Abstract
Pharmaceutical concentration data for Indian surface waters are currently scarce. Sewage often enters Indian rivers without prior treatment, and so previously reported environmental concentrations from regions with routinely implemented sewage treatment cannot simply be used to predict concentrations in Indian surface water. Improved knowledge of pharmaceutical concentrations in Indian waters would enable determination of potential risks posed to aquatic wildlife and human health in this region. The concentrations of five common non-steroidal anti-inflammatory drugs (NSAIDs; diclofenac, ketoprofen, naproxen, ibuprofen, and acetylsalicylic acid) were determined in surface waters from 27 locations of the Kaveri, Vellar, and Tamiraparani Rivers in southern India. The samples were extracted by solid-phase extraction and analyzed by GC-MS. The measured concentrations of four of the five drugs in this reconnaissance were relatively similar to those reported elsewhere (ND–200 ng/l); however, acetylsalicylic acid, the most readily degradable of the investigated drugs, was found at all sites and at considerably higher concentrations (up to 660 ng/l) than reported in European surface waters. This is the first report on the occurrence of NSAIDs in Indian rivers. The finding of elevated concentrations of acetylsalicylic acid is most likely a result of direct discharges of untreated sewage. Therefore, readily degradable pharmaceuticals may present larger concern in regions without consistent sewage treatment. Based on measured environmental concentrations, the risks of direct toxicity to aquatic wildlife and of humans consuming the water are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increased rates of population growth and urbanization are associated with increased use of pharmaceuticals and subsequently an increased load in the receiving aquatic environment due to wastewater discharges. Some pharmaceuticals are almost completely removed during sewage treatment, whereas others are hardly removed at all (Santos et al. 2007, 2009; Lin et al. 2009; Gros et al. 2010; Suarez et al. 2010; Behera et al. 2011). Usually, individual pharmaceutical compounds in treated sewage effluents are found at concentrations of less than 1 μg/l. In contrast, concentrations approaching several milligrams per liter have been measured in effluents from treatment plants receiving waste from pharmaceutical manufacturing facilities (Larsson et al. 2007) and in surface water contaminated by industrial effluents (Fick et al. 2009). Physical and biological processes occurring in aquatic environments may attenuate the degradation of many pharmaceutical compounds. Trace concentrations of active pharmaceutical ingredients and their metabolites have been detected in surface water, ground water, and drinking water (Moldovan 2006; Togola and Budzinski 2007; Peng et al. 2008; Yoon et al. 2010; Zhao et al. 2010; Fram and Belitz 2011). Studies on pharmaceuticals from Indian aquatic environments are, however, still scarce (Larsson et al. 2007; Fick et al. 2009; Diwan et al. 2009; Ramaswamy et al. 2011; Kristiansson et al. 2011).
Pharmaceuticals often exert their effects at relatively low concentrations and through relatively specific modes of action. Partly because many human drug targets are conserved across species, pharmaceuticals can also affect non-target organisms, provided they are exposed to sufficiently high concentrations (Gunnarsson et al. 2008). Perhaps the clearest example is the severely reduced vulture population on the Indian subcontinent, a consequence of exposure to diclofenac via the ingestion of contaminated carcasses (Oaks et al. 2004). Ethinylestradiol is likely an important contributor to the feminization of fish downstream from sewage treatment works (Desbrow et al. 1998; Larsson et al. 1999; Thorpe et al. 2003; Kidd et al. 2007).
Analgesics and anti-inflammatory drugs are widely used in India and comprise the two largest groups of over-the-counter drugs in urban areas (Kamala and Dinesh Kumar 2006). These drugs are generally used to treat inflammation and mild to moderate pain and fever. Because of their high use worldwide and at least partial resistance to removal during sewage treatment, these chemicals are frequently found in the aquatic environment (Brown et al. 2007; Zhang et al. 2008; Fick et al. 2010; Sanchez-Prado et al. 2010). Diclofenac is particularly difficult to remove during sewage treatment (Gros et al. 2010; Suarez et al. 2010); this drug affects hepatic gene expression (Mehinto et al. 2010; Cuklev et al. 2011) and is reported to cause histological changes in several organs of fish at concentrations of approximately 1 μg/l (Schwaiger et al. 2004; Triebskorn et al. 2004), although a recent study did not report any histological changes at these concentrations (Memmert et al. 2013). Acetylsalicylic acid (2-acetoxybenzoic acid) is frequently used as an analgesic and antipyretic agent in India and elsewhere. In addition, low-dose acetylsalicylic acid is employed as an anti-thrombotic agent. Normally, acetylsalicylic acid is very efficiently removed during sewage treatment and easily degraded into salicylic acid (Ternes et al. 2001; Sim et al. 2010).
In India, wastewaters from hospitals, residential areas, and industrial activities are often directly released into surface waters without prior treatment. One can therefore hypothesize that pharmaceuticals would be present at higher concentrations in Indian surface waters than in the surface waters of countries with well-developed infrastructure for sewage treatment and disposal. We hypothesize that this phenomenon would be particularly evident for pharmaceuticals that are removed efficiently by sewage treatment. However, environmental sampling data to evaluate this hypothesis are largely unavailable in India. Hence, the present study aimed to determine the concentrations of five common NSAIDs (diclofenac, ketoprofen, naproxen, ibuprofen, and acetylsalicylic acid) in surface water from three Indian river systems. The detected concentrations were then compared with known toxicity data to evaluate the potential risk for toxicity in aquatic organisms and humans.
Materials and methods
Study area and sample collection
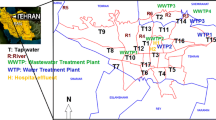
Water samples were collected from the Kaveri, Vellar, and Tamiraparani rivers (Fig. 1) during July 2010. These rivers run through residential and commercial areas; therefore, urban and industrial wastewaters represent a significant input into these rivers, although these rivers also receive runoff from agricultural areas. In total, 27 sites were selected. All sampling sites were outside of the immediate effluent plume from any local wastewater discharges. The sampling reconnaissance covered 13 locations, including sites within 346 km along the Kaveri river, 6 km along the Vellar estuary, and 112 km along the Tamiraparani river. Grab samples of water (two per site) were collected in 2-l pre-cleaned, amber glass bottles that had been pre-rinsed with sample water three times prior to sample collection. Then, the samples were transported on ice to the laboratory and stored at 4 °C until chemical analysis. Further details of the sampled rivers have been published previously (Ramaswamy et al. 2011). Briefly, the River Kaveri (Cauvery) is a major river in India with many tributaries and a basin area of approximately 72,000 km2 that flows through three states in southern India (Karnataka, Kerala, and Tamil Nadu) before emptying into the Bay of Bengal. The Vellar River originates from the Chitteri hills (11°25′N, 79°31′E) in Tamil Nadu state, flows along the southeastern coast, and forms an estuarine system with the Bay of Bengal at Parangipettai. The Pichavaram mangrove forest is located between the Vellar River and Coleroon tributary estuaries. The Tamiraparani River is one of the perennial rivers of the southern peninsular India, originating from the Western Ghats at an altitude of 2,000 m in Tamil Nadu state and flowing into the Gulf of Mannar, a Marine Bioreserve of the Bay of Bengal near Tuticorin, after draining an area of 5,369 km2. These rivers are the lifelines of southern India, supporting irrigated agriculture for centuries as well as serving as drinking water sources.
Chemicals and standards
Standards for ibuprofen, naproxen, ketoprofen, diclofenac, acetylsalicylic acid, and the derivatizing reagent N-methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) were purchased from Sigma-Aldrich (St. Louis, USA). Phenanthrene-d10, an internal standard, was procured from Cambridge Isotope Laboratories Inc. (Andover, USA). Acetone, n-hexane, and ethyl acetate of HPLC grade were procured from Thermo Fisher Scientific India Pvt. Ltd. (Mumbai, India). Solid-phase extraction (SPE) cartridges (Oasis HLB 60 mg/3 ml) were obtained from Waters Corporation (Milford, USA). Glass vials (2 ml capacity) were purchased from Agilent Technologies (Boeblingen, Germany). The purified water was obtained using an ELGA water purification system (Greifswald, UK). Individual standard preparations were described previously (Ramaswamy et al. 2011).
Chemical extractions
Water samples were processed to extract the target NSAIDs, including acetylsalicylic acid, according to the method of Togola and Budzinski (2007) with some modifications. Briefly, the water samples were filtered using glass fiber filters, and the pH of the water (500 ml) was adjusted to 2 with 3.5 M HCl using a pH meter (Elico, National Scientific Suppliers, Chennai, India). Oasis HLB SPE cartridges (60 mg/3 ml) were conditioned with 3 ml each of 1:1 (v/v) ethyl acetate/acetone, methanol, and ultrapure water (pH 2). Water samples were loaded onto the cartridges using a vacuum manifold and a flow rate of 5 ml/min. Then, the cartridges were washed with 5 ml of 5 % methanol in water and dried under vacuum pressure for 1 h. Subsequently, the analytes were eluted with 10 ml of 1:1 (v/v) ethyl acetate/acetone, and the collected extracts were evaporated under a gentle stream of nitrogen gas. The analytes were reconstituted in 500 μl ethyl acetate. Finally, 30 μl of MSTFA was added to each sample and incubated at 65 °C for 35 min for complete derivatization, which resulted in increased analyte volatility and improved chromatographic response, i.e., well-resolved peaks. Water quality parameters were analyzed using the American Public Health Association standard methods for the examination of water and wastewater (APHA 1998).
Instrumental analysis
Analyses of NSAIDs, including acetylsalicylic acid, were performed using a gas chromatograph (GC-2010) interfaced with a quadrupole mass spectrometer (QP-2010; Shimadzu Corporation, Japan). Detailed instrumental conditions were published previously (Shanmugam et al. 2010), and the monitoring mass ions for each compound are listed in Table S1 (Supplementary data).
Quality assurance/quality control
Ultrapure water (500 ml) and surface water (500 ml) were spiked with 100 ng/ml of ibuprofen, naproxen, ketoprofen, diclofenac, and acetylsalicylic acid standards, and extracted as mentioned in “Chemical extractions” section. The recovery, precision, and limit of detection (LOD) are summarized in Table S1. The mean recovery percentages of pharmaceuticals, measured in triplicate, ranged from 80.7 ± 14.8 to 95.0 ± 8.3 and 70.4 ± 1.8 to 120 ± 3.6 in ultrapure and surface water matrices, respectively. The precision (%RSD) ranged from 8.7 to 18.3 %. Duplicate analyses of samples were conducted at random to confirm the accuracy and precision of the sampling data. Phenanthrene-d10 (100 ng/ml) was spiked into final sample extracts before analysis as control for GC-MS sensitivity. A blank sample was measured for each batch of five samples to verify that samples were free from any potential contamination. The linearity of the instrument measurement was good for all analytes, with correlation coefficients equal to or greater than 0.988 in the concentration range measured in this study (0.5–200 ng/ml; Table S1). The LOD and LOQ values were calculated as the minimum amount of analyte standard that produced a chromatographic peak with a signal-to-noise ratio of three and ten times the background chromatographic noise, respectively, at the lowest spiking level of the standard in the validation study. LOQ values for the PPCPs were between 0.16 and 0.31 ng/l.
Ecotoxicological risk assessment
The potential environmental risk posed by the contaminants in aquatic systems was assessed in two ways. First, standard toxicity test data for different organisms (see Table 1) from the literature were used to generate hazard quotients (HQs; European Agency for the Evaluation of Medicinal Products 2006). Chronic toxicity data were used preferably; however, if chronic toxicity data were unavailable, acute toxicity data were considered. Predicted no-effect concentrations (PNECs) for aquatic organisms were derived by dividing either the EC50 by an assessment factor (AF) of 1,000 or by dividing the NOEC values by an AF of 10 (European Commission 2003; Ferrari et al. 2004; Hernando et al. 2006; Gros et al. 2010). The HQs for aquatic organisms were calculated for each sampling area and drug, using the highest measured environmental concentrations (MECs) and the lowest PNECs. Due to limitations in the availability of chronic toxicity data, we used a second approach designed to predict the potential to cause a target-related effect in fish from human potency data via application of the critical environmental concentration (CEC; Fick et al. 2010). The CEC is the water concentration of a drug predicted to achieve blood plasma levels in exposed fish equal to that of humans taking the medication. This approach assumes a similar potency at the same target in humans and in fish and estimates the risk that pharmacological interactions will occur in wildlife but may not predict off-target toxicity. The MEC values for each drug were compared with estimated CEC values to generate a margin of safety. The CECs used were 4,560, 48,978, 827,999, 194,711, and 3.4 × 108 ng/l for diclofenac, ketoprofen, naproxen, ibuprofen, and acetylsalicylic acid, respectively (Fick et al. 2010).
Human health risk assessment
A human health risk assessment was performed using estimated daily intake (assuming consumption of 2 l of water per day) and estimated lifetime consumption over 70 years as predictors of total exposure, using the highest concentrations of drugs found in the river water (I 70; Vulliet and Cren-Olive 2011). The calculated daily intake (DI) was compared with the daily therapeutic dose (TD; WHO 2013) for an adult to derive the margin of safety (MOS) for a therapeutic effect.
Results and discussion
Basic physicochemical parameters for the sampled river waters are given in Table S2 (supplementary data); the observed values were within the permissible limits defined by the Central Pollution Control Board (CPCB 1993). Overall, the concentrations of the investigated drugs, except for acetylsalicylic acid, (Fig. 2, Table S3), were in accordance with previous report from other regions (Togola and Budzinski 2007; Helenkar et al. 2010; Komori et al. 2012; Vystavna et al. 2012). The elevated concentrations of acetylsalicylic acid, measured at higher concentrations than other NSAID concentrations in most of the samples, suggest an ongoing release of untreated sewage into the sampled rivers. Acetylsalicylic acid is a very commonly prescribed drug in India (Paul and Chauhan 2005), but local consumption data (kilograms per capita) are not available. It is also a very frequently used drug in Europe, but concentrations in European surface waters are generally measured at lower concentrations than in this study (Moldovan 2006; Baranowska and Kowalski 2011). Thus, even without detailed consumption data, a reasonable interpretation is that the considerably higher concentrations found in Indian rivers are not simply a result of higher usage, but the lack of efficient sewage treatment is a major cause.
Of note, the two substances that are most difficult to remove during sewage treatment, diclofenac and ketoprofen, were consistently found in the Kaveri River at relatively high concentrations, whereas the measured concentrations in the Vellar and Tamiraparani Rivers were considerably lower (Fig. 2; Table S3). Similar to these drugs, carbamazepine in these rivers were reported with 100 % detection frequency (mean of 28.3 ng/l) in our previous study (Ramaswamy et al. 2011) which is also known to be highly recalcitrant. For the two most readily degradable drugs studied, particularly including acetylsalicylic acid but also ibuprofen, the pattern was quite different, with measured concentrations in the Tamiraparani River that were greater than or equal to those found in the Kaveri River. Higher levels of acetylsalicyclic acid and lower levels of ibuprofen observed may be attributed to the prescription pattern in India as given by Paul and Chauhan (2005). They reported that acetylsalicylic acid was prescribed more by 98 % when compared to only 38 % of ibuprofen, by the physicians.
The detected concentrations of naproxen, which is usually removed with an efficiency similar to the removal of ketoprofen, were moderate, as the concentrations in the Kaveri River were on average higher than in the Tamiraparani River and in the Vellar River estuary. Taken together, these data suggest that sites investigated in the Tamiraparani River were more heavily influenced by exposure to untreated sewage but that the overall sewage load in this river is likely lower than that in the Kaveri River. The degradation of chemical contaminants at the sampling sites, the extent of local sewage treatment, and the distance between the sampling sites and wastewater discharge points may all have influenced measured drug concentrations.
Diclofenac concentrations ranged from not detectable (ND) to 103 ng/l in the collected samples, with a maximum concentration observed at Tiruchirappalli, followed by a site near another major town, Thanjavur (approximately 0.2 million residents; 56.3 ng/l) along the Kaveri River. The measured diclofenac concentrations were comparable with those reported in Asia and Europe, despite different degrees of development in sewage infrastructure. Zhao et al. (2010) reported diclofenac concentrations of 8.3 to 114 ng/l at sites along the Pearl River system in China, and Aydin and Talinli (2013) reported concentrations from BDL (below the detectable limit) to 55.7 ng/l from sites in the Buyukcekmece watershed in Turkey. Vystavna et al. (2012) observed diclofenac at 240 ng/l in samples from the Lopan River in Ukraine. Although India and Pakistan banned diclofenac for veterinary purposes in 2006, increased concentrations of diclofenac (700–440 ng/l) were reported in rivers near Karachi, Pakistan (Scheurell et al. 2009), concentrations that are 40 times higher than the maximum concentration observed in the samples from Indian rivers in this study. The diclofenac concentrations reported in rivers from countries with generally well-developed sewage infrastructure, such as Spain, France, Germany, Canada, Italy, Japan, and Taiwan, were comparable to each other (ND–172 ng/l; Table 2). However, rivers in Poland and Hungary (Danube River) demonstrated maximum concentrations of 429 and 930 ng/l, respectively. The present level of diclofenac in accordance with countries of well-developed sewage infrastructure support our previous report on carbamazepine which also concluded/suggested that concentration of more persistent compounds would not depend as much on the levels of sewerage infrastructure.
The concentration of ketoprofen (ND–100 ng/l) was comparable to reported concentrations in rivers in countries with less-developed infrastructure, e.g., ketoprofen was reported at 15 ng/l in the Lopan River, Ukraine (Vystavna et al. 2012), and as BDL in Slovenian rivers (Kosjek et al. 2005). These ketoprofen concentrations were comparable to reported concentrations from rivers in countries with more advanced sewage infrastructure, such as France, Canada, Portugal, Italy, Japan, and Taiwan, with detected ketoprofen concentrations of up to 150 ng/l (Table 2). However, observed concentrations were higher in the Ebro River basin in Spain, with a maximum concentration of 1,060 ng/l (Silva et al. 2011), and in rivers in Poland (258 ng/l; Baranowska and Kowalski 2011) and Sweden (364 ng/l; Daneshvar et al. 2012). It should be stressed that the relative contribution of sewage discharges to the river water is often not reported in these studies but is expected to differ greatly between individual rivers and sampling sites.
The measured naproxen concentrations in this study ranged from ND to 28 ng/l, which is comparable to concentrations measured in Slovenian rivers (ND–80 ng/l; Kosjek et al. 2005) and the Lopan River (15 ng/l) of Ukraine (Vystavna et al. 2012; Table 2). Much higher concentrations were measured in samples from Turkey in the Buyukeekmece watershed (12,300 ng/l; Aydin and Talinli 2013) and in the Karachi River system of Pakistan (32,000 ng/l; Selke et al. 2010). These higher concentrations suggest that there could be additional sources of naproxen entering surface waters other than human usage. Peng et al. (2008) also reported higher naproxen concentrations in the Pearl River, China (ND–328 ng/l). In most countries with well-developed sewage infrastructure, the concentrations of naproxen are generally measured at a few hundred nanograms per liter (Table 2), with the exception at one sampled site in Canada (ND–4,500 ng/l; Brun et al. 2006).
Ibuprofen concentrations in Indian rivers (ND to 200 ng/l) were comparable to those found in several regions, including the Pearl River system of China (ND–113 ng/l; Zhao et al. 2010), the Somes River of Romania (ND–115 ng/l; Moldovan 2006), and the Buyukcekmece watershed of Turkey (BDL–263). However, ibuprofen was measured at considerably higher concentrations in the Nairobi River basin in Kenya (10,000–30,000 ng/l; Koreje et al. 2012). Somewhat higher concentrations were also found in the surface waters of Asian countries with relatively well-developed infrastructure, such as the Sindian River in Taiwan (4,350 ng/l; Lin et al. 2010) and the Mankyung River in South Korea (414 ng/l; Kim et al. 2009). River samples from Canada, France, Sweden, Germany, and Spain demonstrated concentrations ranging from 541 to 6,400 ng/l, which are higher than the concentrations observed in the present study (Table 2).
Acetylsalicylic acid was found in all of the water samples, with the highest concentration found in samples from Thanjavur (660 ng/l), followed by samples from the Pichavaram mangrove forest on the Kaveri River (640 ng/l). Higher concentrations were observed at Pabanasam (540 ng/l) and Tenkasi (520 ng/l), both sites downstream of Servalar in the Tamiraparani river and Kutrallam Waterfalls in the Tamiraparani tributary, respectively. Unlike the other NSAIDs, the concentrations of acetylsalicylic acid quantified in samples in this study were much higher than in European rivers, such as the Somes River in Romania (ND–37.2 ng/l; Moldovan 2006), the Seine River estuary of France (<1.6–85.8 ng/l; Togola and Budzinski 2007), and rivers in Poland (ND–0.4 ng/l; Baranowska and Kowalski 2011). Since we have only sampled once, higher as well as lower levels of the investigated drugs may be present at different times.
Hazard quotients for each drug in the three rivers, based on the highest MEC, were assessed and are summarized in Table 1. Most reported PNEC values were much higher than the environmental concentrations observed. The HQ values for NSAIDs suggest low risk to the aquatic environment. This analysis suggests that the aquatic organisms from the three Indian rivers sampled in this study are not expected to be adversely affected by these drugs individually. In reality, however, sentinel organisms are exposed to all of these drugs together as a mixture. Currently, the potential risks posed by the chemicals as a mixture are unknown.
If we take into account non-standardized tests and endpoints reported in the literature, NSAIDs may pose larger risks. For instance, Schwaiger et al. (2004) reported the LOEC for diclofenac in a species of fish, Oncorhynchus mykiss, as 5 μg/l, a concentration that induced both renal lesions and alterations in gills. Even lower concentrations of diclofenac (1–1.6 μg/l) induced cytological alterations and differential expression of genes in major tissues, including the liver, kidney, gills, and plasma of O. mykiss (Triebskorn et al. 2004; Mehinto et al. 2010; Cuklev et al. 2011). However, these diclofenac LOECs are higher than the MECs of diclofenac in samples from the investigated Indian rivers. A recent study reported perturbations in reproductive function in fish exposed to ibuprofen at concentrations as low as 1 μg/l (Ji et al. 2013). The concentrations of ibuprofen found in this study (up to 200 ng/l) were below 1 μg/l, but the margin of safety is still relatively small.
By comparing the maximum concentration detected of each pharmaceutical to its CEC value (Fick et al. 2010), margin of safety ratios were derived, as follows: diclofenac, 44; ketoprofen, 489; naproxen, 29,571; ibuprofen, 973; and, acetylsalicylic acid, 515,151. Again, with the possible exception of diclofenac, the risk of target-related pharmacological interactions, at least in fish, appears minor. The very high margin of safety for acetylsalicylic acid is a reflection of its much lower log K ow (1.2) and diminished bioaccumulation potential compared with the other NSAIDs.
The estimated daily intake value through human consumption of river water was highest for acetylsalicylic acid (1.32 μg/day) and lowest for naproxen (0.056 μg/day; Table 3). The maximum lifetime intake (I 70) of acetylsalicylic acid was 33,726 μg, which is equivalent to approximately 1 % of a single daily intentional dose by an adult. In case of other NSAIDs, the I 70 was as high as about 5 % of a single daily intentional dose for diclofenac and the lowest was observed for naproxen (0.2 %). Having said this, lifetime intake is most likely an irrelevant risk measure for chemicals that are as rapidly eliminated from the human body as the drugs investigated here. The estimated margin of safety to reach the therapeutic dose would be between 100,000 and 8 million, clearly indicating an apparent lack of risk from the investigated drugs via water consumption from the rivers sampled in this study. Similar human health risk assessment exercises have been performed for drinking water sources in France (Vulliet and Cren-Olive 2011) and Germany (Webb et al. 2003).
Conclusions
We conclude that in India, a country with inconsistent sewage control, more readily degradable pharmaceuticals may be present at elevated concentrations in the environment. Indeed, in the three sampled rivers in this study, acetylsalicylic acid, which is very easily removed in modern sewage treatment plants, was found at elevated concentrations in all surface water samples analyzed. Further, the lack of removal of easily degradable pharmaceuticals or emerging chemicals may pose environmental risks. As reliable pharmaceutical usage data are difficult to obtain in India, measured environmental concentrations are critically important for performing risk assessments. The calculated HQs in this study suggest a low risk to the aquatic environment from the investigated NSAIDs. No human health risks from river water consumption were identified for the drugs sampled in this study. To our knowledge, this is the first report on the occurrence and risk assessment of anti-inflammatory drugs in surface water in India.
References
American Public Health Association (1998) Standard methods for the examination of water and waste water. American Public Health Association, Washington
Aydin E, Talinli I (2013) Analysis, occurrence and fate of commonly used pharmaceuticals and hormones in the Buyukcekmece watershed, Turkey. Chemosphere 90:2004–2012
Baranowska I, Kowalski B (2011) Using HPLC method with DAD detection for the simultaneous determination of 15 drugs in surface water and wastewater. Polish J Environ stud 20:21–28
Behera SK, Kim HW, Oh JE, Park HS (2011) Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci Total Environ 409:435–4360
Brown JN, Paxeus N, Forlin L, Larsson DGJ (2007) Variations in bioconcentration of human pharmaceuticals from sewage effluents into fish blood plasma. Environ Toxicol Pharmacol 2:267–274
Brun GL, Bernier M, Losier R, Doe K, Jackman P, Lee HB (2006) Pharmaceutically active compounds in Atlantic Canadian sewage treatment plant effluents and receiving waters, and potential for environmental effects as measured by acute and chronic aquatic toxicity. Environ Toxicol Chem 25:2163–2176
Central Pollution Control Board (1993) Accessed from http://www.hppcb.gov.in/eiasorang/spec.pdf on 04/03/2013
Cleuvers M (2004) Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicol Environ Saf 59:309–315
Cuklev F, Kristiansson K, Fick J, Asker N, Forlin L, Larsson DGJ (2011) Diclofenac in fish–blood plasma levels similar to human therapeutic levels affect global hepatic gene expression. Environ Toxicol Chem 30:2126–2134
Daneshvar A, Svanfelt J, Kronberg L, Weyhenmeyer G (2012) Neglected sources of pharmaceuticals in river water footprints of a Reggae festival. J Environ Monit 14:596–603
Desbrow C, Routledge EJ, Brighty GC, Sumpter JP, Waldock M (1998) Identification of estrogenic chemicals in STW effluents. 1. Chemical fractionation and in vitro biological screening. Environ Sci Technol 32:1549–1558
Diwan V, Tamhankar AJ, Aggarwal M, Sen S, Khandal RK, Lundborg CS (2009) Detection of antibiotics in hospital effluents in India. Current 97:12–15
European Agency for the Evaluation of Medicinal Products (2006) Committee for Medicinal Products for Human Use Guideline on the environmental risk assessment of medicinal products for human use. Ref EMEA/CRMP/SWP/4447/00. London, UK
European Commission (2003) Technical Guidance Document in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances and Commission Regulation (EC) No 1488/94 on Risk Assessment for Existing Substances, Part II. Brussels, Belgium
Farre ML, Ferrer I, Ginebreda A, Figueras M, Olivella L, Tirapu L, Vilanova M, Barcelo D (2001) Determination of drugs in surface water and wastewater samples by liquid chromatography-mass spectrometry: methods and preliminary results including toxicity studies with Vibrio fischeri. J Chromatogr A 938:187–197
Ferrari B, Mons R, Vollat B, Fraysse B, Paxeus N, Lo Giudice R, Pollio A, Garric J (2004) Environmental risk assessment of six human pharmaceuticals: are the current environmental risk assessment procedures sufficient for the protection of the aquatic environment? Environ Toxicol Chem 23:1344–1354
Fick J, Soderstrom H, Lindberg RH, Phan C, Tysklind M, Larsson DGJ (2009) Contamination of surface, ground, and drinking water from pharmaceutical production. Environ Toxicol Chem 28:2522–2527
Fick J, Lindberg RH, Tysklind M, Larsson DGJ (2010) Predicted critical environmental concentrations for 500 pharmaceuticals. Regul Toxicol Pharmacol 58:516–523
Fram MS, Belitz K (2011) Occurrence and concentrations of pharmaceutical compounds in groundwater used for public drinking-water supply in California. Sci Total Environ 409:3409–3417
Gros M, Petrovic M, Ginebreda A, Barcelo D (2010) Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ Int 36:15–26
Gunnarsson L, Jauhiainen A, Kristiansson E, Nerman O, Larsson DGJ (2008) Evolutionary conservation of human drug targets in organisms used for environmental risk assessments. Environ Sci Technol 42:5807–5813
Helenkar A, Sebok A, Zaray G, Molnar Perl I, Vasanits-Zsigrai A (2010) The role of the acquisition methods in the analysis of the non-steroidal anti-inflammatory drugs in Danube river by gas chromatography–mass spectrometry. Talanta 82:600–607
Hernando MD, Mezcua M, Fernandez-Alba AR, Barcelo D (2006) Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 69:334–342
Ji K, Liu X, Lee S, Kang S, Kho Y, Giesy JP, Choi K (2013) Effects of non-steroidal anti-inflammatory drugs on hormones and genes of the hypothalamic-pituitary-gonad axis, and reproduction of zebrafish. J Hazard Mater 254–255:242–251
Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, Flick RW (2007) Collapse of a fish population after exposure to a synthetic estrogen. Proc Natl Acad Sci USA 104:8897–8901
Kamala K, Dinesh Kumar B (2006) Drug consumption pattern in urban and rural areas of India and their health implications. Proceedings of symposium on primary health care—new initiatives. Nutrition Foundation of India, New Delhi, pp 140–148
Kim JW, Jang HS, Kim JG, Ishibashi H, Hirano M, Nasu K (2009) Occurrence of pharmaceutical and personal care products (PPCPs) in surface water from Mankyung River, South Korea. J Health Sci 55:249–258
Komori K, Suzuki Y, Minamiyama M, Harada A (2012) Occurrence of selected pharmaceuticals in river water in Japan and assessment of their environmental risk. Environ Monit Assess. doi:10.1007/s1066101228864
Koreje K, Demeestere K, Wispelaere PD, Vergeynst L, Dewulf J, Langenhove HV (2012) From multiresidue screening to target analysis of pharmaceuticals in water: development of a new approach based on magnetic sector mass spectrometry and application in the Nairobi River basin, Kenya. Sci Tot Environ 437:153–164
Kosjek T, Heath E, Krbavcic A (2005) Determination of non-steroidal anti-inflammatory drug (NSAIDs) residues in water samples. Environ Inter 31:679–685
Kristiansson E, Fick J, Janzon A, Grabic R, Rutgersson C, Weijdegard B, Soderstrom H, Larsson DGJ (2011) Pyrosequencing of antibiotic-contaminated river sediments reveals high levels of resistance and gene transfer elements. PLoS One 6:e17038
Larsson DGJ, Adolfsson-Erici M, Parkkonen J, Pettersson M, Berg H, Olsson PE, Forlin L (1999) Ethinyloestradiol—an undesired fish contraceptive? Aquat Toxicol 45:91–97
Larsson DGJ, de Pedro C, Paxeus N (2007) Effluent from drug manufactures contains extremely high levels of pharmaceuticals. J Hazard Mater 148:751–755
Lin AY, Yu TH, Lateef SK (2009) Removal of pharmaceuticals in secondary wastewater treatment processes in Taiwan. J Hazard Mater 167:1163–1169
Lin AYC, Panchangam SC, Chen HY (2010) Implications of human pharmaceutical occurrence in the Sindian river of Taiwan: a strategic study of risk assessment. J Environ Monit 12:261–270
Mehinto AC, Hill EM, Tyler CR (2010) Uptake and biological effects of environmentally relevant concentrations of the nonsteroidal anti-inflammatory pharmaceutical diclofenac in rainbow trout (Oncorhynchus mykiss). Environ Sci Technol 44:2176–2182
Memmert U, Peither A, Burri R, Weber K, Schmidt T, Sumpter JP (2013) Diclofenac: new data on chronic toxicity and bioconcentration in fish. Environ Toxicol Chem 32:442–452
Meyer B, Pailler JY, Guignard C, Hoffmann L, Krein A (2011) Concentrations of dissolved herbicides and pharmaceuticals in a small river in Luxembourg. Environ Monit Assess 180:127–146
Moldovan Z (2006) Occurrences of pharmaceutical and personal care products as micropollutants in rivers from Romania. Chemosphere 64:1808–1817
Oaks JL, Gilbert M, Virani MZ, Watson RT, Meteyer CU, Rideout BA, Shivaprasad HL, Ahmed S, Chaudhry MJI, Arshad M, Mahmood S, Ali A, Khan AA (2004) Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 427:630–633
Patrolecco L, Ademollo N, Grenni P, Tolomei A, Barra Caracciolo A, Capri S (2013) Simultaneous determination of human pharmaceuticals in water samples by solid phase extraction and HPLC with UV-fluorescence detection. Microchem J 107:165–171
Paul AD, Chauhan CK (2005) Study of usage pattern of nonsteroidal anti-inflammatory drugs (NSAIDs) among different practice categories in Indian clinical setting. Eur J Clin Pharmacol 60:889–892
Peng X, Yu Y, Tang C, Tan J, Huang Q, Wang Z (2008) Occurrence of steroid estrogens, endocrine-disrupting phenols, and acid pharmaceutical residues in urban riverine water of the Pearl River Delta, South China. Sci Total Environ 397:158–166
Quinn B, Gagne F, Blaise C (2008) An investigation into the acute and chronic toxicity of eleven pharmaceuticals (and their solvents) found in wastewater effluent on the cnidarians, Hydra attenuata. Sci Total Environ 389:306–314
Ramaswamy BR, Shanmugam G, Velu G, Rengarajan B, Larsson DGJ (2011) GC-MS analysis and ecotoxicological risk assessment of triclosan, carbamazepine and parabens in Indian Rivers. J Hazard Mater 186:1586–1593
Sanchez-Prado L, Garcia-Jares C, Llompart M (2010) Microwave-assisted extraction: application to the determination of emerging pollutants in solid samples. J Chromatogr A 1217:2390–2414
Santos JL, Aparicio I, Alonso E (2007) Occurrence and risk assessment of pharmaceutically active compounds in wastewater treatment plants. A case study: Seville city (Spain). Environ Int 33:596–601
Santos JL, Aparicio I, Callejon M, Alonso E (2009) Occurrence of pharmaceutically active compounds during 1-year period in wastewaters from four wastewater treatment plants in Seville (Spain). J Hazard Mater 164:1509–1516
Scheurell M, Franke S, Shah RM, Huhnerfuss H (2009) Occurrence of diclofenac and its metabolites in surface water and effluent samples from Karachi, Pakistan. Chemosphere 77:870–876
Schwaiger J, Ferling H, Mallow U, Wintermayr H, Negele RD (2004) Toxic effects of the non-steroidal anti-inflammatory drug diclofenac. Part I. Histopathological alterations and bioaccumulation in rainbow trout. Aquat Toxicol 68:141–150
Selke S, Scheurell M, Raza M, Hühnerfuss H (2010) Identification and enantioselective gas chromatographic mass-spectrometric separation of O-desmethylnaproxen, the main metabolite of the drug naproxen, as a new environmental contaminant. J Chromatogr A 1217:419–423
Shanmugam G, Ramaswamy BR, Radhakrishnan V, Tao H (2010) GC-MS method for the determination of paraben preservatives in the human breast cancerous tissue. Microchem J 96:391–396
Silva BFD, Jelic A, Lopez Serna R, Mozeto AA, Petrovic M, Barcelo D (2011) Occurrence and distribution of pharmaceuticals in surface water, suspended solids and sediments of the Ebro river basin, Spain. Chemosphere 85:1331–1339
Sim WJ, Lee JW, Oh JE (2010) Occurrence and fate of pharmaceuticals in wastewater treatment plants and rivers in Korea. Environ Pollut 158:1938–1947
Sousa MA, Goncalves C, Cunha E, Hajslova J, Alpendurada MF (2011) Cleanup strategies and advantages in the determination of several therapeutic classes of pharmaceuticals in wastewater samples by SPE–LC–MS/MS. Anal Bioanal Chem 399:807–822
Suarez S, Lema JM, Omil F (2010) Removal of pharmaceutical and personal care products (PPCPs) under nitrifying and denitrifying conditions. Water Res 44:3214–3224
Ternes T, Bonerz M, Schmidt M (2001) Determination of neutral pharmaceuticals in wastewater and rivers by liquid chromatography–electrospray tandem mass spectrometry. J Chromatogr A 938:175–185
Thorpe KL, Cummingsm RI, Tutchinson TH, Scholz M, Brighty G, Sumpter JP (2003) Relative potencies and combination effects of steroidal estrogens in fish. Environ Sci Technol 37:1142–1149
Togola A, Budzinski H (2007) Analytical development for analysis of pharmaceuticals in water samples by SPE and GC-MS. Anal Bioanal Chem 388:627–635
Triebskorn R, Casper H, Heyd A, Eikemper R, Kohler HR, Schwaiger J (2004) Toxic effects of the non-steroidal anti-inflammatory drug diclofenac. Part II: Cytological effects in liver, kidney, gills and intestine of rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 68:151–166
Vulliet E, Cren-Olive C (2011) Screening of pharmaceuticals and hormones at the regional scale, in surface and groundwaters intended to human consumption. Environ Pollut 159:292–2934
Vystavna Y, Huneau F, Grynenko V, Vergeles Y, Celle-Jeanton H, Tapie N, Budzinski H, Coustumer PL (2012) Pharmaceuticals in rivers of two regions with contrasted socio-economic conditions: occurrence, accumulation, and comparison for Ukraine and France. Water Air Soil Pollut 223:2111–2124
Webb S, Ternes T, Gibert M, Olejniczak K (2003) Indirect human exposure to pharmaceuticals via drinking water. Toxicol Lett 142:157–167
World Health Organization (2013) Accessed from http://www.whocc.no/atc_ddd_index/ on 04/03/2013
Xu Y, Luo F, Pal A, Gin KY, Reinhard M (2011) Occurrence of emerging organic contaminants in a tropical urban catchment in Singapore. Chemosphere 83:963–969
Yoon Y, Ryu J, Oh J, Choi BG, Snyder SA (2010) Occurrence of endocrine disrupting compounds, pharmaceuticals, and personal care products in the Han River (Seoul, South Korea). Sci Total Environ 408:636–643
Zhang Y, Geissen SU, Gal C (2008) Carbamazepine and diclofenac: removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 73:1151–1161
Zhao JL, Ying GG, Liu YS, Chen F, Yang JF, Wang L, Yang XB, Stauber JL, Warne MS (2010) Occurrence and a screening-level risk assessment of human pharmaceuticals in the Pearl River system, South China. Environ Toxicol Chem 29:1377–1384
Acknowledgments
The authors wish to thank the United Nations University, Tokyo, Japan; SHIMADZU Corporation, Japan for the GC-MS facility established at Bharathidasan University through the project titled, “POPs Monitoring in Asian Coastal Hydrosphere;” and Bharathidasan University. SG is grateful to the University Grant Commission (UGC), New Delhi for the Research Fellowship award through a non-SAP grant to the Department of Environmental Biotechnology, Bharathidasan University, India; the Swedish Research Council; and the Swedish Foundation for Strategic Environmental Research (MISTRA).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hongwen Sun
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 34 kb)
Rights and permissions
About this article
Cite this article
Shanmugam, G., Sampath, S., Selvaraj, K.K. et al. Non-steroidal anti-inflammatory drugs in Indian rivers. Environ Sci Pollut Res 21, 921–931 (2014). https://doi.org/10.1007/s11356-013-1957-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1957-6