Abstract
Recalcitrant pollutants mainly come from industrial processes such as mineral extraction, metal casting and coating, discharge of effluents, and nuclear fuel generation. These pollutants are introduced into food chain resulting in their accumulation in human body. Even at low concentration they pose serious health effects. A low-cost biotechnological alternative for remediation of recalcitrant pollutants is phytoremediation. Plants are capable of degrading, immobilizing, stabilizing, and extracting toxic elements from soil, sediment, or water environs. Furthermore, this chapter summarizes the information obtained by scientific sources about the use of plants for cleaning up of recalcitrant pollutants from disturbed environs. Reviews of 80 plants used in remediation of heavy metals and 13 to remove organic compounds have been discussed in this chapter. In addition a case study pertaining to phytoremediation potential of Typha latifolia was discussed in lucid manner. The study revealed that T. latifolia is a hyperaccumulator for Pb and removes other heavy metals at good rate in disturbed environs. Therefore, the present investigation suggests that plants are capable of degrading toxic substances and should be employed for restoration of contaminated environs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
Water is a fundamental substance for the sustenance and reproduction of life since it is indispensable for the development of biological processes (Mongue 2004). However, we currently find that various water sources such as wells and rivers are contaminated with large amounts of recalcitrant’s pollutants (Wong Argüelles 2009). The term recalcitrant applies to compounds whose presence in the environment is prolonged due to their difficult degradation. These compounds can be from simple halogenated hydrocarbons to complex polymers. The recalcitrants can persist even in effluents that have received a previous treatment, which causes the presence of these compounds in the receiving environments causing cause health and environmental problems (Gupta and Thakur 2017). The low concentrations of recalcitrant in the environment make its treatment and elimination difficult using conventional treatments such as activated carbon, chemical precipitation, exchange resins, and filtration (Sandhya et al. 2005).
This chapter summarizes the information obtained, by scientific sources, about plants used in the phytoremediation of organic and inorganic recalcitrant contaminants. In addition, a study we conducted with Typha latifolia in the removal of heavy metals is presented.
11.2 Main Recalcitrants Pollutants
The recalcitrant pollutants are a variety of compounds such as heavy metals, chlorinated phenolic compounds (CPCs), complex dyes, explosive compounds, poly-aromatic hydrocarbons (PAH), polychlorinated biphenyls (PCBs), organochlorine pesticides (OCPs), pharmaceutical active compounds (PhACs) (Ahmadi et al. 2017) (Fig. 11.1).
Heavy Metals
Heavy metals are natural components of the Earth’s crust, and some are indispensable for cell growth and biological mechanisms, however, when released into the environment, and exceed permitted limits are considered toxic. Most heavy metals come from industrial sources and are considered hazardous soil contaminants because they have chronic and acute toxicity (Abdel-Shafy and Mansour 2018). Heavy metals have a prolonged persistence because they do not degrade, which causes them to accumulate in living organisms causing toxic and potential adverse effects on humans and ecosystems (Khatoon et al. 2017). The major toxic heavy metals are Hg (I), Cd (II), Pb (II), Cr (IV), Fe (III), and Cu (II) (Sud et al. 2008).
Complex Dyes
Most synthetic dyes are toxic and highly resistant to degradation due to their complex chemical structures. The textile industry is one of the largest generators of liquid effluent contaminants due to the high amounts of water used in dyeing processes. In recent years, interest in environmental control of dyes has increased due to their toxicity and carcinogenicity, as it contains carcinogenic compounds such as benzidine and other aromatic compounds (Ali 2010).
Explosive Compounds
Explosives are highly stable compounds and have the tendency to bind with soil organic matter and contaminate it. Explosive materials are energy substances that, when released, present toxic hazards to the environment and biota. Around the world, soils are contaminated by such pollutants, whether due to manufacturing operations, military activities, conflicts of different levels, open burning/open detonation, ammunition discharge, etc. (Chatterjee et al. 2017).
Poly-Aromatic Hydrocarbons (PAHs)
They are considered as one of the most dangerous chemical pollutants in the environment. These chemicals have carcinogenic, toxic, and mutagenic effects on the living body. In addition to their carcinogenic activity, these PAHs are recalcitrant, ubiquitous, and have bioaccumulative potential. Diesel and all hydrocarbon fuels are composed of an excessive amount of PAHs. Therefore, PAHs cause abundant pollution to ecosystems (Abdel-Shafy and Mansour 2018).
Polychlorinated Biphenyls (PCBs)
They are among one of these groups of notorious contaminants. Polychlorinated biphenyls (PCBs) are members of chlorinated organic chemicals that theoretically can contain 209 different congeners. Highly chlorinated congeners are more stable and tend to have lower solubility in aqueous solution and also have high octanol water partition coefficients (KOW) than low molecular weight PCBs. The high KOW is partly responsible for their persistence and allows them to absorb strongly in the soil. Due to their environmental and ecological impacts, PBCs have been included in the Stockholm Convention as priority persistent organic pollutants (POPs), which must be removed from the environment by 2025.
Pharmaceutically Active Compounds (PhACs)
In aquatic environments, PhACs have become an important issue that attracts the attention of the public due to their pseudo-persistence, possible toxic effects on microorganisms, and the widespread occurrence in surface waters (Yan et al. 2016). The aromatic side-chain compounds used in the pharmaceutical industry are difficult to biodegrade due to their high persistence in the environment and their resistance to degradation. These compounds reach water systems from different sources, such as human excretion (wastewater), improper disposal, landfill leaching, water drainage, or industries. It has been found that PhACs have the potential to cause aquatic toxicity , development of resistance in pathogenic microbes, genotoxicity, and endocrine alteration (Couto et al. 2019).
Chlorinated Phenolic Compounds (CPCs)
The contamination of the environment by chlorinated aromatic compounds is a serious problem due to its large-scale applications such as biocides, lubricants, solvents, etc. and its high stability to biodegradation (Colomban et al. 2014). CPCs are the precursors or intermediates in many process industries, such as the dye, resin, and plastic, pharmaceutical, and pulp and paper industries. CPCs are irritating at low levels and have a very negative impact on the central respiratory and nervous systems at higher doses. They also have adverse effects of acute toxicity and carcinogenic properties (Miran et al. 2017).
Organochlorine Pesticides (OCPs)
These compounds are of environmental concern due to their recalcitrance in soils and sediments, global distribution, and toxicity. The OCPs had been applied to the soil to protect the crops until they were banned. Due to their properties, they could bind strongly to organic matter and become trapped within the soil matrix, which would lead to persistence several years after their last use. The presence of pesticides released into the soil represents a risk to the environment since they can migrate through the profile; be absorbed by biota and transported to aquatic systems by erosion (Mitton et al. 2012).
11.3 Phytoremediation Technology
Phytoremediation is a term that applies to a group of technologies that use plants to reduce, eliminate, degrade, or immobilize toxic compounds in the environment through biological processes that develop the same plants or through synergistic plant-microorganism processes that develop in the rhizosphere, with the objective of restoring the areas to a condition in which they are usable (Manahan 2007; Peer et al. 2006). This technology is used to remove heavy metals, radionuclides, nutrients (nitrate, phosphate, etc.), solvents, explosives, crude oil and organic pollutants such as persistent organic pollutants (POPs), polycyclic aromatic hydrocarbons (PAHs), and pesticides from wastewater and soils (Anand et al. 2019).
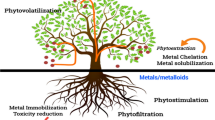
Phytoremediation of contaminated sites has gained popularity as a non-invasive, relatively inexpensive, and esthetically pleasing technology to the public compared to alternative remediation strategies that involve excavation, removal or stabilization, and chemical conversion in situ (Peer et al. 2006). The phytoremediation is a sustainable technology for cleaning up of recalcitrant pollutants from disturbed environs. The phytoremediation strategies have a number of promising advantages in comparison with other remediation techniques, in particular (Fig. 11.2).
There are factors that influence plant phytoremediation, which include the ability of the plant to absorb pollutants through its roots and transfer it to the shoots; the level at which plants tolerate contaminants and the available concentration of contaminants so that the plant can absorb them; and the rate of transforming toxic substances into less toxic by plants. Other factors that also influence phytoremediation are the root length of the plants, the types and concentration of the target metals, the environmental condition of the soil, and the interaction of the plants and associated soil microorganisms (Naila et al. 2019).
11.4 Plants Used in Phytoremediation Technology to Cleaning Up of Recalcitrant Pollutants
This chapter provides information of plants considered hyperaccumulants of recalcitrant contaminants mainly heavy metals in Table 11.1 and other recalcitrant pollutants are presented in Table 11.2. The information was searched by consulting the following electronic sources: ScienceDirect, SCOPUS, Web of Science, SpringerLink, SCIELO, PubMed, and Google Scholar finding 77 studies with different heavy metal accumulator plants such as Cd, Pb, Hg, Mn, Ni, B, Zn, Tl, Co, and Cu. Table 11.1 shows the plant species and the recalcitrant pollutant that removes the part where it accumulates.
Table 11.2 presents the 13 studies with plants removing other recalcitrant pollutants such as polycyclic aromatic hydrocarbons, oil hydrocarbons, phenol, nitrogen compounds.
11.5 Typha latifolia Plant Used in Phytoremediation Technology
Typha latifolia (cattail) is an emergent macrophyte; it is a perennial plant belonging to the Thyphaceae family, which grows in swamps, lakes, and lagoons. In addition, it has the advantage of growing in diverse climatic conditions and in environments contaminated with heavy metals (Alonso-Castro et al. 2009). The aquatic macrophyte presents rapid growth, easy spreading, high-pollutant uptake capacity, and easy harvesting, properties required for aquatic plants used in treatment system (Boyd 1970).
The accumulation of toxic elements by T. latifolia is a gradual process and depends on the concentration of elements and time of exposure. Additionally, this plant presents characteristics desirable for the remediation of aquatic sites such as a well-developed root–rhizome system, easy harvesting, and high biomass production. Further analyses are necessary to characterize the cellular mechanisms involved in the internalization of these elements in this plant. Previous research conducted by our research group indicated that T. latifolia presents the ability to remove and accumulate As, Cd, Pb, Ni, Cr, Mn, and Fe from in situ (an artificial lagoon) and controlled conditions (Carranza Álvarez 2005; Alonso-Castro et al. 2009; Carranza Álvarez et al. 2008; Leura Vicencio et al. 2013). In addition, promoter bacteria were isolated from the root of T. latifolia, finding that these exert a protective role in the phytoextraction of heavy metals. Nevertheless, the response to the toxic elements stress depends on the plant species.
11.5.1 Typha latifolia as Biosorbent
The heavy metals present in waters come mainly from industrial processes such as mineral extraction, wastewater treatment, metal casting and coating, nuclear and nuclear generation industries, among others. Heavy metals are introduced into food chains resulting in accumulation in the human body. Even at low concentrations they have important health effects such as stunted growth, spread of cancer, damage to organs, deterioration of the nervous system, and, in more severe cases, death (Aghababai and Beni 2020).
Lead is a non-essential element in the human body and its accumulation can cause some damage to the brain, leading to decreased learning and memory. It also affects bone metabolism, causing genetic mutations (Chu et al. 2019). There are several processes that can be applied in water treatment to reduce the concentration of heavy metals and other pollutants such as ion exchange, membrane separation, electrodialysis, reverse osmosis, solvent extraction, evaporation, etc. These methods are expensive and ineffective. Adsorption has become an economical and effective method for treating heavy metal wastewater due to its easy availability of raw materials and simple operation (Elhafez et al. 2017). Biosorption, considered as a type of adsorption using biomass, is a promising method for the treatment of Pb (II) contaminated water due to its low cost and minimization of toxic sludge (Liu et al. 2014). The removal of heavy metals using biomass is attributed to a stable and insoluble matrix with active groups on the surface mainly carboxyl, amino, and hydroxyl groups.
There are a large number of reported studies on metal removal using various biosorbents, but few have focused on the use of cattails as the basis for adsorbent materials. Cattails are perennial, herbage plants with high adaptability and large biomass that are usually used in the construction of wetlands for removing nitrogen, phosphorous, and organics from wastewater (Tang et al. 2017). These aquatic plants have taken an interest in the remediation of aquatic ecosystems contaminated by heavy metals. Typha latifolia, Typha angustifolia and Salix matsudana are low-cost materials that have been used directly or as precursors of activated carbons for the removal of heavy metals such as Pb(II), Cd(II), Cr(VI), Cu(II), Mn(II), Zn(II), among others. However, few studies evaluate the physicochemical properties of biosorbents and their relationship with the removal of the metal of interest (Bukhari et al. 2013; Kumari and Tripathi 2015; Liu et al. 2014; Rajaei et al. 2013; Song et al. 2013; Tang et al. 2017).
Therefore, the main objective of the present work was to use the root of Typha latifolia as an adsorbent material for the removal of Pb(II) from water as a low-cost alternative, as well as to relate its physicochemical properties and adsorption capacity.
11.5.2 Preparation of Typha latifolia Root as Biosorbent
T. latifolia samples were collected from a temporary lagoon located in the municipality of Ciudad Valles, San Luis Potosí, Mexico, by means of a meticulous collection of plants with similar characteristics such as height between 30 and 50 cm and degree of robustness of their root and rhizome between 10 and 20 cm long and 5 cm wide. Once collected, they were treated using a process of acclimatization at room temperature in a plant nursery for 30 days. During this acclimatization process, the plants were provided with nutrients (K, P, N) and enough water to promote root growth. Next, the plants with the largest amount of root were selected and washed with abundant water to remove impurities, the roots were cut and washed again using distilled water, then introduced into the stove for drying at 70 °C for 72 h. The roots were then crushed and pulverized using an analytical mill. Once the root was pulverized, it was thoroughly washed with deionized water and sieved to an average particle size of 0.16 mm. Finally, the root was dried at 80 °C for 24 h. The biosorbent obtained was packaged and stored in a plastic container for later use.
11.5.3 Characterization of T. latifolia Root
The morphology of the surface of the natural root of T. latifolia was examined by means of a scanning electron microscope (SEM), Philips, model XL-30-SFEG, equipped with a Link/ISIS-OXFORD microanalysis system of dispersed energy (EDS) which allows to identify the elements present on the surface of the material used as biosorbent. The functional groups present in the natural root of T. latifolia were identified by infrared spectroscopy (IR) . The IR spectra of the root were obtained using an infrared spectrophotometer, Thermo-Scientific, model Nicolet iS10 that has the technique of attenuated total reflectance (ATR). The active sites were determined by the acid–base titration method proposed by Boehm (1994). The total acid and basic sites were neutralized with 0.01N NaOH and HNO3 solutions, respectively. Surface charge and zero point charge (pHPZC) were determined by the procedure proposed by Kuzin and Loskutov (1996).
11.5.4 Determination of the Concentration of Pb(II) in Aqueous Solution
The concentration of Pb(II) in aqueous solution was determined using an atomic absorption spectrophotometer, Varian model SpectrAA-20 and the concentration in aqueous solution was estimated using a calibration curve with standard Pb(II) solutions.
11.5.5 Adsorption Isotherms of Pb(II) on T. latifolia Root
Experimental data on the adsorption equilibrium of Pb(II) on the root of T. latifolia were obtained in batch adsorbers as follows. In a volumetric flask of 50 mL a solution of a known concentration of Pb (II) was prepared from a 1000 mg L−1 standard solution and a buffer solution prepared by mixing NaOH and HNO3 solutions was gauged. Next, an initial sample of 10 mL was taken and subsequently analyzed to corroborate the initial concentration. To the batch adsorber, a certain root mass and 40 mL of the initial concentration solution were added. The adsorber was placed in a constant temperature bath, and the root and solution were left in contact until equilibrium was reached. In previous experiments, it was found that 5 days was enough time to achieve equilibrium. Once a day, the adsorbers were mixed in an orbital agitator at 200 rpm for 30 min.
The pH of the solution was measured periodically with a potentiometer and kept constant by adding drops of solutions 0.01, 0.1, and 1N of HNO3 and NaOH, as necessary. The volumes of these solutions were recorded to calculate the final total volume of the solution. Once equilibrium was reached, a 10 mL sample was taken and analyzed to determine the final concentration of the solution. The mass of Pb (II) adsorbed on the root was calculated by means of a mass balance which is mathematically represented as follows:
where q (mg g−1) is the mass of Pb(II) adsorbed per unit root mass, V (L) is the volume of the Pb(II) solution, m (g) is the mass of the root, C0 (mg L−1) is the initial concentration of Pb(II), and Ce (mg L−1) is the concentration of Pb(II) in the equilibrium.
11.5.6 Results and Discussion About the Study of Typha latifolia as a Biosorbent
11.5.6.1 SEM, EDX, and IR Analyses of the T. latifolia
The surface, morphology, and particle distribution of T. latifolia root were observed by scanning electron microscope (SEM) and the elemental chemical composition of the surface was determined by microanalysis (EDX). Figure 11.3a, b shows the photomicrographs of fragments of T. latifolia root. Figure 11.3a shows a micrograph of the root increased to 36×, in which the irregular and rough surface is observed. Figure 11.3b is at 3500× and it is shown in great detail that the surface of the root does not have a uniform topography, also can be seen in it some irregular particles which are attributed to Pb, these in size range between ±5 μm.
Figure 11.4 presents the X-ray fluorescence (EDX) microanalysis of the T. latifolia exposed to Pb. It can be seen that C and O are the main elements, since it is a lignocellulosic material. The presence of Al, Si, and S was also detected in smaller quantities, as well as the characteristic peak of Pb.
The results of the IR spectra (not shown) revealed that the root of T. latifolia exposed to Pb(II) presents a slight change in the band of the functional groups O–H, C–H, C–O, and C–N. The results of the IR spectra revealed that the root of T. latifolia exposed to Pb(II) presents a slight change in the band of the functional groups O–H, C–H, C–O, and C–N. However, the increase in the intensity for the groups C=O and C=C was more noticeable and is a clear indication of the interaction between the organic compounds of T. latifolia and Pb(II) in solution.
It is very important to mention that the influence of the surface chemistry of adsorbent materials confers a certain degree of selectivity, for example, the presence of carboxylic, lactonic, and hydroxylic phenolic groups is responsible for the acidic surface properties of activated carbon (Böehm 2002). These specific functional groups are essential in the adsorption of heavy metals due to their chelating quality forming complexes (Monser and Adhoum 2002; Kikuchi et al. 2006).
11.5.6.2 Adsorption Isotherms
The experimental Pb(II) adsorption equilibrium data were adjusted to the Freundlich and Langmuir isothermal models. These models are mathematically represented with the following equations:
The parameters K (L mg−1) and qm (mg g−1) are the Langmuir constants for Pb(II) related to adsorption energy and maximum adsorption capacity, respectively. The parameters k (L1/n mg1-1/n g−1) and n are the Freundlich constants for Pb(II) related to adsorption capacity and intensity, respectively. The parameters of these isotherms were estimated by a method of least squares based on the Rosenbrock–Newton optimization algorithm which uses the following objective function:
On the other hand, the average absolute percentage deviation %Dev was estimated using the following equation and the results are reported in Table 11.3.
Table 11.3 shows that the isotherm that best adjusted the adsorption equilibrium data was the Freundlich isotherm since the %Dev was lower than the Langmuir isotherm for all cases. The %Dev was lower than 17.2 and 11.5% for the Langmuir and Freundlich isotherms, respectively. Therefore, it was considered that Freundlich isotherm better interpreted the experimental data. It should be noted that the model of the Freundlich isotherm suggests that the surface of the adsorbent is heterogeneous. However, it is important to clarify that the fit of the isotherm model to the adsorption equilibrium data is a statistical mathematical method and not a corroboration that the adsorption mechanism occurs according to the fundamentals that support the model that best adjusted the data.
11.5.6.3 Effect of Solution pH on the Adsorption Capacity
The solution pH is probably the most important factor in the adsorption of ions in aqueous solution onto porous solids. The pH has a considerable influence on the adsorption equilibrium since the surface charge of the adsorbent and the species or ionic complexes formed by the adsorbate are a function of the pH. The effect of solution pH on the adsorption capacity of Pb(II) in aqueous solution on the root of T. latifolia was investigated by determining adsorption isotherms at pH 2, 3, and 5 and T = 25 °C. The effect of pH on the adsorption capacity of Pb(II) in aqueous solution on the root of T. latifolia was investigated by determining adsorption isotherms at pH 2, 3, and 5 and T = 25 °C. Figure 11.5 shows the effect of pH on the adsorption capacity of T. latifolia root and it can be seen that pH significantly affects adsorption capacity.
The results revealed that maximum adsorption capacity occurs at pH 5 and decreases as it decreases from 5 to 3 and from 3 to 2. On the other hand, the averages of Typha root adsorption capacities were 0.96, 1.72, and 7.74 mg g−1 at pH 2, 3, and 5, respectively. This is a clear indication that the adsorption capacity shown by the T. latifolia root increases considerably with increasing pH. The effect of the pH of the solution on the adsorption capacity can be explained considering that the pHPZC of the biosorbent determined in this study was 5.6 which indicates that its character is acid and that the surface charge can be positive, neutral, or negative when the pH < pHPZC, pH = pHPZC, or pH > pHPZC, respectively.
It is important to mention that during the adsorption of Pb(II), the pH of the solution always remained variable during the adsorption progress and to keep it constant it was necessary to add solutions 0.1 and 0.01N of NaOH or HNO3. In the experiments carried out at pH 5.0, the solution varied considerably, increasing with the days until reaching equilibrium. On the other hand, in the experiments performed at pH 2 and 3, the pH of the solution hardly changed. This could indicate that the H+ ions from the root surface were transferred to the solution and the Pb(II) cations in solution were diffused to the T. latifolia root surface. In other words, Pb(II) was adsorbed by ion exchange.
11.5.6.4 Effect of Solution Temperature on the Adsorption Capacity
The effect of the temperature of the solution on the adsorption of Pb(II) in the root of T. latifolia was analyzed by isotherms of Pb(II) adsorption at temperatures of 15, 25, and 35 °C, at pH of 5. In Fig. 11.6 it is observed that the capacity of the root to adsorb Pb(II) increased as the temperature increased. These results indicate that the adsorption of Pb(II) was endothermic. The effect of temperature can be checked by calculating the heat of adsorption (ΔHads), which when occurring at the same mass of adsorbed Pb(II), but at different temperatures is known as isosteric heat of adsorption. This calculation is made by means of the following equation (Medellin-Castillo et al. 2017):
where (∆Hads)q (J mol−1) is the isosteric heat of adsorption, R (8314 J mol−1 K−1) is the universal constant of the ideal gases, C1 (mg L−1) is the concentration of Pb(II) to T1 (K) at the same value of q in equilibrium, and C2 (mg L−1) is the concentration of Pb(II) to T2 (K) at the same value of q in equilibrium. Thus, at a mass of Pb(II) adsorbed on the root of T. latifolia of q = 14.0 mg g−1, the concentrations of Pb(II) in equilibrium were C1 = 32.0 mg L−1 and C2 = 14.8 mg L−1, at temperatures of T1 = 288.15 K and T2 = 308.15 K, respectively. The estimated isosteric heat was 28.5 KJ mol−1 indicating that the biosorption process of Pb(II) on the root of T. latifolia is endothermic (ΔH > 0).
Experimental data from Pb(II) adsorption isotherms on T. latifolia root were correlated by Freundlich and Langmuir isotherms. The isotherm of Freundlich better adjusted the data under the criterion of the lowest percentage deviation. The study of the effect of pH and temperature in the Pb(II) adsorption isotherm on the root of T. latifolia revealed that the adsorption capacity of this biosorbent to remove lead is considerably dependent on pH and the temperature of the solution, increasing with increasing pH and temperature. The biosorption of Pb(II) on the root of T. latifolia is an endothermic process, with an isosteric heat of adsorption of 28.5 KJ mol−1. Finally, it was concluded that Pb(II) can be efficiently removed from aqueous solutions by means of Typha latifolia root, which is widely distributed in the world and can be easily found in the Huasteca region in San Luis Potosi, Mexico and therefore can be considered a viable and low-cost option for the treatment of water intended for human consumption.
11.6 Conclusions About the Study of Typha latifolia as a Biosorbent
Experimental data from Pb(II) adsorption isotherms on T. latifolia root were correlated by Freundlich and Langmuir isotherms. The isotherm of Freundlich better adjusted the data under the criterion of the lowest percentage deviation. The study of the effect of pH and temperature in the Pb(II) adsorption isotherm on the root of T. latifolia revealed that the adsorption capacity of this biosorbent to remove lead is considerably dependent on pH and the temperature of the solution, increasing with increasing pH and temperature. The biosorption of Pb(II) on the root of T. latifolia is an endothermic process, with an isosteric heat of adsorption of 28.5 KJ mol−1. Finally, it was concluded that Pb(II) can be efficiently removed from aqueous solutions by means of Typha latifolia root, which is widely distributed in the world and can be easily found in the Huasteca region in San Luis Potosi, Mexico and therefore can be considered a viable and low-cost option for the treatment of water intended for human consumption.
11.7 Conclusion
Fitorremediation is a sustainable, low-cost technology because it uses the natural capacity of plants to remove contaminants. Plants are able to remove and accumulate in their plant tissues a wide variety of recalcitrant contaminants (organic and inorganic). To facilitate phytoremediation, native plants with a large amount of biomass and rapid growth such as Typha latifolia should be used. T. latifolia is a suitable plant for cleaning contaminated environments. T. latifolia is solution for cleaning up of recalcitrant pollutants from disturbed and can be used in situ or as a biosorbent material.
References
Abdel-Shafy HI, Mansour MSM (2018) Phytoremediation for the elimination of metals, pesticides, PAHs, and other pollutants from wastewater and soil. In: Phytobiont and ecosystem restitution. Springer, Singapore, pp 101–136. https://doi.org/10.1007/978-981-13-1187-1_5
Aghababai A, Beni EA (2020) Biosorption an efficient method for removing heavy metals from industrial effluents: A review. Environ Technol Innov 17:100503. https://doi.org/10.1016/j.eti.2019.100503
Ahmadi M, Jorfi S, Kujlu R, Ghafari S, Darvishi Cheshmeh Soltani R, Jaafarzadeh Haghighifard N (2017) A novel salt-tolerant bacterial consortium for biodegradation of saline and recalcitrant petrochemical wastewater. J Environ Manag 191:198–208. https://doi.org/10.1016/j.jenvman.2017.01.010
Ali H (2010) Biodegradation of synthetic dyes - a review. Water Air Soil Pollut 213:251–273. https://doi.org/10.1007/s11270-010-0382-4
Alonso-Castro AJ, Carranza-Álvarez C, Alfaro-De la Torre MC, Chávez-Guerrero L, García-De la Cruz RF (2009) Removal and accumulation of cadmium and lead by Typha latifolia exposed to single and mixed metal solutions. Arch Environ Contam Toxicol 57:688–696. https://doi.org/10.1007/s00244-009-9351-6
Alves WS, Manoel EA, Santos NS, Nunes RO, Domiciano GC, Soares MR (2018) Phytoremediation of polycyclic aromatic hydrocarbons (PAH) by cv. Crioula: a Brazilian alfalfa cultivar. Int J Phytoremediation 20(8):747–755. https://doi.org/10.1080/15226514.2018.1425663
Anand S, Bharti SK, Kumar S, Barman SC, Kumar N (2019) Phytoremediation of heavy metals and pesticides present in water using aquatic macrophytes. In: Phyto and rhizo remediation. Springer, Singapore. https://doi.org/10.1007/978-981-32-9664-0_4
Anyasi RO, Atagana HI (2018) Profiling of plants at petroleum contaminated site for phytoremediation. Int J Phytoremediation 20(4):352–361. https://doi.org/10.1080/15226514.2017.1393386
Barbafieri M, Morelli E, Tassi E, Pedron F, Remorini D, Petruzzelli G (2018) Overcoming limitation of “recalcitrant areas” to phytoextraction process: the synergistic effects of exogenous cytokinins and nitrogen treatments. Sci Total Environ 639:1520–1529. https://doi.org/10.1016/j.scitotenv.2018.05.175
Bech J, Roca N, Tume P, Ramos-Miras J, Gil C, Boluda R (2016) Screening for new accumulator plants in potential hazards elements polluted soil surrounding Peruvian mine tailings. Catena 136:66–73. https://doi.org/10.1016/j.catena.2015.07.009
Bhat IUH, Mauris EN, Khanam Z (2016) Phytoremediation of iron from red soil of tropical region by using Centella asiatica. Int J Phytoremediation 18(9):918–923. https://doi.org/10.1080/15226514.2016.1156637
Boehm HP (1994) Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 32:759–769. https://doi.org/10.1016/0008-6223(94)90031-0
Böehm HP (2002) Surface oxides on carbon and their analysis: a critical assessment. Carbon 40:145–149. https://doi.org/10.1016/S0008-6223(01)00165-8
Bouman R, van Welzen P, Sumail S, Echevarria G, Erskine PD, van der Ent A (2018) Phyllanthus rufuschaneyi: a new nickel hyperaccumulator from Sabah (Borneo Island) with potential for tropical agromining. Bot Stud 59(1):9. https://doi.org/10.1186/s40529-018-0225-y
Boyd C (1970) Chemical analyses of some vascular aquatic plants. Arch Hydrobiol 67:78–85. https://doi.org/10.1007/BF02860642
Bukhari IH, Shabbir G, Rehman J, Riaz M, Rasool N, Zubair M, Ain QU, Munir S, Shaheen MA (2013) Biosorption of Pb(II), Cu(II) and Mn(II) metal ions from aqueous solutions by using Typha latifolia waste biomass. J Environ Prot Ecol 14:453–462
Carranza Álvarez C (2005) Fitoextracción de Pb, Cr, Mn, Fe por plantas de Scirpus americanus (Tule) y Typha latifolia (Espadaña) en el Tanque Tenorio. Tesis de Maestría en Ciencias Químicas. FCQ-UASLP
Carranza Álvarez C, Alonso Castro A, Alfaro de la Torre MC, García de la Cruz RF (2008) Accumulation and distribution of heavy metals in Scirpus americanus and Typha latifolia from an artificial Lagoon in San Luis Potosí, México. Water Air Soil Pollut 188:297–309. https://doi.org/10.1007/s11270-007-9545-3
Çelik J, Aksoy A, Leblebici Z (2018) Metal hyperaccumulating Brassicaceae from the ultramafic area of Yahyalı in Kayseri province, Turkey. Ecol Res 33(4):705–713. https://doi.org/10.1007/s11284-018-1606-0
Chamba I, Gazquez MJ, Selvaraj T, Calva J, Toledo JJ, Armijos C (2016) Selection of a suitable plant for phytoremediation in mining artisanal zones. Int J Phytoremediation 18(9):853–860. https://doi.org/10.1080/15226514.2016.1156638
Chandra R, Kumar V (2017) Phytoextraction of heavy metals by potential native plants and their microscopic observation of root growing on stabilised distillery sludge as a prospective tool for in situ phytoremediation of industrial waste. Environ Sci Pollut Res 24(3):2605–2619. https://doi.org/10.1007/s11356-016-8022-1
Chatterjee S, Deb U, Datta S, Walther C, Gupta DK (2017) Common explosives (TNT, RDX, HMX) and their fate in the environment: Emphasizing bioremediation. Chemosphere 184:438–451. https://doi.org/10.1016/j.chemosphere.2017.06.008
Cheng H, Liu Q, Ma M, Liu Y, Wang W, Ning W (2019) Cadmium tolerance, distribution, and accumulation in Taraxacum ohwianum Kitam. as a potential Cd-hyperaccumulator. Int J Phytoremediation 21(6):541–549. https://doi.org/10.1080/15226514.2018.1537240
Chu Y, Zhu S, Xia M, Wang F, Lei W (2019) Methionine-montmorillonite composite – a novel material for efficient adsorption of lead ions. Adv Powder Technol. https://doi.org/10.1016/j.apt.2019.11.026
Colomban C, Kudrik EV, Afanasiev P, Sorokin AB (2014) Degradation of chlorinated phenols in water in the presence of H2O2 and water-soluble μ-nitrido diiron phthalocyanine. Catal Today 235:14–19. https://doi.org/10.1016/j.cattod.2014.03.016
Couto CF, Lange LC, Amaral MCS (2019) Occurrence, fate and removal of pharmaceutically active compounds (PhACs) in water and wastewater treatment plants—a review. J Water Process Eng 32:100927. https://doi.org/10.1016/j.jwpe.2019.100927
Das S, Goswami S (2017) Copper phytoextraction by Salvinia cucullata: biochemical and morphological study. Environ Sci Pollut Res 24(2):1363–1371. https://doi.org/10.1007/s11356-016-7830-7
Delgado M, Valle S, Barra PJ, Reyes-Díaz M, Zúñiga-Feest A (2019) New aluminum hyperaccumulator species of the Proteaceae family from southern South America. Plant Soil. https://doi.org/10.1007/s11104-019-04289-2
Dubchak S, Bondar O (2018) Bioremediation and phytoremediation: best approach for rehabilitation of soils for future use. In: Remediation measures for radioactively contaminated areas. Springer, New York, pp 201–221. https://doi.org/10.1007/978-3-319-73398-2_9
Dudai N, Tsion I, Shamir SZ, Nitzan N, Chaimovitsh D, Shachter A, Haim A (2018) Agronomic and economic evaluation of Vetiver grass (Vetiveria zizanioides L.) as means for phytoremediation of diesel polluted soils in Israel. J Environ Manag 211:247–255. https://doi.org/10.1016/j.jenvman.2018.01.013
Eid EM, Shaltout KH (2016) Bioaccumulation and translocation of heavy metals by nine native plant species grown at a sewage sludge dump site. Int J Phytoremediation 18(11):1075–1085. https://doi.org/10.1080/15226514.2016.1183578
Elhafez SE, Hamad H, Zaatout A, Malash GF (2017) Management of agricultural waste for removal of heavy metals from aqueous solution: adsorption behaviors, adsorption mechanisms, environmental protection, and techno-economic analysis. Environ Sci Pollut Res 24(2):1397–1415. https://doi.org/10.1007/s11356-016-7891-7
Escarré J, Lefèbvre C, Raboyeau S, Dossantos A, Gruber W, Cleyet Marel JC, Frérot H, Noret N, Mahieu S, Collin C, Fvan Oort F (2010).Heavy metal concentration survey in soils and plants of the les malines mining district (Southern France): Implications for soil restoration. Water Air Soil Pollut. 216:485–504. https://doi.org/10.1007/s11270-010-0547-1
Fan Y, Li Z, Zhou T, Zhou S, Wu L, Luo Y, Christie P (2019) Phytoextraction potential of soils highly polluted with cadmium using the cadmium/zinc hyperaccumulator Sedum plumbizincicola. Int J Phytoremed. https://doi.org/10.1080/15226514.2018.1556592
Fernando ES, Quimado MO, Doronila AI (2014) Rinorea niccolifera (Violaceae), a new, nickel-hyperaccumulating species from Luzon Island, Philippines. PhytoKeys, 37, 1–13. https://doi.org/10.3897/phytokeys.37.7136
Gupta A, Thakur IS (2017) Treatment of organic recalcitrant contaminants in wastewater. In: Biological wastewater treatment and resource recovery. IntechOpen, London. https://doi.org/10.5772/66346
Jia YL, Xiao TF, Zhou GZ, Ning ZP (2013) Thallium at the inter-face of soil and green cabbage (Brassica oleracea L. var. capitataL.): soil-plant transfer and influencing factors. Sci Total Environ. 450–451:140–147.
Khatoon H, Pant A, Rai JPN (2017) Plant adaptation to recalcitrant chemicals. In: Plant adaptation strategies in changing environment. Springer, Cham, pp 269–290. https://doi.org/10.1007/978-981-10-6744-0_11
Kikuchi Y, Quian QR, Machida M, Tatsumoto H (2006) Effect of ZnO loading to activated carbon on Pb (II) adsorption from aqueous solution. Carbon 44:195–202. https://doi.org/10.1016/j.carbon.2005.07.040
Kozhevnikova AD, Seregin IV, Gosti F, Schat H (2017) Zinc accumulation and distribution over tissues in Noccaea сaerulescens in nature and in hydroponics: a comparison. Plant Soil 411(1–2):5–16. https://doi.org/10.1007/s11104-016-3116-6
Kumari M, Tripathi BD (2015) Efficiency of Phragmites australis and Typha latifolia for heavy metal removal from wastewater. Ecotoxicol Environ Saf 112:80–86. https://doi.org/10.1016/j.ecoenv.2014.10.034
Kuzin IA, Loskutov AI (1996) J. Appl. Chem, USSR 39
Lange B, Pourret O, Meerts P, Jitaru P, Cancès B, Grison C, Faucon MP (2016) Copper and cobalt mobility in soil and accumulation in a metallophyte as influenced by experimental manipulation of soil chemical factors. Chemosphere 146:75–84. https://doi.org/10.1016/j.chemosphere.2015.11.105
Leura-Vicencio A, Alonso-Castro AJ, Carranza-Álvarez C, Loredo-Portales R, Alfaro de la Torre MC (2013) Removal and accumulation of As, Cd and Cr by Typha latifolia. Bull Environ Contam Toxicol 90(6):650–653. https://doi.org/10.1007/s00128-013-0962-2
Li X, Zhang X, Yang Y, Li B, Wu Y, Sun H, Yang Y (2016) Cadmium accumulation characteristics in turnip landraces from China and assessment of their phytoremediation potential for contaminated soils. Front Plant Sci 7:01862. https://doi.org/10.3389/fpls.2016.01862
Liu J, Shang W, Zhang X, Zhu Y, Yu K (2014) Mn accumulation and tolerance in Celosia argentea Linn.: a new Mn-hyperaccumulating plant species. J Hazard Mater 267:136–141. https://doi.org/10.1016/j.jhazmat.2013.12.051
Liu K, Yu F, Chen M, Zhou Z, Chen C, Li M, Shun ZJ (2016) A newly found manganese hyperaccumulator—Polygonum lapathifolium Linn. Int J Phytoremediation 18(4):348–353. https://doi.org/10.1080/15226514.2015.1109589
Liu Z, Chen W, He X (2018) Evaluation of hyperaccumulation potentials to cadmium (Cd) in six ornamental species (compositae). Int J Phytoremediation 20(14):1464–1469. https://doi.org/10.1080/15226514.2018.1501343
Lu G, Wang B, Zhang C, Li S, Wen J, Lu G et al (2018) Heavy metals contamination and accumulation in submerged macrophytes in an urban river in China. Int J Phytoremediation 20(8):839–846. https://doi.org/10.1080/15226514.2018.1438354
Manahan SE (2007) Introducción a la química ambiental. In: Reverté ediciones (primera). Retrieved from https://books.google.com.mx/books?id=5NR8DIk1n68C&printsec=frontcover&dq=manahan+2007+phytoremediation&hl=es-419&sa=X&ved=0ahUKEwjrzIzj44jmAhUOCawKHXYgBRAQ6AEITjAE#v=onepage&q&f=false
Medellin-Castillo NA, Padilla-Ortega E, Regules-Martínez MC, Leyva-Ramos R, Ocampo-Pérez R, Carranza-Alvarez C (2017) Single and competitive adsorption of Cd(II) and Pb(II) ions from aqueous solutions onto industrial chili seeds (Capsicum annuum) waste. Sustainable Environ Res 27:61–69. https://doi.org/10.1016/j.serj.2017.01.004
Mesjasz-Przybylowicz J, Przybylowicz W, Barnabas A, van der Ent A (2016) Extreme nickel hyperaccumulation in the vascular tracts of the tree Phyllanthus balgooyi from Borneo. New Phytol 209(4):1513–1526. https://doi.org/10.1111/nph.13712
Miran W, Nawaz M, Jang J, Lee DS (2017) Chlorinated phenol treatment and in situ hydrogen peroxide production in a sulfate-reducing bacteria enriched bioelectrochemical system. Water Res 117:198–206. https://doi.org/10.1016/j.watres.2017.04.008
Mitton FM, Gonzalez M, Peña A, Miglioranza KSB (2012) Effects of amendments on soil availability and phytoremediation potential of aged p,p’-DDT, p,p’-DDE and p,p’-DDD residues by willow plants (Salix sp.). J Hazard Mater 203:62–68. https://doi.org/10.1016/j.jhazmat.2011.11.080
Mojiri A, Ziyang L, Tajuddin RM, Farraji H, Alifar N (2016) Co-treatment of landfill leachate and municipal wastewater using the ZELIAC/zeolite constructed wetland system. J Environ Manag 166:124–130. https://doi.org/10.1016/j.jenvman.2015.10.020
Mongue C (2004) La naturaleza del agua como recurso. Perspectiva social, económica e institucional de una gestión integral. Congreso Ibérico sobre gestión y planificación del agua. Tortosa, Esp. http://congreso.us.es/ciberico/archivos_acrobat/zaracomun5segura.pdf
Monser L, Adhoum N (2002) Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater. Sep Purif Technol 26:137–146. https://doi.org/10.1016/S1383-5866(01)00155-1
Moreira ITA, Oliveira OMC, Triguis JA, dos Santos AMP, Queiroz AFS, Martins CMS et al (2011) Phytoremediation using Rhizophora mangle L. in mangrove sediments contaminated by persistent total petroleum hydrocarbons (TPH’s). Microchem J 99(2):376–382. https://doi.org/10.1016/j.microc.2011.06.011
Naila A, Meerdink G, Jayasena V, Sulaiman AZ, Ajit AB, Berta G (2019) A review on global metal accumulators—mechanism, enhancement, commercial application, and research trend. Environ Sci Pollut Res 26:26449–26471. https://doi.org/10.1007/s11356-019-05992-4
Nie J, Liu Y, Zeng G, Zheng B, Tan X, Liu H et al (2016) Cadmium accumulation and tolerance of Macleaya cordata: a newly potential plant for sustainable phytoremediation in Cd-contaminated soil. Environ Sci Pollut Res 23(10):10189–10199. https://doi.org/10.1007/s11356-016-6263-7
Nkrumah PN, Echevarria G, Erskine PD, van der Ent A (2018) Nickel hyperaccumulation in Antidesma montis-silam: from herbarium discovery to collection in the native habitat. Ecol Res 33(3):675–685. https://doi.org/10.1007/s11284-017-1542-4
Palutoglu M, Akgul B, Suyarko V, Yakovenko M, Kryuchenko N, Sasmaz A (2018) Phytoremediation of cadmium by native plants grown on mining soil. Bull Environ Contam Toxicol 100(2):293–297. https://doi.org/10.1007/s00128-017-2220-5
Pavithra M, Hina K (2018) In-vitro regeneration and propagation of Pistia stratiotes as a biotechnological approach for phytoremediation of textile effluent. Res J Chem Environ 22(12):75–79
Peer WA, Baxter IR, Richards EL, Freeman JL, Murphy AS (2006) Phytoremediation and hyperaccumulator plants. Top Curr Genet 14:299–340. https://doi.org/10.1007/4735_100
Pritchard JC, Cho YM, Ashoori N, Wolfand JM, Sutton JD, Carolan ME et al (2018) Benzotriazole uptake and removal in vegetated biofilter mesocosms planted with Carex praegracilis. Water 10(11):1605. https://doi.org/10.3390/w10111605
Rajaei G, Aghaie H, Zare K et al (2013) Adsorption of Cu(II) and Zn(II) ions from aqueous solutions onto fine powder of Typha latifolia L. root: kinetics and isotherm studies. Res Chem Intermed 39:3579–3594. https://doi.org/10.1007/s11164-012-0864-7
Ravanbakhsh M, Ronaghi AM, Taghavi SM, Jousset A (2016) Screening for the next generation heavy metal hyperaccumulators for dryland decontamination. J Environ Chem Eng 4(2):2350–2355. https://doi.org/10.1016/j.jece.2016.04.013
Romero-Hernández JA, Amaya-Chávez A, Balderas-Hernández P, Roa-Morales G, González-Rivas N, Balderas-Plata MÁ (2017) Tolerance and hyperaccumulation of a mixture of heavy metals (Cu, Pb, Hg, and Zn) by four aquatic macrophytes. Int J Phytoremediation 19(3):239–245. https://doi.org/10.1080/15226514.2016.1207610
Saad-Allah KM, Elhaak MA (2017) Hyperaccumulation activity and metabolic responses of Solanum nigrum in two differentially polluted growth habitats. J Saudi Soc Agric Sci 16(3):227–235. https://doi.org/10.1016/j.jssas.2015.08.001
Saha P, Shinde O, Sarkar S (2017) Phytoremediation of industrial mines wastewater using water hyacinth. Int J Phytoremediation 19(1):87–96. https://doi.org/10.1080/15226514.2016.1216078
Salihaj M, Bani A, Shahu E, Benizri E, Echevarria G (2018) Metal accumulation by the ultramafic flora of Kosovo. Ecol Res 33(4):687–703. https://doi.org/10.1007/s11284-018-1635-8
Sandhya S, Padmavathy S, Swaminathan K, Subrahmanyam YV, Kaul SN (2005) Microaerophilic-aerobic sequential batch reactor for treatment of azo dyes containing simulated wastewater. Process Biochem 40(2):885–890. https://doi.org/10.1016/j.procbio.2004.02.015
Shao Z, Wenlong L, Jamal N, Jinjing Z, Li Y (2019) Growth responses and accumulation characteristics of three ornamentals under copper and lead contamination in a hydroponic-culture experiment. Bulletin of Environmental Contamination and Toxicology. 103. 10.1007/s00128-019-02724-9.
Sidhu GPS, Singh HP, Batish DR, Kohli RK (2017) Tolerance and hyperaccumulation of cadmium by a wild, unpalatable herb Coronopus didymus (L.) Sm. (Brassicaceae). Ecotoxicol Environ Saf 135:209–215. https://doi.org/10.1016/j.ecoenv.2016.10.001
Siebert SJ, Schutte NC, Bester SP, Komape DM, Rajakaruna N (2018) Senecio conrathii N.E.Br. (Asteraceae), a new hyperaccumulator of nickel from serpentinite outcrops of the Barberton Greenstone Belt, South Africa. Ecol Res 33(3):651–658. https://doi.org/10.1007/s11284-017-1541-5
Singh S, Melo JS, Eapen S, D’Souza SF (2008) Potential of vetiver (Vetiveria zizanioides L. Nash) for phytoremediation of phenol. Ecotoxicol Environ Saf 71(3):671–676. https://doi.org/10.1016/j.ecoenv.2007.10.023
Song J, Zhang R, Li K, Li B, Tang C (2013) Adsorption of copper and zinc on activated carbon prepared from Typha latifolia L. Clean Soil Air Water 43:79–85. https://doi.org/10.1002/clen.201300533
Sud D, Mahajan G, Kaur MP (2008) Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions – a review. Bioresour Technol, 99, pp. 6017–6027
Tang C, Shu Y, Zhang R, Li X, Song J, Li B, Zhang J, Ou D (2017) Comparison of the removal and adsorption mechanisms of cadmium and lead from aqueous solution by activated carbons prepared from Typha angustifolia and Salix matsudana. RSC Adv 7:16092–16103. https://doi.org/10.1039/C6RA28035H
Torbati S, Movafeghi A, Khataee AR (2015) Biodegradation of C.I. acid blue 92 by nasturtium officinale: study of some physiological responses and metabolic fate of dye. Int J Phytoremediation 17(4):322–329. https://doi.org/10.1080/15226514.2014.910165
Urbaniak M, Wyrwicka A, Zieliński M, Mankiewicz-Boczek J (2016) Potential for phytoremediation of PCDD/PCDF-contaminated sludge and sediments using cucurbitaceae plants: a pilot study. Bull Environ Contam Toxicol 97(3):401–406. https://doi.org/10.1007/s00128-016-1868-6
Van Der Ent A, Van Balgooy M, Van Welzen P (2016) Actephila alanbakeri (Phyllanthaceae): a new nickel hyperaccumulating plant species from localised ultramafic outcrops in Sabah (Malaysia). Bot Stud 57:40529. https://doi.org/10.1186/s40529-016-0122-1
Van Der Ent A, Callahan DL, Noller BN, Mesjasz-Przybylowicz J, Przybylowicz WJ, Barnabas A, Harris HH (2017) Nickel biopathways in tropical nickel hyperaccumulating trees from Sabah (Malaysia). Sci Rep 7:41861. https://doi.org/10.1038/srep41861
Van Der Ent A, Mak R, De Jonge MD, Harris HH (2018) Simultaneous hyperaccumulation of nickel and cobalt in the tree Glochidion cf. sericeum (Phyllanthaceae): elemental distribution and chemical speciation. Sci Rep 8(1):9683. https://doi.org/10.1038/s41598-018-26891-7
Wong Argüelles C (2009) Estudio de organismos acuáticos macrobentónicos como indicadores de la contaminación por metales pesados en ríos de la huasteca potosina. Facd. Ciencias Químicas, Ingeniería y Medicina. UASLP, San Luis Potosí
Wu M, Luo Q, Zhao Y, Long Y, Liu S, Pan Y (2018) Physiological and biochemical mechanisms preventing Cd toxicity in the new hyperaccumulator Abelmoschus manihot. J Plant Growth Regul 37(3):709–718. https://doi.org/10.1007/s00344-017-9765-8
Yamaguchi T, Tomioka R, Takenaka C (2017) Accumulation of cobalt and nickel in tissues of Clethra barbinervis in a metal dosing trial. Plant Soil 421(1–2):273–283. https://doi.org/10.1007/s11104-017-3455-y
Yan Q, Feng G, Gao X, Sun C, Guo JS, Zhu Z (2016) Removal of pharmaceutically active compounds (PhACs) and toxicological response of Cyperus alternifolius exposed to PhACs in microcosm constructed wetlands. J Hazard Mater 301:566–575. https://doi.org/10.1016/j.jhazmat.2015.08.057
Yarahmadi Z, Baharlouei J, Shokoohi R, Alikhani MY, Shirmohammadi-Khorram N (2017) The efficiency of Lolium perenne for phytoremediation of anthracene in polluted soils in the presence of Bacillus aerophilus. Pet Sci Technol 35(7):647–652. https://doi.org/10.1080/10916466.2016.1252771
Yu F, Liu K, Ye P, Zhou Z, Chen C, Li Y (2019) Manganese tolerance and accumulation characteristics of a woody accumulator Camellia oleifera. Environ Sci Pollut Res 26(21):21329–21339. https://doi.org/10.1007/s11356-019-05459-6
Zhong L, Lin L, Liao M, Wang J, Tang Y, Sun G et al (2019) Phytoremediation potential of Pterocypsela laciniata as a cadmium hyperaccumulator. Environ Sci Pollut Res 26(13):13311–13319. https://doi.org/10.1007/s11356-019-04702-4
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ponce-Hernández, A., Maldonado-Miranda, J.J., Medellin-Castillo, N.A., Alonso-Castro, A.J., Carranza-Alvarez, C. (2020). Phytoremediation Technology: Sustainable Solution for Cleaning Up of Recalcitrant Pollutants from Disturbed Environs. In: Bhat, R., Hakeem, K., Saud Al-Saud, N. (eds) Bioremediation and Biotechnology, Vol 3. Springer, Cham. https://doi.org/10.1007/978-3-030-46075-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-46075-4_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-46074-7
Online ISBN: 978-3-030-46075-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)