Abstract
Patients with Hodgkin lymphoma (HL) are generally young, and high cure rates can be achieved. Thus, HL diagnosis and therapy frequently occur at a time of life when family planning plays an important role. It is therefore of major importance for the patients to discuss this subject and to consider fertility preservation techniques as early as possible after diagnosis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Patients with Hodgkin lymphoma (HL) are generally young, and high cure rates can be achieved. Thus, HL diagnosis and therapy frequently occur at a time of life when family planning plays an important role. It is therefore of major importance for the patients to discuss this subject and to consider fertility preservation techniques as early as possible after diagnosis.
2 Gonadal Dysfunction in Men
2.1 Male Reproductive Physiology
Sperm production in males is stimulated via secretion of follicle-stimulating hormone (FSH) by the pituitary gland, regulated by a negative feedback mechanism via inhibin produced from the Sertoli cells and/or seminiferous tubules. Impaired or absent sperm production can be anticipated based on progressive elevation of FSH levels. Testicular androgen production is regulated by pituitary secretion of luteinizing hormone (LH) and controlled by a comparable feedback mechanism via testosterone production of the testicular Leydig cells.
Gonadal function can be evaluated by measuring FSH and LH together with the morning testosterone level. A semen analysis is a more definitive test of fertility, with normal values of >15 × 106/mL, a total sperm motility of >40 %, and with >3 % of normal forms.
2.2 Hodgkin Lymphoma and Male Gonadal Dysfunction
Seventy to eighty percent of male HL patients have inadequate pretreatment semen quality due to the lymphoma itself [1–4]. The mechanisms involved are still unknown; however, possible factors include damage to the germinal epithelium, disturbances in the hypothalamic–hypophysial axis, immunological processes associated with cancer that impair spermatogenesis, and the impact of cytokines [2, 5–9]. In a study by the German Hodgkin Study Group (GHSG), male fertility was assessed in a total of 243 patients. In pretreatment semen analysis, only 20 % of patients had normal sperms. Azoospermia was observed in 11 % of patients and dysspermia in 69 % [3].
2.3 Treatment-Related Gonadal Dysfunction
Post-treatment gonadal damage is most often associated with chemotherapy regimens that include alkylating agents such as cyclophosphamide and procarbazine. The degree of damage and recovery of spermatogenesis depends on the choice of drugs and the dose given. In multiple analyses, the rate of azoospermia after cyclophosphamide, vincristine, procarbazine, and prednisone (COPP); Mustargen, vincristine, procarbazine, and prednisone (MOPP); or cyclophosphamide, vincristine, procarbazine, prednisone, Adriamycin, bleomycin, vinblastine, and dacarbazine (COPP/ABVD) is high, ranging from 80 to 100 % [4, 10–14]. Recovery of spermatogenesis can occur and has been recorded in 11–14 % of males after these regimens [4, 13–15]. This rate was 40 % when dysspermia was included [4]. Da Cunha and colleagues assessed MOPP-induced gonadotoxicity, demonstrating a significantly higher rate of azoospermia in patients treated with more than five cycles of MOPP compared to those receiving three or fewer cycles [16]. Newer and more intensive alkylating agent–based combinations such as bleomycin, etoposide, Adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) are highly gonadotoxic in males. A study of the GHSG performing post-treatment sperm analyses at a median of 17.4 months after the end of therapy revealed azoospermia in 64 % of patients, other forms of dysspermia in 30 %, and normal sperm analysis results in only 6 % of cases [3]. Thirty-eight patients with advanced-stage disease were examined, and 89 % were azoospermic after treatment. None of these patients had a normal sperm status. There was no statistically significant difference in the post-treatment fertility status between a group of patients treated with eight cycles of BEACOPP baseline (with a cumulative cyclophosphamide dose of 5,200 mg/m2) and a group treated with eight cycles of BEACOPP escalated regimens (with a cumulative cyclophosphamide dose of 10,000 mg/m2) [3].
In contrast, ABVD is less gonadotoxic, with gonadal damage that might be only transient [13, 17, 18]. However, more detailed data in advanced-stage patients receiving eight cycles of ABVD is needed.
Pelvic radiotherapy is now infrequently used in the management of HL. The testes are highly sensitive to irradiation in a dose-dependent manner. Doses above 4–6 Gy can result in permanent azoospermia, and doses of more than 6 Gy have a significant risk of this complication. Direct testicular radiation is usually not necessary in HL patients, and scattered radiation can be reduced by shielding the testes.
2.4 Predictive Factors for Gonadal Dysfunction and Damage
In a multivariate analysis of HL patients at initial diagnosis, Rueffer and colleagues described an elevated erythrocyte sedimentation rate (ESR) and poor prognostic risk groups as predictive for severe dysspermia [2]. A comparable study by Gandini and colleagues evaluated the semen quality in 106 untreated HL patients and showed a significant decrease in sperm concentration, total sperm count, and forward motility in the later stages of HL (stage III–IV) compared to early stages (stage I–II). Interestingly, of 53 patients with elevated ESR, 79.2 % had a normal sperm count, suggesting this parameter was not predictive for semen quality or potential infertility [19]. In an analysis of the GHSG, risk groups, extranodal involvement, and treatment with chemotherapy and BEACOPP were predictive factors for post-treatment azoospermia only in a univariate model. The fertility status prior to therapy was not predictive for post-treatment fertility [4, 20].
2.5 Hormonal Analyses to Assess Testicular Function After Therapy
Achievement of paternity and sperm counts provide the strongest evidence of male fertility; however, gonadotropin measurement can also provide useful surrogate information. Most studies in male patients show that the FSH levels correlate with testicular function after treatment [3, 4, 11, 18, 21]. In a study by van der Kaaij and colleagues, FSH was measured in a total of 355 patients with early-stage disease at least 12 months after the end of treatment. FSH was elevated in 35 % of all patients and in 3 % of those receiving radiotherapy only. In contrast, 60 % of patients treated with alkylating agents had elevated FSH levels, whereas this was observed in only 8 % of patients receiving chemotherapy without alkylators. Recovery of fertility was also poorer in patients treated with alkylating agent–containing chemotherapy [21]. Kreuser and colleagues reported increased FSH levels in 80 % of patients after treatment with COPP/ABVD [11]. In a retrospective GHSG analysis, abnormal FSH levels after chemotherapy were found in 79 %. In this group, the majority of patients were azoospermic (78 %; p = 0.001), suggesting an indirect correlation between FSH level and testicular dysfunction after therapy [3]. In contrast, normal levels of LH and testosterone were found in 86 and 63 % of patients after treatment. This underlines the hypothesis that spermatogonia cells are sensitive, whereas Leydig cells are more resistant to the toxic effects of cytostatic drugs [3, 11, 14]. Another important hormone in the assessment of infertility in men is inhibin B, which is produced by the Sertoli cells. Some studies support the use of inhibin B and inhibin B/FSH ratios as markers of male infertility [22, 23]. According to the results of a study by van Casteren and colleagues, 65 % of male cancer survivors had low inhibin B values as compared to 26 % in the control group [24]. Inhibin B levels significantly correlated with sperm concentration [24–26]. In a recent GHSG study, fertility status in men was assessed using hormonal levels of FSH and inhibin B. A total of 761 male survivors younger than 50 years at diagnosis were analyzed after a mean observation time of 48 months. Inhibin B and FSH values significantly correlated with chemotherapy intensity. Half of the survivors after early-stage treatment (2-4xABVD or 2xBEACOPPescalated + 2xABVD) had FSH and inhibin B levels corresponding to proven fertile men, whereas 88.8 % of survivors after advanced-stage treatment had levels indicating oligospermia. An effect of follow-up time on inhibin B and FSH levels was found in men after 2xBEACOPPescalated + 2x ABVD, suggesting a recovery up to 4 years after intermediate aggressive treatment. In contrast to the dose-dependent effect of chemotherapy on spermatogenesis, mean testosterone levels were within the normal range [27].
2.6 Endocrine Hypogonadism After Chemotherapy in Men
Little is known on the endocrine status of men after chemotherapy for HL. A recent study by Kiserud and colleagues investigated post-treatment exocrine and endocrine gonadal function in 165 HL and 129 non-Hodgkin lymphoma (NHL) patients. In almost one-third of the patients, the hormone levels were compatible with endocrine hypogonadism, defined as low testosterone with or without elevated LH or elevated LH and normal testosterone. Interestingly, only three patients were receiving testosterone replacement at the time of analysis [28]. Comparable findings after chemotherapy for testicular cancer in young males were linked with a subsequent risk of developing metabolic syndrome [29].
According to the results of the GHSG study, aging male symptoms were not different between patients in the trials and reference values [27].
2.7 Fertility Preservation in Men: Preventative Pretreatment Strategies and Management After Chemotherapy
Sperm banking is a widely available and successful pretreatment preventative strategy [30]. All postpubertal males should thus be offered sperm banking prior to potentially gonadotoxic chemotherapy. This also needs to include patients planned for ABVD, although this regimen has a lower risk of treatment-related infertility. The reason for this is that in the event of early relapse, sperm quality and quantity might not have recovered, rendering banking impossible prior to gonadotoxic salvage treatment. Sperm should be banked regardless of count as intracytoplasmic sperm injection can be successfully used as part of in vitro fertilization (IVF) where counts are low. If azoospermia is present and time permits, testicular sperm retrieval can be successful, particularly in the presence of a normal or only modestly elevated FSH level.
Cryopreservation of testicular tissue in prepubertal boys is still highly experimental, and pregnancies in humans have not been achieved. However, due to recent success in animal models [31], this technique is already offered in specialized centers to boys, expecting that the scientific progress will allow using the tissue to generate sperm or to reactivate the testes in the future.
3 Gonadal Dysfunction in Women
3.1 Female Reproductive Physiology
In premenopausal menstruating women, ovarian function is controlled by pituitary secretion of FSH and LH. FSH activates the granulosa cells of growing ovarian follicles which in turn begin to proliferate and to produce estradiol. This reduces the FSH levels by feedback inhibition, maintaining them at low levels. A mid-cycle LH surge induces ovulation following the formation of the luteal body that produces progesterone. Follicle development takes place over several months prior to ovulation. The growing follicles produce not only estradiol but also inhibin, which prevents the growth of too many follicles by downregulating FSH.
At puberty, approximately 300,000 follicles are present in the ovary. This number declines with age to around 1,000 at menopause (around 50–52 years of age), when FSH levels are insufficiently suppressed due to declining estrogen levels and therefore rise. The decline accelerates after the age of 35.
The number of follicles present in the ovary is known as the ovarian reserve and reflects reproductive capacity. Anti-Müllerian hormone (AMH) is produced by early, developing follicles, and its levels vary slightly during the menstrual cycle. It acts directly on other follicles in the ovary and inhibits the growth of too many follicles. The levels of this hormone are increasingly used in clinical studies to assess long-term gonadal damage and ovarian reserve.
3.2 Treatment-Related Infertility
While the mechanisms underlying the ovariotoxic effects of cytostatic drugs are still largely unknown, it is clear that the development of primary ovarian failure after chemotherapy is caused by accelerated attrition of the ovarian primordial follicles. As described above, this is age-dependent and relates to the ovarian reserve. For alkylating agents, a direct dose-dependent cytotoxic effect has been described. Acute toxicity reduces the number of follicles, whereas chronic toxicity affects the quality of follicles resulting in early atresia [32].
Very similar to male patients, alkylating agents are most commonly involved in female gonadal damage. This is well documented after treatment with older chemotherapy regimens such as MOPP or MVPP (Mustargen, vinblastine, procarbazine, and prednisone). In an early study, only 17 of 44 women maintained regular menses when either of these regimens was used [33]. In a similar study, Schilsky and colleagues investigated ovarian function after treatment with MOPP and documented persistent amenorrhea in 11 of 24 women [34]. Similarly, after treatment with alternating COPP/ABVD for advanced-stage HL, therapy-induced ovarian failure was described in 17 of 22 women (77 %) [11]. A further analysis included a total of 84 female patients with HL and NHL treated with at least three cycles of chemotherapy including alkylating agents. Premature ovarian insufficiency (POI) was defined as persistent amenorrhea for at least 2 years after the end of chemotherapy and elevated FSH levels. After a median follow-up of 100 months, 31 (37 %) women with preserved fertility achieved natural pregnancy; in 34 women (40.5 %), premature ovarian insufficiency was reported [35]. A study by Haukvik and colleagues reported POF defined as persistent amenorrhea before the age of 41 in 37 % of women after HL treatment. This occurred more commonly in alkylating-agent-treated patients [36]. In a retrospective GHSG analysis, the menstrual status after HL treatment of 405 female patients younger than 40 years was analyzed. With a median follow-up of 3.2 years, 51.4 % of women who received eight cycles of escalated BEACOPP had continuous amenorrhea. Amenorrhea was significantly less common in women treated with two cycles of ABVD (3.9 %), two cycles of alternating COPP/ABVD (6.9 %), four cycles of alternating COPP/ABVD (37.5 %), or eight cycles of BEACOPP baseline (22.6 %). In a multivariate analysis, amenorrhea was most pronounced in women with advanced-stage HL, women older than 30 years of age at treatment, and women who did not take oral contraceptives during chemotherapy [37]. In a more recent analysis of the GHSG, hormonal levels and fertility questionnaires were analyzed in a total of 562 female survivors after a mean observation time of 46 months. Women were younger than 40 years at HL diagnosis. Normal mean AMH levels (>2 μg/L) were observed in women younger than 30 years after two to four cycles of ABVD early-stage treatment, but AMH levels were compromised in survivors ≥30 years old. After treatment with six to eight cycles of BEACOPP, mean AMH levels were 0 μg/L in both age groups, and highest FSH levels were measured in women older than 30 years. Regular menstrual cycle was reported by more than 90 % of women after early-stage treatment and was mostly completed within 1 year. In contrast, after advanced-stage treatment, age at therapy onset was a decisive factor, and time to resumption of menstrual activity was considerably longer (Table 26.1). The risk of sustained amenorrhea 4 years after chemotherapy was 25 % in 25-year-old women and 50 % in 30-year-old women [27].
After ABVD alone, chemotherapy-induced ovarian failure is less likely, especially when women are younger than 30 years at the time of treatment [17, 38–41]. Older women have a significantly lower likelihood of ovarian recovery than those of younger age [11, 27, 33–35, 37, 42, 43].
Interestingly, the study by Haukvik and colleagues demonstrated a high cumulative percentage of POI in the youngest group of women. Compared to women diagnosed at the age of 30 years or older, those younger than 30 years developed POI approximately 5 years later. These findings suggest that younger age at HL treatment delays the development of POI but that the lifetime risk of POI is not decreased [36].
3.3 Post-treatment Assessment of Ovarian Reserve with Anti-Müllerian Hormone Levels
In the literature, the definition of gonadal toxicity varies. As described in the prior section, gonadal toxicity is defined by amenorrhea only in some reports, whereas in others also hormonal parameters such as FSH or LH were used. However, all of these parameters only measure the ovarian reserve indirectly and have little sensitivity. Recent studies suggested that AMH is the most sensitive marker of gonadal function. This hormone is produced by the granulosa cells of early developing preantral and antral follicles in the ovary. The serum AMH levels can be used as a marker for the number of growing follicles – the levels decrease when the number of follicles declines. The AMH levels are not influenced by the day of the menstrual cycle. They are therefore a potentially convenient and useful marker [44–46].
However, several recent studies have revealed that AMH is currently of limited use as a routine parameter due to high fluctuations of AMH concentrations in different AMH assays and laboratories. The introduction of an automated and reproducible immunoassay for anti-Müllerian hormone is expected soon. Until then, AMH concentrations should be interpreted with care.
3.3.1 Hypogonadism in Women
In the study of the GSHG, hypogonadism was analyzed using the menopause rating scale (MRS). Results demonstrated an age-dependent raise in severe menopausal symptoms for all HL stages and therapies. Severe menopausal symptoms in women >30 years were three- to fourfold higher than in an older (45–60 years) German reference population [27].
3.4 Radiation Therapy
Due to the increasing use of combined modality or chemotherapy-only approaches, infradiaphragmatic radiation is rarely used in the treatment of HL. According to a mathematic model described by Wallace and colleagues, the dose of radiation required to destroy approximately 50 % of oocytes has been estimated to be less than 2 Gy [47]. The estimated effective sterilizing radiation dose to the ovary at birth is 20.3 Gy, at the age of 10 years is 18.5 Gy, at the age of 20 is 16.5 Gy, and at the age of 30 is 14.3 Gy [48].
The uterus is more radioresistant than are the ovaries. Nonetheless, partial or complete uterine irradiation, though rarely required, can result in uterine fibrosis with an increased rate of miscarriage. Gonadal and organ damage can be reduced by shielding and other techniques, and pretreatment oophoropexy may also have a role in this process.
3.5 Preventative Treatment Strategies in Women
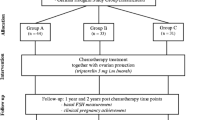
After HL diagnosis, strategies for ovarian protection should be offered to all women who have not completed their family planning. Women should be referred to an experienced center for counseling on protective procedures, after which management approaches should also be discussed with the attending oncologist. Figure 26.1 summarizes the options to preserve fertility in women with HL [49].
Fertility preservation in women with HL (Modified by Demeestere et al. [47])
3.6 Pharmacological Prevention of Gonadal Damage
Gonadotoxic chemotherapy destroys ovarian follicles and leads to decreased estrogen and inhibin secretion. Due to the negative feedback mechanism, the FSH levels increase and induce an increased recruitment of follicles, which are also potentially destroyed by chemotherapy. Pharmacological methods to protect fertility aim at suppressing pituitary gonadotropin secretion and cyclic ovarian function with the use of GnRH agonists, antagonists, and oral contraceptives.
The following putative protective mechanisms using GnRH analogues have been suggested [50]:
-
1.
Creating a prepubertal, hypogonadotropic milieu: Injected GnRH analogues cause an initial stimulation (“flare up”) of the pituitary LH and FSH secretion. As a consequence of the downregulation of the pituitary GnRH receptors, the FSH and LH secretion then declines to low, prepubertal serum levels. This mechanism prevents the FSH levels from increasing and can stop the enhanced recruitment of follicles, thereby rescuing them from accelerated atresia.
-
2.
Decreased utero-ovarian perfusion: Due to the hypoestrogenic milieu, utero-ovarian perfusion is decreased. This may lead to a lower total cumulative exposure of the ovaries to gonadotoxic chemotherapy.
-
3.
A direct effect on GnRH receptors: GnRH-a may directly decrease gonadotoxicity of chemotherapy.
-
4.
Possible role of sphingosine-1-phosphate: Spingosine-1-phosphate (S-1-P) is a lipid mediator of cell growth, survival, invasion, vascular maturation, and angiogenesis. Those processes are involved in cell viability and cancer progression. It has been speculated that GnRH-a may increase intragonadal S-1-P, thus preventing the ovarian follicles from destruction.
-
5.
Possible protection of ovarian stem cells: It is speculated that GnRH-a may protect undifferentiated germ line stem cells that are capable of generating de novo primordial follicles.
Others have challenged the putative protective effect of GnRH-a, as the primordial follicle growth is an FSH-independent process and alkylating agents are not cell-cycle specific. Thus, they might damage resting primordial follicles. Thus, GnRH-a might halt the growth of developing follicles, resulting in a resumption of the menstrual cycle in the short term. This might give the false impression that ovarian function is preserved [51].
Recently, two meta-analyses including women with different types of cancer [52] and women with lymphoma [53] have been published.
Del Masto et al. [52] included nine randomized trials in the meta-analysis with 225 events of POI occurring in 765 analyzed patients. The pooled OR estimate indicated a highly significant reduction in the risk of POF (OR = 0.43; 95 % CI 0.22–0.84; p = 0.013) in patients receiving GnRH-a. There was neither a statistically significant heterogeneity among studies (I2 = 55.8 %; p = 0.012) nor evidence of publication bias. Subgroup analyses showed that the protective effect of GnRH-a against POI was similar in subgroups of patients defined by age and timing of POF assessment, while it was present in breast cancer but unclear in ovarian cancer and lymphoma patients. The authors concluded that GnRH-a significantly reduces the risk of chemotherapy-induced POF in young cancer patients.
Zhang et al. [53] identified three randomized and four case control studies with lymphoma patients. They suggested that GnRH-a may be effective in protecting ovarian function during chemotherapy in lymphoma patients. However, due to the limited number of randomized studies, they also indicated that well-designed prospective studies are needed to improve further understanding of this topic.
Between 2004 and 2007, the GHSG conducted a prospective randomized trial (PROFE) to analyze the protective effect of GnRH-a. This trial was designed for young female patients (18–40 years) with advanced-stage HL receiving eight cycles of escalated BEACOPP. Patients were randomly assigned either to daily oral contraceptives (OC) or the GnRH-analogue (GnRH-a) goserelin, given monthly during eight cycles of polychemotherapy with escalated BEACOPP. The study was closed early after an interim analysis of 23 patients. Twelve patients were enrolled into arm A (OC) and 11 into arm B (GnRH-a). The women’s median age was 26 years in arm A and 25 years in arm B. The AMH level after at least 12 months was reduced in all women. Combining both treatment arms, the respective ovarian follicle preservation rate was 0 % (95 % CI 0–12 %); thus, continuation of the study was not justified [54].
Results of a retrospectively performed study of the GHSG demonstrated that the prophylactic use of GnRH-a during therapy was followed by significantly more pregnancies after therapy for early unfavorable HL stages. This finding suggests a protective effect in women receiving less toxic chemotherapy [55].
Clinically relevant side effects of GnRH-a include menopausal symptoms such as hot flushes, headaches, mood changes, and decreased bone density.
3.7 Cryopreservation of Oocytes/Ovarian Tissue
There have been remarkable advances in recent years in the field of cryopreservation of oocytes and ovarian tissue. But which technique (if any) should be recommended to a young woman before chemotherapy? This depends on the treatment to be used, age, availability of a partner, and the clinical condition of the patient and time available. It should be emphasized that results are likely to significantly improve during the reproductive span of patients currently undergoing harvest and storage.
3.7.1 Ovarian Stimulation and Cryopreservation of Fertilized and Unfertilized Oocytes
A minimum period of 2 weeks is required for both procedures. This is largely due to the time needed for ovarian stimulation. Modified stimulation regimens requiring 2 weeks have been successfully evaluated [56]. The cryopreservation of fertilized oocytes is a well-established method. If a sufficient number of oocytes can be retrieved and all cryopreserved fertilized oocytes are transferred, the average cumulative pregnancy rate can be up to 40 %. The success rate of the cryopreservation of unfertilized oocytes has significantly improved due to the introduction of the vitrification freezing technique. It has been shown by specialized centers that the pregnancy rates after vitrification of oocytes are similar to oocytes without cryopreservation [57].
3.7.2 Cryopreservation of Ovarian Tissue
Cryopreservation of ovarian tissue is an alternative especially for young patients without a partner. This method requires little or no preparative time but does require a laparoscopy. A combination of this technique with other invasive methods is possible.
The ovarian tissue is retrieved from one ovary and subsequently prepared and preserved using cryoprotective agents. If ovarian function insufficiency develops while relapse-free on follow-up, the cryopreserved tissue can be transplanted orthotopically to the remaining ovary or heterotopically. Currently, 30–40 live births and several ongoing pregnancies have been reported using this approach [58]. Work in mice models led to concern about possible tumor reimplantation from ovaries infiltrated with lymphoma [59]. In practice, however, HL rarely involves the ovaries; the tumor cells are extremely fragile, and so far there are no recorded events of tumor cell reimplantation [60].
3.8 Premature Menopause
Early onset of menopause in female patients after treatment for childhood cancer is well described [61, 62] showing higher cumulative incidence of premature menopause by the age of 40 for survivors compared to control siblings (8 vs. 0.8 %) [63]. Alkylating-agent-based combination chemotherapy will very likely lead to premature menopause in female patients. It is important to note that occasionally transient cessation of menses, with or without hot flushes, can occur. Hormone replacement may be indicated to reduce symptoms and prevent osteoporosis. If fertility is desired in younger women and conventional low-dose HRT is used, it is possible to monitor ovarian recovery with FSH levels. If oral contraceptives are used, treatment breaks with re-evaluation of ovarian function may be reasonable.
4 Conclusions
Remarkable advances have occurred in the management of HL, and today cure can be anticipated for the vast majority of young adults. When alkylating-agent-based combination chemotherapy was first devised in the 1960s, almost any late effect on fertility was acceptable in the context of the hitherto grim prognosis of HL, particularly in advanced stages. Then, regimens such as ABVD proved to be equivalent or superior, inducing less gonadotoxic effects. After the introduction of highly effective alkylating-agent-based therapy such as BEACOPP, impressive tumor control and overall survival rates were achieved but were associated with substantial gonadal toxicity, necessitating the development of adjunctive fertility supporting technology. Current trials evaluate risk-adapted treatment, reserving more effective but more toxic treatment for subgroups of patients with poorer prognosis as judged by positron emission tomography (PET) scanning.
The remarkable advances in the management of HL are paralleled by advances in fertility preservation techniques. It is of particular importance that these are considered and discussed as early as possible after diagnosis in the context of the patient’s wishes with regard to treatment and future fertility.
References
Lee SJ et al (2006) American society of clinical oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 24(18):2917–2931
Rueffer U et al (2001) Male gonadal dysfunction in patients with Hodgkin’s disease prior to treatment. Ann Oncol 12(9):1307–1311
Sieniawski M et al (2008) Assessment of male fertility in patients with Hodgkin’s lymphoma treated in the German Hodgkin Study Group (GHSG) clinical trials. Ann Oncol 19(10):1795–1801
Viviani S et al (1991) Testicular dysfunction in Hodgkin’s disease before and after treatment. Eur J Cancer 27(11):1389–1392
Agarwal A, Allamaneni SS (2005) Disruption of spermatogenesis by the cancer disease process. J Natl Cancer Inst Monogr 34:9–12
Barr RD, Clark DA, Booth JD (1993) Dysspermia in men with localized Hodgkin’s disease. A potentially reversible, immune-mediated disorder. Med Hypotheses 40(3):165–168
Dousset B et al (1997) Seminal cytokine concentrations (IL-1beta, IL-2, IL-6, sR IL-2, sR IL-6), semen parameters and blood hormonal status in male infertility. Hum Reprod 12(7):1476–1479
Huleihel M et al (1996) Distinct expression levels of cytokines and soluble cytokine receptors in seminal plasma of fertile and infertile men. Fertil Steril 66(1):135–139
Redman JR et al (1987) Semen cryopreservation and artificial insemination for Hodgkin’s disease. J Clin Oncol 5(2):233–238
Chapman R, Sutcliffe S, Malpas J (1981) Male gonadal dysfunction in Hodgkin’s disease. A prospective study. JAMA 245(13):p1323–p1328
Kreuser E et al (1992) Long-term gonadal dysfunction and its impact on bone mineralization in patients following COPP/ABVD chemotherapy for Hodgkin’s disease. Ann Oncol 3(Suppl 4):105–110
Kreuser ED et al (1987) Reproductive and endocrine gonadal capacity in patients treated with COPP chemotherapy for Hodgkin’s disease. J Cancer Res Clin Oncol 113(3):260–266
Viviani S et al (1985) Gonadal toxicity after combination chemotherapy for Hodgkin’s disease. Comparative results of MOPP vs ABVD. Eur J Cancer Clin Oncol 21(5):p601–p605
Waxman J et al (1982) Gonadal function in Hodgkin’s disease: long-term follow-up of chemotherapy. Br Med J (Clin Res Ed) 285(6355):1612–1613
Andrieu J et al (1981) Male fertility in Hodgkin’s disease before and after chemotherapy (author’s transl). Nouv Presse Med 10(25):2085–2088
da Cunha MF et al (1984) Recovery of spermatogenesis after treatment for Hodgkin’s disease: limiting dose of MOPP chemotherapy. J Clin Oncol 2(6):571–577
Bonadonna G et al (1984) Gonadal damage in Hodgkin’s disease from cancer chemotherapeutic regimens. Arch Toxicol Suppl 7:140–145
Kulkarni S et al (1997) Gonadal function following ABVD therapy for Hodgkin’s disease. Am J Clin Oncol 20(4):354–357
Gandini L et al (2003) Testicular cancer and Hodgkin’s disease: evaluation of semen quality. Hum Reprod 18(4):796–801
Sieniawski M et al (2008) Fertility in male patients with advanced Hodgkin lymphoma treated with BEACOPP: a report of the German Hodgkin Study Group (GHSG). Blood 111(1):71–76
van der Kaaij MA et al (2007) Gonadal function in males after chemotherapy for early-stage Hodgkin’s lymphoma treated in four subsequent trials by the European Organisation for Research and Treatment of Cancer: EORTC Lymphoma Group and the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 25(19):2825–2832
Andersson AM et al (2004) Serum inhibin B and follicle-stimulating hormone levels as tools in the evaluation of infertile men: significance of adequate reference values from proven fertile men. J Clin Endocrinol Metab 89(6):2873–2879
Bordallo MA et al (2004) Decreased serum inhibin B/FSH ratio as a marker of Sertoli cell function in male survivors after chemotherapy in childhood and adolescence. J Pediatr Endocrinol Metab 17(6):879–887
van Casteren NJ et al (2009) Effect of childhood cancer treatment on fertility markers in adult male long-term survivors. Pediatr Blood Cancer 52(1):108–112
Kumanov P et al (2006) Inhibin B is a better marker of spermatogenesis than other hormones in the evaluation of male factor infertility. Fertil Steril 86(2):332–338
van Beek RD et al (2007) Inhibin B is superior to FSH as a serum marker for spermatogenesis in men treated for Hodgkin’s lymphoma with chemotherapy during childhood. Hum Reprod 22(12):3215–3222
Behringer K et al (2013) Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin Study Group HD13 to HD15 trials. J Clin Oncol 31(2):231–239
Kiserud CE et al (2009) Gonadal function in male patients after treatment for malignant lymphomas, with emphasis on chemotherapy. Br J Cancer 100(3):455–463
Nuver J et al (2005) The metabolic syndrome and disturbances in hormone levels in long-term survivors of disseminated testicular cancer. J Clin Oncol 23(16):3718–3725
van der Kaaij et al (2014) Cryopreservation, semen use and the likelihood of fatherhood in male Hodgkin lymphoma survivors: an EORTC-GELA Lymphoma Group cohort study. Hum Reprod 29(3):525–533
Jahnukainen K et al (2012) Autologous ectopic grafting of cryopreserved testicular tissue preserves the fertility of prepubescent monkeys that receive sterilizing cytotoxic therapy. Cancer Res 72:5174–5178
Familiari G et al (1993) Ultrastructure of human ovarian primordial follicles after combination chemotherapy for Hodgkin’s disease. Hum Reprod 8(12):2080–2087
Whitehead E et al (1983) The effect of combination chemotherapy on ovarian function in women treated for Hodgkin’s disease. Cancer 52(6):988–993
Schilsky RL et al (1981) Long-term follow up of ovarian function in women treated with MOPP chemotherapy for Hodgkin’s disease. Am J Med 71(4):552–556
Franchi-Rezgui P et al (2003) Fertility in young women after chemotherapy with alkylating agents for Hodgkin and non-Hodgkin lymphomas. Hematol J 4(2):116–120
Haukvik UK et al (2006) Treatment-related premature ovarian failure as a long-term complication after Hodgkin’s lymphoma. Ann Oncol 17(9):1428–1433
Behringer K et al (2005) Secondary amenorrhea after Hodgkin’s lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin’s Lymphoma Study Group. J Clin Oncol 23(30):7555–7564
Andre M et al (1997) Results of three courses of adriamycin, bleomycin, vindesine, and dacarbazine with subtotal nodal irradiation in 189 patients with nodal Hodgkin’s disease (stage I, II and IIIA). Hematol Cell Ther 39(2):59–65
Bonadonna G (1994) Modern treatment of malignant lymphomas: a multidisciplinary approach? The Kaplan Memorial Lecture. Ann Oncol 5(Suppl 2):5–16
Brusamolino E et al (2000) Treatment of early-stage Hodgkin’s disease with four cycles of ABVD followed by adjuvant radio-therapy: analysis of efficacy and long-term toxicity. Haematologica 85(10):1032–1039
Hodgson DC et al (2007) Fertility among female Hodgkin lymphoma survivors attempting pregnancy following ABVD chemotherapy. Hematol Oncol 25(1):11–15
Horning SJ et al (1981) Female reproductive potential after treatment for Hodgkin’s disease. N Engl J Med 304(23):1377–1382
Howell SJ, Shalet SM (2002) Fertility preservation and management of gonadal failure associated with lymphoma therapy. Curr Oncol Rep 4(5):443–452
Tsepelidis S et al (2007) Stable serum levels of anti-Mullerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod 22(7):1837–1840
van Beek RD et al (2007) Anti-Mullerian hormone is a sensitive serum marker for gonadal function in women treated for Hodgkin’s lymphoma during childhood. J Clin Endocrinol Metab 92(10):3869–3874
Visser JA et al (2006) Anti-Mullerian hormone: a new marker for ovarian function. Reproduction 131(1):1–9
Wallace WH, Thomson AB, Kelsey TW (2003) The radiosensitivity of the human oocyte. Hum Reprod 18(1):117–121
Wo JY, Viswanathan AN (2009) Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys 73(5):1304–1312
Demeestere I et al (2007) Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin’s disease. Oncologist 12(12):1437–1442
Blumenfeld Z, von Wolff M (2008) GnRH-analogues and oral contraceptives for fertility preservation in women during chemotherapy. Hum Reprod Update 14(6):543–552
Oktay K et al (2007) Absence of conclusive evidence for the safety and efficacy of gonadotropin-releasing hormone analogue treatment in protecting against chemotherapy-induced gonadal injury. Oncologist 12(9):1055–1066
Del Mastro L et al (2014) Gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in cancer women: Systematic review and meta-analysis of randomized trials. Cancer Treat Rev 40(5):675-683. doi: 10.1016/j.ctrv.2013.12.001. [Epub 2013 Dec 8].
Zhang Y et al (2013) Gonadotropin-releasing hormone for preservation of ovarian function during chemotherapy in lymphoma patients of reproductive age: a summary based on 434 patients. PLoS One 28:8(11)
Behringer K et al (2010) No protection of the ovarian follicle pool with the use of GnRH-analogues or oral contraceptives in young women treated with escalated BEACOPP for advanced-stage Hodgkin lymphoma. Final results of a phase II trial from the German Hodgkin Study Group. Ann Oncol 21(10):2052–2060
Behringer K et al (2012) Fertility and gonadal function in female survivors after treatment of early unfavorable Hodgkin lymphoma (HL) within the German Hodgkin Study Group HD14 trial. Ann Oncol 23(7):1818–1825
von Wolff M et al (2009) Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase. Fertil Steril 92(4):1360–1365
Rienzi L et al (2010) Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod 25:66–73
Donnez J et al (2013) Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril 99(6):1503–1513
Shaw JM et al (1996) Fresh and cryopreserved ovarian tissue samples from donors with lymphoma transmit the cancer to graft recipients. Hum Reprod 11(8):1668–1673
Seshadri T et al (2006) Lack of evidence of disease contamination in ovarian tissue harvested for cryopreservation from patients with Hodgkin lymphoma and analysis of factors predictive of oocyte yield. Br J Cancer 94(7):1007–1010
Byrne J (1999) Infertility and premature menopause in childhood cancer survivors. Med Pediatr Oncol 33(1):24–28
Larsen EC et al (2003) Reduced ovarian function in long-term survivors of radiation-and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab 88(11):5307–5314
Sklar CA et al (2006) Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst 98(13):890–896
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing
About this chapter
Cite this chapter
Behringer, K., von Wolff, M. (2015). Gonadal Dysfunction and Fertility Preservation in Hodgkin Lymphoma Patients. In: Engert, A., Younes, A. (eds) Hodgkin Lymphoma. Hematologic Malignancies. Springer, Cham. https://doi.org/10.1007/978-3-319-12505-3_26
Download citation
DOI: https://doi.org/10.1007/978-3-319-12505-3_26
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-12504-6
Online ISBN: 978-3-319-12505-3
eBook Packages: MedicineMedicine (R0)