Abstract
As one group of important bioactive compounds in Salvia miltiorrhiza, water-soluble phenolic acids own a variety of bioactivities including anti-oxidation, anti-inflammatory, and anti-cancer. Due to the degradation of genetic resources and low content of phenolic acids in traditionally cultured S. miltiorrhiza, limited phenolic acid production cannot meet the increasing market demand. It is extremely important to use modern biotechnology methods to increase the yield of phenolic acids. Here, we summarize pharmacological activities of phenolic acids in S. miltiorrhiza, as well as various biological methods including culturing hairy roots, callus, suspension cells, and endophytic fungi for producing phenolic acids and using elicitors treatment, metabolic engineering and transcriptional regulation for increasing the production of phenolic acids.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
11.1 Introduction
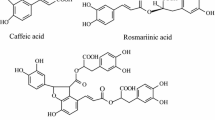
Phenolic acids possessing various pharmacological activities such as anti-cancer, anti-oxidant, anti-bacterial, and anti-inflammatory activities are widely distributed in nature, especially in some commonly used traditional Chinese medicines such as Salvia miltiorrhiza (Zhou et al. 2011, 2012). S. miltiorrhiza has the functions of relieving pain, promoting blood circulation, regulating menstruation, and nourishing the heart so that it is widely used in the treatment of cardiovascular diseases (Wang and Cao 2016). There are more than 20 phenolic acids in S. miltiorrhiza including rosmarinic acid (RA), salvianolic acid B (SAB), salvianolic acid A (SAA), danshensu (DSU), caffeic acid, cinnamic acid, ferulic acid, and lithospermic acid (Fig. 11.1) (Xing et al. 2018a, b). SAB and RA in crude S. miltiorrhiza account for the largest content (Sun et al. 2016).
In recent years, RA, an ester of caffeic acid and 3,4-dihydroxyphenyllactic acid, has been proved to be used for prophylaxis and treatment of neuropathic pain for its anti-apoptotic and anti-inflammatory effects (Pezeshki and Petersen 2018; Rahbardar et al. 2018). SAB and DSU isolated from S. miltiorrhiza are extremely effective anti-oxidants and have stronger oxygen free radical scavenging activity than vitamin C. Moreover, SAB shows better anti-oxidant activity than DSU (Zhao et al. 2008). It has been reported that SAB has a protective effect on emphysema-like lung cell death and protect against ischemia/reperfusion-induced cerebral injury (Dhapare and Sakagami 2018; Fan et al. 2018). SAB can effectively inhibit the growth of cultured MDA-MB-231 cells and tumor xenografts via a ceramide-mediated pathway. SAB also enhances apoptosis by regulating ceramide glycosylase and reduces TNBC cell proliferation (Sha et al. 2018). SAA possesses extensive pharmacological activities like treating liver disease, which prevents chronic ethanol-induced liver damage via SIRT1-mediated autophagosome–lysosome fusion recovery (Shi et al. 2018).
With the rapid development of biotechnology, the biosynthetic pathway of phenolic acids in S. miltiorrhiza has been gradually revealed (Petersen and Simmonds 2003; Di et al. 2013; Ma et al. 2015). Due to its wide medicinal value, the depletion of wild resources and the low yield of phenolic acid, genetic engineering and metabolic engineering have been used to enhance the production of phenolic acids in S. miltiorrhiza, which has become a research hotspot. Here we summarize the research progress of the main substances, pharmacological activities, biosynthetic pathways, and in vitro synthesis of phenolic acids in S. miltiorrhiza, in order to more efficiently produce pharmacological phenolic acids and discuss their prospects.
11.2 Medicinal Properties of Phenolic Acids
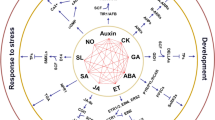
Phenolic acids are the major active components in S. miltiorrhiza, which exerts a variety of pharmacological activities including anti-oxidant, cardio-protective, neuro-protective, anti-platelet, anti-cancer, anti-inflammatory, reno-protective, anti-diabetic properties (summarized in Fig. 11.2). The pharmacological activities of SAA and SAB are listed in Table 11.1.
11.2.1 Anti-oxidant Activity
Phenolic acids have been found to possess potent anti-oxidative ability due to their polyphenolic structure. SAA could enhance the activities of superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase in an established 5/6 nephrectomized (5/6Nx) rat model, and decreased reactive oxygen species (ROS) in H2O2-induced HK-2 cells. The protective effects of SAA on oxidative stress were suggested to be related to the modulation of Akt/GSK-3β/Nrf2 and the NF-κB signaling pathway (Zhang et al. 2019a). In addition, SAA decreased malondialdehyde (MDA) but increased SOD level in angiotensin II-incubated macrophages (Li et al. 2016a). SAB protected against subarachnoid hemorrhage-induced oxidative damage in vivo through activating the SIRT1 and Nrf2 signaling pathway (Zhang et al. 2018a). The levels of antioxidase SOD and GPx were decreased in patients with cysteine stone, which were associated with oxidative stress. Inversely, SAB could prevent cysteine stone formation, protect against oxidative injury (Zhang et al. 2019b). Moreover, SAB and DSU showed potent scavenging activities against HO−, O2−, DPPH, and ABTS radicals than vitamin C (Zhao et al. 2008). SAB also protected the mice from radiation injury through nuclear factor (erythroid-derived 2)-like 2 protein/BTB-mediated anti-oxidant effect (Zhou et al. 2019).
11.2.2 Cardio-protective Activity
Myocardial reperfusion during infarction causes damage in cardiomyocytes. Previous reports indicated that total salvianolic acid injection (TSI) of S. miltiorrhiza improved ischemia/reperfusion (I/R)-induced myocardial injury, decreased apoptosis, and reduced infarct size in Sprague-Dawley rats (Huang et al. 2019). Recent studies demonstrated that SAA could attenuate apoptosis and prevent I/R injury in cardiomyocytes through the PI3K/Akt, GSK-3β, JNK, and ERK1/2 pathways, and probably via the JNK-ERK1/2 crosstalk (Qian et al. 2019). Mitochondrial dysfunction contributes to the heart diseases such as coronary heart disease and heart failure. However, pretreatment with SAA could maintain normal mitochondrial function and biogenesis, alleviate the damage, and protect against arsenic trioxide-induced cardiac injury in vivo (Zhang et al. 2018c). Furthermore, treatment with SAB showed protective effect against doxorubicin-induced cardiac injury, which is a common clinical syndrome that causes severe pain to cancer patients (Chen et al. 2017).
11.2.3 Neuro-protective Activity
Previously, the TSI has been approved by Chinese State Food and Drug Administration (SFDA) for the treatment of ischemic stroke (Han et al. 2017). Besides, SAA was confirmed to recover the neurological function, improve the motor ability, and inhibit apoptosis-related proteins in spinal cord injury rats (Yu et al. 2017). In another middle cerebral artery occlusion mice model, SAA administration ameliorated neuronal damage and decreased infarcted volume via the inhibition of eNOS uncoupling and peroxynitrite formation (Mahmood et al. 2017). Elevated ROS level involves in stroke and other neurodegenerative diseases. However, pretreatment with SAA increased cell survival against ROS-induced neuronal damage (Zhang et al. 2012). Treatment with SAB inhibited oxidative damage, prevented neurologic impairment, and improved cell viability in a rat subarachnoid hemorrhage model and cultured neurons, which was associated with the activation of Nrf2 and SIRT1 signaling pathway (Zhang et al. 2018e). Subchronic SAB administration (10 mg/kg) ameliorated the memory impairment by decreasing the expression of nitric oxide synthase and cyclooxygenase-2 in Aβ25-35 peptide-induced Alzheimer’s disease mouse (Lee et al. 2013). In addition, SAB also affected vasculature and cognitive function. Studies revealed that SAB could recover the angiogenesis and cognitive deficits in cerebral small vessel disease rat though modulation of STAT3/VEGF pathway (Wang and Hu 2018). Other studies have indicated that SAB has beneficial effects on stroke, brain injury, and Parkinson’s disease (Lv et al. 2015; Chen et al. 2011; Zhou et al. 2014).
11.2.4 Anti-platelet Activity
Salvianolate, the bioactive constituents of S. miltiorrhiza, has been approved by Chinese SFDA for the treatment of coronary artery disease since 2005. Clinical research found salvianolate enhanced the anti-platelet activity of standard aspirin plus clopidogrel therapy in acute coronary syndrome patients. Further in vitro studies demonstrated that SAB, the main component of salvianolate (>85%), suppressed thrombin, arachidonic acid, collagen, and U46619-induced platelet aggregation via inhibiting phosphodiesterase and antagonizing P2Y12 receptor (Liu et al. 2014). The beneficial effect of SAB on thrombosis was related to direct peroxide scavenge or indirect adhesion molecules suppression (Wang et al. 2009). SAA inhibited platelet aggregation and attenuates arterial thrombus formation by inhibiting the PI3K expression (Huang et al. 2010). Additionally, intravenous administration of SAA (2.5–10 mg/kg) showed anti-thrombotic activity in vivo. It modulated the hemorheology without influence on the coagulation function and was presumed to be related to the cAMP induction (Fan et al. 2010).
11.2.5 Anti-cancer Activity
SAA exhibited anti-cancer activities against various carcinomas such as acute myeloid leukemia, oral squamous cell, and lung cancer (Pei et al. 2018; Fang et al. 2018; Bi et al. 2013). Moreover, SAA inhibited GRP78 secretion and angiogenesis in tumor microenvironment (Yang et al. 2019). Chemotherapy resistance is a major challenge in cancer treatment. Studies revealed that SAA enhanced the efficacy of cisplatin in lung cancer A549 cells by inhibiting the AKT/mTOR signaling pathway (Tang et al. 2017). Besides, SAA treatment selectively attenuated the growth of multidrug-resistant MCF-7 breast cancer cells, which was correlated with ROS production (Wang et al. 2015b). Overexpression of transgelin 2 increased the resistance of cancer cells to paclitaxel therapy. However, SAA treatment could reverse resistance, induce apoptosis, and inhibit invasion in paclitaxel-resistant breast cancer cells (Cai et al. 2014; Zheng et al. 2015). It was indicated that SAB could reverse the multidrug resistance in colon cancer cells and promote apoptosis in triple-negative breast cancer (Sha et al. 2018; Guo et al. 2018). Autophagy functions as a death executioner that induces autophagic cell death. SAB was demonstrated to be a novel autophagy inducer that mediated colorectal cancer cells autophagy through modulation of the AKT/mTOR signaling pathway (Jing et al. 2016). Evidence also showed that autophagy as well as apoptosis was involved in SAB-induced hepatocellular carcinoma cell death. While pretreatment with autophagy inhibitors attenuates the effects induced by SAB (Gong et al. 2016).
11.2.6 Anti-inflammatory Activity
SAB decreased the levels of inflammatory cytokines (IL-1β, IL-6, and TNF-α) and increased anti-oxidant enzyme (SOD, CAT, and GSH) activities in collagen-induced rheumatoid arthritis rat (Xia et al. 2018). Treatment with SAB markedly ameliorated LPS-induced injury on MH7 A cells via the modulation of NF-κB and JNK pathways, suggesting the potential anti-inflammatory capacity (Meng et al. 2019). Also, SAB decreased the level of pro-inflammatory cytokines in liver injury (Zhao et al. 2019). SAA protected blood-brain barrier by reducing inflammation response and NF-κB inactivation (Zhang et al. 2018d). Liu and colleagues demonstrated that all the phenolic acids isolated from S. miltiorrhiza exhibited anti-inflammatory activity in LPS-stimulated THP-1 cells, among which the inhibitory effect of lithospermic acid was the strongest and similar to that of SAB (Liu et al. 2018).
11.2.7 Reno-protective Activity
Nephrotic syndrome is a common nephrology disorder accompanied by heavy proteinuria, hypoalbuminemia, and hyperlipidaemia. Recent studies demonstrated that SAA administration ameliorated histological damages, podocyte injury, and improved hemorheology in doxorubicin-induced nephropathy (Fan et al. 2015). Intraperitoneal with SAA at 10 mg/kg per day relieved urinary proteins and TNF-α level, alleviated pathological lesions in the kidney of 5/6Nx rats (Zhang et al. 2018e). SAA could also protect against early stage diabetic nephropathy, restored glomerular endothelial permeability through suppressing AGE-RAGE pathway (Hou et al. 2017). Furthermore, combination of SAA with prednisone relieved urinary proteins, improved renal function indices including blood urea nitrogen and serum creatinine level in rats (Wang et al. 2019). SAB showed reno-protective activity in a renal I/R rat model by attenuating inflammatory process and oxidative stress through activating the PI3K/Akt signaling pathway (Ma et al. 2017).
11.2.8 Anti-atherosclerosis Activity
SAB acted as a CD36 antagonist that inhibited lipid uptake in macrophages, leading to the inhibition of atherosclerotic lesions formation in ApoE knockout mice (Bao et al. 2012). SAA attenuated TNF-α-induced CC chemokine ligand-20 (CCL-20) secretion, which plays a crucial role in atherogenesis (Zhang et al. 2014c). In addition, DSU prevented atherosclerosis through inhibiting the expression of adhesion molecules in arterial endothelia (Yang et al. 2010).
11.2.9 Anti-diabetic Activity
Diabetic rats treated with SAB (40 mg/kg) for three weeks showed decreased serum glucose and MDA levels as well as increased serum insulin level. Meanwhile, SAB could protect the pancreatic islet cells against cytotoxicity partly through the inhibition of apoptosis and oxidative stress (Raoufi et al. 2015). In alloxan-induced type 1 and high-fat diet with low-dose streptozotocin-induced type 2 diabetic animal models, SAA administration improved mitochondrial function in liver and skeletal muscle, increased ATP production through activating AMPK/CaMKKβ signaling pathway (Qiang et al. 2015).
11.2.10 Other Activities
SAA was potential anti-allergic therapy that reduced the number of eosinophils and secretion of inflammatory IL-4 and IL-13 in the lung tissue of ovalbumin-induced allergic asthma mice (Heo and Im 2019). SAB showed anti-rheumatoid arthritis activity on collagen-induced rat model (Xia et al. 2018). In addition, SAB exhibited anti-infective property against human pathogen Neisseria meningitidis by inhibiting meningococcal binding (Huttunen et al. 2016). Protocatechualdehyde and SAB were major components in S. miltiorrhiza that possess anti-bacterial properties (Kong et al. 2017). Besides, the combination of salvianolic acid V, a new salvianolic acid, with Colistin sulfate or Levofloxacin showed effects on MRSA or Acinetobacter baumannii (Zhang et al. 2018b). Protocatechuic aldehyde markedly inhibited hepatitis B virus replication in vitro and reduced viremia in virus-infected ducks (Zhou et al. 2007).
11.3 Biosynthesis of Phenolic Acids in S. miltiorrhiza
11.3.1 Biosynthetic Pathway of Phenolic Acids
Phenolic acids are biosynthesized via two pathways including the phenylpropanoid pathway and the tyrosine-derived pathway in S. miltiorrhiza (Xiong et al. 2010; Ma et al. 2015). In the phenylpropanoid pathway, l-phenylalanine produces 4-coumaroyl-CoA under the catalysis of phenylalanine ammonia-lyase (PAL), cinnamic acid 4-hydroxylase (C4H), hydroxycinnamate coenzyme A ligase (4CL) (Di et al. 2013; Shi et al. 2019). In tyrosine-derived pathway, l-tyrosine produces 4-hydroxyphenylpyruvic acid under oxidative deamination of tyrosine aminotransferase (TAT), following 4-hydroxyphenylpyruvate reductase (HPPR) and forming 3,4-dihydroxyphenyllactic acid at last (Di et al. 2013; Shi et al. 2019).
Finally, 3,4-dihydroxyphenyllactic acid and 4-coumaroyl-CoA are catalyzed by the RAS to form the precursor substance 4-coumaroyl-3′,4′-dihydroxyphenyllactic acid (4C-DHPL), and finally, the CYP98A14 enzyme synthesizes salvianolic acid (Di et al. 2013). In S. miltiorrhiza, 4C-DHPL is regarded as the major intermediate for RA biosynthesis involved in this pathway (Fig. 11.3). Meanwhile, RA is also considered to be a precursor of biosynthesis of SAB which is a structural dimer of RA, but the enzyme involved in catalysis is still unclear (Zhang et al. 2014a, b; Ma et al. 2015).
11.3.2 Related Genes Involved in Phenolic Acid Biosynthesis
With the extensive study of secondary metabolic pathways in S. miltiorrhiza, several key enzyme genes involved in these two pathways of phenolic acids biosynthesis have been identified, including SmPAL, SmC4H, Sm4CL, SmTAT, SmHPPR, SmRAS, and CYP98A14 (Table 11.2; Fig. 11.4) (Ma et al. 2015; Shi et al. 2019).
11.3.2.1 Genes in the Phenylpropanoid Pathway
Phenylalanine ammonia-lyase (PAL), the first point enzyme to catalyze in the phenylpropanoid pathway, plays a significant role in primary metabolism and secondary metabolism regulation. Three members in SmPALs have been revealed from S. miltiorrhiza, including SmPAL1, SmPAL2, and SmPAL3. Gene expression pattern shows that SmPAL1 and SmPAL3 highly express in both roots and leaves of S. miltiorrhiza, while SmPAL2 is mainly expressed in stems and flowers. The full-length ORF of SmPAL1 is 2827 bp, encoding a 711-amino-acid peptide. Meanwhile, SmPAL1 has been revealed to be induced by abscisic acid (ABA), wounding, polyethylene glycol (PEG), methyl jasmonate (MJ), salicylic acid (SA), Ca2+, gibberellin (GA), and ethylene (Ma et al. 2015; Hou et al. 2013). SmPAL2 contains 2127 bp ORF encoding a 683-amino-acid peptide, and SmPAL3 has 2283 bp ORF encoding 760 amino acid, which are induced by PEG and MJ (Ma et al. 2015; Song and Wang 2009). Interestingly, all three SmPALs are regulated by MeJA, and the expression of SmPAL1 and SmPAL3 is drastically raised at 6 h after MeJA treatment. Meanwhile, RNAi of SmPAL1 has been found to cause a significant decrease in the content of RA and SAB in S. miltiorrhiza, indicating that SmPAL1 plays a more significant role in phenylpropanoid pathway (Wang et al. 2015a; Song and Wang 2011).
Cinnamate 4-hydroxylase (C4H) is a cytochrome P450 enzyme involved in the catalysis of the production of p-coumaric acid by trans-cinnamic acid produced by PAL (Huang et al. 2008a). The full length of SmC4H1 is 1512 bp encoding a 504-amino-acid protein. SmC4H1 is highly expressed in roots and stems; meanwhile, it is induced by elicitor such as MJ, ABA, Ag+, and UV-B radiation. SmC4H2 encoding a 397-amino-acid protein is absent ER-targeting peptide, probably causing by N-terminal deletion of gene (Shi et al. 2019; Wang et al. 2015a, b). The tissue expression of SmC4H2 is the same to SmC4H1, expressed highly in stem and root (Ma et al. 2015). However, SmC4H2 does not seem to be involved in MJ regulation because of their insensitive to MJ, but its promoter contains some elements that respond to other stresses including fungal attack and salicylic acid (Wang et al. 2015a, b).
4-Coumarate: CoA ligase (4CL) catalyzing 4-coumaroyl acid to form 4-coumaroyl-CoA in phenylpropanoid pathway has ten members such as Sm4CL1, Sm4CL2, Sm4CL3, Sm4CL-like 1, Sm4CL-like 2, Sm4CL-like 3, Sm4CL-like 4, Sm4CL-like 5, Sm4CL-like 6, and Sm4CL-like 7. Sm4CL1 has been found highly expressed in stem, low in leaves and rear in roots, while the expression levels of Sm4CL2, Sm4CL3, Sm4CL-like1, and Sm4CL-like4 are high in roots. Furthermore, the expression of Sm4CL1 is affected by elicitors like MJ and YE, and Sm4CL2 is induced by MJ, YE and Ag+, implying that Sm4CL1 may be involved in the biosynthesis of phenolic acids in the stems and leaves, while Sm4CL2 may participate in the biosynthesis of phenolic acids in roots of S. miltiorrhiza (Jin et al. 2012; Shi et al. 2019; Wang et al. 2015a; Zhao et al. 2006).
11.3.2.2 Genes in the Tyrosine-Derived Pathway
Tyrosine aminotransferase (TAT) and 4-hydroxyphenylpyruvate reductase (HPPR) are two main key enzymes which are involved in forming 3,4-dihydroxyphenyllactic acid (DHPL) in tyrosine-derived pathway. Tyrosine aminotransferase (TAT) is the first enzyme in the tyrosine-derived pathway of phenolic acids biosynthesis. SmTAT1 contains an ORF of 1233 bp encoding 411 amino acid (Huang et al. 2008b). The expression patterns show that SmTAT1 is expressed higher in stem compared to root and leaf. Meanwhile, SmTAT1 responds to MJ, ABA, SA, UV-B, GA, ethylene, Ag+, YE (Xing et al. 2015; Yan et al. 2006; Liang et al. 2013). SmTAT2 and SmTAT3 are found to express in flower, stem, and root, respectively, also have been cloned from S. miltiorrhiza. SmTAT1 and SmTAT3 are grouped in the same branch in the phylogenetic tree analysis, but SmTAT2 is divided into another branch, indicating that SmTAT1 and SmTAT3 may play a similar role in the phenolic acid synthesis pathway (Wang et al. 2015a).
4-Hydroxyphenylpyruvate reductase (HPPR) is the second enzyme in tyrosine-derived pathway, catalyzing 4-hydroxyphenylpyruvic acid to form 4-hydroxyphenyllactic acid. Three HPPRs are designed as SmHPPR1, SmHPPR2, and SmHPPR3. SmHPPR1 and SmHPPR2 encode 313 amino acid, and SmHPPR3 encodes a 319-amino-acid protein. Promoter region of SmHPPR1 expressed highest in stem which possesses many stress-responsive elements. At the same time, SmHPPR1 has been found that it can be induced by MJ, SA, GA3, ABA, Ag+, and UV-B radiation (Xing et al. 2015; Wang et al. 2015a; Xiao et al. 2011).
11.3.2.3 Genes Involved in Rosmarinic Acid (RA) Biosynthesis
Rosmarinic acid synthase (RAS) is regard as a rate-limiting enzyme catalyzing 4-hydroxyphenyllactic acid to form precursor 4-coumaroyl-3′,4′-dihydroxyphenyllactic acid (4C-DHPL). Seven SmRASs including SmRAS1, SmRAS1-like, and five SmHCTs have been revealed so far (Ma et al. 2015; Shi et al. 2019). SmRAS1 containing 1284 bp ORF and encoding a 426-amino-acid polypeptide has been proved expressed predominantly in roots and stems, and induced by MJ, Ag+ (Di et al. 2013; Ma et al. 2015). SmRAS-like is expressed higher in stem than other tissues and sensitive to Pseudomonas lachrymans, MJ, light, and SA (Song. 2010; Ma et al. 2015). SmHCTs include SmHCT1, SmHCT2, SmHCT3, SmHCT4, and SmHCT5, encoding 341, 425, 426, 439, 427 amino acid, respectively. SmHCT2, SmHCT3, SmHCT4, and SmHCT5 are highly expressed in stem, while SmHCT1 is highly expressed in root. Meanwhile, SmHCT3, SmHCT4, and SmHCT5 were responsive to MJ elicitation (Wang et al. 2015a; Ma et al. 2015).
Cytochrome P450-dependent monooxygenase (CYP98A14) in S. miltiorrhiza participates the synthesis of RA by catalyzing 4C-DHPL. SmCYP98A14 has 1525 bp ORF encoding a 508-amino-acid protein, and its expression is higher in roots than that in stems and leaves; moreover, SmCYP98A14 is sensitive to MJ and Ag+ (Di et al. 2013; Wang et al. 2015a; Ma et al. 2015).
11.4 Biotechnological Approaches to Improve the Production of Phenolic Acids
Due to the serious degradation of the quality of traditionally cultivated S. miltiorrhiza, slow growth cycle and low yield of active ingredients, the yield and quality of S. miltiorrhiza cannot meet the growing market demand. Phenolic acids are active ingredients with important economic and medicinal properties; its synthetic route has been basically clear, how to improve the amount of phenolic acid compounds in S. miltiorrhiza has become a research hotspot and difficulty. The application of plant biotechnology in improving biological activity and the required ingredients is more attractive and efficient than traditional methods. Here, we will introduce several methods which were reported to enhance the production of PAs including elicitors, metabolic engineering, and transcriptional regulation.
11.4.1 Elicitation Treatment to Increase the Production of Phenolic Acids
Elicitors are usually an agent that stimulates a plant defense response. Simulating biotic and abiotic stresses, elicitors classified into biotic elicitor and abiotic elicitor can stimulate plants and plant cultures to respond to them and lead to the accumulation of secondary metabolites in plants and plant cultures, which is important to produce valuable pharmaceutical ingredients (Wang and Wu 2013). Some elicitors have been utilized to enhance the production of secondary metabolites such as phenolic acids in the hairy roots or cell culture of S. miltiorrhiza, including methyl jasmonate (MeJA), yeast extract (YE), abscisic acid (ABA), silver ions (Ag+), gibberellic acid (GA), salicylic acid (SA), polyamines, and ethylene. For example, treating with MeJA (0.1 mM), RA and lithospermic acid B accumulation were significantly increased. Meanwhile, several RA biosynthesis genes were induced by 0.1 mM MeJA, including phenylalanine ammonia-lyase, cinnamic acid 4-hydroxylase, tyrosine aminotransferase, 4-hydroxyphenylpyruvate reductase, and 4-hydroxyphenylpyruvate dioxygenase (Xiao et al. 2009). SA was utilized to treat suspension cultures of S. miltiorrhiza, which lead to a significant increase in the RA content. Furthermore, increase of the PAL activity was measured with SA treatment (Jiao et al. 2012). As the most studied heavy metal ion elicitor, low concentration of Ag+ can stimulate 3-O-glucosylresveratrol production, which do not affect cell growth at the same time (Cai et al. 2013). With Ag+ treatment, the total phenolic acids of S. miltiorrhiza hairy roots were increased and the tyrosine aminotransferase (TAT) activity showed a remarkably rise (Yan et al. 2006). It was found that accumulation of RA on day 6 after treatment of S. miltiorrhiza hairy roots with Ag+ treatment was significantly increased to 1.3 times than that of the control and productions of caffeic acid and ferulic acid were also increased with Ag+ treatment. However, productions of DSU, cinnamic acid, and lithospermic acid B (LAB) were significantly dropped by Ag+ treatment. The results of qRT-PCR showed that expression of five key enzymes genes in RA biosynthesis pathways was significantly up-regulated by Ag+ (Xing et al. 2015). YE was reported much more effective than Ag+ to enhance the content of RA and accumulation of total phenolic acids. The activity of PAL showed a notable repression with YE treatment, while the activity of TAT was enhanced by YE (Yan et al. 2006). It was reported that exogenous ABA and polyamines increased the production of salvianolic acids in hairy root cultures of S. miltiorrhiza. The results of HPLC showed that contents of SAB and SAA enhanced 2.0-fold and 3.3-fold, respectively, after 80 μmol L−1 ABA treatment in S. miltiorrhiza hairy roots. Meanwhile, PAL activity also was detected, which increased 1.8-fold after ABA treatment (Hao et al. 2012). Liang et al. (2013) found that three significant phytohormones including abscisic acid (ABA), gibberellin (GA), and ethylene (Eth) could enhance the production of phenolic acids and activities of PAL and TAT in S. miltiorrhiza hairy roots.
11.4.2 Metabolic Engineering for Production of Phenolic Acids in S. miltiorrhiza
The use of modern biotechnology and molecular biology methods regulates the content of phenolic acids in S. miltiorrhiza. The first is the regulation of key enzyme genes in its synthetic pathway. Hairy roots have the advantages of high genetic stability, rapid growth, etc., which is considered as a promising system to generate pharmacological active ingredient of traditional Chinese medicine plant (Guillon et al. 2006; Kai et al. 2012; Shi et al. 2019, 2014). In order to improve the synthesis of pharmacologically active ingredients such as phenolic acids in S. miltiorrhiza, researchers attempted to overexpress one or more key enzymes of the phenolic acid synthesis pathway in hairy roots of S. miltiorrhiza. Previous research revealed that the content of rosmarinic acid (RA) was ~3.6-fold more than control in SmC4H transgenic lines. Furthermore, the concentration of LAB enhanced 11.1-fold of that in control (EV) when overexpressed SmC4H in hairy roots. Overexpression of SmTAT and SmHPPR in hairy root also showed an increase in RA and LAB levels. It is worth noting that the SmTAT-SmHPPR co-transformed lines had the highest level of metabolites that the content more than 16.1 and 18.8 times that in EV line (Xiao et al. 2011). Overexpressed genes such as Sm4CL, SmRAS, and SmCYP98A14 in the biosynthesis pathway of phenolic acids are also a strategy to be used for enhancing the yields of phenolic acids in the future. Not only endogenous genes, but also foreign genes can be involved in the regulation of phenolic acid synthesis. It was reported that the accumulation of phenolic acids in S. miltiorrhiza, especially SAB, was affected by overexpressing the foreign gene AtPAP1 (Zhang et al. 2010).
11.4.3 Transcriptional Regulation of Phenolic Acids Biosynthesis in S. miltiorrhiza
The effects of overexpressing one or more critical enzyme genes of synthetic pathway to enhance the target product are limited, and the increase in the yield of the target product is often limited. The regulatory functions and mechanisms of transcription factors for plant secondary metabolism have become research hotspots. As research continues, it has been found that the use of upstream transcription factors to activate the entire metabolic regulatory network is often more efficient than simply transferring to one or several rate-limiting enzyme genes. JA is involved in plant growth and development regulation as a plant hormone, as well as response to stress and leads to accumulation of secondary metabolites (Wasternack and Hause 2013; Namdeo. 2007). With the continuous advancement of sequencing technology, transcription factors mainly including ERF, WRKY, bHLH, MYB, and other transcription factors in responsive to JAs have been discovered and cloned for secondary metabolic regulation in S. miltiorrhiza (Zhou and Memelink 2016; Yu et al. 2018; Cao et al. 2018; Du et al. 2018; Ding et al. 2017).
Members of the AP2/ERF TF family responding to JA are significant biosynthesis of secondary metabolites in plant (Zhou and Memelink 2016). SmERF115, most sensitive to MeJA, has been isolated and characterized. The phenolic acids production of hairy roots in S. miltiorrhiza is enhanced when SmERF115 overexpressed, while silencing of SmERF115 leads to the decreased of phenolic acids. Meanwhile, SmERF115 is binding directly the promoter of SmRAS and up-regulating the expression of SmRAS to mediate the yield of phenolic acids (Sun et al. 2019).
Basic helix-loop-helix (bHLH) transcription factor, one of the largest families of transcription factors in plants, plays an important role in plant growth and development and secondary metabolism. SmbHLH37, SmbHLH51, and SmbHLH148 in S. miltiorrhiza were revealed to participate the regulation of phenolic acids. SmbHLH37 was reported binding the promoter regions of SmTAT and SmPAL to repress their expression, inhibiting the SAB synthesis pathway and resulting in decreased SAB production. In contrast, SmbHLH51 and SmbHLH148 played a positive role in mediating the pathway of phenolic acid biosynthesis. The content of SmbHLH51-OE lines increased 2.19-fold and 1.59-fold, respectively, and overexpressed SmbHLH148 significantly improved three salvianolic acid levels including caffeic acid, rosmarinic acid, and salvianolic acid B (Du et al. 2018; Wu et al. 2018; Xing et al. 2018a, b). As a special member of the bHLH family, the MYC2 transcription factor has been widely studied. MYC2 is not only the core of the response MJ in plants, but also plays a significant role in secondary metabolism and various growth and development processes (Kazan and Manners 2013; Gangappa et al. 2010). For example, it was reported that SmMYC2 regulated the generation of phenolic acids by activating both primary and secondary metabolic pathways in S. miltiorrhiza. Overexpressed SmMYC2 transgenic plants showed higher SAB content which was 1.88-fold higher than in control lines (Yang et al. 2017).
As the largest transcription factor family, MYB TFs are widely found in plant. The MYB transcription factors are divided into four subfamilies, called 1R-MYB, 2R-MYB, 3R-MYB, and 4R-MYB, respectively, depending on the number of incomplete repeats (one, two, three, or four) in the DNA-binding domain (Katiyar et al. 2012; Zhang et al. 2013; Li and Lu 2014). MYB transcription factors have been shown to be involved in primary and secondary metabolism, cell fate and traits, developmental processes, and responses to biotic and abiotic stresses (Dubos et al. 2010; Katiyar et al. 2012). As a R2R3-MYB transcription factor, SmMYB36 was in involved in tanshinones and phenolic acids biosynthesis regulation. In the overexpressing SmMYB36 hairy roots, the content of SAB, RA, and total phenolic acid decreased significantly compared with the control lines, while the content of tanshinone increased in overexpressing lines (Ding et al. 2017). It also was reported that SmMYB111 promoted accumulation of SAB and RA. The concentrations of RA in SmMYB111-OE lines were 3.05 and 3.10 times higher than that in control lines. Meanwhile, the content of SAB in SmMYB111-OE lines was about 3.54- and 2.50-fold higher than control (Li et al. 2018).
JASMONATE ZIM-DOMAIN (JAZ) transcriptional repressor plays an important role in the JA signaling pathway. Meanwhile, JAZs can interact with MYC2 transcription factor and repress it function when MJ absent (Thines et al. 2007). SmJAZ8 was found involved in biosynthesis of phenolic acids and tanshinones in S. miltiorrhiza hairy roots. The production of phenolic acids was decreased when overexpressed SmJAZ8, but increased in RNAi transgenic lines. Meanwhile, the content of tanshinones also declined or enhanced in SmJAZ8-OE lines or RNAi lines, respectively (Pei et al. 2017).
The above results indicate that it is feasible to increase phenolic acid production at the level of transcription factor regulation. Altering the expression of a transcription factor can directly or indirectly result in a change in the expression of one or more key enzyme genes in the phenolic acid biosynthetic pathway to accumulate or reduce phenolic acid. The production of phenolic acids can be increased to meet needs of markets by constructing single plants or hairy roots that overexpress transcription factors that positively regulate phenolic acid synthesis.
11.4.4 Callus Cultures of S. miltiorrhiza for Enhancing Production of Phenolic Acids
Hairy roots have been previously introduced for the mass production of valuable secondary metabolites, especially some slow-growing medicinal plants. However, studies on using callus and cell suspension cultures in S. miltiorrhiza to generate secondary metabolite, especially important pharmacological components, are limited. In 1996, Taxus cell cultures were reported that could be an alternative source of paclitaxel and related taxane production. A significant increase in paclitaxel and baccatin III levels was observed in cultured cells of Taxus species after treatment with MeJA (Yukimune et al. 1996). In recent years, callus cultures of S. miltiorrhiza were reported to be used for producing RA and SAB. Callus cultures were cultivated in MS medium using stem and leaf explants; subsequently, the active ingredients of the extracts from callus cultures were analyzed by high-performance liquid chromatography coupled to DAD and MS (HPLC-DAD-MS). The results showed that extraction of callus cultures from stem produced higher amounts of RA and SAB than callus leaves. The content of RA in stem was 1.27 ± 0.38% but 0.28 ± 0.02% in leaves, meanwhile, the SAB production in stem and leaves was 0.87 ± 0.20% and 0.07 ± 0.03%, respectively (Wu et al. 2016).
Plant callus and cell suspension cultures possessing high-value secondary metabolites are a promising potential alternative source for industrial production of medicinal ingredients like phenolic acids. Cell cultures are insensitive to the external environment and rapidly produce metabolites with pharmacological active ingredients (Shi et al. 2019). How to use the suspension cells and callus of S. miltiorrhiza to carry out the production of secondary metabolites of pharmaceutical ingredients needs further investigation.
11.4.5 Making Use of Endophytic Fungus in S. miltiorrhiza to Produce Phenolic Acids
Making use of endophytic fungus to produce plant secondary metabolites is a novel technology. Phoma glomerata D14, an endophytic fungus isolated from S. miltiorrhiza, was also found to produce salvianolic acid C (SAC). However, HPLC analysis found that the production of salvianolic acid C in extract of mycelium and broth was very low, 47.67 ± 0.04 μg/g and 0.054 μg/mL, respectively (Li et al. 2016b). Furthermore, Fusarium proliferatum SaR-2 and Alternaria alternata SaF-2 were found to exhibit higher levels of phenolics than plant roots. Results of total phenol production detection showed that total phenolic content of F. proliferatum SaR-2 and A. alternata SaF-2 was 21.75 ± 0.11 mg/g and 20.53 ± 0.08 mg/g, respectively (Li et al. 2015). Trichoderma atroviride D16, an endophytic fungus isolated from roots of S. miltiorrhiza, has been reported to produce tanshinone I and tanshinone IIA, which was a potential source for industrially production of tanshinone I and tanshinone IIA to meet pharmaceutical needs (Lou et al. 2013). Chaetomium globosum D38 isolated from S. miltiorrhiza roots could enhance the production of tanshinones, especially for dihydrotanshinone I and cryptotanshinone. Furthermore, this endophytic fungus could co-exist with the root of S. miltiorrhiza without toxicity. Both live fungus and its mycelia extract were revealed that could increase the production of tanshinones (Zhai et al. 2018). Due to the low variety and low content of endophytic fungus-producing phenolic acids, more endophytic fungus that produce phenolic acid should be explored and culture conditions optimized to increase phenolic acids production in the future.
11.5 Conclusions and Prospects
S. miltiorrhiza is an important traditional Chinese herbal medicine, which has been widely used in the treatment of cardiovascular and cerebrovascular diseases. Phenolic acid is one group of biologically active compounds in S. miltiorrhiza. It has great curative effect and pharmacological activity. However, due to the market demand is increasing, the germplasm resources of S. miltiorrhiza are degraded, and the content of phenolic acids in traditionally cultured S. miltiorrhiza is low. How to improve the production of phenolic acid has become an urgent problem to be solved.
It was a strategy that the use of modern biological means to regulate the synthesis of phenolic acids, through the expression of genes leading to the accumulation of secondary metabolites. However, its mechanism of action and regulation mode needs further exploration due to its complex secondary metabolic network. Combined with molecular biology, transcriptomics, and metabolomics, the expression of secondary metabolite-related genes and their mechanisms are important for understanding the synthesis and regulation mechanisms of phenolic acids. Currently, the upstream pathway of RA synthesis and the key enzymes involved in regulation have been basically understood, including SmPAL, SmC4H, Sm4CL, SmTAT, SmHPPR, SmRAS, and SmCYP98A14. However, the synthetic pathways of other phenolic acids such as SAB downstream of RA are still unclear. Therefore, exploring the synthetic pathways downstream of RA to other phenolic acids, discovering the key enzymes involved in catalysis and further researching the metabolic mechanism of metabolites, will be beneficial to promote the production of phenolic acids and drug development in S. miltiorrhiza.
To date, several types of transcription factors such as bHLH, MYB, JAZ, and AP2/EARF have been reported to regulate phenolic acid biosynthesis. Through the genome and transcriptome data of S. miltiorrhiza, it is possible to dig deeper and isolate more genes involved in the regulation of phenolic acids. The strategy of constructing a “transcription factor-biosynthetic pathway critical gene-metabolite” network will contribute to the improved synthesis of phenolic acids in S. miltiorrhiza. Meanwhile, comprehensive utilization of synthetic biology, elicitors, hairy roots and transgenic plants, endophytic fungus, genetic engineering and transcriptional regulation to enhance phenolic acid production in S. miltiorrhiza is also the direction of future research.
References
Bao Y, Wang L, Xu Y, Yang Y, Wang L, Si S, Cho S, Hong B (2012) Salvianolic acid B inhibits macrophage uptake of modified low density lipoprotein (mLDL) in a scavenger receptor CD36-dependent manner. Atherosclerosis 223(1):152–159
Bi L, Chen J, Yuan X, Jiang Z, Chen W (2013) Salvianolic acid A positively regulates PTEN protein level and inhibits growth of A549 lung cancer cells. Biomed Rep 1(2):213–217
Cai Z, Kastell A, Speiser C, Smetanska I (2013) Enhanced resveratrol production in Vitis vinifera cell suspension cultures by heavy metals without loss of cell viability. Appl Biochem Biotech 171(2):330–340
Cai J, Chen S, Zhang W, Zheng X, Hu S, Pang C, Lu J, Xing J, Dong Y (2014) Salvianolic acid A reverses paclitaxel resistance in human breast cancer MCF-7 cells via targeting the expression of transgelin 2 and attenuating PI3K/Akt pathway. Phytomedicine 21(12):1725–1732
Cao WZ, Wang Y, Shi M, Hao XL, Wang Y, Zhao WW, Wang Y, Ren J, Kai GY (2018) Transcription factor SmWRKY1 positively promotes the biosynthesis of tanshinones in Salvia miltiorrhiza. Front Plant Sci 9:554
Chen T, Liu W, Chao X, Zhang L, Qu Y, Huo J, Fei Z (2011) Salvianolic acid B attenuates brain damage and inflammation after traumatic brain injury in mice. Brain Res Bull 84(2):163–168
Chen RC, Sun GB, Ye JX, Wang J, Zhang MD, Sun XB (2017) Salvianolic acid B attenuates doxorubicin-induced ER stress by inhibiting TRPC3 and TRPC6 mediated Ca2+ overload in rat cardiomyocytes. Toxicol Lett 276:21–30
Dhapare S, Sakagami M (2018) Salvianolic acid B as an anti-emphysema agent I: in vitro stimulation of lung cell proliferation and migration, and protection against lung cell death, and in vivo lung STAT3 activation and VEGF elevation. Pulm Pharmacol Ther 53:107–115
Di P, Zhang L, Chen JF, Tan H, Xiao Y, Dong X, Zhou X, Chen WS (2013) 13C tracer reveals phenolic acids biosynthesis in hairy root cultures of Salvia miltiorrhiza. ACS Chem Biol 8(7):1537–1548
Ding K, Pei TL, Bai ZQ, Jia YY, Ma PD, Liang ZS (2017) SmMYB36, a novel R2R3-MYB transcription factor, enhances tanshinone accumulation and decreases phenolic acid content in Salvia miltiorrhiza hairy roots. Sci Rep 7(1):5104
Du TZ, Niu JF, Su J, Li SS, Guo XR, Li L, Cao XY, Kang JF (2018) SmbHLH37 functions antagonistically with SmMYC2 in regulating jasmonate-mediated biosynthesis of phenolic acids in Salvia miltiorrhiza. Front Plant Sci 9:1720
Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L (2010) MYB transcription factors in Arabidopsis. Trends Plant Sci 15(10):573–581
Fan HY, Fu FH, Yang MY, Xu H, Zhang AH, Liu K (2010) Antiplatelet and antithrombotic activities of salvianolic acid A. Thromb Res 126(1):e17–e22
Fan HY, Yang MY, Qi D, Zhang ZK, Zhu L, Shang-Guan XX, Liu K, Xu H, Che X (2015) Salvianolic acid A as a multifunctional agent ameliorates doxorubicin-induced nephropathy in rats. Sci Rep 5:12273
Fan Y, Luo QP, Wei JJ, Lin RH, Lin LL, Li YK, Chen ZR, Chen Q, Lin W (2018) Mechanism of salvianolic acid B neuroprotection against ischemia/reperfusion induced cerebral injury. Brain Res 1679:125–133
Fang CY, Wu CZ, Chen PN, Chang YC, Chuang CY, Lai CT, Yang SF, Tsai LL (2018) Antimetastatic potentials of salvianolic acid A on oral squamous cell carcinoma by targeting MMP-2 and the c-Raf/MEK/ERK pathway. Environ Toxicol 33(5):545–554
Gangappa SN, Prasad VBR, Chattopadhyay S (2010) Functional interconnection of MYC2 and SPA1 in the photomorphogenic seedling development of Arabidopsis. Plant Physiol 154(3):1210–1219
Gong L, Di C, Xia X, Wang J, Chen G, Shi J, Chen P, Xu H, Zhang W (2016) AKT/mTOR signaling pathway is involved in salvianolic acid B-induced autophagy and apoptosis in hepatocellular carcinoma cells. Int J Oncol 49(6):2538–2548
Guillon S, Trémouillaux-Guiller J, Pati PK, Rideau M, Gantet P (2006) Hairy root research: recent scenario and exciting prospects. Curr Opin Plant Biol 9(3):341–346
Guo P, Wang J, Gao W, Liu X, Wu S, Wan B, Xu L, Li Y (2018) Salvianolic acid B reverses multidrug resistance in nude mice bearing human colon cancer stem cells. Mol Med Rep 18(2):1323–1334
Han JY, Li Q, Ma ZZ, Fan JY (2017) Effects and mechanisms of compound Chinese medicine and major ingredients on microcirculatory dysfunction and organ injury induced by ischemia/reperfusion. Pharm Ther 177:146–173
Hao GP, Ji HW, Li YL, Shi RJ, Wang JM, Feng L, Huang LQ (2012) Exogenous ABA and polyamines enhanced salvianolic acids contents in hairy root cultures of Salvia miltiorrhiza Bge. f. alba. Plant Omics 5(5):446
Heo JY, Im DS (2019) Anti-allergic effects of salvianolic acid A and tanshinone IIA from Salvia miltiorrhiza determined using in vivo and in vitro experiments. Int Immunopharmacol 67:69–77
Hou X, Shao F, Ma Y, Lu S (2013) The phenylalanine ammonia-lyase gene family in Salvia miltiorrhiza: genome-wide characterization, molecular cloning and expression analysis. Mol Biol Rep 40(7):4301–4310
Hou BY, Qiang GF, Zhao YR, Yang XY, Chen X, Yan Y, Wang XB, Liu C, Zhang L, Du GH (2017) Salvianolic acid A protects against diabetic nephropathy through ameliorating glomerular endothelial dysfunction via inhibiting AGE-RAGE signaling. Cell Physiol Biochem 44(6):2378–2394
Huang BB, Duan YB, Yi B, Sun LN, Lu B, Yu XH, Sun H, Zhang W, Chen WS (2008a) Characterization and expression profiling of cinnamate 4-hydroxylase gene from Salvia miltiorrhiza in rosmarinic acid biosynthesis pathway. Russ J Plant Physiol 55(3):390
Huang BB, Yi B, Duan YB, Sun LN, Yu XH, Guo J, Chen WS (2008b) Characterization and expression profiling of tyrosine aminotransferase gene from Salvia miltiorrhiza (Dan-shen) in rosmarinic acid biosynthesis pathway. Mol Biol Rep 35(4):601–612
Huang ZS, Zeng CL, Zhu LJ, Jiang L, Li N, Hu H (2010) Salvianolic acid A inhibits platelet activation and arterial thrombosis via inhibition of phosphoinositide 3-kinase. J Thromb Haemost 8(6):1383–1393
Huang D, Wei X, Mu H, Pan C, Li Q, Hu B, Chang X, Yan L, Fan J, Liu Y, Luo J, Han J (2019) Total salvianolic acid injection prevents ischemia/reperfusion-induced myocardial injury via antioxidant mechanism involving mitochondrial respiratory chain through the upregulation of sirtuin1 and sirtuin3. Shock 51(6):745–756
Huttunen S, Toivanen M, Liu C, Tikkanen-Kaukanen C (2016) Novel anti-infective potential of salvianolic acid B against human serious pathogen Neisseria meningitidis. BMC Res Notes 9(1):25
Jiao M, Cao R, Chen H, Hao W, Dong JE (2012) Effects of salicylic acid on synthesis of rosmarinic acid and related enzymes in the suspension cultures of Salvia miltiorrhiza. Chin J Biotechnol 28(3):320–328
Jin XQ, Chen ZW, Tan RH, Zhao SJ, Hu ZB (2012) Isolation and functional analysis of 4-coumarate: coenzyme A ligase gene promoters from Salvia miltiorrhiza. Biol Plant 56(2):261–268
Jing Z, Fei W, Zhou J, Zhang L, Chen L, Zhang X, Liang X, Xie J, Fang Y, Sui X, Han W, Pan H (2016) Salvianolic acid B, a novel autophagy inducer, exerts antitumor activity as a single agent in colorectal cancer cells. Oncotarget 7(38):61509–61519
Kai GY, Zhang A, Guo YY, Li L, Cui LJ, Luo XQ, Liu C, Xiao JB (2012) Enhancing the production of tropane alkaloids in transgenic Anisodus acutangulus hairy root cultures by over-expressing tropinone reductase I and hyoscyamine-6β-hydroxylase. Mol BioSyst 8(11):2883–2890
Katiyar A, Smita S, Lenka SK, Rajwanshi R, Chinnusamy V, Bansal KC (2012) Genome-wide classification and expression analysis of MYB transcription factor families in rice and Arabidopsis. BMC Genom 13(1):544
Kazan K, Manners JM (2013) MYC2: the master in action. Mol Plant 6(3):686–703
Kong WJ, Zhang SS, Zhao YL, Wu MQ, Chen P, Wu XR, Ma XP, Guo WY, Yang MH (2017) Combination of chemical fingerprint and bioactivity evaluation to explore the antibacterial components of Salvia miltiorrhiza. Sci Rep 7(1):8112
Lee YW, Kim DH, Jeon SJ, Park SJ, Kim JM, Jung JM, Lee HE, Bae SG, Oh HK, Son KH, Ryu JH (2013) Neuroprotective effects of salvianolic acid B on an Abeta25-35 peptide-induced mouse model of Alzheimer’s disease. Eur J Pharmacol 704(1–3):70–77
Li CL, Lu SF (2014) Genome-wide characterization and comparative analysis of R2R3-MYB transcription factors shows the complexity of MYB-associated regulatory networks in Salvia miltiorrhiza. BMC Genom 15(1):277
Li YL, Xin XM, Chang ZY, Shi RJ, Miao ZM, Ding J, Hao GP (2015) The endophytic fungi of Salvia miltiorrhiza Bge. f. alba are a potential source of natural antioxidants. Bot Stud 56(1):5
Li L, Xu T, Du Y, Pan D, Wu W, Zhu H, Zhang Y, Li D (2016a) Salvianolic acid A attenuates cell apoptosis, oxidative stress, Akt and NF-κB activation in angiotensin-II induced murine peritoneal macrophages. Curr Pharm Biotechnol 17(3):283–290
Li XQ, Zhai X, Shu ZH, Dong RF, Ming QL, Qin LP, Zheng CJ (2016b) Phoma glomerata D14: an endophytic fungus from Salvia miltiorrhiza that produces salvianolic acid C. Curr Microbiol 73(1):31–37
Li SS, Wu YC, Kuang J, Wang HQ, Du TZ, Huang YY, Zhang Y, Cao XY, Wang ZZ (2018) SmMYB111 is a key factor to phenolic acid biosynthesis and interacts with both SmTTG1 and SmbHLH51 in Salvia miltiorrhiza. J Agric Food Chem 66(30):8069–8078
Liang ZS, Ma YN, Xu T, Cui BM, Liu Y, Guo ZX, Yang DF (2013) Effects of abscisic acid, gibberellin, ethylene and their interactions on production of phenolic acids in Salvia miltiorrhiza Bunge hairy roots. PLoS ONE 8(9):e72806
Liu L, Li J, Zhang Y, Zhang S, Ye J, Wen Z, Ding J, Kunapuli SP, Luo X, Ding Z (2014) Salvianolic acid B inhibits platelets as a P2Y12 antagonist and PDE inhibitor: evidence from clinic to laboratory. Thromb Res 134(4):866–876
Liu H, Ma S, Xia H, Lou H, Zhu F, Sun L (2018) Anti-inflammatory activities and potential mechanisms of phenolic acids isolated from Salvia miltiorrhiza f. alba roots in THP-1 macrophages. J Ethnopharmacol 222:201–207
Lou JF, Fu LY, Luo RY, Wang XH, Luo HY, Zhou LG (2013) Endophytic fungi from medicinal herb Salvia miltiorrhiza Bunge and their antimicrobial activity. Afr J microbiol Res 7(47):5343–5349
Lv HD, Wang L, Shen JC, Hao SJ, Ming AM, Wang XD, Su F, Zhang ZC (2015) Salvianolic acid B attenuates apoptosis and inflammation via SIRT1 activation in experimental stroke rats. Brain Res Bull 115:30–36
Ma XH, Ma Y, Tang JF, He YL, Liu YC, Ma XJ, Shen Y, Cui GH, Lin HX, Guo J, Huang LQ (2015) The biosynthetic pathways of tanshinones and phenolic acids in Salvia miltiorrhiza. Molecules 20(9):16235–16254
Ma ZG, Xia HQ, Cui SL, Yu J (2017) Attenuation of renal ischemic reperfusion injury by salvianolic acid B via suppressing oxidative stress and inflammation through PI3K/Akt signaling pathway. Braz J Med Biol Res 50(6):e5954
Mahmood Q, Wang GF, Wu G, Wang H, Zhou CX, Yang HY, Liu ZR, Han F, Zhao K (2017) Salvianolic acid A inhibits calpain activation and eNOS uncoupling during focal cerebral ischemia in mice. Phytomedicine 25:8–14
Meng D, Li J, Li H, Wang K (2019) Salvianolic acid B remits LPS-induced injury by up-regulating miR-142-3p in MH7A cells. Biomed Pharmacother 115:108876
Namdeo AG (2007) Plant cell elicitation for production of secondary metabolites: a review. Pharmacogn Rev 1(1):69–79
Pei T, Ma P, Ding K, Liu S, Jia Y, Ru M, Dong J, Liang ZS (2017) SmJAZ8 acts as a core repressor regulating JA-induced biosynthesis of salvianolic acids and tanshinones in Salvia miltiorrhiza hairy roots. J Exp Bot 69(7):1663–1678
Pei R, Si T, Lu Y, Zhou JX, Jiang L (2018) Salvianolic acid A, a novel PI3K/Akt inhibitor, induces cell apoptosis and suppresses tumor growth in acute myeloid leukemia. Leuk Lymphoma 59(8):1959–1967
Petersen M, Simmonds MS (2003) Rosmarinic acid. Phytochemistry 62(2):121–125
Pezeshki S, Petersen M (2018) Rosmarinic acid and related metabolites. In: Biotechnology of natural products. Springer, Cham, pp 25–60
Qian W, Wang Z, Xu T, Li D (2019) Anti-apoptotic effects and mechanisms of salvianolic acid A on cardiomyocytes in ischemia-reperfusion injury. Histol Histopathol 34(3):223–231
Qiang G, Yang X, Shi L, Zhang H, Chen B, Zhao Y, Zu M, Zhou D, Guo J, Yang H, Zhang L, Du G (2015) Antidiabetic effect of salvianolic acid A on diabetic animal models via AMPK activation and mitochondrial regulation. Cell Physiol Biochem 36(1):395–408
Rahbardar MG, Amin B, Mehri S, Mirnajafi-Zadeh SJ, Hosseinzadeh H (2018) Rosmarinic acid attenuates development and existing pain in a rat model of neuropathic pain: an evidence of anti-oxidative and anti-inflammatory effects. Phytomedicine 40:59–67
Raoufi S, Baluchnejadmojarad T, Roghani M, Ghazanfari T, Khojasteh F, Mansouri M (2015) Antidiabetic potential of salvianolic acid B in multiple low-dose streptozotocin-induced diabetes. Pharm Biol 53(12):1803–1809
Sha W, Zhou YF, Ling ZQ, Xie GQ, Pang XW, Wang P, Gu XB (2018) Antitumor properties of salvianolic acid B against triple-negative and hormone receptor-positive breast cancer cells via ceramide-mediated apoptosis. Oncotarget 9(91):36331
Shi M, Luo XQ, Ju GH, Yu XH, Hao XL, Huang Q, Xiao JB, Cui LJ, Kai GY (2014) Increased accumulation of the cardio-cerebrovascular disease treatment drug tanshinone in Salvia miltiorrhiza hairy roots by the enzymes 3-hydroxy-3-methylglutaryl CoA reductase and 1-deoxy-d-xylulose 5-phosphate reductoisomerase. Func Integr Genomics 14(3):603–615
Shi X, Sun RM, Zhao Y, Fu R, Wang RW, Zhao HY, Wang ZC, Tang F, Zhang N, Tian XF, Yao JH (2018) Promotion of autophagosome–lysosome fusion via salvianolic acid A-mediated SIRT1 up-regulation ameliorates alcoholic liver disease. RSC Adv 8(36):20411–20422
Shi M, Huang FF, Deng CP, Wang Y, Kai GY (2019) Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit Rev Food Sci 59(6):953–964
Song J (2010) Function analysis of the genes involved in rosmarinic acid biosynthesis pathway in Salvia miltiorrhiza Bunge. Unpublished Ph.D. thesis, Shaanxi Normal University, Xian, China
Song J, Wang ZZ (2009) Molecular cloning, expression and characterization of a phenylalanine ammonia-lyase gene (SmPAL1) from Salvia miltiorrhiza. Mol Biol Rep 36(5):939
Song J, Wang ZZ (2011) RNAi-mediated suppression of the phenylalanine ammonia-lyase gene in Salvia miltiorrhiza causes abnormal phenotypes and a reduction in rosmarinic acid biosynthesis. J Plant Res 124(1):183–192
Sun WY, Tong L, Miao JZ, Huang JY, Li DX, Li YF, Xiao HT, Sun H, Bi KS (2016) Separation and analysis of phenolic acids from Salvia miltiorrhiza and its related preparations by off-line two-dimensional hydrophilic interaction chromatography × reversed-phase liquid chromatography coupled with ion trap time-of-flight mass spectrometry. J Chromatogr A 1431:79–88
Sun MH, Wang Y, Huang Q, Yuan TP, Wang Q, Wang C, Zhou W, Kai GY (2019) The biosynthesis of phenolic acids is positively regulated by the JA-responsive transcription factor ERF115 in Salvia miltiorrhiza. J Exp Bot 70(1):243–254
Tang XL, Yan L, Zhu L, Jiao DM, Chen J, Chen QY (2017) Salvianolic acid A reverses cisplatin resistance in lung cancer A549 cells by targeting c-met and attenuating Akt/mTOR pathway. J Pharmacol Sci 135(1):1–7
Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He S, Howe G, Browse J (2007) JAZ repressor proteins are targets of the SCF COI1 complex during jasmonate signalling. Nature 448(7154):661
Wang YM, Cao JL (2016) Advances in the chemical and pharmacological studies of phenolic acids in Salvia miltiorrhiza. World Chin Med 11(6):1126–1130
Wang W, Hu W (2018) Salvianolic acid B recovers cognitive deficits and angiogenesis in a cerebral small vessel disease rat model via the STAT3/VEGF signaling pathway. Mol Med Rep 17(2):3146–3151
Wang JW, Wu JY (2013) Effective elicitors and process strategies for enhancement of secondary metabolite production in hairy root cultures. Adv Biochem Eng Biotechnol 134:55–89
Wang F, Liu YY, Liu LY, Zeng QJ, Wang CS, Sun K, Yang JY, Guo J, Fan JY, Han JY (2009) The attenuation effect of 3,4-dihydroxy-phenyl lactic acid and salvianolic acid B on venular thrombosis induced in rat mesentery by photochemical reaction. Clin Hemorheol Microcirc 42(1):7–18
Wang B, Sun W, Li QS, Li Y, Luo HM, Song JY, Sun C, Qian J, Zhu YJ, Hayward A, Xu HB (2015a) Genome-wide identification of phenolic acid biosynthetic genes in Salvia miltiorrhiza. Planta 241(3):711–725
Wang X, Wang CY, Zhang LJ, Li YJ, Wang SJ, Wang JD, Yuan CY, Niu J, Wang CS, Lu GM (2015b) Salvianolic acid A shows selective cytotoxicity against multidrug-resistant MCF-7 breast cancer cells. Anticancer Drugs 26(2):210–223
Wang XK, Qi D, Fu FH, LiX Liu Y, Ji K, Gao ZF, Kong LL, Yu C, Xie H, Yue G, Zhu H, Liu K, Fan HY (2019) Therapeutic and antiproteinuric effects of salvianolic acid A in combined with low-dose prednisone in minimal change disease rats: Involvement of PPARγ/Angptl4 and Nrf2/HO-1 pathways. Eur J Pharmacol. https://doi.org/10.1016/j.ejphar.2019.04.023
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot 111(6):1021–1058
Wu CF, Karioti A, Rohr D, Bilia AR, Efferth T (2016) Production of rosmarinic acid and salvianolic acid B from callus culture of Salvia miltiorrhiza with cytotoxicity towards acute lymphoblastic leukemia cells. Food Chem 201:292–297
Wu YC, Zhang Y, Li L, Guo XR, Wang B, Cao XY, Wang ZZ (2018) AtPAP1 interacts with and activates SmbHLH51, a positive regulator to phenolic acids biosynthesis in Salvia miltiorrhiza. Front Plant Sci 9:1687
Xia ZB, Yuan YJ, Zhang QH, Li H, Dai JL, Min JK (2018) Salvianolic acid B suppresses inflammatory mediator levels by downregulating NF-κB in a rat model of rheumatoid arthritis. Med Sci Monit 24:2524–2532
Xiao Y, Gao SH, Di P, Chen JF, Chen WS, Zhang L (2009) Methyl jasmonate dramatically enhances the accumulation of phenolic acids in Salvia miltiorrhiza hairy root cultures. Physiol Plant 137(1):1–9
Xiao Y, Zhang L, Gao SH, Saechao S, Di P, Chen JF, Chen WS (2011) The c4h, tat, hppr and hppd genes prompted engineering of rosmarinic acid biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. PLoS ONE 6(12):e29713
Xing BC, Yang DF, Guo WL, Liang ZS, Yan XJ, Zhu YH, Liu Y (2015) Ag+ as a more effective elicitor for production of tanshinones than phenolic acids in Salvia miltiorrhiza hairy roots. Molecules 20(1):309–324
Xing BC, Liang LJ, Liu L, Hou ZN, Yang DF, Yan KJ, Zhang XM, Liang ZS (2018a) Overexpression of SmbHLH148 induced biosynthesis of tanshinones as well as phenolic acids in Salvia miltiorrhiza hairy roots. Plant Cell Rep 37(12):1681–1692
Xing BC, Yang DF, Liu L, Han RL, Sun YF, Liang ZS (2018b) Phenolic acid production is more effectively enhanced than tanshinone production by methyl jasmonate in Salvia miltiorrhiza hairy roots. PCTOC 134(1):119–129
Xiong W, Liu LX, Wu C, Yang C, Wu QY (2010) 13C-tracer and gas chromatography-mass spectrometry analyses reveal metabolic flux distribution in the oleaginous microalga Chlorella protothecoides. Plant Physiol 154(2):1001–1011
Yan Q, Shi M, Ng J, Wu JY (2006) Elicitor-induced rosmarinic acid accumulation and secondary metabolism enzyme activities in Salvia miltiorrhiza hairy roots. Plant Sci 170(4):853–858
Yang RX, Huang SY, Yan FF, Lu XT, Xing YF, Liu Y, Liu YF, Zhao YX (2010) Danshensu protects vascular endothelia in a rat model of hyperhomocysteinemia. Acta Pharmacol Sin 31(10):1395–1400
Yang N, Zhou WB, Su J, Wang XF, Li L, Wang LR, Cao XY, Wang ZZ (2017) Overexpression of SmMYC2 increases the production of phenolic acids in Salvia miltiorrhiza. Front Plant Sci 8:1804
Yang YF, Zhang LC, La XQ, Li ZY, Li HQ, Guo SJ (2019) Salvianolic acid A inhibits tumor-associated angiogenesis by blocking GRP78 secretion. Naunyn Schmiedebergs Arch Pharmacol 392(4):467–480
Yu DS, Wang YS, Bi YL, Guo ZP, Yuan YJ, Tong SM, Su RC, Ge LH, Wang J, Pan YL, Guan TT, Cao Y (2017) Salvianolic acid A ameliorates the integrity of blood-spinal cord barrier via miR-101/Cul3/Nrf2/HO-1 signaling pathway. Brain Res 1657:279–287
Yu HZ, Guo WL, Yang DF, Hou ZN, Liang ZS (2018) Transcriptional profiles of SmWRKY family genes and their putative roles in the biosynthesis of tanshinone and phenolic acids in Salvia miltiorrhiza. Int J Mol Sci 19(6):1593
Yukimune Y, Tabata H, Higashi Y, Hara Y (1996) Methyl jasmonate-induced overproduction of paclitaxel and baccatin III in Taxus cell suspension cultures. Nat Biotechnol 14(9):1129–1132
Zhai X, Luo D, Li X, Han T, Jia M, Kong ZY, Ji JC, Rahman K, Qin LP, Zheng CJ (2018) Endophyte Chaetomium globosum D38 promotes bioactive constituents accumulation and root production in Salvia miltiorrhiza. Front Microbiol 8:2694
Zhang Y, Yan YP, Wang ZZ (2010) The Arabidopsis PAP1 transcription factor plays an important role in the enrichment of phenolic acids in Salvia miltiorrhiza. J Agric Food Chem 58(23):12168–12175
Zhang HA, Gao M, Zhang L, Zhao Y, Shi LL, Chen BN, Wang YH, Wang SB, Du GH (2012) Salvianolic acid A protects human SH-SY5Y neuroblastoma cells against H2O2-induced injury by increasing stress tolerance ability. Biochem Biophys Res Commun 421(3):479–483
Zhang SC, Ma PD, Yang DF, Li WJ, Liang ZS, Liu Y, Liu FH (2013) Cloning and characterization of a putative R2R3 MYB transcriptional repressor of the rosmarinic acid biosynthetic pathway from Salvia miltiorrhiza. PLoS ONE 8(9):e73259
Zhang SC, Yan Y, Wang BQ, Liang ZS, Liu Y, Liu FH, Qi ZH (2014a) Selective responses of enzymes in the two parallel pathways of rosmarinic acid biosynthetic pathway to elicitors in Salvia miltiorrhiza hairy root cultures. J Biosci Bioeng 117(5):645–651
Zhang XC, Chen JQ, Li B (2014b) Salvianolic acid A suppresses CCL-20 expression in TNF-α-treated macrophages and ApoE-deficient mice. J Cardiovasc Pharmacol 64(4):318–325
Zhang Y, Yan YP, Wu YC, Hua WP, Chen C, Ge Q, Wang ZZ (2014c) Pathway engineering for phenolic acid accumulations in Salvia miltiorrhiza by combinational genetic manipulation. Metab Eng 21:71–80
Zhang X, Wu Q, Lu Y, Wan J, Dai H, Zhou X, Lv S, Chen X, Zhang X, Hang C, Wang J (2018a) Cerebroprotection by salvianolic acid B after experimental subarachnoid hemorrhage occurs via Nrf2- and SIRT1-dependent pathways. Free Radical Biol Med 124:504–516
Zhang JL, Zhang X, Zhang JB, Li MY, Chen DJ, Wu T (2018b) Minor compounds of the high purity salvianolic acid B freeze-dried powder from Salvia miltiorrhiza and antibacterial activity assessment. Nat Prod Res 32(10):1198–1202
Zhang JY, Wang M, Wang RY, Sun X, Du YY, Ye JX, Sun GB, Sun XB (2018c) Salvianolic acid A ameliorates arsenic trioxide-induced cardiotoxicity through decreasing cardiac mitochondrial injury and promotes its anticancer activity. Front Pharmacol 9:487
Zhang W, Song JK, Zhang X, Zhou QM, He GR, Xu XN, Yan R, Zhou WX, Du GH (2018d) Salvianolic acid A attenuates ischemia reperfusion induced rat brain damage by protecting the blood brain barrier through MMP-9 inhibition and anti-inflammation. Chin J Nat Med 16(3):184–193
Zhang H, Wang Y, Gao C, Gu Y, Huang J, Wang J, Wang J, Zhang Z (2018e) Salvianolic acid A attenuates kidney injury and inflammation by inhibiting NF-κB and p38 MAPK signaling pathways in 5/6 nephrectomized rats. Acta Pharmacol Sin 39(12):1855–1864
Zhang HF, Wang JH, Wang YL, Gao C, Gu YT, Huang J, Wang JH, Zhang Z (2019a) Salvianolic acid A protects the kidney against oxidative stress by activating the Akt/GSK-3β/Nrf2 signaling pathway and inhibiting the NF-κB signaling pathway in 5/6 nephrectomized rats. Oxid Med Cell Longev 2019:2853534
Zhang YF, Xu LW, Liang K, Zhou LH, Ge YZ, Jia RP (2019b) Protective effect of salvianolic acid B against oxidative injury associated with cystine stone formation. Urolithiasis. https://doi.org/10.1007/s00240-019-01114-4
Zhao SJ, Hu ZB, Liu D, Leung FC (2006) Two divergent members of 4-coumarate: coenzyme A ligase from Salvia miltiorrhiza Bunge: cDNA cloning and functional study. J Integr Plant Biol 48(11):1355–1364
Zhao GR, Zhang HM, Ye TX, Xiang ZJ, Yuan YJ, Guo ZX, Zhao LB (2008) Characterization of the radical scavenging and antioxidant activities of danshensu and salvianolic acid B. Food Chem Toxicol 46(1):73–81
Zhao J, Yang XC, Fujino M, Ichimaru N, Que W, Li XK, Takahara S (2019) Salvianolic acid B ameliorates liver injury in a murine aGvHD model by decreasing inflammatory responses via upregulation of HO-1. Transpl Immunol. https://doi.org/10.1016/j.trim.2019.03.002
Zheng X, Chen S, Yang Q, Cai J, Zhang W, You H, Xing J, Dong Y (2015) Salvianolic acid A reverses the paclitaxel resistance and inhibits the migration and invasion abilities of human breast cancer cells by inactivating transgelin 2. Cancer Biol Ther 16(9):1407–1414
Zhou ML, Memelink J (2016) Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnol Adv 34(4):441–449
Zhou Z, Zhang Y, Ding XR, Chen SH, Yang J, Wang XJ, Jia GL, Chen HS, Bo XC, Wang SQ (2007) Protocatechuic aldehyde inhibits hepatitis B virus replication both in vitro and in vivo. Antiviral Res 74(1):59–64
Zhou YQ, Li WZ, Xu L, Chen L (2011) In Salvia miltiorrhiza, phenolic acids possess protective properties against amyloid β-induced cytotoxicity, and tanshinones act as acetylcholinesterase inhibitors. Environ Toxicol Pharmacol 31(3):443–452
Zhou XL, Chan SW, Tseng HL, Deng Y, Hoi PM, Choi PS, Or PM, Yang JM, Lam FF, Lee SM, Leung GP, Kong SK, Ho HP, Kwan YW, Yeung JHK (2012) Danshensu is the major marker for the antioxidant and vasorelaxation effects of Danshen (Salvia miltiorrhiza) water-extracts produced by different heat water-extractions. Phytomedicine 19(14):1263–1269
Zhou J, Qu XD, Li ZY, Wei J, Liu Q, Ma YH, He JJ (2014) Salvianolic acid B attenuates toxin-induced neuronal damage via Nrf2-dependent glial cells-mediated protective activity in Parkinson’s disease models. PLoS ONE 9(7):e101668
Zhou R, Long H, Zhang B, Lao Z, Zheng Q, Wang T, Zhang Y, Wu Q, Lai X, Li G, Lin L (2019) Salvianolic acid B, an antioxidant derived from Salvia militarize, protects mice against γ-radiation-induced damage through Nrf2/Bach1. Mol Med Rep 19(2):1309–1317
Acknowledgements
This work was supported by National Natural Science Fund of China (81522049, 31571735, 31270007), the “Dawn” Program of Shanghai Education Commission (16SG38), Shanghai Science and Technology Committee Project (17JC1404300), Zhejiang Provincial Ten Thousands Program for Leading Talents of Science and Technology Innovation, Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kai, G., Liu, S., Shi, M., Han, B., Hao, X., Liu, Z. (2019). Biochemistry, Biosynthesis, and Medicinal Properties of Phenolic Acids in Salvia miltiorrhiza. In: Lu, S. (eds) The Salvia miltiorrhiza Genome. Compendium of Plant Genomes. Springer, Cham. https://doi.org/10.1007/978-3-030-24716-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-24716-4_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-24715-7
Online ISBN: 978-3-030-24716-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)