Abstract
Three species from the Yungas (Cedrela balansae, C. angustifolia, C. saltensis) and one from the Alto Paraná Rainforest (C. fissilis) represent the genus in Argentina. In this chapter, we present the state-of-the-art in their domestication, including afforestation experiences in block and in enrichment strips. The moth called “Meliaceae borer” is the main natural threat for the cultivation of the genus. Its population dynamics and control treatments are described. The adaptive variation of the genus has been studied through the analysis of physiological, chemical, and growth traits. Species and provenances were evaluated in relation to water stress and low temperatures. First steps toward the breeding of the genus in Argentina have been initiated with the establishment of a network of species, provenance, and progeny field trials. In addition, plus trees were selected in the natural forest and propagated by grafting. Clonal seed orchards of C. balansae, C. angustifolia, and C. fissilis were installed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Commercial plantations
- Natural forest enrichment
- Hypsipyla
- Variation in quantitative traits
- Provenance and progeny tests
- Seed orchards
1 Domestication of Cedrela sp. in Argentina
Trees of the genus Cedrela are among the native forest species of Argentina with the highest wood price in the domestic market. At the end of 2018, average prices of round wood on trucks in the forest were 240 U$S/m3 for Cedrela balansae and 270 U$S/m3 for C. saltensis in the Yungas.Footnote 1 Likewise, for C. fissilis , in the Alto Paraná Rainforest, average prices of 61 U$S/m3 have been reported.Footnote 2 The wood of this genus is known as Spanish Cedar in the world market, originally referring to Cedrela odorata , a species exploited since colonial periods in Central America. According to the International Tropical Timber Organization (ITTO), the price of Spanish Cedar wood dried in a kiln and placed in the Port of Callao, Peru, is over 950 U$S/m3.
Noteworthy, so far, all Cedrela wood sold in Argentina comes from natural forests. Its domestic market is concentrated in the large urban centers of Buenos Aires, Córdoba, and Santa Fe, where it is mainly processed into carpentries for the production of furniture and openings. It is also used in uprights, crossbars, wooden or parquet floors, and the manufacture of various domestic utensils. It is highly appreciated for its excellent aesthetics and workability. It is straight-grained with a medium texture and a subdued luster. When first cut, the heartwood is pale creamy, turning to pinkish brown upon exposure to light and air. The density varies according to the species from lightweight (C. balansae 450 kg/m3) to moderately heavy (C. fissilis 550 kg/m3) (Tortorelli 2009). It also presents a pleasant perfume and tolerance to insect attack once cut (Grau et al. 2006), as well as a good durability. This is endorsed by many still standing colonial churches in Central America and the Andes, whose doors, ceilings, and indoor decoration are made of Cedrela sp. wood (Pennington and Muellner 2010).
Due to its history, wood quality and price, together with the reduction of natural forests resources, the interest in the commercial cultivation of native Cedrela species has increased in Argentina during the last three decades. The experience of cultivation of C. odorata in block plantations in various tropical regions around the world, such as Central and South America, South Africa, and Southeast Asia, represents an outstanding example to imitate. Cozzo (1995) has reported that C. odorata plantations in Ecuador, with a density of 1125 trees/ha, reached 18 m in height and 24 cm in DBH on average at 6 years of age, and 25 m in height and 50 cm in DBH at 18 years, with a yield of 21 m3/ha/year.
However, block plantations in the subtropical region exhibit failures due to the occurrence of frost. In the Yungas, the most sheltered sites of the piedmont region are used for short-cycle and high-yield agricultural crops and are scarcely available for forest cultivation. Thus, it would be useful to cultivate these species in natural forest enrichment systems, especially in forest degraded by overexploitation and extensive cattle grazing. In such sites, the remaining vegetation provides the seedlings of Cedrela sp. protection against eventual frosts during the first years, allowing their establishment.
Following this reasoning, the Instituto Nacional de Tecnología Agropecuaria (INTA) proposed, for both the Yungas and the Alto Paraná Rainforest, the cultivation of Cedrela native species in enrichment systems in areas of intermediate conservation value (“yellow areas”) according to Law No. 26,331. About 1.5 million hectares of “yellow areas” have been identified in the provinces of Jujuy, Tucumán, Salta, and Misiones with suitable conditions for Cedrela sp. cultivation under enrichment systems (Fornes 2012).
Two types of enrichment are proposed: (1) in clumps, without tree cover, and (2) in strips, with tree side cover (Fig. 14.1a and b). The first case involves forests implanted in clearings of 500 to 1000 m2, previously made for crops production (later abandoned) or by fire. If the surface of the clearings exceeds 1000 m2, it is difficult to achieve frost protection. In the second case, the strips are opened with machetes, axes, and chainsaws in the natural vegetation by removing the undergrowth and trees of species of low commercial value. They are about 5 m wide, with a length determined by the characteristics of the area, such as the slope, exposure, soil, and vegetation. One or two lines are planted in each strip according to their width, with a distance of 4–5 m between seedlings within the line. The natural seedlings and saplings of tree species of high commercial value are preserved. The distance between strips is 10–20 m in order to achieve a general density of about 200–250 trees/ha. At the time of plantation, the optimum size of the seedlings is from 0.80 to 1.50 m high, which is reached in the nursery 12–15 months after sowing. Planting should be done during the rainy season (November to March). The use of individual tree shelters is recommended to avoid damage from herbivores such as rabbits and hare. Fertilization is usually unnecessary in these kinds of environments. Weeding must be carried out two or three times a year to prevent the invasion of weeds in the strips (Del Castillo et al. 2004).
According to the Chamber of Forest Industry of the Province of Tucumán,Footnote 3 the estimated cost for the production system in strips is 1142 U$S/ha, with the 52% of expenses concentrated in the first year and 25% corresponding to the task of strip opening. Of the total amount, 80% is commonly covered by the National State in accordance with Law No. 25,080 on investment in cultivated forests, which reimburses the money 1 or 2 years after planting, upon verification of the survival of 200 seedlings per hectare. There are also provincial laws that provides some indirect benefits, such as the Tucumán Law No. 7021, which exempts the payment of the tax on the gross income of these productive activities, the real estate tax, and the service of irrigation of forested lands, among others.
The technology for seedling production of Argentinian Cedrela’s species has been adjusted efficiently. The fruits are capsules and mature in the forest between June and August depending on the species. When they acquire a light-brown color, it is the adequate moment for collection, before their dehiscence. For C. angustifolia , it should be taken into consideration that it presents long periods without fruit production (Aschero 2006); therefore, seed supply can be a “bottleneck.” To extract the seeds, the capsules are placed under the sun in a dry environment until they open (Lorenzi 1992). The seeds are winged and of an easy handling size, 1000 seeds weight 18–33 g for C. balansae, 19.5 g for C. saltensis, and 42.5 g for C. fissilis, and they have a germination rate of over 88% for the first two species and 59% for the third one (Del Castillo et al. 2002). The viability of the seeds decreases up to 50% from 1 year to the next, so it is convenient to use new seeds every year (Fornes et al. 2016). Using as substrate an inert mixture of perlite, coconut fiber, and blonde peat, with slow release fertilizer, seedlings of adequate size for planting are obtained in 3 months. However, it is convenient to raise them for 12–15 months in order to reach the field with larger and more vigorous plants. To do this, when they reach 5 cm in height and have adult leaves, the seedlings are placed in pots 20–25 cm high and 6–7 cm in diameter, filled with soil and forest litter in a ratio of 2:1 (Monteverde 2006). The most vigorous seedlings should be selected in the nursery to increase the survival in the field (Grignola 2014).
The first forest enrichment plantations in Argentina date from the early 1980s. Actually, in the Northwest of Argentina, there are about 1000 ha intervened with this productive system, mostly with species of the genus Cedrela, but also with other native and exotic species, such as Jacaranda mimosifolia, Tipuana tipu, Enterolobium contortisiliquum, and Toona ciliata var. australis. There have been reported 530 ha in Jujuy, 345 ha in Tucumán, and 74 ha in Salta enriched with Cedrela sp. specifically (Fornes et al. 2016). The good results obtained are evidence of the success of this production system. For example, in an experimental plantation of C. balansae located in Yuto (Province of Jujuy), the plants reached a DBH of 31 cm and a height of 14 m at 15 years of age. Likewise, in an experimental plantation of C. angustifolia in El Naranjo (Province of Tucumán), DBH of 36 cm and heights of 19 m were obtained at the age of 31 years.Footnote 4 It is important to know at which age each Cedrela species form the heartwood, since the value of its wood resides in that portion of the trunk. It has been reported the early formation of heartwood in individuals of C. angustifolia, C. balansae, and C. fissilis in plantations, with values between 80 and 97% of the trunk at the ages between 12 and 30 years.
An alternative for small- and medium-sized producers is the development of agroforestry systems, where high-value timber species are combined with consociated crops that allow a profitability anticipated to the cutting cycle of the trees. Through the combination of agricultural and forestry production, the best functions of the production of forests and food crops can be achieved. There are environmental as well as socioeconomic advantages of integrated systems over agriculture and/or tree monocultures (Wiersum 1981). In Alto Paraná Rainforest, native forest species are combined with Ilex paraguariensis, Carica papaya, or Manihot esculenta. In Yungas, a combination of Cedrela sp. can be carried out with native fruit species such as Solanum betaceum, Physalis spp., and Eugenia uniflora, among others. These species also require half shade, especially in the juvenile stages. As they grow, openings in the canopy should be made, allowing more light to enter.
2 A Tiny Enemy Threatens the Cultivation of Cedrela sp.
The moth known as “Meliaceae borer,” Hypsipyla grandella Zeller, (order Lepidoptera, family Pyralidae) is the main pest affecting cedar species, limiting their potential in commercial forestations. It is specific of Cedrela and Swietenia genus in the American continent. In Argentina, it affects all native cedar species. The incidence of this pest is verified in the native forest, but in plantations (either in blocks or enrichment strips), it becomes more notorious. It can also affect plants in nurseries.
The main damage is caused by the larva, which destroys the main and lateral terminal buds, drilling the apices and making tunnels in the young stems. In the affected plants, the loss of apical dominance and its subsequent sprouts result in numerous lateral branches and consequently malformed trees, which lose their value for the production of wood (Fig. 14.2).
The biology of H. grandella has been well studied in Argentina (Tapia 2012). It lays the eggs externally both on the leaves and on the stem of the host plant. Between 48 and 72 h later, the larvae are born and penetrate the axillary bud first and move by boring the terminal non-lignified stem, the axillary bud, or the tip of the branches (Briceño Vergara 1997). The feeding continues generally in the marrow, consuming the bark, phloem, and leaf. Inside the plant, the insect meets between 5 and 7 larval stages and pupa until the adult emerges, which has nocturnal habits and a wingspan of 22–23 mm. The average life cycle is 5 weeks, depending on weather conditions and food availability.
Knowing the duration of the first generation of the year of H. grandella is of great importance to understand the population dynamics of this insect and implement strategies aimed at not allowing the exponential growth of the population over time. Baca et al. (2013) determined in the north of the Province of Salta the temporal distribution of H. grandella attacks in C. balansae saplings. They observed the first adult in September and the second in mid-November, although the largest adult capture was recorded during December and January. The first attacks were observed 8 weeks after the beginning of cedar foliation. The largest number of attacks was observed in mid-December, coinciding with the adult population peak. The first 10% of the observed attacks were concentrated in the term of 22 days (November–December), while the remaining 90% was concentrated within 71 days (December–February). These observations are important because there is a period of 20–30 days since the presence of the first adults of H. grandella and the first damages.
On the other hand, Baca et al. (2013) determined that a generation of H. grandella in field conditions lasted 44 ± 8 days. These results agree with the information obtained in laboratory conditions, where the species completed the total life cycle in 4–7 weeks (average 5 weeks), depending on the conditions and availability of food. The knowledge of life cycle duration allows determining the appropriate control time (Tapia 2012).
There are numerous studies regarding chemical control, mainly in Central America. In Argentina, different evaluated insecticides have proven to be effective. Generally, the effective products are hazardous and require numerous applications. Tapia (2012) conducted a series of control trials with chemicals. The experiments were carried out in commercial plantations of C. balansae, C. angustifolia, C. saltensis, and C. odorata in Salta, Jujuy, and Tucumán, with biweekly and monthly application frequencies. The products evaluated were Imidacloprid (SC 35% at 10‰), Alphacypermethrin (SC 6% at 20‰), Deltamethrin mixture (EC 10% at 10‰) plus Methyl Ethyl Azinphos (SC 36% at 6‰), and Azadirachtin (EC1% at 5‰), an organic farming insecticide extracted from neem seeds (Azadirachta indica). They obtained near-total control with Alphacypermethrin and the mixture of Deltamethrin and Methyl Ethyl Azinphos (Tapia 2012). These results are consistent with those obtained under nursery conditions by Eskiviski et al. (2010). However, the World Health Organization restricted the use of organophosphorus compounds Methyl Ethyl Azinphos (highly dangerous, Class Ib according to WHO). In contrast, Alphacypermethrin (as well as Deltamethrin) is a low toxicity pyrethroid (WHO Class II), making it the recommended choice (Tapia 2012).
It is important to understand the insect population dynamics in order to decide the appropriate moments of action. Likewise, knowing its natural enemies could be relevant, since this will allow the exploration of research lines based on biological controllers, as part of a pest integrated management. In this regard, Baca et al. (2013) were able to isolate from soil samples a strain of Beauveria sp., an entomopathogenic fungus capable of parasitizing the insect. It presents a good rate of sporulation, being possible its multiplication at large scale on solid substrate. This fungus could be used as a biological control strategy against H. grandella. Similarly, a microhymenoptera of the Chalcididae family was found parasitizing the pupae of H. grandella . The emergence of this parasitoid was observed in pupae collected in the field in the north of the Province of Salta, which is the first report of its presence on H. grandella in Argentina. It was identified as Brachymeria subconica (Aquino et al. 2015). This opens the door to further research on its potential and effectiveness as a biological controller.
An alternative to reduce the effect of H. grandella attacks would be to explore plants genetic resistance. The effect of provenance was proved to be highly significant in C. odorata and Swietenia macrophylla for the susceptibility to H. grandella . The mean number of attacks per tree ranged from 0.8 to 2.4 and from 0.6 to 1.3 depending on the provenances, in both species, respectively (Newton et al. 1999), that is, some provenances had triple the attacks than others. Likewise, a different susceptibility was observed among cedar species in a field trial carried out in Yuto Experimental Station of INTA, where 100% of C. fissilis saplings were affected while only 38% and 28% of C. saltensis and C. odorata saplings, respectively, were attacked by H. grandella (Del Castillo and Tapia 2005).
So far, chemical treatments with Alphacypermethrin and Deltamethrin are the most effective and with less toxicity. They must be conducted during the first 3 years of planting, and involve 15% of the total costs of cultivating Cedrela spp. (Tapia 2012). Applications should be made from the first year of planting to obtain a trunk length free of deformation of 3.5–4 m. The appropriate moment to start this treatment is defined by the insect population dynamics, and according to the abovementioned studies, it is commonly recommended from the second half of December until February.
3 Variation in Quantitative Traits
First approaches to unravel the variation of the Cedrela genus in quantitative traits concentrated on the species level, particularly considering physiological traits. An assay of thermic stress tolerance was carried out with seedlings of C. fissilis and C. saltensis (Meloni and Martínez 2011). One-month seedlings were transferred to pots containing Hoagland nutrient solution under greenhouse with photoperiod adjusted (12 h) and controlled temperature conditions (10 °C or 25 °C). Modulated fluorescence emission measurements were made daily between 8:30 am and 10:30 am, and concentrations of quercitol (a cyclitol) and catechin (a phenol), two natural compounds with likely cryoprotective action on cell membranes, were determined (Orthen and Popp 2000).
The modulated fluorescence variables evaluated in this study had different behavior in both species. Temperatures of 10 °C produced a significant increase of non-photochemical quenching (NPQ) and decrease Fv/Fm ratio, but the effect resulted more marked in C. fissilis than in C. saltensis (Fig. 14.3). These results indicate that temperatures of 10 °C generated a decrease of the proportion of photosystem II active reaction centers and increased the loss of energy absorbed through dissipation in the form of heat.
Non-photochemical quenching (NPQ), Fv/ Fm ratio, and quercitol and catechin concentrations in C. saltensis and C. fissilis seedlings, grown at 10 °C and 25 °C. (From Orthen and Popp 2000)
The Fv/Fm ratio represents the maximum efficiency of the photosystem II; a low value of Fv/Fm indicates an inefficient use of the absorbed energy and photoinhibition (Murchie and Lawson 2013). The Fv/Fm ratio decreased in C. fissilis plants grown at 10 °C and remained with no changes in C. saltensis (Fig. 14.3). These results indicate that although C. fissilis increased energy dissipation in the form of heat, in the long term, this mechanism was not enough to compensate the deleterious effects of stress. Consequently, irreversible damage occurred in the photochemical stage of photosynthesis and photoinhibition.
From the results obtained on the modulated fluorescence variables, it can be inferred that C. saltensis was able to acclimate at low temperatures, whereas C. fissilis was not. This behavior agrees with the climatic features of the home sites of both species. It suggests that photosynthesis of C. saltensis from Yungas (Calilegua provenance), with minimum temperatures of 12 °C, is more tolerant to low temperatures than photosynthesis of C. fissilis from Alto Paraná Rainforest (San Antonio provenance), with minimum temperatures of 16 °C.
The leaves of C. saltensis grown at 10 °C showed higher concentrations of quercitol and catechin than those grown at 25 °C. Contrary to C. saltensis, in response to low temperatures, C. fissilis kept the concentrations of quercitol and catechin with no changes (Fig. 14.3).
It can be concluded that C. saltensis , with a higher altitudinal niche than C. fissilis, is more tolerant to low temperatures, since it has a stable photosynthesis and the ability to synthesize cryoprotectants in these environmental conditions.
Interspecific variation of water stress tolerance in the genus was also studied (Ruiz et al. 2013). It is well known that the seedling stage is the most critical for trees development (Garkoti et al. 2003), because its limited root system is more vulnerable to water shortage. The understanding of seedling adaptive physiological responses to drought is relevant to predict potential areas of cultivation. The aim of this work was to study the physiological response in greenhouse conditions of seedlings of C. balansae, C. balansae × C. saltensis hybrid (average annual rainfall below 1000 mm at their home sites), and C. fissilis, (average annual rainfall above 2000 mm) under different simulated water regimes. Two provenances of C. balansae (Río Seco and Yuto), one provenance of the hybrid (Pintascayo), and two provenances of C. fissilis (San Antonio and Guaraní) were submitted to four simulated rainfall treatments: 600 mm/year, 800 mm/year, 1000 mm/year, and 1200 mm/year. This factorial trial was installed in Famaillá Experimental Station of INTA (27° 3’ S, 65° 25’ W, 450 m asl) with a completely randomize design of 15 replications (N = 300). One seed per pot was sown in January; pots were 13 cm in diameter and 45 cm in deep and were filled with a local loamy soil. After sowing, the pots were transferred to a greenhouse in order to exclude the natural rainfall. The pots were maintained close to field capacity until the beginning of simulated rainfall treatments and were rotated regularly in their positions to avoid confounding effects of light and temperature gradients. With seedlings emerged, rainfall treatments were applied from March to December, and, from August onward, leaf relative water content (RWC) and water potential (Ψw) were measured. The RWC was calculated according to the following equation:
where FW is fresh weight, DW is dry weight, and TW is turgid weight.
The Ψw was determined with a Schölander chamber at midday, and at the end of the trial, total biomass production and shoot height were measured.
Even though leaf RWC (Fig. 14.4A) showed no clear differentiation among provenances, it was possible to determine different water adjustment responses between species under the tested treatments. Significant differences in the Ψw were detected between the species from Yungas and that from Alto Paraná Rainforest (Fig. 14.4B). The provenances of C. balansae and C. balansae × C. saltensis hybrid were less susceptible to severe water deficit than C. fissilis provenances. Although interspecific differences were found in the physiological responses, it was not possible to separate the behaviors according to their provenances, indicating an intraspecific stability. It is important to highlight that C. balansae Río Seco provenance showed the best behavior under severe stress situation (600 mm).
Leaf relative water content of Cedrela seedlings growing under four simulated annual rainfall regimes: (a) 1200 mm/year, (b) 1000 mm/year, (c) 800 mm/year, and (d) 600 mm/year. Values are means of ten different measurements. (From Ruiz et al. 2013)
Midday leaf water potential (Ψw) throughout the final phase of the experiment in Cedrela seedlings growing under four simulated annual rainfall regimes: (a) 1200 mm/year, (b) 1000 mm/year, (c) 800 mm/year, and (d) 600 mm/year. Values are means of ten different measurements. (From Ruiz et al. 2013)
Nevertheless, the biometric parameters total biomass production (dry weight) (Fig. 14.5a) and shoot height (Fig. 14.5b) showed significant differences among provenances or simulated rainfall treatments. The dry weight (Fig. 14.5a) of C. fissilis Guaraní was significantly affected under higher hydric deficit treatments (800 and 600 mm), while the provenance of C. balansae Río Seco was only significantly affected under 600 mm. An opposite behavior was observed in the provenance of C. balansae × C. saltensis hybrid (Pintascayo), with a significant increase of the dry weight when exposed to 600 mm, with respect to those under 1200 mm treatment. The shoot height (Fig. 14.5b) of C. balansae Río Seco and C. fissilis Guaraní was significantly affected under 600 mm with respect to the 1200 mm treatment. A different behavior was observed in C. balansae × C. saltensis hybrid (Pintascayo), which was not affected by the water deficit, since it presented no significant differences between 600 mm and 1200 mm treatments.
(a) Total dry weight and (b) shoot height at the end of the experiment in Cedrela seedlings growing under four simulated annual rainfall regimes. Values are means of ten different measurements. Vertical bars represent ± standard error (≤ 0.05). (From Ruiz et al. 2013)
The water balance was more efficient in seedlings from natural environments with lower rainfall regimes. Besides, the efficiency was also manifested in the water use, since the provenances from natural drier environments were able to grow more when the water regime treatment was lower. Differences on drought responses among tree ecotypes growing in tropical and subtropical rainforests may be attributed to genetic differences in physiological and morphological adaptive responses (Engelbrecht and Kursar 2003). Plant responses to water deficit, when they are outside their natural environments, depend on the rainfall regimes of their native habitats (Otieno et al. 2005).
In a subsequent trial, all Argentinian species of the genus were compared with respect to growth traits (Grignola 2014). It included five provenances of C. balansae, two of C. fissilis, two of the hybrids between C. saltensis and C. balansae, two of C. saltensis, and, since it was not possible to obtain enough seeds of C. angustifolia from the same provenance, a pool of several sources was used to represent this species. The trial was established on 100 cm3 individual pots filled with inert substrate and slow-release fertilizer in the greenhouse of Famaillá Experimental Station (27° 3’ S, 65° 25’ W, 450 m asl), with seeds from trees selected in the natural forest according to growth, health, and shape criteria. The hybrid character of adult plants of C. saltensis × C. balansae was confirmed through genetic markers.
Seventy days after germination, the total height and collar diameter of 20 randomly chosen plants per treatment were measured (N = 240). Significant differences among species and among provenances were found for both variables, C. fissilis and C. angustifolia being the species with the tallest but thinnest seedlings. Among the provenances, C. fissilis San Antonio and Guaraní had the highest average heights of the trial (33.0 cm and 27.7 cm, respectively) and the lowest diameters (5.5 mm on average), while C. balansae Río Seco and Ledesma (undifferentiated) exhibited the highest mean diameters, with 7.8 and 8.0 mm, respectively (Fig. 14.6).
The status of carbohydrate reserves is a physiological parameter useful to infer vigor and health of a plant and might have an effect on its performance in the field (Birchler et al. 1998). In turn, the adaptation of a species to low temperatures can be evaluated by testing the resistance of its cell membranes to freezing. Such a test can be performed indirectly by subjecting its fresh leaves to freezing, then immersing them in deionized water, and finally measuring the electrical conductivity of the solution formed by the water and solutes released by the leaves. The measured conductivity is a function of the breakage of the cell membranes by freezing (Dexter et al. 1932; Rodríguez-Rey et al. 2000).
From the previous trial, seven seedlings per treatment were transplanted into 3000 cm3 pots 70 days after germination and were grown for 1 year within a greenhouse. In autumn (temperatures between 10 °C and 15 °C), winter (& lt; 10 °C), and summer (~25 °C), leaves of these plants were sampled, and subsequently their content of simple soluble sugar (glucose + fructose) was measured. In addition, another sampling was done in autumn on four dates characterized by a temperature gradient of 5°, from 18.3 °C to 13.6 °C. In each sampling, six leaflets per plant were collected: three were subjected to −10 °C in a test tube for 1 h and then immersed in deionized water for 2 h at 25 °C. The other three leaflets were used for the control treatment. The electrical conductivity was measured in both solutions, thus obtaining the value of index of tissue damage (ITD) as the ratio between the conductivity of the control treatment and the conductivity of the −10 °C treatment (Grignola 2014).
Sugar concentration for the species and provenances studied varied according to the period of the year. For autumn-winter season, significant differences in sugar concentration were observed, but not in the summer season, differentiating C. fissilis from C. balansae, C. angustifolia, C. saltensis, and the hybrid. The highest value was recorded in Guaraní provenance of C. fissilis (22.5 ± 0.7 mg of glucose + fructose per gram of dry leaves) (Grignola 2014). Regarding the damage of the cell membranes due to freezing, significant differences among species were observed at the four sampling dates, with C. fissilis differing from C. balansae, C. saltensis, and the hybrid in three of the four sampling dates. The species from the Alto Paraná Rainforest had the most negative value of ln-ITD, which means that it was the one with the lowest solute release, namely, the one with the least damage to cell membranes. In addition, differences between the origins were demonstrated for the four sampling dates. For the two lowest temperature dates, the two C. fissilis provenances were different from the other ten tested. These results, besides differentiating C. fissilis from the Yungas species, would be indicative of a lower sensitivity to cold conditions for the species of the Alto Paraná Rainforest, at least at the seedling stage (Grignola 2014).
4 First Steps Toward the Breeding of the Genus in Argentina
The first progeny trial of the genus in Argentina corresponds to C. balansae and was installed in April 2001 in Valle Morado (23° 28′ 11.8″ S, 64° 25′ 52″ W, 385 m asl) by Fundación Proyungas (Balducci et al. 2009). It includes 42 open-pollinated families obtained from mass selected trees in the natural forest according to forestry shape and sanitary criteria. Seeds were collected from five provenances: Ingenio Ledesma, Yuto, Campo Chico, Los Naranjos, and Orán. The trial has an area of 2 ha and an experimental design of incomplete blocks, with 20 repetitions of single-tree plots (40 treatments per block; N = 800). It was installed without any coverage.
First evaluations were made at 4 years of age (Horlent and Monteverde 2006). Significant differences were demonstrated for survival: families without mortality were observed throughout the trial, as well as families with up to 47% mortality. No significant differences were found between families in terms of shoot borer resistance. The percentage of plants attacked per family varied between 35% and 79%. Accordingly, differences could not be shown for the stem shape either, a variable closely related to the H. grandella attack. Similarly, families did not show significant differences for the “burning of the bark.” This damage is caused by direct insolation in the north face of the stem. It begins as spots in the bark and progresses to wounds that reach the cambium, which can reduce the value of the wood and allow the entry of pathogens.
At the age of 5 years, height and diameter increments were evaluated (Balducci et al. 2009), and differences between families were demonstrated. The best ten families had a mean DBH of 14.4 cm and a mean height of 7.1 m. Maximum mean annual increment was 3.89 cm for DBH and 1.67 m for height. In addition to the information that it provides, this trial is important as a source of genetically proven material for further advances in the improvement of the species.
In 2006, INTA formally began a breeding program for this genus. The planned strategy was to create clonal seed orchards from mass selected trees from the natural forest, while different genetic trials were carried out. In December 2008, a small network of three trials was established, in order to test species, provenances, and progenies. They were composed by 110 open-pollinated families coming from seeds collected from trees selected in the natural forest according to growth, health, and shape criteria (Grignola 2014). All Argentinian species of the genus were assayed: five provenances of C. balansae, two of C. fissilis, two of C. saltensis, three of the hybrid (C. saltensis × C. balansae), and a pool of several provenances for C. angustifolia as in the previous trial. Seed pools from C. odorata and Toona ciliata (a Meliaceae from Australia) were used as controls. The test sites were La Moraleja (24° 17′ 59″ S, 64° 1′ 19″ W, 375 m asl), La Fronterita (26° 58′ 18″ S, 65° 30′ 7″ W, 655 m asl), and El Siambón (26° 42′ 49″ S, 65° 26′ 35″ W, 1170 m asl), with strong altitudinal and rainfall contrasts (mean annual rainfall: 850 mm, 1400 mm and 1200 mm, respectively). The experimental design was in randomized complete blocks, with 16 repetitions of single-tree plots in the first two sites and with 8 repetitions in El Siambón. All trials were without coverage, with a distance of 3 m × 3 m between plants. The number of provenances and families tested at each site varied according to seedlings availability (Grignola 2014).
A strong mortality was observed at the third year (Grignola 2014), with greater incidence in El Siambón, the site of highest altitude and lowest minimum temperatures. C. balansae, C. fissilis , and the hybrid did not differentiate, with survival values between 20% and 32% depending on the trial. The highest survival rate in El Siambón was for C. fissilis San Antonio provenance, with 43%. In La Moraleja (the other altitudinal extreme), the highest survival rate was for C. balansae Ledesma provenance, with 36%. The planting system without coverage was a determining factor to explain this high mortality.
Species by site interaction was observed for height at the third year, and significant differences between species were found (Fig. 14.7). The tallest plants belonged to the undifferentiated group of C. balansae (147.5 cm ± 6) and the hybrid (136.7 cm ± 10) in La Moraleja, the hybrid (124.4 cm ± 10.5) in La Fronterita, and C. fissilis (102 cm ± 11) in El Siambón. The highest average height was observed for the plants from C. balansae Ledesma, with 153 cm ± 8 in La Moraleja.
Mean height at 3 years of age of the species assayed in the three study sites. Standard errors are presented. Means with the same letter are not significantly different (p > 0.05). (From Grignola 2014)
Another evaluation was performed at the eighth year (Grignola, unpublished data) in which interaction between trial sites and provenances was confirmed (La Moraleja trial unfortunately could not be continued). In El Siambón, the two tested provenances of C. fissilis were those with the highest annual height growth rate (AHGR) (San Antonio 71.6 ± 4 cm/y and Guaraní 60.0 ± 7 cm/y). These provenances did not show significant differences between them, and differed from all those tested for C. balansae and the hybrid. In La Fronterita, on the contrary, three of the four provenances of C. fissilis were the ones with the lowest AHGR (Guaraní 24.0 ± 4 cm/y, Las Marías 13 ± 5 cm/y and Eldorado 11 ± 4 cm/y). They did not show significant differences between them and differed from all the provenances tested for C. balansae, C. saltensis, the hybrid, and the control Toona ciliata (Fig. 14.8). In Fig. 14.9, the species-by-site interaction becomes evident when reducing the analysis to the main species analyzed in both trials.
BLUP analyses allowed identification of the families of the main evaluated species that were superior at both sites, despite the strong interaction between species and sites. In Fig. 14.10, the performance of each essayed family is simultaneously represented for both sites. Linear regression lines contribute to visualize performance trends for each species. In the upper-right quadrant, open-pollinated families who had better performances in terms of height growth for the two sites are observed. The families CM15, CM14, and CM17 of San Antonio provenance of the species C. fissilis stand out. The Families BC05, BC02, BC04, BC01, BC10, and BC13 of the Calilegua provenance of the species C. balansae were located in the same quadrant. The identification of these superior families might serve to go back to their mothers, propagate them vegetatively, and infuse new genetics into existing clonal seed orchards, or simply to create new ones.
Linear regression plot (oblique black lines) for the three species for AHGR BLUPs obtained from families growing in El Siambón vs. La Fronterita trials. All linear regressions were significant (p < 0.05). The dividing gray lines correspond to the averages AHGR BLUPs from each site. (Grignola, data not published)
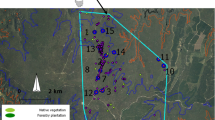
The first basic propagation material of Cedrela in Argentina for the supply of seeds to commercial nurseries is a seed production area of C. balansae planted in 1994 at the Yuto Experimental Station of INTA (23° 35′ 15.95″ S, 64° 30′ 27.69″ W, 350 m asl; Del Castillo, pers. comm.) (Fig. 14.11). It has a surface of 0.5 ha, began production in 2017, and has already been registered with the National Seed Institute (INASE).
The first clonal seed orchards (CSO) of Cedrela in Argentina were established in 2012 in Famaillá Experimental Station of INTA (Fornes et al. 2016): one for C. balansae, other for C. angustifolia, and a third one for C. fissilis. The trees were mass selected in the natural forest by height, stem rectitude, and sanity. Seeds, twigs of sexually mature branches, and leaves were collected from 107 trees belonging to eight natural populations of C. balansae, 52 trees from eight populations of C. fissilis, and 160 trees from 14 populations of C. angustifolia. Seeds were used for the abovementioned trials, twigs for the CSO, and leaves for genetic diversity studies with genetic markers (see Chap. 13). Each specimen was georeferenced and phenotypically described. This way, almost the entire range of the genus Cedrela in Argentina was covered.
The genus can be asexually propagated by several methods (Ortiz Morales and Herrera Tuz 2007). The method used to multiply the trees selected for these three CSOs was grafting by clefting. Scions 10 cm long and 1 cm in diameter were grafted onto rootstocks of the same species. In all three CSOs, a randomized complete block design with single-tree plots was used, and the number of blocks depended on the availability of ramets of each species. In the CSO of C. balansae (Fig. 14.12a), 48 clones were initially planted corresponding to four populations of Salta and two of Jujuy, with up to nine replicates per clone (N = 288). During the subsequent years, several genotypes were added, summing at present up to 66 clones in the seed orchard (N = 314). With a distance between plants of 5 m × 7 m, its total area is 2 ha, and its geographical coordinates are 27° 0′ 55.47″ S, 65° 22′ 16.84″ W (375 m asl).
In the CSO of C. angustifolia, 64 clones were initially planted, corresponding to seven populations of Salta, three of Tucumán, and two of Jujuy, with five replicates per clone (N = 320). Subsequent mortality reduced the number of clones to 44 (N = 85). The initial distance between plants was 4 m × 4 m, thus occupying an area of 0.5 ha (27° 1′ 13.89″ S, 65° 22′ 53.66″ W, 375 m asl).
C. fissilis CSO initially had 42 clones from five populations of Misiones and Corrientes, with nine replicates per clone (N = 378) (Fig. 14.12b). Currently, it consists in 77 clones since genotypes were added over time, although the number of ramets was reduced by mortality to 155. It occupies an area of 1.5 ha with a distance of 5 m × 7 m between plants and is located at 27° 1′ 50.22″ S, 65° 22′ 46.48″ W (365 m asl).
In 2016, seed production started in the orchards of C. fissilis and C. balansae. However, production still fluctuates from year to year, and several clones have not yet came to fruited. A significant portion of the seeds collected still has low germination capacity. It is estimated that production will stabilize in 2022.
Notes
- 1.
Personal Communication: National Direction of Industrial Forestry Development
- 2.
Personal Communication: Misiones College of Forest Engineers
- 3.
Personal Communication: Chamber of Forest Industry of the Province of Tucumán
- 4.
Personal Communication: Adrián Trápani and Miguel Gatto, Programa de Domesticación y Mejoramiento de Especies Forestales Nativas e Introducidas para Usos de Alto Valor (PROMEF)
References
Aquino DA, Tavares Texeira M, Balducci E, Baca V, Quintana de Quinteros S (2015) The microlepidopterous natural enemy Brachymeria subrugosa Blanchard, 1942 (Hymenoptera, Chalcididae): identity, hosts and geographic distribution. Zootaxa 4013:293–300
Aschero V (2006) Biología reproductiva e importancia de la polinización en Cedrela lilloi. In: Pacheco S, Brown A (eds) Ecología y Producción de Cedro (Género Cedrela) en Las Yungas Australes, Tucumán, Argentina, pp 41–50
Baca V, Lucia A, Balducci E, Sanchez E, Malizia L, Quintana de Quinteros S (2013) Dinámica poblacional del barrenador de las Meliaceas, Hypsipyla grandella (Zeller) y su asociación con los ataques ocasionados en plantaciones de Cedro en el norte de la provincia de Salta. 4to Congreso Forestal Argentino y Latinoamericano, 23–27 Sept. 2013, Iguazú.
Balducci E, Arturi M, Goya J, Brown A (2009) Potencial de plantaciones forestales en el pedemonte de las Yungas. Proyungas. Ediciones del Subtrópico, Yerba Buena, pp. 42. http://proyungas.org.ar/wp-content/uploads/2014/12/cartilla-Valle-Morado-1.pdf
Birchler T, Rose WR, Pardos M, Royo A (1998) La planta ideal: Revisión del concepto, parámentros definitorios e implementación práctica. Invest Agr Sist Recur For 7:109–121
Briceño Vergara AJ (1997) Aproximación hacia un manejo integrado del barrenador de las Meliaceas, Hypsipyla grandella (Zeller). Rev For Venez 41:23–28
Cozzo D (1995) Silvicultura de plantaciones maderables. Orientación Gráfica, Buenos Aires. Tomo II, pp. 899.
Del Castillo E, Tapia SN (2005) El barrenador de los brotes: Hypsipyla grandella Zéller, en plantaciones de importancia forestoindustrial en el NOA. Documentos INTA, Yuto. https://inta.gob.ar/sites/default/files/script-tmp-el_barrenador_de_los_brotes.pdf
Del Castillo ME, Zapater MA, Gil MN (2002) El Cedro rosado. Recolección de material genético. Viverización. Ensayos de implantación. SAGPyA, INTA EEA Yuto, pp. 23. https://inta.gob.ar/sites/default/files/script-tmp-cedro_rosado.pdf
Del Castillo EM, Zapater MA, Gil MN (2004) Resultados de plantaciones experimentales con Cedrela Balansae C. DC. (Cedro Orán) en INTA-Yuto: comparación con otras especies forestales nativas y exóticas. https://inta.gob.ar/sites/default/files/script-tmp-comparacin_cedrela_y_otras_especies.pdf
Dexter ST, Tottingham WE, Graber LF (1932) Investigations of the hardiness of plants by measurement of electrical conductivity. Plant Physiol 7:63–78
Engelbrecht BMJ, Kursar TA (2003) Comparative drought resistance of seedlings of 28 species of co-occurring tropical woody plants. Oecologia 136:383–393
Eskiviski E, Tapia S, Fornes L, Agostini J (2010) Evaluación de insecticidas en el control de H. grandella (Zeller) en condiciones de vivero. XIV Jornadas Técnias Forestales y Ambientales. Facultad de Ciencias Forestales. UNaM – INTA EEA Montecarlo, Eldorado, 10–12 junio 2010, pp. 10–14.
Fornes L (2012) Domesticación de especies de alto valor de las selvas subtropicales. Revista Producción Forestal MAGyP 4:28–52
Fornes L, Zelener N, Gauchat ME, Inza MV, Soldati MC, Ruíz V, et al. (2016) Subprograma Cedrela. In: Marcó M, Llavallol C (eds) Domesticación y Mejoramiento de Especies Forestales. Min. Agr. UCAR, pp 137–159
Garkoti S, Zobel D, Singh S (2003) Variation in drought response of sal (Shorea robusta) seedlings. Tree Physiol 23:1021–1030
Grau A, Zapater MA, Neumann RA (2006) Botánica y distribución del género Cedrela en el noroeste de Argentina. In: Pacheco S, Brown A (eds) Ecología y Producción de Cedro (Género Cedrela) en Las Yungas Australes. LIEY-ProYungas, Argentina, pp 19–30
Grignola J (2014) Plasticidad y tolerancia de diferentes especies y procedencias del género Cedrela a las bajas temperaturas. MSc thesis from Universidad Nacional de Córdoba, Argentina, pp. 116.
Horlent M, Monteverde D (2006) Crecimiento de Cedrela balansae en la plantación experimental de Valle Morado. In: Pacheco S, Brown A (eds) Ecología y Producción de Cedro (Género Cedrela) en Las Yungas Australes. LIEY-ProYungas, Argentina, pp 171–178
Lorenzi H (1992) Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas nativas do Brasil. Instituto Plantarum de Estudos da Flora, Odessa, p 352
Meloni DA, Martínez CA (2011) Bases fisiológicas de la tolerancia al estrés térmico en especies del género Cedrela. XIV Reunión Latinoamericana de Fisiología Vegetal. Búzios, 19 y 22 de septiembre de 2011, p. 95.
Monteverde D (2006) Producción de plantines de cedro en vivero. In: Pacheco S, Brown AD (eds) Ecología y Producción de Cedro (Género Cedrela) en Las Yungas Australes. LIEY-ProYungas, Argentina, pp 155–160
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64:3983–3998
Newton AC, Watt AD, Lopez F, Cornelius JP, Mesén JF, Corea EA (1999) Genetic variation in host susceptibility to attack by the mahogany shoot borer, Hypsipyla grandella (Zeller). Agric For Entomol 1:11–18
Orthen B, Popp M (2000) Cyclitols as cryoprotectants for spinach and chickpea thylakoids. Environ Exp Bot 44:125–132
Ortiz Morales ER, Herrera Tuz LG (2007) Cedro (Cedrela odorata L.). Protocolo para su colecta, beneficio y almacenaje. Comisión Nacional Forestal, Programa de Germoplasma Forestal, Estado de Yucatán, pp. 23. http://www.conafor.gob.mx:8080/documentos/docs/19/1299Cedro%20rojo%20Yucat%c3%a1n.pdf
Otieno DO, Schmidt MWT, Adiku S, Tenhunen J (2005) Physiological and morphological responses to water stress in two Acacia species from contrasting habitats. Tree Physiol 25:361–371
Pennington TD, Muellner AN (2010) A monograph of cedrela (Meliaceae). DH Books. The Manse. Chapel Lane, Milborne Port-England, pp. 112.
Rodríguez-Rey JA, Romero E, Gianfrancisco S, David S del C, Amado ME (2000) Evaluación de la capacidad de aclimatamiento a las bajas temperaturas de pimiento Capsicum annuum L. cultivado en invernadero sin calefacción. Rev Fac Agron (LUZ) 17:10–19
Ruiz VE, Meloni DA, Fornes LF, Ordano M, Prado FE, Hilal M (2013) Seedling growth and water relations of three Cedrela species sourced from five provenances: response to simulated rainfall reductions. Agrofor Syst 87:1005–1021
Tapia S (2012) El control del barrenador del brote de los cedros. Experiencias en el NOA. Revista Producción Forestal MAGyP 4:38–42
Tortorelli L (2009) Maderas y bosques argentinos, 2a edición. ed. Editora Orientación Gráfica, Buenos Aires, pp. 1111.
Wiersum KF (1981) Outline of the agroforestry concept. In: Wierzum KF (ed) Viewpoints on agroforestry. Agricultural University, Wageningen, pp 1–23
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Grignola, J. et al. (2021). Breeding Strategy for the Cedrela Genus in Argentina. In: Pastorino, M.J., Marchelli, P. (eds) Low Intensity Breeding of Native Forest Trees in Argentina. Springer, Cham. https://doi.org/10.1007/978-3-030-56462-9_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-56462-9_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-56461-2
Online ISBN: 978-3-030-56462-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)