Abstract
l-Glutamate (Glu) has traditionally not been considered as a nutrient needed in diets for humans and other animals (including swine) due to the unsubstantiated assumption that animals can synthesize sufficient amounts of Glu to meet their needs. The lack of knowledge about Glu nutrition has contributed to suboptimal efficiency of global livestock production. Over the past 25 years, there has been growing interest in Glu metabolism in the pig, which is an agriculturally important species and also a useful model for studying human biology. Because of analytical advances in its analysis, Glu is now known to be a highly abundant free amino acid in milk and intracellular fluid, a major constituent of food and tissue proteins, and a key regulator of gene expression, cell signaling, and anti-oxidative reactions. Emerging evidence shows that dietary supplementation with 2% Glu maintains gut health and prevents intestinal dysfunction in weanling piglets, while enhancing their growth performance and survival. In addition, the inclusion of 2% Glu is required for dietary arginine to maximize the growth performance and feed efficiency in growing pigs, whereas dietary supplementation with 2% Glu reduces the loss of skeletal muscle mass in endotoxin-challenged pigs. Furthermore, supplementing 2% Glu to a corn- and soybean-meal-based diet promotes milk production by lactating sows. Thus, an adequate amount of dietary Glu as a quantitatively major nutrient is necessary to support maximum growth, development, and production performance of swine. These results also have important implications for improving the nutrition and health of humans and other animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
l-Glutamate (Glu) is one of the most abundant amino acids (AAs) in food and animal tissues, and has received growing interest from nutritionists due to its enormous roles in metabolism and physiology (Blachier et al. 2009; Brosnan and Brosnan 2013; Hu et al. 2017; Wu 2018). Historically, the definition of “nutrient requirement” referred to the amount of a nutrient in the diet necessary to prevent metabolic disorders that result in impaired growth and even death of animals (Maynard et al. 1979). To date, in human nutrition, as well as the modern livestock industries (McDonald et al. 2011; Wu 2018), nutrient requirement is assessed to optimize the health, growth, development, and productivity of the organisms. Of particular note, Glu has recently been considered as a nutritionally essential AA for intestinal and whole-body homeostasis in neonates (Hou and Wu 2017; Rezaei et al. 2013a). In the case of animal agriculture, meat and egg producers must also take into account such factors as market weight, feed cost, maintenance cost, feed efficiency, genetic strain, as well as industry and environmental sustainability, when deciding whether to supplement animal feed with Glu or other AAs. An increase in feed efficiency, calculated as body weight (BW) gain/feed intake, can reduce the costs of producing market-weight swine (Patience et al. 2015). Thus, consideration of what constitutes a nutrient requirement includes economic, environmental, and biological or physiologic factors. Because the pig is anatomically, physiologically and biochemically similar to the human, and is also an agriculturally important species, this review focuses on Glu metabolism, nutrition and safety in swine.
Glutamate metabolism in pigs
Knowledge of Glu metabolism in pigs provides a foundation for understanding the basis of Glu nutrition. Glu was traditionally not recognized as a nutritionally essential AA for any age of pigs, because it was thought to be synthesized sufficiently in the body (Maynard et al. 1979). However, modern breeds of pigs grow faster, gain more lean tissues, and gestate more fetuses (Strathe et al. 2017; Weber et al. 2015) and, therefore, have greater requirements for Glu, when compared with previous breeds (Wu et al. 2014). Results of recent studies have shown that: (1) dietary Glu is a major energy substrate in the small intestine of pigs (Reeds et al. 2000; Reeds et al. 1996) and (2) sufficient provision of dietary Glu can enhance villus height and whole-body growth in weanling pigs (Rezaei et al. 2013a). In addition, Glu can improve barrier and anti-oxidative functions in porcine small-intestinal epithelial cells (Jiao et al. 2015). Similarly, dietary supplementation with Glu plus aspartate can alleviate oxidative stress in weaned piglets challenged with hydrogen peroxide (Duan et al. 2016). Furthermore, Kang et al. (2017) reported that dietary supplementation with 2% glutamate alleviated muscle protein loss by activating mechanistic target of rapamycin (mTOR) and inhibiting the expression of proinflammatory factors. Collectively, these findings indicate important nutritional and physiological roles of Glu in pigs. To date, adequate provision of dietary Glu is particularly important in the pig industry, because low-protein diets, which are currently used to reduce the excretion of nitrogenous wastes from swine, do not sufficiently supply Glu or its AA precursors (Hou et al. 2016). Thus, there is an urgent need to reevaluate the dietary Glu requirements of modern breeds of pigs during their nursery, weaning, growing-finishing, gestating and lactating periods. To achieve this goal, it is imperative to fully understand Glu metabolism (synthesis and catabolism) in pigs.
Glutamate synthesis in pigs
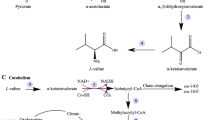
Nearly all cells are capable of forming Glu from Gln, BCAAs, alanine, and aspartate via different enzymes (e.g., phosphate-activated glutaminase, Gln:fructose-6-phosphate transaminase, BCAA transaminase, Glu-pyruvate transaminase; and Glu-oxaloacetate transaminase). However, net synthesis of Glu occurs in a cell- and tissue-specific manner. H.A. Krebs described in 1935 the conversion of Gln into Glu via glutaminase in animal tissues containing mitochondria. In pigs, glutaminase (a mitochondrial enzyme) exists in two isoforms (liver and kidney types) that are encoded by two different genes and differ in biochemical properties. Phosphate-activated glutaminase in extrahepatic tissues and cells is the kidney type (Krebs 1935). Differences in catalytic kinetics and regulation between kidney- and liver-type glutaminases were originally identified by H.A. Krebs in 1935. Specifically, liver-type glutaminase absolutely requires NH3 for activation, has high Km for Gln and high affinity for phosphate, and is not affected by low Glu concentration. In contrast, kidney-type glutaminase does not require NH3 for activation, has low Km for Gln and low affinity for phosphate, is subject to inhibition by low Glu concentration. Another enzyme that can generate Glu from Gln is Gln:fructose-6-phosphate transaminase (cytosolic), which is particularly abundant in red blood cells and endothelial cells (Wu et al. 2001). This reaction may be the major source of Glu in cells (e.g., mammalian red blood cells) that lack mitochondria and do not take up extracellular Glu. The endogenous sources of Glu in pigs are shown in Fig. 1.
Endogenous sources of glutamate in pig tissues. Adapted from Wu (2018). Enzymes that catalyze the indicated reactions are: 1, branched-chain amino acid transaminase; 2, glutamate–oxaloacetate transaminase; 3, glutamate-pyruvate transaminase; 4, phosphate-activated glutaminase; 5, glutamine:fructose-6-phosphate transaminase; 6, enzymes for intracellular protein degradation; and 7, glutamate dehydrogenase. BCAA, branched-chain amino acids; BCKA, branched-chain α-ketoacids; GlcN-6-P, glucosamine-6-phosphate; α-KG, α-ketoglutarate; OAA, oxaloacetate
In animals, including pigs, BCAAs donate an amino group to α-KG to form Glu, with glucose being the major source of α-KG (Li et al. 2009). The activity of BCAA transaminase varies greatly among different cell types, with the skeletal muscle and liver possessing relatively high and very low activity, respectively. Furthermore, the transamination of alanine or aspartate with α-KG generates Glu, and these pathways are widely spread in animal tissues (Wu 2013). Finally, glutamate dehydrogenase can catalyze the synthesis of Glu from α-KG and ammonia. Thus, pigs can form Glu from dietary AAs. However, endogenous synthesis of Glu, which is estimated to be 419 mg/kg BW/day (Fig. 2), may not be sufficient for the maximum growth and feed efficiency of young pigs, particularly under stress conditions such as weaning and infections (Hou et al. 2016).
Whole-body synthesis of glutamate in young pigs. Values are expressed as mg/kg BW/day. The pig (7.92 kg BW) consumes 816 mg Glu/kg BW/day from milk [true ileal digestibility of Glu = 99.7% (Mavromichalis et al. 2001)] and gains 293 g BW/day. The total content of AAs in the milk is 50 g/L (Kim and Wu 2004) and milk intake by piglets is 170 mL/kg BW/days (Wang et al. 2008). True ileal digestibility of non-Glu AAs is 93% (Mavromichalis et al. 2001). There is no net flux of arterial plasma Glu into the small intestine (Wu et al. 1994). Utilization of dietary (milk) Glu by the small intestine in first pass is 773 mg/kg BW/day (814 × 95% = 773) (Wu et al. 2010), with the total use of Glu by the small intestine being 773 mg/kg BW/day. The entry of dietary (milk) Glu into portal vein is 41 mg/kg BW/day (814 × 5% = 41). Utilization of non-Glu AAs in the body is calculated on the basis of the following: intake of non-Glu AAs from milk (7693 mg/kg BW/day; Wu et al. 2010), non-Glu AAs entering large intestine (538 mg/kg BW/day = 7693 × 7% = 538), amount of non-Glu AA available to the small intestine (7155 mg/kg BW/day = 7693 − 538 = 7155), the rate of degradation of non-Glu AAs by the small intestine [10% of non-Glu AAs in the lumen of SI; 716 mg/kg BW/day = 7155 × 10% = 716 (Wu et al. 2011)], need for non-Glu AA for whole-body protein synthesis (5028 mg/kg BW/day), and the amount of non-Glu AA available for oxidation and AA synthesis in the body = 1411 mg/kg/day (7155 − 716 − 5028 = 1411). To meet the needs for Glu for utilization by extraintestinal tissues [460 mg/kg BW/day (Wu et al. 2010)], endogenous synthesis of Glu = 419 mg/kg BW/day (460 − 41 = 419). LI large intestine
Abundance of Glu in sow’s milk and pig tissues
It was recognized in the late 2000s that previous investigators had failed to determine the content of protein-bound Glu in sow’s milk; therefore, studies were initiated to quantify free and protein-bound Glu in sow’s colostrum and milk on days 1–28 of lactation (Haynes et al. 2009). We have shown that concentrations of free Glu in sow’s milk increase from 0.15 mM on day 1 (the day of farrowing) to 1.1 mM on day 8 of lactation, and are relatively constant, thereafter (Wu and Knabe 1994). Consistent with a decline in milk protein content in the first week of lactation, concentrations of protein-bound Glu in sow’s milk also decrease between days 1 and 8 of lactation, and then remain relatively constant through day 28 of lactation (Wu et al. 2011). On all days of lactation, Glu is the second most abundant peptide-bound AA in sow’s milk, only slightly after proline (Haynes et al. 2009; Wu 2013). In post-weaning pigs, Glu intake from typical plant (e.g., corn- and soybean meal)-based diets fed 14–20% crude protein (CP) is the second highest only after Gln (Wu et al. 2014). This is also the case with wheat-based diets (Wu et al. 2011).
Despite the high intake of dietary Glu by pre- and post-weaning pigs, concentrations of this AA in their plasma are relatively low (50–100 µM), when compared with most AAs (Flynn et al. 2000). This is because about 95–97% of dietary Glu is degraded by the small intestine of pigs (Stoll and Burrin 2006; Wu et al. 2010). However, Glu is the third most abundant AA in the body protein of pigs after glycine and proline (Hou et al. 2016). Thus, there must be active pathways for Glu synthesis in all ages of pigs, and these pathways will be discussed in the following section.
Synthesis of Glu from Gln and BCAAs plus α-KG in the extraintestinal tissues of pigs
(a) Small intestine. Although several metabolic pathways can potentially form Glu from Gln and branched-chain AAs (BCAAs) (Fig. 1), Glu merely serves as an intermediate of these reactions in the small intestine. In the case of glutaminase, the Gln-derived Glu is further converted into α-KG, which is oxidized to CO2 in mitochondria. In the case of BCAA transaminase, the Glu that is formed from BCAAs and α-KG is rapidly transaminated with pyruvate or oxaloacetate to form alanine or aspartate, with α-KG being regenerated (Wu 1998). This transamination-coupled reaction does not result in Glu production. There is little net production of Glu by the small intestine of pigs (Hou et al. 2016; Reeds et al. 1996; Wu et al. 1994).
(b) Liver In growing pigs, the liver has net uptakes of basic and most neutral AAs from the portal vein at different proportions of their net portal-vein fluxes, but a net release of Glu (35 g/kg of ingested feed) (Wu 2018). Periportal hepatocytes do not appear to take up extracellular Glu but release this AA. Although perivenous hepatocytes take up extracellular Glu, the liver shows net release of Glu. When pigs are fed a regular diet, their liver receives little dietary Glu from the portal vein as noted previously, but takes up a net amount of Gln (6.4 g/kg of ingested feed) from the portal vein, and releases Glu as a result of hepatic AA metabolism. Hepatic degradation of BCAAs is limited due to a low activity of BCAA transaminase in pigs (Li et al. 2009). In addition, oxidation of Gln at physiological plasma concentrations (e.g., 0.5 to 1 mM) is limited in the liver (Watford 2015). Thus, the major sources of Glu in the liver are neither Gln nor BCAAs, but are likely alanine, proline, phenylalanine and asparagine that are extracted from the portal vein in the largest amounts by the liver (Wu 2018).
(c) Skeletal muscle Skeletal muscle represents 40–45% of the total body weight and, therefore, plays an important role in Glu synthesis in animals. Glu is the second most abundant α-AA in the skeletal muscles of pre- and post-weaning pigs, with concentrations being 5–10 mM (Wu 2013). In muscle, BCAA transaminase produces Glu and BCKAs from BCAAs and α-KG, and glutaminase also hydrolyzes Gln into Glu (Wu et al. 2011). Intramuscular synthesis of Glu is insufficient to meet Glu requirement by muscle, because pig skeletal muscle has a net uptake of Glu (0.49–0.72 µmol/kg BW/min) from the arterial blood to support tissue growth (Ytrebo et al. 2006). In the skeletal muscle, Glu is utilized for the synthesis of proteins, Gln, alanine and aspartate. Thus, exogenous or endogenous provision of a large amount of Glu can promote intramuscular DNA synthesis (Liu et al. 2002) and the rapid gain of lean tissues (Rezaei et al. 2013a) in growing pigs.
(d) Kidneys The kidney has a high rate of glomerular infiltration rate (GFR) and may potentially play a role in Glu synthesis. The kidneys possess BCAA transaminase activity for the production of Glu and BCKAs from BCAAs and α-KG. However, a high renal activity of GDH rapidly converts Glu into ammonia and α-KG to prevent an accumulation of Glu (an inhibitor of renal glutaminase). Based on ammonia assays, Krebs (1935) reported that the porcine kidney homogenates did not have the activity of glutaminase for Glu production. However, Kvamme et al. (1970) identified the presence of phosphate-activated glutaminase in the porcine renal cortex and purified this enzyme. In healthy young pigs (25–30 kg), renal synthesis of Glu is insufficient to meet Glu requirement by the kidneys, because these animals exhibit a net renal uptake of Glu (Ytrebo et al. 2006; Junco et al. 1991).
(e) Mammary gland BCAAs undergo extensive transamination in mammary tissue (Li et al. 2009). Thus, the uptake of BCAAs by porcine mammary glands (76 g/day on Day 13–20 of lactation) is much greater than their excretion in milk protein (46 g/day) (Lei et al. 2012). In contrast, the output of Glu plus Gln in sow’s milk is less than the uptake of these two AAs by the mammary glands (Lei et al. 2012). This necessitates the net production of both Glu and Gln by the mammary glands of lactating sows. Quantitatively, the lactating porcine mammary gland catabolizes 30 g BCAAs/day (40% of the BCAAs taken up from arterial plasma), with nitrogenous products being Glu, Gln, alanine and aspartate (Li et al. 2009). There is evidence that in vivo uptake of BCAAs by mammary tissue is essential for its synthesis of Glu (Matsumoto et al. 2013). The synthesis of Glu and Gln compensates for their inadequate uptake from the arterial blood by the lactating mammary gland and contributes to the high abundance of these two AAs in sow’s milk (Lei et al. 2012).
(f) Placenta The uptake of Glu by the uterus of gestating sows meets at most 46% of Glu required by the growing fetuses during late pregnancy (Hou et al. 2016). Thus, the placenta and fetal tissues must synthesize at least 54% of Glu needed by the fetuses. We found that the porcine placenta (e.g., on days 20–110 of gestation) extensively degrades BCAAs through transamination with α-KG to form Glu (Self et al. 2004), with some of the Glu being released into the fetal circulation (Wu et al. 2013). The synthesis of Glu explains the observation that Glu is the second most abundant free AA and the third most abundant protein-bound AA in the porcine placenta (Wu et al. 2017).
(g) Other tissues Other porcine tissues, including the brain, lungs, heart, adipose tissue and lymphoid organs, generate Glu from: (1) Gln via glutaminase, and (2) BCAAs and α-KG via BCAA transaminase, as noted previously. These tissues, except for the brain, also extract Glu from the arterial blood. Quantitative data on Glu synthesis by the brain, lungs, heart, adipose tissue and lymphoid organs are not available. However, there is evidence that Glu synthesis by the lungs is insufficient to meet their needs for Glu, because the lungs of growing pigs exhibit a net uptake of a large amount of Glu (0.88–1.54 µmol/kg BW/min), which is almost twice the value for the hind-leg muscle (Ytrebo et al. 2006). As noted previously, the liver is a major endogenous source of Glu in the blood circulation of pigs.
Evidence for inadequate Glu synthesis and the need of exogenous Glu by porcine small-intestinal cells
Optimal gut health is fundamental to optimal whole-body health, as well as maximum growth performance and feed efficiency in swine. However, within the first few days post weaning, feed intake is usually limited (< 10% of pre-weaning dry matter intake) and intestinal atrophy along with intestinal oxidative stress occurs. The deficiencies of multiple nutrients, including Glu, contribute to this weaning-associated growth depression syndrome. We have used a porcine enterocyte culture model to determine roles of Glu in intestinal epithelial cells (Jiao et al. 2015). The culture medium contained physiological concentrations of BCAAs, Gln and other AAs found in the pig plasma. We reported that, compared with 0 mM Glu, 0.5-, 1-, and 2 mM Glu enhanced cell growth by 13–37% at 24 h in a concentration-dependent manner. In addition, 0.5 mM Glu increased transepithelial electrical resistance (TEER) by 58% at 24 h and by 98% at 48 h. These effects of Glu were associated with increases in the mRNA abundance of Glu transporter (solute carrier family 1 member 1, SLC1A1) by 30–130% and in the protein abundance of excitatory amino acid transporter 3 (EAAT3) by 19–34%. In response to oxidative stress induced by 1 mM diquat, 0.5 mM Glu enhanced the viability, TEER, and membrane integrity of enterocytes by increasing the abundance of the tight junction proteins, including occludin, claudin-3, zonula occludens (ZO)-2, and ZO-3. Collectively, these results indicate that Glu plays an important role in mucosal barrier function by enhancing cell growth, maintaining membrane integrity, and the expression of tight-junction proteins in response to oxidative stress. Therefore, intestinal synthesis of Glu is inadequate when its substrates [e.g., Gln, BCAAs and glucose (the major source of α-KG)] are limited (e.g., under weaning and other stress conditions), and effective measures should be taken to prevent a dietary deficiency of Glu.
Glutamate catabolism in pigs
Multiple enzymes initiate Glu degradation in a cell- and tissue-specific manner (Wu 2013). Transamination plays an important role in initiating the degradation of Glu to yield α-KG, which is either further oxidized to CO2 and H2O or converted into glucose, depending on the physiological and nutritional status. Glu-pyruvate transaminase and Glu-oxaloacetate transaminase are abundant in both mitochondria and the cytoplasm of most mitochondria-containing cells, particularly hepatocytes, enterocytes, cells of the immune system, and kidneys.
In addition to transamination, dehydrogenation of glutamate by Glu dehydrogenase (GDH) results in the production of α-KG plus ammonia in multiple tissues (Wu 2013). Although GDH catalyzes the interconversion of Glu into α-KG and ammonia, this reaction results in the production of either ammonia or glutamate in animal cells (e.g., hepatocytes and renal tubules) depending on the physiological concentrations of substrates and products (Treberg et al. 2010). GDH is a major enzyme that directly produces ammonia from AA catabolism in animals. Interestingly, this enzyme is allosterically activated by l-leucine, which has important implications for the regulation of Glu catabolism and hormone secretion. In tissues (e.g., skeletal muscle, heart, and small intestine) other than the liver, pancreas and kidneys, GDH activity is low and is not a quantitatively significant source of ammonia.
Decarboxylation of Glu by Glu decarboxylase produces GABA in tissues (Wu 2013). This enzyme is particularly abundant in the brain and the pancreas. In mammals, Glu decarboxylase exists in two isoforms, which are encoded by two different genes: GAD1 (the brain) and GAD2 (the pancreas). GABA is further degraded to either succinate by succinate semialdehyde dehydrogenase or γ-hydroxybutyrate. In the brain, the production and catabolism of GABA occur in neurons and glial cells, respectively. These highly cell-specific events play an important role in neurotransmission. Data on quantitative utilization of plasma Glu in young pigs are summarized in Fig. 3.
Utilization of circulating glutamate by major organs of young pigs fed a milk-protein diet. Values in the figure are expressed as mg Glu/kg BW/day. The pig (7.92 kg BW) consumes 816 mg Glu/kg BW/day from milk [true ileal digestibility of Glu = 99.7% (Mavromichalis et al. 2001)] and gains 293 g BW/day. The total content of AA in the milk is 50 g/L (Kim and Wu 2004). There is no net flux of arterial plasma Glu into the small intestine (Wu et al. 1994). Rate of extraction of Glu by the non-SI portal-drained viscera is 3.4 mg/kg BW/day (Reeds et al. 1996). Glu utilization by skeletal muscle is calculated on the basis of the following: mass of skeletal muscle (3.17 kg; 40% of BW), protein gain in skeletal muscle (2.22 g/kg BW/day), Glu content in muscle protein (8.12 g/100 g protein), concentration of free Glu in skeletal muscle (470 mg/kg wet tissue), and Glu oxidation [0.034 mmol Glu/kg muscle/h; determined at 0.15 mM Glu (G. Wu, unpublished data)]. Glu utilization by kidneys is 184 mg/kg BW/day (Ytrebo et al. 2006). Glu utilization by whole-body lymphocytes is calculated on the basis of 1.43 × 1010 cells/kg BW (Lydyard and Grossi 1989) and the rate of Glu utilization by lymphocytes [0.41 nmol/106 cells/h; determined at 0.15 mM Glu (G. Wu, unpublished data)]. Glu utilization by vascular endothelia is based on the number of endothelial cells [1.5 × 109/kg BW (Wu and Thiagarajan 1996)] and the rate of Glu utilization in endothelial cells [2.86 nmol Glu/106 cells/h; determined at 0.15 mM Glu (G. Wu, unpublished data)]. SI small intestine
(a) Small intestine Besides protein synthesis, the small intestine of pigs utilize Glu via several metabolic pathways (Wu 2013). Studies involving the cannulation of the jejunal artery and jejunal vein of 14- and 21-day-old suckling piglets, as well as 29- to 58-day-old pigs weaned at 21 days of age have shown that their small intestine does not take up Glu from arterial blood (Wu et al. 1994). However, in enterocytes from the small intestine of these pigs, Glu is extensively degraded to mainly CO2, alanine and aspartate via Glu transaminases, and, to a lesser extent, citrulline, proline and arginine via P5C synthase (Wu et al. 1994; Wu and Knabe 1995). Endogenous synthesis of citrulline and arginine in enterocytes, which involves both the mitochondria and the cytosol (Wu and Morris 1998), plays an important role in maintaining arginine homeostasis in milk-fed piglets (Flynn and Wu 1996) and in post-weaning, growing pigs (Wu et al. 1997). Dietary Glu, aspartate, and Gln plus arterial Gln provide approximately 80% of ATP to the small-intestinal mucosa in pigs (Reeds et al. 2000). Blachier et al. (1999) reported that the rate of oxidation of 2 mM Glu to CO2 in enterocytes of 60-kg pigs was similar to that of 2 mM Gln. Consistent with a low-GDH activity, there is limited production of ammonia from Glu or monosodium glutamate (MSG) by pig enterocytes (Blachier et al. 1999).
Results of in vivo isotopic and kinetic studies indicate that 95–97% of the orally administered Glu at a regular intake level (0.5 g Glu/kg BW/day) is utilized by the small intestine of pigs during first-pass metabolism, with only 3–5% of enteral Gln entering the portal vein (Reeds et al. 1996; Wu et al. 2010). The small intestine has such a high capacity to catabolize dietary Glu that even oral administration of Glu at four times its normal dietary intake (Janeczko et al. 2007) or dietary supplementation with 4% MSG (2 g MSG/kg BW/day) (Rezaei et al. 2013a) results in only a transient, approximately 75% increase in circulating levels at 1 h after feeding. Under these conditions, the concentrations of Glu in pig plasma remain low. When MSG alone was rapidly administered to 60-kg pigs (0.33 g MSG/pig/day), an approximately 100% increase in Glu concentrations in arterial and portal vein plasma were observed at 1 h after administration without any adverse effect on pigs (Blachier et al. 1999). This further supports the notion that dietary components can affect Glu catabolism by the small intestine and the whole body (Daabees et al. 1994; Stegink et al. 1973). Due to the extensive utilization of dietary Glu by the pig small intestine, Glu in the body is derived primarily from de novo synthesis in insulin-sensitive tissues (skeletal muscle, heart, liver, and adipose tissue), the brain and kidneys, and, to a much lesser extent, from the diet (Wu 2013; Curthoys and Watford 1995).
(b) Liver In the pig liver, GDH can degrade Glu that is derived from the metabolism of other AAs into ammonia and α-KG (Wu 2018). Ammonia is detoxified as urea via the hepatic urea cycle, and α-KG’s metabolic fate (e.g., for glucose synthesis or ATP production) depends on the physiological state of pigs. Glu is also metabolized to form alanine and aspartate via transamination (Wu 2013). In the liver of growing pigs, the rate of Glu uptake plus synthesis exceeds the rate of Glu degradation, resulting in its release from this organ.
(c) Skeletal muscle The rate of deamination of Glu in skeletal muscle is low. This is because of a low activity of GDH in this tissue. Less than 15% of Glu utilized by the pig skeletal muscle is oxidized to CO2. Production of protein, Gln, alanine and aspartate is the major pathway for Glu utilization in skeletal muscle, which amounts to 0.49–0.72 µmol/kg BW/min (Ytrebo et al. 2006). Thus, the carbon skeleton of Glu is well conserved in the muscle, which may be considered as a major sink of dietary AAs.
(d) Kidneys A high-renal activity of GDH rapidly converts Glu into ammonia and α-KG. Based on the rate of renal Glu extraction by pigs (39 µmol/min per 100 ml GFR per kidney) (Junco et al. 1991) and the GFR of 1.115 mL/kg BW/min per kidney) in pigs (Link et al. 1985), the rate of Glu utilization by both kidneys in pigs is estimated to be 0.87 µmol/kg BW/min. The pathway of GDH contributes to a net release of ammonia (1.8–2.4 µmol/kg BW/min) from the kidneys in growing pigs (Junco et al. 1991; Ytrebo et al. 2006). The Glu-derived α-KG can be oxidized to CO2, which reacts with H2O to form H2CO3 under the action of carbonic anhydrase. H2CO3 is in equilibrium with H+ + HCO3−. The latter is reabsorbed by renal tubules into the blood.
Glutamate nutrition in pigs
Results of recent studies indicate that dietary supplementation with Glu to conventional diets can improve the growth or production performance of modern breeds of pigs. Thus, there have been suggestions that Glu is a conditionally essential AA for weanling pigs (Rezaei et al. 2013a; Watford 2015) and lactating sows (Wu et al. 2014). Based on published work, Wu et al. (2014) and Hou et al. (2016) have recommended the minimal content of Glu in diets for gestating, lactating, nursing, weanling, and growing-finishing swine. Hou and Wu (2017) concluded that the term “nutritionally nonessential AAs” is a misnomer in nutritional sciences and could be replaced with “AAs synthesizable in animals”. This is a major paradigm shift in swine AA nutrition.
Glu nutrition in sow-reared pigs
Quantitative data on Glu utilization by the small intestine and extra-intestinal tissues of sow-reared piglets are summarized in Table 1. About 95% of milk Glu is extracted by the piglet small intestine in the first pass, and only about 5% of milk Glu enters the portal vein. Oxidation to CO2 for ATP production is the major pathway for intestinal Glu metabolism. Skeletal muscle and kidneys are two major extra-intestinal tissues for Glu utilization via protein synthesis and mitochondrial oxidation pathways, respectively, in growing pigs (Table 2). BCAAs and non-BCAA AAs in milk are ultimately nitrogenous substrates for Glu synthesis in suckling piglets (Table 3). Due to no net synthesis of Glu by the small intestine and negligible uptake of Glu by the small intestine from the arterial blood, milk-derived Glu is essential for the growth, integrity and function of this tissue. Oral administration of monosodium glutamate [MSG; 0, 0.06, 0.5, or 1 g/kg BW/day for 21 days] beginning immediately after birth enhanced the expression of Glu receptors and Glu transporters in the stomach and jejunum of sow-reared piglets (Zhang et al. 2013). There are reports that addition of 5 g MSG to 1 kg creep feed (starter diet) can increase the feed consumption of suckling pigs by 36% on Day 18 after farrowing, but does not affect their body weight at weaning (Gatel and Guion 1990). It remains to be determined whether milk-born Glu is sufficient for the maximal growth of sow-reared piglets, particularly low-birth-weight piglets.
Glu nutrition in weanling pigs
There is growing interest in Glu nutrition in weanling pigs. Zimmerman (1975) reported that supplementing 3.36% Glu to a corn- and soybean meal-based low-protein diet (16% CP) for 28 days enhanced daily weight gain by 12% in pigs weaned at 21 days of age without affecting the feed:gain ratio. Of interest, Glu supplementation had no effect on the growth of older pigs weaned at 31 days of age to a corn- and soybean meal-based diet containing 18% CP for 9 days before being fed a 16% CP diet for 21 days (Zimmerman 1975). Thus, a 16% CP diet does not supply sufficient Glu to weanling pigs. Similarly, Gatel and Guion (1990) found that dietary supplementation with 0.5% MSG to the weanling diet increased daily food intake by 10% and daily BW gain by 7%. Interestingly, the effect of MSG was greater in piglets with a weaning weight less than 8.5 kg than those with a weaning weight of ≥ 8.5 kg. This may be because small piglets are more susceptible to stress and metabolic abnormalities than larger piglets at weaning (Wu et al. 2006).
Intestinal dysfunction of weanling piglets is a major concern of swine producers worldwide (Wu et al. 2014). For optimal intestinal health and growth, weanling pigs (21–35 days of age) should receive at least the same quantity of dietary Glu as their age-matched sow-reared counterparts. However, weanling pigs usually have a reduced rate of feed intake during the first week post weaning, compared with suckling piglets (Wu et al. 2014). Thus, the corn- and soybean meal-based weaning diet provides only about 50% of Glu needed by the small intestine of weanling piglets (Table 4). Clearly, dietary supplementation with Glu is necessary for their well-being. This is particularly important for weanling piglets that fed a diet contaminated with mycotoxins (Duan et al. 2014).
Rezaei et al. (2013a) conducted a study to assess the efficacy of dietary supplementation with Glu in postweaning pigs. Piglets were weaned at 21 days of age to a corn- and soybean meal-based diet (21% CP) supplemented with 0, 0.5, 1, 2, or 4% MSG. MSG was added to the basal diet at the expense of cornstarch. At 42 days of age (21 days after weaning), blood samples (10 mL) were obtained from the jugular vein of pigs/group at 1 and 4 h after feeding for AA analysis. Feed intake was not affected by dietary supplementation with 0–2% MSG and was 15% lower in pigs supplemented with 4% MSG, compared with the 0% MSG group. Compared with the control, dietary supplementation with 1, 2 and 4% MSG dose-dependently increased plasma concentrations of Glu, Gln, and many other AAs (including lysine, methionine, phenylalanine and leucine), daily BW gain, and feed efficiency in postweaning pigs. At day 7 post weaning, dietary supplementation with 1–4% MSG also increased jejunal villus height, DNA content, and anti-oxidative capacity. The MSG supplementation dose-dependently reduced the incidence of diarrhea during the first week after weaning. Similar results have been reported by other investigators (Lin et al. 2014). Likewise, supplementing creep feeds with Glu plus Gln can improve intestinal and immunological health in weanling piglets (Cabrera et al. 2013). These data indicate that dietary supplementation with up to 4% MSG improves growth performance in postweaning pigs, and support the view that Glu is a conditionally essential AA for weanling pigs (Table 5).
Glu nutrition in growing-finishing pigs
Reducing dietary intake of protein is an effective means to minimize nitrogen excretion by pigs during the growing and finishing periods. Kirchgessner et al. (1993) conducted experiments to evaluate a role of dietary supplementation with 1–4% Glu to low-protein diets on pig growth and carcass quality. The content of CP in the diets of grower pigs (30–60 kg BW) and finisher pigs (60–90 kg BW) was 12 and 10%, respectively. All diets contained the same amount of nutritionally essential AAs. Compared with the positive control (17% CP in the grower period and 14% CP in the finisher period), daily weight gain and feed conversion were reduced by up to 13% with the low-protein diet during the grower period despite similar feed intake. With increasing the amount of Glu supplementation, weight gains and feed conversion were improved in a dose-dependent manner. However, pigs fed the low protein diet supplemented with 4% Glu still exhibited a lower daily weight gain and a lower feed efficiency, compared with the positive control group. This result suggests that pigs need biosynthesizable AAs other than Glu for achieving maximal growth. Of note, low-protein diet increased fat deposition in the body, but this effect was ameliorated or fully reversed by dietary Glu supplementation. Fickler et al. (1995) reported that the inclusion of 2% Glu was required for dietary arginine to maximize the growth performance and feed efficiency in growing pigs. Likewise, Le Floc’h et al. (1994) found that adding 2.62% Glu plus 1% MSG to a threonine-deficient (0.42% threonine) and low-protein (12.6% CP) diet for growing-finishing pigs (40–100 kg BW) increased daily weight gain and gain:feed ratio by 18% and 10%, respectively, compared with pigs fed the basal diet without Glu or MSG supplementation. Furthermore, as noted previously, dietary supplementation with 2% Glu can reduce the loss of muscle mass in growing pigs challenged with LPS (Kang et al. 2017). These results indicate an important role for Glu in modulating lipid and protein metabolism in swine.
In growing-finishing pigs, a major concern is that excessive amounts of subcutaneous white adipose tissue (e.g., backfat) are naturally deposited in market-weight pigs fed a conventional finishing diet (NRC 2012). Notably, supplementing 3% MSG to a corn- and soybean meal-based diet can beneficially reduce triglyceride concentrations in the plasma and the white adipose tissue of growing pigs (Kong et al. 2015). Likewise, dietary supplementation with 1% Glu beneficially increases the intramuscular fat deposition and improves the meat color without affecting the subcutaneous fat mass in growing-finishing pigs (Hu et al. 2017). This beneficial effect of Glu is achieved likely through increasing lipolysis and reducing lipogenesis in white adipose tissue, as well as stimulating the oxidation of fatty acids and glucose in skeletal muscle (Kong et al. 2015). Additionally, dietary Glu may inhibit the degradation of nutritionally essential AAs in the small intestine (Rezaei et al. 2013a). Thus, Glu can regulate energy partitioning in the body to favor white-fat reduction and lean-tissue gain. This is nutritionally and economically important, as meat production is the main goal of the swine industry.
Glu nutrition in gestating pigs
Modern high prolific sows ovulate 20–30 oocytes, but can deliver only 10–15 live-born piglets at term (Ji et al. 2017; Town et al. 2005). There is a positive relationship between uterine capacity and fetal mortality (Bazer et al. 2014). The greatest restraint on litter size in pigs is placental development and function in early gestation and uterine capacity during all periods of gestation (Bazer et al. 1988). Among domestic animals, pigs exhibit the most severe naturally occurring intrauterine growth restriction, and 76% of these compromised piglets do not survive to weaning (Wu et al. 2006). As noted previously, Glu is a major substrate for the synthesis of arginine in most mammals, including pigs. This is nutritionally and physiologically important for the following reasons. First, both polyamines and nitric oxide (products of Arg) play a key role in placental angiogenesis and growth in mammals (Wu et al. 2013). Second, dietary Arg is insufficient for maximal embryonic/fetal survival or growth in pigs (Wu et al. 2017). Third, Glu contributes an amino group for the conversion of 4-hydroxyproline (a product of collagen degradation) into glycine (a nutritionally essential amino acid for young pigs and possibly gestating dams) in the major tissues of swine (Wu 2018).
The growth of the placenta and the fetus is very rapid during the first-half and second-half of pregnancy, respectively. This requires provision of large amounts of Glu. On day 40 of gestation (term = 114 days), in fetal pig allantoic fluid, Glu (2.4 mM) is the fourth most abundant AA (Wu et al. 1996). This suggests an important role of Glu in porcine fetal growth and development. In modern pig production, maternal feed intake is restricted (e.g., 2–2.5 kg per day) to prevent the development of overweight or obesity during gestation. In gilts fed 2 kg of a corn- and soybean meal-based diet containing 12.2% CP, dietary Glu entering the portal vein can meet only 18% of its uterine uptake during the late gestation, which is associated with a high rate of low-birth-weight piglets (Wu et al. 2006). Endogenous synthesis of AAs, including Glu, may be inadequate for optimal pregnancy outcome in pigs (Ji et al. 2017; Hou et al. 2016). Of note, dietary supplementation with Gln, which is metabolized in maternal tissues and fetuses to form Glu, can alleviate uterine growth restriction in gilts and sows (Wu et al. 2011). Thus, there is a suggestion that Glu is a conditionally essential AA for gestating swine (Watford 2015).
Glu nutrition in lactating sows
A role of dietary Glu in lactation is indicated by the fact that its metabolic product, Arg, is essential to blood flow to mammary glands and milk production by lactating mammals, such as sows (Kim and Wu 2009). Work on Glu nutrition in lactating sows is very limited in the literature. Studies with sows have shown that the lactating mammary gland increases milk production as dietary CP intake is increased from 14 to 18% (Manjarin et al. 2014). The lactating sow fed a corn- and soybean meal-based diet containing 18% CP loses approximately 0.5 kg BW (80 g protein) per day between days 1 and 21 of lactation (Mateo et al. 2008), which is equivalent to 15% of dietary AA entering the portal vein (Hou et al. 2016). Thus, dietary protein intake in the current feeding program is substantially insufficient for milk protein production by prolific sows. Dietary Glu entering the portal vein can meet only 8% of Glu output in porcine milk (Hou et al. 2016). Although the lactating sow mobilizes its protein stores to provide Glu for milk production, this capacity has a physiological limit because an excessive loss of body protein is not compatible with health or survival (Wu et al. 2014). Thus, lactating sows may not be able to synthesize sufficient Glu for supporting maximal milk production. This view is supported by several lines of evidence from studies with lactating sows. First, dietary supplementation with 1% Gln (a precursor of Glu) enhances milk production by sows (Wu et al. 2011), Gln concentration in milk (Manso et al. 2012; Wu et al. 2011), as well as the growth and survival of piglets (Wu et al. 2014). Likewise, supplementing Glu plus Gln to the diet of lactating sows increased the concentrations of lipids in colostrum and mature milk (Santos de Aquino et al. 2014). Second, supplementing 1 and 2% MSG to a corn- and soybean meal-based diet (containing 18% CP) for lactating sows increased: (a) milk production, as well as the concentrations of free and peptide-bound AAs in milk; (b) growth and survival of suckling piglets; and (c) efficiency of feed utilization for lactation (Rezaei et al. 2013b). Third, compared with the control group without any supplementation, supplementing 1.15% MSG to a sorghum- and wheat-based diet (containing 23.5% CP) for lactating sows enhanced milk yield and the preweaning growth of piglets by 9% (Hewitt and van Barneveld 2012).
Safety of Glu supplementation in pigs
Dietary Glu is extensively catabolized in young, growing-finishing, gestating, and lactating pigs (Hou et al. 2016). We found that 7- to 21-day-old low-birth-weight and normal-birth-weight piglets reared by sows could well tolerate the oral administration of 2 g of Glu/kg BW/day during a 2-week experimental period (Wu G, unpublished data). Rezaei et al. (2013a) assessed the safety of Glu supplementation in pigs between 21 and 42 days of age, based on general observations (e.g., behavior, skin health, and hair), feed intake, growth, body composition, as well as hematological and blood chemistry tests. In this study, piglets were fed a typical corn- and soybean meal-based diet (containing 1.91% Glu) supplemented with 0, 0.5, 1, 2 and 4% MSG (equivalent to 0, 0.432, 0.864, 1.73 and 3.46% Glu, respectively) for 21 days. The supplemental doses of 0, 0.5, 1, 2, and 4% MSG provided pigs with 0, 175, 332, 659 and 1263 mg Glu/kg BW/day, respectively, beyond the amount of Glu in the basal diet (710–789 mg/kg BW/day) at the feed intake of 36.5–41.3 g/kg BW/day. Hematological variables at 1 and 4 h after feeding were: (a) the numbers of white blood cells, red blood cells, and platelets; (b) blood hemoglobin, blood pH, plasma protein, and fibrinogen; (c) mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and mean corpuscular volume; and (d) percentages of neutrophils, lymphocytes, monocytes, and eosinophils. Serum chemistry at 1 and 4 h after feeding included the concentrations of: (a) total serum protein, albumin, and globulins; (b) total bilirubin, amino acids, glucose, urea, ammonia, creatinine, free fatty acids, triglyceride, and cholesterol; (c) sodium, chloride, calcium, phosphorus, and magnesium; (d) alkaline phosphatase, alanine transaminase, aspartate transaminase, creatine kinase, and lactate dehydrogenase. All the variables in standard hematology and clinical chemistry tests, as well as gross and microscopic structures, did not differ among all the five groups of pigs. These results indicate that dietary supplementation with up to 4% MSG is safe in post-weaning pigs, while improving their growth performance and feed efficiency.
In studies involving 10- to 20-kg (Chung and Baker 1992) and 20- to 50-kg pigs (Wang and Fuller 1989) fed purified diets containing 10% glutamic acid, no adverse effects on their growth or health were observed. Long-term supplementation with 1–4% Glu to diets for growing-finishing pigs (30–90 kg BW) was also safe (Kirchgessner et al. 1993). Likewise, no adverse effects were reported for post-weaning pigs, growing-finishing pigs, gestating sows, or lactating sows fed diets supplemented with various amounts of Glu or MSG for 14–120 days (Table 5). Based on these results, we suggest that pigs at all production stages can tolerate well dietary supplementation with at least 2% Glu without any adverse effects.
In summary, extensive research over the past 25 years has identified Glu as one of the most abundant AAs in sow’s milk and swine diets. However, nearly all of the dietary Glu is catabolized by the small intestine during the first pass, and endogenous synthesis via inter-organ metabolism of AAs is crucial for maintaining Glu homeostasis in the whole body. At the cellular level, Glu is physiologically essential for the synthesis of proteins and other nitrogenous substances (including glutathione and arginine) with key metabolic functions in the body. Thus, this nutrient plays an important role in improving the health, survival, growth, development, lactation, and reproduction of swine. Compelling evidence shows that Glu is a nutritionally essential AA for weanling pigs to both maintain normal intestinal physiology and enhance efficiency in the utilization of dietary protein for gut and whole-body growth. Additionally, recent findings indicate that adequate amounts of dietary Glu are necessary to support maximum lactation and reproduction performance in pigs. Glu is truly a functional AA and a dietarily essential AA in swine nutrition. All of this new knowledge should be taken into consideration in revising the current version of NRC (2012)-recommended requirements of AAs for swine to formulate balanced diets in various phases of production. These results also have important implications for improving the nutrition of humans and other animals.
Abbreviations
- AA:
-
Amino acid
- BW:
-
Body weight
- CP:
-
Crude protein
- GDH:
-
Glutamate dehydrogenase
- Gln:
-
l-Glutamine
- Glu:
-
L-Glutamate
- α-KG:
-
α-Ketoglutarate
- mTOR:
-
Mechanistic target of rapamycin
- NRC:
-
National Research Council
References
Bazer FW, Thatcher WW, Martinat-Botte F et al (1988) Conceptus development in Large White and prolific Chinese Meishan pigs. J Reprod Fert 84:37–42
Bazer FW, Wu G, Johnson GA et al (2014) Environmental factors affecting pregnancy: endocrine disrupters, nutrients and metabolic pathways. Mol Cell Endocrinol 398:53–68
Bignell H (2014) Maternal ingestion of glutamine and glutamate during sow pregnancy and lactation: lipid profile analysis of milk and neonatal adipose tissues. M.S. Thesis, Rutgers University, New Brunswick, New Jersey
Blachier F, Guihot-Joubrel G, Vaugelade P et al (1999) Portal hyperglutamatemia after dietary supplementation with monosodium glutamate in pigs. Digestion 60:349–357
Blachier F, Boutry C, Bos C et al (2009) Metabolism and functions of l-glutamate in the epithelial cells of the small and large intestines. Am J Clin Nutr 90:814S–821S
Brosnan JT, Brosnan ME (2013) Glutamate: a truly functional amino acid. Amino Acids 45:413–418
Cabrera RA, Usry JL, Arrellano C et al (2013) Effects of creep feeding and supplemental glutamine or glutamine plus glutamate (Aminogut) on pre- and post-weaning growth performance and intestinal health of piglets. J Anim Sci Biotech 43:29
Chung TK, Baker DH (1992) Ideal amino acid pattern for ten kilogram pigs. J Anim Sci 70:3102–3111
Curthoys NP, Watford M (1995) Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr 15:133–159
Daabees TT, Andersen DW, Zike WL et al (1994) Effect of meal components on peripheral and portal plasma glutamate levels in young pigs administered large doses of monosodium-l-glutamate. Metabolism 22:58–67
Duan J, Yin J, Wu M et al (2014) Dietary glutamate supplementation ameliorates mycotoxin-induced abnormalities in the intestinal structure and expression of amino acid transporters in young pigs. PLoS ONE 9(11):e112357
Duan J, Yin J, Ren W et al (2016) Dietary supplementation with l-glutamate and l-aspartate alleviates oxidative stress in weaned piglets challenged with hydrogen peroxide. Amino Acids 48:53–64
Fickler VJ, Kirchgessner M, Roth FX (1995) The effect of dietary arginine supply on the N balance of piglets. 4th. communication on the importance of non-essential amino acids for protein retention. J Anim Physiol Anim Nutr 73:159–168
Flynn NE, Wu G (1996) An important role for endogenous synthesis of arginine in maintaining arginine homeostasis in neonatal pigs. Am J Physiol 271:R1149–R1155
Flynn NE, Knabe DA, Mallick BK et al (2000) Postnatal changes of plasma amino acids suckling pigs. J Anim Sci 78:2369–2375
Gatel F, Guion P (1990) Effects of monosodium l-glutamate on diet palatability and piglet performance during the suckling and weaning periods. Anim Prod 50:365–372
Haynes TE, Li P, Li XL et al (2009) l-Glutamine or l-alanyl-l-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids 37:131–142
Hewitt RJE, van Barneveld RJ (2012) Supplementation of lactating sow diets with glutamine to improve milk yield and growth of piglets. http://apri.com.au/2D-132_Final_report_120504.pdf. Accessed 23 Oct 2017
Hou YQ, Wu G (2017) Nutritionally nonessential amino acids: a misnomer in nutritional sciences. Adv Nutr 8:137–139
Hou YQ, Yao K, Yin YL et al (2016) Endogenous synthesis of amino acids limits growth, lactation and reproduction of animals. Adv Nutr 7:331–342
Hu CJ, Jiang QY, Zhang T et al (2017) Dietary supplementation with arginine and glutamic acid modifies growth performance, carcass traits, and meat quality in growing-finishing pigs. J Anim Sci 95:2680–2689
Janeczko MJ, Stoll B, Chang X et al (2007) Extensive gut metabolism limits the intestinal absorption of excessive supplemental dietary glutamate loads in infant pigs. J Nutr 137:2384–2390
Ji Y, Wu Z, Dai Z et al (2017) Fetal and neonatal programming of postnatal growth and feed efficiency in swine. J Anim Sci Biotech 8:42
Jiao N, Wu Z, Ji Y et al (2015) l-Glutamate enhances barrier and antioxidative functions in intestinal porcine epithelial cells. J Nutr 145:2258–2264
Junco E, Perez R, Jofre R et al (1991) Acute and chronic metabolic acidosis in the pig: renal metabolism and ammoniagenesis. Contrib Nephrol 92:18–30
Kang P, Wang X, Wu H et al (2017) Glutamate alleviates muscle protein loss by modulating TLR4, NODs, Akt/FOXO and mTOR signaling pathways in LPS-challenged piglets. PLoS ONE 12(8):e0182246
Kim SW, Wu G (2004) Dietary arginine supplementation enhances the growth of milk-fed young pigs. J Nutr 134:625–630
Kim SW, Wu G (2009) Regulatory role for amino acids in mammary gland growth and milk synthesis. Amino Acids 37:89–95
Kirchgessner M, Roth FX, Paulicks BR (1993) Effects of adding glutamic acid to low protein diets for fattening pigs on criteria of growth and carcass composition. Agribiol Res 46:346–358
Kong XF, Zhou XL, Feng ZM et al (2015) Dietary supplementation with monosodium l-glutamate modifies lipid composition and gene expression related to lipid metabolism in growing pigs fed a normal-or high-fat diet. Livest Sci 180:247–252
Krebs HA (1935) Metabolism of Amino Acids. IV. The synthesis of glutamine from glutamic acid and ammonia and the enzymic hydrolysis of glutamine in animal tissues. Biochem J 29:19511969
Kvamme E, Tveit B, Svenneby G (1970) Glutaminase from pig renal cortex. I. Purification and general properties. J Biol Chem 245:1871–1877
Le Floc'h N, Sève B, Henry Y (1994) The addition of glutamic acid or protein to a threonine-deficient diet differentially affects growth performance and threonine dehydrogenase activity in fattening pigs. J Nutr 124:1987–1995
Lei J, Feng DY, Zhang YL et al (2012) Nutritional and regulatory role of branched-chain amino acids in lactation. Front Biosci 17:2725–2739
Li P, Knabe DA, Kim SW et al (2009) Lactating porcine mammary tissue catabolizes branched-chain amino acids for glutamine and aspartate synthesis. J Nutr 139:1502–1509
Li XL, Rezaei R, Li P et al (2011) Composition of amino acids in feed ingredients for animal diets. Amino Acids 40:1159–1168
Lien KA, Sauer WC, Fenton M (1997) Mucin output in ileal digesta of pigs fed a protein-free diet. Z Ernährungswiss 36:182–190
Lin M, Zhang B, Yu C et al (2014) L-Glutamate supplementation improves small intestinal architecture and enhances the expressions of jejunal mucosa amino acid receptors and transporters in weaning piglets. PLoS ONE 9(11):e111950
Link L, Weidmann P, Probst P et al (1985) Renal handling of norepinephrine and epinephrine in the pig. Pflugers Arch 405:66–69
Liu T, Peng J, Xiong Y et al (2002) Effects of dietary glutamine and glutamate supplementation on small intestinal structure, active absorption and DNA, RNA concentrations in skeletal muscle tissue of weaned piglets during d 28 to 42 of age. Asian Aust J Anim Sci 15:238–242
Lydyard P, Grossi C (1989) Cells involved in the immune system. In: Roitt I, Brostoff J, Male D (eds) Immunology. Gower Medical Publishing, New York, pp 2.1–2.18
Manjarin R, Bequette BJ, Wu G et al (2014) Linking our understanding of mammary gland metabolism to amino acid nutrition. Amino Acids 46:2447–2462
Manso HE, Filho HC, de Carvalho LE et al (2012) Glutamine and glutamate supplementation raise milk glutamine concentrations in lactating gilts. J Anim Sci Biotechnol 3(1):2
Mateo RD, Wu G, Moon HK et al (2008) Effects of dietary arginine supplementation during gestation and lactation on the performance of lactating primiparous sows and nursing piglets. J Anim Sci 86:827–835
Matsumoto T, Nakamura E, Nakamura H et al (2013) The production of free glutamate in milk requires the leucine transporter LAT1. Am J Physiol 305:C623–C631
Mavromichalis I, Parr TM, Gabert VM et al (2001) True ileal digestibility of amino acids in sow’s milk for 17-day-old pigs. J Anim Sci 79:707–713
Maynard LA, Loosli JK, Hintz HF et al (1979) Animal nutrition. McGraw-Hill, New York
McDonald P, Edwards RA, Greenhalgh JFD et al (2011) Animal nutrition, 7th edn. Prentice Hall, New York
National Research Council (NRC (2012) Nutrient requirements of swine. National Academy Press, Washington, DC
Nichols NL, Bertolo RF (2008) Luminal threonine concentration actually affects intestinal mucosal protein and mucin synthesis in piglets. J Nutr 138:1298–1303
Patience JF, Rossoni-Serão MC, Gutiérrez NA (2015) A review of feed efficiency in swine: biology and application. J Anim Sci Biotechnol 6(1):33
Reeds PJ, Burrin DG, Jahoor F et al (1996) Enteral glutamate is almost completely metabolized in first pas by the gastrointestinal tract of infant pigs. Am J Physiol 270:E413–E418
Reeds PJ, Burrin DG, Stoll B et al (1997) Enteral glutamate is the preferential source for mucosal glutathione synthesis in fed piglets. Am J Physiol 273:E408–E415
Reeds PJ, Burrin DG, Stoll B et al (2000) Intestinal glutamate metabolism. J Nutr 130:978S–982S
Rezaei R, Knabe DA, Tekwe CD et al (2013a) Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids 44:911–923
Rezaei R, Jia SC, San Gabriel A et al (2013b) Monosodium glutamate supplementation to the diet for lactating sows enhances growth performance and survival of suckling piglets. Amino Acids 45:596–597
Santos de Aquino R, Dutra Junior WM, Manso HECC et al (2014) Glutamine and glutamate (AminoGut) supplementation influences sow colostrum and mature milk composition. Livest Sci 169:112–117
Satchithanandam S, Vargofcak-Apker M, Calvert RJ et al (1990) Alteration of gastro-intestinal mucin by fibre feeding in rats. J Nutr 120:1179–1184
Self JT, Spencer TE, Johnson GA et al (2004) Glutamine synthesis in the developing porcine placenta. Biol Reprod 70:1444–1451
Stegink LD, Filer LJ Jr, Baker GL (1973) Monosodium glutamate metabolism in the neonatal pig: effect of load on plasma, brain, muscle and spinal fluid free amino acid levels. J Nutr 103:1138–1145
Stoll B, Burrin DG (2006) Measuring splanchnic amino acid metabolism in vivo using stable isotopic tracers. J Anim Sci 84(E. Suppl):E60–E72
Stoll B, Burrin DG, Henry J et al (1999) Substrate oxidation by the portal drained viscera of fed pigs. Am J Physiol 277:E168–E175
Strathe AV, Bruun TS, Hansen CF (2017) Sows with high milk production had both a high feed intake and high body mobilization. Animal 11:1913–1921
Town SC, Patterson JL, Pereira CZ et al (2005) Embryonic and fetal development in a commercial dam-line genotype. Anim Reprod Sci 85:301–316
Treberg JR, Brosnan ME, Watford M et al (2010) On the reversibility of glutamate dehydrogenase and the source of hyperammonemia in the hyperinsulinism/hyper-ammonemia syndrome. Adv Enzyme Regul 50:34–43
Wang TC (1989) Fuller MF (1989), The optimum dietary amino acid patterns for growing pigs. 1. Experiments by amino acid deletion. Br J Nutr 62:77–89
Wang JJ, Chen LX, Li P et al (2008) Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr 138:1025–1032
Watford M (2015) Glutamine and glutamate: nonessential or essential amino acids? Animal Nutrition 1:119–122
Weber EK, Stalder KJ, Patience JF (2015) Wean-to-finish feeder space availability effects on nursery and finishing pig performance and total tract digestibility in a commercial setting when feeding dried distillers grains with solubles. J Anim Sci 93:1905–1915
Wu G (1998) Intestinal mucosal amino acid catabolism. J Nutr 128:1249–1252
Wu G (2013) Amino Acids: Biochemistry and Nutrition. CRC Press, Boca Raton
Wu G (2018) Principles of animal nutrition. CRC Press, Boca Raton
Wu G, Knabe DA (1994) Free and protein-bound amino acids in sow’s colostrums and milk. J Nutr 124:415–424
Wu G, Knabe DA (1995) Arginine synthesis in enterocytes of neonatal pigs. Am J Physiol 269:R621–R629
Wu G, Morris SM Jr (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17
Wu KK, Thiagarajan P (1996) Role of endothelium in thrombosis and hemostasis. Annu Rev Med 47:315–331
Wu G, Borbolla AG, Knabe DA (1994) The uptake of glutamine and release of arginine, citrulline and proline by the small intestine of developing pigs. J Nutr 124:2437–2444
Wu G, Bazer FW, Tuo W et al (1996) Unusual abundance of arginine and ornithine in porcine allantoic fluid. Biol Reprod 54:1261–1265
Wu G, Davis PK, Flynn NE et al (1997) Endogenous synthesis of arginine plays an important role in maintaining arginine homeostasis in postweaning growing pigs. J Nutr 127:2342–2349
Wu G, Haynes TE, Yan W et al (2001) Presence of glutamine:fructose-6-phosphate amidotransferase for glucosamine-6-phosphate synthesis in endothelial cells: effects of hyperglycaemia and glutamine. Diabetologia 44:196–202
Wu G, Bazer FW, Wallace JM et al (2006) Intrauterine growth retardation: implications for the animal sciences. J Anim Sci 84:2316–2337
Wu G, Bazer FW, Davis TA et al (2007) Important roles for the arginine family of amino acids in swine nutrition and production. Livest Sci 112:8–22
Wu G, Bazer FW, Burghardt RC et al (2010) Functional amino acids in swine nutrition and production. In: Doppenberg J (ed) Dynamics in animal nutrition. Wageningen Academic Publishers, The Netherlands, pp 69–98
Wu G, Bazer FW, Johnson GA et al (2011) Important roles for l-glutamine in swine nutrition and production. J Anim Sci 89:2017–2030
Wu G, Bazer FW, Johnson GA et al (2013) Maternal and fetal amino acid metabolism in gestating sows. Soc Reprod Fertil Suppl 68:185–198
Wu G, Bazer FW, Dai ZL et al (2014) Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci 2:387–417
Wu G, Bazer FW, Johnson GA et al (2017) Functional amino acids in the development of the pig placenta. Mol Reprod Dev 84:879–882
Ytrebo LM, Sen S, Rose C et al (2006) Interorgan ammonia, glutamate, and glutamine trafficking in pigs with acute liver failure. Am J Physiol 291:G373–G381
Zhang J, Yin Y, Shu XG et al (2013) Oral administration of MSG increases expression of glutamate receptors and transporters in the gastrointestinal tract of young piglets. Amino Acids 45:1169–1177
Zimmerman DR (1975) Glutamic acid and tryptophan additions to a low-protein pig starter. J Anim Sci 40:871–874
Acknowledgements
This work was supported, in part, by Grants from the National Key R&D Program of China (2016YFD0501210), Natural Science Foundation of Hubei Province (2016CFA070), Hubei Provincial Technology and Innovation Program (2016ABA121), the Program of National Agricultural Research Outstanding Talents of China (2015), Hubei Hundred Talent program, Agriculture and Food Research Initiative Competitive Grants (2014-67015-21770 and 2015-67015-23276) from the USDA National Institute of Food and Agriculture, and Texas A&M AgriLife Research (H-8200).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
This article reviews published studies and does not require either the approval of animal use or human consent.
Additional information
Handling Editor: J. D. Wade.
Rights and permissions
About this article
Cite this article
Hou, Y., Wu, G. l-Glutamate nutrition and metabolism in swine. Amino Acids 50, 1497–1510 (2018). https://doi.org/10.1007/s00726-018-2634-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-018-2634-3