Abstract
Purpose
Higher levels of physical activity have been associated with improved survival after breast cancer diagnosis. However, no previous studies have considered the influence of the social and built environment on physical activity and survival among breast cancer patients.
Methods
Our study included 4,345 women diagnosed with breast cancer (1995–2008) from two population-based studies conducted in the San Francisco Bay Area. We examined questionnaire-based moderate/strenuous recreational physical activity during the 3 years before diagnosis. Neighborhood characteristics were based on data from the 2000 US Census, business listings, parks, farmers’ markets, and Department of Transportation. Survival was evaluated using multivariable Cox proportional hazards models, with follow-up through 2009.
Results
Women residing in neighborhoods with no fast-food restaurants (vs. fewer fast-food restaurants) to other restaurants, high traffic density, and a high percentage of foreign-born residents were less likely to meet physical activity recommendations set by the American Cancer Society. Women who were not recreationally physically active had a 22 % higher risk of death from any cause than women that were the most active. Poorer overall survival was associated with lower neighborhood socioeconomic status (SES) (p trend = 0.02), whereas better breast cancer-specific survival was associated with a lack of parks, especially among women in high-SES neighborhoods.
Conclusion
Certain aspects of the neighborhood have independent associations with recreational physical activity among breast cancer patients and their survival. Considering neighborhood factors may aide in the design of more effective, tailored physical activity programs for breast cancer survivors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, breast cancer is the most common cancer and the leading cause of cancer death for women [1]. Although 5-year relative survival after breast cancer is approximately 90 % [2], survivors have been found to be at an increased risk of recurrence, second cancers, and premature death [3–5]. Therefore, it is critical to identify modifiable factors that can reduce morbidities and improve survival for all women after breast cancer diagnosis. Higher levels of physical activity have been associated with a 30 % reduction in mortality risk after breast cancer diagnosis [6], as well as reduced risk of recurrence, and improved quality of life and physical functioning [7]. We previously reported that any (vs. no) recreational activity during the 3 years before breast cancer diagnosis was associated with a 34 % lower risk of death for women with estrogen receptor-positive tumors [8].
Research to date suggests that elements of the social and built environment influence physical activity levels [9–12]. The built environment comprises the man-made, physical attributes of a person’s surroundings, such as spatial configuration of streets, the transportation structure, commuting patterns, availability of health-promoting resources (e.g., parks, farmers’ markets), and the number of walkable destinations. These attributes can provide opportunities and/or barriers for healthful behaviors (e.g., physical activity and diet) that can influence health outcomes [13]. The social environment includes the socioeconomic and demographic aspects of a neighborhood and has been associated with opportunities for education, employment, social support, stress and coping, factors that can shape health behaviors and outcomes [14–17]. To our knowledge, no previous studies have considered the associations between recreational physical activity and survival after breast cancer while accounting for measures of the social and built environment. Identifying the environmental barriers and facilitators to physical activity may help to inform and improve interventions for increasing physical activity levels.
Combining interview data from San Francisco Bay Area breast cancer patients with data on neighborhood characteristics, we examined the relationship between recreational physical activity and measures of the neighborhood environment. Additionally, we examined associations of physical activity and neighborhood environment with survival after breast cancer diagnosis.
Materials and methods
Subjects
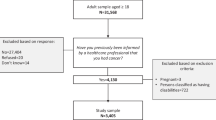
This analysis includes data from two population-based studies that were harmonized and pooled to create the Neighborhoods and Breast Cancer Study (NABC). The two studies include the San Francisco Bay Area Breast Cancer Study (SFBCS), a case–control study of breast cancer in African American, Hispanic, and non-Hispanic white women [18, 19]; and the Northern California site of the Breast Cancer Family Registry (NC-BCFR) [20, 21]. Both studies identified women newly diagnosed with a first primary invasive breast cancer through the Greater Bay Area Cancer Registry and screened cases by telephone to establish study eligibility and self-identified race/ethnicity (participation was 84 % in the SFBCS and 83 % in the NC-BCFR among cases contacted).
In SFBCS, eligible cases were aged 35–79 years who lived in Alameda, Contra Costa, San Mateo, San Francisco, or Santa Clara counties at the time of diagnosis. They included all Hispanics diagnosed between 1 April 1995 and 30 April 2002, all African Americans diagnosed between 1 April 1995 and 30 April 1999, and a random 10 % sample of non-Hispanic whites diagnosed between 1 April 1995 and 30 April 1999. Of 2,571 cases selected into the case–control study, 2,258 (88 %) completed the in-person interview.
In NC-BCFR, eligible cases were aged 18–64 years who lived in Alameda, Contra Costa, Marin, San Mateo, San Francisco, Santa Clara, Santa Cruz, and Monterey counties at the time of diagnosis. They included cases of any race/ethnicity diagnosed from 1 January 1995 to 30 September 1998; Hispanic, African American, and Asian American (Chinese, Filipina, and Japanese) cases diagnosed from 1 October 1998 to 30 April 2002; Hispanic and African American cases diagnosed from 1 May 2002 to 31 December 2008. Cases were enrolled in the NC-BCFR if they had indicators of increased genetic susceptibility [20, 21]. Cases not meeting these criteria were randomly sampled (2.5 % of non-Hispanic whites and 33 % of other racial/ethnic groups). Of 4,708 cases selected into NC-BCFR, 3,631 (77 %) completed the in-person interview.
For cases that participated in both studies (n = 339), we used data from the SFBCS interview. We limited our analytic sample to cases with a first primary invasive breast cancer, who completed the questionnaire themselves within 5 years of diagnosis, had a geocodeable address and had follow-up information from the cancer registry. We excluded cases with Native American or mixed race/ethnicity (n = 10) or unknown physical activity (n = 8). The remaining 4,345 cases were interviewed on average 20.2 months (standard deviation = 8.8 months; range = 0.3–60.0 months) after diagnosis. Study participants provided written informed consent and all protocols were approved by the Institutional Review Board of the Cancer Prevention Institute of California.
Data collection
For both studies, trained interviewers administered similar, structured questionnaires at the participant’s home in English, Spanish, or Chinese. Data were harmonized according to common definitions and included age at diagnosis, race/ethnicity, education, first-degree family history of breast cancer, personal history of benign breast disease, years since last pregnancy, pre-diagnosis oral contraceptive use, pre-diagnosis menopausal hormone therapy use, grams per day of alcohol intake 1 year prior to diagnosis, and body mass index (BMI) 1 year prior to diagnosis.
Assessment of physical activity has been described elsewhere [8, 19]. Briefly, lifetime histories of strenuous and moderate recreational activities were available from both studies and combined for these analyses. SFBCS assessed lifetime recreational physical activity performed at least 1 h per week for at least 4 months per year; for each activity, information was collected on type of activity, the age the activity started and stopped, months per year performed, and hours per week performed. NC-BCFR assessed lifetime histories of moderate (e.g., brisk walking, hiking, cycling on level streets) and strenuous (e.g., swimming laps, aerobics, running, cycling on hills) activities; for each type of activity, information was collected on the average number of hours per week (0.5, 1, 1.5, 2, 3, 4–6, 7–10, ≥11, or do not know) and months per year (1–3, 4–6, 10–12, or don’t know) at specific ages, including during the 3 years before diagnosis. We estimated average recreational physical activity during the 3 years before diagnosis because prior research has shown associations between recent physical activity and survival [22], and this time frame corresponded most closely with the measurement of our neighborhood characteristics. Three physical activity variables were considered. For the first variable, the average hours per week and months per year of moderate and strenuous physical activities during the 3 years before diagnosis were summed and categorized into the following: (1) meeting physical activity recommendations by the American Cancer Society (ACS) [23] (at least 150 min of moderate intensity, 75 min of strenuous intensity, or an equivalent combination of moderate/strenuous activity per week); (2) not meeting the recommendations, but performing some moderate or strenuous physical activity; or (3) performing no moderate or strenuous physical activity. For the second variable, recent moderate and strenuous activities were weighted by metabolic equivalents (MET) (8.5 for strenuous activity and 5.4 for moderate activity) [24] and summed to obtain MET hours per week of activity during the 3 years before diagnosis, as done previously [8]. For the third variable, we considered hours per week of moderate and strenuous activity not weighted by MET.
For each participant, we obtained cancer registry information routinely abstracted from the medical record at diagnosis [25], including tumor histological subtype (ductal, lobular, or mixed/other), histological grade, estrogen receptor (ER) and progesterone receptor (PR) status, AJCC (American Joint Committee on Cancer) stage, time to first and second subsequent tumors, first-course treatment (chemotherapy, radiation, and surgery), marital status and vital status (routinely determined by the cancer registry through hospital follow-up and database linkages) as of 31 December 2009 and, for the deceased, the underlying cause of death.
Geocoding
Residential address at the time of diagnosis was geocoded to a latitude/longitude coordinate and then assigned a 2000 census block group. Addresses were standardized to conform to U.S. Postal Service specifications using ZP4 software (ZP4. Monterey, CA: Semaphore Corp., 2011). Batch geocoding was performed using ArcGIS with both current address point and street geocoding reference files (ArcGIS. Redlands, CA: Environmental Systems Research Institute, Inc., 2011). Manual review was performed to geocode addresses that did not batch geocode, resulting in 97 % of addresses being assigned a latitude and longitude.
Social environment
For neighborhood-level socioeconomic status (SES), we used a previously validated composite SES measure of seven indicator variables at the census block group (education index, median household income, percent living 200 % below poverty level, percent blue-collar workers, percent older than 16 in workforce without job, median rent, median house value) [26]. Neighborhood immigrant population was characterized at the block group level with percentage of foreign-born residents.
Built environment
We derived information on neighborhood amenities including business listings from Walls & Associates’ National Establishment Time-Series Database (which utilizes data from Dunn and Bradstreet) [27], farmers’ markets listings from the California Department of Food and Agriculture [28], and parks from the NavTeq’s NavStreets database [29]. Using ArcGIS software, neighborhood amenities within a 1,600-m network distance [30] from a case’s residence at diagnosis were summed. To do this, we first constrained the search space by selecting all neighborhood amenities within a 1,600-m linear distance of residence, then computed the actual network distance between the residence and every neighborhood amenity. If the network distance was ≤1,600 m, then the neighborhood amenity was included in analyses. We considered businesses active 1 year before diagnosis, during the year of diagnosis, or 2 years after diagnosis. The number of recreational facilities included places where recreational activities could take place (e.g., fitness centers). The Restaurant Environment Index is the ratio of the number of fast-food restaurants to other restaurants, and the Retail Food Environment Index [31] is the ratio of the number of convenience stores, liquor stores, and fast-food restaurants to supermarkets and farmers’ markets. Parks included beaches, recreation areas, and parks. We determined the number of parks with an access point (e.g., vehicular entry point/parking lot or edge of smaller park) within 1,600-m network distance of a participant’s residence.
Neighborhood density was characterized at the census block group level by population density (the number of people/m2) and percentage of total housing units that are not single-family dwellings. Population-based commuting characteristics from the census were summarized by typical travel time to work (% traveling ≥60 min/day). Data on traffic counts from the California Department of Transportation [32] were used to obtain traffic density within a 500-m buffer of each case’s residence, using methods described previously [33].
Statistical analysis
To evaluate the association of personal and neighborhood characteristics with physical activity guidelines [23], we used logistic regression to calculate odds ratios (ORs) and 95 % confidence intervals (CIs). To evaluate the association of physical activity and neighborhood characteristics with overall and breast cancer-specific survival, we used Cox proportional hazards regression to calculate hazard ratios (HRs) and 95 % CI. Base models for both logistic and Cox proportional hazards regression models included age at diagnosis, study, race/ethnicity, stage at diagnosis, and clustering by block group. Neighborhood factors that were associated with survival or physical activity in the base models were included in the fully adjusted model. Logistic models also included potential confounding variables significantly associated with physical activity (education, pre-diagnosis BMI, pre-diagnosis menopausal hormone therapy use, pre-diagnosis alcohol intake). In the fully adjusted Cox models, the confounding variables included personal factors (marital status, education, history of benign breast disease, years since last full-term pregnancy, pre-diagnosis oral contraceptive use, pre-diagnosis menopausal hormone therapy use, pre-diagnosis alcohol intake, pre-diagnosis BMI), tumor characteristics, and treatment. Correlation among the neighborhood variables was assessed, and only uncorrelated neighborhood factors (<0.50 Spearman correlation coefficient) that were significantly associated with the outcomes in univariate models were included in the multivariate models. There were insufficient numbers of cases within each neighborhood unit (block group) to warrant multilevel modeling (55 % of block groups had one case and 80 % of block groups had two or fewer cases).
For deceased cases, survival time was measured in days from the date of interview to the date of death of any cause for overall survival and to the date of death from breast cancer for breast cancer-specific survival. For breast cancer-specific survival, patients who died from other causes were censored at the time of death. Patients alive at the study end date (31 December 2009) were censored at this date or at date of last follow-up (i.e., last known contact). The proportional hazards assumption was tested for physical activity and neighborhood variables using significance tests of interactions with the timescale, and visual examination of scaled Schoenfeld residual plots; there was no evidence that these variables violated the assumption of proportional hazards.
We performed stratified analyses according to age at diagnosis (<50 or ≥50 years), BMI (<25.0, 25.0–29.9, ≥30 kg/m2), ER status (ER+, ER−, unknown), and race/ethnicity. Tests for heterogeneity across strata were conducted using likelihood ratio tests comparing models with and without an interaction term between physical activity and the stratified variable; no significant interactions were found (data not shown). Tests for trend were used to evaluate associations between survival and increasing physical activity and ordinal categories of neighborhood characteristics. p values <0.05 were considered statistically significant, and all tests of significance were two-sided. Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
Results
The majority of breast cancer cases were between 40 and 64 years of age, Hispanic, college educated, and had a normal pre-diagnostic BMI (<25 kg/m2; Table 1). Sixty-six percent of the cases reported moderate or strenuous recreational physical activity during the 3 years prior to diagnosis, with 38 % reporting some recreational strenuous physical activity and 45 % meeting the physical activity recommendations set by the ACS. More than half of the cases lived in high-SES (quintiles 4 and 5) or more densely populated neighborhoods (quartiles 3 and 4), similar to the underlying population of incident breast cancer cases in this region (data not shown). The majority of women lived in neighborhoods where there were few convenience or liquor stores, and fast-food restaurants in comparison with supermarkets and farmers’ markets, as well as few fast-food restaurants in comparison with other types of restaurants. Most women had at least one park within a 1-mile (1.6 km) walking distance to their residence at diagnosis.
Recent recreational physical activity
In the fully adjusted model, not meeting recreational activity recommendations was associated with diagnosis with AJCC stage IV disease, Hispanic and Asian American race/ethnicity, lower education, being obese, and residence in neighborhoods with a high percentage of foreign-born residents, no fast-food restaurants (vs. fewer fast-food restaurants) to other restaurants, and high traffic density (Table 2). There was also borderline significant evidence that meeting recreational physical activity recommendations was associated with being overweight and residing in neighborhoods without convenience or liquor stores and fast-food restaurants (vs. neighborhoods with all types of retail food environment, including supermarkets and farmer’s markets). Number of parks and recreational facilities were not associated with physical activity levels in the fully adjusted model.
Overall survival
After adjustment for personal, tumor, treatment, and neighborhood characteristics (Table 3), Hispanic and Asian American women had better survival than non-Hispanic white women, whereas African American and non-Hispanic white women had similar overall survival. Women who were not recreationally physically active in the 3 years prior to diagnosis had a 22 % HR 1.22, 95 % CI 0.98–1.52) higher risk of death from any cause than women that were the most active. Results were similar and statistically significant when considering hours per week of moderate or strenuous physical activity not weighted for MET [fully adjusted HR 1.27 (95 % CI 1.02–1.59); comparing women with no physical activity to those with the most activity] and meeting physical activity recommendations [fully adjusted HR 1.22 (95 % CI 1.03–1.44) comparing women with no physical activity to those meeting physical activity recommendations] (data not shown in tables). Poorer overall survival was associated with lower neighborhood SES (p trend = 0.02) and residence in more densely populated neighborhoods, although the trend for population density was not significant in the fully adjusted model. Associations of better overall survival with residence in neighborhoods with no parks were only marginally significant in the fully adjusted models. Additional analyses stratified by neighborhood SES showed that the inverse association was present only among women who resided in high-SES (quintiles 4, 5) neighborhoods [HR 0.68 (95 % CI 0.49–0.96) comparing no parks vs. ≥3 parks], while no association was seen among women in lower-SES (quintiles 1–3) neighborhoods [HR 1.05 (95 % CI 0.67–1.25)] (data not shown in tables).
Breast cancer-specific survival
Although the associations with neighborhood characteristics were generally similar for overall and breast cancer-specific survival, there was no association between recreational physical activity and breast cancer-specific survival (Table 4). Results were similar for hours per week of moderate or strenuous physical activity not weighted by MET [fully adjusted HR 1.04 (95 % CI 0.80–1.37) comparing women with no physical activity to those with the most activity] and meeting physical activity recommendations [fully adjusted HR 1.06 (95 % CI 0.85–1.31) comparing women with no physical activity to those meeting physical activity recommendations] (data not shown in tables). Hispanic women had better survival than non-Hispanic white women. Although the trends were not statistically significant, residence in more densely populated neighborhoods was associated with poorer survival, whereas residence in neighborhoods with more foreign born was associated with better survival. Living in neighborhoods without parks was associated with better breast cancer-specific survival. As with overall survival, the association was only seen among women living in high-SES neighborhoods [HR 0.54 (95 % CI 0.34–0.85) comparing no parks vs. ≥3 parks], while no association was seen among women living in lower-SES neighborhoods [HR 1.28 (95 % CI 0.52–1.21)] (data not shown in tables).
Discussion
In the present study, certain aspects of the neighborhood environment have independent associations with physical activity among breast cancer patients and their survival. Among a racially/ethnically diverse population of women with breast cancer, meeting recreational physical activity recommendations varied by neighborhood factors, including percentage of foreign-born residents, the presence of fast-food restaurants and convenience or liquor stores, and traffic density, as well as race/ethnicity, education level, and BMI, as reported previously [12, 34–37]. After adjustment for neighborhood, personal, tumor, and treatment characteristics, recent recreational physical activity was associated with better overall survival after breast cancer diagnosis. While in fully adjusted multivariable models, physical activity, and neighborhood associations with survival were attenuated, our findings suggest that residing in neighborhoods of lower SES, which are more densely populated, or with more parks may be associated with poorer survival. Given the recognized benefits of physical activity among breast cancer survivors, our results support the importance of considering and understanding the role of specific neighborhood social and built environment factors on physical activity and survival.
Our findings of poorer survival among women residing in neighborhoods with lower SES are consistent with numerous studies [38–40]. However, ours is the first study to suggest that higher population density may be associated with poorer survival. Higher population density has been associated with bladder [41] and lung [42] cancer mortality; both traffic-related air pollution, which has been associated with mortality [43], and noise have been cited as potential contributing factors.
In contrast to our hypothesis that greater numbers of parks would be associated with better survival, we found that women residing in neighborhoods without parks had better survival, but this association was limited to women living in high-SES neighborhoods. We also hypothesized that parks and other recreational facilities would provide opportunities for exercise; however, we did not find that the number of parks and recreational facilities were associated with meeting physical activity recommendations, consistent with some [36, 37], but not all [44, 45] studies. While no prior study has considered an association between parks and survival after breast cancer diagnosis, it is possible that the quality, safety, and type of park, factors we could not measure, are more relevant to physical activity and survival. Additional research into the specific attributes of parks and other, unmeasured neighborhood factors associated with the number of parks is needed to understand this unexpected association with survival.
We also found that women residing in neighborhoods with more foreign-born residents had better breast cancer-specific survival, even after adjusting for race/ethnicity and individual-level nativity of study participants (data not shown). In previous analyses specific to Asian American and Hispanic breast cancer patients, we did not find survival to be associated with living in an ethnic enclave, a composite measure that includes percentage of foreign-born residents [39, 40]. Neighborhoods with a higher percentage of foreign born, however, have been found to have healthier food environments, but worse environments related to physical activity (lower walkability and safety and fewer resources for recreational exercise), suggesting that attributes of these neighborhoods both hinder and facilitate healthy behaviors [46]. This study also found lower weekly physical activity levels among Hispanics, but not Chinese, living in these neighborhoods, consistent with our finding of lower physical activity among those who live in neighborhoods with the highest percentage of foreign-born residents [46]. Therefore, future studies will need to explore specific factors that influence survival in neighborhoods with a high percentage of foreign-born residents.
We also found that higher traffic density was associated with not meeting recreational physical activity recommendations, possibly because higher traffic density may reduce pedestrian safety. Previous studies, however, have not found consistent associations with traffic volume and speed; it is hypothesized that the combination of high traffic volume and speed, factors that are difficult to measure, poses a barrier for physical activity [47]. In terms of retail food environment, women living in neighborhoods without unhealthy retail food outlets (no convenience, liquor stores, and fast food) versus a mix of retail food outlets that include supermarkets and farmers’ markets were more likely to meet physical activity recommendations. On the other hand, living in a neighborhood with only healthier restaurants (no fast-food restaurants) was associated with not meeting physical activity recommendations, a finding counter to our hypothesis. When considering these neighborhoods further, we found that neighborhoods with fewer fast-food restaurants to other restaurants had a much larger number of total restaurants (mean = 21 restaurants) than neighborhoods with no fast-food (mean = 2 restaurants) or neighborhoods with a higher ratio of fast food to other restaurants (mean = 5 restaurants), suggesting that neighborhoods with a large variety of predominately healthy restaurants may promote physical activity. Additionally, as with parks, further research that can incorporate information on the quality of restaurants and supermarkets (i.e., cost, availability of fresh produce) and account for other attributes of neighborhoods associated with these types of food establishments is warranted.
Our finding of recent, pre-diagnosis recreational physical activity being associated with better overall survival is consistent with prior studies [6, 22, 48–51]. A meta-analysis found that pre-diagnosis physical activity reduced all-cause mortality by 18 %, but not breast cancer-specific mortality [22], similar to the findings in our study. We did not find differences by ER status, unlike our previous report that included a subset of the breast cancer cases included in this analysis, [8] or by BMI, as found in a meta-analysis [22]. We also found that the poorer survival often seen in African Americans compared to non-Hispanic whites [38, 52] did not persist after adjustment for personal, tumor, treatment, and neighborhood characteristics. While it was not physical activity or neighborhood characteristics that attenuated the African American/non-Hispanic white survival differences (data not shown), it was beyond the scope of this paper to determine what factors attenuated these differences and will be the focus of future analyses. We also found that Hispanics and Asian Americans generally had better survival than non-Hispanic whites, consistent with an analysis in elderly women that controlled for treatment, screening, co-morbidities, and tumor severity [53].
Several potential limitations need to be considered when interpreting our results. We assessed self-reported, pre-diagnosis recreational physical activity rather than physical activity after diagnosis, which has been found to have stronger associations with survival than physical activity prior to diagnosis [22]. In addition, the time frame of neighborhood data did not correspond exactly to the time frame of the physical activity measurement, but was nevertheless relevant to the survival period. Some data suggest that, for most women, levels of activity after treatment are similar to their pre-diagnostic levels [54, 55], although declines in activity have been noted 10 years after diagnosis [56]. If women in the most active group decreased their physical activity levels over time, then our physical activity findings could have been underestimated. The focus on recreational activity may have introduced some exposure misclassification, given that non-recreational physical activity may be more common in Hispanics and African Americans [19]. In the SFBCS, we found no association of occupational or household-related activity with survival (data not shown). While we cannot rule out misclassification of physical activity levels, either by race/ethnicity or by longer time between diagnosis and study interview, a self-reported lifetime physical activity questionnaire comparable to the one in the SFBCS study [19] was found to be reliable (correlation of 0.72) [57]. A further limitation is that our results may not be generalizable to other geographic regions, although our study population is representative of women diagnosed with breast cancer in the San Francisco Bay Area. We also lacked information on co-morbidities, which could influence all-cause mortality [58, 59].
Although the databases we utilized to determine neighborhood amenities allowed us to feasibly characterize neighborhoods, there is limited information on the quality and validity of these data. In addition, as no prior studies, to our knowledge, have used NavTeq data for parks, we cannot rule out misclassification of the parks data. Similar to most studies on this topic, we did not have longitudinal data to consider residential mobility or information on why individuals selected to live in neighborhoods with certain attributes. We also had no information on perceived built environment (e.g., safety), which is likely to affect the behavior [11]. Lastly, even though we did not find heterogeneity of neighborhood associations by race/ethnicity, women in different racial/ethnic groups may be more likely to engage in certain types of activities, for which particular neighborhood characteristics (e.g., utilitarian walking in neighborhoods with more destinations) may be more relevant.
Despite these limitations, our study is among the first to consider physical activity, a potentially modifiable prognostic factor, and measures of the social and built environment with survival after breast cancer diagnosis. The study’s strengths include the inclusion of large number of racial/ethnically diverse women from two population-based breast cancer studies. The two studies asked about many of the same exposures, and findings did not differ by study (data not shown). Our study utilized individual-level interview data, clinical cancer registry data, and neighborhood data that allowed us to consider types of breast cancer, subgroups of patients, and a number of potential confounding variables. We considered a large number of established and objectively measured elements of the neighborhood social and built environment that were not subject to recall bias. Bias due to differential follow-up was minimized by linking data from both studies to the population-based cancer registry. Further, we adjusted for any survival bias by left-truncating all cases at the time of interview.
In our study, elements of the social and built environment have independent associations with recent recreational physical activity in a diverse cohort of breast cancer patients and their survival. Meeting recreational physical activity recommendations varied by the percentage of foreign-born residents, the presence of fast-food restaurants and convenience or liquor stores, and traffic density in neighborhoods. In addition, residing in lower SES or more densely populated neighborhoods may be associated with poorer survival and residing in neighborhoods with more foreign-born residents and no parks may be associated with better survival. The associations of social and built environment with recent recreational physical activity, which was associated with better overall survival, highlight the importance of considering aspects of the social and built environment in future studies of physical activity and breast cancer survival and in future efforts to design more effective physical activity programs for breast cancer survivors.
References
Stewart BW, Kleihues P (eds) (2003) World cancer report. International Agency for Research on Cancer. IARC Press, Lyon
Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) (2012) SEER cancer statistics review, 1975–2009 (Vintage 2009 populations). National Cancer Institute, Bethesda, MD; posted to the SEER web site, April 2012
Sunga AY, Eberl MM, Oeffinger KC, Hudson MM, Mahoney MC (2005) Care of cancer survivors. Am Fam Physician 71(4):699–706
Stein KD, Syrjala KL, Andrykowski MA (2008) Physical and psychological long-term and late effects of cancer. Cancer 112(11 Suppl):2577–2592
Geiger AM, Thwin SS, Lash TL, Buist DS, Prout MN, Wei F et al (2007) Recurrences and second primary breast cancers in older women with initial early-stage disease. Cancer 109(5):966–974
Patterson RE, Cadmus LA, Emond JA, Pierce JP (2010) Physical activity, diet, adiposity and female breast cancer prognosis: a review of the epidemiologic literature. Maturitas 66(1):5–15. doi:10.1016/j.maturitas.2010.01.004
Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL et al (2012) Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 62(4):243–274. doi:10.3322/caac.21142
Keegan TH, Milne RL, Andrulis IL, Chang ET, Sangaramoorthy M, Phillips KA et al (2010) Past recreational physical activity, body size, and all-cause mortality following breast cancer diagnosis: results from the Breast Cancer Family Registry. Breast Cancer Res Treat 123(2):531–542. doi:10.1007/s10549-010-0774-6
Sallis JF, Floyd MF, Rodriguez DA, Saelens BE (2012) Role of built environments in physical activity, obesity, and cardiovascular disease. Circulation 125(5):729–737. doi:10.1161/CIRCULATIONAHA.110.969022
Saelens BE, Handy SL (2008) Built environment correlates of walking: a review. Med Sci Sports Exerc 40(7 Suppl):S550–S566
Brownson RC, Hoehner CM, Day K, Forsyth A, Sallis JF (2009) Measuring the built environment for physical activity: state of the science. Am J Prev Med 36(4 Suppl):S99 e12–S123 e12. doi:10.1016/j.amepre.2009.01.005
Keegan TH, Hurley S, Goldberg D, Nelson DO, Reynolds P, Bernstein L et al (2012) The association between neighborhood characteristics and body size and physical activity in the California teachers study cohort. Am J Public Health 102(4):689–697. doi:10.2105/AJPH.2011.300150
Northridge ME, Sclar ED, Biswas P (2003) Sorting out the connections between the built environment and health: a conceptual framework for navigating pathways and planning healthy cities. J Urban Health 80(4):556–568. doi:10.1093/jurban/jtg064
Diez Roux AV, Mair C (2010) Neighborhoods and health. Ann NY Acad Sci 1186:125–145. doi:10.1111/j.1749-6632.2009.05333.x
Yen IH, Michael YL, Perdue L (2009) Neighborhood environment in studies of health of older adults: a systematic review. Am J Prev Med 37(5):455–463. doi:10.1016/j.amepre.2009.06.022
Meijer M, Rohl J, Bloomfield K, Grittner U (2012) Do neighborhoods affect individual mortality? A systematic review and meta-analysis of multilevel studies. Soc Sci Med 74(8):1204–1212. doi:10.1016/j.socscimed.2011.11.034
Krieger N (2001) Theories for social epidemiology in the 21st century: an ecosocial perspective. Int J Epidemiol 30(4):668–677
John EM, Phipps AI, Davis A, Koo J (2005) Migration history, acculturation, and breast cancer risk in Hispanic women. Cancer Epidemiol Biomarkers Prev 14(12):2905–2913
John EM, Horn-Ross PL, Koo J (2003) Lifetime physical activity and breast cancer risk in a multiethnic population: the San Francisco Bay Area Breast Cancer Study. Cancer Epidemiol Biomarkers Prev 12(11 Pt 1):1143–1152
John EM, Hopper JL, Beck JC, Knight JA, Neuhausen SL, Senie RT et al (2004) The Breast Cancer Family Registry: an infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res 6(4):R375–R389
John EM, Miron A, Gong G, Phipps AI, Felberg A, Li FP et al (2007) Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA 298(24):2869–2876. doi:10.1001/jama.298.24.2869
Ibrahim EM, Al-Homaidh A (2011) Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol 28(3):753–765. doi:10.1007/s12032-010-9536-x
Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV et al (2012) American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 62(1):30–67. doi:10.3322/caac.20140
Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ et al (2000) Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 32(9 Suppl):S498–S504
California Cancer Registry (2009) Cancer reporting in California: abstracting and coding procedures for hospitals, California Cancer Registry Volume I, Data Standards and Data Dictionary Ninth ed
Yost K, Perkins C, Cohen R, Morris C, Wright W (2001) Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 12(8):703–711
(2008) National Establishment Time-Series (NETS) Database 2009 ed. Walls & Associates, Oakland
Irwin ML, Aiello EJ, McTiernan A, Baumgartner RN, Baumgartner KB, Bernstein L et al (2006) Pre-diagnosis physical activity and mammographic density in breast cancer survivors. Breast Cancer Res Treat 95(2):171–178. doi:10.1007/s10549-005-9063-1
NAVSTREETS Street Data Reference Manual v3.7. 1 July 2010 ed: NavTeq
Thornton LE, Pearce JR, Kavanagh AM (2011) Using Geographic Information Systems (GIS) to assess the role of the built environment in influencing obesity: a glossary. Int J Behav Nutr Phys Act 8:71. doi:10.1186/1479-5868-8-71
Designed for disease: the link between local food environments and obesity and diabetes. California Center for Public Health Advocacy, PolicyLink, and the UCLA Center for Health Policy Research, April 2008
Highway performance and monitoring system, 2004 ed: California Department of Transportation
Gunier RB, Hertz A, Von Behren J, Reynolds P (2003) Traffic density in California: socioeconomic and ethnic differences among potentially exposed children. J Expo Anal Environ Epidemiol 13(3):240–246. doi:10.1038/sj.jea.7500276
U.S. Department of Health and Human Services Centers for Disease Control and Prevention National Center for Health Statistics (2008) Early release of selected estimates based on data from the January–June 2008 National Health Interview Survey. In: Leisure Time Physical Activity. Hyattsville, MD. http://www.cdc.gov/nchs/data/nhis/earlyrelease/200812_07.pdf
Centers for Disease Control and Prevention (2009) Differences in prevalence of obesity among black, white, and Hispanic adults—United States, 2006–2008. MMWR 58(27):740–744
King WC, Belle SH, Brach JS, Simkin-Silverman LR, Soska T, Kriska AM (2005) Objective measures of neighborhood environment and physical activity in older women. Am J Prev Med 28(5):461–469. doi:10.1016/j.amepre.2005.02.001
Yang W, Spears K, Zhang F, Lee W, Himler HL (2012) Evaluation of personal and built environment attributes to physical activity: a multilevel analysis on multiple population-based data sources. J Obes 2012:548910. doi:10.1155/2012/548910
Vona-Davis L, Rose DP (2009) The influence of socioeconomic disparities on breast cancer tumor biology and prognosis: a review. J Womens Health (Larchmt) 18(6):883–893. doi:10.1089/jwh.2008.1127
Keegan TH, Quach T, Shema S, Glaser SL, Gomez SL (2010) The influence of nativity and neighborhoods on breast cancer stage at diagnosis and survival among California Hispanic women. BMC Cancer 10:603. doi:10.1186/1471-2407-10-603
Gomez SL, Clarke CA, Shema SJ, Chang ET, Keegan TH, Glaser SL (2010) Disparities in breast cancer survival among Asian women by ethnicity and immigrant status: a population-based study. Am J Public Health 100(5):861–869. doi:10.2105/AJPH.2009.176651
Colli J, Lee BR, Thomas R (2012) Population densities in relation to bladder cancer mortality rates in America from 1950 to 1994. Int Urol Nephrol 44(2):443–449. doi:10.1007/s11255-011-0018-7
Chaix B, Rosvall M, Lynch J, Merlo J (2006) Disentangling contextual effects on cause-specific mortality in a longitudinal 23-year follow-up study: impact of population density or socioeconomic environment? Int J Epidemiol 35(3):633–643. doi:10.1093/ije/dyl009
Raaschou-Nielsen O, Andersen ZJ, Jensen SS, Ketzel M, Sorensen M, Hansen J et al (2012) Traffic air pollution and mortality from cardiovascular disease and all causes: a Danish cohort study. Environ Health 11(1):60. doi:10.1186/1476-069X-11-60
Diez Roux A, Evenson K, McGinn A, Brown D, Moore L, Brines S et al (2007) Availability of recreation resources and physical activity in adults. Am J Public Health 97(3):493–499
Gordon-Larsen P, Nelson MC, Page P, Popkin BM (2006) Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics 117(2):417–424. doi:10.1542/peds.2005-0058
Osypuk TL, Roux AV, Hadley C, Kandula NR (2009) Are immigrant enclaves healthy places to live? The Multi-ethnic Study of Atherosclerosis. Soc Sci Med 69(1):110–120. doi:10.1016/j.socscimed.2009.04.010
McGinn AP, Evenson KR, Herring AH, Huston SL, Rodriguez DA (2007) Exploring associations between physical activity and perceived and objective measures of the built environment. J Urban Health 84(2):162–184
West-Wright CN, Henderson KD, Sullivan-Halley J, Ursin G, Deapen D, Neuhausen S et al (2009) Long-term and recent recreational physical activity and survival after breast cancer: the California Teachers Study. Cancer Epidemiol Biomarkers Prev. doi:10.1158/1055-9965.EPI-09-0538
Bertram LA, Stefanick ML, Saquib N, Natarajan L, Patterson RE, Bardwell W et al (2011) Physical activity, additional breast cancer events, and mortality among early-stage breast cancer survivors: findings from the WHEL Study. Cancer Causes Control 22(3):427–435. doi:10.1007/s10552-010-9714-3
Beasley JM, Kwan ML, Chen WY, Weltzien EK, Kroenke CH, Lu W et al (2012) Meeting the physical activity guidelines and survival after breast cancer: findings from the after breast cancer pooling project. Breast Cancer Res Treat 131(2):637–643. doi:10.1007/s10549-011-1770-1
Cleveland RJ, Eng SM, Stevens J, Bradshaw PT, Teitelbaum SL, Neugut AI et al (2012) Influence of prediagnostic recreational physical activity on survival from breast cancer. Eur J Cancer Prev 21(1):46–54. doi:10.1097/CEJ.0b013e3283498dd4
Ooi SL, Martinez ME, Li CI (2011) Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat 127(3):729–738. doi:10.1007/s10549-010-1191-6
Curtis E, Quale C, Haggstrom D, Smith-Bindman R (2008) Racial and ethnic differences in breast cancer survival: how much is explained by screening, tumor severity, biology, treatment, comorbidities, and demographics? Cancer 112:171–180
Rhodes RE, Courneya KS, Bobick TM (2001) Personality and exercise participation across the breast cancer experience. Psychooncology 10(5):380–388
Courneya KS, Friedenreich CM (1997) Relationship between exercise during treatment and current quality of life among survivors of breast cancer. J Psychoso Oncol 15:35–57
Mason C, Alfano CM, Smith AW, Wang CY, Neuhouser ML, Duggan C et al (2013) Long-term physical activity trends in breast cancer survivors. Cancer Epidemiol Biomarkers Prev 22(6):1153–1161. doi:10.1158/1055-9965.EPI-13-0141
Friedenreich CM, Courneya KS, Bryant HE (1998) The lifetime total physical activity questionnaire: development and reliability. Med Sci Sports Exerc 30(2):266–274
Satariano WA, Ragland DR (1994) The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med 120(2):104–110
Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D (2005) Comorbidity and survival disparities among black and white patients with breast cancer. JAMA 294(14):1765–1772
Acknowledgments
The authors thank David Nelson, Sarah Shema, and Kristine Winters for their contributions to these analyses. This work was supported by National Cancer Institute funds from R21CA133255 (T.H.M.K.) and R01CA140058 (S.L.G.). The Breast Cancer Family Registry (BCFR) was supported by Grant UM1 CA164920 from the National Cancer Institute. The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the BCFR, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the BCFR. The San Francisco Bay Area Breast Cancer Study was supported by National Cancer Institute Grants R01 CA63446 and R01 CA77305; by the U.S. Department of Defense (DOD) Grant DAMD17-96-1-6071; and by the California Breast Cancer Research Program (CBCRP) Grants 4JB-1106 and 7PB-0068. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under Contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, Contract HHSN261201000035C awarded to the University of Southern California, and Contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under Agreement U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keegan, T.H.M., Shariff-Marco, S., Sangaramoorthy, M. et al. Neighborhood influences on recreational physical activity and survival after breast cancer. Cancer Causes Control 25, 1295–1308 (2014). https://doi.org/10.1007/s10552-014-0431-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-014-0431-1