Abstract
Phytoremediation is an important process that uses plants, green vegetation, trees, aquatic plants, and grasses to remove, stabilize, transfer, and/or destroy toxic pollutants from surface water, groundwater, wastewater, sediments, soils, and/or external atmosphere. The phytoremediation mechanisms include phytoextraction (i.e., phytoaccumulation), enhanced rhizosphere biodegradation, phytostabilization, and phytodegradation. Certain plant species have the tendency and the ability to accumulate and store pollutants such as metals and organic contaminants in their roots. The remediation of pollutants includes translocation, accumulation, transpiration, and possibly metabolization of the organic contaminants to plant tissue or CO2. They also prevent the flow of groundwater from transferring pollutants away from the site to the deeper.
Many countries have been successfully remediated several million acres of contaminated soil and land by employing soil phytoremediation technology. The biodegradability of given pollutants is affected by several factors, including the physical-chemical properties of the contaminants and how the soil can influence and affect its chemical state. Nevertheless, the high costs of the conventional physical and chemical strategies hindered these efforts. Thus, the use of higher plants, bacteria, microalgae, and fungi is feasible for degrading persistent contaminants. Phytostimulation process is known as “rhizosphere degradation,” in which degradation of the pollutants is achieved by organisms that are associated with the plant roots.
Phytoextraction or phytoaccumulation is the uptake, accumulation, and concentration of pollutants from the contaminated environment by the roots of plant. The pollutants are then translocated/accumulated into the plant biomass. Phytoabsorption, phytosequestration, or phytoaccumulation involves the absorption of pollutants by the plant roots followed by translocation and accumulation in the aerial parts. This took place mainly in the uptake of heavy metals as well as organic compounds. In addition, the hyper-accumulator plants have the tendency to store reasonable concentrations of certain metals in their tissues. On the other hand, the phenomenon of producing chemical compounds by plant to immobilize pollutants at the interface of soil and roots is described as “phytostabilization.” However, the phytovolatilization technique relies and depends on the ability of certain plants to volatilize and absorb some metals/metalloids. Rhizofiltration is the process through which plants concentrate, absorb, and/or precipitate pollutants, such as heavy metals and/or radioactive elements, from an aqueous medium.
The importance of the phytoremediation process is that it is efficient for the removal of toxic organic aromatic pollutants, polycyclic aromatic hydrocarbons (PAHs), explosives (RDX, TNT, HMX), pesticide, landfill leachates, as well as herbicide contamination. Phytoremediation process is also efficient for wastewater and improving quality of water. Thus, employing phytoremediation in constructed wetland (CW) technology offers a low-cost treatment system for wastewater. Thus, CWs are perfect for the decentralized treatment of wastewater for offering great potential for the phytoremediation of contaminants and removal of pathogens and toxic substances. In conclusion, phytoremediation is an emerging “green bioengineering technology” that uses plants to remediate environmental problems. Green plants (both aquatic and terrestrial) have the wonderful properties of environmental restoration, such as decontamination of polluted soil and water. In general, the phytoremediation technology has several advantages and disadvantages that should be considered when applying such process. The low cost is one of the most important advantages. However, the time needed to observe the necessary achievement can be long. The concentration of contaminants should also be considered.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
5.1 Introduction
Phytoremediation is an important process that uses plants, green vegetation, trees, aquatic plants, and grasses, to remove, stabilize, transfer, and/or destroy toxic pollutants from surface water, groundwater, wastewater, sediments, soils, and/or external atmosphere. The phytoremediation mechanisms include phytoextraction (i.e., phytoaccumulation), enhanced rhizosphere biodegradation, phytostabilization, and phytodegradation (Singh and Jain 2003).
Phytoremediation is applicable for the uptake and remediating metals, hazard organic pollutants (i.e., pesticides, PAHs, crude oil), explosives, solvents, and landfill leachates. Certain plant species have the tendency and the ability to accumulate and store pollutants such as metals in their roots. These plant species can be transplanted to filter metals and pollutants from contaminated water or wastewater. Once the roots become saturated with contaminants and/or metals, they should be harvested. Usually, the hyper-accumulator plants are able to remove, accumulate, and store remarkable amount of the metallic pollutants. Trees are under examination, currently, to investigate their ability in removing the organic pollutants from groundwater. The study includes the translocation, accumulation, transpiration, and possibly metabolization of the organic contaminants to plant tissue or CO2. Meanwhile, plants help prevent wind, dust, and rain. They also prevent the flow of groundwater from transferring pollutants away from the site to deeper underground and/or the surrounding areas.
5.2 Phytoremediation of Soil
As the anthropogenic activities increase around the world, contamination of soil and remediation of the polluted sites become a worldwide concern with the environmental threat (Bundschuh et al. 2012). In Europe, the contaminated sites are about 340,000. About 15% out of them have been remediated (EEA 2014). It was reported by the Office of Solid Waste and Emergency Response (OSWER), of the United States of America, that they have been successfully remediated over 540,000 sites and 23 million acres of contaminated soil and land (Treasury Board of Canada Secretariat 2014). In Australia, agricultural activities and industrial processing including mineral mining, petroleum refinery, and chemical manufacturing have caused soil contamination with pesticides, nutrient elements, heavy metals, mineral salts, hydrocarbons, particulates, etc. The estimated contaminated sites across that country were 80,000 according to DECA (2010). In China, in 2014, the Ministry of Land and Resources and the Chinese Ministry of Environmental Protection released their first bulletin survey concerning the nationwide soil pollution. They estimated about 20 million hectares of land were contaminated with heavy metals. This contaminated land may cause reduction in food supplies exceeding 10 million tons/year in China (Wei and Chen 2001).
The biodegradability of given pollutants is affected by several factors, including the physical-chemical properties of the contaminants and how the soil can influence and affect its chemical state. Diverse soils, generally, vary in their ability to overcome the contamination. This may be due to the behavior of insoluble organic pollutants. There are an extensive efforts and enormous developing strategies for the remediation of environmental pollutants. Nevertheless, the high costs of the physical and chemical strategies hindered these efforts. Thus, the use of biological strategies is a more feasible and applicable. These employed biological methods are using different biological agents such as higher plants, bacteria, microalgae, and fungi to degrade persistent contaminants. The process of soil bioremediation is to utilize the metabolic activities of microorganisms to remove and clean up the hazard contaminants. The main aim is to convert the organic pollutants, completely to harmless compounds, such as water and CO2. The time factor is also an important factor. It is important that microorganisms are capable to achieve the desired degradation within a reasonable period of time (Fu and Secundo 2016).
5.3 Soil Phytoremediation Process
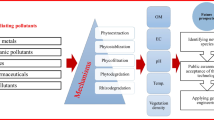
The technical processes of the phytoremediation are varied widely. According to Schnoor (1997), this variation depends on the properties and the chemical nature of the pollutants (if it is subject to degradation in the soil or in the plant or it is volatile or inert) and the characteristics of plant as illustrated in Figs. 5.1 and 5.2.
Schematic representation of phytoremediation techniques. (Schnoor 1997)
Pathway of pollutants inside the plant cell wall. (Schnoor 1997)
5.3.1 Phytostimulation (Biodegradation of Enhanced Rhizosphere)
Phytostimulation process is known as rhizosphere degradation, and it is also known as “Rhizo degradation”, in which enhancement of soil microbial activity for contaminants degradation. Such degradation of the pollutants is achieved by organisms that are associated with the plant roots. Enhanced rhizosphere biodegradation is carried out in the soils that are immediately surrounding plant roots. The microorganisms are supplied by nutrients via the natural substances released by plant roots. Meanwhile, the microorganisms enhance the biological activities of the plant roots; the latter also loosen the soil and then die, leaving paths for transporting of aeration and water. This process also causes to pull water to the surface zone of the soil and tends to dry the lower saturated zones. Plant roots, thus, stimulate soil microbial activity for the degradation of pollutants in the rhizosphere or soil root zone. According to Dowling et al. (2009), plant roots stimulate microbial activity in the following different ways:
-
1.
They enhanced aerobic transformations through oxygenate rhizosphere.
-
2.
They increase the bioavailability of organic carbon.
-
3.
Root exudates possess enzymes, carbohydrates, sugars, amino acids, etc. that enrich the microbes.
-
4.
Degradation of the organic contaminants by the mycorrhizae fungi that grow within rhizosphere.
-
5.
An ideal habitat is provided by the roots to increase the microbe populations. This process is applicable in the containment of organic pollutants from soil like aromatics, poly-aromatic hydrocarbons (PAHs), and pesticides.
In addition, the growing roots promote and enhance the proliferation of degrading rhizosphere microorganisms. The latter utilize metabolites and exudates of plants as a source of energy and carbon. Plants, however, may exude biodegrading enzymes themselves. The phytostimulation application is limited to organic pollutants only (Prasad 2004). In the rhizosphere, the microbial community is heterogeneous due to the different spatial distribution of nutrients. Nevertheless, the genus Pseudomonas species are the predominant organisms, and they are associated with roots (Ali et al. 2013).
Besides, phytostimulation can involve aquatic plants to support active populations of the microbial degraders. Similarly, the stimulation of atrazine degradation was carried out by hornwort (Rupassara et al. 2002). In the phytoremediation projects, the most commonly used flora is poplar trees. This is primarily because the trees are fast growing and they can survive in a very broad range of climates. Relative to other plant species, poplar trees can draw large amounts of water, mainly because water passes directly from an aquifer or through soil. These trees may draw greater quantities of dissolved contaminants from polluted media. They reduce and consume the amount of waters that are originally from an aquifer or pass through soil. Therefore, these trees are reducing the amount of pollutants that pass though the soil or out of aquifer (ITRC 2009) as shown in Fig. 5.3.
Rhizo-degradation of organic compounds from contaminated media. (ITRC 2009)
5.3.2 Phytoextraction or Phytoaccumulation
Phytoextraction is the uptake, accumulation, and concentration of pollutants from the contaminated environment by the roots of plant. The pollutants are then translocated/accumulated into the plant biomass (i.e., shoots and leaves). Phytoabsorption, phytosequestration, or phytoaccumulation involves the absorption of pollutants by the plant roots followed by translocation and accumulation in the shoots and leaves (i.e., aerial parts). This took place mainly in the uptake of heavy metals such as Cd, Ni, Cu, Zn, and Pb (Abdel-Shafy et al. 1986). Other heavy metals including Se, As, Ni-56, and Co and organic compounds can also be eliminated (Abo-El-Souad et al. 1994). The hyper-accumulator plants are used to concentrate specific metals in their aerial parts. These hyper-accumulator plants have the tendency to store reasonable concentrations of certain metals in their tissues (i.e., 0.01–1% dry weights). The amount of accumulated metals varied from one metal to the other (Abdel-Shafy and Farghaly 1995). Pteris vittata, Elsholtzia splendens, Thlaspi caerulescens, and Alyssum bertolonii are hyper-accumulator plants. They are known for the uptake of Cu, Ni, Zn/Cd, and As (Vander Ent et al. 2013).
The aerial parts of plants (i.e., above ground) can be harvested and burnt to obtain energy and regain metals from ash as recycling. This technique has been extensively used for bioremediation and elimination of heavy metals including Pb, Zn, Cu, Ni, and Cd using plants such as Thlaspi caerulescens, sunflower (Helianthus spp.), Indian mustard (Brassica juncea), and vascular plants (Abdel-Shafy et al. 1994a, b). Effective phytoextraction and elimination of certain pollutants require hyper-accumulator plants. These plants are able to accumulate metals at the rate of more than 100 times as compared to non-accumulator plants. In this respect, nickel hyper-accumulator Berkheya coddii was utilized to remove Ni from land near the Rustenburg smelter, South Africa in 1990s (Vatamaniuk 2001). In addition, sunflower (Helianthus annuus) or bracken fern, as hyper-accumulators, was employed to clean up arsenic. The leaves of bracken fern are able to store arsenic as much as 200 times as compared to soil. Similarly, lead was phytoextracted by hemp dogbane, ragweed, or Indian mustard (Fig. 5.4) (Taiz and Zeiger 2010).
(a) Schematic presentation of chelate-assisted phytoremediation of metals, (b) continuous phytoremediation and uptake of metals. Metal concentration is presented by solid line and shoot biomass is presented by dashed line. (Taiz and Zeiger 2010)
In the root zone, phytochemical complexation reduces the fraction of the bioavailable organic pollutants. Transport protein inhibition, located on the root membrane, prevents organic pollutants from entering the plant. Vacuolar storage in the root cells, as phytoaccumulation of organic compounds, in which the organic pollutants can be sequestered into the vacuoles of root cells (ITRC 2009) as shown in (Fig. 5.5).
Phytoaccumulation of organic contaminants. (ITRC 2009)
5.3.3 Phytodegradation (Phytotransformation)
The metabolism of pollutants within plant tissues is known as phytodegradation. Enzymes including oxygenase and dehalogenase are produced by plants. These enzymes help catalyze the degradation process. Several researches are proceeding to investigate if both chlorinated aliphatic and aromatic organic compounds are amenable to phytodegradation by plants. Organic pollutants are degraded, mineralized, or metabolized inside plant cells via specific enzymes. These enzymes are laccases (degradation of anilines), dehalogenases (degradation of chlorinated solvents and pesticides), and nitro-reductases (degradation of nitro-aromatic compounds). Plants such as Myriophyllum spicatum and Populus species are examples that have these enzymatic systems. It was reported by Rylott and Bruce (2008) that chemical modification of environmental substances resulted from plant metabolism and often induces the degradation (phytodegradation), inactivation, or immobilization (phytostabilization) as shown in Fig. 5.6.
Phytodegradation of organic compounds by plant. (ITRC 2009)
In the case of organic contaminants, certain plants, such as Canna, render some pollutants including industrial chemicals, pesticides, solvents, explosives, and other xenobiotic substances, as nontoxic by their metabolism (Kvesitadze et al. 2006). In other cases, however, microorganisms that live and associate with plant roots may metabolize such contaminants in soil or water. The plant tissue metabolism of these recalcitrant compounds and contaminants cannot be broken down to basic molecules (carbon dioxide, water, etc.). Thus, the term phytotransformation is representing the change that took place in the chemical structure of pollutants without complete breakdown of their compound structure.
5.3.4 Phytostabilization (Phytoimmobilization)
The phenomenon of producing chemical compounds by plant to immobilize pollutants at the interface of soil and roots is described as phytostabilization. This phenomenon of phytostabilization reduces the mobility of substances in the environment. This can be achieved by limiting the leaching of substances from soil. Inorganic or organic contaminants are incorporated into humus or into the lignin of the cell wall of roots. By the direct action of root exudates, metals are precipitated as insoluble forms; subsequently metals are trapped in the soil matrix.
The main aim is to avoid the mobilization of the pollutants and to limit their presence in soluble form as well as to avoid their diffusion in the soil. It was reported by Ali et al. (2013) and Berti and Cunningham (2000) that some genera species including Alyssum, Haumaniastrum, Eragrostis, Gladiolus, and Ascolepis are examples of plants that are cultivated for this purpose. The study focused on the elimination of contaminants in soil adjacent to the roots for the purpose of reducing their bioavailability. The pollutants are rendered insoluble, immobile, and less toxic through accumulation and adsorption by the roots. Thus, they precipitate or exudate within the soil adjacent to the roots and root zone. Phreatophytic trees that are characterized with fibrous roots are extensively used for hydraulic control and for soil erosion control. These trees are being used also for the uptake of heavy metals such as Cd, Pb, Zn, Cu, As, U, and Se. Besides, they are utilized for the removal of hydrophobic non-biodegradable organic compounds (Fig. 5.7).
Phytostabilization of organic and inorganic compounds. (ITRC 2009)
5.3.5 Phytovolatilization
The phytovolatilization technique relies and depends on the ability of certain plants to volatilize and absorb some metals/metalloids. Certain elements such as Hg, Se, As, and others from groups IIB, VA, and VIA of the periodic table can be absorbed by the roots, converted into nontoxic forms, and finally released into the atmosphere air. Similarly, selenium can be absorbed by the species of Stanleya pinnata and Astragalus bisulcatus and/or transgenic plants (with bacterial genes) of Arabidopsis thaliana. Meanwhile, Hg can be absorbed by Brassica napus, Liriodendron tulipifera, or Nicotiana tabacum. The same technique can also be employed for the absorbance of organic compounds (Pilon-Smits and Le Duc 2009).
Contaminants are, thus, absorbed from soil or water to be released into the air to less polluting and/or volatile substances as a result of phytotransformation. In this respect, the contaminants are uptaken and/or absorbed by the plant roots and translocated to the leaves to be volatilized through plant stomata (i.e., they are the sites for gaseous exchange as the plant transpiration). These sites are used for the containment of volatile organic compounds. Phreatophytic trees are used for capturing of organic compounds from groundwater (Fig. 5.8). Similarly, Fig. 5.9 represents the uptake of Hg, Se, and As by Brassica juncea in the wetland treatment system (ITRC 2009).
Phytovolatilization of organic contaminants by plant. (ITRC 2009)
Illustration of phytovolatilization of heavy metals as plant uptake. (ITRC 2009)
5.3.6 Rhizofiltration (Phytofiltration)
Rhizofiltration is the process through which plants concentrate, absorb, and/or precipitate pollutants, such as heavy metals and/or radioactive elements, from an aqueous medium. This process is achieved through plant root system and other submerged organs. The contaminants remain adsorbed on or absorbed in the roots. Rhizofiltration of plants is also the process where mass of plant roots could filter groundwater to remove toxic substances and contaminants. It is employed to reclaim groundwater rather than soil remediation. Here how plants are prepared: firstly, the plants are allowed to grow in clean water to achieve a large root system. After developing a mass of sizeable root system, contaminated water is supplied to the plant root to adapt them. Plants are, then, placed in contaminated area where the root mass system accumulates and uptakes pollutants from water. Once the root mass become saturated with the contaminants, the plants are to be harvested and disposed in a safe way. The plants, thus, are kept in a hydroponic system. The effluents or the aqueous media pass and are “filtered” through the roots and/or other organs. The latter is to absorb and concentrate the pollutants (Dhote and Dixit 2009). It is worth to mention that plants with more accumulation capacity, high absorption surface, high root biomass, (aquatic hyper-accumulators) and/or tolerance to pollutants achieve the best accumulation results. The best promising results were achieved by Fontinalis antipyretica, Helianthus annuus, Phragmites australis, Brassica juncea, and several species of Callitriche, Salix, Lemna, and Populus (Favas et al. 2012). It was reported that Phragmites australis was very effective in the removal of contaminants from wastewater (Abdel-Shafy et al. 2017). In addition, water hyacinths proved to be a promising plant for the uptake of heavy metals from the aquatic system (Abdel-Shafy et al. 2016; Fayed and Abdel-Shafy 1985).
Water is filtered through a mass of roots to remove the toxic contaminants or excess nutrients (Favas et al. 2012; Fayed and Abdel-Shafy 1986). It was reported that aquatic plants, submergent plants (algae, Hydrilla spp., stonewort), and emergent plants (coontail, bullrush) are used for the bioremediation of heavy metals including Cd, Pb, Zn, and Cu (Fayed and Abdel-Shafy 1985; Abdel-Shafy and Farghaly 1995). These plants are also used for the bioremediation of hydrophobic organics and radionuclides. The sunflower roots (Helianthus annuus) proved to be good candidate as rhizofilter for the uptake of pollutants including heavy metals, namely, Pb, Cu, Zn, and Cr (Abdel-Shafy and Dewedar 2012). Several common plants are known for being a good accumulator of pollutants and are considered by researchers as remediation. These plants include ragweed (Ambrosia artemisiifolia), sea pink or sea thrift (Armeria maritima), Indian mustard (Brassica juncea), turnip (Brassica napus), rape, rutabaga, cabbage, broccoli (Brassica oleracea), and flowering/ornamental kale. Similarly, other plants are considered as good phytofiltration such as corn (Zea mays), blue sheep fescue (Festuca ovina), wheat (scout) (Triticum aestivum), pennycress (Thlaspi rotundifolium), and sunflower (Helianthus annuus), (Paz-Alberto and Sigua 2013). Figure 5.10 represents the rhizofiltration process of the sunflower in a hydroponic system.
Rhizofiltration process of the sunflower in a hydroponic system. (Paz-Alberto and Sigua 2013)
5.4 Phytoremediation of Soil: Advantages and Limitations
The phytoremediation technology has several advantages and disadvantages that should be considered when applying such process. The low cost is one of the most important advantages. However, the time needed to observe the necessary achievement can be long. The concentration of pollutants as well as the presence of other contaminants should also be considered. Meanwhile, selecting plant species that could be efficient is not easy and could limit the process. These limitations could hinder the advantages of this technology. The possibility of these plants to enter the food chains should be considered carefully and should be taken into account (Burken et al. 2011).
5.4.1 Advantages of Phytoremediation Process
-
The technique can be implemented in situ as well as ex situ.
-
Sunrays are the energy for the phytoremediation process.
-
The process reduces the diverse impact of the environment.
-
It contributes to the landscape view.
-
The phytoremediation view is highly accepted by the public.
-
The system provides good habitat for the animal life.
-
Protect the concern area from contaminated winds and the dispersion of dust.
-
Reducing the surface runoff.
-
Reduces the mobilization and/or leaching of pollutants to the soil.
-
Harvesting of the organs or plants that accumulated pollutants can easily be achieved.
-
The harvested organs or biomass can be economically used.
-
Plant phytoremediation process is easy to be controlled than employing the microorganisms for remediation.
-
It is possible to reuse and recover any valuable metals by the “phytomining” companies that are specialized in this field.
-
The phytoremediation is an eco-friendly process as it protects the environment in a more natural state.
-
Monitoring of plants to follow up the phytoremediation process can be easily achieved.
5.4.2 Limitations of the Phytoremediation Technology
-
The mass of plant root is mostly at variable depths in which the treatment zone is determined as phytoremediation. In most cases, however, pollutants are localized at the surface (<5 m).
-
High concentrations of the contaminants are mostly hazardous and toxic to plants.
-
Mass transfer through the phytoremediation process is limited similar to other bio-treatments.
-
This process may be limited to seasonal variation according to the location.
-
In some cases the phytoremediation transfers pollutants from soil to air.
-
The process is not efficient to eliminate the strong sorbet (such as PCBs).
-
The bioavailability and toxicity of biodegradation products cannot always be predicted.
-
Bioaccumulation of the products by animals or mobilization into groundwater is possible.
-
The technology is still in the developing and demonstrating stages.
-
The phytoremediation technology is still unfamiliar to regulators.
-
Generally, plants are selective in terms of metal uptake in the phytoremediation process.
-
The phytoremediation process is much slower than the conventional physicochemical techniques.
-
At contaminated sites, the phytoremediation plants may not always adapt to the environmental and climatic conditions.
-
To allow the application of phytoremediation techniques in a contaminated area, this area should be large enough for cultivation.
-
The degradation products and the bioavailability of the contaminants remain, mostly unknown.
-
Efficiency of the phytoremediation is limited only down to the depth occupied by the roots.
-
The growth of plants is slow, and the formation of the biomass requires a long-term commitment.
-
Preventing the leaching of pollutants into the groundwater completely is not possible.
-
Survival and efficiency of the plants are greatly affected by the condition of the soil and the toxicity of the pollutants.
-
The bioaccumulation of the pollutants by plants requires a careful and safe disposal of these plant materials to protect the environment.
5.5 Phytoremediation for the Removal of Heavy Metals: Concept and Application
Heavy metals, in the environment, form an important polluting group. These metals naturally occur at low concentrations in the environment. They rarely occur naturally at toxic level. Heavy metals, mostly, come from the industrial local sources such as iron and steel, nonferrous industries, chemical industries, and power plants. Soil contamination with heavy metals is a critical and environmental concern due to their toxic and potential adverse effects to the ecology. Due to their widespread occurrence, heavy metals are considered hazard soil contaminants as they possess chronic and acute toxicity. Heavy metals are strongly persistent in the environment. They are non-biodegradable pollutants and non-thermo-degradable. Unlike organic pollutants they persisted and cannot be degraded into smaller constitutes. They cannot be broken down; thus, they can be accumulated to toxic levels. They cannot also be degraded to harmless or small molecules. The long-term effect might be toxic to the biosphere. Heavy metals are known for having toxic effect (Abdel-Shafy and El-Saharty 2015). Besides, they tend to accumulate in all living organisms with a permanent toxic and carcinogenic effect (Ben Chekroun and Baghour 2013).
Furthermore, heavy metal pollutants are difficult to remediate and/or remove from water, soil, and air. Toxic heavy metals such as and zinc, copper, lead, cadmium, arsenic, and mercury are immutable through all biochemical reactions (Kramer and Chardonnens 2001).
On the other hand, the use of wastewater for irrigation as well as dumping sewage sludge and solid wastes on soils has been a widespread practice in agricultural areas (Abdel-Shafy et al. 2003). The wastewater includes industrial, municipal, and household liquid waste. The source of heavy metals to the agriculture area comes from irrigation with polluted water, use of sewage sludge, contaminated manure, pesticides containing heavy metals, as well as the use of mineral fertilizers especially phosphates (Abdel-Shafy et al. 2005). Other sources of heavy metals are burning of fossil fuels, waste incineration, and road traffic. Several investigators studied the impact of using sewage effluent, drainage water and industrial wastewater in irrigation, and their diverse effect on water, soil, and plants related to heavy metal contamination (Abdel-Sabour et al. 2001; El-Bahy et al. 2005). Their study included the accumulation of heavy metals in soil, in plant, in animals, and lastly in the food product (Khan et al. 2010).
Heavy metals and metalloids, including mercury, cadmium, arsenic, selenium, and lead, are released into the environment by human activities such as industry, mining, and agriculture. These activities are threatening the environment as well as human health (Danik et al. 2006; Kamal et al. 2010). In the United States alone, there are more than 50,000 sites contaminated with heavy metals. Many of these sites are under Superfund, and they should be remediated (Bennett et al. 2003). There is a stinging need to adapt low-cost, efficient, and sustainable technology to detoxify or remove such acute and toxic contaminants from the environment. The phytoremediation process as plant-based approaches is the most convenient. These processes are performed in situ, are relatively inexpensive, and are solar-driven (LeDuc and Terry 2005).
Most researches on the phytoremediation practically aim to extract and remove the pollutants from wastewater, soil, and sediment. The pollutants are to be transported aboveground and to be concentrated by orders of magnitude. Similarly, plants can also assist with the cleanup of surface water polluted with elemental contaminants. In this term, phytoremediation has important advantages over the traditional physical remediation processes. The latter relocates capping or postpones the problem. Plants have the ability to enhance the activity of rhizosphere and extract the contaminants aboveground. Thus, plants could both support and amend fungal and bacterial remediation schemes for metal pollutants. In addition, plants can be applied to the bioremediation of various airborne pollutants. This is mainly due to their natural capacity to extract nutrients including nitrogen, carbon dioxide, and sulfur from air (Meagher and Heaton 2005).
The plant employed in the phytoremediation process must be selected carefully. They should have a considerable capacity of heavy metal absorption and accumulation. The time is an important factor for decontamination and removal of pollutants from the ecosystem (Mudgal et al. 2010). Plants proved to be able to uptake and accumulate different heavy metals from the environment (Baghour et al. 2002; Abdel-Shafy et al. 1994a, b). Tolerance of heavy metal in plants could be referring to their immobilization in the plant cell wall (Davis et al. 2003). It may be also referring to their conferred by compartmentalization in the vacuoles (Ben Chekroun and Baghour 2013). Some algae exhibit high capacity for the accumulation of heavy metals (Abdel-Shafy and Farghaly 1995). This capacity is the result of the tolerance mechanisms. Many algae synthesize metallothioneins and phytochelatins that can form strong complexes with metals and, thus, translocate them into the vacuoles (Abdel-Shafy and Farghaly 1995; Ben Chekroun and Baghour 2013). So far, the main success for phytoremediation application is the removal of toxic metals and trace elements from soil (Chaudhry et al. 2005).
5.6 Origin and Source of Heavy Metals to Aquatic Ecosystems
Aquatic ecosystems, in many countries, have been subjected to the discharge of industrial wastewater. Domestic, sewage sludge, and agricultural drainage have been discharged also to several waterways in many countries. These wastes are important source of inorganic and organic contaminants including heavy metals and pesticides. These contaminations lead to widespread of pollutants to both groundwater and surface water by runoff. Meanwhile, weathering of rocks and soil from volcanic eruptions introduces metals to the aquatic ecosystems. Varieties of human activities including industrial processing and mining are important sources of heavy metals to the environment (Jain 2004). The input of these metals may also be derived from remobilization of natural soils as a result of the changes in local environmental redox conditions. Additional source of heavy metals is the corrosion of metallic subsurface engineering structures that resulted from prolonged submergence under acidic groundwater (Leung and Jiao 2006).
González et al. (2007) studied the origin and bioavailability of heavy metals from the Nador Lagoon sediments (Morocco). They found that the most important trace element anomalies including Mn, Zn, Cu, Co, Pb, Cd, and As were mainly found around old mining and industrial activities. Such industrial activities are the main contributors of very high concentrations of heavy metal into the environment. Their contributions are between 100- and 1000-fold higher than those in the Earth’s crust (Ben Chekroun and Baghour 2013).
In a study by Ben Chekroun and Baghour (2013) on a river polluted by base metal mining, they found that cadmium was potentially bioavailable and the most mobile metal. They also reported that Cd was primarily scavenged by organic matter, non-detrital carbonate minerals, and iron-manganese oxide minerals. On the other hand, mercury is always present in the environment as naturally occurring metal at very low concentrations. Global human activity, however, has led to a remarkable increase of this metal in land and aquatic environment and is released into the atmosphere (Dietz et al. 2009). Wang et al. (2004) mentioned that the most important anthropogenic sources of Hg contamination in aquatic environment are mining, the atmospheric deposition, agricultural material runoff, urban discharges, industrial discharges, fossil fuel use, pharmaceutical production, and burning of coal. It was reported by Mohiuddin et al. (2010) that heavy elements may be immobilized in the stream sediments. Thus, these trace elements could be involved in complex formation, coprecipitation, and absorption (Okafor and Opuene 2007). Trace elements, sometimes, are co-adsorbed along with other elements in the form of hydroxides or oxides of Fe and Mn. They may, also, occur in particulate form (Mwiganga and Kansiime 2005). However, treatment of the metal-contaminated wastewaters before their discharge into the environment is essential. This way protection of the ecosystem can be achieved to control the levels of heavy metal in the environment.
5.7 Mechanism of Phytoremediation for the Uptake of Heavy Metals
Mechanism of the phytoremediation of trace elements/metalloids in soils is illustrated in Fig. 5.11. Plants accumulate metals from different media such as water, wastewater, and soil. These media act as bioavailable pool of trace metals as well as plant nutrients. Factors including pH, competitive cations, root exudates, microbial biomass, and organic matter can affect the availability of trace metals in environment (Sarwar et al. 2010). A specific trace elements once accumulated by plant roots may be either uptaken in root tissues (phytoimmobilization). Metals may also be translocated to the aerial shoot parts of plant through xylem vessels. The latter is achieved via symplastic and/or apoplastic pathways. Generally, metals in the aerial part are accumulated in vacuoles (cellular organelles with low metabolic activity). In the hyper-accumulator plants, this might be an important tolerance mechanism. The purpose is to keep deleterious metals away from important cellular metabolic processes. The mechanism of the phytoextraction has the following five major steps: metal mobilization in rhizosphere, metal uptake by plant roots, translocation toward aerial plant parts, metal sequestration in plant tissues, and trace metal tolerance (Ali et al. 2013). Tolerance of trace metals is a prerequisite for the process of phytoremediation. As the more the plant is tolerant to trace metal stress, the more will be the metal accumulation in plant tissues. This will result with minimum adverse effects on plant health. Metal tolerance potential of a plant depends on mechanisms like active transport of metal ion into the vacuoles, cell wall metal binding, complex formation, and chelation of metal ions with proteins and peptides (Memon and Schreoder 2009).
Mechanisms involved in phytoremediation of trace metals/metalloids in soils. (Memon and Schreoder 2009)
5.8 Role of Algae in Phytoremediation and Heavy Metal Removal
Phytoremediation is considered the most favorable technology in which plants are used for removal of environmental contaminants and/or detoxify these pollutants to make them harmless (Ben Chekroun and Baghour 2013). Several living organisms are able to concentrate and accumulate certain toxicants to their body to level much higher than their environments (Fayed and Abdel-Shafy 1986; Kord et al. 2010). As a result, the use of plants for the removal of heavy metals has become attracted because of the various problems associated with the removal of pollutants using traditional methods. Bioremediation including phytoremediation strategies has been used as an attractive alternative simple technology due to their low cost, low energy, and high efficiency (Abdel-Shafy et al. 2017; Mejare and Bulow 2001).
Recently, there has been great interest in using algae for determining the eutrophication through the biomonitoring of organic and inorganic contaminants. It was possible to estimate the total nitrogen content spectrophotometrically in water of the aquatic systems (Ben Chekroun and Baghour 2013). This was achieved by determining the chlorophyll formation by the algae (Abe et al. 2004). This can give us clear idea about the levels on eutrophication. The high biomass production by algae is one of the advantages of using it in phytoremediation. These species have high tendency to absorb and accumulate heavy metals (Baghour et al. 2002).
It was reported that removal of heavy metal by the conventional treatment involves variable mechanisms which include increasing the pH, flocculation, settling, sedimentation, precipitation, cation and anion exchange, oxidation/reduction, complexation, adsorption, and microbiological activity/microalgae (Abdel-Shafy 2015). The latter can remove heavy metals from contaminated water via two major mechanisms. The first mechanism is the metabolism of metal uptake into their cells at low concentrations. The second mechanism is the bio-sorption of metals which is a non-active adsorption process (Fayed et al. 1983; Ben Chekroun and Baghour 2013).
The algae are ideal candidates for concentrating and removing heavy metals from the environment. They have many features to make them so ideal including large surface area/volume ratios, high tolerance to heavy metals, phytochelatin and phototaxy expression, potential for genetic manipulation, and ability to grow both autotrophically and heterotrophically (Ben Chekroun and Baghour 2013). Macroalgae have been used extensively to determine the environmental contamination and marine pollution with heavy metal in marine ecosystem locations in the world (Abdel-Shafy 2015). Recently, green algal species, namely, Cladophora and/or Enteromorpha, have been used to determine the level of heavy metals in different parts of the world (Al-Homaidan et al. 2011; Abdel-Shafy and Farghaly 1995). Due to their efficiency in accumulating heavy metals and concentrating them in their tissues, the macroalgae have become widespread, used as biomonitors of metal in aquatic and marine ecosystems (Gosavi et al. 2004, Abdel-Shafy and Farghaly 1995). It was reported that Cyanophyta and Chlorophyta are hyper-accumulators and hyper-absorbents for boron and arsenic. They can accumulate and absorb these elements from the environment to concentrate them in their bodies (Ben Chekroun and Baghour 2013). Thus, these algae are considered hyper-phytoremediators. On the other hand, the Phaeophyta as brown algae are efficient in accumulating heavy metals. This is mainly due to the high levels of alginates and sulfated polysaccharides within their cell walls. Thus, heavy metals show a strong affinity to these Phaeophyta brown algae (Davis et al. 2003). Nielsen et al. (2005), afterward, proposed that brown algae including fucus spp. mostly dominate the heavy metal-contaminated vegetation habitats.
5.9 Phytoremediation for the Removal of Toxic Organic Aromatic Pollutants
Poly-aromatic hydrocarbons (PAHs) are identified as one of the most hazardous chemical pollutants in the environment. These chemicals have carcinogenic, toxic, and mutagenic effects on living body (Abdel-Shafy and Mansour 2016). Beside their carcinogenic activity, these polyaromatic hydrocarbons (PAHs) are recalcitrant, ubiquitous occurring and have bioaccumulating potential. The diesel and all hydrocarbon fuels composed of excessive quantity of PAHs. Thus, they cause abundant ecosphere pollution of the PAHs (Ruma et al. 2007). The level of PAHs in the environment varies widely depending on the mode of PAHs transported in the environment, the level of industrial pollution, and the nature of the contaminated sites. It was reported by Kanaly and Harayama (2000) that the concentrations of PAHs in sediment and soil vary from 1 μg/kg to over 300 g/kg. The PAHs are colorless, white/pale yellow solids, and low soluble in water and have high boiling and melting points with low vapor pressure.
The remediation of contaminated sites with PAHs has become the first priority for society due to the increase in the awareness of environmental issues as well as the quality of life standards. During the past few decades, there are increasing interest to develope the in situ strategies to remediate the increasing amount of environmental pollution. For this purpose, several tools of the biological remediation have become more interesting for the removal of these persistent contaminants. Particular interest of employing such biological remediation tools is the high economic cost of the physicochemical processes.
A specific type of phytoremediation is the rhizoremediation. It involves both plants and their associated rhizospheric microbes. The latter is the creative biotechnological approach as an important tool of the phytoremediation technique. Thus, the rhizoremediation of PAHs has advantages over other bioremediation strategies for being bioaugmentating, natural attenuating, and phytoremediating. It was reported that various bacterial species are active in degrading different types of PAHs (Bisht et al. 2015). Most of these bacterial species are isolated from contaminated soil. Nevertheless, few of these bacteria can be also isolated from non-contaminated soil. Pseudomonas aeruginosa, Mycobacterium spp., Pseudomonas fluorescens, Rhodococcus spp., Paenibacillus spp., and Haemophilus spp. are some of these bacteria that were commonly studied for the degradation of PAHs (Bisht et al. 2010). Moreover, investigating the molecular communication between microbes and plants to discover such communication is important to achieve better results for the purpose of pollutant elimination. This area of research could be a fascinating point of research for future perspective.
5.10 Phytoremediation: Remedial Strategy for Polycyclic Aromatic Hydrocarbons (PAHs)
A diverse range of hazardous contaminants can be treated efficiently via phytoremediation process. This process can be employed in much larger scale to clean up the pollution. It is easy to implement to contaminated soil, and it is an environmental friendly eco-technology. Besides, it can be visually attractive sites and sustainable technology for the polluted sites. Earlier, different studies investigated the effects of plants on removal of contaminants from spiked water or soil from polluted sites (Huang et al. 2004; Abdel-Shafy et al. 1986). Most of these studies provided valuable results concerning the insights of phytoremediation mechanisms for the removal of organic contaminants and heavy metals (Reed and Glick 2005; Abdel-Shafy and Farghaly 1995). Organic contaminants that have been successfully removed by phytoremediation include herbicides (atrazine), organic solvents such as trichloroethylene (TCE), polychlorinated biphenyls (PCBs), explosives such as trinitrotoluene (TNT), PHC, the fuel additive as methyl tertiary butyl ether (MTBE), PAHs, and BTEX – mono-aromatic hydrocarbons (Pilon-Smits 2005). In addition, the phytoremediated inorganic pollutants include phosphate, nitrate, radioactive isotopes, and plant macronutrients (Harvey et al. 2002). The finding of their researches and experiments allowed the scientists to explore processes for overcoming pollutant stress, without the effects of environmental condition including nutrient limitation and weather. However, it was concluded by Harvey et al. (2002) that the only problem of phytoremediation lies in the removal of high level of pollutants. The latter tends to inhibit the growth of plant, including their root, as well as the oxidative stress. These two factors limit the rate of removal and the efficiency of the phytoremediation (Huang et al. 2005).
5.10.1 Rhizosphere Technology: For Bioremediation of Polycyclic Aromatic Hydrocarbons (PAHs)
In the rhizosphere technique, the microbial communities are used for biodegradation of contaminants. It was reported, earlier, that the endophytic and rhizospheric bacteria were used for rhizoremediation of PAHs in the environment by employing Populus sp. The latter species, namely, Populus sp., was used in the soil as an inoculation system (Bisht et al. 2010; Bisht et al. 2014). In correlation with the bioaugmentation, nevertheless, Populus sp. in rhizosphere bacteria assisted phytoremediation technology. The rhizoremediation is effective for the degradation and/or removal of organic pollutants from contaminated soils. Particular efficiency can be achieved when the rhizoremediation is used in combination with appropriate agronomic techniques (Zhuang et al. 2007). This is mainly due to the fact that the chemical condition of the rhizosphere greatly differs from bulk soil. Consequently, various processes are induced by the roots of plant along with the rhizobacteria (Marschner 2001).
It is greatly agreeable that the contribution of microbes is of great importance for the degradation of contaminants. The phytoremediation alone, without the microbial contribution, is not an efficient technology for many PAH degradation (Chaudhry et al. 2005). Rhizoremediation term describes the combined beneficial interaction of both the plant and the rhizobacteria. The combined interaction of microbial-plant in the rhizosphere offers very useful tools for PAH environmental remediation (Chaudhry et al. 2005). The flavonoids and other compounds are released naturally by roots to stimulate the activity and growth of the degrading bacteria of PAHs (i.e., rhizoremediation occurs naturally in the environment) (Leigh et al. 2006). However, there are several other factors that can improve the efficiency of the rhizosphere process. As mentioned previously, depending on plant species, the composition of root exudates changes as the plant stage developed. These variations, thus, exert obvious effects on the community of the rhizospheric (Garbeva et al. 2004). In many phytorhizoremediation investigation, Salix supplants are used as they produce salicylic acid and other related compounds. According to De Cárcer et al. (2007), these produced compounds assist in the degradation of both PCBs and PAHs.
5.11 Phytoremediation of Explosives, Pesticide, and Herbicide Contamination
5.11.1 Phytoremediation of Explosives (RDX, TNT, HMX)
There is a large-scale production of munitions worldwide. This has led to severe environmental contamination in different parts of the world. These explosive compounds include RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine), TNT (2,4,6-trinitrotoluene), and HMX (octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine). Explosives consist of these mutagenic and toxic xenobiotics. They are recalcitrant to remediation and stable in the environment. Thus, special technologies can be used including adsorption, incineration, chemical reduction, advanced oxidations processes, etc.). These technologies are very expensive to implement. Besides, they cause additional environmental problems. The most popular technologies, recently, are the biotechnological methods, including the phytoremediation. The latter is relatively low cost, eco- friendly, low energy, and a highly accepted solution by administration and society. The usage of genetically modified plants, in combination with the ability of bacterial genes, is a very promising technology. This combination is able to detoxify compounds via the phytoremediation-modified plants (Panz and Miksch 2012). Effective phytoremediation could also be achieved through transgenic plants with the expression and introduction of bacterial cytochrome p450 genes and nitro-reductases. Meanwhile, effective phytoremediation could be enhanced by introducing pnrA gene from Pseudomonas putida “superbug”, encoding nitro-reductase into rapidly growing aspen tree (Aken 2009).
According to Clark and Boopathy (2007), the concentrations of RDX and HMX in soil samples are generally lower than that of TNT. Concentration of RDX and HMX in soil ranges from 800 to 1900 mg.kg−1 and 600–900 mg.kg−1, respectively. The maximum detected concentrations were 74,000 mg.kg−1 and 5700 mg.kg−1, successively (Clark and Boopathy 2007). It is worth mentioning that these compounds are, generally, less toxic than trinitrotoluene. The earthworm studies by Robidoux et al. (2002) at concentrations lower than 711 mg.kg−1 for HMX and 167.3 mg.kg−1 for RDX in the forest soil showed that oligochaete survived and no change in biomass was observed. Nevertheless, low concentrations of HMX (15.6 mg.kg−1) and RDX (46.7 mg.kg−1) exhibited a significant decline in earthworm reproduction, particularly cocoon and juvenile production (Robidoux et al. 2002).
5.11.2 Phytoremediation for the Removal of TNT
The typical concentration of trinitrotoluene (TNT) in soil is 4000 and 10,000 mg kg−1, and the highest recorded concentration was 87,000 mg kg−1 (Vila et al. 2008). The TNT compound and its transformation products are known for being toxic. Acute toxicity test was carried out by an earthworm using forest soil that was spiked with TNT. The studied assay by Lachance et al. (2004) proved that the TNT at concentration of 143 mg kg−1 is the lethal (L50) for half of the oligochaete population. The compound 2, 4, 6-trinitrotolune is known by the United States Environmental Protection Agency (US EPA 2014) as a potential human carcinogen (class C). It was confirmed by Vila et al. (2007a) that a direct contact with this chemical compound can cause skin irritation, liver disorder, immune system damage, or anemia.
The aquatic and terrestrial plants proved to be able to uptake the trinitrotoluene in hydroponic culture (Wang et al. 2003). Short-term experimental studies were conducted with TNT-contaminated soil. The results showed that the aquatic plants can absorb great percentage of the TNT from the contaminated water. This study showed that the aquatic plant species could remove the concentration of 681 mg L−1 (0.003 mM) trinitrotoluene in groundwater as initial concentration, to 94–100% removal during a period of 10 days. The plant used in most of the previous research is “Parrot feather water milfoil” (Myriophyllum aquaticum) (Wang et al. 2003). This plant was able to perform efficiently for decreasing the concentration of TNT even when the initial concentration was 0.11 mM (Wang et al. 2003). Meanwhile, vetiver grass was efficient in removing the TNT from contaminated soil. At TNT initial concentration of 40 mg kg−1, it was reported that vetiver grass was able to uptake 97% of trinitrotoluene from the contaminated soil after only 3 days of incubation (Das et al. 2010).
5.11.3 Phytoremediation of RDX
RDX is most widely used as military explosive. Using the transgenic plants, the RDX could be phytoremediated. Arabidopsis thaliana plants were reported of being genetically manipulated to express bacterial gene, XplA, that encodes a RDX degrading fused flavodoxin-cytochromeP450-like enzyme. In a laboratory bench-scale study, pure cultures of Rhodococcus rhodochrous strain 11Y demonstrated around 30% mineralization of radiolabelled RDX. These pure cultures of Rhodococcus rhodochrous strain 11Y are the donors for the abovementioned gene and are isolated from RDX-polluted areas. By denitrification, the bacterium could degrade RDX followed by ring cleavage and the release of small aliphatic metabolites. The liquid cultures of A. thaliana expressing XplA were able to remove between 32% and 100% of RDX. By non-transgenic plants, however, only less than 10% was removed. It was suggested by Aken (2009) that transgenic plants were competent of efficient phytoremediation of RDX pollutant. Previous study was conducted by Vila et al. (2007a) to determine the ability of soybean (Glycine max), corn (Zea mays), rice (Oryza sativa), and wheat (Triticum aestivum) for the uptake RDX from contaminated soil (138 mg.kg−1). Further investigation was carried out by Vila et al. (2007b), on the efficiency of rice for the uptake of RDX from the soil at high concentrations (2000 mg.kg−1). They found that the uptake level of RDX in the studied rice rose from 0 to 31.5 mg.g−1 to 0–1000 mg.kg−1 as dry weight, respectively. Later study by Chen et al. (2011) confirmed the founding obtained earlier by Vila et al. (2007a). Both investigations studied the abilities of sorghum, wheat, corn, and soybean on the accumulation and uptake of RDX. The most effective plant for the uptake of RDX was wheat seedling. The soybean, on the other hand, could not survive in the contaminated soil.
5.11.4 Phytoremediation of HMX
The efficiency of ryegrass (L. perenne) for the uptake of heterocyclic nitramines from contaminated soil was studied by Rocheleau et al. (2007). The decrease in the concentration of HMX concentration in the contaminated soil was less than 10%. The study showed that the HMX compound was translocated in the plant from the root to the shoots and concentrated in the aerial parts in an unchanged form. A 3-m-tall tropical plant known as kenaf plant (Hibiscus cannabinus) assimilated HMX from the soil to the plant. According to study on the plant mass balance, for the uptake of octogen from soil, about 9% of the initial concentration was taken by the plants and was accumulated mainly in the aerial parts. The highest efficiency for the uptake of HMX was recorded when dendroremediation (phytoremediation by trees) was employed. In a study on the uptake of octogen from solution, 44.58% was removed by poplar seedlings (Populus deltoides x nigra, DN-34) according to Yoon et al. (2002). After a 65-day period of incubation, the studied plants were examined by radiochromatographic methods. This examination revealed that 70% of the assimilated HMX were translocated to the leaves, without any HMX transformation products.
5.12 Phytoremediation of Pesticide Residues
Environmental threat and serious environmental risk are arising due to the presence of pesticide residues in water and soil. Thus, pollution with pesticides becomes one of the most serious concerns of scientists and environmentalists. Pesticides could be accumulated by plants, soil, and organisms. Such pollutants may be distributed into the water, food chain, and soil. In addition, pesticide could induce reduction in the productivity of the polluted soil. The point and nonpoint pollution are the immediate sources of contamination to soil and the environment. The point source pollution of pesticides is originated from certain agriculture activities, spills, formulation facilities, manufacturer, and agrochemical dealerships. On the other hand, the nonpoint source of pollution is considered, mostly, as the essential source of pesticide contamination. Several studies demonstrated that several plants can increase the dissipation rate of the investigated pesticides from contaminated soil through accumulation into the plant tissue. Additionally, plant vegetation in the contaminated soil may reduce leaching by decreasing the rate of distribution and movement of the organic pollutants through the column of such soil (Belden et al. 2004).
During the last few years, significant investigations were carried out on the interaction between plants in the rhizosphere and microorganisms in terms of the potential to implement this for the remediation of contaminated media with pesticide (Tripathy et al. 2014). Additional studies were conducted using Kochia sp. concentrated on the phytoremediation of pesticide from contaminated groundwater and soils (Erdei et al. 2005). Results indicated that plants show strong evidence of an intrinsic ability to detoxify certain xenobiotic compounds. However, these plants require catabolic pathways, generally, to execute mineralization/degradation. The transfer of genes, therefore, involves in catabolism of xenobiotics from eukaryotes or microbes to plants. This will, further, augment their potential for remediation of the contaminants (Eapen et al. 2007). Tobacco, the transgenic plant, is capable to remove pentachlorophenol through bringing into being laccase and secreting into the rhizosphere. For the purpose of decontamination and removal of organ phosphorus pesticide, a bacterial organophosphorus hydrolase (OPH) gene in the plants of tobacco has been executed. The study showed that transgenic plants after 14 days of growth were able to degrade more than 99% of methyl parathion. A multiplicity of plant enzymes, including peroxidase, peroxygenases, glutathione-S-transferases, cytochrome P450, carboxylesterases, O- and N- malonyltransferases, and O- and N- glucosyltransferases, are involved in the phytotransformation of xenobiotics in the cells of plant (Karavangeli et al. 2005). It was further reported that P450-dependent monooxygenase activity of the transgenic potato plants was found to range from 3.5 to 4.2 times higher than those of the control plants (Tripathy et al. 2014).
5.13 Phytoremediation of Herbicide
Herbicide is classified as one of the serious pollutants in the environment. However, herbicide plays vital and important roles in agriculture. For enhanced phytoremediation, development of herbicide-tolerant transgenic plants is under investigation recently. For removal of atrazine, a herbicide, cytochrome P450 (CYP) was introduced into rice plant. Similarly, for efficient removal of alachlor, g-glutathione synthetase gene was introduced into poplar. Developing transgenic plants, for remediation, can be achieved by increasing root mass bacterial 1- aminocyclopropane-1-carboxylate deaminase, and this can be implemented via introducing bacterial gene atrazine chlorohydrolase.
Cytochromes P450 (CYP) enzymes, from heme proteins superfamily, are involved in the metabolism and the removal of xenobiotics, including substances of abuse and therapeutic drugs, herbicides, and industrial contaminants, as illustrated in Fig. 5.12. They are responsible for safe clearance of around 80% of marketed drugs that is achieved in the liver by conversion into relatively hydrophilic compounds. Due to their broad substrate specificity, CYP enzymes are being used as biocatalysts for remediation. Herbicides atzZ (Paz Alberto and Sigua 2013).
Genetic manipulation of CYP enzymes for overexpression and better activity and stability and cloning of engineered CYP enzymes (e.g., CYP2B6) in plants. This is followed by growth of transgenic plants in contaminated areas which has the potential to detoxify the lands. (Paz Alberto and Sigua 2013)
5.14 Applications of Phytoremediation for Wastewater and Improving Quality of Water
Increasing urbanization, industrialization, and population in Benin City, Nigeria, are responsible for the huge and various discharges of enormous forms of industrial, human, and animal wastewaters into the local nearby surface waters including lakes, rivers, streams, and ponds (Aisien et al. 2010). Such discharged wastewaters contain enormous and various amount of different pollutants that are threatening the environment. Besides, these wastewaters have serious health hazard impact on man, animals, plants, and microorganisms. Thus, it leads to severe environmental challenges (Aisien et al. 2009). One of these wastewater is that produced from abattoir processes including both white and red meat. The slaughter animal’s blood is the main organic pollutants from abattoir wastewater (Osibajo and Adie 2007). The white meat is poultry, and the red meat is beef, mutton, and pork. The abattoir wastewater consists of dissolved pollutants, such as blood and urine, as well as high concentration of total suspended solids, including pieces of flesh tissue, grease, fat, feathers, hair, grit, manure, and undigested feeds. Such insoluble and slowly biodegradable pollutants represented 50% of the contamination load.
Abattoir wastewater contains millions bacterial colonies of total coliform, fecal coliform, and Streptococcus groups. Most of the time, wastewater contains pathogens, such as Salmonella and Shigella bacteria, amoebic cysts, and parasite eggs. The continuous discharge of untreated abattoir wastewater into the nearby surface waters induces increase in algal growth, leads to eutrophication, and reduces aquatic plant and animal growth. It also increases odor of water, color, foaming, temperature, and electric conductivity as well as increases level of heavy metal. Thus, the impact of wastewater discharge to water bodies will contribute to deteriorate the quality of the surface water (Osibajo and Adie 2007). Many physical, chemical, and biological techniques have been studied concerning the degradation, transformation, and consumption of several organic matter and nutrient elements of plants within the constructed wetland treatment system (Aisien et al. 2010). The reuse of treated effluent in non-potable purposes including aquaculture/agriculture is highly encouraged to minimize the demands of freshwater.
Erdei et al. (2005) conducted a study to the potential of plants that can be employed in the phytoremediation of trace metals in Nueva Ecija, Philippines. Samples of water and plant were taken near the discharge sites, about 500 m distance away from the creek. Analysis of water samples indicated that the dumpsite and Panlasian Creek were slightly contaminated with phosphate at considerable amount. Analysis of plant samples showed that Hydrocharitaceae (Ottelia alismoides L.) and kangkong (Ipomea aquatica) were both able to uptake Pb in the phytoremediation process. The study showed also that the concentrations of Pb in Hydrocharitaceae (Ottelia alismoides L.) and morning glory (Ipomea violacea L.) were about 210% more than the concentration of Pb in the water (Erdei et al. 2005).
The potential of water hyacinth plant (Eichhornia crassipes) for the removal of phosphorus pesticide ethion was conducted by Xia and Ma (2006). The results showed that disappearance rate constants of ethion in culture solutions were as follows: −0.01059 for the non-sterile planted, −0.00930 for sterile planted, −0.00294 for non-sterile unplanted, and −0.00201 for sterile unplanted treatment. Furthermore, the uptake of ethion in live water hyacinth plant decreased by 74–81% in roots and 55–91% in shoots after the plant grows for 1 week in ethion-free culture solutions. The results suggested that the plant accumulation and phytodegradation are the most dominant processes for ethion uptake by the water hyacinth. According to this promising finding of this investigation, water hyacinth plant could be employed as an economical, efficient, and eco-friendly alternative to accelerate the degradation and treatment of agro-industrial wastewater contaminated with ethion.
In addition, earlier investigators (Fayed and Abdel-Shafy 1985) confirmed that water hyacinth has the potential to accumulate toxic trace metals including Cd, Zn, Co, and Pb within the plant tissues. The investigators concluded that the water hyacinth plant can be a biological indicator for heavy metal contamination in the environment. This conclusion was also confirmed by other scientists (Abdel-Haleem et al. 1992). According to other investigators, the reported accumulation of metals by water hyacinth was in the following ranges: 63–277 for Fe, 220–280 for Mn, 55–60 for Zn, 5–10 for Cu, and 0–<5 mg/l for Pb (Abdel-Shafy et al. 1994a, b).
Recently, water hyacinth samples were collected from the Nile River, Egypt. The plants were portioned into leaves, stems, and roots and dried. General analyses including crude protein, ash, crude fat, fibers, nitrogen, and free extract of sugars and carbohydrates were determined in the dried plant samples. In addition, level of heavy metals in these samples was also investigated (Abdel-Shafy et al. 2016). Stem of the plants showed maximum reducing sugar content. Leaves showed the highest level of both nonreducing and total sugars. The analysis revealed that metals such as Na, K, Mg, and Ca were found in relatively higher concentration. Trace metals, namely, Fe, Zn, Mn, Cu, Pb, and Cd, were mostly accumulated in the plant roots. The overall results indicated that water hyacinth plant contains considerable amount of nutrient elements. It was then confirmed that water hyacinth has the potential of accumulating metals from the surrounding environment. The level of trace metals in the plant depends on their concentration in the surrounding aquatic media (Abdel-Shafy et al. 2016).
Letachowicz et al. (2006) investigated the phytoremediation capacity in terms of trace metal accumulation by different parts of Typha latifolia L. plant. The plant samples were collected from different seven water bodies in the Nysa region in Poland. Trace metals including Fe, Mn, Zn, Ni, Cu, Pb, and Cd were detected in the Typha latifolia. The results concluded that Typha latifolia species have the potential of absorbing trace metals. Thus, this plant can be utilized as bioindicator of pollution. This plant is linked with nutritious water as well as organic or inorganic contents in the bottom sediments. In addition, Typha latifolia plant is a strongly expansive species. It can control water space because of their intensive growth of rhizomes; besides they often create mono-species group. Meanwhile, this plant can also be found in numerous groups of rushes.
5.15 Phytoremediation for Tertiary Treatment of Sewage
5.15.1 Treatment of Wastewater via Constructed Wetland
Constructed wetlands (CW) offer a low-cost system for wastewater treatment (Masi et al. 2010). Besides, the CWs have low consumption in energy, low-cost operation and maintenance, and low investment and are eco-friendly and simple in construction using phytoremediation (Abdel-Shafy et al. 2017). The operation and maintenance of constructed wetlands do not need high-skilled personnel. A study of CWs showed that the pollution parameters can be removed efficiently including pathogens. Additional advantage of CWs over the traditional treatment systems is that they can be implemented in the same place where the wastewater is produced, maintained by relatively untrained personnel (EPA 2000; Abdel-Shafy et al. 2009). Thus, CWs are perfect for the decentralized treatment of wastewater (Abdel-Shafy and El-Khateeb 2013).
Wetlands offer great potential for the phytoremediation of contaminants and toxic substances. Constructed wetlands are shallow manmade systems (typically between 0.6 and 1.0 m) or bodies of slow-moving wastewater (Fig. 5.13). The CW is planted with dense and tolerant vascular plants including Phragmites australis, cattails, elephant grass, bulrushes, or reed plants. These bodies are artificially created, and they are usually long, narrow channels or trenches (Bennett et al. 2003).
Wastewater treatment via constructed wetlands. (ITRC 2009)
There are several designs for constructing wetlands. These designs can be categorized according to their topology as free water surface (FWS), horizontal subsurface flow (HF or SSF), vertical flow (VF), and the hybrid wetland system (Abdel-Shafy et al. 2017). The main factors of designing are type of wastewater, the required quality of the treated effluent, and the available land area for construction (Masi et al. 2010). The wetland hybrid system is mainly combinations of two or three designs: HF, VF, and FWS (Masi and Martinuzz 2007). Each design of construction (HF, VF, or FWS systems) specifies certain function treatment efficiencies (i.e., the selected design is an important controlling factor). In fact, the hybrid wetland design is able to combine the advantages of each constructed topology. Thus, it minimizes the drawback of single design system. The hybrid wetland system could be one of the following combinations (EPA 2000; Abdel-Shafy et al. 2017):
-
HF + VF: Horizontal subsurface flow has the efficiency of eliminating most of organic load as well as the suspended solids. The subsurface vertical flow ensures the oxidation process and acts for an efficient nitrification of the wastewater without any clogging problems. Through this design, recycling the effluent was carried out to flow again at the beginning of the system for the purpose of more efficient denitrification.
-
VF + HF: Vertical subsurface flow followed by horizontal subsurface flow system. This combined design is aiming to obtain effluent with more efficient denitrification through the vertical treatment system.
-
HF + VF + HF + FWS: The horizontal subsurface systems followed by vertical, horizontal, and finally free water flow system, in which removal of suspended solids and denitrification are efficiently enhanced in the wastewater. The free water surface is able to remove almost all the nitrogen compounds and the entire microbial load.
The aquatic plants present main catalytic role in the purification process of the wetlands. This role is a combination of microbial, biological, and physical activities. The aquatic plants (Fig. 5.14) contribute in the removal of N, P, nutrient elements, and organic matters as well as accumulation of heavy metals (Abdel-Shafy et al. 1994a, b). Furthermore, the attached aerobic bacterial colonies and their continuous building up on the plant rhizomes offer an efficient degradation of the organic pollutants in the wastewater. Meanwhile, there is the convection mechanism that is responsible to pump air from the leaves to the root zone (Masi and Martinuzz 2007; Abdel-Shafy et al. 2017) (Fig. 5.15).
Wastewater treatment via constructed wetland depicting the various methods of phytoremediation. (ITRC 2009)
5.16 Conclusions
Phytoremediation is an important “green bioengineering technology.” It uses green plants for remediating environmental contaminants.
Both aquatic and terrestrial green plants have wonderful characteristics for environmental restoration, including eliminating contaminants from polluted water and soil.
The use of higher plants, bacteria, microalgae, and fungi is feasible for degrading persistent contaminants.
Phytoremediation technology is eco-friendly, solar energy driven, green in nature, cost-effective, nonintrusive, and a safe alternative for removal of environmental contaminants.
This green technology is capable to accumulate, absorb, tolerate, transfer, assimilate, degrade, and stabilize highly toxic materials from the polluted soil and water. These toxic materials are heavy metals, polycyclic aromatic hydrocarbons, pesticides, explosives, crude oil, and organics such as solvents.
The aquatic plants, microorganisms, and algae have the ability to remove organic and inorganic matter, nutrients, pathogens, heavy metals, and other pollutants from wastewater in an eco-friendly natural way. These plant species include reeds, bulrushes, cattails, Phragmites australis, and aquatic plants such as water hyacinths, duckweed, and pennywort.
The importance of the phytoremediation process is that it is efficient for the removal of toxic organic aromatic pollutants, polycyclic aromatic hydrocarbons (PAHs), explosives (RDX, TNT, HMX), pesticide, landfill leachates, as well as herbicide contamination.
Thus, employing phytoremediation in constructed wetland (CW) technology offers a low-cost, eco-friendly treatment system for wastewater.
The phytoremediation technology has several advantages and disadvantages. The low cost is one of the most important advantages. However, the time needed to observe the necessary achievement can be long.
References
Abdel-Haleem AS, Abdel-Sabour MF, Zaghloul RA (1992) The use of water hyacinth as biological indicator of environmental contamination by heavy metals. In: Proceedings conference in environmental contamination. CEP, Consultant Ltd, UK, pp 263–265
Abdel-Sabour MF, Abdel-Shafy HI, Mosalem TM (2001) Heavy metals and plant growth yield as effected by sewage sludge and water hyacinth compost applied to sandy soil. J Environ Prot Eng 27(2):43–53
Abdel-Shafy HI (2015) Chemical treatment for removal of heavy metals from industrial wastewater. Egypt J Chem 58(1):1–12
Abdel-Shafy HI, Dewedar A (2012) Constructed wetlands for urban wastewater treatment in Egypt. J Sustain Sanitation Pract 12:27–32
Abdel-Shafy HI, El-Khateeb MA (2013) Integration of septic tank and constructed wetland for the treatment of wastewater in Egypt. J Desalination Water Treat 51(16–18):3539–3546
Abdel-Shafy HI, El-Saharty AA (2015) Short-term toxicity of mercury and cadmium to Sparus Aurata fry, South Mediterranean Sea, Egypt. Egypt J Chem 58(5):537–553
Abdel-Shafy HI, Farghaly MS (1995) Level of metals in the benthic algae of the Suez Canal. J Environ Prot Eng 21(1–4):5–14
Abdel-Shafy HI, Mansour MSM (2016) A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Pet 25:107–123
Abdel-Shafy HI, Cooper WJ, Handley-Raven LL, Casey LS (1986) Short-term fate of heavy metals in the gravel bed hydroponics wastewater treatment system. J Environ Prot Eng 12(1):61–80
Abdel-Shafy HI, Hegemann W, Teiner A (1994a) Accumulation of metals by vascular plants. J Environ Manag Health 5(2):21–24
Abdel-Shafy HI, Abdel-Sabour MF, Farid MR (1994b) Distribution pattern of metals in the environment of the Little Lake. J Environ Prot Eng 20(1–4):5–16
Abdel-Shafy HI, Anwer S, Hassan SSA, Yahia OTM (2003) Risk assessment of sewage reuse on the sandy soil of Abu-Rawash Desert, Egypt. J Environ Prot Eng 29(1):5–19
Abdel-Shafy HI, Aly RO, Sahab AF, El-Wattar NM (2005) Fate of metalloid fungicide in subjected water, soil and plant in Egypt. J Environ Prot Eng 31(1):69–80
Abdel-Shafy HI, El-Khateeb MA, Regelsberger M, El-Sheikh R, Shehata M (2009) Integrated system for the treatment of black-water and greywater via UASB and constructed wetland in Egypt. Desalin Water Treat 8:1–7
Abdel-Shafy HI, Farid MR, Shams El-Din AM (2016) Water-hyacinth from Nile River: chemical contents, nutrient elements and heavy metals. Egypt J Chem 59(2):131–143
Abdel-Shafy HI, El-Khateeb MA, Shehata M (2017) Blackwater treatment via combination of sedimentation tank and hybrid wetlands for unrestricted reuse in Egypt. J Desalination Water Treat 71:145–151
Abe K, Takizawa H, Kimura S, Hirano M (2004) Characteristics of chlorophyll formation of the aerial microalga Coelastrella striolata var. multistriata and its application for environmental biomonitoring. J Biosci Bioeng 98(1):34–39
Abo-El-Souad MA, Abdel-Sabour MF, Abdel-Shafy HI (1994) Studies on plants grown in soil polluted with cobalt and nickel-56. J Environ Manag Health 5(4):16–21
Aisien SO, Ogoannah SO, Imasuen AA (2009) Helminth parasites of amphibians from the rainforest reserve in south-western Nigeria. Afr Zool 44(1):1–7
Aisien FA, Faleye O, Aisien ET (2010) Phytoremediation of heavy metals in aqueous solutions. Leonardo J Sci 17:37–46
Aken BV (2009) Transgenic plants for enhanced bioremediation of toxic explosives. Curr Opin Biotechnol 20:231–236
Al-Homaidan AA, Al-Ghanayem AA, Areej AH (2011) Green algae as bioindicators of heavy metal pollution in Wadi Hanifah Stream, Riyadh, Saudi Arabia. Int J Water Resour Arid Environ 1(1):10–15
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals–concepts and applications. Chemosphere 91:869–881
Baghour M, Moreno DA, Hernandez J, Castilla N, Romero L (2002) Influence of thermal regime of soil on the sulfur (S) and selenium (Se) concentration in potato plants. J Environ Sci Health. Part A. Toxic Hazard Subst Environ Eng 37:1075–1085
Belden JB, Phillips TA, Coats JR (2004) Effect of prairie grass on the dissipation, movement, and bioavailability of selected herbicides in prepared soil columns. Environ Toxicol Chem 23(1):125–132
Ben Chekroun K, Baghour M (2013) The role of algae in phytoremediation of heavy metals: a review. J Mater Environ Sci 4(6):873–880
Bennett LE, Burkhead JL, Hale KL, Terry N, Pilon M, Pilon-Smits EAH (2003) Analysis of transgenic Indian mustard plants for phytoremediation of metal-contaminated mine tailings. J Environ Qual 32:432–440
Berti WR, Cunningham SD (2000) Phytostabilization of metals. In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals using plants to clean up the environment. Wiley, New York, p 7188
Bisht S, Pandey P, Sood A et al (2010) Biodegradation of anthracene and napthlene by chemotactically active rhizobacteria of Populus deltoids. Braz J Microbiol 41:922–930
Bisht S, Pandey P, Aggarwal H et al (2014) Utilization of endophytic strain Bacillus sp. SBER3 for biodegradation of polycyclic aromatic hydrocarbons (PAH) in soil model system. Eur J Soil Biol 60:67–76
Bisht S, Pandey P, Bhargava B, Sharma S, Kumar V, Sharma KD (2015) Bioremediation of polycyclic aromatic hydrocarbons (PAHs) using rhizosphere technology. Braz J Microbiol 46(1):7–21
Bundschuh J, Litter MI, Parvez F, Román-Ross G, Nicolli HB, Jean J, Liu C, López D, Armienta MA, Guilherme LRG, Cuevas AG, Cornejo L, Cumbal L, Toujaguez R (2012) One century of arsenic exposure in Latin America: a review of history and occurrence from 14 countries. Sci Total Environ 429:2–35
Burken J, Vroblesky D, Balouet JC (2011) Phytoforensics, dendrochemistry, and phytoscreening: new green tools for delineating contaminants from past and present. Environ Sci Technol 45 (15): 6218–6226
Chaudhry Q, Blom-Zandstra M, Gupta S et al (2005) Utilizing the synergy between plants and rhizosphere microorganisms to enhance breakdown of organic pollutants in the environment. Environ Sci Pollut Res 12:34–48
Chen D, Liu ZL, Banwart W (2011) Concentration-dependent RDX uptake and remediation by crop plants. Environ Sci Pollut Res 18:908–917
Clark B, Boopathy R (2007) Evaluation of bioremediation methods for the treatment of soil contaminated with explosives in Louisiana Army Ammunition plant, Minden, Louisiana. J Hazard Mater 143:643–648
Danik JS, Rifai N, Buring JE et al (2006) Lipoprotein(a), measured with an assay independent of Apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially healthy women. J Am Med Assoc 296(11):1363–1370
Das P, Datta R, Makris KC, Sarkar D (2010) Vetiver grass is capable of removing TNT from soil in the presence of Urea. Environ Pollut 158(5):1980–1983
Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37:4311–4330
De Cárcer D, Martín M, Karlson U et al (2007) Changes in bacterial populations and in biphenyl dioxygenase gene diversity in a polychlorinated biphenyl-polluted soil after introduction of willow trees for rhizoremediation. Appl Environ Microbiol 73:6224–6232
DECA (Department of Environment and Conservation, Australia) (2010) Assessment levels for soil, sediment and water. https://www.der.wa.gov.au/images/documents/your-environment/contaminated-sites/guidelines/2009641
Dhote S, Dixit S (2009) Water quality improvement through macrophytes – a review. Environ Monit Assess 152:149–153
Dietz R, Outridge PM, Hobson KA (2009) Anthropogenic contributions to mercury levels in present-day Arctic animals – a review. Sci Total Environ 407(24):6120–6131
Dowling DK, Maklakov AA, Friberg U, Hailer F (2009) Applying the genetic theories of ageing to the cytoplasm: cytoplasmic genetic covariation for fitness and lifespan. J Evol Biol 2(2):818–827
Eapen S, Singh S, D’Souza SF (2007) Advances in development of transgenic plants for remediation of xenobiotic pollutants. Biotechnol Adv 25:442–451
EEA (European Environment Agency) (2014) Progress in management of contaminated sites. http://www.eea.europa.eu/data-and-maps/indicators/progress-in-management-of-contaminated-sites-3/assessment
El-Bahy GS, Ahmed MA, Abdel-Shafy HI, Ibrahim MA (2005) Role of industrial wastewater on the contamination of River Nile in Greater Cairo. Egypt J Chem 48(3):355–363
Erdei LG, Osi M, Mécs I, Vass I, Fôglein F, Bulik L (2005) Phytoremediation as a program for decontamination of heavy-metal polluted environment. Acta Biol Szeged 49:75–76
Favas PJC, Pratas J, Prasad MNV (2012) Accumulation of arsenic by aquatic plants in large scale field conditions: opportunities for phytoremediation and bio indication. Sci Total Environ 433:390–397
Fayed SE, Abdel-Shafy HI (1985) Accumulation of Cu, Zn Cd and Pb by aquatic macrophytes. J Environ Int 11:77–87
Fayed SE, Abdel-Shafy HI (1986) Accumulation of Cd, Cu, Pb and Zn by algae. Egypt J Microbiol 21(2):263–274
Fayed SE, Abdel-Shafy HI, Khalifa NM (1983) Accumulation of Cu, Zn, Cd, and Pb by Scenedesmus obliquus under non-growth conditions. J Environ Int 9(5):409–414
Fu P, Secundo F (2016) Algae and their bacterial consortia for soil bioremediation. Chem Eng Trans 49:427–432
Garbeva P, Van Veen JA, Van Elsas JD (2004) Microbial diversity in soil: selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annu Rev Phytopathol 42:243–270
González I, Águila E, Galán E (2007) Partitioning, bioavailability and origin of heavy metals from the Nador lagoon sediments (Morocco) as a basis for their management. Environ Geol 52:1581–1593
Gosavi K, Sammut J, Gifford S, Jankowski J (2004) Macro-algal-bio-monitors of trace metal contamination in acid sulfate soil aquaculture ponds. Sci Total Environ 324:25–39
Harvey PJ, Campanella BF, Castro PM et al (2002) Phytoremediation of polycyclic aromatic hydrocarbons, anilines and phenols. Environ Sci Pollut Res Int 9:29–47
Huang XD, El-Alawi YS, Penrose D et al (2004) A multi-process phytoremediation system for removal of polycyclic aromatic hydrocarbons from contaminated soils. Environ Pollut 130:465–476
Huang XD, El-Alaw YS, Gurska IJ et al (2005) A multi-process phytoremediation system for decontamination of persistent total petroleum hydrocarbons (TPHs) from soils. Microchem J 8:139–147
ITRC (The Interstate Technology and Regulatory Council) (2009) Phytotechnology technical and regulatory guidance and decision trees. Revised. Phytotechnologies Team, Washington, DC 20001, U.S.
Jain CK (2004) Metal fractionation study on bed sediments of river Yamuna. India Water Res 38:569–578
Kamal S, Prasad R, Varma A (2010) Soil microbial diversity in relation to heavy metals. In: Sherameti I, Varma A (eds) Soil heavy metals, vol 19. Springer-Verlag, Berlin Heidelberg, pp 31–64
Kanaly RA, Harayama S (2000) Biodegradation of high molecular weight polycyclic aromatic hydrocarbons by bacteria. J Bacteriol 182:2059–2067
Karavangeli M, Labrou NE, Clonis YD, Tsaftaris A (2005) Development of transgenic tobacco plants overexpressing maize glutathione S-transferase I for chloroacetanilide herbicides phytoremediation. Biomol Eng 22:121–128
Khan S, Hesham AEL, Qiao M, Rehman S, He JZ (2010) Effects of Cd and Pb on soil microbial community structure and activities. Environ Sci Pollut Res 17:288–296
Kord B, Mataji A, Babaie S (2010) Pine (Pinus eldarica Medw.) needles as indicator for heavy metals pollution. Int. J. Environ Sci Technol 7(1):79–84
Kramer U, Chardonnens AN (2001) The use of transgenic plants in the bioremediation of soils contaminated with trace elements. Appl Microbiol Biotechnol 55:661–672
Kvesitadze G et al (2006) Biochemical mechanisms of detoxification in higher plants. Springer, Berlin. isbn:9783540289968
Lachance B, Renoux AY, Sarrazin M, Hawari J, Sunahara GI (2004) Toxicity and bioaccumulation of reduced TNT metabolites in the earthworm Eisenia Andrei exposed to amended forest soil. Chemosphere 55:1339–1348
LeDuc DL, Terry N (2005) Phytoremediation of toxic trace elements in soil and water. J Ind Microbiol Biotechnol 32:514–520
Leigh MB, Prouzova P, Macek MT et al (2006) Polychlorinated biphenyl (PCB)-degrading bacteria associated with trees in a PCB contaminated site. Appl Environ Microbiol 72:2331–2342
Letachowicz B, Krawczyk J, Klink A (2006) Accumulation of heavy metals in organs of Typha latifolia. Pol J Environ Stud 15(2a):407–409
Leung CM, Jiao JJ (2006) Heavy metal and trace element distributions in groundwater in natural slopes and highly urbanized spaces in mid-levels area. Hong Kong Water Res 40:753–767
Marschner H (2001) Mineral nutrition of higher plants, vol 889. Academic, London
Masi F, Martinuzz N (2007) Constructed wetlands for the Mediterranean countries: hybrid systems for water reuse and sustainable sanitation. Desalination 215:44–55
Masi F, El-Hamouri B, Abdel-Shafy HI, Baban A, Ghrabi A, Regelsberger M (2010) Treatment of segregated black/grey domestic wastewater using constructed wetlands in the Mediterranean basin – the Zer0-m experience. Water Sci Technol 61(1):97–105
Meagher RB, Heaton ACP (2005) Strategies for the engineered phytoremediation of toxic element pollution: mercury and arsenic. J Ind Microbiol Biotechnol 32:502–513
Mejare M, Bulow L (2001) Metal-binding proteins and peptides in bioremediation and phytoremediation of heavy metals. Trends Biotechnol 19:67–73
Memon AR, Schr€oder P (2009) Implications of metal accumulation mechanisms to phytoremediation. Environ Sci Pollut Res 16:162–175
Mohiuddin KM, Zakir HM, Otomo K, Sharmin S, Shikazono N (2010) Geochemical distribution of trace metal pollutants in water and sediments of downstream of an urban river. Int J Environ Sci Technol 7:17–28
Mudgal V, Madaan N, Mudgal A (2010) Heavy metals in plants: phytoremediation: plants used to remediate heavy metal pollution. Agric Biol J N Am 1(1):40–46
Mwiganga M, Kansiime F (2005) The impact of Mpererwe landfill in Kampala–Uganda, on the surrounding environment. Phys Chem Earth 30(11):744–750
Nielsen HD, Burridge TR, Brownlee C, Brown MT (2005) Prior exposure to Cu contamination influences the outcome of toxicological testing of Fucus serratus embryos. Mar Pollut Bull 50(12):1675–1680
Okafor EC, Opuene K (2007) Preliminary assessment of trace metals and polycyclic aromatic hydrocarbons in the sediments. Int J of Environ Sci Technol 4(2):233–240
Osibanjo O, Adie GU (2007) Impact of effluent from Bodija abattoir on the physicochemical parameters of Oshunkaye stream in Ibadan City, Nigeria. Afr J Biotechnol 6(15):1806–1811
Panz K, Miksch K (2012) Phytoremediation of explosives (TNT, RDX, HMX) by wild-type and transgenic plants. J Environ Manag 113:85–92
Paz Alberto AM, Sigua GC (2013) Phytoremediation: a green technology to remove environmental pollutants. Am J Clim Chang 2:7186
Pilon-Smits E (2005) Phytoremediation. Ann Rev Plant Biol l56:15–39
Pilon-Smits EAH, Le Duc DL (2009) Phytoremediation of selenium using transgenic plants. Curr Opin Biotechnol 20:207–212
Prasad MNV (2004) Phytoremediation of metals and radionuclides in the environment: the case for natural hyper accumulators, metal transporters, soil amending chelators and transgenic plants. In: Prasad MNV (ed) Heavy metal stress in plants: from biomolecules to ecosystems. Springer, Berlin, pp 345–391
Reed MLE, Glick BR (2005) Growth of canola (Brassica napus) in the presence of plant growth-promoting bacteria and either copper or polycyclic aromatic hydrocarbons. Can J Microbiol 51:1061–1069
Robidoux PY, Hawari J, Bardai G, Paquet L, Ampleman G, Thiboutot S, Sunahara GI (2002) TNT, RDX, and HMX decrease earthworm (Eisenia andrei) life-cycle responses in a spiked natural forest soil. Arch Environ Contam Toxicol 43:379–388
Rocheleau S, Lachance B, Kuperman RG, Hawari J, Thiboutot S, Ampleman G, Sunahara GI (2007) Toxicity and uptake of cyclic nitramine explosives in ryegrass Lolium Perenne. Environ Pollut 156:199–206
Ruma R, Raja R, Ranjana C et al (2007) Degradation of polyaromatic hydrocarbons by mixed culture isolated from oil contaminated soil-A bioprocess engineering study. Indian J Biotechnol 6:107–113
Rupassara SI, Larson RA, Sims GK, Marley KA (2002) Degradation of atrazine by hornwort in aquatic systems. Biorem J 6(3):217–224
Rylott EL, Bruce NC (2008) Plants disarm soil: engineering plants for the phytoremediation of explosives. Trends Biotechnol 27(2):7381
Sarwar N, Saifullah, Malhi SS, Zia MH, Naeem A, Bibi S, Farid G (2010) Role of plant nutrients in minimizing cadmium accumulation by plant. J Sci Food Agric 90:925–937
Schnoor JL (1997) Phytoremediation. Technology overview report. Ground-Water Remediation Technologies Analysis Center, Series E, Vol. 1, October
Singh OV, Jain RK (2003) Phytoremediation of toxic aromatic pollutants from soil. Appl Microbiol Biotechnol 63:128–135
Taiz L, Zeiger E (2010) Plant physiology, 5th edn. Sinauer Associates, Sunderland
Treasury Board of Canada Secretariat (2014) Contaminants and media. http://www.tbs-sct.gc.ca/fcsi-rscf/cmeng.aspx?qid=1200518
Tripathy S, Paul B, Khalua RK (2014) Phytoremediation: proficient to prevent pesticide pollution. Int J Innov Sci Eng Technol 1(10):282–287
US EPA (US Environmental Protection Agency) (2000) Constructed wetlands treatment of municipal wastewaters. EPA 625/R99/010, Cincinnati, OH
US EPA (US Environmental Protection Agency) (2014) Technical Fact Sheet –2, 4, 6-Trinitrotoluene (TNT). EPA 505-F-14-009
Vander Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hyper-accumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362:319–334
Vatamaniuk O et al (2001) A new pathway for heavy metal detoxification in animals: phytochelatin synthase is required for cadmium tolerance in Caenorhabditis elegans. J Biol Chem 276(24):20817
Vila M, Lorber-Pascal S, Laurent F (2007a) Fate of RDX and TNT in agronomic plants. Environ Pollut 148:148–154
Vila M, Mehier S, Lorber-Pascal S, Laurent F (2007b) Phytotoxicity to and uptake of RDX by rice. Environ Pollut 145:813–817
Vila M, Lorber-Pascal S, Laurent F (2008) Phytotoxicity to and uptake of TNT by rice. Environ Geochem Health 30:199–203
Wang C, Lyon DY, Hughes JB, Bennett GN (2003) Role of hydroxylamine intermediates in the phytotransformation of 2,4,6-trinitrotoluene by Myriophyllum aquaticum. Environ Sci Technol 37:3595–3600
Wang Q, Kim D, Dionysiou DD, Soriala GA, Timberlake D (2004) Sources and remediation for mercury contamination in aquatic systems – a literature review. Environ Pollut 131:323–336
Wei CY, Chen TB (2001) Hyper accumulators and phytoremediation of heavy metal contaminated soil: a review of studies in China and abroad. Acta Ecol Sin 21:1196–1203
Xia H, Ma X (2006) Phytoremediation of ethion by water hyacinth (Eichhornia crassipes) from water. Bioresour Technol 97(8):1050–1054
Yoon JM, Oh BT, Just CL, Schnoor JL (2002) Uptake and leaching of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine by hybrid poplar trees. Environ Sci Technol 36:4649–4655
Zhuang X, Chen J, Shim H et al (2007) New advances in plant growth-promoting rhizobacteria for bioremediation. Environ Int 33:406–413
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Abdel-Shafy, H.I., Mansour, M.S.M. (2018). Phytoremediation for the Elimination of Metals, Pesticides, PAHs, and Other Pollutants from Wastewater and Soil. In: Kumar, V., Kumar, M., Prasad, R. (eds) Phytobiont and Ecosystem Restitution. Springer, Singapore. https://doi.org/10.1007/978-981-13-1187-1_5
Download citation
DOI: https://doi.org/10.1007/978-981-13-1187-1_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1186-4
Online ISBN: 978-981-13-1187-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)