Abstract
Bone substitutes are used in wide range of orthopaedic application. An ideal bone substitute should exhibit superior osteoinductive and osteoconductive properties. Neither bio-derived materials nor synthetic materials can meet the needs of an ideal bone substitute. Preparation of composite materials is a promising way to improve properties of biomaterial. In this study, the porous poly lactic acid (PLA)/demineralized bone matrix (DBM) composite biomaterials prepared by supercritical CO2 technique were implanted to repair rabbit radius segmental bone defect. By comparing with PLA and bone autograft, the X-ray result and histological analysis showed the repair effect of PLA/DBM porous composite materials is significantly better than that of the PLA group and the blank control group, and is similar to autologous bone. The PLA/DBM can promote the healing of bone defects and can be used as a kind of ideal alternative materials to repair bone defects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bone defects may result secondary to the treatment of a variety of pathologies, including traumatic injury and benigh and malignant bone tumors. Bone defect repair is an important topic for the orthopedic surgeon (Sidell et al. 2012). An ideal bone substitute material is crucial to the success of bone defect repair. Bone autografts have the distinct advantage of histocompatibility without the risk of disease transfer and are considered the optimal material for bone repair. However, their limited availability necessitates the development of alternative bone substitutes (Kim et al. 2006). Bone substitute materials applied widely in clinic include bio-derived materials (such as bone allograft, bone xenograft) and synthetic materials (such as HA, PLA, PGA, Calcium Sulfate). However, application of neither meets the needs of an ideal bone substitute due to the instinct disadvantages. It is very valuable to prepare a kind of composite materials with good osteoinduction, osteoconduction and biocompatibility.
Bone allograft has been widespread applied in bone regeneration with good osteoinduction and osteoconduction. It is thought to be the best ideal substitute of bone autograft owing to its chemical components and physical structure similarity to the bone autograft. Approximately one-third of the bone grafts used in North America are allografts (Campana et al. 2014). However, the clinical application of bone allografts, especially cortical bone allografts, have been limited because of their difficulty of shaping, slow degradation rate and poor porosity. In order to improving use of cortical bone allograft, demineralized bone matrix (DBM) is produced by acid extraction of bone allograft. It contains type-1 collagen, non-collagenous proteins and osteoinductive growth factors. There are numerous DBM formulations in market based on the manufacturing process, including powder, granules, gel, putty and paste. However, all of the formulations have poor mechanical properties and porosity (Bakhshalian et al. 2014).
Poly lactic acid (PLA) is a synthetic material possessing controllable biodegradation rate, good biocompatibility and mechanical properties (Lee et al. 2001). It can be degraded to lactic acid in vivo as a natural intermediate in carbohydrate metabolism. PLA has practical medical applications as dissolvable sutures, as matrices for drug delivery, and bone fracture internal fixation devices in surgery. However, the poor osteoinduction limits its application in bone repair.
The use of biodegradable polymer/DBM composites could be an effective solution to these problems (Kim et al. 2006). Composited biomaterials would be promising biomaterials for bone repair and have been extensively investigated (Venkatesan et al. 2014; Bormann et al. 2010). The addition of PLA to DBM would allow for better manipulation and control over both the macrostructure and microstructure in shaping composites to fit bone defects. Meanwhile the use of DBM would improve osteoinduction and osteoconduction of composite materials.
Most of the previous methods for fabricating composite biomaterials, such as the particulate leaching, solvent casting and phase separation, need introduce organic solvents or high temperature in the process which destroy the biocompatibility of materials and some growth factors. Supercritical carbon dioxide (SC-CO2) is a novel method for polymer preparation without introduction of high temperature and organic solvent in the process of preparation and particularly suitable for the preparation of bioactive materials containing growth factors.
In the previous experiments, the PLA/DBM porous composite biomaterial has been prepared by SC-CO2 and confirmed to be good physical and chemical properties, biocompatibility and osteoinduction. In this study, the bone repair ability of PLA/DBM in vivo was be evaluated further.
Materials and methods
Preparation of PLA/DBM and PLA
The bone allografts procured from the qualified donor were processed into bone powder with the size of 50–200 µm according to the processing standards of bone allograft of Shanxi Provincial Tissue Bank. Then the powder was processed into DBM by defatting and demineralization as described by Li et al. (2006). Porous PLA/DBM composite biomaterials were fabricated by the previously described method (Zhang et al. 2007). PLA/DBM composites were prepared with 7:3 PLA particles (diameter = 100–200 µm, molecular weight = 100,000 Da) and DBM (diameter = approximately 100–200 µm) in volume. The sodium chloride particles (sigma, USA) were sieved to yield a range of sizes from 100 to 200 µm. The PLA/DBM and NaCl volume was 4:1. The mixture of PLA, DBM and NaCl was loaded into a cylinder mold (diameter = 1 cm) of the SC-CO2 equipment. Tightly closing the reactor kettle and CO2 under 0 °C was pumped into kettle untill the pressure reached 20 MPa. The mixture was kept in (20 ± 0.1) MPa and the temperature in (36 ± 0.5) °C for 30 min to saturate the polymer with the gas. Decreasing the gas pressure to ambient pressure at the rate of 5 MPa/min. After opening the reacting kettle and taking out the samples, the samples were leached in distilled water to remove the NaCl particles. PLA porous biomaterials without DBM were also fabricated by the same method and used as a control. All of PLA/DBM (Fig. 1) and PLA samples were packaged and sterilized by radiation.
Experimental animals
Thirty male New Zealand White rabbits (NWRs) with an average weight of 2.5 kg were maintained in a temperature-regulated environment (24 °C) on a 12-h light/dark cycle. The animals were housed individually in rabbit cages under the same feeding and management conditions. The animals were acclimatized for 1 week prior to the experiments.
Animals were divided into two groups randomly (Table 1). In one group, no material was implanted in defect site on the left ridius as the blank control group (Blank group) and the bone autograft cut was re-implanted on the right ridius in situ invertedly (Bone allograft group). In another group, PLA/DBM composite material was filled on the left (PLA/DBM group) and PLA material was done on the right (PLA group).
Animal experiments were approved by the Institutional Animal Care and Use Committee at China Institute for Radiation Protection.
Surgical technique
Prior to operation, Anesthesia was achieved with intraperitoneal injection of 3 % sodium pentobarbital anesthesia (1.2 ml/kg). Under the aseptic operating conditions, the diaphysis of both radius were exposed through a longitudinal extensile incision (Fig. 2a), the periosteum was elevated circumferentially, and the 12 mm long middle shaft of the radius was cut with a power-driven oscillating saw (Fig. 2b). After the resulting defect was then implanted with biomaterials according to the groups (Fig. 2c), the wound was sutured. Five animals each group were sacrificed at 4, 8 and 12 weeks following surgery respectively. The defect sites were removed for histological analysis.
Gross observation
Daily wound site care was applied in all subjects, and all subjects were daily assessed with respect to wound site infection, wound swelling and wound site dehiscence. After the implanted site being exposed and removed of soft tissue, the texture of the defect and healing were observed.
Radiographic observation and evaluation
Following the performance of surgical procedures on the subjects, Radiographs were conducted when the animals were sacrificed at postoperative weeks 4, weeks 8 and weeks 12 respectively. The situation of bone reconstruction were observed and numerically scored based on the Criteria of Lane’s X-ray Scores (Table 2).
Histological observation
The implanted samples from rabbits were harvested and fixed in formalin solution. The dehydrated samples were embedded with plastic and sectioned longitudinally by 5 mm thickness, stained with hematoxylin and eosin. The new bone formation and the degradation of the material were observed under light microscope.
Statistical analysis
Data analyzed by SPSS 10.0 statistical package. The normal distribution of the data was evaluated with the factorial design analysis of variance. Evaluation among groups adopted one-way ANOVA. If the variance is homogeneity, Least Significance Difference (LSD) will be used in multiple comparison. If variance is heterogeneity, Tamhane’s T2 method will be adopted. Significance levels were set at P < 0.05 for all statistical measures reported. All data was reported as mean ± standard error of the mean.
Result
Gross observation
The subjects were observed on a daily basis following the surgical procedure in term of wound site infection and wound site dehiscence. Varying degrees of swelling observed in forelegs of all animal postoperatively and the symptoms disappeared in a week. Bone dehiscence was observed in one of subjects in the blank control group and two in the PLA group. No serious complication was found in the bone allograft group and PLA/DBM group. The postoperative bone defect at each time point were filled by fiber scar tissue without new bone tissue, and the broken bone ends sclerosis was demonstrated by gross in the blank control group. In the PLA group, the boundaries between the host bone and the implanted materials were relatively clear. Some fiber connective tissue grew into the implanted bone and increased over time. In the PLA/DBM group, the boundaries between the host bone and the implanted bone were not easy to discern. The implanted material and the host bone combined closely. Bone callus crept into the implanted materials from the host bone. In the bone autograft group, the implanted bone and the host bone were bridged with bone callus and the boundaries were blurred.
Radiographic observation
At postoperative week 4, radiography of the blank control group showed sharp shadow at both ends of the defects, no bone callus formation and bone end sclerosis. In the PLA/DBM group, some bone callus occurred around the both ends, high density shadow appeared in the defect, and the bone defect areas were difficult to discern. In the PLA group, a little low density of callus formed at the both ends of the defects and mist shadow lower than the host bone was observed. The implanted bone in the autologous bone group was still clearly visible with a small amount of callus formation.
At weeks 8 postsurgery, the radiographs showed some low density callus formed in both ends of the defects, the defect area reduced and can be still discerned. In PLA/DBM group, the both ends connected and the defect area was filled with high density shadow. In the PLA group, the both ends near to ulna were bridged with high density bone callus. The bone defect far to ulna was still visible. In the bone autograft, the implanted bone connected with the ends of host bone. There was high density shadow at the boundary and bone callus on the shaft.
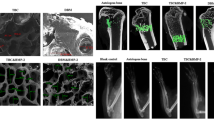
After 12 weeks, in the blank control group, some discontinuous callus formed at the both ends of host bone, the defect area reduced, and sclerosis appeared at the both ends of marrow cavity (Fig. 3a). In the PLA/DBM group, high-density uniform shadow connected the both ends of host bone, the cortical bone kept continuation and cavitas medullaris recanalized (Fig. 3b). In the PLA group, the both ends were connected with uneven shadow, little callus formed circumferentially and the cavitas medullaris did not recanalize (Fig. 3c). In the autograft group the both ends were bridged with uneven high-density callus and the avitas medullaris recanalized (Fig. 3d).
Radiographic images of repairing bone defect at week 12 after operation. a In the blank control group, some callus formed at the both ends of host bone and the defect became shorter; b in the PLA/DBM group, the both ends of host bone connected with uniform high-density shadow, the cortical bone kept continuation and medullary cavity recanalized; c in the PLA group, the both ends were connected with uneven shadow, little callus formed circumferentially and the medullary cavity did not recanalize; d in the autograft group, the both ends were bridged with uneven high-density callus and the medullary cavity recanalized
X-ray scores
The mean ± SD values of X-ray scoring obtained by radiography are presented (Table 3). All the groups showed increase in the score from weeks 4 to 12. It revealed progression of bone regeneration and early evidence of remodeling. No significant differences were observed between the PLA/DBM group and the bone autograft group at 4, 8 and 12 weeks (P = 0.834, 0.436, 0.675). The bone repair of the PLA/DBM and the bone autograft group were better than that of the PLA group and the blank control group (P < 0.05). The PLA groups showed better bone repair than the blank control group only at 8 weeks (P = 0.007).
Histological analysis
At 4 weeks postimplantation, the defect was filled with fibrous tissue and no new bone formed in the blank control group. In the PLA group there was not any new bone formation but a little fibrous connective tissue ingrowth with some inflammative cells. In the PLA/DBM group some fibrous tissue with a few inflammative cells grew in the interior of materials and a little new bone formed at the interface. Some new bone formed in the gap between host bone and the implanted bone and little inflammation can be seen in the autograft group.
At 8 weeks, the defect was filled with a great deal of vascularized fibrous tissue in the blank control group. The PLA material was enveloped with fibrous tissue and little new bone formed in the PLA group. There has been bone trabecula formed between the PLA/DBM and the host bone, osteogenic cell grew briskly into the implanted material in the PLA/DBM group. In the autograft group a great deal of waven bone formed.
At 12 weeks after implantation, the defect was filled with fiber connective tissue and little new bone formed at the ends of host bone in the blank control group (Fig. 4a). In the PLA group, the fiber connective tissue inserted between PLA and host bone. The central PLA was not still absorbed (Fig. 4b). In the PLA/DBM group the residue DBM can be observed and the mesenchymal tissue was turning into osteoid (Fig. 4c). In the autograft group the host bone and the implanted bone has been bridged with new bone and the continuous cancellous bone were observed in the implanted bone (Fig. 4d).
Histological images at 12 weeks after implantation. a In the blank control group, the defect was filled with fiber connective tissue and little new bone formed at the ends of host bone; b in the PLA group, the fiber connective tissue inserted between PLA and host bone, the central PLA was not still absorbed; c in the PLA/DBM group, the residue BMG can be seen and the mesenchymal tissue was turning into osteoid; d in the autograft group, the continuous cancellous bone can be seen in the implanted bone
Discussion
A novel method to fabricate biodegradable composite scaffolds for bone repair was developed in this study. Highly porous PLA/DBM composite scaffolds were fabricated by the SC-CO2. The PLA/DBM have been confirmed be biocompatible and osteoinductive in the previous studies (Zhang et al. 2007). However, the final goal of research and development of bone substitute material is to repair bone defect. It is more valuable to evaluate capability of bone repair in vivo.
Owing to gentle, easy operation and low cost, the experimental model of segmental bone defect of rabbit radius is one of the most common animal models to evaluate bone substitute materials to repair bone defect (Kang et al. 2014; Rathbone et al. 2014; Roohani-Esfahani et al. 2012). The rabbit radius defect model can avoid experimental interference factors without load-bearing and additional fixation after the operation. Critical sized defects (CSDs) (Pereira-Júnior et al. 2007) have been widely applied to evaluate oseteinduction and osteoconduction of bone substitutes. It is actually an important index to evaluate the bone defect model (Sanders et al. 2014). Scarless tissue repair is a remarkable characteristic for bone repair. However, when the size of bone defect is too large, more than bone repair ability, the self-healing process of bone will be broken and bone healing will be caused. Defect threshold refers to the critical value of a long bone defects. Bone defect more than the critical value is considered to be not heal without intervene. It is generally believed defect reaching 1.5–2 times of the diameter of the long bone can be considered CSDs. In this experiment, the diameter of rabbit radius are around 4–5 mm. The segmental bone defect of 12 mm created, more than two times of the radial diameter, is unable to heal itself. Results in the blank control group at 12 weeks also confirmed that 12 mm rabbit radius segmental bone defect model can objectively evaluate the ability of a material to repair bone defect.
X-ray detection can assess on the effect of materials to repair bone defect accurately and is a kind of simple and feasible method of experimental observation. In order to more accurately evaluate the effect of implant materials for bone defect repair, scholars such as the Lane (Lane and Sandhu 1987) make a subjective and comprehensive X-ray criteria reflect the dynamic process of reconstruction of bone defects. The criteria get a general X-ray score according to bone formation, bone connection and bone model in bone defect respectively. X-ray and histological observation showed the repair effect of PLA/DBM porous composite materials was significantly better than that of the PLA group and the blank control group, and was similar to autologous bone. It confirmed that addition of DBM significantly improved osteoconduction and osteoinduction. The PLA/DBM can promote the healing of bone defects and can be used as a kind of ideal alternative materials to repair bone defect.
DBM is an osteoconductive and osteoinductive allograft product that was found to be safe as an option of bone grafting. It has been used to induce bone formation in various procedures. However, the poor mechanical properties and porosity limited its widely application. Synthetic materials such as PLA and beta tricalcium phosphate (β-TCP) have different properties from DBM: the former is osteoconductive, whereas the latter is osteoinductive (Giannoudis et al. 2005; Zimmermann and Moghaddam 2011). A material fully equipped with all of these properties could provide better outcomes. Therefore, composite materials with different properties might enhance bone healing. Some researchers mixed DBM and other biomaterials to improved properties of composite materials. Jemin et al. (2015) proved a HA/DBM mixture inside a PEEK cage can provide noninferior outcomes compared to a HA/TCP mixture in ACDF. Shalash et al. (2013) evaluated the effectiveness of β-TCP alone compared to β-TCP and DBM in regenerating localized horizontal maxillary alveolar ridge deficiencies. The results showed the combination of DBM and β-TCP can be used effectively in cases exhibiting minimal alveolar ridge defects. Our result is also in agreement with evidence from the former studies that the composite material have the better ability to support new bone formation. The following maybe the reason: DBM works through both osteoinduction and osteoconduction. The composite biomaterials with DBM can enrich mesenchymal cells and differentiate mesenchymal cells to osteoblasts at the graft site. The presence of PLA which supports osteoblasts adhesion, combined with DBM at the defect site, can explain the better bone healing and more bone gain that was found in the PLA/DBM group.
References
Bakhshalian N, Nowzari H, Ahn KM, Arjmandi BH (2014) Demineralized dentin matrix and bone graft: a review of literature. J West Soc Periodontol Periodontal Abstr 62(2):35–38
Bormann N, Pruss A, Schmidmaier G, Wildemann B (2010) In vitro testing of the osteoinductive potential of different bony allograft preparations. Arch Orthop Trauma Surg 130:143–149
Campana V, Milano G, Pagano E, Barba M, Cicione C, Salonna G, Lattanzi W, Logroscino G (2014) Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med 25(10):2445–2461
Giannoudis PV, Dinopoulos H, Tsiridis E (2005) Bone substitutes: an update. Injury 36(Suppl 3):S20–S27
Jemin Y, Gun Woo L, Woo Dong N, Kye Young H, Myung-Ho K, Jong Won K, Jonghwa W, Seong Wan K, Won N, Jin SY (2015) A prospective randomized clinical trial comparing bone union rate following anterior cervical discectomy and fusion using a polyetheretherketone cage: hydroxyapatite/B-tricalcium phosphate mixture versus hydroxyapatite/demineralized bone matrix mixture. Asian Spine J 9(1):30–38
Kang SH, Chung YG, Oh IH, Kim YS, Min KO, Chung JY (2014) Bone regeneration potential of allogeneic or autogeneic mesenchymal stem cells loaded onto cancellous bone granules in a rabbit radial defect model. Cell Tissue Res 355(1):81–88
Kim SS, Sun Park M, Jeon O, Yong Choi C, Kim BS (2006) Poly (lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 27:1399–1409
Lane JM, Sandhu HS (1987) Current approaches to experimental bone grafting. Orthop Clin North Am 18(2):213–225
Lee SJ, Park YJ, Park SN, Lee YM, Seol YJ, Ku Y, Chung CP (2001) Molded porous poly(L-lactide) membranes for guided bone regeneration with enhanced effects by controlled growth factor release. J Biomed Mater Res 55:295–303
Li X, Jin L, Balian G, Laurencin CT, Anderson DG (2006) Demineralized bone matrixgelatin as scaffold for osteochondral tissue engineering. Biomaterials 27(11):2426–2433
Pereira-Júnior OC, Rahal SC, Iamaguti P, Felisbino SL, Pavan PT, Vulcano LC (2007) Comparison between polyurethanes containing castor oil (soft segment) and cancellous bone autograft in the treatment of segmental bone defect induced in rabbits. J Biomater Appl 21(3):283–297
Rathbone CR, Guda T, Singleton BM, Oh DS, Appleford MR, Ong JL, Wenke JC (2014) Effect of cell-seeded hydroxyapatite scaffolds on rabbit radius bone regeneration. J Biomed Mater Res A 102(5):1458–1466
Roohani-Esfahani SI, Dunstan CR, Davies B, Pearce S, Williams R, Zreiqat H (2012) Repairing a critical-sized bone defect with highly porous modified and unmodified baghdadite scaffolds. Acta Biomater 8(11):4162–4172
Sanders DW, Bhandari M, Guyatt G, Heels-Ansdell D, Schemitsch EH, Swiontkowski M, Tornetta P 3rd, Walter S, Investigators SPRINT (2014) Critical-sized defect in the tibia: is it critical? Results from the SPRINT trial. J Orthop Trauma 28(11):632–635
Shalash MA, Rahman HA, Azim AA, Neemat AH, Hawary HE, Nasry SA (2013) Evaluation of horizontal ridge augmentation using beta tricalcium phosphate and demineralized bone matrix: a comparative study. J Clin Exp Dent 5(5):e253–e259
Sidell DR, Aghaloo T, Tetradis S, Lee M, Bezouglaia O, DeConde A, St John MA (2012) Composite mandibulectomy a novel animal model. Otolaryngol Head Neck Surg 146:932–937
Venkatesan J, Bhatnagar I, Manivasagan P, Kang KH, Kim SK (2014) Alginate composites for bone tissue engineering: a review. Int J Biol Macromol 11(72C):269–281
Zhang YM, Li BX, Li J, Ma SY, Zhao YP, Wang JR, Yuan L (2007) Fabrication of porous poly lactic acid-bone matrix gelatin composite bioactive material and its osteoinductive activity. Chin J Repar Reconstr Surg 21(2):135–138
Zimmermann G, Moghaddam A (2011) Allograft bone matrix versus synthetic bone graft substitutes. Injury 42(Suppl 2):S16–S21
Acknowledgments
This work was funded by the “Natural Science Foundation for Young Scientists of Shanxi Province (2006021050)” and by the “Scientific Research Fund for the Doctoral Young Scholars, SXTCM”. The authors are grateful to Shanxi Provincial Tissue Bank and China Institute for Radiation Protection for the support provided for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Wang, J., Wang, J. et al. Preparation of porous PLA/DBM composite biomaterials and experimental research of repair rabbit radius segmental bone defect. Cell Tissue Bank 16, 615–622 (2015). https://doi.org/10.1007/s10561-015-9510-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-015-9510-0