Abstract
This article reviews the mechanical processes associated with digestion in decapod crustaceans. The decapod crustacean gut is essentially an internal tube that is divided into three functional areas, the foregut, midgut, and hindgut. The foregut houses the gastric mill apparatus which functions in mastication (cutting and grinding) of the ingested food. The processed food passes into the pyloric region of the foregut which controls movement of digesta into the midgut region and hepatopancreas where intracellular digestion takes place. The movements of the foregut muscles and gastric mill are controlled via nerves from the stomatogastric ganglion. Contraction rates of the gastric mill and foregut muscles can be influenced by environmental factors such as salinity, temperature, and oxygen levels. Gut contraction rates depend on the magnitude of the environmental perturbation and the physiological ability of each species. The subsequent transit of the digesta from the foregut into the midgut and through the hindgut has been followed in a wide variety of crustaceans. Transit rates are commonly used as a measure of food processing rates and are keys in understanding strategies of adaptation to trophic conditions. Transit times vary from as little as 30 min in small copepods to over 150 h in larger lobsters. Transit times can be influenced by the size and the type of the meal, the size and activity level of an animal and changes in environmental temperature, salinity and oxygen tension. Ultimately, changes in transit times influence digestive efficiency (the amount of nutrients absorbed across the gut wall). Digestive efficiencies tend to be high for carnivorous crustaceans, but somewhat lower for those that consume plant material. A slowing of the transit rate allows more time for nutrient absorption but this may be confounded by changes in the environment, which may reduce the energy available for active transport processes. Given the large number of articles already published on the stomatogastric ganglion and its control mechanisms, this area will continue to be of interest to scientists. There is also a push towards studying animals in a more natural environment or even in the field and investigation of the energetic costs of the components of digestion under varying biotic and environmental conditions will undoubtedly be an area that expands in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The digestive system of crustaceans has been the focus of studies for over 150 years. Earlier works concentrated on the comparative anatomical and morphological features of the gut (e.g. Huxley 1880; Mocquard 1883; Pearson 1908; Calman 1909; Balss 1944). In the past four decades, physiological processes such as enzyme activity, the transport and fate of nutrients, and neural and hormonal control mechanisms have received more attention (e.g. Barker and Gibson 1977, 1978; Lovett and Felder 1989; Christie et al. 1995, 2010; Mente 2003; Johnston and Freeman 2005; Marder and Bucher 2007; Linton and Greenaway 2007; Linton et al. 2009; Stein 2009).
The decapod crustacean digestive system is essentially an internal tube that is divided into three functional areas, the foregut, midgut, and hindgut. The foregut is a highly specialized structure that functions in both mechanical and extracellular digestion. The midgut regulates movement of digesta into the hepatopancreas where intracellular digestion takes place, while rhythmic peristaltic movements of the hindgut expel the peritrophic membrane containing the feces (Dall and Moriarty 1983; Icely and Nott 1992; Conklin 1995; Factor 1995). The muscles of the foregut and gastric mill apparatus are innervated by the stomatogastric nervous system (STNS). The fact that the large, readily identifiable neurons of the STNS can be easily removed and studied in vitro, means that it has become a model system for investigating the functioning of central pattern generators in both invertebrates and vertebrates. Studies of the crustacean STNS have resulted in over 1,000 papers as well as several excellent review articles (Selverston et al. 1976; Selverston and Moulins 1987; Hartline et al. 1988; Harris-Warrick and Marder 1991; Harris-Warrick et al. 1992; Marder and Bucher 2007; Stein 2009). In addition to the nervous control of digestion, there are a number of papers and book chapters that provide an excellent overview of nutrition and digestion in crustaceans (Dall and Moriarty 1983; Icely and Nott 1992; Conklin 1995; Factor 1995; Ceccaldi 1997; Johnston 2007; Saborowski 2013). Rather than reproducing these works here, the goal of this review is to provide a basic overview of the structure and function of the gut to aid in understanding the mechanical mechanisms of gastric processing.
This review focuses on crustaceans from the order Decapoda, but relevant examples from other crustacean groups are also discussed. Two main areas are addressed: first, the mechanical processing of food in the foregut is reviewed. This area focuses on the contraction rates and patterns of gastric mill activity as well as regulation of movement of digesta (pyloric sac activity) into the midgut region. The second area covers the transit/clearance rates of digesta from the system and digestive efficiencies. Gut transit rates are a useful tool for assessing food consumption, allowing the estimation of feeding rates and food conversion (Loya-Javellana et al. 1995). The different methodologies for determining transit rates and the movements of digesta between the three functional areas are reviewed. Finally, the modulatory effects of changes in the physicochemical and biotic environment on the digestive physiology of crustaceans are discussed.
The functional anatomy of the decapod crustacean gut
The crustacean gut is essentially an internal tube opening at the esophagus and ending as the anus in the telson of the abdomen. It is divided into the foregut, midgut, and hindgut (Fig. 1a, b). The mouth opens into a short esophagus, the walls of which are lined with tegumental glands. These glands secrete mucus that lubricates the food as muscular waves propel it towards the foregut. The foregut is lined with cuticle and separated into an anterior cardiac chamber, with a smaller pyloric chamber lying posteriorly (Barker and Gibson 1977, 1978; Icely and Nott 1992; Heeren and Mitchell 1997). The cardiac chamber is a large distensible sac that functions as an area for storage and processing of food. The internal structure of the cardiac chamber varies between species. In most decapods, it is lined with numerous calcified ossicles which function in mechanical digestion. Filtration grooves in the walls of the cardiac chamber channel the food towards the posterior of the chamber which contains the gastric mill apparatus (Felgenhauer and Abele 1983, 1989). The gastric mill is composed of large ossified teeth which masticate the food (Maynard and Dando 1974). Not all decapod crustaceans possess these highly calcified teeth, they may totally absent in some of the lower decapods and replaced by folds with uncalcified ossicles (Felgenhauer and Abele 1983, 1989; Brosing 2010). In these species, the mouthparts tend be more complex and take over the job of the gastric mill (Icely and Nott 1992; Allardyce and Linton 2010). Depending on the species and arrangement of the teeth, the gastric mill cuts, crushes, and/or mixes the food with digestive enzymes. The processed food is then pushed posteriorly towards to the pyloric chamber by rhythmic contraction of the muscles of the foregut (Heinzel 1988). At the junction of the two chambers, cardiopyloric setae in conjunction with the cardiopyloric valve regulate movement of digesta between the cardiac and pyloric chambers. Any coarse unprocessed material captured by the cardiopyloric setae is pushed back into the cardiac chamber and gastric mill for further mechanical digestion. The smaller pyloric chamber is situated posteriorly and ventrally to the cardiac chamber (Fig. 1a, b). Its primary function is sorting of food for subsequent transport into the midgut region (Heinzel 1988; Heinzel et al. 1993). Gland filters in the pyloric region filter out particulate matter, so that only the liquid form of digesta reaches the hepatopancreas. The rhythmic pumping of the pyloric sac also functions in propelling food along the midgut region (Hopkin and Nott 1980; Icely and Nott 1992).
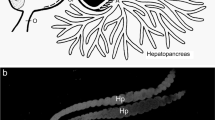
a Anatomy of the digestive system of a decapod crustacean, showing the foregut, midgut, and hindgut regions (modified from Wallace and Taylor 1992). b Schematic diagram of digestive processes occurring in the decapod crustacean gut (modified with permission from Conklin 1995: In The Lobster Homarus americanus. Ed. J. Factor)
The midgut starts at the junction with the pyloric sac and ends in a coiled tube, the posterior midgut caecum at the junction between the carapace and abdomen (Fig. 1a,b; Smith 1978). Unlike the foregut and hindgut which are lined with cuticle, the walls of the midgut are lined with glandular columnar epithelium (Icely and Nott 1992; Johnston 2007). The midgut varies in length depending on species: it tends to be short in some species of crayfish, but extends into the abdomen in the brachyuran crustaceans (Smith 1978; Lovett and Felder 1989). Ducts arise at the junction of the midgut and pyloric sac and branch extensively as blind-ending tubules within the digestive gland or hepatopancreas (Factor 1995). The hepatopancreas (also known as the digestive gland) is a large organ occupying most of the dorsal region of the cepaholathorax and may extend backwards into the abdomen (Gibson and Barker 1979). It is here that enzymatic digestion continues and absorption of food occurs (Hopkin and Nott 1980). The hepatopancreatic ducts contain musculature suggesting that their contraction aids in movement of the digesta through the hepatopancreas (Dall 1967; Johnston et al. 1998). Inert particles are filtered out at the entrance of the ducts and only liquid and particles less than 100 nm in diameter enter the hepatopancreas (Smith 1978; Hopkin and Nott 1980). The hepatopancreas has a number of specialized cells (B, E, F and R cells) within the walls of the tubules (Momin and Rangneker 1974). These are involved with enzyme production and recycling, and absorption of nutrients and water. It is here that intracellular digestion and protein synthesis begin and can continue for up to 2–3 days (Houlihan et al. 1990; Mente 2003; Mente et al. 2003). Most nutrients are absorbed across the tubules of the hepatopancreas, although some may be absorbed directly across the midgut wall. In some crustaceans, blind ending extensions of the midgut, the anterior and posterior midgut caeca are evident (Smith 1978). Their small size suggests that they only play a minor role in digestion, possibly activation or production of some enzymes, maintenance of pH balance or accommodation of volume changes as the foregut contracts (Holliday et al. 1980). They may also play a role in ion and water regulation (Lovett and Felder 1990).
The hindgut arises behind the posterior midgut caecum and runs the length of the abdomen to the anus (Maynard and Dando 1974). It is usually a simple cuticle lined tube, surrounded by outer layers of longitudinal and circular striated muscle (Barker and Gibson 1977, 1978; Johnston and Alexander 1999). It functions in expelling the muco-peritrophic membrane and its contents by rhythmic contractions along its length (Dall and Moriarty 1983). Tegumental glands along the length of the hindgut secrete mucus to lubricate the walls (Gibson 1983). Some digesta may remain in the hindgut and is not voided until a subsequent meal is ingested (Hopkin and Nott 1980). As with other arthropods, the hindgut may also be involved in active ion uptake and transport (Mykles 1979; Factor 1995).
Mechanical processing in the foregut
The muscles and gastric mill apparatus of the foregut are innervated by the stomatogastric nervous system (STNS). The nerves of the STNS synapse on over 40 different skeletal muscles that control the movements of the esophagus (peristalsis), cardiac sac (storage), gastric mill (cutting/grinding), and pyloric sac (filtration of digesta) (Stein 2009; Fig. 2). The STNS consists of four connected ganglia. The prominent stomatogastric ganglion (STG) is located inside the anterior aorta on the dorsal surface of the cardiac stomach. In addition to the STG, there is an esophageal ganglion and the paired commissural ganglia. The muscles surrounding the gastric mill and pyloric sac are innervated by the STG, while the cardiac sac and esophageal pattern generators are located in the commissural ganglia and the esophageal ganglion, respectively (Bucher et al. 2006; Marder and Bucher 2007; Fig. 2).
Diagram of the decapod foregut showing the major muscles involved in moving the gastric mill and the pyloric sac. The main neurons and ganglia of the stomatogastric nervous system (STNS) are also shown (redrawn from Bucher et al. 2006)
Although the anatomy and morphology of the stomach and gastric mill varies somewhat between species (Ngoc-Ho 1984; Allardyce and Linton 2010), common neural networks are evident (Marder and Bucher 2007). However, the interactions, in particular those between the gastric mill and pyloric circuit pattern generators, vary between species (Kilman and Marder 1996; Bucher et al. 2006). All the STG neurons possess multiple receptors and their rhythms are controlled by a variety of pericardial or sinus gland neurohormones and/or STG neurotransmitters (Marder 1976; Marder and Eisen 1984; Weimann et al. 1991; Marder and Weimann 1992; Rezer and Moulins 1992; Weimann 1992; Christie et al. 1995, 2010; Marder et al. 2005; Stein 2009; Christie 2011). The pyloric rhythm is almost continuously expressed in the animal, with a burst frequency of around 1–2 s, although this varies depending on the species and environment (Rezer and Moulins 1983, 1992; Clemens et al. 1998a). In contrast, the gastric mill rhythms are slower and more sporadic (typically 8–20 s) compared with those of the pyloric sac, and become activated in response to feeding (Clemens et al. 1998b, c). The pyloric and gastric rhythms are not controlled separately; instead, there is significant interaction between the two groups of neurons. There can be switching between the neurons that control the two rhythms such that they reset the underlying frequency of either the gastric or pyloric contractions (Weimann et al. 1991; Weimann and Marder 1994; Thuma et al. 2003).
Electromyographic recordings have shown how the neural patterns generated in the STNS manifest themselves on the foregut muscles (e.g. Hartline and Maynard 1975; Hermann and Dando 1977; Rezer and Moulins 1983; Rivera and Marder 1996; Clemens et al. 1998a, b; Stein et al. 2006). However, these articles did not investigate the actual movements of the gastric teeth, but rather relied on the contractions of the muscles themselves to interpret how food was processed in the foregut. To date, there is still very little information how the pattern generators correspond to the actual movements of the gastric mill apparatus in the intact animal.
Heinzel (1988) used an endoscope to study the functioning of the foregut, in vivo, in the lobster, Panulirus interruptus. Two distinct contractions of the gastric mill apparatus are described with intervals of activity ranging between 4 and 70 s. The first “squeezing” motion occurs when the two lateral teeth move towards the midline to contact the medial tooth that is retracting. The lateral teeth do not meet completely and do not contact the rasped surface of the medial tooth, instead only the cusps of each tooth touch (Fig. 3a, b). This movement, which is fairly rapid, squeezes the fluid out of the food and may break hard items such as shell and bone (Heinzel 1988). The second “cutting and grinding” action is more complex (Fig. 3a, c). An inwards movement of the lateral teeth occurs, so that the serrated edges eventually contact one another, performing a cutting motion. During this phase, the medial tooth remains retracted. The lateral teeth then move backwards along the medial tooth. At the same time, the rasped surface of medial tooth moves forwards over the entire surface of the lateral teeth; this action grinds the food (Fig. 3a, c; Heinzel 1988; Heinzel et al. 1993). The cutting and grinding actions are characterized by a longer contraction period than the squeezing movement. The grinding action eventually breaks the food down into a liquid phase which is then passed back across the setal filters into the pyloric region of the foregut (Heinzel et al. 1993).
a Photo of the decapod crustacean gastric mill apparatus showing the left (LL) and right lateral teeth (RL) and the single medial tooth (MT) (reproduced with permission from Marder and Bucher 2007). b Schematic diagram depicting the squeezing motion of the gastric teeth. The cusps of the lateral teeth move inwards to contact the cusp of the medial tooth; at the end of the cycle the lateral teeth move outwards and the medial tooth moves downwards (redrawn from Heinzel 1988). c Schematic diagram showing the cut and grind motion of the gastric mill. The teeth start closer together compared with the squeezing motion. The two lateral teeth move inwards so that the serrated edges contact one another, cutting the food. The lateral teeth remain closed and move backwards over the rasped surface of the medial tooth, at the same time the medial tooth moves forwards, this action grinds the food. The medial tooth retracts and the lateral teeth open at the end of the cycle (redrawn from Heinzel 1988)
Despite the success of the endoscopic method for determining the links between neural pathways and actual gastric mill movements, it was suggested that insertion of the endoscope into the foregut could potentially disrupt some of the activity patterns of the gastric mill (Heinzel 1988; Heinzel et al. 1993). Our lab developed a fluoroscopy method allowing observation of gut contractions in unrestrained crustaceans, in vivo (McGaw 2006). This method substantiates the fact that contractions of the cardiac region of the gut are sporadic across a variety of decapod species (Morris and Maynard 1970; Powers 1973; Heinzel 1988; Heinzel et al. 1993; Clemens et al. 1998a; McGaw 2006, 2007a, 2008, Curtis and McGaw 2010). However, these observations tended to be conducted over fairly short time periods or on unfed animals which may have accounted for the lack of discernible patterns of cardiac stomach activity. If digestion is followed from ingestion of food until the cardiac stomach is emptied (18–24 h), an activity pattern emerges (Fig. 4; McGaw Unpublished data). A pulsating-type movement is observed in the center of the gastric mill (recorded as gastric activity rate) which is overlaid by larger, less frequent contractions of the entire cardiac sac (Fig. 4). The pulsating movement likely represents the small amplitude in and out movement of the accessory teeth, which are controlled by the inferior cardiac and ventricular dilator neurons (Heinzel et al. 1993). These accessory teeth are mechanically coupled with the cardiopyloric valve, which regulates movement of digesta into the pyloric sac (Heinzel et al. 1993). The actual contraction phase of the gastric mill is characterized by a pumping type action, followed by the prolonged contraction of the entire foregut. This pre-pumping, described as an incomplete closure, is termed “pyloric timed pumping of the lateral teeth”, and is controlled by the pyloric pattern generators in the lateral and medial gastric neurons (Heinzel et al. 1993). Foregut contractions increase immediately after feeding, then decrease in frequency over the following 3–6 h (Fig. 4). During this time (3–6 h), the contractions of the cardiac sac occur approximately every 70–100 s and typically last for 1–3 s. As the time since ingestion of the meal increases (12–24 h), these contractions of the cardiac stomach become more frequent and of longer duration. Towards the end of the digestion phase, when the cardiac sac is nearly empty, contractions occur every 15–20 s and the contraction phase lasts between 3 and 10 s. Although the graphs suggest that the pulsatile movement of the accessory teeth decreases with time, this is an artifact due to the decreased time interval between each cardiac stomach contraction; the accessory teeth contractions actually remain fairly steady at about 30–50 min−1 (Fig. 4). The observed changes in gastric activity following feeding are reflected by an increased discharge rate of the neurons supplying the foregut muscles of the gastric mill (Clemens et al. 1998c). Although there is a strong interaction between the gastric and pyloric generator rhythms in unfed animals, these two pattern generators separate following feeding and do not interact again for 24–48 h, which corresponds with the emptying time of the foregut (Clemens et al. 1998b). This allows separation of regional roles of the cardiac and pyloric sac in processing of the food (Clemens et al. 1998a, b).
Patterns of accessory teeth and cardiac stomach contraction in an individual graceful crab, Cancer gracilis, and an individual Dungeness crab, Cancer magister measured using a Lixi® fluoroscope. Foregut activity was assessed (for 300 s) in each species immediately following feeding and at regular intervals until digesta was cleared. The number of accessory teeth movements (defined in text as “pulsatile” movements) are shown over the time interval they were counted. Accessory teeth movements were interrupted by sporadic contractions of the entire cardiac sac which are shown as zero values over the time period that the gut was contracting (McGaw unpublished data). For example, on the first graph, the labelled accessory teeth movements are 52 contractions over a 65 s period, the entire cardiac sac contracts for 2 s (0 value), before the accessory teeth contractions resume again
Contractions of the pyloric sac regulate movement of food into the midgut (Icely and Nott 1992) and are more stable and rapid compared with those of the cardiac region (Heinzel 1988; Clemens et al. 1998a, b). In the fluoroscope preparations, the pyloric contractions appear as small amplitude “pumping” type movements. Contraction rates of 40–80 min−1 agree with burst frequency of the pyloric muscle neurons in a number of decapods (Morris and Maynard 1970; Powers 1973; Heinzel 1988; Heinzel et al. 1993; McGaw 2006, 2007a, 2008; Curtis and McGaw 2010). Pyloric contractions are elevated immediately following the consumption of a meal, but decrease significantly within 1–4 h, stabilizing thereafter (Morris and Maynard 1970; Rezer and Moulins 1983; McGaw 2006, 2007a, 2008; Curtis and McGaw 2010). Activity in the pyloric sac does not cease completely and it continues to contract, albeit at a lower rate, even when the stomach is empty (Clemens et al. 1998a, b). While a suite of neuromodulators is known to affect the STNS (and thus the contraction of the gut), the effect of exogenous sources on the STNS is less well documented. These can include the presence of food material (Powers 1973; Rezer and Moulins 1983; Clemens et al. 1998b) and changes in internal oxygen levels (Massabuau and Meyrand 1996; Clemens et al. 1998b). In addition, the fluoroscopy method has shown that several environmental parameters can modulate the pyloric sac rhythm in intact decapod crustaceans, often in a dose-dependent manner.
The STG lies inside the anterior aorta where it is directly bathed in arterial hemolymph, and changes in internal oxygen levels have been implicated in controlling the STNS. Hemolymph PO2 appears to act in a neuromodulatory fashion and the increase in PO2 associated with feeding causes an acceleration of both gastric and pyloric networks, while the interprandial decreases may allow the system to recover (Massabuau and Meyrand 1996; Clemens et al. 1998b). The Dungeness crab, Cancer magister, is very tolerant of hypoxia (Airriess and McMahon 1994; McGaw 2005). Nevertheless, when the external oxygen tension drops below 5 kPa (25 % O2 saturation), they are unlikely to be able to meet the increased metabolic demands associated with digestion (McGaw 2005). This results in a drop of pyloric contraction rates by about 25 % and a subsequent slowing of the passage of digesta through the gut (McGaw 2008; Fig. 5a). Interestingly, the range of oxygen tensions where a significant decrease in pyloric contraction is observed corresponds closely with their P crit level (McGaw 2008). A similar pattern occurs during short-term emersion in the red rock crab, Cancer productus. During a 6 h period of aerial exposure, the hemolymph PO2 falls from approximately 9 to 2 kPa (McGaw et al. 2009) and pyloric contractions drop from 55–65 min−1 to about 10–15 min−1 (McGaw 2007a). Although the pyloric sac continues to contract slowly, the transit of food through the midgut and hindgut is essentially halted (McGaw 2007a). A higher mortality rate occurs in these postprandial C. productus (compared with unfed animals) due to a decrease in hemolymph pH and PO2 and a rapid build-up of ammonia (McGaw et al. 2009). Consequently, when C. productus is exposed to air, it often regurgitates the contents of the foregut (McGaw 2007a). Rapid waves of contraction start in the posterior region of the cardiac sac forcing food upwards, while simultaneous opening of the esophagus allows expulsion of the food (Fig. 6). This pattern of regurgitation has also been observed in Cancer gracilis exposed to low salinities (McGaw 2006). It is hypothesized that this may be a protective mechanism, removing food that would otherwise be destined for digestion which would put an added physiological stress on the animal (McGaw 2006, 2007a).
Changes in pyloric sac contraction rates (min−1) in three crab species; data represent the means ±SE of 7–10 individuals in each treatment. a Dungeness crabs, Cancer magister were maintained in water of 12 °C and an oxygen tension of 18–21 kPa (85–100 % oxygen saturation), following feeding oxygen tension was dropped to 5 kPa (25 % oxygen saturation) or 1.5 kPa (7.5 % oxygen saturation) and gut parameters followed for a further 20 h (McGaw 2008). b Green crabs, Carcinus maenas, were acclimated to a temperature of 20 °C and fed a radio labeled meal. The temperature was then changed (over 1 h) to either 5, 15 or 25 °C and changes in pyloric contraction rates followed until the foregut was cleared (McGaw and Whiteley 2012). c Graceful crabs, Cancer gracilis, were fed in 100 % seawater (SW), following which the water was changed to 100, 80 or 60 % SW, by the addition of freshwater of ambient temperature. Pyloric contraction rates were followed for a total period of 18 h (McGaw 2006). Letters denote significant differences, like letters are not significantly different from one another (P > 0.05)
Fluoroscope images (dorsal view) of the foregut of a red rock crab, Cancer productus, showing regurgitation of food during aerial exposure. The process starts with vigorous contraction of the foregut muscles squeezing digesta inwards and upwards (arrows). The esophagus (ES) then opens, followed by waves of contraction that force the digesta into the esophagus and out of the mouth (CS = cardiac stomach, PS = pyloric sac) (from McGaw 2007a)
Temperature is one of the most important environmental factors influencing metabolism and would therefore also be expected to influence gastric processing (Robertson et al. 2002; Willmer et al. 2005). In Carcinus maenas acclimated to 10 or 20 °C, pyloric contraction rates are 56–59 and 71–74 min−1, respectively, representing a Q 10 of approximately 1.3. When the crabs are subsequently transferred to either, 5, 15, or 25 °C, the change in rate corresponds directly with the direction and magnitude of the temperature change (Fig. 5b; McGaw and Whiteley 2012). Acute temperature experience, rather than the acclimation temperature, appears to have a more pronounced effect on gastric processing in this species (McGaw and Whiteley 2012).
Low salinity can also modulate gastric activity, and the effects appear to be directly related to the osmoregulatory ability of an animal: the poorer the osmoregulatory ability, the greater the effect on pyloric contraction and thus transit rates. In an efficient osmoregulator such as the blue crab, Callinectes sapidus, pyloric contraction rates are typically 55–65 min−1 and there is no significant change in these rates when animals are transferred to 100, 50, or 25 % seawater (SW) (Curtis and McGaw 2010). In contrast, the Dungeness crab, C. magister, which is classified as a weak regulator (Hunter and Rudy 1975), exhibits a slowing of pyloric contraction rates in salinities below 75 % SW, and it will not feed in salinities below 40 % SW (Curtis and McGaw 2010; Curtis et al. 2010). The graceful crab, C. gracilis, is an osmoconformer and cannot survive in salinities below 55 % SW (Curtis et al. 2007). It exhibits a step-wise decrease in pyloric contraction rates as the salinity decreases, with rates in 60 % SW dropping to about a third of the levels measured in 100 % SW (Fig. 5c; McGaw 2006). It in unclear whether these changes in environmental parameters directly influence the STNS, or whether the changes in gastric processing are the result of the animal being unable to balance the metabolic demands of several physiological processes operating simultaneously (McGaw 2006, 2007b; Curtis and McGaw 2010).
In addition to the contraction of the pyloric sac, peristaltic contractions of the muscles of the midgut and hindgut aid movement of digesta through the intestines (Winlow and Laverack 1972a). However, much less is known about the control mechanisms of the midgut and hindgut compared with the foregut. Coordinated peristaltic contractions of the hindgut are controlled by the central nervous system via nerves originating in the 6th and 7th abdominal segment ganglia (Winlow and Laverack 1972a, b, c). The nerves are not essential for contraction, but rather are used for modulation, as the hindgut also has myogenic pacemaker cells which exhibit spontaneous contractions in vitro (Ebara 1969; Winlow and Laverack 1972a; Brenner and Wilkens 2001). A variety of neurotransmitters and neuropeptides originating in the hindgut can influence the rate and amplitude of contraction of the hindgut (Florey 1961; Jones 1962; Mercier et al. 1997; Mercier and Lee 2002; Wrong et al. 2003; Musolf et al. 2009). The longitudinal muscles primarily influence the rate and amplitude of contraction, while longer sustained contractions are controlled by the circular muscles. The slower tonic-type contractions last for 30–60 s (Wrong et al. 2003). This sustained slow contraction is ideal for movement of wastes along the hindgut and expulsion of feces (Brenner and Wilkens 2001; Wrong et al. 2003). Distinct physiological regions of the hindgut are evident that respond in different ways to neuromodulators (Musolf et al. 2009). For example, the posterior portion of the hindgut acts not only to expel feces but also functions in ion and water uptake (Mykles 1979; Factor 1995) and this region of the gut can undergo forward or reverse peristalsis depending on the animal’s needs (Ebara 1969; Mercier and Lee 2002). Small-scale contractions of the midgut have been observed in vivo, but it is unclear whether or not these are simply vibrations from the upstream contraction of the pyloric sac or actual peristaltic contractions of the midgut (McGaw 2006). Occasional large scale contractions of the whole midgut occur, although these tend to be infrequent, with no discernible pattern (1–2 h−1) (McGaw 2006, 2007a, b, 2008).
Methodology used to monitor gastric processes
A variety of methods have been employed to measure the amount and type of food consumed and its subsequent passage through the digestive system. In herbivorous copepods, a fluorescence method was devised, whereby the decline of chlorophyll a and phaeophyta pigments in the gut are measured (Mackas and Bohrer 1976). Briefly, copepods that have been fed phytoplankton are removed at set intervals (usually in groups), washed, and homogenized with acetone. The filtrate is collected and read on a fluorometer equipped for chlorophyll detection, applying a correction factor for background fluorescence from unfed animals (Ellis and Small 1989; Tirelli and Mayzaud 2005). The decline in pigment levels corresponds to the evacuation of the phytoplankton meal from the gut. Although this method has been applied in numerous studies on zooplankton feeding and egestion rates (e.g. Mackas and Bohrer 1976; Baars and Oosterhuis 1984; Kiorboe et al. 1985; Dagg and Walser 1987; Dam and Peterson 1988; Ellis and Small 1989; Pasternak 1994; Perissinotto and Pakhomov 1996; Tirelli and Mayzaud 2005), it has met with some criticism. Transit and clearance rates may be under-estimated in some cases because the pigments may be degraded, rather than cleared from the gut, and this rate may vary with the type or concentration of the food material and the temperature (Baars and Oosterhuis 1984; Dagg and Walser 1987; Pasternak 1994). The fluorescence method has been validated somewhat by carrying out experiments in conjunction with other methods such as the addition of charcoal tracer particles, enzyme activity, and radioactive (Germanium68) decay rates (Ellis and Small 1989; Peterson et al. 1990; Perissinotto and Pakhomov 1996).
In small, transparent decapod crustaceans, the transit of food through the gut and production rate of feces can be measured by visual observation. In the shrimps, Palaemonetes pugio, Farfantepenaeus aztecus, Litopenaeus setiferus and Macrobrachium borellii, the passage of the meal can be viewed directly through the translucent body (Hoyt et al. 2000; Beseres et al. 2005, 2006; Carvalho et al. 2011). Visualization is often enhanced with some type of marker in the food, such as inert latex fluorescent beads of 2–5 μm (Hoyt et al. 2000; Beseres et al. 2005, 2006), fluorescent dye (Serrano 2012), or bright red copepod prey items (Murtaugh 1984). This method not only allows total gut passage time (first production of feces) to be determined but also transit time through regions of the gut to be calculated as the markers are readily visible in the gut without having to resort to extensive disturbance to, or dissection of the animals (Beseres et al. 2005, 2006).
In larger decapod crustaceans, the thickened carapace does not allow direct observation of the gut. Gastric clearance rates have been measured with a radioactive marker (CO57). Small spheres (15 μm diameter) of the marker are suspended in solution and introduced into the crab’s stomach just before feeding. The resultant feces are collected and transit times calculated by measuring the excretion of radioactive material (0.5–99.5 %) in the feces (Greenaway and Linton 1995; Greenaway and Raghaven 1998). However, this method only gives a time for clearance of the entire gut system. Dall (1967) used radioactive silver (Ag110) particles of 1–5 μm which were ground up with food to form pellets. The radioactivity levels in the animal were then measured with a scintillation counter. The animal was placed over the counter, a small hole was drilled in a lead brick and the animal maneuvered over the hole. This allowed the passage of the radioactive material to be followed through different regions of the gut. However, the primary method that has been used for determining the passage of food in larger decapod crustaceans involves sacrificing the animal with the subsequent removal of the gut and examination of the contents. Originally, this method was used to determine the type and quantity of prey items in the diet of animals collected in the field (Hill 1976; Williams 1981; Choy 1986; Cockcroft and McLachlan 1986; Maynou and Cartes 1998; Cristo 2001). It has subsequently been modified in the lab; here, a serial slaughter technique is used to determine how rapidly certain food items are degraded in the gut. Groups of animals are fed and digestion is then stopped at set intervals by chilling the animals in iced seawater (Hill 1976; Wassenberg and Hill 1987, 1993; Loya-Javellana et al. 1995; Nunes and Parsons 2000; Soares et al. 2005; Waddington 2008), by separation of abdomen and thorax (Joll 1982), or immersing the animals in 10 % formalin (Cristo 2001). The gut is then dissected out from the animal and gut fullness is estimated visually using a stomach repletion (percent fullness) index (Reymond and Lagardtre 1990; Nunes et al. 1997) or by weighing the contents (Hill 1976; Wolcott and Wolcott 1984, 1987; Sarda and Valladares 1990). The actual evacuation rate of the stomach can be calculated using an exponential equation derived by Sarda and Valladares (1990) and modified by Waddington (2008).
where W t is the mass of stomach contents at time t, W O is the mass of the food ingested, R is the evacuation rate and t is the time in hours.
Although serial slaughter has been used extensively, it involves sacrificing the animals and only gives a “snapshot” of what is happening at a set time. An X-ray method, originally used for fish (Talbot and Higgins 1983; Carter et al. 1995; Jobling et al. 1995), has been modified for use with crustaceans (McGaw and Reiber 2000; Thomas et al. 2002; Ahvenharju and Ruohonen 2005; McGaw 2006). Here, a contrast medium (barium sulfate, electrolytic iron powder, Ballatoni lead glass beads) is added to the food. The amount of food eaten and the passage of the marker can then be traced over time. Unlike fish which consume whole food items, decapod crustaceans are messy feeders that tear the food apart before ingestion (Barker and Gibson 1977; Ahvenharju and Ruohonen 2005). Therefore, the contrast medium and prey items have to be mixed together with a binding agent such as gelatin or agar. The animals consume this meal and pass it through the entire gastric system (Thomas et al. 2002; Ahvenharju and Ruohonen 2005; McGaw 2006, 2008). The contrast markers are small enough that they are not sorted out by the pyloric setal filter, and if used in low enough concentration (<15 % by mass), the marker appears to have no effect on the gastric transit rates (McGaw and Reiber 2000). The animals can either be sacrificed and a still image taken using standard X-ray machine (Bayer et al. 1979; McGaw and Reiber 2000) or the meal can be traced in real time using a fluoroscope (Thomas et al. 2002; Ahvenharju and Ruohonen 2005; McGaw 2006). The use of a fluoroscope allows the meal to be followed through entire gut system of individual, undisturbed animals. In addition to transit of the digesta, contractions of the gut can also be measured (Bayer et al. 1979; McGaw 2006, 2007a, b, 2008; Curtis and McGaw 2010; McGaw and Whiteley 2012).
While the above methods are useful for determining food intake and transit and evacuation rates, they are limited in that they only follow the solid phase of digestion through the gut. In crustaceans, the final stages of extracellular and intracellular digestion take place in the hepatopancreas and only the liquid phase of digesta and particles smaller than 100 nm pass into the hepatopancreas (Bayer et al. 1979; Hopkin and Nott 1980). In order to follow the time course of digestive processes within the hepatopancreas, Hopkin and Nott (1980) used colloidal particles of gold (5–30 nm) and thorium oxide (10–20 nm). The feed was mixed with a solution of colloidal gold or thorium oxide and left for 2 days to allow the label to permeate the food, before being fed to the animals. The gold and thorium particles are taken up into the tubules of the hepatopancreas. The crabs are sacrificed by injection of a fixative and the samples are then examined using an X-ray microanalyzer under a transmission electron microscope (Hopkin and Nott 1980). This allows the timing of food processing in the hepatopancreas and the subsequent removal of waste from the hepatic tubules, back into the midgut and hindgut to be measured.
Transit and evacuation rates
The terms gastric or gut evacuation, emptying, residence, retention, passage, transit, and clearance times have been used interchangeably to describe the rate of processing and movement of ingested food through the stomach or gut (Loya-Javellana et al. 1995). For crustaceans, clearance of food from the stomach and/or clearance times of the entire system have typically been quantified. Gastric transit and evacuation rates have been used extensively for assessing food consumption, and calculating feeding and food conversion rates (Loya-Javellana et al. 1995). Penry and Jumars (1986, 1987) modeled copepod guts as chemical reactors and indicated that gut transit time is one of the key processes to study in order to understand strategies of adaptation to trophic conditions. These measures are also important indicators of absorption and assimilation rates. Animals may be able to alter transit rates, slowing the passage of food to extract nutrients or speeding up transit rates if a meal is nutrient poor (Mitra and Flynn 2007). Thus, changes in gut transit times maximize energy uptake and growth rate (Taghon 1981; Ahrens et al. 2001). Environmental factors, animal size, meal size, amount or quality of prey, and the activity levels of the crustaceans can all influence these processes (Loya-Javellana et al. 1995).
There is a large variance in stomach clearance times between species and even within a species (Table 1), ranging from as little as 30 min in copepods to over 120 h in lobsters (Dam and Peterson 1988; Tirelli and Mayzaud 2005; McGaw and Curtis, in preparation). Undoubtedly, the type of meal plays a large part in determining transit times (Joll 1982; Choy 1986; Sarda and Valladares 1990; Greenaway and Raghaven 1998; Beseres et al. 2005; Simon and Jeffs 2008; Waddington 2008; Carvalho et al. 2011). The softer parts of prey items such as muscle tissue are cleared at a faster rate, while harder parts take longer to digest and may be present in the stomach for 2–5 days after feeding (Hill 1976; Choy 1986, Sarda and Valladares 1990; Waddington 2008). In some cases, materials such as shell and bone fragments are eventually regurgitated, rather than being passed through the system (Hill 1976; Choy 1986). The size of the meal will also affect relative transit times, and for crustaceans, the general trend is that the larger the meal, the faster it moves through the gut system (Murtaugh 1984; Dagg and Walser 1987; Haddon and Wear 1987; Tirelli and Mayzaud 2005). There does not appear to be a common pattern of meal transit times in relation to animal size. Gut transit times are faster in smaller euphausiids, Meganyctiphanes norvegica (Heyraud 1979) and in the crabs Ucides cordatus (Nordhaus et al. 2006) and Ovalipes catharus (Haddon and Wear 1987), whereas transit times are longer in large spiny lobsters, Jasus edwardsii (Simon and Jeffs 2008) and in larger juvenile Homarus gammarus (Kurmaly et al. 1990). In crayfish, Cherax quadricarinatus (Loya-Javellana et al. 1995), prawn, Penaeus subtilis (Nunes and Parsons 2000) and the shrimps Farfantepenaeus paulensis and Farfantepenaeus aztecus (Soares et al. 2005; Beseres et al. 2006), no difference in transit rate as a function of body size is evident.
Many decapod crustaceans develop through a series of planktonic larval stages before settling on the benthos; the different feeding strategies and nutrient requirements of these larval stages also influence transit times. The general trend is that herbivorous larvae clear the gut at a significantly faster rate than carnivorous species (Jones et al. 1997; LeVay et al. 2001; Serrano 2012). In herbivorous species, high ingestion rates, coupled with a greater production of enzymes and a fast turnover of food, maximize the energy extraction from the substrate poor meal (LeVay et al. 2001). For carnivorous species where the prey is more limiting, slower transit rates maximize assimilation of the high quality meal (Jones et al. 1997; LeVay et al. 2001). Differences in transit rates also occur within a species: the zoeal stages of the mud crab, Scylla serrata, have a lower retention time of 80 min compared with 120–135 min measured for the megalopa and first crab instar (Genodepa et al. 2006; Serrano 2012). Serrano (2012) suggested that since each of the larval stages of S. serrata were consuming similar meals that a genetically determined component influences transit times during development.
Transit times may also be influenced by the experimental set-up and methodology used to record the transit time (Wolcott and Wolcott 1984, 1987), sex-related differences (McGaw and Reiber 2000; Curtis and McGaw 2010), starvation (Kurmaly et al. 1990), or differing activity levels (McGaw 2007b). During increased activity, resources are diverted away from the gastric organs and towards the muscles (DeWachter and McMahon 1996). This results in a doubling in gut clearance times for active versus inactive crabs (McGaw 2007b).
In addition to internal biotic factors, environmental changes have a noticeable effect on gut transit rates. Temperature is one of the most important factors governing both metabolic reactions and animal distribution (Willmer et al. 2005), and is probably the most important external factor influencing transit rates (Tables 1, 2). A direct relationship exists between the temperature and transit rates, with meals evacuated at a faster rate at higher temperatures (Table 2). In O. catharus, an 11 °C increase in temperature results in a 3–6 fold increase in foregut clearance times (Haddon and Wear 1987; Table 2), while for C. maenas, gut clearance rates are 6–7 times faster after a 15 °C rise in temperature (McGaw and Whiteley 2012; Table 2). A partial metabolic compensation occurs in a number of different taxa during acclimation to cooler temperatures: metabolic enzymes are up-regulated, this results in a higher metabolic rate when the animal subsequently experiences an acute temperature increase (Hochachka and Somero 2002; Gamperl 2011). C. maenas is no exception, and metabolic rates of 10 °C acclimated crabs are higher than 20 °C acclimated crabs when transferred to water of 15 or 25 °C (McGaw and Whiteley 2012). Even though temperature acclimation has a large effect on the metabolism of Carcinus maenas, it does not appear to affect clearance rates in the gut (McGaw and Whiteley 2012; Table 2). This may be explained by the fact that although the activities of digestive enzymes are temperature dependent, they are regulated via different pathways than metabolic enzymes (Somero 1978; Dittrich 1992). Intracellular enzymes regulating metabolic rates may be incorporated into multiple pathways or physiological systems, and will therefore be highly sensitive to temperature acclimation. However, the digestive enzymes are somewhat different. They do not function in regulated, multi-enzyme pathways, rather digestive enzymes are released in a pulse shortly after ingestion of food. Unlike intracellular metabolic enzymes which function over prolonged periods and have their activity altered in response to changing metabolic demands, digestive enzymes are activated when needed and must achieve their maximal catabolic properties very rapidly in response to changing temperature conditions. Thus, the need for breakdown of food rather than regulatory activity may lead to different selective pressures on digestive versus regulatory metabolic enzymes (Somero 1978; McGaw and Whiteley 2012).
Crustaceans inhabiting shallow coastal zones are likely to experience hyposaline exposure from either freshwater run-off or within an estuarine environment. The effects of low salinity on transit rates are interesting because they depend not only on the actual level of salinity experienced but also on the osmoregulatory ability of the species (Table 2; Fig. 8). The graceful crab, Cancer gracilis is classified as an osmoconformer and cannot survive in salinities below about 60 % SW (Curtis et al. 2007). In 100 % SW, the gastric system is cleared within 40 h (Figs. 7, 8a). When the salinity is reduced slightly to 80 % SW, the passage of digesta into the midgut and hindgut regions is slowed (Fig. 7), resulting in a doubling of gut clearance times (Fig. 8a). During exposure to 60 % SW, although some food may be regurgitated (Fig. 7; 60 % SW), there is a considerable slowing of the passage of digesta, with crabs taking over 96 h to clear the gut (Fig. 8a). The Dungeness crab, C. magister is classified as a weak osmoregulator; it can survive short-term exposure to salinities as low as 25 % SW (Hunter and Rudy 1975; McGaw et al. 1999), but will not feed in salinities below 40 % SW (Curtis et al. 2010; Curtis and McGaw 2011). Clearance rates of the fore, mid, and hindgut regions are quite rapid in 100 and 75 % SW, but gut contractions and thus clearance rates are 2.5 times longer in 50 % SW (Fig. 8b). Although increased temperature has a pervasive effect on transit rates (Table 2), when an increased temperature is combined with low salinity exposure, the low salinity, not the temperature is more important in determining the gut clearance rates in C. magister (Table 2; Curtis 2009). In contrast to the two Cancer species, the blue crab, C. sapidus, is a very efficient osmoregulator inhabiting estuaries where it can spend extended periods in full freshwater (Mangum and Amende 1967; McGaw et al. 1999). In order to feed and digest efficiently, it would be expected that low salinity should have a minimal impact on nutrient uptake. This is the case, and contraction rates of the gut, clearance of digesta and digestive efficiency are unaltered during hyposaline exposure (Fig. 8c; Curtis 2009; Curtis and McGaw 2010).
Fluoroscope images showing the transit of a radio-labeled meal through the digestive system of the graceful crab, Cancer gracilis, in 100 % seawater (SW), and in reduced salinities of 80 and 60 % SW. Following feeding (1 h), the foregut appears as a large heart shaped mass in the anterior area of each image. Food enters the midgut region after 3 h in 100 and 80 % SW, and is visible in the hindgut as a coiled tube thereafter (100 % SW at 9 h). In the individual crab in 60 % SW digesta does not appear to enter the midgut or hindgut, yet the contents of the foregut are significantly reduced at 9 h. This is due to regurgitation of foregut contents in this individual crab (McGaw 2006)
Clearance times of digesta from the foregut, midgut and hindgut regions of a Cancer gracilis, an osmoconformer b Cancer magister, a weak osmoregulator and c Callinectes sapidus, an efficient osmoregulator, exposed to different salinity regimes. Data shown are mean values (±SE) of 7–10 animals. Different letters indicate significant differences at the P < 0.05 level. Data were adapted from McGaw (2006) and Curtis and McGaw (2010)
Hypoxia is also prevalent in aquatic environments (Diaz and Rosenburg 1995). Changes in arterial oxygen levels act in a neuromodulatory fashion on the STNS of the lobster, Homarus americanus, leading to a slowing and uncoupling of the pyloric and gastric pattern generator rhythms (Massabuau and Meyrand 1996; Clemens et al. 1998b). Although the Dungeness crab, C. magister, is very tolerant of low oxygen, when oxygen levels fall below its P crit level, there is a decrease in contraction of the foregut (Fig. 5a), leading to a substantial increase in clearance time of the gut (McGaw 2008; Table 2). Likewise, when the red rock crab, C. productus is aerially exposed, a rapid drop in hemolymph PO2 ensues (McGaw et al. 2009) and the passage of digesta through the gut is essentially halted (McGaw 2007a). This suggests that maintenance of an optimal hemolymph PO2 level is required for efficient contraction of the gut and subsequent absorption of nutrients.
Digestive efficiency
Changes in transit rates of digesta will ultimately affect the digestive or assimilation efficiency of the food, a measure of how much of the nutrients have been absorbed across the gut wall; in many crustaceans, there is a strong correlation between the transit time and digestive efficiency (LeVay et al. 2001; Serrano 2012). It is often referred to as apparent digestive efficiency because there are some endogenous sources of nutrients such as the peritrophic membranes and degraded enzymes that may lead to the underestimation of digestion (Leavitt 1985). Digestive efficiencies can be measured gravimetrically (total collection) or by use of an inert marker in the food (Leavitt 1985). Both require collection of food and feces and subsequent measurement of their components. For the gravimetric method, the intake rate of food and total output of feces are measured. A sample of the food and the feces are dried and total energy content is determined using a bomb calorimeter (Romero et al. 2006). The digestive efficiency (total energy) is expressed as a percentage and calculated as:
where I is the caloric content of ingested food, gi is the dry mass of the food consumed, F is the caloric content of the feces, and gf is the dry mass of all feces produced (Romero et al. 2006).
Using the gravimetric method can be problematic if only a small amount of feces is produced or if the feces disintegrates in water; in such cases the use of a marker is more appropriate (Smith and Tabrett 2004). Inert substances such as chromic oxide, ytterbium oxide, or dry ash are typically used as markers in the feed. While each marker has its caveats, an ideal marker cannot be absorbed across the gut and must have an homogenous distribution in the digesta and feces (Newman et al. 1982, Leavitt 1985; Jones and De Silva 1997, 1998; Smith and Tabrett 2004; Simon and Jeffs 2008; Cox et al. 2009). Since the concentration of marker in the food, not the total amount eaten is measured, and it is assumed that the marker is passed through and out of the gut at a constant rate, only a sample of food and feces needs to be collected (Jones and De Silva 1998). The concentration of the CrO3 and YbO3 markers in the food and feces are determined spectrophotometrically or, if dry ash is analyzed, the amount is determined after combustion at 550 °C in a muffle furnace (Newman et al. 1982; Leavitt 1985; Jones and De Silva 1997, 1998; Smith and Tabrett 2004; Simon and Jeffs 2008; Cox et al. 2009) The following equation is an example of those used to determine assimilation rates when using markers:
where M FI and M F are the concentration of marker in the food and feces, respectively, and A FI and A F are the concentration of the nutrient (or analyte) in the food and feces, respectively (Smith and Tabrett 2004).
There are a large number of articles on nutrient assimilation in commercial species of crustaceans: these concentrate on the assimilation of both natural and artificial feeds with respect to survival and growth rates. Here, not only the overall digestive (energy) efficiency is measured but also the assimilation of proteins, lipids, carbohydrates, dry matter and individual amino acids is quantified (e.g. Landry et al. 1984; Kurmaly et al. 1990; Brunson et al. 1997; Kumlu 1999; Ward et al. 2003; Cruz-Suárez et al. 2009). Discussion of this area of aquaculture production is beyond the scope of the present review; here, we concentrate on digestive efficiency pertaining to total energy assimilation only, and how this relates to transit rates. Digestive efficiencies tend to be relatively high for crustaceans (Romero et al. 2006); meals of animal material are assimilated at levels of approximately 75 % or higher (Table 3). Although the amount of energy absorbed is influenced by the intake rate (how much is eaten), the actual components of the food are more important. Assimilation tends to decrease as the content of ash and fiber increase and with an increase in the C/N ratio (low protein levels), thus digestive efficiency tends to be considerably lower for herbivorous decapods (Wolcott and Wolcott 1984; 1987; Dye and Lasiak 1987; Kwok and Lee 1995; Nordhaus and Wolff 2007; Table 3). Protein digestion is usually high and in general the higher the protein content the higher the digestive efficiency (Brunson et al. 1997). However, the efficiency of protein digestion depends on its quality and the amount of non-protein energy available (protein/energy content) such as the levels of lipids and to a lesser degree the levels of carbohydrates in the meal (Bautista 1986; Hewitt and Irving 1990; Ward et al. 2003). These substances have a sparing effect and the proteins do not have to be catabolized as an energy source.
The slowing of the passage of a meal through the gut allows more time for absorption of nutrients and it is expected that digestive efficiency will be higher for an animal with slower transit rates (Kurmaly et al. 1990; LeVay et al. 2001; Mitra and Flynn 2007). In C. maenas, the slowing of food passage at lower temperatures does allow for more nutrient extraction, and digestive efficiency is higher at lower temperatures (McGaw and Whiteley 2012; Table 3). In contrast, Newman et al. (1982) found no effect of a temperature range of 22–34 °C on digestive efficiency in the prawn, Macrobrachium rosenbergii. These authors suggest that the enzymes in this species function equally well over this temperature range, nevertheless the digestive efficiencies may have been confounded somewhat by differences in the amount of food ingested at the different temperature regimes (Newman et al. 1982). A slowing of transit rates also occurs in the Dungeness crab, C. magister, when exposed to 50 % SW, resulting in a slight increase in digestive efficiency (Curtis 2009; Curtis and McGaw 2010). However, the correlation between transit rates and digestive efficiency does not hold true for all cases. During exposure to severe hypoxia (<5 kPa oxygen), C. magister exhibits a twofold increase in transit time, which is associated with a decrease rather than an increase in digestive efficiency (McGaw 2008). In this case, although transit rates are slowed, the delivery of oxygen to the tissues may not be sufficient enough to support the increased energy demand needed for active transport of nutrients across the gut wall (McGaw 2008).
The age and size of an animal can also influence the absorption of nutrients. Digestive efficiencies increase during larval development of the lobster, Homarus gammarus, the crab, Mennipe mercenaria (Mootz and Epifanio 1974; Kurmaly et al. 1990), and through the juvenile stages (V-XII) of H. americanus (Logan and Epifanio 1978). In the shrimp, Penaeus stylirostris, the smaller size shrimps also have lower digestive efficiencies (Fenucci et al. 1982). In contrast, small Litopenaeus vannamei tend to process proteins more efficiently and grow faster than their medium or large counterparts (Smith et al. 1985). Differences in digestive efficiency related to sex have also been noted; female galatheid crabs, Munida gregaria, are more efficient at processing nutrients. This is possibly related to the extra nutrients required for egg production and maintenance (Romero et al. 2006).
Conclusions and future directions
Judging by the large number of articles already generated, and the continued interest in the STNS and foregut control mechanisms as a model system, this area of research will undoubtedly continue to expand and develop over the coming years. In particular, determining interactions between hormonal and neural control pathways will continue to be important (Marder and Bucher 2007; Christie et al. 2010, 2011). Data on cardiac and pyloric contraction rates obtained in vivo by fluoroscopy would suggest that the STNS of different species may have different thresholds or different responses to environmental change which is dependent on the physiological ability of the species. Nevertheless, the articles on the STNS are restricted to comparatively few species, and only a handful of articles have investigated the effects of environmental change on the central pattern generators (e.g. Massabuau and Meyrand 1996; Clemens et al. 1998b) or functioning of the gastric mill in situ (Heinzel 1988; Heinzel et al. 1993). Thus, studies with a wider array of species in differing environmental conditions will be important. This area could expand as we devise new ways to investigate functioning of the nervous system in vivo. In conjunction with studies on the STNS, development of new remote sensing methods and miniaturization of equipment could be used for investigation of animal feeding and digestion in a more natural environment, or even in the field (Costa and Sinervo 2004; Goldstein and Pinshow 2006; Grans et al. 2009; Mykles et al. 2010).
The ingestion and subsequent processing of food is associated with a general increase in metabolism, usually measured as an increase in oxygen uptake. This is termed the specific dynamic action of food or SDA (Secor 2009). In crustaceans, this results in a 2–4 fold increase in oxygen uptake that can remain elevated for over 3 days (Houlihan et al. 1990; McGaw and Reiber 2000; Whiteley et al. 2001; Robertson et al. 2002; Romero et al. 2006; Curtis and McGaw 2010; McGaw and Whiteley 2012). These increases in metabolism are connected with the increased activity associated with feeding, the mechanical cost of processing the meal, up-regulation and release of enzymes, nutrient transport and absorption, and the subsequent intracellular protein synthesis (Carefoot 1990; Houlihan et al. 1990; Mente et al. 2003). Although protein synthesis is thought to account for the bulk of the increase in oxygen uptake (Houlihan et al. 1990; Mente et al. 2003; Whiteley and Faulkner 2005), to date, the energetic cost of the individual components has not been fully worked out for any species (Secor 2009). Thus, calculation of how the metabolic costs associated with mechanical breakdown, nutrient absorption, and transport, and the subsequent intracellular digestion that are allocated under varying biological and environmental conditions represents a pressing issue for digestive physiologists.
References
Ahrens MJ, Hertz J, Lamoureux EM, Lopez GR, McElroy AE, Brownawell BJ (2001) The role of digestive surfactants in determining bioavailability of sediment-bound hydrophobic organic contaminants to two deposit-feeding polychaetes. Mar Ecol Prog Ser 212:145–157
Ahvenharju T, Ruohonen K (2005) Individual food intake measurement of freshwater crayfish (Pacifastacus leniusculus Dana) juveniles. Aquac Res 36:1304–1312
Airriess CN, McMahon BR (1994) Cardiovascular adaptations enhance tolerance of environmental hypoxia in the crab Cancer magister. J Exp Biol 190:23–41
Aldrich JC (1974) Studies on energy relationships in the spider crab Libinia emarginata (Leach). Biol Bull 147:257–273
Allardyce BJ, Linton SM (2010) Functional morphology of the gastric mills of carnivorous, omnivorous, and herbivorous land crabs. J Morphol 271:61–72
Al-Mohanna SY (1983) The structure and function of the hepatopancreas of the shrimp Penaeus semisulcatus De Haan (Crustacea Decapoda). PhD Thesis, University of Wales, pp 1–391
Baars MA, Oosterhuis SS (1984) Diurnal feeding rhythms in North Sea copepods measured by gut fluorescence, enzyme activity and grazing on labelled food. Neth J Sea Res 18:97–119
Balss H (1944) Crustacea, Decapoda. In: Klassen und Ordnungen des Tierreiches. Band 5, Abteilung 1, Buch 7, Lieferung 3, pp 419–470
Barker PL, Gibson R (1977) Observations on the feeding mechanism, structure of the gut and digestive physiology of the European lobster Homarus gammarus (L.). (Decapoda: Nephropidae). J Exp Mar Biol Ecol 26:234–297
Barker PL, Gibson R (1978) Observations on the structure of the mouthparts, histology of the alimentary tract and digestive physiology of the mud crab Scylla serrata (Forskal) (Decapoda: Portunidae). J Exp Mar Biol Ecol 32:177–196
Bautista MN (1986) The response of Penaeus monodon juveniles to varying protein/energy ratios in test diets. Aquaculture 53:229–242
Bayer RC, Gallagher ML, Leavitt DF, Rittenburg JH (1979) A radiographic study of the lobster (Homarus americanus) alimentary canal. In: Proceedings of 10th Annual World Mariculture Society, Baton Rouge, LA, pp 561–564
Beseres JJ, Lawrence AL, Feller RJ (2005) Variation in fiber, protein and lipid content of shrimp feed—effects of gut passage time measured in the field. J Shell Res 24:301–308
Beseres JJ, Lawrence AL, Feller RJ (2006) Practical equivalence of laboratory and field measurements of gut passage time in two penaeid shrimp species. Mar Ecol Prog Ser 309:221–231
Brenner TL, Wilkens JL (2001) Physiology and excitation contraction coupling in the intestinal muscle of the crayfish Procambarus clarkii. J Comp Physiol 171:613–621
Brosing A (2010) Recent developments on the morphology of the brachyuran foregut ossicles and gastric teeth. Zootaxa 2510:1–44
Brunson JF, Romaire RP, Reigh RC (1997) Apparent digestibility of selected ingredients in diets for white shrimp Penaeus setiferus L. Aquac Nutr 3:9–16
Bucher D, Taylor AL, Marder E (2006) Central pattern generating neurons simultaneously express fast and slow rhythmic activities in the stomatogastric ganglion. J Neurophysiol 95:3617–3632
Calman WT (1909) Crustacea. Part 7. Third fascicle. In: Lankester ER (ed) A treatise on zoology. Adam and Black, London, p 346
Carefoot TH (1990) Specific dynamic action (SDA) in the supralittoral isopod, Ligia pallasii: identification of components of apparent SDA and effects of dietary amino acid quality and content on SDA. Comp Biochem Physiol 95A:309–316
Carter CG, McCarthy ID, Houlihan DF, Fonesca N, Perera WMK, Sillah ABS (1995) The application of radiograph to the study of fish nutrition. J Appl Icthy 11:231–239
Carvalho DA, Collins PA, De Bonis CJ (2011) Gut Evacuation time of Macrobrachium borellii (Caridea: Palaemonidae) feeding on three types of prey from the littoral-benthic community. J Crust Biol 31:630–634
Ceccaldi HJ (1997) Anatomy and physiology of the digestive system. In: Abramo LR, Conklin DE, Akiyama DM (eds) Advances in world aquaculture, Crustacean nutrition. The world aquaculture society, vol 6. Louisiana state university, Baton Rouge, pp 261–291
Choy SC (1986) Natural diet and feeding habits of the crabs Liocarcinus puber and L. holsatus (Decapoda, Brachyura, Portunidae). Mar Ecol Prog Ser 31:87–99
Christie AE (2011) Crustacean neuroendocrine systems and their signaling agents. Cell Tiss Res 120:1011–1023
Christie AE, Skiebe P, Marder E (1995) Matrix of neuromodulators in neurosecretory structures of the crab Cancer borealis. J Exp Biol 198:2431–2439
Christie AE, Stemmler EA, Dickinson PS (2010) Crustacean neuropeptides. Cell Mol Life Sci 67:4135–4169
Clemens S, Combes D, Meyrand P, Simmers J (1998a) Long term expression of two interacting motor pattern generating networks in the stomatogastric system of freely behaving lobster. J Neurophysiol 79:1396–1408
Clemens S, Massabuau JC, Legeay A, Meyrand P, Simmers J (1998b) In vivo modulation of interacting central pattern generators in lobster stomatogastric ganglion: influence of feeding and partial pressure of oxygen. J Neurosci 18:2788–2799
Clemens S, Meyrand P, Simmers J (1998c) Feeding induced changes in temporal patterning of muscle activity in the lobster stomatogastric system. Neurosci Lett 254:65–68
Cockcroft A, McLachlan A (1986) Food and feeding habits of the surf zone penaeid prawn Macropetasma africanus (Balss). PSZNI Mar Ecol 7:345–357
Conklin DE (1995) Digestive Physiology and nutrition. In: Factor J (ed) The lobster Homarus americanus. Academic Press, San Diego, pp 441–464
Costa DP, Sinervo B (2004) Field physiology: physiological insights from animals in nature. Ann Rev Physiol 66:209–238
Cox SL, Bruce MP, Ritar AJ (2009) Ingestion of artificial diets with different textures as determined by the inert marker ytterbium oxide during culture of early stage pyllostoma of the spiny lobster Jasus edwardsii. Aquac Nutrit 17:152–158
Cristo M (2001) Gut evacuation rates in Nephrops norvegicus, (L. 1758), Laboratory and field estimates. Sci Mar 65:341–346
Cruz-Suárez LE, Tapia-Salazar M, Villarreal-Cavazos D, Beltran-Rocha J, Nieto-López MG, Lemme A, Ricque-Marie D (2009) Apparent dry matter, energy, protein and amino acid digestibility of four soybean ingredients in white shrimp Litopenaeus vannamei juveniles. Aquaculture 292:87–94
Curtis DL (2009) Integrated responses of crustaceans living in estuaries to the challenges of feeding and digestion in low salinity. PhD Thesis, University of Nevada, Las Vegas
Curtis DL, McGaw IJ (2010) Respiratory and digestive responses of postprandial Dungeness crabs, Cancer magister and blue crabs, Callinectes sapidus during hyposaline exposure. J Comp Physiol B 180:189–198
Curtis DL, McGaw IJ (2011) A possible feeding control mechanism in Dungeness crabs during hyposaline exposure. J Crust Biol 31:313–316
Curtis DL, Jensen EK, McGaw IJ (2007) Behavioural influences on the physiological responses of the graceful crab, Cancer gracilis during hyposaline exposure. Biol Bull 212:222–231
Curtis DL, Vanier CH, McGaw IJ (2010) The effects of starvation and acute low salinity exposure on food intake in the Dungeness crab, Cancer magister. Mar Biol 157:603–612
Dagg MJ, Walser WEJ (1987) Ingestion, gut passage, and egestion by the copepod Neocalanus plumchrus in the laboratory and in the subarctic Pacific Ocean. Limnol Oceanogr 32:178–188
Dall W (1967) The functional anatomy of the digestive tract of a shrimp Metapaneus bennettae Racke and Dall (Crustacean, Decapoda, Penaeidae). Aust J Zool 15:699–714
Dall W, Moriarty DJW (1983) Nutrition and digestion. In: Bliss DE (ed) The Biology of Crustacea Vol. 5. Internal anatomy and physiological regulation. Academic Press, New York, pp 215–261
Dam HJ, Peterson WT (1988) The effect of temperature on the gut clearance rate constant of planktonic copepods. J Exp Mar Biol Ecol 123:1–14
DeWachter B, McMahon BR (1996) Haemolymph flow distribution, cardiac performance and ventilation during moderate walking activity in Cancer magister (Dana) (Decapoda, Crustacea). J Exp Biol 199:627–633
Diaz RJ, Rosenburg R (1995) Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr Mar Biol Annu Rev 33:245–303
Dittrich BU (1992) Life under extreme conditions: aspects of evolutionary adaptation to temperature in crustacean proteases. Polar Biol 12:269–274
Dye AH, Lasiak TA (1987) Assimilation efficiencies of fiddler crabs and deposit-feeding gastropods from tropical mangrove sediments. Comp Biochem Physiol 87A:341–344
Ebara A (1969) Spontaneous activity of crayfish intestine. Annot Zool Jpn 42:169–175
Ellis SG, Small LF (1989) Comparison of gut evacuation rate of feeding and non-feeding Calanus marshallae. Mar Biol 103:175–181
Factor JR (1995) The digestive system. In: Factor JR (ed) Biology of the Lobster Homarus americanus. Academic Press, San Diego, pp 395–440
Felgenhauer BE, Abele LG (1983) Phylogenetic relationships among the shrimp-like decapods. In: Schram F (ed) Crustacean Phylogeny. Crustacean Issues 1, pp 291–311
Felgenhauer BE, Abele LG (1989) Evolution of the foregut in lower Decapoda. In: Felgenhauer BE, Watling L, Thistle AB (eds) Functional morphology of feeding and grooming in Crustacea. Crustacean Issues 6, pp 205–219
Fenucci JL, Fenucci AC, Lawrence AL, Zein-Eldin ZP (1982) The assimilation of protein and carbohydrate from prepared diets by the shrimp, Penaeus stylirostris. Proc World Maricult Soc 13:134–145
Florey E (1961) A new test preparation for bio-assay of factor I and gamma-aminobutyric acid. J Physiol (Lond) 156:1–7
Forster JRM (1972) Some methods of binding prawn diets and their effects on growth and assimilation. J Cons Int Explor Mer 34:200–216
Forster JRM, Gabbott PA (1971) The assimilation of nutrients from compounded diets by the prawns Palaemon serratus and Pandalus platyceros. J Mar Biol Assoc UK 51:943–961
Gamperl AK (2011) Integrated responses of the circulatory system to temperature. In: Farrell AP (ed) Encyclopedia of fish physiology. Elsevier, Amsterdam
Genodepa J, Southgate PC, Zeng C (2006) Determining ingestion of microbound diet particles by mud crab, Scylla serrata, larvae. J Fish Aquatic Sci 1:244–252
Gibson R (1983) Feeding and digestion in decapod crustaceans. In: Pruder G, Langdon CJ, Conklin DE (eds) Proceedings of the 2nd international conference on aquaculture nutrition: Biochemical and physiological approaches to shellfish nutrition. Louisiana State University, Baton Rouge, pp 59–70
Gibson R, Barker PL (1979) The Decapod hepatopancreas. Oceanogr Mar Biol Ann Rev 17:285–346
Goldstein DL, Pinshow P (2006) Taking physiology to the field: using physiological approaches to answer questions about animals in their environments. Physiol Biochem Zool 79:237–241
Grans A, Axelsson M, Pitsillides K, Olsson C, Hojesjo J, Kaufman RC, Cech JJ Jr (2009) A fully implantable multi-channel biotelemetry system for measurement of blood flow and temperature: a first evaluation in the green sturgeon. Hydrobiologia 619:11–25
Greenaway P, Linton SM (1995) Dietary assimilation and food retention time in the herbivorous terrestrial crab Gecarcoidea natalis. Physiol Zool 68:1006–1028
Greenaway P, Raghaven S (1998) Digestive strategies in two species of leaf-eating land crabs (Brachyura: Gecarcinidae) in a rain forest. Physiol Zool 71:36–44
Haddon M, Wear RG (1987) Biology of feeding in the New Zealand paddle crab Ovalipes catharus (Crustacea, Portunidae). New Zealand J Mar Fresh Res 21:55–64
Harris-Warrick RM, Marder E (1991) Modulation of neural networks for behavior. Annu Rev Neurosci 14:39–57
Harris-Warrick RM, Marder E, Selverston AI, Moulins M (1992) Dynamic biological networks: the stomatogastric nervous system. MIT Press, Cambridge
Hartline DK, Maynard DM (1975) Motor patterns in the stomatogastric ganglion of the lobster Panulirus argus. J Exp Biol 62:405–420
Hartline DK, Russell DK, Raper JA, Graubard K (1988) Special cellular and synaptic mechanisms in motor pattern generation. Comp Biochem Physiol C 91:115–131
Heeren T, Mitchell BD (1997) Morphology of the mouthparts, gastric mill and digestive tract of the giant crab Pseudocarcinus gigas (Milne Edwards) (Decapoda, Oziidae). Mar Freshwater Res 48:7–18
Heinzel HG (1988) Gastric mill activity in the lobster. I. Spontaneous modes of chewing. J Neurophysiol 59:528–550
Heinzel HG, Weimann JM, Marder E (1993) The behavioral repertoire of the gastric mill in the crab, Cancer pagurus: an in situ endoscopic and electrophysiological examination. J Neurosci 13:1793–1803
Hermann A, Dando MR (1977) Mechanism of command fibre operation onto bursting pacemaker neurones in the stomatogastric ganglion of the crab, Cancer pagurus. J Comp Physiol 114A:15–33
Hewitt DR, Irving MG (1990) Oxygen consumption and ammonia excretion of the brown tiger prawn Penaeus esculentus fed diets of varying protein content. Comp Biochem Physiol 96A:379–381
Heyraud M (1979) Food ingestion and digestive transit time in the euphausiid Meganyctiphanes norvegica as a function of animal size. J Plank Res 4:301–311
Hill BJ (1976) Natural food, foregut clearance rate and activity of the crab Scylla serrata. Mar Biol 34:109–116
Hochachka PW, Somero GN (2002) Biochemical adaptation: mechanism and process in physiological evolution. Oxford University Press, Oxford
Holliday CW, Mykles DL, Terwilliger RC, Dangott LJ (1980) Fluid secretion by the midgut caeca of the crab, Cancer magister. Comp Biochem Physiol 67A:259–263
Hopkin SP, Nott JA (1980) Studies of the digestive cycle of the shore crab Carcinus maenas with special reference to the B cells in the hepatopancreas. J Mar Biol Assoc UK 60:867–877
Houlihan DF, Waring CP, Mathers E, Gray C (1990) Protein synthesis and oxygen consumption of the shore crab Carcinus maenas after a meal. Physiol Zool 63:735–756
Hoyt M, Fleeger JW, Siebeling R, Feller RJ (2000) Serological estimation of prey-protein gut-residence time and quantification of meal size for grass shrimp consuming meiofaunal copepods. J Exp Mar Biol Ecol 248:105–119
Hunter CH, Rudy PR (1975) Osmotic and ionic regulation in the Dungeness crab, Cancer magister dana. Comp Biochem Physiol 51:439–447
Huxley TH (1880) The crayfish. An introduction to the study of zoology. International Scientific Series No. 28, C. Keagan Paul and Co., London, p 371
Icely JD, Nott JA (1992) Digestion and absorption: digestive system and associated organs. In: Harrison FW, Humes AG (eds) Microscopic anatomy of the invertebrates Vol 10. Decapod Crustacea. Wiley, New York, pp 147–201
Jobling M, Arnesen AM, Baardvik BM, Christiansen JS, Jorgensen EH (1995) Monitoring feeding behaviour and food intake: methods and application. Aquacult Nutr 1:131–143
Johnston DJ (2007) Feeding, morphology and digestive system of slipper lobsters. In: Lavalli KL and Spanier E (eds) Biology and fisheries of the slipper lobster. CRC Press, Boca Raton, pp 111–132
Johnston DJ, Alexander CG (1999) Functional morphology of the mouthparts and alimentary tract of the slipper lobster Thenus orientalis (Decapoda: Scyllaridae). Mar Freshwater Res 50:213–223
Johnston D, Freeman J (2005) Dietary preference and digestive enzymes activities as indicators of trophic resource utilization by six species of crab. Biol Bull 208:36–46
Johnston DJ, Alexander CG, Yellowlees D (1998) Epithelial cytology and function in the digestive gland of Thenus orientalis (Decapoda: Scyllaridae). J Crust Biol 18:271–278
Joll LM (1982) Foregut evacuation of four foods by the western lobster Panulirus cygnus in aquaria. Aus J Mar Freshwater Res 33:939–943
Jones HC (1962) The action of l-glutamic acid and of structurally related compounds on the hind gut of the crayfish. J Physiol (Lond) 164:295–300
Jones PL, De Silva SS (1997) Influence of differential movement of the marker chromic oxide and nutrients on digestibility estimations in the Australian freshwater crayfish Cherax destructor. Aquaculture 154:323–336
Jones PL, De Silva SS (1998) Comparison of internal and external markers in digestibility studies involving the Australian freshwater crayfish Cherax destructor Clark (Decapoda, Parastacidae). Aquac Res 29:487–493
Jones DA, Kumlu M, LeVay L, Fletcher DJ (1997) The digestive physiology of herbivorous, omnivorous and carnivorous crustacean larvae: a review. Aquaculture 155:285–295
Kilman VL, Marder E (1996) Ultrastructure of the stomatogastric ganglion neuropil of the crab, Cancer borealis. J Comp Neurol 374:362–375
Kiorboe T, Mohlenberg F, Riisgard HU (1985) In situ feeding rates of planktonic copepods: a comparison of four methods. J Exp Mar Biol Ecol 88:67–81
Kumlu M (1999) Feeding and digestion in larval decapod crustaceans. Tr J Biol 23:215–229
Kurmaly K, Jones DA, Yule AB (1990) Acceptability and digestion of diets fed to larval stages of Homarus gammarus and the role of dietary conditioning behaviour. Mar Biol 106:181–190
Kwok PW, Lee SY (1995) The growth performances of two mangrove crabs, Chiromanthes bidens and Parasesarma plicata under different leaf litter diets. Hydrobiologia 295:141–148
Landry MR, Hassett RP, Fagerness V, Downs J, Lorenzen CJ (1984) Effect of food acclimation on assimilation efficiency of Calanus pacificus. Limnol Oceanogr 29:361–364
Lasenby DC, Langford RR (1973) Feeding and assimilation of Mysis relicta. Limnol Oceanogr 18:280–285
Leavitt DF (1985) An evaluation of gravimetric and inert marker techniques to measure digestibility in the American lobster. Aquaculture 47:131–142
LeVay L, Jones DA, Puello-Cruz AC, Sangha RS, Ngamphongsai C (2001) Digestion in relation to feeding strategies exhibited by crustacean larvae. Comp Biochem Physiol 128A:623–630
Linton SM, Greenaway P (2007) A review of feeding and nutrition of herbivorous land crabs: adaptations to low quality plant diets. J Comp Physiol B 177:269–286
Linton SM, Allardyce BA, Hagen W, Wencke P, Saborowski R (2009) Food utilisation and digestive ability of aquatic and semi-terrestrial crayfishes, Cherax destructor and Engaeus sericatus (Astacidae, Parastacidae). J Comp Physiol B 179:493–507
Logan D, Epifanio C (1978) A laboratory energy balance for the larvae and juveniles of the American lobster Homarus americanus. Mar Biol 47:381–389
Lovett DL, Felder DL (1989) Ontogeny of gut morphology in the white shrimp Penaeus setiferus (Decapoda, Penaeidae). J Morphol 201:253–272
Lovett DL, Felder DL (1990) Ontogenic changes enzyme distribution and midgut function in developmental stages of Penaeus setiferus (Crustacea, Decapoda, Penaeidae). Biol Bull 178:144–159
Loya-Javellana GN, Fielder DR, Thorne MJ (1995) Foregut evacuation, return of appetite and gastric fluid secretion in the tropical freshwater crayfish, Cherax quadricarinatus. Aquaculture 134:295–306
Mackas D, Bohrer R (1976) Fluorescence analysis of zooplankton gut contents and an investigation of diel feeding patterns. J Exp Mar Biol Ecol 25:77–85
Mangum CP, Amende LM (1967) Blood osmotic concentration of blue crabs (Callinectes sapidus, Rathbun), found in freshwater. Chesapeake Sci 13:318–320
Marder E (1976) Cholinergic motor neurones in the stomatogastric system of the lobster. J Physiol 257:63–86
Marder E, Bucher D (2007) Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol 69:291–316
Marder E, Eisen JS (1984) Transmitter identification of pyloric neurons: electrically coupled neurons use different neurotransmitters. J Neurophysiol 51:1345–1361
Marder E, Weimann JM (1992) Modulatory control of multiple task processing in the stomatogastric nervous system. Pergamon Press, New York
Marder E, Bucher D, Schulz DJ, Taylor AL (2005) Invertebrate central pattern generation moves along. Curr Biol 15:R685–R699
Marte CL (1980) The food and feeding habit of Penaeus monodon Fabricius collected from Makato River, Aklan, Philippines (Decapoda Natantia). Crustaceana 38:225–236
Massabuau JC, Meyrand P (1996) Modulation of a neural network by physiological levels of oxygen in lobster stomatogastric ganglion. J Neurosci 16:3950–3959
Maynard DM, Dando MR (1974) The structure of the stomatogastric neuromuscular system in Callinectes sapidus, Homarus americanus, and Panulirus argus (Decapoda Crustacea). Philos Trans Roy Soc Lond B 268:161–220
Maynou F, Cartes JE (1998) Daily ration estimates and comparative study of food consumption in nine species of deep-water decapod crustaceans of the NW Mediterranean. Mar Ecol Prog Ser 171:221–231
McGaw IJ (2005) Does feeding limit cardiovascular modulation in the Dungeness crab Cancer magister during hypoxia? J Exp Biol 208:83–91
McGaw IJ (2006) Feeding and digestion in low salinity in an osmoconforming crab, Cancer gracilis. II Gastric motility and evacuation. J Exp Biol 209:3777–3785
McGaw IJ (2007a) Gastric processing and evacuation during emersion in the red rock crab Cancer productus. Mar Fresh Behav Physiol 40:117–131
McGaw IJ (2007b) The interactive effects of exercise and feeding on oxygen uptake, activity levels and gastric processing in graceful crab, Cancer gracilis. Physiol Biochem Zool 80:335–343
McGaw IJ (2008) Gastric processing in the Dungeness crab, Cancer magister, during hypoxia. Comp Biochem Physiol 150A:458–463
McGaw IJ, Reiber CL (2000) Integrated physiological responses during feeding and digestion in the blue crab Callinectes sapidus. J Exp Biol 203:359–368
McGaw IJ, Whiteley NM (2012) Effects of acclimation and acute temperature change on specific dynamic action and gastric processing in the green shore crab, Carcinus maenas. J Therm Biol 37:570–578
McGaw IJ, Reiber CL, Guadagnoli JA (1999) Behavioral physiology of four crab species in low salinity. Biol Bull 196:163–176
McGaw IJ, Curtis DL, Ede JD, Ong KJ, van Breukelen F, Goss GG (2009) Physiological responses of postprandial red rock crabs, Cancer productus, during emersion. Can J Zool 87:1158–1169
Mente E (2003) Nutrition, physiology and metabolism of crustaceans. Science Publishers Inc, Enfield, p 160
Mente E, Legeay A, Houlihan DF, Massabuau JC (2003) Influence of oxygen partial pressure on protein synthesis in feeding crabs. Am J Physiol Regul Integr Comp Physiol 284:R500–R510
Mercier AJ, Lee J (2002) Differential effects of neuropeptides on circular and longitudinal muscles of the crayfish hindgut. Peptides 23:1751–1757
Mercier AJ, Lange AB, TeBrugge V, Orchard I (1997) Evidence for proctolin-like and RFamide-like neuropeptides associated with the hindgut of the crayfish Procambarus clarkii. Can J Zool 75:1208–1225
Mitra A, Flynn KJ (2007) Importance of interaction between food quality, quantity and gut transit time on consumer feeding growth and trophic dynamics. Am Nat 169:632–646
Mocquard F (1883) Recherches anatomiques sur l’estomac des Crustacés Podophtalmaires. Thése Fac Sc Paris 505:315
Momin MA, Rangneker PV (1974) Histochemical localization of acid and alkaline phosphatases and glucose-6-phosphatase of the hepatopancreas of the crab, Scylla serrata, (Forskal). J Exp Mar Biol Ecol 14:1–16
Mootz CA, Epifanio CE (1974) An energy budget for Menippe mercenaria larvae fed artemia nauplii. Biol Bull 146:44–55
Moriarty DJW (1976) Quantitative studies on bacteria and algae in the food of the mullet Mugil cephalus L. and the prawn Metapenaeus bennettae (Racek and Dall). J Exp Mar Biol Ecol 22:131–143
Morris J, Maynard DM (1970) Recordings from the stomatogastric system of intact lobsters. Comp Biochem Physiol 33:969–974
Murtaugh PA (1984) Variable gut residence time: problems in inferring feeding rate from stomach fullness of a mysid crustacean. Can J Fish Aquat Sci 41:1287–1293
Musolf BE, Spitzer N, Antonsen BL, Edwards DH (2009) Serotonergic modulation of crayfish hindgut. Biol Bull 217:50–64
Mykles DL (1979) Ultrastructure of alimentary epithelia of lobsters, Homarus americanus and H. gammarus, and crab Cancer magister. Zoo-Morphologie 92:201–215
Mykles DL, Ghalambor CK, Stillman JH, Tomanek L (2010) Grand challenges in comparative physiology: integration across disciplines and across biological levels of organization. Integ Comp Biol 50:6–16
Newman MW, Lutz PL, Snedaker SC (1982) Temperature effects on feed digestion and assimilation efficiency of nutrients by the Malaysian prawn Macrobrachium rosenbergii (de Man). J World Maricul Soc 13:95–103
Ngoc-Ho N (1984) The functional anatomy of the foregut of Povcelfunu plutychehs and a comparison with Gulutheu squumifevu and Upogebia deltuuru (Crustacea: Decapoda). J Zool Lond 203:511–535
Nordhaus I, Wolff M (2007) Feeding ecology of the mangrove crab Ucides cordatus (Ocypodidae): food choice, food quality and assimilation efficiency. Mar Biol 151:1665–1681
Nordhaus I, Wolff M, Diele K (2006) Litter processing and population food intake of the mangrove crab Ucides cordatus in a high intertidal forest in northern Brazil. Est Coast Shelf Sci 67:239–250
Nunes AJP, Parsons GJ (2000) Size-related feeding and gastric evacuation measurements for the Southern brown shrimp Penaeus subtilis. Aquaculture 187:133–151
Nunes AJP, Gesteira TCV, Goddard S (1997) Food consumption and assimilation by the Southern brown shrimp Penaeus subtilis under semi-intensive culture in NE Brazil. Aquaculture 149:121–136
Pasternak AF (1994) Gut fluorescence in herbivorous copepods: an attempt to justify the method. Hydrobiologia 292(293):241–248
Pearson M (1908) Cancer, the edible crab. Liverpool Mar Biol Assoc Memoirs 16:1–217
Penry DL, Jumars PA (1986) Chemical reactor analysis and optimal digestion. Bioscience 36:310–315
Penry DL, Jumars PA (1987) Modeling animal guts as chemical reactors. Am Nat 129:69–96
Perissinotto R, Pakhomov EA (1996) Gut evacuation rates and pigment destruction in the Antarctic krill Euphausia superba. Mar Biol 125:47–54
Peterson W, Painting S, Barlow R (1990) Feeding rates of Calanoides carinatus: a comparison of five methods including evaluation of the gut fluorescence method. Mar Eco Prog Ser 63:85–92
Powers LW (1973) Gastric mill rhythms in intact crabs. Comp Biochem Physiol 46A:767–783
Reymond H, Lagardtre JP (1990) Feeding rhythms and food of Penaeus japonicus Bate (Crustacea, Penaeidae) in salt water ponds: role of halophilic entomofauna. Aquaculture 81:125–143
Rezer E, Moulins M (1983) Expression of the crustacean pyloric pattern generator in the intact animal. J Comp Physiol 153:17–28
Rezer E, Moulins M (1992) Humoral induction of pyloric rhythmic in lobster stomatogastric ganglion: in vivo and in vitro studies. Biol Bull 163:209–230
Rivera JC, Marder E (1996) TNRNFLRFamide and SDRNFLRFamide modulate muscles of the stomatogastric system of the crab Cancer borealis. J Comp Physiol A 179:741–751
Robertson RF, Meagor J, Taylor EW (2002) Specific dynamic action in the shore crab, Carcinus maenas (L.), in relation to acclimation temperature and to the onset of the emersion response. Physiol Biochem Zool 75:350–359
Romero MC, Lovrich GA, Tapella F (2006) Seasonal Changes in dry mass and energetic content of Munida subrugosa (Crustacea Decapoda) in the Beagle Channel, Argentina. J Shell Res 25:101–106
Saborowski R (2013) Nutrition and digestion. In: Chang ES, Thiel M (eds) The natural history of Crustaceans, vol 4 (in press)
Sarda F, Valladares FJ (1990) Gastric evacuation of different foods by Nephrops norvegicus (Crustacea: Decapoda) and estimation of soft tissue ingested, maximum food intake and cannabalism in captivity. Mar Biol 104:25–30
Schwamborn R, Criales MM (2000) Feeding strategy and daily ration of pink shrimp (Farfantepenaeus duorarem) in a south Florida seagrass bed. Mar Biol 137:139–147
Secor SM (2009) Specific dynamic action: a review of the postprandial metabolic response. J Comp Physiol B 179:1–56
Selverston AI, Moulins M (1987) The crustacean stomatogastric system. Springer, Berlin
Selverston AI, Russell DF, Miller JP, King DG (1976) The stomatogastric nervous system: structure and function of a small neural network. Prog Neurobiol NY 7:215–290
Serrano AE (2012) Changes in gut evacuation time of larval mud crab Scylla serrata (Crustacea: Portunidae) fed on artificial plankton or live food. AACL Bioflux 5:240–248
Simon CJ, Jeffs A (2008) Feeding and gut evacuation of cultured juvenile spiny lobsters, Jasus edwardsii. Aquaculture 280:211–219
Smith RI (1978) The midgut caeca and the limits of the hindgut of brachyura: a clarification. Crustaceana 35:195–205
Smith DM, Tabrett SJ (2004) Accurate measurement of in vivo digestibility in shrimp feeds. Aquaculture 46(232):563–580
Smith LL, Lee PG, Lawrence AL, Strawn K (1985) Growth and digestibility by three sizes of Penaeus vannamei Boone: effects of dietary protein level and protein source. Aquaculture 46:85–96
Soares R, Wasielesky W, Peixotob S, D’Incao F (2005) Food consumption and gastric emptying of Farfantepenaeus paulensis. Aquaculture 250:283–290
Somero GN (1978) Temperature adaptation of enzymes: biological optimization through structure-function compromises. Ann Rev Ecol Syst 9:1–29
Stein W (2009) Modulation of stomatogastric rhythms. J Comp Physiol A 195:989–1009
Stein W, Smarandache CR, Nickmann M, Hedrich UB (2006) Functional consequences of activity-dependent synaptic enhancement at a crustacean neuromuscular junction. J Exp Biol 209:1285–1300
Taghon GL (1981) Beyond selection: optimal ingestion rate as a function of food value. Am Nat 118:202–214
Talbot C, Higgins PJ (1983) A radiographic method for feeding studies on fish using metallic iron powder as a marker. J Fish Biol 23:211–220
Thomas CW, Carter CG, Crear BJ (2002) Potential use of radiography for measuring feed intake of southern rock lobster (Jasus edwardsii). J Exp Mar Biol Ecol 273:289–298
Thuma JB, Morris LG, Weaver AL, Hooper SL (2003) Lobster (Panulirus interruptus) pyloric muscles express the motor patterns of three neural networks, only one of which innervates the muscles. J Neurosci 23:8911–8920
Tirelli V, Mayzaud P (2005) Relationship between functional response and gut transit time in the calanoid copepod Acartia clausi: role of food quantity and quality. J Plank Res 27:557–568
Waddington K (2008) Variation in evacuation rates of different foods skew estimates of diet in the western rock lobster Panulirus cygnus. Mar Fresh Res 59:347–350
Wallace RL, Taylor WK (1992) Invertebrate zoology, a laboratory manual, 6th edn. Prentice Hall, Upper Saddle River, p 356
Ward LR, Carter CG, Crear BJ, Smith DM (2003) Optimal dietary protein level for juvenile southern rock lobster, Jasus edwardsii, at two lipid levels. Aquaculture 217:483–500
Wassenberg TJ, Hill BJ (1987) Feeding by the sand crab Portunus pelagicus on material discarded from prawn trawlers in Moreton Bay, Australia. Mar Biol 95:387–393
Wassenberg TJ, Hill BJ (1993) Diet and feeding behaviour of juvenile and adult banana prawns Penaeus merguiensis in the Gulf of Carpentaria, Australia. Mar Ecol Prog Ser 94:287–295
Weimann JM (1992) Multiple task processing in neural networks: numerous central pattern generators in the stomatogastric nervous system of the crab Cancer borealis. PhD Thesis, Brandies University
Weimann JM, Marder E (1994) Switching neurons are integral members of multiple oscillatory networks. Curr Biol 4:896–902
Weimann JM, Meyrand P, Marder E (1991) Neurons that form multiple pattern generators: identification and multiple activity patterns of gastric/pyloric neurons in the crab stomatogastric system. J Neurophys 65:111–122
Whiteley NM, Faulkner LS (2005) Temperature influences whole-animal rates of metabolism but not protein synthesis in a temperate intertidal isopod. Physiol Biochem Zool 78:227–238
Whiteley NM, Robertson RF, Meagor J, El Haj AJ, Taylor EW (2001) Protein synthesis and specific dynamic action in crustaceans: effects of temperature. Comp Biochem Physiol 128A:595–606
Wilcox JR, Jeffries HP (1974) Feeding habits of the sand shrimp Crangon septemspinosa. Biol Bull 146:424–434
Wilde JE, Linton SM, Greenaway P (2004) Dietary assimilation and the digestive strategy of the omnivorous anomuran land crab Birgus latro (Coenobitidae). J Comp Physiol B 174:299–308
Williams MJ (1981) Methods for the analysis of natural diet in the portunid crabs (Crustacea: Decapoda: Portunidae). J Exp Mar Biol Ecol 52:103–113
Willmer P, Stone G, Johnston I (2005) Environmental physiology of animals, 2nd edn. Blackwell, Malden, p 754
Winlow W, Laverack MS (1972a) The control of hindgut motility in the lobster, Homarus gammarus (L.) 1. Analysis of hindgut movements and receptor activity. Mar Behav Physiol 1:1–27
Winlow W, Laverack MS (1972b) The control of hindgut motility in the lobster, Homarus gammarus (L.) 2. Motor output. Mar Behav Physiol 1:29–47
Winlow W, Laverack MS (1972c) The control of hindgut motility in the lobster Homarus gammarus (L.) 3. Structure of the sixth abdominal ganglion (6 A.G.) and associated ablation and microelectrode studies. Mar Behav Physiol 1:93–121
Wolcott DL, Wolcott TG (1984) Food quality and cannibalism in the red land crab, Gecarcinus lateralis. Physiol Zool 57:318–324
Wolcott DL, Wolcott TG (1987) Nitrogen limitation in the herbivorous land crab Cardisoma guanhumi. Physiol Zool 60:262–268
Wrong AD, Sammahin M, Richardson R, Mercier AJ (2003) Pharmacological properties of l-glutamate receptors associated with the crayfish hindgut. J Comp Physiol A 189:371–378
Acknowledgments
We would like to thank the Director and staff of the Bamfield Marine Sciences Centre for use of facilities. This work was supported by and NSERC Discovery grant to IJM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.D. Hume.
Rights and permissions
About this article
Cite this article
McGaw, I.J., Curtis, D.L. A review of gastric processing in decapod crustaceans. J Comp Physiol B 183, 443–465 (2013). https://doi.org/10.1007/s00360-012-0730-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-012-0730-3