Abstract
The Pearl River Estuary (PRE) is vulnerable due to the increasingly serious environmental pollution, such as phthalate esters (PAEs) contaminants, from the Pearl River Delta (PRD). The concentrations of six US Environmental Protection Agency (USEPA) priority PAEs in water and surface sediments collected from the PRD’s six main estuaries in spring, summer, and winter 2013 were measured by GC-MS. Total PAEs (∑6PAEs) concentrations were from 0.5 to 28.1 μg/L and from 0.88 to 13.6 μg/g (dry weight (DW)) in water and surface sediments, respectively. The highest concentration was detected in summer. Higher concentrations of PAEs were found in Yamen (YM) and Humen (HM) areas than the other areas. Bis(2-ethylhexyl)phthalate (DEHP) and dibutyl phthalate (DBP) were the dominant PAEs in the investigated areas, contributing between 61 and 95 % of the PAEs in water and from 85 to 98 % in surface sediments. Based on risk quotients (RQs), DEHP posed greater ecological risks to the studied aquatic environments than other measured compounds. Little human health risk from the target PAEs was identified.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Pearl River is the third largest river in China. Where it meets the sea, the river forms the complex, branched Pearl River Delta (PRD) which is subtropical monsoon climate whose feature is the largest amount of rainfall in summer while the least in winter. The PRD region covers an area of ∼54,000 km2, within which a population of more than 42 million people lives. As one of the most rapidly developing and densely populated area in southern China, there is concern about trace organic chemical contamination by polybrominated diphenyl ethers (PBDEs), endocrine-disrupting chemicals (ECDs), polycyclic aromatic hydrocarbons (PAHs), etc. (Chen et al. 2013; Feng et al. 2012; Zhang et al. 2011). Phthalate esters (PAEs) are widely used in plastic products, building materials, pesticides, automobile parts, cosmetics, and food packaging (Gómez-Hens and Aguilar-Caballos 2003; Hu et al. 2003). In China, the amount of the production and consumption of PAEs is about 1.3 million t/year (He et al. 2013), or at least 20 % of worldwide consumption (more than 6 million t/year; Arbeitsgemeinschaft and Umwelt 2006; Zhiyong et al. 2007). PAEs are easily leached into the environment (Dargnat et al. 2009) and have been found in the atmosphere, sediments, water, soil, and biota, including humans worldwide (Cai et al. 2008; Hongjun et al. 2013; Liu et al. 2014; Sun et al. 2013; Wang et al. 2012).
Aquatic environments may be polluted by PAEs through various ways including discharge wastewater, surface run-off (Adeniyi et al. 2011; Fan et al. 2008; Fromme et al. 2002; He et al. 2013; Liu et al. 2014; Sun et al. 2013; Yuan et al. 2002; Zeng et al. 2008), and because they bio-accumulate and recalcitrance in aquatic organisms, PAEs pose great threats to aquatic ecosystems (Adeniyi et al. 2011; Liu et al. 2014; Sun et al. 2013). Xu et al. (2013a) showed that some PAEs, such as DBP and diethyl phthalate (DEP), have potential neurotoxicity to embryos of zebra fish by inhibiting the activity of acetyl cholinesterase. Other studies have shown that most of PAEs produce endocrine-disrupting effects, for example, developmental and reproductive toxicities in mammals (Chen et al. 2011; Gray et al. 2000; Harris et al. 1997; Horn et al. 2004; Matsumoto et al. 2008). Consequently, there is concern that humans may be exposed to PAEs not only by consuming contaminated surface water but also by feeding on aquatic organisms.
The USEPA has classified DEP, DBP, BBP, DEHP, dimethyl phthalate (DMP), and di-n-octyl phthalate (DnOP) as top-priority contaminations (Keith and Telliard 1979). Li et al. (2015) suggested that the six priority PAEs pose different levels of ecological risk to aquatic ecosystems and that the different sensitivities of different aquatic species are neglected when assessing risk. Indeed, the human health risk of the six priority PAEs from the PRE is still unknown.
In this context, the major objectives of the paper were (a) to investigate the seasonal variation and spacial distribution of top-priority PAEs in the water and surface sediments in the estuaries of PRD, (b) to assess the risk of the dissolved PAEs to aquatic environment in the sampling sites for three different kinds of aquatic organism, and (c) to evaluate noncarcinogenic and carcinogenic risks of studied PAEs in water for female and male residents via bathing and drinking water routes.
Material and methods
Chemicals and reagents
Analytical standard mixture including dimethyl phthalate (DMP), diethyl phthalate (DEP), di(2-ethylhexyl) phthalate (DEHP), di-n-octyl phthalate (DnOP), butylbenzyl phthalate (BBP), di-n-butyl phthalate (DBP) in isooctane at 1 g/L each, and surrogate standards, consisting of diisophenyl phthalate, di-n-phenyl phthalate, di-n-benzyl phthalate, and an internal standard (benzyl benzoate) in acetone at 0.5 mg/L each were purchased from Dr Ehrenstorfer GmbH (Germany). HPLC grade n-hexane was obtained from Secco romeo Corporation (Tianjin, China). Neutral silica gel (100–200 mesh) was activated at 500 °C for 8 h and then deactivated by adding 5 % distilled water. Anhydrous sodium sulfate was baked at 420 °C for 12 h and stored in sealed glass jars. All the glassware used for organic compounds analyses was burned at 450 °C for 6 h prior to use.
Sample collection and pretreatment
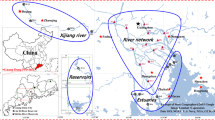
There are six major estuaries in the PRD, namely the Humen (HM), Jiaomen (JM), Hongqimen (HQ), Modaomen (MD), Jitimen (JT), and Yamen (YM).
Sampling campaigns of surface sediments and water samples at the six major estuaries (seven riverine outlets), as shown in Fig. 1, of the PRD were carried out in spring (2–4 April 2013), summer (on 25–27 June 2013), and winter (on 10–15 January 2013). A clean brown glass bottles (1 L) were used to collect water samples 500 mm below the surface; these were closed with Teflon lids. Samples of surface sediments (0–5 cm) were collected with a gravity sampler and then stored in clean aluminum foil envelopes. To avoid tidal influences, three samples were collected during the falling tide at each investigated areas. Water samples were filtered through glass fiber filters (Whatman GF/F, 0.45-μm pore sizes), the pH adjusted to 2, then kept at 4 °C freezer prior to further treatment, usually within 3 days. The sediment samples were stored at −20 °C until further analyses.
The pretreatment methods for water and sediment samples were similar to those of Liu et al. (2010) and Zeng et al. (2008), with only minor modification. Briefly, the filtered water was spiked with 20 μL of a surrogate standards stock solution and then extracted with 90 mL (3 × 30 mL) dichloromethane (DCM). The extracts were dried with anhydrous sodium sulfate and evaporated to approximately 2.0 mL using a rotary evaporator. The sediment samples were freeze-dried, ground, homogenized, and then spiked with 20 μL of 0.1 mg/L surrogate standards into 20 g of sediment samples. Activated copper granules were added to the samples to remove elemental sulfur prior to extraction using acetone/n-hexane (1:1, v:v). Extracting was repeated three times. The supernatants were merged, concentrated until nearly dry, and then inverted into 10 mL n-hexane. The hexane solution was then further reduced to 2.0 mL using a rotary evaporator. Both water and sediment concentrates were further purified on a prepared silica gel glass column filled (from bottom to top) with 1.5 g anhydrous sodium sulfate, 10-g neutral silica gel, and 1.5 g anhydrous sodium sulfate. The column was washed by 40 mL n-hexane and PAEs eluted by 80 mL of diethyl ether/n-hexane (3:7, v/v). The eluent was evaporated to 1 mL by a rotary evaporator and then further reduced to almost dryness under a stream of pure N2. Redissolve the extracts in 1 mL of HPLC grade n-hexane for analysis. Two replicate samples were performed.
Instrumental analysis
A Thermo Trace DSQ GC-MS with a TG-1MS capillary column (30 m × 0.25 mm, 0.25-μm film thickness) in electron impact and selective ion monitoring (SIM) mode were used to determine PAEs in water and surface sediments extracts. The transfer line temperature was kept at 280 °C, and the ion source was maintained at 250 °C. Initial oven temperature was set at 40 °C for 2.0 min, then increased to 110 °C at 35 °C/min, ramped to 130 °C at 15 °C/min, then at 10 °C/min up to 270 °C (1.0 min held), and finally increased at 20 °C/min to 280 °C (10.0 min held) finally. The sample extracts (1.0 μL) were injected into GC-MS at an injector temperature of 250 °C in splitless mode. Helium gas with a speed of 1 mL/min was used as a carrier. The solvent delay was 4.0 min. The benzyl benzoate as internal calibration method was used to quantity individual PAEs based on a five-point calibration curve. Concentrations of PAEs in surface sediments were normalized to dry weight (DW).
Quality control and quality assurance
The QA/QC procedures that included analysis of spiked blanks, procedural blanks, matrix spiked, solvent blanks, and sample duplicates were conducted. Only low concentrations of DBP (0.022 μg/L) and DEHP (0.042 μg/L) were detected in the procedural blanks. The blank values were used to correct the results. The relative standard deviations (RSD) of all samples were below 10 % and the recoveries of six PAEs were within acceptable limits ranging from 76 to 106 %. Instrumental detection limits (IDL) were based on three times the signal-to-noise ratio. The calculated IDL (mean) values for DEP, DMP, DnOP, DEHP, DBP, and BBP were 0.078, 0.041, 0.063, 0.12, 0.020, and 0.054 ng, respectively. The method detection limits (MDLs) for the six PAEs were ranged from 0.38 to 3.68 ng/L in water and 0.11 to 4.31 ng/g (dw).
Risk assessment
Risk quotients (RQs) calculation of individual PAEs on the basis of the European technical guidance document about risk evaluation were used to estimate the ecological risk for the three sensitive aquatic species (EC, 2003). The assessment was based on the measured environmental concentration (MEC) in water samples compared to the predicted no-observed-effect concentrations (PNEC). PNEC was calculated based on no observed effect concentration (NOEC) or median effective concentrations (EC50), which were divided by an assessment factor (AF) (Gros et al. 2010). The average concentration of PAEs and three types of aquatic organisms (fish, crustaceans, algae) were used to assess the risk. The toxicity data and AF of the selection for six PAEs were collected from literatures and the database of US EPA (http://cfpub.epa.gov/ecotox/; Table 1) The estimated environment risk could be considered negligible if RQ < 0.1, low within the 0.1–1, moderate from 1 to 10, and high if RQ > 10 (Cristale et al. 2013).
Human health risk assessment
Human health risk assessment was performed assuming that residents may be exposed to phthalate esters in several ways with oral ingestion from drinking water considered to be the most important route (Das et al. 2014). The carcinogenic risk of DEHP and BBP, and the noncarcinogenic risks of individual PAEs were estimated based on the methods proposed by US EPA (2013) and Hamidin et al. (2008). The equation of hazard index (HI) of noncarcinogenic compounds risk is as follows:
where RfD is defined as the maximum acceptable concentration of pollutants everyday (mg/kg/day) and E is the average daily doses of contaminants (mg/kg/day) via drinking water or bathing. The value is calculated applying two approaches as follows:
where E 1 is the exposure to water via the oral ingestion and E 2 is for bathing, CC is the conversion coefficient, RM is the residual ratio factor of individual PAEs after waterworks, C is the highest detected concentration of PAEs in the water (mg/L), and IRw is the daily intake rate. EF, ED, BW, and H represent the exposure frequency, the exposure duration, body weight (kg), and body height (cm), respectively. AT is the average length of life (days), A sb is the body surface area (cm2), TE is the time of bathing (h), FE is the frequency of bathing (/day), f is the intestinal absorption coefficient, k is the skin absorption parameters (cm/h), and τ is the detention time for individual PAEs in the body (1 h).
The carcinogenic risk (R) of DEHP and BBP was calculated using following equation:
where SF is the slope factor ([mg/kg/day]−1). The values of each parameter for the model are listed in Table 2. The acceptable level of carcinogenic risk (R) of chemical contaminates was less than 1 × 10−6. For the noncarcinogenic hazard index (HI), the limit was 1.0. The standards were suggested by USEPA.
Statistical analysis
All data were indicated as means ± standard error of replicates. The statistical analyses were conducted with Origin 8.0 for Windows.
Results and discussion
Seasonal variation and spatial distribution of ∑6PAEs concentrations both in water and surface sediments
The ∑6PAEs concentrations in the water were 7.6, 16.5, and 2.0 μg/L (range from 2.78 to 20.7, 1.27 to 28.1, and 0.50 to 5.03 μg/L) in spring, summer, and winter, respectively (Table 3). That the highest concentrations of total PAEs were found in summer and the lowest levels were in winter is in accordance with data on other pollutants in this region, with urban stormwater runoff contributing PAEs during summer into the estuary and atmospheric deposition (Feng et al. 2009; Gevao et al. 2008; Seung-Kyu and Kurunthachalam 2007; Xu et al. 2013b).
The highest concentration of ∑6PAEs was found at site S7 (2.05–28.1 μg/L; Fig. 2a), particular in summer, mainly due to that its location was in the downstream of emerging industrial cities such as Zhongshan and Jiangmen. For similar reasons, S6 also had relatively high contamination by PAEs (1.53–23.5 μg/L) in summer. However, after S7, site S2 had the highest concentrations of ∑6PAEs (3.35–25.9 μg/L) perhaps because of the influence of Guangzhou and Dongguan, which are densely populated, heavy industrial, and commercial cities discharging a great amount of PAEs (Pei et al. 2013; Peng et al. 2008). Relatively low levels of total PAEs were found in S4 (0.53–7.59 μg/L) and S5 (0.50–9.72 μg/L), since their location was in Nansha scenic region where there are fewer emission sources.
In sediments, the average concentration of ∑6PAEs was 1.82 μg/g (dw), 5.31 μg/g (dw), and 2.97 μg/g (dw) in spring, summer, and winter, respectively (Table 3). Sediments exhibit different seasonal trends in ∑6PAEs compared to water samples, with the highest levels in summer and the lowest levels in spring. The distribution of PAEs was similar to that observed in rivers elsewhere (Adeniyi et al. 2011; Mackintosh et al. 2006; Sha et al. 2007). This might be because water temperature in spring was higher than that in winter and the biodegradation of PAEs was accelerated during spring (Liu et al. 2010).
Generally, the concentration of ∑6PAEs in sediments was higher in western outlets than eastern ones, except for site S1 (Fig. 2b), because the rate of deposition of sediments in the west of the PRD is faster than the east and may absorb more PAEs (Gan et al. 2002). An interesting phenomenon was observed in S4 where the total concentration of PAEs was highest in sediments and lowest in water in spring. That can be explained by the attenuation/exchange process of the daily tides from the SCS. The relationship maybe as a result of the PAEs’ balanced/unbalanced distribution activity among water, particles phase, and sediments (Doong and Lin 2004; Shi et al. 2005).
PAEs congeners distribution in water and surface sediments
Of the six PAEs studied, DBP (0.06–14.8 μg/L) and DEHP (0.81–12.5 μg/L) were ubiquitously in the water samples (Table 3; Fig. 3). Other main congeners found in water were DEP (n.d.–0.95 μg/L) and DMP (n.d.–12.1 μg/L). The order of average content of six PAEs was DEHP > DBP > DMP > BBP > DnOP > DEP in total three seasons; a similar distribution was found by Feng et al. (2009). The concentrations of DEHP in spring and summer at S2 and the contents of DBP in summer at the same site exceeded the acceptable values for these chemicals in surface water in China (8 μg/L for DEHP and 3 μg/L for DBP, respectively; Ministry of Environmental Protection of the People’s Republic of China and General Administration of Quality Supervision, 2002).
In the sediments, DEHP (0.47–8.53 μg/g (dw)), DBP (0.05–4.66 μg/g (dw)), and DMP (n.d.–3.39 μg/g (dw)) were the dominant species (Table 3; Fig. 3). In this context, the concentration of both DEHP and DBP in the surface sediments in the investigated areas was similar to those in topsoil of Bincheng District of Yellow River Delta, lower than those in the rivers in Taiwan, Yellow River, and Yangtze River, but higher than Jianghan Plain (Fan et al. 2008; Hongjun et al. 2013; Liu et al. 2010; Sha et al. 2007; Yuan et al. 2002). Correlation analysis of target compounds in water and surface sediments was conducted. Pearson correlation coefficient values of DBP and DEHP (p < 0.05, r ≥ 0.779), and DnOP and DEP (p < 0.05, r ≥ 0.814) indicated the significant correlations in between. Furthermore, individual PAE congener in water and surface sediments was highly correlated to the∑6PAEs concentration. The value between DBP and the ∑6PAEs concentration in water was 0.897, and between DEHP and the ∑6PAEs concentration in surface sediments was 0.953 (p < 0.01). It was indicated that DEHP and DBP were predominant in surface sediments and water of the PRE, respectively (Fig. 3).
Ecological risk assessment of PAEs in water
The average concentrations of individual PAEs in water were used to assess the aquatic toxicity by risk quotient (RQ) calculation. Overall, DMP and DEP posed no or very low risk to aquatic environment based on fish, invertebrates, and algae RQ values that were almost all lower than 0.1 (Fig. 4). This is consistent with Selvaraj et al. (2015) and Zhang et al. (2015). Of the remaining four PAEs, DEHP may pose the most significant potential adverse effects on aquatic organisms with 71 and 29 % of samples in the high and medium risk categories, respectively. Accordingly, the ecological risks of PAE congeners were DEHP > DBP > DnOP > BBP > DMP > DEP. This ranking may be contrary to an evaluation of PAEs in surface water of the Lake Chaohu (He et al. 2013), because the pollution is different in the different areas.
Noncarcinogenic and carcinogenic risk assessment of PAEs in seven investigated areas
Both noncarcinogenic risks and carcinogenic risks via the bathing routes were far less than the risks through the oral ingestion (Table S1; Table S2). In addition, females are more susceptible to PAEs. DEHP and BBP are classified as a possible human carcinogen defined by the USEPA, so the carcinogenic risks posed by DEHP and BBP via oral intake were calculated (Table 4). The carcinogenic risks for DEHP (3.70 × 10−9–5.11 × 10−8) were higher than BBP (3.67 × 10−10–3.05 × 10−9). Fortunately, their carcinogenic risk was much lower than 10−6, suggesting that the carcinogenic risk from these chemicals is very low.
The noncarcinogenic hazard risk of the PAEs for female and male via the oral intake was generally below 1 (Fig. 5), indicating that PAEs would cause little noncarcinogenic risk.
The samples with the highest health risks were found in sites S1 and S2, respectively.
Conclusion
All six selected PAEs were detected in water and surface sediments samples from the seven investigated areas of the PRE. DEHP, the most abundant compound, was found at the highest levels in S1 with the relative contribution of total PAEs that was 55 % in the water and 69 % in surface sediments. The seasonal distribution of Σ6PAEs suggested that the highest levels were observed in summer both in water and sediments, which might be mainly attributed to urban stormwater runoff. DEHP and DBP posed much greater potential adverse effects on aquatic ecosystem than the other studied PAE congeners as indicated by risk quotients (RQs). There is little health risk posed by the PAE congeners to the residents in the PRD at the concentrations observed in water samples.
References
Adams WJ, Biddinger GR, Robillard KA, Gorsuch JW (1995) A summary of the acute toxicity of 14 phthalate esters to representative aquatic organisms. Environ Toxicol Chem 14:1569–1574
Adeniyi AA, Okedeyi OO, Yusuf KA (2011) Flame ionization gas chromatographic determination of phthalate esters in water, surface sediments and fish species in the Ogun river catchments, Ketu, Lagos, Nigeria. Environ Monit Assess 172:561–569
Arbeitsgemeinschaft PVC, Umwelt EV (2006) Plasticizers Market Data
Cai Q-Y, Mo C-H, Wu Q-T, Katsoyiannis A, Zeng Q-Y (2008) The status of soil contamination by semivolatile organic chemicals (SVOCs) in China: a review. Sci Total Environ 389:209–224. doi:10.1016/j.scitotenv.2007.08.026
Chen X et al (2011) The combined toxicity of dibutyl phthalate and benzo(a)pyrene on the reproductive system of male Sprague Dawley rats in vivo. J Hazard Mater 186:835–841. doi:10.1016/j.jhazmat.2010.11.078
Chen SJ, Feng AH, He MJ, Chen MY, Luo XJ, Mai BX (2013) Current xlevels and composition profiles of PBDEs and alternative flame retardants in surface sediments from the Pearl River Delta, southern China: comparison with historical data. Sci Total Environ 444:205–211
Cristale J, Garcia Vazquez A, Barata C, Lacorte S (2013) Priority and emerging flame retardants in rivers: occurrence in water and sediment, Daphnia magna toxicity and risk assessment. Environ Int 59:232–243. doi:10.1016/j.envint.2013.06.011
Dargnat C, Blanchard M, Chevreuil M, Teil MJ (2009) Occurrence of phthalate esters in the Seine River estuary (France). Hydrol Process 23:1192–1201. doi:10.1002/hyp.7245
Das MT, Ghosh P, Thakur IS (2014) Intake estimates of phthalate esters for South Delhi population based on exposure media assessment. Environ Pollut 189:118–125. doi:10.1016/j.envpol.2014.02.021
Doong RA, Lin YT (2004) Characterization and distribution of polycyclic aromatic hydrocarbon contaminations in surface sediment and water from Gao-ping River, Taiwan. Water Res 38:1733–1744
EC, 2003. European Commission Technical Guidance Document in Support of Commission Directive 93//67/EEC on Risk Assessment for New Notified Substances and Commission Regulation (EC): No 1488/94 on Risk Assessment for Existing Substance, Part II. In: Commission, E. (Ed.) pp. 100e103
Fan W, Xia X, Sha Y (2008) Distribution of Phthalic Acid Esters in Wuhan section of the Yangtze River, China. J Hazard Mater 154:317–324
Feng Z et al (2009) Seasonal distribution of phthalate esters in surface water of the urban lakes in the subtropical city, Guangzhou, China. J Hazard Mater 169:719–725
Feng AH, Chen SJ, Chen MY, He MJ, Luo XJ, Mai BX (2012) Hexabromocyclododecane (HBCD) and tetrabromobisphenol A (TBBPA) in riverine and estuarine sediments of the Pearl River Delta in southern China, with emphasis on spatial variability in diastereoisomer- and enantiomer-specific distribution of HBCD. Mar Pollut Bull 64:919–925
Fromme H, Küchler T, Otto T, Pilz K, Müller J, Wenzel A (2002) Occurrence of phthalates and bisphenol A and F in the environment. Water Res 36:1429–1438
Gan Z, Andrew P, Alan H, Bixian M, Xiangdong L, Yuehui K, Zhishi W (2002) Sedimentary records of DDT and HCH in the Pearl River Delta, South China. Environ Sci Technol 36:3671–3677
Gevao B, Muzaini S, Helaleh M (2008) Occurrence and concentrations of polybrominated diphenyl ethers in sewage sludge from three wastewater treatment plants in Kuwait. Chemosphere 71:242–247
Gledhill WE, Kaley RG, Adams WJ, Hicks O, Michael PR, Saeger VW, Leblanc GA (1980) An environmental safety assessment of butyl benzyl phthalate. Environ Sci Technol 14:301–305
Gómez-Hens A, Aguilar-Caballos MP (2003) Social and economic interest in the control of phthalic acid esters. TrAC Trends Anal Chem 22:847–857, http://dx.doi.org/10.1016/S0165-9936(03)01201-9
Gray LE, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L (2000) Perinatal Exposure to the Phthalates DEHP, BBP, and DINP, but Not DEP, DMP, or DOTP, Alters Sexual Differentiation of the Male Rat. Toxicol Sci 58:350–365
Gros M, Petrovic M, Ginebreda A, Barcelo D (2010) Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ Int 36:15–26. doi:10.1016/j.envint.2009.09.002
Hamidin N, Yu QJ, Connell DW (2008) Human health risk assessment of chlorinated disinfection by-products in drinking water using a probabilistic approach. Water Res 42:3263–3274. doi:10.1016/j.watres.2008.02.029
Harris CA, Henttu P, Parker MG, Sumpter JP (1997) The estrogenic activity of phthalate esters in vitro. Environ Health Perspect 105:802–811
He W et al (2013) Spatio-temporal distributions and the ecological and health risks of phthalate esters (PAEs) in the surface water of a large, shallow Chinese lake. Sci Total Environ 461–462:672–680. doi:10.1016/j.scitotenv.2013.05.049
Hongjun Y, Wenjun X, Qing L, Jingtao L, Hongwen Y, Zhaohua L (2013) Distribution of phthalate esters in topsoil: a case study in the Yellow River Delta, China. Environ Monit Assess 185:8489–8500. doi:10.1007/s10661-013-3190-7
Horn O, Nalli S, Cooper D, Nicell J (2004) Plasticizer metabolites in the environment. Water Res 38:3693–3698. doi:10.1016/j.watres.2004.06.012
Hu XY, Wen B, Shan XQ (2003) Survey of phthalate pollution in arable soils in China. J Environ Monit 5:649–653. doi:10.1039/b304669a
Keith LH, Telliard WA (1979) Priority Pollutants: I. A Perspective View. Environ Sci Technol 13:416–423
Li T, Yin P, Zhao L, Wang G, Yu QJ, Li H, Duan S (2015) Spatial–temporal distribution of phthalate esters from riverine outlets of Pearl River Delta in China. Water Sci Technol 71:183–190
Liu Y et al (2009) Toxicity of seven phthalate esters to embryonic development of the abalone Haliotis diversicolor supertexta. Ecotoxicology 18:293–303. doi:10.1007/s10646-008-0283-0
Liu H, Liang H, Liang Y, Zhang D, Wang C, Cai H, Shvartsev SL (2010) Distribution of phthalate esters in alluvial sediment: a case study at JiangHan Plain, Central China. Chemosphere 78:382–388
Liu X, Shi J, Bo T, Zhang H, Wu W, Chen Q, Zhan X (2014) Occurrence of phthalic acid esters in source waters: a nationwide survey in China during the period of 2009–2012. Environ Pollut 184:262–270. doi:10.1016/j.envpol.2013.08.035
Mackintosh CE, Maldonado JA, Ikonomou MG, Gobas FAPC (2006) Sorption of phthalate esters and PCBs in a marine ecosystem. Environ Sci Technol 40:3481–3488
Matsumoto M, Hirata-Koizumi M, Ema M (2008) Potential adverse effects of phthalic acid esters on human health: a review of recent studies on reproduction Regulatory. Toxicol Pharmacol 50:37–49
Ministry of Environmental Protection of the People’s Republic of China and General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. 2002. Environmental quality standard for surface water. GB 3838e2002. Beijing
Ortiz-Zarragoitia M, Trant JM, Cajaraville MP (2006) Effects of dibutylphthalate and ethynylestradiol on liver peroxisomes, reproduction, and development of zebrafish (Danio rerio). Environ Toxicol Chem 25:2394–2404
Pei XQ, Song M, Guo M, Mo FF, Shen XY (2013) Concentration and risk assessment of phthalates present in indoor air from newly decorated apartments. Atmos Environ 68:17–23
Peng X, Yu Y, Tang C, Tan J, Huang Q, Wang Z (2008) Occurrence of steroid estrogens, endocrine-disrupting phenols, and acid pharmaceutical residues in urban riverine water of the Pearl River Delta, South China. Sci Total Environ 397:158–166
Rhodes JE, Adams WJ, Biddinger GR, Robillard KA, Gorsuch JW (1995) Chronic toxicity of 14 phthalate esters to Daphnia magna and rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 14:1967–1976
Selvaraj KK, Sundaramoorthy G, Ravichandran PK, Girijan GK, Sampath S, Ramaswamy BR (2015) Phthalate esters in water and sediments of the Kaveri River, India: environmental levels and ecotoxicological evaluations. Environ Geochem Health 37:83–96
Seung-Kyu K, Kurunthachalam K (2007) Perfluorinated acids in air, rain, snow, surface runoff, and lakes: relative importance of pathways to contamination of urban lakes. Environmental science & technology 41:8328–8334
Sha Y, Xia X, Yang Z, Huang GH (2007) Distribution of PAEs in the middle and lower reaches of the Yellow River, China. Environ Monit Assess 124:277–287
Shi Z et al (2005) Contamination of rivers in Tianjin, China by polycyclic aromatic hydrocarbons. Environ Pollut 134:97–111
Shi W et al (2012) Occurrence of thyroid hormone activities in drinking water from eastern China: contributions of phthalate esters. Environ Sci Technol 46:1811–1818. doi:10.1021/es202625r
Sun J, Huang J, Zhang A, Liu W, Cheng W (2013) Occurrence of phthalate esters in sediments in Qiantang River, China and inference with urbanization and river flow regime. J Hazard Mater 248–249:142–149. doi:10.1016/j.jhazmat.2012.12.057
US EPA (United States Environmental Protection Agency), 2013. Mid Atlantic Risk Assessment. Regional Screening Level (RSL) Summary Table. Washington DC
Wan YW, Kang TF, Zhou ZL, Peng-Na LI (2009) Health Risk Assessment of Volatile Organic Compounds in Water of Beijing Guanting Reservoir. Research of Environmental Sciences. 150–154
Wang W, Zhang Y, Wang S, Fan CQ, Xu H (2012) Distributions of phthalic esters carried by total suspended particulates in Nanjing, China. Environ Monit Assess 184:6789–6798. doi:10.1007/s10661-011-2458-z
Xu H et al (2013a) Effects of Di-n-butyl Phthalate and Diethyl Phthalate on Acetylcholinesterase Activity and Neurotoxicity Related Gene Expression in Embryonic Zebrafish. Bull Environ Contam Toxicol 91:635–639
Xu W et al (2013b) Antibiotics in riverine runoff of the Pearl River Delta and Pearl River Estuary, China: concentrations, mass loading and ecological risks. Environ Pollut 182C:402–407
Yuan SY, Liu C, Liao CS, Chang BV (2002) Occurrence and microbial degradation of phthalate esters in Taiwan river sediments. Chemosphere 49:1295–1299, http://dx.doi.org/10.1016/S0045-6535(02)00495-2
Zeng F et al (2008) Occurrence of phthalate esters in water and sediment of urban lakes in a subtropical city, Guangzhou, South China. Environ Int 34:372–380
Zhang K, Wang JZ, Liang B, Zeng EY (2011) Occurrence of polycyclic aromatic hydrocarbons in surface sediments of a highly urbanized river system with special reference to energy consumption patterns. Environ Pollut 159:1510–1515
Zhang L, Liu J, Liu H, Wan G, Zhang S (2015) The occurrence and ecological risk assessment of phthalate esters (PAEs) in urban aquatic environments of China. Ecotoxicology 24:1–18
Zhiyong X, Ralf E, Christian T, Rainer L, Armando C, Wolfgang R (2007) Occurrence and air-sea exchange of phthalates in the Arctic. Environ Sci Technol 41:4555–4560
Acknowledgments
This work was supported by the National fund-joint fund key projects in Guangdong under Grant number U1133003, Industry-university-research combination project of Guangdong Province under Grant number 2013B090600009, and Science and Technology Program of Guangdong under Grant number 2014A020217007.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Hongwen Sun
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 53 kb)
Rights and permissions
About this article
Cite this article
Li, X., Yin, P. & Zhao, L. Phthalate esters in water and surface sediments of the Pearl River Estuary: distribution, ecological, and human health risks. Environ Sci Pollut Res 23, 19341–19349 (2016). https://doi.org/10.1007/s11356-016-7143-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7143-x