Abstract
The present study investigated the effect of copper on photosynthesis, antioxidant potential, and anatomical response of aquatic fern, Salvinia cucullata, with a view to ascertain its phytoremediation potential. Plants were exposed in hydroponics for 21 days to different Cu concentrations (10, 15, 20, and 30 mg/L). Significant declines in chlorophyll, carotenoids, and soluble proteins, as a function of Cu proportion were observed. Lipid peroxidation was also evident, which implied reactive oxygen species (ROS) generation. However, both root and leaf tissues responded remarkably to the ROS produced, by inducing superoxide dismutase (1.6–6.5 times), catalase (1.5–5.4 times), guaicol peroxidase (1.5–7.2 times), and ascorbyl peroxidase (1.3–4.7 times) over the control. The plant showed best phytoremedial activity within Cu range of 10–15 mg/L, with maximum accumulation of 2956 ± 82.6 μg/g dw., at 15 mg Cu/L and showed efficient root to shoot translocation (translocation factor, TF > 1) at this range, which is the stipulated minimum requirement to be a hyperaccumulator. The capacity of metal extraction from environment to leaf (extraction coefficient, EC) was also high (EC = 73–197). However, at higher doses (20–30 mg/L), the plant resorted to an exclusion strategy, whereby, more metal accumulation was observed in root than in leaf. The plant conferred suitable remediation attributes by showing minimal root and leaf anatomical damages along with high Ca peaks in both the tissues, and rapid leaf stomatal closure, all of which probably helped in the Cu induced stress mitigation. Due to its widespread availability, fast growth, ability to grow in myriads of polluted environment, and having hardy physiology, this plant can be suggested for use as a suitable Cu phytoremediator.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With an annual emissions of approximately 3,400,000 tons, copper (Cu) has an ubiquitous presence in aquatic environment (Peng et al. 2004); predominantly due to the widespread use of Cu based fungicide (Brunetto et al. 2014), algaecide (Fabrizio and Coccioni 2012), fertilizers (Alloway 2008), leaching from mining, smelting, metal production as well as domestic wastewaters (Kabata-Pendias 2011). Cu is an essential micronutrient for aquatic plants, but excess of Cu shows adverse effects on its physiology (Monferrán et al. 2009). However, some aquatic plants like Trapa natans (Sweta et al. 2015), Lemna gibba (Perreault et al. 2014), Pistia stratiotes (Lu et al. 2011), Ceratophyllum demersum (Ostroumov and Shestakova 2009), Potamogeton pectinatus (Badr and Fawzy 2008), Scirpus maritimus, Juncus maritimus (Almeida et al. 2006) and Eichhornia crassipes (Liao and Chang 2004), can accumulate Cu and in turn, act as bio filters, remediating the water systems efficiently. Aquatic plants eliminate metals, mostly, by means of extraction and accumulation (Valipour et al. 2011). Use of these techniques, also called phytoremediation, is both cost effective and eco-friendly way of remediation of polluted sites (Garbisu and Alkorta 2001). As Cu is known to be redox active, and generates free radicles in aquatic plants (Monferrán et al. 2009), an efficient Cu remediator should be able to cope with the metal excess via antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), guaicol peroxidase (GPx), and ascobyl peroxidase (APx) (Goswami and Das 2016). Further, such aquatic plants can adapt structurally and physiologically to contaminated environment, where they are in (Hondulas 1994). Since, both root and leaf are in contact with water surface, studying their anatomy would generate valuable data on adaptive role played by these structures during metal uptake.
The free-floating water fern, Salvinia cucullata Roxb. Ex Bory, occurs naturally in Asian countries and often occurs in nutrient rich water bodies where they are considered as weed (McFarland et al. 2004). Apart from nutrient removal with S. cucullata (Jampeetong et al. 2012), a few studies on Cr removal by S. minima (Prado et al. 2010), S. herzogii (Suñé et al. 2007) and S. natans (Olguín et al. 2002); Pb (Leal-Alvarado et al. 2016) and Ni (Fuentes et al. 2014) removal by S. minima are available. However, no significant works on this species are available, either on Cu accumulation, or on the physiological and morphological aspects beneficial for ascertaining its remediation potential. Therefore, the present study aims at analyzing the remediation capacities, and the physiological and anatomical response of this plant to Cu excess.
Materials and methods
Macrophyte and experimental set-up
S. cucullata of uniform size was collected from a clean, unpolluted, freshwater pond. The plants were rinsed thrice with deionized water and put in 30 % Hoagland solution (HS), for acclimatization for 3 weeks, before exposing to copper solutions (CuSO4.5H2O, Himedia, India). The experiments were carried out for 21 days in 1 L plastic containers with Cu concentrations prepared in 1 L of 30 % HS. The doses selected were between 10 and 30 mg Cu L−1, which were the critical concentrations of Cu in plants (Macnicol and Beckett 1985). It was supposed that to be an able hyperaccumulators, and the plants should tolerate this range of Cu concentrations. A total of six replicates were run for each treatment, with one plant in each dose. The control were similarly maintained. The loss of water volume due to evapo-transpiration were maintained by adding deionized water. The plants were grown under natural photoperiod and temperature. Various morphological parameters of S. cucullata on day 0 and after 21 days of Cu treatments were shown in Table 1.
Estimation of chlorophyll, carotenoid, and leaf soluble protein
Plants were removed after 21 days and washed thoroughly with distilled water. The pigment contents were determined in young leaves as per Lichtenthaler (1987). Leaf soluble protein contents was estimated using BSA as standard (Lowry et al. 1951).
Determination of malondialdehyde contents and antioxidant enzyme activity
Lipid peroxidation, as malondialdehyde content, was measured indirectly by thiobarbituric acid reaction (Heath and Packer 1968). Briefly, 50 mg of fresh tissues (root or leaf) were homogenized in 50 mM L−1 Tris-HCl (pH 7.4) including 1.0 mM L−1 EDTA and 2 % (w/v) polyvinylpyrrolidone and centrifuged for 30 min in 10,000×g at 4 °C. Equal amounts of extract and 0.5 % TBA in 20%TCA (w/v) were mixed and heated at 95 °C for 30 min. The reaction was stopped on ice and further centrifuged at 10,000×g for 15 min and absorbance was read at 532 nm. The unspecific turbidity was corrected by subtracting the value of absorbance at 600 nm.
Extraction of enzymes
800 mg of leaf/root tissues were extracted (3:1 buffer volume: fresh weight) in 50 mM L−1 sodium phosphate buffer (pH 7.8) and centrifuged at 12,000×g for 20 min at 4 °C. The supernatant was used to measure the activities of SOD, CAT, GPx, and APx activities. The protein content in the supernatant was estimated according to Lowry et al. (1951).
SOD (EC 1.15.1.1) was estimated as per Beauchamp and Fridovich (1971) following the photo reduction of nitroblue tetrazolium (NBT). One SOD unit per mg of protein was the enzyme activity that inhibited the photo-reduction of NBT to blue formazan by 50 %.
CAT (EC 1.11.1.6) was determined as per Beer and Sizer (1952). The decrease of H2O2 was monitored at 240 nm and quantified by its molar extinction coefficient, ɛ =0.036 mM−1 cm−1, and the results expressed as CAT mM/ mg protein/ min.
The activity of GPX (EC 1.11.1.7) was evaluated as per Souza and MacAdam (1998) using extinction coefficient of 26.6 mM L−1 cm−1 and stated as the decomposition of guaiacol (mM/ mg protein/min).
The activity of APX (EC 1.11.1.11) was evaluated as per Nakano and Asada (1981), detecting the rate of ascorbate oxidation (extinction coefficient of 2.8 mM L−1 cm−1), and the enzyme activity was expressed as mM ascorbate oxidized/ mg protein/ min.
Metal analysis
The plants were washed meticulously by distilled water. The root and leaves were separated, cut into pieces and oven dried at 90 °C for 16 h. 0.05 g of dried parts were crushed to fine dust, which were used for the estimation of Cu after digesting in an acidic mixture of (HNO3:HClO4) as per standard protocol for AAS (APHA 2005) using a GF-AAS.
The standard reference material of Cu procured from Merck, Germany was used for calibration. Quality of the obtained data was certified with frequent analysis of samples, and the outcomes were within the recommended limits. Instrument recognition limit for Cu was 0.01 μg g−1.
Extraction coefficient (EC) and Translocation factor (TF) was calculated as per Goswami and Das (2015).
EC = Cu in plant /Cu in water. TF = Cu in leaf (μg g−1)/ Cu in root (μg g−1).
Scanning electron microscopy (SEM) coupled to energy-dispersive X-ray microanalysis (EDX)
Root and leaf were rinsed with distilled water. For SEM, 3–4 mm pieces of tissues were fixed in 3 % glutaraldehyde (in 0.05 M phosphate buffer) for 1 h and 30 min, followed by post fixation for 30 min in 2 % osmium tetroxide (in 0.01 M sodium cacodylate buffer) (Sandalio et al. 2001). The samples were dehydrated in graded acetone concentrations. SEM photograph were carried for the samples, using SEM model JEOL-JSM-6390 LV attach with energy dispersive X-ray unit, with an accelerating voltage of 20 kV.
EDX analysis with an INCA- 7582 (Oxford Instruments, Abingdon, Oxfordshire, UK) energy dispersive X-ray microanalyzer equipped with a Si (Li) high-resolution detector attached to the JSM-6360 (JEOL) SEM. Quantitative analysis was carried out using the ZAF-4/FLS program. Instructions were given to normalize all elements analyzed to 100 %, and thus the relative percentage of elements was known.
Statistical analysis
One-way Analysis of Variance (ANOVA) was used to compare more than two means followed by Tukey-LSD test for comparison of individual means using SAS JMP Pro11 statistical software for windows.
Results
S. cucullata accumulated Cu in both root and leaf (Table 2). In fact, at 10 mg/L and 15 mg/L exposure, concentrations in leaf were greater than that of root. At 20 and 30 mg/L, progressive decline in leaf metal concentration was observed. The highest leaf and root metal accumulation was at 15 (2.9 mg/g dw.), and 30 mg/L (3.8 mg/g dw.) Cu exposure, respectively. Extraction coefficient (EC), which is the comparison of metal in plant parts to the original metal conc. in external solution was presented in Table 2. The EC maxima for leaf (197 ± 5.5) and root (184 ± 1.15) was observed, respectively, at 15 and 10 mg/L Cu exposure. The leaf to root metal ratios, that is, the translocation factor (TF) of >1 was achieved for only 10 and 15 mg/L Cu treatments (Table 2).
All the Cu doses registered significant declines in Chl a, Chl b, and carotenoid contents (Table 3). There were 9, 22, 40, and 55 % declines in Chl a at 10, 15, 20, and 30 mg/L Cu doses, respectively. While, Chl b declined by 12, 44, 60, and 67 %, respectively, for the similar doses of exposure. Carotenoid contents declined by 26, 42.5, 65.6, and 69 %, respectively, at 10, 15, 20, and 30 mg/L Cu doses. There was a general tendency of increase in Chl a: Chl b till 20 mg Cu/L, then at 30 mg Cu/L, there was a decline in this ratio.
Significant (p < 0.05) elevations in lipid peroxidation, as evidenced by the rise in MDA contents were observed at all the Cu treatment groups. Concomitantly, there were significant declines in leaf soluble proteins (Table 4). For root at 10–30 mg/L Cu exposure, there was 50–247 % increase in lipid peroxidation. Similarly, for leaf, at the same exposure range, there was 41–243 % increase in MDA production, respectively. Leaf soluble protein contents at 10, 15, 20, and 30 mg/L Cu decreased by around 12, 23, 32, and 44 %, respectively, after 21 days of remediation period.
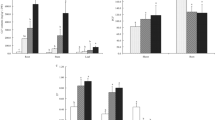
Significant augmentation of all the major antioxidant enzymes were observed after 21 days of treatments with various Cu doses (Fig. 1). For the SOD activities in root, there were 1.6, 3, 4.5, and 6.5 times increase in enzyme activities, respectively, at 10, 15, 20, and 30 mg/L Cu exposures, compared to control. Similarly, in the leaf, there were 1.8, 2.8, 4.3, and 6 times increase in SOD activities, respectively, at the same exposure doses. For the CAT activities in root, there were 2–5.4 times increase in enzyme activities at 10–30 mg/L Cu exposure, when compared to the control. Likewise, for the leaf, CAT activities increased 1.5, 2.3, 3.4, and 4.8 times over the control, at the same Cu exposures, respectively. For the root GPx activities, there was 1.6, 2.3, 3.3, and 7 times increase over the control at 10, 15, 20, and 30 mg Cu/L, respectively. For the GPx activities in the leaf, there were 1.5, 2.3, 3.7, and 7.2 times increase over the control at the same exposure doses, respectively. Similarly, APx in the root increased 1.4, 1.9, 2.8, and 4.7 times, and in the leaf, increased 1.3, 1.8, 2.8, and 4.6 times, at 10, 15, 20, and 30 mg Cu/L, respectively, over the control.
Table 5 depicted the weight % of EDX peaks of both the root and the leaf after exposure to 10 and 15 mg/L Cu. These two doses were selected, because, at this dose, the plants showed maximum metal accumulations in the leaf as against the root, with TF > 1. Significant increase in Ca peaks, over the control, was observed, for both root and leaf. The Ca peaks also elevated with the increase in Cu doses. Further, weight % for Ca peaks were more in root compared to leaf. The quantitative analysis of the elemental composition by weight indicated that in the control spectra, for both leaf and root, approximately 95 % of weight was contributed by carbon, oxygen and potassium. Besides, control tissues showed no peaks for Cu. In contrast, distinct Cu peaks were observed in both root and leaf of treated plants, with peaks in leaf more than that of root. Around 1 % Fe was also seen in the control root only, and not in the leaf.
The gross root morphology showed little signs of external phytotoxicity, either to the control or to the Cu exposed plants (Fig. 2). In fact, the root lengths of Salvinia at 10 mg/L were slightly longer than that of the control. The scanning micrographs of the control root surface showed dense root hairs with no Cu deposition over the surface, the EDX showed Ca peak (2.11 %) and no Cu (Fig. 2a) . The SEM of root at 10 mg Cu/L revealed unaltered root hair densities, but occurrence of Cu deposits (EDX weight % 9.4) over the surface along with significant Ca peaks (5 %) (Fig. 2b). At 15 mg Cu/L, the root hair densities slightly declined (arrow) and showed dense deposit of Cu on the surface with 18.3 % EDX peak. Likewise, high Ca peaks (10.42 %) were also observed at this dose (Fig. 2c).
The gross plant foliar morphology showed little signs of external phytotoxicities at 10 mg/L Cu exposure, barring a slight sign of chlorosis in the leaves, at 15 mg Cu/L (Fig. 2). The SEM of control abaxial leaf surface showed open stomata, with well-defined guard cells. The EDX peaks showed slight (0.63 %) Ca peaks and no Cu deposits in the stomata (Fig. 3a). At 10 mg/L Cu exposure, there was a tendency towards closure of stomata in the leaf and Ca peaks of about 1.21 % in the stomata were observed (Fig. 3b), but at 15 mg Cu/L, almost closed stomata with shrunken guard cells and high Ca peaks (7.82 %) could be observed (Fig. 3c).
Discussion
In this study, S. cucullata accumulated more than 1000 μg/g Cu in its tissues, the minimum requirement to be a Cu hyperaccumulator (Vamerali et al. 2010). Besides, the plants showed efficient root to shoot translocation between 10 and 15 mg Cu/L exposure, with TF > 1, which is the stipulated minimum requirement to be a hyperaccumulator (Garbisu and Alkorta 2001). However, at higher doses (20–30 mg/L), the plant resorted to an exclusion strategy, whereby, more metal accumulation was observed in root than in leaf (Rascio and Navari-Izzo 2011). This advocates that the plant tissues might have reached their maximum capacity to tolerate, accumulate, and consequently, phytoextract Cu. Further, Cu extraction coefficient (EC) for this plant was also >1, and hence, the plant can be deemed as a Cu hyperaccumulator, with the best efficiency between 10 and 15 mg Cu/L exposure. The accumulation of Cu by aquatic plants, both for short and longer durations, has also been described in Ceratophyllum demersum, which accumulated 314.6 μg Cu/ g dw. after 24-h exposure to 4 mM Cu (Devi and Prasad 1998) or in Potamogeton pusillus, which accumulated 162 μg Cu/ g dw. after 7-days exposure to 0.1 mg/ L Cu (Monferrán et al. 2009). Further, Cu accumulations were reported under field conditions in Typha angustifolia (Demirezen and Aksoy 2004) and Myriophyllum spicatum (Samecka-Cymerman and Kempers 2004). Heavy metal accumulation in Salvinia is usually quick and comprises of passive uptake through adsorption of metal ions, onto the plant surface and/or active uptake into plant cells (Suñé et al. 2007).
Previously, it has been observed that Cu targets the photosynthetic apparatus of aquatic plants (Monferrán et al. 2009) and induces a decline in plant pigments as seen in Pistia stratiotes (Upadhyay and Panda 2009). Similarly, this study also showed a decline in Chl a, b, and carotenoid contents after Cu exposure. Such degradation of plant pigments may ultimately reduce photosynthetic competence in plants by changing the osmotic potential and water contents, resulting in a nutritional disparity and a consequential decrease in growth (Abdel Latef 2011). Chl a/Chl b ratio is an indicator of metal stress in plants (Manios et al. 2003). We found an increase in this ratio up to 20 mg Cu/L exposure. This occurs due to a faster hydrolysis of chl b compared with chl a. Chl b synthesis levels have a major impact on the light harvesting and thermal energy dissipation processes, along with linear electron transport and repair processes in grana (Voitsekhovskaja and Tyutereva 2015). Therefore, a decline in Chl b might point towards Cu stress.
Present study showed a decline in soluble proteins in Salvinia, which might be attributed to the high affinity of Cu for sulfhydryl groups and binds to and deactivates proteins (Ruttkay-Nedecky et al. 2013).
Our results showed rise in MDA production with increased extraneous Cu conc. Roots, especially, had higher MDA induction than leaf. Such induction of MDA production in Salvinia, is an indication of peroxidative damage to membranes, which in turn is an indicator of ROS formation (Perreault et al. 2014).
Aquatic plants have an inherent capacity to counter ROS via key antioxidant enzymes (Monferrán et al. 2009). The superoxide radical (O2 ·), is scavenged in plants by SOD, which converts it to hydrogen peroxide (H2O2). H2O2 is scavenged directly by CAT, converting it to water and O2. APX and GPX also scavenge H2O2 indirectly by combining it with antioxidant compounds such as ascorbate and guaiacol (Rascio and Navari-Izzo 2011). Our study found augmentation of the major enzyme repertoire in response to Cu. Such augmentation may be inferred as a beneficial tool to counter ROS and confer better phytoremedial efficiency. Contradictory results of induction Ceratophyllum demersum, (Devi and Prasad 1998) as well as inhibition Pistia stratiotes, (Upadhyay and Panda 2009) of SOD, CAT, GPx in Cu excess have been reported in aquatic plants. Further, induction of enzymes followed by inhibition was also observed in some Potamogeton pusillus (Monferrán et al. 2009).
Morphological damages associated with Cu excess in plant have been described in Pistia stratiotes (Upadhyay and Panda 2009) and in Vitis labrusca (Ambrosini et al. 2015). Apart from the slight declines in root densities, not much significant damage to the root morphology was observed in this study. However, the leaf which showed some signs of chlorosis and stomata had a tendency towards closure. Cu is known to cause chlorosis in plants (Mateos-Naranjo et al. 2013). EDX showed calcium peaks in both root and leaf, in response to Cu. Ca is a well-known intracellular signaling molecule that plays significant part in ion uptake in plants (Yang and Poovaiah 2003). Increase in Ca negates the effects of Cu on the root by decreasing Cu transportation to the shoots and augmenting plant biomass (Juang et al. 2014). Further, the elevations of cytosolic Ca2+ in the guard cells appear to be the major regulator of stomatal functioning and, therefore, of plant water status (Webb et al. 1996). We also found a concomitant rise of Ca levels with increase in extraneous Cu. Such increase of Ca content might stimulate Ca-oxalate crystals formation, that can combine Cu into their matrix, thus minimizing the toxic effects (Franceschi and Nakata 2005), which aptly explains the phytoremediation potential of Salvinia optimally within treatment ranges of 10–15 mg Cu/L.
Conclusions
We conclude that S. cucullata showed the best phytoremedial activity within the range of 10–15 mg Cu/L, with efficient root to shoot translocation of metal. The plant also showed various beneficial traits, including augmentation of major antioxidant enzymes and minimal root and leaf anatomical damages. The plants showed rapid stomatal closure,and high Ca peaks in both leaf and root, all of which probably helped in the mitigation of Cu stress. We, however, felt that the role of Ca signals in plants need further investigation, especially to ascertain its role in the alleviation of Cu stress. The plant, nonetheless, could be conferred suitable for remediation owing to its widespread availability, fast growth, ability to grow in myriads of polluted environment, and having a hardy physiology.
References
Abdel Latef AA (2011) Influence of arbuscular mycorrhizal fungi and copper on growth accumulation of osmolyte, mineral nutrition and antioxidant enzyme activity of pepper (Capsicum annuum L.). Mycorrhiza 21:495–503
Alloway BJ (2008) Copper and zinc in soils: too much or too much? In: New Zealand Trace Elements Group Conference, 13–15 Feb. University of Waikato, Hamilton, p 10
Almeida CMR, Mucha AP, Vasconcelos MTSD (2006) Comparison of the role of the sea club-rush Scirpus maritimus and the sea rush Juncus maritimus in terms of concentration, speciation and bioaccumulation of metals in the estuarine sediment. Environ Pollut 142:151–159
Ambrosini VG, Rosa DJ, Prado JPC, Borghezan M, de Melo GWB, de Sousa Soares CRF, Comin JJ, Simáo DG, Brunetto G (2015) Reduction of copper phytotoxicity by liming: a study of the root anatomy of young vines (Vitis labrusca L.). Plant Physiol Bioch 96:270–280
APHA (American Public Health Association) (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington, DC, p. 1368
Badr NBE, Fawzy M (2008) Bioaccumulation and biosorption of heavy metals and phosphorous by Potamogeton pectinatus L. And Ceratophyllum demersum L. In two Nile delta lakes. Fresenius Environ Bull 17:282–292
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beers RF, Sizer IW (1952) Colorimetric method for estimation of catalase. J Biol Chem 195:133–139
Brunetto G, Miotto A, Ceretta CA, Schmitt DE, Heinzen J, Moraes MP, Canton L, Tiecher TL, Comin JJ, Girotto E (2014) Mobility of copper and zinc fractions in fungicide amended vineyard sandy soils. Arch Agron Soil Sci 60:609–624
Demirezen D, Aksoy A (2004) Accumulation of heavy metals in Typha angustifolia (L.) and Potamogeton pectinatus (L.) living in sultan marsh (Kayseri, Turkey). Chemosphere 56:685–696
Devi SR, Prasad MNV (1998) Copper toxicity in Ceratophyllum demersum L. (Coontail), a free floating macrophyte: response of antioxidant enzymes and antioxidants. Plant Sci 138:157–165
Fabrizio F, Coccioni R (2012) The response of benthic foraminiferal assemblages to copper exposure: a pilot mesocosm investigation. J Environ Prot 3:342–352
Franceschi VR, Nakata PA (2005) Calcium oxalate in plants: formation and function. Annu Rev Plant Biol 56:41–71
Fuentes II, Espadas-Gil F, Talavera-May C, Fuentes G, Santamaría JM (2014) Capacity of the aquatic fern (Salvinia minima baker) to accumulate high concentrations of nickel in its tissues, and its effect on plant physiological processes. Aquat Toxicol 155:142–150
Garbisu C, Alkorta I (2001) Phytoextraction: a cost effective plant-based technology for the removal of metals from the environment. Bioresource Technol 77:229–236
Goswami S, Das S (2015) A study on cadmium phytoremediation potential of Indian mustard, Brassica juncea. Int J Phytoremediat 17:583–588
Goswami S, Das S (2016) Copper phytoremediation potential of Calandula officinalis L. and the role of antioxidant enzymes in metal tolerance. Ecotox Environ Safe 126:211–218
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts I. Kinetic and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hondulas JL (1994) Treatment of polluted water using wetland plants in a floating habitat. US patent 5337516 A.
Jampeetong A, Brix H, Kantawanichkul S (2012) Effects of inorganic nitrogen forms on growth, morphology, nitrogen uptake capacity and nutrient allocation of four tropical aquatic macrophytes (Salvinia cucullata, Ipomoea aquatica, Cyperus involucratus and Vetiver iazizanioides). Aquat Bot 97:10–16
Juang KW, Lee YI, Lai HY, Chen BO (2014) Influence of magnesium on copper phytotoxicity to and accumulation and translocation in grapevines. Ecotox Environ Safe 104:36–42
Kabata-Pendias A (2011) Trace elements in soils and plants, fourth edn. CRC Press, Boca Raton
Leal-Alvarado DA, Espadas-Gil F, Sáenz-Carbonell L, Talavera-May C, Santamaría JM (2016) Lead accumulation reduces photosynthesis in the lead hyper-accumulator Salvinia minima baker by affecting the cell membrane and inducing stomatal closure. Aquat Toxicol 171:37–47
Liao S, Chang N (2004) Heavy metal phytoremediation by water hyacinth at constructed wetlands in Taiwan. J Aquatic Plant Manag 42:60–68
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. In: Sies H, Douce R, Colowick N, Kaplan N (eds) Methods in enzymology-plant cell membranes, vol 148. Academic, San Diego, pp. 350–381
Lowry OH, Rosenberg NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Lu Q, He ZL, Graetz DA, Stoffella PJ, Yang X (2011) Uptake and distribution of metals by water lettuce (Pistia stratiotes L.). Environ Sci Pollut R 18:978–986
Macnicol RD, Beckett PHT (1985) Critical tissue concentrations of potentially toxic elements. Plant Soil 85:107–129
Manios T, Stentiford EI, Millner PA (2003) The effect of heavy metals accumulation on the chlorophyll concentration of Typha latifolia plants, growing in a substrate containing sewage sludge compost and watered with metaliferus water. Ecol Eng 20:65–74
Mateos-Naranjo E, Andrades-Moreno L, Cambrollé J, Perez-Martin A (2013) Assessing the effect of copper on growth, copper accumulation and physiological responses of grazing species Atriplex halimus: ecotoxicological implications. Ecotox Environ Safe 90:136–142
McFarland DG, Nelson LS, Grodowitz MJ, Smart RM, Owens CS (2004) Salvinia molesta D.S. Mitchell (Giant Salvinia) in the United States: a review of species ecology and approaches to management. Environmental laboratory ERDC/EL SR-04-2. US Army Corps of Engineer, Engineer Research and Development Center, Washington, DC, pp. 1–33
Monferrán MV, Sánchez Agudoa JA, Pignata ML, Wunderlin DA (2009) Copper-induced response of physiological parameters and antioxidant enzymes in the aquatic macrophyte Potamogeton pusillus. Environ Pollut 157:2570–2576
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Olguín EJ, Hernández E, Ramos I (2002) The effect of both different light conditions and the pH value on the capacity of Salvinia minima baker for removing cadmium, lead and chromium. Acta Biotechnol 22:121–131
Ostroumov SA, Shestakova TV (2009) Decreasing the measurable concentrations of Cu, Zn, Cd, and Pb in the water of the experimental systems containing Ceratophyllum demersum: the phytoremediation potential. Doklady Biol Sci 428:444–447
Peng SL, Du WB, Li ZA (2004) A review of heavy metal accumulation and tolerance by plants of different ecotype. J Jishou Univ (Nat Sci Ed) 35:19–26
Perreault F, Popovic R, Dewez D (2014) Different toxicity mechanisms between bare and polymer-coated copper oxide nanoparticles in Lemna gibba. Environ Pollut 185:219–227
Prado C, Rodríguez-Montelongo L, González JA, Pagano EA, Hilal M, Prado FE (2010) Uptake of chromium by Salvinia minima: effect on plant growth, leaf respiration and carbohydrate metabolism. J Hazard Mater 177:546–553
Rascio N, Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci 180:169–181
Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T, Stiborova M, Adam V, Kizek R (2013) The role of metallothionein in oxidative stress. Int J Mol Sci 14:6044–6066
Samecka-Cymerman A, Kempers AJ (2004) Toxic metals in aquatic plants surviving in surface water polluted by copper mining industry. Ecotox Environ Safe 59:64–69
Sandalio LM, Dalurzo HC, Goımeź M, Romero-Puertas MC, del Rio LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52:2115–2126
Souza IRP, MacAdam JW (1998) A transient increase in apoplastic peroxidase activity precedes decrease in elongation rate of B73 maize (Zea mays L.) leaf blades. Physiol Plantarum 104:556–562
Suñé N, Sánchez G, Caffaratti S, Maine MA (2007) Cadmium and chromium removal kinetics from solution by two aquatic macrophytes. Environ Pollut 145:467–473
Sweta BK, Singh R, Singh RP (2015) The suitability of Trapa natans for phytoremediation of inorganic contaminants from the aquatic ecosystems. Ecol Eng 83:39–42
Upadhyay RK, Panda SK (2009) Copper-induced growth inhibition, oxidative stress and ultrastructural alterations in freshly grown water lettuce (Pistia stratiotes L.). C R Biologies 332:623–632
Valipour A, Raman VK, Ghole VS (2011) Application of patent bio-rack wetland system using phragmites sp. for domestic wastewater treatment in the presence of high total dissolved solids (TDS) and heavy metal salts. J Environ Sci Eng 53:281–288
Vamerali T, Bandiera M, Mosca G (2010) Field crops for phytoremediation of metal-contaminated land, a review. Environ Chem Lett 8:1–17
Voitsekhovskaja OV, Tyutereva EV (2015) Chlorophyll b in angiosperms: functions in photosynthesis, signaling and ontogenetic regulation. J Plant Physiol 189:51–64
Webb AAR, McAinsh MR, Mansfield TA, Hetherington AM (1996) Carbon dioxide induces increases in guard cell cytosolic free calcium. The Plant J 9:297–304
Yang TB, Poovaiah BW (2003) Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci 8:505–512
Acknowledgment
We thank the Sophisticated Analytical Instrumentation Facility, North Eastern Hill University, Shillong, India, for the SEM-EDX facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Das, S., Goswami, S. Copper phytoextraction by Salvinia cucullata: biochemical and morphological study. Environ Sci Pollut Res 24, 1363–1371 (2017). https://doi.org/10.1007/s11356-016-7830-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7830-7