Abstract
Sentinel lymph node (SLN) biopsy is a standard procedure for axillary staging among patients with clinically node-negative breast cancer. Both the radioisotope and blue dye methods are well established for SLN identification. The indocyanine green (ICG) fluorescence method reveals subcutaneous lymphatic flow and enables the surgeon to navigate and perform sequential dissection of SLNs. The ICG fluorescence method has an equal or higher SLN identification rate compared to conventional methods, and there was no significant difference in the detection of positive SLNs between the ICG fluorescence and radioisotope methods. Hence, the ICG fluorescence method is an acceptable alternative to conventional methods for SLN detection. However, the accuracy of the ICG fluorescence method after preoperative systemic therapy has not yet been systematically evaluated; therefore, long-term follow-up data about survival and adverse events after the application of the ICG fluorescence method are needed to confirm the clinical significance of this method. The recent technological innovation in SLN mapping enables physicians to perform real-time navigation surgery for SLN biopsy. Here, we introduce the technical details and current data of SLN biopsy using the ICG fluorescence method, and describe the technical innovation of SLN mapping.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Sentinel lymph node (SLN) biopsy is part of the standard of care for patients with primary clinically node-negative breast cancer [1] . Clinical trials and meta-analyses have shown that axillary node dissection for SLN-negative patients has no survival benefit [2,3,4]. Recent studies have shown that omission of axillary node dissection for patients with limited positive SLNs has no adverse impact on survival and decreases the incidence of lymphedema [5].
Both the radioisotope (RI) and blue dye methods are well-established techniques and are used worldwide. However, the RI method involves nuclear medicine, which limits its use to relatively high-volume centers, while the blue dye method requires special surgical skill to obtain sufficient accuracy and has a risk of anaphylactic reactions.
To overcome these problems, an indocyanine green (ICG) fluorescence method was developed in 2005 [6]. A near-infrared fluorescence imaging (NIR) system reveals subcutaneous lymphatic flow and enables the surgeon to navigate and perform sequential dissection of SLNs. Several clinical trials have shown that the ICG fluorescence method has an equal or higher SLN identification rate compared to conventional methods [7,8,9]. Meta-analyses have shown that there was no significant difference between ICG fluorescence and RI for SLN detection [10, 11].
In this chapter, we introduce the technical details and current data of SLN biopsy using the ICG fluorescence method, and describe the technical innovation of SLN mapping.
Indications
SLN biopsy using the ICG fluorescence method is indicated for patients with clinically node-negative breast cancer. However, ICG is contraindicated for patients with hypersensitivity or allergy to ICG or iodine, and the safety of ICG for pregnant or lactating women has not been established.
We recommend the combined use of RI with the ICG fluorescence method, especially for obese patients or patients undergoing preoperative systemic therapy (PST). Although only preliminary data have been obtained about the accuracy of the ICG fluorescence method after PST, our exploratory analysis showed that the identification rate was 100% among patients who underwent PST (N = 70) [7]. Although 42.8% of the sentinel lymphatic pathways changed owing to PST, the locations of the SLNs were not affected by PST [12]. The accuracy of the ICG fluorescence method after PST among patients with node-positive breast cancer has not yet been systematically evaluated.
Technical Description of the Procedure
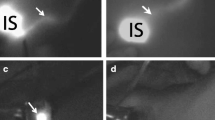
ICG (5 mg in 1 mL; Diagnogreen®; Daiichi Sankyo Co., Tokyo, Japan) was injected intradermally at the edge of the areola. Fluorescent subcutaneous lymphatics were visualized using the NIR system and traced to the axilla where occasional nodes could be observed percutaneously (Fig. 25.1). SLNs detected by the NIR system were then excised. All these procedures were performed with the operating lights turned off, as per the manufacturer’s recommendations. After SLN biopsy using the NIR system, residual SLNs were removed using RI, if used.
The indocyanine green (ICG) fluorescence technique for sentinel lymph node (SLN) biopsy. (a) After intradermal injection of ICG at the edge of the areola, subcutaneous lymphatic vessels were visualized using the near-infrared fluorescence imaging (NIR) system (images on the right side). (b, c) Fluorescence signals from SLNs can be easily recognized, after skin incision. (d) All the excised SLNs were examined using the NIR system
Interpretation
A previous meta-analysis showed that the identification rate of the ICG fluorescence method ranged from 88.6% to 100%, and there was no significant difference between the ICG fluorescence and RI methods for SLN detection [11]. There was no significant difference in the detection of positive SLNs between the ICG fluorescence and RI methods. Although SLNs identified using RI and ICG fluorescence generally overlapped, some of them are divergent [7]. Although the ICG fluorescence method may be an acceptable alternative to SLN detection using the RI method, ICG can function in a complementary manner to maximize the diagnostic performance of RI.
Currently, it is ethically difficult to evaluate the false-negative rate of the ICG fluorescence method. In addition, only few studies have investigated long-term survival after the application of ICG fluorescence method. Our retrospective study showed that axillary recurrence was observed in 6 of 1132 patients (0.53%) who underwent the ICG method between May 2007 and December 2015, and most of the patients with axillary recurrence were undertreated because of the patients’ preference or age (data not shown). Long-term follow-up data regarding the survival or adverse events after the application of the ICG fluorescence method are required to confirm the clinical significance of this method.
Pitfalls
The detectable depth of the ICG fluorescence signal is limited. We usually use a plastic capsule to push and make the subcutaneous tissue thinner to help in the recognition of the fluorescence signals from SLNs percutaneously.
If the fluorescence signals from the SLNs cannot be detected percutaneously, surgeons should follow the lymphatic vessels and then reach the deeply located SLNs. Therefore, it is important not to disrupt the lymphatic vessels during procedures. If the lymphatic vessels get damaged, SLNs can still be recognized as green lymph nodes, as observed in the blue-dye method.
The number of SLNs identified using the ICG method is relatively higher than those identified using the conventional method (range 2.3–3.4) [7, 8]. In our study, skip metastasis to second or third metastasis was observed in 6 of 28 patients with positive SLNs [13]. Hence, we recommend that four-node resection could provide precise information on the nodal status, but the number of SLNs removed should be optimized based on individual patient and tumor characteristics.
Perspectives
Although the ICG fluorescence method is highly sensitive and easy to perform, current NIR systems have several technical issues that need to be improved. The current NIR systems display the fluorescence image on remote monitors, because the fluorescence signals are invisible under direct observation of the surgical field. Therefore, surgeons have to look away from the surgical field to confirm the location of the fluorescence signals. These systems also require dimming of lights within the operative field to prevent white light contamination of images. These factors can disrupt surgical workflow and may increase the duration of surgery.
Hence, we developed a novel device, the medical imaging projection system (MIPS), to visualize fluorescence images directly on the surgical field using the projection mapping technique (Fig. 25.2; Video 25.1) [14]. The MIPS has a projection head that uses a half mirror to match the optical axis of the NIR camera and the projector. The MIPS obtains the location of the fluorescence ICG emission and projects its image onto the location of the fluorescence emission in real time, irrespective of shifting and deformation of the organ. The projector of the MIPS can also illuminate the surgical field, allowing surgeons to perform the procedure without the need for operating lights. Our exploratory study showed that the identification rate of SLNs using this device was 100% (95% CI: 94–100%). This device could be useful in real-time navigation surgery for SLN biopsy.
The medical imaging projection system (MIPS). Photograph of the MIPS, showing the projection head and pole components. The MIPS has a projection head that uses a half mirror to match the optical axis of the near-infrared fluorescence imaging camera and the projector. The MIPS obtains the location of the fluorescence indocyanine green emission and projects its image onto the location of the fluorescence emission in real time, regardless of shifting and deformation of the organ. The projector of the MIPS can also illuminate the surgical field, allowing surgeons to perform the procedure without the need for operating lights
Conclusions
SLN biopsy using the ICG fluorescence method is easy to perform and has an equal or higher SLN identification rate compared to conventional methods, making the ICG fluorescence method an acceptable alternative for SLN detection. Long-term follow-up data about survival or adverse events after the application of the ICG fluorescence method will confirm the clinical significance of this method. Nevertheless, recent technological innovation for SLN mapping enables us to perform real-time navigation surgery for SLN biopsy.
References
Lyman GH, Somerfield MR, Bosserman LD, Perkins CL, Weaver DL, Giuliano AE. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(5):561–4.
Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer. 2006;106(1):4–16.
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927–33.
Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349(6):546–53.
Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–75.
Kitai T, Inomoto T, Miwa M, Shikayama T. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer. 2005;12(3):211–5.
Sugie T, Kinoshita T, Masuda N, Sawada T, Yamauchi A, Kuroi K, et al. Evaluation of the clinical utility of the ICG fluorescence method compared with the radioisotope method for sentinel lymph node biopsy in breast cancer. Ann Surg Oncol. 2016;23(1):44–50.
Sugie T, Sawada T, Tagaya N, Kinoshita T, Yamagami K, Suwa H, et al. Comparison of the indocyanine green fluorescence and blue dye methods in detection of sentinel lymph nodes in early-stage breast cancer. Ann Surg Oncol. 2013;20(7):2213–8.
Wishart GC, Loh SW, Jones L, Benson JR. A feasibility study (ICG-10) of indocyanine green (ICG) fluorescence mapping for sentinel lymph node detection in early breast cancer. Eur J Surg Oncol. 2012;38(8):651–6.
Ahmed M, Purushotham AD, Douek M. Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol. 2014;15(8):e351–e62.
Sugie T, Ikeda T, Kawaguchi A, Shimizu A, Toi M. Sentinel lymph node biopsy using indocyanine green fluorescence in early-stage breast cancer: a meta-analysis. Int J Clin Oncol. 2017;22(1):11–7.
Tsuyuki S, Yamaguchi A, Kawata Y, Kawaguchi K. Assessing the effects of neoadjuvant chemotherapy on lymphatic pathways to sentinel lymph nodes in cases of breast cancer: usefulness of the indocyanine green-fluorescence method. Breast. 2015;24(3):298–301.
Takeuchi M, Sugie T, Abdelazeem K, Kato H, Shinkura N, Takada M, et al. Lymphatic mapping with fluorescence navigation using indocyanine green and axillary surgery in patients with primary breast cancer. Breast J. 2012;18(6):535–41.
Takada M, Takeuchi M, Suzuki E, Sato F, Matsumoto Y, Torii M, et al. Real-time navigation system for sentinel lymph node biopsy in breast cancer patients using projection mapping with indocyanine green fluorescence. Breast Cancer. 2018;25:650.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Electronic Supplementary Material
Video of sentinel lymph node (SLN) biopsy using the medical imaging projection system (MIPS). Subcutaneous lymph channels (blue) visualized using the MIPS. Real-time fluorescence imaging helps to identify the fluorescent SLN after the skin incision is made. The ICG fluorescence signal visualized using a color scale indicating the strength of the signal (red = strong; yellow = medium; blue = weak). The projected fluorescence image is automatically adjusted upon deformation of the organ (MP4 150248 kb)
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Takada, M., Toi, M. (2020). Sentinel Lymph Node Mapping. In: Aleassa, E., El-Hayek, K. (eds) Video Atlas of Intraoperative Applications of Near Infrared Fluorescence Imaging. Springer, Cham. https://doi.org/10.1007/978-3-030-38092-2_25
Download citation
DOI: https://doi.org/10.1007/978-3-030-38092-2_25
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-38091-5
Online ISBN: 978-3-030-38092-2
eBook Packages: MedicineMedicine (R0)