Abstract
Peritoneal carcinomatosis (PC) is a severe oncological condition originating from the mesothelium or, more frequently, from gastrointestinal or gynecological tumors. The PC is believed to be a terminal phase of the oncological disease and, if left untreated, has a median survival of approximately 6 months after diagnosis. PC originating from colorectal cancer is often a metachronous disease, and only 10–15% of patients with colorectal cancer show PC at the time of primary diagnosis. However, the peritoneum is involved up to 50% of cases in patients with colorectal cancer who develop tumor recurrence after potentially curative surgery of the primary tumor; and in 10–35% of cases it is the only site of tumor recurrence. The only potentially curative treatment in primary and metastatic peritoneal carcinomatosis is cytoreductive surgery associated with intraperitoneal hyperthermic chemotherapy (HIPEC) with a 5-year survival rate of 30–48%, in selected cases. One of the most critical problems in PC treatment is represented by the correct diagnosis of the peritoneal nodules and identification of smaller lesions. In recent years, new technologies have allowed surgeons to cope better with these limits. Intraoperative fluoroscopy (FI) is a recently revised imaging modality that could improve PC detection. Indocyanine green (ICG), a near-infrared contrast agent that may become fluorescent, has been shown to selectively accumulate in the tumor tissue, thus increasing diagnostic detection of PC.
This study was carried out to investigate the role of FI with ICG (ICG-FI) in the detection of peritoneal carcinomatosis from colorectal cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Peritoneal carcinomatosis
- Fluorescence imaging
- Indocyanine green
- Peritonectomy
- Cytoreductive surgery
- Colorectal cancer
- Peritoneal index
Introduction
Peritoneal carcinomatosis (PC) is a severe oncological condition originating from the mesothelium (primary PC) or, more frequently, from gastrointestinal or gynecological tumors (secondary PC). Every year, peritoneal carcinomatosis affects about 25,000 people in Italy [1].
This condition is interpreted as a terminal stage of disease and, if not treated, allows a median survival of ≤6 months after diagnosis [2, 3]. Indeed, peritoneal involvement is considered the most serious event in tumor progression [4]. Since it is difficult to treat it, peritoneal diffusion is often the main cause of morbidity and mortality due to tumors affecting the peritoneal serosa. Even in patients resected for intra-abdominal carcinoma, PC is the most frequent cause of death [5,6,7]. Interestingly, PC often develops as a “local” disease in the absence of hematogenous or distant metastases [8]. Particularly, peritoneal metastases occur in 30–40% of patients with colorectal carcinoma (CRC), and they are the only metastases in 25% of patients [9, 10].
In abdominal neoplasms, peritoneal dissemination may be present at the time of diagnosis, but, more often, it occurs as a life-threatening condition after surgical treatment of the primary tumor [5].
In gastric cancer, 10–20% of patients who are candidates for potentially curative resection and 40% of those in advanced stages have peritoneal involvement at the time of abdominal exploration [11]. Furthermore, 20–50% of patients undergoing potentially curative surgery will show a peritoneal recurrence in the future [12]. In the case of advanced gastric cancer, the intracavitary spread of neoplastic cells is responsible up to 54% of deaths due to recurrence after surgery [13]. The greatest risks of peritoneal recurrence have been demonstrated in patients with diffuse or mixed histologic carcinoma (69% at 5 years) and, even more so, those with positive peritoneal cytology at the time of the resective intervention (80% at 5 years) [14, 15]. In addition, the peritoneal cavity is the only place of diffusion in the 40–60% of the recurrences of gastric cancer [16].
The PC originating from colorectal cancer is frequently a metachronous disease, and only 10–15% of colorectal cancer patients show PC at the time of primary diagnosis; however, as observed in gastric cancer, peritoneum is involved up to 50% of cases in colorectal cancer patients who develop tumor recurrence after potentially curative surgery of the primary tumor [17], and, in 10–35% of the cases is the only site of tumor relapse [18]. The mucinous carcinomas of the colon and the carcinoma of the appendix, especially if there is a positive peritoneal cytology, show the highest rates of peritoneal dissemination [12].
Pathogenic Mechanisms
The pathogenic mechanisms that regulate carcinomatosis are multifactorial, but essentially consist of:
-
1.
Peritoneal dissemination of free tumor cells, which exfoliate as a result of the direct invasion of the serosa of the organ involved by primary neoplasm [19] and subsequent implantation on the peritoneal surface through molecules of cell adhesion [5, 8]
-
2.
Passage of malignant cells through the lymphatic lacunae and venous vessels of the peritoneum [19]
-
3.
Insemination after trauma and surgical manipulation [19]
In particular, in low-grade malignant tumors it is assumed that the PC originates from a transparietal spread, and that the dissemination follows a migration path called “neoplastic redistribution.” This migration is governed by a “non-random” redistribution process that is not dependent from the intrinsic biological aggressiveness of the tumor but primarily is related to physical mechanisms, such as the effect of gravity in relation to the site of the primary tumor, and the presence or less of intra-abdominal fluid (ascites, mucus, etc.) [5, 8, 20], as well as the characteristic viscosity of the same. Tumor cells, which move freely within the peritoneal cavity, generally aggregate into well-defined areas due to gravity concentrating in the normal reabsorption sites of the peritoneal fluids, such as the lymphatic lacunae of the small and large omentum and the diaphragm, in particular of the right hemidiaphragm. This generally involves the development of disease, especially in the pelvis, in the subphrenic space, in the parietocolic groove and in Morrison’s pouch [5, 8], or in anfractuous regions where the peritoneal fluid circulates at low flow. When the tumor does not produce fluids, the malignant cells have more limited motility and implant more frequently near the site of the primary tumor. While a liquid vehicle is present within the abdominal cavity, sites more distant than the primary tumor may also be affected, such as the Treitz ligament and the small omentum in the case of ovarian carcinoma. Likewise, as a result of physical mechanisms, the PC does not occur, at least in the initial stages, on the mesenteric surface and on the serosa of the small intestine due to the active peristaltic movements. In contrast, relatively fixed intestinal areas, such as the duodenum and the ileocecal and rectum-sigmoid conjunctions, are often infiltrated by carcinomatosis.
Treatment
Just as the metastatic involvement of the liver by CRC is currently considered susceptible of hepatic resection for curative purposes, the treatment of PC could be considered as potentially curative considering that, in selected cases and within certain limits, the involvement of the peritoneal serosa may represent the extreme margin of diffusion of the neoplasm.
In the last 20 years, the growing and renewed interest in the malignant tumors of the peritoneum, and the increase of the knowledge on the biology of these neoplasms, has led to the search for new and increasingly aggressive therapeutic techniques. There is sufficient consent that the only potentially curative treatment in primary and metastatic peritoneal carcinomatosis is cytoreductive surgery (CS) associated with hyperthermic intraperitoneal chemotherapy (HIPEC) with a 5-year survival rate ranging from 30% to 48% in selected cases [21, 22].
The HIPEC was introduced in 1980 for the treatment of the PC and was initially used alone [23]. Since 1995 some procedures of peritonectomy were associated [24] in several world centers which have reported their experiences using different HIPEC protocols showing encouraging results [2]. This innovative and aggressive treatment modality, directed to the entire abdominal-pelvic area, is able, despite the high rate of morbidity, to significantly reduce and sometimes completely eliminate carcinomatosis, improving long-term survival [24, 25]. The logic that underlies the HIPEC is essentially based on both the direct cytotoxicity of hyperthermia on neoplastic cells, increased rate of the cytotoxicity of some chemotherapeutic agents determined by hyperthermia itself, and, finally, pharmacokinetic advantage obtained by the administration of intraperitoneal chemotherapy [5, 6].
The plasma-peritoneum-barrier (i.e., a physiologic barrier that limits the resorption of drugs from the peritoneal cavity into the blood) guarantees, at the regional site, high concentrations of some cytostatic drugs (including cisplatin, mitomycin c, oxaliplatin, adriblastine) limiting systemic toxicity. Multimodal treatment combining HIPEC and cytoreductive surgery (CRS) with peritonectomy [24] finds space in the treatment of primary peritoneal malignant tumors (abdominal sarcomatosis, peritoneal mesothelioma) [26], pseudomyxoma peritonei, and CR peritoneal carcinomatosis from colorectal [27, 28], ovarian [29], and gastric carcinoma [13]. On the contrary, HIPEC is contraindicated in the case of peritoneal carcinomatosis from neoplasia showing high biological aggressiveness (pancreatic adenocarcinoma and neoplasia of the esophagus), extra-abdominal metastasis, extensive retroperitoneal or lymph node disease, coexistence of important pathologies (cardiorespiratory, neurological, and renal), multiple and diffuse or otherwise unresectable hepatic metastases, previous side effects, and poor response after systemic chemotherapy.
Prognostic Factors
To define the extent of cytoreduction, Jaquet and Sugarbaker [30] introduced the so-called completeness of cytoreduction (CCR) score which provides an assessment of the amount of residual disease after cytoreductive surgery. CCR-0 indicates that no macroscopic disease remains after cytoreduction. CCR-1 indicates that tumor nodules with a diameter of less than 2.5 mm remain after surgery. Finally, CCR-2 and CCR-3 indicate that tumor nodules between 2.5 mm and 2.5 cm, and tumor nodules with a diameter greater than 2.5 cm remain after surgical treatment, respectively.
Since PC should be considered as a regional metastasis and being impossible to remove all microscopic residues, the concept of radicality is relative, so that not only the complete cytoreduction, CCR-0, but also CCR-1 (residual tumor ≤2.5 mm) is deemed acceptable [31]. To assess the extension of the resection in the PC treatment, the CCR score appears to be the most reliable system compared to the R (resection) stage that is traditionally used for primary neoplasms in the tumor node metastasis staging system [4]. In PC, it is generally believed that it is not possible to obtain an R0 state, and therefore CCR-0 is equivalent to R1 (no gross residual disease). R2a indicates that minimal tumor nodules of less than 5 mm remain. R2b indicates that coarse tumor nodules exceeding 5 mm and up to 2 cm remain. R2c indicates that it remains an extended disease of over 2 cm [28].
The diagnosis of peritoneal carcinomatosis is challenging, both pre- and intraoperatively. The gold standard for PC staging is still the direct laparotomic or laparoscopic visualization. Computed tomography (CT) and positron emission tomography (PET) provide the best results before surgery, but underestimation of the disease phase is frequently reported [32]. During laparotomy, surgeons depend on visual inspection and palpation to determine PC extension and extent of resection. However, some subclinical peritoneal lesions may escape intraoperative detection. The peritoneal cancer index (PCI) is of fundamental importance in treatment planning and is closely correlated with the prognosis after CS + HIPEC [5]. The Peritoneal Cancer Index (PCI) is the most accepted metric to quantify the extent of peritoneal disease and is evaluated with the utmost accuracy at the time of surgery, as it has been shown that sensitivity in the detection of peritoneal disease by computerized tomographic scan (CT) turns out to be 41.1% and the specificity 89%. PCI is calculated by evaluating the size of peritoneal lesions in each of the 13 abdomino-pelvic regions. The lesion size (LS) is evaluated with a score of 0–3 for each of the 13 regions and summed to obtain a score from 0 to 39. In patients with PC from CRC, a PCI of 10–20 means extensive carcinomatosis and therefore a worse prognosis. It is believed that only palliative cure should be offered to such patients [17, 33,34,35].
Intraoperative Fluoroscopy and Indocyanine Green
As outlined above, one of the most critical trouble in PC treatment is represented by both correct diagnosis of peritoneal nodules and identification of smaller lesions. In recent years, new technologies have allowed surgeons to better address such limitations [36, 37]. Intraoperative fluoroscopy (FI) is a recently introduced imaging modality that can improve PC detection [38]. Indocyanine green (ICG), a near-infrared (NIR) contrast agent becoming fluorescent if excited by light with a wavelength of 800–900 nm, has been recently proposed for FI due to its special affinity for the cancerous tissue [39, 40]. Approximately 95% of the ICG molecules bind rapidly to intravascular macromolecules, such as albumin and lipoproteins, after intravenous injection. Since in tumor tissue neoangiogenesis is responsible for the presence of immature and permeable vessels, the ICG, like these macromolecules, permeates the endothelial lesions and is retained in the cancerous tissue due to the altered lymphatic drainage (permeability and advanced retention [EPR]) of the lesion [41, 42]. The extravascular ICG accumulation is responsible for the hyperfluorescence observed in the tumor tissue in contrast to the surrounding normal tissue [41, 42].
The detection of tumor tissue depends on the tumor-background relationship (TBR), which is the ratio between the intensity of the fluorescence, expressed in arbitrary units, of the tumor tissue and of the surrounding normal tissue [43]. The ICG has a half-life of 150–180 s, is metabolized by the liver microsomes, and excreted through the bile. Overall, its toxicity can be classified as low. Occasionally, in 1 out of 42,000 cases, mild side effects have been reported in humans such as sore throats and hot flashes. Effects such as anaphylactic shock, hypotension, tachycardia, dyspnea, and urticaria have occurred only in individual cases [44]. The mortality rate is 1:300,000.
Currently ICG is a non-specific fluorescent probe registered and approved by the FDA for optical imaging in clinical settings [45]. ICG is recognized as a safe and economical NIR fluorescent probe. The properties of the ICG, which is a water-soluble amphiphilic molecule with a molecular weight of 775 Dalton and a hydrodynamic diameter of 1.2 nm, make it an excellent vascular and lymphatic contrast agent when injected intravenously (IV) and in the system lymphatic (e.g., by subcutaneous injection), respectively. The intravascular compartmentalization of the ICG before its rapid clearance explains its angiographic properties. Therefore, ICG is used in ophthalmology for retinoscopy and in plastic surgery to evaluate the vascularization of the reconstruction flap [46,47,48]. Furthermore, as it is excreted exclusively from the liver into the bile, it can also be used to evaluate liver function in cirrhotic patients before undergoing liver surgery [49, 50], or during cholecystectomy as a cholangiographic agent [51,52,53]. Moreover, in colorectal surgery for oncological and non-oncological indications, ICG-FI is expected to become a useful application for the evaluation of the vascularization of colorectal anastomoses [54,55,56,57]. After subcutaneous injection, free ICG is a small molecule that can rapidly enter the small lymphatic vessels and serves as a good marker of the lymphatic system. Recently, ICG-FI has emerged as a potential tool in surgical oncology for detection of sentinel lymph nodes (SLN) in various cancers such as breast [58], skin [59], gastric [60], and colorectal cancers [61,62,63,64,65,66,67]. Furthermore, FI after IV injection of ICG has been described as a novel imaging technique to assist surgeons in the intraoperative detection of hepatocellular carcinoma (HCC) [68, 69], cholangiocarcinoma [70], hepatoblastoma [71], and hepatic metastases [72]. Several reviews have reported the role of optical imaging using ICG [38, 73,74,75,76,77,78], but none of these has specifically focused on the role of ICG-FI for the detection of carcinomatosis in colorectal cancer. ICG-FI represents a wide potential field for the clinical application of this emerging imaging technique.
FI-guided surgery with ICG (ICG-FI), both in vivo (ICG-IF intraoperative) and ex vivo (on the ICG-FI table), seems to be particularly suitable for detecting PC in which superficial lesions are present. However, data on ICG-guided surgery in PC CRC treatment are still poor and the technique has not yet been standardized for this use.
Our Experience
At the Division of Surgical Oncology of the University of Naples “Luigi Vanvitelli,” a prospective study was conducted to evaluate the role of ICG-FI in the improvement of outcome in patients affected by peritoneal carcinomatosis from CRC and undergoing CS + HIPEC (Video 21.1). Inclusion criteria for CS + HIPEC were age of 18–70 years, PCI ≤20 at preoperative diagnosis, tumor limited to the peritoneal cavity without other distant metastases, and absence of serious comorbidity with the performance status ≤1. Overall, seven patients with PC from CRC were admitted. Three patients were excluded from surgical treatment due to high PCI (29 and 31, respectively), or poor general conditions (one patient). Ultimately, four patients underwent surgical exploration. All patients had previously been successfully submitted to a potentially curative resection for stage III colorectal adenocarcinoma. All patients underwent adjuvant chemotherapy with 5-fluorouracil plus oxaliplatin, and they were followed at 3-month intervals until tumor recurrence [79].
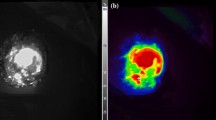
All operations were performed through open median relaparotomy. After clinical exploration of the entire peritoneal cavity and evaluation of the feasibility of CC-0 or CC-1 cytoreduction and localization of metastatic nodules, a dose of 0.25 mg/kg ICG (PULSION Medical Systems SE, FeldKirchen, Germany) was injected intravenously. FI-guided imaging was performed in vivo on the entire peritoneal cavity using Fluobeam® (Fluoptics Imaging Inc., Cambridge, MA, USA), an open system for in vivo infrared fluorescence imaging. At Fluobeam® examination, the peritoneum appears as a large gray area striped with very thin bright lines corresponding to vascular structures. With black and white vision, a hyperfluorescent peritoneal nodule appeared as a well-defined area of intense bright light with clear margins (Fig. 21.1a). This area, with the color vision allowed by Fluobeam®, appeared as a red area with ultraviolet margins (Fig. 21.1b). Visible and/or palpable nodules showing no clear dissimilarity from the gray peritoneal serosa (with black and white vision) or colors ranging from green to ultraviolet were defined as hypofluorescent nodules. All peritoneal sites were checked again at the end of surgical resection to evaluate residual fluorescence. Finally, all specimens were observed ex vivo with Fluobeam® to confirm their previous appearance and investigate the margins of resected tissue.
(a) Patient #3. ICG-FI guided surgery (black and white vision). Hyperfluorescent nodule visible as an intense bright light surrounded by a gray area. (b) Same case (color vision). The nodule appeared as a red area with ultraviolet margins. (From Lieto et al. [79]; used with permission)
HIPEC was performed through a closed technique by using oxaliplatin (400 mg/m2) in 5 L of 5% glucose solution for 30 min at 42 °C.
Patient characteristics are reported in Table 21.1. A cytoreductive surgery classified as CCR-0 followed by HIPEC was performed in all patients. No patient had serious postoperative complications and all were discharged on postoperative days 9–11. Peritoneal exploration was performed at a median time of 50 min after ICG injection (range 30–60; IQR 35–60 min). The ICG-FI required on average 20 min (range 10–30, IQR 15–25 min), and all images collected by Fluobeam® were converted into pictures and videos. The operation time ranged from 240 to 360 min (median 280 min, IQR 250–330 min).
A total of 69 nodules were collected (median diameter 2.7 cm, range 0.2–5.0 cm, IQR 1.2–3.8 cm). With conventional techniques, such as CT and PET scans, 30 nodules had been preoperatively discovered (median diameter 3.8 cm, range 1.5–5.0 cm, IQR 3.5–4.4 cm). At intraoperative exploration further 22 peritoneal nodules (median diameter 2.3 cm, interval 1.3–3.1 cm, IQR 1.8–2.8 cm) were identified by the surgical team. Out of these 52 nodules, 47 (90%) were hyperfluorescent on examination with Fluobeam®. Finally, ICG-FI identified 17 additional hyperfluorescent nodules with a median diameter of 0.5 mm (range 0.2–0.7 cm, IQR 0.3–0.6 cm) (Fig. 21.2). All samples were also examined with Fluobeam® ex vivo, namely on the table in the operating room. There was a complete correspondence between in vivo and ex vivo observations (Fig. 21.3a, b). In addition, a hypofluorescent tissue boundary was identified around each lesion. Postoperative histopathology showed that two nodules detected in the intraoperative phase and two nodules detected intraoperatively were not metastatic. Of the 64 hyperfluorescent nodules, 1 (false-positive) was non-cancerous; of the remaining 5 hypofluorescent nodules, 2 (false-negatives) turned out to be metastatic tissues. In all cases, the hypofluorescent tissue around each lesion was negative for metastatic tissue. Of the 65 metastatic peritoneal nodules, the ICG-FI allowed to identify 16 nodules not diagnosed with conventional procedures, adding a 25% diagnostic improvement. Overall, the sensitivity of current diagnostic procedures (CT and PET) was 43.1% preoperatively and 76.9% intraoperatively (visual examination and palpation). With the ICG-FI sensitivity increased to 96.9%, ICG-FI showed the highest specificity and positive and negative predictive values. The accuracy of the test, that is, the global prognostic performance of the procedure, was 43.4%, 75.3%, and 95.6% for preoperative, intraoperative, and ICG-FI, respectively (Table 21.2).
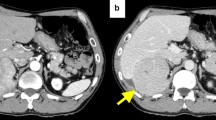
Patient #1. Diffuse pelvic peritoneal carcinomatosis (hyperfluorescent areas–black arrows) clearly visible. In addition, ICG-FI revealed a hyperfluorescent peritoneal nodule (visible between the two branches of the surgical clamp). (From Lieto et al. [79]; used with permission)
(a) Patient #2. The ICG-FI revealed a second sub-centimetric hyperfluorescent nodule (visible between the two branches of the surgical clamp). (b) Same case. ICG-FI guided surgery ex vivo, on the table, to confirm the radicality of the surgical resection. (From Lieto et al. [79]; used with permission)
Prior to ICG-FI, median PCI was 7 (range 2–12, IQR 4–10), but after ICG-FI, PCI increased significantly to a median of 10 (range 3–15, IQR 5–13; p < 0.001). However, the worsening of PCI did not prevent a complete cytoreduction in all patients.
Cytological examination of the peritoneal liquid was positive for malignant cells in three out four cases before HIPEC and negative in all cases after HIPEC.
Considerations
Cytoreductive surgery achieving CCR-0 or at least CC-1 cytoreduction should be the gold standard in the treatment of primary and metastatic peritoneal carcinomatosis, including PC from colorectal cancer. A technique that improves intraoperative detection of PC nodules would help to achieve complete cytoreduction and avoid resection of non-cancerous lesions. Despite the limited number of patients and PC nodules that are limitations of the present study, our results with intraoperative ICG-FI appear promising. Using this imaging technique, it was possible to correctly map the metastatic areas with a sensitivity of 97%, a test accuracy of 95.6%, and an improvement of almost 25% in the identification of the disease. Particularly, the 16 malignant lesions identified intraoperatively with Fluobeam® had not been detected during conventional abdominal exploration.
In 2016, Liberale et al. and Barabino et al., both from Europe, have reported their results in 17 and 10 patients, respectively, showing PC from colorectal cancer [33, 40]. Our results differ from those reported by Barabino [40]. In contrast to our results showing that the PCI score has improved significantly from 7 (with conventional methods) to 10 (with PCI-FI), Barabino et al. reported a non-significant difference between conventional and ICG-FI-guided surgery [40]. They also reported false-positive and false-negative rates of 40% and 27%, respectively, while our rates were 25% and 3%, respectively. The explanation of the authors of these high percentages includes preoperative chemotherapy and the limitations of the EPR effect of ICG. However, Barabino and colleagues administered ICG 24 h before surgery and it could have impaired their results. In our experience, 50 min after ICG intravenous injection, the intraoperative view with Fluobeam® of the fluorescent areas in the abdomen was optimal. Our decision to perform the intraoperative injection of the fluorescent probe was influenced by our previous experience with ICG-FI guided surgery for liver cancers [80]. In order to consistently reduce physiological hepatic uptake and allow the drug to concentrate in the tumor, the injection of ICG had been performed 24 h before surgery [81, 82]. In these cases, no peritoneal fluorescence occurred at the time of the operation. Interestingly, as in our observations, Liberale et al. did not detect fluorescence in peritoneal metastatic nodules in the first two patients who received ICG 24 h before surgery. In contrast, in the remaining patients who received ICG intraoperative injection, all peritoneal nodules were hyperfluorescent [33]. Establishing the optimal ICG dosage and injection times are important for the standardization of the technique. Some authors [42] propose a dose of 0.5 mg/kg 12–24 h before surgery, others [33] a total dose of 5 mg administered intraoperatively. In the case of the PC, in which numerous, small, hypervascularized nodules are to be detected, an intraoperative ICG injection is suitable since the ICG disappears from the plasma at a rate of 18–25% per minute [83].
A new interesting tool is represented by prophylactic HIPEC in CRCs at high risk of developing peritoneal metachronous carcinomatosis, such as tumors invading serosa (pT4a) or with positive peritoneal lavage [84]. In these patients, current clinical and imaging techniques do not have sufficient diagnostic sensitivity [85], and ICG-FI-guided surgery could identify small undiagnosed peritoneal metastatic nodules. Furthermore, this technique would be an excellent tool to improve sensitivity for second-level laparoscopy in high-risk patients and could be practice-changing.
Although all the available studies show some limitations particularly related to the small number of investigated patients, ICG-FI-guided surgery appears to be a promising tool to improve the radicality of CS in PC originating from CRC. Further studies are needed to standardize the technique and determine its role in this patient population.
References
Pontiggia P, Pontiggia E. Immunità e ipertermia nella cura dei tumori. ETS edizioni 8; 2016.
Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004;5:219–28.
Chua TC, Yan TD, Saxena A, et al. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure: a systematic review of morbidity and mortality. Ann Surg. 2009;249:900–7.
Jessup MJ, Goldberg RM, Asara EA, et al. Colon and rectum. In: American Joint Committee on Cancer, editor. AJCC cancer staging manual. 8th ed. Berlin: Springer; 2017. p. 251–74.
Sugarbaker PH. Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin Surg Oncol. 1998;14:254–61.
Van der Speeten K, Stuart OA, Sugarbaker PH. Pharmacokinetics and pharmacodynamics of perioperative cancer chemotherapy in peritoneal surface malignancy. Cancer J. 2009;15(3):216–24.
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:359–86.
Sugarbaker PH. Observations concerning cancer spread within the peritoneal cavity and concepts supporting an ordered pathophysiology. Cancer Treat Res. 1996;82:79–100.
Chu DZ, Lung NP, Thompson C, et al. Peritoneal carcinomatosis in non-gynecologic malignancies: a prospective study of prognostic factors. Cancer. 1989;63:364–7.
Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis in non-gynecologic malignancies: results of EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–63.
Kodera Y, Yamamura Y, Shimizu Y, et al. Peritoneal washing cytology: prognostic value of positive findings in patients with gastric carcinoma undergoing a potentially curative resection. J Surg Oncol. 1999;72:60–4.
Roviello F, Marrelli D, Neri A, et al. Treatment of peritoneal carcinomatosis by cytoreductive surgery and intraperitoneal hyperthermic chemoperfusion (IHCP): postoperative outcome and risk factors for morbidity. World J Surg. 2006;30:2033–40.
Fujimoto S, Takahashi M, Mutou T, et al. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer. 1999;85:529–34.
Marrelli D, Roviello F, De Manzoni G, Italian Research Group for Gastric Cancer, et al. Different patterns of recurrence in gastric cancer depending on Lauren’s histological type: longitudinal study. World J Surg. 2002;26:1160–5.
Roviello F, Marrelli D, de Manzoni G, Italian Research Group for Gastric Cancer, et al. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg. 2003;90:1113–9.
Al-Shammaa HA, Li Y, Yonemura Y. Current status and future strategies of cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. World J Gastroenterol. 2008;14:1159–66.
Portilla AG, Sugarbaker PH, Chang D. Second look surgery after cytoreductive and intraperitoneal chemotherapy for peritoneal–29 carcinomatosis from colorectal cancer: analysis of prognostic features. World J Surg. 1999;23:23–9.
Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–63.
Stewart JH 4th, Shen P, Levine EA. Intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: current status and future directions. Ann Surg Oncol. 2005;12(10):765–77.
Yonemura Y, Endo Y, Yamaguchi T, et al. Mechanisms of the formation of the peritoneal dissemination in gastric cancer. Int J Oncol. 1996;8(4):795–802.
Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with preoperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284–92.
Blackham AU, Russell GB, Stewert JH 4th, et al. Metastatic colorectal cancer: survival comparison of hepatic resection versus cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2014;21:2667–74.
Spratt JS, Adcock RA, Muskovin M, et al. Sistema di somministrazione clinica per la chemioterapia ipertermica intraperitoneale. Cancer Res. 1980;40:256–60.
Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42.
Esquivel J, Sugarbaker PH. Second aspect surgery in patients with peritoneal dissemination from appendicular neoplasia: analysis of prognostic factors in 98 patients. Ann Surg. 2001;234:198–205.
Deraco M, Nonaka D, Baratti D, et al. Prognostic analysis of clinicopathologic factors in 49 patients with diffuse malignant peritoneal mesothelioma treated with cytoreductive surgery and intraperitoneal hyperthermic perfusion. Ann Surg Oncol. 2006;13:229–37.
Moran B, Baratti D, Yan TD, et al. Consensus statement on the loco-regional treatment of appendiceal mucinous neoplasms with peritoneal dissemination (pseudomyxoma peritonei). J Surg Oncol. 2008;98:277–82.
Esquivel J, Sticca R, Sugarbaker P, Society of Surgical Oncology Annual Meeting, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Society of Surgical Oncology. Ann Surg Oncol. 2007;14:128–33.
Raspagliesi F, Kusamura S, Campos Torres JC, et al. Cytoreduction combined with intraperitoneal hyperthermic perfusion chemotherapy in advanced/recurrent ovarian cancer patients: the experience of National Cancer Institute of Milan. Eur J Surg Oncol. 2006;32:671–5.
Jaquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–74.
Begossi G, Gonzales-Moreno S, Ortega Perez G, et al. Cytoreduction and intraperitoneal chemotherapy for the management of peritoneal carcinomatosis, sarcomatosis and mesothelioma. Eur J Surg Oncol. 2002;28:80–7.
Dromain C, Leboulleux S, Auperin A, et al. Staging of peritoneal carcinomatosis: enhanced CT vs. PET/CT. Abdom Imaging. 2008;33:87–9.
Liberale G, Vankerckhove S, Caldon MG, et al. Fluorescence imaging after indocyanine green injection for detection of peritoneal metastases in patients undergoing cytoreductive surgery for peritoneal carcinomatosis from colorectal cancer: a pilot study. Ann Surg. 2016;264:1110–5.
Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol. 1999;43:15–25.
Berthet B, Sugarbaker TA, Chang D, et al. Quantitative methodologies for selection of patients with recurrent abdominopelvic sarcoma for treatment. Eur J Cancer. 1999;3:413–9.
Kim S, Lim YT, Soltesz EG, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22:93–7.
Schaafsma BE, Mieog JSD, Hutteman M, et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol. 2011;104:323–32.
Polom K, Murawa D, Rho Y, et al. Current trends and emerging future of indocyanine green usage in surgery and oncology: a literature review. Cancer. 2011;117:4817–22.
Fox IJ, Wood EH. Indocyanine green: physical and physiologic properties. Proc Staff Meet Mayo Clin. 1960;35:732–44.
Barabino G, Klein JP, Porcheron J, et al. Intraoperative near-infrared fluorescence imaging using indocyanine green in colorectal carcinomatosis surgery: proof of concept. Eur J Surg Oncol. 2016;42:1931–7.
Maeda H, Wu J, Sawa T, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–84.
Bekheit M, Vibert E. Fluorescent-guided liver surgery: Paul Brousse experience and perspectives. In: Dip FD, editor. Fluorescence imaging for surgeons: concepts and applications, vol. 11. Cham: Springer; 2015. p. 117–26.
Frangioni J. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–34.
Sabapathy MV, Mentam J, Jacob PM, et al. Non invasive optical imaging and in vivo cell tracking of indocyanine green labeled human stem cells transplanted at superficial or in-depth tissue of SCID. Stem Cells Int. 2015;2015:606415. https://doi.org/10.1155/2015/606415.
FDA. Product Insert: Indocyanine Green (IC-GreenTM); 2016. http://www.accessdata.fda.gov/drugsatfda_docs/label/2006/011525s017bpdf.
Stanga PE, Lim JI, Hamilton P. Indocyanine green angiography in chorioretinal diseases: indications and interpretation: an evidence-based update. Ophthalmology. 2003;110:15–23.
Holm C, Tegeler J, Mayr M, et al. Monitoring free flaps using laser-induced fluorescence of indocyanine green: a preliminary experience. Microsurgery. 2002;22:278–87.
Munabi NC, Olorunnipa OB, Goltsman D, et al. The ability of intra-operative perfusion mapping with laser-assisted indocyanine green angiography to predict mastectomy flap necrosis in breast reconstruction: a prospective trial. J Plast Reconstr Aesthet Surg. 2014;67:449–55.
Makuuchi M, Kosuge T, Takayama T, et al. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298–304.
Nanashima A, Abo T, Tobinaga S, et al. Indocyanine green retention rate at 15 minutes by correlated liver function parameters before hepatectomy. J Surg Res. 2011;169:119–25.
Ishizawa T, Bandai Y, Kokudo N. Fluorescent cholangiography using indocyanine green for laparoscopic cholecystectomy: an initial experience. Arch Surg. 2009;144:381–2.
Ishizawa T, Bandai Y, Ijichiet M, et al. Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg. 2010;97:1369–77.
Ishizawa T, Tamura S, Masuda K, et al. Intraoperative fluorescent cholangiography using indocyanine green: a biliary road map for safe surgery. J Am Coll Surg. 2009;208:1–4.
Hellan M, Spinoglio G, Pigazzi A, et al. The influence of fluorescence imaging on the location of bowel transection during robotic left-sided colorectal surgery. Surg Endosc. 2014;28:1695–702.
Boni L, Fingerhut A, Marzorati A, et al. Indocyanine green fluorescence angiography during laparoscopic low anterior resection: results of a case-matched study. Surg Endosc. 2017;31:1836–40.
Boni L, David G, Dionigi G, et al. Indocyanine green-enhanced fluorescence to assess bowel perfusion during laparoscopic colorectal resection. Surg Endosc. 2016;30:2736–42.
Jafari MD, Wexner SD, Martz JE, et al. Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg. 2015;220:82–92.
Kitai T, Inomoto T, Miwa M, et al. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer. 2005;12:211–5.
Tanaka R, Nakashima K, Fujimoto W, et al. Sentinel lymph node detection in skin cancer using fluorescence navigation with indocyanine green. J Dermatol. 2009;36:468–70.
Nimura H, Narimiya N, Mitsumori N, et al. Infrared ray electronic endoscopy combined with indocyanine green injection for detection of sentinel nodes of patients with gastric cancer. Br J Surg. 2004;91:575–9.
Nagata K, Endo S, Hidaka E, et al. Laparoscopic sentinel node mapping for colorectal cancer using infrared ray laparoscopy. Anticancer Res. 2006;26:2307–12.
Kusano M, Tajima Y, Yamazaki K, et al. Sentinel lymph node mapping guided by indocyanine green fluorescence imaging: a new method for sentinel lymph node navigation surgery in gastrointestinal cancer. Dig Surg. 2008;25:103–8.
Noura S, Ohue M, Seki Y, et al. Feasibility of a lateral region sentinel lymph node biopsy of lower rectal cancer guided by indocyanine green using a near-infrared camera system. Ann Surg Oncol. 2010;17:144–51.
Hirche C, Mohr Z, Kneif S, et al. Ultrastaging of colon cancer by sentinel node biopsy using fluorescence navigation with indocyanine green. Int J Colorectal Dis. 2012;27:319–24.
Cahill RA, Anderson M, Wang LM, et al. Near-infrared (NIR) laparoscopy for intraoperative lymphatic road-mapping and sentinel node identification during definitive surgical resection of early-stage colorectal neoplasia. Surg Endosc. 2012;26:197–204.
Van der Pas MH, Ankersmit M, Stockmann HB, et al. Laparoscopic sentinel lymph node identification in patients with colon carcinoma using a near-infrared dye: description of a new technique and feasibility study. J Laparoendosc Adv Surg Tech A. 2013;23:367–71.
Liberale G, Vankerckhove S, Galdon MG, et al. Sentinel lymph node detection by blue dye versus indocyanine green fluorescence imaging in colon cancer. Anticancer Res. 2016;36:4853–8.
Ishizawa T, Fukushima N, Shibahara J, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer. 2009;115:2491–504.
Gotoh K, Yamada T, Ishikawa O, et al. A novel image-guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation. J Surg Oncol. 2009;100:75–9.
Harada N, Ishizawa T, Muraoka A, et al. Fluorescence navigation hepatectomy by vizualization of localized cholestasis from bile tumor infiltration. J Am Coll Surg. 2010;210:2–6.
Yamamichi T, Oue T, Yonekura T, et al. Clinical application of indocyanine green (ICG) fluorescent imaging of hepatoblastoma. J Pediatr Surg. 2015;50:833–6.
Yokoyama N, Otani T, Hashidate H, et al. Real-time detection of hepatic micrometastases from pancreatic cancer by intraoperative fluorescence imaging: preliminary results of a prospective study. Cancer. 2012;118:2813–9.
Frangioni JV. New technologies for human cancer imaging. J Clin Oncol. 2008;26:4012–21.
Velde EA, Veerman T, Subramaniam V, et al. The use of fluorescent dyes and probes in surgical oncology. Eur J Surg Oncol. 2009;36:6–15.
Rao J, Dragulescu-Andrasi A, Yao H. Fluorescence imaging in vivo: recent advances. Curr Opin Biotechnol. 2007;18:17–25.
Gioux S, Choi HS, Frangioni JV. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol Imaging. 2010;9:237–55.
Xiong L, Gazyakan E, Yang W, et al. Indocyanine green fluorescence-guided sentinel node biopsy: a meta-analysis on detection rate and diagnostic performance. Eur J Surg Oncol. 2014;40:843–9.
Lim C, Vibert E, Azoulay D, et al. Indocyanine green fluorescence imaging in the surgical management of liver cancers: current facts and future implications. J Visc Surg. 2014;151:117–24.
Lieto E, Auricchio A, Cardella F, et al. Fluorescence-guided surgery in the combined treatment of peritoneal carcinomatosis from colorectal cancer: preliminary results and considerations. World J Surg. 2018;42:1154–60.
Lieto E, Galizia G, Cardella F, et al. Indocyanine green fluorescence imaging-guided surgery in primary and metastatic liver tumors. Surg Innov. 2018;25:62–8.
Miyashiro I, Miyoshi N, Hiratsuka M, et al. Detection of sentinel node in gastric cancer surgery by indocyanine green fluorescence imaging: comparison with infrared imaging. Ann Surg Oncol. 2008;15:1640–3.
Takahashi H, Zaidi N, Berber E. An initial report on the intraoperative use of indocyanine green fluorescence imaging in the surgical management of liver tumors. J Surg Oncol. 2016;114:625–9.
Faybik P, Hetz H. Plasma disappearance rate of indocyanine green in liver dysfunction. Transplant Proc. 2006;38:801–2.
Honoré C, Goéré D, Souadka A, et al. Definition of patients presenting a high risk of developing peritoneal carcinomatosis after curative surgery for colorectal cancer: a systematic review. Ann Surg Oncol. 2013;20:183–92.
Cortes-Guiral D, Elias D, Cascales-Campos PA, et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy for patients with colorectal cancer at high risk of peritoneal carcinomatosis: does it really save lives. World J Gastroenterol. 2017;23:377–81.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Electronic Supplementary Material
The video shows our experience with fluorescence imaging guided surgery with indocyanine green. Diagnosis and treatment of peritoneal carcinomatosis, HCC, and liver metastasis from colorectal cancer are shown. (MP4 13157 kb)
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Galizia, G. et al. (2020). The Role of Near-Infrared Fluorescence Imaging in the Assessment of Peritoneal Carcinomatosis from Colorectal Cancer. In: Aleassa, E., El-Hayek, K. (eds) Video Atlas of Intraoperative Applications of Near Infrared Fluorescence Imaging. Springer, Cham. https://doi.org/10.1007/978-3-030-38092-2_21
Download citation
DOI: https://doi.org/10.1007/978-3-030-38092-2_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-38091-5
Online ISBN: 978-3-030-38092-2
eBook Packages: MedicineMedicine (R0)