Abstract
Crustaceans (e.g., shrimp and crabs) are a good source of protein-rich foods for human consumption. They are the second largest aquaculture species worldwide. Understanding the digestion of dietary protein, as well as the absorption, metabolism and functions of amino acids (AAs) and small peptides is essential to produce cost-effective and sustainable aquafeeds. Hepatopancreas (the midgut gland) is the main site for the digestion of dietary protein as well as the absorption of small peptides and AAs into the hemolymph. Besides serving as the building blocks of protein, AAs (particularly aspartate, glutamate, glutamine and alanine) are the primary metabolic fuels for the gut and extra-hepatopancreas tissues (e.g., kidneys and skeletal muscle) of crustaceans. In addition, AAs are precursors for the syntheses of glucose, lipids, H2S, and low-molecular-weight molecules (e.g., nitric oxide, glutathione, polyamines, histamine, and hormones) with enormous biological importance, such as physical barrier, immunological and antioxidant defenses. Therefore, both nutritionally essential and nonessential AAs are needed in diets to improve the growth, development, molt rate, survival, and reproduction of crustaceans. There are technical difficulties and challenges in the use of crystalline AAs for research and practical production due to the loss of free AAs during feed processing, the leaching of in-feed free AAs to the surrounding water environment, and asynchronous absorption with peptide-bounded AAs. At present, much knowledge about AA metabolism and functions in crustaceans is based on studies of mammals and fish species. Basic research in this area is necessary to lay a solid foundation for improving the balances and bioavailability of AAs in the diets for optimum growth, health and wellbeing of crustaceans, while preventing and treating their metabolic diseases. This review highlights recent advances in AA nutrition and metabolism in aquatic crustacean species at their different life stages. The new knowledge is expected to guide the development of the next generation of their improved diets.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Crustaceans (including shrimp and crabs) are low-fat, good sources of high-quality protein, free amino acids (AAs), small peptides, and polyunsaturated fatty acids for human consumption (Bhavan et al. 2010; Wu et al. 2016; Wu 2020). Therefore, they are healthy seafoods worldwide. Crustacean farming has been an economically important enterprise in either a marine or a freshwater environment as the second largest aquaculture species (e.g., 7.86 million tons and US$ 57.1 billion in 2016; Tacon 2018). Twenty-seven (27) species of aquatic crustaceans have been reported, which include mainly shrimps, crabs, and crawfish (Tacon 2018).
Crustaceans have particularly high requirements for dietary protein, which ranges from 60% of the diet for some post-larvae to 30–50% of the diet for juvenile shrimp, crabs and lobsters (Unnikrishnan and Paulraj 2010; Jin et al. 2013; Mente 2006). High-protein diets lead to the excretion of a large amount of nitrogen and low water quality. Traditionally, fishmeal has been the major protein source for crustaceans due to its high levels of digestible protein and balanced AA profiles (Unnikrishnan and Paulraj 2010). However, fishmeal is an unsustainable protein source due to its limited source and high price (Hardy 2010). In the culture of crustaceans, the cost of feeds represents more than 50% of the production costs (Mente 2006). Therefore, continued expansion of crustaceans is not unsustainable if fishmeal is their sole or primary protein source. In addition, disease and animal health have been a major limiting factor for the culture of shrimps, crabs, and crawfish (Mente 2006; Stentiford et al. 2012). Knowledge of their optimum requirements for nutrients, particularly AAs, is key to solving this problem, because many AAs regulate key metabolic pathways that are crucial to the maintenance, growth, reproduction, and immune responses of animals (Li et al. 2007, 2009b; Wu 2010; Wu et al. 2014).
Understanding the digestion of dietary protein, as well as the absorption, metabolism and functions of small peptides and AAs are essential to manufacture environmentally-oriented aquafeeds and reduce feed costs in animal production (Li et al. 2009b). Such diets can improve the health and wellbeing of crustaceans, while preventing and treating their metabolic diseases. Although a wide range of dietary AA requirements has been reported for aquatic animals in the literature, our knowledge about AA metabolism and functions in crustaceans is limited. The crustaceans belong to the suborders of the Decapoda with different metabolic, physiological, and immunological characteristics, when compared with other animals such as fish and mammals (NRC 2011; Vazquez et al. 2009). The major objective of this article is to highlight current knowledge about AA nutrition and metabolism in shrimps, crabs, and crawfish at their different stages of lives. This will help to advance the field of protein nutrition and guide the development of future crustacean feeds.
9.2 Protein Digestion and the Absorption of Small Peptides and Free AAs in Crustaceans
The diets of crustaceans contain high concentrations of protein (NRC 2011). The digestive tract of crustaceans is essentially an internal tube and generally divided into three functional segments: foregut (a tubular esophagus and a stomach), midgut (a simple tubule with associated ceca and the hepatopancreas), and hindgut (rectum and anus; Fig. 9.1). The esophagus joins the mouth to the stomach [an anterior chamber (the gastric mill apparatus) and a posterior chamber]. The anterior chamber functions in mastication (cutting and grinding) of the ingested food, whereas the posterior chamber keeps food particles from leaving the stomach until the gastric mill has reduced them into a small size (McGaw et al. 2013). Secretion of HCl by gastric epithelial cells results in acidic conditions in stomach fluids (pH = ~ 4 or higher) during digestion. The food particles leave the posterior chamber of the stomach to enter the anterior midgut and then the hepatopancreas (also called the midgut gland or digestive gland; a branching array of blind-ended tubules lined with an epithelium) that connects to the anterior midgut via ducts (Ceccaldi and Ceccaldi 1989). The hepatopancreas secretes digestive enzymes that flow into the midgut and then retrograde into the stomach. Much extracellular digestion of foods and absorption of digestion products (free AAs, as well as di- and tri-peptides) or simple nutrients into the hemolymph occur within the hepatopancreas (Buarque et al. 2009, 2010; Fernández et al. 1997; Saborowski et al. 2006). The midgut plays a relatively minor role in the digestion and absorption of nutrients. Di- and tri-peptides (the major products of protein digestion) are taken up by the epithelial cells of the hepatopancreas via the apical-membrane peptide transporter-1, whereas free AAs are taken up by these cells via various sodium-dependent and independent transporters (Wu 2013). Within the absorptive cells, the small peptides are hydrolyzed by peptidases (including proline peptidases) to free AAs. AAs that are not metabolized by the hepatopancreatic cells enter the hemolymph. Undigested food particles and unabsorbed nutrients from the terminal midgut enter the rectum to form feces, which leaves the gut through the anus.
Scheme of the digestive tract of crustaceans. The digestive tract of crustaceans consists of the foregut (a tubular esophagus and a stomach), midgut (a simple tubule with associated ceca and the hepatopancreas), and hindgut (rectum and anus). Cutting and grinding of the ingested foods, as well as their initial digestion (by digestive enzymes from the hepatopancreas) occurs in the anterior chamber of the stomach (the gastric mill apparatus). The food particles enter the anterior midgut and then the joining hepatopancreas. The hepatopancreas secretes digestive enzymes and is the major site for the extracellular digestion of foods and absorption of digestion products or simple nutrients into the hemolymph. Undigested foods and unabsorbed nutrients enter the rectum and exit the gut through the anus
Studies with the southern brown shrimp Farfantepenaeus subtilis have shown the highest activity of aminopeptidase in the presence of alanine-, arginine-, lysine- or leucine-β-naphthylamide as a substrate (Buarque et al. 2010). Proteinases and peptidases activities in crustaceans are modulated by several internal and external factors (Saborowski et al. 2006). These enzymes have an optimum pH around 8 (Buarque et al. 2009; Dionysius et al. 1993). Moreover, the enzyme activities are also influenced by ontogenetic events (Lemos et al. 2000), life stages (Lee et al. 1984), hormones (Gorell and Gilbert 1969; Thomson et al. 1971), the molting cycle (Gimenez et al. 2001, 2002), and diet composition such as protein levels and sources (Lee et al. 1984; Brito et al. 2000; Muhlia-Almazan et al. 2003). All of these results indicate that crustaceans can adapt to changes in their diets and physiological states.
Crustaceans have a high ability to digest a wide range of animal- and plant-source proteins. In whitelegs shrimp, the digestibilities of AAs are greater than 92% (Cruz-Suárez et al. 2009). Proteins from animal resources are better digested than plant proteins in several crustacean species (Forster and Gabbott 1971; Fenucci et al. 1982). A decrease in the digestibility of AAs was observed with an increase in the graded dietary level of rice protein concentrate from 0% to 100% (i.e., 25, 50, 75, and 100%) (Oujifard et al. 2012). The low digestibility of AAs in plant ingredients results from the presence of inhibitors of proteinases and peptidases (Garcia-Carreo et al. 1997; Oujifard et al. 2012). To solve this problem, heating and fermentation are the common ways to remove or reduce these anti-nutritive factors in plant-source feedstuffs (NRC 2011). Moreover, feed additives, such as organic acids and enzymes, can be added to crustacean feeds to improve the utilization of alternative dietary protein sources. In whiteleg shrimp, dietary organic acids can modify the activities of digestive enzymes and the digestibility of dietary protein possibly due to changes in gastric pH and intestinal microbes (Silva et al. 2016). Supplementation with proteases to low fishmeal diets has been reported to improve the growth or feed utilization of some shrimp (Li et al. 2016; Song et al. 2017) and crab (Chowdhury et al. 2018) species.
9.3 The Free AA pool in Crustacean Tissues
Crustaceans have an open circulatory system, where nutrients, oxygen, hormones, and cells are distributed in the hemolymph. Therefore, all of their blood is not contained within vessels, but rather blood is drawn into the heart through holes called the ostia, pumped out again to circulate through tissues, and return to the heart (Wirkner and Stefan 2013). After the hepatopancreas absorb small peptides and free AAs through its single-cell layer of epithelial cells into the hemolymph, AAs participate in metabolic pathways in the whole body as the building blocks of proteins and peptides, substrates for ATP production, and precursors for the syntheses of low-molecular-weight bioactive substances (e.g., NO, neurotransmitters, and thyroid hormones), signaling molecules (Li et al. 2007; Wu 2013). The concentrations of free AAs in most crustacean tissues are higher than those in vertebrate tissues. Table 9.1 shows the concentrations of AAs in the hemolymph of shrimp. The major free AAs in crustaceans are glycine, glutamine, alanine, arginine, and taurine, which may vary among different species (Fig. 9.2; Shinji and Wilder, 2012; Miyagawa et al., 1990). All of these AAs are abundant in animal-source feedstuffs (Li and Wu 2018; Li and Wu 2020a), whereas all plant-source feedstuffs lack taurine and contain low concentrations of glycine (Hou et al. 2019; Li and Wu 2020a; Li et al. 2011a). Of note, arginine phosphate is present in some crustaceans, such as shrimp. Concentrations of free AAs in their tissues are affected by diets and environmental factors, such as salinity (Shinji and Wilder 2012), ammonia levels (Chen et al. 1994), temperature (Rao and Ramachandra 1961), and intracellular protein turnover (Wu 2013). Free AAs in tissues are in dynamic equilibrium with the protein pool. On the molar basis, glycine is the most abundant free AA in the hemolymph (a fluid analogous to the blood in vertebrates) and the whole body of the whiteleg shrimp (Litopenaeus vannamei), followed by alanine, taurine, arginine, glutamine and proline in the hemolymph and by arginine, taurine, proline, glutamine, and alanine in the whole body, in descending order (Table 9.1). Of note, in the whole body of the shrimp, most of free AAs represent about 5% (g/g) of their corresponding total AAs (free plus peptide-bound), but free glycine and free arginine account for 30.5% and 23.3% of the total AAs, respectively. In the whole body of the whiteleg shrimp, the ratio of total free proteinogenic AAs (72.7 mg/g of dry weight) to the total proteinogenic AAs (721.5 mg/g of dry matter) is 1.0:10.0 (Table 9.1). The high abundance of free AAs is consistent with their important role in the maintenance of osmolality and metabolism in shrimp.
Different metabolic pathways for the catabolism of amino acids converge to common intermediates that feed into the Krebs cycle, lipogenesis, and glucogenesis pathways in crustaceans. G6Pase glucose-6-phosphatase, PCL pyruvate carboxylase, PEPCK phosphoenolpyruvate carboxykinase, PDH pyruvate dehydrogenase, PK pyruvate kinase, LDH lactate dehydrogenase, HK hexokinase, PFK-1 phospho-fructokinase-1, G-3-P glyceradehyde-3-phosphate, α-KG α-ketoglutarate, OAA oxaloacetate
9.4 Protein Synthesis in Crustacean Tissues
The process of protein synthesis in both crustaceans and other animals include five steps: (1) gene transcription; (2) initiation of translation; (3) peptide elongation; (4) termination, and (5) posttranslational modifications (Wu 2013). In crustaceans, the rate of protein synthesis is generally higher in the hepatopancreas, followed by the heart, gill, tail and claw muscle in descending order (Houlihan et al. 1990; Mente et al. 2011). Among these tissues, protein synthesis in skeletal muscle is crucial for shrimp growth and production. A postecdysial increase in muscle fiber length and the associated increase in the sarcomere number are accompanied by an increase in muscle protein synthesis (Carter and Mente 2014). The rate of muscle protein synthesis (Ks, the percentage of the protein mass synthesized per day) is 1.26%/day at 27 °C in whitelegs shrimp (Mente et al. 2002), 1.15%/day at 15 °C for shore crabs (Carcinus maenas; El Haj and Houlihan 1987), and 0.9–1.4%/day at 30 °C in brown tiger prawn (Penaeus esculentus; Hewitt 1992). For comparison, the rate of protein synthesis is lower at 0.3–0.4%/day in the claw, leg and abdominal muscles of the American lobster (Homarus Americanus, Haj et al. 1996). The rate of muscle protein synthesis also varies with muscle fiber type and muscle type. Slow-type tonic muscle fibers have a rate of protein synthesis that is 2.1 times greater than fast-type phasic fibers (El Haj and Houlihan 1987). Protein synthesis plays a vital role in the growth, development, health and survival of animals (Carter and Mente 2014; Li et al. 2020c). For example, vitellogenesis (synthesis of vitellogenin as a precursor protein of egg yolk in the blood or hemolymph of females) occurs in the ovary and hepatopancreas to support reproduction (Tseng et al. 2001). Increases in protein synthesis in the midgut gland after feeding enhance the secretions of digestive enzymes for the digestion of dietary nutrients (Houlihan et al. 1990).
The growth of crustaceans depends on ecdysis (also known as molt), which refers to the replacement of their rigid carapace with a new and larger one generated underneath the former exoskeleton that consists primarily of chitosan (Comeau and Savoie 2001). Therefore, protein synthesis is highly related to the molt cycle. The highest rate of protein synthesis occurs during the premolt stages in shore crabs (El Haj and Houlihan 1987). Moreover, protein synthesis is also influenced by several abiotic and biotic factors, such as hormones (Carter and Mente 2014), starvation and re-feeding (Pellegrino et al. 2013), dietary composition, hypoxia, hyperoxia, temperature, salinity, and other environmental factors (Intanai et al. 2009; Mente et al. 2002, 2003). For example, the rates of protein synthesis, survival, and specific growth are higher in shrimp fed diets with high quality proteins than in shrimp fed low quality proteins (Mente et al. 2002). Of note, muscle protein synthesis is substantially higher in brown tiger prawn (Penaeus esculentus) fed a 50%-protein diet than a 30%- or 40%-protein diet (Hewitt 1992). Similar to other animals, protein synthesis requires a large amount of energy in crustaceans and accounts 20% to 37% of oxygen consumption in the shore crab (Houlihan et al. 1990). Therefore, starch and lipids are often included in artificial diets for crustacean as an energy source to spare protein and improve protein deposition. The protein-sparing effect of dietary digestible carbohydrate has been reported in Litopenaeus vannamei (Wang et al. 2015). In crabs fed a high-digestible carbohydrate diet, the rate of muscle protein synthesis measured with 14C-leucine has been reported to be 2.3-fold greater than that in crabs fed a high protein diet (Pellegrino et al. 2013). This conclusion, however, may not be valid because leucine is extensively catabolized by skeletal muscle and therefore, is not an appropriate tracer for the measurement of its protein synthesis (Wu 2013).
Substantial amounts of collagens are present in tissues of crustaceans, including the shell (consisting of 22–24% dry matter) and skeletal muscles of shrimp. For example, shrimp shell consists of the following (dry matter basis): 25–40% protein, 15–20% chitin, 45–50% calcium carbonate, and 15–40 mg astaxanthin/kg, with the protein comprising of 60–75% collagen, 4–5% elastine, and 20–35% keratine (Immaculada et al. 2009). Kimura and Tanaka (1986) reported that the collagen content in the skeletal muscles of three species of crustaceans (giant river prawn, fleshy prawn and spiny lobster) was 2.4% to 2.6% of total protein. The content of collagen as the percentage of total protein in the muscles of crustaceans is as follows: 1.1–2.2% in the shrimp (Trachypenaeus curvirostris, Palaemon paucidens, and Pandalus borealis), 2.6–2.9% in prawn (Penaeus japonicas), 2.5–2.7% in lobster (Panulirus Iongipes), 0.2–0.8% in crabs (Charybdis japonica, Portunus trituberculatus, Chionoecetes opilio ♂, Chionoecetes opilio ♀, and Erimacrus isenbeckii), 3.4% in crayfish (Procambarus clarkia), and 5.9–6.2% in squilla (quilla Oratosquilla oratoria) (Yoshinaka et al. 1989). For comparison, collagen represents 2% of total protein in beef skeletal muscle (Wu et al. 2016). The AA composition and solubility of the major collagen in the crustacean muscles are similar to those of Type V collagen in vertebrate skeletal muscles (Yoshinaka et al. 1989). As a major constituent of the connective tissue, collagen supports the structure, locomotion, mechanical strength of the muscles, bones and fin in crustaceans. Based on the content of 4-hydroxyproline in the whole body of shrimp (Table 9.1), the abundance of collagen in the whole body of shrimp appears to be 66% lower than that in vertebrates (Wu 2013).
9.5 Catabolism of Energy Substrates for ATP Production in Crustacean Tissues
The requirement of crustaceans for dietary protein has been reported to be 30–60%, depending on their species, developmental stage, and production conditions (Halver and Hardy 2002; Cuzon et al. 2004; Unnikrishnan and Paulraj 2010; Jin et al. 2013; Mente 2006). However, the rate of retention of dietary nitrogen is only about 17–30%, which is even lower than that for some fish species (Bulbul et al. 2016; Panini et al. 2017; Qiu et al. 2017). In addition, the oxygen:nitrogen ratio (the ratio of oxygen consumed to nitrogen excreted; O/N, mol/mol) is often employed in energetic studies as an indicator for the use of organic substrates (i.e., lipids, carbohydrates or proteins) as metabolic fuels. An oxygen:nitrogen ratio in shrimp is < 40 (Coelho et al. 2019; Comoglio et al. 2004; Zhang et al. 2019), indicating AAs may be their predominant energy substrates. The limited utilization of glucose by penaeid shrimp has been reported in some studies, and the recommended levels of digestible carbohydrates starch in diets are generally less than 20% (Guo et al. 2006). Rosas et al. (2002) have suggested that shrimp (Litopenaeus vannamei) are well adapted to dietary protein as a source of energy because of its limited ability to use high carbohydrate. In crabs (Neohelice granulate), dietary proteins have been suggested as an important source of energy (Pellegrino et al. 2013). AAs (especially alanine) are important substrates in the gill tissue of the blue crab, and appears to play a role in both short-term cell volume regulation and long-term osmoregulatory processes (Pressley and Graves 1983).
In all animals, individual AAs have their own catabolic pathways because of their different structures (Wu 2013). However, the catabolism of many AAs shares a number of common steps to generate pyruvate, oxaloacetate (OAA), α-Ketoglutarate (α-KG), fumarate, succinyl-CoA, and acetyl-CoA (Fig. 9.2). For example, the carbon backbones of some AAs are converted to α-KG by glutamate dehydrogenase (GDH) and transaminases. Aminotransferases have been reported in the skeletal muscle, gill and hepatopancreas of crabs (Carcinus maenas; Chaplin et al. 1967). The catabolism of glutamine involves its deamination by phosphate-activated glutaminase to produce glutamate and ammonia. The major end product of AA metabolism in crustaceans is ammonia, which represents more than 50% of their nitrogenous wastes (Regnault 1987). Free AAs are the second most important nitrogenous waste since they account for 10–25% of the total excreted nitrogen in different species (Regnault 1987). Urea and uric acid are nitrogenous end-products but are usually excreted by crustaceans in small amounts (< 10%).
To generate ATP, the carbon backbone of glutamate, alanine, and aspartate are converted into α-KG, pyruvate, and oxaloacetate by GDH, glutamate-pyruvate transaminase (GPT), and glutamate-oxaloacetate transaminase (GOT), respectively (Wu 2013; Richard et al. 2010; Lu et al. 2015). We found that in both whiteleg shrimp (Litopenaeus vannamei) and blue crabs (Callinectes sapidus), AAs, such as aspartate, glutamine and glutamate, provide the bulk of energy but the oxidation of glucose for ATP production is very limited in their skeletal muscle and ovaries (Table 9.2 and Fig. 9.3). In both animal species, aspartate is the predominant metabolic fuel among the AAs (Fig. 9.3). Similarly, both GPT and GOT are present in different tissues (hemolymph, hepatopancreas, gills and skeletal muscle) of shrimp (Fenneropenaeus indicus), with the activity of GOT being 2–3 times higher than that of GPT in the same tissue (Mohankumar and Ramasamy 2006). GDH is largely responsible for the production of ammonia from AAs in crustaceans (Fernández-Urruzola et al. 2011). In whiteleg shrimp (Litopenaeus vannamei, the activity of GDH increases with increasing the dietary protein level from 25% to 50% (Li et al. 2011b). The measurement of GDH activity in the crude homogenates of the shrimp (Crangon crangon) suggests that the oxidative deamination of glutamate by GDH may account for all the ammonia excretion by this species (Batrel and Regnault, 1985). GDH transcripts are detected in most tissues of Chinese mitten crabs (Eriocheir sinensis; Wang et al. 2012), freshwater prawn (Macrobrachium rosenbergii; Chakrapani et al. 2017), whiteleg shrimp (Litopenaeus vannamei; Li et al. 2009a), and mud crabs (Scylla paramamosain; Lu et al. 2015).
ATP production from the oxidation of individual substrates in tissues (the midgut, hepatopancreas, gill plus skeletal muscle) of the 15-g whiteleg shrimp Litopenaeus vannamei (Panel A) and the 150-g swimming crab Portunus trituberculatus (Panel B). The rates of ATP production were calculated from the data in Table 9.1, as described by Li et al. (2020b).1 The contribution of an individual substrate to total ATP production in tissues incubated in the presence of a mixture of substrates. Glc glucose, Pa palmitate, Glu glutamate, Gln glutamine, Ala alanine, Asp aspartate, Leu leucine
Although AAs are the major energy sources for crustaceans, the rates of their oxidation to CO2 vary among different tissues and species. For example, the specific activity of GPT in the skeletal muscle and gills of black tiger shrimp (Penaeus monodon) is about 3-times the value measured in the hepatopancreas (Richard et al. 2010). The activity of GDH is also relatively low in the hepatopancreas of black tiger shrimp, suggesting a minor role of this tissue in glutamate catabolism (Richard et al. 2010). Likewise, although GDH is expressed in the skeletal muscle, epithelium, eyestalk, hepatopancreas, and gill of Pacific white shrimp, its enzymatic activity in the hepatopancreas is much lower than that in the other four tissues (Li et al. 2009a). Similarly, the rate of CO2 production from aspartate is 3–6 times higher than that from glucose in the intestine and skeletal muscle of whiteleg shrimp, but the rate of oxidation of these two substrates is quantitatively comparable in the hepatopancreas (Table 9.2). Of particular note, in blue crabs, palmitate is the primary energy source for the midgut and hepatopancreas, with the rate of its oxidation being substantially higher than that of any AA substrates (Table 9.2). In both whiteleg shrimp and blue crabs, AAs are the most important energy substrates for ATP production in skeletal muscle. Richard et al. (2010) also reported that skeletal muscle has high activities of GPT and GDH for glutamate catabolism in black tiger shrimp.
Phosphate-activated glutaminase may be quantitatively the major enzyme for initiating glutamine catabolism in crustaceans. For example, in a fresh-water crab (Paratelphusa hydrodromus), a high correlation between glutaminase activity and ammonia excretion rate has been observed at various salinity levels (Krishnamoorthy and Srihari, 1973). In whiteleg shrimp, the rate of the oxidation of glutamine is similar to or even higher than that of glutamate in various tissues (Table 9.2). However, the organs (except for the gill) of blue crabs oxidize much more glutamate than glutamine, which may be attributed to the low glutaminase activity. The gill of blue crabs oxidized both glutamate and glutamine at relatively high rates. This is in agreement with a previous report that glutaminase activity is most active in the gills of three crab species, indicating that this organ is an active site of glutamine hydrolysis and glutamate degradation (King et al. 1985). Skeletal muscles of crabs have a high activity of glutamine synthetase and may be the major site for glutamine synthesis in the body (King et al. 1985). Interestingly, the activities of GDH and glutaminase are undetectable or very low in the hepatopancreas of the three crab species studied (King et al. 1985). Similarly, our results indicated that glutamine and other AAs are not the primary energy substrates in the hepatopancreas of blue crabs. To date, our knowledge of AA catabolism in crustaceans is very limited (Table 9.3).
9.6 Glucogenesis and Lipogenesis in Crustaceans
AAs can be the precursors for glucose and lipid syntheses to provide the body with glucose and lipids (Fig. 9.2). Gluconeogenesis and its related key enzymes [e.g., phosphoenolpyruvate carboxykinase (PEPCK)] have been demonstrated in different tissues of crustacean species, such as the skeletal muscle, hepatopancreas, and gill (Reyes-Ramos et al. 2018; Vinagre and Da Silva 2002; Schein et al. 2004). The conversion of 14C-alanine and 14C-glycine into glucose occurred in the hepatopancreas, gill and skeletal muscle of crabs (Chasmagnathus granulate; Oliveira et al. 1997; 2004; Vinagre and Da Silva, 2002; Martins et al. 2011). The in vitro experiments also showed that these tissues were able to incorporate 14C-glycine to lipids (Vinagre and Da Silva 2002; Martins et al. 2011). The presence of gluconeogenesis from AAs in the skeletal muscle of crabs is interesting, because such a biochemical pathway is absent from terrestrial mammals and birds (Wu 2018).
Glucose and lipids are important energy sources for crustaceans under certain physiological conditions or stresses (Reyes-Ramos et al. 2018). For example, intramuscular lipids are used for ATP production in crabs in the fall and winter (Kucharski and Da Silva 1991). Dietary AAs are converted into lipids in skeletal muscle when crabs (N. granulate) are fed diets with high protein content, and the intramuscular lipids serve as an important energy reserve for the animals during osmoregulation and in the winter (Pellegrino et al. 2013). Moreover, gluconeogenesis and lipogenesis contribute to the adjustment of the intracellular concentration of nitrogenous compounds to withstand changes in the salinity of the surrounding water (Martins et al. 2011). Therefore, both gluconeogenesis and lipogenesis from AAs are important for the growth and health of crustaceans exposed to different levels of salinity. Previous experiments indicated that the incorporation of [14C]alanine into glucose in the jaw muscles of crabs submitted to a hyperosmotic shock increased by 77% over the control group (Schein et al. 2004). In the posterior gills of N. granulata subjected to hyper- and hypo-osmotic stresses, the formation of 14C-lipids from 14C-glycine increased at 72 h after the treatment, but the activity of PEPCK (a rate-controlling enzyme for glucose synthesis) decreased (Martins et al. 2011). Similarly, the rate of lipid synthesis in shrimp exposed to both hypo- or hyper-osmotic conditions was slightly enhanced with an increase in FAS activity, when compared with a normo-osmotic condition (Chen et al. 2014). Thus, the partition of AAs toward the synthesis of either lipids or glucose in crustaceans, depending on nutritional, physiological and environmental factors.
9.7 Syntheses of Bioactive Metabolites in Crustaceans
In addition to the syntheses of proteins, lipids and glucose, AAs are the precursors of many low-molecular-weight substances with important and diverse biological roles in animals (Wu 2013, 2018). These products of AAs include NO, billirubin, carnosine and related dipeptides, carnitine, catecholamines, neurotransmitters, creatine, glucosamine, glutathione, heme, histamine, polyamines (putrescine, spermidine and spermine), purines, and pyrimidines, and are produced in a tissue-specific manner (He and Wu 2020; Wu 2013). Polyamines, which are synthesized from methionine and arginine, play vital roles in chromatin structure, gene transcription and translation, DNA stabilization, signal transduction, cell growth, and proliferation in animals. Polyamines are also involved in the regulation of osmotic and ionic homeostasis by interacting directly with the Na +, K+-ATPase enzyme in crabs (Lovett and Watts 1995). GSH is formed from cysteine, glutamate, and glycine via two ATP-dependent enzymes in the cytosol: γ-glutamyl-cysteine synthetase and glutathione synthetase (Wu 2013). Glutathione exerts both growth-promoting and immunostimulatory effects in Litopenaeus vannamei (Xia and Wu 2018). L-Phosphoarginine (arginine phosphate), which is generated from arginine and ATP by arginase kinase, exists in skeletal muscles from various invertebrate animals. Of particular note, concentrations of L-phosphoarginine in the skeletal muscles of some crustacean species (e.g., crayfish) can be up to 83 to 100 mM (Ennor et al. 1956; Marcus and Morrison 1964). We found that the concentration of phosphoarginine in the hemolymph of Litopenaeus vannamei was about 40 nmol/ml. The main function of phosphoarginine is to store biological energy like phosphocreatine in animals (Wu 2013). Phosphoarginine also plays a role in the metabolic support of the gill’s function to regulate osmoregulation in crustaceans (Holt and Kinsey 2002; Kotlyar et al. 2000). However, knowledge about the metabolism and functions of these AA metabolites in most crustacean species is limited.
9.8 Functions of AAs in the Culture of Crustacean Species
9.8.1 Molt and Survival
The growth of crustaceans occurs through the shedding of an old exoskeleton (shell) and the formation of a new exoskeleton, and is greatly influenced by the extended intermolt period (molt frequency) and the molt increment (carapace and body weight growth at molt). Moreover, the survival of some crustacean species is highly dependent on the molting processes. For example, many deaths are due to the presence of calcium deposits embedded on and in the inner surface of the exuvial exoskeleton, which is known as the molt death syndrome (Bowser and Renée 1981; Wang et al. 2016). The molting process is under the control of several regulatory hormones, environmental factors (Hosamani et al. 2017), and diets (Kibria 1993; Millikin 1980). The cumulative molts in crabs are strongly affected by voluntary feed and protein intakes, indicating that AAs are required for tissue growth especially during the postmolt period (Nguyen et al. 2014). AAs have been suggested as important factors for molting processes through energy provision for ecdysis, osmoregulation, collagen synthesis, and the removal of the exokeleton (Dooley et al. 2002), as well as the regulation of hormone release (Qi et al. 2019). For example, free proline and glycine may be used as metabolic fuels during ecdysis (Claybrook 1983) and substrates for the synthesis of the new exoskeleton in the later premoult (Yamaoka and Skinner 1976). Concentrations of a molt hormone, ecdysterone, are increased in the serum of crab (Eriocheir sinensis) receiving dietary supplementation with arginine (Qi et al. 2019). The same species have higher survival rates and molt frequency when fed diets containing adequate lysine and arginine (Jiang et al. 2005; Qi et al. 2019). More details about the functions of AAs in the molting of shrimp are presented in Table 9.4.
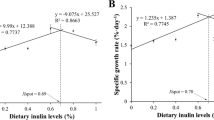
Osmoregulation is an essential physiological process for the majority of aquatic crustaceans since many of them have been widely farmed in inland and oceans with different environmental conditions (Romano and Zeng 2012). As a result, the crustaceans usually are faced with numerous stresses such as low or high salinity, high density, and hot or cold temperatures. Free AAs in the hemolymph appear to play important roles in ATP production (Pressley and Graves 1983). Their levels generally increase in the hemolymph under various stress conditions (Shinji and Wilder 2012). Of particular note, some free AAs (e.g., glutamate, proline, glycine, alanine, taurine and arginine) are known to be involved in the active adjustment of intracellular osmoregulation in marine invertebrates (Tan et al. 1981; Chen and Chen 2000; Liu et al. 2012; 2018; Chakrapani et al. 2017). A recent review has indicated that an increase in protein levels in the diet of Litopenaeus vannamei is a practical method of nutritional modulation to increase their production at extreme high and low salinities (Li et al. 2015). After an acute salinity change, the survival of whiteleg shrimp is increased with increasing the dietary glycine level from 2.26% to 2.70% (Xie et al. 2014).
AAs play an important role in controlling osmoregulation in crustaceans because their metabolic enzymes such as transaminase (Koyama et al. 2018), GDH (Lu et al. 2015) and arginine kinase (Holt and Kinsey, 2002; Kotlyar et al. 2000) are regulated by salinity levels. In the abdominal muscle of the kuruma shrimp, the concentrations of alanine and glutamine are elevated in response to increased salinity in association with a decrease in GPT gene expression and an increase in GDH gene expression (Koyama et al. 2018). Acute salinity stress increases GDH expression, as well as the syntheses of glutamate, proline and alanine in the muscle of the Chinese mitten Crab (Eriocheir Sinensis) to meet the demand for osmoregulation at hyperosmotic conditions (Wang et al. 2012). Consistent with this finding, a reduction in 14C-alanine oxidation appears to be one of the mechanisms responsible for the increase of the free AA pool in the hepatopancreas of crabs (Chasmagnathus granulate; Schein et al. 2005) during hyperosmotic stress. A hyperosmotic stimulus also induces proline synthesis from glutamate in Tigriopus californicus (Burton 1991).
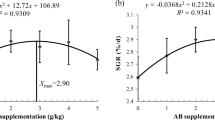
Much evidence shows that AAs play a central adaptive role in crustaceans during exposure to cold, starvation and ammonia (Chen et al. 1994, 2000; Zhou et al. 2011). For example, the accumulation of proline and alanine in the hepatopancreas seems to be a common response to cold stress in some invertebrates (Hanzal and Jegorov 1991; Fields et al. 1998; Liu et al. 2018). Increasing the content of proline from 2.02% to 2.6% in low (15%) fishmeal diets improved the tolerance of Litopenaeus vannamei to ammonia stress (Xie et al. 2015a, b). Moreover, shrimp fed diets with a deficiency of lysine had the greatest incidence and severity of neural lesions when they were challenged with subsequent stress exercises (Katzen et al. 1984). Clearly, it is imperative to study the functions of specific AAs in crustaceans exposed to different stresses.
9.8.2 Growth and skeletal muscle development
AAs have been traditionally classified as essential (EAAs) or nonessential (NEAAs) for animals, including crustaceans. The diets of crustacean species must contain ten EAAs for survival and growth: arginine, methionine, valine, threonine, isoleucine, leucine, lysine, histidine, phenylalanine, and tryptophan, all of which are not synthesized de novo by eukaryotic cells (NRC, 2011). These AAs are considered as limiting nutrients in commercial feed formulas and are indispensable for the growth, development and survival of the animals. If one of the EAAs is deficient, it will limit the use of all AAs for intracellular protein synthesis, therefore increasing their oxidation to CO2. For example, a low rate of retention of dietary protein in the Litopenaeus vannamei results from a deficiency of lysine (Xie et al. 2012) or threonine (Zhou et al. 2013) in their diets. Purified or semi-purified diets have been employed to determine both qualitative and quantitative requirements of crustaceans for dietary EAAs. Lysine, arginine, and methionine are regarded as the most limiting factors for whole-body growth. Most of these studies were based on the growth performance of select crustaceans as shown in Table 9.5. To date, NEAAs have been recommended to be included in the diets of all animals (Wu 2013). This revises the classical “ideal protein” concept to formulate balanced diets for improving protein accretion, feed efficiency, and health in animals (Wu 2018). A recent study indicated that weight gains and specific growth rates were increased in juvenile Pacific white shrimp receiving dietary supplementation with glycine (Xie et al. 2014). Many factors, such as feeding regime, stocking density, water quality, and other rearing conditions, may affect the requirements of aquatic organisms for dietary AAs (Façanha et al. 2016; Zhang et al. 2018).
AAs can promote muscle development and protein synthesis by either providing the building blocks or stimulating signaling pathways. In mammals, dietary supplements with branched-chain amino acid (BCAAs) alone elicits an anabolic response (e.g., muscle protein synthesis; Wolfe 2017; Wu 2013). An evolutionally conserved protein kinase, mechanistic target of rapamycin (mTOR), is the master regulator of protein synthesis and cytoskeleton remodeling, as well as intracellular protein degradation via autophagy (Wu 2013). AAs, such as leucine, arginine, glutamine, glycine, tryptophan and valine, activate the mTOR cell signaling to initiate protein synthesis in skeletal muscle and intestine (Li et al. 2011c; Wu 2018). The mTOR plays an important role in the regulation of growth, molting, cell differentiation, and nutrient metabolism in crustacean species (Abuhagr et al. 2014; Shyamal et al. 2018; Wu et al. 2019). In the Chinese white shrimp (Fenneropenaeus chinensis), intraperitoneal administration of leucine and arginine stimulated the expression of fch-TOR and activated the mTOR signaling pathway in skeletal muscle (Sun et al. 2015a). Functional AAs are expected to enhance the growth, survival, and productivity of crustaceans, as reported for terrestrial mammals and birds (Wu 2018).
9.8.3 Release of Hormones
Similar to terrestrial animals, hormones in crustaceans are messengers that help to regulate their physiological states and functions, such as temperature, satiety, nutrient and energy metabolism, growth, development, and reproduction. For example, AAs regulate muscle growth not only through direct actions on myogenic regulatory factors and mTOR signaling, but also indirectly via the growth hormone/insulin-like growth factor (IGF) axis. Growth hormone in serum and the expression of IGF2 in the hepatopancreas of the Chinese mitten crab (Eriocheir sinensis) were significantly enhanced by dietary supplementation with arginine (Qi et al. 2019). In addition, the concentrations of insulin and neuropeptide Y in the blood of Litopenaeus vannamei were increased in response to dietary supplementation with GABA (Xie et al. 2015b). Tryptophan is the precursor of the monoaminergic neurotransmitter serotonin (5-hydroxytryptamine). In mud crabs, tryptophan supplementation contributed to a significant increase of serotonin in the hemolymph, thus suppressing the agonistic behavior of mud crabs during aggressive encounters and improving their survival (Laranjia et al. 2010). In the Chinese mitten crab (Eriocheir sinensis), dietary supplementation of tryptophan can promote limb regeneration by regulating regeneration-related gene expression and the digestion of foods within the hepatopancreas, which may be related to the enhanced levels of melatonin and the binding of serotonin and dopamine to their corresponding receptors (Zhang et al. 2019). In the juvenile Litopenaeus vannamei, dietary supplementation with tryptophan was beneficial to improve its growth performance possibly by mediating serotonin and GABA signaling pathways (Sun et al. 2015b).
9.8.4 Immune and Antioxidant Responses
Proper nutrition is critical not only to achieve optimal growth rates but also to maintain the health of cultured aquatic animals (Pohlenz and Gatlin 2014). AAs are essential components of the cells and tissues of the immune system, and play a vital role in the immunity of mammals, fish and crustacean species (Trichet 2010; Li et al. 2007). Like other invertebrates, crustaceans lack adaptive immune systems and depend solely on the innate immune system to defend against infectious pathogens (Vazquez et al. 2009). The prophenoloxidase activating system (the proPO-system) and associated factors are important mediators of immunity in crustaceans. The proPO is activated by substances of microbial origins (e.g., β-1,3-glucans, lipopolysaccharides, and peptidoglycans) to stimulate the circulating hemocytes (large granular hemocytes, small granular hemocytes, and hyaline cells). These cells play important roles not only through direct sequestration and killing of infectious agents but also by synthesis and exocytosis of a battery of bioactive molecules (Söderhäll and Cerenius 1992). Along with hemocytes, crustaceans possess plasma proteins or humoral factors, such as lectin, α-2 macroglobulin responsible for clotting, lipopolysaccharide-binding protein, β-glucan-binding protein, antimicrobial peptides, and lysosomes (Trichet, 2010; Vazquez et al. 2009). As the nitrogenous precursor for NO, arginine has a beneficial effect on tissue oxygenation and immune function for animals (Wu et al. 2009), including crustaceans (Qi et al. 2019; Zhu et al. 2009). Thus, increasing the dietary arginine content from 1.72% to 3.72% improved the growth, feed efficiency survival, immunity, and disease resistance to Aeromonas hydrophila in the juvenile Chinese mitten crab (Qi et al. 2019). Similarly, dietary supplementation with tryptophan to Chinese mitten crabs increases their survival after a challenge with pathogens (Yang et al. 2019).
Reactive oxygen species (ROS) are highly reactive molecules that may contribute to radiation-induced cytotoxicity (e.g., chromosome aberrations, protein oxidation, and muscle injury), as well as metabolic and morphologic changes (e.g., increased muscle proteolysis and dysfunction of the central nervous system) in animals (Fang et al. 2002). Endogenous antioxidant defenses are crucial for the control of ROS production and the prevention of oxidative damage in cells. The principal defense systems against oxygen free radicals are superoxide dismutase, glutathione, glutathione peroxidases, glutathione reductase, catalase (a heme-containing enzyme), and antioxidant nutrients (Fig. 9.4). AAs and their derivatives are important antioxidant nutrients for crustacean species, as for terrestrial animals (Fang et al. 2002). For example, glutathione, which is the most abundant thiol-containing substance of low molecular weight in cells, is synthesized from glutamate, cysteine, and glycine. Dietary supplementation with glutathione to Litopenaeus vannamei enhances immunity and antioxidant defenses (Xia and Wu 2018). Glycine supplementation also improves the resistance of the shrimp to acute salinity challenge (Xie et al. 2014). More details about the functions of AAs and their derivatives in immunity and antioxidant responses are summarized in Table 9.6. Adequate AA nutrition plays a crucial role in protecting crustaceans from infectious and metabolic diseases, such as the white spot syndrome caused by viral infection (Corteel 2013), bacterial infection (Zhang et al. 2018), and oxidant-induced tissue damage (Dong et al. 2018; Li et al. 2020a,c).
Roles of amino acids and their metabolites as antioxidants in crustaceans. Cys cystine, CAT catalase, Gln glutamine, Glu glutamate, Gly glycine, GPx glutathione peroxidase, GR glutathione reductase, GSH glutahione, GSSG oxidized glutathione, Lys lysine, Leu leucine, LH lipids (unsaturated fatty acids), LOOH lipid hydroperoxide, Met methionine, SOD superoxide dismutase. (Adapted from Fang et al. 2002)
9.8.5 Spawning and Larval Development in Crustaceans
Most crustaceans have separate sexes. The weight of the gonad of maturing shrimp or crabs increase during their reproductive development, which prepares sufficient nutrients needed for the formation of egg yolk or spermatogenesis. This process is important to sustain the normal development of the embryos and the production of pre-feeding larvae in crustacean species (Islam et al. 2010). Optimum development of ovaries is necessary for maximum crab production as they are a popular edible tissue (Wu et al. 2020). Vitellogenesis is the process of yolk formation, which plays the central role in ovarian development and reproduction (Subramoniam 2011). Vitellogenin is an egg yolk precursor protein and is synthesized in the hepatopancreas and gonad tissues in decapod crustaceans (Tsukimura 2001). Its synthesis is under the control of estradiol-17β and other neuropeptidic precursors from the nervous system (Fig. 9.5). Furthermore, the hepatopancreas is an important site for the syntheses of vitellogenin and sex steroid hormones. Therefore, the crustacean hepatopancreas is crucial for maximum growth and optimum maturation of ovaries. An unbalanced or incomplete diet causes poor reproductive performance or may even stop animals from reproducing (Woulters et al. 2001). As noted previously, the release of some hormones can be influenced by dietary AA intake. By augmenting the syntheses of egg yolk proteins, hormone peptides and enzymes during maturation and reproduction, AAs are also essential to ovarian development. Indeed, we found that AAs, particularly aspartate and glutamate, are important metabolic fuels in the ovaries of blue crabs (Table 9.2). Thus, increasing dietary provision of AAs (particularly aspartate and glutamate) may beneficially improve reproduction in crustaceans.
Roles of internal and external factors in the regulation of reproduction in female crustaceans. DA Dopamine, 5-HT 5-hydroxytryptamine (serotonin), VIH vitellogenesis-inhibiting hormone, MOIH Mandibular organ-inhibiting hormone, CHH crustacean hyperglycemic hormone, MIH molt-inhibiting hormone, B & TG brain and thoracic ganglia, GOSH gonad stimulating hormone, MO mandibular organ, MF methyl farnesoate, E2, 17β-estradiol, Vg vitellogenin ‘+’ and ‘-’ denotes activation and inhibition, respectively. (Adapted from Pamuru (2019) and Subramoniam (2011), (2017))
Protein and AAs are the main components of dry matter in invertebrate eggs, and support embryonic survival, growth and development (Heras et al. 2000; Xu et al. 2013). Moreover, broodstock nutrition can significantly affect the biochemical profiles of embryos and, therefore, embryogenesis, the quality of larvae and post-larvae (Calado et al. 2005; Harrison 1990). Shrimp (Litopenaeus setiferus) fed a 35%-protein diet had a lower sperm quality than shrimp fed a 45%-protein diet, indicating that dietary AAs are important for its reproductive performance (Gonimier et al. 2006). There are suggestions that a deficiency of dietary protein or certain AAs can induce Daphnia pulex to enter a resting, non-reproductive state (Koch et al. 2009, 2011). Arginine and histidine can enhance not only the number of eggs, but also the development of subitaneous eggs in Daphnia pulex (Fink et al. 2011).
Crabs and shrimp must initiate exogenous feeding after yolk nutrients are no longer sufficient to support the metabolic demand of their larvae. The diet for the larvae relies on either live food (algae and zooplankton) or artificial micro-diets, depending on the life stage. Free AAs are important for the metamorphosis of crustacean larvae by providing them with energy, enhancing protein synthesis in their tissues, and promoting their rapid growth (Bahabadi et al. 2018; Rønnestad et al. 2000). For example, feeding Litopenaeus vannamei larvae taurine-enriched rotifers improved their survival and development (Jusadi et al. 2011). Likewise, the enrichment of Artemia with lysine increased the survival, growth performance, and stress resistance capacity of Litopenaeus vannamei post-larvae (Bahabadi et al. 2018).
9.9 Conclusion and Perspectives
Both EAAs and NEAAs play vital roles in the production of aquatic crustacean species. AAs are substrates for ATP production, as well as the syntheses of lipids, glucose, protein and other bioactive molecules (e.g., NO, creatine, polyamines, GABA, catecholamines, and glutathione). In addition, AAs increase the ability of crustaceans to resist various adverse factors (such as hyperosmotic, ammonia, hot and cold stresses), improve their immune and antioxidant defense systems, and regulate their hormone release, metabolic pathways and osmotic homeostasis. Thus, dietary AAs are vital to the growth, development, reproduction, health, and survival of these aquatic animals. Dietary protein and AAs may also play important roles in spawning and larval development, although traditional studies have focused on the nutrition of lipids.
Based on the recent advances in our understanding of AA metabolism and nutrition in shrimp and crabs, considerations should be given on the use of crystalline AAs (particularly aspartate, glutamate, glutamine, leucine, and glycine) and their alternative sources to feed crustaceans for enhancing their survival and productivity (Huo et al. 2017; Wu 2018). At present, there are several technical difficulties and challenges in the use of crystalline AAs to formulate diets for crustacean species. We would also like to propose solutions to solve the problems.
First, although heating can increase the digestibility of native proteins in plants by unfolding the polypeptide chains and removing the intrinsic protease inhibitors, overheated meals or feeds are undesirable because they have reduced biological values in animals (Wu 2018). The Maillard reaction during the feedstuff heating process damages protein and AAs, leading to reductions in the digestibility of dietary protein and the bioavailability of AAs in feeds (Deng et al. 2005). Animal-source feedstuffs, which contain large amounts and proper balances of AAs (Li and Wu 2020a), can be used as the major source of dietary AAs to reduce the inclusion of plant-source ingredients and fishmeal in the diets of crustaceans.
Second, leaching can lead to the loss of nutrients (including protein and AAs) from the diets fed to crustacean species such as shrimp and crabs, particularly because most of them are slow and continuous eaters. These animals can pick up a feed pellet, cradle it with their maxillipeds (an appendage modified for feeding in crustaceans that is situated in pairs behind the maxillae), and begin to tear and crush the end of a pellet with their mandibles (Obaldo et al. 2006). Therefore, nutritional studies with shrimp and crabs have met with the difficulties of enhancing feeding efficiencies due to the leaching of nutrients before feed pellets are consumed by the animals. If crustaceans are fed an experimental diet with a high leaching rate, their estimated requirements for dietary AAs may be inaccurate. To optimize the utilization of crystalline AAs, a possible approach is to coat AAs with lipids (Alam et al. 2004b; Gu et al. 2013).
Third, crystalline AAs in diets enter the systemic circulation of crustaceans more rapidly than the protein-bound AAs, possibly resulting in the asynchronous absorption of dietary AAs and a suboptimal efficiency of utilization of dietary AAs (Lovell 1991; Guo et al. 2020). For example, there are higher percentages of AAs lost in the urine (e.g., 13.6% for His; 17.6% for phenylalanine; and 8–10% for isoleucine, leucine, lysine and valine) when shrimp fed diets with crystalline AAs in comparison with diets with proteins (Liou et al. 2005). Similarly, a previous study showed that shrimp fed diets with coated crystalline methionine grew more rapidly than those fed diets with uncoated crystalline methionine (Chi et al. 2011). Therefore, it is necessary to systematically evaluate the efficiency of utilization of different free AAs (either coated vs crystalline) to define an appropriate replacement level of protein-bound AAs by crystalline AAs. Some studies with pigs (Gahl et al. 1994) and rainbow trout (Tran et al. 2007) demonstrated that the efficiencies of utilization of supplemented crystalline AAs varied with diets, depending on protein sources especially at suboptimal dietary levels of AAs. This means that AAs with the same quantity and quality may yield different effects on the growth of animals when they are supplemented to diets with various feedstuff ingredients.
Fourth, there are no standardized diets or AAs as isonitrogenous controls for nutritional research in crustaceans. Due to the inadequate understanding of NEAAs in the past decades, glutamate, glycine and aspartate have long been used as an isonitrogenous control in nutritional experiments. This is inappropriate based on recent studies with terrestrial animals (Hou and Wu 2018; Wu 2018), fish (Li et al. 2020a), and crustaceans (Xie et al. 2014, Xie et al. 2015a, b) indicating that these AAs have nutritional or physiological effects in the animals. We suggest that L-alanine be used as the isonitrogenous control in nutritional studies with crustaceans where it is not a test AA.
Fifth, there is limited knowledge about the cell- and tissue-specific metabolism of AAs in different aquatic crustaceans (e.g. crabs and shrimp). For example, GPT and GOT are abundant in both the mitochondria and the cytoplasm of hepatocytes of many animal species (Wu 2013). Thus, the activities of these two enzymes in serum are often determined to assess hepatic integrity in human medicine. Similarly, both enzymes in the hemolymph of giant tiger prawn and Pacific white shrimp have been regarded as important indicators of the hepatopancreatic injury (Pan et al. 2003; Liu et al. 2019). This, however, it may be not valid for all species of shrimp and crabs. For example, the activity of GPT and GDH in the hepatopancreas of black tiger shrimp (Penaeus monodon) is either very low or undetectable (Richard et al. 2010). Furthermore, in blue crabs, the hepatopancreas is not a main site for the catabolism of AAs (Table 9.2).
Finally, although there has been active research to determine the dietary requirements of crustaceans for crude protein over the past 50 years (Table 9.5), much emphasis should be directed to studies of the dietary requirements of these animals for NEAAs. Nutritionists should move away from the traditional concept of crude protein toward all AAs with nutritional and physiological functions in the animals. The composition of AAs in the diets with various protein sources for crustaceans may differ substantially even though the diets have the same crude-protein level. Dietary requirements of crustaceans for all AAs (including AAs that are synthesized in animal cells, such as glutamate, glutamine and glycine) should be defined to optimize dietary formulations for both health and growth performance. Research on the metabolism and functions of AAs is fundamental to achieve this goal so as to manufacture future environment-friendly aquafeeds and reduce feed costs in crustacean production. The new nutritional concepts of “dietary requirements of animals for NEAAs” and “functional AAs”, which were originally proposed on the basis of basic and applied studies with terrestrial animals (Wu 2010), are expected to transform nutritional studies with shrimp and crabs, as well as feeding practices in the global crustacean production (Xie et al. 2014, 2015a,b).
Abbreviations
- AA:
-
amino acid
- EAA:
-
nutritionally essential amino acid
- GABA:
-
γ-aminobutyrate
- GDH:
-
glutamate dehydrogenase
- GOT:
-
glutamate-oxaloacetate transaminase
- GPT:
-
glutamate-pyruvate transaminase
- mTOR:
-
mechanistic target of rapamycin
- NEAA:
-
nutritionally nonessential amino acid
- NO:
-
nitric oxide
- NRC:
-
National Research Council
- ROS:
-
reactive oxygen species
References
Abuhagr AM, MacLea KS, Chang ES, Mykles DL (2014) Mechanistic target of rapamycin (mTOR) signaling genes in decapod crustaceans: cloning and tissue expression of mTOR, Akt, Rheb, and p70 S6 kinase in the green crab, Carcinus maenas, and blackback land crab, Gecarcinus lateralis. Comp Biochem Physiol A 168:25–39
Alam MS, Teshima SI, Ishikawa M, Hasegawa D, Koshio S (2004a) Dietary arginine requirement of juvenile kuruma shrimp Marsupenaeus japonicus (bate). Aquac Res 35:842–849
Alam MS, Teshima S, Koshio S, Ishikawa M (2004b) Effects of supplementation of coated crystalline amino acids on growth performance and body composition of juvenile kuruma shrimp Marsupenaeus japonicus. Aquac Nutr 10:309–316
Aparicio-Simón B, Piñón M, Racotta R, Racotta IS (2010) Neuroendocrine and metabolic responses of Pacific whiteleg shrimp Litopenaeus vannamei exposed to acute handling stress. Aquaculture 298:308–314
Assaad H, Yao K, Tekwe CD, Feng S, Bazer FW, Zhou L, Carroll RJ, Meininger CJ, Wu G (2014) Analysis of energy expenditure in diet-induced obese rats. Frontiers in bioscience (Landmark edition) 19:967
Bahabadi NM, Mozanzadeh MT, Agh N, Ahmadi A, Yaghoubi M (2018) Enriched artemia with L-lysine and DL-methionine on growth performance, stress resistance, and fatty acid profile of Litopenaeus vannamei postlarvae. J Appl Aquac 30:325–336
Batrel Y, Regnault ML (1985) Metabolic pathways of ammoniogenesis in the shrimp Crangon crangon L.: possible role of glutamate dehydrogenase. Comp Biochem Physiol B 82:217–222
Bhavan PS, Radhakrishnan S, Seenivasan C, Shanthi R, Poongodi R, Kannan S (2010) Proximate composition and profiles of amino acids and fatty acids in the muscle of adult males and females of commercially viable prawn species Macrobrachium rosenbergii collected from natural culture environments. Int J Biol 2(2):107
Bowser PR, Rosemark R (1981) Mortalities of cultured lobsters, Homarus, associated with a molt death syndrome. Aquaculture 23:11–18
Brito R, Chimal ME, Gaxiola G, Rosas C (2000) Growth, metabolic rate, and digestive enzyme activity in the white shrimp Litopenaeus setiferus early postlarvae fed different diets. J Exp Marine Biol Ecol 255:21–36
Buarque DS, Castro PF, Santos FMS, Lemos D, Júnior LBC, Bezerra RS (2009) Digestive peptidases and proteinases in the midgut gland of the pink shrimp Farfantepenaeus paulensis (Crustacea, Decapoda, Penaeidae). Aquac Res 40:861–870
Buarque DS, Castro PF, Santos FMS, Amaral IPG, Oliveira SM, Alves KB, Carvalho LB Jr, Bezerra RS (2010) Digestive proteinases and peptidases in the hepatopancreas of the southern brown shrimp (Farfantepenaeus subtilis) in two sub-adult stages. Aquac Nutr 16:359–369
Bulbul M, Kader MA, Asaduzzaman M, Ambak MA, Chowdhury AJK, Hossain MS, Ishikawa M, Koshio S (2016) Can canola meal and soybean meal be used as major dietary protein sources for kuruma shrimp, Marsupenaeus japonicus? Aquaculture 452:194–199
Burton RS (1991) Regulation of proline synthesis during osmotic stress in the copepod Tigriopus californicus. J Exp Zool 259:166–173
Calado R, Rosa R, Nunes ML, Narciso L (2005) Amino and fatty acid dynamics of Lysmata seticaudata (Decapoda: Hippolytidae) embryos during early and late reproductive season. Mar Biol 147:341–351
Carter CG, Mente E (2014) Protein synthesis in crustaceans - a review focused on feeds and feeding. Cent Eur J Biol 9:1–10
Caskey JL, Watson GM, Bauer RT (2009) Studies on contact sex pheromones of the caridean shrimp Palaemonetes pugio: II. The role of glucosamine in mate recognition. Invertebrate Reprod Dev 53:105–116
Ceccaldi H, Ceccaldi HJ (1989) Anatomy and physiology of digestive tract of crustaceans decapods reared in aquaculture. In: Advances in tropical aquaculture, Tahiti, French Polynesia, pp 243–259
Chakrapani V, Rasal KD, Mohapatra SD, Rasal AR, Jayasankar P, Barman HK (2017) Molecular characterization, computational analysis and transcript profiling of glutamate dehydrogenase (gdh) gene of Macrobrachium rosenbergii exposed to saline water. Gene Reports 8:37–44
Chaplin AE, Huggins AK, Munday KA (1967) The distribution of L-α-aminotransferases in Carcinus maenas. Comp Biochem Physiol 20:195–198
Chen JM, Chen JC (2000) Study on the free amino acid levels in the hemolymph, gill, hepatopancreas and muscle of Penaeus monodon exposed to elevated ambient ammonia. Aquatic Toxicol 50:27–37
Chen HY, Leu YT, Roelants I (1992) Quantification of arginine requirements of juvenile marine shrimp, Penaeus monodon, using microencapsulated arginine. Mar Biol 114:229–233
Chen JC, Cheng SY, Chen CT (1994) Changes of haemocyanin, protein and free amino acid levels in the haemolymph of Penaeus japonicus exposed to ambient ammonia. Comp Biochem Physiol A 109:339–347
Chen K, Li E, Gan L, Wang X, Xu C, Lin H, Qin JG, Chen L (2014) Growth and lipid metabolism of the pacific white shrimp Litopenaeus vannamei at different salinities. J Shellfish Res 33:825–832
Chi SY, Tan BP, Lin HZ, Mai KS, Ai QH, Wang XJ, Zhang WB, Xu W, Liufu ZG (2011) Effects of supplementation of crystalline or coated methionine on growth performance and feed utilization of the pacific white shrimp, Litopenaeus vannamei. Aquac Nutr 17:e1–e9
Chowdhury MAK, Zhu J, Cai C, Ye Y, He J (2018) Dietary protease modulates nutrient retention efficiency and hepatopancreatic protease activity in juvenile Chinese mitten crab Eriocheir sinensis. Aquac Nutr 24:911–917
Claybrook DL (1983) Nitrogen metabolism in: the biology of Crustacea, mantel LH. Academic, NY, pp 163–213
Coelho RTI, Yasumaru FA, Passos MJACR, Gomes V, Lemos D (2019) Energy budgets for juvenile Pacific whiteleg shrimp Litopenaeus vannamei fed different diets. Braz J Oceanogr 67:e19243
Comeau M, Savoie F (2001) Growth increment and molt frequency of the American lobster (Homarus americanus) in the southwestern gulf of St. Lawrence. J Crustacean Biol 21:923–936
Comoglio LI, Gaxiola G, Roque A, Cuzon G, Amin O (2004) The effect of starvation on refeeding, digestive enzyme activity, oxygen consumption and ammonia excretion in juvenile white shrimp Litopenaeus vannamei. J Shellfish Res 23:243–250
Corteel M (2013) In: Ghent University (ed) White spot syndrome virus infection in P. vannamei and M. rosenbergii: experimental studies on susceptibility to infection and disease. PhD Dissertation, Gent, Belgium
Cruz-Suárez LE, Tapia-Salazar M, Villarreal-Cavazos D, Beltran-Rocha J, Nieto-López MG, Lemme A, Ricque-Marie D (2009) Apparent dry matter, energy, protein and amino acid digestibility of four soybean ingredients in white shrimp Litopenaeus vannamei juveniles. Aquaculture 292:87–94
Cuzon G, Lawrence A, Gaxiola G, Rosas C, Guillaume J (2004) Nutrition of Litopenaeus vannamei reared in tanks or in ponds. Aquaculture 235:513–551
Deng DF, Hemre GI, Storebakken T, Shiau SY, Hung SS (2005) Utilization of diets with hydrolyzed potato starch, or glucose by juvenile white sturgeon (Acipenser transmontanus), as affected by Maillard reaction during feed processing. Aquaculture 248:103–109
Dionysius DA, Hoek KS, Milne JM, Slaitery SL (1993) Trypsin-like enzyme from sand crab (Portunus pelagicus): purification and characterization. J Food Sci 58:780–784
Do Huu H, Tabrett S, Hoffmann K, Köppel P, Lucas JS, Barnes AC (2012) Dietary nucleotides are semi-essential nutrients for optimal growth of black tiger shrimp (Penaeus monodon). Aquaculture 366:115–121
Do Huu H, Tabrett S, Hoffmann K, Köppel P, Barnes AC (2013) The purine nucleotides guanine, adenine and inosine are a dietary requirement for optimal growth of black tiger prawn, P. monodon. Aquaculture 408:100–105
Dong J, Cheng R, Yang Y, Zhao Y, Wu G, Zhang R, Zhu X, Li L, Li X (2018) Effects of dietary taurine on growth, non-specific immunity, anti-oxidative properties and gut immunity in the Chinese mitten crab Eriocheir sinensis. Fish Shellfish Immunol 82:212–219
Dooley PC, Crouch PJ, West JM (2002) Free amino acids in claw muscle and haemolymph from Australian freshwater crayfish at different stages of the moult cycle. Comp Biochem Physiol A 131:625–637
El Haj AJ, Houlihan DF (1987) In vitro and in vivo protein synthesis rates in a crustacean muscle during the moult cycle. J Exp Biol 127:413–426
Ennor AH, Morrison JF, Rosenberg H (1956) The isolation of phosphoarginine. Biochem J 62:358–361
Façanha FN, Oliveira-Neto AR, Figueiredo-Silva C, Nunes AJP (2016) Effect of shrimp stocking density and graded levels of dietary methionine over the growth performance of Litopenaeus vannamei reared in a green-water system. Aquaculture 463:16–21
Fang YZ, Yang S, Wu G (2002) Free radicals, antioxidants, and nutrition. Nutrition 18:872–879
Feng Z, Dong C, Wang L, Hu Y, Zhu W (2013) Optimal content and ratio of lysine to arginine in the diet of Pacific white shrimp, Litopenaeus vannamei. Chin J Oceanol Limnol 31:789–795
Fenucci JL, Fenucci AC, Lawrence AL, Zein-Eldin ZP (1982) The assimilation of protein and carbohydrate from prepared diets by the shrimp Penaeus stylirostris. J World Mariculture Soc 13:134–145
Fernández I, Oliva M, Carrillo O, Van Wormhoudt A (1997) Digestive enzyme activities of Penaeus notialis during reproduction and moulting cycle. Comp Biochem Physiol A 118:1267–1271
Fernández-Urruzola I, Packard TT, Gómez M (2011) GDH activity and ammonium excretion in the marine mysid, Leptomysis lingvura: effects of age and starvation. J Exp Marine Biol Ecol 409:21–29
Fields PG, Fleurat-Lessard F, Lavenseau L, Febvay G, Peypelut L, Bonnot G (1998) The effect of cold acclimation and deacclimation on cold tolerance, trehalose and free amino acid levels in Sitophilus granarius and Cryptolestes ferrugineus (Coleoptera). J Insect Physiol 44:955–965
Fink P, Pflitsch C, Marin K (2011) Dietary essential amino acids affect the reproduction of the keystone herbivore Daphnia pulex. PLoS One 6(12):e28498
Forster JRM, Gabbott PA (1971) The assimilation of nutrients from compounded diets by the prawns Palaemon serratus and Pandalus platyceros. J Marine Biol 51:943–961
Gahl MJ, Crenshaw TD, Benevenga NJ (1994) Diminishing returns in weight, nitrogen, and lysine gain of pigs fed six levels of lysine from three supplemental sources. J Anim Sci 72:3177–3187
Garcia-Carreo FL, Navarrete del Toro A, Ezquerra M (1997) Digestive shrimp proteases for evaluation of protein digestibility in vitro. I: effect of protease inhibitors in protein ingredients. J Marine Biotechnol 5:36–40
Gimenez AF, Garcıa-Carreno FL, Del Toro MN, Fenucci JL (2001) Digestive proteinases of red shrimp Pleoticus muelleri (Decapoda, Penaeoidea): partial characterization and relationship with molting. Comp Biochem Physiol B 130:331–338
Gimenez AF, Garcıa-Carreno FL, Del Toro MN, Fenucci JL (2002) Digestive proteinases of Artemesia longinaris (Decapoda, Penaeidae) and relationship with molting. Comp Biochem Physiol B 132:593–598
Goimier Y, Pascual C, Sánchez A, Gaxiola G, Sánchez A, Rosas C (2006) Relation between reproductive, physiological, and immunological condition of Litopenaeus setiferus pre-adult males fed different dietary protein levels (Crustacea; Penaeidae). Anim Reprod Sci 92:193–208
Gorell TA, Gilbert LI (1969) Stimulation of protein and RNA synthesis in the crayfish hepatopancreas by crustecdysone. Gen Comp Endocrinol 13:308–310
Gu M, Zhang WB, Bai N, Mai KS, Xu W (2013) Effects of dietary crystalline methionine or oligo-methionine on growth performance and feed utilization of white shrimp (Litopenaeus vannamei) fed plant protein-enriched diets. Aquac Nutr 19:39–46
Guo R, Liu YJ, Tian LX, Huang JW (2006) Effect of dietary cornstarch levels on growth performance, digestibility and microscopic structure in the white shrimp, Litopenaeus vannamei reared in brackish water. Aquacu Nutr 12:83–88
Guo J, Duan M, Qiu X, Masagounder K, Davis DA (2020) Characterization of methionine uptake and clearance in the hemolymph of Pacific white shrimp Litopenaeus vannamei. Aquaculture 526:735351
Haj A, Clarke S, Harrison PAUL, Chang E (1996) In vivo muscle protein synthesis rates in the American lobster Homarus americanus during the moult cycle and in response to 20-hydroxyecdysone. J Exp Biol 199:579–585
Halver JE, Hardy RW (2002) Fish nutrition, 3rd edn. Academic, San Diego, California
Hanzal R, Jegorov A (1991) Changes in free amino acid composition in haemolymph of larvae of the wax moth, Galleria mellonella L., during cold acclimation. Comp Biochem Physiol A 100:957–962
Hardy RW (2010) Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal. Aquac Res 41:770–776
Harrison KE (1990) The role of nutrition in maturation, reproduction and embryonic development of decapod crustaceans: a review. J Shellfish Res 9:1–28
He W, G Wu (2020) Metabolism of amino acids in the brain and their roles in regulating food intake. Adv Exp Med Biol 1265:167–185
Heras H, Gonzalez-Baró MR, Pollero RJ (2000) Lipid and fatty acid composition and energy partitioning during embryo development in the shrimp Macrobrachium borellii. Lipids 35:645–651
Hewitt DR (1992) Response of protein turnover in the brown tiger prawn Penaeus esculentus to variation in dietary protein content. Comp Biochem Physiol A 103:183–187
Holt SM, Kinsey ST (2002) Osmotic effects on arginine kinase function in living muscle of the blue crab Callinectes sapidus. J Exp Biol 205:1775–1785
Hosamani N, Reddy SB, Reddy RP (2017) Crustacean molting: regulation and effects of environmental toxicants. J Marine Sci Res Dev 7:236
Hou YQ, Wu G (2018) L-glutamate nutrition and metabolism in swine. Amino Acids 50:1497–1510
Hou YQ, He WL, Hu SD, Wu G (2019) Composition of polyamines and amino acids in plant-source foods for human consumption. Amino Acids 51:1153–1165
Houlihan DF, Waring CP, Mathers E, Gray C (1990) Protein synthesis and oxygen consumption of the shore crab Carcinus maenas after a meal. Physiol Zool 63:735–756
Huo YW, Jin M, Sun P, Hou YM, Li Y, Qiu H, Zhou QC (2017) Effect of dietary leucine on growth performance, hemolymph and hepatopancreas enzyme activities of swimming crab, Portunus trituberculatus. Aquac Nutr 23:1341–1350
Immaculada A, Mengibar M, Harris R, Panos I, Miralles B, Acosta N, Galed G, Heras A (2009) Functional characterization of chitin and chitosan. Curr Chem Biol 3:203–230
Intanai I, Taylor EW, Whiteley NM (2009) Effects of salinity on rates of protein synthesis and oxygen uptake in the post-larvae and juveniles of the tropical prawn Macrobrachium rosenbergii (de man). Comp Biochem Physiol A 152:372–378
Islam MS, Kodama K, Kurokora H (2010) Ovarian development of the mud crab Scylla paramamosain in a tropical mangrove swamps, Thailand J Sci Res 2:380–389
Jia S, Li X, Zheng S, Wu G (2017) Amino acids are major energy substrates for tissues of hybrid striped bass and zebrafish. Amino Acids 49:2053–2063
Jiang H, Li K, Chew L, Wang Q, Liu Q, Liu L (2005) Nutritional requirement of the Chinese mitten-handed crab Eriocheir sinensis juvenile for arginine and lysine. J World Aquac Soc 36:515–520
Jiang G, Yu R, Zhou M (2006) Studies on nitric oxide synthase activity in haemocytes of shrimps Fenneropenaeus chinensis and Marsupenaeus japonicus after white spot syndrome virus infection. Nitric Oxide 14:219–227
Jin M, Zhou QC, Zhang W, Xie FJ, ShenTu JK, Huang XL (2013) Dietary protein requirements of the juvenile swimming crab, Portunus trituberculatus. Aquaculture 414:303–308
Jin M, Wang MQ, Huo YW, Huang WW, Mai KS, Zhou QC (2015a) Dietary lysine requirement of juvenile swimming crab, Portunus trituberculatus. Aquaculture 448:1–7
Jin M, Wang M, Huo Y, Huang W, Hou Y, Ding L, Zhou Q (2015b) Dietary methionine requirement of juvenile swimming crab (Portunus trituberculatus). Chinese J Anim Nutr 27:3457–3467
Jin M, Zhou QC, Wang MQ, Huo YW, Huang WW, Mai KS (2016) Dietary arginine requirement of juvenile swimming crab, Portunus trituberculatus. Aquac Nutr 22:1174–1184
Jusadi D, Ruchyani S, Ekasari J (2011) Improvement of survival and development of Pacific white shrimp Litopenaeus vannamei larvae by feeding taurine enriched rotifers. J Akuakultur Indonesia 10:131–136
Katzen S, Salser BR, Ure J (1984) Dietary lysine effects on stress-related mortality of the marine shrimp, Penaeus stylirostris. Aquaculture 40:277–281
Kibria G (1993) Studies on molting, molting frequency and growth of shrimp Penaeus monodon fed on natural and compounded diets. Asian Fish Sci 6:203–211
Kimura S, Tanaka H (1986) Partial characterization of muscle collagens from prawns and lobster. J Food Sci 51:330–332
King FD, Cucci TL, Bidigare RR (1985) A pathway of nitrogen metabolism in marine decapod crabs. Comp Biochem Physiol B 80:401–403
Koch U, Von Elert E, Straile D (2009) Food quality triggers the reproductive mode in the cyclical parthenogen Daphnia (Cladocera). Oecologia 159:317–324
Koch U, Martin-Creuzburg D, Grossart HP, Straile D (2011) Single dietary amino acids control resting egg production and affect population growth of a key freshwater herbivore. Oecologia 167:981–989
Kotlyar SIMON, Weihrauch DIRK, Paulsen RS, Towle DW (2000) Expression of arginine kinase enzymatic activity and mRNA in gills of the euryhaline crabs Carcinus maenas and Callinectes sapidus. J Exp Biol 203:2395–2404
Koyama H, Mizusawa N, Hoashi M, Tan E, Yasumoto K, Jimbo M, Ikeda D, Yokoyama T, Asakawa S, Piyapattanakorn S, Watabe S (2018) Changes in free amino acid concentrations and associated gene expression profiles in the abdominal muscle of kuruma shrimp (Marsupenaeus japonicus) acclimated at different salinities. J Exp Biol 221:jeb168997
Krishnamoorthy RV, Srihari K (1973) Changes in the excretory patterns of the fresh-water field crab Paratelphusa hydrodromous upon adaptation to higher salinities. Mar Biol 21:341–348
Kucharski LCR, Da Silva RSM (1991) Seasonal variation in the energy metabolism in an estuarine crab, Chasmagnathus granulata (Dana, 1851). Comp Biochem Physiol A 100:599–602
Laranja JLQ Jr, Quinitio ET, Catacutan MR, Coloso RM (2010) Effects of dietary L-tryptophan on the agonistic behavior, growth and survival of juvenile mud crab Scylla serrata. Aquaculture 310:84–90
Lee PG, Smith LL, Lawrence AL (1984) Digestive proteases of Penaeus vannamei Boone: relationship between enzyme activity, size and diet. Aquaculture 42:225–239
Lemos D, Ezquerra JM, Garcia-Carreno FL (2000) Protein digestion in penaeid shrimp: digestive proteinases, proteinase inhibitors and feed digestibility. Aquaculture 186:89–105
Li P, Wu G (2018) Roles of dietary glycine, proline and hydroxyproline in collagen synthesis and animal growth. Amino Acids 50:29–38
Li P, Wu G (2020a) Composition of amino acids and related nitrogenous nutrients in feedstuffs for animal diets. Amino Acids 52:523–542
Li XY, Wu G (2020b) Dietary supplementation with microbial biomass (NovacqTM) improves whiteleg shrimp growth. Aquaculture America Annual Meeting, Honolulu, Hawaii, Feb 9-12, 2020
Li P, Yin YL, Li D, Kim SW, Wu G (2007) Amino acids and immune function. Br J Nutr 98:237–252
Li E, Arena L, Chen L, Qin JG, Van Wormhoudt A (2009a) Characterization and tissue-specific expression of the two glutamate dehydrogenase cDNAs in Pacific white shrimp, Litopenaeus vannamei. J Crustacean Biol 29:379–386
Li P, Mai K, Trushenski J, Wu G (2009b) New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids 37:43–53
Li XL, Rezaei R, Li P, Wu G (2011a) Composition of amino acids in feed ingredients for animal diets. Amino Acids 40:1159–1168
Li E, Arena L, Lizama G, Gaxiola G, Cuzon G, Rosas C, Chen L, Van Wormhoudt A (2011b) Glutamate dehydrogenase and Na+-K+ ATPase expression and growth response of Litopenaeus vannamei to different salinities and dietary protein levels. Chin J Oceanol Limnol 29:343–349
Li F, Yin Y, Tan B, Kong X, Wu G (2011c) Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids 41:1185
Li E, Wang X, Chen K, Xu C, Qin JG, Chen L (2015) Physiological change and nutritional requirement of Pacific white shrimp Litopenaeus vannamei at low salinity. Rev Aquac 9:57–75
Li CY, Wang YJ, Huang SW, Cheng CS, Wang HC (2016) Replication of the shrimp virus WSSV depends on glutamate-driven anaplerosis. PLoS One 11(1):e0146902
Li XL, Zheng SX, Wu G (2020a) Nutrition and metabolism of glutamate and glutamine in fish. Amino Acids 52:671–691
Li XL, Zheng SX, Jia SC, Song F, Zhou CP, Wu G (2020b) Oxidation of energy substrates in tissues of largemouth bass (Micropterus salmoides). Amino Acids 52:1017–1032
Li XY, Zheng SX, Wu G (2020c) Nutrition and functions of amino acids in fish. Adv Exp Med Biol 1285:133–168
Lin H, Chen Y, Niu J, Zhou C, Huang Z, Du Q, Zhang J (2015) Dietary methionine requirements of pacific white shrimp Litopenaeus vannamei of three different sizes. Israeli J Aquac 67:1163
Liou CH, Lin SC, Cheng JH (2005) Urinary amino acid excretion by marine shrimp, Penaeus monodon, in response to orally administrated intact protein and crystalline amino acids. Aquaculture 248:35–40
Liu HY, Sun WW, Tan BP, Chi SY, Dong XH, Yang QH (2012) Molecular cloning and expression of hepatopancreas glutamine synthetase in the Pacific white shrimp, Litopenaeus vannamei, induced by acute hypo-osmotic stress. Aquaculture 362:80–87
Liu Z, Zhou Z, Wang L, Li M, Wang W, Yi Q, Huang S, Song L (2018) Dopamine and serotonin modulate free amino acids production and Na+/K+ pump activity in Chinese mitten crab Eriocheir sinensis under acute salinity stress. Front Physiol 9:1080
Liu T, Zhang G, Feng Y, Kong C, Ayisi CL, Huang X, Hua X (2019) Dietary soybean antigen impairs growth and health through stress-induced non-specific immune responses in Pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol 84:124–129
Lovell RT (1991) Nutrition of aquaculture species. J Anim Sci 69:4193–4200
Lovett DL, Watts SA (1995) Changes in polyamine levels in response to acclimation salinity in gills of the blue crab Callinectes sapidus Rathbun. Comp Biochem Physiol B 110:115–119
Lu JY, Shu MA, Xu BP, Liu GX, Ma YZ, Guo XL, Liu Y (2015) Mud crab Scylla paramamosain glutamate dehydrogenase: molecular cloning, tissue expression and response to hyposmotic stress. Fish Sci 81:175–186
Marcus F, Morrison JF (1964) The preparation of phosphoarginine: a comparative study. Biochem J 92:429–435
Martin GG, Castro C, Moy N, Rubin N (2003) N-acetyl-D-glucosamine in crustacean hemocytes; possible functions and usefulness in hemocyte classification. Invertebrate Biol 122:265–270
Martins TL, Chittó ALF, Rossetti CL, Brondani CK, Kucharski LC, Da Silva RS (2011) Effects of hypo-or hyperosmotic stress on lipid synthesis and gluconeogenic activity in tissues of the crab Neohelice granulata. Comp Biochem Physiol A 158:400–405
McGaw IJ, Daniel L, Curtis DL (2013) A review of gastric processing in decapod crustaceans. J Comp Physiol B 183:443–465
McLeay L, Doubell M, Roberts S, Dixon C, Andreacchio L, James C, Luick J, Middleton J (2015) Prawn and crab harvest optimisation: a bio-physical management tool. South Australian Research and Development Institute (Aquatic Sciences), West Beach
Mente E (2006) Protein nutrition in crustaceans. CAB reviews: perspectives in agriculture, veterinary science, nutrition and natural resources. CAB International, Wallingford
Mente E, Coutteau P, Houlihan D, Davidson I, Sorgeloos P (2002) Protein turnover, amino acid profile and amino acid flux in juvenile shrimp Litopenaeus vannamei: effects of dietary protein source. J Exp Biol 205:3107–3122
Mente E, Legeay A, Houlihan DF, Massabuau JC (2003) Influence of oxygen partial pressures on protein synthesis in feeding crabs. Am J Physiol 284:R500–R510
Mente E, Carter CG, Barnes RK, Karapanagiotidis IT (2011) Protein synthesis in wild-caught Norway lobster (Nephrops norvegicus L.). J Exp Marine Biol Ecol 409:208–214
Millamena OM, Bautista-Teruel MN, Kanazawa A (1996) Methionine requirement of juvenile tiger shrimp Penaeus monodon Fabricius. Aquaculture 143:403–410
Millamena OM, Bautista-Teruel MN, Reyes OS, Kanazawa A (1998) Requirements of juvenile marine shrimp, Penaeus monodon (Fabricius) for lysine and arginine. Aquaculture 164:95–104
Millikin MR, Biddle GN, Siewicki TC, Fortner AR, Fair PH (1980) Effects of various levels of dietary protein on survival, molting frequency and growth of juvenile blue crabs (Callinectes sapidus). Aquaculture 19:149–161
Miyagawa M, Tabuchi Y, Yamane K, Matsuda H, Watabe S, Hashimoto K, Katakai R, Otsuka Y (1990) Changes in the free amino acid profile of snow crab Chionoecetes opilio muscle during storage in ice. Agric Biol Chem 54:359–364
Mohankumar K, Ramasamy P (2006) Activities of membrane bound phosphatases, transaminases and mitochondrial enzymes in white spot syndrome virus infected tissues of Fenneropenaeus indicus. Virus Res 118:130–135
Muhlia-Almazan A, Garcıa-Carreno FL, Sanchez-Paz JA, Yepiz-Plascencia G, Peregrino-Uriarte AB (2003) Effects of dietary protein on the activity and mRNA level of trypsin in the midgut gland of the white shrimp Penaeus vannamei. Comp Biochem Physiol B 135:373–383
Nguyen NTB, Chim L, Lemaire P, Wantiez L (2014) Feed intake, molt frequency, tissue growth, feed efficiency and energy budget during a molt cycle of mud crab juveniles, Scylla serrata (Forskål, 1775), fed on different practical diets with graded levels of soy protein concentrate as main source of protein. Aquaculture 434:499–509
NRC (2011) Nutrient requirements of fish and shrimp. The. National Academies Press, Washington, DC
Obaldo LG, Masuda R (2006) Effect of diet size on feeding behavior and growth of Pacific white shrimp, Litopenaeus vannamei. J Appl Aquac 18:101–110
Oliveira GT, Da Silva RS (1997) Gluconeogenesis in hepatopancreas of Chasmagnathus granulata crabs maintained on high-protein or carbohydrate-rich diets. Comp Biochem Physiol A 118:1429–1435
Oliveira GT, Rossi IC, Kucharski LC, Da Silva RS (2004) Hepatopancreas gluconeogenesis and glycogen content during fasting in crabs previously maintained on a high-protein or carbohydrate-rich diet. Comp Biochem Physiol A 137:383–390
Oujifard A, Seyfabadi J, Kenari AA, Rezaei M (2012) Growth and apparent digestibility of nutrients, fatty acids and amino acids in Pacific white shrimp, Litopenaeus vannamei, fed diets with rice protein concentrate as total and partial replacement of fish meal. Aquaculture 342:56–61
Palma J, Andrade JP, Lemme A, Bureau DP (2015) Quantitative dietary requirement of juvenile a tlantic ditch shrimp Palaemonetes varians for lysine, methionine and arginine. Aquac Res 46:1822–1830
Pamuru RR (2019) Endocrinology of reproduction in crustaceans. In: Narayan EJ (ed) Comparative endocrinology of animals. IntechOpen. https://doi.org/10.5772/intechopen.83018
Pan CH, Chien YH, Hunter B (2003) The resistance to ammonia stress of Penaeus monodon Fabricius juvenile fed diets supplemented with astaxanthin. J Exp Marine Biol Ecol 297:107–118
Panini RL, Freitas LEL, Guimarães AM, Rios C, da Silva MFO, Vieira FN, Fracalossi DM, Samuels RI, Prudêncio ES, Silva CP, Amboni RD (2017) Potential use of mealworms as an alternative protein source for Pacific white shrimp: digestibility and performance. Aquaculture 473:115–120
Pellegrino R, Martins TL, Pinto CB, Schein V, Kucharski LC, Da Silva RSM (2013) Effect of starvation and refeeding on amino acid metabolism in muscle of crab Neohelice granulata previously fed protein-or carbohydrate-rich diets. Comp Biochem Physiol A 164:29–35
Pohlenz C, Gatlin DM III (2014) Interrelationships between fish nutrition and health. Aquaculture 431:111–117
Pressley TA, Graves JS (1983) Increased amino acid oxidation in the gills of blue crabs acclimated to dilute seawater. J Exp Zool 226:45–51
Qi C, Wang X, Han F, Jia Y, Lin Z, Wang C, Lu J, Yang L, Wang X, Li E, Qin JG (2019) Arginine supplementation improves growth, antioxidant capacity, immunity and disease resistance of juvenile Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol 93:463–473
Qiu X, Davis DA (2017) Effects of dietary phytase supplementation on growth performance and apparent digestibility coefficients of Pacific white shrimp Litopenaeus vannamei. Aquac Nutr 23:942–951
Rao KP, Ramachandra R (1961) Effect of acclimatization to high temperature on the blood chloride, free amino acids and osmotic pressure in the freshwater field crab Paratelphusa sp. and the freshwater mussel Lamellidens marginalis. J Exp Biol 38:29–34
Rao MS, Rajitha B, Pavitra E, Anjaneyulu N (2008) Changes of copper and protein profiles in hepatopancreas and hemolymph tissues during different molt stages of white shrimp, Litopenaeus vannamei (Boone, 1931). Biotechnol 7:153–156
Regnault M (1987) Nitrogen excretion in marine and fresh-water crustacea. Biol Rev 62:1–24
Reyes-Ramos CA, Peregrino-Uriarte AB, Cota-Ruiz K, Valenzuela-Soto EM, Leyva-Carrillo L, Yepiz-Plascencia G (2018) Phosphoenolpyruvate carboxykinase cytosolic and mitochondrial isoforms are expressed and active during hypoxia in the white shrimp Litopenaeus vannamei. Comp Biochem Physiol B 226:1–9
Richard L, Vachot C, Brèque J, Blanc PP, Rigolet V, Kaushik S, Geurden I (2010) The effect of protein and methionine intake on glutamate dehydrogenase and alanine aminotransferase activities in juvenile black tiger shrimp Penaeus monodon. J Exp Marine Biol Ecol 391:153–160
Romano N, Zeng C (2012) Osmoregulation in decapod crustaceans: implications to aquaculture productivity, methods for potential improvement and interactions with elevated ammonia exposure. Aquaculture 334:12–23
Rønnestad I, Conceição LE, Aragão C, Dinis MT (2000) Free amino acids are absorbed faster and assimilated more efficiently than protein in postlarval Senegal sole (Solea senegalensis). J Nutr 130:2809–2812
Rosas C, Cuzon G, Gaxiola G, Pascual C, Taboada G, Arena L, van Wormhoudt A (2002) An energetic and conceptual model of the physiological role of dietary carbohydrates and salinity on Litopenaeus vannamei juveniles. J Exp Marine Biol Ecol 268:47–67
Saborowski R, Thatje S, Calcagno JA, Lovrich GA, Anger K (2006) Digestive enzymes in the ontogenetic stages of the southern king crab, Lithodes santolla. Mar Biol 149:865–873
Safari O, Atash MMS, Paolucci M (2015) Effects of dietary L-carnitine level on growth performance, immune responses and stress resistance of juvenile narrow clawed crayfish, Astacus leptodactylus leptodactylus Eschscholtz, 1823. Aquaculture 439:20–28
Schein V, Waché Y, Etges R, Kucharski LC, van Wormhoudt A, Da Silva RS (2004) Effect of hyperosmotic shock on phosphoenolpyruvate carboxykinase gene expression and gluconeogenic activity in the crab muscle. FEBS Lett 561:202–206
Schein V, Chittó ALF, Etges R, Kucharski LC, Van Wormhoudt A, Da Silva RS (2005) Effect of hyper or hypo-osmotic conditions on neutral amino acid uptake and oxidation in tissues of the crab Chasmagnathus granulata. Comp Biochem Physiol B 140:561–567
Shinji J, Wilder MN (2012) Dynamics of free amino acids in the hemolymph of Pacific whiteleg shrimp Litopenaeus vannamei exposed to different types of stress. Fish Sci 78:1187–1194
Shyamal S, Das S, Guruacharya A, Mykles DL, Durica DS (2018) Transcriptomic analysis of crustacean molting gland (Y-organ) regulation via the mTOR signaling pathway. Sci Rep 8(1):7307
Silva BC, Nolasco-Soria H, Magallón-Barajas F, Civera-Cerecedo R, Casillas-Hernández R, Seiffert W (2016) Improved digestion and initial performance of whiteleg shrimp using organic salt supplements. Aquac Nutr 22:997–1005
Söderhäll K, Cerenius L (1992) Crustacean immunity. Annu Rev Fish Dis 2:3–23
Song HL, Tan BP, Chi SY, Liu Y, Chowdhury MK, Dong XH (2017) The effects of a dietary protease-complex on performance, digestive and immune enzyme activity, and disease resistance of Litopenaeus vannamei fed high plant protein diets. Aquac Res 48:2550–2560
Stentiford GD, Neil DM, Peeler EJ, Shields JD, Small HJ, Flegel TW, Vlak JM, Jones B, Morado F, Moss S, Lotz J (2012) Disease will limit future food supply from the global crustacean fishery and aquaculture sectors. J Invertebrate Pathol 110:141–157
Subramoniam T (2011) Mechanisms and control of vitellogenesis in crustaceans. Fish Sci 77:1–21
Subramoniam T (2017) Steroidal control of vitellogenesis in Crustacea: a new understanding for improving shrimp hatchery production. Proc Indian Natl Sci Acad 83:595–610
Sun SJ, Wang BJ, Jiang KY, Sun J, Liu M, Wang L (2015a) Target of rapamycin (TOR) in Fenneropenaeus chinensis: cDNA cloning, characterization, tissue expression and response to amino acids. Aquac Nutr 21:1–9
Sun YP, Guan LZ, Xiong JH, Xi QY, Zhang YL (2015b) Effects of L-tryptophan-supplemented dietary on growth performance and 5-HT and GABA levels in juvenile Litopenaeus vannamei. Aquac Int 23:235–251
Tacon AG (2018) Global trends in aquaculture and compound aquafeed production. World Aquaculture 49:33–46
Tan CH, Choong KY (1981) Effect of hyperosmotic stress on hemolymph protein, muscle ninhydrin-positive substances and free amino acids in Macrobrachium rosenbergii (De man). Comp Biochem Physiol A 70:485–489
Thomson JA, Kinnear JF, Martin MD, Horn DHS (1971) Effects of crustecdysone (20-hydroxyecdysone) on synthesis, release, and uptake of proteins by the larval fat body of Calliphora. Life Sc 10:203–211
Tran TNT, Parkouda C, De Saeger S, Larondelle Y, Rollin X (2007) Comparison of the lysine utilization efficiency in different plant protein sources supplemented with L-lysine center dot HCl in rainbow trout (Oncorhynchus mykiss) fry. Aquaculture 272:477–488
Trichet VV (2010) Nutrition and immunity: an update. Aquac Res 41:356–372
Tseng DY, Chen YN, Kou GH, Lo CF, Kuo CM (2001) Hepatopancreas is the extraovarian site of vitellogenin synthesis in black tiger shrimp, Penaeus monodon. Comp Biochem Physiol A 129:909–917
Tsukimura B (2001) Crustacean vitellogenesis: its role in oocyte development. Am Zool 41:465–476
Tuan VV (2016) Antibacterial and antiviral activity of different haemocyte subpopulations of Litopenaeus vannamei. Ph.D. dissertation. Ghent University; Gent, Belgium
Unnikrishnan U, Paulraj R (2010) Dietary protein requirement of giant mud crab Scylla serrata juveniles fed iso-energetic formulated diets having graded protein levels. Aquac Res 41:278–294
Vazquez L, Alpuche J, Maldonado G, Agundis C, Pereyra-Morales A, Zenteno E (2009) Immunity mechanisms in crustaceans. Innate Immun 15:179–188
Vinagre AS, Da Silva RS (2002) Effects of fasting and refeeding on metabolic processes in the crab Chasmagnathus granulata (Dana, 1851). Can J Zool 80:1413–1421
Wang Y, Li E, Yu N, Wang X, Cai C, Tang B, Chen L, Van Wormhoudt A (2012) Characterization and expression of glutamate dehydrogenase in response to acute salinity stress in the Chinese mitten crab, Eriocheir sinensis. PLoS One 7(5):e37316
Wang XD, Li EC, Wang SF, Qin JG, Chen XF, Lai QM, Chen K, Xu C, Gan L, Yu N, Du ZY (2015) Protein-sparing effect of carbohydrate in the diet of white shrimp Litopenaeus vannamei at low salinity. Aquac Nutr 21:904–912
Wang JT, Han T, Li XY, Hu SX, Jiang YD, Wang CL (2016) Effects of dietary phosphatidylcholine (PC) levels on the growth, molt performance and fatty acid composition of juvenile swimming crab, Portunus trituberculatus. Anim Feed Sci Technol 216:225–233
Wirkner CS, Richter S (2013) Circulatory system and respiration. Natural history of Crustacea, Watling L and Thiel M, eds. Oxford University Press, Oxford, pp 376–412
Wolfe RR (2017) Branched-chain amino acids and muscle protein synthesis in humans: myth or reality? J Int Soc Sports Nutr 14:1–7
Wongprasert K, Asuvapongpatana S, Poltana P, Tiensuwan M, Withyachumnarnkul B (2006) Serotonin stimulates ovarian maturation and spawning in the black tiger shrimp Penaeus monodon. Aquaculture 261:1447–1454
Wouters R, Lavens P, Nieto J, Sorgeloos P (2001) Penaeid shrimp broodstock nutrition: an updated review on research and development. Aquaculture 202:1–21
Wu G (2010) Functional amino acids in growth, reproduction, and health. Adv Nutr 1:31–37
Wu G (2013) Amino acids: biochemistry and nutrition. CRC Press, Boca Raton, Florida
Wu G (2016) Dietary protein intake and human health. Food Funct 7:1251–1265
Wu G (2018) Principles of animal nutrition. CRC Press, Boca Raton, Florida
Wu G (2020) Important roles of dietary taurine, creatine, carnosine, anserine and hydroxyproline in human nutrition and health. Amino Acids 52:329–360
Wu G, Meininger CJ (2008) Analysis of citrulline, arginine, and methylarginines using high-performance liquid chromatography. Methods Enzymol 440:177–189
Wu G, Bazer FW, Davis TA, Kim SW, Li P, Rhoads JM, Satterfield MC, Smith SB, Spencer TE (2009) Yin Y. arginine metabolism and nutrition in growth, health and disease. Amino Acids 37:153–168
Wu G, Bazer FW, Dai Z, Li D, Wang J, Wu Z (2014) Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci 2:387–417
Wu G, Cross HR, Gehring KB, Savell JW, Arnold AN, McNeill SH (2016) Composition of free and peptide-bound amino acids in beef chuck, loin, and round cuts J Anim Sci 94:2603–2613
Wu P, Yang W, Dong Y, Wang Y, Zhang Y, Zou X, Ge H, Hu D, Cui Y, Chen Z (2019) Feasibility of cultivation of Spinibarbus sinensis with coconut oil and its effect on disease resistance (nonspecific immunity, antioxidation and mTOR and NF-kB signaling pathways). Fish Shellfish Immunol 93:726–731
Wu H, Ge M, Chen H, Jiang S, Lin L, Lu J (2020) Comparison between the nutritional qualities of wild-caught and rice-field male Chinese mitten crabs (Eriocheir sinensis). LWT – Food Sci Technol 117:108663
Xia Z, Wu S (2018) Effects of glutathione on the survival, growth performance and non-specific immunity of white shrimps (Litopenaeus vannamei). Fish Shellfish Immunol 73:141–144
Xie F, Zeng W, Zhou Q, Wang H, Wang T, Zheng C, Wang Y (2012) Dietary lysine requirement of juvenile Pacific white shrimp, Litopenaeus vannamei. Aquaculture 358:116–121
Xie SW, Tian LX, Jin Y, Yang HJ, Liang GY, Liu YJ (2014) Effect of glycine supplementation on growth performance, body composition and salinity stress of juvenile Pacific white shrimp, Litopenaeus vannamei fed low fishmeal diet. Aquaculture 418:159–164
Xie SW, Tian LX, Li YM, Zhou W, Zeng SL, Yang HJ, Liu YJ (2015a) Effect of proline supplementation on anti-oxidative capacity, immune response and stress tolerance of juvenile Pacific white shrimp, Litopenaeus vannamei. Aquaculture 448:105–111
Xie SW, Li YT, Zhou WW, Tian LX, Li YM, Zeng SL, Liu YJ (2015b) Effect of γ-aminobutyric acid supplementation on growth performance, endocrine hormone and stress tolerance of juvenile Pacific white shrimp, Litopenaeus vannamei, fed low fishmeal diet. Aquac Nutr 23:54–62
Xu X, Liu X, Tao J (2013) Changes in biochemical composition and digestive enzyme activity during the embryonic development of the marine crab, Charybdis japonica (Crustadea: Decapoda). Zool Sci 30:160–166
Yamaoka LH, Skinner DM (1976) Free amino acid pools in muscle and hemolymph during the molt cycle of the land crab, Gecarcinus lateralis. Comp Biochem Physiol A 55:129–134
Yang X, Xu M, Huang G, Zhang C, Pang Y, Cheng Y (2019) Effect of dietary L-tryptophan on the survival, immune response and gut microbiota of the Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol 84:1007–1017
Ye J, Wang Y, Guo J, Chen J, Pan Q, Shen B (2010) Lysine, methionine and arginine requirements of juvenile Chinese mitten crab (Eriocheir sinensis). J Fish China 34:1541–1548
Yoshinaka R, Sato K, Itoh Y, Nakajima S, MamoruSato S (1989) Content and partial characterization of collagen in crustacean muscle. Comp Biochem Physiol B 94:219–223
Zhang C, Zhang Q, Song X, Pang Y, Song Y, Cheng Y, Yang X (2018) Dietary L-tryptophan modulates the hematological immune and antibacterial ability of the Chinese mitten crab, Eriocheir sinensis, under cheliped autotomy stress. Front Immunol 9:2744
Zhang C, Zhang Q, Song X, Pang Y, Song Y, Wang Y, He L, Lv J, Cheng Y, Yang X (2019) L-tryptophan promotes the cheliped regeneration of Chinese mitten crab (Eriocheir sinensis) through melatonin, serotonin and dopamine involvement. Aquaculture 511:734205
Zhou M, Wang AL, Xian JA (2011) Variation of free amino acid and carbohydrate concentrations in white shrimp, Litopenaeus vannamei: effects of continuous cold stress. Aquaculture 317:182–186
Zhou QC, Zeng WP, Wang HL, Wang T, Wang YL, Xie FJ (2012) Dietary arginine requirement of juvenile Pacific white shrimp, Litopenaeus vannamei. Aquaculture 364:252–258
Zhou QC, Wang YL, Wang HL, Tan BP (2013) Dietary threonine requirements of juvenile Pacific white shrimp, Litopenaeus vannamei. Aquaculture 392:142–147
Zhou M, Wu Z, Liang R, Gu N (2017) Effects of dietary taurine, carnitine and cholesterol supplementation on growth performance and immunological status of Litopenaeus vannamei under cold exposure. Aquac Res 48:1279–1290
Zhu CH, Li GL, Wu TL, Shi SL, Wu ZH (2009) Effects of L-arginine on the humoral immune factors of Litopenaeus vannamei. Mar Sci 33:55–59
Acknowledgments
We thank our students, research assistants and colleagues for helpful discussions. This work was supported by Texas A&M AgriLife Research (H-8200) and funds from Guangdong Yuehai Feeds Group Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Li, X., Han, T., Zheng, S., Wu, G. (2021). Nutrition and Functions of Amino Acids in Aquatic Crustaceans. In: Wu, G. (eds) Amino Acids in Nutrition and Health. Advances in Experimental Medicine and Biology, vol 1285. Springer, Cham. https://doi.org/10.1007/978-3-030-54462-1_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-54462-1_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-54461-4
Online ISBN: 978-3-030-54462-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)