Abstract
Objective
Existing information regarding the impact of physical activity after a cancer diagnosis on all-cause mortality is limited. We examined the association between different types of physical activity (domestic, walking, sports) and mortality in 293 participants (65.5% women) with a cancer registration prior to the baseline assessment.
Methods
Participants were drawn from the Scottish Health Surveys (1995, 1998, 2003) that were linked to a national database of cancer registrations and deaths. The main outcome was all-cause mortality during a mean follow-up period of 5.9 ± 3.2 years. Cox proportional hazards models were used to estimate the risk of all-cause mortality by levels of physical activity.
Results
There were 78 deaths during follow-up. The lowest risks for all-cause mortality were seen in sports activity groups [multivariable-adjusted hazard ratio (HR) for any compared with groups of no sports: 0.47, 95% CI 0.23–0.96, p = 0.039] although light and moderate activity such as domestic activity (HR = 1.04, 0.60–1.80) and regular walking (HR = 0.95, 0.57–1.56) did not confer protection.
Conclusion
Participation in an average of more than three sessions of vigorous exercise per week for at least 20 min/session was associated with the lowest risks of all-cause mortality following a cancer diagnosis. Vigorous physical activity could therefore be a more important determinant of survival than duration or total volume of exercise in cancer survivors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Continued advances in early-detection and effective treatments have resulted in the hope for a longer survival and even cure for many cancer patients. Despite an increase in UK cancer incidence in the 10-year period between 1994 and 2003, overall mortality from cancer decreased [1]. Overall, 2.1% of the general population of men and 2.9% of women in Scotland are recovering from curative cancer treatment, and for patients diagnosed during 2001–2004, 42% of male and 51% of female patients survived to five years after diagnosis relative to the life expectancy of the general population [2]. With the increasing aging population, the number of elderly cancer survivors is expected to double over the next 50 years [3], which means that disease-free survival is becoming an increasingly important issue in the management of cancer patients.
Studies have shown that physical activity is associated with higher levels of physical functioning and cardiorespiratory fitness, reduced feelings of fatigue, and improved health-related quality of life in cancer patients [3–6]. Hence, a physically active lifestyle is now considered an important factor for promoting overall health, quality of life, and longevity during recovery from cancer treatment [7]. Cancer survivors are encouraged to engage in at least 30 min of moderate-to-vigorous physical activity, and preferably 45–60 min of intentional physical activity (above usual activities), for five or more days per week [7]. These recommendations, however, are largely based on guidelines for cancer prevention and are similar to UK physical activity recommendations for the primary prevention of the chronic disease [8]. Evidence that they are relevant to disease-free survival and mortality in cancer survivors is very limited.
Currently, the amount of physical activity required to promote improved survival after cancer treatment is unclear. Recently published studies of breast [9, 10] and colorectal cancer [11, 12] patients show that physical activity after a cancer diagnosis can reduce the risk of cancer-specific and all-cause mortality. A further study of colorectal cancer patients showed that low physical activity levels before diagnosis were associated with poorer overall and disease-specific survival [13]. In these studies, the volume and intensity of physical activity that was associated with decreased risk of mortality were quite different. Thus, there is a conflicting evidence regarding the optimal exercise prescription for promoting improved survival after a cancer diagnosis.
The aim of the present study was to examine the association between different types of physical activity (domestic, walking, sports) and mortality in men and women after a cancer diagnosis over 5.9 years of follow-up. We hypothesized that vigorous sporting activity would provide the greatest protection against mortality risk.
Materials and methods
Participants
The Scottish Health Survey (SHS) is a periodic survey (typically every 3–5 years) that draws a nationally representative sample of the general population living in households. The sample was drawn using multistage stratified probability sampling with postcode sectors selected at the first stage and household addresses selected at the second stage. Different samples were drawn for each survey. The present analyses combined data from the 1995, 1998, and 2003 SHS. Participants gave full informed consent to participate in the study, and ethical approval was obtained from the London Research Ethics Council.
Baseline assessment
Survey interviewers visited eligible households and collected data on demographics, personal characteristics, and health behaviors. On a separate visit, nurses collected information on medical history (doctor-diagnosed conditions), and took anthropometric variables. Detailed information on the survey method can be found elsewhere [14].
Physical activity
Physical activity interviews inquired about participation in the four weeks prior to the interview (1998 and 2003) or during a typical week (1995). Frequency of participation (for at least 20 min/occasion) was assessed across three domains of activity: leisure time sports (e.g., cycling, swimming, running, aerobics, dancing, and ball sports such as football and tennis), walking for any purpose, and domestic physical activity (e.g., heavy housework, home improvement activities, manual and gardening work). The criterion validity of these questions is supported by the results of a recent study on 106 British adults from the general population (45 men) where the output of accelerometers (worn for two non-consecutive weeks over a month period) was compared against the questionnaire output (unpublished observations, manuscript under preparation). The questionnaire appeared to be a valid measure of time spent in moderate-to-vigorous physical activity; correlation coefficients were 0.47 in men (p = 0.03) and 0.43 in women (p = 0.02). In terms of test–retest reliability, the coefficients of time spent in moderate-to-vigorous physical activity were 0.89 for men (p < 0.001) 0.76 in women (p < 0.001).
Cancer diagnosis and outcome
The surveys were linked to the Scottish Cancer Registry (Information Services Division [ISD] Scotland [2]) that receives notification of cancer from hospital systems, including discharges, radiotherapy, oncology, hematology and pathology records, and prospective audit datasets. The ISD database has demonstrated high levels of accuracy and completeness when samples of computerized records from the Scottish national database are compared with the original patient casenotes [2]. Information on deaths between baseline measurement and September 2006 was ascertained from the General Registrar Office for Scotland. Classification of the underlying cause on the death is based on information collected on the medical certificate of cause of death together with any additional information provided subsequently by the certifying doctor. The present analyses consisted of 293 participants (65.5% women, aged 64.1 ± 10.5 years) with a cancer registration before the baseline examination. The mean time since cancer diagnosis was 4.9 ± 1.5 years. Among these participants, 47.8% were diagnosed with breast cancer, 21.2% with bowel cancer, 12.6% with bladder cancer, and the rest with cancer of the lung/trachea (3.8%), prostate (9.2%), stomach (1.7%), and ovaries (3.8%).
Statistical analysis
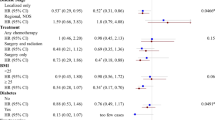
Cox proportional hazards models were used with months as the timescale to estimate the risk of all-cause mortality by levels of physical activity. The participants who survived the data were censored to September 2006. Physical activity variables were frequency-based and were expressed as number of weekly episodes lasting for at least 20 continuous minutes. Participation in different types of physical activity was converted into two categories (none versus any reported activity). We also devised a combined recreational activity group consisting of walking and sports that was categorized into three groups (<1, 1–3, >3/week). The proportional hazards assumption was examined by comparing the cumulative hazard plots grouped on exposure, although no appreciable violations were noted. In multivariate models, we adjusted for age, gender, socioeconomic group using the Registrar General Classification (professional/intermediate, skilled nonmanual, skilled manual, part-skilled/unskilled), marital status (single/never married, married, widowed, separated/divorced), body mass index category (underweight <18.5 kg/m2, normal weight 18.5–25.0 kg/m2, overweight 25.01–30.0 kg/m2, obese >30.0 kg/m2), smoking (never, ex-smoker, current smoker), alcohol intake (number of units consumed in the last seven days), doctor-diagnosed diabetes and hypertension, hospital admissions for cardiovascular events, type of cancer diagnosis, number of cancer recurrences, and survey year. We used chi-square tests to examine univariable relationships of the confounders with the exposure and outcome variables. All analyses were performed using SPSS (version 14), and all tests of statistical significance were based on two-sided probability.
Results
Participants reported an average weekly participation rate of 4.7 ± 2.4 sessions of any type of physical activity lasting at least 20 min. In general, walking was the most popular form of activity, with 40% of the sample reporting any walking of at least 20 min in comparison with a 21% participation rate in any sports and exercise, and 32% in any domestic activity. Table 1 presents characteristics of the sample in relation to physical activity behavior. The physically inactive group were older, contained a relatively higher proportion of men, were of lower socioeconomic status, and were more likely to be smokers, although there were no differences in any co-morbidities. Levels of recreational physical activity in the highest activity group equated to approximately three to four sessions of sport and two sessions of walking per week of at least 20 min in duration, whereas in the medium activity group walking was the predominant activity with no sports (see Table 1).
There were a total of 78 deaths (49 from neoplasms and 11 from cardiovascular causes) over an average follow-up period of 5.9 ± 3.2 years. There was an inverse association between recreational physical activity and all-cause mortality and adjustments for covariates strengthened these associations (see Table 2). In particular, greater than 3 sessions/week of recreational activity was associated with a 52% reduction in risk of death. When we examined specific activities, in models mutually adjusted for activity type, there was no association between all-cause mortality and domestic activity or walking when comparing any activity with none (referent group). In further analysis, we also categorized these activities into tertiles, although no associations were evident in the highest exposure groups. There was, however, a 53% reduction in the risk of death associated with participating in any sports activity after adjustment for potential confounders.
Given that lower physical activity levels among cancer survivors might reflect occult cancer recurrence or impending death, we excluded eight deaths that had occurred in the first year of follow-up, although this did not appreciably alter the results for sports participation (HR = 0.49, 95% CI 0.24–1.00, p = 0.053). In addition, we explored the possibility that lower physical activity might be related to recent cancer treatment. When we excluded participants who had a cancer diagnosis within 12 months prior to the baseline assessment (n = 54, deaths = 19), the association between sports participation and all-cause mortality was attenuated (fully adjusted HR = 0.62, 0.29–1.31), although the point estimate was less reliable, which possibly reflected a reduction in statistical power. However, there was no difference in physical activity levels between participants with a cancer diagnosis less than and greater than one year prior to baseline, thus partly excluding the possibility of reverse causality. Approximately 50% of the participants had their first cancer diagnosis more than five years prior to baseline. Because many cancers have the highest risk of recurrence within the first five years, we also examined whether there were any notable differences in physical activity or other baseline characteristics between participants diagnosed with cancer less than and greater than five years prior to baseline, but there was none. Cancer recurrence was, however, a significant independent predictor of mortality (HR = 2.09, 1.26–3.45).
Discussion
We have demonstrated an inverse association between physical activity and mortality in cancer survivors followed up over an average of 5.9 years. This association was largely explained by engaging in vigorous activities such as sports and not by light and moderate activities that included walking and domestic activity. This is an important finding and suggests that the current minimum physical activity recommendations for cancer survivors maybe inappropriate for reducing the risk of all-cause mortality.
A limited number of prospective studies have investigated the association between physical activity participation and all-cause mortality in cancer survivors. Some of these studies have quantified physical activity in terms of a composite measure of duration and intensity (MET-h week−1), but have not independently examined associations with vigorous physical activity. In breast cancer survivors, the Nurses Health Study reported optimal risk reduction for cancer-specific and all-cause mortality in the intermediate physical activity category (equivalent of walking 3–5 h/week at an average pace), although when walking and vigorous exercise were compared, lower risks were observed in women who undertake any vigorous exercise [9]. In contrast, Holick et al. [10] reported a dose–response association between total recreational physical activity and mortality in breast cancer survivors but there was no association for vigorous physical activity. In stage I–III colon cancer patients, the equivalent of 6 h of moderate intensity physical activity was associated with a 49–57% risk reduction for mortality [11, 12]. Evidence from two other large cohort studies [15, 16] shows that participation in the equivalent of 6–8 h/week of moderate physical activity or 4–5 h of vigorous physical activity is associated with a 31–67% reduction in the risk of advanced or aggressive prostate cancer, suggesting that this amount of physical activity is necessary to slow disease progression and have a tangible impact on prostate cancer mortality. Considered together, evidence from these studies suggests that current recommendations for minimal levels of physical activity in cancer survivors (30 min of moderate intensity physical activity on five or more days per week) [7] may be insufficient for reducing the risk of all-cause mortality in some cancer groups. Furthermore, our data suggest that at least three weekly bouts of vigorous recreational activity are required for improved survival.
Although body mass index was similar across physical activity tertiles and did not independently predict mortality, physical activity might have influenced all-cause mortality through its effects on insulin sensitivity or body composition, particularly central adiposity, and the impact this can have on putative risk factors for cancer, including circulating levels of sex steroid hormones, adipokines, and insulin-like growth factors [17, 18]. Being overweight at the time of diagnosis and weight gain after diagnosis has been linked with poorer outcome in breast, colorectal, and prostate cancer patients [13, 19–22]. The amount of estrogen produced via aromatase activity is larger in obese than the leaner postmenopausal women [23] and may explain the increased risk of breast cancer recurrence in overweight postmenopausal women. Elevated levels of adipokines such as leptin, interleukin-6 and tumor necrosis factor-alpha, and the inflammatory mediator C-reactive protein, as well as reduced levels of adiponectin are also associated with adiposity [17, 24] and can be modified by weight-loss interventions involving physical activity [25].
Participation in vigorous sports activities may have also influenced cardiovascular mortality in our patient cohort, which accounted for 14% of deaths. Physical activity evokes improvements in cardiovascular fitness in cancer patients and survivors [6], and higher intensity exercise is associated with greater risk reduction for coronary heart disease (CHD) in middle-aged men and postmenopausal women [26, 27]. Vigorous exercise which promotes improvements in CHD risk profile could be particularly important in patients receiving androgen deprivation therapy for localized prostate cancer, as this treatment has been associated with increased risk of cardiovascular mortality [28]. Other possible mechanisms underpinning the observed inverse association between physical activity and all-cause mortality include improvements in the functioning of immune cells [29], an increased antioxidant defense capacity [30], and exercise-induced alterations in gene function or apoptosis [31, 32]. Although lines of evidence exist for all these putative mechanisms, prospective intervention studies are needed to more firmly establish biological plausibility for this association and to provide the evidence of causality needed to inform optimal exercise prescription.
Strengths and weaknesses
This is the first study, to our knowledge, that has assessed the impact of physical activity on all-cause mortality following a cancer diagnosis in a representative sample from the UK. Physical activity data for the SHS have been validated using accelerometry, which overcomes some of the limitations of self-reported physical activity, and high levels of accuracy and completeness have been demonstrated for the ISD database. In addition, we were able to demonstrate the independent effects of sports participation (a surrogate measure of vigorous exercise) on the association between physical activity and all-cause mortality, which has not always been considered in previous cohort studies.
A number of study limitations should be considered. The present study was not sufficiently empowered to examine causes of death and specific cancer sites; thus we are unable to comment on the association between physical activity and cancer mortality. The design of our study did not permit us to collect physical activity data before cancer diagnosis or during follow-up; thus we cannot exclude the possibility that changes in physical activity behavior over time could have influenced our results. Given that lower levels of physical activity among cancer survivors could reflect occult cancer recurrence, impending death, or side-effects from cancer treatment, we cannot discount reverse causality. We did, however, attempt to examine these potential biases by performing various sensitivity analyses. The exclusion of deaths in the first year of follow-up did not appreciably alter the results. When we removed participants with a cancer diagnosis in the first year prior to baseline, the association between sports participation and risk of death was slightly attenuated, albeit with a reduced statistical power. However, we found no evidence for reduced levels of physical activity in participants with a recent cancer diagnosis, thus partly refuting the possibility of reverse causality.
Future research and conclusions
Our analysis is consistent with other recent reports showing an inverse association between physical activity and all-cause mortality after a cancer diagnosis, although further studies with larger sample sizes are required to replicate and extend our findings. In addition, well-controlled intervention studies are now needed to provide more robust data on the frequency, intensity, duration, and type of physical activity which confers the greatest risk reduction in relation to disease-free survival and mortality in patients recovering from different cancer treatments. A number of primary prevention trials are investigating the biological mechanisms which could underpin the observed inverse associations between physical activity and cancer risk [33]. Similar trials in cancer survivors would help to further elucidate biological mechanisms which underpin the observed associations between physical activity and all-cause mortality following a cancer diagnosis and treatment.
In summary, our data show that cancer survivors who participated on average in more than three sessions of vigorous exercise per week for at least 20 min/session demonstrated the lowest risks of all-cause mortality. This suggests that vigorous physical activity could be a more important determinant of survival than duration or total volume of exercise in cancer survivors.
References
Cancer Research UK. UK Cancer incidence statistics. http://info.cancerresearchuk.org/cancerstats. Accessed Feb 2008
Information Services Division. Cancer in Scotland (2007) NHS National Services Scotland. http://www.isdscotland.org/isd/5323.html. Accessed Feb 2008
Yancik R, Ries LA (2000) Aging and cancer in America. Demographic and epidemiologic perspectives. Hematol Oncol Clin North Am 14:17–23. doi:10.1016/S0889-8588(05)70275-6
Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G (2005) Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. J Clin Oncol 23:3830–3842. doi:10.1200/JCO.2005.02.148
Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R (2005) Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 14:1588–1595. doi:10.1158/1055-9965.EPI-04-0703
McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS (2006) Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ 175:34–41. doi:10.1503/cmaj.051073
Doyle C, Kushi LH, Byers T, Courneya KS, Demark-Wahnefried W, Grant B, McTiernan A, Rock CL, Thompson C, Gansler T, Andrews KS, The 2006 Nutrition, Physical Activity and Cancer Survivorship Advisory Committee; American Cancer Society (2006) Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin 56:323–353
Department of Health (2004) At least five a week: evidence on the impact of physical activity and its relationship to health. A report from the Chief Medical Officer
Holmes MD, Chen WY, Feskanich D et al (2005) Physical activity and survival after breast cancer diagnosis. JAMA 293:2479–2486. doi:10.1001/jama.293.20.2479
Holick CN, Newcomb PA, Trentham-Dietz A, Titus-Ernstoff L, Bersch AJ, Stampfer MJ, Baron JA, Egan KM, Willett WC (2008) Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev 17:379–386. doi:10.1158/1055-9965.EPI-07-0771
Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Thomas J, Nelson H, Whittom R, Hantel A, Schilsky RL, Fuchs CS (2006) Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol 24:3535–3541. doi:10.1200/JCO.2006.06.0863
Meyerhardt JA, Giovannucci EL, Holmes MD et al (2006) Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol 24:3527–3534. doi:10.1200/JCO.2006.06.0855
Haydon AM, Macinnis RJ, English DR, Giles GG (2006) Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut 55:62–67. doi:10.1136/gut.2005.068189
The Scottish Government Statistics. Scottish Health Survey Publications. http://www.scotland.gov.uk/Topics/Statistics/Browse/Health/scottish-healthsurvey/Publications. Accessed Nov 2007
Giovannucci EL, Liu Y, Leitzmann MF, Stampfer MJ, Willett WC (2005) A prospective study of physical activity and incident and fatal prostate cancer. Arch Intern Med 165:1005–1010. doi:10.1001/archinte.165.9.1005
Patel AV, Rodriguez C, Jacobs EJ, Solomon L, Thun MJ, Calle EE (2005) Recreational physical activity and risk of prostate cancer in a large cohort of U.S. men. Cancer Epidemiol Biomarkers Prev 14:275–279. doi:10.1158/1055-9965.EPI-04-0583
Campbell KL, McTiernan A (2007) Exercise and biomarkers for cancer prevention studies. J Nutr 137:161S–169S
Saxton JM (2006) Diet, physical activity and energy balance and their impact on breast and prostate cancers. Nutr Res Rev 19:197–215. doi:10.1017/S095442240720294X
Kroenke CH, Chen WY, Rosner B, Holmes MD (2005) Weight, weight gain and survival after breast cancer. J Clin Oncol 23:1370–1378. doi:10.1200/JCO.2005.01.079
Bastarrachea J, Hortobagyi GN, Smith TL, Kau SW, Buzdar AU (1994) Obesity as an adverse prognostic factor for patients receiving adjuvant chemotherapy for breast cancer. Ann Intern Med 120:18–25
Obermair A, Kurz C, Hanzal E et al (1995) The influence of obesity on the disease-free survival in primary breast cancer. Anticancer Res 15:2265–2269
Bassett WW, Cooperberg MR, Sadetsky N, Silva S, DuChane J, Pasta DJ, Chan JM, Anast JW, Carroll PR, Kane CJ (2005) Impact of obesity on prostate cancer recurrence after radical prostatectomy: data from CaPSURE. Urology 66:1060–1065. doi:10.1016/j.urology.2005.05.040
McTiernan A, Rajan KB, Tworoger SS, Irwin M, Bernstein L, Baumgartner R, Gilliland F, Stanczyk FZ, Yasui Y, Ballard-Barbash R (2003) Adiposity and sex hormones in postmenopausal breast cancer survivors. J Clin Oncol 21:1961–1966. doi:10.1200/JCO.2003.07.057
Visser A, Bouter LM, McQuillan GM, Wener MH, Harris TB (1999) Elevated C-reactive protein levels in overweight and obese adults. JAMA 282:2131–2135. doi:10.1001/jama.282.22.2131
Hamer M (2007) The relative influences of fitness and fatness on inflammatory factors. Prev Med 44:3–11. doi:10.1016/j.ypmed.2006.09.005
Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Siscovick DS (2002) Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med 347:716–725. doi:10.1056/NEJMoa021067
Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB (2002) Exercise type and intensity in relation to coronary heart disease in men. JAMA 288:1994–2000. doi:10.1001/jama.288.16.1994
Tsai HK, D’Amico AV, Sadetsky N, Chen MH, Carroll PR (2007) Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst 99:1516–1524. doi:10.1093/jnci/djm168
Hutnick NA, Williams NI, Kraemer WJ, Orsega-Smith E, Dixon RH, Bleznak AD, Mastro AM (2005) Exercise and lymphocyte activation following chemotherapy for breast cancer. Med Sci Sports Exerc 37:1827–1835. doi:10.1249/01.mss.0000175857.84936.1a
Meijer EP, Goris AH, van Dongen JL, Bast A, Westerterp KR (2002) Exercise-induced oxidative stress in older adults as a function of habitual activity level. J Am Geriatr Soc 50:349–353. doi:10.1046/j.1532-5415.2002.50069.x
Campbell KL, McTiernan A, Li SS, Sorensen BE, Yasui Y, Lampe JW, King IB, Ulrich CM, Rudolph RE, Irwin ML, Surawicz C, Ayub K, Potter JD, Lampe PD (2007) Effect of a 12-month exercise intervention on the apoptotic regulating proteins Bax and Bcl-2 in colon crypts: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev 16:1767–1774. doi:10.1158/1055-9965.EPI-07-0291
Leung PS, Aronson WJ, Ngo TH, Golding LA, Barnard RJ (2004) Exercise alters the IGF axis in vivo and increases p53 protein in prostate tumor cells in vitro. J Appl Physiol 96:450–454. doi:10.1152/japplphysiol.00871.2003
McTiernan A (2008) Mechanisms linking physical activity with cancer. Nat Rev Cancer 8:205–211. doi:10.1038/nrc2325
Acknowledgments
The authors received grant funding from the British Heart Foundation, UK (MH), and the National Institute for Health Research, UK (ES). The Scottish Health Survey is funded by the Scottish Executive. The views expressed in this article are those of the authors and not necessarily of the funding bodies. We declare that the funders played no role in the concept and design of the study, analysis or interpretation of data, or drafting and critical revision of the manuscript.
Contributors
All authors were responsible for the study concept and design, interpretation of data, and drafting and critical revision of the manuscript. ES was responsible for the acquisition of the data. Statistical analysis and preparation of the data was performed by MH and ES. All authors approved the final version of the article. MH is the guarantor.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamer, M., Stamatakis, E. & Saxton, J.M. The impact of physical activity on all-cause mortality in men and women after a cancer diagnosis. Cancer Causes Control 20, 225–231 (2009). https://doi.org/10.1007/s10552-008-9237-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-008-9237-3