et al., 2014, 2015, 2021; Shanmugam et al., 2014a, 2014b; Vimalkumar et al., 2018, 2021). However, until now phthalates have not been studied in the Tamiraparani and Vellar Rivers in Tamil Nadu, India. Therefore, this study aims to investigate the distribution profile and risk assessment of six phthalates in water and sediment gathered from 30 locations from the Vellar and Tamiraparani Rivers along with the Kaveri River. This is the first report on the occurrence of phthalates in the Tamiraparani and Vellar Rivers of Tamil Nadu, India.
2 Materials and Methods
2.1 Study Area and Sample Collection
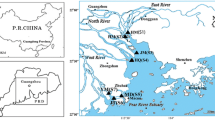
The surface water and sediment samples were collected from 30 locations (Fig. 1) in three rivers (Kaveri: 16; Vellar: 3; and Tamiraparani: 11) during dry season (1–15 September 2017) and wet season (19 November–11 December 2016). See Table 1 for details of the sampling locations. Amber glass bottles (1 l) were washed consecutively before sampling with liquid detergent, running tap water, ultrapure deionized water, and sample water prior to water sampling. Bags made of polypropylene were used for grab sampling of the sediment. The sampling station was particularly far from wastewater/effluent mixing point in the river stream to get realistic contamination levels. The samples obtained were put in iceboxes and then transported to the laboratory, where water samples were kept at 4 °C and analyzed within a week, and sediment samples were kept in deep freezer at −20 °C up to chemical extraction.
2.2 Preparation of Stock Standards and Chemicals
Stock solution of phthalates was made by dissolving EPA phthalate esters mix (4S8231) consisting of dimethyl phthalate (DMP), diethyl phthalate (DEP), dibutyl phthalate (DBP), benzyl butyl phthalate (BBP), bis(2-ethylhexyl) phthalate (DEHP), and di-n-octyl phthalate (DnOP) in solvent ethyl acetate. EPA phthalate esters mix was procured from Supelco Analytical (Philadelphia, USA). Phenanthrene d-10 was used as internal standard which was bought from Cambridge Isotope Laboratories (Andover, USA). Ethyl acetate, hexane, and methanol used during experiments were of HPLC grade, whereas other chemicals used were of analytical grade. Solid phase extraction cartridges Strata C18-E (1000 mg/6 ml) were bought from Phenomenex (California, USA), whereas, glass vials (2 ml) for GC-MS analysis were obtained from Agilent Technologies (Waldbronn, Germany). Water purification system (ELGA, UK) was used to get ultrapure water for analytical experiments. Silica gel (60–120 mesh), sodium sulfate , and glass wool were dried overnight in hot air oven (Heco, Chennai, India) at 200 °C prior to use.
2.3 Standards Preparation
The purchased standard mix of phthalates was considered as stock solution. Working solutions were prepared by diluting the stock solution at appropriate concentrations in ethyl acetate. These were used for instrumental calibration and procedural recovery check. Standard solutions were stored in deep freezer at −20 °C.
2.4 Preparation of Glassware
Initially the glassware was washed with 10% liquid detergent solution (Laboline, Fischer Scientific, Mumbai, India) followed by tap water, 50% hydrochloric acid, and ultrapure water (ELGA, USA). After air drying, the glassware was wrapped in aluminum foil (Hindalco, Mumbai) and baked overnight in a hot-air oven (Heco, Chennai, India) at 200 °C. To decrease the background concentration of phthalates, safety measures were taken to evade plastics contact.
2.5 Extraction of Phthalates from Water by Solid Phase Extraction
Phthalates were extracted from water samples based on the method developed by Selvaraj et al. (2015). C18-E cartridges were preconditioned and 500 ml water sample (pH 7) was passed at the rate of 5 ml/min using a vacuum manifold. The cartridge was then air dried and eluted with 20 ml of ethyl acetate. Sodium sulfate (anhydrous) was added to the eluate to remove the moisture content. Then the eluent was condensed using rotary vacuum evaporator (Buchi) and stored in teflon-lined screw cap glass vials until GC–MS analysis.
2.6 Extraction of Phthalates from Sediment by Ultrasonication
Sediment samples were extracted for phthalates by following the method established in our laboratory (Selvaraj et al., 2015). The sediment samples were air dried and homogenized using mortar and pestle. One gram sediment sample was taken in an amber glass bottle; 20 ml of ethyl acetate was added to it and ultrasonicated for 30 min. The extract was dehydrated (using sodium sulfate) and condensed using rotary vacuum evaporator to dryness. Finally, the analyte was put back together with 1 ml of ethyl acetate and the column clean-up was performed to remove interfering molecules.
2.7 Column Clean-Up
The glass column was plugged with glass wool at its base. Silica gel (2 g) slurry was prepared using 15 ml of ethyl acetate and added to the glass column. Silica gel was layered (1 cm) with anhydrous sodium sulfate; 20 ml of ethyl acetate was then passed through the column to wash away the impurities. Subsequently, the sediment extract was eluted with 20 ml of ethyl acetate. The eluate was condensed to 1 ml using rotary vacuum evaporator. Then, it was stored in 2 ml glass vials with teflon-lined screw cap until GC–MS analysis.
2.8 Instrumental Parameters
Phthalates were identified and quantified by gas chromatograph mass spectrometer (GCMS QP-2010) (Shimadzu Corporation, Japan) equipped with electron impact mode operating at 70 eV; 1 μl of the final extract was loaded onto the column in splitless mode using auto injector (AOC-20i). RTX-PCB fused silica capillary column (60 m × 0.25 mm i.d., 0.25 μm film thickness) (Restek, Pennsylvania, USA) was used to resolve the analytes. The injection port temperature was 280 °C. The column temperature program was as follows: 150 °C for 1 min and then at the rate of 8 °C/min raised to 300 °C and further to 320 °C at the rate of 7 °C/min and maintained for 10 min. The transfer line temperature was maintained at 270 and ion source temperature was 230 °C. Ultrapure (99.999%) helium gas was used as the carrier phase (flow rate – 2.25 ml/min). Identification of the analytes were performed based on the retention time (RT) and mass spectrum obtained in full scan acquisition mode (m/z 50–500). Individual peak for six phthalates and internal standard is shown in Fig. 2. Quantitative analysis was performed using the selected-ion monitoring (SIM) mode where one target ion and two characteristic ions (Table 2) were used for quantification. Additionally, external calibration method was adopted for quantification purpose.
2.9 Quality Assurance and Quality Control
Validation of the solid phase extraction method for water was performed by spiking 1000 ng of phthalate standard mix in 500 ml of river water. Correspondingly, ultrasonic extraction method for sediment was validated by spiking 100 ng of phthalate standard mix to 1 g of sediment. The recovery rate was satisfactory for both the extraction methods. Linear correlations (R2 > 0.999) were obtained for six phthalates and internal standard using external calibration method in the range of 1–200 μg/l. The limit of detection (LOD) and limit of quantification (LOQ) for each of the phthalates were assessed with a signal-to noise ratio of 3 and 10 times, respectively. For every set of five samples, one solvent blank was analyzed to look for interference and/or cross-contamination. The target ions, recovery, retention time, LOD, and LOQ are shown in Table 2, whereas, Fig. 2 shows the standard and sample chromatograms .
2.10 Freshwater and Sediment Risk Assessment
For selective organisms which are representative of the ecosystem of interest, ecological risk assessment as risk quotient (RQ) was conducted. RQ was estimated as the ratio of the measured environmental concentration (MEC) to the predicted no effect concentration (PNEC) of aquatic organisms as per formula (1). The methodology is given in detail elsewhere in our previous studies (Ramaswamy, 2015; Ramaswamy et al., 2011; Selvaraj et al., 2015; Selvaraj et al., 2014; Shanmugam et al., 2014b).
The PNEC values were calculated from the chronic NOEC toxicity values of algae, invertebrates, and fish retrieved from literature. An assessment factor of 10 was applied to NOEC values. Further, PNEC values for sediment was adopted from the European Chemicals Agency (ECHA) website (echa.europa.eu). The RQ value greater than one indicates associated risk for the respective chemical of concern, whereas the RQ value less than one indicates no associated risk.
3 Result and Discussion
3.1 Phthalates in River Water
3.1.1 Dry Season
Phthalates are the primary category of plasticizers used globally, so knowing their fate in riverine ecosystem is very much warranted. Aiming to know the distribution of phthalates, water samples collected from 30 locations at the Kaveri, Vellar, and Tamiraparani Rivers and tested for six major phthalates. Phthalates, namely, DMP, DEP, DBP, BBP, DEHP, and DnOP were detected ranging from ND (not detected) to 3,58,694 ng/l as shown in Fig. 3a. The detection frequency for these compounds varied between 0% and 100% in Kaveri, Vellar, and Tamiraparani: three major rivers of Tamil Nadu. Furthermore, total phthalates in all the samples were found between ND and 5,34,324 ng/l with mean level of 2,51,376 ng/l.
The concentration of phthalates in the Tamil Nadu river waters (Fig. 3a) indicates that DEHP, DBP, and DEP were ubiquitous in the Kaveri and Vellar River water samples, whereas BBP was detected only in 75% and 27% of Kaveri and Tamiraparani River water samples, respectively. Among 30 locations, 18 locations were found with all the phthalates, signifying widespread occurrence of phthalates in the riverine environment. The calculated mean level of DBP (1,52,489 ng/l) and BBP (1,03,617 ng/l) were two orders greater in magnitude than DEHP (6016 ng/l) and DnOP (4467 ng/l), whereas four orders of magnitude higher than DMP (22 ng/l). From the above mean values, it is understood that DBP (57%) and BBP (39%) contribute more for total phthalates than DEHP (2%) and DnOP (2%). The contribution of other phthalates (DMP and DEP) was not significant (<1%).
The total phthalates in the Kaveri River were quantified in the range of 128–5,34,324 ng/l which was three orders greater in magnitude than our previous report (ND–822 ng/l) indicative of the increased use over the period of 5 years (Selvaraj et al., 2015). The mean total (1,03,354 ng/l) was comparable to the Vellar River (1,39,874 ng/l) and one order greater in magnitude than in the Tamiraparani (24,444 ng/l) River. The major contributing phthalates were DBP (51%), followed by BBP (44%) and DnOP (4%) which were comparable to our earlier study BBP (92%) and DBP (67%) (Selvaraj et al., 2015). Among the individual phthalates, DBP was quantified very high up to 3,58,694 ng/l in Mettur (Stanley reservoir) and up to 95,348 ng/l in Muthupet. Similarly, BBP also quantified more at Mettur (1,68,200 ng/l) and Muthupet (1,11,358 ng/l). It could be because chemical effluents and household discharge were dumped into the river at several sites near these places. Other emerging pollutants, such as Bisphenol A and nonyl phenol, have already been identified at higher rates in Muthupet (136 ng/l and 2200 ng/l, respectively) compared to other locations of the Kaveri River (Selvaraj et al., 2014).
In the Vellar River, the total phthalates were observed in the range of 1,22,344–1,69,089 ng/l (Fig. 3a). All the phthalates were quantified in all the locations (i.e., 100% detection frequency). Mean concentrations of all the phthalates except DnOP calculated in the Vellar River was comparable to the Kaveri River. The major contributing phthalates were DBP (61%), followed by BBP (38%) as in the case of the Kaveri River. Like the Kaveri River, DBP was quantified at higher concentration (1,05,366 ng/l) followed by BBP (59,917 ng/l) at Railadi.
In the Tamiraparani River, the distribution of phthalates was slightly different from the other two rivers with DBP (68%) and DEHP (25%) followed by trivial contribution from BBP (4%) and DnOP (2%) (Fig. 3a). It was in agreement with the finding of Selvaraj (2015) who reported contribution of DBP and DEHP as 56% and 31%, respectively, in the Tamiraparani River. The total phthalates were observed ranging from ND to 43,857 ng/l, which reveals the Tamiraparani River is less contaminated compared to other two rivers. This may be due to the fairly low population of the region. Similar to the Kaveri and Vellar Rivers, DBP was quantified highest in Tenkasi (35,525 ng/l), whereas, DEHP was quantified maximum in Tirunelveli (11,769 ng/l) among all the river locations. Ramaswamy et al. (2011) also reported higher concentration of a well-known antibacterial agent, triclosan (3800 ng/l) in Tirunelveli. Apart from DEHP, concentration of DEP found in Tenkasi (765 ng/l), Kutralam (583 ng/l), Srivaikuntam (684 ng/l), and Aeral (624 ng/l) was indeed greater than any other river locations.
3.1.2 Wet Season
The total phthalates in all the water samples collected during wet season were found between 6 and 25,511 ng/l (mean: 18,993 ng/l). Individual phthalates were detected ranging from ND (not detected) to 24,946 ng/l as shown in Fig. 3b. This concentration was less than dry season, the similar trend was also observed by Lee et al. (2019) while studying distribution of phthalates in waters of the Asan Lake of South Korea. Wet deposition and runoff will greatly influence the concentration of phthalates in water (Gao & Wen, 2016). Furthermore, the detection frequency of these compounds varied between 17% and 100% in three rivers. The occurrence of phthalates during wet season (Fig. 3b) indicates that DBP was ubiquitous in the Kaveri and Tamiraparani River water samples, whereas DEP was omnipresent in the Vellar and Tamiraparani River samples. Moreover, DMP and DnOP were among the least detected compounds (33–56%). Unlike the dry season, among 30 locations only 9 locations were found with all the phthalates. The mean level of DBP (25,029 ng/l) was two orders greater in magnitude than DEP (407 ng/l), BBP (453 ng/l), and DEHP (892 ng/l), whereas three orders greater in magnitude than DMP (15 ng/l) and DnOP (11 ng/l). Based on the above results, DBP (93%) was found to be the single most contributor to total phthalates relative to other compounds.
The Kaveri River exhibited total phthalates between 45 and 25,511 ng/l (Fig. 3b). This was one order of magnitude less than the amounts recorded in the dry season with the mean total concentration of 9296 ng/l. The major contributing phthalate was DBP (93%), followed by DEHP (3%), DEP (2%), and BBP (4%) consisting the minor portion. Among the phthalates, DBP (24,946 ng/l) and BBP (414 ng/l) were quantified at elevated level in Pichavaram mangrove forest area.
Total phthalates in the Vellar River were detected between 6 and 15,696 ng/l (Fig. 3b). This was one order magnitude lower than the levels observed during dry season. The detection frequency of phthalates varied between 33% and 100%. DEP was detected at all three sampling stations of the Vellar River. Similar to the dry season, mean concentrations of all the phthalates in the Vellar River was comparable to the Kaveri River. The key contributing phthalate was DBP (94%), while other five phthalates comprising the negligible portion. Highest individual phthalate concentrations were recorded at Railadi for DBP (14,593 ng/l), DEHP (528 ng/l), DEP (359 ng/l), BBP (205 ng/l), DMP (7 ng/l), and DnOP (4 ng/l).
Unlike dry season, phthalate distribution in the Tamiraparani River was similar to the other two rivers with DBP (91%) followed by slight contribution from DEP (4%), BBP (3%), and DEHP (2%) (Fig. 3b). The total phthalates were within in the range of 327–23,011 ng/l, which was comparable to the other two rivers . Similar to the Kaveri and Vellar Rivers, DBP was quantified highest in Punnaikayal (21,901 ng/l). In the harbor city of Punnaikayal, DEP and BBP were also reported at highest levels of 620 ng/l and 416 ng/l, respectively. These levels were higher than the ones recorded at the Kaveri and Vellar Rivers.
3.2 Phthalates in River Sediment
3.2.1 Dry Season
The distribution and composition of six major phthalates in southern Indian rivers were recorded in this study. The study of chemicals in sediment provides clues on their prolonged occurrence in environment than water where the concentration flux is high. Phthalate concentration in the Tamil Nadu river sediments during dry season (Fig. 4a) specifies that DBP was ubiquitous in all the samples, whereas DEHP was pervasive in the samples collected from the Vellar and Tamiraparani Rivers. The same pattern was also observed by Selvaraj et al. (2015) with DBP (100%) and DEHP (96%) occurring predominantly. The levels of individual phthalates ranged from ND to 673 ng/g dw, whereas the total phthalates were in the range of 6–725 ng/g dw. In comparison, DEHP (mean 99 ng/g dw) was quantified at higher concentrations at several of the sites, followed by DBP (66 ng/g dw), DMP (62 ng/g dw), BBP (11 ng/g dw), DEP (9 ng/g dw), and DnOP (5 ng/g dw). As observed, the phthalate that contributed most to the total concentration was as follows: DEHP (39%) > DBP (26%) > DMP (25%) > DEP (4%) > BBP (4%) > DnOP (2%). The total mean phthalates were found in the decreasing in the order of Kaveri (112 ng/g dw) > Tamiraparani (81 ng/g dw) > Vellar (32 ng/g dw) Rivers.
Among the locations in the Kaveri River, all the phthalates were detected only at Ammapettai. The major contributing phthalates for total concentration were DEHP (39%), followed by DBP (26%) and DMP (25%) (Fig. 4a). The minimum and maximum concentration of total phthalates were found at Pichavaram (6 ng/g dw) and Ammapettai (725 ng/g dw), respectively. Not only total phthalates, DEHP (673 ng/g dw) and DEP (8 ng/g dw) (highest among all the rivers) were also recorded maximum at Ammapettai. In mangrove forest area like Picharavam, the overall phthalates were found to be 6 ng/g dw with maximum input from DBP (4 ng/g dw). This could be attributed to the discharge of domestic waste into the mangrove waters.
The total phthalates quantified in the Vellar River ranged from 27 to 39 ng/g dw. DEP, DBP, and DEHP were detected at all three locations, whereas DMP, BBP, and DnOP were not detected in sediments of the Vellar River. The maximum phthalate concentration was observed for DBP at Railadi (30 ng/g dw) which much lower than the Kaveri River. The major composition of phthalates consisted more of DBP (53%) and DEHP (34%) with less DEP (13%) (Fig. 4a). The Vellar River sediment showed lower concentration of phthalates than the Kaveri and Tamiraparani Rivers.
The maximum concentration of overall phthalates was witnessed at Tenkasi (205 ng/g dw) on the Tamiraparani River with major contribution from DBP (182 ng/g dw), whereas Ambasamudram was lowest at 27 ng/g dw. The phthalate composition consisted of DBP (56%) contributing higher than DEHP (30%) and BBP (11%), whereas, DMP and DnOP were not detected in sediment samples of the Tamiraparani River. The mean of DBP, DEHP, BBP, and DEP was calculated as 51 ng/g dw, 27 ng/g dw, 10 ng/g dw, and 2 ng/g dw. The signature of phthalates (mean: 59 ng/g dw) in Punnaikayal signifies the potential load of phthalates drained into the estuary and thereby into the open sea.
3.2.2 Wet Season
Total phthalates in all the sediment samples collected during wet season were found between 12 and 748 ng/g dw (Fig. 4b) with an average level of 236 ng/g dw which was comparable with the dry season. Individual phthalates were detected in the range of ND to 705 ng/g dw. Furthermore, the detection frequency for phthalates varied between 0% and 100% in the Kaveri, Vellar, and Tamiraparani Rivers. Phthalate occurrence indicates that DBP and DEHP were ubiquitous in all three rivers unlike DMP which was not reported during wet season. DMP was more frequently detected in water than in sediments, which can be due to its relatively higher water solubility (4000 mg/L) (Howard et al., 1985).
Furthermore, no location had reported all the phthalates in sediment samples. DEHP (60%) was the contributor for total phthalates followed by DBP (28%). The mean concentration of DEHP and DBP was 149 and 70 ng/g dw, respectively.
The Kaveri River showed total phthalates in the range of 12–748 ng/g dw (Fig. 4b) with the average total concentration of 115 ng/g dw. The major contributing phthalates were DEHP (73%) followed by DBP (16%) with DEP (4%), BBP (5%), and DnOP (2%) consisting of the small portion. Among the phthalates, DEHP (705 ng/g dw), DEP (12 ng/g dw), BBP (8 ng/g dw), and DnOP (3 ng/g dw) were quantified highest in Tanjore area. Furthermore, DnOP was only detected at Tanjore.
Total phthalates in the Vellar River ranged from 45 to 97 ng/g dw. Similar to the dry season, DEHP and DBP were detected across all three locations, while DMP and DnOP were not detected in the Vellar River sediments. Furthermore, like dry season, the maximum phthalate concentration was observed for DBP at Railadi (60 ng/g dw) which was much lower than the Kaveri River. The composition of phthalates comprised more of DBP (56%) and DEHP (26%) with a smaller amount of DEP (11%) (Fig. 4b). Similar to the dry season, the Vellar River sediments showed lower concentration of phthalates than the Kaveri and Tamiraparani Rivers.
In the Tamiraparani River, total phthalates ranged from 21 to 670 ng/g dw (Fig. 4b) with the average total concentration of 152 ng/g dw. Similar to the Kaveri River, the main contributing phthalates were DEHP (75%) followed by DBP (17%). DEHP was quantified at highest concentration (636 ng/g dw) at Kutralam. Ramaswamy et al. (2011) also reported significant presence of triclosan and parabens in Kutralam.
For the most part, the recent sources of phthalates are indicated by the levels of phthalates in water phase, while the sediment trend demonstrates the accumulation/persistence of phthalates over the years. Therefore, the concurrent occurrence of phthalates in water and sediment at higher level in most of the locations indicates contamination of rivers for years.
3.2.3 Phthalates Comparison with Indian and Global Studies
The concentration of total phthalates recorded in the rivers Kaveri, Vellar, and Tamiraparani was comparable to the studies carried out around the world (Table 3). The Kaveri River water reported the highest concentration of total phthalates (5,34,324 ng/l) which was greater than other studies globally. Net et al. (2015b) reported the highest level (1,79,100 ng/l) of total phthalates (n = 6) at Nivelles on the Scarpe River which was comparable to the Vellar River (1,69,089 ng/l). Upper levels reported in the Tamiraparani River (43,857 ng/l) were comparable to the levels reported (28,000 ng/l) in Cochin estuary, Kerala, India (Ramzi et al., 2020). The total phthalate concentration of the water and sediment samples of the Yellow River in Lanzhou was ND to 6040 ng/l and ND to 9898 ng/g dw, respectively, with the concentrations of two priority phthalates (DBP and DEHP) higher in all samples (Zhao et al., 2020). This observation is similar to the present study. In case of sediment, the levels reported in the Kaveri River (6–748 ng/g dw) and the Tamiraparani River (21–670 ng/g dw) were comparable with the levels reported by (Selvaraj et al., 2015) in the Kaveri River (2–1438 ng/g dw) and recently reported levels in the Adyar River (52–1796 ng/g dw) and the Cooum River (28–856 ng/g dw). Concentrations reported in the Vellar River (27–97 ng/g dw) were lower compared to the Kaveri and Tamiraparani Rivers, which were comparable with the levels seen in eight urban lakes (17–242 ng/g dw) of Guangzhou, China (Zhang et al., 2018).
3.2.4 Ecotoxicological Risk Assessment
Risk assessment for freshwater organisms was performed based on RQ calculated from MEC and PNEC. To determine the ecological toxicity by risk quotient, the highest determined environmental concentration of phthalates in the rivers was used. List of organisms, end point, and NOEC for phthalates were given in Table 4.
The risk to aquatic habitat from phthalates was assessed for invertebrates, fish, and algae as shown in Fig. 5. The calculated RQs of DMP and DEP in the Kaveri, Vellar, and Tamiraparani Rivers for Daphnia magna, Oncorhynchus mykiss, and Selenastrum capricornutum were well below unity, whereas RQs of DBP for Oncorhynchus mykiss were in decreasing order of the Kaveri River (35.9) > the Vellar River (10.5) > the Tamiraparani River (3.6). The RQ of DBP for Daphnia magna was 3.7 and 1.1 for the Kaveri and Vellar Rivers, respectively. Furthermore, the RQ of DBP for algae Chlorella emersoni was 1.3 in the Tamiraparani River. The RQs of BBP in the Kaveri and Vellar Rivers for Daphnia magna, Oncorhynchus mykiss, and Selenastrum capricornutum ranged from 2.1 to 16.8 indicating risk to all three taxonomic groups. Higher levels of DEHP in the Tamiraparani River mirrored the risk for Daphnia magna (RQ 1.5) and Selenastrum capricornutum (RQ 1.2). In case of DnOP, Daphnia magna was found to be at risk in the Kaveri River (RQ 1.5). Therefore, these results indicate that DMP and DEP pose little or negligible risk, and potential risk can be anticipated due to higher water concentrations of DBP, BBP, DEHP, and DnOP in South Indian rivers. In Cochin estuary, India, parallel trend was observed where DBP and DEHP risk quotient values were large enough to pose moderate to high risk to aquatic biota (Ramzi et al., 2020).
Sediment risk assessment of phthalates showed all the calculated RQs did not exceed unity (Fig. 6). This assessment determines that phthalates are not expected to pose a risk to environmentally sensitive benthic communities in sediments.
4 Conclusion
Phthalates were ubiquitously detected in three major rivers of southern India. Furthermore, this study reports these contaminants for the first time in the Vellar and Tamiraparani Rivers, confirming the anthropogenic influence throughout the river stretch. Among the contaminants analyzed, higher levels of DBP were observed in water. Among the rivers, Kaveri seems to be contaminated at higher proportion than others. Aquatic risk evaluation indicated potential vulnerability because of DBP, BBP, DEHP, and DnOP. The results indicate the need for water quality guidelines to manage the discharge of domestic/industrial sewage effluents containing estrogenic compounds into the river system. In addition, this study will also act as basic data for future environmental monitoring, fate, and toxicological investigation initiatives relating to emerging contaminants.
References
Adams, W. J., Biddinger, G. R., Robillard, K. A., & Gorsuch, J. W. (1995). A summary of the acute toxicity of 14 phthalate esters to representative aquatic organisms. Environmental Toxicology and Chemistry, 14, 1569–1574. https://doi.org/10.1002/etc.5620140916
Chen, H., Mao, W., Shen, Y., Feng, W., Mao, G., Zhao, T., Yang, L., Yang, L., Meng, C., Li, Y., & Wu, X. (2019). Distribution, source, and environmental risk assessment of phthalate esters (PAEs) in water, suspended particulate matter, and sediment of a typical Yangtze River Delta City, China. Environmental Science and Pollution Research, 26, 24609–24619. https://doi.org/10.1007/s11356-019-05259-y
Defoe, D. L., Holcombe, G. W., Hammermeister, D. E., & Biesinger, K. E. (1990). Solubility and toxicity of eight phthalate esters to four aquatic organisms. Environmental Toxicology and Chemistry, 9, 623–636. https://doi.org/10.1002/etc.5620090509
Diamanti-Kandarakis, E., Bourguignon, J.-P., Giudice, L. C., Hauser, R., Prins, G. S., Soto, A. M., Zoeller, R. T., & Gore, A. C. (2009). Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocrine Reviews, 30, 293–342. https://doi.org/10.1210/er.2009-0002
EU. (2018). Official Journal of the European Union [WWW Document]. Europa.eu. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2018:322:FULL&from=EN. Accessed 1 Apr 2019.
EUROPA. (2011). Phthalates and their alternatives: Health and environmental concerns [WWW Document]. Europa.eu. http://ec.europa.eu/environment/aarhus/pdf/35/Annex_11_report_from_Lowell_Center.pdf. Accessed 2 Jan 2019.
Gao, D.-W., & Wen, Z.-D. (2016). Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Science of the Total Environment, 541, 986–1001. https://doi.org/10.1016/j.scitotenv.2015.09.148
Gao, H.-T., Xu, R., Cao, W.-X., Di, Q.-N., Li, R.-X., Lu, L., Xu, Q., & Yu, S.-Q. (2018). Combined effects of simultaneous exposure to six phthalates and emulsifier glycerol monosterate on male reproductive system in rats. Toxicology and Applied Pharmacology, 341, 87–97. https://doi.org/10.1016/j.taap.2018.01.013
Gu, S., Zheng, H., Xu, Q., Sun, C., Shi, M., Wang, Z., & Li, F. (2017). Comparative toxicity of the plasticizer dibutyl phthalate to two freshwater algae. Aquatic Toxicology, 191, 122–130. https://doi.org/10.1016/j.aquatox.2017.08.007
Holland, M. (2018). Socio-economic assessment of phthalates. OECD Publ. Paris, OECD Environment Working Papers No. 133. https://doi.org/10.1787/a38a0e34-en
Howard, P. H., Banerjee, S., & Robillard, K. H. (1985). Measurement of water solubilities, octanol/water partition coefficients and vapor pressures of commercial phthalate esters. Environmental Toxicology and Chemistry, 4, 653–661. https://doi.org/10.1002/etc.5620040509
Lee, Y.-M., Lee, J.-E., Choe, W., Kim, T., Lee, J.-Y., Kho, Y., Choi, K., & Zoh, K.-D. (2019). Distribution of phthalate esters in air, water, sediments, and fish in the Asan Lake of Korea. Environment International, 126, 635–643. https://doi.org/10.1016/j.envint.2019.02.059
Li, X., Yin, P., & Zhao, L. (2016). Phthalate esters in water and surface sediments of the Pearl River Estuary: Distribution, ecological, and human health risks. Environmental Science and Pollution Research, 23, 19341–19349. https://doi.org/10.1007/s11356-016-7143-x
Li, R., Liang, J., Gong, Z., Zhang, N., & Duan, H. (2017). Occurrence, spatial distribution, historical trend and ecological risk of phthalate esters in the Jiulong River, Southeast China. Science of the Total Environment, 580, 388–397. https://doi.org/10.1016/j.scitotenv.2016.11.190
Liu, H., Cui, K., Zeng, F., Chen, L., Cheng, Y., Li, H., Li, S., Zhou, X., Zhu, F., Ouyang, G., Luan, T., & Zeng, Z. (2014). Occurrence and distribution of phthalate esters in riverine sediments from the Pearl River Delta region, South China. Marine Pollution Bulletin, 83, 358–365. https://doi.org/10.1016/j.marpolbul.2014.03.038
M’Rabet, C., Pringault, O., Zmerli-Triki, H., Ben Gharbia, H., Couet, D., & Kéfi-Daly Yahia, O. (2018). Impact of two plastic-derived chemicals, the Bisphenol A and the di-2-ethylhexyl phthalate, exposure on the marine toxic dinoflagellate Alexandrium pacificum. Marine Pollution Bulletin, 126, 241–249. https://doi.org/10.1016/j.marpolbul.2017.10.090
Mathieu-Denoncourt, J., Wallace, S. J., de Solla, S. R., & Langlois, V. S. (2015). Plasticizer endocrine disruption: Highlighting developmental and reproductive effects in mammals and non-mammalian aquatic species. General and Comparative Endocrinology, 219, 74–88. https://doi.org/10.1016/j.ygcen.2014.11.003
McCarthy, J. F., & Whitmore, D. K. (1985). Chronic toxicity of di-n-butyl and di-n-octyl phthalate to Daphnia magna and the fathead minnow. Environmental Toxicology and Chemistry, 4, 167–179. https://doi.org/10.1002/etc.5620040206
Melin, C., & Egnéus, H. (1983). Effects of di-n-butyl phthalate on growth and photosynthesis in algae and on isolated organelles from higher plants. Physiologia Plantarum, 59, 461–466. https://doi.org/10.1111/j.1399-3054.1983.tb04230.x
Meng, F. X., Li, M., Song, M. Z., Yuan, L. X., Gong, Y. F., Qian, Y. X., Shi, G., & Wang, R. X. (2018). Di-2-ethylhexyl phthalate (DEHP) exposure disturbs lipid metabolism in juvenile yellow catfish Tachysurus fulvidraco. Journal of Fish Biology, 92, 85–93. https://doi.org/10.1111/jfb.13493
Mukhopadhyay, M., Sampath, S., Muñoz-Arnanz, J., Jiménez, B., & Chakraborty, P. (2020). Plasticizers and bisphenol A in Adyar and Cooum riverine sediments, India: Occurrences, sources and risk assessment. Environmental Geochemistry and Health, 42(9), 2789–2802. https://doi.org/10.1007/s10653-020-00516-3
Net, S., Delmont, A., Sempéré, R., Paluselli, A., & Ouddane, B. (2015a). Reliable quantification of phthalates in environmental matrices (air, water, sludge, sediment and soil): A review. Science of the Total Environment, 515–516, 162–180. https://doi.org/10.1016/j.scitotenv.2015.02.013
Net, S., Rabodonirina, S., Sghaier, R. B., Dumoulin, D., Chbib, C., Tlili, I., & Ouddane, B. (2015b). Distribution of phthalates, pesticides and drug residues in the dissolved, particulate and sedimentary phases from transboundary rivers (France–Belgium). Science of the Total Environment, 521–522, 152–159. https://doi.org/10.1016/j.scitotenv.2015.03.087
NIH. (2019). Phthalates: Your environment, your health | National Library of Medicine [WWW Document]. Tox town. https://toxtown.nlm.nih.gov/chemicals-and-contaminants/phthalates. Accessed 2 Jan 2019.
Oehlmann, J., Schulte-Oehlmann, U., Kloas, W., Jagnytsch, O., Lutz, I., Kusk, K. O., Wollenberger, L., Santos, E. M., Paull, G. C., Look, K. J. W. V., & Tyler, C. R. (2009). A critical analysis of the biological impacts of plasticizers on wildlife. Philosophical Transactions of the Royal Society B: Biological Sciences, 364, 2047. https://doi.org/10.1098/rstb.2008.0242
Paluselli, A., Aminot, Y., Galgani, F., Net, S., & Sempéré, R. (2018). Occurrence of phthalate acid esters (PAEs) in the northwestern Mediterranean Sea and the Rhone River. Progress in Oceanography, 163, 221–231. https://doi.org/10.1016/j.pocean.2017.06.002
Patil, N. N., Selvaraj, K. K., Krishnamoorthy, V., Elaiyaraja, A., & Ramaswamy, B. R. (2015). Organochlorine pesticide contamination in the Kaveri (Cauvery) River, India: A review on distribution profile, status, and trends. In ‘Water challenges and solutions on a global scale’ ‘ACS symposium series’ (pp. 115–128). American Chemical Society. https://doi.org/10.1021/bk-2015-1206.ch007
Praveena, S. M., Teh, S. W., Rajendran, R. K., Kannan, N., Lin, C.-C., Abdullah, R., & Kumar, S. (2018). Recent updates on phthalate exposure and human health: A special focus on liver toxicity and stem cell regeneration. Environmental Science and Pollution Research, 25, 11333–11342. https://doi.org/10.1007/s11356-018-1652-8
Ramaswamy, B. R. (2015). Environmental risk assessment of personal care products. In M. S. Díaz-Cruz & D. Barceló (Eds.), ‘Personal care products in the aquatic environment’ ‘The handbook of environmental chemistry’ (pp. 139–163). Cham: Springer International Publishing. https://doi.org/10.1007/698_2014_297
Ramaswamy, B. R., Shanmugam, G., Velu, G., Rengarajan, B., & Larsson, D. G. J. (2011). GC–MS analysis and ecotoxicological risk assessment of triclosan, carbamazepine and parabens in Indian rivers. Journal of Hazardous Materials, 186, 1586–1593. https://doi.org/10.1016/j.jhazmat.2010.12.037
Ramzi, A., Gireeshkumar, T. R., Habeeb Rahman, K., Manu, M., Balachandran, K. K., Chacko, J., & Chandramohanakumar, N. (2018). Distribution and contamination status of phthalic acid esters in the sediments of a tropical monsoonal estuary, Cochin – India. Chemosphere, 210, 232–238. https://doi.org/10.1016/j.chemosphere.2018.06.182
Ramzi, A., Gireeshkumar, T. R., Habeeb Rahman, K., Balachandran, K. K., Shameem, K., Chacko, J., & Chandramohanakumar, N. (2020). Phthalic acid esters – A grave ecological hazard in Cochin estuary, India. Marine Pollution Bulletin, 152, 110899. https://doi.org/10.1016/j.marpolbul.2020.110899
Rhodes, J. E., Adams, W. J., Biddinger, G. R., Robillard, K. A., & Gorsuch, J. W. (1995). Chronic toxicity of 14 phthalate esters to Daphnia magna and rainbow trout (Oncorhynchus mykiss). Environmental Toxicology and Chemistry, 14, 1967–1976. https://doi.org/10.1002/etc.5620141119
Schmidt, N., Castro-Jiménez, J., Fauvelle, V., Ourgaud, M., & Sempéré, R. (2020). Occurrence of organic plastic additives in surface waters of the Rhône River (France). Environmental Pollution, 257, 113637. https://doi.org/10.1016/j.envpol.2019.113637
Selvaraj, K. K. (2015). Study on the contamination status of Alkylphenol Ethoxylates, Bisphenol A and Phthalates in major rivers of Tamil Nadu, India and its ecotoxicological risk assessment. Bharathidasan University, Tiruchirappalli, Tamil Nadu, India.
Selvaraj, K. K., Shanmugam, G., Sampath, S., Joakim Larsson, D. G., & Ramaswamy, B. R. (2014). GC–MS determination of bisphenol A and alkylphenol ethoxylates in river water from India and their ecotoxicological risk assessment. Ecotoxicology and Environmental Safety, 99, 13–20. https://doi.org/10.1016/j.ecoenv.2013.09.006
Selvaraj, K. K., Sundaramoorthy, G., Ravichandran, P. K., Girijan, G. K., Sampath, S., & Ramaswamy, B. R. (2015). Phthalate esters in water and sediments of the Kaveri River, India: Environmental levels and ecotoxicological evaluations. Environmental Geochemistry and Health, 37, 83–96. https://doi.org/10.1007/s10653-014-9632-5
Selvaraj, K. K., Murugasamy, M., Nikhil, N. P., Elaiyaraja, A., Sampath, S., Krishnamoorthi, V., ... & Ramaswamy, B. R. (2021). Investigation of distribution, sources and flux of perfluorinated compounds in major southern Indian rivers and their risk assessment. Chemosphere, 277, 130228. https://doi.org/10.1016/j.chemosphere.2021.130228
Shanmugam, G., Ramasamy, K., Selvaraj, K. K., Sampath, S., & Ramaswamy, B. R. (2014a). Triclosan in fresh water fish Gibelion Catla from the Kaveri River, India, and its consumption risk assessment. Environmental Forensics, 15, 207–212. https://doi.org/10.1080/15275922.2014.930940
Shanmugam, G., Sampath, S., Selvaraj, K. K., Larsson, D. G. J., & Ramaswamy, B. R. (2014b). Non-steroidal anti-inflammatory drugs in Indian rivers. Environmental Science and Pollution Research, 21, 921–931. https://doi.org/10.1007/s11356-013-1957-6
Shariati, S., Pourbabaee, A. A., Alikhani, H. A., & Rezaei, K. A. (2019). Assessment of phthalic acid esters pollution in Anzali wetland, north of Iran. International journal of Environmental Science and Technology, 16, 7025–7036. https://doi.org/10.1007/s13762-018-2110-3
Srivastava, A., Sharma, V. P., Tripathi, R., Kumar, R., Patel, D. K., & Mathur, P. K. (2010). Occurrence of phthalic acid esters in Gomti River Sediment, India. Environmental Monitoring and Assessment, 169, 397–406. https://doi.org/10.1007/s10661-009-1182-4
Sun, G., & Li, Y. (2019). Exposure to DBP induces the toxicity in early development and adverse effects on cardiac development in zebrafish (Danio rerio). Chemosphere, 218, 76–82. https://doi.org/10.1016/j.chemosphere.2018.11.095
Sun, J., Huang, J., Zhang, A., Liu, W., & Cheng, W. (2013). Occurrence of phthalate esters in sediments in Qiantang River, China and inference with urbanization and river flow regime. Journal of Hazardous Materials, 248–249, 142–149. https://doi.org/10.1016/j.jhazmat.2012.12.057
US-CPSC. (2018). Phthalates business guidance & small entity compliance guide [WWW Document]. CPSC.gov. https://www.cpsc.gov/Business%2D%2DManufacturing/Business-Education/Business-Guidance/Phthalates-Information. Accessed 4 Jan 2019.
Vimalkumar, K., Arun, E., Krishna-Kumar, S., Poopal, R. K., Nikhil, N. P., Subramanian, A., & Babu-Rajendran, R. (2018). Occurrence of triclocarban and benzotriazole ultraviolet stabilizers in water, sediment, and fish from Indian rivers. Science of The Total Environment, 625, 1351–1360. https://doi.org/10.1016/j.scitotenv.2018.01.042
Vimalkumar, K., Nikhil, N. P., Arun, E., Mayilsamy, M., & Babu-Rajendran, R. (2021). Synthetic musks in surface water and fish from the rivers in India: Seasonal distribution and toxicological risk assessment. Journal of Hazardous Materials, 414, 125558. https://doi.org/10.1016/j.jhazmat.2021.125558
Wang, F., Xia, X., & Sha, Y. (2008). Distribution of phthalic acid esters in Wuhan section of the Yangtze River, China. Journal of Hazardous Materials, 154, 317–324. https://doi.org/10.1016/j.jhazmat.2007.10.028
Weizhen, Z., Xiaowei, Z., Peng, G., Ning, W., Zini, L., Jian, H., & Zheng, Z. (2020). Distribution and risk assessment of phthalates in water and sediment of the Pearl River Delta. Environmental Science and Pollution Research, 27, 12550–12565. https://doi.org/10.1007/s11356-019-06819-y
Xu, H., Shao, X., Zhang, Z., Zou, Y., Wu, X., & Yang, L. (2013). Oxidative stress and immune related gene expression following exposure to di-n-butyl phthalate and diethyl phthalate in zebrafish embryos. Ecotoxicology and Environmental Safety, 93, 39–44. https://doi.org/10.1016/j.ecoenv.2013.03.038
Yang, G. C. C., Wang, C.-L., & Chiu, Y.-H. (2015). Occurrence and distribution of phthalate esters and pharmaceuticals in Taiwan river sediments. Journal of Soils and Sediments, 15, 198–210. https://doi.org/10.1007/s11368-014-1003-4
Yang, W.-K., Chiang, L.-F., Tan, S.-W., & Chen, P.-J. (2018). Environmentally relevant concentrations of di(2-ethylhexyl)phthalate exposure alter larval growth and locomotion in medaka fish via multiple pathways. Science of The Total Environment, 640–641, 512–522. https://doi.org/10.1016/j.scitotenv.2018.05.312
Zhang, Z., Zhang, C., Huang, Z., Yi, X., Zeng, H., Zhang, M., & Huang, M. (2018). Residue levels and spatial distribution of phthalate acid esters in water and sediment from urban lakes of Guangzhou, China. Journal of Environmental Science and Health, Part A, 54(2), 127–135. https://doi.org/10.1080/10934529.2018.1530539
Zhang, Z.-M., Zhang, J., Zhang, H.-H., Shi, X.-Z., Zou, Y.-W., & Yang, G.-P. (2020). Pollution characteristics, spatial variation, and potential risks of phthalate esters in the water–sediment system of the Yangtze River estuary and its adjacent East China Sea. Environmental Pollution, 265, 114913. https://doi.org/10.1016/j.envpol.2020.114913
Zhao, X., Shen, J., Zhang, H., Li, X., Chen, Z., & Wang, X. (2020). The occurrence and spatial distribution of phthalate esters (PAEs) in the Lanzhou section of the Yellow River. Environmental Science and Pollution Research, 27, 19724–19735. https://doi.org/10.1007/s11356-020-08443-7
Acknowledgments
The authors wish to thank United Nations University, Tokyo, Japan, and SHIMADZU Corporation, Japan, for the GC/MS facility established at Bharathidasan University, and also thank the funding agencies RUSA 2.0 (Thematic Research Project in Biological Sciences), DBT (BT/PR18836/BCE/8/1368), UGC-SAP, DST (FIST & PURSE), New Delhi, India, for providing research grants/facilities. Nikhil Nishikant Patil thanks Bharathidasan University (15183/URF/K7/2017Dt:13.09.2017) and E. Arun thanks DBT for the fellowship to carry out their doctoral research.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Patil, N.N., Arun, E., Vimalkumar, K., Ramaswamy, B.R. (2022). Phthalate Esters and Their Ecotoxicological Risks from the Rivers of India. In: Vasanthy, M., Sivasankar, V., Sunitha, T.G. (eds) Organic Pollutants. Emerging Contaminants and Associated Treatment Technologies. Springer, Cham. https://doi.org/10.1007/978-3-030-72441-2_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-72441-2_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-72440-5
Online ISBN: 978-3-030-72441-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)