Abstract
The keratinocytes of the skin are unique in being not only the primary source of vitamin D for the body, but also possessing the enzymatic machinery to metabolize vitamin D to active metabolites [in particular, 1,25 dihydroxyvitamin D (1,25(OH)2D)] and the vitamin D receptor (VDR) that enables the keratinocytes to respond to the 1,25(OH)2D they produce. Numerous functions of the skin are regulated by vitamin D and/or its receptor: these include inhibition of proliferation, stimulation of differentiation including formation of the permeability barrier, promotion of innate immunity, regulation of the hair follicle cycle, and suppression of tumor formation. Regulation of these actions is exerted by a number of different coregulators including the coactivators DRIP and SRC, a less well known inhibitor, hairless, and β-catenin. Different coregulators appear to be involved in different VDR-regulated functions. This review examines the various functions of vitamin D and its receptor, and to the extent known explores the mechanisms by which these functions are regulated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The epidermis is the major source of vitamin D for the body. Under the influence of sunlight [ultraviolet radiation (UVR), action spectrum 280–320 nM or UVB], 7-dehydrocholesterol (7-DHC) in the epidermis is converted to vitamin D. However, the major cell of the epidermis, the keratinocyte, also possesses the enzymes to further metabolize vitamin D to its active form, 1,25 dihydroxyvitamin D (1,25(OH)2D), and the vitamin D receptor (VDR) capable of responding to the 1,25(OH)2D so produced [1]. 1,25(OH)2D, acting through the VDR, regulates epidermal proliferation in the basal layer (stratum basale) and promotes the sequential differentiation of keratinocytes as they form the upper layers of the epidermis. Loss of VDR or loss of the capacity to produce 1,25(OH)2D (CYP27b1 mutations/deletion) disrupts differentiation of the epidermis and results in hyperproliferation of the basal layers. The keratinocytes lining the outer layer of the hair follicle (the outer root sheath, or ORS) also possess VDR. Loss of VDR function either by inactivating mutations or bioengineered deletions leads to loss of hair follicle cycling and alopecia. In this case, it is less obvious that the VDR requires 1,25(OH)2D for its activity in that deletion of CYP27b1 does not produce alopecia. Whether regulation of hair follicle cycling is truly a ligand-independent action of VDR or whether an as yet to be identified other ligand is required is unclear. VDR also functions as a tumor suppressor. Mice lacking the VDR are susceptible to tumor formation in the skin induced either by chemical means or by UVR. As for hair follicle cycling, the role of 1,25(OH)2D in this tumor suppressor function is not clear, although some studies support a protective role. The specificity of VDR action within the skin for the different functions it regulates is attributed at least in part to the different coregulators that modulate its genomic actions. In the proliferating keratinocytes of the epidermis and hair follicle, the vitamin D receptor interacting protein complex (DRIP complex), also known as Mediator, is the dominant coregulator. In the more differentiated keratinocytes of the epidermis, the SRC (steroid receptor coactivator) complexes (SRC2 and -3) dominate VDR function. In the hair follicle, the coregulator hairless (Hr) plays an important role. For 1,25(OH)2D regulated VDR actions, Hr acts as a cosuppressor. However, its interaction with VDR in regulating hair follicle cycling, an action of VDR independent of 1,25(OH)2D, is less clear. In this review, we examine these different actions of vitamin D and its receptor, emphasizing the many roles vitamin D plays in regulating epidermal proliferation and differentiation, hair follicle cycling, and tumorigenesis.
Vitamin D regulation of epidermal proliferation and differentiation
The epidermis is composed of four layers of keratinocytes at different stages of differentiation (Fig. 1). The basal layer (stratum basale, SB) rests on the basal lamina separating the dermis and epidermis. Within this layer are the stem cells. These cells proliferate, providing the cells for the upper differentiating layers. They contain an extensive keratin network comprised of keratins K5 and K14. By a process that we are only beginning to understand, cells migrate upward from this basal layer, acquiring the characteristics of a fully differentiated corneocyte, which is eventually sloughed off. The layer above the basal cells is the spinous layer (stratum spinosum, SS). These cells initiate the production of the keratins K1 and K10, which are the keratins characteristic of the more differentiated layers of the epidermis. Cornified envelope precursors such as involucrin also appear in the spinous layer, as does the enzyme transglutaminase K, responsible for the ε-(γ-glutamyl)lysine cross-linking of these substrates into the insoluble cornified envelope. The granular layer (stratum granulosum, SG), lying above the spinous layer, is characterized by electron-dense keratohyalin granules, which are of two types. The larger of the two granules contains profilaggrin, the precursor of filaggrin, a protein thought to facilitate the aggregation of keratin filaments; the smaller granule contains loricrin, a major component of the cornified envelope. The granular layer also contains lamellar bodies, lipid-filled structures that fuse with the plasma membrane, divesting their contents into the extracellular space between the SG and stratum corneum (SC) where the lipid contributes to the permeability barrier of skin. As the cells pass from the granular layer to the cornified layer (SC), they undergo destruction of their organelles with further maturation of the cornified envelope into an insoluble, highly resistant structure surrounding the keratin–filaggrin complex and linked to the extracellular lipid milieu. The outer layer of the epidermis provides not only a barrier to water loss (permeability barrier) but a barrier to invasion by infectious organisms via its expression of the innate immune system. In particular, disruption of the barrier triggers the induction of defensins such as cathelicidin that provide the initial defense in killing such organisms.
The different layers of the epidermis and the functions within those layers regulated by the vitamin D receptor (VDR) and its coactivators. The basal layer of the epidermis (stratum basale) contains the stem cells that through proliferation provide the cells for the upper layers. As the keratinocytes leave the basal layer differentiation takes place, with K1, K10, involucrin, and transglutaminase being expressed in the SS, and filaggrin and loricrin being expressed in the stratum granulosum (SG). Lamellar bodies forming in the stratum granulosum inject their lipid content into the intercellular spaces between the stratum granulosum and stratum corneum to provide the water proofing for the permeability barrier. DRIP205 is most highly expressed in the stratum basale and spinosum where it participates with VDR in regulating proliferation in partnership with the wnt/β-catenin signaling pathway. Cyclin D1 and Gli1 (a transcriptional factor for the hedgehog pathway) are regulated by VDR, DRIP205, and β-catenin. SRC3, on the other hand, is found in highest concentration in the SG, where it participates with VDR in the regulation of terminal differentiation. SRC3 is critical for lipid processing and lamellar body formation required for formation of the permeability barrier as well as 1,25(OH)2D induction of cathelicidin and CD14 required for innate immunity
As already noted, the keratinocytes of the epidermis are unique in their ability to produce vitamin D3 from the precursor 7-DHC and to convert the vitamin D produced to the active metabolite 1,25(OH)2D. 1,25(OH)2D increases involucrin, transglutaminase activity, loricrin, filaggrin, and cornified envelope formation at subnanomolar concentrations [2–7] while inhibiting proliferation, at least at concentrations above 1 nM. The antiproliferative effects are accompanied by a reduction in the mRNA levels for c-myc [8] and cyclin D1 and an increase in the cell-cycle inhibitors p21cip and p27kip. In addition, 1,25(OH)2D and its receptor regulate the processing of the long-chain glycosylceramides that are critical for permeability barrier formation [9] and induce the receptors, toll-like receptor 2 (TLR2) and its coreceptor CD14, that initiate the innate immune response in skin [10]. Activation of these receptors leads to the induction of CYP27b1 (the enzyme that produces 1,25(OH)2D), which in turn induces cathelicidin, resulting in the killing of invasive organisms [10, 11].
The availability of mice lacking either the VDR [12] or CYP27b1 [13] has expanded our understanding of the role of 1,25(OH)2D in epidermal differentiation. Although the most striking feature of the VDR null mouse is the development of alopecia (also found in many patients with mutations in the VDR, referred to as hereditary vitamin D resistance), these mice also exhibit a defect in epidermal differentiation as shown by reduced levels of involucrin and loricrin and loss of keratohyalin granules [14, 15]. Furthermore, these mice show a reduction in the lipid content of the lamellar bodies concomitant with a reduction in glucosylceramide production and transport into the lamellar bodies leading to a defective permeability barrier [9]. The CYP27b1 null mouse also shows a reduction in levels of the epidermal differentiation markers and altered permeability barrier [13]. However, the CYP27b1 null mice do not have a defect in hair follicle cycling.
Role of VDR coactivators in epidermal proliferation and differentiation
The process of epidermal differentiation is sequential. 1,25(OH)2D and VDR regulate all steps from the control of proliferation in the SB, to the regulation of K1, K10, involucrin, and transglutaminase production in the SS, to the regulation of loricrin and filaggrin production in the SG, to the synthesis of lipids required for the permeability barrier in the SC, and to the development of the innate immune system [1, 7, 10]. A major question being addressed in our laboratory is how this occurs? Although CYP27b1 and VDR are found in highest concentration in the SB, they are both distributed throughout the epidermis [16–18], so this does not provide an obvious explanation for the sequential induction of genes involved in the differentiation process. However, VDR requires the binding of coactivators to stimulate transcription. As already mentioned, the two major coactivator complexes in the epidermis are DRIP (Mediator) and SRC [19, 20]. An important clue for understanding the sequential induction of epidermal differentiation by 1,25(OH)2D and VDR came from our demonstration that in proliferating keratinocytes DRIP dominated the binding to VDR, whereas SRC dominated VDR binding in differentiated keratinocytes [19, 21]. These results are consistent with our finding that in the epidermis DRIP205 (Med1) is expressed in highest concentration in the SB and SS, whereas SRC3 is expressed in highest concentration in the SG [21, 22]. The DRIP complex is anchored to VDR via DRIP205 [23, 24]. The SRC complex is anchored to VDR with one of three homologous proteins, SRC1, -2, and -3 [25], but only SRC2 and -3 are found in keratinocytes [19]. These coactivator complexes interact with the C-terminal (AF2) domain of VDR following ligand binding via LxxLL motifs (NR boxes). They do not bind to VDR at the same time, competing as they do for the same region of the VDR [24]. The SRC coactivators have three NR boxes whereas DRIP205 has two. Our recent studies [26] indicate that VDR binds most strongly to the second and third NR box of SRC1 and -2, the third NR box of SRC3, and the second NR box of DRIP205. Different nuclear hormone receptors differ in the affinity for the different NR boxes of the different coactivators, suggesting some degree of specificity [27]. SRC recruits CREB-binding protein (CBP) or P300 and other histone acetyl transferases (HATs) and methyltransferases (MeTs) to the VDR, resulting in a multisubunit complex [28]. The HAT and MeT activity of the SRC complex is thought to destabilize the interaction between DNA and the histone core, enabling transcription to occur. The DRIP complex does not have HAT or MeT activity but functions, at least in part, through recruitment of RNA polymerase II to the transcription start site [24, 29]. Some studies suggest that the order of coactivator binding to its nuclear hormone receptor is sequential with different kinetics, generally with SRC binding preceding and being required for DRIP binding [27]. Other studies indicate that the specificity of coactivator binding to VDR depends on the gene being regulated [30, 31], the ligand being evaluated [32], and the cellular context [33, 34]. Our data, summarized below, indicate that the sequential action of 1,25(OH)2D and its receptor on keratinocyte differentiation is caused by the differential expression and distribution of these coactivators according to the differentiation status of the cell, coupled with the selectivity of genes regulated by VDR for one or the other of the coactivator complexes.
DRIP205 is expressed in proliferating keratinocytes, and its expression decreases with differentiation, as the expression of SRC3 is increased [19]. Knockdown of DRIP205 using siRNA results in increased keratinocyte proliferation, similar to that seen by knocking down VDR itself, but knockdown of SRC3 does not show such an effect [21]. Inhibition of DRIP205 binding to VDR in proliferating keratinocytes blocks VDR transcriptional activity with a vitamin D response element (VDRE) reporter construct, but such inhibition is not seen in differentiated keratinocytes [19]. However, this inhibition of transcriptional activity turns out to be gene specific. For example, knockdown of DRIP205 using siRNA methodology has a greater impact on keratins 1, 10, and involucrin expression than does knockdown of SRC3, although depletion of both coactivators profoundly reduces loricrin and filaggrin expression [7]. On the other hand, SRC3 knockdown, not DRIP205 knockdown, reduces glucosylceramide production and lamellar body formation similar to that of VDR knockdown [9], and prevents 1,25(OH)2D-induced cathelicidin expression [22]. Thus, our hypothesis is that SRC facilitates the ability of VDR to regulate the more differentiated functions of the keratinocyte, whereas DRIP facilitates the ability of VDR to regulate proliferation and early keratinocyte differentiation, although some overlap in coactivator function is observed.
Role of VDR in hair follicle cycling
The hair follicle cycle is divided into three main stages: anagen, catagen, and telogen (Fig. 2). Anagen is the stage of hair follicle growth; catagen is the stage of regression; and telogen is the resting stage. Only the proximal or dermal portion of the hair follicle cycles; the distal or epidermal portion does not. The duration of these stages in a given species varies from location to location on the body and between genders. Furthermore, there are two types of cycles: developmental and postnatal. The developmental cycle is initiated during embryogenesis. The follicle develops from specific regions of the epidermis called placodes. The development, number, and placement of these placodes are under the control of a number of factors, to which we will return. The follicle is induced to grow by its interaction with a collection of specialized mesenchymal cells in the dermis called the dermal papilla. Wnt signaling (β-catenin) appears to be necessary to maintain the ability of the dermal papilla to stimulate hair follicle growth [35, 36]. Following the developmental cycle, which leads to the initial coat of hair, the follicle undergoes repetitive cycling until senescence. The length of the hair is dependent on the duration of anagen. During this stage the follicle grows through the dermis into the subcutaneous tissue. As the follicle develops, different cell layers appear. The ORS is a direct extension of the stratum basale, and separates the hair follicle from the surrounding connective tissue sheath (CTS). From outside in are found the companion layer, the three layers of the inner root sheath (IRS)—Henle’s layer, Huxley’s layer, cuticle of the IRS—and the hair shaft itself, including the cuticle of the shaft, shaft cortex, and shaft medulla. Stem cells in the bulge are capable of generating all cells in the hair follicle and epidermis [37]. The keratins produced by the cells of the IRS and hair shaft differ from those expressed by epidermal keratinocytes [38]. Of particular interest, these hair keratins have β-catenin/LEF1 binding sites in their promoters that regulate their expression [39]. Following anagen, the follicle enters catagen, during which massive apoptosis occurs primarily in the cells of the proximal follicle (the dermal portion), and the hair shaft produced during anagen is generally shed. At the end of catagen the follicle enters telogen, the resting phase. Duration of telogen is highly variable. A new cycle then begins with anagen. The juxtaposition of the dermal papilla to the bulge is critical for this process to begin, and it is associated with increased proliferation of stem cells in the bulge with migration of cells from the bulge into the hair bulb to restart the growth of the hair follicle. The regulatory elements that control the transition from one stage to the next are not well understood.
Hair follicle cycling. The initial developmental phase of hair follicle development terminates with catagen and the first telogen, after which repetitive cycles of anagen (the growth phase), catagen (the regression phase), and telogen (the resting phase) occur throughout the life span of the animal. In general, the hair follicle spends most of its time in anagen, but cycle duration varies according to location, gender, age, and species. The bulge is the source of stem cells for the regenerating hair follicle, responding to signals from the dermal or follicular papilla (FP). ORS outer root sheath, IRS inner root sheath, HS hair shaft, mel. melanin for hair shaft, HM hair matrix, BM basement membrane, SG sebaceous gland, APM arrector pili muscle
Alopecia is a well-known part of the phenotype of many patients with mutations in their VDR [40, 41], a syndrome currently known as hereditary vitamin D-resistant rickets (HVDRR). In that vitamin D deficiency per se or CYP27b1 mutations are not associated with alopecia, the explanation for this phenomenon has remained obscure. Exploration of the link between alopecia and VDR received a major boost with the development of the VDR null mouse by two groups [12, 42]. These mice develop their first coat of hair normally, but reinitiation of anagen following the first cycle or after depilation is impaired [43]. Reconstitution of the VDR to the VDR null mouse skin using a keratinocyte-specific promoter reverses the defect in hair growth without reversing the metabolic defects of skeletal growth retardation, hypocalcemia, and rickets otherwise associated with the VDR null condition [44, 45]. On the other hand, correction of the metabolic abnormalities with a high calcium diet prevents the rickets and hyperparathyroidism but does not prevent the alopecia [46]. Furthermore, it is the lack of VDR in the keratinocyte as opposed to the dermal papilla that is critical. Dermal papilla cells obtained from either VDR null or wild-type mice can initially induce hair growth in a hair reconstitution assay when mixed with epidermal keratinocytes obtained from wild-type or VDR null mice, but if the hair grown with keratinocytes from VDR null mice is then depilated, anagen will not be reinitiated regardless of the source of dermal papilla cells [43].
Hr mutations in both mice [47] and humans [48, 49] and transcriptionally inactivating β-catenin mutations in mice [50, 51] result in phenocopies of the VDR null mouse and some human VDR mutations, respectively, with regard to the morphological changes observed in hair follicle cycling. In these models the abnormality leading to alopecia develops during catagen at the end of the developmental cycle, precluding initiation of anagen in the first postnatal cycle. The dissociation of the dermal papilla from the hair bulb by the end of catagen is thought to account for the failure to initiate the subsequent anagen in both Hr mutant and VDR null mice [15, 47, 52]. Signaling via the hedgehog (Hh) and wnt pathways appears to be important for these mesenchymal/epithelial interactions. Expression of Gli1 and -2 (transcription factors activated by Hh) and LEF1 (transcription factor involved with β-catenin transcriptional activity) is reduced during the latter stages of catagen in the VDR null hair follicle (A. Teichert and D.D. Bikle, unpublished). The distal (epidermal) portion of the hair follicle including the sebaceous gland as well as the interfollicular epidermis is less impacted [14, 47, 52, 53]. Indeed, expression of Hh signaling pathway components is increased in the interfollicular epidermis and utricles of the VDR null mouse (A. Teichert and D.D. Bikle, unpublished data). The large dermal cysts that develop with time contain markers of the differentiated interfollicular epidermis and sebaceous gland [14, 52, 53], suggesting their origin from the distal portion of the hair follicle or epidermis, features similar to that seen in Hr and β-catenin mutant animals [50–52, 54].
The control of hair follicle development and cycling is complex [50, 51], and a large number of factors are implicated in this process. Many of these gene products are involved in placode and/or hair follicle development, and so their overexpression or underexpression results in abnormalities in the developmental cycle of hair growth. These are phenotypes different from that seen in the VDR null mouse. Sonic hedgehog (Shh) is one of these factors [55], but it appears to play an important role in adult hair follicle cycling as well. Disruption of canonical wnt signaling (β-catenin or LEF1 mutations) disrupts both developmental and postnatal hair follicle cycling and is associated with loss of Shh expression [50, 51]. Both Hr null [56] and VDR null mice show loss of Shh expression in the proximal (dermal) portion of the hair follicle (A. Teichert and D.D. Bikle, unpublished data). The Hr null mouse shows an increase in expression of the wnt inhibitor WISE [56], whereas we have shown a reduction of wnt4 in the VDR null hair follicle. Wnt4 is normally expressed in the matrix and precortex and would be expected to have an important role in interactions with the dermal papilla [57]. We have not seen a difference in β-catenin mRNA levels. However, in other cells VDR has been found to bind to β-catenin directly, reducing its interaction with TCF4 or LEF1 and so reducing the transcriptional activity of β-catenin [58–60]. Therefore, function of β-catenin rather than expression may be altered in VDR null hair follicles.
Role of hairless as VDR coregulator
As discussed above, Hr plays an important role in hair follicle cycling. We have found VDR and Hr in the nuclei of keratinocytes in the stratum basale and ORS [15]. However, we found little or no VDR in the IRS and hair bulb or cells of the dermal papilla and CTS, whereas we did find Hr in those locations [15]. However, another study with a different Hr antibody showed a somewhat more restricted distribution (ORS and hair bulb) [56]. Hr has characteristics of a coregulator in that it resides in the nucleus; its structure contains a nuclear localization signal, a putative zinc finger, and three LXXLL motifs [61] resembling that found in coactivators which interact with nuclear hormone receptors such as VDR as well as ΦXXΦΦ motifs (Φ = hydrophobic amino acid) similar to regions in corepressors such as SMRT and NCoR, responsible for the binding of these corepressors to nuclear hormone receptors. In the brain, Hr has been suggested as a corepressor of the thyroid receptor (THRb) in that Hr can bind to THRb and inhibit its transcriptional activity [62]. However, Hr does not appear to regulate thyroid hormone action in the keratinocyte [63]. Rather, VDR appears to be the target [64]. Hsieh et al. [65] demonstrated that Hr could bind to VDR in COS cells. They noted that Hr bound to VDR in the same region predicted for corepressor binding, which differed from the C-terminal region to which coactivators bind. The region of Hr responsible for VDR binding contains one LXXLL motif, but also a ΦXXΦΦ motif. However, only mutations in the ΦXXΦΦ motif altered binding to VDR [65]. Furthermore, when we tested both motifs separately for their binding to VDR, the ΦXXΦΦ motif had the higher affinity regardless of the presence or absence of 1,25(OH)2D [26]. We have shown that the endogenous VDR binds to endogenous Hr in keratinocytes [64]. Binding of Hr to VDR inhibited 1,25(OH)2D stimulation of a CYP24A1 (24-hydroxylase) promoter construct containing the VDRE of this vitamin D target gene. Overexpression of Hr blocks the ability of 1,25(OH)2D to induce differentiation markers in keratinocytes, whereas inhibition of Hr expression enhances the stimulation by 1,25(OH)2D of these markers [64]. The Hr null animal demonstrates upregulation of differentiation markers in the epidermis [54] consistent with a corepressor role for Hr in vitamin D regulated epidermal differentiation. Antibodies to Hr enhance the binding of VDR to VDREs in vitamin D target genes in gel retardation assays [64] suggesting that Hr binding to VDR blocks its binding to VDREs. 1,25(OH)2D displaces Hr from VDREs as it recruits the coactivators DRIP205 and SRC3 to these same VDREs [64]. Thus, at least for 1,25(OH)2D stimulated actions of VDR, Hr is a cosuppressor.
Role of β-catenin as VDR coregulator
Wnt signaling via activation of β-catenin has a complex role in VDR function (Fig. 3). Wnt ligands bind to their seven-transmembrane Frizzled receptors and an LRP5 or LRP6 coreceptor, leading to phosphorylation of disheveled (Dvl) and resulting in disruption of the axin–APC complex and inhibition of the kinase activity of glycogen synthase kinase 3β (GSK-3β), which otherwise phosphorylates the serine(s) within exon 3 of β-catenin, facilitating its degradation by the E3 ubiquitin ligase. Thus, wnt signaling increases the availability of β-catenin in the cytoplasm, which can then bind to transcription factors of the T-cell factor (TCF) and lymphoid enhancer factor (LEF) families to promote expression of genes such as cyclin D1 and c-myc [66] important for proliferation. β-Catenin also forms part of the adherens junction complex with E-cadherin, where it may play an important role in keratinocyte differentiation [67]. Tyrosine phosphorylation of E-cadherin, as occurs after calcium administration to keratinocytes, promotes the binding of β-catenin and other catenins to the adherens junction complex [67, 68], making it less available for transcriptional activity. Cells differ markedly in the components of the β-catenin signaling pathway utilized, as is well illustrated in the keratinocytes of the hair follicle and interfollicular epidermis. LEF1 is the dominant transcription partner for β-catenin in the dermal portion of the hair follicle, which has little E-cadherin. The epidermal keratinocyte, on the other hand, has little LEF1 but much E-cadherin, especially in the differentiated layers [51]. Overexpression and/or overactivating mutations in the β-catenin pathway lead to skin tumors, in this case pilomatricomas or trichofolliculomas (hair follicle tumors) [69–71], indicative of the hyperproliferative response to β-catenin in these cells. Activating mutations of specific serines within exon 3, or deletion of exon 3, block phosphorylation of β-catenin by GSK-3β, phosphorylation that otherwise leads to its proteosomal degradation. As a result, β-catenin levels increase in the nucleus where its transcriptional activity is exerted in association with members of the LEF/TCF family of transcription factors. In colon cancer cells, VDR has been shown to bind to β-catenin and reduce its transcriptional activity in a ligand-dependent fashion [60]. Furthermore, in these cells 1,25(OH)2D has been shown to increase E-cadherin expression, such that β-catenin is redistributed from the nucleus to the plasma membrane where it forms a complex with E-cadherin and other catenins at adherens junctions [58]. However, the suppression of β-catenin signaling by 1,25(OH)2D does not necessarily require E-cadherin [59]. Rather β-catenin binds to VDR in its AF-2 domain, binding that enhances the ability of 1,25(OH)2D to activate the transcriptional activity of the VDR [59] but blocks the transcriptional activity of β-catenin. Mutations in the AF-2 domain that block coactivator binding do not necessarily block β-catenin binding [59]. Whether β-catenin binding alters DRIP205 or SRC3 binding to this same region has not been determined. A recent publication by Palmer et al. [72] evaluated the interaction between VDR and β-catenin in transcriptional regulation in keratinocytes and identified putative response elements for VDR and β-catenin/LEF in a number of genes. These interactions were either positive or negative, depending on the gene being evaluated. The hypothesis put forward is that genes in which the interaction was positive (i.e., stimulated transcription) benefited from β-catenin acting as a coactivator for VDR on VDREs, whereas in situations where the interaction was negative (i.e., suppression of transcription) VDR prevented β-catenin from binding to TCF/LEF required for transcription in those genes. We (Y. Oda and D.D. Bikle, unpublished data) have found in keratinocytes that knockdown of VDR and DRIP205 reduces E-cadherin expression and formation of the β-catenin/E-cadherin membrane complex, resulting in increased β-catenin transcriptional activity, whereas 1,25(OH)2D administration has the opposite effect; this was associated with increased (with VDR and DRIP205 knockdown) or decreased (with 1,25(OH)2D administration) keratinocyte proliferation and cyclin D1 expression, respectively. Other studies have demonstrated that VDR potentiates, not inhibits, β-catenin transcriptional activity. Cianferotti et al. [73] found a reduction in proliferation of keratinocytes in the dermal portion of the hair follicle (below the bulge) in VDR null mice, and no stimulation of proliferation when β-catenin was overexpressed in these cells, in contrast to the stimulation of proliferation in control animals. Thus, VDR/β-catenin interactions can be positive or negative, and this depends on the gene/cell/function being evaluated.
The canonical wnt signaling pathway. Wnts bind to their frizzled receptors (FZ) and coreceptors LRP in the membrane. This binding can be blocked by dickkopf (Dkk) or soluble frizzled related proteins (sFRP). Activation of FZ by wnt results in phosphorylation of disheveled (Dvl), which induces the disruption of the axin/APC/GSK-3β complex and recruitment of axin to the membrane. This complex when active phosphorylates β-catenin, leading to its proteosomal degradation. However, following wnt stimulation, β-catenin is no longer degraded and can enter the nucleus, where in combination with members of the LEF/TCF family it can induce expression of its target genes such as cyclin D1. β-Catenin also binds to the E-cadherin complex in the plasma membrane, a complex stabilized by calcium and induced by 1,25(OH)2D
VDR as a tumor suppressor in the skin
Over 1 million skin cancers occur annually in the United States, 80% of which are basal cell carcinoma (BCC) [16% squamous cell carcinoma (SCC), 4% melanomas], making it by far the most common cancer [74]. UVR is the major etiological agent. UV wavelengths shorter than 280 nm (UVC) are absorbed by the ozone layer and do not reach the earth. UV wavelengths longer than 320 nm (UVA) have limited ability to induce the characteristic mutations in DNA seen in epidermal cancers. Thus, UVB with a spectrum between 280 and 320 nm is the major cause of these cancers [75]. The principal genotoxic lesions induced by UVR are cyclobutane pyrimidine dimers (CPDs) and pyrimidine(6-4)pyrimidone photoproducts (6-4PP), which if not repaired result in C to T or CC to TT mutations, the UVB “signature” lesion [76]. Such mutations in p53 are common (50–90%) in both BCC and SCC [77–80] as well as in actinic keratoses, the precursor lesions to SCC [81]. Precursor lesions for BCC have not been identified, but BCC are thought to arise from interfollicular basal cells, hair follicles, and sebaceous glands. Mutations in ras are much more common in SCC than BCC [82], whereas mutations in the Hh signaling pathway, in particular in patched 1 (Ptch1), characterize BCC [82–86], but can also be found in SCC in patients also susceptible to BCC [87].
Recent studies indicate that vitamin D plays a protective role in the skin with respect to carcinogenesis. Zinser et al. [88] treated VDR null mice bearing medroxyprogesterone pellets with two oral administrations of 7,12-dimethylbenzanthracene (DMBA) at 5.5 and 7 weeks, a protocol designed to induce breast cancers. No breast tumors were observed, at least initially. Instead, they found that 85% of the VDR null mice developed skin tumors within 2 months. No tumors (breast or skin) were found in the wild-type controls. The tumors were mostly sebaceous, squamous, and follicular papillomas, but several BCC were observed. No SCC were reported. These results have been confirmed using topical administration of DMBA/12-O-tetradecanoyl-phorbol-13-acetate (TPA), although only papillomas were seen in the VDR null mice, in contrast to RXR-α null mice, which developed both BCC and SCC [89]. The most recent study [90] likewise confirmed the results by Zinser et al., demonstrated that mice lacking CYP27b1 (and so lacking 1,25(OH)2D) were not prone to DMBA-induced tumors, and further demonstrated that the VDR null mice were also more susceptible to tumor formation induced by UVR. We have confirmed these results, and, similar to DMBA-induced tumor formation, we showed that CYP27b1 null mice are not more susceptible to UVR induced tumor formation than wild-type controls (A. Teichert and D.D. Bikle, unpublished data). UVR-induced tumors include SCC and BCC. The appearance of BCC in these studies is surprising because the typical malignancy induced in mouse skin by UVR, ionizing radiation, or chemical carcinogens is SCC, not BCC [77].
The hedgehog pathway in epidermal tumor formation
The Hh pathway is depicted in Fig. 4. The appearance of BCC is characteristic of tumors formed when Hh signaling is disrupted [91], although activation of Hh signaling (Ptch –/+ mice) also predisposes to UVR-induced SCC formation [92], and a polymorphism of Ptch has been observed to promote Hras-induced SCC formation [93]. We (A. Teichert and D.D. Bikle, unpublished data) have found that the epidermis and epidermal portion (utricles) of the hair follicles of adult VDR null animals overexpress elements of the Hh signaling pathway, unlike the dermal portion of the hair follicle, suggesting that one of the causes of the increased susceptibility of the epidermis to malignant transformation is loss of 1,25(OH)2D and/or VDR regulation of Hh signaling. Of interest is that vitamin D may regulate this pathway, not only via the genomic actions of 1,25(OH)2D acting through its receptor, VDR, but also by direct inhibition by vitamin D independent of its receptor. The latter possibility stems from recent observations that vitamin D itself as well as its precursor 7-DHC can bind to and inhibit the actions of smoothened (Smoh), a critical step in Hh signaling [94].
The sonic hedgehog (Shh) signaling pathway. In the absence of Shh, Ptch1 suppresses signaling by smoothened (Smoh). Binding of Shh to Ptch1 relieves this inhibition. Activation of Smoh leads to the activation and translocation of transcription factors of the Gli family into the nucleus, with subsequent changes in gene expression
Appreciation of the pivotal role of the Hh signaling pathway in BCC development began with the identification of the Ptch1 gene as the site of mutations underlying the rare autosomal dominant heritable basal cell nevus syndrome (BCNS) (Gorlin syndrome), one of whose cardinal features is a high susceptibility to the development of BCCs [84, 85]. The BCCs in these subjects frequently lose function of the inherited wild-type Ptch1 allele, leaving the tumor cells functionally null of Ptch1. Subsequently it has become clear that essentially all BCCs, whether arising in patients with BCNS or sporadically, have mutations in Ptch1 or other alterations in Hh signaling [85]. This appreciation has resulted in the development of the Ptch1+/– (Gorlin) mouse as the first practical model of murine BCCs [86]. Treatment of these mice (in contrast to treatment of Ptch1 wild-type mice) with UVR or ionizing radiation produces BCC as well as SCC [86].
Ptch1 is the membrane receptor for Shh (Fig. 4). In the absence of Shh, Ptch1 inhibits the function of another membrane protein, smoothened (Smoh). Shh reverses this inhibition, freeing Smoh to enable the activation of a family of transcription factors: Gli1, Gli2, and Gli3. Suppressor of fused (Sufu) may maintain these transcription factors in the cytoplasm and/or limit their activity in the nucleus such that the loss of Sufu leads to increased Hh signaling [92, 95]. However, mutations in Sufu have not been clearly shown to result in BCC, although they are associated with medulloblastomas, a feature of the Gorlin syndrome [84]. The role of Gli3 is unclear, and unlike Gli1 and Gli2, it is not elevated in BCC. Gli1 and -2 overexpression in keratinocytes can increase the expression of each other as well as Ptch1, the antiapoptotic factor bcl2, cyclins D1 and D2, E2F1, cdc45 (all of which promote proliferation) while suppressing genes associated with keratinocyte differentiation such as K1, K10, involucrin, loricrin and the VDR [91, 96–99]. 1,25(OH)2D and VDR, on the other hand, have the opposite action on these genes. Mice overexpressing Gli1, Gli2, or Shh in their basal keratinocytes [98–100] or grafted with human keratinocytes overexpressing Shh [101] develop BCC-like lesions. Furthermore, BCC show overexpression of Ptch1, Smoh, Gli1, and Gli2 [102, 103]. Gli2 null mice resemble Shh null animals in phenotype, Gli2 deletion partially rescues Ptch1 null animals, and Gli2 is required for Shh signaling in hair follicle development [104]. Shh, Ptch1, Ptch2, Gli1, and Gli2 have consensus sequences for VDRE in their promoters [72, 105], suggesting that 1,25(OH)2D/VDR may regulate their expression directly. This idea has not yet been demonstrated, but it is under investigation in our laboratory.
β-Catenin signaling in epidermal tumor formation
The appearance of hair follicle elements in many of the tumors forming in the VDR null mouse skin also suggests that the β-catenin pathway is disrupted. As in the Hh pathway, overexpression and/or activating mutations in the β-catenin pathway lead to skin tumors, in this case pilomatricomas or trichofolliculomas (hair follicle tumors), as previously mentioned [69–71]. A recent publication by Palmer et al. [72] evaluated the interaction between VDR and β-catenin in transcriptional regulation and identified putative response elements for VDR and β-catenin/LEF in a number of genes including Shh, Ptch1 and -2, and Gli1 and -2. Furthermore, they found that the ability of β-catenin overexpression to induce trichofolliculomas was blocked by an analogue of 1,25(OH)2D, and in the absence of VDR, BCC were induced rather than trichofolliculomas.
In human tumors, Palmer et al. [72] noted that trichofolliculomas have high nuclear levels of both β-catenin and VDR, whereas BCC have high levels of β-catenin but low levels of VDR. These observations are consistent with their animal data showing that lack of VDR in the skin of animals with activated β-catenin resulted in BCC. Saldanha et al. [106] likewise found nuclear localization of β-catenin in 20 of 86 BCC, which correlated with increased proliferative activity in these tumors, but they did not correlate these results with VDR levels. Thus in humans as well as mice, VDR appears to modulate the differential action of β-catenin signal in the hair follicle and in the epidermis.
The β-catenin and Shh pathways interact [68, 72]; both are required for normal hair follicle development and cycling. Putative β-catenin/LEF response elements have been found in a number of Hh pathway genes [72]. Conditional deletion of β-catenin eliminates Shh expression from the hair follicle [52] and tongue [107], whereas Shh inhibits β-catenin transcriptional activity [107]. The degree to which β-catenin–Shh interactions occur in the formation of skin cancer has not been examined.
Ligand-dependent versus ligand-independent actions of VDR in preventing skin cancer
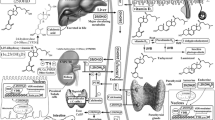
Our working model by which 1,25(OH)2D and its receptor VDR regulate the Hh and β-catenin signaling pathways is shown in Fig. 5. As already discussed, most skin tumors induced by systemic DMBA in mice lacking the VDR contain hair follicle elements and/or are of basal cell origin, tumors characteristic of overexpression of the Hh and β-catenin pathways in keratinocytes. UVR induces mostly SCC but some BCC in VDR null animals [90]. The VDR is found in the ORS and basal layer of the interfollicular epidermis, a postulated source for BCC development [15], but the VDR is also found in the upper layers of the epidermis, where it promotes differentiation. Lack of VDR causes a hyperproliferative response in the keratinocytes of the ORS of the hair follicle and basal layer of epidermis during catagen (days 15–19), with disruption of normal hair follicle cycling and decreased epidermal differentiation [14, 15]. We postulate that increased Hh and β-catenin signaling in the keratinocytes of the VDR null animal predisposes the skin to the development of tumors, in part by stimulating proliferation and reducing differentiation. At this point it is unclear the degree to which the role of VDR is ligand (i.e., 1,25(OH)2D) dependent or independent. Disruption of hair follicle cycling in the VDR null mouse is accompanied by both hyperproliferation and increased apoptosis in the hair follicle [15]; disruption of hair follicle cycling is not found in the CYP27B1 null mouse [13]. Furthermore, we were unable to show that mice lacking 1,25(OH)2D3 production (CYP27B1 null) had increased susceptibility to UVR-induced tumor formation. Thus, 1,25(OH)2D may have only a synergistic role concerning UVR-induced tumor formation in the epidermis.
Regulation of Hh and β-catenin signaling in keratinocytes by 1,25(OH)2D and VDR. The keratinocyte is capable of making its own 1,25(OH)2D from the vitamin D produced from 7-dehydrocholesterol (DHC) under the influence of UVB, as it has both CYP27A1 (which converts vitamin D to 25OHD) and CYP27b1 (which converts 25OHD to 1,25(OH)2D). However, both 25OHD and 1,25(OH)2D are also available from the circulation. The VDR with or without its ligand suppresses Shh expression, may have a direct inhibitory action on Gli transcriptional activity (postulated, not demonstrated), binds β-catenin, and induces E-cadherin expression, reducing the amount of β-catenin available for binding to LEF1. However, VDR may promote the transcriptional activity of β-catenin in a ligand-independent fashion, at least in hair follicle keratinocytes. These actions regulate transcriptional activity of both Gli and β-catenin
Conclusions
Vitamin D and its receptor have many roles in the skin. Some of these roles—induction of genes required for differentiation, suppression of genes involved with proliferation—appear to require both 1,25(OH)2D and VDR. Other roles such as regulation of hair follicle cycling and tumor suppression do not appear to be ligand-dependent, or at least 1,25(OH)2D-dependent, roles of VDR. Different coactivator complexes including DRIP and SRC modulate the actions of VDR, and the choice of coactivator complex in many cases is gene specific. Regulation of proliferation is dependent on DRIP, whereas more differentiated functions including innate immunity and permeability barrier formation are SRC dependent. Hr is a coregulator with profound actions in hair follicle cycling. Although Hr blocks 1,25(OH)2D-regulated VDR functions, its role in VDR-regulated hair follicle cycling is less clear. β-Catenin interactions with VDR function can enhance VDR induction of some genes, whereas VDR can suppress β-catenin induction of other genes. Many genes in the skin contain both putative VDREs and LEF/TCF sites. As is β-catenin, the Hh pathway is critical for hair follicle cycling, and again resembling β-catenin, the Hh pathway is differentially regulated in the interfollicular epidermis compared to the proximal hair follicle. Both β-catenin and Hh pathways are likely mediators of the predisposition of VDR null animals to epidermal tumor formation. At this point we are a long way away from understanding how vitamin D via its active metabolite(s) and receptor (VDR) regulate all the various functions in the skin that it appears to regulate. But, what we have learned indicates that the skin is a fertile area for understanding the mechanisms by which vitamin D regulates so many different physiological processes.
References
Bikle DD, Pillai S (1993) Vitamin D, calcium, and epidermal differentiation. Endocr Rev 14:3–19
Pillai S, Bikle DD (1991) Role of intracellular-free calcium in the cornified envelope formation of keratinocytes: differences in the mode of action of extracellular calcium and 1,25 dihydroxyvitamin D3. J Cell Physiol 146:94–100
Bikle DD, Pillai S, Gee E (1991) Squamous carcinoma cell lines produce 1,25 dihydroxyvitamin D, but fail to respond to its prodifferentiating effect. J Invest Dermatol 97:435–441
Hosomi J, Hosoi J, Abe E, Suda T, Kuroki T (1983) Regulation of terminal differentiation of cultured mouse epidermal cells by 1-alpha, 25-dihydroxyvitamin D3. Endocrinology 113:1950–1957
Smith EL, Walworth NC, Holick MF (1986) Effect of 1-alpha, 25-dihydroxyvitamin D3 on the morphologic and biochemical differentiation of cultured human epidermal keratinocytes grown in serum-free conditions. J Invest Dermatol 86:709–714
McLane JA, Katz M, Abdelkader N (1990) Effect of 1,25-dihydroxyvitamin D3 on human keratinocytes grown under different culture conditions. In Vitro Cell Dev Biol 26:379–387
Hawker NP, Pennypacker SD, Chang SM, Bikle DD (2007) Regulation of human epidermal keratinocyte differentiation by the vitamin D receptor and its coactivators DRIP205, SRC2, and SRC3. J Invest Dermatol 127:874
Matsumoto K, Hashimoto K, Nishida Y, Hashiro M, Yoshikawa K (1990) Growth-inhibitory effects of 1,25-dihydroxyvitamin D3 on normal human keratinocytes cultured in serum-free medium. Biochem Biophys Res Commun 166:916–923
Oda Y, Uchida Y, Moradian S, Crumrine D, Elias PM, Bikle DD (2009) Vitamin D receptor and coactivators SRC2 and 3 regulate epidermis-specific sphingolipid production and permeability barrier formation. J Invest Dermatol 129:1367–1378
Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zugel U, Bikle DD, Modlin RL, Gallo RL (2007) Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest 117:803–811
Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL (2006) Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology 118:509–519
Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB (1997) Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA 94:9831–9835
Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Choi EH, Dardenne O, Xie Z, Arnaud RS, Feingold K, Elias PM (2004) 25 Hydroxyvitamin D 1-alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol 122:984–992
Xie Z, Komuves L, Yu QC, Elalieh H, Ng DC, Leary C, Chang S, Crumrine D, Yoshizawa T, Kato S, Bikle DD (2002) Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol 118:11–16
Bikle DD, Elalieh H, Chang S, Xie Z, Sundberg JP (2006) Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol 207:340–353
Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M (2001) Extrarenal expression of 25-hydroxyvitamin D(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab 86:888–894
Milde P, Hauser U, Simon T, Mall G, Ernst V, Haussler MR, Frosch P, Rauterberg EW (1991) Expression of 1,25-dihydroxyvitamin D3 receptors in normal and psoriatic skin. J Invest Dermatol 97:230–239
Stumpf WE, Clark SA, Sar M, DeLuca HF (1984) Topographical and developmental studies on target sites of 1,25 (OH)2 vitamin D3 in skin. Cell Tissue Res 238:489–496
Oda Y, Sihlbom C, Chalkley RJ, Huang L, Rachez C, Chang CP, Burlingame AL, Freedman LP, Bikle DD (2003) Two distinct coactivators, DRIP/mediator and SRC/p160, are differentially involved in vitamin D receptor transactivation during keratinocyte differentiation. Mol Endocrinol 17:2329–2339
McKenna NJ, Lanz RB, O’Malley BW (1999) Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 20:321–344
Oda Y, Ishikawa MH, Hawker NP, Yun QC, Bikle DD (2007) Differential role of two VDR coactivators, DRIP205 and SRC-3, in keratinocyte proliferation and differentiation. J Steroid Biochem Mol Biol 103:776–780
Schauber J, Oda Y, Buchau AS, Steinmeyer A, Zugel U, Bikle DD, Gallo RL (2008) Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol 128:816–824
Rachez C, Lemon BD, Suldan Z, Bromleigh V, Gamble M, Naar AM, Erdjument-Bromage H, Tempst P, Freedman LP (1999) Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature (Lond) 398:824–828
Rachez C, Gamble M, Chang CP, Atkins GB, Lazar MA, Freedman LP (2000) The DRIP complex and SRC-1/p160 coactivators share similar nuclear receptor binding determinants but constitute functionally distinct complexes. Mol Cell Biol 20:2718–2726
Leo C, Chen JD (2000) The SRC family of nuclear receptor coactivators. Gene (Amst) 245:1–11
Teichert A, Arnold LA, Otieno S, Oda Y, Augustinaite I, Geistlinger TR, Kriwacki RW, Guy RK, Bikle DD (2009) Quantification of the vitamin D receptor-coregulator interaction. Biochemistry 48:1454–1461
Acevedo ML, Lee KC, Stender JD, Katzenellenbogen BS, Kraus WL (2004) Selective recognition of distinct classes of coactivators by a ligand-inducible activation domain. Mol Cell 13:725–738
Christakos S, Dhawan P, Liu Y, Peng X, Porta A (2003) New insights into the mechanisms of vitamin D action. J Cell Biochem 88:695–705
Rachez C, Freedman LP (2000) Mechanisms of gene regulation by vitamin D(3) receptor: a network of coactivator interactions. Gene (Amst) 246:9–21
Carvallo L, Henriquez B, Paredes R, Olate J, Onate S, Van Wijnen AJ, Lian JB, Stein G, Stein JL, Montecino M (2008) 1,25-Dihydroxy vitamin D3-enhanced expression of the osteocalcin gene involves increased promoter occupancy of basal transcription regulators and gradual recruitment of the 1,25-dihydroxy vitamin D3 receptor-SRC-1 coactivator complex. J Cell Physiol 214:740–749
Issa LL, Leong GM, Sutherland RL, Eisman JA (2002) Vitamin D analogue-specific recruitment of vitamin D receptor coactivators. J Bone Miner Res 17:879–890
Bouillon R, Verlinden L, Eelen G, De Clercq PV M, Mathieu C, Verstuyf A (2005) Mechanisms for the selective action of vitamin D analogs. J Steroid Biochem Mol Biol 97:21–30
Maeda Y, Rachez C, Hawel IL, Byus CV, Freedman LP, Sladek FM (2002) Polyamines modulate the interaction between nuclear receptors and vitamin D receptor-interacting protein 205. Mol Endocrinol 16:1502–1510
Peleg S, Ismail A, Uskokovic M, Avnur Z (2003) Evidence for tissue- and cell-type selective activation of the vitamin D receptor by Ro-26–9228, a noncalcemic analog of vitamin D3. J Cell Biochem 88:267–273
Shimizu H, Morgan BA (2004) Wnt signaling through the beta-catenin pathway is sufficient to maintain, but not restore, anagen-phase characteristics of dermal papilla cells. J Invest Dermatol 122:239–245
Kishimoto J, Burgeson RE, Morgan BA (2000) Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev 14:1181–1185
Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G (2004) Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 22:411–417
Langbein L, Rogers MA, Praetzel S, Winter H, Schweizer J (2003) K6irs1, K6irs2, K6irs3, and K6irs4 represent the inner-root-sheath-specific type II epithelial keratins of the human hair follicle. J Invest Dermatol 120:512–522
Zhou P, Byrne C, Jacobs J, Fuchs E (1995) Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev 9:700–713
Hochberg Z, Gilhar A, Haim S, Friedman-Birnbaum R, Levy J, Benderly A (1985) Calcitriol-resistant rickets with alopecia. Arch Dermatol 121:646–647
Marx SJ, Bliziotes MM, Nanes M (1986) Analysis of the relation between alopecia and resistance to 1,25-dihydroxyvitamin D. Clin Endocrinol (Oxf) 25:373–381
Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S (1997) Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 16:391–396
Sakai Y, Demay MB (2000) Evaluation of keratinocyte proliferation and differentiation in vitamin D receptor knockout mice. Endocrinology 141:2043–2049
Kong J, Li XJ, Gavin D, Jiang Y, Li YC (2002) Targeted expression of human vitamin d receptor in the skin promotes the initiation of the postnatal hair follicle cycle and rescues the alopecia in vitamin D receptor null mice. J Invest Dermatol 118:631–638
Chen CH, Sakai Y, Demay MB (2001) Targeting expression of the human vitamin D receptor to the keratinocytes of vitamin D receptor null mice prevents alopecia. Endocrinology 142:5386–5389
Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R, Delling G, Demay MB (1998) Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology 139:4391–4396
Panteleyev AA, Botchkareva NV, Sundberg JP, Christiano AM, Paus R (1999) The role of the hairless (hr) gene in the regulation of hair follicle catagen transformation. Am J Pathol 155:159–171
Miller J, Djabali K, Chen T, Liu Y, Ioffreda M, Lyle S, Christiano AM, Holick M, Cotsarelis G (2001) Atrichia caused by mutations in the vitamin D receptor gene is a phenocopy of generalized atrichia caused by mutations in the hairless gene. J Invest Dermatol 117:612–617
Ahmad W, Faiyaz ul Haque M, Brancolini V, Tsou HC, ul Haque S, Lam H, Aita VM, Owen J, deBlaquiere M, Frank J, Cserhalmi-Friedman PB, Leask A, McGrath JA, Peacocke M, Ahmad M, Ott J, Christiano AM (1998) Alopecia universalis associated with a mutation in the human hairless gene. Science 279:720–724
Millar SE (2002) Molecular mechanisms regulating hair follicle development. J Invest Dermatol 118:216–225
Stenn KS, Paus R (2001) Controls of hair follicle cycling. Physiol Rev 81:449–494
Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W (2001) beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105:533–545
DasGupta R, Rhee H, Fuchs E (2002) A developmental conundrum: a stabilized form of beta-catenin lacking the transcriptional activation domain triggers features of hair cell fate in epidermal cells and epidermal cell fate in hair follicle cells. J Cell Biol 158:331–344
Zarach JM, Beaudoin GM III, Coulombe PA, Thompson CC (2004) The co-repressor hairless has a role in epithelial cell differentiation in the skin. Development (Camb) 131:4189–4200
Chiang C, Swan RZ, Grachtchouk M, Bolinger M, Litingtung Y, Robertson EK, Cooper MK, Gaffield W, Westphal H, Beachy PA, Dlugosz AA (1999) Essential role for sonic hedgehog during hair follicle morphogenesis. Dev Biol 205:1–9
Beaudoin GM III, Sisk JM, Coulombe PA, Thompson CC (2005) Hairless triggers reactivation of hair growth by promoting Wnt signaling. Proc Natl Acad Sci USA 102:14653–14658
Reddy ST, Andl T, Lu MM, Morrisey EE, Millar SE (2004) Expression of Frizzled genes in developing and postnatal hair follicles. J Invest Dermatol 123:275–282
Shah S, Hecht A, Pestell R, Byers SW (2003) Trans-repression of beta-catenin activity by nuclear receptors. J Biol Chem 278:48137–48145
Shah S, Islam MN, Dakshanamurthy S, Rizvi I, Rao M, Herrell R, Zinser G, Valrance M, Aranda A, Moras D, Norman A, Welsh J, Byers SW (2006) The molecular basis of vitamin D receptor and beta-catenin crossregulation. Mol Cell 21:799–809
Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Munoz A (2001) Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol 154:369–387
Djabali K, Aita VM, Christiano AM (2001) Hairless is translocated to the nucleus via a novel bipartite nuclear localization signal and is associated with the nuclear matrix. J Cell Sci 114:367–376
Thompson CC, Bottcher MC (1997) The product of a thyroid hormone-responsive gene interacts with thyroid hormone receptors. Proc Natl Acad Sci USA 94:8527–8532
Engelhard A, Christiano AM (2004) The hairless promoter is differentially regulated by thyroid hormone in keratinocytes and neuroblastoma cells. Exp Dermatol 13:257–264
Xie Z, Chang S, Oda Y, Bikle DD (2006) Hairless suppresses vitamin D receptor transactivation in human keratinocytes. Endocrinology 147:314–323
Hsieh JC, Sisk JM, Jurutka PW, Haussler CA, Slater SA, Haussler MR, Thompson CC (2003) Physical and functional interaction between the vitamin D receptor and hairless corepressor, two proteins required for hair cycling. J Biol Chem 278:38665–38674
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW (1998) Identification of c-MYC as a target of the APC pathway. Science 281:1509–1512
Xie Z, Bikle DD (2007) The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. J Biol Chem 282:8695–8703
Bienz M (2005) beta-Catenin: a pivot between cell adhesion and Wnt signalling. Curr Biol 15:R64–R67
Chan EF, Gat U, McNiff JM, Fuchs E (1999) A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet 21:410–413
Gat U, DasGupta R, Degenstein L, Fuchs E (1998) De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 95:605–614
Xia J, Urabe K, Moroi Y, Koga T, Duan H, Li Y, Furue M (2006) beta-Catenin mutation and its nuclear localization are confirmed to be frequent causes of Wnt signaling pathway activation in pilomatricomas. J Dermatol Sci 41:67–75
Palmer HG, Anjos-Afonso F, Carmeliet G, Takeda H, Watt FM (2008) The vitamin D receptor is a Wnt effector that controls hair follicle differentiation and specifies tumor type in adult epidermis. PLoS ONE 3:e1483
Cianferotti L, Cox M, Skorija K, Demay MB (2007) Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc Natl Acad Sci USA 104:9428–9433
Greenlee RT, Hill-Harmon MB, Murray T, Thun M (2001) Cancer statistics, 2001. CA Cancer J Clin 51:15–36
Freeman SE, Hacham H, Gange RW, Maytum DJ, Sutherland JC, Sutherland BM (1989) Wavelength dependence of pyrimidine dimer formation in DNA of human skin irradiated in situ with ultraviolet light. Proc Natl Acad Sci USA 86:5605–5609
Hussein MR (2005) Ultraviolet radiation and skin cancer: molecular mechanisms. J Cutan Pathol 32:191–205
Daya-Grosjean L, Sarasin A (2005) The role of UV induced lesions in skin carcinogenesis: an overview of oncogene and tumor suppressor gene modifications in xeroderma pigmentosum skin tumors. Mutat Res 571:43–56
Ziegler A, Leffell DJ, Kunala S, Sharma HW, Gailani M, Simon JA, Halperin AJ, Baden HP, Shapiro PE, Bale AE, Brash DE (1993) Mutation hotspots due to sunlight in the p53 gene of nonmelanoma skin cancers. Proc Natl Acad Sci USA 90:4216–4220
Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, Remington L, Jacks T, Brash DE (1994) Sunburn and p53 in the onset of skin cancer. Nature (Lond) 372:773–776
Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, Halperin AJ, Ponten J (1991) A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci USA 88:10124–10128
Bito T, Ueda M, Ahmed NU, Nagano T, Ichihashi M (1995) Cyclin D and retinoblastoma gene product expression in actinic keratosis and cutaneous squamous cell carcinoma in relation to p53 expression. J Cutan Pathol 22:427–434
Reifenberger J, Wolter M, Knobbe CB, Kohler B, Schonicke A, Scharwachter C, Kumar K, Blaschke B, Ruzicka T, Reifenberger G (2005) Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br J Dermatol 152:43–51
Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, Quinn AG, Myers RM, Cox DR, Epstein EH Jr, Scott MP (1996) Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 272:1668–1671
Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E, Unden AB, Gillies S, Negus K, Smyth I, Pressman C, Leffell DJ, Gerrard B, Goldstein AM, Dean M, Toftgard R, Chenevix-Trench G, Wainwright B, Bale AE (1996) Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 85:841–851
Aszterbaum M, Rothman A, Johnson RL, Fisher M, Xie J, Bonifas JM, Zhang X, Scott MP, Epstein EH Jr (1998) Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol 110:885–888
Aszterbaum M, Epstein J, Oro A, Douglas V, LeBoit PE, Scott MP, Epstein EH Jr (1999) Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med 5:1285–1291
Ping XL, Ratner D, Zhang H, Wu XL, Zhang MJ, Chen FF, Silvers DN, Peacocke M, Tsou HC (2001) PTCH mutations in squamous cell carcinoma of the skin. J Invest Dermatol 116:614–616
Zinser GM, Sundberg JP, Welsh J (2002) Vitamin D(3) receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis (Oxf) 23:2103–2109
Indra AK, Castaneda E, Antal MC, Jiang M, Messaddeq N, Meng X, Loehr CV, Gariglio P, Kato S, Wahli W, Desvergne B, Metzger D, Chambon P (2007) Malignant transformation of DMBA/TPA-induced papillomas and nevi in the skin of mice selectively lacking retinoid-X-receptor alpha in epidermal keratinocytes. J Invest Dermatol 127:1250–1260
Ellison TI, Smith MK, Gilliam AC, Macdonald PN (2008) Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J Invest Dermatol 128:2508–2517
Regl G, Kasper M, Schnidar H, Eichberger T, Neill GW, Ikram MS, Quinn AG, Philpott MP, Frischauf AM, Aberger F (2004) The zinc-finger transcription factor GLI2 antagonizes contact inhibition and differentiation of human epidermal cells. Oncogene 23:1263–1274
Barnfield PC, Zhang X, Thanabalasingham V, Yoshida M, Hui CC (2005) Negative regulation of Gli1 and Gli2 activator function by Suppressor of fused through multiple mechanisms. Differentiation 73:397–405
Wakabayashi Y, Mao JH, Brown K, Girardi M, Balmain A (2007) Promotion of Hras-induced squamous carcinomas by a polymorphic variant of the Patched gene in FVB mice. Nature (Lond) 445:761–765
Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP (2006) Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol 4:e232
Svard J, Heby-Henricson K, Persson-Lek M, Rozell B, Lauth M, Bergstrom A, Ericson J, Toftgard R, Teglund S (2006) Genetic elimination of suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev Cell 10:187–197
Regl G, Kasper M, Schnidar H, Eichberger T, Neill GW, Philpott MP, Esterbauer H, Hauser-Kronberger C, Frischauf AM, Aberger F (2004) Activation of the BCL2 promoter in response to Hedgehog/GLI signal transduction is predominantly mediated by GLI2. Cancer Res 64:7724–7731
Regl G, Neill GW, Eichberger T, Kasper M, Ikram MS, Koller J, Hintner H, Quinn AG, Frischauf AM, Aberger F (2002) Human GLI2 and GLI1 are part of a positive feedback mechanism in basal cell carcinoma. Oncogene 21:5529–5539
Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui CC, Dlugosz AA (2000) Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet 24:216–217
Nilsson M, Unden AB, Krause D, Malmqwist U, Raza K, Zaphiropoulos PG, Toftgard R (2000) Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc Natl Acad Sci USA 97:3438–3443
Oro AE, Higgins KM, Hu Z, Bonifas JM, Epstein EH Jr, Scott MP (1997) Basal cell carcinomas in mice overexpressing sonic hedgehog. Science 276:817–821
Fan H, Oro AE, Scott MP, Khavari PA (1997) Induction of basal cell carcinoma features in transgenic human skin expressing sonic hedgehog. Nat Med 3:788–792
Tojo M, Mori T, Kiyosawa H, Honma Y, Tanno Y, Kanazawa KY, Yokoya S, Kaneko F, Wanaka A (1999) Expression of sonic hedgehog signal transducers, patched and smoothened, in human basal cell carcinoma. Pathol Int 49:687–694
Bonifas JM, Pennypacker S, Chuang PT, McMahon AP, Williams M, Rosenthal A, De Sauvage FJ, Epstein EH Jr (2001) Activation of expression of hedgehog target genes in basal cell carcinomas. J Invest Dermatol 116:739–742
Eichberger T, Regl G, Ikram MS, Neill GW, Philpott MP, Aberger F, Frischauf AM (2004) FOXE1, a new transcriptional target of GLI2 is expressed in human epidermis and basal cell carcinoma. J Invest Dermatol 122:1180–1187
Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, Bourdeau V, Konstorum A, Lallemant B, Zhang R, Mader S, White JH (2005) Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol 19:2685–2695
Saldanha G, Ghura V, Potter L, Fletcher A (2004) Nuclear beta-catenin in basal cell carcinoma correlates with increased proliferation. Br J Dermatol 151:157–164
Iwatsuki K, Liu HX, Gronder A, Singer MA, Lane TF, Grosschedl R, Mistretta CM, Margolskee RF (2007) Wnt signaling interacts with Shh to regulate taste papilla development. Proc Natl Acad Sci USA 104:2253–2258
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Bikle, D.D. Vitamin D and the skin. J Bone Miner Metab 28, 117–130 (2010). https://doi.org/10.1007/s00774-009-0153-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-009-0153-8