Abstract
This chapter mainly focuses on microbial fuel cell and applications of nanomaterials in microbial fuel cell. Dumping of waste on environment has increased drastically. It has massive impacts and effects on environment. Also it causes serious problems. Microbial fuel cell is a device that generates sustainable energy from the waste using electrochemically active microorganisms. It is fabricated with electrodes, membranes, and catalysts. It is functioning on the mechanisms like electron transfer mechanism and oxygen reduction reaction. In addition to electricity generation, it has applications like remote power source, fuel gas production (hydrogen and methane), wastewater treatment, water desalination, biosensors, remote sensors, and cleaning of polluted water reservoirs or sources (like ponds, lakes, and rivers). Nanomaterials are emerging as an important materials created by nanotechnology. Particles of these materials are smaller than 100 nanometers in at least one dimension. Their physical and chemical properties often differ from their bulk materials. They are utilized in the fabrication of microbial fuel cell components. Nanomaterials like carbon nanomaterials, nanocomposites, and biogenic inorganic nanoparticles improve the functioning of microbial fuel cell. Their unique property like high surface area supports for the catalytic activity. Also, this chapter discusses about the challenges faced by microbial fuel cell and the factors influencing on the microbial fuel cell output.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Microbial fuel cell
- Nanomaterials
- Nanocomposites
- Biocathode
- Biocatalysts
- Biofilms
- Proton-exchange membranes
- Biogenic inorganic nanoparticles
- Wastewater treatment
- Microfabrication
13.1 Introduction: Microbial Fuel Cells (MFCs)
A fuel cell is an energy-generating device that converts chemical energy into electrical energy. It consists of an electrolytic cell with the electrolyte and two electrodes (cathode and anode) which are connected together with a load resistance. MFC is an electrochemical fuel cell. It is used for sustainable energy generation by wastewater treatment with the help of electrochemically active microorganisms. The microorganisms are fed with organic substances which are called as substrates . They form biofilms over the anode for efficient electron transfer between the substrate and the anode. MFC has potential applications in alternative energy, wastewater treatment, environmental protection, and bio-sensing (for oxygen and pollutants) (Siegert et al. 2019).
Biofilm is a sticky matrix which has conducting nanowires. The microorganisms oxidize the substrate, and electron transfer may occur directly or with the help of mediators. The electrons are used to reduce oxygen into water at the cathode. Zhou et al. reported that the bacterial biofilm produced at anode acts as catalyst. It converts the chemical energy of the organic molecule into electrons during oxygen reduction at cathode and forms water (Zhou et al. 2013). Mustakeem reported the biofilm formation in MFC . It is shown in Fig. 13.1 (Mustakeem 2015). MFCs are classified into various types like single-chamber MFC or two-chamber MFC (with two separate compartments for anode and cathode). In a two-chamber MFC , the chambers are separated by a PEM which allows the protons to permeate through it to participate in redox reactions. They can be used to power small electronic devices like biosensors.
Schematic diagram shows the bacterial biofilm formed at the anode of a typical single-chamber MFC. (a) Effluent inlet. (b) Effluent outlet. (c) Catalyst layer. (d) Diffusion layers at air-cathode. (From Mustakeem, 2015)
In MFC, organic materials and waste materials are the source of biofuels. The ions (anion and cation) in the water and the water-soluble ions of these materials serve as electrolytes. Microorganisms serve as biocatalysts (Khan et al. 2017). When these materials are catalyzed by the microorganisms, electrons and protons are transferred and moved (Moqsud et al. 2015). Initially, electrons (e−) go to the anode electrode. Next, they move to the cathode electrode through the external circuit. In the case of protons (H+ ions), they go to the cathode electrode by the electrolyte. In the cathode, they react with oxygen. Hence, MFC generates electrical power (Ersan et al. 2010; Zhao et al. 2013; Khudzari et al. 2016; Khan et al. 2017; Siegert et al. 2019). Figure 13.2 shows the bioelectricity production from organic and waste materials.
In the report of Buzea et al. (2007), nanomaterials are described as, in principle, materials of which a single unit is sized (in at least one dimension) between 1 and 1000 nanometers (10−9 meter) but usually is 1 and 100 nm. Nanomaterial synthesis has been developed in support of microfabrication research. They often have unique optical, electronic, or mechanical properties (Hubler and Osuagwu 2010). Nanomaterials are being commercialized slowly (Eldridge 2014) and emerging as commodities (McGovern 2010). These materials are applied in various fields, industries, and products including healthcare, electronics, and cosmetics.
Since MFC is working with microorganisms, there is a need for biocompatible materials as electrodes. So, carbon materials are found to be suitable candidates as electrode materials for MFC. Nanotechnology offers many efficient materials like graphene, carbon nanotubes, and their composites with conducting polymers like polyaniline, polypyrrole, and other conducting metal oxides like iron oxide and manganese oxide. Using novel nanomaterials, increasing the power density of MFC is a trend for practical applications. Various instruments are used to study the reaction mechanism in a microbial fuel cell.
Confocal laser scanning microscopy is used to view the growth and thickness of the biofilms on electrodes. Cyclic voltammetry is applied to study the extracellular electron transfer. But it can be used only at low scan rates since the reactions involve living bacteria (Omkar et al., 2017). Bacteria produce power indefinitely as long as there are enough food sources to nurture the bacteria. MFCs are applied in the production of hydrogen fuel cells and desalination of seawater and provide sustainable energy sources for remote areas (Reuben 2018).
Generally, in the MFC, wastes are utilized as a source of substrate due to the dual advantage, i.e., cheap electricity generation and wastewater treatment . Some of the common wastes are domestic wastewater, vegetable wastes, agrowastes, azo dye wastes, and wastewater of various industries like food industries, breweries, cellulose/starch industries, organic industries, and chemical (chromium, selenium, and nitrate) industries. Shewanella oneidensis, Geobacter sulfurreducens, Klebsiella pneumoniae, Rhodoferax ferrireducens, Escherichia coli, Rhodopseudomonas palustris, Desulfovibrio desulfuricans, Acidithiobacillus ferrooxidans, Clostridium cellulolyticum, Enterobacter cloacae, Trichococcus pasteurii, Streptomyces enissocaesilis KNU (K strains), Nocardiopsis sp. KNU (S strain), Pseudomonas species, Parabacteroides, Proteiniphilum, and Catonella are some of the bacteria utilized in the MFC. Apart from bacteria, microorganism like yeast (Saccharomyces cerevisiae, Candida melibiosica, Hansenula anomala, Hansenula polymorpha, Arxula adeninivorans, and Kluyveromyces marxianus) is utilized as biocatalysts.
The power output of the MFC is proportional to the concentration of organic wastes. Hence, MFC can be used as a biochemical oxygen demand sensor (Chang et al. 2005; Kim et al. 2003). Apart from the substrate, many factors including microorganisms alter performance of the MFC. Some higher-level power output of the MFC (using various substrates and microorganisms) is reported: as per Wang et al. report, MFC using rice straw hydrolysate substrate (400 mg/mL concentration) generates the current density 137.6 mA/cm2 (Wang et al. 2014); MFC with Pseudomonas sp. microorganism, peptone substrate, and methylene blue mediator generates the power density 979 mW/m2 (Daniel et al. 2009); current output 2236 mW/m2 is obtained from the MFC with 200 ppm concentration of acid navy blue r (ANB) dye waste (Khan et al. 2015); and 2900 mW/m2 is the output of the MFC with 25 mg/ L concentration of selenium waste (Catal et al. 2009).
Utilizations of aerobic organisms in MFC and wastewater treatment require oxygen. Hence, it is essential to flow oxygen into the system for the survival of bacteria. It leads to increase the cost of the system but anaerobic organisms such as anode-respiring bacteria (ARB) reduces the cost substantially. Anaerobic organisms do not need oxygen. Also, ARB is able to transfer electrons in between the electrodes. It helps to the electrons to flow freely between the electrodes. Alternative form of the MFC, i.e., electrolytic cell using microbial agents, produces hydrogen at the cathode instead of electricity (Waste-management-world.com 2013). Growing of aerobic bacteria in cathode chamber (improves the oxygen reduction rate and inhibits proton transfer) and growing of anaerobic organisms like ARB in anode chamber (supports for electrons flow) will improve the MFC performance.
13.2 Energy Needs of the World
As per the International Energy Agency (IEA), current power demand is 12 billion tonne oil equivalent. It is expected to increase up to 18 billion tonne oil equivalent in the coming decades (Chu and Majumdar 2012). Enormous energy is found in organic matter such as carbohydrates. They are present in the municipal waste. Fuel cells are coming under alternative energy sources having a negligible CO2 emission. Also MFCs do not need any external energy input. This is an additional advantage for the extensive applications of MFCs particularly where electrical amenities less or no (Chandrasekhar et al. 2018).
Biomass and household waste are identified as resources for the bioenergy. Reuse, recovery, and recycling of wastewater can offer a sustainable wastewater management system in freshwater-scarce regions. Microbial fuel cell is the solution for both water and energy needs (Gajda et al. 2016). The world shortage of potash, nitrogen, and phosphorus suggests the usage of treated wastewater for farming. Water and wastewater treatment and distribution consume 1.4% of the total national electricity consumption. Figure 13.3 shows the energy production from nonrenewable energy resources causes environmental pollution and MFC produces clean energy.
13.3 Construction of MFC
A two-chamber MFC usually consists of two chambers in “H” shape separated by a tube with proton-exchange membrane. The anode should be highly conductive, biocompatible, and chemically stable under the conditions present inside the fuel cell. Metals like copper are avoided because of corrosion which has toxic effect on microorganisms. The cathode ought to have good reduction performance, good re-oxidation, and high long-term stability. The salt or agarose bridges used in MFC have high internal resistance. In a MFC aerobic respiration and fermentation should be avoided, and anaerobic respiration must be accelerated (igem.org 2018). Figure 13.4a, b shows the schematic working model of dual-chambered MFC and single-chambered MFC, respectively (Chaturvedi and Verma 2016).
MFC working model. (a) Dual-chambered MFC and (b) single-chambered MFC . (From Chaturvedi and Verma 2016)
13.3.1 Biocathode of MFC
The higher cost , non-environmental friendly, and complexities in the catalyst production are the factors leading to the biocathode development. Microorganisms serve as the catalyst in these biocathodes. The biofilm created on the cathode surface catalyzes the reduction reaction (Harnisch and Schröder 2010). Figure 13.5 illustrates a biocathode with biofilm on the surface. The biofilm catalyzes the reduction of chemical active species such as nitrate and oxygen (Mustakeem 2015).
MFC biocathode : (a) Oxygen and nitrate are reduced by direct electron transfer or by mediator. Transition metal mediators transfer the electrons to oxygen. (b) Reduction of oxygen and chemical reactants. This MFC will generate power and will drive biochemical synthesis reactions. (From Mustakeem 2015)
Biocathodes are categorized as aerobic and anaerobic biocathodes. Oxygen acts as a terminal electron acceptor, and hydrogen peroxide acts as an intermediate in aerobic biocathodes. Transition metals like iron and manganese serve as electron mediators in between the electrode and oxygen. Electrons are transferred from the cathode to the terminal electron acceptor in this method (Park and Zeikus 2003). In anaerobic biocathodes oxygen is not present. In this type, nitrates and sulfates can be utilized as terminal electron acceptors instead of oxygen .
13.3.2 Proton-Exchange Membrane (PEM)
Commercially available membranes (like Nafion) are highly conductive. These membranes have high ion-exchange capacity and proton conductivity due to their structural properties. However Nafion has problems of oxygen leakage from the cathode to the anode, substrate losses and biofouling, conductivity at low water content, and poor mechanical strength at high temperatures. Proton-exchange membrane is synthesized by solution casting method utilizing the materials such as poly(oxyethylene), poly(vinyl alcohol), chitosan, and phosphoric acid. Water absorption capacity is directly proportional to the concentration of PVA. A higher percentage of water uptake means higher ion-exchange capacity of the membrane (Dharmadhikari et al. 2018).
Membranes made of natural materials such as eggshell membrane are used in MFC. Sensor devices with those membranes possess low internal resistances and high sensitivity (Chouler et al. 2017). The membrane must transfer protons from the anode to the cathode and should prevent the transfer of oxygen and substrates. Oxygen penetration to the anode part makes the anode aerobic. Also, it reduces power density and wastewater treatment efficiency. Penetration of oxygen into the cathode substrate leads to the reduction of power out and microorganisms’ efficiency in chemical oxygen demand (COD) removal (Ghasemi et al. 2015).
MFC uses expensive proton-exchange membrane for separating anode and cathode. Recently some inexpensive clay is studied as an ion-exchange medium. They are known as cation-exchange membranes. They have structurally stronger properties. Both proton-exchange membrane and cation-exchange membrane reduce oxygen diffusion in the anode chamber of the cell. But proton-exchange membrane cannot be reused and is difficult to adapt to desired shapes. Clay mixed with kaolin and bentonite and finally fired designed a system. This kind of clay system produced an open-circuit voltage up to 1.5 volts. Figure 13.6 shows the diagram of double-chamber MFC designed with cation-exchange membrane (Reuben 2018). Similarly an inexpensive nanocomposite membrane based on cotton fabric is designed. A highest proton conductivity of PVAc-g-PVDF-coated cotton fabric of (1.5 ± 0.2) × 10−2 S/cm at 25 °C and lowest glucose permeability of (12 ± 1) × 10−6 cm2/s are obtained (Zhang et al. 2017a).
A simple double-chamber MFC design . It has porous pot anode chamber and copper cathode chamber. Porous pot is cation-exchange membrane. (From Reuben 2018)
13.3.3 Microorganisms as Biocatalysts
Microorganisms produce bacterial flagellar-like structures that are made up of several proteins which possess metallic-like conductivity. This type of extracellular electrically conductive protein nanofilaments are identified in Geobacter sulfurreducens called as microbial nanowires. These wires are also formed in organisms like Rhodopseudomonas palustris, Desulfovibrio desulfuricans, and Acidithiobacillus ferrooxidans. The electrical conductivity of these microbial nanowires is comparable to carbon nanotubes and some organic conductors. Proteins contain several aromatic amino acids which are responsible for electrical conduction. So, these microorganisms found applications in the field of bioremediation of heavy metals like uranium, arsenic, and chromium and in bioelectronics since these wires are having enough mechanical strength (Young’s modulus ~1 GPa) for construction of electronic devices (Sure et al. 2016).
Microorganisms can transfer electrons from the substrate to the anode without the help of mediators or exoelectrogens. They produce specific proteins or genes for their inevitable performance toward electricity generation in MFCs. The mechanisms involved in this process are biofilm formation, metabolism, and electron transfer. The expression of certain genes for outer membrane multiheme cytochromes, redox-active compounds, and conductive pili participate in the exoelectrogenic activity of microorganism genus such as Geobacter and Shewanella (Kumar et al. 2015).
The anode-respiring bacteria (ARB) can thrive in oxygen-free environments, and they are called as anaerobic organisms. These anode-respiring bacteria are able to transfer electrons to the negative terminal, thus generating useable current in the process. They attach themselves to the anode of a battery. They consume an organic substrate for food and release excess electrons as part of their metabolic pathway. The biofilm formed is a living matrix of protein and sugar. Geobacter is an ARB which is useful in the production of high current densities. ARB like only one compound, i.e., acetate, which is a fatty acid (Waste-management-world.com 2013).
Direct transfer of electrons takes place from bacteria to anode. Sometimes mediators like thionine, methyl viologen, and humic acid are used for electron transfer. These mediators are more expensive and toxic to the microorganisms. Bacteria like Shewanella oneidensis and Geobacter sulfurreducens produce electrically conductive appendages called bacterial nanowires. These nanowires facilitate direct transfer of electrons to the anode, thus increasing the efficiency and reducing the cost (Lal Deeksha 2013). Escherichia coli are often used for power generation because they can grow fast and are quite robust regarding cultivation conditions. Electrons are transported through nanowires called pili, which possess delocalized electronic states to function as protein wires with metallic-like conductivity (Omkar et al. 2017).
Internal mediators have advantages more than the external mediators. They are cheap and have no toxic effect on the microorganism . Figure 13.7 shows some examples for the internal and external mediators with their advantages and disadvantages. Several yeast strains like Saccharomyces cerevisiae, Candida melibiosica, Hansenula anomala, Hansenula polymorpha, Arxula adeninivorans, and Kluyveromyces marxianus have been studied as biocatalysts in MFC. Figure 13.8 shows the electron transfer during the metabolism of the organic materials (cellular respiration process) in the yeast cell (Sayed and Abdelkareem 2017).
External and internal mediators in MFCs. (From Sayed and Abdelkareem 2017)
Illustration for the possibilities of electrons’ origin and transfer of yeast cells into MFC. (From Sayed and Abdelkareem 2017)
13.4 Functions and Mechanisms of MFC
13.4.1 Mimicking and Resemblance of MFC
MFC mimics the interactions of microbes found in nature. Bacteria like Listeria monocytogenes deliver electrons. The mechanism involved in the energy production from wastes by MFC somewhat resembles the metabolism process by the gut flora of humans and other animals (including insects). Microbes generate energy from glucose by cellular respiration process. In aerobic cellular respiration, glucose is broken down into carbon dioxide and water using oxygen. In the case of anaerobic cellular respiration, nitrate (NO3) or other molecules are utilized instead of oxygen.
Extracellular electron transfer (EET) are the microbial-bioelectrochemical processes which transfer electrons. Food-borne pathogen L. monocytogenes is a fermentative gram-positive bacterium which decays organic matter. It uses flavin-based mechanism to deliver electrons into the surrounding environments. The characterization of a flavin-based EET mechanism establishes new avenues for the electrochemical activities and bioenergetic applications (Light et al. 2018). L. monocytogenes can be utilized to improve MFC.
13.4.2 Electron Transfer Mechanism
The electron transfer rate has controls on the output power of MFC. Mustakeem (2015) reported that the extracellular transport of electrons to electrodes takes place in three mechanisms, i.e., direct electron transfer, electron transfer through mediators, and electron transfer through nanowires. Apart from these mechanisms, another mechanism of electron transport is suggested by Schaetzle and co-workers. They have reported that the electron transport is done by the oxidation of the excreted catabolites (Schaetzle et al. 2009).
Some bacteria genera like Geobacter and Shewanella deliver electrons from the oxidative metabolic pathways to the external environment. They are called as exoelectrogens (Reguera et al. 2005). They directly transfer the electrons to the electrode surface (Lies et al. 2005). Also, they transfer electron through mediators, i.e., conductive appendages or nanowires (El-Naggar et al. 2010; Gorby et al. 2006). The electronic conductivity of these nanowires is higher than the synthetic metallic nanostructure (Malvankar et al. 2011). Few bacteria genera like Shewanella and Pseudomonas secrete some chemicals (like flavins). These chemicals transfer electrons from the bacteria to electrodes (Yang et al. 2012; Schroder 2007).
13.4.3 Oxygen Reduction Reaction (ORR)
The performance of MFC is influenced by the kinetics of the electrode reactions. The performance of the electrodes depends on the materials from which the electrodes are made. A wide range of materials have been tested to improve the performance of MFCs. Carbon-based nanomaterials, composite materials, and various transition metal oxides have emerged as promising materials for both anode and cathode constructions. These materials have the potential as alternatives to conventional expensive metals like platinum particularly for the oxygen reduction reaction (Mustakeem 2015).
Electrode materials play a key role in MFC. Carbon material like graphite can be used as both anode and cathode (Mashkour and Rahimnejad, 2015). The electrode material selection is vital for the performance of MFC. It determines the various factors of the MFC including bacterial adhesion, electron transfer, and electrochemical efficiency (Logan 2010). The biocompatibility of the electrode increases the property of bacterial adhesion. However, carbon-based materials have poor catalytic activity. An additional catalyst is required to increase the ORR of these materials (Mustakeem 2015).
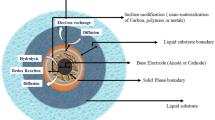
A low-level ORR at a neutral pH and low temperature reduces the MFC performance. In MFC, the ORR takes place at the three-phase interface of air (gas), electrolyte (liquid), and electrode (solid). The cathode has three layers, viz., diffusion layer, conducting support material, and catalyst. Though the identical materials are utilized to make anode and cathode, a robust cathode should have mechanical strength, catalytic property, and electronic and ionic conductivity properties at higher level. Figure 13.9a shows the ORR at the interface of gas, liquid, and solid. Figure 13.9b shows ORR at the three-phase interface of the cathode layers, i.e., catalyst layer, electrode base material, and oxygen diffusion layer (Mustakeem 2015).
Schematic diagram shows the cathode reaction . The reaction occurs at the triple-phase interface of air, solution, and catalyst. (a) Oxygen reduction reaction (ORR) at the interface of air, electrolyte, and electrode produces water as product. (b) ORR at three-phase interface of cathode layers. (From Mustakeem 2015)
A catalyst increases the ORR rate by decreasing the activation energy barrier (Lim and Wilcox 2012). In acidic medium, ORR occurs in two different electron pathways. The 4-electron and the 2-electron pathways are expressed in Eqs. 1 and 2 (Erable et al. 2012).
Various literatures report that MnO2-catalyzed cathode increases the ORR . The output power density of the MnO2 cathode is much higher than the cathode materials such as carbon black, carbon nanotubes (CNTs), graphite, and stainless steel. MnO2 is a two-dimensional (2D) material. Zhang et al. confirm that the MnO2 deposited over glassy carbon using polyvinylidene fluorine (PVDF) as binder performs similar to a platinum catalyst. In the ORR, initially, MnO2 is reduced to MnOOH by electrons. Oxidation occurs in the next step. The current density of ORR from MnO2 deposited cathode is ten times higher than the glassy carbon cathode . It indicates the increased ORR catalytic property of MnO2 (Zhang et al. 2009).
13.5 Nanomaterials in MFC
13.5.1 Carbon Nanomaterials in MFC
Carbon nanomaterials are highly conductive and mechanically stable with larger surface area and higher electrochemical catalytic activity (Ghasemi et al. 2013). Carbon nanomaterials can work both as anode and cathode. As the anode, carbon materials can promote microbial colonization and accelerate the formation of biofilms, thus increasing the power density. As the cathode, carbon-based materials can function as catalysts for the oxygen reduction reaction even reaching the performance of Pt catalysts (Li et al. 2017). The performances of different carbon materials like carbon felt, carbon cloth, and carbon paper are analyzed and compared. The power output depends not only on the material but also on the surface features. Based on this, carbon felt is superior in treating winery wastewater which is a highly organic-loaded waste to the other two materials (Penteado et al. 2017).
Pretreatment and modification of the anode improve the performance of the anode. The carbon brush is heated at various temperatures (300 °C, 450 °C, and 500 °C), and the performance of the MFC is analyzed. Figure 13.10a, b, and c show the results related to power density, electrode potential, and power curves of the preheated carbon brush anode. Figure 13.10a shows the maximum power densities obtained from the reactor with the 300 °C and 500 °C anode are 1160 ± 82 and 1149 ± 11 mW/m2, respectively. The 450 °C anode generates higher-power density, i.e., 1305 ± 67 mW/m2, at 6.1 ± 0.2 A/m2 current density. Figure 13.10b indicates that the cathode potentials remain consistent but variations occur in the anode potentials of different preheating anodes like power densities. As per Fig. 13.10c power density curves (results obtained from the linear sweep voltammetry analysis), the maximum power densities are 1450 (300 °C), 1607 (450 °C), and 1561 (500 °C) mW/m2 (Yang Qiao et al. 2017).
Carbon brush anodes of MFCs treated with different temperatures (300 °C, 450 °C, and 500 °C) by variable external resistance method. (a) Power densities, (b) electrode potentials, (c) power curves by linear sweep voltammetry. (From Yang Qiao et al. 2017)
Carbon nanomaterials are also prepared by carbonization of the biological materials at high temperatures (Yuan et al. 2013). Nitrogen-enriched carbon nanomaterials have potentials to prepare anode materials of MFC. The maximum power density obtained was found to be 1600 ± 50 mW m−2 when nitrogen-doped carbon nanotubes (NCNTs) are used as cathodic catalysts. Further the bamboo-shaped and vertically aligned NCNTs had lower internal resistance and higher cathode potentials (Feng et al. 2011).
Graphene-based composite materials are used as cathode in MFCs. As per report of Valipour et al., RGOHI-AcOH (highly conductive graphene material) cathode gives a power density of 1683 ± 23 mW/m2, and RGO/Ni (graphene/nickel) nanoparticle composite cathode gives a power density of 1015 ± 28 mW/m2. The catalytic activity of RGOHIHI-AcOH is mainly due to the high surface area and degree of graphitization. The double loading of catalyst offers stable power generation and long-term operation of MFCs (Valipour et al. 2016).
In MFCs, low hydrophilicity property of graphene adversely affects the performance of the graphene-modified anodes (G anodes). To elevate the hydrophilicity and performance of the graphene anode, different amounts (0.15 mg·mg−1 to 0.2 mg·mg−1 and 0.25 mg·mg−1) of graphene oxide (GO) are doped. In this case, the contact angle decreases considerably. The G anode (doped with GO 0.2 mg·mg−1) produces the static water contact angle (θc) value as 64.6 ± 2.75°. It exhibits the optimal performance. MFC with this anode generates the maximum power density (Pmax) 1100.18 mW·m−2 which is 1.51 times higher than the bare graphene anode. The results are shown in Fig. 13.11a and b. Also, it has COD removal efficiency, and coulomb efficiency (CE) higher than the other MFCs with GO-doped anode as well as G anode. It has COD removal efficiency 82.78 ± 0.45% and CE 33.76 ± 0.43%, but the MFC with G anode exhibits 79.69 ± 0.65% and 30.24 ± 0.46%, respectively (Yang Na et al. 2016b).
Graphene oxide (GO) doped graphene (G) anode. (a) Schematic diagram of G-GO anode and G anode with static water contact angle (θc) values. (b) Power densities and current densities of the MFCs with G and G-GO anodes. MFC with G-GO anode (0.2 mg·mg−1 GO doped) generates power density higher than the MFCs with G anode and other doped anodes. (From Yang Na et al. 2016b)
When carbon nanotubes are mixed with wastewater (10 mg L−1 to 200 mg L−1 concentration), it produces positive effect on power generation. It stimulates the power generation due to the increased conductivity of the MWCNTs. Also COD removal efficiency is also enhanced from 74.2% to 84.7% (Miran et al. 2016). When compared to the conventional carbon cloth electrode, the intertwined CNT-textile fibers have larger biofilm. This film facilitates electron transfer from exoelectrogens to the CNT-textile anode (Xie et al. 2010). Graphene and CNT are coated on carbon cloth and stainless steel mesh electrodes to improve their performance (Tsai et al. 2015; Hsu et al. 2017).
High-strength wastewaters such as landfill leachate contains dissolved extracts and suspended matter. When it is treated with MFC, ammonia is separated from the leachate. Activated carbon performs well in this treatment as anode. The percentages of ammonia removal of activated carbon, zinc, and black carbon are found to be 96.6%, 66.6%, and 92.8%, respectively (Alabiad et al. 2017). In MFC, graphite nanofibers with diameter 6–10 μm also work as an efficient anode. Their surface area to volume ratio is 15,000 m2/m3. The activated carbon air-cathode is an alternative for Pt-based cathode. While utilizing it as cathode, it works almost equal to the platinum catalyst and performs well for long duration.
It is observed that coating of nanomaterials like multilayer graphene (MG) and CNTs on the stainless steel mesh (SSM) electrode improves the power density and reduces the internal resistance of a MFC system. The surface modification of anode or cathode with CNTs and graphene increases the power generation by approximately 3–7 and 1.5–4.5 times, respectively. When comparing to the MFC with an untreated anode, the internal resistances of MFCs with CNTs and MG-modified anodes are in the reduced level, i.e., 18 and 30%, respectively. SSM cathode surface modification is done by the coating of MG and MWCNTs mixed PTFE solution. SEM images of the MG and MWCNTs mixed PTFE are shown in Fig. 13.12a and Fig. 13.12b, respectively. It is observed from these figures that the surface roughness of the MWCNTs mixed PTFE is more than the MG mixed PTFE (Hsu et al. 2017).
SEM images (5000× and 10,000× magnification) of cathodes covered with treated PTFE . (a) PTFE mixed with multilayer graphene. (b) PTFE mixed with MWCNTs. (From Hsu et al. 2017)
Carbon cloth electrodes are modified with carbon nanotube and graphene to investigate the performance of single-chamber MFC. In this investigation, Escherichia coli HB101 is used as catalyst in an air-cathode MFC. It is observed from the results that the carbon cloth electrodes modified with both materials, i.e., CNT and graphene, improve the power density of MFCs. The internal resistance of normal electrodes decreases dramatically from 377 kΩ to 5.6 kΩ (while using both electrodes modified by graphene with a cathodic catalyst). Among the all electrodes, graphene-modified electrode exhibits superior performance. When comparing to the modified cathode, the modified anode exhibits greater performance (Tsai et al. 2015).
The single-chamber MFC is fabricated using polymethyl methacrylate (PMMA) which is shown in Figure 13.13a. The cathode electrode is fixed to the air side , and the anode electrode is fixed opposite side. The reactor volume is 75 mL and the surface area of electrode is 12.57 cm2. SEM images of MWCNTs and graphene coated on the carbon cloth are shown in Fig. 13.13b, c, respectively. Both materials are uniformly distributed on the carbon cloth which improves the specific surface area of the electrode. Specific surface area of graphene-coated electrode is two times more than the CNT-coated electrode (Tsai et al. 2015). This improved specific surface area leads to the superior performance of graphene-coated electrode.
Schematic diagram of air-cathode MFC : (a) The single-chamber MFC has PMMA chamber as the air-cathode, modified or unmodified carbon cloth as electrodes, glucose as fuel, and Escherichia coli as anode’s catalyst. Copper wires are used to connect with external resistance. (b) and (c) SEM images of MWCNTs and graphene coated on the carbon cloth, respectively. (From Tsai et al. 2015)
Metals and carbon felt are used as electrodes. Metals rapidly corrode, and carbon felt is porous and prone to clogging. So, the carbon felt is replaced with paper coated with carbon paste. Lamberg and Bren reported about the carbon paste paper electrodes (CPPEs). These electrodes are fabricated by coating of carbon paste on a paper strip followed by polyaniline coating. The carbon paste is made by the mixture of graphite powder and mineral oil. The CPPEs are assessed as anodes in bioelectrochemical cells (BECs). In this assessment, Shewanella oneidensis MR-1 bacteria is utilized to donate the electrons through extracellular electron transfer. When comparing to the polyaniline-modified carbon felt electrode (CFE), the BEC using the CPPE anode works better. It generates a current density (maximum value of 2.2 A m−2) after 24 hours of the inoculation. It is two times more than the BEC with CFE anode. It generates current continuously 4 days without the need for additional fuel (lactate). It is confirmed from this assessment that CPPE is a simple, low-cost, and promising new bioelectrode material for microbial fuel cells (Lamberg and Bren 2016).
13.5.2 Nanocomposites in MFC
To improve the cathode kinetics of MFC , activated carbon (AC), graphene nanosheet (GNS), and iron-aminoantipyrine (Fe-AAPyr), catalyst materials are integrated as an alternative cathode catalyst material. The air-breathing cathode made with GNS and Fe-AAPyr materials generates higher power. The power generated from the MFC utilizing these cathode materials is enumerated in Table.13.1. Figure 13.14a, b shows the SEM images of Fe-AAPyr material and graphene nanosheet, respectively (Kodali et al. 2018).
SEM images of cathode catalyst materials. (a) Iron-aminoantipyrine (Fe-AAPyr) catalyst (b) Graphene nanosheets. (From Kodali et al. 2018)
Anode is prepared by using materials like conductive polymers and carbon nanomaterial composites. Qiao Yan and co-workers tested a composite of carbon nanotube and polyaniline in the electrochemical impedance spectroscopy. In this test, Escherichia coli bacteria are utilized as the microbial catalyst with 20 wt. % CNT composite anode. The obtained power density (maximum) is 42 mW m−2 (Qiao et al. 2007).
Deng et al. explain the interaction between the exoelectrogens and their induced GO reduction in the high-performance MFC application . During the in situ preparation of graphene/exoelectrogen composite biofilm electrode, graphene oxide is reduced by E. coli bacteria. This reduction provides a rough surface and three-dimensional structure to the electrode which leads to larger specific surface area for microbes to settle. Graphene-modified anode increases the height of biofilm thickness largely. The increased level of the total protein in microbial reduction of graphene oxide (mrGO)-modified anode demonstrates the attachment of more bacteria on the anode surface. After five electricity production cycles, the power density of the mrGO-modified MFC reaches a maximum value of 1140.63 mW m−2 (Deng et al. 2017).
Two-dimensional porous anodes have small pore sizes. So, bacteria clog on the surface and are inaccessible to the interior of the anode. This seriously limits the anode efficiency. The problems associated with the 3D structures include low specific surface area (due to lack of microscopic or nanoscopic structures), small pore sizes for bacteria penetration, poor conductivity, and disruption of bacterial membrane by sharp nanomaterials. While using the novel 3D macroporous anode, these problems are avoided. This anode is designed by the freestanding, flexible, conductive, and monolithic graphene foam decorated with the conductive polymer, i.e., PANI. The 3D graphene is synthesized by chemical vapor deposition with nickel foam as the substrate and using ethanol as the carbon source. The pore size of graphene foam is much larger than the size of bacteria (1–2 μm). Hence, bacteria can easily diffuse inside and colonize. The MFC equipped with carbon cloth anode generates the power density ∼110 mW/m2 at 6 h. MFC (with 3D graphene/PANI foam anode) generates the same power density at 6 h, but at 24 h, the power density is higher than at 6 h, i.e.,190 mW/m2 (Yong et al. 2012).
13.5.3 Biogenic Inorganic Nanoparticles
Nanomaterials are having more surface area which allows a very good adhesion of microorganisms at the anode. Some biogenic inorganic nanoparticles facilitate extracellular electron transfer in MFCs. Jiang et al. studied the performance of MFC with iron sulfide nanoparticles and Shewanella bacteria. In this study, it is observed that iron sulfide nanoparticles are in intimate contact with the cell membrane by uniform coating. The charge transport occurs in the presence of live Shewanella. It improves the electron transfer at cell/electrode interface and the cellular networks which leads to the enhanced current output (Jiang et al. 2014). Nano-CeO2 is utilized for the modification of the carbon felt anode in the MFC. The modified anode obtains the higher closed circuit voltage resulting from the lower anode potential. The MFC with nano-CeO2-modified carbon felt anode generates maximum power density 2.94 W m−2 with lower internal resistance 77.1 Ω (Yin et al. 2016).
In MFCs, the electrodes should be immersed in electrolytes in neutral pH. In such conditions, Au core- and Pd shell-type bimetallic nanoparticles exhibit better ORR due to the enhanced catalytic properties. The lattice strain produced in between the core and shell regions leads to the enhanced catalytic properties. The maximum power density produced in the membrane less single-chamber MFC using wastewater and Au-Pd core-shell cathode catalysts is ca. 16 W m−3. It is stable for more than 150 days. Optimization of Au core size and Pd shell thickness enhances the core-shell properties. Au-Pd core-shell analyses results and images related to the single-chamber MFC are shown in Figs.13.15 and 13.16, respectively (Yang Gaixiu et al. 2016a).
Images of Au-Pd core-shell prepared in oleylamine at elevated temperature. (a) TEM image. Insert HRTEM image of a single Au-Pd particle. (b) STEM image. (c) EDX analysis. (d) Elemental profiles in STEM mode. (e–h) Nanoscale element mappings of (e) the formation of core-shell Au-Pd structure and (f–h) distributions of Au, Pd, and bimetallic nanoparticles. (From Yang Gaixiu et al. 2016a)
Construction of single-chamber MFC to assess the ORR catalytic properties of the bimetallic core-shell Au-Pd nanoparticles. (a) The practical construction. (b) Schematic illustration shows the parts of single-chamber MFC (carbon cloth cathode, carbon felt anode, silicon gasket, sample chamber, and sampling ports). (From Yang Gaixiu et al. 2016a)
The performance of MFC using core-shell Au-Pd nanoparticle cathode is compared with the MFC using hollow Pt nanostructure air-cathode catalyst. When comparing the performance of both cathode catalysts, the core-shell Au-Pd cathode shows better performance. It has higher ORR catalytic performance. The maximum power density and cathode voltages of core-shell Au-Pd nanoparticles are higher than the hollow structured Pt cathode. The maximum power density of the core-shell Au-Pd cathode and the hollow structured Pt cathode are 16.0 W m−3 and 7.1 W m−3, respectively. The observed results clearly manifest that the core-shell Au-Pd nanoparticle cathode is an alternative to the Pt-based cathode catalysts. These nanoparticles can be used in MFC for the production of electricity without losing power efficiency and stability. Figure 13.17a, b shows the power density and polarization curves of both cathodes, respectively (Yang Gaixiu et al. 2016a).
Performance graph of MFC using core-shell Au-Pd nanoparticles cathode and MFC using hollow Pt nanostructures air-cathode catalyst. (a) Power density comparison of the both cathodes. (b) Electrode polarization (voltage) graph. (From Yang Gaixiu et al. 2016a)
MFC is a renewable and clean energy-generating system . Bacterial growth is the most important thing in this system. Composts rich in organic substances are commonly used for such growth. In addition, non-inert (metal) and inert (usually carbon-based) electrodes are used in MFC. In this study, zinc anode is evaluated for MFC, and it is observed that zinc is a corrosion-resistant material. Compost mixed with MFC having zinc anode and graphite cathode produces high-power density (5.33 W/m2). According to the measurements and calculations of this study, zinc has promising electrode technology with good electrochemical and biochemical performances (Nurettin 2017). Various metal oxide nanoparticles like titanium dioxide and iron oxide along with conducting polymer like polyaniline are utilized as bioanodes in MFC.
Anodic electron transfer is the main process of electricity generation in MFCs. The anodic biofilm formation and electron transfer can be accelerated by adding biosurfactants to the anolyte. Rhamnolipid biosurfactant with the quantity of 40 mg/L, 80 mg/L, and 120 mg/L is added to the anolyte. It increases the abiotic capacitance from 15.12 F/m2 (control) to 16.54 F/m2, 18.00 F/m2, and 19.39 F/m2, respectively. After 7th day of the inoculation, anodic biofilm formation is facilitated for dosing 40 mg/L of rhamnolipids, with anodic biofilm coverage from 0.43% to 42.51% and the power density from 6.92 ± 1.18 W/m3 to 9.93 ± 0.88 W/m3. Rhamnolipid concentration with 80 mg/L and 120 mg/L blocks the electron transfer. This analysis reveals that rhamnolipids facilitate the enrichment of exoelectrogen (Zhang et al. 2017b).
Nanostructured hexagonal klockmannite copper selenide (CuSe) grown on the hybrid material exhibits the superior ORR catalytic performance. It also has more positive onset potential, higher current density, smaller Tafel slope, and excellent stability. The hybrid material is the mixture of reduced graphene oxide (rGO) and carbon nanotubes. MFC equipped with CuSe@rGO-CNTs cathode exhibits larger energy output than the MFC with carbon-supported platinum (Pt/C) catalyst (Tan et al. 2016).
13.6 Microfabrication in MFC
Microfabrication technology is employed in semiconductor manufacturing industries for thin film deposition, photolithography, etching, microelectromechanical system, and lab-on-a-chip toward batch fabrication with low expense and precisely controlled geometry. Miniaturized MFC in lab-on-a-chip devices find applications in biosensors for toxic chemical detection (Ren and Chae 2015). Micro-sized MFCs are miniature energy scavengers. They can be useful power sources for lab-on-a-chip applications and integrated onto chips for low-power electronic devices or sensors (Rojas and Hussain 2015).
Miniaturized energy-harvesting devices can be built using advanced microfabrication techniques. Micro-sized MFC is constructed using single-chamber MFC concept with an air-cathode. In this system, proton-exchange membrane is removed. MFCs are fabricated as system-on-chip functionality. This paves the way for MFC applications in sensors, watches, and mobile phones. Ni, Au, and MWCNT as anode are designed with air-cathode (Mink and Hussain 2013).
The micro-sized MFC has advantages like utilization of less electrode area and less liquid fuel volume. Justine E Mink et al. (2014) fabricate a mobile and inexpensive micro-sized MFC that using human saliva as fuel. This 25 μl MFC has graphene as an anode for efficient current generation and an air-cathode for enabling the use of the oxygen present in air. This system makes the entire operation completely mobile without using any laboratory chemicals. It produces the higher current densities of 1190 Am−3. The graphene anode generated 40 times power more than carbon cloth anode. Also, test results (using acetate as organic material instead of saliva) demonstrate a linear relationship between the organic loading and current. Findings of this report lead to the applications of saliva-powered fuel cell technology for lab-on-a-chip devices or portable point-of-care diagnostic devices (Mink et al. 2014).
Micro-sized MFC can be utilized as an ovulation predictor based on the conductivity of a woman’s saliva. It is observed that before the 5 days of ovulation period, a sharp decrease in the conductivity of saliva occurs. It is caused by a high level of estrogen and low level of electrolyte concentration in saliva (Huang et al. 1997). This micro-sized MFC analyzes the conductivity changes of saliva which help to identify the fertility period and to maintain woman’s health. Also, it helps for better family planning in a noninvasive method .
MFC can be used as a potential power source for implanted bioMEMS devices. The MFC is biocatalyzed by Saccharomyces cerevisiae. This microorganism converts chemical energy stored in glucose of the blood stream. The MFC has 0.2 μm thickness gold evaporated polydimethylsiloxane (PDMS) anode and cathode separated by a Nafion 117 proton-exchange membrane. MFC with this type of micropillar structure shows excellent performance than silicon micro-machined MFCs, i.e., 4.9 times higher average current density and 40.5 times higher average power density. The MFC uses 15 μL of human plasma containing 4.2 mM glucose. It produces maximum open-circuit potential (OCP) of 488.1 mV, maximum current density of 30.2 μA/cm2, and a maximum power density of 401.2 nW/cm2. During continuous operation for 60 minutes, it produces an average OCP of 297.4 mV, average current density of 4.3 μA/cm2, and average power density of 42.4 nW/cm2 at 1 k Ω load. The coulombic efficiency of electron conversion from blood glucose is 14.7% (Chiao 2008).
13.7 Wastewater Treatment in MFC
MFC technology utilizes wastewater effectively to generate energy (Logan and Regan 2006a, b). Agrowaste materials produced during various agricultural operations are rich in COD. Some of them are useful in bioelectricity generation as well as wastewater treatment. MFC can be utilized for Cr(VI) wastewater treatment. It is observed from the reports that the MFC system that has mixed cultures of bacteria yields output better than the MFC system with single-culture bacteria. Also, reports indicate that cassava mill wastewater has potential to generate electricity from MFCs. During the wastewater treatment process using microorganisms, clean energy, i.e., hydrogen production , is also possible.
Wastewater from paper industries contains water-insoluble materials such as cellulose . The cellulosic waste materials are the attractive source of energy for electricity production in MFCs. However, this process requires anaerobes that can degrade cellulose and transfer electrons to the electrode (exoelectrogens). MFC with two-chamber system avoids oxygen contamination of the anode. Single-chamber MFC with air-cathode produces higher-power densities than aqueous catholyte MFC due to less internal resistance. Also, it avoids energy input for the cathodic reaction. While examining the changes in the bacterial consortium in a single-chamber, air-cathode MFC fed cellulose, it is observed that the main genera developed after extended operation of the MFC are Parabacteroides, Proteiniphilum, Clostridium, and Catonella. These results confirm that different bacteria evolve in single-chamber air-cathode MFC than the two-chamber reactors. Details of bacteria abundance in MFC are in Fig. 13.18. Polarization and power density curves obtained from the MFC are illustrated in Fig. 13.19 (Toczyłowska-Maminska et al. 2018).
Relative abundance of dominating bacteria genera in fresh inoculum and in single-chamber, air-cathode MFC fed cellulose system (after the operation). (From Toczyłowska-Maminska et al. 2018)
Polarization and power density curves of single-chamber air-cathode cellulose-fed MFC. After 1 month of the MFC operation, the maximum current produced is 331 mA/m2 (R = 100 Ω), and the maximum power produced is 44 mW/m2 (R = 1000 Ω). (From Toczyłowska-Maminska et al. 2018)
The amount of energy needed for the treatment of wastewater is very high in the present situations. MFC can be a very good solution because the energy generated is sufficient for the system to run. Yue Dong et al. reported that energy self-sufficiency is essential for the sustainable wastewater treatment. They combine a microbial fuel cell and an intermittently aerated biological filter (MFC-IABF) to treat the effluent with self-sufficient energy. Schematic diagram of the MFC-IABF system and its wastewater treatment performance are shown in Figs. 13.20 and 13.21, respectively (Dong Yue et al. 2015).
Schematic diagram of (a) MFC reactor for electricity generation and COD removal. (b) Two-stage combined MFC-IABF system. This combined system operates with self-sufficient energy and treats the wastewater more efficiently than MFC reactor. Air is flowed inside the IABF system by the aerator. (From Dong Yue et al. 2015)
Wastewater treatment performance of MFC and two-stage combined MFC-IABF system . The value inside the bars indicates the SCOD and TCOD removal rate (influent COD concentration is 1000 mg/L). (From Dong Yue et al. 2015)
In the combined MFC-IABF system , synthetic wastewater (COD = 1000 mg L−1) is fed continuously for more than 3 months using a capacitor-based circuit. This system is operated at room temperature. As the output of this work, the MFC generates electricity and supplies to IABF along with COD removal. It is observed that the MFC produces energy (0.27 kWh m−3) which is sufficient to the pumping system (0.014 kWh m−3) and aeration system (0.22 kWh m−3). The IABF works in the intermittent aeration mode (aeration rate 1000 ± 80 mL h−1), removes the wastes, and improves the water quality (HRT = 7.2 h). This combined system removes SCOD 93.9% and TCOD 91.7% (effluent SCOD = 61 mg L−1 and TCOD = 82.8 mg L−1). These results confirm that the combined MFC-IABF system operates in an energy self-sufficient manner and treats the wastewater efficiently (Dong Yue et al. 2015).
Ren et al. have obtained the maximum power density 143 mW/m2 with 1 g/L carboxymethyl cellulose (CMC) from a dual-chambered MFC. In this process, a binary culture of cellulose-degrading bacteria Clostridium cellulolyticum and electrochemically active bacteria Geobacter sulfurreducens is utilized (Ren et al. 2008). Rezaei et al. have enriched the cellulose-degrading bacteria Enterobacter cloacae strain FR in MFC with wastewater. This MFC produces maximum power density of 4.9 mW/m2 using cellulose 4 g/L (Rezaei et al. 2009). Sedky et al. have utilized cellulose as substrate in a MFC. In this setup, cellulose-degrading bacteria Nocardiopsis sp. KNU and Streptomyces enissocaesilis KNU are employed for cellulose degradation in anode. The cathode has 50 mM ferricyanide. The maximum power density produced from this culture is 188 mW/m2 consuming 1 g/L cellulose (Hassan et al. 2012).
A high amount of starch-rich wastewater is released during the starch production from cassava. It has high chemical oxygen demand (COD), biochemical oxygen demand (BOD), total solids, and cyanoglycosides. These cyanoglycosides form cyanide. Many wastewaters have cyanide concentration up to 200 mg/L. Hence, proper treatment of the cassava wastewater is essential prior to its release into the environment. Cassava wastewater sludge has a high organic content of 16,000 mg/L. MFC utilizes cassava wastewater, removes COD approximately 88% within 120 h, and generates maximum power 1771 mW/m2 (Kaewkannetra et al. 2009). Prasertsung et al. (2012) have identified that increasing the pH of anode chamber in a MFC increases the production of electricity. The generated maximum power density was 22.19 W/m3 at pH 9 from a single-chambered MFC that was used with cassava mill wastewater. COD of the wastewater was 1086 mg/L, and the initial pH was 5.0 (Prasertsung et al. 2012).
Chromium has industrial applications like leather tanning, metallurgy, electroplating, and wood preservatives. It exists in the aqueous solution either as hexavalent chromium [Cr(VI)] or trivalent chromium [Cr(III)]. Cr(VI) is considered as more hazardous material because of its mutagenic and carcinogenic properties (Humphries et al. 2004). Hence, Cr(VI) wastewater treatment can be coupled with electricity generation using MFC. In this method, Cr(VI) is reduced in the cathode of an MFC by the microorganisms Trichococcus pasteurii and Pseudomonas aeruginosa. Acetate and bicarbonate are added into the anode and cathode compartments, respectively. Cr(VI) is reduced by microbial activity utilizing the electrons and protons generated from the oxidation of acetate. It generates current and power density of 123.4 mA/m2 and 55.5 mW/m2, respectively (Tandukar et al. 2009).
A more efficient biocathode is designed with reticulated vitreous carbon (RVC) and carbon nanotube (CNT) to use in MFC for Cr(VI) removal application. It is prepared by the electrophoretic deposition of CNT on RVC. The material RVC is cheap and commercially available. It is an open-pore foam carbon material that is used in MFCs. The approximate power density of the MFC using RVC-CNT electrode is 132.1 ± 2.8 mWm−2. This device removes 80.9% of Cr(VI) within 48 h of the operation (Fei et al. 2017).
MFC can be used for the production of catholyte within the reactor and for recovering of nitrogen from wastewater (shown in the Fig. 13.22). An electrolyzed basic solution (pH > 11) is produced from the cathode chamber, and the production rate is largely proportional to electrical current generation. This catholyte possess bactericidal properties. The bactericidal effect is confirmed using bacterial kill curves constructed by exposing a bioluminescent Escherichia coli target. The catholyte solution has cleaning properties. It reduces the microbial populations and limits undesired biofilm formation. Hence, it can be utilized as a washing agent in waterless urinals to improve sanitation. The demonstrated self-driven MFC system leads to the development of bioprocesses for sustainable wastewater treatment (Gajda et al. 2016).
Illustration of catholyte production from wastewater treatment using MFC. (a) Photograph shows the formation of droplets and accumulation catholyte solution inside the MFC cylinder. (b) Image shows the gas diffusion side of the cathode of working MFC (loaded) and open-circuit MFC (control). Biofilm growth is present only on the open-circuit MFC, i.e., that do not produce electricity. This indicates that the catholyte inhibits the growth of microorganisms and prevents the biofouling of cathode as well as the membrane. (From Gajda et al. 2016)
13.8 Challenges in Microbial Fuel cells
MFCs have advantages like production of low-cost electricity from waste materials all-round the year (particularly in places where energy production plants are not available) and alternate method for bioremediation. However, they face some challenges. Utilizations of waste material in MFCs produce power density lower than pure carbon sources such as glucose. It is not possible for the utilization of pure sources routinely because it will increase the cost. These challenges hinder the commercialization of MFCs (Chaturvedi and Verma 2016). Also, the economically noncompetitive and high-cost components of MFCs are the major barriers for commercialization.
Some other drawbacks of MFCs are less surface area of the anode, inefficient electron transfer, proton mass transfer, poor oxygen reduction rate, low-power generation, lack of modularity, short life span of biochemical substrate, and very slow performance in wastewater treatment. The anode material should have more surface area and more affinity for microorganisms. The electron transfer mechanism of the anode materials is not understood well. Utilizations of materials like platinum as electrode increases the cost of MFC to higher end. Microorganisms are also one of the great challenges of MFC. The disadvantage of using mixed culture in a MFC is that it may contain pathogenic bacteria. Also, some bacteria may be sensitive to different kinds of stress. As per the report of Joseph Miceli, ARB helps to flow the electrons freely in between the electrodes (Waste-management-world.com 2013).
Materials such as nickel foam, stainless steel wool, platinum-coated stainless steel mesh, and molybdenum disulfide-coated stainless steel mesh electrodes are used as alternative to commercially non-viable electrode material like platinum (Ma Xiaoli et al. 2017). To bring the MFC technology out of the laboratory (for energy production at larger scales), pilot-scale tests are performed. These are good indicators that commercialization of this technology is possible (Logan 2010).
The power output is low in MFCs because of their high internal resistance. Also, it is lower than chemical fuel cells. The internal resistance is due to the proton mass transfer as well as the poor oxygen reduction rate at the cathode. Aerobic bacteria have higher affinity for oxygen than the abiotic cathode materials. Oxygen reduction kinetics and performance of the MFC is improved while using a cathode with aerobic bacteria (including the bacteria developing the corroding biofilm). Addition of the inorganic compounds into the anode chamber as nutrients and development of proton-specific membrane lead to inhibit proton transfer. MFCs should be optimized for its reactor configuration and electrolyte to reduce the internal resistance and to harvest the entire microbial catalytic potential (Kim et al. 2007).
To demonstrate MFC as power generator to supply power to the electronic systems, there is a possibility to use solid-phase organic matter at the anode. Sediment MFCs are designed from marine sediment to utilize as the source of bacteria and organic matter. The synthetic solid anolyte (SSA) is made by dissolving of agar, carbonaceous, and nitrogen sources into diluted seawater. This long-lasting portable SSA-MFC (shown in Fig. 13.23) overcomes problems like hydraulic pump system and biochemical substrate replacement (to sustain bacteria metabolism). It generates the maximum power density 60 mW/m2 on the 7th day of the operation. It has 4 months lifetime without the need of electrolyte replacement and human assistance (Tommasi et al. 2016).
Photograph of SSA-MFC . (a) Two-chamber MFC. The inset shows the SSA in the anode chamber. The anode is sandwiched between the SSA and CEM membrane and immersed in the buffer solution. (b) Photograph of the agar-based SSA embedded with energy storage system for bacteria metabolism. (c) Molecular structure of agar. (From Tommasi et al. 2016)
13.9 Influencing Factors of MFC Output
It is analyzed from the various reports that the output of the MFC depends on factors like substrates, concentration of waste, electrodes, pH, chamber construction, proton-exchange membrane, microorganism species, and their culture types. Some of the factors influence the performance of MFC. They are enumerated below.
In order to enhance the power generation of MFC:
-
Increasing the pH of anode chamber (low ORR occurs at neutral pH)
-
Increasing the electrons flow
-
Increasing the ORR (catalyst increases the ORR rate).
-
Increasing the concentration of organic waste materials (biofuels)
-
Reducing the proton transfer
-
Reducing internal resistance of MFC (by coating of nanomaterials like graphene and carbon nanotubes)
-
Addition of the inorganic nutrients into the anode chamber
-
Development of proton-specific membrane
The factors mentioned below help to improve the overall performance of MFC:
-
Single-chamber air-cathode MFC supports for bacteria development better than the two-chamber MFC.
-
Utilization of mixed bacteria cultures instead of single-culture bacteria increases the MFC output.
-
Growing of anaerobic bacteria in anode chamber. It helps for electrons flow which leads to enhance the MFC performance.
-
Utilization of cathode with aerobic bacteria inhibits proton transfer. It leads to improve the oxygen reduction kinetics as well as MFC performance.
-
Biocompatible electrode increases the adhesion of bacteria which improves the MFC performance.
-
Temperature maintenance is essential (low temperature reduces the MFC performance).
13.10 Conclusion
MFC is a novel wastewater treatment device with energy recovery from the waste. It mimics the interactions of microbes present in the nature. It converts chemical energy into electricity using microorganisms. The energy production mechanism of the MFC resembles the metabolism process by the gut flora. MFC has great potential in alternative energy source, wastewater treatment, environmental protection, bioremediation, and biosensor for oxygen and pollutants. In addition to the various applications, MFCs have certain drawbacks.
The drawbacks hinder in the practical applications of MFCs. Hence, extensive optimization is required for the efficient and wide applications of MFCs and to obtain the maximum microbial potential from them. Production of low-power density is the major drawback. It is rectified by using various nanomaterials and potent microorganisms. Some of the microorganisms have been identified that can transfer the electrons efficiently. Also, limited surface area of the electrodes prevents the microorganisms to adhere. Utilizations of nanomaterials increase the surface area of the electrodes.
Nanomaterials such as carbon-based materials, transition metal oxides, biogenic inorganic nanoparticles, and nanocomposites are widely used in MFCs due to their properties like high conductivity, biocompatibility, and chemical stability. Apart from these factors, they are utilized as alternative materials to reduce the high cost of components. Many methods have been identified to improve the performance of MFCs and to reduce the cost for feasible implementation of MFCs in commercial applications. Although MFCs face some challenges, there are good scopes and future prospects for them.
Abbreviations
- 2D:
-
Two dimension
- 3D:
-
Three dimension
- AC:
-
Activated carbon
- Am−2:
-
Ampere per meter square (unit for current density)
- Am−3:
-
Ampere per cubic meter (unit for current density)
- ANB:
-
Acid navy blue r dye
- ARB:
-
Anode-respiring bacteria
- Au:
-
Gold
- BECs:
-
Bioelectrochemical cells
- bioMEMS:
-
Biomedical microelectromechanical systems
- BOD:
-
Biochemical oxygen demand
- CE:
-
Coulomb efficiency
- CeO2:
-
Ceric oxide (or) ceric dioxide (or) ceria (or) cerium oxide (or) cerium dioxide (or) cerium(IV) oxide
- CFE:
-
Carbon felt electrode
- CMC:
-
Carboxymethyl cellulose
- CNTs:
-
Carbon nanotubes
- COD:
-
Chemical oxygen demand
- CPPEs:
-
Carbon paste paper electrodes
- Cr(III):
-
Trivalent chromium
- Cr(VI):
-
Hexavalent chromium
- CS:
-
Chitosan
- e−:
-
Electrons
- EDX:
-
Energy dispersive X-Ray analyzer
- Fe-AAPyr:
-
Iron-aminoantipyrine
- GNS:
-
Graphene nanosheet
- GO:
-
Graphene oxide
- H+ ions:
-
Protons
- HRTEM:
-
Transmission electron microscope
- kΩ:
-
Kiloohm (unit for resistance)
- MFC:
-
Microbial fuel cells
- MnO2:
-
Manganese oxide (or) manganese dioxide (or) manganese(IV) oxide
- MnOOH:
-
Hydroxy-oxido-oxomanganese
- mV:
-
Millivolt (unit for potential)
- MWCNTs:
-
Multiwall carbon nanotubes
- mW/m2:
-
Milliwatts per square meter (unit for power density)
- Ni:
-
Nickel
- nm:
-
Nanometer
- nW/cm2:
-
Nano-watts per square centimeter (unit for power density)
- ORR:
-
Oxygen reduction reaction
- PA:
-
Phosphoric acid
- PANI:
-
Polyaniline
- Pd:
-
Palladium
- PDMS:
-
Polydimethylsiloxane
- PEM:
-
Proton-exchange membrane
- pH:
-
Power of hydrogen
- PMMA:
-
Polymethyl methacrylate
- POE:
-
Poly(oxyethylene)
- Pt:
-
Platinum
- PTFE:
-
Polytetrafluoroethylene
- PVA:
-
Poly(vinyl alcohol)
- PVAc-g-PVDF:
-
Polyvinyl acetate-polyvinylidene fluoride coated cotton fabric
- rGO:
-
Reduced graphene oxide
- SCOD:
-
Soluble chemical oxygen demand
- SEM:
-
Scanning electron microscope
- STEM:
-
Scanning transmission electron microscope
- TCOD:
-
Total chemical oxygen demand
References
Alabiad I, Ali UFM, Zakarya IA, Ibrahim N, Radzi RW, Zulkurnai NZ, Azmi NH (2017) Ammonia removal via microbial fuel cell (MFC) dynamic reactor. In: IOP conference series: materials science and engineering, vol. 206, no. 1. IOP Publishing, p 012079
Buzea C, Pacheco II, Robbie K (2007) Nanomaterials and nanoparticles: sources and toxicity. Biointerphases 2(4):MR17–MR71
Catal T, Bermek H, Liu H (2009) Removal of selenite from wastewater using microbial fuel cells. Biotechnol Lett 31(8):1211–1216
Chandrasekhar K, Kadier A, Kumar G, Nastro RA, Jeevitha V (2018) Challenges in microbial fuel cell and future scope. In: Microbial fuel cell. Springer, Cham, pp 483–499
Chang IS, Moon H, Jang JK, Kim BH (2005) Improvement of a microbial fuel cell performance as a BOD sensor using respiratory inhibitors. Biosens Bioelectron 20(9):1856–1859
Chaturvedi V, Verma P (2016) Microbial fuel cell: a green approach for the utilization of waste for the generation of bioelectricity. Bioresour and Bioprocess 3(1):38
Chiao M (2008) A microfabricated PDMS microbial fuel cell. J Microelectromech Syst 17(6):1329–1341
Chouler J, Bentley I, Vaz F, O’Fee A, Cameron PJ, Di Lorenzo M (2017) Exploring the use of cost-effective membrane materials for Microbial Fuel Cell based sensors. Electrochim Acta 231:319–326
Chu S, Majumdar A (2012) Opportunities and challenges for a sustainable energy future. Nature 488(7411):294
Daniel DK, Mankidy BD, Ambarish K, Manogari R (2009) Construction and operation of a microbial fuel cell for electricity generation from wastewater. Int J Hydrog Energy 34(17):7555–7560
Deng F, Sun J, Hu Y, Chen J, Li S, Chen J, Zhang Y (2017) Biofilm evolution and viability during in situ preparation of a graphene/exoelectrogen composite biofilm electrode for a high-performance microbial fuel cell. RSC Adv 7(67):42172–42179
Dharmadhikari S, Ghosh P, Ramachandran M (2018) Synthesis of proton exchange membranes for dual-chambered microbial fuel cells. J Serb Chem Soc 83(5):611–623
Dong Y, Feng Y, Qu Y, Du Y, Zhou X, Liu J (2015) A combined system of microbial fuel cell and intermittently aerated biological filter for energy self-sufficient wastewater treatment. Sci Rep 5:18070
Eldridge T (2014) Achieving industry integration with nanomaterials through financial markets. http://www.nanotech-now.com/columns/?article=835. Accessed 21 Nov 2018
El-Naggar MY, Wanger G, Leung KM, Yuzvinsky TD, Southam G, Yang J, Lau WM, Nealson KH, Gorby YA (2010) Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. Proc Natl Acad Sci 107(42):18127–18131
Erable B, Féron D, Bergel A (2012) Microbial catalysis of the oxygen reduction reaction for microbial fuel cells: a review. ChemSusChem 5(6):975–987
Ersan K, Irfan AR, Tukek S (2010) Effect of humidification of gases on first home constructed PEM fuel cell stack potential. Gazi University J Sci 23(1):61–70
Fei K, Song TS, Wang H, Zhang D, Tao R, Xie J (2017) Electrophoretic deposition of carbon nanotube on reticulated vitreous carbon for hexavalent chromium removal in a biocathode microbial fuel cell. R Soc Open Sci 4(10):170798
Feng L, Yan Y, Chen Y, Wang L (2011) Nitrogen-doped carbon nanotubes as efficient and durable metal-free cathodic catalysts for oxygen reduction in microbial fuel cells. Energy Environ Sci 4(5):1892–1899
Gajda I, Greenman J, Melhuish C, Ieropoulos IA (2016) Electricity and disinfectant production from wastewater: microbial fuel cell as a self-powered electrolyser. Sci Rep 6:25571
Ghasemi M, Daud WRW, Hassan SH, Oh SE, Ismail M, Rahimnejad M, Jahim JM (2013) Nano-structured carbon as electrode material in microbial fuel cells: a comprehensive review. J Alloys Compd 580:245–255
Ghasemi M, Halakoo E, Sedighi M, Alam J, Sadeqzadeh M (2015) Performance comparison of three common proton exchange membranes for sustainable bioenergy production in microbial fuel cell. Procedia CIRP 26:162–166
Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, Beveridge TJ, Chang IS, Kim BH, Kim KS, Culley DE (2006) Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci 103(30):11358–11363
Harnisch F, Schröder U (2010) From MFC to MXC: chemical and biological cathodes and their potential for microbial bioelectrochemical systems. Chem Soc Rev 39(11):4433–4448
Hassan SH, Kim YS, Oh SE (2012) Power generation from cellulose using mixed and pure cultures of cellulose-degrading bacteria in a microbial fuel cell. Enzym Microb Technol 51(5):269–273
Hsu WH, Tsai HY, Huang YC (2017) Characteristics of carbon nanotubes/graphene coatings on stainless steel meshes used as electrodes for air-cathode microbial fuel cells. J Nanomater 2017:9875301
Huang Z, Wu Z, Zhou F, Zhou J (1997) Ovulation prediction by monitoring the conductivity of woman’s saliva. In: Engineering in medicine and biology society, 1997. Proceedings of the 19th annual international conference of the IEEE, vol 5. IEEE, pp 2344–2346
Hubler AW, Osuagwu O (2010) Digital quantum batteries: energy and information storage in nanovacuum tube arrays. Complexity 15(5):48–55
Humphries AC, Nott KP, Hall LD, Macaskie LE (2004) Continuous removal of Cr (VI) from aqueous solution catalysed by palladised biomass of Desulfovibrio vulgaris. Biotechnol Lett 26(19):1529–1532
Igem.org (2018) Microbial fuel cell. [Online] Available at: http://2013.igem.org/Team:Bielefeld-Germany/Project/MFC. Accessed 21 Nov 2018
Jiang X, Hu J, Lieber AM, Jackan CS, Biffinger JC, Fitzgerald LA, Ringeisen BR, Lieber CM (2014) Nanoparticle facilitated extracellular electron transfer in microbial fuel cells. Nano Lett 14(11):6737–6742
Kaewkannetra P, Imai T, Garcia-Garcia FJ, Chiu TY (2009) Cyanide removal from cassava mill wastewater using Azotobacter vinelandii TISTR 1094 with mixed microorganisms in activated sludge treatment system. J Hazard Mater 172(1):224–228
Khan MD, Abdulateif H, Ismail IM, Sabir S, Khan MZ (2015) Bioelectricity generation and bioremediation of an azo-dye in a microbial fuel cell coupled activated sludge process. PLoS One 10(10):e0138448
Khan N, Clark I, Bolan N, Meier S, Saint CP, Sánchez-Monedero MA, Shea S, Lehmann J, Qiu R (2017) Development of a buried bag technique to study biochars incorporated in a compost or composting medium. J Soils Sediments 17(3):656–664
Khudzari JM, Tartakovsky B, Raghavan GV (2016) Effect of C/N ratio and salinity on power generation in compost microbial fuel cells. Waste Manag 48:135–142
Kim BH, Chang IS, Gil GC, Park HS, Kim HJ (2003) Novel BOD (biological oxygen demand) sensor using mediator-less microbial fuel cell. Biotechnol Lett 25(7):541–545
Kim BH, Chang IS, Gadd GM (2007) Challenges in microbial fuel cell development and operation. Appl Microbiol Biotechnol 76(3):485
Kodali M, Herrera S, Kabir S, Serov A, Santoro C, Ieropoulos I, Atanassov P (2018) Enhancement of microbial fuel cell performance by introducing a nano-composite cathode catalyst. Electrochim Acta 265:56–64
Kumar R, Singh L, Wahid ZA (2015) Role of microorganisms in microbial fuel cells for bioelectricity production. In: Microbial Factories. Springer, New Delhi, pp 135–154
Lal Deeksha (2013) Microbes to generate electricity. Indian J Microbiol 53(1):120–122
Lamberg P, Bren KL (2016) Extracellular Electron transfer on sticky paper electrodes: carbon paste paper anode for microbial fuel cells. ACS Energy Lett 1(5):895–898
Li S, Cheng C, Thomas A (2017) Carbon-based microbial-fuel-cell electrodes: from conductive supports to active catalysts. Adv Mater 29(8):1602547
Lies DP, Hernandez ME, Kappler A, Mielke RE, Gralnick JA, Newman DK (2005) Shewanella oneidensis MR-1 uses overlapping pathways for iron reduction at a distance and by direct contact under conditions relevant for biofilms. Appl Environ Microbiol 71(8):4414–4426
Light SH, Su L, Rivera-Lugo R, Cornejo JA, Louie A, Iavarone AT, Ajo-Franklin CM, Portnoy DA (2018) A flavin-based extracellular electron transfer mechanism in diverse gram-positive bacteria. Nature 562(7725):140. https://doi.org/10.1038/s41586-018-0498-z
Lim DH, Wilcox J (2012) Mechanisms of the oxygen reduction reaction on defective graphene-supported Pt nanoparticles from first-principles. J Phys Chem C 116(5):3653–3660
Logan BE (2010) Scaling up microbial fuel cells and other bioelectrochemical systems. Appl Microbiol Biotechnol 85(6):1665–1671
Logan BE, Regan JM (2006a) Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol 14(12):512–518
Logan BE, Regan JM (2006b) Microbial fuel cells-challenges and applications. Environ Sci Technol 40(17):5172–5180. https://doi.org/10.1021/es0627592
Ma X, Li Z, Zhou A, Yue X (2017) Energy recovery from tubular microbial electrolysis cell with stainless steel mesh as cathode. R Soc Open Sci 4(12):170967
Malvankar NS, Vargas M, Nevin KP, Franks AE, Leang C, Kim BC, Inoue K, Mester T, Covalla SF, Johnson JP, Rotello VM (2011) Tunable metallic-like conductivity in microbial nanowire networks. Nat Nanotechnol 6(9):573
Mashkour M, Rahimnejad M (2015) Effect of various carbon-based cathode electrodes on the performance of microbial fuel cell. Biofuel Res J 2(4)
McGovern C (2010) Commoditization of nanomaterials. Nanotechnol Perceptions 6(3):155
Mink JE, Hussain MM (2013) Sustainable design of high-performance microsized microbial fuel cell with carbon nanotube anode and air cathode. ACS Nano 7(8):6921–6927
Mink JE, Qaisi RM, Logan BE, Hussain MM (2014) Energy harvesting from organic liquids in micro-sized microbial fuel cells. Npg Asia Mat 6(3):e89
Miran W, Nawaz M, Jang J, Lee DS (2016) Effect of wastewater containing multi-walled carbon nanotubes on dual-chamber microbial fuel cell performance. RSC Adv 6(94):91314–91319
Moqsud MA, Yoshitake J, Bushra QS, Hyodo M, Omine K, Strik D (2015) Compost in plant microbial fuel cell for bioelectricity generation. Waste Manag 36:63–69
Mustakeem M (2015) Electrode materials for microbial fuel cells: nanomaterial approach. Mater Renew Sustain Energy 4:22. https://doi.org/10.1007/s40243-015-0063-8
Nurettin ÇEK (2017) Examination of zinc electrode performance in microbial fuel cells. Gazi University J Sci 30(4):395–402
Omkar S Powar, Lakshminarayana Bhatta KG, Raghavendra Prasad HD, Venkatesh K, Raghu AV (2017) Influence of carbon based electrodes on the performance of the microbial fuel cell. Int J Res-Granthaalayah 5(4: RAST):7–16. https://doi.org/10.5281/zenodo.803412
Park DH, Zeikus JG (2003) Improved fuel cell and electrode designs for producing electricity from microbial degradation. Biotechnol Bioeng 81(3):348–355
Penteado ED, Fernandez-Marchante CM, Zaiat M, Gonzalez ER, Rodrigo MA (2017) Influence of carbon electrode material on energy recovery from winery wastewater using a dual-chamber microbial fuel cell. Environ Technol 38(11):1333–1341
Prasertsung N, Reungsang A, Ratanatamskul C (2012) Alkalinity of cassava wastewater feed in anodic enhance electricity generation by a single chamber microbial fuel cells. Eng J 16(5):17–28
Qiao Y, Li CM, Bao SJ, Bao QL (2007) Carbon nanotube/polyaniline composite as anode material for microbial fuel cells. J Power Sources 170(1):79–84
Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR (2005) Extracellular electron transfer via microbial nanowires. Nature 435(7045):1098
Ren H, Chae J (2015) Microfabricated microbial fuel cells. In Micro Energy Harvesting, Wiley, pp. 347–361. https://doi.org/10.1002/9783527672943
Ren Z, Steinberg LM, Regan JM (2008) Electricity production and microbial biofilm characterization in cellulose-fed microbial fuel cells. Water Sci Technol 58(3):617–622
Reuben Y Tamakloe (2018) PEM-less microbial fuel cells, Proton exchange membrane fuel cell, Tolga Taner, IntechOpen. https://doi.org/10.5772/intechopen.71479. Available from: https://www.intechopen.com/books/proton-exchange-membrane-fuel-cell/pem-less-microbial-fuel-cells
Rezaei F, Xing D, Wagner R, Regan JM, Richard TL, Logan BE (2009) Simultaneous cellulose degradation and electricity production by Enterobacter cloacae in a microbial fuel cell. Appl Environ Microbiol 75(11):3673–3678
Rojas JP, Hussain MM (2015) The role of microfabrication and nanotechnology in the development of microbial fuel cells. Energ Technol 3(10):996–1006
Sayed ET, Abdelkareem MA (2017) Yeast as a biocatalyst in microbial fuel cell. In Old yeasts-new questions. InTechOpen. https://doi.org/10.5772/intechopen.70402
Schaetzle O, Barrière F, Schröder U (2009) An improved microbial fuel cell with laccase as the oxygen reduction catalyst. Energy Environ Sci 2(1):96–99
Schroder U (2007) Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys Chem Chem Phys 9(21):2619–2629
Siegert M, Sonawane JM, Ezugwu CI, Prasad R (2019) Economic Assessment of Nanomaterials in Bio-Electrical Water Treatment. In: Prasad R, Thirugnanasanbandham K (eds) Advanced Research in Nanosciences for Water Technology. Springer International Publishing AG, Cham, pp 5–23
Sure S, Ackland ML, Torriero AA, Adholeya A, Kochar M (2016) Microbial nanowires: an electrifying tale. Microbiology 162(12):2017–2028
Tan L, Li N, Chen S, Liu ZQ (2016) Self-assembly synthesis of CuSe@ graphene–carbon nanotubes as efficient and robust oxygen reduction electrocatalysts for microbial fuel cells. J Mater Chem A 4(31):12273–12280
Tandukar M, Huber SJ, Onodera T, Pavlostathis SG (2009) Biological chromium (VI) reduction in the cathode of a microbial fuel cell. Environ Sci Technol 43(21):8159–8165
Toczyłowska-Maminska R, Szymona K, Król P, Gliniewicz K, Pielech-Przybylska K, Kloch M, Logan BE (2018) Evolving microbial communities in cellulose-fed microbial fuel cell. Energies 11(1):124
Tommasi T, Salvador GP, Quaglio M (2016) New insights in microbial fuel cells: novel solid phase anolyte. Sci Rep 6:29091
Tsai HY, Hsu WH, Huang YC (2015) Characterization of carbon nanotube/graphene on carbon cloth as an electrode for air-cathode microbial fuel cells. J Nanomater 2015:3
Valipour A, Ayyaru S, Ahn Y (2016) Application of graphene-based nanomaterials as novel cathode catalysts for improving power generation in single chamber microbial fuel cells. J Power Sources 327:548–556
Wang Z, Lee T, Lim B, Choi C, Park J (2014) Microbial community structures differentiated in a single-chamber air-cathode microbial fuel cell fueled with rice straw hydrolysate. Biotechnol Biofuels 7(1):9
Waste-management-world.com (2013) Bacteria that turn waste to energy in microbial fuel cells studied. [Online] Available at: https://waste-management-world.com/a/bacteria-that-turn-waste-to-energy-in-microbial-fuel-cells-studied. Accessed 21 Nov 2018
Xie X, Hu L, Pasta M, Wells GF, Kong D, Criddle CS, Cui Y (2010) Three-dimensional carbon nanotube−textile anode for high-performance microbial fuel cells. Nano Lett 11(1):291–296
Yang Y, Xu M, Guo J, Sun G (2012) Bacterial extracellular electron transfer in bioelectrochemical systems. Process Biochem 47(12):1707–1714
Yang G, Chen D, Lv P, Kong X, Sun Y, Wang Z, Yuan Z, Liu H, Yang J (2016a) Core-shell au-Pd nanoparticles as cathode catalysts for microbial fuel cell applications. Sci Rep 6:35252
Yang N, Ren Y, Li X, Wang X (2016b) Effect of graphene-graphene oxide modified anode on the performance of microbial fuel cell. Nano 6(9):174
Yang Q, Liang S, Liu J, Lv J, Feng Y (2017) Analysis of anodes of microbial fuel cells when carbon brushes are preheated at different temperatures. Catalysts 7(11):312
Yin Y, Huang G, Zhou N, Liu Y, Zhang L (2016) Increasing power generation of microbial fuel cells with a nano-CeO2 modified anode. Energy Sources, Part A 38(9):1212–1218
Yong YC, Dong XC, Chan-Park MB, Song H, Chen P (2012) Macroporous and monolithic anode based on polyaniline hybridized three-dimensional graphene for high-performance microbial fuel cells. ACS Nano 6(3):2394–2400
Yuan Y, Zhou S, Liu Y, Tang J (2013) Nanostructured macroporous bioanode based on polyaniline-modified natural loofah sponge for high-performance microbial fuel cells. Environ Sci Technol 47(24):14525–14532
Zhang L, Liu C, Zhuang L, Li W, Zhou S, Zhang J (2009) Manganese dioxide as an alternative cathodic catalyst to platinum in microbial fuel cells. Biosens Bioelectron 24(9):2825–2829
Zhang B, Jiang Y, Han J (2017a) A flexible nanocomposite membrane based on traditional cotton fabric to enhance performance of microbial fuel cell. Fibers and Polym 18(7):1296–1303
Zhang Y, Jiang J, Zhao Q, Gao Y, Wang K, Ding J, Yu H, Yao Y (2017b) Accelerating anodic biofilms formation and electron transfer in microbial fuel cells: role of anionic biosurfactants and mechanism. Bioelectrochemistry 117:48–56
Zhao Y, Collum S, Phelan M, Goodbody T, Doherty L, Hu Y (2013) Preliminary investigation of constructed wetland incorporating microbial fuel cell: batch and continuous flow trials. Chem Eng J 229:364–370
Zhou M, Wang H, Hassett DJ, Gu T (2013) Recent advances in microbial fuel cells (MFCs) and microbial electrolysis cells (MECs) for wastewater treatment, bioenergy and bioproducts. J Chem Technol Biotechnol 88(4):508–518
Acknowledgment
The author expresses thanks to her husband Mr. G. Sankar for his assistances in this work. Also, she acknowledges the assistances of the International Research Center, Kalasalingam Academy of Research and Education (Deemed University), Krishnankoil – 626 126 (India).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Thirugnanasambandan, T. (2019). Nanomaterials in Microbial Fuel Cells and Related Applications. In: Prasad, R. (eds) Microbial Nanobionics. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-16383-9_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-16383-9_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16382-2
Online ISBN: 978-3-030-16383-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)