Abstract
With combinatorial approaches getting stronger to design materials with better functionalities and compatibility for restoring bone tissue, it is becoming important to understand the progress and evolution of existing and newly designed materials. For being clinically usable, they should have features that address the biomechanical, biochemical, and medical requirements.
Their various characteristics determine the cascade of events that take place at the site of bone healing. They should be selected based on the specific purpose with maximum benefit to the patient in long run. The current efforts in this domain are to render the orthopedic procedures minimally invasive and maximally effective.
This chapter encompasses the journey of classes of biomaterials used for their osteoinductive and osteoconductive properties and discusses the challenges for bringing them closer to fulfil the requisites.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
With an increased pace of life and increasing life expectancy, every year the number of bone graft procedures are increasing [1]. For bone repair, substitution, and augmentation, autografts have continued to be the gold standard till date, followed by allografts. Both of these grafts, in spite of having osteogenicity or osteoinductivity, have many shortcomings. Autografts incur high costs, are limited in their availability and cause additional trauma to the patient due to donor site morbidity. Allografts pose issues of potential viral transmission and immunogenicity apart from their limited supply and high cost. Their high cost and limited availability necessitate innovation in artificial novel biomaterials for bone substitution, repair, and augmentation.
To overcome this challenge of substituting bone tissue, researchers must have insight to reproduce the highly hierarchical composite microstructure of bone, which has not been possible yet. Any type of bone tissue engineering biomaterial should be mechanically and biologically compatible, osteoconductive, osteoinductive and allow integration with physicochemical characteristics irrespective of its source of origin [2]. With evolving technology in recent times, bone regenerative engineering is gradually becoming the solution for the new age with a bottom-up approach that incorporates stem cells, biomaterials, and growth factors as required, to regenerate bone tissue as compared to the conventional top-down approach commonly known as bone tissue engineering [3]. The three most commonly used terms pertaining to biologically relevant properties of biomaterials which are sometimes interchangeably used in regenerative medicine are as follows.
Osteoinduction is the regular phenomenon which induces osteogenesis during the bone healing process. The immature and pluripotent stem cells are stimulated to become preosteoblastic cells. Urist identified osteoinduction as the “the mechanism of cellular differentiation towards bone tissue due to the physicochemical effect or contact with another tissue” [4]. Osteoinduction is the major driving force in any bone healing situation as depicted in Fig. 1.
Principles of osteoinductive materials. Principle 1: Osteoinductive materials recruit MSCs to bone graft surfaces by growth factor release. Principle 2: The material promotes MSC differentiation into osteoblasts. Principle 3: Osteoblasts forms ectopic bone in vivo [6]
Osteoconduction is that attribute of a material that permits new bone to grow on the surface. So, any surface that allows bone growth either above it or inside it is an osteoconductive surface. In 1987, Wilson-Hench suggested that the process where the bone is aligned with a material’s surface or contour is regarded as osteoconduction [5]. This property is indispensable for the success of bone implants. Few metals such as Ag, Cu as well as bone cement show little or negligible osteoconduction due to their poor biocompatibility [6].
Osseointegration can be realized as the apposition or interface of connecting bone and the implant. It is explained as “the direct attachment of an implant by the formation of bony tissue around the implant” (Dorland’s illustrated medical dictionary). It can be realized as the “direct functional and structural bridge between an implant and bone” as bone deposition increases temporally.
Mechanism of Osteoinduction by Biomaterials
The real modus operandi of osteoinduction by biomaterials is still under exploration. The real question is whether there are any similarities between the mechanisms of osteoinduction by bone morphogenetic proteins (BMPs) and biomaterials. One of the possibilities is that after implantation, endogenous BMPs accumulate on the biomaterial surface and consequently induce bone formation [7,8,9] but there is no conclusive evidence for this hypothesis. Yuan and co-workers, suggested the role of BMPs in biomaterial-led-osteoinduction but their presence was not indispensable. A statistically lower number of animals under this pilot study makes this conclusions unreliable [7, 10]. There are some key contrasts in the osteoinduction mechanism by BMPs and biomaterials, such as: (a) BMPs generally induce bone through the endochondral pathway [11] while biomaterial-induced bone is always intramembranous [12, 13] ; (b) in smaller organisms like rats, bone is formed with much ease as in the case of BMPs as compared to biomaterials [14,15,16,17,18,19]; and (c) with biomaterials, bone formation happens inside the pores of biomaterials and not on the edge, while bone formation via BMPs happens on the external surface of the carrier, including the soft tissues that are located away from the implant surface [10, 20].
Although major aspects of the osteoinduction process by biomaterials are still unclear and under exploration, many studies in this field have proved its structure (at the macro, micro, and nano-levels) along with its chemical components play the most crucial role in rendering a material osteoinductive. The physicochemical and structural characteristics of osteoinductive biomaterials determine the course and output of bone formation both directly and indirectly (Fig. 2). As their inherent mechanical properties vary a lot, it is quite complicated to fit it with human body requirements (Table 1). Properties like ultrastructure and surface topography can modulate the dynamics of interactions with BMPs, growth factors, and other endogenous proteins which initiate bone formation or result in implant failure if events like stress shielding or infection takes place [21].
Schematic summarizing hypothesized mechanisms of osteoinduction, bone formation, and implant failure. (Figure adapted and redrawn from Barradas et al. [21])
Identification of Osteoinductive Materials [6]
To identify and select suitable osteoinductive biomaterials out of a plethora of materials, there is a specific sequence of biological activity characteristics which is as follows:
-
Recruitment of mesenchymal-type osteoprogenitor cells;
-
Transformation of mesenchymal stem cells (MSCs) into a mature, bone-forming lineage; and
-
Induction of new bone formation when implanted intramuscularly.
Classes of Orthopedic Biomaterials
There are various classes of biomaterials that have been employed as osteoinductive and osteoconductive materials as listed in Table 2 [22].
Metals
Metals are the very first category of materials to be used as implants in ancient times as per reports from Egyptian era. Aluminum, lead, gold, and silver were among the first metals to be used while titanium and its alloys are the most widely used metallic biomaterials in modern times. Owing to their good biocompatibility, strength, non-toxicity, and corrosion resistance, metal alloys are used for replacing joints and fixing fractures but many times, due to their non-biodegradability, these implants need removal. Metallic biomaterials are not bioactive per se. They are generally applied in load-bearing implants which require high strength, sufficient biocompatibility, and low in vivo corrosion rates. Metallic implants also tend to loosen due to stress shielding-led-bone resorption, weak interfacial bonding with bone, and absence of a supporting structure for new tissue.
In recent times, there have been efforts to design and develop metal implants that can mimic the traits and microstructure of the trabecular bone for expanding the scope of the use of metals. Hence, these special materials are termed as open-cell porous metals, metallic scaffolds, metallic foams, or cellular metals with three dimensionally (3D) interconnected pores. The total porosity is 50–75% with pore sizes in the range 200–500 μm [23]. Another method to make multipurpose and bioactive metals consist of: (1) applying a surface coating over the implant with bioactive ceramics or (2) chemically modifying the surface of the metal to bring about the in vivo build-up of a bioactive ceramic, or (3) to trigger the adhesion of proteins and cells toward tissue–material interactions [24]. The major metals used as orthopedic biomaterials are discussed below.
Magnesium (Mg)
The physical characteristics of magnesium are attractive, like high strength-to-mass ratios and an elasticity coefficient that matches with natural bone more as compared to other conventional metals. Apart from resemblance to human bone, they also have functional roles as natural ion content in human physiology and their biodegradation dynamics once inside the body [23]. Though they have been used for making short-term biodegradable implants with load-bearing purposes, their high corrosion rates and low bioactivity are some major challenging problems, which need to be resolved before tapping into their full potential in clinical applications. Various reports suggest the need to develop Mg alloys with modifiable in vivo decomposition rates and mechanical attributes similar to those of bone [25].
Endeavors to limit the corrosion dynamics of Mg have involved many approaches such as purification, alloying, and surface modification. Pure Mg also shows significantly reduced corrosion rates but owing to its low yield strength, it does not find widespread usage in orthopedics and other load-bearing applications. Surface modification is one of the very important strategies that reduces and controls the degradation behavior along with improving its surface biocompatibility. As compared to adjusting its bulk structure and composition, tuning the surface properties is a much simpler approach to adjust surface corrosion resistance. This helps in preserving favorable bulk attributes and is easily implemented on Mg alloys [25].
In one of the landmark studies with Mg alloys, Lee et al. systematically investigated the bone induction mechanism by using a Mg-5wt%Ca-1wt%Zn screw in an extensive clinical investigation. They employed this biodegradable implant and observed changes in elemental composition and crystallinity at the material interface. Controlled degradation of this alloy was followed by the growth of a biologically similar calcification matrix at the degrading surface which aids in initiating bone generation. This phenomenon facilitated faster healing with entirely replaced new bone at the place of the biodegradable implant within 1 year of implantation, as shown in an elaborated clinical study [26].
Despite alloying which is one of the major approaches for metals to tune their properties, its larger electronegative potential (−2.4 V) makes it harder to lower the degradation rate, only with the approach of alloying. Rather, the addition of a ceramic phase has proven to be a successful solution for optimizing Mg’s attributes, such as mechanical properties and corrosion resistance. Witte et al. carried out some initial studies with Mg/hydroxyapatite (HA) composites. They reinforced 20% HA particles to Mg alloy AZ91D matrix and developed a metal matrix composite (MMC). The mechanical, corrosive, and compatibility properties of the composite were studied in vitro. It was revealed that adding HA particles lowered the pH, enhanced the resistance for corrosion in various fluids, and enhanced the cell viability for a variety of cell lines when compared with the AZ91D matrix [27].
It’s important to be cautious while designing and fabricating such composites as these steps may have several hazards involved. One of the possible reactions is that of Mg with calcium phosphate which forms Mg phosphide that forms phosphine (a toxic gas) upon reaction with water. The possibility of this reaction increases with molten Mg and for amalgamations of HA due to hydroxyl groups in HA [28].
There are many commercially available Mg implants available today with various brand names. Cortical bone screws and pins from MAGNEZIX® are shown in Fig. 3. Various forms of Mg such as porous, fine grained, composite, and glassy structures, have been employed for implant fabrication purposes.
Commercially available MAGNEZIX® cortical screw (CS), cortical bone screw (CBS), and pin (Image courtesy www.magnezix.de)
Steel
The first line of effective substitutive joint prosthesis came very late in the 1950s and it was a cemented prosthesis with a stainless steel stem developed by Charnley [29]. Stainless steel materials show resistance to a variety of harsh chemical agents due to their high chromium content (~12%). They also induce the synthesis of a firm coating of Cr2O3 which has self-HA healing and corrosion resistance properties. Austenitic stainless steel is the preferred form of steel among the available versions for implant manufacturing. To behave austenitically at ambient temperature, stainless steel requires austenite stabilizers like Ni or Mn. AISI 316L grade of stainless steel is generally employed for clinical applications and it consists of 17–20 wt% Cr, 12–14 wt% Ni, 0.03 wt% C, and 2–3 wt% Mo as well as traces of N, Mn, P, Si, and S [24].
One of the major problems found with medical-grade steel alloys is the release of nickel ions which pose negative effects for various organs [30]. Nickel is expensive as well as causes serious allergies to the human skin. These issues have compelled advances in creating better nickel-free stainless steels. Nitrogen has been found as one of the great substitutes for nickel for austenite stabilizing and strengthening. Also, there have been developments in bio-composites, such as those made up of nickel-free stainless steels and HA [31,32,33].
As per ASTM standards, two of such nitrogen-containing medical-grade stainless steels have been listed without the presence of nickel: ASTM ID: F2229 and ASTM ID: F2581. A number of research studies have been conducted on these alloys in the recent years, and showed better biocompatibility, osteointegration, and corrosion behavior compared to traditional steel compositions [30, 34,35,36,37].
Popkov et al. studied the impact of nanostructured calcium-phosphate coatings on steel and Ti. They implanted these in the tibial bone marrow of dogs. Stainless steel and Ti wires were administered for the first and second group, and stainless steel and Ti wires of the same diameter coated with HA were implanted for the third and fourth groups. It was found that the HA coated wire implants provided better bioactivity and osteointegration and the nanostructure of HA played a major role in bone formation. These wires may find applications in various orthopedic conditions like fractures, osteogenesis imperfecta , and rectification of bone deformities [38].
Various modifications and coatings over existing steel have been yielding great results. UNS S31254 grade steel is one such version with a higher nitrogen content and lower cost (close to Ti) and has been further improved by HA coating using pulsed laser deposition (PLD). In one of such research studies with this technology, HA columns were successfully coated on a UNS S31254 substrate. Surface roughness of the coating is a desirable property for increased cell growth and proliferation and was found to be increased by atomic force microscopy (AFM) and scanning electron microscopy (SEM). This material had superior antibacterial properties and bioactivity as well as better adhesive strength as compared to the standard. This research paves the way to an alternate route of improving the physiological behavior of conventional metals. The favorable outcomes of this study emphasize the obvious requirements for an improved orthopedic implant material [39].
Titanium
Pure Ti shows relatively poor strength despite having excellent biocompatibility. When it is alloyed, the strength quotient goes up, but the toxicity level also goes up. The superior compatibility of Ti implants is accredited to its surface events (Fig. 4) and the stable 3–10 nm layer of oxide that is spontaneously formed on its exposure to oxygen [40, 41]. This layer creates direct contact with bone while blocking the development of a fibrous capsule outside the implant. At the beginning of Ti(O2) implantation, its non-physiological surface gets exposure of the physiological conditions and it becomes important to create a biomimicking coating onto the Ti surface. This helps in a gradual transformation between the Ti surface and the adjoining bone tissue and results in better bone cell adhesion, growth, and differentiation [42].
Schematic representation of events consecutively taking place at the titanium surface after implantation into living bone tissue. Water binds to the surface, followed by the incorporation of hydrated ions, adsorption and desorption of proteins, eventually leading to cell attachment. After differentiation, mature osteoblasts produce the extracellular matrix (ECM) [42]
Apart from the above-mentioned surface changes, immobilization of proteins, enzymes, peptides, and other biological molecules on implants have been the latest interest in this domain [43,44,45,46]. As compared to the conventional method of inorganic calcium phosphate coatings, the newer approach for surface modification employs purely organic components of bone. The organic and natural mimicking coatings include: (1) extracellular matrix (ECM) proteins or peptide sequences from laminin, fibronectin, or heparin binding domain [47, 48]; (2) cell signalling agents (TGF, BMPs, FGF, IGF, PDGF, etc.) to trigger new bone formation [20, 49, 50], (3) DNA [46, 51, 52] , and (4) enzymes [53].
There have been studies on both organic and inorganic phases over the implant. Xia et al. applied HA and collagen (which constitute the bone) over implant surfaces to enrich the biological reactions at the tissue and implant interface. They successfully prepared a homogenous collagen/apatite coating using a biomimetic technique. These composite layers showed a homogeneous porous structure with fibrous collagen and crystalline apatite. The in vitro studies showed better osteoblast proliferation over a composite coating with higher collagen content than with the pure apatite coating. This kind of organic/inorganic bone mimicking molecular cocktail has a wide scope to be applied on implant surfaces and improve the osteointegration between the implant and adjacent bone [54].
There are certain other surface modification routes as well to simply deposit pure and firm HA over Ti alloy surfaces not including the mediation of organic molecules. The zwitterionic polymer-modified surfaces are nonreactive to biological moieties viz. proteins/cells and have the potential to induce mineralized cluster growth. In one such experiment by Nishida et al., a monolayer of poly(ethylene glycol) methacrylate phosphate (Phosmer PE) was self-assembled on a Ti alloy surface [55] with a zwitterionic monomer (carboxymethyl betaine, CMB) . This poly(CMB) -modified Ti alloy plate suppressed the adsorption of proteins and attachment of cells, and triggered deposition of around two times calcium (Ca2+) as compared to the unaltered Ti alloy plate. In another experiment, the enzyme alkaline phosphatase (ALP), which plays the most crucial role in bone and cartilage mineralization process, was utilized to coat the Ti surface. It is believed to raise the concentration levels of inorganic phosphate as well as to lower the levels of extracellular pyrophosphate which impedes the process of mineralization. These coatings hastened mineralization over the Ti implant surface [53].
In one of the experiments to study surface topography effects, Yu et al. created two periodic microscale functionalized zones on Ti (MZT), i.e., nanoneedle zones and plain (buffer) zones. It was designed to relay spatially controlled topographical signals for better bone regeneration. The alternating buffer zones with no nanoneedle arrays were intercalated as the tips of the nanoneedles were too sharp to act as a contact area for the cells to effectively proliferate. In this way, the MZT displayed zone-wise apatite deposition and protein adsorption in N-zone and cell differentiation, mechanotransduction, and proliferation in the B and B-N zones. Enhanced osteoblast differentiation and nodule deposition was also observed with MZT. The constitution of the bone nodules on untreated Ti and MZT were also found to be different and the process of bone formation was enhanced by the MZT implant [56].
Tantalum
Ta is a transition metal and has recently emerged as a useful biomaterial for orthopedic applications due to its mechanical properties. This material has caught special attention due to properties such as greater volumetric porosity, friction coefficient, and modulus of elasticity that matches with natural compact and spongy bone. It has been successful in several orthopedic applications owing to its exceptional biocompatibility and favorable characteristics. It also possesses similarity with cancellous bone and is safe for in vivo use as verified by its clinical use in orthopedic surgery [57].
Despite its biocompatibility, inertness, and resistance to corrosion, the use of solid Ta in orthopedic implant devices has been restricted due difficulty in its manipulation. This issue led to the advent of designing porous Ta implants. As the technology of making implants with interconnected porosity has been here for a while, porous Ta trabecular metal (PTTM) also entered in the orthopedic domain in early 1990s [58]. “PTTM is commercially available as Trabecular Metal Material (Zimmer, Trabecular Metal Technology, Inc., Parsippany, NJ, USA) with an open-cell porous structure having 3D dodecahedron repeats similar to trabecular bone and the striking similarity can be seen” (Fig. 5). An initial foam-like vitreous carbon general scaffold is fabricated for these open-cell dodecahedron repeats and in due course it becomes the inner framework of the PTTM implant [59].
Scanning electron micrograph of human trabecular bone (a), porous tantalum fabricated by the CVD/CVI (b) or PM (c) method [60]
The pore dimension of PTTM is in the range of 300–600 μm with a porosity of 75–85%. For materials used in dental applications, like Ti and Ti alloys (with an elastic modulus of 106–115 GPa), PTTM is much more suitable due to its relevant properties. Porous Ta is also resistant to corrosion and is biocompatible. It can extensively augment the proliferation and differentiation of primary osteoblasts extracted from aged patients than those on traditional solid Ti [60].
In one of the studies on novel porous Ta implant by Lu et al., its osteocompatibility and efficacy to achieve lumbar interbody fusion (LIF) was evaluated in a rabbit anterior lumbar fusion model. The fusion was accomplished 12 months postoperatively as confirmed both radiographically and histologically in the porous Ta group similar to autologous bone implanted at intervertebral spaces. Implant degradation, wear debris, and osteolysis were found to be nil and no significant local inflammation response was found inside or outside the implant. Even the composites and degradation products of the Ta implant were non-toxic and biocompatible [61].
In an attempt to compare orthopedic implant grade porous Ta (i.e., trabecular metal), the responses of human osteoblast cultures from two groups of females were examined (less than 45 years and more than 60 years) on a titanium fiber mesh (TFM) and tissue culture plastic. In relationship to the cells from older patients, the cell attachment, growth, and mineralization were better in cells derived from the patients of lower age as expected. Among all the three substrates, cell adhesion was not much different on porous Ta than TFM or tissue culture plastic but cell proliferation on porous Ta was found to be highly stimulated. The findings from this study substantiated that porous Ta can find many more clinical applications in degenerative skeletal conditions than titanium [62].
Another study by Lee et al., compared Trabecular Metal™ Dental Implants (TM) and Tapered Screw-Vent® Dental Implants (TSV) for the potential of neo-osteogenesis and trabecular bone microarchitecture in fresh canine extraction sockets. It was revealed that there was more new bone in the TM implant than in the TSV at various healing time points. Histologically, trabecular metal implants exhibit higher amounts of bone with newly woven bone earlier than in the TSV implants [63].
NiTi Alloy: Apart from many of these traditionally used metals, a special alloy with a shape memory effect was identified by Buehler and Wang in 1967 [64] made up of Ni and Ti called as a NiTi alloy . The shape memory effect is a property that brings a material back to the old shape on rising its ambient temperature after it gets “plastically” deformed. Due to their elastic modulus and elastic recoverable strain, their use in load-bearing applications have been found to be more adequate than other metals. They exert compressive stress after the material has recovered from the prestrain of heating and this makes them suitable as spinal correctors, staples for osteotomies, internal fixators for long bone shafts, fracture repair, vertebral spacers and anchoring of prostheses, etc. [65, 66]. Despite these advantages, NiTi alloys show problems of allergy and toxicity due to Ni ion release. The toxicity of Ni and its possible carcinogenicity constraints the use of NiTi alloys in many parts of the world [24].
Ceramics
Ceramics have a very old history of usage as biomaterials and they have evolved many generations from first, second, third to modern generation bioactive ceramics (Fig. 6). They possess high hardness, high melting temperatures, low conduction of electricity and heat along with biocompatibility, resistance to compression and corrosion. Their surface properties are also favorable such as high wetting degrees and surface tensions that facilitate the adhesion of proteins, cells, and other biological moieties. As biomaterials, they are suitable specially for the generation of hard tissue engineering, owing to their chemical and physical properties. However, these biomaterials have some disadvantages, such as brittleness and low strength [67, 68].
Historical evolution of ceramics from routine objects to bioceramics. (a) Changing forms and factors of ceramics and (b) timeline of bioceramics [186]
From bio-inert ceramics such as alumina, zirconia to bioactive bioglasses, mesoporous silica and organic-inorganic composites, bioceramics have come a long way. The major categories of ceramics utilized for bone tissue engineering are as follows.
Hydroxyapatite
Hydroxyapatite (HA) is a natural kind of calcium phosphate and is the largest depot of inorganic constituent of human bones. This is the reason why it is most widely used in bone regeneration [69]. The word apatite stems from the Greek word `απαταω’ (“to deceive”), as it was not easy to be distinguished from other naturally occurring compounds such as aquamarine, amethyst, etc. The general formula for apatite is Ca5(PO4)X, where X can be any mono- and/or divalent anion such as fluoride, hydroxide, or carbonate. They are noticeably similar to the mineral phase of human bones and denture and thus are the molecules of choice for bone tissue engineering [70]. HA is very stable calcium phosphate and doesn’t easily dissolve under in vivo conditions specified by temperature, pH, body fluids, etc. [71, 72] The HA surface acts as a site for nucleation of bone minerals [73, 74] and one of its best features is that it doesn’t induce inflammatory reactions when used in clinical applications. Among apatites, carbonate apatite is the most abundant bioceramic phase of the human system. They can also be modified easily by ionic substitutions such as Ca2+ions with Ba2+, Sr2+or Pb2+, etc. [42]. Although HA has a natural occurrence and can be procured, due to its non-uniform and sometimes defective structures, HA for clinical applications and research needs to be synthesized in aqueous solution systems. HA possesses osteoconductive properties but not osteoinductive properties and the ionic substitution sometimes helps to overcome this drawback. The examples include fluoride ions for anionic substitution and Mg as cationic substitution which resulted in increased stability and favorable biological effects, respectively [74, 75].
Research has shown the potential and biocompatibility of HA with in vivo bone regeneration studies. This material enhances the differentiation and proliferation of MSCs by improving the attachment of osteoblasts [76, 77]. As it is quite brittle and hard, it is not usable for load bearing. It has been used for biomaterial surface coatings and as graft materials and also for bone regenerative applications in many forms, such as granules, cements, and pastes [78,79,80]. They have shown to improve many relevant aspects, such as osteoblast activity, implant contact area, and cellular responses of bone implants. Indirectly, they improve all the aspects of biomaterial performance [81, 82]. The HA coating methods can vary from spraying, sputtering, pulsed laser deposition or sol-gel techniques, etc. Their tunability and spectrum of usage can be further enhanced by combination with other flexible biomaterials like gels. Controlling their pore size and distribution, mechanical properties, biological activity, and user-friendliness can render them much more useful for bone regenerative applications [83,84,85,86].
Tricalcium Phosphate
Tricalcium phosphate (TCP; Ca3(PO4)2) is among the most widely used calcium phosphates. It has two phases (viz. α and β). α-TCP has monoclinic crystal strucures while β-TCP displays rhombohedron structures [87, 88]. β-TCP displays more stability as it takes more time than α-TCP to degrade. All of these reasons make β-TCP more applicable for bone regeneration. Its resorption rate is better and thus is more widely employed to enhance the biocompatibility of other materials [89, 90]. β-TCP also helps the proliferation of osteoblasts and bone marrow stromal cells [91, 92]. The excellent biomineralization and cell adhesion activities are attributed to its nanoporous structure. Due to such favorable properties, β-TCP has actively been examined and used for bone regeneration purposes, such as in bone cements and for bone replacement [93, 94].
For blending the best properties of TCP and HA in one place, special materials have come into existence which are termed as biphasic materials and were first formulated in 1986. These are made in such a manner that their constituents do not become separated owing to homogeneous and intimate submicron level mixing. These biphasic ceramic blends exist as a mixture of HA (with more stability) and β-TCP (with more solubility) [95]. Their bioactivity, bioresorbability, and osteoinductivity have been under evaluation for use in orthopedics and dentistry [96,97,98]. Such biphasic mixtures have shown promising results for the osteogenic differentiation of MSCs, increased cell adhesion, and enhanced mechanical properties [99, 100]. A biodegradable blend of β-TCP matrix and HA nanofibers was designed by Ramay et al. They constructed these microporous nanocomposite scaffolds using gel-polymer methods. Apart from cues to enhance cell growth and neovascularization, they also possessed enhanced mechanical properties which made them apt for use in load-bearing applications for bone tissue engineering [101].
Whitlockite
Whitlockite (WH) is a CaP containing ceramic with Mg content and is represented as Ca9Mg(HPO4) (PO4)6. After HA, it is the second highest concentration of mineral in the human skeleton, with a Ca/P ratio of 1.43 and crystal structure with a rhombohedral space group [102, 103]. It has a negatively charged surface and shows good stability at low pH [104, 105]. It has higher solubility under physiological conditions which leads to the continuous release of ions. As compared to HA, it has a higher compressive strength but because of its difficult synthesis, its research and development has not progressed well [106, 107].
WH is formed in the presence of Mg ions and calcium phosphate under acidic solutions even at low temperatures. The same phenomenon happens in vivo when old bone is resorbed by osteoclasts under acidic conditions [108,109,110]. Jang et al. designed an easy process for the formation of a stable, high-purity WH without toxic by-products. WH has shown to induce elevated expression levels of osteogenic genes than other ceramics [105, 107]. WH with a composite hydrogel promoted better growth and osteogenic activity than HA as shown in a bone regeneration study in rat calvaria [106]. The property of WH to continuously release Mg and PO4-3 seems to be the causal mechanism for osteogenic differentiation and bone growth as the Mg ions also decrease osteoclast activity [111].
Another form of calcium phosphate (viz. octacalcium phosphate (OCP)) that has a natural presence in human teeth is also considered important during the early phases of bone mineralization [112,113,114,115]. It has a very high biocompatibility and has been widely researched for bone implantation and coatings [116,117,118,119,120]. Even the amorphous form of calcium phosphate (ACP) transiently releases calcium and phosphate ions locally and has been utilized in several clinical applications [121,122,123].
The utilization of WH and HAP at a bone-like ratio (1:3) has recently been shown to exhibit remarkable osteogenic activity [124]. Such findings pave a hopeful way that understanding the generation and function of WH within local bone can guide in designing better calcium phosphate based materials. Because of certain mechanical disadvantages in clinical applications, research is being focused on employing calcium phosphate in combination with other materials [125].
In one comparative study with commercially available biomaterials, Ishikawa et al. studied three artificial bone substitutes with different ceramic constitutions viz. HA (Neobone®), carbonate apatite (CO3Ap ; Cytrans® ), and β-tricalcium phosphate (β-TCP; Cerasorb®) [126]. The difference in their ultrastructure can be seen in Fig. 7. Their physicochemical responses along with their tissue responses to bone were studied in hybrid dogs. As per SEM investigations, Neobone® and Cerasorb® were found to be porous, whereas Cytrans® was relatively much dense. As the fabrication of Cytrans® bone substitutes was done through a dissolution–precipitation method, it had a greater specific surface area with smaller crystals when compared to the other two which were fabricated by sintering. After 12 weeks of implantation, CO3Ap (Cytrans®) stimulated a higher new bone volume than HA and β-TCP. A bone-like composition and a higher specific surface area of CO3Ap (Cytrans®) may have caused a better osteogenesis response.
Typical scanning electron microscopy images of Neobone® (HAp) (a–c), Cytrans® (CO3Ap) (d–f), and Cerasorb® (β-TCP) (g–i). Both Neobone® and Cerasorb® displayed a porous structure, where Cerasorb® had much smaller pores compared to Neobone®. By contrast, Cytrans® was dense, and had no pores in the granules. Higher magnification showed that both Neobone® and Cerasorb® had smooth surfaces typical for sintering [126]
Bioactive Glasses
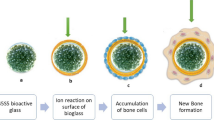
Bioactive glasses (BGs) are surface active glass ceramics. “Bioactive” refers to “a material that elicits a specific biological response at the material surface which results in the formation of a bond between the tissues and the materials” [127]. They are one of the most favorable bioactive scaffold materials for bone regeneration. They have a specific advantage in bone tissue engineering applications due to their capacity to form HA, along with their established osteoconductivity and their strong bonding ability with bone and soft tissues [128]. Since its invention as silicate glass (45S5) by Hench et al. in 1971, it has been a favorite material for exploration in clinical applications. 45S5 Bioglass® has a specific composition of 45% silica (SiO2), 24.5% calcium oxide (CaO), 24.5% sodium oxide (Na2O), and 6% phosphorous pentoxide (P2O5). The University of Florida has the intellectual property rights of this name viz. Bioglass® and it denotes the original 45S5 composition. All the other glasses are termed as bioactive glasses [129]. There are many methods for fabricating BG scaffolds (Fig. 8) and the method directly influences their structural aspects such as porosity and thus functionalities [130]. BGs can be either silicate-, borate-, or phosphate-based systems but silicate-based systems have been most studied and also successfully translated to many commercially available products.
Microstructures of bioactive glass scaffolds fabricated by different methods: (a) sol-gel; (b) thermal bonding (sintering) of particles (microspheres); (c) polymer foam replication technique to create “trabecular” scaffold; (d) grid-like microstructure prepared by robocasting; (e) oriented microstructure prepared by unidirectional freezing of suspensions (plane perpendicular to the orientation direction); and (f) micro-computed tomography image of the oriented scaffolds in (e) [130]
In a study by Bi et al., the influence of the microstructure of BGs on bone regeneration capacity was examined. Three variants of microstructures of borate bioactive glass (1393B3) scaffolds (trabecular, fibrous, and oriented) were fabricated. They were then tested for their bone regeneration potential in a calvarial defect model of rats. The extent of formed and mineralized new bone and angiogenesis was assessed 12 weeks post-implantation by histomorphometry and SEM. HA formed at the site of scaffold at the end of study period had a ratio of Ca to P that matched with that of bone. Out of the three variants, the trabecular microstructure seemed to be the most promising as it showed better neo-osteogenesis, higher osteoinductive ability, and greater blood vessel infiltration than the other two microstructures. Thus, out of the three variants, trabecular microstructure may have the highest potential for bone regeneration using synthetic implants [128].
BGs can also be used to functionalize other scaffolds and improve their physicochemical and osteoinductive properties. Moses et al., investigated copper doped BGs for functionalizing two silk scaffolds (Bombyx mori and Antheraea assama). The sol-gel coating of BG efficiently functionalized silk microfibers. An even, non-crystalline and nanoparticulate coating was formed over the silk microfibers. These functionalized silk microfibers had better physical characteristics like wettability, bulk density, stiffness, etc. These reinforced composite matrices were also better in the terms of their surface area along with open pores and a biomimetic microenvironment that allowed for cellular infiltration. The composite matrices got integrated and fully resorbed with new bone formation in rabbit femur defects. Such a kind of composite matrix opens interesting futuristic avenues to heal complex bone defects [131]. The market of bioactive glasses is constantly emerging and some of the commercially available products are presented in Table 3 [132].
Studies show that the regeneration by BG scaffolds is dependent on many aspects such as its constitution, method of fabrication, ultrastructure, and pore characteristics. Even other factors like pretreatment of the scaffold and the presence of growth factors play a major role. Lastly, the animal model used for the experiment, size of the defect, and implantation time may alter the scaffold behavior and results [129, 133,134,135].
Natural Ceramic (Nacre)
Over the past two decades , there have been major efforts to design bone substitutes, which can be employed for easy and efficient clinical practice. Along with the quest for synthetic materials, the aspect of biomimicking and generating bone-like composites, and natural materials are also under constant exploration. Nacre is also called the mother‐of‐pearl and is one such material that has osteoinductive, osteoconductive, biocompatible, and biodegradable properties. Its usage in the human race as a medical entity dates back to historic times. Nacre is a composite material composed of an organic matrix and calcium carbonate produced by molluscs as the shell. In vivo and in vitro studies confirm its outstanding properties as a potential multipurpose biomaterial as a bone graft substitute [136].
Akilal et al. experimented on Cowrie's shell derived powder (CSDP) and Nacre derived powder (NDP) for bone regeneration (Fig. 9). Structural and physicochemical investigation of CSDP revealed its brick and mortar ultrastructure composed of aragonite crystals. Upon soaking at 37 °C in simulated body fluid (SBF) for a week, these crystals transformed to poorly crystalline B-type carbonate apatite, reflecting bioactive features. Upon culturing stem cells on both substrates, it was found that CSDP supported cell proliferation more than nacre derived powder (NDP) over the study time period.
Scheme of the experiment with cowrie shell derived powder [137]
The morphology of stem cells also seemed flattened over CSDP, indicating superior biocompatibility. The cytoskeletal labeling showed well elongation of fibers on CSDP and relatively flattened cells over NDP after 7 days (Fig. 10). After 21 days, the cells were confluent with highly elongated actin fibers. The study suggests that these naturally derived materials offer an economic and novel hope for bone regenerative medicine [137].
Fluorescent microscopy views of human umbilical cord derived stem cells-cytoskeleton labeled cells after 7 days in presence of (a) CSDP and (b) NDP, respectively [137]
Polymers
As compared to metals and ceramics, polymers offer more flexibility, resilience, low cost, low conductivity, high durability, and moldability. Like all the biomaterial classes, polymers have also evolved in their subsequent generations. The first generation of polymer biomaterials included silicone rubber, polyethylene (PE), acrylic resins, polyurethanes, polypropylene (PP), and polymethylmethacrylate (PMMA). The major issue with first-generation biomaterials was the adsorption of various unspecific proteins on their surface post-implantation. The protein alignment in various conformations led to unspecific signaling pathways in the cellular microenvironment which further resulted in fibrous tissue growth which encapsulated the entire implant [138,139,140].
The second generation of polymer biomaterials was marked by the evolution of resorbable biomaterials that allowed for more control over their chemical breakdown and resorption. Biodegradable polymers of synthetic and natural origin such as polyglycolide (PGA), polylactide (PLA), poly(ϵ-caprolactone) (PCL), polydioxanone (PDS), polyhydroxybutyrate (PHB), chitosan (CS), polyorthoester, poly(2-hydroxyethyl-methacrylate) (PHEMA), polyethylene glycol (PEG), hyaluronic acid and other hydrogels have been researched and developed over this period. The hydrogel structure and 3D network help to hold large volumes of water and play multiple roles when used as an orthopedic biomaterial. Hydrogel polymers have shown potential for the repair of various tissues of the musculoskeletal system [141].
The most important properties for third generation biomaterials are bioactivity and biodegradability. The surfaces of these materials are also bioactivated with specific molecules to allow cell guidance and elicit particular responses. This class of biomaterials aim to closely match the ECM milieu and function by integrating specific cues. In short, they are able to modulate the important phases of cell behavior (viz. cell adhesion, migration, proliferation, and guided differentiation to the desired lineage) [24, 142, 143].
To develop new 3D scaffolds, natural as well as synthetic polymers have been employed for musculoskeletal regenerative medicine. Natural polymers have their own advantages but synthetic biodegradable polymers have drawn special interest because their physicochemical properties can be controlled in a better manner as demonstrated by their successful use in various medical purposes. Polymers that have been studied the most for bone tissue engineering include PLA, PGA, PCL, and PHB. Natural polymers like collagen and silk have been explored for applications in ligament tissue engineering [144, 145]. Composites of PCL and hyaluronic acid have yielded promising results and potential for meniscus tissue engineering [146]. “For applications such as cartilage and intervertebral disc tissue engineering, hyaluronic acid, polyglactin collagen, fibrin, alginates, chondroitin sulphate photo-crosslinked hydrogels and glycosaminoglycans have also been explored.” [147,148,149].
A polymeric membrane of gelatin-CS with inclusions of HA and titania nanoparticles was fabricated with UV radiation as a safe cross-linking agent. It resulted in a homogeneous material with well-distributed nanoparticles. UV cross-linking yielded better biological and mechanical properties of the composite membrane. The osteogenic potential of the gelatin-based material was established by ALP assay with mouse embryonic fibroblasts (MEF) in vitro. This composite is a good substitute to the existing guided bone regeneration membranes [150]. Babitha et al. designed gelatin composites with natural polymer viz. zein but because of its poor osteoinductivity for human periodontal ligament stem cells (hPDLSCs), they added a ceramic phase to it. This resulted in the fabrication of novel zein/gelatin/nanoHA (zein/gelatin/nHAp) nanofibrous membranes. These nanofiber membranes showed better surface wettability and induced better cell proliferation and differentiation of hPDLSCs to osteogenic lineage. It was demonstrated that these composite membranes possess superior biocompatibility and osteoinductive ability for hPDLSCs [151].
The behavior of bone mesenchymal stromal cells (BMSCs) was examined on electrospun CaCl2 treated poly(l-lactide) (PLLA) and gelatin composite fibers (PG-Ca). Mineralization ability for both fibers was also assessed in α minimum essential medium (αMEM) and it was found that the PG-Ca fibers strongly induced HA formation as compared to PG fibers. Apatite depositions were found after culturing BMSCs on both kinds of fibrous mats. Osteogenic differentiation of the proliferating BMSCs was also enhanced despite the absence of extra osteoinductive factors due to the continuous consumption of ions. PG-Ca fibrous mats showed better results than the control group. In essence, it was inferred that designing scaffolds which can infuse bone supportive ions such as Ca+2 and PO4-3 around cells can be a great approach to facilitate bone formation [152].
In an attempt to mimic bone in 3D, Anada et al. used a two-step digital light processing technique and fabricated a spheroid structure with octacalcium phosphate (OCP), spheroids of human umbilical vein endothelial cells (HUVEC), and a gelatin methacrylate (GelMA) hydrogel. The whole construct was designed to mimic the inner architecture of bone wherein the peripheral hydrogel with OCP mimicked the cortical shell and the inner bone marrow-like space was created using HUVEC spheroids embedded in GelMA (Fig. 11). The results showed that evenly embedded OCP stimulated the osteoblastic differentiation of MSCs. The capillary-like structure formation from the spheroids was regulated by the concentration of GelMA. This biomimetic construct with a cell-loaded hydrogel base and dual ring structure seems to be an encouraging model for bone tissue engineering purposes [153].
(a) Diagrammatic representation of fabrication process for 3D hydrogel constructs. (b) A photograph of 3D hydrogel constructs for vascular and bone formation. Bar = 5 mm [153]
Ingavle et al. studied the bone healing capacity of two natural polymer, i.e., alginate and hyaluronate, based biomineralized microspheres with entrapped MSCs. The polymers were altered with the adhesive tripeptide arginine-glycine-aspartic acid (RGD). Both the in vitro and in vivo studies (in Swiss alpine sheep) showed great results in terms of osteogenesis. When assessed against the untreated/acellular gels, the modified polymers had a substantial positive effect on parameters like blood vessel density and bone formation. These results indicated that hydrogels with stem cells can prove useful for bone regeneration in large animal bone defects [154].
Murahashi et al. loaded multi-layered PLLA nanosheets with recombinant human fibroblast growth factor-2 (rhFGF-2) for bone regeneration. They examined its effect in critical-sized mouse femoral defects and it was found that these nanosheets acted as a modified sustained-release carrier and efficiently induced bone regeneration. The nanosheets also induced FGFR1 gene activation and subsequent osteoblast differentiation [155].
Hydrogels are one of the very important classes of polymers that have immense potential as a biomaterial. Their optimization and design strategies must ensure features such as: (1) Non-immunogenicity and non-cytotoxicity, (2) osteoinductivity, osteoconductivity, osteogenicity, as well as osteocompatibility, (3) mimicry of the natural ECM to the extent possible, (4) degradability by endogenous enzymes or hydrolysis to synchronize with neo-osteogenesis, (5) mechanical and structural strength for treating load-bearing defects, (6) correct pore dimensions with interconnected porosity, and (7) injectability for patient compliance to enhance administration ease [156].
Due to their weaker mechanical properties, they are better suited for non-load-bearing applications. Various physical and chemical methods for attaching specific functional groups, hydrogen bonding, electrostatic interaction of the natural materials and inclusion of other phases, etc. are used to strengthen their mechanical traits and improve their bioactivity in order to augment their clinical value [144]. A recent development in the field of hydrogels is called nanogels which is a class of materials with spherical nanoparticles formed by cross-linking 3D polymer networks. They tend to expand in fluids by physical or chemical means. They show a range of hydrogel features like a great biocompatibility and tunable mechanical properties along with desirable dimensions of nanoparticles. Nanogels have great scope in the bone regeneration domain [157]. One more system of polymer hydrogels that can be used for bone regeneration includes microbeads. Microbeads represent an ideal system for entrapping live stem cells and therapeutic molecules with their cross-linking mechanisms. Still more studies are underway to develop perfectly biocompatible, osteoconductive, osteoinductive, and osteogenic microbead models [158].
Along with nanogel and microbead forms, a recent form of hydrogels that can be utilized are hydrogel fibers which may be responsive owing to their greater surface-volume ratio and immobilization capacity. They are made up of fibrous structures with a diameter of a few nanometers to many microns. The advantage of hydrogel fibers over microbeads is that the hydrogel fibers can be arranged axially in the syringe for better administration to the defect site. They can also be expected to stay at the implant site for an extended time as compared to the rounded structures of the microbeads [159].
Onat et al. reported the osteoinductivity of a polymer in their latest research. Most of the time, biodegradable polymers have been largely employed for complexing with and delivering osteoinductive moieties, but not by themselves as osteoinductive agents. This work reported the osteoinductive ability of poly(4-hydroxy-l-proline ester) (PHPE), which is a biodegradable cationic polymer with a specific property for cell penetration. The specific reactions cause PHPE to degrade in vivo and convert to trans 4-hydroxy-l-proline (trans-Hyp), a non-coded amino acid that plays crucial role in the formation and stability of collagen fibrils [160]. It was derived from this study that this polymer is nontoxic for the cells; cell exposure to PHPE induces higher COL1A1 expression leading to more synthesis of collagen, thus secretion in osteoblast-like cells. It also induces ECM mineralization in primary osteoblasts which further promotes ECM mineralization and bone tissue regeneration.
The effect of polymer molecular weight was demonstrated in one study by Davide et al. In bone tissue regeneration, polymers have mostly been used as various composites such as calcium-phosphate-based composites. They utilize the best features of both material classes and help to facilitate osteoinduction. This study examined the potential effects of the polymer phase as its molecular weight regulates fluid uptake, degradation dynamics, and the onset of surface reactions. They developed composites by the extrusion of two different molecular weight L/D, l-lactide copolymers with calcium phosphate apatite, namely M38 and M60. The M38 copolymer permitted higher fluid uptake leading to a better adsorptive ability for proteins in vitro. Figure 12 shows a higher amount of bone generated in the composite formed with lower molecular weight. The underlying reason may be its faster degradation and thus exposure of a rougher surface to trigger stem cells to differentiate osteogenically and cause bone formation [161].
Low magnification scans of composite explants. (a) Heterotopic bone formed in all 5 implants with M38. Bone is indicated by the dark pink areas among the M38 granules. (b) None of the M60 composites induced bone formation as no dark areas can be observed [161]
Composites
Significant efforts have been made to develop favorable bone replacement biomaterials for repairing larger bone defects. Still, the success in achieving perfect bone biomaterials with ideal physicochemical and osteoinductive properties seems far and that is where the role of composites comes in which can blend the best properties for various classes of materials. As the bone is also an organic–inorganic composite, this methodology can prove to be the best road to closely simulate bone. Some of the composite-based commercially available graft materials are listed in Table 4 [162].
Alidadi et al. evaluated the efficacy of xenogeneic demineralized bone matrix (DBM) , with two polymers viz. CS, and PMMA for repairing critical-sized radial bone defects in rats. As compared to DBM, CS and PMMA were found to be slowly degrading, non-compatible polymers with slow biodegradation rates that impeded bone regeneration, though CS is not osteoconductive or osteoinductive alone [163].
Lai et al. fabricated a novel porous composite with a powdered form of Mg, poly (lactide-co-glycolide) (PLGA), and β-tricalcium phosphate (β-TCP). This PLGA/TCP/Mg (PTM) scaffold was evaluated against a PLGA/TCP composite (PT) for its osteogenic and angiogenic properties. New vessels and new bone formation were seen in the PTM group and the results were better than the PT group. These findings divulged the osteogenic and angiogenic abilities of the PTM scaffold due to the presence of three chief orthopedic biomaterials and their concerted synergy which is capable of clinical translation [164]. An experiment to compare the biological qualities and bone regenerative capacity of an uncoated porous PCL scaffold with MgCO3-doped HA particles (PCL_MgCHA) and the same scaffold biomimetically coated with apatite-like crystals (PCL_MgCHAB) was carried out [165]. Both the scaffolds were found to be non-cytotoxic. PCL_MgCHAB displayed higher levels of ALP expression and collagen production. New bone trabeculae growth in PCL_MgCHAB was significantly higher as compared to PCL_MgCHA at the 4th and 12th weeks after implantation.
In a study, the osteogenicity and antimicrobial activity of HA and silver, respectively, were examined in a composite blend of HA/Ag. As the elevated concentrations of silver are known to trigger cytotoxicity, this study aimed to use silver nanoparticles so that even lower concentrations of silver can exhibit improved antimicrobial and anti-inflammatory effects. These particulate HA/Ag nanocomposites showed apatite formation in SBF and parameters like cell viability were found to be unaltered. This HA/Ag nanocomposite expressed bioactivity, and higher antimicrobial activity against Escherichia coli, Staphylococcus aureus, and Candida albicans even at lower concentrations of silver [166].
In one of the latest developments, a novel composite was designed with unique combinatorial chemistry. Strontium, which is known for bone strength enhancement and graphene which is known for its massive surface area, great specific conductance, high tensile modulus, and simple functional groups were amalgamated with the competence of nanotechnology. Chen et al. developed these innovative strontium-graphene oxide (Sr-GO) nanocomposites to release Sr ions in a sustained manner and were used as a reinforcement in collagen scaffolds. The Sr-GO-Col scaffold demonstrated better physicochemical and mechanical properties than the unmodified Col scaffolds. They also facilitated cell adhesion and osteogenic differentiation due to the MAPK signalling pathway stimulation. These Sr-GO-Col composites showed excellent bone regeneration, defect repairing, and angiogenesis results at 12 weeks in a critical-size calvarial bone defects in rats. The residual Sr-GO nanoparticles were found to be phagocytosed and degraded [167].
Apart from the above-mentioned materials, there have been many new explorations, such as with graphene for bone regenerative medicine per se with stem cells and with other molecules like PCL, alginate, gelatin, fibroin, etc. to produce composites and 3D scaffolds [168,169,170,171]. In one of the studies to combine the best of synthetic and naturally derived materials, a porous composite was prepared by PLA and DBM. This was implanted in a rabbit radius segmental bone defect to examine repair. The composite was compared with only PLA and only a bone autograft. The results from X-ray and histology confirmed that the effect of composite materials on bone repair was substantially higher than any of them acting alone [172].
A 3D scaffold with the combination of synthetic polymers and nanoceramics was explored for bone tissue engineering by Carrow et al. using additive manufacturing (AM). They reported the synthesis of a bioactive 3D scaffold nanocomposite from a poly(ethylene oxide terephthalate) (PEOT)/poly(butylene terephthalate) (PBT) (PEOT/PBT) copolymer and 2D nanosilicates. This particular combination was chosen with PEOT/PBT as they boost calcification and increase bone bonding ability, while 2D nanosilicates are known to lead hMSCS to an osteogenic lineage even without relying on osteoinductive agents. The stability and the bioactive properties of the composites were found to be increased. The osteo-related proteins were significantly upregulated along with the production of a mineralized matrix [173].
Role of Topography in Orthopedic Biomaterials
Bone as a Nanocomposite
The unique properties of natural bone are not derived just from its chemistry but also from its microarchitecture that span from nanoscale to macroscopic dimensions, with precise and meticulously engineered interfaces. The nanostructural composition of HA nanocrystals and collagen fibrils is what makes bone exceptional, a feature that could not be precisely reproduced artificially till date. In the stem cell microenvironment also, communications with ECM components exert indirect mechanical forces which influence stem cell behavior. The ECM has a unique composition with various types of polymers with different hierarchical dimensions such as collagen strands at the nanometer level to the micron range [174]. These symphonized spatiotemporal communications between the cells and their surroundings maintain and control their behaviors in the long term.
Cells keep receiving mechanotransducive cues in their natural microenvironment due to nanotopographical structures within the ECM around them. These cues influence their local migration, cell polarization, and other functions. This is the underlying reason how the nanoscale and microscale topographical features of a biomaterial can act as signalling modalities and can control cellular functions [175, 176].
Nanomaterials for Bone Regeneration and Repair
Nanomaterials and nanocomposites have been emerging as potential models for recreating the structure and function of bone and ECM. They serve as a structural framework for cells along with the regulation of key cell phases viz. growth, proliferation, differentiation, and migration that result in organized tissues. There can be various kinds of nanotopographical features on the biomaterial surface that influence cellular processes, some of which are represented in Fig. 13. They possess biomimicking and suitable traits such as high roughness and surface area that leads to higher protein adsorption than traditional biomaterials. Interactions at the interface of cells and a biomaterial are supposed to be moderated by integrin-led pathways that affect cell behavior [177].
Schematic depictions of representative nanotopography geometries. Three basic nanotopography geometries include nanogrooves (a), nanopost array (b), and nanopit array (c). The speculative pathways (d) for cell-shape-directed osteogenic and adipogenic differentiations of MSCs were examined in growth medium. RhoA Ras homolog gene family member A, ROCK Rho-associated protein kinase [185]
Many research groups have been constantly striving to recreate the bone’s mechanical properties (stiffness, strength, and toughness) as tissue engineered constructs through the inclusion of nanostructures in the base matrices to mimic bone’s natural composition [178, 179].
A precise microscale shape pattern has been shown to better regulate the osteogenesis process and nodule deposition as it directly influences cellular differentiation. The periodicity and dimensions of nanotopographical features have been shown to affect cellular differentiation process in many studies [176, 180, 181]. The topographical cues also affect events like protein adsorption and apatite deposition, which subsequently influence bone-mineral deposition [182, 183].
The introduction of advanced small-scale technologies has facilitated the design and development of methods that allows us to closely fathom stem cell behavior and biomechanics. Even the spatial arrangement of nanosized features play a crucial role in determining cell behavior and differentiation. Irregularly placed nanopits were found to be more effective in eliciting hMSC differentiation as demonstrated by higher osteopontin expression (Fig. 14) [184]. Moreover, biologically inspired substrates with a tuned ultrastructure are evolving for better understanding and control of stem cell behavior [185]. In the advent of the third generation of biomaterials, the magnitude of importance of their physical properties has taken central stage along with their chemical composition.
Exploring the effect of spatially different nanotopographies on cell differentiation. (a, b) nanotopographies fabricated by electron beam lithography (EBL). The pits (120 nm diameter, 100 nm depth) (a) in a square arrangement and (b) with increasing disorder (displaced square ±50 nm from true center). The nanoscale disorder stimulates human mesenchymal stem cells to increase the expression of the bone-specific ECM protein osteopontin (d, arrow) as compared to the ordered structure (c) [184]
Conclusion
Apart from natural grafts, metals, ceramics, and polymers are the major classes which have been under investigation as budding candidates for orthopedic regenerative medicine. Each of them has advocated for their unique characteristics but the far superior and unique traits of bone necessitate better combinatorial chemistry from the various material genres. It’s equally important that the material design schemes pay a high level of attention to minimize risks such as those caused by their degradation products to avoid various unforeseen hazards. As the in vitro results show inconsistency when tested in vivo, it’s crucial to understand the host responses after implantation (Table 5).
The natural composition and strength of bone is so unique that despite innumerable scientific attempts, identical ultrastructure and mechanical properties could not be achieved for their replacement and repair. Moreover, bone is constantly remodelled by daily loaded actions, both in static and dynamic conditions and this makes it even more challenging to engineer bone-tissue similes.
The third and upcoming generation of bone replacement materials includes not only biocompatibility, safety, and inertness, but also the capacity to induce or stimulate healing. The ongoing research is incorporating an understanding of the molecular level mechanisms and adding further functionalities and activation strategies to enhance the active participation of biomaterials in osteogenesis and new bone formation. These include major considerations for macro, micro, and nanoscale architectures and geometries, surface topography, biomimicking coatings, compositions, microenvironment tailoring, hierarchically organized structures, enzyme or growth factor release patterns, etc. such that they can directly regulate cellular responses toward favorable outcomes.
The futuristic ideal and smart material for bone regenerative applications will not only need to be osteoinductive and osteoconductive but also “osteo-sensitive” to adapt as per the changing mechanical and biophysical needs. Along with the quest to invent new materials and relevant compounds, parallel learning from nature and integrating both of these approaches seems like the most plausible way to come up with closest possible bone composites and fast translation to the clinics.
References
Laurencin CT, Khan Y, Kofron M et al (2006) THE ABJS NICOLAS ANDRY AWARD: tissue engineering of bone and ligament. Clin Orthop Relat Res. 447:221–236. https://doi.org/10.1097/01.blo.0000194677.02506.45
El-Ghannam A (2005) Bone reconstruction: from bioceramics to tissue engineering. Expert Rev Med Devices. 2(1):87–101. https://doi.org/10.1586/17434440.2.1.87
Ozdemir T, Higgins AM, Brown JL (2013) Osteoinductive biomaterial geometries for bone regenerative engineering. Curr Pharm Des 19(19):3446–3455. http://www.ncbi.nlm.nih.gov/pubmed/23432675. Accessed 11 Apr 2019
Urist MR (1965) Bone: formation by autoinduction. Science (80- ) 150(3698):893 LP–893899. http://science.sciencemag.org/content/150/3698/893.abstract
Wilson-Hench J (1987) Osteoinduction. Prog Biomed Eng 4:29
Miron RJ, Zhang YF (2012) Osteoinduction: a review of old concepts with new standrads. J Dent Res. 91(8):736–744. https://doi.org/10.1177/0022034511435260
Habibovic P, de Groot K (2007) Osteoinductive biomaterials—properties and relevance in bone repair. J Tissue Eng Regen Med. 1(1):25–32. https://doi.org/10.1002/term.5
Ripamonti U. The morphogenesis of bone in replicas of porous hydroxyapatite obtained from conversion of calcium carbonate exoskeletons of coral. 1991. http://wiredspace.wits.ac.za/handle/10539/19185. Accessed 27 Feb 2019.
Ripamonti U (1996) Osteoinduction in porous hydroxyapatite implanted in heterotopic sites of different animal models. Biomaterials. 17(1):31–35. https://doi.org/10.1016/0142-9612(96)80752-6
Yuan H, de Bruijn JD, Zhang X, van Blitterswijk CA, de Groot K (2001) Use of an osteoinductive biomaterial as a bone morphogenetic protein carrier. J Mater Sci Mater Med. 12(9):761–766. https://doi.org/10.1023/A:1013957431372
Reddi AH (1981) Cell biology and biochemistry of endochondral bone development. Coll Relat Res. 1(2):209–226. https://doi.org/10.1016/S0174-173X(81)80021-0
Yuan H, van den Doel M, Li S, van Blitterswijk CA, de Groot K, de Bruijn JD (2002) A comparison of the osteoinductive potential of two calcium phosphate ceramics implanted intramuscularly in goats. J Mater Sci Mater Med. 13(12):1271–1275. https://doi.org/10.1023/A:1021191432366
Ripamonti U (1991) The morphogenesis of bone in replicas of porous hydroxyapatite obtained from conversion of calcium carbonate exoskeletons of coral. J Bone Joint Surg Am. 73(5):692–703. http://www.ncbi.nlm.nih.gov/pubmed/1675218. Accessed 26 Feb 2019
Ohgushi H, Goldberg VM, Caplan AI (1989) Heterotopic osteogenesis in porous ceramics induced by marrow cells. J Orthop Res. 7(4):568–578. https://doi.org/10.1002/jor.1100070415
Goshima J, Goldberg VM, Caplan AI (1991) The osteogenic potential of culture-expanded rat marrow mesenchymal cells assayed in vivo in calcium phosphate ceramic blocks. Clin Orthop Relat Res. 262:298–311. http://www.ncbi.nlm.nih.gov/pubmed/1984928. Accessed 27 Feb 2019
Klein C, de Groot K, Chen W, Li Y, Zhang X (1994) Osseous substance formation induced in porous calcium phosphate ceramics in soft tissues. Biomaterials. 15(1):31–34. https://doi.org/10.1016/0142-9612(94)90193-7
Yang Z, Yuan H, Tong W, Zou P, Chen W, Zhang X (1996) Osteogenesis in extraskeletally implanted porous calcium phosphate ceramics: variability among different kinds of animals. Biomaterials. 17(22):2131–2137. http://www.ncbi.nlm.nih.gov/pubmed/8922598. Accessed 27 Feb 2019
Hari RA (1992) Regulation of cartilage and bone differentiation by bone morphogenetic proteins. Curr Opin Cell Biol. 4(5):850–855. https://doi.org/10.1016/0955-0674(92)90110-X
Wozney JM (1992) The bone morphogenetic protein family and osteogenesis. Mol Reprod Dev. 32(2):160–167. https://doi.org/10.1002/mrd.1080320212
Liu Y, de Groot K, Hunziker E (2005) BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model. Bone. 36(5):745–757. https://doi.org/10.1016/j.bone.2005.02.005
Barradas AMC, Yuan H, Clemens A van B, Habibovic P (2011) Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms. Eur Cells Mater. 21:407–429. https://doi.org/10.22203/eCM.v021a31
Chocholata P, Kulda V, Babuska V, Chocholata P, Kulda V, Babuska V (2019) Fabrication of scaffolds for bone-tissue regeneration. Materials (Basel). 12(4):568. https://doi.org/10.3390/ma12040568
Yazdimamaghani M, Razavi M, Vashaee D, Moharamzadeh K, Boccaccini AR, Tayebi L (2017) Porous magnesium-based scaffolds for tissue engineering. Mater Sci Eng C. 71:1253–1266. https://doi.org/10.1016/j.msec.2016.11.027
Navarro M, Michiardi A, Castaño O, Planell JA (2008) Biomaterials in orthopaedics. J R Soc Interface. 5(27):1137–1158. https://doi.org/10.1098/rsif.2008.0151
Kamrani S, Fleck C (2019) Biodegradable magnesium alloys as temporary orthopaedic implants: a review. BioMetals. 32(2):185–193. https://doi.org/10.1007/s10534-019-00170-y
Lee J-W, Han H-S, Han K-J et al (2016) Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. Proc Natl Acad Sci U S A. 113(3):716–721. https://doi.org/10.1073/pnas.1518238113
Witte F, Feyerabend F, Maier P et al (2007) Biodegradable magnesium–hydroxyapatite metal matrix composites. Biomaterials. 28(13):2163–2174. https://doi.org/10.1016/J.BIOMATERIALS.2006.12.027
Courtenay J, Bryant M (2011) Bone ash replacement product is safer. Alum Times. 57
Charnley J (1960) Anchorage of the femoral head prosthesis to the shaft of the femur. J Bone Joint Surg Br. 42-B:28–30. http://www.ncbi.nlm.nih.gov/pubmed/13855642. Accessed 23 Apr 2019
Yang K, Ren Y (2010) Nickel-free austenitic stainless steels for medical applications. Sci Technol Adv Mater. 11(1):014105. https://doi.org/10.1088/1468-6996/11/1/014105
Younesi M, Bahrololoom ME, Fooladfar H (2010) Development of wear resistant NFSS–HA novel biocomposites and study of their tribological properties for orthopaedic applications. J Mech Behav Biomed Mater. 3(2):178–188. https://doi.org/10.1016/J.JMBBM.2009.08.003
Younesi M, Bahrololoom ME (2010) Formulation of the wear behaviour of nickel-free stainless-steel/hydroxyapatite bio-composites by response surface methodology. Proc Inst Mech Eng Part J J Eng Tribol. 224(11):1197–1207. https://doi.org/10.1243/13506501JET773
Younesi M, Bahrololoom ME, Ahmadzadeh M (2010) Prediction of wear behaviors of nickel free stainless steel–hydroxyapatite bio-composites using artificial neural network. Comput Mater Sci. 47(3):645–654. https://doi.org/10.1016/J.COMMATSCI.2009.09.019
Montanaro L, Cervellati M, Campoccia D, Arciola CR (2006) Promising in vitro performances of a new nickel-free stainless steel. J Mater Sci Mater Med. 17(3):267–275. https://doi.org/10.1007/s10856-006-7313-3
Torricelli P, Fini M, Borsari V et al (2003) Biomaterials in orthopedic surgery: effects of a nickel-reduced stainless steel on in vitro proliferation and activation of human osteoblasts. Int J Artif Organs. 26(10):952–957. https://doi.org/10.1177/039139880302601013
Thomann UI, Uggowitzer PJ (2000) Wear–corrosion behavior of biocompatible austenitic stainless steels. Wear. 239(1):48–58. https://doi.org/10.1016/S0043-1648(99)00372-5
Ren YB, Yang HJ, Yang K, Zhang BC (2007) In vitro biocompatibility of a new high nitrogen nickel free austenitic stainless steel. Key Eng Mater. 342-343:605–608. https://doi.org/10.4028/www.scientific.net/KEM.342-343.605
Popkov AV, Gorbach EN, Kononovich NA, Popkov DA, Tverdokhlebov SI, Shesterikov EV (2017) Bioactivity and osteointegration of hydroxyapatite-coated stainless steel and titanium wires used for intramedullary osteosynthesis. Strateg Trauma Limb Reconstr. 12(2):107–113. https://doi.org/10.1007/s11751-017-0282-x
Das A, Shukla M (2019) Surface morphology, bioactivity, and antibacterial studies of pulsed laser deposited hydroxyapatite coatings on Stainless Steel 254 for orthopedic implant applications. Proc Inst Mech Eng Part L J Mater Des Appl. 233(2):120–127. https://doi.org/10.1177/1464420716663029
Sul Y-T, Johansson CB, Petronis S et al (2002) Characteristics of the surface oxides on turned and electrochemically oxidized pure titanium implants up to dielectric breakdown:: the oxide thickness, micropore configurations, surface roughness, crystal structure and chemical composition. Biomaterials. 23(2):491–501. https://doi.org/10.1016/S0142-9612(01)00131-4
Raikar GN, Gregory JC, Ong JL et al (1995) Surface characterization of titanium implants. J Vac Sci Technol A Vacuum, Surfaces, Film 13(5):2633–2637. https://doi.org/10.1116/1.579462
de Jonge LT, Leeuwenburgh SCG, Wolke JGC, Jansen JA (2008) Organic–inorganic surface modifications for titanium implant surfaces. Pharm Res. 25(10):2357–2369. https://doi.org/10.1007/s11095-008-9617-0
Bierbaum S, Hempel U, Geißler U et al (2003) Modification of Ti6AL4V surfaces using collagen I, III, and fibronectin. II. Influence on osteoblast responses. J Biomed Mater Res Part A. 67A(2):431–438. https://doi.org/10.1002/jbm.a.10084
MacDonald DE, Markovic B, Allen M, Somasundaran P, Boskey AL (1998) Surface analysis of human plasma fibronectin adsorbed to commercially pure titanium materials. J Biomed Mater Res. 41(1):120–130. https://doi.org/10.1002/(SICI)1097-4636(199807)41:1<120::AID-JBM15>3.0.CO;2-R
Roessler S, Born R, Scharnweber D, Worch H, Sewing A, Dard M (2001) Biomimetic coatings functionalized with adhesion peptides for dental implants. J Mater Sci Mater Med. 12(10/12):871–877. https://doi.org/10.1023/A:1012807621414
van den Beucken JJJP, Vos MRJ, Thüne PC et al (2006) Fabrication, characterization, and biological assessment of multilayered DNA-coatings for biomaterial purposes. Biomaterials. 27(5):691–701. https://doi.org/10.1016/J.BIOMATERIALS.2005.06.015
Ferris D, Moodie G, Dimond P, Giorani CW, Ehrlich M, Valentini R (1999) RGD-coated titanium implants stimulate increased bone formation in vivo. Biomaterials. 20(23-24):2323–2331. https://doi.org/10.1016/S0142-9612(99)00161-1
Elmengaard B, Bechtold JE, Søballe K (2005) In vivo study of the effect of RGD treatment on bone ongrowth on press-fit titanium alloy implants. Biomaterials. 26(17):3521–3526. https://doi.org/10.1016/J.BIOMATERIALS.2004.09.039
Liu Y, Hunziker EB, Layrolle P, De Bruijn JD, De Groot K (2004) Bone morphogenetic protein 2 incorporated into biomimetic coatings retains its biological activity. Tissue Eng. 10(1-2):101–108. https://doi.org/10.1089/107632704322791745
Schmidmaier G, Wildemann B, Cromme F, Kandziora F, Haas NP, Raschke M (2002) Bone morphogenetic protein-2 coating of titanium implants increases biomechanical strength and accelerates bone remodeling in fracture treatment: a biomechanical and histological study in rats. Bone. 30(6):816–822. https://doi.org/10.1016/S8756-3282(02)00740-8
van den Beucken JJJP, Walboomers XF, Boerman OC et al (2006) Functionalization of multilayered DNA-coatings with bone morphogenetic protein 2. J Control Release 113(1):63–72. https://doi.org/10.1016/J.JCONREL.2006.03.016
van den Beucken JJJP, Walboomers XF, Leeuwenburgh SCG et al (2007) Multilayered DNA coatings: in vitro bioactivity studies and effects on osteoblast-like cell behavior. Acta Biomater. 3(4):587–596. https://doi.org/10.1016/J.ACTBIO.2006.12.007
De Jonge LT, Leeuwenburgh SCG, van de Beucken J, Wolke JGC, Jansen JA (2008) Electrosprayed enzyme coatings as bio-inspired alternatives to conventional bioceramic coatings for orthopedic and oral implants. Adv Funct Mater 19:755–762
Xia Z, Yu X, Wei M (2012) Biomimetic collagen/apatite coating formation on Ti6Al4V substrates. J Biomed Mater Res Part B Appl Biomater. 100B(3):871–881. https://doi.org/10.1002/jbm.b.31970
Nishida M, Nakaji-Hirabayashi T, Kitano H, Saruwatari Y, Matsuoka K (2017) Titanium alloy modified with anti-biofouling zwitterionic polymer to facilitate formation of bio-mineral layer. Colloids Surfaces B Biointerfaces. 152:302–310. https://doi.org/10.1016/j.colsurfb.2017.01.018
Yu P, Zhu X, Wang X et al (2016) Periodic nanoneedle and buffer zones constructed on a titanium surface promote osteogenic differentiation and bone calcification in vivo. Adv Healthc Mater. 5(3):364–372. https://doi.org/10.1002/adhm.201500461
George N, Nair AB (2018) Porous tantalum: a new biomaterial in orthopedic surgery. Fundam Biomater Met:243–268. https://doi.org/10.1016/B978-0-08-102205-4.00011-8
Edelmann AR, Patel D, Allen RK, Gibson CJ, Best AM, Bencharit S (2019) Retrospective analysis of porous tantalum trabecular metal–enhanced titanium dental implants. J Prosthet Dent. 121(3):404–410. https://doi.org/10.1016/j.prosdent.2018.04.022
Bencharit S, Byrd WC, Altarawneh S et al (2014) Development and applications of porous tantalum trabecular metal-enhanced titanium dental implants. Clin Implant Dent Relat Res. 16(6):817–826. https://doi.org/10.1111/cid.12059
Liu Y, Bao C, Wismeijer D, Wu G (2015) The physicochemical/biological properties of porous tantalum and the potential surface modification techniques to improve its clinical application in dental implantology. Mater Sci Eng C. 49:323–329. https://doi.org/10.1016/j.msec.2015.01.007
Lu M, Xu S, Lei Z-X et al (2019) Application of a novel porous tantalum implant in rabbit anterior lumbar spine fusion model. Chin Med J (Engl). 132(1):51–62. https://doi.org/10.1097/CM9.0000000000000030
Sagomonyants KB, Hakim-Zargar M, Jhaveri A, Aronow MS, Gronowicz G (2011) Porous tantalum stimulates the proliferation and osteogenesis of osteoblasts from elderly female patients. J Orthop Res. 29(4):609–616. https://doi.org/10.1002/jor.21251
Lee JW, Wen HB, Gubbi P, Romanos GE (2018) New bone formation and trabecular bone microarchitecture of highly porous tantalum compared to titanium implant threads: a pilot canine study. Clin Oral Implants Res. 29(2):164–174. https://doi.org/10.1111/clr.13074
Buehler WJ, Wang FE (1968) A summary of recent research on the nitinol alloys and their potential application in ocean engineering. Ocean Eng. 1(1):105–120. https://doi.org/10.1016/0029-8018(68)90019-X
Chu Y, Dai KR, Zhu M, Mi XJ (2000) Medical application of NiTi shape memory alloy in China. Mater Sci Forum. 327-328:55–62. https://doi.org/10.4028/www.scientific.net/MSF.327-328.55
Duerig TW, Pelton AR, Stöckel D (1996) The utility of superelasticity in medicine. Biomed Mater Eng. 6(4):255–266. https://doi.org/10.3233/BME-1996-6404
Vallet-Regí M, Salinas AJ (2019) Ceramics as bone repair materials. Bone Repair Biomater:141–178. https://doi.org/10.1016/B978-0-08-102451-5.00006-8
Arcos D, Izquierdo-Barba I, Vallet-Regí M (2009) Promising trends of bioceramics in the biomaterials field. J Mater Sci Mater Med. 20(2):447–455. https://doi.org/10.1007/s10856-008-3616-x
Yoshikawa H, Myoui A (2005) Bone tissue engineering with porous hydroxyapatite ceramics. J Artif Organs. 8(3):131–136. https://doi.org/10.1007/s10047-005-0292-1
Rey C (1990) Calcium phosphate biomaterials and bone mineral. Differences in composition, structures and properties. Biomaterials. 11:13–15. https://doi.org/10.1016/0142-9612(90)90045-R
Ramselaar MMA, Driessens FCM, Kalk W, De Wijn JR, Van Mullem PJ (1991) Biodegradation of four calcium phosphate ceramics;in vivo rates and tissue interactions. J Mater Sci Mater Med. 2(2):63–70. https://doi.org/10.1007/BF00703460
Rapacz-Kmita A, Ślósarczyk A, Paszkiewicz Z (2005) FTIR and XRD investigations on the thermal stability of hydroxyapatite during hot pressing and pressureless sintering processes. J Mol Struct. 744-747:653–656. https://doi.org/10.1016/J.MOLSTRUC.2004.11.070
Bohner M, Lemaitre J (2009) Can bioactivity be tested in vitro with SBF solution? Biomaterials. 30(12):2175–2179. https://doi.org/10.1016/J.BIOMATERIALS.2009.01.008
Samavedi S, Whittington AR, Goldstein AS (2013) Calcium phosphate ceramics in bone tissue engineering: a review of properties and their influence on cell behavior. Acta Biomater. 9(9):8037–8045. https://doi.org/10.1016/J.ACTBIO.2013.06.014
Ogata K, Imazato S, Ehara A et al (2005) Comparison of osteoblast responses to hydroxyapatite and hydroxyapatite/soluble calcium phosphate composites. J Biomed Mater Res Part A. 72A(2):127–135. https://doi.org/10.1002/jbm.a.30146
Douglas T, Pamula E, Hauk D et al (2009) Porous polymer/hydroxyapatite scaffolds: characterization and biocompatibility investigations. J Mater Sci Mater Med. 20(9):1909–1915. https://doi.org/10.1007/s10856-009-3756-7
Guo H, Su J, Wei J, Kong H, Liu C (2009) Biocompatibility and osteogenicity of degradable Ca-deficient hydroxyapatite scaffolds from calcium phosphate cement for bone tissue engineering. Acta Biomater. 5(1):268–278. https://doi.org/10.1016/J.ACTBIO.2008.07.018
Capilla MV, Olid MNR, Gaya MVO, Botella CR, Romera CZ (2007) Cylindrical dental implants with hydroxyapatite- and titanium plasma spray–coated surfaces: 5-year results. J Oral Implantol 33(2):59–68. https://doi.org/10.1563/1548-1336(2007)33[59:CDIWHA]2.0.CO;2
Zhou W, Liu Z, Xu S, Hao P, Xu F, Sun A (2011) Long-term survivability of hydroxyapatite-coated implants: a meta-analysis. Oral Surg. 4(1):2–7. https://doi.org/10.1111/j.1752-248X.2010.01112.x
Zhou M, Geng Y, Li S et al (2019) Nanocrystalline hydroxyapatite-based scaffold adsorbs and gives sustained release of osteoinductive growth factor and facilitates bone regeneration in mice ectopic model. J Nanomater. 2019:1–10. https://doi.org/10.1155/2019/1202159
Ramires PA, Wennerberg A, Johansson CB, Cosentino F, Tundo S, Milella E (2003) Biological behavior of sol-gel coated dental implants. J Mater Sci Mater Med. 14(6):539–545. https://doi.org/10.1023/A:1023412131314
Albrektsson T (1998) Hydroxyapatite-coated implants: a case against their use. J Oral Maxillofac Surg. 56(11):1312–1326. https://doi.org/10.1016/S0278-2391(98)90616-4
de Oliveira PT, Zalzal SF, Beloti MM, Rosa AL, Nanci A (2007) Enhancement ofin vitro osteogenesis on titanium by chemically produced nanotopography. J Biomed Mater Res Part A. 80A(3):554–564. https://doi.org/10.1002/jbm.a.30955
Göransson A, Arvidsson A, Currie F et al (2009) An in vitro comparison of possibly bioactive titanium implant surfaces. J Biomed Mater Res Part A. 88A(4):1037–1047. https://doi.org/10.1002/jbm.a.31911
Hwang NS, Varghese S, Lee HJ, Zhang Z, Elisseeff J (2013) Biomaterials directed in vivo osteogenic differentiation of mesenchymal cells derived from human embryonic stem cells. Tissue Eng Part A. 19(15-16):1723–1732. https://doi.org/10.1089/ten.tea.2013.0064
Dhivya S, Saravanan S, Sastry TP, Selvamurugan N (2015) Nanohydroxyapatite-reinforced chitosan composite hydrogel for bone tissue repair in vitro and in vivo. J Nanobiotechnology. 13(1):40. https://doi.org/10.1186/s12951-015-0099-z
Dickens B, Schroeder LW, Brown WE (1974) Crystallographic studies of the role of Mg as a stabilizing impurity in β-Ca3(PO4)2. The crystal structure of pure β-Ca3(PO4)2. J Solid State Chem. 10(3):232–248. https://doi.org/10.1016/0022-4596(74)90030-9
Mathew M, Schroeder LW, Dickens B, Brown WE (1977) The crystal structure of α-Ca3(PO4)2. Acta Crystallogr Sect B Struct Crystallogr Cryst Chem. 33(5):1325–1333. https://doi.org/10.1107/S0567740877006037
Horch H-H, Sader R, Pautke C, Neff A, Deppe H, Kolk A (2006) Synthetic, pure-phase beta-tricalcium phosphate ceramic granules (Cerasorb®) for bone regeneration in the reconstructive surgery of the jaws. Int J Oral Maxillofac Surg. 35(8):708–713. https://doi.org/10.1016/J.IJOM.2006.03.017
Yamada S, Heymann D, Bouler J-M, Daculsi G (1997) Osteoclastic resorption of calcium phosphate ceramics with different hydroxyapatite/β-tricalcium phosphate ratios. Biomaterials. 18(15):1037–1041. https://doi.org/10.1016/S0142-9612(97)00036-7
Yao C-H, Liu B-S, Hsu S-H, Chen Y-S, Tsai C-C (2004) Biocompatibility and biodegradation of a bone composite containing tricalcium phosphate and genipin crosslinked gelatin. J Biomed Mater Res. 69A(4):709–717. https://doi.org/10.1002/jbm.a.30045
Liu H, Cai Q, Lian P et al (2010) β-tricalcium phosphate nanoparticles adhered carbon nanofibrous membrane for human osteoblasts cell culture. Mater Lett. 64(6):725–728. https://doi.org/10.1016/J.MATLET.2009.12.050
Kamitakahara M, Ohtsuki C, Miyazaki T (2008) Review paper: behavior of ceramic biomaterials derived from tricalcium phosphate in physiological condition. J Biomater Appl. 23(3):197–212. https://doi.org/10.1177/0885328208096798
Bi L, Cheng W, Fan H, Pei G (2010) Reconstruction of goat tibial defects using an injectable tricalcium phosphate/chitosan in combination with autologous platelet-rich plasma. Biomaterials. 31(12):3201–3211. https://doi.org/10.1016/J.BIOMATERIALS.2010.01.038
Ellinger RF, Nery EB, Lynch KL (1986) Histological assessment of periodontal osseous defects following implantation of hydroxyapatite and biphasic calcium phosphate ceramics: a case report. Int J Periodontics Restorative Dent. 6(3):22–33. http://www.ncbi.nlm.nih.gov/pubmed/3015813. Accessed 28 Apr 2019
Daculsi G (1998) Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute. Biomaterials. 19(16):1473–1478. https://doi.org/10.1016/S0142-9612(98)00061-1
Lobo SE, Livingston Arinzeh T, Lobo SE, Livingston AT (2010) Biphasic calcium phosphate ceramics for bone regeneration and tissue engineering applications. Materials (Basel). 3(2):815–826. https://doi.org/10.3390/ma3020815
Dorozhkin SV (2012) Biphasic, triphasic and multiphasic calcium orthophosphates. Acta Biomater. 8(3):963–977. https://doi.org/10.1016/J.ACTBIO.2011.09.003
Arinzeh TL, Tran T, Mcalary J, Daculsi G (2005) A comparative study of biphasic calcium phosphate ceramics for human mesenchymal stem-cell-induced bone formation. Biomaterials. 26(17):3631–3638. https://doi.org/10.1016/J.BIOMATERIALS.2004.09.035
Amirian J, Linh NTB, Min YK, Lee B-T (2015) Bone formation of a porous Gelatin-Pectin-biphasic calcium phosphate composite in presence of BMP-2 and VEGF. Int J Biol Macromol. 76:10–24. https://doi.org/10.1016/J.IJBIOMAC.2015.02.021
Ramay HR, Zhang M (2004) Biphasic calcium phosphate nanocomposite porous scaffolds for load-bearing bone tissue engineering. Biomaterials. 25(21):5171–5180. https://doi.org/10.1016/J.BIOMATERIALS.2003.12.023
Scotchford CA, Vickers M, Yousuf Ali S (1995) The isolation and characterization of magnesium whitlockite crystals from human articular cartilage. Osteoarthr Cartil. 3(2):79–94. https://doi.org/10.1016/S1063-4584(05)80041-X
Elliott JC (1994) Structure and chemistry of the apatites and other calcium orthophosphates. Elsevier, Amsterdam. https://www.sciencedirect.com/bookseries/studies-in-inorganic-chemistry/vol/18. Accessed 26 Jul 2019
Jang HL, Lee HK, Jin K, Ahn H-Y, Lee H-E, Nam KT (2015) Phase transformation from hydroxyapatite to the secondary bone mineral, whitlockite. J Mater Chem B. 3(7):1342–1349. https://doi.org/10.1039/C4TB01793E
Jang HL, Jin K, Lee J et al (2014) Revisiting whitlockite, the second most abundant biomineral in bone: nanocrystal synthesis in physiologically relevant conditions and biocompatibility evaluation. ACS Nano. 8(1):634–641. https://doi.org/10.1021/nn405246h
Kim HD, Jang HL, Ahn H-Y et al (2017) Biomimetic whitlockite inorganic nanoparticles-mediated in situ remodeling and rapid bone regeneration. Biomaterials. 112:31–43. https://doi.org/10.1016/J.BIOMATERIALS.2016.10.009
Jang HL, Bin ZG, Park J et al (2016) In vitro and in vivo evaluation of whitlockite biocompatibility: comparative study with hydroxyapatite and β -tricalcium phosphate. Adv Healthc Mater. 5(1):128–136. https://doi.org/10.1002/adhm.201400824
Cheng PT, Grabher JJ, LeGeros RZ (1988) Effects of magnesium on calcium phosphate formation. Magnesium. 7(3):123–132. http://www.ncbi.nlm.nih.gov/pubmed/2846970. Accessed 29 Apr 2019
Silver IA, Murrills RJ, Etherington DJ (1988) Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 175(2):266–276. https://doi.org/10.1016/0014-4827(88)90191-7
Teitelbaum SL (2000) Bone resorption by osteoclasts. Science. 289(5484):1504–1508. https://doi.org/10.1126/SCIENCE.289.5484.1504
Kim H-K, Han H-S, Lee K-S et al (2017) Comprehensive study on the roles of released ions from biodegradable Mg-5 wt% Ca-1 wt% Zn alloy in bone regeneration. J Tissue Eng Regen Med. 11(10):2710–2724. https://doi.org/10.1002/term.2166
Zapanta Le Geros R (1974) Variations in the crystalline components of human dental calculus: i. crystallographic and spectroscopic methods of analysis. J Dent Res. 53(1):45–50. https://doi.org/10.1177/00220345740530012801
Barrère F, van Blitterswijk CA, de Groot K (2006) Bone regeneration: molecular and cellular interactions with calcium phosphate ceramics. Int J Nanomedicine. 1(3):317–332. http://www.ncbi.nlm.nih.gov/pubmed/17717972. Accessed 29 Apr 2019
Bodier-Houllé P, Steuer P, Voegel J-C, Cuisinier FJG (1998) First experimental evidence for human dentine crystal formation involving conversion of octacalcium phosphate to hydroxyapatite. Acta Crystallogr Sect D Biol Crystallogr. 54(6):1377–1381. https://doi.org/10.1107/S0907444998005769
Suzuki O, Imaizumi H, Kamakura S, Katagiri T (2008) Bone regeneration by synthetic octacalcium phosphate and its role in biological mineralization. Curr Med Chem. 15(3):305–313. https://doi.org/10.2174/092986708783497283
Barrère F, Layrolle P, van Blitterswijk CA, de Groot K (2001) Biomimetic coatings on titanium: a crystal growth study of octacalcium phosphate. J Mater Sci Mater Med. 12(6):529–534. https://doi.org/10.1023/A:1011271713758
Socol G, Torricelli P, Bracci B et al (2004) Biocompatible nanocrystalline octacalcium phosphate thin films obtained by pulsed laser deposition. Biomaterials. 25(13):2539–2545. https://doi.org/10.1016/J.BIOMATERIALS.2003.09.044
Shelton RM, Liu Y, Cooper PR, Gbureck U, German MJ, Barralet JE (2006) Bone marrow cell gene expression and tissue construct assembly using octacalcium phosphate microscaffolds. Biomaterials. 27(14):2874–2881. https://doi.org/10.1016/J.BIOMATERIALS.2005.12.031
Kikawa T, Kashimoto O, Imaizumi H, Kokubun S, Suzuki O (2009) Intramembranous bone tissue response to biodegradable octacalcium phosphate implant. Acta Biomater. 5(5):1756–1766. https://doi.org/10.1016/J.ACTBIO.2008.12.008
Stefanic M, Krnel K, Pribosic I, Kosmac T (2012) Rapid biomimetic deposition of octacalcium phosphate coatings on zirconia ceramics (Y-TZP) for dental implant applications. Appl Surf Sci. 258(10):4649–4656. https://doi.org/10.1016/J.APSUSC.2012.01.048
ter Brugge PJ, Wolke JGC, Jansen JA (2003) Effect of calcium phosphate coating composition and crystallinity on the response of osteogenic cells in vitro. Clin Oral Implants Res. 14(4):472–480. https://doi.org/10.1034/j.1600-0501.2003.00886.x
Combes C, Rey C (2010) Amorphous calcium phosphates: synthesis, properties and uses in biomaterials. Acta Biomater. 6(9):3362–3378. https://doi.org/10.1016/J.ACTBIO.2010.02.017
Popp JR, Laflin KE, Love BJ, Goldstein AS (2012) Fabrication and characterization of poly(lactic-co-glycolic acid) microsphere/amorphous calcium phosphate scaffolds. J Tissue Eng Regen Med. 6(1):12–20. https://doi.org/10.1002/term.390
Cheng H, Chabok R, Guan X et al (2018) Synergistic interplay between the two major bone minerals, hydroxyapatite and whitlockite nanoparticles, for osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 69:342–351. https://doi.org/10.1016/J.ACTBIO.2018.01.016
Jeong J, Kim JH, Shim JH, Hwang NS, Heo CY (2019) Bioactive calcium phosphate materials and applications in bone regeneration. Biomater Res. 23:4. https://doi.org/10.1186/s40824-018-0149-3
Ishikawa K, Miyamoto Y, Tsuchiya A et al (2018) Physical and histological comparison of hydroxyapatite, carbonate apatite, and β-tricalcium phosphate bone substitutes. Materials (Basel). 11(10):1993. https://doi.org/10.3390/ma11101993
Williams DF (2008) On the mechanisms of biocompatibility. Biomaterials. 29(20):2941–2953. https://doi.org/10.1016/J.BIOMATERIALS.2008.04.023
Bi L, Rahaman MN, Day DE et al (2013) Effect of bioactive borate glass microstructure on bone regeneration, angiogenesis, and hydroxyapatite conversion in a rat calvarial defect model. Acta Biomater. 9(8):8015–8026. https://doi.org/10.1016/j.actbio.2013.04.043
El-Rashidy AA, Roether JA, Harhaus L, Kneser U, Boccaccini AR (2017) Regenerating bone with bioactive glass scaffolds: a review of in vivo studies in bone defect models. Acta Biomater. 62:1–28. https://doi.org/10.1016/j.actbio.2017.08.030
Fu Q (2019) Bioactive glass scaffolds for bone tissue engineering. Biomed Ther Clin Appl Bioact Glas:417–442. https://doi.org/10.1016/B978-0-08-102196-5.00015-X
Moses JC, Nandi SK, Mandal BB (2018) Multifunctional cell instructive silk-bioactive glass composite reinforced scaffolds toward osteoinductive, proangiogenic, and resorbable bone grafts. Adv Healthc Mater. 7(10):1701418. https://doi.org/10.1002/adhm.201701418
Baino F (2018) Bioactive glasses—when glass science and technology meet regenerative medicine. Ceram Int. 44(13):14953–14966. https://doi.org/10.1016/j.ceramint.2018.05.180
Srinivasan S, Jayasree R, Chennazhi KP, Nair SV, Jayakumar R (2012) Biocompatible alginate/nano bioactive glass ceramic composite scaffolds for periodontal tissue regeneration. Carbohydr Polym. 87(1):274–283. https://doi.org/10.1016/j.carbpol.2011.07.058
Gkioni K, Leeuwenburgh SCG, Douglas TEL, Mikos AG, Jansen JA (2010) Mineralization of hydrogels for bone regeneration. Tissue Eng Part B Rev. 16(6):577–585. https://doi.org/10.1089/ten.TEB.2010.0462
Jones JR (2013) Review of bioactive glass: from Hench to hybrids. Acta Biomater. 9(1):4457–4486. https://doi.org/10.1016/j.actbio.2012.08.023
Zhang G, Brion A, Willemin A-S et al (2017) Nacre, a natural, multi-use, and timely biomaterial for bone graft substitution. J Biomed Mater Res Part A. 105(2):662–671. https://doi.org/10.1002/jbm.a.35939
Akilal N, Lemaire F, Bercu NB et al (2019) Cowries derived aragonite as raw biomaterials for bone regenerative medicine. Mater Sci Eng C. 94:894–900. https://doi.org/10.1016/j.msec.2018.10.039
Anderson JM, Rodriguez A, Chang DT (2008) Foreign body reaction to biomaterials. Semin Immunol. 20(2):86–100. https://doi.org/10.1016/j.smim.2007.11.004
Anderson JM (2001) Biological responses to materials. Annu Rev Mater Res. 31:81–110. http://www.bioen.utah.edu/faculty/pat/Courses/biomaterials/BiologicalResponse.pdf. Accessed 7 Mar 2017
Navarro M, Michiardi A, Castano OPA (2008) Biomaterials in orthopaedics. J R Soc Interface. 5:1137–1158
Ambrosio L, De Santis R, Nicolais L (1998) Composite hydrogels as intervertebral disc prostheses. In: Science and technology of polymers and advanced materials. Springer US, Boston, MA, pp 547–555. https://doi.org/10.1007/978-1-4899-0112-5_46
Hasan A, Byambaa B, Morshed M et al (2018) Advances in osteobiologic materials for bone substitutes. J Tissue Eng Regen Med. 12(6):1448–1468. https://doi.org/10.1002/term.2677
Campana V, Milano G, Pagano E et al (2014) Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med. 25(10):2445–2461. https://doi.org/10.1007/s10856-014-5240-2
Guarino V, Caputo T, Altobelli R, Ambrosio L (2015) Degradation properties and metabolic activity of alginate and chitosan polyelectrolytes for drug delivery and tissue engineering applications. AIMS Mater Sci. 2(4):497–502. https://doi.org/10.3934/matersci.2015.4.497
Guarino V, Causa F, Ambrosio L (2007) Bioactive scaffolds for bone and ligament tissue. Expert Rev Med Devices. 4(3):405–418. https://doi.org/10.1586/17434440.4.3.405
Chiari C, Koller U, Dorotka R et al (2006) A tissue engineering approach to meniscus regeneration in a sheep model. Osteoarthr Cartil. 14(10):1056–1065. https://doi.org/10.1016/J.JOCA.2006.04.007
Marijnissen WJC, van Osch GJV, Aigner J et al (2002) Alginate as a chondrocyte-delivery substance in combination with a non-woven scaffold for cartilage tissue engineering. Biomaterials. 23(6):1511–1517. https://doi.org/10.1016/S0142-9612(01)00281-2
Cancedda R, Dozin B, Giannoni P, Quarto R (2003) Tissue engineering and cell therapy of cartilage and bone. Matrix Biol. 22(1):81–91. https://doi.org/10.1016/S0945-053X(03)00012-X
Revell PA, Damien E, Di Silvio L, Gurav N, Longinotti C, Ambrosio L (2007) Tissue engineered intervertebral disc repair in the pig using injectable polymers. J Mater Sci Mater Med. 18(2):303–308. https://doi.org/10.1007/s10856-006-0693-6
Acevedo CA, Olguín Y, Briceño M et al (2019) Design of a biodegradable UV-irradiated gelatin-chitosan/nanocomposed membrane with osteogenic ability for application in bone regeneration. Mater Sci Eng C. 99:875–886. https://doi.org/10.1016/J.MSEC.2019.01.135
Ou Q, Miao Y, Yang F, Lin X, Zhang L-M, Wang Y (2019) Zein/gelatin/nanohydroxyapatite nanofibrous scaffolds are biocompatible and promote osteogenic differentiation of human periodontal ligament stem cells. Biomater Sci 7(5):1973–1983. https://doi.org/10.1039/c8bm01653d
Cao M, Zhou Y, Mao J et al (2019) Promoting osteogenic differentiation of BMSCs via mineralization of polylactide/gelatin composite fibers in cell culture medium. Mater Sci Eng C. 100:862–873. https://doi.org/10.1016/j.msec.2019.02.079
Anada T, Pan C-C, Stahl A et al (1096) Vascularized bone-mimetic hydrogel constructs by 3D bioprinting to promote osteogenesis and angiogenesis. Int J Mol Sci. 20(5):2019. https://doi.org/10.3390/ijms20051096
Ingavle GC, Gionet-Gonzales M, Vorwald CE et al (2019) Injectable mineralized microsphere-loaded composite hydrogels for bone repair in a sheep bone defect model. Biomaterials. 197:119–128. https://doi.org/10.1016/j.biomaterials.2019.01.005
Murahashi Y, Yano F, Nakamoto H et al (2019) Multi-layered PLLA-nanosheets loaded with FGF-2 induce robust bone regeneration with controlled release in critical-sized mouse femoral defects. Acta Biomater. 85:172–179. https://doi.org/10.1016/j.actbio.2018.12.031
Lee S-H, Shin H (2007) Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev. 59(4-5):339–359. https://doi.org/10.1016/J.ADDR.2007.03.016
Vinogradov SV, Bronich TK, Kabanov AV (2002) Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv Drug Deliv Rev 54(1):135–147. https://doi.org/10.1016/S0169-409X(01)00245-9
Bai X, Gao M, Syed S, Zhuang J, Xu X, Zhang X-Q (2018) Bioactive hydrogels for bone regeneration. Bioact Mater. 3(4):401–417. https://doi.org/10.1016/j.bioactmat.2018.05.006
Heo YJ, Shibata H, Okitsu T, Kawanishi T, Takeuchi S (2011) Long-term in vivo glucose monitoring using fluorescent hydrogel fibers. Proc Natl Acad Sci. 108(33):13399–13403. https://doi.org/10.1073/pnas.1104954108
Onat B, Tuncer S, Ulusan S, Banerjee S, Erel GI (2019) Biodegradable polymer promotes osteogenic differentiation in immortalized and primary osteoblast-like cells. Biomed Mater. 14(4):045003. https://doi.org/10.1088/1748-605X/ab0e92
Barbieri D, Yuan H, Luo X, Farè S, Grijpma DW, de Bruijn JD (2013) Influence of polymer molecular weight in osteoinductive composites for bone tissue regeneration. Acta Biomater. 9(12):9401–9413. https://doi.org/10.1016/j.actbio.2013.07.026
Ricciardi BF, Bostrom MP (2013) Bone graft substitutes: claims and credibility. Semin Arthroplasty 24(2):119–123. https://doi.org/10.1053/j.sart.2013.07.002
Alidadi S, Oryan A, Bigham-Sadegh A, Moshiri A (2017) Comparative study on the healing potential of chitosan, polymethylmethacrylate, and demineralized bone matrix in radial bone defects of rat. Carbohydr Polym. 166:236–248. https://doi.org/10.1016/j.carbpol.2017.02.087
Lai Y, Li Y, Cao H et al (2019) Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials. 197:207–219. https://doi.org/10.1016/j.biomaterials.2019.01.013
Veronesi F, Giavaresi G, Guarino V et al (2015) Bioactivity and bone healing properties of biomimetic porous composite scaffold: in vitro and in vivo studies. J Biomed Mater Res Part A. 103(9):2932–2941. https://doi.org/10.1002/jbm.a.35433
Martínez-Sanmiguel JJ, Zarate-Triviño G, Hernandez-Delgadillo R et al (2019) Anti-inflammatory and antimicrobial activity of bioactive hydroxyapatite/silver nanocomposites. J Biomater Appl 33(10):1314–1326. https://doi.org/10.1177/0885328219835995
Chen Y, Zheng Z, Zhou R et al (2019) Developing a strontium-releasing graphene oxide/collagen-based organic-inorganic nanobiocomposite for large bone defect regeneration via MAPK signaling pathway. ACS Appl Mater Interfaces 11(17):15986–15997. https://doi.org/10.1021/acsami.8b22606
Arnold AM, Holt BD, Daneshmandi L, Laurencin CT, Sydlik SA (2019) Phosphate graphene as an intrinsically osteoinductive scaffold for stem cell-driven bone regeneration. Proc Natl Acad Sci U S A 116(11):4855–4860. https://doi.org/10.1073/pnas.1815434116
Narimani M, Teimouri A, Shahbazarab Z (2019) Synthesis, characterization and biocompatible properties of novel silk fibroin/graphene oxide nanocomposite scaffolds for bone tissue engineering application. Polym Bull. 76(2):725–745. https://doi.org/10.1007/s00289-018-2390-2
Wang W, Junior JRP, Nalesso PRL et al (2019) Engineered 3D printed poly(ɛ-caprolactone)/graphene scaffolds for bone tissue engineering. Mater Sci Eng C. 100:759–770. https://doi.org/10.1016/J.MSEC.2019.03.047
Purohit SD, Bhaskar R, Singh H, Yadav I, Gupta MK, Mishra NC (2019) Development of a nanocomposite scaffold of gelatin–alginate–graphene oxide for bone tissue engineering. Int J Biol Macromol. 133:592–602. https://doi.org/10.1016/J.IJBIOMAC.2019.04.113
Zhang Y, Wang J, Wang J et al (2015) Preparation of porous PLA/DBM composite biomaterials and experimental research of repair rabbit radius segmental bone defect. Cell Tissue Bank. 16(4):615–622. https://doi.org/10.1007/s10561-015-9510-0
Carrow JK, Di Luca A, Dolatshahi-Pirouz A, Moroni L, Gaharwar AK (2019) 3D-printed bioactive scaffolds from nanosilicates and PEOT/PBT for bone tissue engineering. Regen Biomater. 6(1):29–37. https://doi.org/10.1093/rb/rby024
Li X, Wang L, Fan Y, Feng Q, Cui F-Z, Watari F (2013) Nanostructured scaffolds for bone tissue engineering. J Biomed Mater Res Part A. 101A(8):2424–2435. https://doi.org/10.1002/jbm.a.34539
Bettinger CJ, Langer R, Borenstein JT (2009) Engineering substrate topography at the micro- and nanoscale to control cell function. Angew Chemie Int Ed. 48(30):5406–5415. https://doi.org/10.1002/anie.200805179
Peng R, Yao X, Ding J (2011) Effect of cell anisotropy on differentiation of stem cells on micropatterned surfaces through the controlled single cell adhesion. Biomaterials. 32(32):8048–8057. https://doi.org/10.1016/j.biomaterials.2011.07.035
McMahon RE, Wang L, Skoracki R, Mathur AB (2013) Development of nanomaterials for bone repair and regeneration. J Biomed Mater Res Part B Appl Biomater. 101B(2):387–397. https://doi.org/10.1002/jbm.b.32823
Pasqui D, Torricelli P, De Cagna M, Fini M, Barbucci R (2014) Carboxymethyl cellulose-hydroxyapatite hybrid hydrogel as a composite material for bone tissue engineering applications. J Biomed Mater Res Part A. 102(5):1568–1579. https://doi.org/10.1002/jbm.a.34810
Gelinsky M, Welzel PB, Simon P, Bernhardt A, König U (2008) Porous three-dimensional scaffolds made of mineralised collagen: Preparation and properties of a biomimetic nanocomposite material for tissue engineering of bone. Chem Eng J. 137(1):84–96. https://doi.org/10.1016/J.CEJ.2007.09.029
Kilian KA, Bugarija B, Lahn BT, Mrksich M (2010) Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci. 107(11):4872–4877. https://doi.org/10.1073/pnas.0903269107
Watari S, Hayashi K, Wood JA et al (2012) Modulation of osteogenic differentiation in hMSCs cells by submicron topographically-patterned ridges and grooves. Biomaterials. 33(1):128–136. https://doi.org/10.1016/j.biomaterials.2011.09.058
Cai K, Frant M, Bossert J, Hildebrand G, Liefeith K, Jandt KD (2006) Surface functionalized titanium thin films: zeta-potential, protein adsorption and cell proliferation. Colloids Surfaces B Biointerfaces. 50:1–8. https://doi.org/10.1016/j.colsurfb.2006.03.016
Palmer LC, Newcomb CJ, Kaltz SR, Spoerke ED, Stupp SI (2008) Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem Rev. 108(11):4754–4783. https://doi.org/10.1021/cr8004422
Dalby MJ, Gadegaard N, Tare R et al (2007) The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater 6(12):997–1003. https://doi.org/10.1038/nmat2013
Gong T, Xie J, Liao J, Zhang T, Lin S, Lin Y (2015) Nanomaterials and bone regeneration. Bone Res. 3:15029. https://doi.org/10.1038/boneres.2015.29
Vallet-Regí M, Salinas AJ (2009) Ceramics as bone repair materials. 2nd. Elsevier, Amsterdam. https://doi.org/10.1533/9781845696610.2.194
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Agrawal, S., Srivastava, R. (2020). Osteoinductive and Osteoconductive Biomaterials. In: Li, B., Moriarty, T., Webster, T., Xing, M. (eds) Racing for the Surface. Springer, Cham. https://doi.org/10.1007/978-3-030-34471-9_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-34471-9_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-34470-2
Online ISBN: 978-3-030-34471-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)