Abstract
Research question
How is ovarian reserve affected by chemotherapy in patients with Hodgkin lymphoma (HL) who undergo fertility preservation (FP)?
Methods
A retrospective study was conducted by reviewing medical records of 105 HL patients referred to the FP unit before starting adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) chemotherapy. Ovarian reserve was evaluated before chemotherapy and at the last follow-up using anti-Müllerian hormone (AMH) and antral follicle count (AFC) measurements. The decrease in AMH was compared with that expected from normograms. AMH was compared between patients who underwent cryopreservation of ovarian tissue and those who underwent cryopreservation of mature oocytes.
Results
After ABVD, 15% of patients required hematopoietic stem cell transplantation. At a median follow-up of 33 months, the median decrease in AMH was 0.88 ng/mL, which was significantly greater than that of the general population of this age group (p < 0.001). Of the 82 women who only had ABVD, 38 underwent FP by cryopreservation of mature oocytes and 44 underwent cryopreservation of the ovarian cortex. There was no significant difference in AMH or AFC at the last follow-up between FP techniques.
Conclusion
Although ABVD is considered to be of low gonadotoxic risk, the decrease in AMH was greater than expected for patients’ age, and 15% of patients needed more aggressive therapy during follow-up. Type of FP was not associated with decline in ovarian reserve. Reproductive-aged women with HL should have the opportunity for FP counseling before starting treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hodgkin lymphoma (HL) is one of the most common malignancies in young adults and has a high cure rate and long survival time [1]. Because of the long survival, short- and long-term side effects of treatment are an important issue, and infertility is a major concern in HL patients. Previous studies demonstrate that the type of treatment, particularly the dose of alkylating agents, and age at diagnosis are the two main risk factors for infertility in reproductive-aged women diagnosed with HL [2, 3].
ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine) is the “gold standard” chemotherapy (CT) for HL and is considered to be of low gonadotoxic risk [4]. Nonetheless, refractory disease and relapses require use of more gonadotoxic chemotherapies. After first-line therapy, 15–20% of patients do not respond to treatment or relapse [5, 6].
After chemotherapy, recovery of normal menstrual cycles does not guarantee normal fertility and does not necessarily correlate with normal ovarian reserve parameters. ABVD is considered a low-risk gonadotoxic treatment, but its impact on patients’ ovarian reserve is not empirically known. We aim to provide data on the true impact of ABVD chemotherapy on ovarian reserve measured by AMH levels during a follow-up period after FP. This information will optimize reproductive counseling for patients with HL, more accurately assessing the impact of CT and fertility preservation techniques on ovarian reserves. The current study evaluated ovarian reserve before and after CT in a cohort of HL patients treated with ABVD who underwent fertility preservation before chemotherapy.
Materials and methods
This retrospective study included HL patients referred to fertility preservation counseling after diagnosis. The study received approval by the Institutional Review Board of our center. A total of 105 patients were included. The main inclusion criteria were the diagnosis of HL, the treatment with ABVD, and the performance of a single FP technique (double techniques were excluded). Patients were referred from hospitals throughout Spain and were granted free access to FP within the Valencian Fertility Preservation Program. Exclusion criteria for funded FP were age > 39 years old, age > 35 years old and AFC < 6 or AMH < 0.84 ng/mL, previous live birth, estimated low survival rate, and absolute contraindication for pregnancy after treatment or due to another medical condition. The FP technique was discussed between a hematologist and an infertility specialist. The choice of FP type depended on maximum time from diagnosis to initiation of curative-intent chemotherapy permitted by the hematologist. Before any procedure, all patients signed an informed consent. Cryopreservation of mature oocytes was the first-line technique if the hematologist agreed to postpone initiation of CT for approximately 2 weeks. FP cycles were performed with a gonadotropin-releasing hormone (GnRH) antagonist-based protocol for controlled ovarian hyperstimulation with a GnRH agonist trigger (0.2 mg triptorelin) 36 h before oocyte retrieval.
Cryopreservation of ovarian tissue was chosen for patients who required immediate treatment and for pre-menarchal patients. Ovarian cortex tissue was removed by laparoscopic single hemi-oophorectomy of one of the ovaries unless contraindicated by an anesthesiologist, in which case, a mini-laparotomy was performed. Our standard protocol reserves complete oophorectomy only for patients in whom the risk of premature ovarian failure (POF) after the treatment is considered high due to gonadotoxic risk (i.e., use of alkylating agents). After extraction, ovarian tissue was transferred to a sterile tube with M199 medium at 4 °C. Tissue was immediately processed, and ovarian medulla was mechanically separated from the cortex by scraping with a curved scalpel. This procedure was performed inside a sterile metal pan placed over an ice plate to keep tissue and medium at 4 °C. After removal of the ovarian medulla, cortex was fragmented into 1 cm × 1 mm pieces and placed in a 20-mL tube with sterile M199 medium, and the tube was sealed with paraffin wax. A small piece of cortex (1 mm × 1 mm) and sample of medulla were sent for pathological examination of malignancy infiltration. Fragments were slow-frozen and stored at Biobank. Vitrification of ovarian tissue preserves a larger population of quiescent follicles than slow freezing after transplantation, maintaining potential fertility [7].
All patients submitted to FP were followed up with clinical evaluation and hormonal (follicle-stimulating hormone, luteinizing hormone, estradiol, progesterone, AMH) and AFC measurements at 6 months and 1 year after the end of CT, and then every 2 years.
Serum AMH was measured from venous blood using a repeated ELISA with a normal range of 1–8 ng/mL; values of < 1 ng/mL were considered severely reduced ovarian reserve. AFC was assessed using a transvaginal probe during early follicular phase (days 2–4 cycle) whenever possible. However, due to time constraints, if the patient started ovarian stimulation during luteal phase AFC before starting stimulation was considered as basal. The presence of at least 6 follicles was considered normal ovarian reserve.
Our main objective was to compare the decline of AMH and AFC after CT in our cohort of patients with that of a standard population of infertile patients using an AMH normogram. We also compared the effect of FP technique on AMH levels. Data processing was carried out using STATA 14.1. Statistical analysis was performed using Mann–Whitney U and χ2 tests. The Kolmogorov–Smirnov test was used to test equality of the median decrease of AMH in our sample with a reference median decrease. Quantile regression was used to analyze the impact of FP technique on AMH and AFC, adjusting for other variables. p < 0.05 was considered statistically significant.
Results
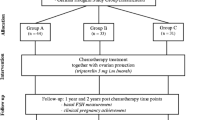
Our study cohort included 105 HL patients who underwent fertility preservation before starting CT (Fig. 1). We verified that there was no association between disease stage and basal AMH (p = 0.47) or basal AFC (p = 0.53). At a median follow-up of 33 months, final AMH and AFC were significantly lower than basal AMH (r = 0.51; p < 0.001) and AFC (r = 0.41; p = 0.001) (see Tables 1 and 2).
We also analyzed AMH levels according to different age categories and to FP technique. As shown in Table 3, AMH levels decreased as female age increased as expected, but there were no differences within each age category on AMH levels even in patients receiving different FP techniques.
Because not all patients had the same follow-up period and AMH decreases with time, we also grouped the patients according to their follow-up period (Table 4). There were no differences within each group depending on FP technique received.
We compared the decrease of AMH (0.88 ng/mL) in our sample with that reported in the literature. Normograms describe a median decrease in AMH of 0.25 ng/mL per year for individuals 26–30 years old [8]. At a follow-up of 2.75 years, the decrease in our sample (0.88 ng/mL) was significantly higher than expected (0.69 ng/mL) (p < 0.001).
In our sample, all patients had ABVD as first-line CT. At a median follow-up of 33 months after diagnosis, 14.3% (15/105) of patients needed hematopoietic stem cell transplant (HSCT) because of refractory disease or relapse (Fig. 1). Patients who needed transplant (15/105) after ABVD had lower final AMH (0.14 ng/mL) than patients who did not require transplant (90/105; 1.5 ng/mL; p < 0.001).
To assess the impact of type of FP technique on ovarian reserve, we analyzed patients who only received ABVD and no other gonadotoxic treatments during follow-up (n = 82). Of these patients, 38 underwent FP by cryopreservation of mature oocytes and 44 underwent cryopreservation of ovarian tissue (Table 2). In our sample, women submitted to cryopreservation of ovarian tissue were significantly younger than those submitted to cryopreservation of mature oocytes (22.6 versus 26 years; p = 0.04). We found no significant difference in basal and final measurements of AMH or AFC by type of FP technique (p > 0.05 for all comparisons).
We performed quantile regression to evaluate the association of type of FP and median decrease of AMH and AFC over the follow-up (Table 5). Adjusting for age and basal AMH and AFC, we found no difference in decrease of AMH and AFC by type of FP technique (p = 0.52; p = 0.38, respectively).
Discussion
We present here the results of our study which add to the existing literature a significant novelty regarding the knowledge of the impact of ABVD treatment and FP technique (i.e., oocyte vitrification and ovarian cortex cryopreservation) on ovarian reserve.
Relative to ovarian reserve, our results indicate that ABVD has a significant impact on ovarian reserve, measured on our sample of patients with HL undergoing FP, there was a greater decrease in AMH levels after ABVD than expected based solely on their age (0.88 ng/mL versus 0.69 ng/mL; p < 0.001). Furthermore, almost 15% of patients who initiated ABVD, which is considered the gold standard for HL and to have low gonadotoxicity, had to undergo HSCT, a much more aggressive treatment causing premature ovarian failure in a high proportion of patients [9]. As expected, the group of patients who needed transplant after ABVD had lower final AMH than patients who did not require transplant (0.14 ng/mL versus 1.5 ng/mL; p < 0.001).
Although ABVD is considered to be of low gonadotoxic risk, the greater than expected age-based decrease in AMH in our sample may reflect negative impact of CT on ovarian reserve or the impact of HL itself. The literature is controversial on this matter.
Azem et al. evaluated patients for 6 months following CT and verified that the ABVD protocol has minimal risk of ovarian damage and diminished ovarian reserve [10]. The main difference in our study is that we evaluated pre-CT AMH, whereas Azem et al. did not provide this data. Other studies have demonstrated that patients treated with low-gonadotoxic therapies have similar ovarian reserves to age-matched controls when evaluated within a few years from the end of therapy but clear impairment over a longer time [11]. A secondary analysis of the RATHL trial [12] demonstrated that recovery of ovarian function after treatment with ABVD is dependent on age, with full recovery of anti-Müllerian hormone seen in participants younger than 35 years, but not in women aged 35 years or older. Our study shows a decline in AMH levels even in younger patients, not confirming the previous results published by Anderson et al. which could have led us to the conclusion that ABVD chemotherapy was almost harmless in patients < 35 years. Interestingly, a study has shown that ovarian reserve is reduced in female patients with HL even before starting CT, suggesting that the disease could contribute to direct lesion of the ovary via cytokines or other intermediators [13]. This reinforces the importance of offering FP immediately after HL diagnosis.
With regard to the impact of the FP technique chosen, theoretically, partial oophorectomy for cryopreservation of ovarian tissue could have a negative effect on ovarian reserve. Studies have demonstrated that laparoscopic ovarian cystectomy contributes to a significant decline in serum AMH levels [14, 15]. In our sample, we found no difference in final AMH or AFC or in the level of decline of these parameters over follow-up according to type of FP technique (see Table 4). To our knowledge, this is the first study to compare impact on ovarian reserve between cryopreservation of ovarian cortex and mature oocytes. The fact that cryopreservation of ovarian cortex did not have an unfavorable impact on ovarian reserve compared with cryopreservation of mature oocytes could be due to the fact that a small sample of ovarian tissue was removed by laparoscopy and, contrary to cystectomy procedures, that may cause some trauma to adjacent ovarian tissue with loss of follicles on healthy ovarian tissue; the procedure for cryopreservation of ovarian cortex aims only to remove a small fragment of ovary with minimal manipulation. Moreover, the tissue is removed with cold scissors and electrocauterization is rarely used, as opposed to conventional cystectomy. Another factor that could explain this difference is the type of surgeon performing the procedure. Ovarian cortex retrievals are performed by fertility specialists who are extremely aware of the effects of ovarian surgery on ovarian reserve, whereas cystectomies can be performed by general gynecologists. The inclusion of two different FP techniques in our study is an important addition to recent publications that bring cryopreservation of the ovarian cortex from an experimental technique to a viable option for fertility preservation [16, 17]. A pregnancy rate of ~ 30% has been described after auto-transplantation of frozen-thawed ovarian cortex [18]. Over the past 10 years, ovarian tissue has been cryopreserved in female patients with hematologic malignancies, and HL is one of the most frequent in this group [19].
The decision as to whether ABVD start can be delayed to allow a window for FP belongs only to the hematologist. There is a paucity of studies to guide the timing of chemotherapy to treat lymphoma [20]. Although some literature suggests that for HL, time from histologic diagnosis to first ABVD treatment of > 4 weeks is not associated with worse overall survival, worse disease-specific survival, or lower progression-free survival, in clinical practice, hematologists make every effort possible to initiate curative-intent chemotherapy as soon as the diagnosis is established, especially for advanced stage or compressive bulky disease [21]. Clinical parameters and staging of the disease at diagnosis do not allow accurate risk stratification since most reports of prognostic factors for HL are retrospective analyses [22]. Since we cannot effectively predict which patients will need more gonadotoxic regimens during follow-up after ABVD, it is important to discuss FP options with all HL patients before CT [23,24,25].
With regard to achievement of pregnancy, a previous study showed that female HL patients who survived without recurrence for at least 3 years and who had attempted pregnancy after ABVD had a pregnancy rate similar to controls [11]. We could not report data on reproductive outcomes for the purposes of this study so we do not know whether this decrease on AMH will translate into fewer pregnancies. We acknowledge this as a limitation discussed below. However, the major justification for FP is that we cannot predict which patients will relapse and need more aggressive therapeutic strategies after ABVD which will involve a very high POF risk.
The main strengths of this study include that we presented and analyzed two different FP strategies, allowing us to compare their impact on ovarian reserve and verifying that the technique was not associated with a significant decrease of ovarian reserve. This is an important addition to the literature and can guide FP in HL requiring immediate onset of CT. Furthermore, all patients analyzed had FP before CT and had the same first-line CT scheme (ABVD). All evaluations of basal AMH were performed before CT administration.
Nonetheless, we acknowledge some limitations of our study. First, the retrospective nature of this study prevented us from having complete and more detailed information about patients, such as ABVD dosage or number of cycles. There was also some missing data on the HL stage at diagnosis. Second, to compare the decrease of AMH after CT, we used an AMH normogram that was built based on the US population referred to infertility centers because there was no validated normogram for our population. Finally, we were unable to assess the reproductive impact of FP and CT because we did not have enough data on pregnancy and live birth rates. It is possible that the significant decline in ovarian reserve may not translate into poorer clinical outcomes. However, this is unlikely, especially because our patients are still young and their ovarian reserves could only decrease over time, meaning that they will reach suboptimal fertility earlier than other women. Nevertheless, we plan to collect this data prospectively for further study.
In conclusion, our results indicate that reproductive-aged women diagnosed with HL should have an opportunity to discuss the possibility of preserving their fertility before CT initiation because (1) up to 20% of them will need more aggressive therapies, not being able to predict which ones at diagnosis and (2) ABVD appears to have a significant impact on ovarian reserve, despite being considered low risk for gonadotoxicity. Whether this has a real impact on fertility outcomes needs to be elucidated with further well-designed studies with longer follow-up.
Abbreviations
- ABVD:
-
Adriamycin, bleomycin, vinblastine, and dacarbazine
- AFC:
-
Antral follicle count
- AMH:
-
Anti-Müllerian hormone
- CT:
-
Chemotherapy
- FP:
-
Fertility preservation
- HL:
-
Hodgkin lymphoma
- HSCT:
-
Hematopoietic stem cell transplant
- POF:
-
Premature ovarian failure
References
Kogel KE, Sweetenham JW. Current therapies in Hodgkin’s disease. Eur J Nucl Med Mol Imaging. 2003;30(Suppl 1):S19–27 [Internet]. [cited 2019 Jun 21]Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s00259-003-1156-7.
Kiserud CE, Fosså A, Holte H, Fosså SD. Post-treatment parenthood in Hodgkin’s lymphoma survivors. Br J Cancer. 2007;96:1442–9.
Franchi-Rezgui P, Rousselot P, Espié M, Brière J, Pierre Marolleau J, Gisselbrecht C, et al. Fertility in young women after chemotherapy with alkylating agents for Hodgkin and non-Hodgkin lymphomas. Hematol J. 2003;4:116–20 [Internet]. [cited 2019 Jun 20]. Available from: http://www.nature.com/doifinder/10.1038/sj.thj.6200248.
Brusamolino E, Lunghi F, Orlandi E, Astori C, Passamonti F, Baraté C, et al. Treatment of early-stage Hodgkin’s disease with four cycles of ABVD followed by adjuvant radio-therapy: analysis of efficacy and long-term toxicity. Haematologica. 2000;85:1032–9 [Internet]. [cited 2019 Jun 20]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11025593.
Diehl V, Franklin J, Pfreundschuh M, Lathan B, Paulus U, Hasenclever D, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N Engl J Med. 2003;348:2386–95.
Duggan DB, Petroni GR, Johnson JL, Glick JH, Fisher RI, Connors JM, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin’s disease: report of an intergroup trial. J Clin Oncol. 2003;21:607–14.
Herraiz S, Novella-Maestre E, Rodríguez B, Díaz C, Sánchez-Serrano M, Mirabet V, et al. Improving ovarian tissue cryopreservation for oncologic patients: slow freezing versus vitrification, effect of different procedures and devices. Fertil Steril. 2014;101:775–784.e1.
Seifer DB, Baker VL, Leader B. Age-specific serum anti-Müllerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril. 2011;95:747–50. [Internet]. Elsevier Ltd. https://doi.org/10.1016/j.fertnstert.2010.10.011.
Socié G, Salooja N, Cohen A, Rovelli A, Carreras E, Locasciulli A, et al. Nonmalignant late effects after allogeneic stem cell transplantation. Blood. 2003;101:3373–85 [Internet]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12511420.
Azem F, Samara N, Cohen T, Ben-Yosef D, Almog B, Lessing JB, et al. Assessment of ovarian reserve following ovarian tissue banking and/or GnRH-a co-treatment prior to chemotherapy in patients with Hodgkin’s disease. J Assist Reprod Genet. 2008;25:535–8.
Di Paola R, Costantini C, Tecchio C, Salvagno GL, Montemezzi R, Perandini A, et al. Anti-Mullerian hormone and antral follicle count reveal a late impairment of ovarian reserve in patients undergoing low-gonadotoxic regimens for hematological malignancies. Oncologist. 2013;18:1307–14.
Anderson RA, Remedios R, Kirkwood AA, Patrick P, Stevens L, Clifton-Hadley L, et al. Determinants of ovarian function after response-adapted therapy in patients with advanced Hodgkin’s lymphoma (RATHL): a secondary analysis of a randomised phase 3 trial. Lancet Oncol. 2018;19:1328–37.
Hodgson DC, Pintilie M, Gitterman L, Dewitt B, Buckley C-A, Ahmed S, et al. Fertility among female hodgkin lymphoma survivors attempting pregnancy following ABVD chemotherapy. Hematol Oncol. 2007;25:11–5 [Internet]. [cited 2019 Jun 20]. Available from: http://doi.wiley.com/10.1002/hon.802.
Kwon SK, Kim SH, Yun SC, Kim DY, Chae HD, Kim CH, et al. Decline of serum antiMüllerian hormone levels after laparoscopic ovarian cystectomy in endometrioma and other benign cysts: a prospective cohort study. Fertil Steril. 2014;101:435–41. [Internet]. Elsevier Inc. https://doi.org/10.1016/j.fertnstert.2013.10.043.
Alborzi S, Keramati P, Younesi M, Samsami A, Dadras N. The impact of laparoscopic cystectomy on ovarian reserve in patients with unilateral and bilateral endometriomas. Fertil Steril. 2014;101:427–34 [Internet]. [cited 2019 Jun 20]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0015028213031658.
Imbert R, Moffa F, Tsepelidis S, Simon P, Delbaere A, Devreker F, et al. Safety and usefulness of cryopreservation of ovarian tissue to preserve fertility: a 12-year retrospective analysis. Hum Reprod. 2014;29:1931–40.
Pacheco F, Oktay K. Current success and efficiency of autologous ovarian transplantation: a meta-analysis. Reprod Sci. 2017;24:1111–20 [Internet]. [cited 2019 Jun 20]. Available from: http://journals.sagepub.com/doi/10.1177/1933719117702251.
Jadoul P, Guilmain A, Squifflet J, Luyckx M, Votino R, Wyns C, et al. Efficacy of ovarian tissue cryopreservation for fertility preservation: lessons learned from 545 cases. Hum Reprod. 2017;32:1046–54 [Internet]. [cited 2019 Jun 21]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28333228.
Meirow D, Baum M, Yaron R, Levron J, Hardan I, Schiff E, et al. Ovarian tissue cryopreservation in hematologic malignancy: ten years’ experience. Leuk Lymphoma. 2007;48:1569–76.
Brooks EG, Connors JM, Sehn LH, Gascoyne RD, Savage KJ, Shenkier TN, et al. Impact of time from diagnosis to initiation of curative-intent chemotherapy on clinical outcomes in patients with classical Hodgkin lymphoma. Leuk Lymphoma. 2016;57:872–9.
Alexander M, Blum R, Burbury K, Coutsouvelis J, Dooley M, Fazil O, et al. Timely initiation of chemotherapy: a systematic literature review of six priority cancers - results and recommendations for clinical practice. Intern Med J. 2017;47:16–34 [Internet]. [cited 2019 Jun 20]. Available from: http://doi.wiley.com/10.1111/imj.13190.
Derenzini E, Younes A. Hodgkin lymphoma: epidemiology, histopathology, staging, and treatment; 2011.
Diehl V, Sextro M, Franklin J, Hansmann M-L, Harris N, Jaffe E, et al. Clinical presentation, course, and prognostic factors in lymphocyte-predominant Hodgkin’s disease and lymphocyte-rich classical Hodgkin’s disease: report from the European Task Force on Lymphoma Project on Lymphocyte-Predominant Hodgkin’s Disease. J Clin Oncol. 1999;17:776 [Internet]. [cited 2019 Jun 20]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10071266.
Banerjee D. Recent advances in the pathobiology of Hodgkin’s lymphoma: potential impact on diagnostic, predictive, and therapeutic strategies. Adv Hematol. 2011;2011:1–19.
Shimabukuro-Vornhagen A, Haverkamp H, Engert A, Balleisen L, Majunke P, Heil G, et al. Lymphocyte-rich classical Hodgkin’s lymphoma: clinical presentation and treatment outcome in 100 patients treated within German Hodgkin’s Study Group Trials. J Clin Oncol. 2005;23:5739–45.
Acknowledgments
We would like to thank Dr César Díaz-García for his invaluable contribution to the design of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Catarina Policiano and Jessica Subirá are joint first authors.
Key message
ABVD treatment for HL reduces ovarian reserve compared with normal population. The technique used for FP does not seem to have an impact on ovarian reserve parameters. This information and its potential consequences on future fertility should be considered when counseling HL patients before FP.
Rights and permissions
About this article
Cite this article
Policiano, C., Subirá, J., Aguilar, A. et al. Impact of ABVD chemotherapy on ovarian reserve after fertility preservation in reproductive-aged women with Hodgkin lymphoma. J Assist Reprod Genet 37, 1755–1761 (2020). https://doi.org/10.1007/s10815-020-01844-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01844-0