Abstract
Background
Rehabilitation seems to promote brain plasticity, but objective measures of efficacy are lacking and there is a limited understanding of the mechanisms underlying functional recovery.
Objective
To study functional and structural brain changes induced by gait rehabilitation.
Methods
We enrolled MS inpatients (EDSS 4.5–6.5) undergoing a 4-week neurorehabilitation. Several clinical measures were obtained, including: 2-min walk test (2MWT), dynamic gait index (DGI), Berg balance scale (BBS). Furthermore, motor-task functional MRI (fMRI) of plantar dorsiflexion, resting state fMRI, and regional diffusion tensor imaging (DTI) metrics were obtained. All the assessments were performed at baseline (T0), after the end of the rehabilitation period (T1) and 3 months later (T2).
Results
Twenty-nine patients were enrolled at T0, 26 at T1, and 16 completed all timepoints. At T1, there was a significant improvement of 2MWT, DGI, and BBS scores, along with a reduced extent of the widespread activation related to the motor task at the fMRI and an increased functional connectivity in the precentral and post-central gyrus, bilaterally. None of these changes were maintained at T2.
Conclusions
Our findings show a short-term beneficial effect of motor rehabilitation on gait performances in MS, accompanied by brain functional reorganization in the sensory-motor network.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a progressive central nervous system (CNS) disease characterized by long-term disability accrual. Available treatments are mainly effective on the inflammatory mechanisms typical of the relapsing–remitting phase of MS, whereas their impact on the neurodegenerative process is limited.
Therefore, neuromotor and cognitive rehabilitation are of crucial importance as they may represent the main approach for treating the progressive forms of MS [1]. However, data on their effect in MS are few and often contradictory, leading to lack of evidence-based practice guidelines [2, 3]. Key issues correspond to the absence of algorithms to provide tailored treatments and a poor understanding of the mechanisms underlying recovery. With respect to the latter, non-conventional MRI techniques such as functional MRI (fMRI) and diffusion tensor imaging (DTI) may be helpful, as they can investigate brain properties and functionality at a microstructural level. In particular, task-oriented fMRI allows to visualize brain areas activated by different types of stimuli, whereas DTI reflects tissue integrity. The functional connectivity of the brain can be investigated also independently of any external stimulus with resting state fMRI.
The ability of the brain to functionally and structurally reorganize itself, so-called brain plasticity, has been demonstrated in MS both following an acute relapse and as a result of a chronic insult [4,5,6,7,8,9]. Whereas cortical reorganization in response to cognitive rehabilitation has been the subject of numerous studies [9,10,11,12,13,14], only few reports have investigated the effect of motor rehabilitation [15,16,17,18,19,20], all of them in an out-patient setting, and mainly with a low frequency of motor sessions/week.
Against this background, we planned the current study to investigate the effect of an intensive motor rehabilitation program on MS inpatients with mild-to-moderate disability, both on clinical outcomes and functional and structural MRI parameters.
Methods
Study design
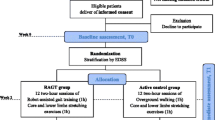
The design of this longitudinal randomized study included two arms of rehabilitation treatment, consisting of either resistance or endurance training, with 32 MS patients in each. Sample sizes were calculated based on power calculations with simulated data, which showed that 30–35 subjects per arm would result in 80% power when mean difference in improvement was greater than a Z score of 0.7 on the 2-min walk test (2MWT). However, due to funding restrictions, we could not recruit the number of subjects originally planned. Accordingly, we were not able to assess the secondary aim of the study, i.e., to explore which rehabilitation treatment had a greater impact on MRI parameters.
Patients enrolled in the study underwent clinical and MRI examinations at baseline (T0), at the end of the 4-week rehabilitation period (T1) and 3 months later (T2). After patient discharge at the end of T1, patients were advised to maintain physical activity to the best of their ability, but were not followed in an out-patient setting for the sake of the protocol.
Subjects
We recruited MS patients admitted for hospital-based rehabilitation. To be included patients had to be relapse- and steroid-free for at least 1 month prior the beginning, right-handed and with an Expanded Disability Status Scale (EDSS) ≤ 6.5. All patients provided written informed consent, and the local ethical committee of our institution approved the study.
Clinical assessments
Clinical and neurological examinations were performed and disability was quantified by means of EDSS. Patients also underwent the 2MWT, the timed 25-foot walk (T25FW); the dynamic gait index (DGI). The following scales or questionnaires were also completed: Berg balance scale (BBS), MS walking scale-12 items (MSWS-12), and modified fatigue impact scale (MFIS). At T1 and T2, patients were assessed by a blinded evaluator not involved in the rehabilitation sessions.
Rehabilitation approach
Participants underwent two treatment sessions/day, 5 days/week, during the 4-week admission period, each session lasting 30–45 min. In the first session, participants were treated to improve global physical functioning, while in the second session, they were randomized to resistance or endurance training. A thorough description of the aforementioned sessions is reported in the Supplementary file.
MRI acquisition
Patients were scanned on a 1.5 T MRI scanner (Siemens Magnetom Avanto, Erlangen, Germany) with a 12-channel head matrix coil. The imaging protocol included the following sequences: (1) three-dimensional T1-weighted magnetization-prepared rapid gradient echo (MP-RAGE) [TR 1900 ms; TE 3.37 ms; inversion time (TI) 1100 ms; flip angle 15; 176 contiguous, 1-mm-thick axial slices; 1-mm2 in-plane resolution]; (2) 2D gradient echo, echo-planar imaging (EPI) for task-based fMRI (TR 3000, TE 40 ms; 40 3-mm-thick axial slices with 0.3-mm gap, 3-mm2 in-plane resolution; 200 volumes, plus eight initial volumes to allow for the system to reach steady state); (3) 2D gradient echo EPI for resting state fMRI (TR 3020 ms, TE 50 ms; 32 3.5-mm-thick axial slices with 0.35 gap; 3.125-mm2 in-plane resolution; 190 volumes); (4) diffusion-weighted (DW) pulsed-gradient spin echo-planar (TR 7000 ms; TE 94 ms; 50 contiguous, 2.5-mm-thick axial slices; 2-mm2 in-plane resolution; diffusion gradients applied in 12 non-collinear directions with a b value 900 s/mm2; number of runs 2). A dual-echo, gradient echo field map sequence (TE1 = 4.76 ms, TE2 = 9.52 ms) was also collected for correcting geometric distortions inherent in EPI sequences. All slices were acquired parallel to the subcallosal plane.
Motor-task fMRI analysis
Patients performed foot movements of plantar dorsiflexion in separate acquisitions (10 blocks of active task versus rest, 30 s each). Left and right feet were moved in separate imaging acquisitions. All patients were trained on the movement prior to as well as after being positioned in the scanner. During the initial setup of the study, considerable intra-subject variability was observed in patient capability. As such, patients were instructed to perform the dorsi-plantar flexion as quickly as possible while still maintaining a fluid movement rather than using a fixed rate. During the acquisition, foot movements were monitored to ensure compliance.
Individual first-level task fMRI data were processed using the functional MRI (fMRI) Expert Analysis Tool (FEAT) Version 6.00, part of the FSL (FMRIB’s Software Library, http://www.fmrib.ox.ac.uk/fsl). Pre-statistics processing was applied as follows: motion correction; correction for susceptibility-induced geometric distortions; brain extraction, spatial smoothing using a Gaussian kernel of full-width half-maximum 5 mm; and high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma 50.0 s). The estimated motion parameters for each participant were included as covariates of no interest to reduce spurious activations due to head motion and scanner drift. The resulting activation maps were normalized via non-linear registration of the MP-RAGE to the MNI 2-mm brain template. Higher level (group) statistical analysis was performed using FLAME (FMRIB’s Local Analysis of Mixed Effects) and maps were thresholded using cluster correction for multiple comparisons with a cluster-forming threshold of Z > 2.3 and cluster-size significance of p < 0.01.
Resting state fMRI analysis
Resting state fMRI data were pre-processed with motion correction, correction for susceptibility-induced geometric distortions and independent component analysis identification using MELODIC software. Next, FMRIB’s ICA-based Xnoiseifier (FIX) [21] was used to automatically classify good and bad components. All FIX outputs were quality controlled to ensure accurate classification. Then, each participant’s cleaned data set was spatially smoothed with a 5-mm full-width half-maximum Gaussian kernel, filtered with a high-pass temporal filter (cut-off frequency of 0.01 Hz), co-registered to its structural scan and normalized to the standard MNI152 space with 2 × 2 × 2 mm3 resampling. Next, the data were temporally concatenated across subjects and sessions and group ICA was performed using MELODIC. Out of 20 independent components obtained with the group ICA, 14 of them were classified as resting state networks and used in the dual regression approach [22] and functional connectivity maps were subtracted from one another to assess changes over the follow-up using a one-sample t test. We limited the assessment to only within the sensorimotor network (defined by group ICA) to reduce the number of statistical comparisons.
Diffusion tensor imaging analysis
Pre-processing of the diffusion data included correction for eddy currents and subject with the eddy_correct tool, which is part of FSL, and correction for susceptibility-induced geometric distortions. The diffusion tensor was then computed on a voxelwise basis and DTI-derived metrics of fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) were obtained. Using non-linear registration of probabilistic atlases, DTI parameters were calculated within the corticospinal tract, cingulum, and callosal body, as previously described [23]. Lesions were excluded from the cost function during registration to reduce their impact on the quality of the spatial normalization. Moreover, atlas probability values were used as weighting factor, such that voxels in the center of the tracts were emphasized and areas with increased variability contributed less to the final measure. This approach overcomes the difficulty of tracking through white matter lesions that can occur when using tractographic fiber bundle reconstruction. As an exploratory analysis, we also performed tract-based spatial statistics (TBSS) and compared changes within the white matter skeleton between timepoints [24]. Nonparametric permutation analysis was used as implemented in the randomise tool (with 5000 permutations).
Statistical analyses
Statistical analyses were performed using SPSS (version 21; IBM Corp., Armonk, NY, USA). Between T0 and T1, changes in clinical outcomes were assessed using paired t tests for patients who completed both timepoints. Repeated measures designs were used in the group of patients that completed all the three timepoints. Baseline differences between patients who completed the study versus those who did not were compared using the Student’s t and Kruskal–Wallis tests, as appropriate. Significance was set at p < 0.05. Correction for multiple comparisons in investigating clinical changes was performed using the Benjamini–Hochberg procedure to control for the false discovery rate.
Prior to enrolling the first patient, reliability of the MRI post-processing pipelines was assessed in three MS patients that were imaged twice in a week. The motor-task fMRI elicited similar patterns and magnitude of activation, the resting state fMRI analysis showed the same networks, and DTI-derived metrics were highly correlated (intraclass correlation coefficients > 0.9). Additional details are provided in the Supplementary material.
Results
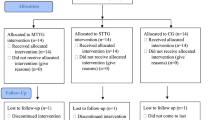
Twenty-nine MS patients (65.5% female) were enrolled. The CONSORT flow diagram of the study is depicted in Fig. 1. At baseline, the mean age was 51.2 (SD 9.5) years, mean disease duration was 17.2 (SD 6.7) years, and median EDSS was 6.0 (range 4.5–6.5). Of the entire cohort, 12 had relapsing–remitting MS (RRMS), 14 secondary progressive MS (SPMS), and 3 primary progressive MS (PPMS). Two RRMS patients had experienced a relapse in 6 months preceding the admission (T0), while 12 progressive MS patients had a neurological deterioration in the same time frame. When considering the interval between 7 and 12 months before T0, 7 progressive patients had experienced a clinical worsening, which continued during the following 6 months in three of them. Ninety percent (26/29) of the patients completed the assessments at T1, and 55% (16/29) completed all the three timepoints. Reasons for patient attrition were: early discharge due to personal reasons (2), patient’s decision to discontinue (3), lost to follow-up (2), and unwillingness to travel long-distance back to the hospital for T2 assessment (3).
Due to the limited sample size, and since no significant group differences at T1 and T2 were found for clinical and MRI results between patients undergoing resistance training (n = 14 at T0, 13 at T1, and 9 at T2) and those who underwent endurance training (n = 15 at T0, 13 at T1, and 7 at T2) (data not shown), findings from the whole study cohort will be reported hereafter.
Clinical results
The scores obtained at the scales/questionnaires at T0 and T1 are reported in Table 1. The static balance, represented by the BBS score, significantly improved after rehabilitation, as well as the walking endurance (2MWT). Changes of DGI, which reflects gait adaptability and fall risk, are also consistent with the above-mentioned results. These results remained significant even after controlling for the false discovery rate. Patient-reported outcomes of walking ability (MSWS-12) and fatigue (MFIS) improved, although their changes did not reach statistical significance. Clinical findings from patients who completed all study timepoints are reported in Table 2. In this reduced sample of the entire cohort, only the BBS score showed a statistically significant improvement at T1, which was not maintained at T2. All the other parameters showed a trend towards improvement at T1, but were either stable or tended to revert back to the initial value at T2. No baseline differences were detected between patients who completed the study and those who did not.
Motor-task fMRI
Examining the plots of head movements following motion correction did not reveal greater motion during the “task active” period compared to the rest period. This was also confirmed with visual inspection of the data.
There was a wide variability in the patterns of activation at T0: some subjects presented a more focal contralateral activation mostly confined to motor areas, while others showed a widespread, bihemispheric activation. Figures 2 and 3 represent the activated areas in response to the motor task for all the individuals who completed it with both feet. At a group level, at T0, patients demonstrated a diffuse activation involving motor and somatosensory areas in both hemispheres for both foot motor tasks (Figs. 2a, 3a). The peak activation, for the motor task of each foot, was in the contralateral precentral gyrus. At T1, the group showed a reduction of the scattered activation with a more focal activation involving motor areas (Figs. 2b, 3b). The direct comparison between subjects who completed the right foot motor task at T0 and T1 showed a significant reduction in the activation of the left precentral gyrus (Fig. 4). The same analysis comparing activation of the left foot at T0 and T1 did not yield any significant results. At T2, the group reverted to the same pattern of activation at baseline, with no significant differences in activation between T0 and T2. The patterns of activation and their changes over time were not related to 2MWT, T25FW, MFIS scores or to the pyramidal EDSS subscore.
Group activation maps of all the subjects completing the motor task (plantar dorsiflexion) with the left foot at T0 (a) and T1 (b). Widespread bilateral activation was observed at T0, with peak activation in the right precentral gyrus. At T1, an overall reduction in the extent and magnitude of activation was seen. The color bar represents the Z score with warmer colors representing higher scores
Group activation maps of all the subjects completing the motor task (plantar dorsiflexion) with the right foot at T0 (a) and T1 (b). Widespread bilateral activation was observed at T0, with peak activation in the left precentral gyrus. At T1, an overall reduction in the extent and magnitude of activation was seen. The color bar represents the Z score with warmer colors representing higher scores
Areas of significantly decreased activation at T1 versus T0 for the motor task (plantar dorsiflexion) of the right foot. As evidenced, the changes were localized to the left precentral and post-central gyri. The color bar represents the Z score with warmer colors representing greater changes in activation
Resting state fMRI
At T1, both the precentral gyrus (representing the primary motor cortex) and the post-central gyrus (site of the primary somatosensory cortex) showed an increase of bilateral connectivity, albeit significance was only at puncorr < 0.01. Neither of these modifications were maintained ad T2.
Diffusion tensor imaging
None of the considered DTI measures showed a statistically significant modification, in any of the analyzed structures, either at T1 or at T2. However, a trend towards an increase in FA was found in both the left (p = 0.061) and right (p = 0.067) cingulum at T1. The exploratory TBSS yielded no areas of significant changes over time. Finally, none of the changes in DTI parameters were related to clinical measures.
Discussion
This study demonstrated a short-term positive effect of intensive neuromotor rehabilitation of the lower limbs on both clinical and functional MRI outcomes in MS patients with mild-to-moderate disability, without any evidence of structural brain modifications.
The traditional concept of the brain as a “static” organ has recently been undermined by the evidence of not only functional, but also structural changes in response to different stimuli. Different studies aimed to evaluate the ability of the brain to gradually adapt and reorganize itself in response to a progressive tissue damage involving both white matter (WM) and grey matter (GM) in MS [4, 5, 8, 25]. All the aforementioned studies described adaptive brain tissue changes that aid in preserving functional abilities. Such effects probably also account for the discrepancy between the degree of disability and the amount of structural damage as quantified by conventional MRI measures [26]. The fMRI analysis of our patients at T0 showed widespread bihemispheric activation of several cortical areas in response to a motor stimulus, i.e., the plantar foot dorsiflexion, although the peak of activation was still, as expected, in the precentral gyrus, site of the primary motor cortex. Considering the level of disability and disease duration of our cohort, these data might be explained by the occurrence of several clinical relapses (i.e., episodes of acute brain damage) in their past history and/or the persistence of a chronic, subtle tissue damage, leading to a progressively broader recruitment of different areas to adequately perform the motor task [6, 7, 27]. At the end of an intense neuromotor rehabilitation program, completed in an inpatient setting, our cohort showed a reduction in the extent of activation, although not statistically significant, with a more focal activation of the areas specifically correlated with the execution of the motor task. This seems to indicate that despite the relatively long disease duration, adaptive mechanisms favoring the recovery of lost or reduced functions are still potentially active, as previously observed [25]. Indeed, we recently demonstrated that intensive rehabilitation in MS can have a significant short-term impact on brain functional reorganization even in the presence of superimposed tissue damage provoked by progressive multifocal leukoencephalopathy [28]. Furthermore, the direct comparison between T1 and T0 of the right foot task showed a significant reduction in the peak activation in the contralateral primary motor cortex. Interestingly, a previous study described the reduction of an abnormally high fMRI activation in the recovery phase from an acute relapse, interpreting it as a positive adaptive mechanism leading towards function recovery [4]. Another study demonstrated greater peak activation in MS patients compared to HCs in the contralateral dorsal premotor cortex as a response to thumb movements [29]. Furthermore, peak activation in HCs was reduced following a 30-min training session in that study. Thus, reduced peak activation at T1 in our study might correspond a form of “recovery” favored by rehabilitation. In this context, attenuated activation might possibly reflect a reduced need for an increased level of activity.
We also applied resting state fMRI to investigate possible modifications of the brain functional connectivity in relation with rehabilitation. There are some reports on the positive functional effect of cognitive training [10, 13] and one describing an improvement in the balance of interhemispheric connectivity in patients with reduced spasticity after undergoing intermittent theta burst stimulation [30], but to the best of our knowledge, this is the first study reporting on resting state fMRI changes in MS related to motor rehabilitation. The increased functional connectivity that we found in the primary motor and sensorimotor cortex at the end of the 4-week rehabilitation period is in line with the clinical findings showing an improvement in several scales and questionnaires involving gait function and balance, as well as with the motor-task fMRI data. However, these results should be interpreted with caution given that they did not survive a more stringent correction for multiple comparisons.
The inpatient setting represents a unique environment in which admitted patients can dedicate time to exercise training every day. This is important as MS subjects are less physically active than non-diseased populations in their daily life [31]. Furthermore, evidence coming both from healthy individuals [32, 33] and MS patients (20) shows that structural changes related to learning new skills or exercise training are generally not maintained over extended periods of time. In these studies, a reversion to baseline was seen after the subjects were no longer regularly exposed to the stimulus. To further support our findings, in a study in which GM changes were still evident after 3 months, the participants had also maintained their performance of the learned task [34]. Therefore, our results could be interpreted considering the difficulty to maintain the same level of physical activity after discharge. However, since only 16 patients completed all three timepoints, we cannot rule out the possibility that the lack of significant differences between T2 and T0 is a result of the small sample size.
Contrarily to previous reports [18, 20], we did not detect any structural modification of the brain tissue in response to physiotherapy, which might be the consequence of the small sample size and the relatively high drop-out rate. Another possibility is that the we did not use tractographic reconstruction as in the above-cited papers, but rather utilized probabilistic atlases. In our cohort, the former approach was not reliable in many subjects due to severe WM pathology. However, a trend towards a bilateral increase of FA in the cingulum was observed at T1, supporting the clinical finding of a significant reduction of the MFIS score. Indeed, recent findings point at the cingulum as a key structure involved in subjective fatigue perception [35].
Our study is not without limits. First, the relatively small sample size, related to budget limitations, along with the high drop-out rate, likely had an impact on our statistical power. This fact also prevented us from performing sub-group analyses to compare subjects with different clinical characteristics (e.g., active versus non-active disease, stable versus worsening disease). Second, foot plantar dorsiflexion is a difficult task to perform and maintain over time, as the presence of muscle weakness, spasticity, and fatigability might interfere with the performance. Nevertheless, our key findings were in fact with task fMRI, whereas changes detected using resting state fMRI and DTI were much weaker. Finally, the lack of a control group of healthy subjects limits our ability to draw firm conclusions about how strong the changes were in terms of reduced activation in the right foot motor task at T1.
In conclusion, our study demonstrates the short-term beneficial effect of intensive neuromotor rehabilitation of the lower limbs on several gait-related functions such as endurance and balance, together with a subject improvement mainly related to a decreased sensation of fatigue. Moreover, we showed the impact of motor rehabilitation on brain plasticity, in terms of favorable functional changes related to function recovery. These results should encourage additional studies to help define evidence-based guidelines for better rehabilitation approaches. In addition, such studies may also identify relevant lifestyle changes that can ensure the persistence of a long-term effect of physiotherapy for MS patients affected by motor disability.
References
Ontaneda D, Thompson AJ, Fox RJ, Cohen JA (2017) Progressive multiple sclerosis: prospects for disease therapy, repair, and restoration of function. Lancet 389(10076):1357–1366
Khan F, Turner-Stokes L, Ng L, Kilpatrick T (2007) Multidisciplinary rehabilitation for adults with multiple sclerosis. Cochrane Database Syst Rev 2:CD006036
Motl RW, Sandroff BM, Kwakkel G, Dalgas U, Feinstein A, Heesen C et al (2017) Exercise in patients with multiple sclerosis. Lancet Neurol. 16(10):848–856
Reddy H, Narayanan S, Arnoutelis R, Jenkinson M, Antel J, Matthews PM et al (2000) Evidence for adaptive functional changes in the cerebral cortex with axonal injury from multiple sclerosis. Brain 123(Pt 11):2314–2320
Lee M, Reddy H, Johansen-Berg H, Pendlebury S, Jenkinson M, Smith S et al (2000) The motor cortex shows adaptive functional changes to brain injury from multiple sclerosis. Ann Neurol 47(5):606–613
Rocca MA, Colombo B, Falini A, Ghezzi A, Martinelli V, Scotti G et al (2005) Cortical adaptation in patients with MS: a cross-sectional functional MRI study of disease phenotypes. Lancet Neurol 4(10):618–626
Mezzapesa DM, Rocca MA, Rodegher M, Comi G, Filippi M (2008) Functional cortical changes of the sensorimotor network are associated with clinical recovery in multiple sclerosis. Hum Brain Mapp 29(5):562–573
Tomassini V, Matthews PM, Thompson AJ, Fuglo D, Geurts JJ, Johansen-Berg H et al (2012) Neuroplasticity and functional recovery in multiple sclerosis. Nat Rev Neurol 8(11):635–646
Prosperini L, Piattella MC, Gianni C, Pantano P (2015) Functional and structural brain plasticity enhanced by motor and cognitive rehabilitation in multiple sclerosis. Neural Plast 2015:481574
De Giglio L, Tona F, De Luca F, Petsas N, Prosperini L, Bianchi V et al (2016) Multiple sclerosis: changes in thalamic resting-state functional connectivity induced by a home-based cognitive rehabilitation program. Radiology 280(1):202–211
Chiaravalloti ND, Wylie G, Leavitt V, Deluca J (2012) Increased cerebral activation after behavioral treatment for memory deficits in MS. J Neurol 259(7):1337–1346
Cerasa A, Gioia MC, Valentino P, Nistico R, Chiriaco C, Pirritano D et al (2013) Computer-assisted cognitive rehabilitation of attention deficits for multiple sclerosis: a randomized trial with fMRI correlates. Neurorehabil Neural Repair 27(4):284–295
Parisi L, Rocca MA, Valsasina P, Panicari L, Mattioli F, Filippi M (2014) Cognitive rehabilitation correlates with the functional connectivity of the anterior cingulate cortex in patients with multiple sclerosis. Brain Imaging Behav 8(3):387–393
Parisi L, Rocca MA, Mattioli F, Copetti M, Capra R, Valsasina P et al (2014) Changes of brain resting state functional connectivity predict the persistence of cognitive rehabilitation effects in patients with multiple sclerosis. Mult Scler 20(6):686–694
Rasova K, Prochazkova M, Tintera J, Ibrahim I, Zimova D, Stetkarova I (2015) Motor programme activating therapy influences adaptive brain functions in multiple sclerosis: clinical and MRI study. Int J Rehabil Res 38(1):49–54
Rasova K, Krasensky J, Havrdova E, Obenberger J, Seidel Z, Dolezal O et al (2005) Is it possible to actively and purposely make use of plasticity and adaptability in the neurorehabilitation treatment of multiple sclerosis patients? A pilot project. Clin Rehabil 19(2):170–181
Tomassini V, Johansen-Berg H, Jbabdi S, Wise RG, Pozzilli C, Palace J et al (2012) Relating brain damage to brain plasticity in patients with multiple sclerosis. Neurorehabil Neural Repair 26(6):581–593
Ibrahim I, Tintera J, Skoch A, Jiru F, Hlustik P, Martinkova P et al (2011) Fractional anisotropy and mean diffusivity in the corpus callosum of patients with multiple sclerosis: the effect of physiotherapy. Neuroradiology 53(11):917–926
Bonzano L, Tacchino A, Brichetto G, Roccatagliata L, Dessypris A, Feraco P et al (2014) Upper limb motor rehabilitation impacts white matter microstructure in multiple sclerosis. Neuroimage 90:107–116
Prosperini L, Fanelli F, Petsas N, Sbardella E, Tona F, Raz E et al (2014) Multiple sclerosis: changes in microarchitecture of white matter tracts after training with a video game balance board. Radiology 273(2):529–538
Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM (2014) Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage 90:449–468
Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM et al (2009) Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA. 106(17):7209–7214
Bergsland N, Lagana MM, Tavazzi E, Caffini M, Tortorella P, Baglio F et al (2015) Corticospinal tract integrity is related to primary motor cortex thinning in relapsing–remitting multiple sclerosis. Mult Scler 21(14):1771–1780
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31(4):1487–1505
Rocca MA, Gavazzi C, Mezzapesa DM, Falini A, Colombo B, Mascalchi M et al (2003) A functional magnetic resonance imaging study of patients with secondary progressive multiple sclerosis. Neuroimage 19(4):1770–1777
Barkhof F (2002) The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol 15(3):239–245
Filippi M, Preziosa P, Rocca MA (2017) Brain mapping in multiple sclerosis: lessons learned about the human brain. Neuroimage. https://doi.org/10.1016/j.neuroimage.2017.09.021
Groppo E, Baglio F, Cattaneo D, Tavazzi E, Bergsland N, Di Tella S et al (2017) Multidisciplinary rehabilitation is efficacious and induces neural plasticity in multiple sclerosis even when complicated by progressive multifocal leukoencephalopathy. Front Neurol 8:491
Morgen K, Kadom N, Sawaki L, Tessitore A, Ohayon J, McFarland H et al (2004) Training-dependent plasticity in patients with multiple sclerosis. Brain 127(Pt 11):2506–2517
Boutiere C, Rey C, Zaaraoui W, Le Troter A, Rico A, Crespy L et al (2017) Improvement of spasticity following intermittent theta burst stimulation in multiple sclerosis is associated with modulation of resting-state functional connectivity of the primary motor cortices. Mult Scler 23(6):855–863
Motl RW, McAuley E, Snook EM (2005) Physical activity and multiple sclerosis: a meta-analysis. Mult Scler 11(4):459–463
Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A (2004) Neuroplasticity: changes in grey matter induced by training. Nature 427(6972):311–312
Boyke J, Driemeyer J, Gaser C, Buchel C, May A (2008) Training-induced brain structure changes in the elderly. J Neurosci 28(28):7031–7035
Filippi M, Ceccarelli A, Pagani E, Gatti R, Rossi A, Stefanelli L et al (2010) Motor learning in healthy humans is associated to gray matter changes: a tensor-based morphometry study. PLoS ONE 5(4):e10198
Pardini M, Bonzano L, Bergamino M, Bommarito G, Feraco P, Murugavel A et al (2015) Cingulum bundle alterations underlie subjective fatigue in multiple sclerosis. Mult Scler 21(4):442–447
Acknowledgements
This study was supported by a grant from Fondazione Italiana Sclerosi Multipla (FISM) (Grant # 2013/R/26).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors report no disclosures.
Ethical statement
The study has been approved by the local ethics committee and has, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
All the subjects recruited in the study gave their written informed consent prior to their inclusion in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tavazzi, E., Bergsland, N., Cattaneo, D. et al. Effects of motor rehabilitation on mobility and brain plasticity in multiple sclerosis: a structural and functional MRI study. J Neurol 265, 1393–1401 (2018). https://doi.org/10.1007/s00415-018-8859-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8859-y