Abstract
Esophageal diverticula are outpouchings of the esophageal mucosa and submucosa and are found throughout the esophagus. Esophageal diverticula classified by location and are divided into traction and pulsion mechanisms. Most types of esophageal diverticula are more common beyond the sixth decade of life and may be diagnosed by endoscopy and/or fluorometric studies. Treatment is largely focused on symptoms, though size alone may be an indication to seek treatment. Treatment approaches differ based on anatomic location of the diverticulum. The best accumulated evidence is for treatment of hypopharyngeal diverticula, more commonly known by their eponymous designation: Zenker’s diverticula. Treatment may be surgical, via direct endoscopy for flexible endoscopic approaches, including tunneled submucosal approaches.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Diverticulum
- Zenker’s diverticulum
- Hypopharyngeal diverticula
- Diverticulopexy
- Cricopharyngeus
- Myotomy
- Killian’s triangle
- Mid-esophageal diverticulum
- Epiphrenic diverticulum
- Trans-oral endoscopic/rigid endoscopic
- Flexible endoscopy
- Submucosal tunneling
Introduction
Esophageal diverticula are outpouchings of esophageal mucosa and submucosa classified based on both anatomic location and presumed mechanism leading to their formation and enlargement. Possible locations include hypoesophageal (pharyngoesophageal), mid-esophageal, or epiphrenic. Mechanisms of formation are traction and pulsion. Traction diverticula result from pulling forces that originate external to the esophagus. Classic examples are inflammatory reactions and scar tissue formation from anterior spinal fixation and stabilization hardware or mediastinal lymphadenopathy. Pulsion diverticula result from a pushing forces created from pressurization of the esophageal lumen, with mucosa herniating through weaknesses in esophageal musculature. The most common esophageal diverticulum is widely known by its eponymous designation, a Zenker’s diverticulum. A Zenker’s diverticulum is a pulsion diverticulum that occurs in the posterolateral esophagus in a natural weakness located superior to the cricopharyngeus muscle and inferior to the thyropharyngeus (both are portions of the inferior constrictor muscle), known as Killian’s triangle. Presentations and symptoms of esophageal diverticula vary somewhat by location within the esophagus. Diagnosis of esophageal diverticula typically follows a similar algorithm and is primarily made using barium contrast esophagrams. Hypopharyngeal diverticula will be discussed in detail, as there has been a wide array of advances in surgical approach over the past two decades and these are the most common. Other esophageal diverticula, including mid-esophageal and epiphrenic diverticula, will be discussed briefly as well. We use the term diverticulum throughout this chapter; however, most diverticula discussed are in fact pseudodiverticula, wherein the mucosa and submucosa are herniated. True diverticula of the esophagus do exist that involve all layers of the esophageal wall. However, these are quite rare and are primarily traction-type diverticula of the proximal and middle esophagus.
Incidence of Esophageal Diverticula
Hypopharyngeal diverticula are the most common diverticula of the esophagus, they and make up more than 70% of all esophageal diverticula. A prototypical patient presenting with a symptomatic hypopharyngeal diverticulum will be a male sexagenarian or septuagenarian. While presentation at a younger age is possible, hypopharyngeal diverticula noted under the age of 40 is virtually unheard of [1, 2]. Most published series report incidence of symptomatic hypopharyngeal diverticula, and as such the true prevalence including asymptomatic individuals is likely unknown. The best estimates of incidence that are published originate from England, with an estimated incidence of roughly 2 hypopharyngeal diverticula per 100,000 persons per year [1, 3]. Prior studies have noted geographic variation in terms of incidence of hypopharyngeal diverticula, which appear to occur with greater frequency in Europe, the United States, and Canada when compared to Japan and Indonesia, which may be related to variations in neck length [1, 4]. Mid-esophageal diverticula comprise 10–15% of esophageal diverticula [5]. As opposed to hypopharyngeal diverticula, mid-esophageal diverticula may arise from both pulsion and traction mechanisms, and as such may also be either pseudodiverticula or true diverticula involving all layers of the esophagus. Mid-esophageal diverticula are often related to other disorders such as mediastinal lymphadenopathy, which may be either extrinsic or intrinsic to the esophagus. Epiphrenic diverticula make up the remainder of esophageal diverticula and are quite rare. Only hundreds of epiphrenic diverticula are reported in the literature [6]. Increased use of upper endoscopy and cross-sectional imaging over the past three decades have led increased detection of smaller, asymptomatic diverticula at all levels of the esophagus.
Pathophysiology of Hypopharyngeal Diverticula
The classically described Zenker’s diverticulum occurs superior to the cricopharyngeus in the previously described Killian’s triangle above the cricopharyngeal muscle and inferior to the thyropharyngeus [7]. This occurs at the level of the sixth cervical vertebrae. Two other rarer types of hypopharyngeal diverticula exist. One is a herniation within the cricopharyngeus muscle where the upper oblique fibers of the cricopharyngeus diverge from the lower transverse fibers (Killian-Jamieson triangle), known as Killian’s diverticulum. The second occurs at the inferior border of the cricopharyngeus and above the confluence of the longitudinal muscle fibers of the esophagus (Larimer’s triangle), known as Larimer’s diverticulum. All produce similar symptoms and are treated similarly. They are collectively referred to as hypopharyngeal diverticula (see Fig. 15.1).
Potential anatomic locations of hypopharyngeal diverticula in relation to musculature of the pharynx and proximal esophagus. (Reproduced from Kroh and Reavis [134]; Fig. 18.1)
The etiologic origin of hypopharyngeal diverticula has been a matter of debate since it was first described by Zenker as a pulsion diverticulum. Specifically, it was unclear if there was an antecedent disorder of coordination, muscle tone, or compliance that initiated the process of diverticulization through over pressurization of the hypopharyngeal region. Contemporary understanding is derived from both videofluorometric and manometric studies and focus on two abnormalities: anatomic weakness of the muscles of the posterior pharynx adjacent to the upper esophageal sphincter, and/or muscular dysfunction of the upper esophageal sphincter. The best evidence comes from studies published in the early 1990s where a 6 cm sleeve catheter for manometry with concurrent videofluorometric recording was performed in patients with symptomatic hypopharyngeal diverticula [8, 9]. The experiments showed that there was full manometric relaxation realized by the upper esophageal sphincter, as defined by the hypopharyngeal wall losing contact with the recording catheter. However, videofluorimetry revealed that relaxation was incomplete, and specifically was reduced in comparison to controls. This resulted in increased pressure through the segment as a food bolus passed, which was termed intrabolus pressure [8, 9]. Thus, incomplete relaxation of the upper esophageal sphincter led to increased hypopharyngeal pressurization, in particular in response to food bolus. Histologic studies and muscle contractility studies of the cricopharyngeus offer additional supporting evidence for this theory [10]. Compared to normal controls, patients with hypopharyngeal diverticula have slower and weaker muscular contraction with delayed relaxation of the cricopharyngeus. This is partially related to more predominate type 1 muscle fibers [10]. Enzymatic function studies of histologic sections have demonstrated decreased acetylcholinesterase and fewer neurofilaments, suggesting denervation may play a role as well [10]. In addition, histologic samples of the cervical mucosa show an increase in overall collagen fibers and a decrease in elastin content in the muscularis mucosa of the cervical esophagus, which may indicate that the mucosa is also less compliant [11]. Worth noting for these studies, however, is that the majority have been performed in patients that have already developed symptomatic hypopharyngeal diverticula, thus it is difficult to determine if noted changes were present and contributed to the formation of diverticula, or occured secondarily after development of the diverticulum. While it is most likely that a constellation of neuromuscular and histological changes must exist to initiate the pathologic process leading to formation of a hypopharyngeal diverticulum, a unifying theory does not yet exist. The ideal study to discover that condition once would have to obtain functional studies, dynamic anatomic studies, and histological samples from the hypopharynx and its associated musculature and regular time points from a population with normal baseline structure and function of their inferior pharynx and proximal esophagus, following by post hoc analysis to compare those that eventually developed diverticula to those that did not. It is unlikely, however, that a study of that nature could be feasibly accomplished.
Theories of impaired musculature relaxation and discoordination do not stand opposed to complementary theories that anatomic variations may also contribute. The hypopharynx has an inherent defect that behaves as a natural point of weakness occurring between the oblique fibers of the thyropharyngeus and the horizontal fibers of the cricopharyngeus, also known as Killian’s triangle. Jos van Overbeek proposed individuals with longer necks may have a larger Killian’s triangle. This may also explain regional variations in incidence, as individuals from Western countries, where hypopharyngeal diverticula are more common, tend to have both longer necks and a longer pharynx [4]. However, this has not been supported by anatomic studies [12].
More recent technological advances shine new light on this subjection. The advent of high-resolution video manometry has led to new classification schema for motility disorders of the esophagus. High-resolution manometry studies detailing the pharyngeal phase of swallowing are currently limited; however, comparison between patients with hypopharyngeal diverticula and normal controls may better elucidate and/or classify the nature of motility disorders associated with hypopharyngeal diverticula. Impedance planimetry may also offer new insight. Impedance planimetry uses an electrode area on a catheter surrounded by a balloon containing a conductive fluid. Signal processing is able to use the change the minor alteration in the electric field produced by the changes in the balloon diameter to reconstruct a profile of the upper esophageal sphincter. Measures of esophageal distensibility, diameter, relaxation time, and pressure may be determined within the upper esophageal sphincter [8, 13, 14].
Symptoms of Hypopharyngeal Diverticula
The predominant symptom of hypopharyngeal diverticula is progressive dysphagia, which is present the vast majority of patients. There are two mechanisms that may underlie dysphagia. The first is the aforementioned impaired relaxation of the upper esophageal sphincter. The second is an effect of content of a food bolus preferentially filling the diverticulum, impeding distension of the esophageal lumen by mass effect. Filling of the diverticulum may also distort the esophagus, as the weight of the contents of diverticulum causes traction and angulation of the esophagus. In addition to dysphagia, regurgitation of undigested food even hours after ingestion may occur. There are numerous reports of pills being found within hypopharyngeal diverticula at the time of operative intervention, which would reduce the efficacy of medications, since they are undigested. Other symptoms include halitosis, belching, cervical borborygmi, globus pharyngeus, and recurrent respiratory infections from unprovoked aspiration events. Boyce’s sign is a physical exam finding of a gurgling mass present in the lateral neck, which, while rare, is pathognomonic for hypopharyngeal diverticula.
Diagnosis of Hypopharyngeal Diverticula
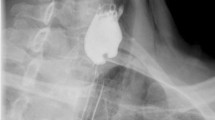
When symptoms are present, diagnosis of hypopharyngeal diverticulum is typically made by barium esophagography (see Fig. 15.2). In most cases flexible endoscopic evaluation is also pursued to rule out other causes for dysphagia, in particular neoplasm (see Fig. 15.3). Given the increased use of both endoscopic investigations and cross-sectional imaging, there is a proportion of hypopharyngeal diverticula that are discovered incidentally in asymptomatic individuals. Diverticula vary in size, and presumably grow slowly over time. Measures of diverticular size from the operating room correlated well with radiographic studies, but do not correlated well with dimensions determined endoscopically [15]. A number of authors have attempted to classify hypopharyngeal diverticula in terms of size and presumed disease severity [7]. These are summarized in Table 15.1, though their utility in a clinical setting is relatively limited.
Endoscopic view of a hypopharyngeal diverticulum. In this image, an endoscopic cap has been attached to the end of the endoscope. The esophageal lumen (E) appears on the left of the image, and the diverticulum (D) on the right, separated by the common wall or septum, which contains the cricopharyngeus muscle
Treatment of Hypopharyngeal Diverticula and Defining Treatment Success
Surgical treatment of hypopharyngeal diverticula is generally reserved for individuals with symptoms. Both open transcervical and transoral options exist to treat hypopharyngeal diverticula, with the best outcomes for smaller diverticula. Increases in the use of cross-sectional imaging and endoscopy have increased the diagnosis of small diverticula that are asymptomatic. While these do not warrant surgical repair when asymptomatic, patients should be followed to expedite repair early, when diverticula are smaller and result in less severe complications. In most studies, the primary outcome has been resolution of symptoms, as this is the most clinically meaningful outcome. However, metrics to track this quantitatively or semiquantitatively are varied by study. There are a number of scoring systems that have been validated that assess severity of dysphagia and degrees of dysfunction. A full review of these scoring systems is outside the scope of this chapter, and they are of greatest utility in the research setting. In general, with current surgical techniques, greater than 95% of patients should expect to garner symptomatic relief after surgical intervention, though durability of symptom resolution will vary.
Recurrence may be defined based on symptoms or radiography. However, similar to paraesophageal hernia, radiographic evidence of recurrence may not correlate with symptomatic recurrence. The authors advise caution when interpreting studies that quote recurrence rates, as they are a function of both follow-up time and the definition used to define recurrence in that particular manuscript. This is particularly important in the literature that has emerged using flexible endoscopic techniques in the past decade, as some patients undergo “multiple sessions” of endoscopic intervention in a short time frame, with the recurrence quoted as the recurrence from the time of full symptom resolution to relapse, as opposed to the first intervention. Also, given that there may be underlying an esophageal motility disorder in conjunction with hypopharyngeal diverticula, some recurrence of symptoms may be attributable to dysmotility and not the presence of a new or recurrent diverticulum.
Historical Perspectives on Surgical Management of Hypopharyngeal Diverticula
An investigation into the management of hypopharyngeal diverticulum includes a history of surgical innovation and ingenuity spanning more than a century. Both open and transoral techniques have been developed and will be detailed. Given the rarity of this condition, the bulk of the accumulated evidence is comprised of case series and retrospective comparison studies. The evidence that is summarized below demonstrates that there is a tremendous heterogeneity in techniques and devices employed both historically and contemporarily. While the past two decades have seen new application of a flexible endoscope, surgical treatment of hypopharyngeal must simultaneously address two separate issues: division of cricopharyngeus muscle to decrease pharyngeal pressurization and eliminating the ability of the pouch to fill. The latter may be accomplished either by resection, inversion, suspension, or elimination of the common wall shared by the diverticulum and esophagus.
Open Surgical Approaches for Hypopharyngeal Diverticula
Open Hypopharyngeal Diverticulectomy
Some of the earliest descriptions of surgical therapy for hypopharyngeal diverticula came in 1830, with a description by Bell (of Bell’s palsy fame). The described technique was the surgical creation of a diverticulocutaneous fistula [12]. The first recorded attempt at this surgical technique came in 1877, and resulted in the patient’s death [12]. The following decade saw the first attempts at surgical resection through a left lateral neck incision, which again resulted in the patient’s death from mediastinal sepsis [12]. Early series of single stage diverticulectomy and esophageal closure were beset by frequent, and often fatal mediastinal sepsis [16]. One of the earliest publications on the surgical treatment of hypopharyngeal diverticula that was published in 1910 included 60 patients from multiple centers and prior publication. The pooled mortality rate reported in that manuscript was 16.6%, with numerous additional complications including bleeding, pneumonia, recurrent laryngeal nerve paralysis, and esophageal stricture [12]. While tragic, these early publications were part of what spurned later innovation, in a century-long drive toward safer operative approaches.
The first innovation, which became the most widely accepted in the early twentieth century was a two-stage approach, introduced by Goldmann in 1909, and practiced and promoted by Charles Mayo and Frank Lahey [12, 17]. In the first stage, a lateral neck incision was made over the anterior border of the sternocleidomastoid, and the diverticulum completely dissected from its surrounding attachments, but not divided. The mobilized diverticulum was then temporarily suspended within the deeper layers of the surgical wound, which was then closed. Patients remained in the hospital for 7–10 days, until the second stage operation was performed 7–10 days later. The same incision was reopened and the neck of the diverticulum ligated and divided, and the esophagus closed. The proposed advantage of this technique was that fascial planes contiguous between the neck and the mediastinum would be obliterated at the time of the second operation, preventing mediastinitis as a consequence of esophageal leak, relegating it rather to the surgical space in the neck where drainage was simpler and complications were decreased [16, 18]. A single stage transcervical approach to hypopharyngeal diverticulectomy was popularized later especially after publications by Sweet in 1956 [19, 20] and Payne [21] in that showed a similar profile of complications compared to the two stage diverticulectomy [17]. However, it should be noted that these series with single stage diverticulectomy were largely performed in the era where antibiotics were more readily available, arming the surgeon with more than scalpel and a drain to treat mediastinal sepsis, and leading to better outcomes [16]. Open, transcervical diverticulectomy continues to be a viable option for operative management of symptomatic hypopharyngeal diverticula, though typically when other approaches are nonfeasible.
Open Hypopharyngeal Diverticulopexy
Schmid proposed diverticulopexy alone as a treatment option in 1912. In this technique, the diverticulum was approached from an identical left lateral neck incision. After complete dissection of the diverticulum, permanent sutures secured the fundus to the prevertebral fascia, inverting the diverticulum, and positioning the fundus cephalad to diverticular neck. This configuration both allows dependent drainage of any accumulated food and inhibits accumulation of new debris [17]. Importantly, diverticulopexy avoided violation of the mucosa and resultant esophageal leak [16]. However, in some patients this leads to a caudally extending diverticulum [16, 17]. Diverticulopexy was later incorporated into combination procedures, but is no longer acceptable as a standalone surgical therapy.
Open Hypopharyngeal Diverticular Invagination
Invagination of a hypopharyngeal diverticulum was reported in 1896 by Girard [16, 17]. In this technique, the diverticulum was approached trough a lateral neck incision and completely dissection. To avoid violation of the mucosa, the fundus of the diverticulum was pushed though the diverticular neck and inverted into the esophageal lumen. The muscular defect was then oversewn. A high recurrence rate made this therapy nonviable, and it is rarely used in clinical practice.
Open Cricopharyngeal Myotomy
Better understanding of role that the cricopharyngeal muscle, including both spasm and impaired relaxation, has in the development of hypopharyngeal diverticula led to a new phase in open surgical management and helped to lay the surgical framework that later supported endoscopic approaches. Cricopharyngeal myotomy was first reported in 1958 by Harrison [17, 22]. Sutherland followed this report in 1962, with an accompanying proposal that myotomy alone may be effective therapy for small diverticula and that resection or inversion of the diverticulum was not necessary [17, 23]. Belsey demonstrated complete radiographic resolution of small diverticula after cricopharyngeal myotomy alone [24]. Several other series that mainly included small hypopharyngeal diverticula noted that cricopharyngeal myotomy was efficacious in reducing symptoms, without addressing the diverticulum itself. Cricopharyngeal myotomy was performed through a lateral neck incision as well, but, because it avoids the extensive dissection that leads to the more severe complications (recurrent laryngeal nerve injury, esophageal leak), there was significantly less morbidity compared to diverticulectomy. However, in a series that included patients with larger pouches, cricopharyngeal myotomy alone failed to resolve symptoms, likely because some of the symptoms were directly derived from the presence of a large pouch and not the underlying dysmotility. Later studies evaluated the influence of the myotomy length on efficacy and duration of symptomatic relief. Lerut et al advocated a 5 cm myotomy based on histologic analysis suggesting muscular abnormalities extended beyond the cricopharyngeus to the esophagus itself among patients with hypopharyngeal diverticula [25]. Using intraoperative manometry, Pera and colleagues demonstrated that esophageal pressurization was significantly reduced after cricopharyngeal myotomy. They additionally demonstrated that extending the myotomy 2 cm further into the hypopharyngeus led to an even greater reduction in esophageal pressurization [26].
Open Combination Procedures: Cricopharyngeal Myotomy with Other Open Techniques
Current understanding supports the notion that symptoms may arise from either the pouch or from the pharyngeal constriction and pressurization from a hypertonic or dysfunctional cricopharyngeus muscle. Simultaneously addressing both the cricopharyngeus and the pouch had guided surgical thought for the past six decades. Reports of both cricopharyngeal myotomy in combination with both diverticulectomy and diverticulopexy were published from several centers beginning in the 1960s. Interestingly, the addition of cricopharyngeal myotomy to transcervical diverticulectomy actually reduced complications, likely because the decreased pressurization of the pharynx did not promote esophageal leaks [16].
There is little comparative literature with regard to open techniques to treat hypopharyngeal diverticula and no randomized trails. However, taken altogether, the literature best supports myotomy alone from pouches <1 cm. Moderate-sized pouches may be treated with combination of cricopharyngeal myotomy and diverticulopexy, while large pouches are best suited with cricopharyngeal myotomy and diverticulectomy if an open technique is pursued [16]. For patients with large pouches, but significant comorbid disease, cricopharyngeal myotomy, and diverticulopexy may be entertained as an option as it may avoid mucosal breach and resultant risk of complications related to diverticulectomy [17]. However, as discussed below, there are currently other, less invasive options that exist, provided the surgeon has experience with these techniques.
Transoral Surgical Approaches for Hypopharyngeal Diverticula
Primarily in an attempt to reduce morbidity and mortality associated with open approaches, surgeons have sought less invasive therapeutic options. Literature published regarding these techniques is highly varied, and suffers from a terminology that has co-evolved with technology. The term endoscopic is frequently used; however, this may refer to an esophagoscope, a rigid diverticuloscope (Weerda-scope), or a flexible endoscope, among others. We have chosen to refer to all these techniques collectively as transoral approaches and divide between rigid and flexible devices. Rigid or fixed devices are traditionally used to position the head and neck and/or provide a conduit to visualize the hypopharynx or diverticulum directly. Flexible endoscopic approaches use a flexible bronchoscope or endoscope more typically used for gastrointestinal endoscopy, where the effector end of the scope is manipulated by hand controls, and the device integrates a channel or channels through which instruments may be introduced. The majority of literature on transoral techniques is retrospective series typically of single centers. Where comparative literature does exist, it is in comparison to open surgical approaches. Comparisons between two or more transoral techniques are generally lacking.
Transoral Hypopharyngeal Diverticulotomy
The first report of a transoral therapy for a hypopharyngeal diverticulum was made in 1917 by Mosher [27]. Using esophagoscopic visualization, he described dividing the common wall between the esophagus and the diverticulum using a scissor punch [12]. However, as the base of the divided wall was unable to be sealed, the risk of leak increased. Unfortunately leaks almost certainly lead to mediastinitis, which proved to be a fatal complication in one of his first patients. As a result, he abandoned the technique. The technique of dividing the common wall, almost certainly accomplished a cricopharyngeal myotomy, a fact that was only recognized in hindsight as the cricopharyngeal myotomy was developed and studied in the open technique.
Before discussing further transoral therapies, we would be remiss to not mention the contributions of Chevalier Jackson on this field. His report in 1915 was the first use of an esophagoscope to aid in diverticulopexy [28, 29]. While his manuscripts did more to support a one-stage approach to transcervical diverticulectomy, he was one of the first surgeons to utilize an endoscope intra-operatively as a tool to facilitate identification of anatomy and to complete an open operation. He used an endoscope to first visualize the diverticulum, then to clear the pouch of its food debris. Advancing the esophagoscope into the pouch, he capitalized on transillumination of the pouch to identify the pouch externally. Then, following resection the endoscope was advanced into the esophageal lumen and the closure performed around the endoscope to ensure the lumen was not narrowed [12]. While it would be difficult to confirm whether this was the first instance of endoscopic assistance for open surgery, it is worth noted that this technique elegantly mirrors contemporary use of a flexible endoscope to perform multiple intraoperative functions.
Transoral Hypopharyngeal Diverticulotomy with Thermal Devices
The next major advance in transoral therapy for hypopharyngeal diverticula was in 1960, by Dohlman and Mattsson. In a report of 100 patients, they described using a modified rigid esophagoscope (Weerda-scope®, Karl Storz, Fig. 15.4) to visualize a hypopharyngeal diverticulum and the common wall, similar to that described by Chevalier Jackson). Dohlman’s advance in terms of technique over that of Mosher was the use of electrocautery to complete the division of the common wall [30]. Based on his own prior radiographic studies, this work both described the importance of the cricopharyngeus muscle and properly described the endoscopic division of this muscle. The series described the “endoscopic esophagodiverticulostomy” included no cases of mediastinitis and a recurrence rate of 7%, which was noteworthy for the time. Additionally, the assertion was made that in the setting of a recurrent diverticulum previously treated with a transoral approach, a transoral reoperation was likely to be considerably easier than repeating an open surgical approach, where natural planes would be obscured [10, 12].
Rigid distracting diverticuloscope, without attached stabilization arm (Weerda® scope, Karl Storz). (Reproduced from Kroh and Reavis [134]; Fig. 18.3)
In the subsequent three decades, a number of authors offered improvements on the Dohlman diverticulotomy technique, using a variety of instruments and new thermal technologies to divide the common wall shared between the diverticulum and esophagus, with division of the cricopharyngeus muscle. This included additional centers reporting us of laparoscopy scissors connected to an electrosurgical unit [31, 32]. In the late 1990s, Lippert introduced a microendoscopic approach using a CO2 laser to divide the mucosa and the cricopharyngeus muscle contained within the common wall [33, 34]. Results were similar; however, electrocautery was still needed often to seal the distal dissection flaps or any mediastinal opening. Another laser (KTP/532 nm) was also adapted in a similar technique by other authors [35]. Taken together, each of these authors used a thermal energy device to expose and divide the diverticular septum and seal the exposed edges. These reports were generally associated with short hospital stays, more rapid resumption of diet, and similar efficacy to trans-cervical surgery. There were more risks of mediastinitis using thermal devices for transoral diverticulotomy, though this was typically less severe than mediastinitis associated with open approaches likely because the defects were small. Mediastinitis after these transoral approaches more often responded to medical therapy alone. Moreover, the risk of recurrent laryngeal nerve injury is decreased with transoral approaches over that of transcervical approaches. The lack of widespread use of laser technology in general limited more general use of those techniques. There was also a general sense among most surgeons treating hypopharyngeal diverticula that the lack of mechanical device to seal the edges increased the risk of mediastinitis and thus “suture-less endoscopic esophagodiverticulostomy” fell out of favor [10].
Transoral Stapled Hypopharyngeal Diverticulotomy
The next major advance in transoral approach to hypopharyngeal diverticula was the use of a stapling device to both divide and seal the common wall between the diverticulum and the esophagus, in conjunction with the cricopharyngeus muscle contained therein. The first of these reports was by Collard and colleagues in 1993 [36], in a contemporary patient cohort to those advocating microendoscopic laser-based approaches. Collard and colleagues modified a rigid transoral diverticuloscope to have narrower lips. They introduced a 12 mm diameter laparoscopic gastrointestinal stapler (30 mm cartridge length) through the diverticuloscope and then use a 5 mm endoscopic camera to verify position prior to firing the stapler (see Fig. 15.5) [36]. They also point out that the divided cricopharyngeus muscle, since it is sealed by the overlying stapled mucosa, provides lateral retraction to the cut edges, creating a wide opening between the diverticulum and the esophagus [36]. Their report included 6 patients, 5 of who had complete resolution of dysphagia and the last was improved. Numerous additional publications replicated these results using very similar techniques [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. One of the challenges of this technique is that the stapling devices have a small portion at the tip, beyond the cut line, which results in a small residual septum. To overcome this, some authors modify the stapler by filing down the end of the anvil to ensure the staple line reaches as far into the diverticulum as possible [36]. Several authors also report placement of temporary traction sutures at either end of the septum to aid in pulling the septum into the stapler jaw and positioning it appropriately. The largest study included a total of 585 patients who were culled from previously published literature between 1995 and 2010 (534 patients) and 51 patients retrospectively identified at the study center (1999–2011) [53]. There were 92.3% of attempted transoral stapling procedures completed, in a mean 22 minutes. Of those with completed operations, 91% reported improved symptoms. Most aborted procedures were due to small diverticula (<3 cm), or inability to adequately extend the neck to position the diverticuloscope. Complications occurred in a total of 9.6% of patients, though most were minor [53]. The most common complication was iatrogenic perforation (n = 26, 4.8%), and most complications were managed with medical therapy alone. There was one death included in that study (0.2%). Recurrence of symptoms was estimated to be 12.8% [53]. However, they stated that restapling with a transoral approach for recurrence can be accomplished with little additional risk compared to index stapling procedures [16, 54]. Similar results to this study were noted in a in a recent systematic review [55]. Transoral stapling may be difficult in patients with diverticula that are small (<2 cm) as the stapler may not be able to engage the short septum, or very large diverticulum, where the stapler may not be able to reach the furthest extent of the common septum [16]. For larger diverticula, more than one staple cartridge may be necessary and are allowable [43]. Patient satisfaction is noted to be higher among patients undergoing transoral stapled diverticulotomy compared to open techniques, though this is likely related to decreased pain, shorter hospitalization and generally shorter convalescent period [16]. Overall, transoral stapled diverticulotomy is likely the most widely practiced transoral technique to treat hypopharyngeal diverticula and is likely the first-line operation practiced at most centers.
Position of laparoscopic linear stapler/cutter in trans-oral stapled diverticulotomy. Image shown without the accompanying rigid diverticuloscope. (Reproduced from Fisichella and Patti [135] ; Fig. 11.5)
Transoral Flexible Endoscopic Hypopharyngeal Diverticulotomy
A major limitation to the rigid transoral techniques is that they are dependent on using a rigid device to visualize the diverticulum and septum. The rigid diverticuloscope functions in a linear fashion, demanding that the neck be extended, to allow a direct line of visualization to the junction between the hypopharynx and the esophagus. Osteoarthritis, prior cervical disk fusion, or kyphosis, which is common in the population among whom hypopharyngeal diverticula are more prevalent, may limit neck extension such that a rigid scope cannot be properly positioned. Advances in flexible endoscopic techniques in general, largely afforded by advances in instrumentation, have led to new approaches to treat hypopharyngeal diverticula. Figure 15.6 depicts barium esophagograms obtained before and after a flexible endoscopic transoral hypopharyngeal diverticulotomy.
(a) A lateral view of the neck obtained from a barium esophagram demonstrating a hypopharyngeal diverticulum filling with contrast. This image was obtained prior to a flexible endoscopic transoral diverticulotomy. (b) An endoscopic view of the cricopharyngeus muscle from a submucosal tunnel. The red line outlines the edges of the muscle, “e” marks the true lumen of the esophagus. (c) Lateral view of the neck obtained from a barium esophagram after a flexible endoscopic transoral diverticulotomy demonstrates that contrast enters the persistent pouch but is still able to empty freely into the esophagus
While the first description of a flexible endoscopic approach to treatment of hypopharyngeal diverticulum was made in 1982 [56], the first case series with follow-up data available were not made until 1995, with reports from two centers using similar techniques [57, 58]. Ishioka and colleagues utilized a needle knife to perform mucosal incision and myotomy, while Mulder and colleagues reported using monopolar coagulation forceps. Both techniques utilize an electrosurgical instrument to both divide and seal the common wall shared between the esophagus and the diverticulum, as well as the underlying cricopharyngeus muscle [57, 58]. In these series, complications were rare and success rates were high in terms of short-term symptomatic relief. However, there were patients included in those (and subsequent) series that needed multiple endoscopic sessions, wherein partial division was accomplished, and then symptoms evaluated. If symptoms persisted, a subsequent endoscopic session was scheduled for further division, separated by at least 1 day [57,58,59]. While there may be some theoretical advantage in titrating the extent of division to the minimum possible to produce symptomatic relief, this is likely offset by the need for multiple rounds of sedation/anesthesia and its associated risks. These early reports were followed by other series utilizing other endoscopic devices, including argon plasma coagulation [59, 60], hook knife [61, 62], and more recently knifes that were developed for endoscopic mucosal resection and endoscopic submucosal dissection, including the Dualknife™ [63], insulated tip knife [64], and the stag beetle knife [65, 66]. Various assistive devices have been reported as well, including endoscopic caps [61, 67, 68] and endoscopic clips to seal mucosal edges, or the inferior extent of resection. However, the most commonly mentioned device is a flexible diverticuloscope (see Fig. 15.7) [63,64,65,66, 69, 70]. Most studies that utilize a flexible diverticuloscope originate from centers in Europe, where commercial devices are more readily available [71]. Other centers have reported modifying the end of endoscopic overtubes to resemble a flexible diverticuloscope. The purpose of the flexible diverticuloscope, which is introduced over the endoscope, is to isolate and stabilize the septum during division. A summary of early manuscripts, endoscopic devices, complications, and reported outcomes is given in Table 15.2.
One of the reported advantages of flexible endoscopic approaches to hypopharyngeal diverticula is that they may be performed without general anesthesia. As is well stated in an anecdote in an editorial by Sakai, “...open surgery has been advised for patients at excessive surgical risk…[however] the same rationale has been used to suggest the opposite, namely flexible endoscopy” [56]. That is to say, patients with severe respiratory comorbidities, such that it may be difficult to wean the patient from intraprocedural mechanical ventilation, may be better suited to a flexible endoscopic approach. This is an inversion of conventional thought, where open transcervical approaches were the most conservative, and reserved for higher risk individuals. Many of the early reports of flexible endoscopic approaches reported use of conscious sedation [57, 58, 60, 72, 74]. This was likely largely driven by provider comfort, in that most authors were endoscopists and were comfortable performing similar procedures under conscious sedation. However, later series used intubation and general anesthesia, and or allowed for selective use of general anesthesia. Procedure times are on the order of 15–25 minutes in most series reporting outcome of flexible endoscopic approaches.
Most published techniques, including all those in the Table 15.2, employ a single incision technique, in which a single liner cut is made from the septum toward the base of the diverticulum. This maneuver replicates the division accomplished via rigid transoral endoscopic stapling. There have been recent publications that have advanced that approach to a two-incision approach, where two parallel incisions are made 1 cm apart and continued to the base of the diverticulum, with the distal extent taken by an endoscopic snare [82, 83]. The resultant wedge-shaped resection does remove a larger portion of tissue and may result in less frequent recurrence, but data are lacking long term [84].
The extent of resection with flexible endoscopic approaches to hypopharyngeal diverticula is also a matter of debate. While few would argue that complete division of the septum is most likely to resolve symptoms and reduce recurrence [70], when facing a screen and deciding which will be the last fibers to cut is another matter entirely. One of the justifications of the step-wise, multisession approach in early publications was likely fear to free perforation into the mediastinum and resultant mediastinitis; however, this leads to a similar problem noted with transoral stapling, where residual septa can contribute to persistent symptoms. Over time, accumulated evidence demonstrates that even when perforations occur, they are generally small and can be managed with endoscopic clips and do not often need surgery. Some even advocate that the dissection can be readily advanced into the neck, up to a centimeter beyond the base of the diverticulum to extend the myotomy onto the esophagus [85]. They note a reduction in postprocedure dysphagia rates when the myotomy is extended [79].
One of the implications of the introduction of the flexible endoscopic platform to treat hypopharyngeal diverticula was a shift in the medical providers that were providing therapy. Transcervical and most rigid transoral techniques were developed by surgeons with specialization in general surgery, otolaryngology, or thoracic surgery. On the other hand, many of the authors of papers involving flexible endoscopy arose from gastroenterology. Gastroenterologists, while facile with endoscope and therapeutic devices that can be introduced through them, are not typically trained in principles of surgical practice. These complementary specializations have a history of collaboration and co-evolution as organized under the auspices of Natural Orifice Trans-Endoscopic Surgery (NOTES). There are some gastroenterologists that remain trepid regarding their specialties’ role in treating hypopharyngeal diverticula [71]. However, as is noted in the next section, the most recent advances in the treatment of hypopharyngeal diverticula, from within the submucosal plane, would have likely not been possible without this history of cross-specialty collaboration.
Transoral Flexible Endoscopic Submucosal Approach Hypopharyngeal Diverticula
As submucosal tunneling technique has become more prevalent for the treatment of various malignant and benign conditions of the gastrointestinal tract, including early stage colon, rectal and gastric cancers, leiomyomas, gastroparesis, and achalasia, submucosal treatment of hypopharyngeal diverticula has begun to be explored as well. In this technique, the cricopharyngeus is approached with a submucosal tunnel [85,86,87,88,89]. The endoscope is oriented such that the septum is vertical, as opposed to horizontal. A mucosal incision is made roughly 3 cm proximal to the septum and extended longitudinally to accommodate the endoscope. A tunnel is made to the septum and then developed on either side of the muscle. The muscle fibers are then divided. This myotomy may be extended as distally as desired, even beyond the base of the diverticula if needed. The mucosal incision is then closed with clips. While currently limited to only select centers which have broad experience with submucosal tunneling, this is an emerging area of application to hypopharyngeal diverticula.
Preoperative Assessment
The diagnosis of hypopharyngeal diverticulum is confirmed using the methods described above. It is often helpful for the surgeon performing the procedure to perform their own endoscopic inspection for operative planning, and is typically best accomplished with a flexible endoscopy. This may also reveal evidence of concomitant disease, including reflux associated esophagitis that may be comorbid in these patients. Physical examination should include an evaluation of active next extension and inspection of the mouth opening. A review of comorbidities, especially cardiac and pulmonary conditions, will guide the provider toward an anesthetic approach; however, we most often prefer general anesthesia.
Postoperative Care
A wide variety of protocols exist regarding postoperative care, without any comparative studies to suggest superiority of one over another. The time to resumption of diet, content of diet, duration of antibiotics if they are used at all, and radiographic esophageal imaging are all variable. We typically use a transoral flexible endoscopic approach, performed in the operating room, under general anesthesia. Our typical approach is to keep patients with only sips of clear liquids on the day of the procedure. On the following day, a contrast esophagram is obtained both to verify no leak and to establish a new baseline should symptoms recur in the future. If there is no leak or perforation and the patient is able to perform the study without bothersome dysphagia, then they are advanced to a thick liquid diet. This diet is continued for 1–2 weeks thereafter to allow healing of the mucosa and to reduce mechanical insult to the tissue. Hospitalization is typically only the night of surgery in our practice.
Contemporary Surgical Techniques to Hypopharyngeal Diverticula: Open Approach to Hypopharyngeal Diverticula
The patient is positioned supine on the operating room table, often with a shoulder roll to facilitate neck extension. In some practices, a preincision flexible endoscopy is performed and the diverticulum packed with gauze to aid in identification of the diverticulum. A nasogastric tube and/or esophageal dilator may also be passed to aid in identification of the true esophageal lumen. An incision is made over the anterior border of the sternocleidomastoid muscle ipsilateral to the diverticulum, typically the left side. Alternately a transverse incision is made in a skin crease between the hyoid and the clavicle. Subplatysmal flaps are developed. Posterolateral retraction of the sternocleidomastoid is performed. As the posterior pharynx is identified, the recurrent laryngeal nerve is identified, as well as the thyroidal vessels. These may need to be ligated depending on the size and position of the diverticulum. The diverticulum is then identified and freed from the surrounding attachments. Once this is completed, the diverticular sac may be resected or suspended. A cricopharyngeal myotomy is nearly always performed as well. Numerous studies have demonstrated that leaving the cricopharyngeus intact leads to frequent recurrence. For small diverticula, cricopharyngeal myotomy may be a sufficient treatment alone.
In early descriptions, the need for multiple interventions and the lack of antibiotics contributed to a high rate of morbidity and even mortality. In contemporary series, open techniques for hypopharyngeal diverticula have an aggregate mortality rate of around 1.5% and morbidity of around 11.5%. Complications include fistula formation, recurrence, recurrent laryngeal nerve injury, mediastinitis, and esophageal strictures [90].
Rigid Transoral Approach to Hypopharyngeal Diverticula
The patient is positioned supine on the operating room table, often with a shoulder roll to facilitate neck extension. General anesthesia is induced and the patient is intubated. A bivalve diverticuloscope is positioned, with the anterior (upper) blade within the esophageal lumen and the posterior (lower) blade within the diverticulum. A telescope is used to visualize the septum and the diverticuloscope is suspended. Temporary traction sutures may be placed at the lateral aspect if desired. Any bezoar is removed from the diverticulum. The endoscopic stapler is then introduced. The stapler cartridge (the hammer portion) of the stapler is often easier to position in the diverticulum, as this is often a larger orifice in this view, and the anvil is positioned in the esophageal lumen. Once the stapler has been fired, the septum is divided and a common opening (diverticulo-esophagostomy) is created in the posterior wall of the esophagus. The edges of this divided tissue included both mucosal edges, and the underlying muscle is sealed by the staple rows. Multiple firings may be necessary depending on the size of the stapler cartridges used and the size of the diverticulum. Because the anvil of the stapler always extends beyond the cutting blade, there may be a small remnant septum. There are descriptions of division of this remaining septum using ultrasonic shears or monopolar forceps. Limitations to this technique include inability to extend neck or position the diverticuloscope due to small mouth opening or prominent dentition. Small diverticula (<3 cm) are difficult to treat using this technique. For both reasons, up to 30% of eligible patients will be not be able to be managed with this approach, and an alternative approach should be sought.
Flexible Endoscopic Approach to Hypopharyngeal Diverticula
The patient is positioned supine on the operating room table, often with a shoulder roll to facilitate neck extension. Numerous publications report performed flexible endoscopic treatment of hypopharyngeal diverticulum under conscious sedation or monitored anesthesia care/deep sedation. While this in an option, we prefer intubation and general anesthesia, in case an open approach is needed. Moreover, as scope stability is the most challenging aspect of this approach, general anesthesia, and resultant minimal patient movement may make the procedure more facile. We would recommend a general anesthetic approach in particular for providers that have less experience with the flexible endoscopic techniques, as prolonged procedure times may not be well tolerated by less sedate patients. A standard gastroscope is used, fitted with a transparent cap, which helps to maintain visualization of the tissue. A diagnostic endoscopy is performed first. We typically place a nasogastric tube into the true esophageal lumen under endoscopic guidance; a guidewire may be used for the same purpose. This helps to maintain orientation and may aid in scope stability as well. While there are numerous reports of using a flexible diverticuloscope, or modified overtube, we do not prefer this approach. The main benefit of the flexible diverticuloscope is scope stabilization, but we often find the resultant limitation in scope mobility to be detrimental. Any bezoar is removed from the diverticulum. We prefer to use a triangular tip knife for the procedure. A cut current is used to divide the mucosa and a coagulation current thereafter. The triangular tip knife is larger than most other endoscopic knives, so caution must be exercised as thermal spread can also be greater. However, the increased surface area allows the knife to be used to push fibers or edges away, and the corners may be used to pull fibers as well; the multiple ways to use the knife may be advantageous in the small space.
There are two approaches we use the first is a cutting only technique. The mucosa overlying the central part of the septum is divided, orthogonal to the long axis of the septum. This is carried distally toward the base of the diverticulum. The pressure applied from the cap will tend to spread the mucosal edges apart and the underlying muscle and connective tissue will appear as a V. If areolar fibers are visualized with no muscle beyond, the mediastinum has been reached. Clips should be placed at the base to help reduce the risk of leak into the mediastinum in case an unnoticed iatrogenic peroration has occurred.
The alternative approach is a submucosal tunneling technique, which exposes the underlying cricopharyngeus muscle while maintaining musical flaps to later close. In this case, the mucosotomy is along the long axis of the septum, long enough to accommodate the endoscopic cap. The loose connective tissue of the submucosal is then carefully teased away to separate the mucosa from the underlying muscle on both sides of the septum. The cricopharyngeus can then be completely divided once its fibers are exposed. The mucosotomy can then be closed with endoscopic clips. In this technique, there may be a partial persistent septum; however, the complete cricopharyngeal myotomy that is accomplished will still produce durable symptomatic relief.
Non-Zenker’s Diverticula of the Esophagus
Mid-esophageal and epiphrenic diverticula occur within the general population, though with less commonality compared to hypopharyngeal diverticulae. Given their more distal location, management differs from hypopharyngeal diverticula, and given their relative rarity, evidence to guide treatment is more limited.
Mid-Esophageal Diverticula
Mid-esophageal diverticula occur in an area with 5 cm above or below the carina and make up roughly 10–17% of all esophageal diverticula [5, 91]. While these anatomic limits are somewhat arbitrary, diverticula in this region are unique, in that they have traditionally demanded transthoracic procedures for treatment. Mid-esophageal diverticula are often related to underlying conditions, in particular conditions leading to paraesophageal inflammation. Early studies linked many mid-esophageal diverticula to tuberculosis-related lymphadenitis, or histoplasmosis. The inflammation of peri-bronchial lymph nodes results in a true traction diverticulum, where all layers of the esophageal wall are pulled into an outpouching. Most often diverticula occurring by this mechanism form on the right side of the esophagus, just below the carina, as the subcarinal lymph nodes lie nearest the esophagus in this position [5]. More contemporary studies have demonstrated that pulsion diverticula may also occur, and may be related to neuromuscular dysfunction of the esophagus [5, 92]. Specifically, hypertonicity or hyperactivity of the distal esophagus may lead to an area of hyperpressurization at the proximal aspect of the abnormal segment [92]. However, it should be noted that most studies that have established hyperpressurization as a causitive factor were performed prior to the availability of high-resolution manometry, which has resulted in substantial changes in classification of some subsets of esophageal motility disorders. There may also be congenital attachments that act as traction lead points in the mid-esophagus and contribution to enlargement of pulsion diverticula [5]. An alternative explanation is that mid-esophageal pulsion diverticula form through a weakness in the esophageal wall where a congenital foregut cyst is present. This also fits with observed patterns of pulsion diverticula occurring primarily on the left side of esophagus [5].
Symptoms of Mid-Esophageal Diverticula
Small mid-esophageal diverticula may be asymptomatic, as they were often small and wide mouthed. This appearance was typical of diverticula associated with tuberculosis. However, complications including hemorrhage, and/or fistulous connections to the aerobronchial or central vasculature have been reported [5]. The association between mid-esophageal diverticula and neuromuscular or motility disorders of the esophagus, may make it difficult to ascertain what if any symptoms arise from the diverticulum as opposed to the underlying motility disorder.
Diagnosis of Mid-Esophageal Diverticula
Diagnosis of mid-esophageal diverticula is typically made radiographically. An esophagram is the best for diagnosis and characterization of mid-esophageal diverticula. Similar to hypopharyngeal diverticula, some mid-esophageal diverticula are discovered incidentally as the use of cross-sectional imaging continues to be more common. Endoscopy may be useful in evaluating complications of diverticula, but it is of little additional diagnostic yield. Manometric analysis may be useful in determining etiology. Manometric catheters may be difficult to place when large diverticula are present, as the catheter may preferential course into the diverticulum; endoscopic guidance may be necessary to ensure passage into the esophageal lumen.
Surgical Treatment of Mid-Esophageal Diverticula
Indications for treatment of mid-esophageal diverticula are symptoms or complications. Traction type diverticula of the mid-esophagus may be treated by diverticulectomy. Traditionally, this was performed via a right-sided thoracotomy, with the assistance of an esophageal bougie to avoid narrowing the esophageal lumen. Myotomy is traditionally performed on the opposite side of the esophagus in this approach, and is associated with a significant decrease in leak rates [93]. The defects were closed in with absorbable sutures in two layers and often buttressed with the pleuropericardial fat pad, or pleura [5]. Alternatives include diverticulopexy with suspension to the prevertebral fascia [5]. Where this is an associated motility disorder, myotomy alone may be sufficient to relieve symptoms. Thoracoscopic approaches are possible, but are likely best performed by surgeons in centers with significant experience with minimally invasive esophageal surgery [91, 94]. Robot-assisted approaches have also been reported. There are also increasing reports of using submucosal tunneled approaches to perform myotomies for mid-esophageal diverticula similar to those discussed above with hypopharyngeal diverticula [86, 95]. Given the likely association with motility disorders for some mid-esophageal diverticula, this therapy is intriguing; however, long-term data are lacking.
Intramural Pseudodiverticula of the Esophagus
Intramural pseudodiverticula appear on barium esophagram. Multiple small (usually <5 mm) cystic structures appear in the esophagus, perpendicular to the esophageal wall [96]. Patients presenting with intramural pseudodiverticula often do not have associated endoscopic abnormalities. However, intramural psuedodiverticula are typically associated with a distal stricture, and may not resolve with treatment of the distal stricture. They do not warrant surgical intervention.
Epiphrenic Diverticula of the Esophagus
Epiphrenic diverticula are pulsion type diverticula that form in the distal portion of the esophagus, typically in the distal 10 cm. They are quite uncommon, with an estimate incidence of 1 in 500,000 per year [97]. Typically epiphrenic diverticula occur on the right side of the esophagus [98]. The first description is attributed Hoxie in 1804 based on anatomical studies, and then radiographically by Zeitstein in 1898 [6]. Mondiere described symptoms attributed to epiphrenic diverticula and was the first to postulate in 1833 that intraluminal pressure played a role in the development epiphrenic diverticula [99]. Current understanding is the epiphrenic diverticula are pulsion-type diverticula that result from herniation of the mucosa and submucosa through an intrinsic weakness in the esophageal wall. Similar to hypopharyngeal diverticula, most epiphrenic diverticula are related to an underlying motility disorder that results in a functional distal obstruction and hyper-pressurization of the esophageal lumen [24, 99, 100]. One group was able to perform barium videoesophagography, upper gastrointestinal endoscopy, and esophageal manometric in patients with symptomatic epiphrenic diverticula [98]. By performing this complement of studies, they were able to demonstrate that all of the patients had a concomitant motor abnormality, and were distributed across five separate diseases: achalasia, diffuse esophageal spasm, hypertensive lower esophageal sphincter, nutcracker esophagus, and vigorous achalasia [98]. However, even in the absence of a diagnosed esophageal motility disorder, patients with epiphrenic diverticula will have alterations in the myenteric plexus leading to poor coordination of muscular contraction [101].
Symptoms of Epiphrenic Diverticula
As with other esophageal diverticula, the association with motility disorders can make it difficult to ascertain what symptoms may be due to an epiphrenic diverticulum. Dysphagia and reflux are the most common symptoms experienced by patients [102]. Regurgitation, chest pain, epigastric pain, reflux, and aspiration pneumonia have been additionally reported. Severity of symptoms and size do not appear to be correlated [102].
Diagnosis of Epiphrenic Diverticula
Most epiphrenic diverticula are diagnosed on barium esophagram. An upper endoscopy should be pursued as well, to rule out associated malignancy or dysplasia. The frequency with which esophageal motility disorders are co-morbid also warrants esophageal manometry. Mobile esophageal monitoring may be necessary and/or illuminating in some patient who only experience symptoms intermittently or with food [98]. Manometry catheters may need to be placed under endoscopic guidance if diverticula significantly distort the distal esophagus. Esophageal manometry is particularly helpful to aid in operative planning regarding the length of myotomy required and whether a simultaneous antireflux procedure should be performed.
Surgical Treatment of Epiphrenic Diverticula
While there is general consensus that surgery should be reserved only for symptomatic patients, even this is not without controversy. While some authors have used a size criteria for indication for operation for epiphrenic diverticula [103], both the poor correlation between size and symptoms and better understanding of the underlying pathophysiology have removed this an indication alone [104]. Additionally, while some authors advocate all patients with a known epiphrenic diverticulum should be operated upon [105], this must be balanced against the significant risk of morbidity, and a mortality rate as high as 11.1% in published series [106]. Moreover, many patients with minimal symptoms do not progress, or at least progress slowly over time [107]. Perhaps the best summary statement was crafted by Orringer, “masterful inactivity is generally the best approach” in describing annual surveillance for patients with minimal symptoms from epiphrenic diverticula [6, 108]. When surgical intervention becomes necessary, frequently diverticulectomy and esophageal myotomy are performed [93, 109].
Surgical approach is also a matter for debate. Classically, the distal esophagus was best accessed from a left thoracotomy, and was the preferred approach, despite the more frequent right-sided location of epiphrenic diverticula. Over the past several decades, there has been a progression toward minimally invasive approaches in published literature, either thoracoscopic, laparoscopic, or both [93, 109, 110]. It is worth noting that even in centers that perform high volumes of minimally invasive esophageal surgery, complications of surgical procedures to address epiphrenic diverticula are substantial [104].
Given that esophageal motility disorders underlie the development of epiphrenic diverticula, myotomy has been advocated as part of the surgical treatment. Myotomy is now considered routine, and is performed in the majority of cases (85.5%) in the reported literature, and is selectively employed without intervention on the epiphrenic diverticulum itself [93]. Myotomy is intended to relief the high pressure zone, similar to cricopharyngeal myotomy for hypopharyngeal diverticula; failure to eliminate the high pressure zone is associated with increased risk of perioperative leak, and recurrence of an epiphrenic diverticula [110]. Accordingly, extension of the myotomy of onto the stomach has been advocated [104, 110, 111]. However, this puts patients at risk for postoperative reflux. Thus, selective myotomy or limited myotomy is employed in some centers, guided by the esophageal manometry results from preoperative testing [98, 112].
An antireflux procedure performed in conjunction with epiphrenic diverticulectomy and myotomy was introduced as an option in the open surgical era [107]. Among published studies, less than 70% of patients have undergone concomitant antireflux operations [93]. Some feel that there is limited risk of reflux, if the lower esophageal sphincter is not divided, which generally implies a transthoracic approach with a limited myotomy [6, 109]. What emerges from the literature is that nonobstructing antireflux procedures are preferred over a full fundoplication. The most common antireflux operations and Dor type (anterior, 180° wrap) or a Belsey-Mark IV (anterior 220–240° wrap with diaphragmatic plication) [93]. Overall, a recent meta-analysis of surgical options for epiphrenic diverticulum concludes that diverticulectomy with myotomy is likely the best operation in most circumstances, with our without an antireflux procedure [93]. Given that open and minimally invasive approaches had similar outcomes in terms of morbidity and mortality, the approach is best left to the discretion of the surgeon, and which approach they are most comfortable (Table 15.3).
Emerging Transoral Endoscopic Treatment of Epiphrenic Diverticula
Given the development of the per-oral esophago-myotomy (POEM) procedure [113] as a treatment for achalasia and other esophageal motility disorders, and that these same motility disorders occur in conjunction with epiphrenic diverticula, it is perhaps not surprising that POEM and submucosal tunneling have been applied to epiphrenic diverticula. There are scattered case reports of this technique and single multicenter study that includes three patients [86, 114]. Initial technical success has been good, with symptomatic relief noted in the short term [86]. We expect there to be more reports similar to this in the coming years, and eagerly look to studies describing selection criteria, correlation with high-resolution manometry pre- and postprocedure, as well as longer term outcomes.
Conclusions
Surgeons evaluating published literature reports results of operative management of esophageal diverticula are left wanting for comparative literature. The rarity of esophageal diverticula and diversity of presentation likely render randomized controlled trials impossible. The heterogeneity of techniques and nonstandardized reporting of symptoms and success rates make direct comparison difficult. No standard set of criteria for preoperative currently exist that comprise symptoms (dysphagia, odynophagia, aspiration, reflux, regurgitation), assessment of symptom severity (dysphagia scoring), indications for operation (size vs symptoms vs comorbidities), definition of clinical success (elimination or symptoms, improvement of symptoms) recurrence (after multiple or single procedures) or time-frame for follow-up (early vs late). There is no way for us to recommend substantial superiority of one procedure in comparison to another. Moreover, operative approaches offered to symptomatic patients are increasingly likely to be a function of the specialty training of the provider. However, given that the majority of the procedures described here have a high degree of efficacy, and fairly low risk of complications, the best approaches are likely best left to the surgeon or endoscopist to determine the best approach based upon their training, experience, and most importantly the patient sitting in front of them.
References
Law R, Katzka DA, Baron TH. Zenker’s Diverticulum. Clin Gastroenterol Hepatol. 2014;12:1773–82; quiz e111-112.
Ferreira LEVVC, Simmons DT, Baron TH. Zenker’s diverticula: pathophysiology, clinical presentation, and flexible endoscopic management. Dis Esophagus. 2008;21:1–8.
Laing MR, Murthy P, Ah-See KW, Cockburn JS. Surgery for pharyngeal pouch: audit of management with short- and long-term follow-up. J R Coll Surg Edinb. 1995;40:315–8.
van Overbeek JJ. Meditation on the pathogenesis of hypopharyngeal (Zenker’s) diverticulum and a report of endoscopic treatment in 545 patients. Ann Otol Rhinol Laryngol. 1994;103:178–85.
Rice TW, Baker ME. Midthoracic esophageal diverticula. Semin Thorac Cardiovasc Surg. 1999;11:352–7.
Allen MS. Treatment of epiphrenic diverticula. Semin Thorac Cardiovasc Surg. 1999;11:358–62.
Scher RL, Puscas L. Chapter 71: Zenker diverticulum. In: Cummings Otolaryngology. 6th ed. Philadelphia: Saunders Elsevier; 2015. p. 1025–34.
Zaninotto G, Costantini M. Chapter 11: Cricopharyngeal dysfunction and Zenker diverticulum. In: Shackelfords surgery alimentary tract. 8th ed. Philadelphia: Elsevier; 2019. p. 157–72.
Cook IJ, Gabb M, Panagopoulos V, Jamieson GG, Dodds WJ, Dent J, Shearman DJ. Pharyngeal (Zenker’s) diverticulum is a disorder of upper esophageal sphincter opening. Gastroenterology. 1992;103:1229–35.
Veenker EA, Andersen PE, Cohen JI. Cricopharyngeal spasm and Zenker’s diverticulum. Head Neck. 2003;25:681–94.
Zainabadi K, Courcoulas AP, Awais O, Raftopoulos I. Laparoscopic revision of Nissen fundoplication to Roux-en-Y gastric bypass in morbidly obese patients. Surg Endosc. 2008;22:2737–40.
Hillel AT, Flint PW. Evolution of endoscopic surgical therapy for Zenker’s diverticulum. Laryngoscope. 2009;119:39–44.
Regan J, Walshe M, Timon C, McMahon BP. Endoflip® evaluation of pharyngo-oesophageal segment tone and swallowing in a clinical population: a total laryngectomy case series. Clin Otolaryngol. 2015;40:121–9.
Regan J, Walshe M, Rommel N, Tack J, McMahon BP. New measures of upper esophageal sphincter distensibility and opening patterns during swallowing in healthy subjects using EndoFLIP®. Neurogastroenterol Motil. 2013;25:e25–34.
Pomerri F, Costantini M, Dal Bosco C, Battaglia G, Bottin R, Zanatta L, Ancona E, Muzzio PC. Comparison of preoperative and surgical measurements of Zenker’s diverticulum. Surg Endosc. 2012;26:2010–5.
Aly A, Devitt PG, Jamieson GG. Evolution of surgical treatment for pharyngeal pouch. Br J Surg. 2004;91:657–64.
Stewart K, Sen P. Pharyngeal pouch management: an historical review. J Laryngol Otol. 2016;130:116–20.
Lahey FH. Pharyngo-esophageal diverticulum: its management and complications. Ann Surg. 1946;124:617–36.
Sweet RH. Pulsion diverticulum of the pharyngo-esophageal junction: technic of the one-stage operation: a preliminary report. Ann Surg. 1947;125:41–8.
Sweet RH. Excision of diverticulum of the pharyngo-esophageal junction and lower esophagus by means of the one stage procedure; a subsequent report. Ann Surg. 1956;143:433–8.
Payne WS. The treatment of pharyngoesophageal diverticulum: the simple and complex. Hepato-Gastroenterology. 1992;39:109–14.
Harrison MS. The aetiology, diagnosis and surgical treatment of pharyngeal diverticula. J Laryngol Otol. 1958;72:523–34.
Sutherland HD. Cricopharyngeal achalasia. J Thorac Cardiovasc Surg. 1962;43:114–26.
Belsey R. Functional disease of the esophagus. J Thorac Cardiovasc Surg. 1966;52:164–88.
Lerut T, van Raemdonck D, Guelinckx P, Dom R, Geboes K. Zenker’s diverticulum: is a myotomy of the cricopharyngeus useful? How long should it be? Hepato-Gastroenterology. 1992;39:127–31.
Pera M, Yamada A, Hiebert CA, Duranceau A. Sleeve recording of upper esophageal sphincter resting pressures during cricopharyngeal myotomy. Ann Surg. 1997;225:229–34.
Mosher H. Webs and pouches of the oesophagus, their diagnosis and tratment. Surg Gynecol Obstet. 1917;25:175.
Gaub O, Jackson C. Pulsion diverticulum of the esophagus: a new operatoin for its cure. Surg Gynecol Obstet. 1915;21:52.
Jackson C, Shallow TA. Diverticula of the oesophagus, pulsion, traction, malignant and congenital. Ann Surg. 1926;83:1–19.
Dohlman G, Mattsson O. The endoscopic operation for hypopharyngeal diverticula: a roentgencinematographic study. AMA Arch Otolaryngol. 1960;71:744–52.
Von Doersten PG, Byl FM. Endoscopic Zenker’s diverticulotomy (Dohlman procedure): forty cases reviewed. Otolaryngol Head Neck Surg. 1997;116:209–12.
Wayman DM, Byl FM, Adour KK. Endoscopic diverticulotomy for the treatment of Zenker’s diverticulum. Otolaryngol Head Neck Surg. 1991;104:448–52.
Lippert BM, Folz BJ, Rudert HH, Werner JA. Management of Zenker’s diverticulum and postlaryngectomy pseudodiverticulum with the CO2 laser. Otolaryngol Head Neck Surg. 1999;121:809–14.
Lippert BM, Folz BJ, Gottschlich S, Werner JA. Microendoscopic treatment of the hypopharyngeal diverticulum with the CO2 laser. Lasers Surg Med. 1997;20:394–401.
Kuhn FA, Bent JP. Zenker’s diverticulotomy using the KTP/532 laser. Laryngoscope. 1992;102:946–50.
Collard JM, Otte JB, Kestens PJ. Endoscopic stapling technique of esophagodiverticulostomy for Zenker’s diverticulum. Ann Thorac Surg. 1993;56:573–6.
Scher RL, Richtsmeier WJ. Long-term experience with endoscopic staple-assisted esophagodiverticulostomy for Zenker’s diverticulum. Laryngoscope. 1998;108:200–5.
Thaler ER, Weber RS, Goldberg AN, Weinstein GS. Feasibility and outcome of endoscopic staple-assisted esophagodiverticulostomy for Zenker’s diverticulum. Laryngoscope. 2001;111:1506–8.
Cook RD, Huang PC, Richstmeier WJ, Scher RL. Endoscopic staple-assisted esophagodiverticulostomy: an excellent treatment of choice for Zenker’s diverticulum. Laryngoscope. 2000;110:2020–5.
Lüscher MS, Johansen LV. Zenker’s diverticulum treated by the endoscopic stapling technique. Acta Otolaryngol Suppl. 2000;543:235–8.
Baldwin DL, Toma AG. Endoscopic stapled diverticulotomy: a real advance in the treatment of hypopharyngeal diverticulum. Clin Otolaryngol Allied Sci. 1998;23:244–7.
Philippsen LP, Weisberger EC, Whiteman TS, Schmidt JL. Endoscopic stapled diverticulotomy: treatment of choice for Zenker’s diverticulum. Laryngoscope. 2000;110:1283–6.
Narne S, Cutrone C, Bonavina L, Chella B, Peracchia A. Endoscopic diverticulotomy for the treatment of Zenker’s diverticulum: results in 102 patients with staple-assisted endoscopy. Ann Otol Rhinol Laryngol. 1999;108:810–5.
Omote K, Feussner H, Stein HJ, Ungeheuer A, Siewert JR. Endoscopic stapling diverticulostomy for Zenker’s diverticulum. Surg Endosc. 1999;13:535–8.
Koay CB, Bates GJ. Endoscopic stapling diverticulotomy for pharyngeal pouch. Clin Otolaryngol Allied Sci. 1996;21:371–6.
Peracchia A, Bonavina L, Narne S, Segalin A, Antoniazzi L, Marotta G. Minimally invasive surgery for Zenker diverticulum: analysis of results in 95 consecutive patients. Arch Surg. 1998;133:695–700.
Raut VV, Primrose WJ. Long-term results of endoscopic stapling diverticulotomy for pharyngeal pouches. Otolaryngol Head Neck Surg. 2002;127:225–9.
Stoeckli SJ, Schmid S. Endoscopic stapler-assisted diverticuloesophagostomy for Zenker’s diverticulum: patient satisfaction and subjective relief of symptoms. Surgery. 2002;131:158–62.
Bonavina L, Aiolfi A, Scolari F, Bona D, Lovece A, Asti E. Long-term outcome and quality of life after transoral stapling for Zenker diverticulum. World J Gastroenterol. 2015;21:1167–72.
Wasserzug O, Zikk D, Raziel A, Cavel O, Fleece D, Szold A. Endoscopically stapled diverticulostomy for Zenker’s diverticulum: results of a multidisciplinary team approach. Surg Endosc. 2010;24:637–41.
Jaramillo MJ, McLay KA, McAteer D. Long-term clinico-radiological assessment of endoscopic stapling of pharyngeal pouch: a series of cases. J Laryngol Otol. 2001;115:462–6.
Morse CR, Fernando HC, Ferson PF, Landreneau RJ, Luketich JD. Preliminary experience by a thoracic service with endoscopic transoral stapling of cervical (Zenker’s) diverticulum. J Gastrointest Surg. 2007;11:1091–4.
Leong SC, Wilkie MD, Webb CJ. Endoscopic stapling of Zenker’s diverticulum: establishing national baselines for auditing clinical outcomes in the United Kingdom. Eur Arch Otorhinolaryngol. 2012;269:1877–84.
Koay CB, Commins D, Bates GJ. The role of endoscopic stapling diverticulotomy in recurrent pharyngeal pouch. J Laryngol Otol. 1998;112:954–5.
Verdonck J, Morton RP. Systematic review on treatment of Zenker’s diverticulum. Eur Arch Otorhinolaryngol. 2015;272:3095–107.
Sakai P. Endoscopic myotomy of Zenker’s diverticulum: lessons from 3 decades of experience. Gastrointest Endosc. 2016;83:774–5.
Ishioka S, Sakai P, Maluf Filho F, Melo JM. Endoscopic incision of Zenker’s diverticula. Endoscopy. 1995;27:433–7.
Mulder CJ, den Hartog G, Robijn RJ, Thies JE. Flexible endoscopic treatment of Zenker’s diverticulum: a new approach. Endoscopy. 1995;27:438–42.
Mulder CJ. Zapping Zenker’s diverticulum: gastroscopic treatment. Can J Gastroenterol. 1999;13:405–7.
Rabenstein T, May A, Michel J, Manner H, Pech O, Gossner L, Ell C. Argon plasma coagulation for flexible endoscopic Zenker’s diverticulotomy. Endoscopy. 2007;39:141–5.
Repici A, Pagano N, Romeo F, Danese S, Arosio M, Rando G, Strangio G, Carlino A, Malesci A. Endoscopic flexible treatment of Zenker’s diverticulum: a modification of the needle-knife technique. Endoscopy. 2010;42:532–5.
Rouquette O, Abergel A, Mulliez A, Poincloux L. Usefulness of the Hook knife in flexible endoscopic myotomy for Zenker’s diverticulum. World J Gastrointest Endosc. 2017;9:411–6.
Laquière A, Grandval P, Arpurt JP, Boulant J, Belon S, Aboukheir S, Laugier R, Penaranda G, Curel L, Boustière C. Interest of submucosal dissection knife for endoscopic treatment of Zenker’s diverticulum. Surg Endosc. 2015;29:2802–10.
Manno M, Manta R, Caruso A, Bertani H, Mirante VG, Osja E, Bassotti G, Conigliaro R. Alternative endoscopic treatment of Zenker’s diverticulum: a case series (with video). Gastrointest Endosc. 2014;79:168–70.
Battaglia G, Antonello A, Realdon S, Cesarotto M, Zanatta L, Ishaq S. Flexible endoscopic treatment for Zenker’s diverticulum with the SB Knife. Preliminary results from a single-center experience. Dig Endosc. 2015;27:728–33.
Antonello A, Ishaq S, Zanatta L, Cesarotto M, Costantini M, Battaglia G. The role of flexible endotherapy for the treatment of recurrent Zenker’s diverticula after surgery and endoscopic stapling. Surg Endosc. 2016;30:2351–7.
Costamagna G, Iacopini F, Tringali A, Marchese M, Spada C, Familiari P, Mutignani M, Bella A. Flexible endoscopic Zenker’s diverticulotomy: cap-assisted technique vs. diverticuloscope-assisted technique. Endoscopy. 2007;39:146–52.
Vogelsang A, Preiss C, Neuhaus H, Schumacher B. Endotherapy of Zenker’s diverticulum using the needle-knife technique: long-term follow-up. Endoscopy. 2007;39:131–6.
Huberty V, El Bacha S, Blero D, Le Moine O, Hassid S, Devière J. Endoscopic treatment for Zenker’s diverticulum: long-term results (with video). Gastrointest Endosc. 2013;77:701–7.
Costamagna G, Iacopini F, Bizzotto A, Familiari P, Tringali A, Perri V, Bella A. Prognostic variables for the clinical success of flexible endoscopic septotomy of Zenker’s diverticulum. Gastrointest Endosc. 2016;83:765–73.
Katzka DA, Baron TH. Transoral flexible endoscopic therapy of Zenker’s diverticulum: is it time for gastroenterologists to stick their necks out? Gastrointest Endosc. 2013;77:708–10.
Hashiba K, de Paula AL, da Silva JG, Cappellanes CA, Moribe D, Castillo CF, Brasil HA. Endoscopic treatment of Zenker’s diverticulum. Gastrointest Endosc. 1999;49:93–7.
Sakai P, Ishioka S, Maluf-Filho F, Chaves D, Moura EG. Endoscopic treatment of Zenker’s diverticulum with an oblique-end hood attached to the endoscope. Gastrointest Endosc. 2001;54:760–3.
Christiaens P, De Roock W, Van Olmen A, Moons V, D’Haens G. Treatment of Zenker’s diverticulum through a flexible endoscope with a transparent oblique-end hood attached to the tip and a monopolar forceps. Endoscopy. 2007;39:137–40.
Evrard S, Le Moine O, Hassid S, Devière J. Zenker’s diverticulum: a new endoscopic treatment with a soft diverticuloscope. Gastrointest Endosc. 2003;58:116–20.
Al-Kadi AS, Maghrabi AA, Thomson D, Gillman LM, Dhalla S. Endoscopic treatment of Zenker diverticulum: results of a 7-year experience. J Am Coll Surg. 2010;211:239–43.
Case DJ, Baron TH. Flexible endoscopic management of Zenker diverticulum: the Mayo Clinic experience. Mayo Clin Proc. 2010;85:719–22.
Halland M, Grooteman KV, Baron TH. Flexible endoscopic management of Zenker’s diverticulum: characteristics and outcomes of 52 cases at a tertiary referral center. Dis Esophagus. 2016;29:273–7.
Pescarus R, Shlomovitz E, Sharata AM, Cassera MA, Reavis KM, Dunst CM, Swanström LL. Trans-oral cricomyotomy using a flexible endoscope: technique and clinical outcomes. Surg Endosc. 2016;30:1784–9.
Perbtani Y, Suarez A, Wagh MS. Techniques and efficacy of flexible endoscopic therapy of Zenker’s diverticulum. World J Gastrointest Endosc. 2015;7:206–12.
Ishaq S, Hassan C, Antonello A, et al. Flexible endoscopic treatment for Zenker’s diverticulum: a systematic review and meta-analysis. Gastrointest Endosc. 2016;83:1076–1089.e5.
Gölder SK, Brueckner J, Ebigbo A, Messmann H. Double incision and snare resection in symptomatic Zenker’s diverticulum: a modification of the stag beetle knife technique. Endoscopy. 2018;50:137–41.
Pang M, Koop A, Brahmbhatt B, Bartel MJ, Woodward TA. Comparison of flexible endoscopic cricopharyngeal myectomy and myotomy approaches for Zenker diverticulum repair. Gastrointest Endosc. 2019;89:880–6.
Ishaq S, Sultan H, Siau K, Kuwai T, Mulder CJ, Neumann H. New and emerging techniques for endoscopic treatment of Zenker’s diverticulum: State-of-the-art review. Dig Endosc. 2018;30:449–60.
Beard K, Swanström LL. Zenker’s diverticulum: flexible versus rigid repair. J Thorac Dis. 2017;9:S154–62.
Yang J, Zeng X, Yuan X, et al. An international study on the use of peroral endoscopic myotomy (POEM) in the management of esophageal diverticula: the first multicenter D-POEM experience. Endoscopy. 2019;51:346–9.
Li Q-L, Chen W-F, Zhang X-C, Cai M-Y, Zhang Y-Q, Hu J-W, He M-J, Yao L-Q, Zhou P-H, Xu M-D. Submucosal tunneling endoscopic septum division: a novel technique for treating Zenker’s diverticulum. Gastroenterology. 2016;151:1071–4.
Brieau B, Leblanc S, Bordacahar B, Barret M, Coriat R, Prat F, Chaussade S. Submucosal tunneling endoscopic septum division for Zenker’s diverticulum: a reproducible procedure for endoscopists who perform peroral endoscopic myotomy. Endoscopy. 2017;49:613–4.
Hernández Mondragón OV, Solórzano Pineda MO, Blancas Valencia JM. Zenker’s diverticulum: submucosal tunneling endoscopic septum division (Z-POEM). Dig Endosc. 2018;30:124.
Chang CY, Payyapilli RJ, Scher RL. Endoscopic staple diverticulostomy for Zenker’s diverticulum: review of literature and experience in 159 consecutive cases. Laryngoscope. 2003;113:957–65.
Palanivelu C, Rangarajan M, Senthilkumar R, Velusamy M. Combined thoracoscopic and endoscopic management of mid-esophageal benign lesions: use of the prone patient position : Thoracoscopic surgery for mid-esophageal benign tumors and diverticula. Surg Endosc. 2008;22:250–4.
Cross FS, Johnson GF, Gerein AN. Esophageal diverticula. Associated neuromuscular changes in the esophagus. Arch Surg. 1961;83:525–33.
Chan DSY, Foliaki A, Lewis WG, Clark GWB, Blackshaw GRJC. Systematic review and meta-analysis of surgicaltreatment of Non-Zenker’s Oesophageal diverticula. J Gastrointest Surg. 2017;21:1067–75.
Fernando HC, Luketich JD, Samphire J, Alvelo-Rivera M, Christie NA, Buenaventura PO, Landreneau RJ. Minimally invasive operation for esophageal diverticula. Ann Thorac Surg. 2005;80:2076–80.
Mou Y, Zeng H, Wang Q, Yi H, Liu W, Wen D, Tang C, Hu B. Giant mid-esophageal diverticula successfully treated by per-oral endoscopic myotomy. Surg Endosc. 2016;30:335–8.
Baker ME, Zuccaro G, Achkar E, Rice TW. Esophageal diverticula: patient assessment. Semin Thorac Cardiovasc Surg. 1999;11:326–36.
Abdollahimohammad A, Masinaeinezhad N, Firouzkouhi M. Epiphrenic esophageal diverticula. J Res Med Sci. 2014;19:795–7.
Nehra D, Lord RV, DeMeester TR, Theisen J, Peters JH, Crookes PF, Bremner CG. Physiologic basis for the treatment of epiphrenic diverticulum. Ann Surg. 2002;235:346–54.
Soares R, Herbella FA, Prachand VN, Ferguson MK, Patti MG. Epiphrenic diverticulum of the esophagus. From pathophysiology to treatment. J Gastrointest Surg. 2010;14:2009–15.
Effler DB, Barr D, Groves LK. Epiphrenic diverticulum of the esophagus: surgical treatment. Arch Surg. 1959;79:459–67.
Rice TW, Goldblum JR, Yearsley MM, Shay SS, Reznik SI, Murthy SC, Mason DP, Blackstone EH. Myenteric plexus abnormalities associated with epiphrenic diverticula. Eur J Cardiothorac Surg. 2009;35:22–7; discussion 27.
Fasano NC, Levine MS, Rubesin SE, Redfern RO, Laufer I. Epiphrenic diverticulum: clinical and radiographic findings in 27 patients. Dysphagia. 2003;18:9–15.
Debas HT, Payne WS, Cameron AJ, Carlson HC. Physiopathology of lower esophageal diverticulum and its implications for treatment. Surg Gynecol Obstet. 1980;151:593–600.
Varghese TK, Marshall B, Chang AC, Pickens A, Lau CL, Orringer MB. Surgical treatment of epiphrenic diverticula: a 30-year experience. Ann Thorac Surg. 2007;84:1801–9; discussion 1801-1809.
Altorki NK, Sunagawa M, Skinner DB. Thoracic esophageal diverticula. Why is operation necessary? J Thorac Cardiovasc Surg. 1993;105:260–4.
Fékéte F, Vonns C. Surgical management of esophageal thoracic diverticula. Hepato-Gastroenterology. 1992;39:97–9.
Benacci JC, Deschamps C, Trastek VF, Allen MS, Daly RC, Pairolero PC. Epiphrenic diverticulum: results of surgical treatment. Ann Thorac Surg. 1993;55:1109–13; discussion 1114.
Orringer MB. Epiphrenic diverticula: fact and fable. Ann Thorac Surg. 1993;55:1067–8.
Tapias LF, Morse CR, Mathisen DJ, Gaissert HA, Wright CD, Allan JS, Lanuti M. Surgical management of esophageal epiphrenic diverticula: a transthoracic approach over four decades. Ann Thorac Surg. 2017;104:1123–30.
Kao AM, Arnold MR, Schlosser KA, Siddiqui SL, Prasad T, Colavita PD, Heniford BT. Epiphrenic diverticulum: 20-year single-institution experience. Am Surg. 2018;84:1159–63.
Klaus A, Hinder RA, Swain J, Achem SR. Management of epiphrenic diverticula. J Gastrointest Surg. 2003;7:906–11.
Streitz JM, Glick ME, Ellis FH. Selective use of myotomy for treatment of epiphrenic diverticula. Manometric and clinical analysis. Arch Surg. 1992;127:585–7; discussion 587–88.
Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265–71.
Demeter M, Bánovčin P, Ďuriček M, Kunda R, Hyrdel R. Peroral endoscopic myotomy in achalasia and large epiphrenic diverticulum. Dig Endosc. 2018;30:260–2.
Hudspeth DA, Thorne MT, Conroy R, Pennell TC. Management of epiphrenic esophageal diverticula. A fifteen-year experience. Am Surg. 1993;59:40–2.
Castrucci G, Porziella V, Granone PL, Picciocchi A. Tailored surgery for esophageal body diverticula. Eur J Cardiothorac Surg. 1998;14:380–7.
Jordan PH, Kinner BM. New look at epiphrenic diverticula. World J Surg. 1999;23:147–52.
van der Peet DL, Klinkenberg-Knol EC, Berends FJ, Cuesta MA. Epiphrenic diverticula: minimal invasive approach and repair in five patients. Dis Esophagus. 2001;14:60–2.
Matthews BD, Nelms CD, Lohr CE, Harold KL, Kercher KW, Heniford BT. Minimally invasive management of epiphrenic esophageal diverticula. Am Surg. 2003;69:465–70; discussion 470.
Tedesco P, Fisichella PM, Way LW, Patti MG. Cause and treatment of epiphrenic diverticula. Am J Surg. 2005;190:891–4.
D’Journo XB, Ferraro P, Martin J, Chen L-Q, Duranceau A. Lower oesophageal sphincter dysfunction is part of the functional abnormality in epiphrenic diverticulum. Br J Surg. 2009;96:892–900.
Melman L, Quinlan J, Robertson B, Brunt LM, Halpin VJ, Eagon JC, Frisella MM, Matthews BD. Esophageal manometric characteristics and outcomes for laparoscopic esophageal diverticulectomy, myotomy, and partial fundoplication for epiphrenic diverticula. Surg Endosc. 2009;23:1337–41.
Soares RV, Montenovo M, Pellegrini CA, Oelschlager BK. Laparoscopy as the initial approach for epiphrenic diverticula. Surg Endosc. 2011;25:3740–6.
Fumagalli Romario U, Ceolin M, Porta M, Rosati R. Laparoscopic repair of epiphrenic diverticulum. Semin Thorac Cardiovasc Surg. 2012;24:213–7.
Zaninotto G, Parise P, Salvador R, Costantini M, Zanatta L, Rella A, Ancona E. Laparoscopic repair of epiphrenic diverticulum. Semin Thorac Cardiovasc Surg. 2012;24:218–22.
Rossetti G, Fei L, del Genio G, et al. Epiphrenic diverticula mini-invasive surgery: a challenge for expert surgeons--personal experience and review of the literature. Scand J Surg. 2013;102:129–35.
Bagheri R, Maddah G, Mashhadi MR, Haghi SZ, Tavassoli A, Ghamari MJ, Sheibani S. Esophageal diverticula: analysis of 25 cases. Asian Cardiovasc Thorac Ann. 2014;22:583–7.
Gonzalez-Calatayud M, Targarona EM, Balague C, Rodriguez-Luppi C, Martin AB, Trias M. Minimally invasive therapy for epiphrenic diverticula: systematic review of literature and report of six cases. J Minim Access Surg. 2014;10:169–74.
Hauge T, Johnson E, Sandstad O, Johannessen H-O, Trondsen E. Surgical treatment of epiphrenic oesophageal diverticulum. Tidsskr Nor Laegeforen. 2014;134:1047–50.
Allaix ME, Borraez Segura BA, Herbella FA, Fisichella PM, Patti MG. Is resection of an esophageal epiphrenic diverticulum always necessary in the setting of achalasia? World J Surg. 2015;39:203–7.
Bowman TA, Sadowitz BD, Ross SB, Boland A, Luberice K, Rosemurgy AS. Heller myotomy with esophageal diverticulectomy: an operation in need of improvement. Surg Endosc. 2016;30:3279–88.
Macke RA, Luketich JD, Pennathur A, Bianco V, Awais O, Gooding WE, Christie NA, Schuchert MJ, Nason KS, Levy RM. Thoracic esophageal diverticula: a 15-year experience of minimally invasive surgical management. Ann Thorac Surg. 2015;100:1795–802.
Brandeis AE, Singhal S, Lee TH, Mittal SK. Surgical management of epiphrenic diverticulum: a single-center experience and brief review of literature. Am J Surg. 2018;216:280–5.
Kroh M, Reavis KM, editors. Chap. 18: Endoscopic interventions for the thoracic esophagus: Zenker’s and other diverticula. In: The SAGES manual: operating through the endoscope. Cham: Springer; 2016. https://doi.org/10.1007/978-3-319-24,145-6_18.
Conigliaro R, Frazzoni M, editors. Chap. 11: Endoscopic and surgical management of Zenker’s diverticulum: new approaches. In: Diagnosis and endoscopic management of digestive diseases. Cham: Springer; 2017. https://doi.org/10.1007/978-3-319-42,358-6_11.
Fisichella P, Patti M, editors. Chap. 11: Operations for Zenker’s diverticulum. In: Atlas of esophageal surgery. Cham: Springer; 2015.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Strong, A.T., Ponsky, J.L. (2021). Esophageal Diverticula. In: Zundel, N., Melvin, W.S., Patti, M.G., Camacho, D. (eds) Benign Esophageal Disease. Springer, Cham. https://doi.org/10.1007/978-3-030-51489-1_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-51489-1_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-51488-4
Online ISBN: 978-3-030-51489-1
eBook Packages: MedicineMedicine (R0)