Abstract
In multiple sclerosis (MS), improvements of structural magnetic resonance imaging (MRI) techniques have offered the possibility to identify and grade the extent of central nervous system (CNS) damage at different stages of the disease, contributing to improve the understanding of the mechanisms responsible for the accumulation of irreversible disability. Despite this, a gap between clinical and MRI measures still remains.
Interindividual variability of response to CNS damage in terms of recovery from tissue damage and functional plasticity can contribute to fill such a gap. Plasticity occurs at multiple levels in MS, from cells to synapses, from myelin to axons, from individual regions to large-scale brain networks. fMRI provides an indirect measure of neural activity, thus representing a powerful tool to measure brain plasticity in vivo. The application of fMRI has shown that functional reorganization occurs after structural injury in MS and can contribute to limit the clinical consequences of widespread structural tissue damage. The failure or exhaustion of CNS adaptive properties might be among the factors responsible for the accumulation of irreversible neurological deficits.
Clearly, identifying adaptive and maladaptive reorganization is an attractive goal which might help develop therapeutic strategies able to promote the individual adaptive capacity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
1.1 General Considerations
Over the last decade, improvements of methods of acquisition and analysis of magnetic resonance imaging (MRI) techniques have extended the knowledge of the mechanisms underlying the clinical manifestations of several neurologic and psychiatric disorders. Structural MRI techniques have been widely used to study patients with multiple sclerosis (MS) with the aim of increasing the understanding of the mechanisms responsible for the accumulation of irreversible disability, including cognitive impairment (Filippi and Rocca 2004; Filippi et al. 2003). Despite this, measures derived from quantitative MR techniques, such as diffusion tensor (DT) and magnetization transfer imaging, to grade the presence and extent of microscopic disease-related structural damage in the central nervous system (CNS) have contributed only partially to explain MS clinical manifestations and variability of disease course (Filippi and Rocca 2004; Filippi et al. 2003).
Interindividual variability of response to CNS damage in terms of recovery from tissue damage (Franklin and Kotter 2008) and functional plasticity (Tomassini et al. 2012) can help explain that gap. Brain plasticity relies on molecular and cellular mechanisms (including increased axonal expression of sodium channels, synaptic changes, increased recruitment of parallel existing pathways or “latent” connections, and reorganization of distant sites) (Waxman 1998) with the potential to induce modifications in system-level functional responses. Functional MRI (fMRI) techniques based on changes in the blood-oxygenation-level-dependent (BOLD) signal provide an indirect measure of neural activity, thus representing a powerful tool to measure brain plasticity in vivo.
The application of fMRI has shown that functional reorganization does occur after CNS white matter (WM) injury of different etiology (including MS), that such functional changes are related to the extent of CNS damage, and that they can contribute to limit the clinical consequences of widespread structural tissue damage (at least in some groups of patients) (Rocca and Filippi 2006, 2007). Conversely, the failure or the exhaustion of the adaptive properties of the cerebral cortex might be among the factors responsible for the accumulation of irreversible neurological deficits or the presence of specific symptoms (e.g., fatigue) in MS patients (Rocca and Filippi 2006, 2007).
While the majority of previous fMRI studies used active fMRI paradigms to investigate the patterns of recruitment within different functional systems (motor, visual, cognitive) in MS patients, the need for obtaining relevant pieces of information on functional reorganization also in patients with severe clinical (and cognitive) impairment has fuelled the use of resting-state (RS) fMRI paradigms. From its seminal description by Biswal et al. (1995), RS fMRI has been widely used in both healthy subjects and patients with various neurologic, neurosurgical, and psychiatric disorders (Fox et al. 2005). As previously mentioned, one of the features that makes RS fMRI particularly attractive is that it is a task-free approach, thus providing the unique opportunity to perform fMRI studies in MS patients who may have difficulty with task instructions and execution, as well as in pediatric MS patients who may have different compliance (not disease-related) when performing an active task.
1.2 Methodological Issues
The RS fMRI analysis examines correlations in slow (<0.1 Hz) spontaneous fluctuations in the BOLD signal (Cordes et al. 2001). There are multiple ways to analyze RS fMRI data, and each approach has different implications in terms of type of information that can be extracted from the data (Lv et al. 2018). Analysis approaches can be broadly grouped into two categories: functional segregation techniques, which rely on the analysis of RS fMRI focusing on local function of specific brain regions, and functional integration techniques, which rely on functional connectivity (FC) analysis looking at the brain as an integrated network (Liu et al. 1999; Tononi et al. 1994).
Methods commonly used for functional segregation analysis include amplitude of low-frequency fluctuations (ALFF) , fractional ALFF, and regional homogeneity (ReHo) . These methods reflect different aspects of regional neural activity. While ALFF is focused on measuring the strength of the activity, ReHo is more specific for coherence and centrality of regional activity (Lv et al. 2018). However, because the brain is more appropriately studied as an integrated network rather than isolated clusters, the excitement for stand-alone functional segregation methods has gradually receded in favor of functional integration methods, which measure the degree of synchrony of the BOLD time-series between different brain regions and can be the result of a direct anatomic connection, an indirect path (Lanting et al. 2014), or may have no anatomic connection. For assessing functional integration features, commonly used computational methods include FC density analysis, region of interest (ROI)-based FC analysis, independent component analysis (ICA), and graph analysis. FC density analysis attempts to identify the highly connected functional hubs, but it does not indicate which regions are connected (Tomasi and Volkow 2010). Seed-based FC, also called ROI-based FC, finds regions correlated with the activity in a seed region, requiring a priori determination of the seeds, which is often based on a hypothesis or prior results. ICA facilitates the effective extraction of distinct RS fMRI networks by employing mathematical algorithms to decompose the signal from whole-brain voxels to spatially and temporally independent components. ICA investigates multiple simultaneous voxel-to-voxel interactions of distinct networks in the brain, thus representing a powerful technique to perform group-level analysis as well as for the same group in different conditions (e.g., different psychological, physiological, and pharmacological conditions) (Smitha et al. 2017). Graph theory analysis of brain FC assesses different aspects of connectivity through different graph parameters (Bullmore and Sporns 2009), which provides measure of both functional integration and segregation.
Starting from this background, this chapter summarizes the major contribution of RS fMRI application to understand the pathophysiology of MS and its clinical manifestations, in terms of clinical disability and cognitive impairment as well as to monitor treatment, mostly in the field of rehabilitation.
2 Within-Network RS fMRI Abnormalities in MS
2.1 Sensorimotor Network
As previously discussed, many studies have applied active fMRI tasks to investigate the patterns of recruitment within the sensorimotor network in patients with MS, mainly focusing on the analysis of the performance of simple motor tasks with the dominant right upper limb (Lee et al. 2000; Reddy et al. 2000, 2002; Filippi et al. 2002b, 2004; Rocca et al. 2002a, b, 2003a, b, c, d, 2004a, b, 2005a, b, 2007a, b, 2008; Pantano et al. 2002a, b; Lowe et al. 2002; Mezzapesa et al. 2008). More recently, sensorimotor network reorganization has been explored using RS fMRI.
In line with the evidence of the one-to-one functional link of motor network regions of one hemisphere to their homolog in the contralateral hemisphere, with a somatotopic organization (van den Heuvel and Hulshoff Pol 2010), a decreased RS FC has been shown, using a ROI approach, between right- and left-hemisphere primary motor cortices in MS patients in comparison to healthy controls (HC) (Lowe et al. 2002). Using ICA, another study demonstrated that motor network functional reorganization occurs relatively early in the course of the disease, already in patients presenting with a clinically isolated syndrome (CIS), and tends to decrease with disease progression, in relapsing-remitting (RR) MS . Compared to HC, CIS patients showed areas of significantly higher RS FC in the sensorimotor network (right premotor and sensory cortices) of nondominant hemisphere as well as a subthreshold increased RS FC in the dominant premotor cortex and in supplementary motor area (Fig. 23.1), whereas no RS FC modifications were detected in RRMS patients (Roosendaal et al. 2010). In the whole group of patients, increased RS FC of the right premotor cortex was significantly correlated with reduction of WM fractional anisotropy (FA) (a measure of microstructural integrity), which was prominent in RRMS and almost absent in CIS patients, suggesting that functional reorganization might be a finite phenomenon, modulated by the progressive accumulation of disease-related structural damage. Additional studies have confirmed the presence of increased sensorimotor network RS FC in RRMS patients at disease onset (Faivre et al. 2012), including pediatric patients (Rocca et al. 2014), as well as stronger functional coupling between the left dorsal premotor cortex and the motor RS network with increasing disability in RRMS (Dogonowski et al. 2013a).
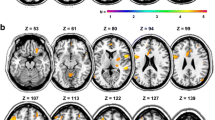
Resting-state (RS) networks identified with independent component analysis in healthy controls (upper rows) and patients with clinically isolated syndromes (lower rows). (a) Executive functioning network; RS functional connectivity (FC) was increased compared to controls in the left mesial prefrontal cortex. (b) Sensorimotor network: increased RS FC compared to controls in the right premotor cortex and inferior parietal gyrus. (c) Ventral and dorsal attention system: increased RS FC compared to controls in the bilateral precuneus. RS FC was also increased compared to relapsing-remitting (RR) multiple sclerosis (MS) patients in the precuneus. (d) Default mode network: increased RS FC compared to RRMS patients in the posterior cingulate gyrus. (e) Right frontoparietal network: increased RS FC compared to RRMS patients in the left inferior temporal gyrus and right superior temporal gyrus. (f) Left frontoparietal network: increased RS FC compared to RRMS patients in the left superior parietal gyrus and the occipital lobe. (g) Visual processing and (h) auditory and language processing: no significant differences between groups. (Reproduced from Roosendaal et al. (2010) with permission)
In progressive MS patients (both primary progressive [PP] and secondary progressive [SP] phenotypes), reduced sensorimotor network RS FC has been shown by several studies, suggesting that functional reorganization might reach a plateau with the progressive accumulation of CNS structural damage and that this might be an additional phenomenon contributing to disability accumulation over time (Rocca et al. 2012, 2018; Basile et al. 2014).
RS fMRI has been also applied to study FC of deep gray matter (DGM) structures, such as the basal ganglia. Compared to HC, MS patients had more widespread motor connectivity in the basal ganglia and a preservation of cortical motor RS FC, which was attributed to a less efficient funneling of neural processing in the motor cortico-basal ganglia-thalamo-cortical loops (Dogonowski et al. 2013b). Subcortical expansion of RS FC did not differ between RRMS and SPMS, suggesting that this altered subcortical motor RS FC could represent a disease-state marker rather than a phenotypical marker (Dogonowski et al. 2013b).
2.2 Default-Mode Network
The default-mode network (DMN) is a medial cortical network including several brain regions (medial prefrontal cortex [mPFC], rostral anterior cingulate cortex [ACC], posterior cingulate cortex [PCC], precuneus, and lateral parietal cortex), which has been found to be active at rest (Raichle et al. 2001) and deactivated when subjects perform attention-demanding or goal-oriented tasks (Shulman et al. 1997). The first observations of DMN alterations in MS come from fMRI studies conducted during active tasks demonstrating that MS patients required greater deactivation of the DMN (and increased prefrontal recruitment) to perform the same cognitive tasks of HC (Sweet et al. 2006; Morgen et al. 2007). This led to the assumption that DMN recruitment could be considered as a marker of cerebral efficiency, which was investigated by subsequent RS fMRI studies.
In the study by Roosendaal et al. (2010), DMN RS FC had the same behavior of the sensorimotor network, with an increased RS FC of the cingulate cortex in CIS patients compared to HC, which was lost in RRMS patients (Fig. 23.1). Reduced DMN RS FC, particularly in the anterior node of the network, contributed to explain the presence of cognitive impairment, both in RRMS (Bonavita et al. 2011) and progressive MS patients (Rocca et al. 2010). In this latter study (Rocca et al. 2010), the extent of DMN RS FC reduction correlated with the severity of structural damage of the cingulum and corpus callosum, measured using DT tractography, pointing to WM structural damage as one of the substrates of the maladaptive functional reorganization occurring in the DMN in progressive MS patients. Differently from adults, pediatric MS patients with cognitive impairment showed decreased RS FC in the posterior regions of the DMN (Rocca et al. 2014), suggesting that onset of the disease in pediatric subjects might impair network maturational trajectories.
Another study compared RRMS to SPMS and found higher RS FC in SPMS patients in the right supramarginal gyrus, left PCC, ITG, and middle temporal gyrus (MTG) (Basile et al. 2013). The previous regions are involved in a number of cognitive processes, including language and visual perception (Cabeza and Nyberg 2000), and fall out the DMN, indicating that the re-allocation of neuronal resources is another mechanism contributing to counteract structural damage in these patients.
A combined DT MRI and RS fMRI study explored alterations of long WM tracts in paired DMN subregions and their RS FC in RRMS patients (Zhou et al. 2014). Structural and functional connectivity measures were significantly correlated in the connection between the PCC/precuneus and mPFC and between PCC/precuneus and bilateral medial temporal lobes. More interesting, three different patterns of structural and functional connectivity relationship were identified: (a) a slightly increased RS FC positively correlated with structural connection damage, (b) significantly increased RS FC negatively correlated with structural connection damage, and (c) a dissociation of structural and functional connectivity coupling. These results contribute to support the previous speculations, demonstrating how in minimally disabled MS patients, increased DMN RS FC may represent a finite compensatory mechanism in response to structural damage.
2.3 Visual Network
Optic neuritis (ON) is one of the most common clinical manifestations of MS. As a consequence, several studies have investigated the effect of ON on visual network reorganization. Studies using active fMRI paradigms have consistently shown relevant and dynamic functional changes taking place in the primary and secondary visual areas following acute ON (Toosy et al. 2005; Rombouts et al. 1998; Werring et al. 2000).
Using RS fMRI, several studies have demonstrated modification of FC within the visual network in CIS and RRMS patients with an acute (Wu et al. 2015; Backner et al. 2018) or chronic (Gallo et al. 2012) ON, mainly characterized by altered RS FC of primary visual processing areas and extra-striate motion processing regions. A collective interpretation of these findings suggests that even a single episode of ON is sufficient to induce RS FC changes within the visual network, resulting in reduced RS FC in the acute stage (Wu et al. 2015), and increased connectivity (probably as a compensatory mechanism) during the recovery stage (Backner et al. 2018). However, also for this system, the compensatory capabilities may tend to decrease over time, as supported by the correlation found in RRMS patients between the number of ON and reduced FC (Gallo et al. 2012).
A recent study (Backner et al. 2018) has investigated visual network anatomical and functional connectivity abnormalities in CIS patients with and without ON. Patients with ON had a higher RS FC within the visual network (calcarine cortex and visual motion processing areas) in the presence of an intact postgeniculate anatomical network, suggesting that functional network changes may be part of the recovery process, independently from structural damage.
2.4 Cognitive Networks
A few studies in MS patients have analyzed modifications of RS FC of networks involved in specific cognitive processes.
The executive control network (ECN), constituted by the medial frontal gyrus (MFG), superior frontal gyrus (SFG), and ACC, is involved in executive functions, such as control processes and working memory. The seminal study by Roosendaal et al. (Roosendaal et al. 2010) found ECN RS FC abnormalities in CIS patients compared to HC, but not in RRMS patients. However, these results have not been confirmed by more recent studies, which detected ECNS alterations also in patients with RRMS (Rocca et al. 2012; Sbardella et al. 2015).
Studies aimed at exploring other networks involved in cognitive functioning, such as the salience network (SN), working memory network (WMN), dorsal attention network (DAN), and frontoparietal network (FPN), are still scanty and provided inconclusive and sometime discordant results (Roosendaal et al. 2010; Faivre et al. 2012; Rocca et al. 2012; Smith et al. 2009).
2.5 Deep Gray Matter RS FC
Structural involvement of DGM structures, in terms of focal lesions, microscopic abnormalities, and atrophy, is frequent and occurs early during the course of MS. Only recently these structures have become topic of functional investigations.
In a large cohort of 295 MS patients (121 early RRMS patients, 122 late RRMS, and 52 SPMS), Meijer et al. (2018) explored whether RS FC abnormalities follow the atrophy pattern observed with disease progression, that is, moving from the DGM toward the cortex. SPMS patients had higher within-DGM connectivity compared with patients with late and early RRMS, higher DGM-cortex connectivity compared with patients with early RRMS and HCs, and lower within-cortex connectivity compared with patients with early RRMS. Late RRMS showed higher within-DGM and DGM-cortex connectivity than early RRMS (Fig. 23.2). These results suggested that RS FC changes might start with disturbances in the interaction between DGM structures that, on turn, could lead to an improper filtering of irrelevant information, resulting in strong and maladaptive connections between the DGM and cortex. After a long period of increasing structural damage and inefficient network connections, RS FC between cortical regions is likely to decrease in SPMS, a sign of incipient cortical network collapse that may be specific to this disease stage.
Resting-state (RS) functional connectivity (FC) within deep gray matter (DGM) structures, between DGM and the cortex, and within cortex in the different stages of relapse-onset multiple sclerosis (MS). (a) Connectivity within DGM. (b) Connectivity between DGM and cortex. (c) Connectivity within the cortex. Positive connectivity z-scores reflect increases in the level of connectivity, whereas negative connectivity z-scores reflect decreases in the level of connectivity. Asterisk (∗) indicates significant differences and error bars reflect standard error of the mean. ERRMS early relapsing-remitting MS, LRRMS late relapsing-remitting MS, SPMS secondary progressive MS. (Reproduced from Meijer et al. (2018) with permission)
Another study (Cui et al. 2017) used a seed-based technique to explore RS FC of six striatal subregions for each hemisphere with the remaining brain regions. Compared to HC, MS patients had a significant increase of RS FC of dorsal caudal putamen with premotor area, dorsal PFC, insula, precuneus, and superior parietal lobule as well as increased RS FC of superior ventral striatum and PCC, indicating the importance of this region in the pathophysiology of MS.
Among DGM structures, the thalamus represents the most frequently studied region, due to its early and clinically relevant involvement in the disease, according to the literature derived from the use of structural MRI techniques. On the other hand, the application of RS fMRI has provided heterogeneous and conflicting results. While some studies described a decreased RS FC between the thalami and several brain regions (Liu et al. 2015; De Giglio et al. 2016), others reported both increased and decreased RS FC between the thalamus and different cortical regions in MS patients (Prosperini et al. 2014). In one study (De Giglio et al. 2016), increased thalamo-cortical RS FC correlated with poor cognitive performance, suggesting that this might reflect a maladaptive mechanism contributing to cognitive deficits.
The heterogeneity of these results might be explained by a recent study that, by using a connectivity-based parcellation of the thalamus, showed that the main thalamic subregions have different RS FC abnormalities in MS patient (d’Ambrosio et al. 2017). In details, compared to HC, MS patients had increased intra- and interthalamic RS FC for almost all thalamic subregions and increased RS FC between all thalamic subregions and the left insula. Reduced RS FC was also found between frontal and motor thalamic subregions and the caudate nucleus as well as between the temporal thalamic subregions and the ipsilateral thalamus, anterior and middle cingulate cortex, and cerebellum. RS FC abnormalities contributed to explain the different clinical features, since lower thalamic RS FC correlated with worse motor performance, whereas higher thalamic RS FC correlated with better motor performance.
2.6 Cerebellar RS FC
The cerebellum plays a crucial role in motor and cognitive functions. Using a ReHo analysis, compared to HC, MS patients had reduced ReHo in the left cerebellar hemisphere, including left superior cerebellar lobe, which was correlated with higher T2 lesion load (Dogonowski et al. 2014). Another study (Sbardella et al. 2017) detected higher RS FC between the dentate nucleus and several frontal and parietal areas in MS patients in comparison to HC. Within the previous areas, higher RS FC correlated with less severe disability, suggesting an adaptive mechanism contributing to preserved clinical function.
Cerebellar dentate nuclei RS FC has been also investigated in pediatric patients with MS (Cirillo et al. 2016), who experienced patterns of both increased and decreased RS FC. Decreased RS FC was correlated with longer disease duration, higher T2 lesion volumes, and cognitive impairment, suggesting a maladaptive mechanism. On the other hand, increased RS FC correlated with shorter disease duration, lower T2 lesion volume, and a better motor performance.
2.7 Spinal Cord RS FC
Using ultra-high field MRI, recent studies have provided evidence of RS FC in the human spinal cord (Barry et al. 2014, 2016). RS FC in spinal cord was highly reproducible within and across human HC and was mainly constituted by two networks: a ventral motor and a dorsal sensory network, whose organization and spatial distribution appeared consistent with those detected with task-based fMRI studies (Maieron et al. 2007; Cadotte et al. 2012; Eippert et al. 2017; Stroman et al. 2012). To date, only one study has assessed RS fMRI abnormalities at the level of the spinal cord in MS patients using a 7 T scanner (Conrad et al. 2018). In HC, a pattern of connectivity among ventral GM regions and a distinct network among dorsal regions were detected, with higher ventral network connectivity in female. No differences were detected between MS patients and controls. However, a significant effect of focal lesions on local alterations in connectivity was found in patients, with differential effects depending on columnar location.
3 Between-Network RS fMRI Abnormalities
Brain functioning requires high level of integration between functionally relevant networks to subserve higher functional processes. In this perspective, in addition to the previous approaches that provide a FC measure for each single network, functional interactions among the RS networks were also explored. In RRMS patients, compared to HC, the ECN had an increased connectivity with the SN and a decreased connectivity with the DMN. An abnormal connectivity between the WMNs and sensory networks was also found (Fig. 23.3) (Rocca et al. 2012). These findings supported the hypothesis that MS alters CNS functioning in a distributed manner.
Internetwork connectivity of resting-state networks in patients with relapsing-remitting multiple sclerosis. Diagram showing resting-state networks with a significantly different pairwise connectivity between healthy controls and patients with relapsing-remitting multiple sclerosis (RRMS), as assessed using a functional network connectivity analysis. Solid arrows indicate increased network connectivity in patients with RRMS vs controls; dotted arrows indicate decreased network connectivity in patients with RRMS vs controls. DMN default mode network, ECN executive control network, SN salience network, WMN working memory network. (Reproduced from Rocca et al. (2012) with permission)
Starting from the observation that the anticorrelation existing between the DMN and the DAN represents an intrinsic aspect of brain functional organization, whose strength varies across individuals and appears determinant for behavioral performance (Kelly et al. 2008), a recent study (Huang et al. 2018) explored the relationship between these two networks in RRMS patients. A stable relationship between DMN and DAN was detected in RRMS patients, with a significantly increased driving connectivity from DAN to DMN, which may represent an adaptive mechanism contributing to the maintenance of a stable interaction by increasing the information transmission capacity.
A distributed pattern of FC abnormalities within large-scale neuronal networks has also been detected in pediatric MS patients and has been shown to be influenced by focal WM lesions and to contribute to their cognitive status (Rocca et al. 2014). To explore whether functional reorganization mechanisms have a protective role over time in pediatric MS patients, another study assessed brain alterations in pediatric-onset MS patients in their early adulthood in comparison with age- and disability-matched adult-onset MS patients. Compared to adult-onset patients and HC, pediatric-onset MS patients had reduced long-range RS FC between DMN and secondary visual network, whose interaction subserves important cognitive functions. They also had more severe structural damage, measured with DT MRI, in clinically eloquent regions for physical disability (Giorgio et al. 2017). These data suggest that occurrence of functional and structural abnormalities early in the disease course may confer higher vulnerability to unfavorable clinical outcome in the long term.
4 Large-Scale Network Connectivity by Means of Graph Analysis
A large body of literature in neuroscience has analyzed brain networks from the perspective of graph theory, where network graphs can be quantified with a wide range of simple yet “neuro-biologically” meaningful measures (Rubinov and Sporns 2010). Considering MS as a disconnection syndrome, graph theoretical analysis is a promising approach to detect functional changes occurring at different stages of the disease, characterized by both global and local network measure alterations.
Graph analysis studies in patients at the earliest stages of MS have so far provided conflicting results. While some authors found no abnormalities of network measures in CIS patients in comparison to HC (Shu et al. 2016), others (Liu et al. 2017; Abidin et al. 2017) found that CIS patients had decreased whole-brain network efficiency, which was, however, less pronounced than what observed in RRMS patients. Conversely, at regional analysis, alterations in nodal efficiency and RS FC detected in CIS patients were similar to those found in MS patients, supporting the hypothesis that regional network degeneration is already present in CIS.
By applying a graph theoretical approach , Rocca et al. (2016) investigated the topological organization of the functional brain connectome in a large cohort of MS patients including RRMS, benign MS (BMS), and SPMS. A disruption of global functional organization was observed in the whole group of MS patients and contributed to distinguish cognitively impaired MS patients from HC, but not the main MS clinical phenotypes. Compared to HC, MS patients also had modifications of regional network properties, which contributed to cognitive impairment and phenotypic variability of MS.
In a large group of MS patients at 6 years from the diagnosis, Schoonheim et al. (2014) reported an increase in centrality in the PCC and decreased centrality in the sensorimotor and ventral stream areas. Since the thalamus, an area exhibiting increased centrality, showed a higher connectivity to areas with decreased centrality, the authors hypothesized a rerouting of thalamic connections as a response to continuous inflammatory activity.
5 Clinical Relevance of Functional Network Abnormalities
5.1 Diagnosis and Differential Diagnosis
The potential of RS fMRI analysis as a tool for diagnosis and differential diagnosis has been only marginally explored. One study has investigated the utility of graph theoretical network measures to differentiate CIS and MS patients from HC (Liu et al. 2017). Among the metrics analyzed, the mean connectivity strength exhibited the highest power in distinguishing MS (AUC = 0.825, P < 0.001) and CIS (AUC = 0.789, P < 0.001) patients from HC, with sensitivity of 88.2% (30 of 34 patients) and 61.8% (21 of 34 patients) and specificity of 66.7% (24 of 36 control subjects) and 91.7% (33 of 36 control subjects), respectively. This yielded an accuracy of 77.1% for the classification of MS patients vs HC and of 77.1% for the classification of CIS patients vs HC.
Another study (Eshaghi et al. 2015) combined RS fMRI, structural MRI, and clinical measures to automatically differentiate MS from neuromyelitis optica (NMO) patients. RS fMRI resulted the second modality (after WM lesions) able to distinguish HC, MS, and NMO patients.
Using a whole brain connectivity analysis, Richiardi et al. (2012) demonstrated that a multivariate approach, based on predictive modeling of brain RS FC, allows a reliable differentiation of minimally disabled MS patients and HCs. In this model, only 4% of the analyzed connections (90 × 90) resulted discriminative. Classification performance yielded a sensitivity of 82% and specificity of 86% to distinguish between MS patients and HC. The most discriminative connectivity changes were found in subcortical and temporal regions, and contralateral connections were more discriminative than ipsilateral ones.
5.2 Phenotype-Specific RS fMRI Patterns and Clinical Disability, Ambulation, and Balance
Many of the studies previously discussed have assesses the correlations between RS FC abnormalities and clinical disability. In the majority of studies, a negative correlation between RS FC strength and clinical impairment, measured with the Expanded Disability Status Scale (EDSS), has been reported (Roosendaal et al. 2010; Faivre et al. 2012; Rocca et al. 2012; Richiardi et al. 2012), whereas a few studies found a positive correlation between increased RS FC strength and more severe clinical impairment (Dogonowski et al. 2013a; Rocca et al. 2010). These discrepancies may be due not only to differences in patient populations and methods of analysis but also to the clinical function considered and the network investigated.
Several studies tried to define whether RS FC modifications might have different expression and might correlated differently with clinical disability according to the clinical phenotype of the disease. Studies in patients with RRMS have consistently shown a correlation between reduced RS FC within selected brain networks and higher EDSS (reflecting more severe disability) (Rocca et al. 2012; Schoonheim et al. 2014; Janssen et al. 2013). A recent study (Rocca et al. 2018) included 215 MS patients with the main disease clinical phenotypes (from CIS to progressive MS) and demonstrated a progressive reduction of RS FC from the earliest to the progressive phenotypes in parietal, frontal, and cerebellar regions of cognitive, sensory, and motor networks, which may reflect an exhaustion of functional plasticity with progressive accumulation of disease-related structural damage.
Other studies focused their analysis to specific clinical deficits, such as ambulation and balance. A study (Bollaert et al. 2018) examined the correlation between RS FC in cortical motor and non-motor networks and walking performance, assessed by timed 25-foot walk (T25FW) in MS patients. T25FW performance correlated with RS FC of brain regions that are part of the sensorimotor network as well as with FC of regions that have a major role in visual perception, spatial orientation, and navigation, indicating that MS clinical manifestations are influenced by functional abnormalities of critical regions part of interconnected networks.
Balance deficits affect almost 75% of MS patients during the course of the disease (Cameron and Lord 2010). Using a seed-based analysis, reduced RS FC between the dentate nucleus and the left caudate nucleus within the cerebellar network was found to correlate with worse balance measures in RRMS patients, suggesting that a functional disconnection between these two regions may impair high-level control of balance (Tona et al. 2018).
5.3 Cognitive Impairment, Depression, and Fatigue
Cognitive impairment affects a large proportion of MS patients, with prevalence ranging from 40% to 70%, depending on the population studied, the tests used, and the cut-off values applied (Chiaravalloti and DeLuca 2008). A prominent involvement of information processing speed and episodic memory domain has been observed, while less frequently impairment in executive functions, including verbal fluency, and word list generation have been described (Benedict et al. 2002; Benedict and Zivadinov 2006).
RS FC of the DMN and its relation with cognitive impairment have been previously discussed (see Sect. 23.2.2). Using a whole-brain connectivity approach, a recent graph analysis study (Eijlers et al. 2017) measured the overall importance for cognition of individual brain regions in 332 MS patients. Both degree and eigenvector centrality were assessed in order to respectively measure the quantity and quality of functional connections. Cognitively impaired (CI) MS patients had widespread centrality increases compared to both HC and cognitively preserved (CP) MS patients mainly in regions making up the DMN, while all patient groups showed decreased centrality in occipital and sensorimotor areas. This study provides evidence that under pressure of disease, the hierarchy of the entire brain network shifts, and the DMN becomes of central importance in CI MS patients.
Despite less frequently object of investigation, depression is another frequent symptom of MS (Feinstein et al. 2014). The clinical manifestations of depression in MS patients are very similar to those of patients with major depressive disorder, but its etiology, potentially related to specific MS pathology, is still poorly understood. Starting from the observation of abnormal RS FC between limbic and frontal regions in patients with major depressive disorder (Northoff et al. 2011), a recent study explored frontolimbic system RS FC in MS patients with and without depression (van Geest et al. 2019). Compared to non-depressed MS patients, MS patients with depression had decreased RS FC between the amygdala and frontal regions, which correlated with higher depression rank scores. These results are in line with those from a previous study, which detected a correlation between higher depression scores and reduced RS FC between the hippocampus and the orbitofrontal cortex (Rocca et al. 2015).
Another study (Colasanti et al. 2016) explored the hippocampus in terms of neuroinflammation and RS FC as possible substrates of depressive symptoms in MS. Hippocampal RS FC to the subgenual cingulate and prefrontal and parietal regions correlated with depression severity and metabolic changes as assessed by positron emission tomography (PET). These results not only confirmed the central role of this set of regions in determining affective disorders in MS patients but also identified chronic neuroinflammation as potential pathogenic substrate of RS FC abnormalities.
Fatigue is one of the most disabling MS symptoms, significantly impacting patients’ daily activities and quality of life and affecting up to 80% of patients. Several studies investigated the anatomical substrates of fatigue, identifying dysfunction in the sensorimotor network and basal ganglia as pathobiological basis of this symptom. Using regions of GM atrophy in fatigued (F) MS patients as seeds, F MS patients had decreased RS FC between the primary motor and somatosensory cortices, which correlated with the severity of fatigue. Non-fatigued (NF) MS patients presented higher RS FC in the premotor cortex compared to F MS and in the primary motor cortex compared to HC (Cruz Gomez et al. 2013). Based on these results, the authors suggested that the increased RS FC observed in NF MS patients could reflect a compensatory mechanism associated with subclinical fatigue. Another study (Bisecco et al. 2018) found that abnormalities of RS FC within both the sensorimotor network and DMN contributed to the presence and severity of fatigue in MS patients.
The investigation of basal ganglia RS FC has been substantiated by neuroimaging studies showing structural and functional changes of this level in F MS patients, including atrophy, reduced perfusion, reduced glucose metabolism, and reduced activation during the performance of motor tasks (DeLuca et al. 2008; Roelcke et al. 1997; Inglese et al. 2007; Filippi et al. 2002a). In RRMS patients, higher fatigue scores were correlated with reduced RS FC of caudate nucleus, putamen, and pallidum with frontal and parietal areas, but also positively correlated with RS FC between caudate nucleus and the motor cortex bilaterally (Finke et al. 2015). This latter finding was interpreted as a compensatory mechanism contributing to maintain a normal function.
By performing a RS FC analysis of the different thalamic subregions, a recent investigation (Hidalgo de la Cruz et al. 2018) demonstrated that regional thalamic RS FC abnormalities may contribute to explain the different components of fatigue in MS patients. In particular, abnormal thalamic connectivity with the precuneus and posterior lobe of the cerebellum explained cognitive fatigue, whereas altered connectivity with the sensorimotor network had a role in explaining physical and psychosocial fatigue.
6 Structural Substrates of RS fMRI Abnormalities
The majority of available RS fMRI studies has included different measures derived from structural MRI (e.g., T2 lesion volume, measures of WM integrity, measures of volume loss of the whole brain or of selected CNS regions) to investigate the correlation between structural and functional MRI abnormalities in patients with MS. Independently from the measure analyzed, all of them have consistently demonstrated that there is a relationship between structural damage and functional reorganization. Studies of patients at different stages of the disease have suggested that such a relationship might have an inverted U-shape, with increased functional reorganization with accumulation of brain structural damage until a certain level of the disease (adaptive functional reorganization), followed by collapse of functional reorganization when a plateauing level is reached. At this stage, functional reorganization becomes maladaptive and contributes, together with progression of structural damage, to clinical (and cognitive) worsening (Roosendaal et al. 2010; Rocca et al. 2010; Hawellek et al. 2011).
A combined analysis of structural and functional network alterations in CIS and RRMS patients has suggested that structural network modifications may precede (and maybe influence) those of network function, as CIS patients only showed structural network abnormalities, whereas RRMS had concomitant structural and functional abnormalities (Fig. 23.4) (Shu et al. 2016).
Structural and functional connectome in clinically isolated syndromes and multiple sclerosis patients. (a) The top panel represents connected networks showing decreased structural connections in CIS vs controls, MS vs CIS, and MS vs controls. The regional pairs showed decreased connections in the patient groups (p < 0.05, corrected). The bottom panel represents the connected networks, showing decreased functional connections in MS vs CIS and MS vs controls (p < 0.05, corrected). Between CIS and controls, no connected components with significant differences were found. (b) Correlation between the structural and functional connection strength within sensorimotor and visual components across all patients while removing the effects of age and gender. (Reproduced from Shu et al. (2016) under a Creative Commons Attribution 4.0 International License)
7 RS fMRI and Treatment
7.1 Pharmaceutical Treatment
Only a few fMRI studies have been performed to monitor the effects of symptomatic treatments in MS patients (Parry et al. 2003; Mainero et al. 2004; Cader et al. 2009), mainly adopting active fMRI paradigms. Recently, the effects of smoke cannabis on cognition, as well as their possible functional and structural MRI correlates, have been investigated (Pavisian et al. 2014). MS patients who smoked cannabis on a regular basis showed more cognitive deficits than MS patients who were drug-free. Cannabis-related cognitive problems were associated with a different pattern of cerebral activation during the N-back on fMRI, whereas no relation was found with structural measures or with RS fMRI measures.
7.2 Motor Rehabilitation
Promoting restoration of function and competence of dysfunctional brain networks is one of the main goals of motor and cognitive rehabilitation. Even though the mechanisms underlying clinical improvement after rehabilitation are not yet fully understood, by applying different rehabilitation procedures, several studies in MS patients have consistently demonstrated that motor and cognitive rehabilitation results in an improvement of the rehabilitated function (i.e., motor functions, attention, memory, and executive function) and that this improvement is somehow mediated by modification of recruitment and/or functional connectivity occurring in function-related networks.
Improved gait performance following 4 weeks of motor rehabilitation was associated with RS FC modifications in the sensorimotor network in MS patients with mild-to-moderate disability (Tavazzi et al. 2018). All these changes disappeared 3 months after the termination of motor rehabilitation, suggesting the need of continuous training (or bouts of training) in order to maintain the benefits of motor rehabilitation.
Recent evidence has suggested that repetitive transcranial magnetic stimulation (rTMS) of the motor cortex may be effective to reduce spasticity and promoting motor function recovery in MS patients (Centonze et al. 2007; Mori et al. 2011). A recent study (Boutière et al. 2017) tested the effect on RS FC of 5-week intermittent theta burst stimulation (iTBS) applied over the primary motor cortex combined with physical rehabilitation. No effects of iTBS on global topology of brain network was observed, while changes of interhemispheric balance were observed in bilateral homologous primary cortices (connectivity degree decreased in the stimulated region of 38% and increased in the contralateral regions of 52%). Based on these findings, the authors concluded that the relative decrease in connectivity of the stimulated primary motor cortex with other brain areas could in turn promote corticospinal descending activity, resulting in improvement of spasticity.
7.3 Cognitive Rehabilitation
In patients with RRMS with selective deficits of attention, information processing, and executive functions, 3 months of computer-assisted cognitive rehabilitation of these functions resulted in cognitive improvement through enhanced recruitment of brain networks subserving the trained functions (Filippi et al. 2012). To investigate the persistence of treatment efficacy after treatment termination and its possible pathobiological substrates, the same cohort of patients was reevaluated 6 months after the termination of rehabilitation (Parisi et al. 2014). The positive effects of cognitive rehabilitation on cognitive tests were still present. There were some additional improvements at depression and quality of life scales, which were not detected immediately after the termination of cognitive rehabilitation. Interestingly enough, measures derived from RS fMRI during the rehabilitation phase of the study were the only predictors of these effects at 6 months, suggesting that cognitive rehabilitation may act by optimizing cognitive network recruitment, resulting in a generalized functional improvement.
Interestingly, since the results of fMRI analysis of the previous study (Filippi et al. 2012) pointed toward a role for the left dorsolateral PFC in improving cognitive functions, this region was selected, in a subsequent study, as a target for an anodal transcranial direct current stimulation, in combination with attention training in MS patients impaired in attention/speed of information processing (Mattioli et al. 2016). In the study of MS patients, the combination of cognitive training with an anodal transcranial direct current stimulation over the left dorsolateral PFC during ten daily sessions fostered improvements in attention and executive function, which persisted up to 6 months after the intervention (Mattioli et al. 2016).
The beneficial effect of cognitive rehabilitation and its modulation of RS FC have been confirmed by several subsequent studies, not only on information processing speed and executive functions (Pareto et al. 2018; Bonavita et al. 2015; Cerasa et al. 2013) but also on memory (Leavitt et al. 2014; Dobryakova et al. 2014). The effects of video-game-based cognitive rehabilitation on RS FC of the thalamus have also been shown (De Giglio et al. 2016).
Starting from the consideration that exercise training has been proposed as an approach for managing the cognitive consequences of MS, RS FC changes after a pilot treadmill walking exercise training intervention for improving cognitive processing speed in MS have been explored (Sandroff et al. 2018). After 12 weeks of treadmill walking exercise training, increase in thalamocortical RS FC, which correlated with cognitive processing speed improvement, was observed.
8 Future Perspectives
All fMRI studies discussed in this chapter have been based on the assumption that brain RS FC is static across the whole duration of image acquisition (usually taking about 10 min), and thus the strength of the interaction between different brain regions is considered to be constant over time. However, RS FC between two or more regions changes dynamically over time (Calhoun et al. 2014; Allen et al. 2014). This has led to a shift from measuring static to measuring time-varying (dynamic) FC between different brain regions (Calhoun et al. 2014). Analysis of dynamic FC allows capturing reoccurring patterns of interaction among intrinsic networks at rest (Calhoun et al. 2014; Allen et al. 2014). Studies that have used dynamic RS FC analysis have shown the utility of this method in shedding light not only on the physiological processes in HC (Allen et al. 2012) but also in diseased subjects, for diagnostic purposes (Jones et al. 2012) or to improve the understanding of their clinical manifestations (Rashid et al. 2014).
A preliminary study (Leonardi et al. 2013) has assessed dynamic RS FC abnormalities in 15 minimally disabled RRMS patients and found altered dynamic FC in a network of connections centered on the DMN. The clinical relevance of these abnormalities, particularly for cognition, deserves further investigations.
9 Conclusions
RS fMRI studies conducted in MS patients have demonstrated that this technique contributes to provide important insights into the role of functional reorganization following CNS structural injury. Being a task-free approach, this tool complements and sometime replaces active fMRI acquisitions, allowing the generalization of the results to the whole spectrum of MS clinical phenotypes or clinical manifestations.
It is now established that clinical and cognitive features of MS patients are likely to represent the output of the complex interplay existing between structural damage and RS FC changes, indicating that the rate of accumulation of disability in MS is a function not only of tissue loss but also of the progressive failure of the adaptive capacity of the brain with increasing tissue damage. All of this is pivotal for the development of intervention strategies for the preservation or restoration of function.
References
Abidin AZ, Chockanathan U, DSouza AM, Inglese M, Wismüller A (2017) Using large-scale granger causality to study changes in brain network properties in the clinically isolated syndrome (CIS) stage of multiple sclerosis. Proc SPIE Int Soc Opt Eng 10137. https://doi.org/10.1117/12.2254395
Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD (2014) Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 24(3):663–676
Allen EA, Erhardt EB, Wei Y, Eichele T, Calhoun VD (2012) Capturing inter-subject variability with group independent component analysis of fMRI data: a simulation study. Neuroimage 59(4):4141–4159
Backner Y, Kuchling J, Massarwa S, Oberwahrenbrock T, Finke C, Bellmann-Strobl J et al (2018) Anatomical wiring and functional networking changes in the visual system following optic neuritis. JAMA Neurol 75(3):287–295
Barry RL, Rogers BP, Conrad BN, Smith SA, Gore JC (2016) Reproducibility of resting state spinal cord networks in healthy volunteers at 7 Tesla. Neuroimage 133:31–40
Barry RL, Smith SA, Dula AN, Gore JC (2014) Resting state functional connectivity in the human spinal cord. Elife 3:e02812
Basile B, Castelli M, Monteleone F, Nocentini U, Caltagirone C, Centonze D et al (2013) Functional connectivity changes within specific networks parallel the clinical evolution of multiple sclerosis. Mult Scler J 20(8):1050–1057
Basile B, Castelli M, Monteleone F, Nocentini U, Caltagirone C, Centonze D et al (2014) Functional connectivity changes within specific networks parallel the clinical evolution of multiple sclerosis. Mult Scler 20(8):1050–1057
Benedict RH, Fischer JS, Archibald CJ, Arnett PA, Beatty WW, Bobholz J et al (2002) Minimal neuropsychological assessment of MS patients: a consensus approach. Clin Neuropsychol 16(3):381–397
Benedict RH, Zivadinov R (2006) Predicting neuropsychological abnormalities in multiple sclerosis. J Neurol Sci 245(1–2):67–72
Bisecco A, Nardo FD, Docimo R, Caiazzo G, d’Ambrosio A, Bonavita S et al (2018) Fatigue in multiple sclerosis: the contribution of resting-state functional connectivity reorganization. Mult Scler 24(13):1696–1705. https://doi.org/10.1177/1352458517730932
Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34(4):537–541
Bollaert RE, Poe K, Hubbard EA, Motl RW, Pilutti LA, Johnson CL et al (2018) Associations of functional connectivity and walking performance in multiple sclerosis. Neuropsychologia 117:8–12
Bonavita S, Gallo A, Sacco R, Corte MD, Bisecco A, Docimo R et al (2011) Distributed changes in default-mode resting-state connectivity in multiple sclerosis. Mult Scler J 17(4):411–422
Bonavita S, Sacco R, Della Corte M, Esposito S, Sparaco M, d’Ambrosio A et al (2015) Computer-aided cognitive rehabilitation improves cognitive performances and induces brain functional connectivity changes in relapsing remitting multiple sclerosis patients: an exploratory study. J Neurol 262(1):91–100
Boutière C, Rey C, Zaaraoui W, Le Troter A, Rico A, Crespy L et al (2017) Improvement of spasticity following intermittent theta burst stimulation in multiple sclerosis is associated with modulation of resting-state functional connectivity of the primary motor cortices. Mult Scler J 23(6):855–863
Bullmore ET, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10(3):186–198
Cabeza R, Nyberg L (2000) Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12(1):1–47
Cader S, Palace J, Matthews PM (2009) Cholinergic agonism alters cognitive processing and enhances brain functional connectivity in patients with multiple sclerosis. J Psychopharmacol 23(6):686–696
Cadotte DW, Stroman PW, Mikulis D, Fehlings MG (2012) A systematic review of spinal fMRI research: outlining the elements of experimental design. J Neurosurg Spine 17(Suppl 1):102–118
Calhoun VD, Miller R, Pearlson G, Adali T (2014) The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron 84(2):262–274
Cameron MH, Lord S (2010) Postural control in multiple sclerosis: implications for fall prevention. Curr Neurol Neurosci Rep 10(5):407–412
Centonze D, Koch G, Versace V, Mori F, Rossi S, Brusa L et al (2007) Repetitive transcranial magnetic stimulation of the motor cortex ameliorates spasticity in multiple sclerosis. Neurology 68(13):1045–1050
Cerasa A, Gioia MC, Valentino P, Nistico R, Chiriaco C, Pirritano D et al (2013) Computer-assisted cognitive rehabilitation of attention deficits for multiple sclerosis: a randomized trial with fMRI correlates. Neurorehabil Neural Repair 27(4):284–295
Chiaravalloti ND, DeLuca J (2008) Cognitive impairment in multiple sclerosis. Lancet Neurol 7(12):1139–1151
Cirillo S, Rocca MA, Ghezzi A, Valsasina P, Moiola L, Veggiotti P et al (2016) Abnormal cerebellar functional MRI connectivity in patients with paediatric multiple sclerosis. Mult Scler 22(3):292–301
Colasanti A, Guo Q, Giannetti P, Wall MB, Newbould RD, Bishop C et al (2016) Hippocampal neuroinflammation, functional connectivity, and depressive symptoms in multiple sclerosis. Biol Psychiatry 80(1):62–72
Conrad BN, Barry RL, Rogers BP, Maki S, Mishra A, Thukral S et al (2018) Multiple sclerosis lesions affect intrinsic functional connectivity of the spinal cord. Brain 141(6):1650–1664
Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH et al (2001) Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol 22(7):1326–1333
Cruz Gomez AJ, Ventura Campos N, Belenguer A, Avila C, Forn C (2013) Regional brain atrophy and functional connectivity changes related to fatigue in multiple sclerosis. PLoS One 8(10):e77914
Cui F, Zhou L, Wang Z, Lang C, Park J, Tan Z et al (2017) Altered functional connectivity of striatal subregions in patients with multiple sclerosis. Front Neurol 8:129
d’Ambrosio A, Hidalgo de la Cruz M, Valsasina P, Pagani E, Colombo B, Rodegher M et al (2017) Structural connectivity-defined thalamic subregions have different functional connectivity abnormalities in multiple sclerosis patients: implications for clinical correlations. Hum Brain Mapp 38(12):6005–6018
De Giglio L, Tona F, De Luca F, Petsas N, Prosperini L, Bianchi V et al (2016) Multiple sclerosis: changes in thalamic resting-state functional connectivity induced by a home-based cognitive rehabilitation program. Radiology 280(1):202–211
DeLuca J, Genova HM, Hillary FG, Wylie G (2008) Neural correlates of cognitive fatigue in multiple sclerosis using functional MRI. J Neurol Sci 270(1–2):28–39
Dobryakova E, Wylie GR, DeLuca J, Chiaravalloti ND (2014) A pilot study examining functional brain activity 6 months after memory retraining in MS: the MEMREHAB trial. Brain Imaging Behav 8(3):403–406
Dogonowski AM, Andersen KW, Madsen KH, Sorensen PS, Paulson OB, Blinkenberg M et al (2014) Multiple sclerosis impairs regional functional connectivity in the cerebellum. Neuroimage Clin 4:130–138
Dogonowski AM, Siebner HR, Soelberg Sorensen P, Paulson OB, Dyrby TB, Blinkenberg M et al (2013a) Resting-state connectivity of pre-motor cortex reflects disability in multiple sclerosis. Acta Neurol Scand 128(5):328–335
Dogonowski AM, Siebner HR, Sorensen PS, Wu X, Biswal B, Paulson OB et al (2013b) Expanded functional coupling of subcortical nuclei with the motor resting-state network in multiple sclerosis. Mult Scler 19(5):559–566
Eijlers AJ, Meijer KA, Wassenaar TM, Steenwijk MD, Uitdehaag BM, Barkhof F et al (2017) Increased default-mode network centrality in cognitively impaired multiple sclerosis patients. Neurology 88(10):952–960
Eippert F, Kong Y, Winkler AM, Andersson JL, Finsterbusch J, Büchel C et al (2017) Investigating resting-state functional connectivity in the cervical spinal cord at 3T. Neuroimage 147:589–601
Eshaghi A, Riyahi-Alam S, Saeedi R, Roostaei T, Nazeri A, Aghsaei A et al (2015) Classification algorithms with multi-modal data fusion could accurately distinguish neuromyelitis optica from multiple sclerosis. Neuroimage Clin 7:306–314
Faivre A, Rico A, Zaaraoui W, Crespy L, Reuter F, Wybrecht D et al (2012) Assessing brain connectivity at rest is clinically relevant in early multiple sclerosis. Mult Scler 18(9):1251–1258
Feinstein A, Magalhaes S, Richard JF, Audet B, Moore C (2014) The link between multiple sclerosis and depression. Nat Rev Neurol 10(9):507–517
Filippi M, Riccitelli G, Mattioli F, Capra R, Stampatori C, Pagani E et al (2012) Multiple sclerosis: effects of cognitive rehabilitation on structural and functional MR imaging measures—an explorative study. Radiology 262(3):932–940
Filippi M, Rocca MA (2004) Magnetization transfer magnetic resonance imaging in the assessment of neurological diseases. J Neuroimaging 14(4):303–313
Filippi M, Rocca MA, Colombo B, Falini A, Codella M, Scotti G et al (2002a) Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. Neuroimage 15(3):559–567
Filippi M, Rocca MA, Comi G (2003) The use of quantitative magnetic-resonance-based techniques to monitor the evolution of multiple sclerosis. Lancet Neurol 2(6):337–346
Filippi M, Rocca MA, Falini A, Caputo D, Ghezzi A, Colombo B et al (2002b) Correlations between structural CNS damage and functional MRI changes in primary progressive MS. Neuroimage 15(3):537–546
Filippi M, Rocca MA, Mezzapesa DM, Ghezzi A, Falini A, Martinelli V et al (2004) Simple and complex movement-associated functional MRI changes in patients at presentation with clinically isolated syndromes suggestive of multiple sclerosis. Hum Brain Mapp 21(2):108–117
Finke C, Schlichting J, Papazoglou S, Scheel M, Freing A, Soemmer C et al (2015) Altered basal ganglia functional connectivity in multiple sclerosis patients with fatigue. Mult Scler J 21(7):925–934
Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102(27):9673–9678
Franklin RJ, Kotter MR (2008) The biology of CNS remyelination: the key to therapeutic advances. J Neurol 255(Suppl 1):19–25
Gallo A, Esposito F, Sacco R, Docimo R, Bisecco A, Della Corte M et al (2012) Visual resting-state network in relapsing-remitting MS with and without previous optic neuritis. Neurology 79(14):1458–1465
Giorgio A, Zhang J, Stromillo ML, Rossi F, Battaglini M, Nichelli L et al (2017) Pronounced structural and functional damage in early adult pediatric-onset multiple sclerosis with no or minimal clinical disability. Front Neurol 8:608
Hawellek DJ, Hipp JF, Lewis CM, Corbetta M, Engel AK (2011) Increased functional connectivity indicates the severity of cognitive impairment in multiple sclerosis. Proc Natl Acad Sci U S A 108(47):19066–19071
Hidalgo de la Cruz M, d’Ambrosio A, Valsasina P, Pagani E, Colombo B, Rodegher M et al (2018) Abnormal functional connectivity of thalamic sub-regions contributes to fatigue in multiple sclerosis. Mult Scler 24(9):1183–1195. https://doi.org/10.1177/1352458517717807
Huang MH, Zhou FQ, Wu L, Wang B, Wan H, Li FJ et al (2018) Synchronization within, and interactions between, the default mode and dorsal attention networks in relapsing-remitting multiple sclerosis. Neuropsychiatr Dis Treat 14:1241–1252
Inglese M, Park SJ, Johnson G, Babb JS, Miles L, Jaggi H et al (2007) Deep gray matter perfusion in multiple sclerosis: dynamic susceptibility contrast perfusion magnetic resonance imaging at 3 T. Arch Neurol 64(2):196–202
Janssen AL, Boster A, Patterson BA, Abduljalil A, Prakash RS (2013) Resting-state functional connectivity in multiple sclerosis: an examination of group differences and individual differences. Neuropsychologia 51(13):2918–2929
Jones DT, Vemuri P, Murphy MC, Gunter JL, Senjem ML, Machulda MM et al (2012) Non-stationarity in the “resting brain’s” modular architecture. PLoS One 7(6):e39731
Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP (2008) Competition between functional brain networks mediates behavioral variability. Neuroimage 39(1):527–537
Lanting CP, de Kleine E, Langers DRM, van Dijk P (2014) Unilateral tinnitus: changes in connectivity and response lateralization measured with fMRI. PLoS One 9(10):e110704
Leavitt VM, Wylie GR, Girgis PA, DeLuca J, Chiaravalloti ND (2014) Increased functional connectivity within memory networks following memory rehabilitation in multiple sclerosis. Brain Imaging Behav 8(3):394–402
Lee M, Reddy H, Johansen-Berg H, Pendlebury S, Jenkinson M, Smith S et al (2000) The motor cortex shows adaptive functional changes to brain injury from multiple sclerosis. Ann Neurol 47(5):606–613
Leonardi N, Richiardi J, Gschwind M, Simioni S, Annoni JM, Schluep M et al (2013) Principal components of functional connectivity: a new approach to study dynamic brain connectivity during rest. Neuroimage 83:937–950
Liu Y, Duan Y, Huang J, Ren Z, Ye J, Dong H et al (2015) Multimodal quantitative MR imaging of the thalamus in multiple sclerosis and neuromyelitis optica. Radiology 277(3):784–792
Liu Y, Gao JH, Liotti M, Pu Y, Fox PT (1999) Temporal dissociation of parallel processing in the human subcortical outputs. Nature 400(6742):364–367
Liu Y, Wang H, Duan Y, Huang J, Ren Z, Ye J et al (2017) Functional brain network alterations in clinically isolated syndrome and multiple sclerosis: a graph-based connectome study. Radiology 282(2):534–541
Lowe MJ, Phillips MD, Lurito JT, Mattson D, Dzemidzic M, Mathews VP (2002) Multiple sclerosis: low-frequency temporal blood oxygen level-dependent fluctuations indicate reduced functional connectivity initial results. Radiology 224(1):184–192
Lv H, Wang Z, Tong E, Williams LM, Zaharchuk G, Zeineh M et al (2018) Resting-state functional MRI: everything that nonexperts have always wanted to know. AJNR Am J Neuroradiol 39(8):1390–1399
Maieron M, Iannetti GD, Bodurka J, Tracey I, Bandettini PA, Porro CA (2007) Functional responses in the human spinal cord during willed motor actions: evidence for side- and rate-dependent activity. J Neurosci 27(15):4182–4190
Mainero C, Inghilleri M, Pantano P, Conte A, Lenzi D, Frasca V et al (2004) Enhanced brain motor activity in patients with MS after a single dose of 3,4-diaminopyridine. Neurology 62(11):2044–2050
Mattioli F, Bellomi F, Stampatori C, Capra R, Miniussi C (2016) Neuroenhancement through cognitive training and anodal tDCS in multiple sclerosis. Mult Scler 22(2):222–230
Meijer KA, Eijlers AJC, Geurts JJG, Schoonheim MM (2018) Staging of cortical and deep grey matter functional connectivity changes in multiple sclerosis. J Neurol Neurosurg Psychiatry 89(2):205–210
Mezzapesa DM, Rocca MA, Rodegher M, Comi G, Filippi M (2008) Functional cortical changes of the sensorimotor network are associated with clinical recovery in multiple sclerosis. Hum Brain Mapp 29(5):562–573
Morgen K, Sammer G, Courtney SM, Wolters T, Melchior H, Blecker CR et al (2007) Distinct mechanisms of altered brain activation in patients with multiple sclerosis. Neuroimage 37(3):937–946
Mori F, Ljoka C, Magni E, Codeca C, Kusayanagi H, Monteleone F et al (2011) Transcranial magnetic stimulation primes the effects of exercise therapy in multiple sclerosis. J Neurol 258(7):1281–1287
Northoff G, Wiebking C, Feinberg T, Panksepp J (2011) The ‘resting-state hypothesis’ of major depressive disorder-a translational subcortical-cortical framework for a system disorder. Neurosci Biobehav Rev 35(9):1929–1945
Pantano P, Iannetti GD, Caramia F, Mainero C, Di Legge S, Bozzao L et al (2002a) Cortical motor reorganization after a single clinical attack of multiple sclerosis. Brain 125(Pt 7):1607–1615
Pantano P, Mainero C, Iannetti GD, Caramia F, Di Legge S, Piattella MC et al (2002b) Contribution of corticospinal tract damage to cortical motor reorganization after a single clinical attack of multiple sclerosis. Neuroimage 17(4):1837–1843
Pareto D, Sastre-Garriga J, Alonso J, Galan I, Arevalo MJ, Renom M et al (2018) Classic block design “pseudo”-resting-state fMRI changes after a neurorehabilitation program in patients with multiple sclerosis. J Neuroimaging 28(3):313–319
Parisi L, Rocca MA, Mattioli F, Copetti M, Capra R, Valsasina P et al (2014) Changes of brain resting state functional connectivity predict the persistence of cognitive rehabilitation effects in patients with multiple sclerosis. Mult Scler 20(6):686–694
Parry AM, Scott RB, Palace J, Smith S, Matthews PM (2003) Potentially adaptive functional changes in cognitive processing for patients with multiple sclerosis and their acute modulation by rivastigmine. Brain 126(Pt 12):2750–2760
Pavisian B, MacIntosh BJ, Szilagyi G, Staines RW, O’Connor P, Feinstein A (2014) Effects of cannabis on cognition in patients with MS: a psychometric and MRI study. Neurology 82(21):1879–1887
Prosperini L, Fanelli F, Petsas N, Sbardella E, Tona F, Raz E et al (2014) Multiple sclerosis: changes in microarchitecture of white matter tracts after training with a video game balance board. Radiology 273(2):529–538
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci U S A 98(2):676–682
Rashid B, Damaraju E, Pearlson GD, Calhoun VD (2014) Dynamic connectivity states estimated from resting fMRI Identify differences among Schizophrenia, bipolar disorder, and healthy control subjects. Front Hum Neurosci 8:897
Reddy H, Narayanan S, Matthews PM, Hoge RD, Pike GB, Duquette P et al (2000) Relating axonal injury to functional recovery in MS. Neurology 54(1):236–239
Reddy H, Narayanan S, Woolrich M, Mitsumori T, Lapierre Y, Arnold DL et al (2002) Functional brain reorganization for hand movement in patients with multiple sclerosis: defining distinct effects of injury and disability. Brain 125(Pt 12):2646–2657
Richiardi J, Gschwind M, Simioni S, Annoni JM, Greco B, Hagmann P et al (2012) Classifying minimally disabled multiple sclerosis patients from resting state functional connectivity. Neuroimage 62(3):2021–2033
Rocca MA, Absinta M, Amato MP, Moiola L, Ghezzi A, Veggiotti P et al (2014) Posterior brain damage and cognitive impairment in pediatric multiple sclerosis. Neurology 82(15):1314–1321
Rocca MA, Agosta F, Colombo B, Mezzapesa DM, Falini A, Comi G et al (2007a) fMRI changes in relapsing-remitting multiple sclerosis patients complaining of fatigue after IFNbeta-1a injection. Hum Brain Mapp 28(5):373–382
Rocca MA, Agosta F, Mezzapesa DM, Falini A, Martinelli V, Salvi F et al (2004a) A functional MRI study of movement-associated cortical changes in patients with Devic’s neuromyelitis optica. Neuroimage 21(3):1061–1068
Rocca MA, Colombo B, Falini A, Ghezzi A, Martinelli V, Scotti G et al (2005b) Cortical adaptation in patients with MS: a cross-sectional functional MRI study of disease phenotypes. Lancet Neurol 4(10):618–626
Rocca MA, Falini A, Colombo B, Scotti G, Comi G, Filippi M (2002b) Adaptive functional changes in the cerebral cortex of patients with nondisabling multiple sclerosis correlate with the extent of brain structural damage. Ann Neurol 51(3):330–339
Rocca MA, Filippi M (2006) Functional MRI to study brain plasticity in clinical neurology. Neurol Sci 27(Suppl 1):S24–S26
Rocca MA, Filippi M (2007) Functional MRI in multiple sclerosis. J Neuroimaging 17(Suppl 1):36S–41S
Rocca MA, Gallo A, Colombo B, Falini A, Scotti G, Comi G et al (2004b) Pyramidal tract lesions and movement-associated cortical recruitment in patients with MS. Neuroimage 23(1):141–147
Rocca MA, Gavazzi C, Mezzapesa DM, Falini A, Colombo B, Mascalchi M et al (2003a) A functional magnetic resonance imaging study of patients with secondary progressive multiple sclerosis. Neuroimage 19(4):1770–1777
Rocca MA, Matthews PM, Caputo D, Ghezzi A, Falini A, Scotti G et al (2002a) Evidence for widespread movement-associated functional MRI changes in patients with PPMS. Neurology 58(6):866–872
Rocca MA, Mezzapesa DM, Falini A, Ghezzi A, Martinelli V, Scotti G et al (2003b) Evidence for axonal pathology and adaptive cortical reorganization in patients at presentation with clinically isolated syndromes suggestive of multiple sclerosis. Neuroimage 18(4):847–855
Rocca MA, Mezzapesa DM, Ghezzi A, Falini A, Agosta F, Martinelli V et al (2003c) Cord damage elicits brain functional reorganization after a single episode of myelitis. Neurology 61(8):1078–1085
Rocca MA, Mezzapesa DM, Ghezzi A, Falini A, Martinelli V, Scotti G et al (2005a) A widespread pattern of cortical activations in patients at presentation with clinically isolated symptoms is associated with evolution to definite multiple sclerosis. AJNR Am J Neuroradiol 26(5):1136–1139
Rocca MA, Pagani E, Absinta M, Valsasina P, Falini A, Scotti G et al (2007b) Altered functional and structural connectivities in patients with MS: a 3-T study. Neurology 69(23):2136–2145
Rocca MA, Pagani E, Ghezzi A, Falini A, Zaffaroni M, Colombo B et al (2003d) Functional cortical changes in patients with multiple sclerosis and nonspecific findings on conventional magnetic resonance imaging scans of the brain. Neuroimage 19(3):826–836
Rocca MA, Pravata E, Valsasina P, Radaelli M, Colombo B, Vacchi L et al (2015) Hippocampal-DMN disconnectivity in MS is related to WM lesions and depression. Hum Brain Mapp 36(12):5051–5063
Rocca MA, Tortorella P, Ceccarelli A, Falini A, Tango D, Scotti G et al (2008) The “mirror-neuron system” in MS: a 3 tesla fMRI study. Neurology 70(4):255–262
Rocca MA, Valsasina P, Absinta M, Riccitelli G, Rodegher ME, Misci P et al (2010) Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology 74(16):1252–1259
Rocca MA, Valsasina P, Leavitt VM, Rodegher M, Radaelli M, Riccitelli GC et al (2018) Functional network connectivity abnormalities in multiple sclerosis: correlations with disability and cognitive impairment. Mult Scler 24(4):459–471
Rocca MA, Valsasina P, Martinelli V, Misci P, Falini A, Comi G et al (2012) Large-scale neuronal network dysfunction in relapsing-remitting multiple sclerosis. Neurology 79(14):1449–1457
Rocca MA, Valsasina P, Meani A, Falini A, Comi G, Filippi M (2016) Impaired functional integration in multiple sclerosis: a graph theory study. Brain Struct Funct 221(1):115–131
Roelcke U, Kappos L, Lechner-Scott J, Brunnschweiler H, Huber S, Ammann W et al (1997) Reduced glucose metabolism in the frontal cortex and basal ganglia of multiple sclerosis patients with fatigue: a 18F-fluorodeoxyglucose positron emission tomography study. Neurology 48(6):1566–1571
Rombouts SA, Lazeron RH, Scheltens P, Uitdehaag BM, Sprenger M, Valk J et al (1998) Visual activation patterns in patients with optic neuritis: an fMRI pilot study. Neurology 50(6):1896–1899
Roosendaal SD, Schoonheim MM, Hulst HE, Sanz-Arigita EJ, Smith SM, Geurts JJ et al (2010) Resting state networks change in clinically isolated syndrome. Brain 133(Pt 6):1612–1621
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52(3):1059–1069
Sandroff BM, Wylie GR, Sutton BP, Johnson CL, DeLuca J, Motl RW (2018) Treadmill walking exercise training and brain function in multiple sclerosis: preliminary evidence setting the stage for a network-based approach to rehabilitation. Mult Scler J Exp Transl Clin 4(1):2055217318760641
Sbardella E, Tona F, Petsas N, Upadhyay N, Piattella MC, Filippini N et al (2015) Functional connectivity changes and their relationship with clinical disability and white matter integrity in patients with relapsing–remitting multiple sclerosis. Mult Scler J 21(13):1681–1692
Sbardella E, Upadhyay N, Tona F, Prosperini L, De Giglio L, Petsas N et al (2017) Dentate nucleus connectivity in adult patients with multiple sclerosis: functional changes at rest and correlation with clinical features. Mult Scler 23(4):546–555
Schoonheim MM, Geurts J, Wiebenga OT, De Munck JC, Polman CH, Stam CJ et al (2014) Changes in functional network centrality underlie cognitive dysfunction and physical disability in multiple sclerosis. Mult Scler 20(8):1058–1065
Shu N, Duan Y, Xia M, Schoonheim MM, Huang J, Ren Z et al (2016) Disrupted topological organization of structural and functional brain connectomes in clinically isolated syndrome and multiple sclerosis. Sci Rep 6:29383
Shulman GL, Corbetta M, Buckner RL, Raichle ME, Fiez JA, Miezin FM et al (1997) Top-down modulation of early sensory cortex. Cereb Cortex 7(3):193–206
Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE et al (2009) Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A 106(31):13040–13045
Smitha KA, Raja KA, Arun KM, Rajesh PG, Thomas B, Kapilamoorthy TR et al (2017) Resting state fMRI: a review on methods in resting state connectivity analysis and resting state networks. Neuroradiol J 30(4):305–317
Stroman PW, Bosma RL, Tsyben A (2012) Somatotopic arrangement of thermal sensory regions in the healthy human spinal cord determined by means of spinal cord functional MRI. Magn Reson Med 68(3):923–931
Sweet LH, Rao SM, Primeau M, Durgerian S, Cohen RA (2006) Functional magnetic resonance imaging response to increased verbal working memory demands among patients with multiple sclerosis. Hum Brain Mapp 27(1):28–36
Tavazzi E, Bergsland N, Cattaneo D, Gervasoni E, Lagana MM, Dipasquale O et al (2018) Effects of motor rehabilitation on mobility and brain plasticity in multiple sclerosis: a structural and functional MRI study. J Neurol 265(6):1393–1401
Tomasi D, Volkow ND (2010) Functional connectivity density mapping. Proc Natl Acad Sci U S A 107(21):9885–9890
Tomassini V, Matthews PM, Thompson AJ, Fuglo D, Geurts JJ, Johansen-Berg H et al (2012) Neuroplasticity and functional recovery in multiple sclerosis. Nat Rev Neurol 8(11):635–646
Tona F, De Giglio L, Petsas N, Sbardella E, Prosperini L, Upadhyay N et al (2018) Role of cerebellar dentate functional connectivity in balance deficits in patients with multiple sclerosis. Radiology 287(1):267–275
Tononi G, Sporns O, Edelman GM (1994) A measure for brain complexity: relating functional segregation and integration in the nervous system. Proc Natl Acad Sci U S A 91(11):5033–5037
Toosy AT, Hickman SJ, Miszkiel KA, Jones SJ, Plant GT, Altmann DR et al (2005) Adaptive cortical plasticity in higher visual areas after acute optic neuritis. Ann Neurol 57(5):622–633
van den Heuvel MP, Hulshoff Pol HE (2010) Specific somatotopic organization of functional connections of the primary motor network during resting state. Hum Brain Mapp 31(4):631–644
van Geest Q, Boeschoten RE, Keijzer MJ, Steenwijk MD, Pouwels PJ, Twisk JW et al (2019) Fronto-limbic disconnection in patients with multiple sclerosis and depression. Mult Scler 25(5):715–726. https://doi.org/10.1177/1352458518767051
Waxman SG (1998) Demyelinating diseases—new pathological insights, new therapeutic targets. N Engl J Med 338(5):323–325
Werring DJ, Bullmore ET, Toosy AT, Miller DH, Barker GJ, MacManus DG et al (2000) Recovery from optic neuritis is associated with a change in the distribution of cerebral response to visual stimulation: a functional magnetic resonance imaging study. J Neurol Neurosurg Psychiatry 68(4):441–449
Wu GF, Brier MR, Parks CA, Ances BM, Van Stavern GP (2015) An eye on brain integrity: acute optic neuritis affects resting state functional connectivity. Invest Ophthalmol Vis Sci 56(4):2541–2546
Zhou F, Zhuang Y, Gong H, Wang B, Wang X, Chen Q et al (2014) Altered inter-subregion connectivity of the default mode network in relapsing remitting multiple sclerosis: a functional and structural connectivity study. PLoS One 9(7):e101198
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rocca, M.A., De Meo, E., Filippi, M. (2020). Resting-State fMRI in Multiple Sclerosis. In: Ulmer, S., Jansen, O. (eds) fMRI. Springer, Cham. https://doi.org/10.1007/978-3-030-41874-8_23
Download citation
DOI: https://doi.org/10.1007/978-3-030-41874-8_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-41873-1
Online ISBN: 978-3-030-41874-8
eBook Packages: MedicineMedicine (R0)