Abstract
Sentinel lymph node (SLN) biopsy is a standard of care for axillary staging in breast cancer. The modalities involving radioisotope (RI) and blue dye are the most widely used for SLN mapping. Near-infrared fluorescence imaging using indocyanine green (ICG) visualizes superficial lymphatic flow from tumor to SLN transcutaneously and directs the surgeon to the tumor-draining SLN in the axillary basin. This novel method achieves a high detection of SLN comparable with the RI method, and the additional use of ICG fluorescence maximizes the detection impact of RI. The ICG fluorescence method is reliable and safe and would be an acceptable alternative to SLN mapping using radioactive tracers in early breast cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The theory of the radical mastectomy with axillary lymph node dissection (ALND) was that removal of breast cancer cells as far as they extended might have benefit of not only local control of breast cancer but also survival. The alternative theory that breast cancer was a systemic disease at inception induced total mastectomy to partial mastectomy in early breast cancer treatment based upon results of randomized control trials. However, data from meta-analyses suggest that inadequate local therapy can increase risk of local recurrence [1]. ALND has been used for the past century as a means of preventing lymph node metastasis of breast cancer. Because ALND confers a high probability of complications such as arm edema (lymphedema) and neuropathy (locomotors disorder, pain, etc.), reduction in patient QOL is a major problem of ALND. In the 1990s, the concept of sentinel lymph nodes (SLNs) directly receiving lymphatic drainage from the tumor was proposed. As SLNs represent metastatic status of regional lymph nodes, removal of SLNs can be alternative to completion of ALND for diagnosis of axillary status.
Since this concept was first applied in 1992 to melanoma patients by Morton et al. [2], its application to various fields has been attempted. In the field of breast cancer, the validity of the SLN biopsy was evaluated in 1993 by Krag et al. [3] using a radioisotope (RI) and during approximately the same period by Giuliana et al. [4] using blue dye. Thereafter, many reports supporting the validity of the SLN biopsy using RI, blue dye, or the combination of both have been published. On the basis of large multicenter clinical studies, the mapping involving RI and/or blue dye becomes currently a standard method used worldwide.
2 SLN Mapping Using Near-infrared (NIR) Fluorescence Imaging Technique
At present, the standard methods achieve a high detection rate of SLN with a low false negative rate. In a meta-analysis by Kim et al., the median detection rate by the standard methods was 96 %, with a false negative rate of 7.3 % [5]. The RI method is advantageous with respect to a high SLN detection rate, but its applicability is restricted in high-volume hospitals that have nuclear medicine departments and radiation protection areas. The blue dye method is advantageous with respect to cost-effectiveness but has some drawbacks, such as a low SLN detection rate and the necessity of trained surgical skill [6].
To overcome the issues of the standard methods, Kitani et al. [7] reported in 2005 the application of indocyanine green (ICG) instead of RI and NIR fluorescence imaging system in breast cancer. The ICG fluorescence method utilizes the optic fluorescent characteristics of ICG within the NIR optic window (700–900 nm). The advantages of the NIR light include high tissue penetration due to less absorbance by water and hemoglobin and low autofluorescence. The ICG fluorescence method is currently adopted at the discretion of the physicians in view of the following advantages: (1) no risk for exposure to radiation, (2) applicable outside of large hospitals because this method does not requires nuclear medicine or a radiation protection area, (3) optimal for intraoperative SLN localization because it enables real-time imaging of lymphatic flow over the skin, and (4) requiring little skill. The technical aspects of SLN mapping using each modality are summarized in Table 9.1.
3 Current Perspective of ICG Fluorescence Method
3.1 Current Devices for ICG Fluorescence Method
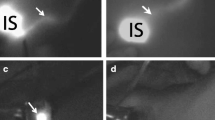
For the detection device, the NIR fluorescence imager, photodynamic eye (PDE, Hamamatsu Photonics, Hamamatsu Co., Japan) device, is widely used in the majority of clinical studies. This handheld device is composed of a light-emitting diode (LED) and a charge couple device (CCD) camera (Fig. 9.1a). The LED produces light at a wavelength of 760 nm to activate ICG, the CCD converts fluorescence light at a wavelength of 830 nm to digital imaging, and the filter of the camera cuts off the region of light at a wavelength below 820 nm. This ICG fluorescence method visualizes subcutaneous lymphatic flow in real-time (Fig. 9.1b) and directs orderly and sequential dissection to harvest SLNs (Fig. 9.1c, d). Another commercially available device is the Hyper Eye Medical System (HEMSTM, Mizuho Co. Toyo Japan). The HEMES can acquire both ICG fluorescence and color video imaging simultaneously, and this real-time imaging can direct the surgeon to the SLNs in the axilla without switching on or off the surgical light [8]. The FLARE and MIN-FLARE systems, which are not commercially available, have two light sources for the visible (400–650 nm) and NIR (760 nm) optic range and it can overlay the NIR signal on the color video image of the operation field [9]. There is no report of a direct comparison among these three devices. Each study, however, has reported highly successful detection rates, demonstrating that the diagnostic performance of ICG fluorescence is reliable and reproducible independent of the device.
3.2 Optimization of the ICG Fluorescence Signal
ICG is an amphiphilic molecule and quickly binds to plasma proteins in the vascular compartment. The protein-bound ICG fluorescence exhibits increased hydrostatic diameter, and this hydrostatic diameter is important for retention in the SLN. In general, there is a nonlinear association between ICG concentration and the intensity of an ICG fluorescence signal, and a high ICG concentration leads to decreased fluorescence intensity by fluorescence quenching. An optimal concentration of ICG absorbed to human serum albumin (ICG: HSA) was investigated for SLN biopsy in breast cancer patients. An ICG: HSA injection of 400–800 μM achieved the highest signal-to-background ratio (SBR) for SLNs compared with higher concentrations [10]. In melanoma, a dose of 600 μM ICG: HAS was optimal to obtain high SBR [11]. An ICG concentration of 0.5 % (5 mg/ml; 6.4 mM) was widely used following the original report in 2005 [7]. Although this dose is approximately tenfold higher than the optimal dose reported, a high detection rate was obtained in subsequent studies. For studies of lymphatic function or intracellular optical imaging, several compounds containing ICG, such as liposomal formulation of ICG or bovine serum albumin-coated polymeric nanocapsules loaded with ICG, have been developed [12, 13]. These new technologies could develop NIR fluorescence imaging for cancer detection and treatment.
3.3 Technical Aspects of SLN Mapping Using ICG Fluorescence
Several technical issues should be addressed to optimize lymphatic mapping for axillary staging. For the site of ICG injection, the subareolar site is acceptable in the case of intradermal or subcutaneous injection because these superficial injections yield a favorable visualization of lymphatic drainage and high fluorescence signals in the axillary basin compared with deep injection in the peritumoral site. It is still unknown whether ICG should be injected in the subareolar and the peritumoral site together or the subareolar site alone. Indeed, injection in the peritumoral site directs ICG to deep lymphatic flow and sometimes reveals extra-axillary drainage in the breast (i.e., inframammary chain). However, the main purpose of SLN biopsy is axillary staging, and we believe that subareolar injection alone is acceptable for the routine SLN mapping irrepective of tumor location [14].
After the induction of general or local anesthesia, 1 ml of ICG at a concentrate of 0.05–0.5 % is injected into the periareolar area, and a brief massage promotes the movement of ICG toward the axilla. As it takes 1–5 min for ICG to reach the axilla, NIR fluorescence imaging must be performed shortly after ICG administration. Fluorescence streaming can be detected on the skin surface by directing PDE onto the breast. During this process, blood vessels are sometimes visualized. The distinction between blood vessels and lymph ducts is easy because the flow rate of ICG through blood vessels is higher than through lymph ducts. ICG fluorescence in blood stream is quickly washed out, whereas it persists in lymphatic stream and visualizes lymphatic ducts longer.
As the fluorescence signal is attenuated by adipose tissue and cannot penetrate more than 2 cm in depth, fluorescent afferent lymphatic channels are interrupted at the site where they enter the axillary space. To press the skin over SLN using a plastic hemisphere decreases the distance between the skin and SLNs and helps to visualize fluorescent SLNs [15]. When SLNs cannot be identified over the skin, the skin incision made 2 cm distal to the site where the fluorescence signal disappears is recommended. While taking care to avoid injury of lymph ducts otherwise spoiling the operation field with spilled ICG, the anatomical and didactic dissection can be achieved in the axillary basin, and the fluorescence-emitting SLNs are exposed below the fascia [16].
Of the fluorescence-emitting lymph nodes, the lymph node to which the lymph ducts enter first is removed as the first SLN. The next fluorescence-emitting lymph nodes, if any, are removed as the second and subsequent SLNs. Furthermore, lymph nodes palpable in the operative field are also resected as SLNs. The ICG fluorescence method leads to this sequential and orderly SLN removal.
3.4 Definition of SLN Using ICG Fluorescence
SLN is defined as the first lymph node(s) to which cancer cells are most likely to spread from a primary tumor. In clinical practice, however, tracer positivity is commonly used as a surrogate for lymphatic drainage, and the identification of SLNs depends heavily on the specificity of the agent(s) used for the mapping. Using NIR fluorescence imaging system, SLNs can be categorized based on both the positivity of the fluorescent signal and the anatomical site in conjunction with lymphatic flow. For the RI method, the hottest SLN represents the definitive SLN, whereas the brightest SLN for fluorescence is only a good candidate for the definitive SLN; however, the quantification of the fluorescence signal has not yet been established. For the ICG fluorescence method, the removed SLN can be classified as follows:
-
1.
Definitive SLN: This single node is the most proximal lymph node along the subcutaneous lymphatic flow and uptakes ICG fluorescence. This node is usually harvested first during the dissection procedure and sometimes exhibits the green color when 0.5 % ICG is used. This first lymph node represents actual lymph node status in the axilla.
-
2.
Probable SLNs: These lymph nodes usually appeared adjacent to the first lymph node with fluorescence signals. One to two nodes are often harvested in the second and/or further tier.
-
3.
Less probable SLNs: Palpable lymph nodes without any fluorescence signal may be included in this category
3.5 Comparison Between ICG Fluorescence and Blue Dye
The cumulative results for the ICG fluorescence method demonstrate that the ICG fluorescence method achieved a higher SLN detection rate (99–100 %) compared with the use of blue dye [17–22]. A large prospective study [23] comparing the ICG fluorescence method and the blue dye method in detection of SLNs reported that the ICG fluorescence method detected a significantly larger number of SLNs than did the blue dye method, and the median difference in the number of SLNs identified between the two methods was one ( range 0–6, p < 0.001). The overall detection rate and the false negative rates for the ICG fluorescence and the blue dye method was 99 % and 78 % (p < 0.001) and 0 % and 30 %, respectively. A recent meta-analysis [24] also confirmed that the ICG fluorescence method is significantly superior to the blue dye method for SLN detection (OR 18.37, 95 % CI 8.63–39.10). When ICG is used under visible light, the SLN detection rate is only 73.8 %, whereas ICG can achieve a high detection rate using NIR imaging system. Van den Vorst and colleagues [25] reported that blue dye did not have any impact on SLN detection when ICG fluorescence is used in combination with radioactivity. On the basis of these results, blue dye can be spared when NIR imaging system is used.
3.6 Comparison Between ICG Fluorescence and RI
Several clinical trials have already demonstrated that the ICG fluorescence method is safe and can achieve a high SLN detection rate comparable with or superior to the RI method. Murawa et al. [26] analyzed the accuracy of SLN biopsy with the RI method and the ICG fluorescence method in 20 patients with breast cancer. In that study, the SLN detection rate for RI and ICG was 85 % and 100 %, the sensitivity in 13 lymph node metastasis-positive cases was 77 % and 92 %, and the false negative rate was 23 % and 8 %, respectively. The prospective direct comparison between the ICG fluorescence method and the RI method revealed that the SLN detection rate with the ICG fluorescence method was higher than that with the RI method (100 % v 91.3 %, p < 0.001) [27]. Other clinical trials [28, 29] also showed clinical utility of the ICG fluorescence method compared with the RI method, although these analyses do not have enough statistical power because of small cohort studies. As summarized in Table 9.2, the recent prospective study [30], which recruited 821 early breast cancer patients with clinically node-negative disease, demonstrated that there was no difference between the ICG fluorescence and the RI method for overall SLN detection rate (97.2 % v 97.0 %, p = 0.88) and for tumor-positive SLN detection rate (93.3 % v 90 %, p = 0.18). However, the additional use of ICG fluorescence with RI significantly improved the detection rate for overall and tumor-positive SLN compared with RI alone (99.8 % v 97.0 %, p < 0.001, 97.2 % v 90 %, p < 0.001, respectively). On the basis of these results, the ICG fluorescence method could be considered an alternative and additional method to SLN detection using RI in breast cancer.
3.7 SLN Biopsy After Preoperative/Neoadjuvant Systemic Therapy
SLN biopsy after preoperative/neoadjuvant systemic therapy (NACT) was previously not recommended because of a high false positive rate. However, several clinical studies have revealed that the sensitivity of SLN biopsy after NACT is not inferior to that before systemic therapy. Recent meta-analyses reported that the conventional RI method provided a detection rate of 90–90.5 % and a false negative rate of 10–12 % [31, 32] and that there is no significant difference in SLN detection between the groups before and after NACT. Based on these findings, the 2014 ASCO guidelines reported that SLN biopsy may be offered after NACT [33]. Chemotherapeutic agents, however, might cause fibrosis and obstruction of lymphatic channels, which leads to a less accurate procedure using the conventional RI method. As the hydrodynamic diameter of ICG (<1 nm) is smaller than that of RI (>50 mm), ICG may potentially reach the first SLN or the further tier more easily than RI. In the SENTIA trial [34], the false negative rate for SLN mapping was 14.2 % for patients who converted from clinically node-positive to node-negative disease after NACT. For the detection technique, the additional use of blue dye tended to improve the false negative rate. This false negative rate was also associated with the number of SLNs harvested, and the accuracy of SLN biopsy was apparently improved when more than two SLNs were harvested. ICG fluorescence yields the mean number of 2.3 SLNs removed [30] and has the potential to localize SLNs even in narrow lymphatic channels after NACT. As ICG would be a suitable method for SLN mapping after NACT, a large-scale clinical trial is required to confirm the clinical utility of ICG after NACT in patients with axillary involvement.
4 Conclusions
The cumulative results clearly demonstrate the advantage of NIR fluorescence imaging using ICG for SLN mapping in breast cancer. The ICG fluorescence method could be considered as an alternative and an accebtable additional method to SLN mapping using RI in breast cancer. This nonradioactive imaging system has the potential to be widely adopted in accordance with efforts to reduce radiation exposure.
References
Halsted CP, Benson JR, Jatoi I (2014) A historical account of breast cancer surgery: beware of local recurrence but be not radical. Future Oncol 10(9):1649–1657
Morton DL, Wen DR, Wong JH et al (1992) Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 127:392–399
Krag DN, Weaver DL, Alex JC, Fairbank JT (1993) Surgical resection and radiolocalization of the sentinel node in breast cancer using a gamma probe. Surg Oncol 2:335–339
Giuliano AE, Kirgan DM, Kuenther JM, Morton DL (1994) Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg 220:398–401
Kim T, Giuliano AE, Lyman GH (2006) Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma. Cancer 106:4–16
Morrow M, Rademaker AW, Bethke KP et al (1999) Learning sentinel node biopsy: results of a prospective randomized trial of two techniques. Surgery 126:714–720
Kitai T, Inomoto T, Miwa M, Shikayama T (2005) Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer 12:211–215
Yamauchi K, Nagafuji H, Nakamura T et al (2011) Feasibility of ICG fluorescence-guided sentinel node biopsy in animal models using the HyperEye Medical System. Ann Surg Oncol 18:2042–2047
Troyan SL, Kinzard V, Gibbs-Strauss SL et al (2009) The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol 16:2943–2952
Mieog JS, Troyan SL, Hutteman M et al (2011) Towards optimization of imaging system and lymphatic tracer for near-infrared fluorescent sentinel lymph node mapping in breast cancer. Ann Surg Oncol 18:2483–2491
van der Vorst JR, Schaafsma BE, Verbeek FP et al (2013) Dose optimization for near-infrared fluorescence sentinel lymph node mapping in patients with melanoma. Br J Dermatol 168:93–98
Proulx ST, Luciani P, Derzsi S et al (2010) Quantitative imaging of lymphatic function with liposomal indocyanine green. Cancer Res 70:7053–7062
Bahmani B, Gupta S, Upadhyayula S et al (2011) Effect of polyethylene glycol coatings on uptake of indocyanine green loaded nanocapsules by human spleen macrophages in vitro. J Biomed Opt 16:051303
Sugie T (2010) Controversy of axillary diagnosis and treatment. In: Toi M, Winer EP (eds) Local and systemic management of primary breast cancers. Kyoto University Press, Kyoto, pp 61–71
Kitai T, Kawashima M (2012) Transcutaneous detection and direct approach to the sentinel node using axillary compression technique in ICG fluorescence-navigated sentinel node biopsy for breast cancer. Breast Cancer 19:343–348
Sugie T, Kassim KA, Takeuchi M et al (2010) A novel method for sentinel lymph node biopsy by indocyanine green fluorescence technique in breast cancer. Cancers 2:713–720
Abe H, Mori T, Umeda T et al (2011) Indocyanine green fluorescence imaging system for sentinel lymph node biopsies in early breast cancer patients. Surg Today 41:197–202
Tagaya N, Yamazaki R, Nakagawa A et al (2008) Intraoperative identification of sentinel lymph node by near-infrared fluorescence imaging in patients with breast cancer. Am J Surg 195:850–853
Takeuchi M, Sugie T, Abdelazeem K et al (2012) Lymphatic mapping with fluorescence navigation using indocyanine green and axillary surgery in patients with primary breast cancer. Breast J 18:535–541
Aoyama K, Kamio T, Ohchi T et al (2011) Sentinel lymph node biopsy for breast cancer patients using fluorescence navigation with indocyanine green. World J Surg Oncol 9:157
Hirano A, Kamimura M, Ogura K et al (2012) A comparison of indocyanine green fluorescence imaging plus blue dye and blue dye alone for sentinel node navigation surgery in breast cancer patients. Ann Surg Oncol 19:4112–4116
Inoue T, Nishi T, Nakano Y et al (2014) Axillary lymph node recurrence after sentinel lymph node biopsy performed using a combination of indocyanine green fluorescence and the blue dye method in early breast cancer. Breast Cancer. doi:10.1007/s12282-014-0573-8
Sugie T, Sawada T, Tagaya N et al (2013) Comparison of the indocyanine green fluorescence and blue dye methods in detection of sentinel lymph nodes in early-stage breast cancer. Ann Surg Oncol 20:2213–2218
Ahmed M, Purushotham AD, Douek M (2014) Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol 15:e351–e362
van der Vorst JR, Schaafsma BE, Verbeek FP et al (2012) Randomized comparison of near-infrared fluorescence imaging using indocyanine green and 99(m)technetium with or without patent blue for the sentinel lymph node procedure in breast cancer patients. Ann Surg Oncol 19:4104–4111
Murawa D, Hirche C, Dresel S, Hünerbein M (2009) Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br J Surg 96:1289–1294
Wishart GC, Loh SW, Jones L, Benson JR (2012) A feasibility study (ICG-10) of indocyanine green (ICG) fluorescence mapping for sentinel lymph node detection in early breast cancer. Eur J Surg Oncol 38:651–656
Schaafsma BE, Verbeek FP, Riebergen DD et al (2013) Clinical trial of combined radio-and fluorescence-guided sentinel lymph node biopsy in breast cancer. Br J Surg 100:137–144
Verbeek FP, Troyan SL, Mieog JS et al (2014) Near-infrared fluorescence sentinel lymph node mapping in breast cancer: a multicenter experience. Breast Cancer Res Treat 143:333–342
Sugie T, Kinoshita T, Masuda N et al (2016) Evaluation of the clinical utility of the ICG fluorescence method compared with the radioisotope method for sentinel lymph node biopsy in breast cancer. Ann Surg Oncol 23:44–50
Kelly AM, Dwamena B, Cronin P, Carios RC (2009) Breast cancer sentinel node identification and classification after neoadjuvant chemotherapy-systematic review and meta analysis. Acad Radiol 16:551–563
van Deurzen CH, Vriens BE, Tjan-Heijnen VC et al (2009) Accuracy of sentinel node biopsy after neoadjuvant chemotherapy in breast cancer patients: a systematic review. Eur J Cancer 45:3124–3130
Lyman GH, Temin S, Edge SB et al (2014) Practice. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 32:1365–1383
Kuehn T, Bauerfeind I, Fehm T et al (2014) Sentinel-lymph node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicenter cohort study. Lancet Oncol 14:609–618
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Sugie, T., Inamoto, T. (2016). Lymphatic Mapping and Optimization of Sentinel Lymph Node Dissection. In: Toi, M., Winer, E., Benson, J., Klimberg, S. (eds) Personalized Treatment of Breast Cancer. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55552-0_9
Download citation
DOI: https://doi.org/10.1007/978-4-431-55552-0_9
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55551-3
Online ISBN: 978-4-431-55552-0
eBook Packages: MedicineMedicine (R0)