Abstract
This chapter provides a critical overview of the methods of biodiesel production from waste oily by-products from edible oil refinery, waste fats, and waste cooking oils with emphasis on factors that impact the synthesis of fatty acids alkyl esters. The aim is to show exploitation possibilities of the mentioned waste materials for making biodiesel. Various technologies such as chemical (homogeneous and heterogeneous) and enzyme catalysis as well as non-catalytic processes have been applied in biodiesel production from waste oils, fats, and cooking oils. The future commercial process of biodiesel production will be a choice among solid catalysts, lipases, and non-catalytic processes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Technological development, global warming, and increasing environmental pollution have directed scientific research toward alternative and ecologically acceptable energy resources. In a group of alternative fuels, which are substitutes for the conventional ones, the most perspective are biofuels among which biodiesel has great significance. Biodiesel is defined as a mixture of long-chain fatty acid alkyl esters (FAAEs) that satisfy specified standards. It is mainly produced by transesterification (alcoholysis) of triacylglycerols (TAGs) from different natural resources, in excess of alcohol, and most commonly in the presence of a catalyst. Annual world biodiesel production is growing rapidly in the last decade, reaching a level of approximately 35–45 million tonnes in 2019 [1]. The world’s largest biodiesel producers in 2019 were the EU and the USA with annual productions of over 14 million tonnes and 5.6 million tonnes, respectively.
There are many advantages in the appliance of biodiesel, such as:
-
It can be used “as is” or in mixture with diesel D-2, without or with minimal engine modifications;
-
It is biodegradable;
-
It can be derived from biologically renewable recourses (vegetable oils and animal fat);
-
During the combustion of biodiesel emission of carbon and sulfur oxides, soot particles and non-combusted hydrocarbons are reduced.
Despite many advantages of biodiesel compared to fossil diesel, high manufacture price is the primary barrier in commercial usage of biodiesel. The manufacture price is determined by feedstock type, production capacity, and applied technology [2, 3]. Research shows that edible vegetable oils, included in current industrial processes, participate with 70–95% in the total price of biodiesel production [4]. Also, the use of edible oils in the biodiesel production process is restricted by their usage in the human diet and food industry. Furthermore, even if the whole amount of available edible vegetable oils were used for biodiesel production, the gained amount of fuel would not satisfy current diesel requirements [5]. As a consequence, attention of the researchers is significantly turned to examining possibilities of new and cheaper oily feedstocks for biodiesel production, such as waste oily by-products from edible oil refinery (called here waste vegetable oils), waste animal fats (WAFs), waste cooking oils (WCOs ), and nonedible oils.
Methods of biodiesel production from waste vegetable oils, WAFs, and WCOs with an overview of factors that impact the synthesis of FAAEs are critically reviewed. The aim is to show exploitation possibilities of the mentioned waste materials for making an economically sustainable and ecologically acceptable product such as biodiesel.
2 Biodiesel Production from Conventional Oily Feedstocks
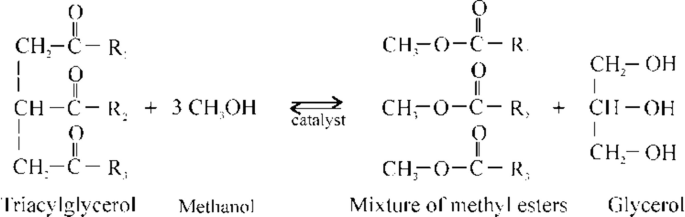
According to its chemical composition , biodiesel is most commonly a mixture of fatty acid methyl esters (FAMEs ) or ethyl esters (FAEEs), obtained from TAGs via transesterification or from free fatty acids (FFAs ) via esterification:
-
Transesterification reaction:
-
Esterification reaction :
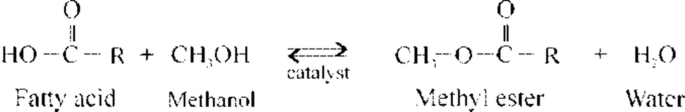
The basic feedstocks for biodiesel production are vegetable oils or animal fats consisting mainly of TAGs. Therefore, transesterification is the main reaction for biodiesel production, whereas esterification is needed for feedstocks having a higher content of FFAs . Transesterification is a sequence of three reversible reactions in which TAGs are gradually converted into diacylglycerols (DAGs ), monoacylglycerols (MAGs ), and glycerol. Esterification is also an equilibrium reaction. In both reactions, to shift the equilibrium to the right, methanol is added in an excess relative to the stoichiometric amount, or an end product is removed out of the reaction system. For instance, because of their immiscibility, FAMEs and glycerol separate easily, and the latter is removed from the reaction mixture enabling a high conversion degree. The rate of both reactions is usually enhanced using a catalyst, which can be an acid, a base, or an enzyme, although the reactions can be conducted in the absence of any catalyst but at higher temperatures and pressures.
According to the type of catalyst employed, the reactions for biodiesel production can be classified into four groups: (a) homogeneously catalyzed, (b) heterogeneously catalyzed, (c) enzymatically catalyzed, and (d) non-catalyzed.
2.1 Homogeneously Catalyzed Reactions
Due to the short reaction time, low demands concerning the quality of equipment, and small investments, transesterification is usually performed in the presence of a homogeneous base catalyst: hydroxides or alkoxides of sodium or potassium. The reason for the massive usage of alkali hydroxides is good catalytic activity, low cost, and simple transport and storage manage. Alkali methoxides are more catalytically active, but more expensive and highly hygroscopic, which makes them much harder to handle [7]. The main limitation of using these catalysts is the quality of oily feedstock, which refers to the contents of FFAs (<1%) [8] and water (<0.1%) [9]. As the transesterification reaction is reversible, the maximal conversion of TAGs is reached with an initial molar ratio alcohol-to-oil higher than the stoichiometrical one. When methanol is used, the optimal ratio, according to most researchers, is 6:1. Acid-catalyzed transesterification is significantly slower but more suitable for oils with a higher content of FFAs . To keep the alcohol liquid, temperatures of up to 100 °C and pressures of up to 5 bars are typically employed in homogeneously acid-catalyzed methanolysis. Therefore, acid catalysts are usually used for esterification reactions. Concentrated sulfuric acid is the best and cheapest acid catalyst for esterification reactions. The major disadvantage of homogenous catalysts is the fact that they cannot be reused. Figure 5.1 shows the process scheme of homogeneously catalyzed biodiesel production process, depending on FFA content [6]. The process includes pre-esterification of a feedstock with high FFA content under acidic conditions, followed by base-catalyzed transesterification.
Process selection and steps for biodiesel production. (Adapted from [6])
One of the best-known industrial methods of synthesizing biodiesel using a homogeneous base catalyst is the Lurgi process [10]. Refined vegetable oils are mainly used as feedstock. A two-stage mixer-settler unit is used to running the process continuously (Fig. 5.2a). The reaction takes place in the mixing section at 60 °C and atmospheric pressure, using sodium methoxide. The FAME light phase is separated from the glycerol-water heavy phase in the settling section. The FAME product was washed by water in a countercurrent washing column and then dried. Methanol contained in the glycerol water is recovered in a rectification column and used again in the process. Glycerol can be recovered from the glycerol water and further purified. Biodiesel plants have an annual capacity in the range between 40,000 and 250,000 tonnes of biodiesel according to EN14214.
2.2 Heterogeneously Catalyzed Reactions
The use of heterogeneous catalysts , both acidic and basic, simplifies the product purification step. Namely, solid catalysts can be recovered by filtration or decantation or be used in fixed-bed reactors, so there are no wastewaters. They can be reused with or without regeneration. Their major drawbacks are mass-transfer limitations in the three-phase reaction system, higher initial methanol-to-oil molar ratios, and the complex catalyst preparation in some cases. The most frequently used heterogeneous base catalysts are alkali metal- and alkaline earth metal oxides and carbonates. The application of calcium oxide seems to be promising because it is an easily available and cheap substance [12]. The other tested solid catalysts are zeolites, ion-exchange resins, Mg-Al hydrotalcites, etc. The future attention of researchers will be focused on discovering bifunctional and superacid solid catalysts that catalyze both esterification of FFAs and transesterification of TAGs .
The only commercial continuous process of biodiesel production based on the use of a non-noble metal solid catalyst is the Esterfip-H process realized by Axens (France) [11]. Suitable feedstocks are virgin and semi-refined vegetable oils. The plant includes two fixed-bad reactors with the catalyst (Fig. 5.2b). Excess methanol is removed after each reactor by partial flash evaporation, while FAMEs and glycerol are separated in a settler. Biodiesel is produced after the final removal of methanol by evaporation under vacuum, and the yield is close to the theoretical one. Salt-free glycerol of high purity (>98%) is also produced. The annual plant capacity is up to 200,000 tonnes of biodiesel .
2.3 Enzyme-Catalyzed Reactions
Lipases from different microorganisms have been tested in biodiesel production in the last decade. These enzymes catalyze both esterification of FFAs and transesterification of TAGs under mild reaction conditions at low initial methanol-to-oil molar ratios. Therefore, feedstocks having high FFAs content can be used without any pretreatment. The separation and purification of the end products are simple, and a minimal amount of wastewater is generated. The basic barriers to performing enzyme-catalyzed processes industrially are the high price of the enzyme, low enzyme activity, and stability in the presence of polar alcohols and the necessity of careful control of process variables. Being better from the ecological point of view, compared to other catalyst types, the enzyme-catalyzed biodiesel production will become more important in the future. A promising strategy is to use lipases immobilized on a carrier, enabling easy enzyme removal and reuse.
2.4 Non-catalyzed Reactions
Transesterification of TAGs with lower alcohols can occur in the absence of a catalyst at high temperatures and pressures (above the critical point for methanol 239 °C and 8.1 MPa). Under supercritical conditions, the reaction takes place in a single phase without mass-transfer limitation. Also, high-purity esters and soap-free glycerol are produced. During this process, esterification, hydrolysis, and methanolysis occur at the same time, which is suitable to produce biodiesel from used and waste materials [13]. However, supercritical processes still have no industrial application due to the high capital investment and high energy consumption, which indicates the necessity of a good design of the process in terms of energy recovery [14]. Because of a high initial methanol-to-oil molar ratio (up to 50:1), most of the energy is spent on the recovery of methanol. A two-step non-catalyzed process, which includes hydrolysis of TAGs into FFAs with an excess of water and subsequent esterification, requires lower amounts of methanol [15].
3 Biodiesel Production from By-products of Edible Oil Refinery Process
Edible oils are primarily obtained from various oilseeds. The first step in producing edible oils is the separation of so-called crude (unrefined) oil by pressing followed by solvent extraction. The major component of the crude oil is TAGs, while the other minor components are MAGs, DAGs, FFAs, phosphatides, sterols, tocopherols, squalene, pigments, glycerol, hydrocarbons, vitamins, glycolipids, metals, etc. The crude oil is refined by employing specific processes in several steps to remove the minor components contributing to undesirable appearance, odor, and flavor. The refinery process results in edible oil as the main product and several by-products (waste vegetable oils) containing primarily TAGs and/or FFAs, which can be used as raw materials for biodiesel production. At first, phospholipids (gums ) are removed by the degumming process, the obtained so-called oil sediments . If the chemical refinery is applied, FFAs are neutralized by a weak alkaline solution, and the by-product obtained is soapstock. Soapstock is usually acidulated by adding mineral acid to liberate FFAs, generating so-called acid oil or acidulated soapstock . Physical refinery processes include vacuum steam distillation to remove FFAs, producing acid oils. In the bleaching step, pigments, residual phosphatides, soaps, and metals are removed by using bleaching earth, producing another solid waste called spent bleaching earth (SBE). Finally, FFAs and odoriferous components are removed in the deodorization step by vacuum steam distillation, producing a by-product called deodorizer distillate (DD). Details on the chemical composition and utilization of the main by-products from edible oil refining processes can be found elsewhere [16, 17].
3.1 Biodiesel from Soapstocks, Oil Sediments, and Acid Oils
When designing processes for biodiesel production from soapstock, one should consider the presence of both acylglycerols (AG) and FFAs, as well as its high-water content and semisolid nature under ambient conditions. Generally, two-step processes are needed, consisting of acid-catalyzed esterification of FFAs followed by a base-catalyzed transesterification of AGs, because the latter reaction is ineffective at esterifying FFAs. Also, water inhibits both transesterification and acid-catalyzed esterification and favors ester hydrolysis, which is unfavorable for biodiesel production. The biodiesel production from acid oils involves less difficulty than that from soapstocks because the former raw material has lower water content. Both raw materials, however, contain other impurities such as phospholipids that, as surfactants, lead to the difficulty in separating methyl esters from glycerol after the washing step. Based on the annual world’s production of the selected edible oils (soybean, rapeseed, sunflower, and palm kernel: 533.7 million metric tonnes) [18] and the estimates suggested by Echim and coworkers [17], the world’s generation of soapstock and acid oils in 2019 can be estimated to be 14.7–19.4 million metric tonnes and 6.7–11.4 million metric tonnes, respectively.
There are two main routes to produce biodiesel from soapstocks (Fig. 5.3), namely direct conversion and pretreatment of soapstocks before conversion either by acidulation to produce acid oils (so-called WCO SSR) or by hydrolysis of neutral oil (hydrolysis route). Another route is the esterification of FFAs with glycerol to AGs (termed glycerolysis) prior to transesterification. The process of direct soapstock conversion into biodiesel is on the laboratory scale, while the only industrial process is undertaken via SSR [17]. Table 5.1 reviews the selected literature related to the use of soapstocks, oil sediments, and acid oils in biodiesel production. Soapstocks originate mainly from the refinery of soybean oil , and methanol is only used as an esterification agent. Processes are usually conducted in batch stirred reactors, although packed-bed and tubular reactors are also applied. Direct esterification of FFAs from soapstocks was catalyzed by either sulfuric acid or lipases after appropriate pretreatment. Different soap-splitting procedures are employed to produce acid oils which are further esterified using sulfuric acid, solid acid catalysts, and lipases. Non-catalyzed esterification and glycerolysis processes have been rarely studied. The final product yield depends on the origin of soapstock or acid oil and the employed process conditions.
Schematic representation of different routes used to convert soapstock to biodiesel. (Adapted from [17])
3.1.1 Direct Conversion of Soapstock
Direct conversion of soapstock “as is” has been seldom studied. Acid-catalyzed [19] and enzyme-catalyzed [20] esterification is employed to convert cotton oil soapstock and soybean oil soapstock to biodiesel, respectively. In the former process, the splitting of the soaps and esterification was performed in the presence of sulfuric acid as a catalyst at 75 °C in 2 h [19]. The latter process employs sequentially a base-catalyzed transesterification and enzyme-catalyzed esterification to convert AGs and FFAs of soapstock to the esters of monohydric alcohols [20]. Water from the soapstock was removed by freeze-drying prior to the transesterification, and the pH of the final reaction mixture was adjusted to pH 6.0 prior to esterification to obtain a significant activity of C. antarctica SP-435 lipase. Only 63% conversion of FFAs was achieved at the ethanol-to-FFA ratio of 20:1 in the presence of water (0.70%) within 39 h. The process combining transesterification and esterification reached the overall conversion of only 81%. Therefore, the enzymatic process was judged as insufficient for achieving complete esterification.
3.1.2 SPR (via Acid Oil as an Intermediate)
3.1.2.1 Chemically Catalyzed Processes
Eaveas et al. [29] converted acidulated soapstock (acid oil) to methyl esters using HCl and Twitchell reagent in a packed column reactor. The optimum conditions for the highest conversion of up to 86% are as follows: the temperature of 110–120 °C, the pressure of 11.34 bar, the methanol-to-FFA ratio of 5:1, the acid catalyst of 3–5% (based on the FFA mass), and the reaction time of 15 min. Passing the acid oil pretreated by hydrolysis twice through the reactor the conversion degree was increased up to 97%.
Haas et al. [21] optimized soybean acid oil esterification with methanol and catalyzed by sulfuric acid at 65 °C using statistical experimental design. Since greater than 15% of the FFAs remaining in the final reaction mixture as free or glycerol-linked, the reaction was inefficient within 26 h and therefore unacceptable for industrial use. In an alternative method, a high acid acid oil was prepared by the complete hydrolysis of AGs from the soapstock. The high acid acid oil was then esterified by sulfuric acid catalysis. The conversion of 89% was reached within 14 h at a methanol/FFAs/catalyst molar ratio of 1.8:1:0.17 at 65 °C. The uncompleted esterification was the result of the action of water formed. The new reaction step following removal of the formed water by centrifugation reduced the content of FFAs to 0.2%. Park et al. [25] esterified a high acid acid oil, obtained by the same procedure as in the previous study, using methanol and Amberlyst-15 and reached a maximum final ester content of 91.7% at the methanol-to-FFAs of 9:1 after double water evaporation during the process. Biodiesel yields reached with Amberlyst-15 and sulfuric acid were similar [26].
Luxem and Troy [30] patented a method where esterification of FFAs and transesterification of AGs occurred simultaneously under pressure (34.5 bar) with methanol in the presence of sulfuric acid at 130 and 150 °C without removing the by-products (glycerol and water). An 82% conversion was achieved within 60 min and 15 min, respectively. At longer reaction times and at higher temperatures, conversion degrees higher than 90% were achieved.
Wang et al. [22] studied biodiesel production from soybean acid oil in a pressurized stirred reactor using methanol and sulfuric acid as a methylation agent and as a catalyst, respectively, at 80 °C for the first 1 h and at 95 °C for the following 4 h. Under the optimal mass ratio of methanol/acid oil/catalyst (1.5:1:0.1), a conversion of 92% was reached within 3–5 h depending on the initial water content and the methanol-to-acid oil ratio. After distillation, the purity of the final biodiesel product was 97.6%, corresponding to a yield of 94% based on total FFA content in the initial raw material.
McNeff et al. [31] used microspheres of metal oxides (Zr, Ti, and Al) as catalysts to convert continuously different raw materials (acid oil among them) to methyl esters in a packed-bed reactor under high pressure and temperatures (300–450 °C). A good conversion of acid oils (90.2%) was achieved without loss of catalytic activity overextended applications.
Jin et al. [23] utilized a mixture of oil sediments and soapstock for producing FAMEs in a three-step process. The mixture of oil sediments and soapstock was first extracted with ethyl ether and, after the addition of saturated sodium chloride solution, it was centrifuged to obtain three phases. Sulfuric acid was added to the soap phase to get FFAs. This high acid acid oil was esterified (conversion degree 92.1% of theoretical) using methanol (5 mol per a mole of FFAs) and sulfuric acid (3%) at 85 °C within 5 h. In the third step, TAGs and phosphatides were extracted from the organic phase with acetone and then transesterified by methanol and sodium hydroxide at 65 °C within 1 h, producing a maximum FAME yield of 94%.
Shao et al. [24] optimized biodiesel production from rapeseed soapstock employing soap-splitting and short-path distillation. A biodiesel yield of 96.45% was achieved from the acid oil within 1.42 h with a methanol-to-acid oil ratio of 0.33:1 v/v and sulfuric acid of 1.44 vol% at 60 °C.
Li et al. [27] employed acidification of a soapstock, molecular distillation to separate FFAs and AGs, followed by acid-catalyzed esterification and base-catalyzed methanolysis, respectively. A solid superacid catalyst, SO42−/ZrO2–TiO2/La3+ (5%), was used in the esterification reaction undertaken at a methanol-to-oil molar ratio of 15:1 at 60 °C; the conversion of 98% was reached in 4 h. Base-catalyzed methanolysis reached a conversion of 97.25% within 30 min at the catalyst (sodium methoxide) loading of 0.6%, the methanol-to-oil molar ratio of 5:1, and 55 °C.
Guo et al. [28] prepared acid oil from a soybean oil soapstock by a process involving the removal of phospholipids and acidification of the soap phase. The acid oil was esterified with methanol in the presence of a solid acid lignin-derived carbonaceous catalyst. This catalyst had 3.5 times higher catalytic activity than sulfuric acid. The best conversion of above 97% was achieved within 5 h with a catalyst loading of 7% at a methanol-to acid oil molar ratio of 9:1 at 70 °C.
Pantoja et al. [29] optimized the FAME production from the buriti (Mauritia flexuosa) oil soapstock via acidulation and esterification using H2SO4 as a catalyst. The best acidulation conditions were the 0.8 molar ratio and the reaction time of 60 min whereas the best esterification conditions were the molar ratio of 18:1, catalyst loading of 4%, and reaction time of 14 h, which provided a yield of 92% and a conversion of 99.9%.
Domingues et al. [33] reported the use of a solid vanadyl phosphate catalyst in the simultaneous esterification of FFAs and transesterification of AGs from rapeseed acid oil with methanol. A mixture contacting 87% of methyl esters and 7.2% of FFAs was obtained within 6 h at 125 °C. The increase in the reaction temperature at 150 °C led to the biodiesel product containing 93.5% of methyl esters and 3.3% of FFAs. Spent catalyst can be regenerated by reoxidation of the reduced vanadium with air.
The production cost of biodiesel obtained from soybean soapstock was shown by an economic analysis to be for 25% less than that estimated for biodiesel produced from refined soybean oil, whereas engine emissions and performance during operation on the former biodiesel were comparable to those on the latter one [48].
3.1.2.2 Enzyme-Catalyzed Processes
Watanabe et al. [34] applied a two-step process including enzyme-catalyzed esterification of FFAs from acid oil and enzyme-catalyzed methanolysis of AGs using immobilized lipase from C. antarctica. In the first step, the esterification degree of 91% was achieved within 24 h at the methanol-to-acid oil molar ratio of 1:1. In the repeated batches, the biodiesel content at 24 h decreased by 24% after the tenth cycle, indicating that the lipase was unstable. At higher methanol-to-acid oil molar ratios (5–7.5:1), the lipase inactivation was avoided, and the esterification of FFAs within 24 h was increased (>96%). The second step included the dehydrated first-step product, refined rapeseed oil, methanol, and glycerol to convert AGs into methyl esters using immobilized lipase. The final product contained 91.1% of methyl esters. The enzyme was successfully employed in 100 cycles.
Shao et al. [35] optimized the biodiesel production from rapeseed soapstock by immobilized enzyme-catalyzed esterification after its saponification and acidification. All four employed parameters (enzyme amount, methanol-to-acid oil molar ratio, water content, and temperature) were found to be statistically important. The best conversion of 63.6% was reached under the optimal conditions. After molecular distillation, the methyl ester yield was increased above 95%.
Chen et al. [36] studied biodiesel production from acid oil using soluble lipases from genetically modified Aspergillus oryzae/Aspergillus niger microorganism. A central composite design showed that the influences of enzyme concentration, methanol-to-acid oil molar ratio, temperature, and agitation speed on the methyl esters yield were statistically significant. Under the optimal conditions, the biodiesel yield was 88.7%.
Chen et al. [37] catalyzed the reaction between a pretreated acid oil and methanol by immobilized Candida lipase in a series of three packed-bed reactors. The influences of lipase, n-hexane and water contents, temperature, and mass flow rate were analyzed. Under the optimum reaction conditions, the best methyl esters yield of 90.2% was obtained. The immobilized enzyme can be recycled with a relatively stable activity after removing glycerol adsorbed.
Tüter et al. [38] performed esterification of corn and sunflower acid oils with several alcohols using lipase Novozym 435 in n-hexane. The highest methyl ester content (6.6%) was obtained within 1.5 h at the methanol-to-acid oil molar ratio of 1:1 and 40 °C using a 15% enzyme. However, higher ester yields (about 70%) were obtained with other primer alcohols (n-propanol, n- and i-butanol, n- and i-amyl alcohol, and n-octanol ).
3.1.2.3 Non-catalyzed Process
Akgün et al. [39] optimized the production of biodiesel from olive acid oil using non-catalyzed esterification with methanol under supercritical conditions in a continuous tubular reactor. The most effective factors were reaction temperature and flow rate of the reactants. The methyl ester yield of 92.3% was obtained under the optimum conditions (pressure of 240 bar, temperature of 380 °C, methanol-to-acid oil molar ratio of 1.12:1 and flow rate of 0.4 mL/min). After treating with bleaching earth and calcium hydroxide at 80 °C and filtering, the resulted product contained 96.6% methyl esters.
3.1.3 Hydrolysis Route
This route consists of hydrolysis (saponification) of all AGs to FFAs, followed by esterification of the obtained product containing primarily FFAs (Fig. 5.3). Alkali- and enzyme-catalyzed saponification was performed to achieve the complete hydrolysis. Acid and enzymatic esterifications were conducted to convert FFAs to methyl esters of fatty acids.
3.1.3.1 Chemically Catalyzed Processes
Haas et al. [40] described a two-step process involving alkaline hydrolysis of AGs and acid-catalyzed esterification of the obtained sodium salts of fatty acids. Although soapstock is already alkaline, sodium hydroxide has to be added to a final total concentration of 4.2% followed by incubation at 100 °C to complete hydrolysis within 2–4 h. Under these conditions, both AGs and phosphoacylglycerols were completely hydrolyzed. Before esterification, water was removed from the product of saponification by freeze-drying. The resulting dried product was converted to methyl esters by reaction with methanol in the presence of sulfuric acid. At the minimum molar ratio of methanol/fatty acids/sulfuric acid of 30:1:5, the resulting product containing more than 99% methyl esters was obtained at 35 °C within 10 min. The process produced biodiesel of high quality, but the product yield was only 60% of the theoretical yield.
3.1.3.2 Enzyme-Catalyzed Processes
A two-step enzymatic process for conversion of acid oil to biodiesel consisting of hydrolysis of AGs by lipase followed by esterification of FFAs with methanol by another lipase has been used [41, 42]. Watanabe et al. [41] used Candida rugosa lipase and immobilized C. antarctica lipases for hydrolysis of acid oil and esterification of FFAs with methanol to biodiesel, respectively. In the first esterification, where the hydrolyzed acid oil and methanol (molar ratio of 1:5) reacted in the presence of the enzyme (1%) at 30 °C, the conversion of 96% was reached within 24 h. The resulting reaction mixture was dehydrated and subjected to the second esterification to reach the total conversion of 99% for 24 h. Over 98% of total conversion was maintained for 40 cycles. Cruz et al. [42] obtained a FAME yield of 94% using the hydrolysis of an acid oil from soapstock of vegetable oil refining (a mixture of seeds) at 35 °C (shaking rate of 200 rpm, 1:0.5 water:oil mass ratio, 24 h) by Thermomyces lanuginosus lipase (3%) and then the esterification of the obtained FFAs with methanol (2:1 mol/mol, 35 °C, 200 rpm, 7 h) by the same lipase (2%).
A research group has been investigating a strategy for reducing the biodiesel production costs by a fermented solid with lipase activity in a solvent-free system in both batch reactor systems [43,44,45]. Lipases are produced by solid-state cultivation of a pathogenic (Burkholderia cepaciaon) [43, 44] or non-pathogenic (Rhizopus microsporus) microorganism [45] on a mixture of sugarcane bagasse and sunflower seed meal or sugarcane bagasse enriched with urea, soybean oil, and a mineral solution, respectively, and the dried fermented solid is directly used as the catalyst in the esterification of fatty acids with ethanol in a solvent-free system. When used in a packed-bed bioreactor in a closed-loop batch system, up to 30% of the reaction medium is sorbed onto the dried fermented solid, and the sorbed medium has a different composition compared to the bulk phase [43]. In further work, this research group develops a combined sorption-kinetic model describing the reaction kinetics for multiphasic ethyl esterification of fatty acids from soybean soapstock acid oil [44]. Botton et al. [45] have improved this reaction system by using the non-pathogenic R. microspores to produce the fermented solid catalyst. The conversion of 86% of the soybean soapstock acid oil hydrolyzed in subcritical water was reached by the esterification reaction with ethanol (10:1 molar ratio, 40 °C, 48 h). The use of a fermented solid produced by a non-pathogenic microorganism and the possibility of using hydrolyzed low-quality fatty raw materials could render the scale-up of the enzymatic biodiesel production via hydro-esterification more feasible and more competitive with the chemically catalyzed processes. These results foster further studies on the scaling-up of the environmentally friendly biodiesel production process.
Choi et al. [46] synthesized FAEEs from acidulated rice bran soapstock via the T. lanuginosus lipase-catalyzed transesterification of acid oil with ethanol in a continuous packed-bed reactor. The water content of the substrate, temperature, and lanuginosus affected considerably the FAEE yield, and the optimum conditions were 4%, 20 °C, and 1:4, respectively, ensuring the maximum yield of 92%. The corresponding composition of the final product was 92% FAEEs, 3% FFAs, and 5% AGs. When glycerol was removed from the reaction mixture by intermittent washing with ethanol, the relative lipase activity was maintained over 82% for 27 cycles.
3.1.4 AG Route
The AG route is conducted by esterification of FFAs with glycerol (termed glycerolysis) to form AGs, which is then transesterified conventionally. High reaction temperatures (up to 250 °C) are required to complete the reaction. For the purpose of decreasing the reaction temperature in the AG route, Luxem and Mirous [49] screened various acid, base, and transition metal catalysts. The glycerolysis reactions between acid oil and crude neutralized glycerol were carried out at 180 °C for 4 h. The amount of catalyst was normalized based on equal equivalents of metal content per mole of acid oil. The most efficient catalysts were organo-metal catalysts, tetrabutyl titanate, dibutyl tin oxide, and tin oxalate. The best conversion of FFAs of 93% was achieved using tin oxalate (1%), whereas dibutyl tin oxide (2%) reached the conversion of 81%. The process was scaled-up using the latter catalyst, and the nearly complete acid conversion was achieved, resulting in the product with a low acid value (0.5 mg KOH/g). The final product of esterification was converted by base-catalyzed transesterification into biodiesel with the overall yield of 95%, which was reduced to 92% after distillation. Felizardo et al. [47] studied the glycerolysis reaction of FFAs from acidulated soybean soapstock using metallic zinc and zinc acetate dihydrate as a catalyst. The best methyl ester yield of 94.7% was obtained with a 0.1% catalyst at 200 °C for 2 h.
The produced methyl esters do not satisfy the specific biodiesel standards and can be used as a biofuel for steam or power generation [17].
3.2 Biodiesel from SBE
Acid-activated bleaching earth is an adsorbent of high capacity that is commonly used in the crude vegetable oil refining process (so-called bleaching process ) to remove coloring pigments, residual phosphatides, soaps, etc. The produced solid waste material is known as SBE. Besides almost all impurities, this material adsorbs crude vegetable oil by up to 20–40% by mass [50]. A large amount of SBE is discarded from edible oil production. Based on 1.2–1.6 kg of the SBE per tonnes of edible oil produced [51] and the world edible oil production of 150.8 million tonnes in 2011 [18], the world generation of SBE is estimated to be about 180,000–240,000 tonnes/year. Most SBE is disposed of by inclusion in animal feeds, incineration, landfilling, or concrete manufacturing [17], and only its small amount is recovered and reused [51]. Disposal at landfills is unacceptable due to the potential environmental hazards and the cost of disposal.
More convenient ways to manage SBE are to utilize it as an alternative raw material and to convert it into valuable products. The adsorbed oil can be recovered from SBE by solvent [50, 52,53,54,55], supercritical carbon dioxide [56, 57], and lye [58] extractions. The extracted vegetable oil can be either recycled to the vegetable oil refining process or sold as a raw material to lubricant and biodiesel industries [51, 59,60,61]. The SBE reactivated by heating treatment (500 °C) and a combination of heating and acid treatment (0.1 M HCl) improves palm oil biodiesel filterability [62]. By physical, chemical, or biochemical treatment, the amount of organics contained by SBE are reduced to nearly zero, and the remaining deoiled solid material (up to 60%) can be freely disposed on landfills, recycled to the oil refining process, or used as a soil conditioner [63].
The conversion of the waste vegetable oil from SBE into biodiesel has already been investigated. A review of the selected literature related to the use of SBE in biodiesel production is presented in Table 5.2. SBE originates from the refinery of palm, soybean, or rapeseed oils. Two possible ways of biodiesel production processes are employed. The first group includes the extraction of waste vegetable oil that is followed by transesterification of the extracted oil, and the second group involves in situ extraction and transesterification of waste oil.
3.2.1 Extraction Followed by Transesterification
The waste vegetable oil absorbed on SBE is usually recovered by solvent (conventional maceration and Soxhlet extraction) and supercritical CO2 extraction. n-Hexane is mainly used as an extracting solvent, although some other solvents are also employed, such as methanol, ethanol, and petroleum ether. The biodiesel production method is performed as a one-step (methanolysis) or two-step (esterification followed by methanolysis) process in agitated batch reactors.
3.2.1.1 One-Step Processes
In this case, base- or enzyme-catalyzed methanolysis is undertaken. Alkali hydroxides (KOH, NaOH), calcium oxide, and Rhizopus oryzae lipase are employed as catalysts. Gűl et al. [64] optimized NaOH-catalyzed methanolysis of the waste vegetable oil extracted from SBE by response surface methodology. Lim et al. [65] compared the effects of CaO and alkali hydroxides as catalysts for methanolysis of the waste vegetable oil. In the CaO-catalyzed reaction, the highest FAME yield of 98.6% was achieved within 2.5 h at the following reaction conditions: the methanol-to-oil mass ratio of 0.5:1, CaO loading of 6%, and reaction temperature of 65 °C. Alkali hydroxides achieved 99% conversion in 1 h at the following optimal reaction conditions: methanol-to-oil mass ratio of 0.25:1, catalyst loading of 1%, and reaction temperature of 65 °C. The use of CaO as a catalyst has several advantages over homogeneous catalysts. CaO can be easily separated from the reaction mixture and reused for several runs without significant deactivation [73]. Aladetuyi et al. [66] used cocoa pod ash as a solid catalyst to produce biodiesel from palm kernel oil recovered from SBE. The biodiesel yield provided by cocoa pod ash was 86% and higher than that achieved by potassium hydroxide (81.2%), respectively. Therefore, this work suggests that agricultural residues could replace alkali catalysts for biodiesel production. Lara Pizarro and Park [67] used R. oryzae lipase to catalyze the methanolysis of extracted waste vegetable oils in a water-containing system. Optimum reaction conditions were the methanol-to-oil molar ratio of 4:1, the water content of 75%, the enzyme amount of 67 IU/g, and the reaction temperature of 35 °C. The highest FAME yield of 55% was reached with palm oil within 96 h of reaction .
3.2.1.2 Two-Step Processes
These processes are used for producing FAMEs from waste vegetable oils having a high content of FFAs, such as waste palm and rapeseed oils. The presence of FFAs strongly affects process performance and economics. If a homogeneous base catalyst is employed, soaps will be produced in the reaction between the base catalyst and FFAs, which inhibits FAME synthesis. If a homogeneous acid catalyst is used, saponification is avoided but the transesterification rate is slow. By applying a two-step process consisting of acid-catalyzed esterification followed by base-catalyzed transesterification, the mentioned disadvantages of homogeneous base and acid catalysts are overcome.
Kheang et al. [61] employed a sulfonated ion-exchange resin and sodium hydroxide as a catalyst to obtain FAMEs from the waste vegetable oil extracted from SBE, which contains more than 11% of FFAs. The esterification step using the resin catalyst (oil-to-resin ratio 10:1) converts most of the FFAs to FAMEs. The conversion of TAGs to FAMEs in the transesterification step using sodium hydroxide was more than 98%. If the content of FFAs is extremely higher, such as in SBE exposed to air for a couple of months, the amount of resin catalyst and the reaction time should be increased. The obtained methyl esters have comparable fuel characteristics as petroleum diesel.
Huang and Chang [51] esterified FFAs from waste oil by methanol in the presence of sodium hydroxide until its content was reduced below 2%, and then, the esterified oil was subjected to methanolysis using again sodium hydroxide when the conversion gave a FAME yield between 85% and 90%. They also performed a financial analysis showing that the production cost of biodiesel from the waste oil was lower than those of diesel or biodiesels obtained from refined oil or WCO.
3.2.2 In Situ Extraction and Transesterification
In situ biodiesel production is a novel method for producing biodiesel from oil-bearing materials in which extraction and transesterification take place simultaneously. It integrates the oil extraction from SBE and the extracted oil conversion into biodiesel in one continuous process so that the process can reduce the time and the cost of biodiesel production [69]. The biodiesel production from SBE containing waste oil can be performed through two consecutive or simultaneous oil extraction and reaction processes; commonly transesterification stage is proceeded by the pre-esterification stage due to a high FFA content of the SBE oil. Extracting solvent may be either alcohol used as esterification/transesterification reagent or an organic solvent. The esterification is usually catalyzed using an acid (sulfuric acid). Only homogeneously and enzyme-catalyzed methanolysis has been investigated so far.
Mat et al. [68] have compared the activity of homogeneous base (potassium hydroxide) and acid (sulfuric acid) catalysts for in situ methanolysis of SBE containing waste palm oil in the presence of n-hexane as an extracting solvent. The use of base catalyst produced a higher FAME yield in a shorter time than the use of acid catalyst, as expected. However, reported FAME yields are too low (below 20%) to be interesting for developing an industrial biodiesel production process.
In situ homogeneous biodiesel production from SBE containing waste palm oil can be carried out through a two-stage process that includes in situ esterification and transesterification [69, 70]. The first esterification stage is catalyzed by an acid (sulfuric acid) whereas the second transesterification is base-catalyzed using an alkali (sodium hydroxide). Sugiharto et al. [69] optimized the in situ transesterification of the pre-esterified SBE palm oil using sodium hydroxide regarding reaction temperature, catalyst concentration, and time. The optimum conditions (64.33 °C, 2.39% NaOH, and 2.32 h) provided a biodiesel yield of 21.45% (biodiesel/SBE). Under the optimum agitation speed (730 rpm), Suryani et al. [70] obtained the biodiesel yield and purity of 84.5% and 99.3%, respectively, for 90 min.
Park and coworkers [63, 71, 72] have investigated in situ transesterification of waste oils catalyzed by lipases of different origin in the presence of different organic solvents. Various primary alcohols were used for transesterification. Of several tested lipases, the most active originates from Candida cylindracea, displaying a conversion of 78% within 4 h of methanolysis reaction in the presence of n-hexane. However, this lipase reached the conversion of 96% in 8 h of reaction in the presence of 1-butanol and n-hexane. Kojima et al. [71] investigated fossil fuels (diesel oil and kerosene) as a solvent for the transesterification of TAGs embedded in SBE. The lipase showed the highest stability in diesel oil. A nearly 100% conversion within 3 h was obtained from SBE using diesel oil as a solvent in the presence of 10% lipase. Kerosene was shown to be as good solvent as n-hexane. These results were utilized to perform lipase-catalyzed biodiesel production from SBE in a 50-L pilot plant [72]. With 1% lipase added to SBE, the conversion reached 97% within 12 h at 25 °C. A mixture of biodiesel and diesel oil at the ratio of 45:55 meets the standard EN 14214.
Schematic presentation of in situ FAME production from SBE based on the use of lipase in the presence of an appropriate solvent is shown in Fig. 5.4. The production process using diesel oil is much simpler than that using n-hexane [71]. When diesel oil is used, a mixture of FAMEs and diesel oil is produced directly the following filtration after the extraction/esterification, while when n-hexane is employed, an additional separation step is needed. The filtration cake consists of oil-free waste solid material, FAMEs, glycerol, solvent, and enzyme. The main product, FAMEs, can be recovered from the filtration cake by extraction with n-hexane. The solvent can be recuperated and reused in the process. However, it is impossible to isolate lipase from the FAME-free waste solid material. This final by-product can be regarded as immobilized lipase that can be recycled to the process so long as the lipase is active, which will decrease the catalyst cost. The repeated production of FAMEs with SBE was demonstrated in solvent-free systems [74]. The repeated batch and fed-batch processes were conducted for nine and six cycles without a significant enzyme inactivation, but the FAME yield was twice higher in the former process .
Schematic diagram of the FAME production from spent bleaching earth using diesel oil (a) and n-hexane (b) as the solvent. (Adapted from [61])
3.3 Biodiesel from DD
DDs are a valuable by-product in the last step of vegetable oil refinery, called deodorization, where odoriferous components and FFAs are removed from the refined oil by vacuum steam distillation. The amount of DD is typically about 0.2–0.5% of the raw material. Based on the annual world’s production of edible oils [18] and the assumptions suggested by Echim and coworkers [17], the world’s generation of DD in 2019 is estimated to be 4.7–8.1 million metric tonnes, respectively. The composition of DD depends on the vegetable oil origin, the refining procedure, and the operating conditions of the distillation plant [17]. Generally, it is rich in FFAs (33–81%), the unsaponifiable matter containing tocopherols (vitamin E), sterols and squalene (6.6–41.2%), and AGs (0.72–13.6%).
DDs are a good source of bioactive compounds (sterols, tocopherols, and squalene). These compounds can be extracted and further used in the pharmaceutical industry, cosmetics, and as food additives. Furthermore, FFAs from DDs are mostly used as additives for animal food, fluidizing agents for lecithin, or as medium-grade soaps. DD have also nonfood applications, such as a biofuel in the mixture with the fuel oil to fire the steam boilers [17].
There are two possible routes to produce biodiesel from DD, namely by direct esterification of FFAs or by conversion of FFAs to AGs by glycerolysis prior to transesterification, as shown in Fig. 5.5. Direct FFA esterification is performed not only for the biodiesel production but also as a preliminary step in the purification of the tocopherols and sterols. Reviews of the literature on biodiesel production from DD via the two routes are given in Table 5.3. DD originates from the refinery of palm, soybean, rapeseed, corn, and canola oils. Direct esterification of FFAs catalyzed by sulfuric acid, ion-exchange resins, or lipases has been much more studied than glycerolysis of FFAs which has been catalyzed either by lipases or was conducted in the absence of any catalyst. Usually batch stirred reactors are employed, although a packed-bed reactor and a continuous stirred tank reactor are also applied. Methanol is mainly used as an esterification agent, whereas other alcohols (ethanol, butanol) are rarely employed. The yield of final reaction products depends on the origin of DD and the reaction conditions applied.
Production of biodiesel, sterols, and tocopherols from deodorizer distillates by direct esterification (a) and production of biodiesel/biofuel via acylglycerols route from deodorizer acid oils or distillates (b). (Adapted from [17])
3.3.1 Direct Esterification
3.3.1.1 Chemically Catalyzed Esterification
Facioli and Arellano [75] described an esterification process catalyzed by concentrated sulfuric acid to obtain FAEEs from soybean DD. The process was statistically optimized, and a conversion degree of 94% was achieved under the optimum conditions: ethanol-to-FFAs molar ratio of 6.4:1 to 11.2:1, H2SO4 amount of 0.9–1.5% and reaction time from 1.3 to 2.6 h. The esterification of FFAs with ethanol was the predominant reaction, while the loss of tocopherols was lower than 5.5%. An excess of ethanol was necessary for obtaining the best conversion.
Verhé et al. [76] reported a process of converting the DD to biodiesel by methanolysis catalyzed by sulfuric acid at 75 °C for 5 h. The methanol-to-FFA weight ratio of 1:1 and 5% sulfuric acid were employed. The crude biodiesel was washed with water, dried, and distilled to increase the quality of the FAMEs. The distillation pitch was processed for obtaining sterols and tocopherols.
Chongkhong et al. [77, 78] studied batch and continuous esterification of palm fatty acid distillate (93% FFAs) with methanol in the presence of sulfuric acid as a catalyst. The conversion higher than 95% was achieved in the batch process with the methanol-to-distillate molar ratio of 4.3:1 with 1.834% of H2SO4 at 90 °C within 2 h, while the optimum conditions for the continuous process were methanol-to-distillate molar ratio of 8:1, 1.834% of H2SO4, 70 °C and retention time of 60 min. The batch esterification yield (99%) was higher than the continuous yield (97%). A further treatment of the obtained product, consisting of FFA neutralization and AG transesterification, was required to obtain biodiesel, which complies with the specifications. The flow diagram for the proposed continuous process operated under mild reaction conditions is shown in Fig. 5.6.
A schematic diagram of a continuous unit for biodiesel production from palm fat acid distillate. (Adapted from [68])
Villardi et al. [79] compared the conversion of FFAs present in soybean DD into FAEEs through the batch esterification reaction using methanol with and without catalyst (sulfuric acid) and free catalyst in a batch reactor. In the presence of the catalyst (3%), the maximum conversion was 99.7% at the ethanol-to-oil molar ratio of 10:1 and 100 °C in 180 min whereas in the absence of the catalyst, the maximum conversion was lower (89.0%) at the same ethanol-to-oil molar ratio at a higher temperature (280 °C) but a shorter reaction time (105 min). These results indicate that the supercritical medium reduces the oil conversion to FAEEs due to the parallel reactions occurring and to the degradation of acids and esters at the required high temperature and pressure. The developed kinetic model based on the one-step reversible second-order reactions agrees well with the experimental data.
Souza et al. [80] tested several solid acid catalysts for the esterification of soybean oil DD with ethanol. The highest conversion (49%) was achieved with 9% of a commercial zeolite type (CBV-780) at 100 °C within 2.5 h. Xi and Cao [81] esterified a palm oil DD using a cation-exchange resin as a catalyst and achieve the conversion of about 82% under the optimum reaction conditions (methanol-to-DD molar ratio of 17.25:1 and 60 °C).
Liu and Wang [82] performed esterification of FFAs from rapeseed oil DD catalyzed by a cation-exchange resin in a packed column reactor. The conversion of over 96% was achieved under the following optimal conditions: the resin catalyst dosage of 18% (based on oil mass), the methanol-to-oil molar ratio of 9:1, the reaction temperature of 60 °C, and the reaction time of 4 h. The catalyst can be regenerated and reused. In ten repeated batch cycles (40 h), biodiesel yield was over 88%. This process was as effective as the process catalyzed by sulfuric acid, but it had no washing step. The process was further improved by including alkali-catalyzed transesterification after the pre-esterification step [83]. The biodiesel yield by KOH-catalyzed transesterification was 97.4% using a methanol-to-oil molar ratio of 4:1 at 60 °C within 1.5 h. Furthermore, biodiesel and tocopherols were co-produced from soybean oil DD combining a pretreatment with supercritical carbon dioxide extraction. The pretreatment included cation-exchange resin-catalyzed esterification, cold recrystallization to removing sterols, and then alkali-catalyzed transesterification.
Yin et al. [84] produced biodiesel from a pre-esterified soybean oil DD using calcined duck eggshell (DES) as an inexpensive and environment-friendly catalyst after calcination (900 °C). The DD pre-esterification with methanol (12:1) was catalyzed by sulfuric acid (1.5%) at 60 °C for 2 h. The process of biodiesel production from pre-esterified DD using the obtained CaO as catalyst was carried out under the optimal conditions (catalyst amount of 10 wt%, methanol-to-oil ratio of 10:1, 60 °C, 80 min) provided the biodiesel yield of 94.6%. The derived catalyst can be reused five times with the biodiesel yield above 80%. The obtained results indicate that catalysts prepared from carbonate-rich waste or natural products are suitable for catalyzing biodiesel production.
Naz et al. [85] prepared a novel solid tin-alginate catalyst was prepared from sodium alginate polymer, which was used for the esterification of corn DD with methanol. High recovery of 97.6% of FAMEs was obtained after eight cycles using the reprocessed catalyst under the optimized reaction conditions. Hence, by replacing the homogeneous acid and base catalysts and ease of catalyst separation, the tin-alginate catalyst has a great potential for green biodiesel production from DD with a high free fatty acid content.
Although the ultrasonic-assisted biodiesel production from a variety of feedstock has been frequently studied, a few studies have focused on DD as feedstock [86, 87]. Biodiesel production from soybean oil DD was enhanced by countercurrent pulsed ultrasound [86], compared to the transesterification under static probe sonication; the values of the rate constant were 0.68 L/mol/min and 0.56 L/mol/min, respectively. Under the optimal conditions (initial temperature 25 °C, methanol-to-oil molar ratio 10:1, flow rate 200 mL/min, catalyst content 1.8%, ultrasound working on/off-time 4 s/2 s and total operating time 50 min), determined using a single-factor experiment design, the biodiesel conversion was 96.1%. The same research group intensified the transesterification of the pre-esterified soybean oil DD by dual-frequency countercurrent pulsed ultrasound, compared to a single-frequency ultrasound-assisted reaction [87]. The highest biodiesel conversion was achieved by the combination of 20/28 kHz. Under the optimum conditions (methanol-to-oil molar ratio 8:1, catalyst content 1.8%, the water content less than 0.4%, the acid value less than 2 mg KOH/g), the biodiesel conversion was 96.3%. The transesterification reactions assisted by single-frequency static and dual-frequency countercurrent (simultaneous mode) pulsed ultrasound are pseudo-second-order with the energy activation of 26.034 kJ/mol and 18.122 kJ/mol, respectively, indicating that the latter is easier to occur than the former.
3.3.1.2 Enzyme-Catalyzed Esterification
Ramamurthi et al. [88] obtained up to 96.5% conversion by methyl esterification of FFAs from canola oil DD (CODD ) using immobilized lipase Randozyme SP-382 as a biocatalyst at temperatures around 50 °C and at a methanol-to-FFA molar ratio between 1.8 and 2.0 with no use of vacuum or water-removing agent. The inhibitory effect of methanol on the lipase activity was reduced by working at the lower temperature (around 50 °C). The esterification was considered to be a preliminary step preceding the recovery of sterols and tocopherols.
Facioli and Barrera-Arellano [89] reported the enzymatic esterification of the FFAs from soybean DD with ethanol using immobilized fungal lipase (Lipozyme IM) as a catalyst. The best conversion (above 88%) was obtained within 2 h with the lipase concentration of 10.7–23.0%, ethanol-to-FFA molar ratio of 1.7–3.2:1, and temperature of 46.4–53.6 °C. During the process, no losses of tocopherols were noticed.
Nagesha et al. [90] showed that supercritical carbon dioxide was a potential medium for esterification of FFAs from hydrolyzed soybean DD with butanol using an immobilized Mucor miehei lipase. Process conditions were optimized by conducting a statistical design method. A pressure of 122 bar, butanol concentration 1.2 M, enzyme concentration 15% (w/w), temperature 36 °C, and incubation time of 3 h were the optimal conditions ensuring 95.2% conversion of FFAs into butyl esters. This esterification process of FFAs is faster than the shake-flask method, where it takes 7 h to reach 88% conversion.
Wang et al. [91] described a process of simultaneous esterification of FFAs (28%) and transesterification of AGs (60%) from soybean DD to alkyl esters. A mixture of two enzymes (3% Lipozyme TL-IM and 2% Novozym 435) was employed in the presence of tert-butanol as cosolvent, which eliminated the negative effects of the methanol excess and glycerol on the enzyme stability. The activity of lipase was stable after 120 cycles. The maximum FAME yield of 84% was achieved with increasing tert-butanol content up to 80% (based on the oil mass). An adsorbent, silica gel or molecular sieve, was added to the reaction mixture (ten times maximum water mass) to control by-product water concentration, ensuring the biodiesel yield of 93% and 97%, respectively.
Du et al. [92] studied the enzymatic esterification of soybean oil DD. The reaction was Novozym 435-catalyzed methanolysis at 40 °C in a solvent-free medium. The lipase could maintain its stability and high activity even with more than 3 M of methanol existing in the reaction system, which was attributed to the presence of FFAs. Lipase tolerance to methanol had an almost linear relationship to free fatty acid content. There was almost no loss in lipase activity after being reused for ten cycles, each cycle of 24 h. The highest conversion of 95% was achieved by adding the molecular sieve to the reaction system.
Zeng et al. [93] produced biodiesel yields of 92.63% for 30 h and 94.36% for 9 h from rapeseed oil DD using liquid forms of Candida rugosa lipase and Rhizopus oryzae lipase, respectively, whereas the synergetic effect between the two lipases enhanced biodiesel yield to 98.16% in 6 h under the optimized conditions (DD-to-lipase ratio 0.84, water content 46%, 34 °C).
Dos Santos Corrêa et al. [94] investigated esterification of FFAs from palm oil DD with short-chain alcohols (methanol and ethanol) using immobilized commercial lipases (Lipozyme RM-IM, Lipozyme TL-IM, and Novozym 435). Among the enzymes studied, Novozym 435 showed the highest conversion using methanol (95%) and ethanol (91%). In the case of this enzyme, stepwise addition had a minor effect on the conversion. No significant increase in the conversion and the initial rate was observed when the amount of Novozym 435 was increased from 0.5% to 9%. A conversion of 86.7% was obtained using only 0.5% of Novozym 435. This enzyme was reused ten times with conversion reaching 88% and 65% after the eleventh batch with ethanol and methanol, respectively.
Rahman Talukder et al. [95] applied an immobilized C. antarctica lipase (Novozym 435) and an acidic styrene-divinylbenzene sulfonated ion-exchange resin (Amberlyst 15) as catalysts for biodiesel production from palm oil acid distillate in the presence and absence of organic solvents. Both catalysts were shown as effective catalysts for the mentioned process, but Amberlyst 15 was more methanol tolerant than Novozym 435. However, Novozym 435 acted fast, its optimal specific activity was 50-fold higher than that of Amberlyst 15, but its maximum biodiesel yield (95%) was somewhat smaller than that of Amberlyst (97%). Also, the minimum amount of Novozym 435 (1% of distillate) required for obtaining maximum biodiesel yield was much lower than that of Amberlyst 15 (30% of distillate). Novozym 435 activities at both 50 and 60 °C were the same and the biodiesel yield reached 90% within 2 h, while Amberlyst 15 was more active at a higher temperature and the biodiesel yield reached a maximum (97%) within 6–8 h. Water inhibited the activity of Amberlyst 15 more considerably than that of Novozym 435. Nonpolar solvent (isooctane, hexane) improved biodiesel yield in the enzymatic system from 90% to 95%, while their impact on the biodiesel yield in the Amberlyst 15 catalytic system was negligible.
3.3.2 Biodiesel Production via AG Route
Esterification of FFAs from DD with glycerol to form AGs as an intermediate step is another approach in the production of biodiesel or biofuels (Fig. 5.5b). This reaction leads to a mixture of MAGs, DAGs, and TAGs as well as unreacted reactants. The composition of the mixture depends on the reaction conditions such as the presence and type of catalyst, temperature, and the FFA-to-glycerol molar ratio.
Pure AGs can be prepared by the direct esterification of glycerol with the use of homogeneous basic (NaOH, KOH) and acidic (p-toluene sulfonic acid) catalysts, although the use of different heterogeneous catalysts has been reported. Enzymes have also an enormous catalytic potential in the processes requiring high regioselectivity [80], but these are not yet competitive at the commercial scale because of the high cost of the enzyme [17]. However, most of the research has been done on the synthetic samples and less on the sidestream refining products. The existing studies of the synthesis of AGs as an intermediate step in the biodiesel/biofuels production include enzymatically or non-catalyzed processes.
3.3.2.1 Enzymatically Catalyzed Process
Lo et al. [96,97,98] reported the synthesis of AGs (mainly DAG)s by lipase-catalyzed esterification of glycerol with FFAs from corn oil, palm oil, and soybean oil DD. Impact of reaction conditions, such as enzyme type and load, substrate-to-glycerol molar ratio, reaction time, temperature, and water content, as well as the effect of a water adsorbent, was studied. Lipozyme RM-IM was the most effective lipase among the lipases screened. Under the optimum reaction conditions (10% catalyst, 2.5:1 FFA-to-glycerol molar ratio, 65 °C and 30% molecular sieves), the AG yields of 70.0%, 52.0%, and 69.9% were achieved from corn oil, palm oil, and soybean oil DD in 5 h, 6 h, and 4 h, respectively.
Tangkam et al. [99] studied the enzymatic preparation of DAGs from DD resulting from the refining of various vegetable oils. A direct glycerolysis of a mixed distillate with Novozym 435 led to moderate proportions (52%) of DAGs. The application of a two-stage reaction involving hydrolysis of DD followed by glycerolysis led to a higher synthesis (62–72%) of DAGs. Furthermore, the high initial concentration of FFAs in the distillate had a positive effect on the concentration of DAGs in the final product (>71%). Short-path vacuum distillation of the esterified product led to a concentrate containing 94% of DAGs, up to 3.9% of TAGs below 1% of FFAs. Reaction temperature strongly increased the esterification rate, whereas the effect of pressure was moderate.
3.3.2.2 Non-catalyzed Process
Smet [100] described the esterification of a fatty acid distillate (93% FFA) with glycerol in a stirred batch reactor at 200 °C and 90 mbar. The novelty of the process is in synthesizing AGs in less than 6 h with no catalyst present in the reaction system. The total AG content of 85.3% was obtained using a glycerol-to-FFA molar ratio of 1:1 in 345 min reaction time. A similar yield of total AGs (86.2%) was obtained at a reduced molar ratio of 1:2. However, at an increased molar ratio of 2:1, the reaction was slowed down and the total AG content was reduced to 64.9%. Because of the high content of FFAs, a distillation step was necessary to increase the purity of the synthesized AGs. The by-products of distillation were further used as reaction products in the synthesis of AGs.
4 Biodiesel Production from WAFs
Animal fats, like vegetable oils, are biological materials (lipids), having similar chemical structures, but a different distribution of fatty acids. Both materials are water-insoluble, hydrophobic, soluble in nonpolar organic solvents, and made up mainly of TAGs, although DAGs, MAGs, and FFAs are also present. Their fatty acids content can be very high [6]. While vegetable oils are generally liquid at ambient temperature, many animal fats and greases tend to be predominantly solid due to their high content of saturated fatty acids (SFAs) [101]. For example, the SFA content in beef tallow is 45.6%, mutton tallow 61.1%, lard 39.3%, and chicken fat 32% [102]. As a result, the synthesis of FAMEs from WAFs can be realized at higher temperatures unlike the processes of WCO conversion [103]. WAFs have not been studied as extensively as sources for biodiesel production as vegetable oils, although their methyl esters have some advantages such as high cetane number and non-corrosivity [104]. The use of WAFs as a feedstock for biodiesel production eliminates the possibility of their disposition and contributes to the biodiesel supply. However, the available amount of WAFs is limited, meaning that these feedstocks will never meet the world’s fuel needs. The main sources of WAFs are meat animal processing facilities, large food processing, service facilities, and the collection and processing of animal mortalities by rendering companies [9]. About 1 million tonnes of biodiesel was produced from inedible rendering by-products in 2018 in the EU with a stable use of category 1 and 2 fats and a slight decrease in category 3 fat [105]. The first biodiesel plants in the world using not only trap grease and WAFs but also, the plants based on WCOs and palm fatty acid distillate were built in the Netherlands and Hong Kong in 2010 and 2011, respectively, both with capacities 100,000 tonnes/year [106].
Different WAFs such as pork lard (rendered pork fat), tallow (beef tallow from domestic cattle and mutton tallow from sheep), chicken fat, and grease are used as feedstocks for biodiesel production [106]. Tallow is a waste final product generated in slaughter, processing facilities, or by rendering operations. Its use is declined in time due to changing feeding habits of people and the soap industry cannot take up all produced excess WAFs. Recycled grease products are referred to as waste grease, which is generally classified based on the FFA level in two categories, yellow grease, and brown grease. Yellow grease is produced from animal fat and vegetable oil that is heated, used for cooking, and collected from commercial or industrial cooking businesses. It should have an FFA content of less than 15%. If the amount of FFAs exceeds 15%, then the grease is classified as brown grease. It sometimes referred as trap grease, a material that is collected in special traps in restaurants to prevent the grease from entering the sanitary sewer system. They are inexpensive material compared to food-grade vegetable oil and hence often cited as a potential feedstock for biodiesel production. One kilogram of most WAFs can be converted to a kilogram of biodiesel. If all the 5300 million tonnes/year of WAFs were converted to biodiesel, it would replace about 5.7 million L of diesel fuel [9].
The problem with the processing of WAFs in biodiesel production is their generally high content of FFAs, which determines the viability of the transesterification process. WAFs can be often converted to biodiesel using a base catalyst, but the great problem is the formation of soaps, which leads to loss of catalyst and ester, prevents separation of two fractions: biodiesel and glycerol and increases production processing costs [107]. An alternative method is to use acid catalysts, which are capable of catalyzing FFA esterification and TAG transesterification at the same time. Although the water content of WAFs is relatively low, it can affect the conversion [108]. For the base-catalyzed process, the conversion is slightly reduced when more water was added, but when the acid catalyst was used, the addition of only 0.1% of water leads to some reduction of the yield of esters. The presence of water has a more negative effect on transesterification than the presence of FFAs. To achieve the best results, the water content of beef tallow should be kept not beyond 0.06% [109].
To exclude the disadvantages of both base and acid catalysts, two-step (acid/base) processes for biodiesel production from WAFs with a high FFA content are developed. They consist of the acid-catalyzed FFA esterification (pretreatment, first step) for reducing the FFAs below 0.5% [109], or to less than 2 mg KOH/g [110, 111] and the base-catalyzed TAG transesterification (second step). In this way, compared to one-step processes, it is possible to achieve high biodiesel yield in short reaction time at mild reaction conditions. The only disadvantage of the two-step process, compared to the one-step process, is the higher production cost.
4.1 One-Step Processes
Different alternative procedures, such as homogeneous and heterogeneous catalysis, enzymatic production, and non-catalytic transesterification, have been studied with the goal of achieving higher conversion and shorter reaction time in the one-step processing of WAFs. The studies on one-step transesterification of different WAFs are reviewed in Table 5.4. Acids, bases, and enzymes are used as catalysts in these processes, although non-catalytic processes are also employed. Therefore, the processes for biodiesel production from WAFs are classified as follows: (a) acid-catalyzed, (b) base-catalyzed, (c) enzyme-catalyzed, and (d) non-catalyzed processes.
4.1.1 Acid-Catalyzed Processes
The use of acid catalysts in transesterification reactions has not only advantages such as the tolerance and less sensitivity toward the high FFA presence in the low-cost feedstocks (>6%) but also disadvantages such as the slower reaction rate, the requirement for higher alcohol-to-oil molar ratio, lower catalyst activity, and higher reaction temperature [151]. Biodiesel yield in homogeneous acid-catalyzed transesterifications is in the range 80–99%, and the reaction time is longer, compared to the base-catalyzed process [112, 113]. Catalyst loading, alcohol quantity, reaction temperature, and time are the factors that influence ester yield [112,113,114]. Ethanol is found to be better than methanol for converting WAFs from restaurants into esters since the former gives lower viscosity and maximum conversion of 78% [114]. The transesterification rate is usually greater at higher alcohol concentrations [113]. Also, with increasing catalyst quantity ester yield firstly increases up to the maximum value and then decreases, independently of WAF type and reaction temperature [112, 113]. This can be explained by the reversible nature of the transesterification reaction [112]. Also, esters produced from WAFs using acid catalysis results in a higher yield, compared to base catalysis [112].
Trap greases can be efficiently used for biodiesel production [115]. Two acid catalysts were employed to optimize the reaction conditions for the esterification of trap grease prior to the conventional base-catalyzed transesterification. Sulfuric acid is a more efficient catalyst than Fe2(SO4)3 in reducing the FFA content of trap grease under identical reaction conditions. Therefore, Montefrio et al. [115] recommended H2SO4 as a catalyst, although Fe2(SO4)3 has some advantages such as insolubility in methanol and grease, easily use and recovery, as well as the possibility of reduction for equipment corrosion. Mixing intensity is a significant parameter in the efficient pretreatment because of the heterogeneous nature of the reaction mixture. The efficiency of esterification increases with mixing intensity much higher in the presence of H2SO4 than in the presence of Fe2(SO4)3 [115].
The type of a heterogeneous catalyst for biodiesel production from WAFs depends on the FFA content in the feedstock. Base solid catalysts are preferable in the case of WAFs with a lower FFA content [137, 139], while acid solid catalysts are used for FAME synthesis from WAFs with high FFA content (>5%) [116,117,118, 120]. Different heterogeneous catalysts (basic, acidic, or mixed materials) can be used for biodiesel production. Most of them, as metal hydroxides, metal complexes, metal oxides such as calcium, magnesium or zirconium oxide, zeolites, hydrotalcites, and supported catalysts, can overcome some of the drawbacks on the use of homogeneous catalysts [152]. Kim et al. [117] showed that ZrO2 supported catalyst was highly active for esterification of brown grease, while Bianchi et al. [118] recommended strongly acidic cation-exchange resin Amberlyst for pretreatment of lard. Zirconium-containing SBA-15 silica (Zr-SBA-15) displayed good catalytic activity in FAME production by methanolysis of low-grade WAFs, accompanied by high stability and reusability after calcination [120]. Also, diarylammonium salts supported onto silica SBA 15 were very effective for the esterification of FFAs in greases [116].
In order to obtain biodiesel from brown greases with high FFA content (40% and 87%, respectively), Ngo et al. [116] and Kim et al. [117] developed new catalyst technologies using different solid catalysts. Silica-supported diarylammonium and ZrO2 supported metaloxide catalysts were very effective in the conversion of waste greases. The long-term activity of the ZnO/ZrO2 catalyst has been also confirmed in a packed-bed continuous flow reactor system for esterification of 90% technical grade oleic acid as a model compound for brown grease with methanol [117]. The FAME yield remained over 97% for 60 days.
Melero et al. [120] showed that for low-grade WAsF, independently of their acid value or unsaponifiable matter content, Zr-SBA-15 catalyst is highly active in the simultaneous esterification of FFAs and transesterification of TAGs with methanol .
4.1.2 Base-Catalyzed Processes
Homo- and heterogeneously base-catalyzed transesterification reactions are often used for biodiesel production from WAFs (Table 5.4). The most important factors which influence the reaction rate and biodiesel yield are the presence of water and FFAs in raw material, type and concentration of catalyst, alcohol-to-fat molar ratio, reaction time, and temperature.
The high biodiesel yield (about or above 90%) was achieved in most of the studies, independently of the type of animal raw material and type of catalyst. For the homogeneously catalyzed methanolysis of lard, the highest ester yield of about 98% was achieved for only 20 min and at the alcohol-to-fat molar ratio 7.5:1 [130]. Also, in the methanolysis reaction of duck tallow (molar ratio 6:1), a high ester yield of 97% was obtained within 3 h [127]. Bhatti et al. [112] showed that the higher FAME yield could be achieved using rather chicken than mutton fats at the same operating conditions. Results of Mata et al. [128] showed that it was viable to produce biodiesel from three different feedstocks (tallow, lard, and poultry fat) at the same operating conditions, whereby the highest yield was obtained using lard (91.4%). Biodiesel B100 (100% biodiesel) from these feedstocks cannot be used in vehicle engines without further additives introduction. Also, the high biodiesel yield was obtained in the presence of solid catalysts using mutton fat [139] and poultry fat [140].
The ester yield can be negatively affected by water and FFAs, so a pretreatment is needed to reduce or eliminate FFAs from WAFs. To reduce water content, the WAFs must be heated over 100 °C. The high acidity can be reduced in many ways, namely by applying acid-catalyzed esterification of FFA, acid-catalyzed transesterification , or heterogeneous catalyst [129]. The water content in the reaction mixture should be kept below 0.06%, while the FFA content should be kept below 0.5%. Beef tallow with 0.3–0.9% FFAs [122, 123], duck tallow with 0.28% FFAs, and lard with 0.33% FFAs [130] were successfully treated by homogeneously base-catalyzed methanolysis, and high biodiesel yields (above 90%) were achieved. On the contrary, Araújo et al. [153] successfully performed transesterification of beef tallow with high acidity (above 3.6%) after heating and preliminary formation of a microemulsion. However, Mutreja et al. [139] reported that catalyst MgO-KOH-20 was effective and tolerant to water or palmitic/oleic acids as FFAs.
The most used base catalysts in homogeneous transesterification are KOH and NaOH. The initial catalyst concentration is a very important factor having an influence on the ester yield. The optimal amount of the base catalyst is in the range 0.5–1% (based on oil weight), which depends on type of WAFs, although some researchers have reported slightly higher catalyst concentrations such as 2% [121]. An increase in catalyst amount increases the ester yield at a constant reaction temperature [112, 124, 127, 130]. However, beyond a certain catalyst concentration, a decrease in the FAME yield was observed due to soap formation [112, 124]. The soap prevents separation of biodiesel from glycerol fraction, increases the biodiesel viscosity, and decreases yield [124]. Comparing the type of catalyst under the same operating conditions, Chung et al. [127] found that the lower ester yield was obtained from duck tallow using CH3ONa (83.6%) and NaOH (81.3%) than KOH (97%). The KOH-catalyzed methanolysis of waste lard from piglet roasting takes part in a pseudo-homogeneous regime, obeying to the irreversible pseudo-first-order reaction law [132]. The reaction rate constant increases with raising the fatty acid unsaturation degree. A higher conversion degree (>97%) was achieved with waste lard within shorter reaction time (3 min) than with palm, sunflower, and waste cooking oils. In the presence of n-hexane as a cosolvent, the FAEE yield in the KOH-catalyzed ethanolysis of a blend of chicken fat and waste chicken oil is enhanced up to about 97% and the biodiesel properties were improved compared to the product of the non-solvent process [133]. This reaction follows also the first-order kinetics. Miladinović et al. [134] have recently shown that the continuous KOH-catalyzed transesterification of waste lard with methanol in a reciprocating plate reactor follows either the irreversible pseudo-first-order reactions or the reactions involving the changing mechanism and TGA mass transfer. The positive characteristics of continuous reciprocating plate reactor, such as frequent renewal of the interfacial contact area, plug flow, and effective mixing between immiscible reactants, shorten residence time (only 10 min) and make this novel reactor promising for upgrading biodiesel production processes using homogeneously catalyzed transesterification reactions.
For the heterogeneously catalyzed reaction, the preparation of basic catalysts is particularly important. It could be carried out by a wet impregnation method with the addition of an aqueous solution of KOH over MgO [139] or Al2O3 [136], followed by calcination of impregnated catalyst at a high temperature. Crystal nanonization is an efficient technique for preparing catalysts for biodiesel production even at room temperature because of reactivity and increased surface area of nanosized oxides [140]. The calcination of hydrotalcite yields mixed oxides, which show high surface areas and pore volumes, affecting positively their catalytic performance [120]. The decrease in the amount of MgO catalyst impregnated with KOH showed an increase in time for completion of the reaction [139]. An increase in catalyst amount increases the ester yield [136, 139], but after a certain limitation in the catalyst concentration, there is a decrease in the ester yield [136]. Mg-Al mixed oxide was found to be thermally and mechanically stable, and no significant difference was observed in particle size and morphology of the used catalyst. The similar Mg-Al ratio of the fresh and used catalyst also confirmed that the catalyst did not leach in the reaction mixture of poultry fat and methanol [141]. To catalyze the transesterification of waste lard from piglet roasting with methanol, Stojković et al. [138] used powdered quicklime (<15 μm, basically CaO) and pure CaO in a batch stirred reactor and quicklime bits (2.0–3.15 mm) in a continuous packed-bed tubular reactor. The kinetic models involving the changing- and first-order reaction rate laws with respect to TAGs and FAMEs, respectively, were verified for both reactors. At the methanol-to-lard molar ratio of 6:1, the catalyst amount of 5% (based on the lard weight) and the reaction temperature of 60 °C, a high FAME concentration in the produced biodiesel (97.5%) for 1 h, were obtained with quicklime in two consecutive batches. Under the same reaction conditions and the residence time of 1 h, the biodiesel yield in the continuous reactor was 97.6% while the FAME concentration in the biodiesel product was 96.5%.
The alcohol-to-fat molar ratio usually used in homogeneously catalyzed transesterification of WAFs is 6:1 [101, 122, 123, 126, 128, 129, 154], although some researchers suggest a higher molar ratio such as 7.5:1 [130] and 9:1 [125]. Some authors [124, 127] showed that the ester yield did not increase when the alcohol-to-fat molar ratio increased above 6:1. The authors generally agreed that the increase in the initial alcohol-to-fat molar ratio up to a certain limit increased the ester yield for both homogeneous [124, 125, 127] and heterogeneous [136, 137, 139, 141] processes. The alcohol-to-fat molar ratios in heterogeneously catalyzed transesterification are higher, for example, 18:1 [137] and 30:1 [141].
Homogeneous base-catalyzed transesterification of WAFs requires about 1–3 h. It was observed that most of the methanolysis process occurred during the second hour [124]. This could be associated with the molecular structure of the feedstock that contains SFAs [124]. The exceptions area much lower reaction time in the case of the lard methanolysis (0.33 h) [130] as well as the beef tallow methanolysis in the presence of ultrasound (0.02 h) [123] and radio-frequency heating (0.083 h) [125]. It was shown that the conversion increased with the reaction time [124, 125, 127]. The required time for heterogeneously base-catalyzed processes is usually longer, up to 8 h [141].
The WAF methanolysis has not been investigated in the wide range of reaction temperature, and the optimal temperature is about 60–65 °C, independently of the type of catalyst. Some researchers recommended lower temperature such as 30 °C for the homogeneously catalyzed methanolysis of chicken and mutton fat [112], but the lower ester yield was achieved. Also, the temperature of 20 °C was suggested for the beef tallow methanolysis using radio-frequency heating [125]. The increase in reaction temperature increases the biodiesel yield so that the average yield could be increased roughly by 5% for every 5 °C for homogeneously catalyzed processes [124]. The proportional increase in ester yield was also observed by the other investigators [127, 130]. The increase in reaction temperature improves the miscibility of poultry fat and methanol in the presence of a heterogeneous catalyst [137, 139, 141].
Da Cunha et al. [122] performed the methanolysis of WAFs using KOH as the catalyst in a continuous pilot plant aiming at the construction of an industrial-scale plant (120,000 kg/day capacity) for biodiesel production from beef tallow. However, it was necessary to introduce two additional steps: a methanol recovery from glycerol and biodiesel and biodiesel separation using a centrifuge.
The solid catalyst could be reused without significant loss of activity [137, 140]. Catalyst nanocrystalline CaO can be successfully recycled three times, but it failed in the fourth cycle [140].
Addition of cosolvent (hexane, toluene, or tetrahydrofuran) could not enhance the conversion of poultry fat using Mg-Al hydrotalcite derived catalyst [141].
The application of ultrasonic irradiation for biodiesel production from waste animal fats has received little attention until recently. This method may be a promising and effective alternative to the conventional method for the production of quality biodiesel from WAFs [123, 131, 136]. The ultrasound-assisted KOH-catalyzed transesterification of chicken fat with methanol provided a similar conversion degree (94.8%) as the conventional method while the reaction time was significantly reduced, making the former method superior to the latter method [131]. A high FAME yield (about 92%) can be achieved in the shorter time, compared to the conventional procedure (1 h), due to a collapse of the cavitation bubbles and ultrasonic jets that impinge methanol to TAGs and cause emulsification. Ultrasonic heating also reduces the reaction time of the solid catalyzed process [136]. Therefore, the TAG methanolysis using ultrasound is feasible, time-saving, and economical method for producing biodiesel. However, ultrasound reduces the activity of a solid catalyst. After the completion of transesterification, the collected solid catalyst could be refreshed by loading an additional catalyst amount and then reused [136].
Beside microwave heating, radio frequency is another dielectric heating technology with a similar mechanism, but simpler, considering the system configuration, and with deeper energy penetration into the material [125]. It is more economical and more suitable to apply in large-scale reactors than microwave heating. A conversion of 96.3% was obtained in the transesterification of beef tallow with NaOH under radio-frequency heating for only 5 min at 20 °C [125].
4.1.3 Enzyme-Catalyzed Processes
Enzymatic catalysts , lipases, are also used in the transesterification reaction of WAFs via a one-step process. They can simultaneously catalyze TAG transesterification and FFA esterification. Lipases are preferred to be used in immobilized form, which allows easy reuse and control of the process. The review of the reaction conditions of the lipase-catalyzed transesterification of WAFs providing a significant ester yields is given in Table 5.4.
Inactivation of the enzyme that leads to the decrease in ester yields mostly depends on the methanol concentration. This problem can be resolved by the stepwise addition of alcohol. Three-step methanolysis is sufficient to convert TAGs from lard to high ester yields [142, 143]. In the first and second steps of alcohol addition, the conversions are low, but methanol is completely soluble in the obtained ester in the third step, making the enzyme-substrate contact more sufficient. Also, Lee et al. [142] applied porous materials, such as silica gel, to keep the lipase active during the reaction when excess methanol was used.
Temperature is an important factor in the enzymatic processes of biodiesel synthesis. Generally, the enzymatic reaction is performed at temperatures between 30 and 50 °C [136, 142, 143, 145, 146]. Higher temperatures denature the enzyme, lead to the loss of solvents through volatilization [143], and decline the product amount [146].
Water content is one of the key parameters in the enzyme-catalyzed process, because it affects the catalytic activity of lipase. According to Lu et al. [143], the FAME yield decreases when the water content is more than 30% due to reduced homogeneity of substrate mixtures. Several organic solvents are indicated for their suitability in the enzymatic production of biodiesel [143]. In the lard methanolysis catalyzed with Candida lipase, the ester yield increases by the addition of n-hexane in the reaction mixture [143], although the immobilized Candida lipase can convert lard effectively to esters in a solvent-free system [142]. Generally, enzyme-catalyzed transesterification is performed with a high lipase amount (about 4–20%). The FAME yield increased rapidly with increasing the amount of lipase up to 20% but slowly above this limit [143].
The main drawback of the enzyme-catalyzed process, the high cost of the lipase, can be reduced by enzyme immobilization, which enables the reuse and easy recovery of the enzyme. Immobilized lipase is operationally stable over seven repeated cycles of the lard methanolysis with no evident decrease in the lipase activity [143]. Also, two immobilized lipases (non-specific Novozym 435 and 1,3 specific Lipozyme TL-IM) were successfully reused for 20 cycles [136]. The combined use of these two lipases is a potential way to reduce the cost of enzyme-catalyzed biodiesel production from lard using methanol as acyl acceptor and tert-butanol as the solvent [136].
The enzymatic approach in the presence of supercritical carbon dioxide (SC-CO2) has been also applied [147, 148]. When WAFs, which have a high melting point close to the denaturation temperature of lipase, are used for biodiesel production, they must be dissolved in a solvent. SC-CO2 can be proposed as an alternative to organic solvents which have a harmful effect on human health. The enzymatic process of WAF transesterification using SC-CO2 has many advantages [148]. Beside low temperature, there is no need for feedstock purification, and lipase is capable of transesterification of TAGs and esterification of FFAs present in the feedstock. However, the optimum ester yields obtained in the presence of lipase Novozym 435 are low (about 50%). By investigating the effects of enzyme loading, reaction temperature, and methanol-to-fat molar ratio on ester yield, Taher et al. [147] showed that FAME yield increased with both enzyme loading and time. The increase in reaction temperature resulted at first in an increase in ester yield because of the increase in rate constants and the reduction in mass-transfer limitations. Further, an increase in temperature resulted in a drop in ester yield because of the denaturation of the enzyme. The critical temperature at which the enzyme starts to deactivate was different, depending on the type of lipase and immobilized surface. As expected, the increase in methanol-to-fat molar ratio from the stoichiometric one resulted in the increased FAME yield to an optimum value, but after that the yield dropped due to inhibition of lipase by methanol [147]. A combined continuous process of extracting fat from meat and ester synthesis using SC-CO2 in an integrated system seems to be economically feasible [148]. The drop in enzyme activity was observed in the third meat replacement cycle of the continuous experiment, compared to that of the first one. The inhibition effect of methanol is clearly seen from the higher drop in enzyme activity with the increase in methanol-to-fat molar ratio.
4.1.4 Non-catalyzed Processes
Recently, the transesterification of WAFs using supercritical methanol has been suggested to overcome the drawbacks of homogeneous catalytic processes. This non-catalytic process is simpler, environmentally friendly, and does not require any pretreatment of inexpensive unrefined WAFs [147, 150]. Furthermore, the presence of water and FFAs do not affect the ester yield because TAG transesterification and FFA esterification occur simultaneously. For example, different waste lard samples containing various FFAs and water contents were treated successfully using a supercritical process [150]. Obtained FAMEs from waste lard with no pretreatment were found to be comparable with those from refined lard. A review of the operating conditions applied in supercritical one-step processes in batch and continuous reactors is given in Table 5.4.
Marulanda et al. [149, 155] investigated the effect of temperature , pressure, alcohol-to-fat molar ratio, and residence time on the chicken fat conversion and the product quality in batch and continuous reactors under supercritical conditions. A preheating of the feedstock at high temperature (350 °C) was used without a significant thermal fat decomposition. Furthermore, it was concluded that the transesterification was not a reverse reaction at 300–400 °C, but by-product (glycerol) was thermally decomposed. Thus, a continuous process with a moderate excess of methanol and in situ glycerol decomposition could be used as very promising for processing WAFs and increasing biodiesel profitability [155].
The reaction pressure does not significantly affect the efficiency of the TAG conversion at high temperature, but slightly changes the composition of product [155]. Usually, a high biodiesel yield can be achieved at a pressure of 20–40 MPa [149, 155]. When the methanol-to-fat molar ratio was increased, the complete conversion was achieved, but excess methanol was also consumed in other thermal reactions [155].
The ester yield in a tubular reactor initially increased as the residence time increased to a maximum value and then decreased at longer residence times, which was attributed to the thermal decomposition of initially formed FAMEs under supercritical conditions [155].
4.2 Two-Step Processes
A review of two-step homo- and heterogeneous transesterification processes employing different WAFs is presented in Table 5.5. Most studies were related to the use of homogeneous catalysts. The important factors affecting the acid value in the first and the ester yield in the second step are the type of feedstock, type and concentration of catalyst, alcohol-to-fat molar ratio, reaction temperature, and time.
The most important property of acid catalysts used in the two-step processing of WAFs is the possibility of simultaneous accomplishment of esterification and transesterification. Independently of the type of WAFs, sulfuric acid is mainly used as an acid catalyst (the required amount varied from 0.5% to 20%) in the first stage of the process. The most used base catalysts in the second stage of the process are KOH, NaOH, or CH3ONa (the required amount varied from 0.4% to 1%). The catalyst amount is the most important factor affecting product quality.
After the addition of a mixture of acid catalyst and methanol into heated WAFs, the initial acid value decreases and then intends to stabilize [113, 156,157,158]. This behavior is attributed to the migration of the catalyst into the accumulated water, so becoming unavailable for the reaction [113]. The increase in base catalyst amount in the second step to the optimal value enhances the ester yield considerably, after which a slight decrease is observed because of soap formation [104, 113, 156, 157]. Actually, there is a desired level of acid or base catalyst amount bellow which the acid value or the ester yield is not reduced.
Among mineral acids applied so far in the first step, sulfuric acid is the most effective. Sulfamic [158] and phosphoric [113] acid are poor catalysts, as they do not reduce the FFA content of the WAFs significantly.
Methanol is the mainly used alcohol in both steps of the WAF processing. Independently of the type of catalyst, the optimal molar ratio of methanol-to-fat in both steps is varied in the range of 6:1 to 40:1. The increase in methanol-to-fat molar ratio in the first step leads to the reduction of the acid value to the optimal level [113, 157, 158], because the excess of methanol promotes reaction completion keeping the acid in methanol phase [113]. With increasing molar ratio methanol-to-esterified fat in the second step, the ester yield continuously increases to the optimal value and then remains the same or slightly rises [104, 157]. Encinar et al. [113] believed that for ratios higher than the optimal one, the excess of methanol could favor slightly the recombination of esters and glycerol to MAGs.
The reaction temperature for the transesterification of WAFs is a particularly important factor because of the high-fat melting point. The optimal reaction temperatures in both process steps are close to the boiling point of alcohol, i.e., in the range of 60–65 °C, when the maximum ester yield was obtained. The increase in reaction temperature decreases the acid value during the reaction time in the first step [113, 162]. If the temperature in the second step was adjusted at 50 °C, the reaction could not be started [104]. The increase in temperature in the range from 62 to 70 °C caused a decrease in the biodiesel yield because of methanol evaporation [104]. In the temperature range of 25–60 °C, the ester yield increases with increasing the reaction temperature [159]. The reaction temperature and time are interactive parameters in the transesterification reaction. The acid value decreases with time at different reaction temperatures [113, 158]. Decreasing is higher in the initial period of the reaction when the esterification of FFAs is almost complete [113, 162].
Several research groups have optimized the two-step biodiesel production from WAFs by conventional [160, 161, 163] and novel methods involving ultrasonication [164] and microwave heating [165]. Chavan et al. [160] optimized the alkaline transesterification step of biodiesel production from chicken fat oil by methanol whereas Keskin et al. [161] conducted the optimization of the esterification step of biodiesel production from broiler rendering fat with methanol in the presence of sulfuric acid. The overall FAME yields from these feedstocks were 89% [160] and 87.4% [161]. While the biodiesel from chicken fat oil contains 97.7% FAME [160], thus satisfying the standard limit, the final product from broiler rendering fat was 95.5%, i.e., below the limit. However, Sarantopoulos et al. [163] optimized both steps of biodiesel production from waste lard under mild conditions. The esterification step is significantly affected by the methanol:FFA ratio and the reaction time, and the feedstock acidity. On the other side, the transesterification reaction is positively affected by the reaction, time, KOH concentration, and methanol:TAG ratio. Furthermore, two empirical models describing the evolution of the two-step biodiesel production process were developed, which could be useful for scaling-up the two-step process. He et al. [164] enhanced biodiesel production from diseased swine fat by an ultrasound-assisted two-step catalyzed process. The response surface methodology provided the following optimal transesterification reaction conditions: the catalyst concentration of 1.11%, reaction temperature of 62.3 °C, methanol-to-oil molar ratio of 7.42:1, and the reaction time of 116.14 min, which ensured the 98.0% biodiesel purity within 176 min, thus shortening the overall process nearly three times compared with the one-step process. Lawan et al. [165] carried out biodiesel production from waste lard in a microwave reactor using calcium oxide (CaO) loaded on zeolite as a catalyst. Under the optimal reaction conditions, the FAME yield of 90.9% from the pre-esterified feedstock was achieved in a shorter time (135 min).
In order to prevent yield losses caused by the dissolution of FAMEs in the glycerol phase, Fröhlich et al. [126] investigated the possibility of esterifying FFAs either before or after base-catalyzed methanolysis of low-grade tallow (FFA content >8%) into biodiesel-grade esters. Under optimum laboratory conditions, base-catalyzed methanolysis followed by esterification of FFAs in the presence of different acids gave almost theoretical yields (about 98%). Considering the relatively large amounts of reagents required for neutralization in that case, it was concluded that the initial esterification of FFAs from tallow was a more convenient process for large-scale biodiesel production. Also, comparing the two- with the one-step base-catalyzed conversion of the same starting material, a much higher ester yield was obtained in the former case.
Ngo et al. [103] demonstrated that the polymer-immobilized catalysts were equally effective as their homogeneous counterparts in esterifying FFAs to esters and were readily recycled and reused at least three cycles for esterification upon reactivation with triflic acid. The resulting ester-AG mixture was then readily converted to total esters by base-catalyzed transesterification. However, when the reactivated catalyst was used for the fourth time under similar reaction conditions, a significant drop in the esterification activity was observed.
5 Biodiesel Production from WCOs
WCOs are promising feedstocks for biodiesel production because of their lower price than that of pure edible vegetable oils and easy availability. Some WCOs are used for fodder making and soap production , but major quantity is disposed of and thrown into landfills causing environmental pollution, such as water contamination . Since 2002, the European Union has prohibited the use of these oils in animal feeding due to the presence of harmful compounds that are formed during oil frying.
The amount of WCOs depends on the amount of edible oil consumption, and it is different in various world regions. For example, the amount of WCOs per year was estimated to be from 0.3 to 0.4 million tonnes in the United States, 0.135 million tonnes in Canada, 0.14 million tonnes in India, and 0.7–1.0 million tonnes in EU countries [166]. Thus, WCOs can be a potential source for biodiesel production. However, there is a lack of information on the overall WCO quantity used for biodiesel production annually in the world. The reported capacities of commercial plants for biodiesel production from WCO range from small (about 1–19 million L/year) [167,168,169,170] to large (16 million tonnes/year) [171].
Biodiesel production from WCOs depends on their physicochemical properties, which differ from “fresh” oils due to thermolytic, oxidative, and hydrolytic reactions occurring during frying. These chemical reactions lead to the formation of undesirable products (oxidized TAGs and DAGs, FFAs, polymers, dimmers), increasing viscosity, density, and tendency to foam, changing in the surface tension and color [172]. Knowing the properties of WCOs, amounts of FFAs and water above all are the first condition for successfully defining the method for its conversion into FAAEs.
The presence of undesirable compounds in WCOs has a negative effect on the course of transesterification reaction and FAAE yield, which makes appropriate pretreatment necessary. Depending on the quality of WCOs, pretreatment includes removal of suspended solid particles by filtration and decrease in moisture and FFA content. To decrease the FFA amount in WCOs, base neutralization [172, 173] and distillation [174] are recommended. Neutralization of FFAs is required especially in the case of base-catalyzed transesterification and can be performed as pretreatment of the oil [173], or simultaneously with transesterification reaction by adding excess catalyst than the amount necessary for catalysis [175]. Decreasing the FFA content prevents soap formation and catalyst consumption. The soap causes gel formation, makes glycerol separation difficult, and reduces ester yield. Water, present in the WCO, hydrolyzes AGs and esters to FFAs which subsequently form soap. Usually, water amount is decreased by heating, adsorption, evaporation, and distillation in vacuum or treatment with magnesium sulfate, silica gel, and calcium chloride [172, 176]. Drying of WCOs in industrial conditions is commonly done by distillation in a vacuum (0.05 bar) and at a temperature from 30 to 40 °C [177]. Simultaneous decrease in FFAs and water amount is achieved by the treatment of WCOs with a mixture of aluminum oxide and magnesium silicate [142] or by steam injection and sedimentation [178].
FAAEs from WCOs are produced in one- and two-step processes, depending mainly on the quality of the oily feedstock. Acid, base, or enzyme catalysts can be used or the transesterification reaction can be performed without catalyst under supercritical alcohol conditions. The application of each type of catalyst has certain advantages and disadvantages, which are influenced mainly by the amounts of FFAs and water in WCOs.
5.1 One-Step Processes
Table 5.6 summarizes the researches on biodiesel production from WCOs in one-step processes, as well as the applied reaction conditions and their optimal values for achieving the highest ester yield.
5.1.1 Acid-Catalyzed Processes
Acid catalysts are insensitive to the presence of FFAs in the oil and can catalyze esterification and transesterification reactions simultaneously, which makes them suitable for the production of biodiesel from low-cost WCOs with high FFA content [107, 185]. The advantage of the acid-catalyzed process is no soap formation. The FFA esterification reaction is relatively fast, while the TAG transesterification is slow and takes a long time. The main disadvantage of acid-catalyzed reaction is a slower reaction rate, compared to the base-catalyzed reaction. Lotero et al. [107] explained the low activity of homogeneous acid catalysts by different reaction mechanisms of acid- and base-catalyzed transesterification reactions. Another drawback of the acid-catalyzed process is the inhibition of the reaction by the water formed in FFA esterification, which stops the reaction before reaching the completion [166]. Also, the homogeneous catalysts are no reusable and their use causes problems with catalyst separation, acidic effluent, and serious environmental problems as well as the high cost of equipment and corrosion-related problems [183, 185, 244]. As already said, the use of heterogeneous acid catalysts could eliminate these problems, offering several benefits compared to homogeneous acid catalysts such as easy separation from the reaction mixture, simple purification of the products, reusability with or without regeneration, less environmental impact, and less corrosion of equipment [188, 244]. Due to its environmentally and economically advantageous, the heterogeneously catalyzed process is referred to as a green process [176].
Commonly used homogeneous acid catalysts in transesterification of WCOs are inorganic acids (sulfuric acid, hydrochloric acid, and phosphoric acid) and sulfonated organic acids. Among them, the most often used is H2SO4 due to its higher catalytic activity [180]. The scheme of biodiesel production from WCOs by H2SO4-catalyzed transesterification is shown in Fig. 5.7. The catalyst amount is variable and significantly influences the ester yield. A low catalyst amount is not suitable because the reaction is incomplete, while the high catalyst amount can cause the water formation and decrease in ester yield. Different optimal H2SO4 loadings have been reported so far (Table 5.6), which ranges (based on the oil weight) from 1.5% [179] to almost 15% (or H2SO4-to-oil molar ratio 1.3:1) [181].
Simplified block flow diagram of the acid-catalyzed process. (Adapted from [91])
Recent investigations of biodiesel production from WCOs are directed toward the use of heterogeneous acid catalysts which have strong potential to replace homogeneous catalysts [185]. Different heterogeneous acid catalysts have been employed (Table 5.6), such as heteropolyacid, cation-exchange resins, 12-tungstophosphoric acid supported on niobium, MoO3, WO3, zinc stearate, zinc ethanoate and 12-tungstophosphoric acid supported on silica or zirconia, cation-exchange resin/polyethersulfone, and polystyrene sulfonic acid/polyvinyl alcohol catalytic membranes. Compared to H2SO4-catalyzed methanolysis, high ester yields are achieved in the presence of higher solid catalyst amounts and in longer reaction times. The optimal catalyst amount and reaction time are influenced by the catalytic activity of the heterogeneous catalyst, active site concentration, specific surface area, as well as pore size and volume [245]. In methanolysis catalyzed by zinc stearate immobilized on silica, the highest FAME yield of 98% was obtained at the catalyst amount of 3% within 10 h [185], while the optimal catalyst amount and time for a cation-exchange resin (NKC-9) catalyzed methanolysis were 18% and 3 h, respectively [195].
One of the most important variables affecting the ester yield is the methanol-to-oil molar ratio. Wide ranges of the methanol-to-oil molar ratio of 4:1 to 250:1 and 1:1 to 110:1 have been used in homogeneously and heterogeneously catalyzed methanolysis, respectively. Generally, different molar ratios at which the maximum ester yield is reached have been reported. For H2SO4-catalyzed methanolysis of WCOs, the optimal methanol-to-oil molar ratio is 6:1 [179] while Zheng et al. [182] suggest a much higher value of 245:1. According to Feng et al. [195], the methanol-to-oil molar ratio of 3:1 provides achieving the highest FFA conversion in WCO methanolysis catalyzed by cation-exchange resin, while Talebian-Kiakalaieh et al. [189] and Cao et al. [190] observe the optimal molar ratio of 70:1 for heteropolyacid-catalyzed methanolysis. It is obvious that the optimal methanol-to-oil molar ratio depends on the catalyst type and other reaction conditions, and it should be established experimentally.
Reaction temperature has no significant influence on final ester yield but higher temperatures increase the reaction rate and consequently decrease the reaction time [150]. Different values of the optimal reaction temperature for the H2SO4-catalyzed methanolysis of WCOs have been reported such as 60 °C [184], 65 °C [179], and 80 °C [182]. The reaction temperature is more crucial in the case of using a solid catalyst because of the existence of a three-phase system which causes mass-transfer limitation, especially in the initial reaction period. High reaction temperature increases rates of both mass transfer and chemical reaction and enables achieving a high ester yield in short reaction time. If the heterogeneous catalyst poses strong acidity, high catalytic activity, and suitable textural properties, such as heteropolyacids [189,190,191], some cation-exchange resins and catalytic membranes [193, 194], the optimal temperature is around the boiling temperature of methanol, although the long reaction time is required for achieving the highest FAME yield. In the case of SO42−/SnO2–SiO2 [187] and 12-tungstophosphoric acid supported on Nb2O5 [192], much higher temperatures are suggested (150 °C and 200 °C, respectively).
Recently, novel carbon-based solid acid catalysts have been developed for biodiesel production from WCOs having high FFA content [196, 246]. They are obtained by sulfonation of incompletely carbonized carbohydrates: d-glucose, sucrose, cellulose, or starch. Incomplete carbonization leads to a rigid carbon material, which after sulfonation becomes a highly stable solid with a high density of active SO3H sites. These, so-called “sugar catalysts,” are characterized by excellent catalytic performance for the methanolysis of WCOs without leaching of SO3H groups during the reaction. According to Lou et al. [196], the best catalytic activity for WCO methanolysis has a starch-derived catalyst providing the 92% ester yield at a methanol-to-oil molar ratio of 30:1, catalyst amount of 10%, and 80 °C. The starch-derived catalyst is recyclable, stable, and promising for the development of an eco-friendly process for biodiesel production.
The possibility of reusing heterogeneous catalysts is another of their advantages, which enables the reduction of the process cost. Before reusing, catalysts are regenerated by washing with methanol [190, 191] or hexane and methanol [185, 189] to remove adsorbed compounds, or only filtered without any treatment [196]. Zinc stearate/SiO2 [185] and H3PW12O40 · 6H2O [189] were reused up to four times and Zr0.7H0.2PW12O40 [191] five times without serious loss in their catalytic activity. The cation-exchange NKC-9 resin exhibited excellent reusability for ten runs, and even an enhancement of catalytic activity was observed, which was attributed to the increase in the surface area due to the breaking of resin particles under agitation [195]. The excellent operational stability was observed for a starch-derived catalyst even after 50 cycles of successive reuse without any treatment of the used catalyst [196].
The reusability and stability of heterogeneous catalysts allow the development of continuous processes, which enable larger biodiesel productivity and reduced production cost, so they are acceptable for industrial biodiesel production. The continuous process for WCO methanolysis catalyzed by a heteropolyacid was developed by Noshadi et al. [188]. The process was conducted in a reactive distillation column, which combines reaction and separation of the products. At a total feed flow rate of 116.23 mol/h and inlet feed temperature of 30 °C, the FAME yield of 93.9% was obtained. Wang et al. [247] proposed a continuous process for biodiesel production from WCO by using the SO42−/TiO2–SiO2 solid acid catalyst. The production process was carried out in a sequence of three reactors with the countercurrent flow of vaporized methanol. Based on this process, an industrial demonstration plant with an annual capacity of 10,000 tonnes of biodiesel was built [247]. Park et al. [248] reported a continuous process for biodiesel production from WCO by using the pellet-typeWO3/ZrO2 catalyst. The process was carried out in a packed-bed reactor. However, the steady-state conversion obtained in a 140 h is 70%, and further improvement of the proposed process is needed. The FAME synthesis from acidified WCO was carried out in a packed-bed reactor with cation-exchange resin NKC-9 [249]. At mild optimal reaction conditions, the achieved FFA conversion was over 98% during 500 h of continuous running, indicating high efficiency and operational stability of the process .
5.1.2 Base-Catalyzed Processes
Base-catalyzed transesterification is the most commonly used method for the production of biodiesel from WCOs with low FFA content (less than 2%). However, if FFA content in the WCOs is more than 6%, the base catalyst is not suitable [107]. Generally, base-catalyzed WCO methanolysis occurs at milder reaction conditions, compared to the acid-catalyzed reaction. Apart from the oil properties, the reaction rate and FAME yield depend on the type and amount of the catalyst, methanol-to-oil molar ratio, reaction temperature, and agitation intensity of the reaction mixture. Homogeneous base catalysts are commonly used in biodiesel production because of their high catalytic activity at mild reaction conditions, achieving high ester yield in short reaction time, easy availability, and low cost. The scheme of the homogeneous base-catalyzed biodiesel production process from WCOs with FFA neutralization as pretreatment is shown in Fig. 5.8 [250]. Applying heterogeneous catalysts in biodiesel synthesis from WCOs is the subject of recent researches, and different compounds were investigated as catalysts .
Scheme of biodiesel production from WCOs by base-catalyzed alcoholysis. (Adapted from [215])
The most commonly used homogeneous base catalysts for WCO transesterification are KOH, NaOH, and CH3ONa. Dorado et al. [198] compared the catalytic activity of KOH and NaOH in transesterification of WCO with FFA content in the range of 2.76% and 4.33% and concluded that the KOH-catalyzed reaction was faster than the NaOH-catalyzed one. Other researchers also considered that KOH is an optimal catalyst [197, 200, 201]. Exceptionally, Dias et al. [172] reported that KOH was less effective than the sodium-based catalysts. Despite a slower reaction rate, NaOH is often used as a catalyst in WCO transesterification [175, 177, 199, 208]. The amount of base catalyst depends on the type of oil used [210] and ranging from 0.6% [177] to 1.26% [198], based on the oil weight, but according to most investigations, the optimal amount is 1%.
Different solid catalysts were used in the methanolysis of WCOs: metal oxides (pure or as oxides mixture), hydrotalcites, resins, and hydroxides loaded on support. As in the case of acid-catalyzed transesterification, high ester yield in the heterogeneously base-catalyzed reaction is achieved in the presence of higher catalyst amounts. The optimal amount depends on the catalyst type, and it is ranging from 3%, based on the oil weight, for activated carbon-supported KF [213] to 100% for calcined waste coral fragments [220].
Ester yield is significantly affected by the used amount of methanol. The homogeneous base-catalyzed methanolysis of WCOs was studied in the range of methanol-to-oil molar ratio from 3:1 to 15:1. Most researchers suggest the methanol-to-oil molar ratio of 6:1 as the optimal one [197, 200, 201, 203, 205, 206, 251], although some researchers suggest somewhat higher mole ratios, such as 7:1 [208], 7:1–8:1 [210], and 12:1 [204]. On the other side, Felizardo et al. [177] found out that the optimal methanol-to-oil molar ratio for NaOH-catalyzed methanolysis of WCO was 4.8:1. For heterogeneously catalyzed reaction, the optimal methanol-to-oil molar ratio is generally higher compared to the homogeneous reaction. According to most researchers, it is higher than 15:1 (Table 5.6), and in some cases, it reaches 30:1 [211] or even 50:1 [217].
Transesterification of WCOs was performed at different temperatures, depending on their properties, catalyst type, and applied reaction conditions. The optimal reaction temperature is in the range from ambient temperature [197, 198, 207] to the boiling temperature of alcohol [177, 200, 201]. The heterogeneously catalyzed methanolysis is performed at a higher temperature, compared to a homogeneously catalyzed reaction. The reaction temperature goes from 60 °C [212] to as high as 200 °C [186] to achieve ester yield more than 96%.
The agitation intensity is of particular importance for the methanolysis rate, especially in the initial reaction period, since the reactants are immiscible and a poor mass transfer between two phases limits the overall process rate. Therefore, intensive mixing is required in order to increase the reaction rate and to short the reaction time [202, 220].
Nowadays, high-efficient catalysts for the methanolysis reaction with CaO as the main component have been obtained from waste materials. Boey et al. [222] used a mixture of CaO from waste mud crab shells and cockleshells and boiler ash from agricultural waste in the methanolysis of WCO and achieved ester yield of 99% under mild reaction conditions. The CaO obtained by calcination of chicken manure was a promising catalyst for WCO methanolysis [226]. Calcined waste coral fragments [220] and calcined snail shell [194, 227] give the FAME yield in WCO methanolysis above 98%. Valuable catalysts for biodiesel synthesis consisting mainly of hydroxyapatite, CaO, and Ca(OH)2 can be derived from waste animal hard tissues using the thermal calcination method. Such catalysts, obtained from waste chicken bones [228] and waste quail beaks [229], were efficient in WCO methanolysis. Effective solid base catalysts for methanolysis reaction were also barium-enhanced waste marble catalyst [223] and potassium-loaded pumice [219].
The catalyst reusing is the subject of many studies in order to obtain high active and stable heterogeneous catalysts which are important for the development of continuous processes. Some catalysts could be reused after the appropriate regeneration method. TiO2–MgO mixed oxide after washing with methanol [217] and calcined layered double hydroxides CaAl2 700 after recalcination [214] were reused in four cycles without considerable change in their activity. Borges et al. [219] reported that potassium supported pumice could be reused up to five times after more complex regeneration consisting of washing with ethanol and new ionic exchange of pumice with KOH solution and calcination. The excellent stability was observed for calcined waste coral fragments, which was reused without regeneration up to five cycles reaching FAME yield more than 94%. The natural availability of this catalyst, together with its high stability and possibility to catalyze the methanolysis of WCO, makes it as promising for large-scale biodiesel production.
Shibasaki-Kitakawa et al. [215] developed a continuous process for biodiesel production using anion-exchange resin, Diaion PA306S, in an expanded-bed reactor, where a FAME yield of 93 mol% was achieved under mild reaction conditions. The methanolysis of WCO was also performed in a pilot plant consisting of a packed-bed reactor with recirculation of the reaction mixture [216]. CaO obtained by calcination of crushed limestone was packed into the reactor, and the obtained FAME yield was over 99%. The process was successfully repeated 17 times, and the FAME yield remained over 96.5% for every run. Zik et al. [230] also used the packed-bed reactor with recirculation for biodiesel production from WCO in the presence of a catalyst consisting of CaO and nanocrystal cellulose (obtained from chicken bone and coconut residues, respectively) supported with polyvinyl alcohol. The highest biodiesel yield (98.4%) was obtained under mild conditions (methanol-to-oil molar ratio 6:1 temperature 65 °C, and catalyst loading 0.5%). The catalyst was reused four cycles with maintaining the biodiesel yield above 90% [230].
5.1.3 Enzyme-Catalyzed Processes
Enzyme-catalyzed methanolysis can be successfully used for FAME synthesis from WCOs because of the insensitivity of the enzymatic reactions to FFAs and water amount, easy recovery of product, mild reaction conditions, and catalyst recycling. However, the main disadvantages of enzyme-catalyzed processes are a low reaction rate and a long reaction time needed for achieving high ester yield. Therefore, researches are directed toward the possible improvement of this process, which mainly involves an increase in lipase catalytic activity by techniques of immobilization, optimization of reaction parameters, and application of new reactor systems. High FAME yield from a WCO (about 93%) was achieved by the application of the immobilized Penicillium expansum lipase in the presence of an adsorbent (silica gel), which efficiently controlled the amount of water and positively affected the ester yield [236]. Recently, four different lipases from C. antarctica, T. lanuginosus, R. miehei, and P. fluorescens are most often used as catalysts for WCO transesterification.
Due to lipase inactivation by methanol, enzyme-catalyzed processes are usually performed at a low methanol-to-oil molar ratio, most often at the stoichiometric amount and with stepwise addition of methanol in accordance with the dynamics of its consumption [237, 252]. In the WCO methanolysis catalyzed by Candida sp. 99–125 lipase immobilized on the cotton membrane, the FAME yield increased five times when methanol was added in three steps, compared to single-step methanol addition [252]. Also, the higher methanol amount can be used if the process is carried out in the presence of ionic liquids and solvents which improve mutual solubility of TAGs and methanol and also protect enzymes from denaturation [38, 232, 233, 253]. The FAME yield obtained in the WCO methanolysis in the solvent-free system and in the presence of n-hexane (20% to the oil) was increased from 65% to 91%.
The organic solvent can ensure a homogeneous reaction mixture, reduce their viscosity and mass-transfer limitation, accelerate the reaction rate, and stabilize the enzyme. On the other side, the use of solvents increases the cost of the purification steps at the end of the production process [254]. Various organic solvents such as n-hexane, n-heptane, cyclohexane, acetone, benzene, chloroform, toluene, petroleum ether, tert-amyl alcohol, tert-butanol, acetonitrile, and isooctane have been used in the enzymatic biodiesel synthesis [233, 237, 252], but n-hexane is the most suitable one [237, 252]. Although hydrophilic solvents are much less effective, tert-butanol ensured high ester yield due to its moderate polarity and possibility to dissolve glycerol and methanol, resulting in high lipase stability [233]. Recently, ionic liquids [232] and supercritical CO2 [253] have also been used as solvents in the enzymatic transesterification.
The lipase activity is influenced by the presence of water in the reaction media [38, 252, 255], since it increases the interfacial area between aqueous and organic phases where lipase acting [242]. However, excess water leads to the hydrolysis reaction and the reduced FAME yield. For example, FAME yield increased from 31% to 91% as water content increased from 0% to 10% of the WCO, and then decreased as water content rose from 10% to 20% [37]. The optimal water amount depends on the feedstock, the lipase, the immobilization support, and the organic solvent employed.
Compared to chemically catalyzed reaction, the transesterification of WCOs catalyzed by lipase is performed at the low reaction temperature, and the optimal one is usually up to 45 °C. Exceptionally, Chesterfield et al. [231] and Dizge et al. [255] recommend a higher reaction temperature (65 °C).
In order to improve the lipase catalytic activity and to increase FAME yield, the researchers have investigated the use of different carriers for the enzyme immobilization such as textile materials (338, 252) and microporous polymeric matrix [255], using a lipase mixture [253], recombinant cells [256], or dual lipase modification procedure composed of cross-linking and protein coating with K2SO4 [257].
The enzymatic biodiesel production is usually performed in a batch stirred tank reactor. The use of continuously operated reactor contributes to the reduction of operational costs and increases the biodiesel productivity. Packed-bed reactors are suitable and most often applicable to biodiesel production [254]. The main disadvantage of using this type of reactor is that the glycerol remains at the reactor bottom and can be adsorbed on the lipase surface and decreased the process efficiency. Therefore, glycerol must be removed during the production process. Recently, several studies reported the application of a packed-bed reactor for the enzyme-catalyzed methanolysis of WCOs. The reaction setup consisting of two packed-bed reactors was used for WCO methanolysis catalyzed by Novozyme 435, and the reaction conditions were optimized for achieving the highest ester yield [258]. The obtained FAME yield was 80%, and it retained longer than 120 h. Nie et al. [252] conducted the WCO methanolysis catalyzed by Candida sp. 99–125 lipase immobilized on the cotton membrane in a series of nine packed-bed reactors with hydrocyclones after each reactor to separate glycerol. The final FAME yield under the optimal condition was 92%, and the lipase operational stability was more than 20 days. This process was recommended for industrial biodiesel production by Nie et al. [252]. The Candida sp. 99–125 lipase immobilized on the textile cloth was used for WCO methanolysis in a three-step packed-bed reactor system. The process was conducted for 100 h, with decreasing the FAME yield by 15.7%, which was attributed to lipase inhibition by glycerol or methanol [37]. A packed-bed reactor integrated with a glycerol-separating system was used for WCO methanolysis, yielding a methyl ester content of 94.3% [259]. This reactor system operated for 22 batches achieving the FAME yield over 92%.
The most efficient process for biodiesel production from WCO in a packed-bed reactor followed by downstream separation was developed by Rodrigues et al. [253]. A mixture of two lipases, C. antarctica (Novozym 435) and T. lanuginosus (Lipozyme TL-IM), in 2:1 mass ratio, was employed, and the reaction was performed in supercritical CO2. The reaction products were separated into two high-pressure separators. At steady-state and under the optimal reaction conditions, the FAME yield was 99% with the 30 s residence time [253].
Nowadays, a static mixer reactor was employed in the enzyme-catalyzed biodiesel production from WCO catalyzed by Candida sp. 99–125 lipase [260]. The main advantages of this reactor are low energy consumption, high mixing efficiency, and better mass transfer, as well as no moving parts. To achieve the highest FAME yield, both the static mixer structure and the process parameters were optimized. The number and length of mixing units, as well as the flow rate of WCO and methanol, were selected based on the pressure drop and methanol volume fraction, which indicated the energy consumption and mixing efficiency, respectively. The lowest pressure drop and the highest methanol volume were observed when six mixing units with a length-diameter ratio of 1.5 and the reactant flow rate of 0.28 m/s were used. Under the optimal reaction conditions, the FAME yield was 82.8% within the reaction time of 12 h, which is twice shorter compared to the reaction time in a batch stirred reactor [260].
Tan et al. [261] have recently reported that a factory in China conducts enzymatic catalysis using WCO as feedstock in a plant with a capacity of 10,000 tonnes. Immobilized lipase Candida sp. is used as a catalyst in a stirred tank reactor. The enzyme dosage is 0.4% (based on the oil mass). FAME yields of 90% are achieved under the optimal conditions.
5.1.4 Non-catalyzed Processes
Non-catalyzed transesterification of WCOs is a potential alternative to the abovementioned catalyzed processes. Although high pressure and temperature are required, this process is attractive due to nearly complete conversion in short reaction time. Since the presence of FFAs and water has a positive effect on the reaction rate due to the faster esterification rate and the water contribution to easier separation [108], non-catalyzed processes are suitable for biodiesel production from WCOs. Because of high capital costs and great energy consumption, the non-catalyzed process is still not employed in industrial biodiesel production. Recently, West et al. [262] have reported that the economics of the non-catalyzed transesterification of WCO was superior to those of chemically catalyzed processes.
Non-catalyzed WCO methanolysis was investigated in wide ranges of reaction conditions (methanol-to-oil molar ratio: 6:1–60:1; temperature: 250–450 °C and pressure: 10–43 MPa). The authors agree that optimal methanol-to-oil molar ratio is from 40:1 to 42:1 [108, 240, 242]. One of the most important variables affecting FAME yield is reaction temperature. Due to the thermal degradation of methyl esters at higher reaction temperatures which lowering the FAME yield [263], recent investigations of non-catalyzed WCO methanolysis are performed at lower temperatures ranging from 240 to 287 °C [240, 241]. The optimal temperature providing FAME yield of almost 100% was reported to be 270 °C [241] and 287 °C [240], despite the longer reaction time (45 min and 30 min, respectively). Contrary, Tan et al. [242] suggested the reaction temperature of 360 °C, but the obtained FAME yield was much lower (80%). The data about the influence of the reaction pressure on the non-catalyzed WCO methanolysis are not available, but it can be expected (as in the case of refined oils) that pressure above 10 MPa does not have a significant influence on the FAME yield [13].
Campanelli et al. [239] used methyl acetate instead of methanol for the non-catalyzed synthesis of FAMEs from WCOs, edible oils, and nonedible oils. Although the reactivity of supercritical methyl acetate was lower than that of methanol, the proposed process produces triacetin, a valuable, active biodiesel component, instead of glycerol. Furthermore, counting the triacetin content, this process led to higher overall biodiesel productivity.
5.2 Two-Step Processes
Two-step processes have been studied during the last years because of their efficiency for achieving higher ester yields from WCOs in shorter reaction times, compared to one-step processes. The most commonly used two-step processes are performed as the acid-catalyzed esterification of FFAs in WCOs (step 1) and the base-catalyzed transesterification of treated oil from the first step (step 2). The use of acid catalysts allows FFA conversion to alkyl esters, thus reducing the FFA content, and the transesterification of the treated oil can then be performed by using a base catalyst. The most commonly used acid catalyst is sulfuric acid, while the KOH is usually used base catalyst, followed by NaOH and CH3ONa. A review of two-step processes for FAAE synthesis from WCOs is given in Table 5.7.
The homogeneous two-step processes for WCO transesterification were investigated in the presence of different catalysts and methanol amounts as well as reaction temperatures. The optimal acid catalyst amount for the first step depends on the FFA content in the WCO. For the WCO with lower acid value (1.45 mg KOH/g), the optimal H2SO4 amount is lower (0.68% based on the oil weight) [264], while in the case of WCO with an acid value of 65 mg KOH/g, optimal H2SO4 amount is 15% [264]. Since the first step of the process provides necessary requirements for caring out the second step of the process, the optimal amount of base catalyst is usually around 1%, a typical value for base-catalyzed transesterification.
The most used alcohol in two-step processes is methanol . Excess of methanol is required to drive the reaction toward the formation of products, but the higher methanol amount in the first step can dilute the system results in a reduction of the H2SO4 efficiency in the first step [264] and makes the recovery of the glycerol difficult [243]. Therefore, the optimal methanol-to-oil molar ratio should be established experimentally. The base-catalyzed step has been investigated in the range of methanol-to-oil molar ratios from 4.5:1 to 35:1, and different optimal values were reported (Table 5.7). For instance, Charoenchaitrakool et al. [264] and Tanawannapong et al. [269] reported the optimal methanol-to-oil molar ratio of 9:1 at the KOH amount of 1%, while Li et al. [267] observed a higher optimal molar ratio (25:1) and a lower KOH amount (0.15%).
Achieving higher ester yield from WCOs with low acid value and meeting the biodiesel quality standard, two-step base-catalyzed processes were developed with an improved ester yield by 20%, compared to the one-step base transesterification [204]. To reduce the reaction time, Hancsok et al. [265] recommended the addition of cosolvent (tetrahydrofuran or dioxane) to the reaction mixture. The main disadvantage of acid/base two-step processes for synthesis is the necessity to remove catalysts in both steps. The removal of acid catalyst from the first step could be done by adding excess base catalyst in the second step [172]. Considering the negative effect of extra-base catalyst (formation of gel, difficulties in product separation), the amount of base catalyst should be carefully chosen. To overcome this problem, Guzatto et al. [271, 272] have recently proposed a base/acid two-step transesterification process (denominated Transesterification Double Step Process—TDSP ) for biodiesel production from vegetable oils. The process involves consecutive homogeneous base- and acid-catalyzed reactions without cooling the reaction mixture and the catalyst removal between steps, which significantly reduces the total process time. The proposed TDSP process is characterized by mild reaction conditions, easy separation of phases, high reaction rate, and conversion efficiency [271, 272]. The TDSP process was used for WCO methanolysis [271] and ethanolysis [272], and the achieved oil conversion was 97% and 98%, respectively.
Another, more widely used method for avoiding catalyst removal in the first stage is the use of heterogeneous catalysts. The researchers have suggested the use of ferric sulfate [183, 243, 276], SiO2 pretreated with HF [273] or 25 wt% 12-tungstophosphoric acid (TPA) supported on Nb2O5 [274] as catalysts for the first step. Compared with sulfuric acid , these catalysts are environmentally friendly, high efficiently, reusable, and easily separable from the reaction mixture. For example, the activity of SiO2(HF) [273] and 25 wt% TPA/Nb2O5 [274] remained rather unaffected after 10 runs and 6 runs, respectively.
Nowadays, researches are directed toward the development of two-step heterogeneous catalyzed processes. The FAME yield of 81.3% was achieved in a two-step process involving ferric sulfate in FFA esterification and CaO in methanolysis reaction [275]. The highest FAME yield (95%) was obtained by using 25 wt% TPA/Nb2O5 in the first step and 20 wt% ZnO/Na-Y in the second one at almost the same reaction temperatures but at the higher methanol-to-oil molar ratio, higher catalyst loading and longer total reaction time [274].
To reduce the biodiesel production cost, the nonconventional, highly active base catalysts obtained from natural and waste materials were used in the two-step biodiesel production processes. The base-catalyzed transesterification of the pre-esterified WCOs was performed in the presence of calcined sea sand, consisting mainly of CaO [277], and calcined waste chicken and fish bones, a combination of CaO, hydroxyapatite, and Ca(OH)2 [278]. Both catalysts were very active in the methanolysis reaction, providing a high FAME yield under mild reaction conditions.
The improvement of two-step processes includes the novel technologies, such as radio-frequency heating [268], ultrasound [270], and use of microtube reactor [269] which are, up to date, applied in the homogeneous processes. The two-step process of WCO methanolysis with radio-frequency heating was completed (FAME yield of 98.8%) in a reaction time of 13 min [268]. Thanh et al. [270] reported that a continuous ultrasonic reactor with a two-step process was an effective method for the biodiesel production from WCO, ensuring an almost total conversion at the residence time of 0.93 min for the entire process. A continuous microtube reactor exhibits excellent performance because of its extremely high mass and heat transfer rates and short molecular diffusion distance [269]. The FAME yield of 91.8% was obtained in this type of reactor for an overall process time of 10 s.
A biodiesel pilot plant production from WCO (acid value from 80 to 120 mg KOH/g) in a two-step process is located in Tianjin, China [279]. A scheme of the process is shown in Fig. 5.9. The capacity of the plant is approximately 3000 kg/day. FFA esterification is performed with methanol (weight ratio to the oil 60%) in the presence of H2SO4 (2%, based on the oil weight) and at 70 °C. The methanolysis reaction is catalyzed by KOH (0.8% based on the oil weight) at the methanol weight ratio to the oil of 30% and the reaction temperature of 60 °C. The biodiesel purification includes washing with acidified water, and heating by thin-film evaporator at 120 °C to eliminate water and residual methanol. This project is a first step for the construction of a big plant for biodiesel production with a capacity of 10,000 tonnes/year [279].
Flow sheet of biodiesel production via two-step process. (Adapted from [241])
6 Fuel Properties of Biodiesel Produced from Waste Oily Feedstocks
Compositional and physical properties of biodiesels produced from conventional vegetable oils, sidestream products of edible oil refining processes, WAFs, and WCOs are presented in Tables 5.8, 5.9, 5.10, and 5.11, respectively. For comparison, the provisional standards for biodiesel according to EN14214 are added. Most of the presented studies were not considered upgrading of crude biodiesel produced from waste oils, WAFs, and WCOs.
Edible-grade vegetable oils are currently the predominant feedstocks for biodiesel production. Based on the predominant oilseeds grown, these are soybean oil in the USA, rapeseed and sunflower oil in Europe, and palm oil in Asia. The oil quality and its fatty acid composition have the most significant influence on the fuel properties of biodiesel. The properties of biodiesel produced from edible oils along with the biodiesel quality standard EN14214 are compared in Table 5.8. Generally, edible oils biodiesel satisfies all standard limits. The exception is iodine value, which is in the case of soybean biodiesel higher than standard limits [280] because of the presence of a large amount of unsaturated fatty acids. Iodine value is dependent on the origin of the vegetable oil, and until recently, it has been believed that it influences the oxidation stability. However, the stability of biodiesel is shown to depend not on content but rather on the position of double bonds in the FAMEs [290]. On the other hand, biodiesel obtained from oils with a high amount of SFAs has a low iodine number, but higher pour and cloud point, as in the case of palm oil biodiesel [283]. This poor cold flow property is one of the most critical obstacles against biodiesel usage in cold climate conditions.
The chemical composition of biodiesel produced from waste vegetable oils can be expected to be essentially identical to that produced from origin vegetable oils as shown for biodiesels from soybean oil and its soapstock [285] and palm oil and SBE from palm oil refining process [61]. The properties of biodiesel produced from waste oily feedstocks are similar to those of biodiesel produced from refined vegetable oils and meet the biodiesel standard quality for all assayed parameters with some exceptions. The density of all biodiesels is within the specified limits, while kinematic viscosity, which is important for the biodiesel quality during the storage, is rarely outside the range specified by the standard. Flash point, carbon residue, cetane number, water content, and acid value are in accordance with the biodiesel standard. However, sulfur and phosphorus contents are not in agreement with the prescribed limits. High sulfur content is undesirable due to the increased emission of sulfur oxides. Most of the biodiesels does not meet the standard value for oxidation stability, which affects the storage of biodiesel. A low iodine value and a high cetane number of the biodiesel produced from an SBE residual oil were explained by a high content of SFAs [51].
When compared to biodiesel from refined vegetable origin, biodiesel from WAFs has the advantage of the higher heating value and higher cetane number and the disadvantage of lower stability to oxidation, because of the absence of natural antioxidants, and higher cold filter plugging point, because of greater content of SFAs [137]. The flash point of WAF biodiesel was significantly higher than that of the standard limit [122, 123, 128, 129, 144, 158, 159]. With a high flash point, biodiesel is safer to handle, transport, and store. However, too high a flash point, as in the case of chicken fat and tallow biodiesel [128, 129, 158, 159], may cause ignition problems in the engine. Pour point (or cold filter plugging point, CFPP) is indicative of a high concentration of saturated fatty esters in the product and important for their use in low temperatures. Biodiesel obtained from beef tallow has a higher CFPP than the limit [122, 123, 128, 129]. The higher cetane number of WAF biodiesel than the specified minimum limit makes them attractive as an alternative fuel [122, 156, 291]. Teixeira et al. [123] observed the higher viscosity of WAF biodiesel than conventional diesel, which causes poor fuel amortization, incomplete combustion, and carbon deposition on the injector. Also, the higher viscosity of beef tallow biodiesel than the established limit is due to the high content of high molecular weight SFAs [122]. Compared to biodiesel obtained from different feedstocks, Mata et al. [128, 129] observed that the kinematic viscosities for lard and chicken fat biodiesel were higher than the standard limit. The same authors also observed that the water content of chicken fat biodiesel was very high, but lard biodiesel presented a low value. However, tallow biodiesel purified with water satisfied the standard maximum limit. Also, only for the purified tallow biodiesel, the amount of Na + K is within the standard limit. This parameter suggests that purification methods were not effective, leaving catalyst residues dissolved in the biodiesel. Comparing homo- and heterogeneously catalyzed processes of lard, Dias et al. [292] concluded that the acid value of the product was significantly lower when the homogeneous catalyst was used, and also, it was smaller than the maximum standard limit. Such differences were due to the fact that the homogeneous catalyst tends to react with FFAs to generated soaps, which reduced the acid value. It was noted that the viscosities of the products were similar independently of the type of process.
The properties of biodiesel produced from WCOs generally meet the biodiesel standard quality with some exception. A somewhat higher value of kinematic viscosity of WCO biodiesel than the standard limit is the result of the presence of dimeric FAMEs, which are formed from polymers incurred during the heating of oil [172] and higher content of unreacted AGs [293]. Phan and Phan [210] reported high carbon residue, which corresponded to the amount of AGs as well as FFAs, soaps, remaining catalyst, polymers (dimeric and polymeric methyl esters), and other impurities [293]. The high total sulfur content of 180 ppm in a WCO biodiesel [180] cannot be compared with standard limit since it includes sulfur and sulfate ash content. The acid value generally meets the biodiesel standard, with the exception of WCO ethyl esters, but its acid value is in the range of ASTM D-6751 biodiesel standard [288]. Density, flash point, cetane number, water content, iodine value, MAGs, DAGs, and TAGs content, as well as phosphorus and Ca + Mg content, are within the biodiesel standard limits. Free and total glycerol amounts are significant for defining the quality of biodiesel. A higher free glycerol content may cause problems during storage because of its separation or can lead to injector fouling or the higher aldehyde emissions [293]. Based on available data on WCO biodiesel characteristics, free and total glycerol are in one case outside of standard ranges [209]. Free glycerol can easily be removed by washing step, while bonded glycerol depends on AG content, and could be lowered by optimization of reaction conditions in order to achieve higher AG conversion or by further distillation of the product. The higher value of Na + K indicated the remaining of catalysts, which can be removed by washing biodiesel [209].
Generally, no change in engine operation was observed during the test in the case of biodiesels derived from soybean soapstock [285] and WCO [172]. Regarding the exhaust emissions, only the NOx emission was increased [172, 285]. Particulate matter emission was significantly higher, and hydrocarbon emission was significantly lower for the soapstock biodiesel, compared to the biodiesel from soybean oil [285]. WAF biodiesel often reduces bot NOx emission and particulate matter and provides greater lubricity [105]. A slight fried food smell was observed, when WCO biodiesel was used on a large scale with diesel fuel [172].
7 Economics of Biodiesel Production from Waste Oily Feedstocks
Various factors affect biodiesel production costs including oily feedstock, other reactants, conversion and purification processes, the scale of production, region, etc. The major economic factor is oily feedstock, which is about 75–80% of the total cost, followed by labor and chemicals (methanol and catalyst) [294]. Although economic considerations are of great importance for employing a process at the industrial scale, a few papers present cost analysis of biodiesel production from waste vegetable oils [48, 51, 78] and WCOs [3, 14, 48, 262, 295]. Process simulation and economic analysis were conducted using HYSYS [3, 262, 295] and Aspen Tech [14, 48, 295] software packages.
Haas [48] assessed the economic viability of biodiesel production from soapstock. An estimate of 0.41 US$/L was obtained from a model of an industrial plant with a capacity of 20–40 million L of biodiesel per year, which was nearly 25% less than the cost of biodiesel from refined soybean oil. Chongkhong et al. [78] estimated the cost of biodiesel production from palm fatty acid distillate of 0.62 US$/kg for the capacity of 72,000 kg/year. The main part (60%) of the overall production cost was the cost of the input raw material. Huang and Chang [51] performed an economic analysis for annual biodiesel production of 1000 tonnes from SBE residual oil and got the cost of 0.37 US$/L, which was lower than the estimated price of biodiesel produced from refined vegetable oils or WCOs (0.8–1.5 US$/L). They showed that the price of crude oil heavily affected the production cost and the investment return period since the chemicals were the predominant cost constituents. The production cost of biodiesel from animal fats is estimated to be 0.36 US$/L and is lower than the price of biodiesel from rapeseed and sunflower oils (0.39 US$/L and 0.62 US$/L, respectively) but higher than the price of soybean biodiesel (0.33 US$/L) [296]. This cost can be decreased if none of the pretreatment capital costs are allocated to the total production costs.
The estimated total production cost for the non-catalyzed process of WCO biodiesel production was 150 US$/tonne, 214 US$/tonne, and 442 US$/tonne (corresponding to biodiesel required selling price of 0.17 US$/L, 0.24 US$/L, and 0.52 US$/L) for plant capacity of 125,000 tonnes/year, 80,000 tonnes/year, and 8000 tonnes/year, respectively [14]. The total production cost of 574 US$/tonne for a plant capacity of 8000 tonnes/year was established by West et al. [262], while Lee et al. [295] reported a somewhat higher value of 725 US$/tonne for plant capacity 40,000 tonnes/year. The manufacturing cost for homogeneous alkali-catalyzed batch processes with a capacity of 7260 tonnes/year was estimated to be 598 US$/tonne in the case of the hot water purification process and 641 US$/tonne for vacuum FAME distillation process [297]. Higher production cost (884 US$/tonne) was established by Zhang et al. [3] for a plant with 8000 tonnes/year capacity. The cost of WCO biodiesel production in a homogeneous pretreated alkali-catalyzed process was reported to be 650 US$/tonne [262] and 875 US$/tonne [295]. The reported values of biodiesel production cost in the homogeneous acid-catalyzed processes are close: 595 US$/tonne [262] and 644 US$/tonne [3]. The biodiesel production cost in the heterogeneous acid-catalyzed process was nearly 18% less than the cost of biodiesel in the homogeneous one in the same plant capacity [262]. Sakai et al. [297] estimated the production cost for batch CaO-catalyzed processes of 584 US$/tonne and 622 US$/tonne for water washing and vacuum FAME distillation processes, respectively, which is almost the same compared to batch homogeneous (KOH) process with the same capacity [297].
8 Conclusion
At present, homogeneously catalyzed processes of edible oils are primarily used in the commercial biodiesel production, although a heterogeneous process is also applied. Due to the competition to the edible oil market, usage in the human diet and food industry, and insufficient quantities of edible oils for biodiesel production, the use of alternative oily feedstocks in biodiesel production has been focused. Therefore, special attention has been paid to cheap, nonedible, and low-quality oily feedstocks, such as waste oily by-products from an edible oil refinery, WAFs, and WCOs. Instead of being disposed into landfills with potential environmental hazards, these materials can be used for making biodiesel as an economically sustainable and ecologically acceptable product. The fuel properties of biodiesel derived from these waste oily materials are similar to those of biodiesel produced from refined vegetable oils and meet the biodiesel standard quality for all assayed parameters with some exceptions. Also, no change in engine operation was observed with biodiesel obtained from waste oily feedstocks. The price of this biodiesel depends on the input waste oily feedstock, but it is generally smaller than the cost of biodiesel from refined vegetable oils.
Although the two-step (acid/base) homogeneously catalyzed process seems to be useful for converting low-quality oily feedstocks having a high FFA content, present investigations of biodiesel production from these feedstocks are focused on developing novel technologies based on the application of solid catalysts, enzymes, or supercritical alcohol conditions. It might be expected that homogeneous catalysis will be replaced by these novel technologies. Future processes will involve, beside low-quality oily feedstocks: (a) cheap, active, stable, bifunctional, no leachable and reusable catalysts, (b) continuous operation, (c) as low power input as possible (lower pressure, temperature and alcohol-to-oil ratio), and (d) no environmental problem. It is probable that the future commercial process of biodiesel production will be a choice among solid catalysts, lipases, and non-catalytic processes. Nowadays, it is claimed that a one-step enzymatically catalyzed process is operated for biodiesel production from WCOs at the pilot scale.
Abbreviations
- AG:
-
Acylglycerols
- DAG:
-
Diacylglycerols
- DD:
-
Deodorizer distillate
- FAAE:
-
Fatty acid alkyl esters
- FAEE:
-
Ethyl esters
- FAME:
-
Fatty acid methyl esters
- FFA:
-
Free fatty acids
- MAG:
-
Monoacylglycerols
- SBE:
-
Spent bleaching earth
- SSR:
-
Soap-splitting route
- TAG:
-
Triacylglycerols
- WAF:
-
Waste animal fats
- WCO:
-
Waste cooking oils
References
Toldrá-Reig F, Mora L, Toldrá F (2020) Trends in biodiesel production from animal fat waste. Appl Sci 10:3644. https://doi.org/10.3390/app10103644
Canakci M, Van Gerpen J (2001) Biodiesel production from oils and fats with high free fatty acids. Trans ASAE 44(6):1429–1436
Zhang Y, Dube MA, McLean DD, Kates M (2003) Biodiesel production from waste cooking oil: 2. Economic assessment and sensitivity analysis. Bioresour Technol 90:229–240
Krawczyk T (1996) Biodiesel: alternative fuel makes inroads but hurdles remain. Inform 7:800–815
Moser BR, Williams A, Haas MJ, McCormick RL (2009) Exhaust emissions and fuel properties of partially hydrogenated soybean oil methyl esters blended with ultra low sulfur diesel fuel. Fuel Process Technol 90:1122–1128
Karmakar A, Karmakar S, Mukherjee S (2010) Properties of various plants and animals feedstocks for biodiesel production. Bioresour Technol 101:7201–7210
Singh A, He B, Thompson J, van Gerpen J (2006) Process optimization of biodiesel production using different alkaline catalysts. Appl Eng Agric 22:597–600
Ma F, Hanna MA (1999) Biodiesel production: a review. Bioresour Technol 70:1–15
Canakci M (2007) The potential of restaurant waste lipids as biodiesel feedstocks. Bioresour Technol 98:183–190
Biodiesel Lurgi. http://lurgi.com/website/fileadmin/user_upload/1_PDF/1_Broshures_Flyer/englisch/0301e_Biodiesel.pdf. Accessed 1 Nov 2016
Bournay L, Casanave D, Delfort B, Hillion G, Chodorge JA (2005) New heterogeneous process for biodiesel production: a way to improve the quality and the value of the crude glycerin produced by biodiesel plants. Catal Today 106:190–192
Veljković VB, Stamenković OS, Todorović ZB, Lazić ML, Skala DU (2009) Kinetics of sunflower oil methanolysis catalyzed by calcium oxide. Fuel 88:1554–1562
Pinnarat T, Savage PE (2008) Assessment of non-catalytic biodiesel synthesis using supercritical reaction conditions. Ind Eng Chem Res 47:6801–6808
Van Kasteren JMN, Nisworo AP (2007) A process model to estimate the cost of industrial scale biodiesel production from waste cooking oil by supercritical transesterification. Resour Conserv Recycl 50:442–458
Kusdiana D, Saka S (2004) Two-step preparation for catalyst-free biodiesel fuel production. Appl Biochem Biotechnol 113–116:781–791
Dumont M-J, Narine SS (2007) Soapstock and deodorizer distillates from North American vegetable oils: review on their characterization, extraction and utilization. Food Res Int 40:957–974
Echim C, Verhe R, De Greyt W, Stevens C (2009) Production of biodiesel from side-stream refining products. Energy Environ Sci 2:1131–1141
Soy Stats (2020) World vegetable oil consumption 2019. In: A reference guide to important soybean facts & figures. American Soybean Association. https://soygrowers.com/wp-content/uploads/2020/05/SoyStats2020_for-WEB.pdf. Accessed 23 May 2020
Keskin A, Gürü M, Altiparmak D, Aydin K (2008) Using of cotton oil soapstock biodiesel–diesel fuel blends as an alternative diesel fuel. Renew Energy 33:553–557
Haas MJ, Scott K (1996) Combined nonenzymatic-enzymatic method for the synthesis of simple alkyl fatty acid esters from soapstock. J Am Oil Chem Soc 73:1393–1401
Haas MJ, Michalski PJ, Runyon S, Nunez A, Scott KM (2003) Production of FAME from acid oil, a by-product of vegetable oil refining. J Am Oil Chem Soc 80:97–102
Wang ZM, Lee JS, Park JY, Wu CZ, Yuan ZH (2007) Novel biodiesel production technology from soybean soapstock. Korean J Chem Eng 24:1027–1030
Jin B, Zhu M, Fan P, Yu L-J (2008) Comprehensive utilization of the mixture of oil sediments and soapstocks for producing FAME and phosphatides. Fuel Process Technol 89:77–82
Shao P, Hea J, Suna P, Jiang S (2009) Process optimization for the production of biodiesel from rapeseed soapstock by a novel method of short path distillation. Biosyst Eng 102:285–290
Park J-Y, Kim D-K, Wang Z-M, Lee J-P, Park S-C, Lee J-S (2008) Production of biodiesel from soapstock using an ion-exchange resin catalyst. Korean J Chem Eng 25:1350–1354
Park J-Y, Wang Z-M, Kim D-K, Lee J-S (2010) Effects of water on the esterification of free fatty acids by acid catalysts. Renew Energy 35:614–618
Li Y, Zhang X-D, Sun L (2010) Fatty acid methyl esters from soapstocks with potential use as biodiesel. Energy Convers Manag 51:2307–2311
Guo F, Xiu Z-L, Liang Z-X (2012) Synthesis of biodiesel from acidified soybean soapstock using a lignin-derived carbonaceous catalyst. Appl Energy 98:47–52
Pantoja SS, Mescouto VA, Costa C, Zamian JR, Rocha Filho G, Nascimento L (2018) High-quality biodiesel production from buriti (Mauritia flexuosa) oil soapstock. Molecules 24:94
Eaves PH, Spadaro JJ, Gastrock EA (1959) Methyl esters directly from acidulated soapstock. J Am Oil Chem Soc 36:230–234
Luxem FJ, Troy WM (2004) Method of making alkyl esters using pressure. US Patent 6768015. Research 550–553:687–692
McNeff CV, McNeff LC, Yan B, Nowlan DT, Rasmussen M, Gyberg AE, Krohn BJ, Ronald LF, Hoye TR (2008) A continuous catalytic system for biodiesel production. Appl Catal A Gen 343:39–48
Domingues C, Correia MJN, Carvalho R, Henriques C, Bordado J, Dias APS (2013) Vanadium phosphate catalysts for biodiesel production from acid industrial by-products. J Biotechnol 164:433–440
Watanabe Y, Pinsirodom P, Nagao T, Yamauchi A, Kobayashi T, Nishida Y, Takagi Y, Shimada Y (2007) Conversion of acid oil by produced in vegetable oil refining to biodiesel fuel by immobilized Candida antarctica lipase. J Mol Catal B Enzym 44:99–105
Shao P, Meng X, He J, Sun P (2008) Analysis of immobilized Candida rugosa lipase catalyzed preparation of biodiesel from rapeseed soapstock. Food Bioprod Process 86:283–289
Chen X, Du W, Liu D (2008) Response surface optimization of biocatalytic biodiesel production with acid oil. Biochem Eng J 40:423–429
Chen Y, Xiao B, Chang J, Fu Y, Lv P, Wang X (2009) Synthesis of biodiesel from waste cooking oil using immobilized lipase in fixed bed reactor. Energy Convers Manag 50:668–673
Tüter M, Ayşe Aksoy H, Elif Gılbaz E, Kurşun E (2004) Synthesis of fatty acid esters from acid oils using lipase B from Candida antarctica. Eur J Lipid Sci Technol 106:513–517
Akgün N, Yaprakci A, Candemir C (2010) Esterification of olive acid oil in supercritical methanol. Eur J Lipid Sci Technol 112:593–599
Haas MJ, Bloomer S, Scott K (2000) Simple, high-efficiency synthesis of fatty acid methyl esters from soapstock. J Am Oil Chem Soc 77:373–379
Watanabe Y, Nagao T, Nishida Y, Takagi Y, Shimada Y (2007) Enzymatic production of fatty acid methyl esters by hydrolysis of acid oil followed by esterification. J Am Oil Chem Soc 84:1015–1021
Cruz M, Almeida MF, Alvim-Ferraz MC, Dias JM (2019) Monitoring enzymatic hydroesterification of low-cost feedstocks by Fourier transform infrared spectroscopy. Catalysts 9:535
Soares D, Serres JDS, Corazza ML, Mitchell DA, Goncalves AG, Krieger N (2015) Analysis of multiphasic behavior during the ethyl esterification of fatty acids catalyzed by a fermented solid with lipolytic activity in a packed-bed bioreactor in a closed-loop batch system. Fuel 159:364–372
Serres JDDS, Balmant W, Soares D, Corazza ML, Krieger N, Mitchell DA (2017) A combined sorption and kinetic model for multiphasic ethyl esterification of fatty acids from soybean soapstock acid oil catalyzed by a fermented solid with lipase activity in a solvent-free system. Biochem Eng J 120:84–92
Botton V, Piovan L, Meier HF, Mitchell DA, Cordova J, Krieger N (2018) Optimization of biodiesel synthesis by esterification using a fermented solid produced by Rhizopus microsporus on sugarcane bagasse. Bioprocess Biosyst Eng 41:573–583
Choi N, Kim Y, Lee J, Kwak J, Lee J, Kim I-H (2016) Synthesis of fatty acid ethyl ester from acid oil in a continuous reactor via an enzymatic transesterification. J Am Oil Chem Soc 93:311–318
Felizardo P, Machado J, Vergueiro D, Correia MJN, Gomes JP, Bordado JM (2011) Study on the glycerolysis reaction of high free fatty acid oils for use as biodiesel feedstock. Fuel Process Technol 92:1225–1229
Haas MJ (2005) Improving the economics of biodiesel production through the use of low value lipids as feedstocks: vegetable oil soapstock. Fuel Process Technol 86:1087–1096
Luxem FJ, Mirous BK (2008) Biodiesel from acidulated soapstock. In: Hou CT, Shaw JF (eds) Biocatalysis and bioenergy. John Wiley & Sons, Inc., Hoboken, NJ, pp 115–129
Ong JTL (1983) Oil recovery from spent bleaching earth and disposal of the extracted material. J Am Oil Chem Soc 60:314–315
Huang Y-P, Chang JI (2010) Biodiesel production from residual oils recovered from spent bleaching earth. Renew Energy 35:269–274
Aziz AR, Harcharan S, Elkanzi EM, Lam LS, Liew SH (2001) Feasibility study of oil recovery from used bleaching earth using waste solvents. In: Porim International Palm Oil Congress (PIPOC) Proceedings, pp 126–133
Kalam A, Joshi J (1988) Regeneration of spent earth by wet oxidation. J Am Oil Chem Soc 65:1536–1540
Loh SK, Cheng SF, Choo YM, Ma AN (2006) A study of residual recovered from spent bleaching earth: their characteristics and applications. Am J Appl Sci 3:2063–2067
Yoo CK, Lin SW (2004) Regeneration of spent bleaching clay. MPOB TT No. 230. Malaysian Palm Oil Board, Ministry of Plantation Industries and Communities, Kuala Lumpur
King JW, List GR, Johnson JH (1992) Supercritical carbon dioxide extraction of spent bleaching clays. J Supercrit Fluids 5:38–41
Waldmann C, Eggers R (1991) De-oiling contaminated bleaching clay by high-pressure extraction. J Am Oil Chem Soc 68:922–930
Chang JI, Tai HS, Huang TH (2006) Regeneration of spent bleaching earth by lye extraction. Environ Prog 25:373–378
Basiron Y, Weng CK (2004) The oil palm and its sustainability. J Palm Oil Res 16:1–10
Boukerroui A, Ouali MS (2000) Regeneration of a spent bleaching earth and its reuse in the refining of an edible oil. J Chem Technol Biotechnol 75:773–776
Kheang LS, Foon CT, May CY, Ngan MA (2006) A study of residual oils recovered from spent bleaching earth: their characteristics and applications. Am J Appl Sci 3:2063–2067
Plata V, Rojas O, Gauthier-Maradei P (2020) Improvement of palm oil biodiesel filterability by treatment with reactivated spent bleaching earths. Fuel 260:116198
Lara PV, Park EY (2004) Potential application of waste activated bleaching earth on the production of fatty acid methyl esters using Candida cylindracea lipase in organic solvent system. Enzym Microb Technol 34:270–277
Gül ÖF, Tüter M, Aksoy HA (2010) The utilization of waste activated bleaching earth in biodiesel production: optimization by response surface methodology. Energy Source A 32:1812–1820
Lim BP, Maniam GP, Hamid SA (2009) Biodiesel from adsorbed waste oil on spent bleaching clay using CaO as a heterogeneous catalyst. Eur J Sci Res 33:347–357
Aladetuyi A, Olatunji G, Ogunniyi D, Odetoye T, Oguntoye S (2014) Production and characterization of biodiesel using palm kernel oil; fresh and recovered from spent bleaching earth. Biofuel Res J 1:134–138
Lara Pizarro AV, Park EY (2003) Lipase-catalyzed production of biodiesel fuel from vegetable oils contained in waste activated bleaching earth. Process Biochem 38:1077–1082
Mat R, Ling OS, Johari A, Mohamed M (2011) In situ biodiesel production from residual oil recovered from spent bleaching earth. Bull Chem React Eng Catal 6:53–57
Sugiharto R, Hidayati S, Cholik R (2019) Application of response surface methodology to evaluate biodiesel production from spent bleaching earth by in situ transesterification process. IOP Conf Ser Earth Environ Sci 230:012074
Suryani A, Mubarok Z, Suprihatin, Romli M, Yunira EN (2017) Process design of in situ esterification-transesterification for biodiesel production from residual oil of spent bleaching earth (SBE). IOP Conf Ser Earth Environ Sci 65:012040
Kojima SK, Du D, Sato M, Park EY (2004) Efficient production of fatty acid methyl ester from waste activated bleaching earth using diesel oil as organic solvent. J Biosci Bioeng 98:420–424
Park EY, Sato M, Kojima S (2008) Lipase-catalyzed biodiesel production from waste activated bleaching earth as raw material in a pilot plant. Bioresour Technol 99:3130–3135
Granados ML, Poves MDZ, Alonso DM, Mariscal R, Galisteo FC, Moreno-Tost R, Santamaría J, Fierro JLG (2007) Biodiesel from sunflower oil by using activated calcium oxide. Appl Catal B 73:317–326
Du D, Sato M, Mori M, Park EY (2006) Repeated production of fatty acid methyl ester with activated bleaching earth in solvent-free system. Process Biochem 41:1849–1853
Facioli NL, Barrera-Arellano D (2002) Optimization of direct acid esterification process of soybean oil deodorizer distillate. Grasas Aceites 53:206–212
Verhé R, Van Hoed V, Echim C, Stevens C, De Greyt W, Kellens M (2008) Production of biofuel from lipids and alternative resources. In: Hou CT, Shaw JF (eds) Biocatalysis and bioenergy. John Wiley & Sons, Inc., Hoboken, NJ, pp 185–195
Chongkhong S, Tongurai C, Chetpattananondh P, Bunyakan C (2007) Biodiesel production by esterification of palm fatty acid distillate. Biomass Bioenergy 31:563–568
Chongkhong S, Tongurai C, Chetpattananondh P (2009) Continuous esterification for biodiesel production from palm fatty acid distillate using economical process. Renew Energy 34:1059–1063
Villardi H, Ferreira Leal M, Andrade P, Pessoa F, Salgado A, Gomes de Oliveira A (2017) Catalytic and non-catalytic esterification of soybean oil deodorizer distillate by ethanol: kinetic modelling. Chem Eng Trans 57:1999–2004
Souza MS, Aguieiras ECG, da Silva MAP, Langone MAP (2009) Biodiesel synthesis via esterification of feedstock with high content of free fatty acids. Appl Biochem Biotechnol 154:253–267
Xi L, Cao S (2011) Synthesis of biodiesel from by-product of plant oil by the catalysis of solid acid in fixed bed. Adv Mater Res 322:15–20
Liu Y, Wang L (2009) Biodiesel production from rapeseed deodorizer distillate in a packed column reactor. Chem Eng Process 48:1152–1156
Liu Y, Wang L, Yan Y (2009) Biodiesel synthesis combining pre-esterification with alkali catalyzed process from rapeseed oil deodorizer distillate. Fuel Process Technol 90:857–862
Yin XL, Duan XL, You QH, Dai CH, Tan ZB, Zhu XY (2016) Biodiesel production from soybean oil deodorizer distillate using calcined duck eggshell as catalyst. Energy Convers Manag 112:199–207
Naz S, Kara H, Sherazi STH, Aljabour A, Talpur FN (2014) A green approach for the production of biodiesel from fatty acids of corn deodorizer distillate. RSC Adv 4:48419–48425
Yin X, You Q, Ma H, Dai C, Zhang H, Li K, Li Y (2015) Biodiesel production from soybean oil deodorizer distillate enhanced by counter-current pulsed ultrasound. Ultrason Sonochem 23:53–58
Yin X, Zhang X, Wan M, Duan X, You Q, Zhang J, Li S (2017) Intensification of biodiesel production using dual-frequency counter-current pulsed ultrasound. Ultrason Sonochem 37:136–143
Ramamurthi S, Bhirud PR, McCurdy AR (1991) Enzymatic methylation of canola oil deodorizer distillate. J Am Oil Chem Soc 68:970–975
Facioli NL, Barrera-Arellano D (2001) Optimisation of enzymatic esterification of soybean oil deodoriser distillate. J Sci Food Agric 81:1193–1198
Nagesha GK, Manohar B, Udaya Sankar K (2004) Enzymatic esterification of free fatty acids of hydrolyzed soy deodorizer distillate in supercritical carbon dioxide. J Supercrit Fluids 32:137–145
Wang L, Du W, Liu D, Li L, Dai N (2006) Lipase-catalyzed biodiesel production from soybean oil deodorizer distillate with absorbent present in tert-butanol system. J Mol Catal B Enzym 43:29–32
Du W, Wang L, Liu D (2007) Improved methanol tolerance during Novozym435-mediated methanolysis of SODD for biodiesel production. Green Chem 9:173–176
Zeng L, He Y, Jiao L, Li K, Yan Y (2017) Preparation of biodiesel with liquid synergetic lipases from rapeseed oil deodorizer distillate. Appl Biochem Biotechnol 183:778–791
Dos Santos Corrêa IN, de Souza SL, Catran M, Bernardes OL, Portilho MF, Langone MAP (2011) Enzymatic biodiesel synthesis using a byproduct obtained from palm oil refining. Enzyme Res 2011:814507. https://doi.org/10.4061/2011/814507
Rahman Talukder MM, Wu JC, Lau SK, Cui LC, Shimin G, Lim A (2009) Comparison of Novozym 435 and Amberlyst 15 as heterogeneous catalyst for production of biodiesel from palm fatty acid distillate. Energy Fuel 23:1–4
Lo SK, Baharin BS, Tan CP, Lai OM (2004) Enzyme-catalyzed production and chemical composition of diacylglycerols from corn oil deodorizer distillate. Food Biotechnol 18:265–278
Lo SK, Baharin BS, Tan CP, Lai OM (2004) Diacylglycerols from palm oil deodoriser distillate. Part 1 – Synthesis by lipase-catalysed esterification. Food Sci Technol Int 10:149–156
Lo SK, Baharin BS, Tan CP, Lai OM (2004) Lipase-catalysed production and chemical composition of diacylglycerols from soybean oil deodoriser distillate. Eur J Lipid Sci Technol 106:218–224
Tangkam K, Weber N, Wiege B (2008) Solvent-free lipase-catalyzed preparation of diglycerides from co-products of vegetable oil refining. Grasas Aceites 59:245–253
Smet P (2008) Valorisatie van vetzuurdestillaten als biobrandstof door herverestering met glycerol. Master thesis. Karel de Grote-Hogeschool. Cited according to Echim et al. (17)
Öner C, Altun S (2009) Biodiesel production from inedible animal tallow and an experimental investigation of its use as alternative fuel in a direct injection diesel engine. Appl Energy 86:2114–2120
Thamsiriroj T, Murphy JD (2010) How much of the target for biofuels can be met by biodiesel generated from residues in Ireland? Fuel 89:3579–3589
Ngo HL, Zafiropoulos NA, Foglia TA, Samulski ET, Lin W (2008) Efficient two-step synthesis of biodiesel from greases. Energy Fuel 22:626–634
Gϋrϋ M, Artukoğlu BD, Keskin A, Koca A (2009) Biodiesel production from waste animal fat and improvement of its characteristics by synthesized nickel and magnesium additive. Energy Convers Manag 50:498–502
Dobbelaere D (2019) Statistical overview of the animal by-products industry in the EU in 2018. In: The 19th EFPRA Congress, La Baule, France, 14 June 2019. https://www.efpralabaule2019.com/docs/dobbelaere.pdf. Accessed 26 May 2020
Banković-Ilić IB, Stojković IJ, Stamenković OS, Veljković VB, Hung Y-T (2014) Waste animal fats as feedstocks for biodiesel production. Renew Sust Energy Rev 32:238–254
Lotero E, Liu Y, Lopez DE, Suwannakarn K, Bruce DA, Goodwin JG (2005) Synthesis of biodiesel via acid catalysis. Ind Eng Chem Res 44:5353–5363
Kusdiana D, Saka S (2004) Effects of water on biodiesel fuel production by supercritical methanol treatment. Bioresour Technol 91:289–295
Ma F, Clements LD, Hanna MA (1998) The effects of catalyst, free fatty acids and water on transesterification of beef tallow. Trans Am Soc Agric Eng 41:1261–1264
Mittelbach M, Pokits B, Silberholz A (1992) Production and fuel properties of fatty acid methyl esters from used frying oil. In: Liquid Fuels from Renewable Resources. Proceedings of an Alternative Energy Conference. ASAE Publication, Nashville, TN, USA, pp 74–78
Liu K (1994) Preparation of fatty acid methyl esters for gas-chromatographic analysis of lipids in biological materials. J Am Oil Chem Soc 71(11):1179–1187
Bhatti HN, Hanif MA, Qasim M, Rehman A (2008) Biodiesel production from waste tallow. Fuel 87:2961–2966
Encinar JM, Sánchez N, Martínez G, García L (2011) Study of biodiesel production from animal fats with high free fatty acid content. Bioresour Technol 102:10907–10914
Tashtoush GM, Mohamad I, Al-Widyan M, Al-Jarrah M (2004) Experimental study on evaluation and optimization of conversion of waste animal fat into biodiesel. Energy Convers Manag 45:2697–2711
Montefrio MJ, Xinwen T, Obbard JP (2010) Recovery and pre-treatment of fats, oil and grease from grease interceptors for biodiesel production. Appl Energy 87:3155–3161
Ngo HL, Zafiropoulos NA, Foglia TA, Samulski ET, Lin W (2010) Mesoporous silica-supported diarylammonium catalysts for esterification of free fatty acids in greases. J Am Oil Chem Soc 87:445–452
Kim M, DiMaggio C, Yan S, Wang H, Salley SO, Ng KYS (2011) Performance of heterogeneous ZrO2 supported metaloxide catalysts for brown grease esterification and sulfur removal. Bioresour Technol 102:2380–2386
Bianchi CL, Boffito DC, Pirola C, Ragaini V (2010) Low temperature de-acidification process of animal fat as a pre-step to biodiesel production. Catal Lett 134:179–183
Soldi RA, Oliveira ARS, Ramos LP, César-Oliveira MAF (2009) Soybean oil and beef tallow alcoholysis by acid heterogeneous catalysis. Appl Catal A Gen 361:42–48
Melero JA, Bautista LF, Iglesias J, Morales G, Sánchez-Vázquez R (2012) Zr-SBA-15 acid catalyst: optimization of the synthesis and reaction conditions for biodiesel production from low-grade oils and fats. Catal Today 195(1):44–53
Morales MSA, Krause LC, da Cunha ME, Faccini CS, de Menezes EW, Veses RC, Rodrigues MRA, Caramão EB (2008) Tallow biodiesel: properties evaluation and consumption tests in a diesel engine. Energy Fuel 22:1949–1954
Da Cunha ME, Krause LC, Moraes MSA, Faccini CS, Jacques RA, Almeida SR, Rodrigues MRA, Caramão EB (2009) Beef tallow biodiesel produced in a pilot scale. Fuel Process Technol 90:570–575
Teixeira LSG, Assis JCR, Mendonça DR, Santos ITV, Guimarães PRB, Pontes LAM, Teixeira JSR (2009) Comparison between conventional and ultrasonic preparation of beef tallow biodiesel. Fuel Process Technol 90:1164–1166
Hoque ME, Singh A, Chuan YL (2011) Biodiesel from low cost feedstocks: the effects of process parameters on the biodiesel yield. Biomass Bioenergy 35:1582–1587
Liu S, Wang Y, Oh JH, Herring JL (2011) Fast biodiesel production from beef tallow with radio frequency heating. Renew Energy 36:1003–1007
Fröhlich A, Rice B, Vicente G (2010) The conversion of low grade tallow into biodiesel-grade methyl ester. J Am Oil Chem Soc 87:825–833
Chung KH, Kim J, Lee KY (2009) Biodiesel production by transesterification of duck tallow with methanol on alkali catalysts. Biomass Bioenergy 33:155–158
Mata TM, Cardoso N, Ornelas M, Neves S, Caetano NS (2010) Sustainable production of biodiesel from tallow, lard and poultry fat and its quality evaluation. Energy Fuel 25:4756–4762
Mata TM, Cardoso N, Ornelas M, Neves S, Caetano NS (2011) Evaluation of two purification methods of biodiesel from beef tallow, pork lard and chicken fat. Chem Eng Trans 9:13–18
Jeong GT, Yang HS, Park DH (2009) Optimization of transesterification of animal fat ester using response surface methodology. Bioresour Technol 100:25–30
Fayyazi E, Ghobadian B, Najafi G, Hosseinzadeh B, Mamat R, Hosseinzadeh J (2015) An ultrasound-assisted system for the optimization of biodiesel production from chicken fat oil using a genetic algorithm and response surface methodology. Ultrason Sonochem 26:312–320
Stojković IJ, Banković-Ilić IB, Veličković AV, Avramović JM, Stamenković OS, Povrenović DS, Veljković VB (2016) Waste lard methanolysis catalyzed by KOH at moderate temperatures. Chem Eng Technol 39:741–750
Fadhil AB, Saeed IK, Saeed LI, Altamer MH (2016) Co-solvent ethanolysis of chicken waste: optimization of parameters and characterization of biodiesel. Energy Source A 38:2883–2890
Miladinović MR, Stojković IJ, Veličković AV, Stamenković OS, Banković-Ilić IB, Veljković VB (2019) Optimization and kinetic modeling of waste lard methanolysis in a continuous reciprocating plate reactor. Chin J Chem Eng 27:2481–2490
Dias JM, Alvim-Ferraz MCM, Almeida MF (2008) Mixtures of vegetable oils and animal fat for biodiesel production: influence on product composition and quality. Energy Fuel 22:3889–3893
Huong LTT, Tan PM, Hoa TTV (2011) Biodiesel production from fat of Tra catfish via heterogeneous basic-catalyzed transesterification using ultrasonic mixing. e J Surf Sci Nanotech 9:477–481
Dias JM, Alvim-Ferraz MCM, Almeida MF, Díaz JDM, Polo MS, Utrilla JR (2012) Selection of heterogeneous catalysts for biodiesel production from animal fat. Fuel 94:418–425
Stojković IJ, Miladinović MR, Stamenković OS, Banković-Ilić IB, Povrenović DS, Veljković VB (2016) Biodiesel production by methanolysis of waste lard from piglet roasting over quicklime. Fuel 182:454–466
Mutreja V, Singh S, Ali A (2011) Biodiesel from mutton fat using KOH impregnated MgO as heterogeneous catalysts. Renew Energy 36:2253–2258
Reddy CRV, Oshel R, Verkade JV (2006) Room-temperature conversion of soybean oil and poultry fat to biodiesel catalyzed by nanocrystalline calcium oxides. Energy Fuel 20:1310–1314
Liu Y, Lotero E, Goodwin JG Jr, Mo X (2007) Transesterification of poultry fat with methanol using Mg–Al hydrotalcite derived catalysts. Appl Catal A Gen 331:138–148
Lee KT, Foglia TA, Chang KS (2002) Production of alkyl ester as biodiesel from fractionated lard and restaurant grease. J Am Oil Chem Soc 79:191–195
Lu J, Nie K, Xie F, Wang F, Tan T (2007) Enzymatic synthesis of fatty acid methyl esters from lard with immobilized Candida sp. 99-125. Process Biochem 42:1367–1370
Huang Y, Zheng H, Yan Y (2010) Optimization of lipase-catalyzed transesterification of lard for biodiesel production using response surface methodology. Appl Biochem Biotechnol 160:504–515
Da Rós PCM, Silva GAM, Mendes AA, Santos JC, de Castro HF (2010) Evaluation of the catalytic properties of Burkholderia cepacia lipase immobilized on non-commercial matrices to be used in biodiesel synthesis from different feedstocks. Bioresour Technol 101:5508–5516
Aryee ANA, Simpson BK, Cue RI, Phillip LE (2011) Enzymatic transesterification of fats and oils from animal discards to fatty acid ethyl esters for potential fuel use. Biomass Bioenergy 35:4149–4157
Taher H, Al-Zuhair S, Al-Marzouqui A, Hashim I (2011) Extracted fat from lamb meat by supercritical CO2 as feedstock for biodiesel production. Biochem Eng J 55:23–31
Al-Zuhair S, Hussein A, Al-Marzouqi AH, Hashim I (2012) Continuous production of biodiesel from fat extracted from lamb meat in supercritical CO2 media. Biochem Eng J 60:106–110
Marulanda VF, Anitescu G, Tavlarides LL (2010) Investigations on supercritical transesterification of chicken fat for biodiesel production from low-cost lipid feedstocks. J Supercrit Fluids 54:53–60
Shin HY, Lee SH, Ryu JH, Bae SY (2012) Biodiesel production from waste lard using supercritical methanol. J Supercrit Fluids 61:134–138
Vyas AP, Verma JL, Subrahmanyam N (2010) A review on FAME production processes. Fuel 89:1–9
Semwal S, Arora AK, Badoni RP, Tuli DK (2011) Biodiesel production using heterogeneous catalysts. Bioresour Technol 102:2151–2161
Araújo BQ, da Rocha Nunes RC, de Moura CVR, de Moura EM, das Gracas Lopes Cito AM, dos Santos Junior JR (2010) Synthesis and characterization of beef tallow biodiesel. Energy Fuel 24:4476–4480
Dias JM, Alvim-Ferraz MCM, Almeida MF (2008) Comparison of the performance of different homogeneous alkali catalysts during transesterification of waste and virgin oils and evaluation of biodiesel quality. Fuel 87:3572–3578
Marulanda VF, Anitescu G, Tavlarides LL (2010) Biodiesel Fuels through a continuous flow process of chicken fat supercritical transesterification. Energy Fuel 24:253–260
Panneerselvam SI, Miranda LR (2011) Biodiesel production from mutton tallow. Int J Renew Energy Res 1:45–49
Alptekin E, Canakci M, Sanli H (2012) Evaluation of leather industry wastes as a feedstock for biodiesel production. Fuel 95:214–220
Alptekin E, Canakci M (2010) Optimization of pretreatment reaction for methyl ester production from chicken fat. Fuel 89:4035–4039
Alptekin E, Canakci M (2011) Optimization of transesterification for methyl ester production from chicken fat. Fuel 90:2630–2638
Chavan SB, Yadav M, Singh R, Singh V, Kumbhar RR, Sharma YC (2017) Production of biodiesel from three indigenous feedstocks: optimization of process parameters and assessment of various fuel properties. Environ Prog Sust Energy 36(3):788–795
Keskin A (2018) Two-step methyl ester production and characterization from the broiler rendering fat: the optimization of the first step. Renew Energy 122:216–224
Dias JM, Alvim-Ferraz MCM, Almeida MF (2009) Production of biodiesel from acid waste lard. Bioresour Technol 100:6355–6361
Sarantopoulos I, Chatzisymeon E, Foteinis S, Tsoutsos T (2014) Optimization of biodiesel production from waste lard by a two-step transesterification process under mild conditions. Energy Sust Dev 23:110–114
He C, Mei Y, Zhang Y, Liu L, Li P, Zhang Z, Jing Y, Li G, Jiao Y (2020) Enhanced biodiesel production from diseased swine fat by ultrasound assisted two-step catalyzed process. Bioresour Technol 304:123017
Lawan I, Garba ZN, Zhou W, Zhang M, Yuan Z (2020) Synergies between the microwave reactor and CaO/zeolite catalyst in waste lard biodiesel production. Renew Energy 145:2550–2560
Math MC, Kumar SP, Chetty SV (2010) Technologies for biodiesel production from used cooking oil - a review. Energy Sust Dev 14:339–345
Go-Green (2012) McDonald’s going eco-friendly. http://www.go-green.ae/greenstory_view.php?storyid=1690. Accessed 26 May 2020
REM (2012) Neutral Fuels to expand bio-fuels operations to Australia. Renew Energy Mag. https://www.renewableenergymagazine.com/biofuels/neutral-fuels-to-expand-biofuels-operations-to-20121219. Accessed 26 May 2020
Okada M (2012) Overview of production process and utilization of biodiesel fuel. JIME 47:45–50
Businesswire (2013) SeQuential Pacific biodiesel produces 20 millionth gallon of fuel, teams up with University of Oregon. https://www.businesswire.com/news/home/20130828006134/en/SeQuential-Pacific-Biodiesel-Produces-20-Millionth-Gallon. Accessed 26 May 2020
Biofuels International (2012) New Agri biodiesel plant opens in Liverpool. https://biofuels-news.com/news/new-agri-biodiesel-plant-opens-in-liverpool/. Accessed 26 May 2020
Kulkarni MG, Dalai AK (2006) Waste cooking oil – an economical source for biodiesel: a review. Ind Eng Chem Res 45:2901–2913
Cvengroš J, Cvengrošova Z (2004) Used frying oils and fats and their utilization in the production of methyl esters of higher fatty acids. Biomass Bioenergy 27:173–181
Yuan X, Liu J, Zeng G, Shi J, Tong J, Huang G (2008) Optimization of conversion of waste rapeseed oil with high FFA to biodiesel using response surface methodology. Renew Energy 33:1678–1684
Lertsathapornsuk V, Pairintra R, Aryusuk K, Krisnangkura K (2008) Microwave assisted in continuous biodiesel production from waste frying palm oil and its performance in a 100 kW diesel generator. Fuel Process Technol 89:1330–1336
Refaat AA (2010) Different techniques for the production of biodiesel from waste vegetable oil. Int J Environ Sci Technol 7:183–213
Felizardo P, Correia MJ, Raposo I, Mendes JF, Berkemeier R, Bordado JM (2006) Production of biodiesel from waste frying oils. Waste Manag 26:487–494
Supple B, Holward-Hildige R, Gonzalez-Gomez E, Leahy JJ (2002) The effect of steam treating waste cooking oil on the yield of methyl ester. J Am Oil Chem Soc 79:175–178
Ning N, Ren L (2012) Process of biodiesel made from waste cooking oil via acid catalyst. Adv Mater Res 518–523:3427–3431
Al-Widyan MI, Al-Shyoukh AO (2002) Experimental evaluation of the transesterification of waste palm oil into biodiesel. Bioresour Technol 85:253–256
Zhang Y, Dube MA, McLean DD, Kates M (2003) Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour Technol 89:1–16
Zheng S, Kates M, Dubé MA, McLean DD (2006) Acid-catalyzed production of biodiesel from waste frying oil. Biomass Bioenergy 30:267–272
Wang Y, Ou SY, Liu PZ, Xue F, Tang S (2006) Comparison of two different processes to synthesize biodiesel by waste cooking oil. J Mol Catal A Chem 252:107–112
Dhawane SH, Karmakar B, Ghosh S, Halder G (2018) Parametric optimisation of biodiesel synthesis from waste cooking oil via Taguchi approach. J Environ Chem Eng 6:3971–3980
Jacobson K, Gopinath R, Meher LC, Dalai AK (2008) Solid acid catalyzed biodiesel production from waste cooking oil. Appl Catal B Environ 85:86–91
Yan S, Salley SO, Simon Ng KY (2009) Simultaneous transesterification and esterification of unrefined or waste oils over ZnO-La2O3 catalysts. Appl Catal A Gen 353:203–212
Lam MK, Lee KT (2011) Mixed methanol–ethanol technology to produce greener biodiesel from waste cooking oil: a breakthrough for SO42–/SnO2–SiO2 catalyst. Fuel Process Technol 92:1639–1645
Noshadi I, Amin NAS, Parnas RS (2012) Continuous production of biodiesel from waste cooking oil in a reactive distillation column catalyzed by solid heteropolyacid: optimization using response surface methodology (RSM). Fuel 94:156–164
Talebian-Kiakalaieh A, Amin NAS, Zarei A, Noshadi I (2013) Transesterification of waste cooking oil by heteropoly acid (HPA) catalyst: optimization and kinetic model. Appl Energy 102:283–292
Cao F, Chen Y, Zhai F, Li J, Wang J, Wang X, Wang S, Zhu W (2008) Biodiesel production from high acid value waste frying oil catalyzed by superacid heteropolyacid. Biotechnol Bioeng 101:93–100
Zhang X, Li J, Chen Y, Wang J, Feng L, Wang X, Cao F (2009) Heteropolyacid nanoreactor with double acid sites as a highly efficient and reusable catalyst for the transesterification of waste cooking oil. Energy Fuel 23:4640–4646
Srilatha K, Issariyakul T, Lingaiah N, Sai Prasad PS, Kozinski J, Dalai AK (2010) Efficient esterification and transesterification of used cooking oil using 12-tungstophosphoric acid (TPA)/Nb2O5 catalyst. Energy Fuel 24:4748–4755
Zhang H, Ding J, Zhao Z (2012) Microwave assisted esterification of acidified oil from waste cooking oil by CERP/PES catalytic membrane for biodiesel production. Bioresour Technol 123:72–77
Zhu M, He B, Shi W, Feng Y, Ding J, Li J, Zeng F (2010) Preparation and characterization of PSSA/PVA catalytic membrane for biodiesel production. Fuel 89:2299–2304
Feng Y, He B, Cao Y, Li J, Liu M, Yan F, Liang X (2010) Biodiesel production using cation-exchange resin as heterogeneous catalyst. Bioresour Technol 101:1518–1521
Lou W-Y, Zong M-H, Duan Z-Q (2008) Efficient production of biodiesel from high free fatty acid-containing waste oils using various carbohydrate-derived solid acid catalysts. Bioresour Technol 99:8752–8758
Tomašević AV, Šiler-Marinković SS (2003) Methanolysis of used frying oil. Fuel Process Technol 81:1–6
Dorado MP, Ballesteros E, Mittelbach M, Lopez FJ (2004) Kinetic parameters affecting the alkali-catalyzed transesterification process of used olive oil. Energy Fuel 18:1457–1462
Çetinkaya M, Karaosmanoğlu F (2004) Optimization of base-catalyzed transesterification reaction of used cooking oil. Energy Fuel 18:1888–1895
Encinar JM, Gonzalez JF, Rodriguez-Reinares A (2005) Biodiesel from used frying oil. Variables affecting the yields and characteristics of the biodiesel. Ind Eng Chem Res 44:5491–5499
Refaat AA, Attia NK, Sibak HA, El Sheltawy ST, El Diwani GI (2008) Production optimization and quality assessment of biodiesel from waste vegetable oil. Int J Environ Sci Technol 5:75–82
Alcantara R, Amores J, Canoira L, Fidalgo E, Franco MJ, Navarro A (2000) Catalytic production of biodiesel from soy-bean oil, used frying oil and tallow. Biomass Bioenergy 18:515–527
Agarwal M, Chauhan G, Chaurasia SP, Singh K (2012) Study of catalytic behavior of KOH as homogeneous and heterogeneous catalyst for biodiesel production. J Taiwan Inst Chem Eng 43:89–94
Encinar JM, Juan F, Gonzalez JF, Rodriguez-Reinares A (2007) Ethanolysis of used frying oils: biodiesel preparation and characterization. Fuel Process Technol 88:513–522
Azcan N, Yilmaz O (2013) Microwave assisted transesterification of waste frying oil and concentrate methyl ester content of biodiesel by molecular distillation. Fuel 104:614–619
Chen K-S, Lin Y-C, Hsu K-H, Wang H-K (2012) Improving biodiesel yields from waste cooking oil by using sodium methoxide and a microwave heating system. Energy 38:151–156
Thanh LT, Okitsu K, Sadanaga Y, Takenaka N, Maeda Y, Bandow H (2013) A new co-solvent method for the green production of biodiesel fuel – optimization and practical application. Fuel 103:742–748
Leung DYC, Guo Y (2006) Transesterification of neat and used frying oil: optimization for biodiesel production. Fuel Process Technol 87:883–890
Chhetri AB, Watts KC, Islam MR (2008) Waste cooking oil as an alternate feedstock for biodiesel production. Energies 1:3–18
Phan AN, Phan TM (2008) Biodiesel production from waste cooking oils. Fuel 87:3490–3496
Dehkordi AM, Ghasemi M (2012) Transesterification of waste cooking oil to biodiesel using Ca and Zr mixed oxides as heterogeneous base catalysts. Fuel Process Technol 97:45–51
Guan G, Kusakabe K, Yamasaki S (2009) Tri-potassium phosphate as a solid catalyst for biodiesel production from waste cooking oil. Fuel Process Technol 90:520–524
Hameed BH, Goh CS, Chin LH (2009) Process optimization for methyl ester production from waste cooking oil using activated carbon supported potassium fluoride. Fuel Process Technol 90:1532–1537
Sankaranarayanan S, Antonyraj CA, Kannan S (2012) Transesterification of edible, non-edible and used cooking oils for biodiesel production using calcined layered double hydroxides as reusable base catalysts. Bioresour Technol 109:57–62
Shibasaki-Kitakawa N, Tsuji T, Kubo M, Yonemoto T (2011) Biodiesel production from waste cooking oil using anion-exchange resin as both catalyst and adsorbent. Bioenergy Res 4:287–293
Kouzu M, Hidaka J-S, Komichi Y, Nakano H, Yamamoto M (2009) A process to transesterify vegetable oil with methanol in the presence of quick lime bit functioning as solid base catalyst. Fuel 88:1983–1990
Wen Z, Yu X, Tu S-T, Yan J, Dahlquist E (2010) Biodiesel production from waste cooking oil catalyzed by TiO2–MgO mixed oxides. Bioresour Technol 101:9570–9576
Brito A, Borges ME, Garín M, Hernández A (2009) Biodiesel production from waste oil using Mg-Al layered double hydroxide catalysts. Energy Fuel 23:2952–2958
Borges ME, Díaz L, Alvarez-Galván MC, Brito A (2011) High performance heterogeneous catalyst for biodiesel production from vegetal and waste oil at low temperature. Appl Catal B Environ 102:310–315
Roschat W, Kacha M, Yoosuk B, Sudyoadsuk T, Promarak V (2012) Biodiesel production based on heterogeneous process catalyzed by solid waste coral fragment. Fuel 98:194–202
Birla A, Singh B, Upadhyay SN, Sharma YC (2012) Kinetics studies of synthesis of biodiesel from waste frying oil using a heterogeneous catalyst derived from snail shell. Bioresour Technol 106:95–100
Boey P-L, Ganesana S, Maniamb GP, Khairuddean M (2012) Catalysts derived from waste sources in the production of biodiesel using waste cooking oil. Catal Today 190:117–121
Balakrishan K, Olutoye MA, Hameed BH (2013) Synthesis of methyl esters from waste cooking oil using construction waste material as solid base catalyst. Bioresour Technol 128:788–791
Baskar G, Aiswarya R (2015) Biodiesel production from waste cooking oil using copper doped zinc oxide nanocomposite as heterogeneous catalyst. Bioresour Technol 188:124–127
Hsiao M-C, Kuo J-Y, Hsieh S-A, Hsieh P-H, Hou S-S (2020) Optimized conversion of waste cooking oil to biodiesel using modified calcium oxide as catalyst via a microwave heating system. Fuel 266:117114
Maneerung T, Kawi S, Dai Y, Wang C-H (2016) Sustainable biodiesel production via transesterification of waste cooking oil by using CaO catalysts prepared from chicken manure. Energy Convers Manag 123:487–497
Kaewdaeng S, Sintuya P, Nirunsin R (2017) Biodiesel production using calcium oxide from river snail shell ash as catalyst. Energy Procedia 138:937–942
Farooq M, Ramli A, Naeem A (2015) Biodiesel production from low FFA waste cooking oil using heterogeneous catalyst derived from chicken bones. Renew Energy 76:362–368
Khan HM, Iqbal T, Ali CH, Yasin S, Jamil F (2020) Waste quail beaks as renewable source for synthesizing novel catalysts for biodiesel production. Renew Energy 154:1035–1043
Zik NAFA, Sulaiman S, Jamal P (2020) Biodiesel production from waste cooking oil using calcium oxide/nanocrystal cellulose/polyvinyl alcohol catalyst in a packed bed reactor. Renew Energy 155:267–277
Chesterfield DM, Rogers PL, Al-Zaini EO, Adesina AA (2012) Production of biodiesel via ethanolysis of waste cooking oil using immobilised lipase. Chem Eng J 207–208:701–710
De los Ríos AP, Hernández Fernández FJ, Gómez D, Rubio M, Víllora G (2011) Biocatalytic transesterification of sunflower and waste cooking oils in ionic liquid media. Process Biochem 46:1475–1480
Halim SFA, Kamaruddin AH (2008) Catalytic studies of lipase on FAME production from waste cooking palm oil in a tert-butanol system. Process Biochem 43:1436–1439
Yagiz F, Kazan D, Akin AN (2007) Biodiesel production from waste oils by using lipase immobilized on hydrotalcite and zeolites. Chem Eng J 134:262–267
Chen G, Ying M, Li W (2006) Enzymatic conversion of waste cooking oils into alternative fuel—biodiesel. Appl Biochem Biotechnol 132(129):911–921
Li N-W, Zong M-H, Wu H (2009) Highly efficient transformation of waste oil to biodiesel by immobilized lipase from Penicillium expansum. Process Biochem 44:685–688
Charpe TW, Rathod VK (2011) Biodiesel production using waste frying oil. Waste Manag 31:85–90
Ali CH, Qureshi AS, Mbadinga SM, Liu J-F, Yang S-Z, Mu B-Z (2017) Biodiesel production from waste cooking oil using onsite produced purified lipase from Pseudomonas aeruginosa FW_SH-1: central composite design approach. Renew Energy 109:93–100
Campanelli P, Banchero M, Manna L (2010) Synthesis of biodiesel from edible, non-edible and waste cooking oils via supercritical methyl acetate transesterification. Fuel 89:3675–3682
Demirbaş A (2009) Biodiesel from waste cooking oil via base-catalytic and supercritical methanol transesterification. Energy Convers Manag 50:923–927
Lee S, Posarac D, Ellis N (2012) An experimental investigation of biodiesel synthesis from waste canola oil using supercritical methanol. Fuel 91:229–237
Tan KT, Lee KT, Mohamed AR (2011) Potential of waste palm cooking oil for catalyst-free biodiesel production. Energy 36:2085–2088
Patil P, Deng S, Rhodes JI, Lammers PJ (2010) Conversion of waste cooking oil to biodiesel using ferric sulfate and supercritical methanol processes. Fuel 89:360–364
Lam MK, Lee MK, Mohamed AR (2010) Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: a review. Biotechnol Adv 28:500–518
Zabeti M, Wan Daud WMA, Aroua MK (2009) Activity of solid catalysts for biodiesel production: a review. Fuel Process Technol 90:770–777
Zong M-H, Duan Z-Q, Lou W-Y, Smith TJ, Wu H (2007) Preparation of a sugar catalyst and its use for highly efficient production of biodiesel. Green Chem 9:434–437
Wang JF, Peng BX, Wang JL, Wang GR, Wang RD (2007) A continuous process for producing biodiesel from feedstocks with high contents of free fatty acids by heterogenous catalysis. Chinese Patent CN101067091
Park Y-M, Lee D-W, Kim D-K, Lee J-S, Lee K-Y (2008) The heterogeneous catalyst system for the continuous conversion of free fatty acids in used vegetable oils for the production of biodiesel. Catal Today 131:238–243
Feng Y, Zhang A, Li J, He B (2011) A continuous process for biodiesel production in a fixed bed reactor packed with cation-exchange resin as heterogeneous catalyst. Bioresour Technol 102:3607–3609
Enweremadu CC, Mbarawa MM (2009) Technical aspects of production and analysis of biodiesel from used cooking oil—a review. Renew Sust Energy Rev 13:2205–2224
Saifuddin N, Chua KH (2004) Production of ethyl ester (biodiesel) from used frying oil: optimization of transesterification process using microwave irradiation. Malays J Chem 6:77–82
Nie K, Xie F, Wang F, Tan T (2006) Lipase catalyzed methanolysis to produce biodiesel: optimization of the biodiesel production. J Mol Catal B Enzym 43:142–147
Rodrigues AR, Paiva A, da Silva MG, Simões P, Barreiros S (2011) Continuous enzymatic production of biodiesel from virgin and waste sunflower oil in supercritical carbon dioxide. J Supercrit Fluids 56:259–264
Gog A, Roman M, Toşa M, Paizs C, Irimie FD (2012) Biodiesel production using enzymatic transesterification – current state and perspectives. Renew Energy 39:10–16
Dizge N, Aydiner C, Imer DY, Bayramoglu M, Tanriseven A, Keskinler B (2009) Biodiesel production from sunflower, soybean, and waste cooking oils by transesterification using lipase immobilized onto a novel microporous polymer. Bioresour Technol 100:1983–1991
Kim SH, Kim S-j, Park S, Kim HK (2013) Biodiesel production using cross-linked Staphylococcus haemolyticus lipase immobilized on solid polymeric carriers. J Mol Catal B Enzym 85–86:10–16
Yan J, Yan Y, Liu S, Hu J, Wang G (2011) Preparation of cross-linked lipase-coated micro-crystals for biodiesel production from waste cooking oil. Bioresour Technol 102:4755–4758
Halim SFA, Kamaruddin AH, Fernando WJN (2009) Continuous biosynthesis of biodiesel from waste cooking palm oil in a packed bed reactor: optimization using response surface methodology (RSM) and mass transfer studies. Bioresour Technol 100:710–716
Hama S, Yoshida A, Tamadani N, Noda H, Kondo A (2013) Enzymatic production of biodiesel from waste cooking oil in a packed-bed reactor: an engineering approach to separation of hydrophilic impurities. Bioresour Technol 135:417–421
Gong H, Gao L, Nie K, Wang M, Tan T (2020) A new reactor for enzymatic synthesis of biodiesel from waste cooking oil: a static-mixed reactor pilot study. Renew Energy 154:270–277
Tan T, Lu J, Nie K, Deng L, Wang F (2010) Biodiesel production with immobilized lipase: a review. Biotechnol Adv 28:628–634
West AH, Posarac D, Ellis N (2008) Assessment of four biodiesel production processes using HYSYS.Plant. Bioresour Technol 99:6587–6601
Imahara H, Minami E, Hari S, Saka S (2008) Thermal stability of biodiesel in supercritical methanol. Fuel 87:1–6
Charoenchaitrakool M, Thienmethangkoon J (2011) Statistical optimization for biodiesel production from waste frying oil through two-step catalyzed process. Fuel Process Technol 92:112–118
Hancsók J, Kovács F, Krár M (2004) Production of vegetable oil fatty acid methyl esters from used frying oil by combined acidic/alkali transesterification. Petrol Coal 46:36–44
Jain S, Sharma MP, Rajvanshi S (2011) Acid base catalyzed transesterification kinetics of waste cooking oil. Fuel Process Technol 92:32–38
Li J, Zhou H, Cao Y (2012) Transesterification of waste cooking oil to produce biodiesel using acid and alkaline catalyst. Adv Mater Res 518–523:3566–3572
Liu S, McDonald T, Wang Y (2010) Producing biodiesel from high free fatty acids waste cooking oil assisted by radio frequency heating. Fuel 89:2735–2740
Tanawannapong Y, Kaewchada A, Jaree A (2013) Biodiesel production from waste cooking oil in a microtube reactor. J Ind Eng Chem 19:37–41
Thanh LT, Okitsu K, Sadanaga Y, Takenaka N, Maeda Y, Bandow H (2010) A two-step continuous ultrasound assisted production of biodiesel fuel from waste cooking oils: a practical and economical approach to produce high quality biodiesel fuel. Bioresour Technol 101:5394–5401
Guzatto R, de Martini TL, Samios D (2011) The use of a modified TDSP for biodiesel production from soybean, linseed and waste cooking oil. Fuel Process Technol 92:2083–2088
Guzatto R, Defferrari D, Reiznautt QB, Cadore ÍR, Samios D (2012) Transesterification double step process modification for ethyl ester biodiesel production from vegetable and waste oils. Fuel 92:197–203
Corro G, Tellez N, Jimenez T, Tapia A, Banuelos F, Vazquez-Cuchillo O (2011) Biodiesel from waste frying oil. Two step process using acidified SiO2 for esterification step. Catal Today 166:116–122
Srilatha K, Prabhavathi Devi BLA, Lingaiah N, Prasad RBN, Sai Prasad PS (2012) Biodiesel production from used cooking oil by two-step heterogeneous catalyzed process. Bioresour Technol 119:306–311
Wan Omar WNN, Nordin N, Mohamed M, Amin NAS (2009) A two-step biodiesel production from waste cooking oil: optimization of pre-treatment step. J Appl Sci 9:3098–3103
Wang Y, Ou S, Liu P, Zhang Z (2007) Preparation of biodiesel from waste cooking oil via two-step catalyzed process. Energy Convers Manag 48:184–188
Muciño GG, Romero R, Ramírez A, Martínez SL, Baeza-Jiménez R, Natividad R (2014) Biodiesel production from used cooking oil and sea sand as heterogeneous catalyst. Fuel 138:143–148
Tan YH, Abdullah MO, Kansedo J, Mubarak NM, Chan YS, Nolasco-Hipolito C (2019) Biodiesel production from used cooking oil using green solid catalyst derived from calcined fusion waste chicken and fish bones. Renew Energy 139:696–706
Guangrui L, Guanyi C (2012) Pilot plant of biodiesel production from waste cooking oil. Adv Mater Res 550–553:687–692
Joshi H, Moser BR, Toler J, Walker T (2010) Preparation and fuel properties of mixtures of soybean oil methyl and ethyl esters. Biomass Bioenergy 34:14–20
Cvengroš J, Paligová J, Cvengrošova Z (2006) Properties of alkyl esters base on castor oil. Eur J Lipid Sci Technol 108:629–635
Rashid U, Anwar F (2008) Production of biodiesel through optimized alkaline-catalyzed transesterification of rapeseed oil. Fuel 87:265–273
Cheng SF, Choo YM, Yung CL, Ma AN, Basiron Y (2005) Palm biodiesel: gearing towards Malaysian biodiesel standards. Malaysia Palm Oil Board. Palm Oil Developments, Kuala Lumpur. Issue POD 42
Yaşar F (2020) Comparison of fuel properties of biodiesel fuels produced from different oils to determine the most suitable feedstock type. Fuel 264:116817
Haas MJ, Scott KM, Alleman TL, McCormick RL (2001) Engine performance of biodiesel fuel prepared from soybean soapstock: a high quality renewable fuel produced from a waste feedstock. Energy Fuel 15:1207–1212
Kulkarni BM, Pujar BG, Shanmukhappa S (2008) Investigation of acid oil as a source of biodiesel. Ind J Chem Technol 15:467–471
Yingming C, Jidong L, Bo X, Jie C, Yan F, Xuewei W (2008) Lipase-catalyzed synthesis of biodiesel from acid oil in fixed bed reactor. Res J Biotech 3:5–12
Issariyakul T, Kulkarni MG, Dalai AK, Bakhshi N (2007) Production of biodiesel from waste fryer grease using mixed methanol/ethanol system. Fuel ProcessTechnol 88:429–436
Meng X, Chena G, Wang Y (2011) Biodiesel production from waste cooking oil via alkali catalyst and its engine test. Fuel Process Technol 89:851–857
Canakci M, Sanli H (2008) Biodiesel production from various feedstocks and their effects on the fuel properties. J Ind Microbiol Biotechnol 35:431–441
Jagadale SS, Jugulkar LM (2012) Production and analysis of chemical properties of chicken fat based biodiesel and its various blends. Int J Eng Res Dev 1(7):34–37
Dias JM, Alvim-Ferraz MCM, Almeida MF, Diaz JDM, Polo MS, Utrilla JR (2013) Biodiesel production using calcium manganese oxide as catalyst and different raw materials. Energy Convers Manag 65:647–653
Mittelbach M (1996) Diesel fuel derived from vegetable oils, VI: specifications and quality control of biodiesel. Bioresour Technol 56:7–11
Demirbas A (2010) Social, economic, environmental and policy aspects of biofuels. Energy Educ Sci Technol B 2:75–109
Lee S, Posarac D, Ellis N (2011) Process simulation and economic analysis of biodiesel production processes using fresh and waste vegetable oil and supercritical methanol. Chem Eng Res Des 89:2626–2642
Demirbas A (2009) Progress and recent trends in biodiesel fuels. Energy Convers Manag 50:14–34
Sakai T, Kawashima A, Koshikawa T (2009) Economic assessment of batch biodiesel production processes using homogeneous and heterogeneous alkali catalysts. Bioresour Technol 100:3268–3276
Acknowledgment
Serbian authors are thankful to the Ministry of Education, Science and Technological Development of the Republic of Serbia, which supports their work under the Project III 45001.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Glossary
- Biodiesel
-
Biodiesel is a form of diesel fuel derived from plants or animals and consisting of long-chain fatty acid esters.
- Catalysis
-
Catalysis is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst.
- Cooking oils
-
Cooking oil is plant, animal, or synthetic fat used in frying, baking, and other types of cooking.
- Enzyme catalysis
-
Enzyme catalysis is the increase in the rate of a process by a biological molecule, an “enzyme.”
- Esterification
-
Esterification is the general name for a chemical reaction in which two reactants (typically an alcohol and an acid) form an ester as the reaction product.
- Fats
-
Fat is a type of nutrient.
- Transesterification
-
Transesterification is the process of exchanging the organic group R″ of an ester with the organic group R′ of an alcohol.
- Vegetable oils
-
Vegetable oils, or vegetable fats, are oils extracted from seeds, or less often, from other parts of fruits.
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Veljković, V.B., Banković-Ilić, I.B., Stamenković, O.S., Hung, YT. (2021). Waste Vegetable Oils, Fats, and Cooking Oils in Biodiesel Production. In: Wang, L.K., Wang, MH.S., Hung, YT. (eds) Integrated Natural Resources Research. Handbook of Environmental Engineering, vol 22. Springer, Cham. https://doi.org/10.1007/978-3-030-61002-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-61002-9_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-61001-2
Online ISBN: 978-3-030-61002-9
eBook Packages: EngineeringEngineering (R0)