Abstract
By 2019, there had been at least 145 studies published that examined some aspect of the association between physical activity and survival. There is now strong and consistent evidence that both pre- and post-diagnosis physical activity improve survival after breast, colorectal, and prostate cancer. There is also evidence that physical activity can improve outcomes for female reproductive cancers, glioma, hematologic cancers, and kidney, liver, lung, and stomach cancers. While both pre- and post-diagnosis activity are associated with improved survival after cancer, somewhat greater benefit was observed with post-diagnosis activity. Preliminary evidence suggests that all population subgroups, as defined by race/ethnicity, as well as different clinicopathologic characteristics, can benefit from physical activity after cancer diagnosis. There is also emerging evidence that both aerobic and resistance exercise can improve cancer outcomes from both observational studies and exercise trials conducted in cancer patients during and posttreatment. Cardiorespiratory fitness, as a separate indicator of physical activity, has been found in a limited number of studies to be positively associated with improved survival after cancer. With additional research, it will be possible for more evidence-based and precise recommendations for physical activity that specify the optimal type, dose, and timing of activity for each cancer site and for specific population subgroups.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Physical activity
- Exercise
- Survival
- Mortality
- Cancer
- Meta-analyses
- Review
- Resistance training
- Cardiorespiratory fitness
- Intervention
- Observational studies
- Randomized controlled trials
Introduction
Current physical activity guidelines generated by the World Health Organization (WHO) recommend that the general population participate in at least 150 minutes of moderate aerobic physical activity per week (equivalent to 75 minutes of vigorous aerobic physical activity) in bouts of 10 minutes or more [1]. Guidelines for cancer survivors produced by the World Cancer Research Fund/American Institute for Cancer Research recommend that cancer survivors engage in regular physical activity following guidelines for the general population and further recommend that survivors should return to normal daily activities as soon as possible following diagnosis [2,3,4]. In 2018, the Clinical Oncology Society of Australia delivered a position statement on exercise in cancer care in which they recommend that exercise should be “embedded as part of standard practice in cancer care and to be viewed as an adjunct therapy that helps counteract the adverse effects of cancer and its treatment” [5]. This position statement raised some concerns in the exercise oncology community since the state of evidence regarding the feasibility, suitability, type, and dose of activity that should be recommended for all cancer patients and survivors remains unclear. Recognition exists, nonetheless, regarding the vital role that physical activity has during treatment, rehabilitation, and survival for cancer despite a lack of evidence in some areas. Furthermore, the field of precision medicine has also been applied to physical activity and cancer in a seminal paper led by Jones (2016) in which a framework for precision exercise oncology was provided [6]. In this chapter, we review the evidence on physical activity and survival in cancer populations while also providing insight into some elements of precision exercise oncology in efforts to discern which cancer patient and survivor groups could experience particular survival benefits with regular physical activity. The primary reviews of the evidence will focus on physical activity as it was reported in the included studies.
Physical Activity and Cancer Survival: Epidemiologic Research
The first study investigating the relationship between physical activity and survival outcomes in cancer survivors was published in 1992 [7]. However, this area of research did not garner much traction until 2005. Since then, there has been an exponential increase in the number of studies published evaluating the association between physical activity and mortality outcomes among cancer survivors. By 2019, identified through searches in PubMed, EMBASE, and SportDiscus, over 145 studies had been published on this topic. These studies provide sufficient data to permit the completion of meta-analyses that seek to quantify the direction and magnitude of association between physical activity and cancer survival.
The relationship between physical activity and survival outcomes following cancer has been most commonly investigated in breast [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49], colorectal [8, 47, 48, 50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69], and prostate cancers [47, 48, 70,71,72,73,74,75,76,77,78,79] and all cancer sites combined [7, 8, 47, 48, 69, 79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117]. Nonetheless, in more recent years, findings from studies involving other single cancer sites, including bladder [79, 118], brain [79], childhood [119], esophagus [47, 79, 120, 121], female reproductive (endometrial, ovarian, and cervical) [47, 48, 79, 122,123,124,125,126,127,128,129], glioma [130], head and neck [47, 79], hematologic (leukemia, lymphoma, myeloma, and other hematopoietic cancers) [69, 79, 80, 131,132,133,134], kidney [79, 135], liver [8, 47], lung [8, 47, 69, 79, 80, 136, 137], melanoma [138], pancreatic [47, 69, 79, 80, 139,140,141,142], and stomach [47, 69, 79, 80, 120, 121] cancers, have also been published. Data collected for these analyses have most commonly been derived from prospective follow-up of cohorts of cancer cases identified either in case-control or cohort studies. There were also four randomized controlled exercise intervention trials that conducted long-term follow-up of trial participants for mortality outcomes [32, 33, 49, 132].
Summary of the Study Designs and Methods
Of the 145 studies published to date, cohorts from mostly developed countries have been investigated and include North America (Canada, Puerto Rico, and the USA), Europe (Denmark, Finland, Germany, Italy, the Netherlands, Norway, Scotland, Sweden, Switzerland, the United Kingdom,) Australia/New Zealand, and Asia (China, Japan, Korea, Taiwan, Thailand, and Singapore). Contributing sample sizes varied widely, ranging from 103 to 1,290,000, as did the timing and method by which physical activity was assessed and reported before or after a cancer diagnosis. Only a few studies included repeated assessments that covered both pre- and post-diagnosis periods. The methods for assessing physical activity included self-administered physical activity questionnaires, interview-administered questionnaires, or direct measures of activity through accelerometers or exercise logs/diaries (used in the randomized controlled intervention trials); the majority relied on data from one of several self-administered physical activity questionnaires. In addition, 21 studies examined cardiorespiratory fitness (a potential surrogate measure of physical activity) and its association with cancer survival [7, 110, 114, 143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160]. The type of physical activity measured was primarily recreational activity or total physical activity, with a minority of studies assessing occupational and household activities as separate domains in addition to recreational activity. For most studies, the frequency, intensity, and duration of activities were assessed which permitted an estimation of the total energy expenditure in MET-hours/week. Overall, there is clear heterogeneity in the methods used for physical activity assessment, which needs to be considered when assessing findings derived from these studies.
Evidence Synthesis Methods
We used random effects DerSimonian and Laird models to assess the strength of the associations between physical activity and cancer survival for these studies. Specifically, we estimated the associations by time period of physical activity assessment (pre- or post-diagnosis) and by type of outcome (cancer-specific survival or all-cause mortality). For simplicity of presentation, and given the level of evidence for some cancer sites for which five or fewer studies have been published, we created five categories for the associations ranging from >20% statistically significant decreased mortality risks to >10% non-statistically significant increased risks. In addition, we examined each study to assess if the dose-response relationship between physical activity and mortality outcomes was investigated and if there was evidence of a statistically significant dose-response effect. We further reviewed the degree of consistency in the evidence across studies and categorized it as follows: (1) yes (with at least 10 different contributing estimates for which at least 75% had similar findings); (2) moderate (at least five contributing estimates with at least 75% demonstrating similar results or ≥ 10 estimates with 50–75% demonstrating similar findings); (3) limited (two to five contributing estimates with 75% displaying similar findings or more than five estimates with 50–75% showcasing similar findings); (4) or no (there was a lack of consistency (≤50% with similar findings) and/or too few estimates (i.e., only one contributing estimate) to determine consistency). These results were summarized for 18 cancer sites for which at least one study had been published that examined either pre- or post-diagnosis in association with either cancer-specific or all-cause mortality in cancer survivors (Table 3.1).
Overall Results
Findings presented in Table 3.1 support that the highest versus lowest levels of physical activity were associated with statistically significant decreases of >20% in cancer-specific or all-cause mortality outcomes in studies that assessed all cancer sites combined and ten other specific tumor sites, including breast, colorectal, female reproductive, glioma, hematologic, kidney, liver, lung, prostate, and stomach cancers. The strongest and most consistent evidence was observed for all cancer sites combined and breast, colorectal, and prostate cancers with data supporting an effect for all associations examined (i.e., pre- and post-diagnosis physical activity and cancer-specific and all-cause mortality).
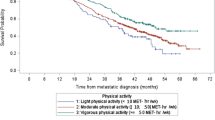
We also examined the question of timing of physical activity in relation to mortality outcomes (Figs. 3.1, 3.2, 3.3, 3.4, 3.5, and 3.6). When all cancer sites combined, breast and colorectal cancers were considered, reductions in mortality risks were observed for both pre- and post-diagnosis activity. However, post-diagnosis physical activity was generally more protective (HR ~0.60) compared with pre-diagnosis physical activity (HR ~0.80) for both cancer-specific and all-cause mortality estimates.
Population Subgroups: Effects by Race
The emerging area of precision oncology has raised interest in determining whether patient sociodemographic characteristics or clinical and pathologic characteristics might predict populations or subgroups within a specific cancer population that could particularly benefit from physical activity. Unfortunately, to date, there exist only a limited number of studies available to contribute to meta-analyses that seek to evaluate the relationships between subgroups within a study population and physical activity. While this lack of evidence adversely influences the strength of statements that can be drawn from our findings, several noteworthy findings are worth consideration. First, breast cancer is the only cancer site to have investigations completed on racial subgroups [17, 23, 38, 39]. Yet the established survival disparities by race for most cancers highlight the importance of determining whether or not race is a potential effect modifier of the association between physical activity and cancer survival. Furthermore, physical activity levels and types of physical activity undertaken have been shown to differ by race. For example, African-American women are less likely to meet physical activity guidelines compared with white women; Hispanic women most frequently report walking and household activities, while non-Hispanic white women are more likely to report participation in sport-based activities [161]. Preliminary findings from our meta-analyses, using data from the few breast cancer studies that provided race-specific estimates, suggest that physical activity (pre- and post-diagnosis) is at least as beneficial for breast cancer-specific and all-cause mortality for African-American, Hispanic, and Asian-American women, as it is for white women. More data within and beyond breast cancer cohorts are required to improve the consistency and strength of these findings.
Precision Oncology: Effects by Hormone Receptor Status
The differences in etiology, treatment, and prognosis for hormone receptor-positive and hormone receptor-negative cancers have also raised questions regarding the potential effect of physical activity in providing survival benefits among these subgroups [162]. Nine studies, involving women with breast cancer, presented stratified estimates for effect of physical activity by hormone receptor status [9, 13, 17, 20, 25, 29, 31, 38, 41], enabling us to explore these relationships in meta-analyses. We found statistically significant reductions of risk for both hormone receptor-positive and hormone receptor-negative breast cancers associated with post-diagnosis physical activity and both cancer-specific and all-cause mortality outcomes (cancer-specific HR+ 0.58 (0.45–0.75), HR− 0.59 (0.42–0.83); all-cause HR+ 0.66 (0.51–0.84), HR− 0.57 (0.42–0.78)). Hence, physical activity seems to confer survival benefits regardless of hormone receptor status in breast cancer survivors. To date, there are an insufficient number of studies examining triple-negative breast cancers in association with physical activity and survival to draw any conclusions for this patient population.
Precision Oncology: Effects by Cancer Stage
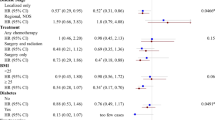
Another important predictor of survival after cancer is stage at diagnosis. As such, we examined the results from studies with data stratified by stage (Fig. 3.7), with studies involving colorectal and breast cancers providing sufficient data for this analysis. No clear patterns were identified for the association between physical activity and survival. Overall, there was evidence of a risk reduction of mortality outcomes for all cancer stages, with one notable exception. Specifically, findings from one colorectal cancer study with estimates for patients with stage IV disease suggested that post-diagnosis physical activity was associated with an increased risk of mortality, though this estimate should be interpreted with caution because of its small sample size and consequently wide confidence intervals.
Other important population subgroups warranting attention in future research include differences by sociodemographic characteristics and clinicopathologic characteristics, such as molecular tumor markers. To date, there have been an insufficient number of studies conducted on these population subgroups to provide any summaries.
Other Cancer Survival Outcomes and Physical Activity
While cancer-specific and all-cause mortality outcomes are the most commonly reported outcomes to consider, other survival outcomes have been investigated. For cancer recurrence or progressions, the following outcomes have been assessed: first recurrence or progression, late recurrence (>5 years), non-relapse mortality, progression-free survival, recurrence, recurrence-free interval, recurrence-free survival, progression or new primary cancer, recurrence-free period, recurrent/progressive primary cancer, relapse/disease-specific mortality, and time to recurrence. Unfortunately, the inconsistency in the definitions of these additional survival outcomes made it particularly challenging to compare findings within and between cancer sites. Acknowledging these limitations, by combining categories of first recurrence or progression, recurrence, progression or new primary cancer, and recurrent/progressive primary cancer, we found seven studies investigating the effects of physical activity (either pre- or post-diagnosis) on specifically first recurrence or progression in breast cancer [9, 13, 18, 19, 24, 28, 41], one in prostate cancer [76], and one in childhood cancers [119]. The pooled hazards ratios for each of these cancer sites are 0.90 (0.78–1.04), 1.05 (0.80–1.39), and 0.83 (0.59–1.17), respectively. While the lack of statistical significance in these findings, alongside potential heterogeneity in the outcome measures, highlights the need for caution in interpreting these findings, it seems plausible that a trend toward a potentially protective effect of physical activity is emerging, at least in relation to breast cancer recurrence/progression. The use of standardized endpoint definitions, such as those provided by the STEEP guidelines, in future observational epidemiologic studies would facilitate future and important summaries [163].
Change in Physical Activity and Cancer Outcomes
Following a diagnosis of cancer, patients are often motivated to seek out and implement positive changes to their behavior, for multiple reasons, which include to improve coping, rehabilitation, quality of life, and survival [164]. From a public health and patient perspective, it would be particularly useful to understand whether or not changes in physical activity from pre- to post-diagnosis also influence survival. We found nine studies that reported on the relationship between physical activity changes and cancer-specific or all-cause mortality in cancer survivors, with either “unchanged levels” or “inactive” defined as the reference category for comparisons across subgroups [17, 26, 50, 53, 74, 76, 126, 134, 135]. Overall, increasing physical activity from pre- to post-diagnosis levels was associated with decreased risk of mortality (HR, 0.79, 0.69–0.92). When stratified by type of survival outcome (all-cause and cancer-specific mortality), results remained relatively unchanged for all-cause mortality (HR, 0.76, 0.64–0.90), but the magnitude of effect was attenuated, and the estimate was no longer statistically significant for cancer-specific survival (HR, 0.84, 0.65–1.08) (Fig. 3.8). These results indicate that increasing physical activity levels post-diagnosis, irrespective of meeting levels consistent with physical activity guidelines, may have positive effects on survival. However, the heterogeneity which exists relating to the method of determining physical activity changes (including differences in what constitutes a change and timing of the change) highlights the need for caution in interpreting statistical significance and clinical relevance of findings, as well as the need for more research in this area.
Resistance Training and Cancer Survival
The studies included in this review have primarily examined the effects of aerobic physical activity or total physical activity on survival outcomes in cancer populations. Despite the more recent inclusion of resistance exercise in physical activity guidelines for people with cancer [1], there is limited information pertaining to the effect of resistance training, or muscle-strengthening activity, on survival outcomes in cancer populations. Specifically, six studies identified in our review assessed this association in populations consisting of all cancer survivors, as well as independently for colorectal, breast, endometrial, and prostate cancer survivors (Table 3.2) [48, 56, 100, 102, 108, 112]. These studies varied in their definitions of participation in muscle-strengthening activities from assessing lifetime resistance training as a dichotomous variable of yes versus no to assessing if participants met the strength training guidelines. Overall, while there was a trend, based on effect size for decreases in both all-cause and cancer-specific mortality outcomes, for cancer survivors who reported engaging in the highest versus lowest categories of strength training (HR range, 0.46–1.15), however, confidence intervals often crossed the null value. Of particular note are findings derived from a cancer cohort study that compared individuals who met neither aerobic nor muscle-strengthening guidelines to individuals who met either one or both guidelines [48]. Results suggested that meeting both strength training and aerobic activity guidelines had a compounding effect, wherein stronger improvements in survival were observed when both components of physical activity guidelines are met, compared to when only one component of guidelines is met. These represent important findings, particularly relevant to physical activity guidelines promoted to those with cancer, but also require confirmation in future epidemiological and clinical trial research. Since there is currently only one study that we identified which investigated the compounded effect of meeting both aerobic and strength training physical activity guidelines, future research is warranted to determine whether or not these findings are observed by others. Mechanistically, there is biologic plausibility for an association between strength training and improved survival. Strength training is more effective than aerobic exercise at leading to increases or preservation over time in muscle mass and strength, higher muscle mass and strength are associated with improved physical functioning and quality of life, and higher muscle mass and strength have independently been associated with improved survival [165].
Cardiorespiratory Fitness and Cancer Survival
Cardiorespiratory fitness (CRF) , also known as exercise tolerance or physical fitness, refers to the ability of the circulatory, respiratory, and musculoskeletal systems to supply oxygen during sustained physical activity [166]. While higher physical activity levels have been associated with higher physical fitness, these terms are not synonymous, and it is possible that an individual may report high levels of physical activity but have low physical fitness. Consequently, exploring the relationship between physical fitness and survival outcomes following cancer is relevant. Physical activity is defined as any bodily movement produced by skeletal muscles that results in energy expenditure [167, 168]. In contrast, physical fitness (as captured by CRF ) represents the capacity to which an individual is able to achieve or perform physical activity [168]. With these distinctions made, it is important to also investigate the utility of using cardiorespiratory fitness as a predictor of cancer survival.
Schmid and Leitzmann completed a systematic review and meta-analysis on this topic in 2015 [169], which identified six studies capturing physical fitness information on 71,654 individuals and 2002 cases of total cancer mortality [7, 143,144,145,146,147]. From this review, these authors found that compared to low levels of cardiorespiratory fitness, intermediate levels and high levels of cardiorespiratory fitness were associated with statistically significant decreased risks of total cancer mortality (relative risks, 0.80 [0.67–0.97] and 0.55 [0.47–0.65], respectively). Several studies were not included in their review that examined total cancer mortality and cardiorespiratory fitness [110, 114, 148,149,150,151,152,153,154,155], and since publication of their review, there have been additional studies reporting on the relationship between cardiorespiratory fitness and site-specific cancer mortality [156,157,158,159,160]. These additional articles support and strengthen Schmid and Leitzmann’s findings with growing evidence to suggest that intermediate and high levels of cardiorespiratory fitness compared to low levels of cardiorespiratory fitness are associated with survival benefits in cancer populations.
There are some limitations to this body of research, including that the majority of studies have been restricted to male samples [110, 114, 147, 149, 150, 152, 153, 155,156,157,158, 160] and that a high proportion of the published findings have used data from the Aerobics Center Longitudinal Study [153, 155, 157,158,159,160], both of which limit the generalizability of results to the wider cancer population. With these caveats taken into consideration, the evidence does suggest that, overall, there are inverse and statistically significant associations between cardiorespiratory fitness and improved survival outcomes in cancer populations.
Exercise and Cancer Survival: Evidence from Clinical Trials Research
Besides the observational epidemiologic studies reviewed thus far, preliminary clinical trial evidence on the potential effect of participation in an exercise intervention, during or following treatment for cancer, on survival outcomes is also emerging. Data derived for these analyses have come from cohorts with breast cancer [32, 49], lymphoma, and leukemia [132, 170] and patients with bone metastasis following a range of cancers [171] (sample size range across the five trials, 60–337), with >65% of participants in all studies having completed or currently receiving chemotherapy during the intervention period. Interventions evaluated have involved aerobic-based only, resistance-based only, and aerobic- and resistance-based exercise, commencing during or post-active adjuvant therapy, with varying durations (range, 12–32 weeks) and mixed degree of supervision. Due to the small number of trials, and degree of heterogeneity between the samples and interventions evaluated, we provide here a narrative summary of the findings (rather than results from meta-analyses). A beneficial effect of exercise on all-cause mortality (HR, 0.45–0.71) was found in three of the five trials, with results remaining relatively unchanged following adjustment for other prognostic characteristics [32, 49, 132]. However, no effect of exercise on all-cause mortality (HR, 1.06 and 1.10) was reported in the remaining two trials (involving patients with metastatic disease and patients with lymphoma) [170, 171].
Comparison of findings from the two breast cancer trials warrants particular attention [32, 49]. First, sample characteristics, including age, body mass index, and stage of disease, were relatively similar between the two trials. In addition, of the five trials published to date on this topic, these two trials had the largest sample sizes (242 [32] and 337 [49]), evaluated the longest intervention (approximately 17 weeks in one trial [32] and 32 weeks for the other [49]), and longest time to follow up of survival data (89 [32] and 100 months [49]), minimizing some of the heterogeneity that would limit comparisons of findings. Further, the findings derived from these two trials for the effect of exercise on improving overall survival and disease-free survival were remarkably similar (HRs for overall survival, 0.60 [32] and 0.45 [49]; HRs for disease-free survival, 0.68 [32] and 0.66 [49]). Finally, despite the exploratory nature of the analyses undertaken (with limited power), the effect sizes observed are consistent with those observed in observational breast cancer studies, which suggest that improvements in survival of greater than 20% can be accrued through participation in physical activity post-diagnosis. These exciting, albeit preliminary, findings suggest that influencing physical activity behavior through exercise intervention may be beneficial for cancer outcomes.
Ongoing and Future Research
There is now not only a clear need for investigating causal associations between physical activity and cancer survival in adequately powered, randomized controlled trials but also the necessary evidence to support trial design, implementation, and evaluation. In addition, the recognized limitations in previous observational epidemiologic studies need to be addressed in future cohort studies. Progress in science addressing this gap is already happening. For example, the Alberta Moving Beyond Breast Cancer (AMBER) cohort study involves objective assessment of physical activity, sedentary behavior, health-related fitness, and breast cancer outcomes (target sample size, 1500) [172]. These design features address limitations of existing cohort studies evaluating physical activity and cancer survival outcomes, which have relied heavily on self-reported assessments of dose and type of physical activity, without concurrent assessment of fitness and sedentary behavior. Further, there now exist at least four randomized controlled exercise intervention trials, with target sample sizes providing adequate power for survival analyses following colon, metastatic prostate, ovarian, and allogeneic hematopoietic stem cell transplant patients [173,174,175,176]. Together these studies provide the ideal platform for improvements in knowledge needed to transform cancer care practice. Current and future research that seeks to explore optimal exercise dosage, modes of delivery, timing and duration of interventions, and characteristics that influence ability and capacity for a physiological and psychological response to exercise will ensure the workforce is equipped to prescribe evidence-based exercise to the growing cancer survivorship population.
Summary
Rapidly accumulating evidence from observational epidemiologic studies and follow-ups from randomized controlled exercise intervention trials supports recommendations to maintain and increase physical activity after cancer diagnosis for improved survival outcomes. This review found evidence for improved cancer-specific and all-cause mortality outcomes for 11 different cancer sites (10 specific cancer sites and all cancer sites combined), as well as preliminary evidence for decreased risk of recurrence and progressions. Increasing activity from pre- to post-diagnosis may also improve these outcomes after cancer. Population subgroups that might most benefit from physical activity remain unclear given the paucity of evidence to date. All stages of cancer appear to benefit equally from physical activity done either before or after cancer. More research is needed that focuses on these subgroups defined by sociodemographic characteristics as well as clinical and pathologic tumor characteristics. Additional research also needs to clarify the appropriate type, dose, and timing of physical activity that are most beneficial for improved survival outcomes by cancer site. This future research will help address the remaining gaps in understanding on the appropriate physical activity recommendations that can be provided to improve survival after cancer.
References
2018 Physical Activity Guidelines Advisory Committee Scientific Report (2018). 2018 Physical Activity Guidelines Advisory Committee. U.S. Department of Health and Human Services, Washington, D.C.
World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018: diet, nutrition, physical activity and cancer: a global perspective. 2018.
Canadian Physical Activity Guidelines 2012 Scientific Statements (2012).
Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, Gapstur S, Patel AV, Andrews K, Gansler T, American Cancer Society N, Physical Activity Guidelines Advisory C. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30–67. https://doi.org/10.3322/caac.20140.
Cormie P, Atkinson M, Bucci L, Cust A, Eakin E, Hayes S, McCarthy S, Murnane A, Patchell S, Adams D. Clinical Oncology Society of Australia position statement on exercise in cancer care. Med J Aust. 2018;209(4):184–7.
Jones LW. Precision oncology framework for investigation of exercise as treatment for cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33(35):4134–7. https://doi.org/10.1200/JCO.2015.62.7687.
Arraiz GA, Wigle DT, Mao Y. Risk assessment of physical activity and physical fitness in the Canada Health Survey mortality follow-up study. J Clin Epidemiol. 1992;45(4):419–28.
Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, Chan HT, Tsao CK, Tsai SP, Wu X. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378(9798):1244–53. https://doi.org/10.1016/S0140-6736(11)60749-6.
Beasley JM, Kwan ML, Chen WY, Weltzien EK, Kroenke CH, Lu W, Nechuta SJ, Cadmus-Bertram L, Patterson RE, Sternfeld B, Shu XO, Pierce JP, Caan BJ. Meeting the physical activity guidelines and survival after breast cancer: findings from the after breast cancer pooling project. Breast Cancer Res Treat. 2012;131(2):637–43.
Rohan TE, Fu W, Hiller JE. Physical activity and survival from breast cancer. Eur J Cancer Prev. 1995;4(5):419–24.
Borugian MJ, Sheps SB, Kim-Sing C, Van Patten C, Potter JD, Dunn B, Gallagher RP, Hislop TG. Insulin, macronutrient intake, and physical activity: are potential indicators of insulin resistance associated with mortality from breast cancer? Cancer Epidemiol Biomarkers Prev. 2004;13(7):1163–72.
Enger SM, Bernstein L. Exercise activity, body size and premenopausal breast cancer survival. Br J Cancer. 2004;90(11):2138–41.
Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–86. https://doi.org/10.1001/jama.293.20.2479.
Abrahamson PE, Gammon MD, Lund MJ, Britton JA, Marshall SW, Flagg EW, Porter PL, Brinton LA, Eley JW, Coates RJ. Recreational physical activity and survival among young women with breast cancer. Cancer. 2006;107(8):1777–85. https://doi.org/10.1002/cncr.22201.
Dal Maso L, Zucchetto A, Talamini R, Serraino D, Stocco CF, Vercelli M, Falcini F, Franceschi S, Prospective Analysis of Case-control studies on Environmental f, health study g. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 2008;123(9):2188–94. https://doi.org/10.1002/ijc.23747.
Holick CN, Newcomb PA, Trentham-Dietz A, Titus-Ernstoff L, Bersch AJ, Stampfer MJ, Baron JA, Egan KM, Willett WC. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomark Prev. 2008;17(2):379–86.
Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, Baumgartner RN, Baumgartner KB, Bernstein L. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26(24):3958–64.
Friedenreich CM, Gregory J, Kopciuk KA, Mackey JR, Courneya KS. Prospective cohort study of lifetime physical activity and breast cancer survival. Int J Cancer. 2009;124(8):1954–62.
Sternfeld B, Weltzien E, Quesenberry CP Jr, Castillo AL, Kwan M, Slattery ML, Caan BJ. Physical activity and risk of recurrence and mortality in breast cancer survivors: findings from the LACE study. Cancer Epidemiol Biomarkers Prev. 2009;18(1):87–95. https://doi.org/10.1158/1055-9965.Epi-08-0595.
West-Wright CN, Henderson KD, Sullivan-Halley J, Ursin G, Deapen D, Neuhausen S, Reynolds P, Chang E, Ma H, Bernstein L. Long-term and recent recreational physical activity and survival after breast cancer: the California Teachers Study. Cancer Epidemiol Biomarkers Prev. 2009;18(11):2851–9. https://doi.org/10.1158/1055-9965.EPI-09-0538.
Emaus A, Veierod MB, Tretli S, Finstad SE, Selmer R, Furberg AS, Bernstein L, Schlichting E, Thune I. Metabolic profile, physical activity, and mortality in breast cancer patients. Breast Cancer Res Treat. 2010;121(3):651–60.
Hellmann SS, Thygesen LC, Tolstrup JS, Gronbaek M. Modifiable risk factors and survival in women diagnosed with primary breast cancer: results from a prospective cohort study. Eur J Cancer Prev. 2010;19(5):366–73. https://doi.org/10.1097/CEJ.0b013e32833b4828.
Keegan TH, Milne RL, Andrulis IL, Chang ET, Sangaramoorthy M, Phillips KA, Giles GG, Goodwin PJ, Apicella C, Hopper JL, Whittemore AS, John EM. Past recreational physical activity, body size, and all-cause mortality following breast cancer diagnosis: results from the Breast Cancer Family Registry. Breast Cancer Res Treat. 2010;123(2):531–42. https://doi.org/10.1007/s10549-010-0774-6.
Bertram LA, Stefanick ML, Saquib N, Natarajan L, Patterson RE, Bardwell W, Flatt SW, Newman VA, Rock CL, Thomson CA, Pierce JP. Physical activity, additional breast cancer events, and mortality among early-stage breast cancer survivors: findings from the WHEL study. Cancer Causes Control. 2011;22(3):427–35. https://doi.org/10.1007/s10552-010-9714-3.
Chen X, Lu W, Zheng W, Gu K, Matthews CE, Chen Z, Zheng Y, Shu XO. Exercise after diagnosis of breast cancer in association with survival. Cancer Prev Res (Phila). 2011;4(9):1409–18. https://doi.org/10.1158/1940-6207.CAPR-10-0355.
Irwin ML, McTiernan A, Manson JE, Thomson CA, Sternfeld B, Stefanick ML, Wactawski-Wende J, Craft L, Lane D, Martin LW, Chlebowski R. Physical activity and survival in postmenopausal women with breast cancer: results from the women’s health initiative. Cancer Prev Res. 2011;4(4):522–9.
Cleveland RJ, Eng SM, Stevens J, Bradshaw PT, Teitelbaum SL, Neugut AI, Gammon MD. Influence of prediagnostic recreational physical activity on survival from breast cancer. Eur J Cancer Prev. 2012;21(1):46–54. https://doi.org/10.1097/CEJ.0b013e3283498dd4.
Schmidt ME, Chang-Claude J, Vrieling A, Seibold P, Heinz J, Obi N, Flesch-Janys D, Steindorf K. Association of pre-diagnosis physical activity with recurrence and mortality among women with breast cancer. Int J Cancer. 2013;133(6):1431–40.
Tao MH, Hainaut P, Marian C, Nie J, Ambrosone C, Edge SB, Trevisan M, Dorn J, Shields PG, Freudenheim JL. Association of prediagnostic physical activity with survival following breast cancer diagnosis: influence of TP53 mutation status. Cancer Causes Control. 2013;24(12):2177–86.
Williams PT. Breast cancer mortality vs. exercise and breast size in runners and walkers. PLoS One. 2013;8(12):e80616. https://doi.org/10.1371/journal.pone.0080616.
Bradshaw PT, Ibrahim JG, Khankari N, Cleveland RJ, Abrahamson PE, Stevens J, Satia JA, Teitelbaum SL, Neugut AI, Gammon MD. Post-diagnosis physical activity and survival after breast cancer diagnosis: the Long Island Breast Cancer Study. Breast Cancer Res Treat. 2014;145(3):735–42.
Courneya KS, Segal RJ, McKenzie DC, Dong H, Gelmon K, Friedenreich CM, Yasui Y, Reid RD, Crawford JJ, Mackey JR. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sports Exerc. 2014;46(9):1744–51. https://doi.org/10.1249/MSS.0000000000000297.
de Glas NA, Fontein DB, Bastiaannet E, Pijpe A, De Craen AJ, Liefers GJ, Nortier HJ, de Haes HJ, van de Velde CJ, van Leeuwen FE. Physical activity and survival of postmenopausal, hormone receptor-positive breast cancer patients: results of the Tamoxifen Exemestane Adjuvant Multicenter Lifestyle study. Cancer. 2014;120(18):2847–54. https://doi.org/10.1002/cncr.28783.
Keegan TH, Shariff-Marco S, Sangaramoorthy M, Koo J, Hertz A, Schupp CW, Yang J, John EM, Gomez SL. Neighborhood influences on recreational physical activity and survival after breast cancer. Cancer Causes Control. 2014;25(10):1295–308. https://doi.org/10.1007/s10552-014-0431-1.
Williams PT. Significantly greater reduction in breast cancer mortality from post-diagnosis running than walking. Int J Cancer. 2014;135(5):1195–202. https://doi.org/10.1002/ijc.28740.
Bao PP, Zhao GM, Shu XO, Peng P, Cai H, Lu W, Zheng Y. Modifiable lifestyle factors and triple-negative breast cancer survival: a population-based prospective study. Epidemiology. 2015;26(6):909–16.
Borch KB, Braaten T, Lund E, Weiderpass E. Physical activity before and after breast cancer diagnosis and survival – the Norwegian women and cancer cohort study. BMC Cancer. 2015;15:967. https://doi.org/10.1186/s12885-015-1971-9.
Lu Y, John EM, Sullivan-Halley J, Vigen C, Gomez SL, Kwan ML, Caan BJ, Lee VS, Roh JM, Shariff-Marco S, Keegan TH, Kurian AW, Monroe KR, Cheng I, Sposto R, Wu AH, Bernstein L. History of recreational physical activity and survival after breast cancer: the California Breast Cancer Survivorship Consortium. Am J Epidemiol. 2015;181(12):944–55. https://doi.org/10.1093/aje/kwu466.
Pinkston CM, Baumgartner RN, Connor AE, Boone SD, Baumgartner KB. Physical activity and survival among Hispanic and non-Hispanic white long-term breast cancer survivors and population-based controls. J Cancer Surviv Res Pract. 2015;9(4):650–9.
Ammitzboll G, Sogaard K, Karlsen RV, Tjonneland A, Johansen C, Frederiksen K, Bidstrup P. Physical activity and survival in breast cancer. Eur J Cancer. 2016;66:67–74.
Jones LW, Kwan ML, Weltzien E, Chandarlapaty S, Sternfeld B, Sweeney C, Bernard PS, Castillo A, Habel LA, Kroenke CH, Langholz BM, Queensberry CP, Dang C, Weigelt B, Kushi LH, Caan BJ. Exercise and prognosis on the basis of clinicopathologic and molecular features in early-stage breast cancer: the LACE and pathways studies. Cancer Res. 2016;76(18):5415–22.
McCullough LE, Chen J, Cho YH, Khankari NK, Bradshaw PT, White AJ, Teitelbaum SL, Terry MB, Neugut AI, Hibshoosh H, Santella RM, Gammon MD. Modification of the association between recreational physical activity and survival after breast cancer by promoter methylation in breast cancer-related genes. Breast Cancer Res. 2017;19(1) (no pagination):19.
Cifu G, Arem H. Adherence to lifestyle-related cancer prevention guidelines and breast cancer incidence and mortality. Ann Epidemiol. 2018;28(11):767–773.e761. https://doi.org/10.1016/j.annepidem.2018.09.002.
Maliniak ML, Patel AV, McCullough ML, Campbell PT, Leach CR, Gapstur SM, Gaudet MM. Obesity, physical activity, and breast cancer survival among older breast cancer survivors in the Cancer Prevention Study-II Nutrition Cohort. Breast Cancer Res Treat. 2018;167(1):133–45.
Palesh O, Kamen C, Sharp S, Golden A, Neri E, Spiegel D, Koopman C. Physical activity and survival in women with advanced breast cancer. Cancer Nurs. 2018;41(4):E31–e38. https://doi.org/10.1097/ncc.0000000000000525.
Parada H Jr, Sun X, Tse CK, Olshan AF, Troester MA. Lifestyle patterns and survival following breast cancer in the carolina breast cancer study. Epidemiology. 2019;30(1):83–92. https://doi.org/10.1097/EDE.0000000000000933.
Jee Y, Kim Y, Jee SH, Ryu M. Exercise and cancer mortality in Korean men and women: a prospective cohort study. BMC Public Health. 2018;18(1):761. https://doi.org/10.1186/s12889-018-5669-1.
Tarasenko YN, Linder DF, Miller EA. Muscle-strengthening and aerobic activities and mortality among 3+ year cancer survivors in the U.S. Cancer Causes Control. 2018;29(4–5):475–84.
Hayes SC, Steele ML, Spence RR, Gordon L, Battistutta D, Bashford J, Pyke C, Saunders C, Eakin E. Exercise following breast cancer: exploratory survival analyses of two randomised, controlled trials. Breast Cancer Res Treat. 2018;167(2):505–14. https://doi.org/10.1007/s10549-017-4541-9.
Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, Fuchs CS. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24(22):3527–34.
Asia Pacific Cohort Studies C, Huxley R, Ansary-Moghaddam A, Huxley R, Lam TH, Ueshima H, Gu DF, Kim HC, Woodward M, Fang X, Gu DF, Imai Y, Pan WH, Rodgers A, Suh I. The role of lifestyle risk factors on mortality from colorectal cancer in populations of the Asia-Pacific region. Asian Pac J Cancer Prev. 2007;8(2):191–8.
Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W, Fuchs CS. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009;169(22):2102–8.
Baade PD, Xingqiong M, Youl PH, Aitken JF, Dunn J, Chambers SK. The impact of body mass index and physical activity on mortality among patients with colorectal cancer in Queensland, Australia. Cancer Epidemiol Biomark Prev. 2011;20(7):1410–20.
Morrison DS, Batty GD, Kivimaki M, Davey Smith G, Marmot M, Shipley M. Risk factors for colonic and rectal cancer mortality: evidence from 40 years’ follow-up in the Whitehall I study. J Epidemiol Community Health. 2011;65(11):1053–8. https://doi.org/10.1136/jech.2010.127555.
Kuiper JG, Phipps AI, Neuhouser ML, Chlebowski RT, Thomson CA, Irwin ML, Lane DS, Wactawski-Wende J, Hou L, Jackson RD, Kampman E, Newcomb PA. Recreational physical activity, body mass index, and survival in women with colorectal cancer. Cancer Causes Control. 2012;23(12):1939–48.
Boyle T, Fritschi L, Platell C, Heyworth J. Lifestyle factors associated with survival after colorectal cancer diagnosis. Br J Cancer. 2013;109(3):814–22.
Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31(7):876–85.
Pelser C, Arem H, Pfeiffer RM, Elena JW, Alfano CM, Hollenbeck AR, Park Y. Prediagnostic lifestyle factors and survival after colon and rectal cancer diagnosis in the National Institutes of Health (NIH)-AARP Diet and Health Study. Cancer. 2014;120(10):1540–7.
Arem H, Pfeiffer RM, Engels EA, Alfano CM, Hollenbeck A, Park Y, Matthews CE. Pre- and postdiagnosis physical activity, television viewing, and mortality among patients with colorectal cancer in the national institutes of health-AARP diet and health study. J Clin Oncol. 2015;33(2):180–8.
Hardikar S, Newcomb PA, Campbell PT, Aung Ko W, Lindor NM, Buchanan DD, Makar KW, Jenkins MA, Potter JD, Phipps AI. Prediagnostic physical activity and colorectal cancer survival: overall and stratified by tumor characteristics. Cancer Epidemiol Biomark Prev. 2015;24(7):1130–7.
Romaguera D, Ward H, Wark PA, Vergnaud AC, Peeters PH, van Gils CH, Ferrari P, Fedirko V, Jenab M, Boutron-Ruault MC, Dossus L, Dartois L, Hansen CP, Dahm CC, Buckland G, Sanchez MJ, Dorronsoro M, Navarro C, Barricarte A, Key TJ, Trichopoulou A, Tsironis C, Lagiou P, Masala G, Pala V, Tumino R, Vineis P, Panico S, Bueno-de-Mesquita HB, Siersema PD, Ohlsson B, Jirstrom K, Wennberg M, Nilsson LM, Weiderpass E, Kuhn T, Katzke V, Khaw KT, Wareham NJ, Tjonneland A, Boeing H, Quiros JR, Gunter MJ, Riboli E, Norat T. Pre-diagnostic concordance with the WCRF/AICR guidelines and survival in European colorectal cancer patients: a cohort study. BMC Med. 2015;13(1) (no pagination):107.
Mok Y, Jeon C, Lee GJ, Jee SH. Physical activity level and colorectal cancer mortality. Asia Pac J Public Health. 2016;28(7):638–47. https://doi.org/10.1177/1010539516661761.
Thong MS, Kaptein AA, Vissers PA, Vreugdenhil G, van de Poll-Franse LV. Illness perceptions are associated with mortality among 1552 colorectal cancer survivors: a study from the population-based PROFILES registry. J Cancer Surviv Res Pract. 2016;10(5):898–905.
Ratjen I, Schafmayer C, di Giuseppe R, Waniek S, Plachta-Danielzik S, Koch M, Burmeister G, Nothlings U, Hampe J, Schlesinger S, Lieb W. Postdiagnostic physical activity, sleep duration, and TV watching and all-cause mortality among long-term colorectal cancer survivors: a prospective cohort study. BMC Cancer. 2017;17(1) (no pagination):701.
Walter V, Jansen L, Knebel P, Chang-Claude J, Hoffmeister M, Brenner H. Physical activity and survival of colorectal cancer patients: population-based study from Germany. Int J Cancer. 2017;140(9):1985–97.
Jayasekara H, English DR, Haydon A, Hodge AM, Lynch BM, Rosty C, Williamson EJ, Clendenning M, Southey MC, Jenkins MA, Room R, Hopper JL, Milne RL, Buchanan DD, Giles GG, MacInnis RJ. Associations of alcohol intake, smoking, physical activity and obesity with survival following colorectal cancer diagnosis by stage, anatomic site and tumor molecular subtype. Int J Cancer. 2018;142(2):238–50. https://doi.org/10.1002/ijc.31049.
Phipps AI, Qian S, Zemla TJ, Dotan E, Gill S, Goldberg RM, Hardikar S, Jahagirdar B, Limburg PJ, Newcomb PA, Shields A, Sinicrope FA, Sargent DJ, Alberts SR. Physical activity and outcomes in patients with stage III colon cancer: a correlative analysis of phase III trial NCCTG N0147 (Alliance). Cancer Epidemiol Biomark Prev. 2018;27(6):696–703.
Van Blarigan EL, Fuchs CS, Niedzwiecki D, Zhang S, Saltz LB, Mayer RJ, Mowat RB, Whittom R, Hantel A, Benson A, Atienza D, Messino M, Kindler H, Venook A, Ogino S, Giovannucci EL, Ng K, Meyerhardt JA. Association of survival with adherence to the American Cancer Society Nutrition and Physical Activity Guidelines for Cancer survivors after colon cancer diagnosis: the CALGB 89803/Alliance Trial. JAMA Oncol. 2018;4(6):783–90. https://doi.org/10.1001/jamaoncol.2018.0126.
Batty GD, Shipley MJ, Marmot M, Smith GD. Physical activity and cause-specific mortality in men: further evidence from the Whitehall study. Eur J Epidemiol. 2001;17(9):863–9.
Nilsen TI, Romundstad PR, Vatten LJ. Recreational physical activity and risk of prostate cancer: a prospective population-based study in Norway (the HUNT study). Int J Cancer. 2006;119(12):2943–7. https://doi.org/10.1002/ijc.22184.
Crespo CJ, Garcia-Palmieri MR, Smit E, Lee IM, McGee D, Muti P, Figueroa Valle NR, Ramierez-Marrero FA, Freudenheim JL, Sorlie P. Physical activity and prostate cancer mortality in Puerto Rican men. J Phys Act Health. 2008;5(6):918–29.
Orsini N, Bellocco R, Bottai M, Pagano M, Andersson SO, Johansson JE, Giovannucci E, Wolk A. A prospective study of lifetime physical activity and prostate cancer incidence and mortality. Br J Cancer. 2009;101(11):1932–8. https://doi.org/10.1038/sj.bjc.6605404.
Batty GD, Kivimaki M, Clarke R, Davey Smith G, Shipley MJ. Modifiable risk factors for prostate cancer mortality in London: forty years of follow-up in the Whitehall study. Cancer Causes Control. 2011;22(2):311–8. https://doi.org/10.1007/s10552-010-9691-6.
Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29(6):726–32.
Bonn SE, Sjolander A, Lagerros YT, Wiklund F, Stattin P, Holmberg E, Gronberg H, Balter K. Physical activity and survival among men diagnosed with prostate cancer. Cancer Epidemiol Biomarkers Prev. 2015;24(1):57–64. https://doi.org/10.1158/1055-9965.EPI-14-0707.
Friedenreich CM, Wang Q, Neilson HK, Kopciuk KA, McGregor SE, Courneya KS. Physical activity and survival after prostate cancer. Eur Urol. 2016;70(4):576–85.
Tai SY, Hsieh HM, Huang SP, Wu MT. Hair dye use, regular exercise, and the risk and prognosis of prostate cancer: multicenter case-control and case-only studies. BMC Cancer. 2016;16:242. https://doi.org/10.1186/s12885-016-2280-7.
Yang W-S, Fu W-X, Wang X, Deng Q, Wang L, Wang L-Y, Zhao H, Fan W-Y, Huang S-X. Comprehensive assessments of long-term sleep habits in epidemiological study: validity and reliability of sleep factors questionnaire (SFQ) among Chinese women. J Psychosom Res. 2017;95:12–8. https://doi.org/10.1016/j.jpsychores.2017.02.005.
Arem H, Moore SC, Park Y, Ballard-Barbash R, Hollenbeck A, Leitzmann M, Matthews CE. Physical activity and cancer-specific mortality in the NIH-AARP Diet and Health Study cohort. Int J Cancer. 2014;135(2):423–31. https://doi.org/10.1002/ijc.28659.
Davey Smith G, Shipley MJ, Batty GD, Morris JN, Marmot M. Physical activity and cause-specific mortality in the Whitehall study. Public Health. 2000;114(5):308–15.
Kampert JB, Blair SN, Barlow CE, Kohl HW 3rd. Physical activity, physical fitness, and all-cause and cancer mortality: a prospective study of men and women. Ann Epidemiol. 1996;6(5):452–7.
Rosengren A, Wilhelmsen L. Physical activity protects against coronary death and deaths from all causes in middle-aged men. Evidence from a 20-year follow-up of the primary prevention study in Goteborg. Ann Epidemiol. 1997;7(1):69–75.
Kilander L, Berglund L, Boberg M, Vessby B, Lithell H. Education, lifestyle factors and mortality from cardiovascular disease and cancer. A 25-year follow-up of Swedish 50-year-old men. Int J Epidemiol. 2001;30(5):1119–26.
Hu G, Tuomilehto J, Silventoinen K, Barengo NC, Peltonen M, Jousilahti P. The effects of physical activity and body mass index on cardiovascular, cancer and all-cause mortality among 47 212 middle-aged Finnish men and women. Int J Obes. 2005;29(8):894–902. https://doi.org/10.1038/sj.ijo.0802870.
Schnohr P, Lange P, Scharling H, Jensen JS. Long-term physical activity in leisure time and mortality from coronary heart disease, stroke, respiratory diseases, and cancer. The Copenhagen City Heart Study. Eur J Cardiovasc Prev Rehabil. 2006;13(2):173–9. https://doi.org/10.1097/01.hjr.0000198923.80555.b7.
Matthews CE, Jurj AL, Shu XO, Li HL, Yang G, Li Q, Gao YT, Zheng W. Influence of exercise, walking, cycling, and overall nonexercise physical activity on mortality in Chinese women. Am J Epidemiol. 2007;165(12):1343–50. https://doi.org/10.1093/aje/kwm088.
Orsini N, Bellocco R, Bottai M, Pagano M, Michaelsson K, Wolk A. Combined effects of obesity and physical activity in predicting mortality among men. J Intern Med. 2008;264(5):442–51. https://doi.org/10.1111/j.1365-2796.2008.01985.x.
van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ. 2008;337:a1440. https://doi.org/10.1136/bmj.a1440.
Hamer M, Stamatakis E, Saxton JM. The impact of physical activity on all-cause mortality in men and women after a cancer diagnosis. Cancer Causes Control. 2009;20(2):225–31.
Autenrieth CS, Baumert J, Baumeister SE, Fischer B, Peters A, Doring A, Thorand B. Association between domains of physical activity and all-cause, cardiovascular and cancer mortality. Eur J Epidemiol. 2011;26(2):91–9. https://doi.org/10.1007/s10654-010-9517-6.
Borch KB, Braaten T, Lund E, Weiderpass E. Physical activity and mortality among Norwegian women – the Norwegian Women and Cancer Study. Clin Epidemiol. 2011;3:229–35. https://doi.org/10.2147/CLEP.S22681.
Laukkanen JA, Rauramaa R, Makikallio TH, Toriola AT, Kurl S. Intensity of leisure-time physical activity and cancer mortality in men. Br J Sports Med. 2011;45(2):125–9. https://doi.org/10.1136/bjsm.2008.056713.
McCullough ML, Patel AV, Kushi LH, Patel R, Willett WC, Doyle C, Thun MJ, Gapstur SM. Following cancer prevention guidelines reduces risk of cancer, cardiovascular disease, and all-cause mortality. Cancer Epidemiol Biomarkers Prev. 2011;20(6):1089–97.
Lin CC, Li CI, Liu CS, Lin WY, Fuh MM, Yang SY, Lee CC, Li TC. Impact of lifestyle-related factors on all-cause and cause-specific mortality in patients with type 2 diabetes: the Taichung Diabetes Study. Diabetes Care. 2012;35(1):105–12. https://doi.org/10.2337/dc11-0930.
Mok Y, Won S, Kimm H, Nam C, Ohrr H, Jee SH. Physical activity level and risk of death: the severance cohort study. J Epidemiol. 2012;22(6):494–500.
Parekh N, Lin Y, Craft LL, Vadiveloo M, Lu-Yao GL. Longitudinal associations of leisure-time physical activity and cancer mortality in the Third National Health and Nutrition Examination Survey (1986–2006). J Obes. 2012;2012:518358. https://doi.org/10.1155/2012/518358.
Inoue-Choi M, Robien K, Lazovich D. Adherence to the WCRF/AICR guidelines for cancer prevention is associated with lower mortality among older female cancer survivors. Cancer Epidemiol Biomarkers Prev. 2013;22(5):792–802.
Vergnaud AC, Romaguera D, Peeters PH, van Gils CH, Chan DS, Romieu I, Freisling H, Ferrari P, Clavel-Chapelon F, Fagherazzi G, Dartois L, Li K, Tikk K, Bergmann MM, Boeing H, Tjonneland A, Olsen A, Overvad K, Dahm CC, Redondo ML, Agudo A, Sanchez MJ, Amiano P, Chirlaque MD, Ardanaz E, Khaw KT, Wareham NJ, Crowe F, Trichopoulou A, Orfanos P, Trichopoulos D, Masala G, Sieri S, Tumino R, Vineis P, Panico S, Bueno-de-Mesquita HB, Ros MM, May A, Wirfalt E, Sonestedt E, Johansson I, Hallmans G, Lund E, Weiderpass E, Parr CL, Riboli E, Norat T. Adherence to the World Cancer Research Fund/American Institute for Cancer Research guidelines and risk of death in Europe: results from the European Prospective Investigation into Nutrition and Cancer cohort study1,4. Am J Clin Nutr. 2013;97(5):1107–20. https://doi.org/10.3945/ajcn.112.049569.
Wang N, Zhang X, Xiang YB, Li H, Yang G, Gao J, Zheng W, Shu XO. Associations of Tai Chi, walking, and jogging with mortality in Chinese men. Am J Epidemiol. 2013;178(5):791–6. https://doi.org/10.1093/aje/kwt050.
Yu R, Leung J, Woo J. Housework reduces all-cause and cancer mortality in Chinese men. PLoS One. 2013;8(5):e61529. https://doi.org/10.1371/journal.pone.0061529.
Gunnell AS, Knuiman MW, Divitini ML, Cormie P. Leisure time physical activity and long-term cardiovascular and cancer outcomes: the Busselton Health Study. Eur J Epidemiol. 2014;29(11):851–7. https://doi.org/10.1007/s10654-014-9963-7.
Hardee JP, Porter RR, Sui X, Archer E, Lee IM, Lavie CJ, Blair SN. The effect of resistance exercise on all-cause mortality in cancer survivors. Mayo Clin Proc. 2014;89(8):1108–15.
Hastert TA, Beresford SA, Sheppard L, White E. Adherence to the WCRF/AICR cancer prevention recommendations and cancer-specific mortality: results from the Vitamins and Lifestyle (VITAL) Study. Cancer Causes Control. 2014;25(5):541–52. https://doi.org/10.1007/s10552-014-0358-6.
Lee IM, Wolin KY, Freeman SE, Sattlemair J, Sesso HD. Physical activity and survival after cancer diagnosis in men. J Phys Act Health. 2014;11(1):85–90.
Wanner M, Tarnutzer S, Martin BW, Braun J, Rohrmann S, Bopp M, Faeh D, Swiss National C. Impact of different domains of physical activity on cause-specific mortality: a longitudinal study. Prev Med. 2014;62:89–95. https://doi.org/10.1016/j.ypmed.2014.01.025.
Brown JC, Harhay MO, Harhay MN. The prognostic importance of frailty in cancer survivors. J Am Geriatr Soc. 2015;63(12):2538–43.
Kabat GC, Matthews CE, Kamensky V, Hollenbeck AR, Rohan TE. Adherence to cancer prevention guidelines and cancer incidence, cancer mortality, and total mortality: a prospective cohort study. Am J Clin Nutr. 2015;101(3):558–69. https://doi.org/10.3945/ajcn.114.094854.
Kraschnewski JL, Sciamanna CN, Poger JM, Rovniak LS, Lehman EB, Cooper AB, Ballentine NH, Ciccolo JT. Is strength training associated with mortality benefits? A 15year cohort study of US older adults. Prev Med. 2016;87:121–7. https://doi.org/10.1016/j.ypmed.2016.02.038.
Lee JY, Ryu S, Cheong E, Sung KC. Association of physical activity and inflammation with all-cause, cardiovascular-related, and cancer-related mortality. Mayo Clin Proc. 2016;91(12):1706–16.
Robsahm TE, Falk RS, Heir T, Sandvik L, Vos L, Erikssen JE, Tretli S. Measured cardiorespiratory fitness and self-reported physical activity: associations with cancer risk and death in a long-term prospective cohort study. Cancer Med. 2016;5(8):2136–44. https://doi.org/10.1002/cam4.773.
Gunnell AS, Joyce S, Tomlin S, Taaffe DR, Cormie P, Newton RU, Joseph D, Spry N, Einarsdottir K, Galvao DA. Physical activity and survival among long-term cancer survivor and non-cancer cohorts. Front Public Health. 2017;5:19. https://doi.org/10.3389/fpubh.2017.00019.
Kamada M, Shiroma EJ, Buring JE, Miyachi M, Lee IM. Strength training and all-cause, cardiovascular disease, and cancer mortality in older women: a cohort study. J Am Heart Assoc. 2017;6(11). https://doi.org/10.1161/JAHA.117.007677.
O’Donovan G, Lee IM, Hamer M, Stamatakis E. Association of “weekend warrior” and other leisure time physical activity patterns with risks for all-cause, cardiovascular disease, and cancer mortality. JAMA Intern Med. 2017;177(3):335–42. https://doi.org/10.1001/jamainternmed.2016.8014.
Vainshelboim B, Muller J, Lima RM, Nead KT, Chester C, Chan K, Kokkinos P, Myers J. Cardiorespiratory fitness, physical activity and cancer mortality in men. Prev Med. 2017;100:89–94. https://doi.org/10.1016/j.ypmed.2017.04.014.
Dohrn IM, Sjostrom M, Kwak L, Oja P, Hagstromer M. Accelerometer-measured sedentary time and physical activity-A 15 year follow-up of mortality in a Swedish population-based cohort. J Sci Med Sport. 2018;21(7):702–7. https://doi.org/10.1016/j.jsams.2017.10.035.
Liu Y, Wen W, Gao YT, Li HL, Yang G, Xiang YB, Shu XO, Zheng W. Level of moderate-intensity leisure-time physical activity and reduced mortality in middle-aged and elderly Chinese. J Epidemiol Community Health. 2018;72(1):13–20. https://doi.org/10.1136/jech-2017-209903.
Patel AV, Hildebrand JS, Leach CR, Campbell PT, Doyle C, Shuval K, Wang Y, Gapstur SM. Walking in relation to mortality in a large prospective cohort of older U.S. adults. Am J Prev Med. 2018;54(1):10–9. https://doi.org/10.1016/j.amepre.2017.08.019.
Liss MA, White M, Natarajan L, Parsons JK. Exercise decreases and smoking increases bladder cancer mortality. Clin Genitourin Cancer. 2017;15(3):391–5.
Scott JM, Li N, Liu Q, Yasui Y, Leisenring W, Nathan PC, Gibson T, Armenian SH, Nilsen TS, Oeffinger KC, Ness KK, Adams SC, Robison LL, Armstrong GT, Jones LW. Association of exercise with mortality in adult survivors of childhood cancer. JAMA Oncol. 2018. https://doi.org/10.1001/jamaoncol.2018.2254.
Sundelof M, Lagergren J, Ye W. Patient demographics and lifestyle factors influencing long-term survival of oesophageal cancer and gastric cardia cancer in a nationwide study in Sweden. Eur J Cancer. 2008;44(11):1566–71.
Okada E, Ukawa S, Nakamura K, Hirata M, Nagai A, Matsuda K, Ninomiya T, Kiyohara Y, Muto K, Kamatani Y, Yamagata Z, Kubo M, Nakamura Y, Tamakoshi A. Demographic and lifestyle factors and survival among patients with esophageal and gastric cancer: the Biobank Japan Project. J Epidemiol. 2017;27(3S):S29–35.
Kim MK, Sim JA, Yun YH, Bae DS, Nam JH, Park CT, Cho CH, Lee JM, Park SY. Health-related quality of life and sociodemographic characteristics as prognostic indicators of long-term survival in disease-free cervical cancer survivors. Int J Gynecol Cancer. 2016;26(4):743–9.
Arem H, Park Y, Pelser C, Ballard-Barbash R, Irwin ML, Hollenbeck A, Gierach GL, Brinton LA, Pfeiffer RM, Matthews CE. Prediagnosis body mass index, physical activity, and mortality in endometrial cancer patients. J Natl Cancer Inst. 2013;105(5):342–9.
Arem H, Chlebowski R, Stefanick ML, Anderson G, Wactawski-Wende J, Sims S, Gunter MJ, Irwin ML. Body mass index, physical activity, and survival after endometrial cancer diagnosis: results from the Women’s Health Initiative. Gynecol Oncol. 2013;128(2):181–6.
Arem H, Pfeiffer RM, Moore SC, Brinton LA, Matthews CE. Body mass index, physical activity, and television time in relation to mortality risk among endometrial cancer survivors in the NIH-AARP Diet and Health Study cohort. Cancer Causes Control. 2016;27(11):1403–9.
Yang L, Klint A, Lambe M, Bellocco R, Riman T, Bergfeldt K, Persson I, Weiderpass E. Predictors of ovarian cancer survival: a population-based prospective study in Sweden. Int J Cancer. 2008;123(3):672–9. https://doi.org/10.1002/ijc.23429.
Moorman PG, Jones LW, Akushevich L, Schildkraut JM. Recreational physical activity and ovarian cancer risk and survival. Ann Epidemiol. 2011;21(3):178–87. https://doi.org/10.1016/j.annepidem.2010.10.014.
Zhou Y, Chlebowski R, LaMonte MJ, Bea JW, Qi L, Wallace R, Lavasani S, Walsh BW, Anderson G, Vitolins M, Sarto G, Irwin ML. Body mass index, physical activity, and mortality in women diagnosed with ovarian cancer: results from the Women’s Health Initiative. Gynecol Oncol. 2014;133(1):4–10. https://doi.org/10.1016/j.ygyno.2014.01.033.
Abbott SE, Camacho F, Peres LC, Alberg AJ, Bandera EV, Bondy M, Cote ML, Funkhouser E, Moorman PG, Peters ES, Qin B, Schwartz AG, Barnholtz-Sloan J, Terry P, Schildkraut JM. Recreational physical activity and survival in African-American women with ovarian cancer. Cancer Causes Control. 2018;29(1):77–86.
Ruden E, Reardon DA, Coan AD, Herndon JE 2nd, Hornsby WE, West M, Fels DR, Desjardins A, Vredenburgh JJ, Waner E, Friedman AH, Friedman HS, Peters KB, Jones LW. Exercise behavior, functional capacity, and survival in adults with malignant recurrent glioma. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(21):2918–23. https://doi.org/10.1200/jco.2011.34.9852.
Schmid D, Behrens G, Arem H, Hart C, Herr W, Jochem C, Matthews CE, Leitzmann MF. Pre- and post-diagnosis physical activity, television viewing, and mortality among hematologic cancer survivors. PLoS One. 2018;13(1) (no pagination):(e0192078).
Wiskemann J, Kleindienst N, Kuehl R, Dreger P, Schwerdtfeger R, Bohus M. Effects of physical exercise on survival after allogeneic stem cell transplantation. Int J Cancer. 2015;137(11):2749–56.
Boyle T, Connors JM, Gascoyne RD, Berry BR, Sehn LH, Bashash M, Spinelli JJ. Physical activity, obesity and survival in diffuse large B-cell and follicular lymphoma cases. Br J Haematol. 2017;178(3):442–7. https://doi.org/10.1111/bjh.14702.
Pophali PA, Ip A, Larson MC, Rosenthal AC, Maurer MJ, Flowers CR, Link BK, Farooq U, Feldman AL, Allmer C, Slager SL, Witzig TE, Habermann TM, Cohen JB, Cerhan JR, Thompson CA. The association of physical activity before and after lymphoma diagnosis with survival outcomes. Am J Hematol. 2018;93(12):1543–50. https://doi.org/10.1002/ajh.25288.
Schmid D, Matthews CE, Leitzmann MF. Physical activity and sedentary behavior in relation to mortality among renal cell cancer survivors. PLoS One. 2018;13(6) (no pagination):(e0198995).
Jones LW, Hornsby WE, Goetzinger A, Forbes LM, Sherrard EL, Quist M, Lane AT, West M, Eves ND, Gradison M, Coan A, Herndon JE, Abernethy AP. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer. 2012;76(2):248–52.
Sloan JA, Cheville AL, Liu H, Novotny PJ, Wampfler JA, Garces YI, Clark MM, Yang P. Impact of self-reported physical activity and health promotion behaviors on lung cancer survivorship. Health Qual Life Outcomes. 2016;14 (1) (no pagination):66.
Schwitzer E, Orlow I, Zabor EC, Begg CB, Berwick M, Thomas NE, Jones LW, Busam KJ, Reiner AS, Roy P, Sharma A, Pilla EL, Luo L, White K, Paine S, Armstrong BK, Kricker A, Cust AE, Venn A, Dwyer T, Tucker P, Gallagher RP, Kan D, Marrett LD, Theis E, From L, Zanetti R, Rosso S, Anton-Culver H, Ziogas A, Gruber SB, Johnson T, Sturgeon D, Millikan RC, Ollila DW, Conway K, Groben PA, Edmiston SN, Hao H, Parrish E, Frank JS, Gibbs DC, Bramson JI, Rebbeck TR, Kanetsky PA, Taylor JL. No association between prediagnosis exercise and survival in patients with high-risk primary melanoma: a population-based study. Pigment Cell Melanoma Res. 2017;30(4):424–7.
Lee IM, Sesso HD, Oguma Y, Paffenbarger RS Jr. Physical activity, body weight, and pancreatic cancer mortality. Br J Cancer. 2003;88(5):679–83.
Lin Y, Kikuchi S, Tamakoshi A, Yagyu K, Obata Y, Inaba Y, Kurosawa M, Kawamura T, Motohashi Y, Ishibashi T, Group JS. Obesity, physical activity and the risk of pancreatic cancer in a large Japanese cohort. Int J Cancer. 2007;120(12):2665–71. https://doi.org/10.1002/ijc.22614.
Stevens RJ, Roddam AW, Spencer EA, Pirie KL, Reeves GK, Green J, Beral V, Million Women Study C. Factors associated with incident and fatal pancreatic cancer in a cohort of middle-aged women. Int J Cancer. 2009;124(10):2400–5. https://doi.org/10.1002/ijc.24196.
Nakamura K, Nagata C, Wada K, Tamai Y, Tsuji M, Takatsuka N, Shimizu H. Cigarette smoking and other lifestyle factors in relation to the risk of pancreatic cancer death: a prospective cohort study in Japan. Jpn J Clin Oncol. 2011;41(2):225–31. https://doi.org/10.1093/jjco/hyq185.
Evenson KR, Stevens J, Cai J, Thomas R, Thomas O. The effect of cardiorespiratory fitness and obesity on cancer mortality in women and men. Med Sci Sports Exerc. 2003;35(2):270–7. https://doi.org/10.1249/01.MSS.0000053511.02356.72.
Farrell SW, Cortese GM, LaMonte MJ, Blair SN. Cardiorespiratory fitness, different measures of adiposity, and cancer mortality in men. Obesity (Silver Spring). 2007;15(12):3140–9. https://doi.org/10.1038/oby.2007.374.
Farrell SW, Finley CE, McAuley PA, Frierson GM. Cardiorespiratory fitness, different measures of adiposity, and total cancer mortality in women. Obesity (Silver Spring). 2011;19(11):2261–7. https://doi.org/10.1038/oby.2010.345.
Laukkanen JA, Pukkala E, Rauramaa R, Makikallio TH, Toriola AT, Kurl S. Cardiorespiratory fitness, lifestyle factors and cancer risk and mortality in Finnish men. Eur J Cancer. 2010;46(2):355–63. https://doi.org/10.1016/j.ejca.2009.07.013.
Sawada SS, Muto T, Tanaka H, Lee IM, Paffenbarger RS Jr, Shindo M, Blair SN. Cardiorespiratory fitness and cancer mortality in Japanese men: a prospective study. Med Sci Sports Exerc. 2003;35(9):1546–50. https://doi.org/10.1249/01.MSS.0000084525.06473.8E.
Kim Y, White T, Wijndaele K, Westgate K, Sharp SJ, Helge JW, Wareham NJ, Brage S. The combination of cardiorespiratory fitness and muscle strength, and mortality risk. Eur J Epidemiol. 2018;33(10):953–64. https://doi.org/10.1007/s10654-018-0384-x.
Pletnikoff PP, Laukkanen JA, Tuomainen TP, Kurl S. The joint impact of prediagnostic inflammatory markers and cardiorespiratory fitness on the risk of cancer mortality. Scand J Med Sci Sports. 2018;28(2):613–20. https://doi.org/10.1111/sms.12952.
Vainshelboim B, Lima RM, Shuval K, Pettee Gabriel K, Myers J. Pre-cancer diagnosis cardiorespiratory fitness, physical activity and cancer mortality in men. J Sports Med Phys Fitness. 2018. https://doi.org/10.23736/S0022-4707.18.08989-2.
Wang Y, Chen S, Zhang J, Zhang Y, Ernstsen L, Lavie CJ, Hooker SP, Chen Y, Sui X. Nonexercise estimated cardiorespiratory fitness and all-cancer mortality: the NHANES III Study. Mayo Clin Proc. 2018;93(7):848–56. https://doi.org/10.1016/j.mayocp.2018.01.004.
Vainshelboim B, Chen Z, Lee YN, Sorayya A, Kokkinos P, Nead KT, Chester C, Myers J (2017) Cardiorespiratory fitness, adiposity, and cancer mortality in men. Obesity (Silver Spring). 25 Suppl 2:S66–71. https://doi.org/10.1002/oby.22009.
Lakoski SG, Willis BL, Barlow CE, Leonard D, Gao A, Radford NB, Farrell SW, Douglas PS, Berry JD, DeFina LF, Jones LW. Midlife cardiorespiratory fitness, incident cancer, and survival after cancer in men: the Cooper Center Longitudinal Study. JAMA Oncol. 2015;1(2):231–7. https://doi.org/10.1001/jamaoncol.2015.0226.
Sawada SS, Lee IM, Naito H, Kakigi R, Goto S, Kanazawa M, Okamoto T, Tsukamoto K, Muto T, Tanaka H, Blair SN. Cardiorespiratory fitness, body mass index, and cancer mortality: a cohort study of Japanese men. BMC Public Health. 2014;14:1012. https://doi.org/10.1186/1471-2458-14-1012.
Lee CD, Blair SN. Cardiorespiratory fitness and smoking-related and total cancer mortality in men. Med Sci Sports Exerc. 2002;34(5):735–9.
Jensen MT, Holtermann A, Bay H, Gyntelberg F. Cardiorespiratory fitness and death from cancer: a 42-year follow-up from the Copenhagen Male Study. Br J Sports Med. 2017;51(18):1364–9. https://doi.org/10.1136/bjsports-2016-096860.
Sui X, Lee DC, Matthews CE, Adams SA, Hebert JR, Church TS, Lee CD, Blair SN. Influence of cardiorespiratory fitness on lung cancer mortality. Med Sci Sports Exerc. 2010;42(5):872–8. https://doi.org/10.1249/MSS.0b013e3181c47b65.
Peel JB, Sui X, Matthews CE, Adams SA, Hebert JR, Hardin JW, Church TS, Blair SN. Cardiorespiratory fitness and digestive cancer mortality: findings from the aerobics center longitudinal study. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1111–7. https://doi.org/10.1158/1055-9965.EPI-08-0846.
Peel JB, Sui X, Adams SA, Hebert JR, Hardin JW, Blair SN. A prospective study of cardiorespiratory fitness and breast cancer mortality. Med Sci Sports Exerc. 2009;41(4):742–8. https://doi.org/10.1249/MSS.0b013e31818edac7.
Thompson AM, Church TS, Janssen I, Katzmarzyk PT, Earnest CP, Blair SN. Cardiorespiratory fitness as a predictor of cancer mortality among men with pre-diabetes and diabetes. Diabetes Care. 2008;31(4):764–9. https://doi.org/10.2337/dc07-1648.
Slattery ML, Sweeney C, Edwards S, Herrick J, Murtaugh M, Baumgartner K, Guiliano A, Byers T. Physical activity patterns and obesity in Hispanic and non-Hispanic white women. Med Sci Sports Exerc. 2006;38(1):33–41.
Chen WY, Colditz GA. Risk factors and hormone-receptor status: epidemiology, risk-prediction models and treatment implications for breast cancer. Nat Clin Pract Oncol. 2007;4(7):415–23. https://doi.org/10.1038/ncponc0851.
Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman JA, Sparano JA, Hunsberger S, Enos RA, Gelber RD, Zujewski JA. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(15):2127–32. https://doi.org/10.1200/JCO.2006.10.3523.
Karvinen K, Bruner B, Truant T. The teachable moment after cancer diagnosis: perceptions from oncology nurses. Oncol Nurs Forum. 2015;42(6):602–9. https://doi.org/10.1188/15.ONF.602-609.
Volaklis KA, Halle M, Meisinger C. Muscular strength as a strong predictor of mortality: a narrative review. Eur J Intern Med. 2015;26(5):303–10. https://doi.org/10.1016/j.ejim.2015.04.013.
Lee DC, Artero EG, Sui X, Blair SN. Mortality trends in the general population: the importance of cardiorespiratory fitness. J Psychopharmacol. 2010;24(4 Suppl):27–35. https://doi.org/10.1177/1359786810382057.
Organization WH. Global recommendations on physical activity for health. Geneva: World Health Organization; 2010.
Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–31.
Schmid D, Leitzmann MF. Cardiorespiratory fitness as predictor of cancer mortality: a systematic review and meta-analysis. Ann Oncol. 2015;26(2):272–8. https://doi.org/10.1093/annonc/mdu250.
Courneya KS, Friedenreich CM, Franco-Villalobos C, Crawford JJ, Chua N, Basi S, Norris MK, Reiman T. Effects of supervised exercise on progression-free survival in lymphoma patients: an exploratory follow-up of the HELP trial. Cancer Causes Control. 2015;26(2):269–76. https://doi.org/10.1007/s10552-014-0508-x.
Rief H, Bruckner T, Schlampp I, Bostel T, Welzel T, Debus J, Forster R. Resistance training concomitant to radiotherapy of spinal bone metastases – survival and prognostic factors of a randomized trial. Radiat Oncol (London, England). 2016;11:97. https://doi.org/10.1186/s13014-016-0675-x.
Courneya KS, Vallance JK, Culos-Reed SN, McNeely ML, Bell GJ, Mackey JR, Yasui Y, Yuan Y, Matthews CE, Lau DC, Cook D, Friedenreich CM. The Alberta moving beyond breast cancer (AMBER) cohort study: a prospective study of physical activity and health-related fitness in breast cancer survivors. BMC Cancer. 2012;12:525. https://doi.org/10.1186/1471-2407-12-525.
Wiskemann J, Kuehl R, Dreger P, Huber G, Kleindienst N, Ulrich CM, Bohus M. Physical Exercise Training versus Relaxation in Allogeneic stem cell transplantation (PETRA study) – rationale and design of a randomized trial to evaluate a yearlong exercise intervention on overall survival and side-effects after allogeneic stem cell transplantation. BMC Cancer. 2015;15:619. https://doi.org/10.1186/s12885-015-1631-0.
Hayes S, Friedlander M, Obermair A, Mileshkin L, Janda M, Gordon L, Barnes E, Beesley V, Eakin E, Sommeijer D, Martyn J, Stockler M, Gebski VAL, Naumann F, Schmitz K, Webb P. Exercise during chemotherapy for ovarian cancer (ECHO): study design features and outcomes of a cancer Australia and cancer council Australia funded randomised, controlled trial. Int J Gynecol Cancer. 2014;4:200–1.
Newton RU, Kenfield SA, Hart NH, Chan JM, Courneya KS, Catto J, Finn SP, Greenwood R, Hughes DC, Mucci L, Plymate SR, Praet SFE, Guinan EM, Van Blarigan EL, Casey O, Buzza M, Gledhill S, Zhang L, Galvao DA, Ryan CJ, Saad F. Intense Exercise for Survival among Men with Metastatic Castrate-Resistant Prostate Cancer (INTERVAL-GAP4): a multicentre, randomised, controlled phase III study protocol. BMJ Open. 2018;8(5):e022899. https://doi.org/10.1136/bmjopen-2018-022899.
Courneya KS, Booth CM, Gill S, O’Brien P, Vardy J, Friedenreich CM, Au HJ, Brundage MD, Tu D, Dhillon H, Meyer RM. The Colon Health and Life-Long Exercise Change trial: a randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr Oncol. 2008;15(6):279–85.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Friedenreich, C.M., Stone, C.R., Hayes, S.C. (2020). Physical Activity and Cancer Survival. In: Schmitz, K. (eds) Exercise Oncology. Springer, Cham. https://doi.org/10.1007/978-3-030-42011-6_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-42011-6_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-42010-9
Online ISBN: 978-3-030-42011-6
eBook Packages: MedicineMedicine (R0)