Abstract
Hodgkin’s lymphoma occurs frequently in younger age. Fertility preservation is an important issue as survival rates are very high and some of the treatments are highly gonadotoxic.
Two relevant chemotherapy protocols are performed in Hodgkin’s lymphoma: ABVD and escalated BEACOPP. Some patients also receive local radiotherapy. Risk of amenorrhoea is >20% in women ≥30 years receiving any escalated BEACOPP therapy, and in women <30 years receiving ≥6 cycles escalated BEACOPP. Oligospermia can be detected among male patients independent of chemotherapy regimens and radiation. Azoospermia depends on intensity of the chemotherapy regimen. Therefore, women and men who are treated with these regimens should be advised to undergo fertility preservation therapy. The treatments which can be offered are freezing of oocytes, freezing of ovarian tissue and GnRHa, alone or in combination, as well as freezing of sperm or testicular tissue.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Stage-Dependent Prognosis
Hodgkin’s lymphoma occurs worldwide with an annual incidence of 2–3/100,000, with men being affected slightly more frequently in a ratio of 3:2 [1].
A total of 2490 new cases were recorded in Germany in 2016. Young people are frequently affected and up to ¾ of patients are under 60 years of age at diagnosis [2]. For young patients, family planning is often not yet complete at the time of the initial diagnosis or has not been addressed at all. About 30% of women counselled in the FertiPROTEKT network suffer from lymphoma, predominantly Hodgkin’s lymphoma [3].
In recent decades, Hodgkin’s lymphoma has developed from an incurable disease to one of the best treatable oncological diseases in adulthood with outstanding 5-year survival rates (Tables 1 and 2).
In a large retrospective study by Glimelius et al [4], the outcome of a total of 1947 Swedish Hodgkin’s lymphoma patients diagnosed in the period 1992–2009 and aged between 15 and 59 years at diagnosis was recorded. The age distribution in the patient-collective showed the expected age distribution with 36.4% in the youngest age group of 18–29 years, 21.2% aged 30–39 years and 14.2% aged 40–49 years. The outcome in terms of age is shown with the 5- and 15-year (relative) survival rates and shows a clear age-dependent outcome. The 15-year survival rate is 94% in the youngest age group of 18–29 years, and 87% in the 40–49 years age group.
This is also the result of an evaluation by Pulte et al. [5], in which the 5-year (relative) survival of a total of 5300 patients suffering from Hodgkin’s lymphoma in Germany from 1997 to 2006 is evaluated. The 5-year survival rate also decreased with increasing age.
As a limitation, it should be mentioned that there were major therapeutic advances in the studied period of 18 or 10 years, and that the patient collective was treated with various radiation and chemotherapy regimens. The cure rate also depends on the stage, the response to therapy and the risk factors (Table 2). Overall, it is between 80 and 95%, and the number of long-term survivors is steadily increasing [6].
After staging, patients are divided into three risk groups using the Ann Arbor classification (describes the involvement of the lymph node regions) and the presence or absence of certain risk factors:
-
1.
Clinical stages (CS) I-IIA and B without risk factors are considered early, prognostically favourable stages.
-
2.
CS IA and B and CS IIA with ≥1 risk factor or CS IIB with the risk factors: accelerated blood sedimentation rate and/or ≥3 affected lymph node areas are listed as an intermediate patient group.
-
3.
CS II B with the risk factors: large mediastinal tumour mass and/or extranodal tumour foci as well as CS III/IV are classified as advanced stages.
According to the German Swiss Austrian S3 guideline “Diagnosis, treatment and follow-up care of Hodgkin’s lymphoma in adult patients” from June 2018, the stage-adapted treatment recommendation for patients ≤60 years of age is as follows [10]:
-
In the early stages, two cycles of ABVD (adriamycin, bleomycin, vinblastine, dacarbazine) and then involved field (IF)—RT with 20 Gray are administered.
-
In the intermediate stages, the recommended treatment regimen consists of combination chemotherapy consisting of two cycles of escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisolone) and two ABVD (2 + 2) cycles, as well as subsequent IF-RT with 30 Gray.
-
In advanced stages, intermediate staging with PET/CT is carried out after two BEACOPP cycles. PET-negative patients receive two additional escalated BEACOPP cycles, for a total of four cycles. PET/CT positive patients receive four further escalated BEACOPP cycles, for a total of six cycles. If PET-positive residual lymphoma tissue of ≥2.5 cm is still present after the end of chemotherapy, localized radiotherapy with 30 Gray is carried out.
In the analyses of the HD13 trial by the German Hodgkin’s Study Group (GHSG) for the early stages, HD14 for the intermediate stages and HD15 and HD18 for the advanced stages, the current standard treatments showed excellent outcome results in all stages. The values collected refer to a follow-up period of 5 years, in which the following outcome criteria are recorded: “freedom from treatment failure” (FFTF), “progression-free survival” (PFS), which records patients who survived 5 years progression-free/relapse-free and 5-year overall survival (OS), which represents overall survival after 5 years. There was a stage-dependent outcome with a 5-year PFS and OS of 93.5% and 97.6% in the early stages versus 90.3% and 95.3% in the advanced stages.
Treatment Gonadotoxicity
Chemotherapy and radiotherapy always carry a risk of gonadotoxicity. The chemotherapy regimens administered for Hodgkin’s lymphoma show dose- and substance-dependent gonadotoxicity, with the more intensive escalated BEACOPP regime having a higher gonadotoxic effect than the ABVD regimen. Of the active ingredients administered, the alkylating agents procarbazine and cyclophosphamide play a decisive role [11,12,13].
Data on fertility after Hodgkin’s lymphoma treatment were obtained in two studies by the German Hodgkin’s Study Group (GHSG). The results published by Behringer et al. [14] looked at 405 patients who were under 40 years of age at first diagnosis and were treated in the third study generation (HD7–nine studies) between 1994 and 1998. The more recent data from Behringer et al. [15] were obtained from a follow-up of a total of 1323 male and female patients from the fifth study generation (HD13–15 studies).
Treatment Gonadotoxicity in Women
The publication by Behringer et al. [14], with a mean follow-up time of 3.2 years, showed that 51.4% of patients who received eight cycles of escalated BEACOPP suffered from amenorrhea. The most frequent reports of amenorrhea were from women in advanced stages, when the age at initial diagnosis was ≥30 years and when no oral contraceptives were taken during chemotherapy.
With a mean follow-up time of 46 months in a total of 562 female Hodgkin’s lymphoma survivors who were <40 years old at initial diagnosis, the evaluation by Behringer et al. [15] also showed a clear difference with regard to age at initial diagnosis (</≥30 years) and the treatment regimen administered (ABVD or escalated BEACOPP) (Tables 3 and 4).
The measured values for follicle-stimulating hormone (FSH) and anti-Müllerian hormone (AMH) were in women ≥30 years and were significantly worse after treatment with escalated BEACOPP, consistent with damage to the ovarian reserve. The occurrence of regular menstruation was reported by more than 90% of women after early-stage therapy. In most cases, it started within the first year after treatment. A recent paper by Weibull et al. showed similar conclusions [16]. Fortunately, a comparable birth rate between the examined 449 relapse-free Hodgkin lymphoma patients (all stages) and the normal population was shown 3 years after diagnosis. The birth rate was 22.5% in the group of former patients.
Data from Behringer et al. [15] showed a longer time for ovarian function recovery after treatment with six to eight cycles of escalated BEACOPP, which was strongly dependent on the age of the patient at initial diagnosis. The risk of persistent amenorrhoea after 4 years was 25% in 25-year-old patients, while it increased to 50% in 30-year-old patients.
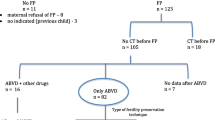
Table 5 summarises the risks of amenorrhoea after a mean follow-up period of 46 months after chemotherapy [15] and thus the risk of POI depending on the chemotherapy used and the age of the woman and, derived from this, the recommendations for the implementation of a fertility preservation measure are shown in Fig. 1.
Treatment Gonadotoxicity in Men
Radiochemotherapy mainly affects spermatogenesis in men, which is often limited even before the start of therapy, especially in the advanced stages [17]. The effects of treatment on testosterone production, however, are small. In the study data collected by Behringer et al. [15], the mean values for testosterone were within the limits after all treatment intensities.
Behringer et al. [15] examined hormone parameters depending on the chemotherapy regimen (Table 6). Seven hundred and sixty-one male Hodgkin’s lymphoma survivors who were younger than 50 years at the time of initial diagnosis were examined after a mean follow-up period of 48 months. Inhibin B and FSH levels correlated significantly with the intensity of chemotherapy (Table 6). After treatment in the early stages, 50% of the men showed an Inhibin B/FSH ratio corresponding with definite fertility (inhibin B/FSH ratio > 23.5 ng/U). However, the highest proportion of inhibin B/FSH values, which correlated with oligospermia, was found after six to eight cycles of escalated BEACOPP therapy (88.8%).
Paoli et al. [18] examined sperm parameters and azoospermia rates depending on different chemotherapy regimens (Table 7). Chemotherapy according to the ABVD regimen led to a significant reduction in sperm concentration, which normalised within 24 months. Other chemotherapy regimens and radiotherapy often lead to long-term azoospermia (Table 6). Although the cohorts in the study by Paoli et al. are small, they show a clear correlation between the intensity of the chemotherapy regimen and sometimes long-term azoospermia rates.
Table 7 summarises the risks of long-term azoospermia 2 or 3 years after treatment [18], depending on the chemotherapy used. Recommendations for the implementation of a fertility-preservation measure are shown in Fig. 2.
Probability of Gonadal Metastasis
The data available for estimating gonadal metastasis are limited.
Several studies have systematically attempted to detect tumour cells in cryopreserved ovarian tissue. Table 8 shows the studies known to the authors and published to date. Overall, there is almost never any evidence of Hodgkin’s lymphoma cells in ovarian tissue, even in higher grade tumour stages. However, a case report with ovarian involvement of the lymphoma has been published [21]. Because of this case report and since it is never possible to examine the tissue to be transplanted, only an ovarian biopsy, a tissue sample should be examined histologically during cryopreservation or at the latest before transplantation.
Based on these examinations and the large number of ovarian tissue transplants without evidence of recurrence, Hodgkin’s lymphoma was classified as a disease with a low risk of metastasis (see chapter “Removal of Ovarian Tissue”, Table 2).
Effectiveness and Risks of Fertility Preservation
Effectiveness
Women and men with Hodgkin’s lymphoma are usually of a younger age. Women therefore usually have a good ovarian reserve and oocyte quality, and fertility-protective measures such as cryopreservation of ovarian tissue and oocytes are also very effective.
However, there are studies which suggest a lower ovarian reserve in women with Hodgkin’s lymphoma. The number of oocytes obtained during ovarian stimulation is 1.2–1.4 times lower in women with Hodgkin’s lymphoma [3, 23]. Lawrenz et al. [24] demonstrated a 35% lower serum concentration of AMH in women with Hodgkin’s lymphoma, which explains the lower response to ovarian stimulation.
However, it is questionable whether the lower AMH concentration also leads the fertility-preservation measures being less effective. If oocytes are to be cryopreserved, the stimulation dose can be adjusted because the women are usually younger. If ovarian tissue is cryopreserved, the AMH concentration plays a minor role. What is important, however, is the actual ovarian reserve, i.e. the density of primordial follicles, which—in contrast to the AMH concentration—is not reduced in women with Hodgkin’s lymphoma [25].
It should be noted that sperm quality is reduced in men with lymphomas. Caponecchia et al. [17] found an average sperm concentration of 34.5 million/mL in men with lymphoma (Hodgkin’s and non-Hodgkin’s) compared to fertile men with 46.5 million/mL. However, motility and morphology were not affected.
Risks
Fertility-preservation measures are usually only associated with minor risks in patients with Hodgkin’s lymphoma. The tumour cells are not hormone-dependent and the time available for carrying out all procedures is usually sufficient. The risk of lymphoma cells appearing in the gonadal tissue also appears to be low, as no tumour cells have been detected to date.
It should be noted, however, that Hodgkin’s lymphomas are often accompanied by mediastinal involvement, which means that intubation and extubation can be risky if the patient undergoes laparoscopy. In these cases, ovarian tissue should only be removed if the anaesthetists assess the sedation and intubation risk as being low. Alternatively, hormonal stimulation for cryopreservation of unfertilised or fertilised oocytes may be considered, as intubation anaesthesia is not required for follicle puncture.
References
Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116(19):3724–34. https://doi.org/10.1182/blood-2010-05-282632.
Robert-Koch-Institut (2016). 3.28 Morbus Hodgkin. Krebs Deutschl. 122–5.
von Wolff M, Bruckner T, Strowitzki TGA. Fertility preservation: ovarian response to freeze oocytes is not affected by different malignant diseases-an analysis of 992 stimulations. J Assist Reprod Genet. 2018;35(9):1713–9.
Glimelius I, Ekberg S, Jerkeman M, Chang ET, Björkholm M, Andersson TML, et al. Long-term survival in young and middle-aged Hodgkin lymphoma patients in Sweden 1992-2009-trends in cure proportions by clinical characteristics. Am J Hematol. 2015;90(12):1128–34. https://doi.org/10.1002/ajh.24184.
Pulte D, Jansen L, Gondos A, Emrich K, Holleczek B, Katalinic A, et al. Improved population level survival in younger Hodgkin lymphoma patients in Germany in the early twenty-first century. Br J Haematol. 2014;164(6):851–7. https://doi.org/10.1111/bjh.12722.
Skoetz N, Trelle S, Rancea M, Haverkamp H, Diehl V, Engert A, et al. Effect of initial treatment strategy on survival of patients with advanced-stage Hodgkin’s lymphoma: a systematic review and network meta-analysis. Lancet Oncol. 2013;14(10):943–52. https://doi.org/10.1016/S1470-2045(13)70341-3.
Behringer K, Goergen H, Hitz F, Zijlstra JM, Greil R, Markova J, et al. Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin’s lymphoma (GHSG HD13): an open-label, randomised, non-inferiority trial. Lancet. 2014;385(9976):1418–27. https://doi.org/10.1016/S0140-6736(14)61469-0.
Von Tresckow B, Plütschow A, Fuchs M, Klimm B, Markova J, Lohri A, et al. Dose-intensification in early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin study group HD14 trial. J Clin Oncol. 2012;30(9):907–13. https://doi.org/10.1200/JCO.2011.38.5807.
Engert A, Haverkamp H, Kobe C, Markova J, Renner C, Ho A, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379(9828):1791–9. https://doi.org/10.1016/S0140-6736(11)61940-5.
S3-Leitlinie Diagnostik, Therapie und Nachsorge des Hodgkin Lymphoms bei erwachsenen. Patienten. 2019: 1–183.
van Beek RD, Smit M, van den Heuvel-Eibrink MM, de Jong FH, Hakvoort-Cammel FG, van den Bos C, et al. Inhibin B is superior to FSH as a serum marker for spermatogenesis in men treated for Hodgkin’s lymphoma with chemotherapy during childhood. Hum Reprod. 2007;22(12):3215–22. https://doi.org/10.1093/humrep/dem313.
Kiserud CE, Fosså A, Bjøro T, Holte H, Cvancarova M, Fosså SD. Gonadal function in male patients after treatment for malignant lymphomas, with emphasis on chemotherapy. Br J Cancer. 2009;10:455–63. https://doi.org/10.1038/sj.bjc.6604892.
Kulkarni S, et al. Gonadal function following ABVD therapy for Hodgkin‘ s disease. Am J Clin Oncol. 1997;20(4):354–7.
Behringer K, Breuer K, Reineke T, May M, Nogova L, Klimm B. Secondary amenorrhea after Hodgkin’s lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin’s lymphoma study group. J Clin Oncol. 2005;23(30):7555–64. https://doi.org/10.1200/JCO.2005.08.138.
Behringer K, Mueller H, Goergen H, Thielen I, Eibl AD, Stumpf V, et al. Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin study group HD13 to HD15 trials. J Clin Oncol. 2013;31(2):231–9. https://doi.org/10.1200/JCO.2012.44.3721.
Weibull CE, Eloranta S, Smedby KE, Bjorkholm M, Kristinsson SY, Johansson AL, et al. Pregnancy and the risk of relapse in patients diagnosed with Hodgkin lymphoma. J Clin Oncol. 2016;34(4):337–44. https://doi.org/10.1200/JCO.2015.63.3446.
Caponecchia L, Cimino G, Sacchetto R, Fiori C, Sebastianelli A, Salacone P, et al. Do malignant diseases affect semen quality? Sperm parameters of men with cancers. Andrologia. 2016;48(3):333–40.
Paoli D, Rizzo F, Fiore G, Pallotti F, Pulsoni A, Annechini G, et al. Spermatogenesis in Hodgkin’s lymphoma patients: a retrospective study of semen quality before and after different chemotherapy regimens. Hum Reprod. 2016;31(2):263–72. https://doi.org/10.1093/humrep/dev310.
Seshadri T, Gook D, Lade S, Spencer A, Grigg A, Tiedemann K, et al. Lack of evidence of disease contamination in ovarian tissue harvested for cryopreservation from patients with Hodgkin lymphoma and analysis of factors predictive of oocyte yield. Br J Cancer. 2006;94(7):1007–10. https://doi.org/10.1038/sj.bjc.6603050.
Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra’anani H, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23(5):1007–13. https://doi.org/10.1093/humrep/den055.
Bittinger SE, Nazaretian SP, Gook DA, Parmar C, Harrup RA, Stern CJ. Detection of Hodgkin lymphoma within ovarian tissue. Fertil Steril. 2011;95(2):803e3–6. https://doi.org/10.1016/j.fertnstert.2010.07.1068.
Hoekman EJ, Smit VTHBM, Fleming TP, Louwe LA, Fleuren GJ, CGJM H. Searching for metastases in ovarian tissue before autotransplantation: a tailor-made approach. Fertil Steril. 2015;103(2):469–77. https://doi.org/10.1016/j.fertnstert.2014.11.001.
Lekovich J, Lobel ALS, Stewart JD, Pereira N, Kligman I, Rosenwaks Z. Female patients with lymphoma demonstrate diminished ovarian reserve even before initiation of chemotherapy when compared with healthy controls and patients with other malignancies. J Assist Reprod Genet. 2016;33(5):657–62. https://doi.org/10.1007/s10815-016-0689-1.
Lawrenz B, Fehm T, Von Wolff M, Soekler M, Huebner S, Henes J, et al. Reduced pretreatment ovarian reserve in premenopausal female patients with Hodgkin lymphoma or non-Hodgkin-lymphoma—evaluation by using antimüllerian hormone and retrieved oocytes. Fertil Steril. 2012;98(1):141–4. https://doi.org/10.1016/j.fertnstert.2012.04.021.
Liebenthron J, Reinsberg J, van der Ven K, Saenger N, Kruessel JS, von Wolff M. Serum anti-Mullerian hormone concentration and follicle density throughout reproductive life and in different diseases—implications in fertility preservation. Hum Reprod. 2019;34(12):2513–22. https://doi.org/10.1093/humrep/dez215.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bürkle, C., von Wolff, M., Behringer, K. (2020). Hodgkin’s Lymphoma. In: von Wolff, M., Nawroth, F. (eds) Fertility Preservation in Oncological and Non-Oncological Diseases. Springer, Cham. https://doi.org/10.1007/978-3-030-47568-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-47568-0_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-47567-3
Online ISBN: 978-3-030-47568-0
eBook Packages: MedicineMedicine (R0)