Abstract
The wide range of inherent and tunable physicochemical properties of polymers made them ubiquitous and unavoidable materials in the physical and biological world. Both natural and synthetic polymers find their wide range of applications from everyday life to technical innovations. The unique physicochemical properties dependent on the morphology of the polymers have led its wide range of applications in therapies and diagnostic options. The inherent and tunable surface characteristics of polymers are used for technical applications with specific surface specialties. The polymers used for biomaterial application must meet some criteria, such as non-cytotoxicity, high drug loading capacity and improved cellular internalization. However, the polymer may have some special surface properties due to its performance according to its application design. In these perspectives, the surface functionalization of polymers is necessary to take advantage of their properties and achieve the selective and specific recognition required for biomedical applications. The surface functionalization of polymers is a promising technique to introduce and monitor some specific surface properties, such as adhesion, adsorption, biocompatibility, electrical conductivity, optical properties and wettability to target their corresponding applications. In this chapter, the chemistry, scope and current trends of surface functionalization will be discussed in terms of chemical, physical, and nanotechnological methods.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction: Fundamentals of Surface Functionalization of Polymer
Various polymeric substances have been designed in different nano-forms (polymeric NPs, nanofibers, nanorods, nanospheres, nanotubes, electro-spun fibers, etc.) and have been used in various aspects of research and commercial applications (Bhattarai et al. 2019b, 2020). The properties of the polymeric nanomaterial are the synergistic effect of its bulk properties, surface properties, formulation and engineering. The nanomaterials endowed with the intended bulk and surface properties could be the material chosen for their applications as advanced materials with desirable characteristics. The high surface/volume ratio of nanomaterials and quantum confinement have triggered its scope and dynamics in various research fields, as well as trades and commerce. A wide range of materials and their performance depend largely on their surface characteristics. In biological settings, surface functionalization of polymer is intended to influence on the implant biocompatibility, thus reducing thrombogenicity and tuning the adhesion of proteins and cells (Tang et al. 2008). Some properties such as thermal stability, mechanical strength, behavior towards solvent, polymer degradation, swelling/deswelling properties are more related with bulk properties of polymer, while other properties such as surface adhesion, chemistry and wettability, as well as behavior towards harsh chemical/environmental agents are more concerned with surface characteristics. In most cases, the polymers of choice may be lower than the intended properties required for particular applications. To fill these gaps, the polymerization surface functionalization strategy can be a way of implementation (Penn and Wang 1994).
The understanding of the material surfaces and their engineering has increased significantly with a greater capacity to tailor and modulate the surface characteristics for specific application. Especially, such surface modifications of polymers via surface functionalization would change the fate of surface hydrophilicity, cellular behavior and physicochemical platforms such as antibacterial or photothermal properties (Tiwari et al. 2019).
Different types of polymers are used for several types of physical applications. The electric charge transfer process through the polymer film and metal electrode interfaces plays a key role in many polymeric optoelectronic devices, including light-emitting diodes, photovoltaic cells and thin film transistors. Polymers such as PBTP, PC, PDMS, PMMA, polyolefins (e.g. PE and PP), etc. can be used in the manufacture of thermosetting materials (Zarrintaj et al. 2019). However, the polymers used can capture some specific types compounds from the solution passing through it. In order to inhibit such undesired molecular adsorptions, it is required to functionalize the surface of such polymers (Rohr et al. 2003).
Polymers, in different forms and designs, are being used in different fields of bio-applications. Whatever the polymeric materials or their design, it is the surface of the biomaterials which comes into contact with physiological environment immediately after their accommodation into the body (Dai et al. 2003). To improve the surface effectiveness of the engineered material, it is a common practice to carry out surface functionalization of the material. However, the approaches of surface functionalization might be different. The surface of electro-spun fibers can be modified or functionalized by various ways to introduce new functionalities. In some cases, even after surface functionalization, cell-recognizable ligands can be chemically immobilized on the substrate surface for anchoring (Gutiérrez et al. 2018). The surface functionalization of polymers can be linked to different fields of applications including drug delivery, gene delivery and various aspects of tissue engineering. The high surface area of nanomaterials could provide platforms for the immobilization of bioactive molecules such as antibodies, DNA, enzymes and peptides (Kim and Park 2006). In view of the bio-application of any polymer, the cellular interaction and cytotoxicity of the materials depends on the physicochemical properties and the surface modification of material (Dowding et al. 2013). Once nanomaterials enter the body’s administration, they come into immediate contact with various plasma constitutes. The initial interaction of nanomaterials with body environment induces the formation of protein coronas which influences the subsequent immunological cascade and can either stimulate or mitigate the immune response. For this reason, in vivo therapeutics requires the evaluation of the nanomaterial/protein corona formation and their subsequent destination towards the physicochemical properties of the substrate (Lee et al. 2015). The surface of polymeric substrates (electro-spun mats, NPs, nanorods, etc.) can be chemically or physically modified with bioactive molecules (carbohydrates, nucleic acid and proteins) and cell-recognizable ligands (Yoo et al. 2009). Such types of biologically modified synthetic nanofibers can further support the specific cell phenotype and cell organization due to diverse biochemical signals on the cell contact surface.
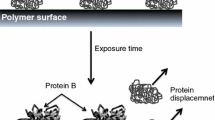
The chemical functionalities of the surface influence the adsorption of proteins, cell behavior and subsequent tissue responses. Common functionalities in relation to biomaterial interactions are the amino (–NH2), carboxyl (–COOH), hydroxyl (–OH) and methyl (–CH3) groups. The hydrophilic surface provides low interfacial free energy resulting in less protein adsorption and cell adhesion. Reduction of protein adsorption influence on subsequent cellular responses. The interactions between protein and biomaterial can be modulated by the nature of functional groups and their density. Increasing the density of –OH and –NH2 groups onto the polymeric substrate can improve the surface hydrophilicity and the influence on immunoglobulin G (IgG) adsorption from serum. Fibrinogen can be linked to the hydroxyl and amino surfaces, as well as fibrinogen can be linked to methyl-functionalized surface via hydrophobic interactions (Tang et al. 2008). The functional group of polymeric surfaces characterized by hydrophilic nature, presence of hydrogen bond acceptors (no hydrogen bond donor) and electrically neutral charge can resist the protein adsorption (Ostuni et al. 2001).
All in all, the inherent surface properties of the polymer may not meet all the requirements for specific physical or biomedical applications. However, it is possible to induce and monitor the desired interfacial properties of a substrate by some chemical or physical means. In this context, it has been commendable to modify the outermost layers of polymer by introducing some types of functional groups onto the polymer surface. The approaches of surface functionalization may be different for each case and particularly depends on the interplay between functionalized materials and anchor molecules. For example, aminolysis, hydrolysis, and plasma treatment can be adopted for surface functionalization of non-degradable synthetic polymers (Croll et al. 2004).
2.2 Scope of Surface Functionalization of Polymers
Surface functionalization of polymers paves the way for their novel applications in different research fields, including biomedical applications, electronics, protective coatings, textiles, thin film technology and others (Fig. 2.1) (Iijima et al. 2016). Different fields of research involving surface functionalization of polymers are described briefly below.
2.2.1 Surface Functionalization of the Polymer in Microfluidic Channels
Surface chemistry plays an important role in the interactions of flowing liquid to the inner surface of the microfluidic channels . The polymers commonly used in microfluidic channels are PC, PDMS, PMMA, etc. These polymers exhibit hydrophobic nature and can capture some specific molecules during the solution flow through the channels. The capture of specific molecules can change the concentration of the solution by denying the precise quantitation of the analytes, as a result, the adsorbed molecules on the wall of channels continuously change the surface chemistry, thus affecting the reliability of quantitative assays. Appropriate functionalization of the polymeric microchannel surface could control the undesired adsorption process causing better reliability in the tests (Rohr et al. 2003).
2.2.2 Surface Functionalization of the Polymer in Ultrafiltration
Ultrafiltration is a kind of membrane filtration that is used to separate high molecular weight solute (1–1000 kD, retentate) from low molecular weight solute and water (permeate) through a semipermeable membrane under the effect of gradient of pressure or concentration. Adsorption and permeation are two main events that can occur in biological filtration. During the filtration process, the protein molecules are adsorbed and deposited onto the porous membrane. Researches have shown that hydrophilic polymers adsorbed on the surface of membranes relieve fouling of proteins during the time of ultrafiltration and microfiltration (Chen and Belfort 1999). In such cases, the polymer layers can be functionalized on the surface to obtain more stability and durable surface properties to support the ultrafiltration process.
2.2.3 Surface Functionalization of the Polymer in Drug Delivery
Recent studies have suggested that the surface functionality of drug delivery vehicles influence on rate and mechanism of cellular absorption. Polymeric nanomaterials can be a good drug delivery depot and form a vehicle for slow drug molecule release, thus obtaining a sustained therapeutic effect. The surface functionalization of polymeric nanomaterials by hydrophilic polymer (e.g. PEG) can increase the residence time of polymeric NPs in the systemic circulation. The tailor of tissue-recognizable ligands can also help drug delivery to targets (Patil et al. 2009; Otsuka et al. 2003). Surface functionalization of the polymer can complement some additional tunable characteristics of polymeric materials and can make them responsive to external stimuli such as light (e.g. near infrared (NIR) and ultraviolet (UV) radiation), pH, temperature, ultrasound, etc. to trigger the controlled release of drugs. Surface functionalization can significantly change the biological response beyond the mere performance of the polymer’s core structure (Masserini 2013). The pH-responsive drug delivery systems can mediate the drug release rate according to the pathophysiological need demand. For example, Jiang et al. (2014) reported that PDA coating can load and release the rate of charged molecules from PCL nanofibers with different pH values. In this work, the positively charged molecules triggered the quick release of the drug in acidic medium compared to the neutral and basic medium under the same incubation conditions. In addition, polymer NPs must be biodegradable and biocompatible, as well as non-inflammatory and non-immunogenic for medical use. They should be nontoxic to bloodstream cells or to stationary cells. Figure 2.2 shows the graphic representation of surface modified NPs with drugs for brain drug delivery (Masserini 2013; Jiang et al. 2014).
2.2.4 Surface Functionalization of the Polymer in Electronics
Uniform dispersion of NPs into polymer nanocomposites for polymer matrix formulation in order to tune the desired electrical, mechanical opto-electronic and thermal properties is a challenging task. One of the solutions for such complications could be surface functionalization of particles with organic surfactant molecules to develop compatible polarity in mixing solutions. Polymer composite materials with low temperature processability and various functionalities are good materials for optoelectronic applications. For example, functionalized GO nanosheets with hydrophobic brushes (e.g. PtBuA-grafted GO nanosheet) can be tailored onto an electroactive polymer matrix for switching behavior of bistable electrical conductivity (Jiang et al. 2006; Li et al. 2010). Dopamine-functionalized PMMA has also been shown to improve dielectric properties compared to pristine PMMA films (Thakur et al. 2014). Functionalized conductive polymers could be used to improve performance, selectivity and sensitivity of sensors beyond the level of their pristine form (Anantha-Iyengar et al. 2019).
2.2.5 Surface Functionalization of the Polymer in Textiles
Superhydrophobic surfaces offer a self-cleaning mechanism in compliance with lotus effect. The concept of superhydrophobicity arose in the field of textile industry since about 1940. For example, amine functionalized SiO2 coating onto cotton fibers followed by dipping into epoxy functionalized silica (particles solution) was performed to avail epoxy group for surface grafting (Hao et al. 2012). Stearic acid grafting can also impart superhydrophobic property to the cotton fabric (Gao and McCarthy 2006; Xue et al. 2008). Zemljič et al. (2018) also reported the encapsulation of iodine with chitosan (Cs) on cellulose viscose fabrics to exhibit an antimicrobial and antioxidative function.
2.2.6 Surface Functionalization of the Polymer in Food Packaging Process
The application of novel technique in the food packaging process could reduce discomfort related to operating conditions and processing costs, which reduces environmental and financial costs. Plasma-induced effects can exploit the surface functionalization of food packaging polymers to promote adhesion or anti-adhesion, printing and sealing capacity, adhesion of antibacterial coatings. PP is a polymer commonly used to develop packaging films which are often coated with acrylic to improve durability, flexibility and resistance to degradability caused by UV rays (Vukušić et al. 2018). PEGylated CNCs via PDA chemistry have been shown to significantly improve the crystallization, mechanical and barrier properties of PLLA bio-nanocomposites, thus exhibiting great promise for potential applications in packaging fields (Li et al. 2019).
2.2.7 Surface Functionalization of the Polymer in Protective Coatings
Only a polymer coating on a substrate may not satisfy the anticorrosive action, but the surface functionalization of polymer could support this. PDA, which contains the catechol and amine groups, can be used to modify a wide variety of material surface to support protein immobilization (Kang et al. 2010; Cho et al. 2009). Keeping this in view, Tian et al. (2016) demonstrated that the PEO/PCL coating on the AZ31 magnesium alloy can improve bioactivity, cytocompatibility and corrosion resistivity of the substrate.
2.2.8 Surface Functionalization of the Polymer to Influence Particle Stability
Zeta potential value of NPs allows to know the fate of the stability of the particles (Bhattarai et al. 2019b). Some polymeric NPs need to improve their stability for their sound stability under a physiological pH value. Under such requirements, a greater zeta potential magnitude can be achieved by surface functionalization. The research results shown, e.g. that the zeta potential magnitude of PLGA NPs can be increased by surface functionalization using heparin- or Cs-pluronic conjugates (Chung et al. 2010). Zeta potential of polymeric NPs can also be tuned by varying the nature of surfactants (anionic, cationic or non-ionic) and, consequently, changes the stability of the particles (Sis and Birinci 2009).
2.2.9 Surface Functionalization of the Polymer in Biomedical Applications
The applications of polymer varieties in different forms and functions are ubiquitous and wonderful in the biological world. It would be commendable in most of the cases to functionalize the polymer surface by inducing some desired physicochemical properties. The surface functionalization of polymers for bio-application can be multipurpose. For example, the surface functionalization of polymers is carried out not only to develop hydrophilicity, or to develop a conducive environment to protein adhesion, but also to develop antimicrobial and antifouling properties (Zeng et al. 2018).
Metallic implants are prone to corrosion under physiological environment. It is usual to develop a coating of anticorrosive polymer on the substrate to solve the corrosion problem. Under such conditions, the surface functionalization of polymers plays a crucial and promising role in the field of materials science. Surface functionalization of polymers for biomedical applications is considered under the tuning possibilities of the physicochemical properties of the polymer capable of working within a physiological niche. The reduced dimensions, the high surface area/volume ratio and the high drug loading efficiency of biocompatible NPs also make them attractive for drug delivery (Wang et al. 2017). These NPs may have the ability to respond quickly to environmental stimuli, such as magnetic field, pH, temperature or ultrasound. Different synthetic polymers such as PGA, PLA and PLGA copolymers have been used for the manufacture of NPs to control drug release due to its established safety profile, its long history of clinical applications and its well-understood degradation mechanism. Figure 2.3 shows the multiple nanocarriers for the encapsulation and release of growth factors.
Multiple nanocarriers for the encapsulation and release of growth factors. Adapted with permission from Wang et al. (2017)
2.3 Classification of Surface Functionalization of Polymer
Surface functionalization of polymer can be classified into different bases. Some of the classification approaches are given below:
2.3.1 Classification According to the Surface Charge
-
Cationic surface functionalization:
In this type of functionalization, cationic moieties are tailored onto the polymer surfaces. As an example, a large number of hydroxyl groups present onto CNCs make it a space for diverse chemical functionalization possibilities with the generation of relevant surface properties. In addition, the extent of surface functionalization and the nature of tailored functional group onto the substrate can influence the surface charge density and impact the physic-chemical properties of the substrate. Wide varieties of surface functionalization of polymers have been made possible to interact with the corresponding biomolecules for multiple biomedical applications. With this in mind, Despres et al. (2019) obtained a CNC-PAPMA polymer with cationic surface functionalization. The cationic or anionic surface charge of the synthesized polymer can be determined by zeta potential measurements, while functionalized chemical structure can be determined by Fourier transform infrared (FTIR), energy-dispersive X-ray (EDX), X-ray photoelectron spectra (XPS) or nuclear magnetic resonance (NMR) analysis. Such types functionalized polymer on different surfaces behave differently towards cell interaction.
-
Anionic surface functionalization:
In this type of surface modification, the anionic groups are tailored onto the polymer surface. This can be done by acid hydrolysis process or by some other methods. For example, hydrolysis of cellulose by using sulphuric acid incorporates anionic sulphate half-ester (OSO3−) (Fig. 2.4) (Sunasee and Hemraz 2018) and hydrolysis by using phosphoric acid incorporates phosphate groups onto the surface of CNCs (Eyley and Thielemans 2014). In such cases, the extent of the sulphate or phosphate groups onto the polymer can be estimated by elemental detection or functional group identification. Similarly, CNC-PNIPAAm is a polymer with anionic surface functionalization.
-
Zwitter-ionic surface functionalization:
Zwitterion is a chemical moiety that has at least one positive and one negative electric charge. The net charge of the entire chemical species is zero. The surface charge of such molecules is a function of the pH value of the solution. It means that they behave like cationic or anionic molecules below or above a particular pH value. However, the molecule behaves like a neutral molecule at a particular pH value, called an isoelectric point. At the isoelectric point, the zwitterionic molecule does not migrate at all, even under the application of electric field. Such zwitterionic molecules can be exploited to functionalize the polymer surface. Wu et al. (2018) reported the grafting of zwitterionic PSBMA onto the cotton fabrics via intermediate reaction of reactive dye and a surface-initiated atom transfer radical polymerization method to develop a non-fouling property.
Hydrolysis of cellulose through the use of sulfuric acid incorporating anionic sulfate half-ester (OSO3−). Adapted with permission from Sunasee and Hemraz (2018)
2.3.2 Classification on the Basis of Functional Group Modification
-
Amine functionalization:
It is the tailoring of the amine groups onto the polymer surface. For example, the amine functionalized PS NPs can be synthesized by a copolymerization of styrene miniemulsion and a different amount of amine functionalized monomer such as 2-aminoethylmethacrylate hydrochloride (Fig. 2.5) (Holzapfel et al. 2005).
-
Amide functionalization (Amidation):
It is the tailoring or insertion of amide groups onto the polymer surface. Amide-functionalized polymers have shown substantial electron mobility and have gained the most encouraging device performance in the field of organic optoelectronics and polymer semiconductors (Guo et al. 2014). Suresh et al. (2014) synthesized amide-functionalized microporous organic polymers from trimesic acid and p-phenylenediamine in the presence of thionyl chloride. The amide group in the polymer helps, e.g. to adsorb carbon dioxide molecules via Lewis acid-base type interaction.
-
Carboxylate functionalization:
It is the tailoring of carboxylate groups onto the polymer surface. For example, the carboxylate functionalized PS NPs can be synthesized by mini-emulsion copolymerization of styrene and a different amount of carboxylic functionalized monomers such as AA (Holzapfel et al. 2005). Murphy et al. (2005) reported the increased stability of polythiophene against oxidative doping due to the addition of an electron-withdrawing alkyl carboxylate substitute.
-
Ester functionalization (esterification):
Esterification is the process of introducing the ester group onto the polymer surface. Esterification by substituting hydroxyl groups of polymers introduce less polar ester group onto the polymer surface. The hydroxyl substituted esterification is applied to hydrophobize polymer. For bio-application, natural α-hydroxy acids such as citric acid, glyceric acid, glycolic acid, lactic acid, malic acid, mandelic acid, tartaric acid, etc. can be used to catalyze the esterification of CNC (hydroxyl rich molecule). CNCs can, for example, be esterified by treatment with acetic anhydride in presence of citric acid. Here, the hydroxyl group of α-hydroxy acid presumably induce a nucleophilic attack on the acylating agent (Ávila Ramírez et al. 2014, 2017).
-
Surface acetylation:
The acetyl group can be introduced onto the polymer surface by an acetylation process. For example, surface acetylation of CNC can be carried out by treating the CNC with acetic anhydride in presence of pyridine (Eyley and Thielemans 2014).
-
Surface acylation:
It is the tailoring of acyl (RCO-) group onto the polymer by chemical method. For example, alkenyl succinic anhydride can be used as an acylating agent for polysaccharide nanocrystals in non-polar media (Gopalan Nair et al. 2003; Angellier et al. 2005).
-
Catechol functionalization:
Catechol group of a molecule imparts water solubility, which makes it a useful candidate in the design of biomimetic materials. Catechol functionalization onto polymer such as Cs increases the bio-adhesiveness and antioxidant properties (Zhang et al. 2018; Ryu et al. 2015; Faure et al. 2013).
-
Oxidation:
The oxidation modification of the functional groups on the surface significantly affects the surface charge density, hydrophilicity/hydrophobicity of polymer which influences the cellular interactions accordingly. For example, the primary hydroxyl (hydroxymethyl) group of cellulose into carboxyl group is possible by 2,2,6,6-tetramethylpiperidinyloxyl radical-mediated oxidation process (Habibi et al. 2006). However, the oxidation of CNCs by sodium periodate can develop aldehyde functional group (Sun et al. 2015).
Amine-functionalized PS NPs. Adapted with permission from Holzapfel et al. (2005)
2.3.3 Classification Based on the Polymer Grafting/Spraying
-
Polymer grafting:
The results of current research have suggested the grafting of conjugated polymers into biopolymers to introduce novel functionalities with new features. Conductive polymer grafting over the specified polymer introduces conductivity in the polymer composite. Surface functionalization of cellulose can, for example, be performed by direct grafting of P3HT via oxidative polymerization. The grafting of P3HT onto the cellulose surface increases the hydrophobic properties with lotus effect and also increases conductivity (Hai and Sugimoto 2018).
-
Spray coating:
The antibacterial or antimicrobial properties can be developed onto a polymer by a spray coating method. For example, mussel-inspired polyglycerol (MI-PG) can be successfully functionalized to impart antibacterial activities via the spray coating of an aqueous solution of silver nitrate (Schlaich et al. 2018).
-
PEGylation:
PEG is an amphiphilic polymer composed of a repetitive unit of ethylene glycol. PEG is activated for polymer conjugation by substituting its terminal –OH functional group by appropriate group retaining its biological activities. The activated PEG may be PEG-aldehyde, PEG-alkyne, PEG-amine, PEG-hydrazine, PEG-NHS ester, PEG-thiol, etc. (Giorgi et al. 2014). This process links the PEG polymer chain to the target molecules via covalent or non-covalent bonding. PEGylation is most commonly adopted to functionalize proteins and anticancer drugs. Generally, PEGylation is carried out to improve the water solubility of substances such as drugs, proteins, etc. and to improve colloidal stability to polymers (Lin et al. 2008; Veronese and Pasut 2005; Pelaz et al. 2015).
-
Silylation (silane grafting) :
It is the process of introducing a substituted silyl group (R3Si) to a molecule such as alcohol, amine, carboxylic acid, phosphate, thiol, etc. of a particular polymer. The silane group can be introduced onto CNCs to increase hydrophobicity. APTES is a commonly used silane, and can functionalize the CNC surface for silylation. For example, Khanjanzadeh et al. (2018) linked the APTES hydrocarbon chain to the surface of the CNC via an Si-O-C bond by the condensation reaction between the hydroxyl group of CNC and the silanol group of APTES. This type of surface modification promotes the dispersion of the polymer into non-polar media. The surface properties of polymers can change with the incorporation of functionalized nanomaterials and, consequently, improve the effectiveness of the material for a specific application (Pokharel and Lee 2014; Pokharel et al. 2015a; Pant et al. 2015; Pokharel and Truong 2014). Figure 2.6 shows the schematic diagram for grafting of PU chains onto the APTES-treated GO surface (Pokharel et al. 2015b). The epoxide groups of GO react with the amine group of aminosilane in aqueous medium via SN2 nucleophilic displacement (Pokharel et al. 2015b). Following Pokharel et al. (2015b), the GO-grafted APTES methoxy groups underwent hydrolysis and this was responsible for increasing the number of hydroxyl groups onto the GO surface. The hydroxyl and carboxyl groups onto GO surface can also react with the -NCO groups of MDI and the PU pre-polymer (Pokharel et al. 2015c, 2019; Pokharel and Choi 2015; Pokharel 2014). As a result, the electrical, mechanical, surface and thermal properties of the nanocomposite are completely changed during the preparation of PU nanocomposites by in-situ polymerization (Pokharel et al. 2015c, 2019; Pokharel and Choi 2015; Pokharel 2014).
Schematic diagram to illustrate the functionalization of polymers during the preparation of GO-based PU nanocomposites. BD 1,4-butanediol, FGSs functionalized graphene sheets, MDI 4,4′-methylene diphenyl diisocyanate, PTMEG poly(tetramethylene glycol) (average Mw = 1000 g/mol). Reprinted with permission from Pokharel et al. (2015b)
2.4 Methods of Surface Functionalization of Polymers
The surface functionalization of polymer depends on several factors, such as the material used in the surface functionalization, the functional group intended to bind onto the polymer and purpose of the specific application. Although several methods of surface functionalization of polymers are theoretically possible, practically some methods are limited by some types of technical problems and aspects of applicability. Basically, the surface modification/functionalization of polymer can be carried out by covalent fixation of chemical moieties, plasma treatment, wet chemical oxidation, and functionalization based on classical organic chemistry (Penn and Wang 1994). Some commonly used methods of the surface functionalization of polymers are given below:
2.4.1 Plasma Treatment
Plasma is considered the fourth state of matter, it is a mixture of highly-excited molecular, atomic, ionic and radical species (Chu et al. 2002). Plasma treatment can be used to etch the polymer surface, develop a certain functional group and thin layer coat of polymer on the substrate. For surface functionalization of polymer, plasma sources are selected to direct a particular functional group on the polymer surface to introduce the desired functionality without compromising their bulk properties. Plasma treatment with oxygen, ammonia or air can generate amine and carboxyl groups on the surface of the substrate. Air, water vapor and oxygen plasma are used to introduce oxygen-containing functional groups, while alkyl amine, ammonia, nitrogen, etc. are used to introduce nitrogen-containing functional groups.
Plasma treatment can also modify the polymer surface via different ways such as activation, cleaning, crosslinking, etching, etc. Chemical etching is promoted by radicals such as O2 and CF4 discharge, while physical etching is promoted by strong ion bombardment (e.g. argon plasma). Plasma treatment on polymer refines the surface and also leaves some post-reaction-active sites (Hegemann et al. 2003). Plasma treatment can increase or decrease the surface hydrophilicity of polymer. For example, oxygen plasma (mixing several reactive oxygen species) can improve the hydrophilicity while fluorocarbon treatment increases the surface hydrophobicity to the polymer. According to Park et al. (2007a) the hydrophilicity of electro-spun PLGA nanofibers can be increased by the gas plasma treatment method. The increase in hydrophilicity could be associated with the intrusion of polar functional moieties (Park et al. 2007a), whereas air plasma treatment on PCL nanofibers can, for example, introduce –COOH functional groups which can help gelatin grafting in the presence of carbodiimide cross-linker (Ma et al. 2005a).
Plasma treatment followed by grafting of hydrophilic moieties could also be an alternative approach to polymer surface modification. For example, the surface of biodegradable polymers such as PGA, PLGA and PLLA can be chemically modified by an oxygen plasma treatment which makes a room for grafting hydrophilic acid such as AA. Such grafting of hydrophilic moieties can be evidenced by a higher ratio of oxygen to carbon, a lower contact angle and the presence of a carboxylic acid group. Such surface-functionalized scaffolds improve the cell attachment and proliferation (Park et al. 2007b). The surface of the electro-spun PVDF fibers can also be treated to argon plasma followed by graft-copolymerization with methacrylic acid, thus increasing material hydrophilicity (Kaur et al. 2007). Hetemi and Pinson (2017) showed that the surface functionalization of S-Nitrosated PLGA-cysteine (PLGH-cysteine) by water plasma treatment without disturbing the active NO group can also decrease water contact angle values from 116° to 0° (Fig. 2.7).
Surface functionalization of S-Nitrosated PLGA-cysteine (PLGH-cysteine) by water plasma treatment without disturbing the active NO group. Adapted with permission from Hetemi and Pinson (2017)
2.4.2 UV Treatment
Surface functionalization of polymer can be made feasible by UV treatment (~200–400 nm of radiation). The effect of UV treatment depends on the UV irradiation parameters (distance, power and time of UV irradiation), room temperature, concentration of functionalizing and oxidizing agent (Gutiérrez and González 2016). UV treatment can cause surface functionalization of polymer via radical formations, or oxidation of the polymer or some types of bond cleavage (Hetemi and Pinson 2017). For example, surface functionalization of PES nanofibers can be carried out by UV irradiation into the PES nanofibers immersed in an aqueous solution of functionalizing agent (e.g. AA) in the presence of oxidant (e.g. sodium periodate solution) (Chua et al. 2006). The functionalization of AA onto the substrate introduces a functional group of carboxylic acid, while the poly(acrylic acid)-grafted PES can further be conjugated with other chemical moieties (such as the amino groups) by using different spacers (butylene, ethylene, hexylene, etc.) ethylene diamine employing carbodiimide crosslinking technique (Chua et al. 2007).
2.4.3 Ozone Treatment
Surface functionalization of polymer by ozone treatment method is also a type of oxidation process. This can develop the peroxide formation on or within the polymer, making a space for the binding of the active group. For example, an active group can be linked onto PE via ozone treatment (Hetemi and Pinson 2017).
2.4.4 Electrospinning
The desired functional group in the polymer can be introduced by electrospinning of the mixing solution. For example, the primary amino groups can be introduced onto the polymer surface by electrospinning of the PLGA and PLGA-b-PEG-NH2 diblock copolymer mixture. Such surface-aminated polymeric fibers offer rich sites for enzyme or peptide immobilization, which lead to greater cell attachment and proliferation (Kim and Park 2006).
2.4.5 Enzyme Immobilization
In some cases, polymer nanofibers are activated and processed for a chemical reaction with enzyme such as lipase. For example, hydrogen chloride gas can be bubbled into the PAN fiber immersed in an absolute ethanol solution to introduce imidoester (R-CNH-OR′) functionalities which impart the sites for conjugation with amine moieties of lipase (Fig. 2.8) (Li et al. 2007).
Modification of PAN fiber by enzymatic immobilization of lipase. Adapted with permission from Li et al. (2007)
2.4.6 Hydrolysis
Hydrolysis of the esters produces carboxylic acid and hydroxyl groups. The carboxylic acid groups can be introduced into the ester bond polymer by hydrolysis, which would be beneficial for further processing. For example, partial hydrolysis of PCL fibers develops carboxylate groups which promotes the nucleation of calcium ions during the biomineralization process (Fig. 2.9) (Araujo et al. 2008). Similarly, the hydrolysis of PET produces a polymer surface with functionality of the carboxyl and hydroxyl groups (Chen and McCarthy 1998).
Hydrolysis of PCL using sodium hydroxide. Adapted with permission from Araujo et al. (2008)
2.4.7 Grafting
It is the method of chemical immobilization of molecular moieties or polymer chain onto the polymer surface. The polymer chain can be linked onto the polymer either by graft polymerization (synthesis of polymer chains from the reactive sites of the polymer substrate) or by polymer grafting (attachment of polymer segment previously synthesized onto the polymer substrate). Proteins or some types of monomers are covalently conjugated onto the material surface to alter surface chemistry. The polymer surfaces are activated with some types of reactive groups followed by grafting of the desired functionality. The polymer surface activation can be carried out by ozone exposure, plasma treatment, UV irradiation, etc. Chemical grafting method is more prone to toxicity compared to the physical adsorption method (Tang et al. 2008).
2.4.8 Chemical Reduction
The functional groups of some polymer can be reduced to other types by treating the polymer with some types of reducers such as NaBH4. For example, the ketone functional group of PEEK polymer can be reduced to hydroxyl functional group by treating the polymer with NaBH4 in DMSO at 120 °C (Fig. 2.10) (Park et al. 2007b). The –OH group can further be transformed into other desired functionalities by some types of chemical treatment (Hetemi and Pinson 2017; Diez-Pascual et al. 2009). Similarly, the alcohol functionality can be introduced onto PET by reducing it with lithium aluminum hydride (Chen and McCarthy 1998).
Surface functionalization of PEEK polymers by transforming its carbonyl group into hydroxyl group by the reducing action of NaBH4 in DMSO medium. Adapted with permission from Hetemi and Pinson (2017)
Some specific polymers and approaches for surface functionalization, as well as their advantages are briefly presented in Table 2.1.
2.5 Analytical Techniques for Surface Characterization
The surface functionalization of polymer causes some types of chemical and physical changes associated to surface chemistry, morphology and topography, as well as hydrophilicity or hydrophobicity, crystallinity and functional group on the polymer surface. Accurate evaluations of such modifications are required in relation to physicochemical characterizations and their targeted objectives. However, the characterization is a bit sensitive because the mass or volume of the functionalizing species is extremely low in relation to bulk mass. In addition, the spectroscopic signal from the bulk mass overwhelms the contribution from the surface. No single method of characterization can provide a complete evaluation of the surface. Many of the characterizations in concert can give supporting evidence on surface characterization. The techniques commonly used for the assessment of surface functionalization of polymers are given in Table 2.2.
2.6 Recent Trends in the Surface Functionalization of Polymers
Several approaches of surface functionalization of polymers have been practiced for some decades. In this sense, surface functionalization of polymer should be carried out without compromising their inherent biocompatibility, biodegradability concerns, non-cytotoxicity and bulk properties. Until now, the surface functionalization of polymers has expanded the various promising aspects of the polymer, from physical applications to biomedical applications. Currently, research is not limited to only reducing or increasing the hydrophilicity of polymers, but are expanding for multipurpose applications. In addition, modulation of the degree of surface functionalization has been achieved based on the polymer nature and the flux of reactive plasma species (Vesel and Mozetic 2017). Selective synchrotron radiations can be applied to functionalize the polymer in presence of reactive gas species (Weibel 2010). Medical implants are more susceptible to microbial attack and embedding. Polymer-coated implants must retain certain specific properties for their targeted applications. Sterilization and other material processing events should not deteriorate the properties of polymers. For this reason, surface functionalization of polymer should be seen as a holistic approach rather than as a stereotype. Today, surface functionalization of polymers has developed new properties on the simple state of the polymer. For example, Tiwari et al. (2018) functionalized the PCL electro-spun mat by using poly(pyrrole) via chemical polymerization to induce photothermal property for hyperthermia applications targeted at cancer treatment. PDA surface functionalization onto PCL mat unveils an efficient photothermal chemotherapy route (Tiwari et al. 2019).
Although there have been several types of surface functionalization approaches in practice, surface functionalization by plasma treatment has played a leading role among many other methods. The reasons behind the main role of plasma treatment in surface functionalization is that plasma treatment can introduce various functional groups with different concentrations (Zille et al. 2015; Kajjout et al. 2019). For example, oxygen plasma treatment can introduce several oxygenated functional groups, such as carbonyl (>C=O), ether (C–O–C), hydroxyl (–OH), peroxy (–C–O–O–), etc. As plasma treatment does not involve the maximum use of harsh chemicals, it is more applicable in industrial sectors.
2.7 Conclusion
Regarding the various aspects of surface functionalization of polymers, the crucial issue is to tune the surface functionalities without compromising the inherent bulk properties, targeted for a specific application. Specifically, the related nanotoxicology and cytotoxicity issues are the main concerns regarding their bioapplication in clinical trials. However, it may get some exemption of toxicity concerns for physical applications. To date, significant progress has been made in the field of surface functionalization of polymers. A large number of polymer surface functionalization approaches have been designed, formulated and employed in recent decades. Successes in surface functionalization of polymers has led to progress in the design and formulation of multifunctional platforms to recapitulate the complex functioning process both in biological and physical fields. The surface functionalization of polymer has not only modified the polymer surface, but also provides efficiencies of differential immobilization and release kinetics of growth factors and drugs. The surface functionalized polymer can be used for a wide range of nanocarriers to improve the therapeutic efficacy. Future research directions related to surface functionalization of polymer should be focused on a comprehensive understanding of surface functionalized polymer-tissue interactions and optimization for long-term performance.
Change history
15 December 2020
The country in Dr. Deval Prasad Bhattarai affiliation is incorrectly mentioned as “India”.
Abbreviations
- AA:
-
Acrylic acid
- APTES:
-
3-Aminopropyltriethoxysilane
- CNC:
-
Cellulose nanocrystal
- DMSO:
-
Dimethylsulphoxide
- GO:
-
Graphene oxide
- HAp:
-
Hydroxyapatite
- MDI:
-
4,4′-methylene diphenyl diisocyanate
- NaBH4:
-
Sodium borohydride
- NPs:
-
Nanoparticles
- P3HT:
-
Poly(3-hexylthiophene)
- PAN:
-
Poly(acrylonitrile)
- PAPMA:
-
Poly(N-3-aminopropylmethacrylamide)
- PBTP:
-
Poly(butylene terephthalate)
- PC:
-
Poly(carbonate)
- PCL:
-
Poly(ε-caprolactone)
- PDA:
-
Poly(dopamine)
- PDMS:
-
Poly(dimethylsiloxane)
- PE:
-
Poly(ethylene)
- PEEK:
-
Polyetheretherketone
- PEG:
-
Poly(ethylene glycol)
- PEO:
-
Poly(ethylene oxide)
- PES:
-
Poly(ether sulfone)
- PET:
-
Poly(ethylene terephthalate)
- PGA:
-
Poly(glycolide)
- PLA:
-
Poly(lactide)
- PLGA:
-
Poly(lactic-co-glycolic acid)
- PLGA-b-PEG-NH2:
-
Poly(d,l-lactic-co-glycolic acid)-poly(ethylene glycol)-NH2
- PLLA:
-
Poly(l-lactic acid)
- PLLC:
-
Poly(l-lactide-co-caprolactone)
- PMAA:
-
Poly(methacrylic acid)
- PMMA:
-
Poly(methyl methacrylate)
- PNIPAAm:
-
Poly(N-isopropylacrylamide)
- PP:
-
Poly(propylene)
- PS:
-
Poly(styrene)
- PSBMA:
-
Poly(sulfobetaine methacrylate)
- PtBuA:
-
Poly(tert-butyl acrylate)
- PU:
-
Poly(urethane)
- PVDF:
-
Poly(vinylidene fluoride)
References
Anantha-Iyengar, G., Shanmugasundaram, K., Nallal, M., Lee, K. P., Whitcombe, M. J., Lakshmi, D., & Sai-Anand, G. (2019). Functionalized conjugated polymers for sensing and molecular imprinting applications. Progress in Polymer Science, 88, 1–129. https://doi.org/10.1016/j.progpolymsci.2018.08.001.
Angellier, H., Molina-Boisseau, S., Belgacem, M. N., & Dufresne, A. (2005). Surface chemical modification of waxy maize starch nanocrystals. Langmuir, 21(6), 2425–2433. https://doi.org/10.1021/la047530j.
Araujo, J. V., Martins, A., Leonor, I. B., Pinho, E. D., Reis, R. L., & Neves, N. M. (2008). Surface controlled biomimetic coating of polycaprolactone nanofiber meshes to be used as bone extracellular matrix analogues. Journal of Biomaterials Science, Polymer Edition, 19(10), 1261–1278. https://doi.org/10.1163/156856208786052335.
Ávila Ramírez, J. A., Suriano, C. J., Cerrutti, P., & Foresti, M. L. (2014). Surface esterification of cellulose nanofibers by a simple organocatalytic methodology. Carbohydrate Polymers, 114, 416–423. https://doi.org/10.1016/j.carbpol.2014.08.020.
Ávila Ramírez, J. A., Fortunati, E., Kenny, J. M., Torre, L., & Foresti, M. L. (2017). Simple citric acid-catalyzed surface esterification of cellulose nanocrystals. Carbohydrate Polymers, 157, 1358–1364. https://doi.org/10.1016/j.carbpol.2016.11.008.
Bhattarai, D. P., Tiwari, A. P., Maharjan, B., Tumurbaatar, B., Park, C. H., & Kim, C. S. (2019a). Sacrificial template-based synthetic approach of polypyrrole hollow fibers for photothermal therapy. Journal of Colloid and Interface Science, 534, 447–458. https://doi.org/10.1016/j.jcis.2018.09.047.
Bhattarai, D. P., Awasthi, G. P., Maharjan, B., Lee, J., Kim, B. S., Park, C. H., & Kim, C. S. (2019b). Synthesis of polythiophene nanoparticles by surfactant-free chemical oxidative polymerization method: Characterization, in vitro biomineralization, and cytotoxicity evaluation. Journal of Industrial and Engineering Chemistry, 77, 243–252. https://doi.org/10.1016/j.jiec.2019.04.045.
Bhattarai, D. P., Hwang, T. I., Kim, J. I., Lee, J. H., Chun, S., Kim, B. S., Park, C. H., & Kim, C. S. (2020). Synthesis of polypyrrole nanorods by sacrificial removal of aluminum oxide nanopore template: A study on cell viability, electrical stimulation and neuronal differentiation of PC12 cells. Materials Science and Engineering: C, 107, 110325. https://doi.org/10.1016/j.msec.2019.110325.
Chen, H., & Belfort, G. (1999). Surface modification of poly(ether sulfone) ultrafiltration membranes by low-temperature plasma-induced graft polymerization. Journal of Applied Polymer Science, 72(13), 1699–1711. https://doi.org/10.1002/(sici)1097-4628(19990624)72:13<1699::aid-app6>3.0.co;2-9.
Chen, W., & McCarthy, T. J. (1998). Chemical surface modification of poly(ethylene terephthalate). Macromolecules, 31(11), 3648–3655. https://doi.org/10.1021/ma9710601.
Cheon, Y. W., Lee, W. J., Baek, H. S., Lee, Y. D., Park, J.-C., Park, Y. H., Ki, C. S., Chung, K.-H., & Rah, D. K. (2010). Enhanced chondrogenic responses of human articular chondrocytes onto silk fibroin/wool keratose scaffolds treated with microwave-induced argon plasma. Artificial Organs, 34(5), 384–392. https://doi.org/10.1111/j.1525-1594.2009.00871.x.
Cho, S. H., White, S. R., & Braun, P. V. (2009). Self-healing polymer coatings. Advanced Materials, 21(6), 645–649. https://doi.org/10.1002/adma.200802008.
Chu, P. K., Chen, J. Y., Wang, L. P., & Huang, N. (2002). Plasma-surface modification of biomaterials. Materials Science & Engineering R: Reports, 36(5–6), 143–206. https://doi.org/10.1016/s0927-796x(02)00004-9.
Chua, K. N., Chai, C., Lee, P. C., Tang, Y. N., Ramakrishna, S., Leong, K. W., & Mao, H. Q. (2006). Surface-aminated electrospun nanofibers enhance adhesion and expansion of human umbilical cord blood hematopoietic stem/progenitor cells. Biomaterials, 27(36), 6043–6051. https://doi.org/10.1016/j.biomaterials.2006.06.017.
Chua, K.-N., Chai, C., Lee, P.-C., Ramakrishna, S., Leong, K. W., & Mao, H.-Q. (2007). Functional nanofiber scaffolds with different spacers modulate adhesion and expansion of cryopreserved umbilical cord blood hematopoietic stem/progenitor cells. Experimental Hematology, 35(5), 771–781. https://doi.org/10.1016/j.exphem.2007.02.002.
Chung, Y.-I., Kim, J. C., Kim, Y. H., Tae, G., Lee, S.-Y., Kim, K., & Kwon, I. C. (2010). The effect of surface functionalization of PLGA nanoparticles by heparin-or chitosan-conjugated Pluronic on tumor targeting. Journal of Controlled Release, 143(3), 374–382. https://doi.org/10.1016/j.jconrel.2010.01.017.
Croll, T. I., O’Connor, A. J., Stevens, G. W., & Cooper-White, J. J. (2004). Controllable surface modification of poly(lactic-co-glycolic acid)(PLGA) by hydrolysis or aminolysis I: Physical, chemical, and theoretical aspects. Biomacromolecules, 5(2), 463–473. https://doi.org/10.1021/bm0343040.
Dai, L., He, P., & Li, S. (2003). Functionalized surfaces based on polymers and carbon nanotubes for some biomedical and optoelectronic applications. Nanotechnology, 14(10), 1081–1097. https://doi.org/10.1088/0957-4484/14/10/305.
Despres, H. W., Sabra, A., Anderson, P., Hemraz, U. D., Boluk, Y., Sunasee, R., & Ckless, K. (2019). Mechanisms of the immune response cause by cationic and anionic surface functionalized cellulose nanocrystals using cell-based assays. Toxicology In Vitro, 55, 124–133. https://doi.org/10.1016/j.tiv.2018.12.009.
Diez-Pascual, A. M., Martinez, G., & Gomez, M. A. (2009). Synthesis and characterization of poly(ether ether ketone) derivatives obtained by carbonyl reduction. Macromolecules, 42(18), 6885–6892. https://doi.org/10.1021/ma901208e.
Dowding, J. M., Das, S., Kumar, A., Dosani, T., McCormack, R., Gupta, A., Sayle, T. X. T., Sayle, D. C., von Kalm, L., Seal, S., & Self, W. T. (2013). Cellular interaction and toxicity depend on physicochemical properties and surface modification of redox-active nanomaterials. ACS Nano, 7(6), 4855–4868. https://doi.org/10.1021/nn305872d.
Eyley, S., & Thielemans, W. (2014). Surface modification of cellulose nanocrystals. Nanoscale, 6(14), 7764–7779. https://doi.org/10.1039/c4nr01756k.
Faure, E., Falentin-Daudré, C., Jérôme, C., Lyskawa, J., Fournier, D., Woisel, P., & Detrembleur, C. (2013). Catechols as versatile platforms in polymer chemistry. Progress in Polymer Science, 38(1), 236–270. https://doi.org/10.1016/j.progpolymsci.2012.06.004.
Gao, L., & McCarthy, T. J. (2006). “Artificial lotus leaf” prepared using a 1945 patent and a commercial textile. Langmuir, 22(14), 5998–6000. https://doi.org/10.1021/la061237x.
Giorgi, M. E., Agusti, R., & de Lederkremer, R. M. (2014). Carbohydrate PEGylation, an approach to improve pharmacological potency. Beilstein Journal of Organic Chemistry, 10(1), 1433–1444. https://doi.org/10.3762/bjoc.10.147.
Gopalan Nair, K., Dufresne, A., Gandini, A., & Belgacem, M. N. (2003). Crab shell chitin whiskers reinforced natural rubber nanocomposites. 3. Effect of chemical modification of chitin whiskers. Biomacromolecules, 4(6), 1835–1842. https://doi.org/10.1021/bm030058g.
Guo, X., Facchetti, A., & Marks, T. J. (2014). Imide-and amide-functionalized polymer semiconductors. Chemical Reviews, 114(18), 8943–9021. https://doi.org/10.1021/cr500225d.
Gutiérrez, T. J., & González, G. (2016). Effects of exposure to pulsed light on surface and structural properties of edible films made from cassava and taro starch. Food and Bioprocess Technology, 9(11), 1812–1824. https://doi.org/10.1007/s11947-016-1765-3.
Gutiérrez, T. J., Ollier, R., & Alvarez, V. A. (2018). Chapter 5. Surface properties of thermoplastic starch materials reinforced with natural fillers. In V. K. Thakur & M. K. Thakur (Eds.), Functional biopolymers (pp. 131–158). Cham: Editorial Springer International. EE.UU. ISBN: 978-3-319-66416-3. eISBN: 978-3-319-66417-0. https://doi.org/10.1007/978-3-319-66417-0_5.
Habibi, Y., Chanzy, H., & Vignon, M. R. (2006). TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose, 13(6), 679–687. https://doi.org/10.1007/s10570-006-9075-y.
Hai, T. A. P., & Sugimoto, R. (2018). Surface functionalization of cellulose with poly(3-hexylthiophene) via novel oxidative polymerization. Carbohydrate Polymers, 179, 221–227. https://doi.org/10.1016/j.carbpol.2017.09.067.
Hao, L., An, Q., & Xu, W. (2012). Facile fabrication of superhydrophobic cotton fabric from stearyl methacrylate modified polysiloxane/silica nanocomposite. Fibers and Polymers, 13(9), 1145–1153. https://doi.org/10.1007/s12221-012-1145-1.
Hegemann, D., Brunner, H., & Oehr, C. (2003). Plasma treatment of polymers for surface and adhesion improvement. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 208, 281–286. https://doi.org/10.1016/s0168-583x(03)00644-x.
Hetemi, D., & Pinson, J. (2017). Surface functionalisation of polymers. Chemical Society Reviews, 46(19), 5701–5713. https://doi.org/10.1039/c7cs00150a.
Holzapfel, V., Musyanovych, A., Landfester, K., Lorenz, M. R., & Mailänder, V. (2005). Preparation of fluorescent carboxyl and amino functionalized polystyrene particles by miniemulsion polymerization as markers for cells. Macromolecular Chemistry and Physics, 206(24), 2440–2449. https://doi.org/10.1002/macp.200500372.
Iijima, K., Nagahama, H., Takada, A., Sawada, T., Serizawa, T., & Hashizume, M. (2016). Surface functionalization of polymer substrates with hydroxyapatite using polymer-binding peptides. Journal of Materials Chemistry B, 4(21), 3651–3659. https://doi.org/10.1039/c6tb00624h.
Jia, J., Duan, Y.-Y., Yu, J., & Lu, J.-W. (2008). Preparation and immobilization of soluble eggshell membrane protein on the electrospun nanofibers to enhance cell adhesion and growth. Journal of Biomedical Materials Research Part A, 86(2), 364–373. https://doi.org/10.1002/jbm.a.31606.
Jiang, H., Moon, K. S., Li, Y., & Wong, C. P. (2006). Surface functionalized silver nanoparticles for ultrahigh conductive polymer composites. Chemistry of Materials, 18(13), 2969–2973. https://doi.org/10.1021/cm0527773.
Jiang, J., Xie, J., Ma, B., Bartlett, D. E., Xu, A., & Wang, C. H. (2014). Mussel-inspired protein-mediated surface functionalization of electrospun nanofibers for pH-responsive drug delivery. Acta Biomaterialia, 10(3), 1324–1332. https://doi.org/10.1016/j.actbio.2013.11.012.
Kajjout, M., Lemmouchi, Y., Jama, C., Rolando, C., Villasmunta, F., Heinrich, F., & Mazzah, A. (2019). Grafting of amine functions on cellulose acetate fibers by plasma processing. Reactive and Functional Polymers, 134, 40–48. https://doi.org/10.1016/j.reactfunctpolym.2018.11.004.
Kang, S. M., You, I., Cho, W. K., Shon, H. K., Lee, T. G., Choi, I. S., Choi, I. S., & Lee, H. (2010). One-step modification of superhydrophobic surfaces by a mussel-inspired polymer coating. Angewandte Chemie International Edition, 49(49), 9401–9404. https://doi.org/10.1002/anie.201004693.
Kaur, S., Ma, Z., Gopal, R., Singh, G., Ramakrishna, S., & Matsuura, T. (2007). Plasma-induced graft copolymerization of poly(methacrylic acid) on electrospun poly(vinylidene fluoride) nanofiber membrane. Langmuir, 23(26), 13085–13092. https://doi.org/10.1021/la701329r.
Khanjanzadeh, H., Behrooz, R., Bahramifar, N., Gindl-Altmutter, W., Bacher, M., Edler, M., & Griesser, T. (2018). Surface chemical functionalization of cellulose nanocrystals by 3-aminopropyltriethoxysilane. International Journal of Biological Macromolecules, 106, 1288–1296. https://doi.org/10.1016/j.ijbiomac.2017.08.136.
Kim, T. G., & Park, T. G. (2006). Surface functionalized electrospun biodegradable nanofibers for immobilization of bioactive molecules. Biotechnology Progress, 22(4), 1108–1113. https://doi.org/10.1021/bp060039t.
Kim, T. G., & Park, T. G. (2008). Biodegradable polymer nanocylinders fabricated by transverse fragmentation of electrospun nanofibers through aminolysis. Macromolecular Rapid Communications, 29(14), 1231–1236. https://doi.org/10.1002/marc.200800094.
Lee, J. H., Park, J. W., & Lee, H. B. (1991). Cell adhesion and growth on polymer surfaces with hydroxyl groups prepared by water vapour plasma treatment. Biomaterials, 12(5), 443–448. https://doi.org/10.1016/0142-9612(91)90140-6.
Lee, H., Dellatore, S. M., Miller, W. M., & Messersmith, P. B. (2007). Mussel-inspired surface chemistry for multifunctional coatings. Science, 318(5849), 426–430. https://doi.org/10.1126/science.1147241.
Lee, Y. K., Choi, E. J., Webster, T. J., Kim, S. H., & Khang, D. (2015). Effect of the protein corona on nanoparticles for modulating cytotoxicity and immunotoxicity. International Journal of Nanomedicine, 10, 97–113. https://doi.org/10.2147/ijn.s72998.
Li, S.-F., Chen, J. P., & Wu, W.-T. (2007). Electrospun polyacrylonitrile nanofibrous membranes for lipase immobilization. Journal of Molecular Catalysis B: Enzymatic, 47(3–4), 117–124. https://doi.org/10.1016/j.molcatb.2007.04.010.
Li, G. L., Liu, G., Li, M., Wan, D., Neoh, K. G., & Kang, E. T. (2010). Organo-and water-dispersible graphene oxide-polymer nanosheets for organic electronic memory and gold nanocomposites. The Journal of Physical Chemistry C, 114(29), 12742–12748. https://doi.org/10.1021/jp102640s.
Li, L., Bao, R. Y., Gao, T., Liu, Z. Y., Xie, B. H., Yang, M. B., & Yang, W. (2019). Dopamine-induced functionalization of cellulose nanocrystals with polyethylene glycol towards poly(L-lactic acid) bionanocomposites for green packaging. Carbohydrate Polymers, 203, 275–284. https://doi.org/10.1016/j.carbpol.2018.09.057.
Lin, C. A. J., Sperling, R. A., Li, J. K., Yang, T. Y., Li, P. Y., Zanella, M., Chang, W. H., & Parak, W. J. (2008). Design of an amphiphilic polymer for nanoparticle coating and functionalization. Small, 4(3), 334–341. https://doi.org/10.1002/smll.200700654.
Ma, Z., He, W., Yong, T., & Ramakrishna, S. (2005a). Grafting of gelatin on electrospun poly(caprolactone) nanofibers to improve endothelial cell spreading and proliferation and to control cell orientation. Tissue Engineering, 11(7–8), 1149–1158. https://doi.org/10.1089/ten.2005.11.1149.
Ma, Z., Kotaki, M., Yong, T., He, W., & Ramakrishna, S. (2005b). Surface engineering of electrospun polyethylene terephthalate (PET) nanofibers towards development of a new material for blood vessel engineering. Biomaterials, 26(15), 2527–2536. https://doi.org/10.1016/j.biomaterials.2004.07.026.
Masserini, M. (2013). Nanoparticles for brain drug delivery. ISRN Biochemistry, 238428. 18 pages. https://doi.org/10.1155/2013/238428.
Murphy, A. R., Liu, J., Luscombe, C., Kavulak, D., Fréchet, J. M., Kline, R. J., & McGehee, M. D. (2005). Synthesis, characterization, and field-effect transistor performance of carboxylate-functionalized polythiophenes with increased air stability. Chemistry of Materials, 17(20), 4892–4899. https://doi.org/10.1021/cm050911d.
Ostuni, E., Chapman, R. G., Holmlin, R. E., Takayama, S., & Whitesides, G. M. (2001). A survey of structure-property relationships of surfaces that resist the adsorption of protein. Langmuir, 17(18), 5605–5620. https://doi.org/10.1021/la010384m.
Otsuka, H., Nagasaki, Y., & Kataoka, K. (2003). PEGylated nanoparticles for biological and pharmaceutical applications. Advanced Drug Delivery Reviews, 55(3), 403–419. https://doi.org/10.1016/s0169-409x(02)00226-0.
Pant, H. R., Baek, W. I., Nam, K. T., Jeong, I. S., Barakat, N. A., & Kim, H. Y. (2011). Effect of lactic acid on polymer crystallization chain conformation and fiber morphology in an electrospun nylon-6 mat. Polymer, 52(21), 4851–4856. https://doi.org/10.1016/j.polymer.2011.08.059.
Pant, H. R., Pokharel, P., Joshi, M. K., Adhikari, S., Kim, H. J., Park, C. H., & Kim, C. S. (2015). Processing and characterization of electrospun graphene oxide/polyurethane composite nanofibers for stent coating. Chemical Engineering Journal, 270, 336–342. https://doi.org/10.1016/j.cej.2015.01.105.
Park, H., Lee, K. Y., Lee, S. J., Park, K. E., & Park, W. H. (2007a). Plasma-treated poly(lactic-co-glycolic acid) nanofibers for tissue engineering. Macromolecular Research, 15(3), 238–243. https://doi.org/10.1007/bf03218782.
Park, K., Ju, Y. M., Son, J. S., Ahn, K. D., & Han, D. K. (2007b). Surface modification of biodegradable electrospun nanofiber scaffolds and their interaction with fibroblasts. Journal of Biomaterials Science, Polymer Edition, 18(4), 369–382. https://doi.org/10.1163/156856207780424997.
Patil, Y. B., Toti, U. S., Khdair, A., Ma, L., & Panyam, J. (2009). Single-step surface functionalization of polymeric nanoparticles for targeted drug delivery. Biomaterials, 30(5), 859–866. https://doi.org/10.1016/j.biomaterials.2008.09.056.
Pelaz, B., del Pino, P., Maffre, P., Hartmann, R., Gallego, M., Rivera-Fernández, S., de la Fuente, J. M., Ulrich Nienhaus, G., & Parak, W. J. (2015). Surface functionalization of nanoparticles with polyethylene glycol: Effects on protein adsorption and cellular uptake. ACS Nano, 9(7), 6996–7008. https://doi.org/10.1021/acsnano.5b01326.
Penn, L. S., & Wang, H. (1994). Chemical modification of polymer surfaces: A review. Polymers for Advanced Technologies, 5(12), 809–817. https://doi.org/10.1002/pat.1994.220051207.
Pokharel, P. (2014). High performance polyurethane nanocomposite films prepared from a masterbatch of graphene oxide in polyether polyol. Chemical Engineering Journal, 253, 356–365. https://doi.org/10.1016/j.cej.2014.05.046.
Pokharel, P., & Choi, S. (2015). The effect of hard segment length on the thermal and mechanical properties of polyurethane/graphene oxide nanocomposites. Composites Part A: Applied Science and Manufacturing, 69, 168–177. https://doi.org/10.1016/j.compositesa.2014.11.010.
Pokharel, P., & Lee, D. S. (2014). Thermal and mechanical properties of reduced graphene oxide/polyurethane nanocomposite. Journal of Nanoscience and Nanotechnology, 14(8), 5718–5721. https://doi.org/10.1166/jnn.2014.8824.
Pokharel, P., & Truong, Q. T. (2014). Multi-step microwave reduction of graphite oxide and its use in the formation of electrically conductive graphene/epoxy composites. Composites Part B: Engineering, 64, 187–193. https://doi.org/10.1016/j.compositesb.2014.04.013.
Pokharel, P., Bae, H., Lim, J.-G., Lee, K. Y., & Choi, S. (2015a). Effects of titanate treatment on morphology and mechanical properties of graphene nanoplatelets/high density polyethylene nanocomposites. Journal of Applied Polymer Science, 132(23). https://doi.org/10.1002/app.42073.
Pokharel, P., Pant, B., Pokhrel, K., Pant, H. R., Lim, J. G., Kim, H. Y., & Choi, S. (2015b). Effects of functional groups on the graphene sheet for improving the thermomechanical properties of polyurethane nanocomposites. Composites Part B: Engineering, 78, 192–201. https://doi.org/10.1016/j.compositesb.2015.03.089.
Pokharel, P., Lee, S. H., & Lee, D. S. (2015c). Thermal, mechanical, and electrical properties of graphene nanoplatelet/graphene oxide/polyurethane hybrid nanocomposite. Journal of Nanoscience and Nanotechnology, 15(1), 211–214. https://doi.org/10.1166/jnn.2015.8353.
Pokharel, P., Xiao, D., Erogbogbo, F., & Keles, O. (2019). A hierarchical approach for creating electrically conductive network structure in polyurethane nanocomposites using a hybrid of graphene nanoplatelets, carbon black and multi-walled carbon nanotubes. Composites Part B: Engineering, 161, 169–182. https://doi.org/10.1016/j.compositesb.2018.10.057.
Prabhakaran, M. P., Venugopal, J., Chan, C. K., & Ramakrishna, S. (2008). Surface modified electrospun nanofibrous scaffolds for nerve tissue engineering. Nanotechnology, 19(45), 455102. https://doi.org/10.1088/0957-4484/19/45/455102.
Rohr, T., Ogletree, D. F., Svec, F., & Fréchet, J. M. (2003). Surface functionalization of thermoplastic polymers for the fabrication of microfluidic devices by photoinitiated grafting. Advanced Functional Materials, 13(4), 264–270. https://doi.org/10.1002/adfm.200304229.
Ryu, J. H., Hong, S., & Lee, H. (2015). Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomaterialia, 27, 101–115. https://doi.org/10.1016/j.actbio.2015.08.043.
Schlaich, C., Li, M., Cheng, C., Donskyi, I. S., Yu, L., Song, G., Osorio, E., & Haag, R. (2018). Mussel-inspired polymer-based universal spray coating for surface modification: Fast fabrication of antibacterial and superhydrophobic surface coatings. Advanced Materials Interfaces, 5(5), 1701254. https://doi.org/10.1002/admi.201701254.
Sis, H., & Birinci, M. (2009). Effect of nonionic and ionic surfactants on zeta potential and dispersion properties of carbon black powders. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 341(1–3), 60–67. https://doi.org/10.1016/j.colsurfa.2009.03.039.
Sun, H., & Önneby, S. (2006). Facile polyester surface functionalization via hydrolysis and cell-recognizing peptide attachment. Polymer International, 55(11), 1336–1340. https://doi.org/10.1002/pi.2090.
Sun, B., Hou, Q., Liu, Z., & Ni, Y. (2015). Sodium periodate oxidation of cellulose nanocrystal and its application as a paper wet strength additive. Cellulose, 22(2), 1135–1146. https://doi.org/10.1007/s10570-015-0575-5.
Sunasee, R., & Hemraz, U. D. (2018). Synthetic strategies for the fabrication of cationic surface-modified cellulose nanocrystals. Fibers, 6(1), 15. https://doi.org/10.3390/fib6010015.
Suresh, V. M., Bonakala, S., Atreya, H. S., Balasubramanian, S., & Maji, T. K. (2014). Amide functionalized microporous organic polymer (Am-MOP) for selective CO2 sorption and catalysis. ACS Applied Materials & Interfaces, 6(7), 4630–4637. https://doi.org/10.1021/am500057z.
Tang, L., Thevenot, P., & Hu, W. (2008). Surface chemistry influences implant biocompatibility. Current Topics in Medicinal Chemistry, 8(4), 270–280. https://doi.org/10.2174/156802608783790901.
Thakur, V. K., Vennerberg, D., Madbouly, S. A., & Kessler, M. R. (2014). Bio-inspired green surface functionalization of PMMA for multifunctional capacitors. RSC Advances, 4(13), 6677–6684. https://doi.org/10.1039/c3ra46592f.
Tian, P., Xu, D., & Liu, X. (2016). Mussel-inspired functionalization of PEO/PCL composite coating on a biodegradable AZ31 magnesium alloy. Colloids and Surfaces B: Biointerfaces, 141, 327–337. https://doi.org/10.1016/j.colsurfb.2016.02.004.
Tiwari, A. P., Joshi, M. K., Lee, J., Maharjan, B., Ko, S. W., Park, C. H., & Kim, C. S. (2017). Heterogeneous electrospun polycaprolactone/polyethylene glycol membranes with improved wettability, biocompatibility, and mineralization. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 520, 105–113. https://doi.org/10.1016/j.colsurfa.2017.01.054.
Tiwari, A. P., Hwang, T. I., Oh, J. M., Maharjan, B., Chun, S., Kim, B. S., Joshi, M. K., Park, C. H., & Kim, C. S. (2018). pH/NIR-responsive polypyrrole-functionalized fibrous localized drug-delivery platform for synergistic cancer therapy. ACS Applied Materials & Interfaces, 10(24), 20256–20270. https://doi.org/10.1021/acsami.7b17664.
Tiwari, A. P., Bhattarai, D. P., Maharjan, B., Ko, S. W., Kim, H. Y., Park, C. H., & Kim, C. S. (2019). Polydopamine-based implantable multifunctional nanocarpet for highly efficient photothermal-chemo therapy. Scientific Reports, 9(1), 2943. https://doi.org/10.1038/s41598-019-39457-y.
Veronese, F. M., & Pasut, G. (2005). PEGylation, successful approach to drug delivery. Drug Discovery Today, 10(21), 1451–1458. https://doi.org/10.1016/s1359-6446(05)03575-0.
Vesel, A., & Mozetic, M. (2017). New developments in surface functionalization of polymers using controlled plasma treatments. Journal of Physics D: Applied Physics, 50(29), 293001. https://doi.org/10.1088/1361-6463/aa748a.
Vukušić, T., Vesel, A., Holc, M., Ščetar, M., Jambrak, A., & Mozetič, M. (2018). Modification of physico-chemical properties of acryl-coated polypropylene foils for food packaging by reactive particles from oxygen plasma. Materials, 11(3), 372. https://doi.org/10.3390/ma11030372.
Wang, Z., et al. (2017) Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Materials, 9(10), e435–e435.
Weibel, D. E. (2010). Polymer surface functionalization using plasma, ultraviolet and synchrotron radiation. Composite Interfaces, 17(2–3), 127–136. https://doi.org/10.1163/092764410x490545.
Wu, J., Zhang, C., Xu, S., Pang, X., Cai, G., & Wang, J. (2018). Preparation of zwitterionic polymer-functionalized cotton fabrics and the performance of anti-biofouling and long-term biofilm resistance. Colloid and Interface Science Communications, 24, 98–104. https://doi.org/10.1016/j.colcom.2018.02.001.
Xue, C. H., Jia, S.-T., Zhang, J., Tian, L.-Q., Chen, H.-Z., & Wang, M. (2008). Preparation of superhydrophobic surfaces on cotton textiles. Science and Technology of Advanced Materials, 9(3), 035008. https://doi.org/10.1088/1468-6996/9/3/035008.
Yang, J., Shi, G., Bei, J., Wang, S., Cao, Y., Shang, Q., Yang, G., & Wang, W. (2002). Fabrication and surface modification of macroporous poly(L-lactic acid) and poly(L-lactic-co-glycolic acid)(70/30) cell scaffolds for human skin fibroblast cell culture. Journal of Biomedical Materials Research, 62(3), 438–446. https://doi.org/10.1002/jbm.10318.
Yang, F., Wolke, J. G. C., & Jansen, J. A. (2008). Biomimetic calcium phosphate coating on electrospun poly(ɛ-caprolactone) scaffolds for bone tissue engineering. Chemical Engineering Journal, 137(1), 154–161. https://doi.org/10.1016/j.cej.2007.07.076.
Yoo, H. S., Kim, T. G., & Park, T. G. (2009). Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Advanced Drug Delivery Reviews, 61(12), 1033–1042. https://doi.org/10.1016/j.addr.2009.07.007.
Zarrintaj, P., Jouyandeh, M., Ganjali, M. R., Hadavand, B. S., Masoud Mozafari, M., Sheiko, S. S., Vatankhah-Varnoosfaderani, M., Gutiérrez, T. J., & Saeb, M. R. (2019). Thermo-sensitive polymers in medicine: A review. European Polymer Journal, 117, 402–423. https://doi.org/10.1016/j.eurpolymj.2019.05.024.
Zemljič, L. F., Peršin, Z., Šauperl, O., Rudolf, A., & Kostić, M. (2018). Medical textiles based on viscose rayon fabrics coated with chitosan-encapsulated iodine: Antibacterial and antioxidant properties. Textile Research Journal, 88(22), 2519–2531. https://doi.org/10.1177/0040517517725117.
Zeng, Q., Zhu, Y., Yu, B., Sun, Y., Ding, X., Xu, C., Wu, Y.-W., Tang, Z., & Xu, F.-J. (2018). Antimicrobial and antifouling polymeric agents for surface functionalization of medical implants. Biomacromolecules, 19(7), 2805–2811. https://doi.org/10.1021/acs.biomac.8b00399.
Zhang, H., Zhao, T., Newland, B., Liu, W., Wang, W., & Wang, W. (2018). Catechol functionalized hyperbranched polymers as biomedical materials. Progress in Polymer Science, 78, 47–55. https://doi.org/10.1016/j.progpolymsci.2017.09.002.
Zille, A., Oliveira, F. R., & Souto, A. P. (2015). Plasma treatment in textile industry. Plasma Processes and Polymers, 12(2), 98–131. https://doi.org/10.1002/ppap.201400052.
Acknowledgments
D. Xiao sincerely acknowledges the Jacob F. Buckman Chair endowment fund at the University of New Haven. P. Pokharel and D. Xiao gratefully acknowledge the Higasket Research Fund.
Conflicts of Interest The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bhattarai, D.P., Pokharel, P., Xiao, D. (2020). Surface Functionalization of Polymers. In: Gutiérrez, T.J. (eds) Reactive and Functional Polymers Volume Four. Springer, Cham. https://doi.org/10.1007/978-3-030-52052-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-52052-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-52051-9
Online ISBN: 978-3-030-52052-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)