Abstract
Purpose
The purpose of this study is to investigate if female patients with lymphoma demonstrate diminished ovarian reserve prior to initiation of the lymphoma treatment.
Methods
Sixty-four patients with newly diagnosed lymphoma undergoing controlled ovarian hyperstimulation for fertility preservation were compared with 365 healthy controls undergoing elective oocyte cryopreservation (controlled ovarian hyperstimulation (COH)) and 128 patients with other types of malignancy prompting fertility preservation. The data of all lymphoma patients, all elective, and all the patients with other types of malignancy who met the inclusion criteria and underwent COH for fertility preservation during the study period were retrospectively analyzed. Primary outcomes included serum anti-Müllerian hormone (AMH) levels (ng/mL) and antral follicle count (AFC).
Results
Patients in the lymphoma group demonstrated significantly lower AMH levels and AFC and had less oocytes harvested and cryopreserved when compared to healthy controls as well as patients with other malignancies.
Conclusion
Patients with lymphoma demonstrate diminished ovarian reserve when compared with healthy controls and patients with other malignancies. This should be taken into consideration when deciding on the dose for COH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is estimated that approximately 80,000 new cases of Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL) will be diagnosed in the USA in 2015 [1]. Due to recent treatment advances, 5-year survival rate is approximately 70 % [1]. Treatment for these conditions commonly begins with chemotherapy using alkylating agents and total body irradiation, both of which carry a substantial risk of premature gonadal failure due to their gonadotoxicity [2, 3].
In postpubertal women of reproductive age diagnosed with a lymphoma in whom treatment can be delayed, the International Society for Fertility Preservation recommends cryopreservation of oocytes and/or embryos, as appropriate to their circumstances, including religious beliefs, prior to initiation of treatment [4] via controlled ovarian stimulation, egg retrieval, and in vitro fertilization (IVF) when applicable. An oocyte/embryo cryopreservation cycle can require treatment to be postponed for 2–6 weeks.
Several studies have demonstrated decreased sperm quality in men diagnosed with both HL and NHL, even before initiation of chemotherapy [5, 6]. Moreover, the literature suggests diminished ovarian reserve in patients with inherited bone marrow failure syndromes, which are associated with the development of lymphoma and leukemia [7]. We sought to investigate if female patients with lymphoma undergoing controlled ovarian hyperstimulation (COH) for fertility preservation prior to initiation of chemotherapy demonstrate gonadal dysfunction similar to men with these diseases.
Materials and methods
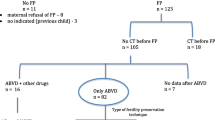
We performed a retrospective chart analysis of all lymphoma patients who underwent oocyte cryopreservation at the Ronald O. Perelman and Claudia Cohen Center for Reproductive Medicine from January 2010 until September 2013. Patients were excluded if they had a history of oophorectomy/ovarian cystectomy, documented endometriosis, prior chemotherapy, suffered from any long-standing chronic medical conditions, or were older than 40 years of age. The characteristics of the first cycle at our center were included in the final analysis for each patient (mostly because the lymphoma as well as the patients with other malignancies only underwent one oocyte cryopreservation cycle before the initiation of chemotherapy).
Sixty-four patients met the study criteria: 37 with HL and 27 with NHL. This study group was compared with a control group composed of 365 healthy women who underwent elective oocyte cryopreservation during the study period. Finally, in order to control for the previously suggested potential negative effect of malignant disease on COH response in general [8, 9], the study group was also compared with 128 patients with newly diagnosed non-lymphoma malignant disease (cancer control group) who simultaneously underwent oocyte cryopreservation prior to initiation of cancer treatment (Table 1). Primary outcomes included anti-Müllerian hormone (AMH) levels at the beginning of cycle, antral follicle count (AFC), and the number of harvested and cryopreserved oocytes. Secondary outcomes included the total amount of gonadotropins utilized for stimulation and the number of days of stimulation.
Protocols for stimulation and oocyte retrieval were conducted according to previously described standard protocols [10]. Pure human chorionic gonadotropin (hCG), pure leuprolide acetate, or the combination of the two (dual trigger) was used for final oocyte maturation based on estradiol levels and patient’s weight. Transvaginal oocyte retrieval under ultrasound guidance was performed 35 h after final oocyte maturation trigger administration. In the interest of time, especially among lymphoma patients and patients with other malignancies, COH was occasionally initiated in the luteal phase of the cycle, while in majority of patients, we initiated COH on cycle day 2. Only mature oocytes in meiosis II were cryopreserved, using vitrification.
Serum AMH levels were determined using ELISA (Gen II, Beckmann Coulter, NJ) and expressed in nanograms per milliliter. Intra- and inter-assay coefficients of variation were <5.5 and <9.0 %, respectively. Progesterone levels on the day of the initiation of COH were used to determine the phase of the cycle at the start (progesterone level <1.5 ng/mL indicated a follicular and ≥1.5 ng/mL a luteal phase start).
Student’s T test was used for comparison of continuous variables. Mann-Whitney U was used for comparison of non-parametric data. Chi-square and Fisher’s exact tests were used for comparison of categorical variables as indicated. Logistic regression model was used to control for age. P value <0.05 was considered statistically significant for all comparisons. The Weill Cornell Medical College institutional review board approved this study.
Results
Patients in the lymphoma group were significantly younger than the patients in the healthy control group (30.5 years (range 17–37, interquartile range (IQR) 28–34) vs. 37 years (range 20–40, IQR 35–39), P < 0.001), but had a comparable BMI and did not differ in terms of type of trigger used for final oocyte maturation (Table 2). A greater proportion of patients in the lymphoma group initiated stimulation in the luteal phase of the menstrual cycle (6.25 vs. 1.09 %, P = 0.04), and they were less commonly to use oral contraceptive pills (OCPs) for birth control immediately prior to initiation of COH (14.05 vs. 30.13 %, P = 0.006).
Patients in the lymphoma group demonstrated significantly lower baseline AMH levels when compared to the healthy controls (1.08 ± 0.74 ng/mL vs. 2.03 ± 1.93, P < 0.001) (Table 2). AFC was also decreased in the study group (9.41 ± 4.77 vs. 10.29 ± 3.74, P = 0.09; crude OR = 0.94; 95 % CI = 0.87–1.01), and this difference was statistically significant when adjusted for age (P = 0.01, adjusted OR = 0.87, 95 % CI = 0.82–0.95). The number of harvested and frozen oocytes was also significantly lower in the study group when adjusted for age (P = 0.03, adjusted OR = 0.94, 95 % CI = 0.89–0.97, and P = 0.01, adjusted OR = 0.72, 95 % CI = 0.66–0.77 respectively). Similar amounts of gonadotropins were used for a similar number of days (Table 2).
Lymphoma patients were younger than the patients with other malignancies (30.5 years (IQR 28–34) vs. 33 years (IQR 30–36), P = 0.02). The groups were otherwise comparable in terms of trigger used for final oocyte maturation, as well as proportion of patients on oral contraceptive pills (OCPs) prior to COH initiation and the number of those who initiated COH in the luteal phase (Table 3). The study group demonstrated significantly lower AMH levels (1.08 ± 0.74 vs. 2.21 ± 1.62 ng/mL, P < 0.001) and AFC than patients with other malignancies (9.41 ± 4.77 vs. 12.03 ± 4.26, P < 0.001), as well as lower yield of retrieved and frozen oocytes (Table 3). The study group had a shorter mean duration of stimulation (10.03 ± 2.20 vs. 10.92 ± 2.17 days, P = 0.01).
Discussion
Our study shows that the patients with lymphoma demonstrate lower baseline ovarian reserve (in the form of lower AMH levels and AFC) even before the initiation of chemotherapy, as well as poorer response to COH when similar amounts of gonadotropins are used when compared to healthy controls and patients with a different type of malignancy.
Our findings are consistent with those of Lawrenz et al. [11] who demonstrated decreased AMH levels and harvested oocyte yield in lymphoma patients. AFC was not used as a measurement for ovarian reserve in this study. On the other hand, Decanter et al. [12] demonstrated normal AMH levels in lymphoma patients before initiation of chemotherapy when compared with previously reported levels in healthy ovulatory women of reproductive age. While Beringer et al. also described adequate pre-treatment ovarian reserve in lymphoma patients, that study used the proportion of patients having children [13] and regular menstrual bleeding [14] prior to chemotherapy as indirect measures of ovarian reserve, which might not necessarily reflect its diminished state. It has been previously shown that recent OCP use, especially if prolonged, can be associated with decreased AMH levels and AFC when compared to non-users [15, 16]. Paradoxically, our healthy control group had a higher proportion of OCP users than the study group, yet demonstrated significantly higher mean AMH levels and AFC, after adjustment for age. There was the exact opposite finding, even though not statistically significant, when lymphoma patients were compared with patients with other malignancies (more OCPs users in the lymphoma group). Finally, one should keep in mind that the AMH levels tend to vary with age, the highest being in infancy, then increasing from adolescence to mid-20s when they peak after which point they start to inversely correlate with age [17, 18]. This phenomenon has been taken into account when analyzing AMH levels in our lymphoma group in comparison to both healthy controls and patients with other types of cancer; however, there was only one adolescent patient in our lymphoma group (age 17) and a total of eight patients younger than 25, and two 17-year-old patients in our cancer control group (total of 4 patients younger than 25) which would not account for this difference between the groups. Besides, it would also not explain the low AFC in the lymphoma group and the overall lower response to stimulation. So, even though in the current study there were not many adolescents, the fact that AMH levels tend to be lower in adolescence and increase towards mid-20s should be kept in mind when treating lymphoma patients knowing that a great proportion of them will be adolescents at the time of diagnosis.
Even though there was a higher proportion of luteal phase starts in the lymphoma group compared to controls, it was comparable to the group with a different type of malignancy. This may not be relevant, as the body of evidence suggests no difference in the number of retrieved and mature oocytes or the cycle length between the luteal and follicular phase COH initiation [19–22], and we therefore have no reason to believe that this difference would account for poorer response to COH in the lymphoma group.
Suppression of hypothalamic-pituitary-gonadal axis and consequent hypopituitarism has been reported in patients with lymphoma, however more commonly with either intracranial (specifically pituitary) involvement [23, 24] or with intravascular lymphocytosis [25, 26]. While none of our patients had either of these two clinical entities, we analyzed LH levels on the start day of their IVF cycle and found that the lymphoma patients had higher overall LH levels than healthy controls and that there were no differences between the lymphoma and the cancer control groups. The difference in the first comparison might be due to the fact that more patients in the control group were on OCPs just prior to initiation of the controlled ovarian stimulation and were less likely to start the stimulation in the luteal phase. LH levels in our lymphoma patients did not demonstrate pituitary suppression that could theoretically be attributable to lower response to stimulation.
The duration of ovarian stimulation was longer in the cancer control group. Seventy-seven percent of our cancer control group were breast cancer patients, of which the vast majority was treated using letrozole with concomitant administration of gonadotropins to reduce peak estradiol levels during stimulation [27], thus increasing the safety of this protocol [28]. Letrozole has been associated with increased rate of oocyte immaturity [27, 29], and increasing the follicular diameter trigger threshold to approximately 20 mm has been suggested. This effect probably explains the longer COH duration in this group when compared to lymphoma patients. Additionally, we would not expect the letrozole protocol to be the cause of better response to COH in the cancer group, as at least one retrospective study of 16 IVF centers observed significantly lower oocyte yield in these patients [30].
Sklavos et al. [7] have recently demonstrated diminished ovarian reserve in patients with inherited bone marrow failure syndromes including Fanconi anemia, dyskeratosis congenita, and Diamond-Blackfan anemia, which are all associated with increased risk of leukemia and lymphoma. While the information regarding the presence of these pre-existing conditions in our lymphoma patients was not available to us, it is possible that persons predisposed to bone marrow diseases have concomitant (possibly genetic) predisposition to diminished ovarian reserve. Even though controversial, Johnson at al [31] demonstrated the existence of germline stem cells in the postnatal mammalian ovary, and moreover, the same group identified bone marrow as a potential source of these stem cells [32]. This could potentially explain the mechanism of diminished ovarian reserve in these patients. Alternatively, the disease may be associated with the production of gonadotoxic cytokines [6, 33] or infiltration of ovarian tissue with inflammatory or malignant cells, even though primary ovarian lymphoma and secondary ovarian involvement are uncommon due to lack of lymphatic tissue in the ovary [34, 35].
A major weakness of our study is its retrospective nature. Our groups were also not age-matched, as a great proportion of the lymphoma patients were in their late teens or early twenties, and attempting to have an age-matched control would result in the exclusion of these patients and diminishing of our sample size. We used logistic regression to control for age instead. It would also be interesting to see if AMH levels were decreased in our lymphoma patients prior to diagnosis of malignancy; however, the majority of these patients did not have fertility issues and ovarian reserve testing in the past. That information would help to determine whether diminished ovarian reserve in this population results from a genetic, congenital, or early-acquired predisposition prior to lymphoma, or if it is directly caused by it. Future research should focus on prospectively following patients with diminished ovarian reserve and determining the proportion of those who later on develop lymphoma. Lastly, given possible diminished ovarian reserve as well as reduced response to COH in these patients, it would be reasonable to (a) consider more generous gonadotropin dosing, however with great deal of caution while weighing against the risk of primary ovarian hyperstimulation syndrome, (b) insist on fertility cryopreservation earlier rather than later regardless of the magnitude of gonadotoxicity of chemotherapy planned, as well as (c) setting up patients’ expectations for the cycle outcome.
References
U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2012 incidence and mortality Web-based report. Atlanta: Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2015. Available at: www.cdc.gov/uscs.
Fleischer RT, Vollenhoven BJ, Weston GC. The effects of chemotherapy and radiotherapy on fertility in premenopausal women. Obstet Gynecol Surv. 2011;66(4):248–54.
Wo JY, Viswanathan AN. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys. 2009;73(5):1304–12.
Jadoul P, Kim SS, ISFP Practice Committee. Fertility considerations in young women with hematological malignancies. J Assist Reprod Genet. 2012;29(6):479–87.
Viviani S, Ragni G, Santoro A, Perotti L, Caccamo E, Negretti E, et al. Testicular dysfunction in Hodgkin's disease before and after treatment. Eur J Cancer. 1991;27(11):1389–92.
Rueffer U, Breuer K, Josting A, Lathan B, Sieber M, Manzke O, et al. Male gonadal dysfunction in patients with Hodgkin's disease prior to treatment. Ann Oncol. 2001;12:1307–11.
Sklavos MM, Stratton P, Giri N, Alter BP, Savage SA, Pinto LP. Reduced serum levels of anti-Müllerian hormone in females with inherited bone marrow failure syndromes. J Clin Endocrinol Metabol. 2015;100:E197–203.
Quintero RB et al. Ovarian stimulation for fertility preservation in patients with cancer. Fertil Steril. 2010;93:865–8.
Johnson LN et al. Response to ovarian stimulation in patients facing gonadotoxic therapy. Reprod Biomed Online. 2013;26:337–44.
Davis OK, Rosenwaks Z. Superovulation strategies for assisted reproductive technologies, 2001.19:207-12.
Lawrenz B, Fehm T, von Wolff M, Soekler M, Huebner S, Henes J, et al. Reduced pretreatment ovarian reserve in premenopausal female patients with Hodgkin lymphoma or non-Hodgkin-lymphoma—evaluation by using antimüllerian hormone and retrieved oocytes. Fertil Steril. 2012;98(1):141–4.
Decanter C, Morschhauser F, Pigny P, Lefebvre C, Gallo C, Dewailly D. Anti-Müllerian hormone follow-up in young women treated by chemotherapy for lymphoma: preliminary results. Reprod Biomed Online. 2010;20:280–5.
Behringer K, Mueller H, Goergen H, Thielen I, Eibl AD, Stumpf V, et al. Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin Study Group HD13 to HD15 trials. J Clin Oncol. 2013;31(2):231–9.
Behringer K, Breuer K, Reineke T, May M, Nogova L, Klimm B, et al. Secondary amenorrhea after Hodgkin's lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin's lymphoma study group. J Clin Oncol. 2005;23:7555–64.
Johnson LN, Sammel MD, Dillon KE, Lechtenberg L, Schanne A, Gracia CR. Antimüllerian hormone and antral follicle count are lower in female cancer survivors and healthy women taking hormonal contraception. Fertil Steril. 2014;102:774–81.
Bentzen JG, Forman JL, Pinborg A, Lidegaard Ø, Larsen EC, Friis-Hansen L, et al. Ovarian reserve parameters: a comparison between users and non-users of hormonal contraception. Reprod Biomed Online. 2012;25:612–9.
Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ, et al. Serum anti-Müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97(12):4650–5.
Hagen CP, Aksglaede L, Sørensen K, Main KM, Boas M, Cleemann L, et al. Serum levels of anti-Müllerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. J Clin Endocrinol Metab. 2010;95(11):5003–10.
Checa MA, Brassesco M, Sastre M, et al. Random-start GnRH antagonist for emergency fertility preservation: a self-controlled trial. Int J Womens Health. 2015;7:219–25.
Kuang Y, Hong Q, Chen Q, et al. Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles. Fertil Steril. 2014;101(1):105–11.
Nayak SR, Wakim AN. Random-start gonadotropin-releasing hormone (GnRH) antagonist-treated cycles with GnRH agonist trigger for fertility preservation. Fertil Steril. 2011;96(1):e51–4.
Ozkaya E, San Roman G, Oktay K. Luteal phase GnRHa trigger in random start fertility preservation cycles. J Assist Reprod Genet. 2012;29(6):503–5.
Moore JA, Moore MB, Samaniego F, Pinnix CC, Moore Jr DF. Small lymphocytic lymphoma presenting with hypopituitarism. Am J Med. 2016;129(1):e9–e10.
Valeros KA, Khoo E. Anterior panhypopituitarism in diffuse large B-cell stage IV lymphoma. J Clin Neurosci. 2014;21(8):1464–6.
Schleinitz N, Bernit E, Mazodier K, Charbonnier A, Horchowski N, Andrac-Meyer L, et al. Two cases of intravascular lymphomatosis disclosing with hypopituitarism. Haematologica. 2002;87(6):ECR21.
Mourand I, Menjot de Champfleur N, Bauchet L, Dumontel T, Corlobé A, Quittet P, et al. Reversible hypothalamic-pituitary axis involvement in a patient with intravascular lymphomatosis. J Neuroradiol. 2014;41(5):360–2.
Shapira M, Raanani H, Meirow D. IVF for fertility preservation in breast cancer patients—efficacy and safety issues. J Assist Reprod Genet. 2015 Jul 1.
Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26:2630–5.
Kim JH et al. Efficacy of random-start controlled ovarian stimulation in cancer patients. J Korean Med Sci. 2015;30:290–5.
Revelli A et al. Is letrozole needed for controlled ovarian stimulation in patients with estrogen receptor-positive breast cancer? Gynecol Endocrinol. 2013;29:993–6.
Johnson J, Canning J, Keneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–50.
Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–15.
Hill JA, Haimovici D, Politich JA, Anderson DJ. Effects of soluble products of activated lymphocytes on macrophages (lymphokines and monokines) on human sperm motion parameters. Fertil Steril. 1987;47:460–5.
Hu R, Miao M, Zhang R, Li Y, Li J, Zhu K, et al. Ovary involvement of diffuse large B cell lymphoma. Am J Case Rep. 2012;13:96–8.
Chorlton I, Norris HJ, King FM. Malignant reticuloendothelial disease involving the ovary as a primary manifestation: a series of 19 lymphomas and 1 granulocytic sarcoma. Cancer. 1974;34(2):397–407.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Weill Cornell Medical College institutional review board approved this study.
Conflict of interest
The authors declare that they have no competing interests.
Financial support
Institutional
Additional information
Capsule Female patients with lymphoma demonstrate diminished ovarian reserve when compared with healthy controls as well as patients with other type of malignancy even before initiation of chemotherapy.
Rights and permissions
About this article
Cite this article
Lekovich, J., Lobel, A.L.S., Stewart, J.D. et al. Female patients with lymphoma demonstrate diminished ovarian reserve even before initiation of chemotherapy when compared with healthy controls and patients with other malignancies. J Assist Reprod Genet 33, 657–662 (2016). https://doi.org/10.1007/s10815-016-0689-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0689-1