Abstract
Microbial fuel cell (MFC) technologies have been globally noticed as one of the most promising sources for alternative renewable energy, due to its capability of transforming the organics in the wastewater directly into electricity through catalytic reactions of microorganisms under anaerobic conditions. In this chapter, the state of the art of review on the various emerging technological aspects of nanotechnology for the development of nanomaterials to make the existing microbial fuel cell technology as more sustainable and reliable in order to serve the growing energy demand. Initially, a brief history of the development and the current trends of the microbial fuel cells along with its basic working mechanism, basic designs, components, fascinating derivative forms, performance evaluation, challenges and synergetic applications have been presented. Then the focus is shifted to the importance of incorporation of the nanomaterials for the sustainable development of MFC technology by means of advancements through anode, cathode, and proton exchange membranes modifications along with the various ultimate doping methods. The possibilities of applied nanomaterials and its derivatives in various places in MFCs are discussed. The nanomaterials in MFCs have a significant contribution to the increased power density, treatment efficiency, durability, and product recovery due to its higher electrochemical surface area phenomenon, depending on the fuel cell components to get modified. The promising research results open the way for the usage of nanomaterials as a prospective material for application and development of sustainable microbial fuel cells. Though the advances in nanomaterials have opened up new promises to overcome several limitations, but challenges still remain for the real-time and large-scale applications. Finally, an outlook for the future development and scaling up of sustainable MFCs with the nanotechnology is presented with some suggestions and limitations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Energy demand
- Nanomaterials
- Sustainable microbial fuel cells
- Microbial fuel cells anode modification
- Microbial fuel cells cathode modification
- Microbial fuel cells PEM modification
8.1 Introduction
The demand for energy, globally, continues to grow faster than predicted, thereby leading to the energy crisis and environmental pollution. Due to the availability of fossil fuels in the range of finite and depleting amount, it became an unsustainable and unreliable source of energy. Nowadays research has been targeted toward the alternative, renewable, and carbon-neutral sources of energy, which are the need of the hour for economic and sustainable development (Parkash 2016).
With this backdrop, microbial fuel cell (MFC) technologies have been universally observed as one of the most promising substitute sources of renewable energy (Xia et al. 2018).
The technology of generating electricity through bacteria was found 100 years ago, but it did not gain much attention due to the production very less power (Rabaey et al. 2004; Reguera et al. 2006). Because of its ability in converting available chemical energy into electrical energy, MFCs got several applications such as wastewater treatment, electricity generation, biosensor, resource recovery, biohydrogen generation, etc. (Logan et al. 2005, 2006; Reguera et al. 2006). Consequently, the number of research studies in the area of MFCs became greater than before since the early 1990s (Xia et al. 2018).
The innovative idea of employing microbes to generate electricity was conceived and attributed to Potter in 1911 (Potter 1911); moreover, the concept of “animal electricity” dates back to the eighteenth century when Galvani was experimenting with frog legs for electricity generation (Piccolino 2008). Later on, the concepts and practical improvements were explored since, with Cohen’s 35-unit setup in 1931. The investigations on the development of the catalyst in the 1960s by Karube et al. (1976) and more in the recent times of the 1980s–1990s, with the work of Bennetto et al. on synthetic mediators gave rise to the development of the so-called analytical MFC that is still in use to date. From those primary instances, major progress on the understanding of electron transfer mechanisms, improvement of efficient bioelectrocatalytic interfaces, and development of unique, low-cost, and durable electrode materials has been attained; however, ample room for improvement and work have to be done for the industrialization of MFCs (Rahimnejad et al. 2015; Santoro et al. 2017a; Trapero et al. 2017).
There has been a substantial shift of focus toward the renewable energy technologies for many decades like wind, wave, solar, and also nuclear technology.
As a promising alternative to the other potential area of renewable energy sources, MFCs has resurfaced in recent times as modern technology which accommodate and exhibit potential for generating power using bacterial populations (Juan and Nixon 2013).
The emerging areas of science are nanoscience and nanotechnology playing a lead role in diversified fields like electrical, environmental engineering, medical etc., due to technological and scientific upgradation. Nanoscience is a study of atoms, molecules, and objects which possess size on the nanometer level. In nanotechnology, the manipulation of matter for a specific application with the help of nano-sized dimensions in the range of 1–100 nm with the peculiar features through physical and chemical processes is explored. The nanotechnological approaches can be incorporated in various forms in order to increase the overall performance of the microbial fuels, which may satisfy the energy needs of the electrical appliances.
The enormous significance is being provided by human beings to generate energy in many ways, which is considered as the main resource for human activities. Due to the substantial growth in the global human population, there is no hope for the decrease in the energy demand other than the drastic increase in the need. For the futuristic energy needs, the modern nanotechnology is one of the promising solutions to gain a more effective and efficient procedure to generate more energy.
Furthermore, huge importance should be given to nanotechnology in the microbial fuel cells energy systems in order to attain greater energy production with high efficiency, lower production cost, and ease during real-time applications.
8.2 MFCs’ Working Principle, Components, and Designs
8.2.1 Working Principle of MFC
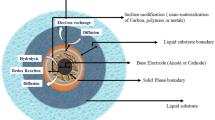
Microbial fuel cell (MFC) is a bioreactor incorporated with an electrochemical system that converts chemical energy in the chemical bonds in organic pollutants in the wastewater to electrical energy through catalytic reactions of microorganisms under anaerobic conditions (Fig. 8.1).
MFC can be constructed using a variety of materials and in an ever-increasing diversity of configurations.
In the bioreactor, the bacterial populations will oxidize the provided wastewater as substrates in the anodic chamber to produce free electrons and protons, while carbon dioxide will be an oxidation product. Electrons attached on the anode (negative terminal) flow to the cathode (positive terminal) through an external circuit. Free protons migrate across the proton/cation exchange membrane to combine with electrons to form water if oxygen is provided or to form ferrocyanide if ferricyanide is provided (Xia et al. 2018).
8.2.2 Electron Transport Mechanism (ETM) in MFCS
Electron transport mechanism in microbial fuel cell deals with the transfer of liberated electrons from the degraded organic matter to the electrodes through several irreversible and reversible electrochemical reactions of the electron transport chain and finally ends up with the generation of electricity by transferring those electrons from cell to MFC circuit (Busalmen et al. 2008; Bhunia and Dutta 2018).
In order to achieve the reversible interfacial reactions at the anode surface, the electrons have to depend on shuttles such as cytochromes, proteins (such as PQQ), and bound or soluble redox mediators to reach at least within a proximity of 10 A˚ near the anode (Labelle and Bond 2009; Bhunia and Dutta 2018).
The proposed three pathways like (i) direct membrane complex mediated electron transfer (Bond and Lovley 2003), (ii) Indirect or redox shuttle mediated electron transfer (Rabaey et al. 2005a), and (iii) electron transfer through conductive pili or nanowires (Reguera et al. 2005) are the very often reported modes of electron transport (Fig. 8.2).
8.2.2.1 Direct Electron Transfer
In the direct electron transport mechanism, a monolayer of microorganisms will be formed over the anode surface, which permits a direct transfer of electrons to the anode surface through a cell membrane or a membrane organelle.
Notably, certain Fe (III)-reducing bacteria (S. putrefaciens) would be able to transfer electrons to the electrode even with the absence of synthetic mediators (Mahadevan et al. 2014; Bhunia and Dutta 2018).
It is evident that the presence of cytochrome (a redox protein) over the outer membrane of the cell might be responsible for direct electron transfer through a reduction of the water-soluble Fe (III). As a consequence, due to the negligible gap between the microbes and the electrode, this phenomenon results in the lowest extracellular potential losses (Bhunia and Dutta 2018).
The direct transport mechanism through the cytochrome has been clearly demonstrated with mutants of G. suilfurreducens through experiments wherein the gene encoding for the cytochrome C proteins has been deleted or overexpressed (Lovley 2006a; Santoro et al. 2017a).
Bond and Lovley (2003) reported that cathodephillic microorganisms such as Thiobacillus ferrooxidans which forms a biofilm over the cathode and finally the cathode act as the electron donor. These microbes results with a potential difference in cathode driving to a suitable reaction at the anode by acidophilic microorganisms to generate the electricity (Tekle and Demeke 2015).
8.2.2.2 Indirect or Mediated Electron Transfer
The extensive study about an indirect or mediated electron transport mechanism is being carried out for electricity production in MFCs due to the sufficient weakness of the direct electron transport mechanism (Schröder 2007; Bhunia and Dutta 2018).
The mechanism of transporting electrons by artificial mediators is referred to as electron shuttles. These chemical compounds offer the opportunity for microorganisms to produce the reduced products that are more electrochemically active than utmost fermentation products. These electron shuttles or mediators are usually capable of crossing cell membranes, accepting electrons from one or more electron carriers within the cell, exiting the cell in the reduced form, and then transferring electrons onto the electrode surface (Lovley 2006b; Tekle and Demeke 2015).
In an indirect or mediated electron transfer mechanism, the microbes produce/require indigenous soluble organic redox mediators (quinone and flavin) or artificial exogenous mediators (dye or toxic metal complexes) to transport electron between terminal respiratory enzyme and anode surface (Park and Zeikus 2000; Das and Mangwani 2010).
To enhance the mediated electron transfer mechanism, there is a wide range of mediators like microbial electroactive metabolic mediators and synthetic mediators which are categorized and listed in the following section (Bhunia and Dutta 2018).
The redox mediators like phenazines, phenothiazines, phenoxazines, and quinones will be employed initially to conduct electron transfer mechanism in MFCs. The active microorganisms release the electroactive metabolites in bulk solution/over the formed biofilm and finally interact at the electrode surface (Bhunia and Dutta 2018).
Most remarkably, the primary metabolites like H2 and H2S generated through microbial catabolic oxidation of fuel (anaerobic respiration and fermentation) have been productively used as redox mediators (Straub et al. 2004; Sekoai and Gueguim Kana 2014) (Fig. 8.3). Some of the secondary metabolites, namely, phenazine-1-carboxamide, pyocyanine (Pseudomonas aeruginosa), neutral red, humic acid, anthraquinone-2,6-disulfonate (AQDS), thionine, methyl viologen, methyl blue, and 2-amino-3-carboxy-1,4-naphthoquinone (ACNQ) (Bifidobacterium longum), are extensively involved in indirect electron transfer mechanism in MFCs (Mahadevan et al. 2014; Bhunia and Dutta 2018).
The synthetic mediators like methylene blue, neutral red, thionine, Meldola’s blue, and Fe (III) EDTA possess high redox potentials, but the problem is their toxicity which limits their use in MFCs (Davis and Higson 2007). In the current state of knowledge, cyclic voltammetry (CV) study can reveal the mechanism of electron transfer that takes place through redox enzymes, namely, ferricyanide reductase and lactate dehydrogenase, at the outer membrane of the microbial cell, whether it is a mobile redox shuttles transfer or direct electrons transfer mechanism through the membrane-associated compounds (Mahadevan et al. 2014; Bhunia and Dutta 2018).
8.2.2.3 Electron Transfer via Conductive Pili or Nanowires
The mechanism of electron transfer to the anode surface by bacteria requires physical contact with the electrode’s surface which involves outer membrane-bound cytochromes or putative conductive pili called nanowires (Coates and Wrighton 2009; Das and Mangwani 2010).
More remarkably in 2005, an additional type of electron transfer mimicking the direct electron transport mechanism has been described, which proceeds via extracellular conductive connections called the conductive pili or bacterial nanowires (Reguera et al. 2005; Santoro et al. 2017a).
In a study, Geobacter sulfurreducens with its electrically conductive bacterial appendages named as pili or nanowires have been revealed to be electrically conductive through scanning tunneling electron microscopy (Reguera et al. 2005; Bhunia and Dutta 2018).
These nanowires are a flexible structure made with proteins, which assist the bacteria to hold the surface of the anode and as distinguished materials in its surroundings. The internal networks of conductive pilus may overcome the restrictions of the earlier discussed direct electron transfer mechanism. More specifically, the pili consist of a cluster of aromatic acids and subunit of proteins, which permit them to play a significant role in electrical conductivity (Reguera et al. 2005; Bhunia and Dutta 2018).
Surprisingly, Shewanella oneidensis involves in the direct electron transfer mechanism as well as conductive pilus-like nanowires for electron transfer through the c-type cytochrome (Reguera et al. 2005; Bhunia and Dutta 2018).
The conductivity of the nanowire can be measured through the equation as given:
where G is the conductance of the biofilm, L is the length of the electrode, g is the biofilm thickness, and a is the nonconducting gap width between two working electrodes (anode) (Malvankar et al. 2011; Bhunia and Dutta 2018).
On the other hand, bacterial nanowires are positive findings toward a long range of electron transfer on the electrode, but the attractive feature of the pili that eases metallike conductivity, notably other microbial species, does not possess this provisions, and still in-depth investigations are needed (Mahadevan et al. 2014).
8.2.3 Adequate Components of MFC
An ideal MFC reactor may consist of two chambers (anodic and cathodic) made with polycarbonate, polyacrylic sheet, or glass, with the desired electrodes like graphite felt, carbon paper, graphite, carbon cloth, reticulated vitreous carbon (RVC), carbon black, etc. Both these chambers will be separated by proton exchange membrane (PEM) like Nafion® or Ultrex® with the phenomenon of only permitting the ions to get diffused than the substrate crossover (Fig. 8.4 and Table 8.1). The organic substrates are filled in the anodic chamber employed with the microbes for degradation of organics and production of free electrons and protons; on the other side, the cathode chamber is filled with a potential electron acceptor to complete the circuit and get the electricity. An ideal electron acceptor like oxygen can be used as a preferred oxidizing reagent due to its nontoxic effect and simplified operation. For the very high performance with optimal power density, ferricyanide can be used as the potential electron acceptor. The various designs of the MFC are only dependent on the assembly of anode and cathode chambers. Besides the simple MFC prototypes like two-chamber or single chamber, numerous alterations have been made to obtain other prototypes of MFC design and structure (Das and Mangwani 2010).
8.2.3.1 Biocatalysts for Substrate Utilization and Electricity Generation
Generally, the microbial cells facilitate the electron transfer from the substrate to the electrode through the mechanism of electron shuttling; this effect is called as bioelectrocatalysis.
Our understanding regarding the electrochemically active exoelectrogenic microbes is still in its beginning, but evidently, a whole new field of microbial ecology is getting developed that is based on the knowledge about the anodophilic bacteria and its potential interspecies electron transfer mechanism. These bacteria with the ability to release the electrons exocellularly are called as exoelectrogens (Table 8.2). Earlier an understanding of electron transfer mechanism by bacteria to electrodes came to light from the studies of dissimilatory metal-reducing bacteria such as Geobacter and Shewanella species, which can generate electricity in MFCs (Bond and Lovley 2003; Gil et al. 2003). Various genetic and biochemical characterizations directed that outer membrane cytochromes can be involved in exogenous electron transfer (Myers and Myers 2001). Furthermore, some microbes may produce and use soluble electron shuttles which exclude the need for direct contact between the cell and an electron acceptor (Turick et al. 2002; Logan and Regan 2006).
The substrate is also the key factor for an efficient production of electricity from an MFC: from the range of simple to a complex mixture of organic matters present in the substrate/wastewater can be employed. The simple mixture substrate like acetate and glucose are most recommended for immediate output and the complex mixtures substrate for the growth of diverse active microbial populations. Another promising and most used substrate is brewery wastewater with growth-promoting organic matter and lacking inhibitory substances (Feng et al. 2008). Other unconventional substrates like synthetic wastewater, landfill leachates, and dye wastewater are used for the generation of electricity (Das and Mangwani 2010; Pant et al. 2010).
8.2.3.2 Electrocatalyst for Oxygen Reduction Reaction (ORR)
The role of the electrocatalyst is very crucial in the cathode chamber, where the synthesized free protons and electrons recombine and reduce an electron acceptor to end up the electrical circuit. Oxygen is the paramount suitable electron acceptor for an MFC owing to its high oxidation potential, abundance, low cost, sustainability, and the lack of a chemical waste product (water is formed as the only end product). However, the reaction is very slow with the oxygen as an electron acceptor; hence the need for a catalyst arises. Most of the MFCs use platinum as the catalyst; on the other hand, this is exceptionally expensive. Chemical electron acceptors like ferricyanide and potassium permanganate have been used effectively with results similar to those achieved with platinum. These chemicals are far less expensive than platinum, but the disadvantage is that they are much toxic and getting consumed in the reaction (He and Angenent 2006). Due to the abovementioned drawbacks, which affect the viability of the MFC directly, these chemicals should be avoided by replacing them with an equally efficient but less expensive catalyst through extensive research (Tekle and Demeke 2015).
8.3 Designs of MFC
8.3.1 Dual Compartment MFC System
Generally, this dual compartment MFC system has an anodic and cathodic compartment connected by a proton exchange membrane (PEM) that mediates only the transfer of free protons from the anode to cathode compartment while blocking the diffusion of oxygen into the anode and also the crossover of the substrate into the cathode compartment (Fig. 8.5). This type of MFC is commonly employed for treatment of wastewater with simultaneous electricity generation. But scaling up of this MFC system to industrial size is quite challenging, due to the necessity of aeration in the cathodic chamber.
The mode of operation of the double chamber MFC system will be batch and fed-batch mode (Parkash 2016).
8.3.2 Single Air-Cathode Chamber MFC System
The earliest single air-cathode chamber MFC system was designed and reported the possibility that an oxygen gas diffusion electrode could be used as a cathode in a bioelectrochemical fuel cell (Liu et al. 2005a). However, initially, this single air-cathode setup has not drawn much attention in MFC research until Liu reported the air-cathode MFC possibly will generate much greater power than typical dual chamber aqueous-cathode MFCs (Fig. 8.6). They advanced the air-cathode configuration with and without the PEM, reported with the maximum power output of 262 mW/m2 using glucose with PEM and 494 mW/m2 without PEM (Liu et al. 2005a; Parkash 2016).
Furthermore, Liu et al.’s study was attempted with acetate and butyrate in the same reactor in the absence of the CEM. Other studies have also been performed to examine the power outputs in single air-cathode MFCs, and shown the power density results in 506 mW/m2 with acetate and 305 mW/m2 for butyrate in the same air-cathode setup without any membrane (Liu et al. 2005a).
The single air-cathode MFCs design is expected to optimize some of the characteristics of dual chamber MFCs, such as high cost of cathode catalysts , less relative power output and energy requirement for intensive air/oxygen sparing . An additional advantage of the single air-cathode MFCs over the dual chamber is the reduction of the high internal resistance of MFCs, which is a significant factor to improve electricity production (Parkash 2016).
8.3.3 Tubular/Upflow MFC System
The generated voltage by MFCs remained limited and unable to exceed theoretical open circuit voltage of 1.14 V as based on the NADH (−0.32 V) and pure oxygen (+0.82) redox potentials even ignoring the internal resistances (Logan and Regan 2006). Tubular/upflow architecture is the well-known and highly optimized MFCs setup (Cheng and Hamelers 2008). Chang et al. (2006) have designed a tubular reactor MFC setup working in continuous flow mode; the flow was moving through an anode chamber and then directed up into the cathode chamber in the same column (Fig. 8.7). They claimed that the upflow reactor had several advantages over other conventional designs, on the basis of higher affinity for oxygen with cathode (Lovley 2006a), combining the benefits of the upflow anaerobic sludge blanket system with two-chamber MFC (Parkash 2016).
Typical upflow MFC. (Figure drawn with modifications after Jang et al. 2004)
8.3.4 Stacks
The idea of connecting several microbial fuel cells in series may result in the added voltages, whereas connecting several MFCs in parallel may result in the enhanced current outputs. Due to these reasons, the existence of the stacked MFCs came into practice (Fig. 8.8). The application of the stacked MFCs for the treatment of the wastewater treatment can result in the improved chemical oxygen demand removal when compared to the single MFC (Fig. 8.9a). This type of MFC setup also got optimized at the level of configurations and components aimed at the reduced internal resistance and increased cell power outputs (Chae et al. 2009; Parkash 2016).
Typical stack MFC. (Figure drawn with modifications after Aelterman et al. 2006)
8.4 Advancements and Fascinating Derivative Forms of MFC
Microbial fuel cells (MFCs) are comparatively unique bioelectrochemical systems (BESs) that directly transform microbial metabolic energy into electricity (ElMekawy et al. 2017).
There are numerous forms of bioelectrochemical systems (BESs) that have been proposed, and they are classified on the basis of their applications. Most of the BESs can be employed to extract the chemical energy from the provided complex organic substrates in the anodic chamber and transform it into useful electrical energy. Other forms of the BESs have been developed to generate valuable products like hydrogen gas (Escapa et al. 2016; Kadier et al. 2016), acetate (Xafenias and Mapelli 2014), methane gas (Villano et al. 2011; Babanova et al. 2017), formate (Nevin et al. 2010), desalinate water (Cao et al. 2009), etc. other than generating the electricity from the wastewater (Werner et al. 2013; Watson et al. 2015; Santoro et al. 2017a).
8.4.1 Microbial Electrolysis Cell (MEC)
Among the BESs, one of the most remarkable and well investigated is the microbial electrolysis cell (MEC), which was first introduced in 2005 and related publications on MEC increased over time (Santoro et al. 2017a). MFC is a galvanic process that generates electricity, while MEC is an electrolytic process which requires extra potential. This process occurs in a device similar to that of the microbial fuel cell (MFC) (Rozenfeld et al. 2018).
An illustration of the working principle of the microbial electrolysis cell is presented (Fig. 8.9b). MEC is of a specific interest ever since hydrogen is an expensive gas produced and essentially needed for the upcoming hydrogen energy economy (Winter 2005; Rizzi et al. 2014).
MEC consist of a single- or dual-glass compartment separated by a proton exchange membrane. The anode chamber contains exoelectrogenic bacterial cells which oxidize organic material to free electrons and protons which recombine on the cathode to produce biohydrogen.
However, since the overall free energy of this process is positive, the MEC device operates under a low external voltage of about 0.15–0.8 V, which is much lower than the theoretical and practical values of 1.5 and above in the general water electrolysis system (Liu et al. 2010).
Numerous progress and developments in relation to the increase in production of hydrogen gas, improvements in aspects of cell design, membrane removal and replacement, and utilization of microbial catalysts in the optimal amount or Pt-free catalysts have been productively revealed. Notable work was reported with the scale-up studies dealing with the hydrogen gas production from industrial winery wastewater and other sources of wastewater (Santoro et al. 2017a).
These scale-up studies on production of hydrogen gas from the industrial wastewater clearly signposted the intentions of the scientists taking this technology to the next level by researching on the limitations related to the increase in reactor’s working volume (Santoro et al. 2017a).
8.4.2 Microbial Desalination Cell (MDC)
Remarkably, microbial desalination cell (MDC) has been magnificently advanced in the longtime objectives of treating wastewater, desalinating water, and generating electricity simultaneously (Cao et al. 2009; Santoro et al. 2017a).
A general schematic of the microbial desalination cell is here presented (Fig. 8.9c). There are numerous research works reported on the main configurations adopted in microbial desalination cells, along with the implementation of air-breathing cathodes , biocathodes, and osmotic membranes (Santoro et al. 2017a).
There is a rising attention in developing innovative processes to reduce the energy and chemical costs connected with the conventional nitrification-denitrification process. The very first proof of concept study proved that ANAMMOX mechanism can be advantageous to deliver simultaneous removal of carbon and nitrogen compounds from the source wastewater in MDCs along with bioelectricity generation (Kokabian et al. 2018).
This ANAMMOX mechanism is considered as ideal candidates which significantly reduce the required energy and chemical inputs. Anammox bacteria are much capable of attaining anaerobic ammonia oxidation (ANAMMOX) which results in the anaerobic transformation of ammonium to nitrogen gas resulting in the substantial energy and cost savings (Kokabian et al. 2018).
The electrons produced from the oxidation of wastewater at the anode chamber will be utilized by a biocathode to drive the nitrite/nitrate reduction. The MDC is gaining huge interests in the recent years for addressing water and energy nexus issues in a single process configuration potentially. This system can be adapted for treating the wastewater, water reuse, and desalination in water-scarce regions (Kokabian et al. 2018).
The important parameters such as recirculating anolyte and catholyte, stacking the cells, and using capacitive materials for deionization were investigated for improving the performance of the microbial desalination cells. A pilot-scale MDC system of 105 L was also presented in the recent (Santoro et al. 2017a).
8.4.3 Microbial Electrosynthesis Device (MES)
With the results of recent research, BESs have been presented with the as microbial electrosynthesis device, where the specific bacterial population or operating conditions can be utilized for the production of highly valuable resources as the products from CO2 or other compounds, including gas transformation or reduction (Fig. 8.9d). This absolute new direction is of high interest due to the possible utilization of renewable energy when separated from the main power lines distribution.
Fascinatingly, this MEC device can utilize the CO2, and it can be converted to methane (Rabaey and Rozendal 2010; Lu and Ren 2016), acetate (Rabaey and Rozendal 2010), formate (Srikanth et al. 2014), and other compounds (Rabaey and Rozendal 2010; Roy et al. 2015; ElMekawy et al. 2016; Huang et al. 2016; Bajracharya et al. 2017). While the feasibility of the process has been reported in several cases, numerous challenges have yet to be overcome.
The most challenging problems to address are selectivity of the product, product separation from the solution, low reaction kinetics, and cell design. Regardless of these challenges, the results are quite promising and deserve further investigations (Santoro et al. 2017a).
8.5 Performance Evaluation by Electrochemical and Electroanalytical Techniques
8.5.1 Power/Voltage Generation
Power density is one of the normalized characteristic of the reactor in order to make it possible to compare the power output of the various types of fuel cells systems. The choice of the parameter that is generally used for the normalization depends on the application. Generally, the power output is usually normalized to three ways, the power density (P An, W/m 2) based on the projected anode surface area (A An) (Park et al. 1999; Park and Zeikus 2003; Liu et al. 2004; Rabaey et al. 2004).
The power density in the case of anode consists of a material which can be challenging to express the surface area of the granular material (Rabaey et al. 2005b) instead the area of the cathode (A Cat) can alternatively be used to obtain a power density (P Cat).
In order to perform engineering calculations for size and costing of reactors, the power is normalized to the reactor volume, or where PV is the volumetric power (W/m3) (Bullen et al. 2006),
8.5.2 Coulombic Efficiency
The coulombic efficiency (CE) is known as the ratio of total coulombs actually transferred to the anode against that theoretically present in the organic substrate for current generation over the time period. The energy conversion efficiency of MFCs describes which region of the energy present in the provided organic substrate ends up as an electrical energy.
The total coulombs obtained are determined by integrating the current over time, so that the coulombic efficiency for an MFC run in fed-batch mode, εc b, evaluated over a period of time tb, is calculated (Rabaey et al. 2005b; Cheng et al. 2006), where M is the molecular weight of oxygen, F is Faraday’s constant, b is the number of electrons exchanged (4) per mole of oxygen, v An is the volume of liquid in the anode compartment, and ΔCOD is the change in COD over time (t b). In the case of continuous flow through the system, the coulombic efficiency (εcb or CE) is calculated on the basis of current generated under steady conditions as where q is the volumetric influent flow rate and ΔCOD is the difference in the influent and effluent COD (Logan et al. 2006).
8.5.3 Electrokinetics and Mass Transfers
The examination of electron transfer kinetics is not only great interest but also even greater complexity. The kinetic parameters can be identified from the separation of the peak potentials and peak currents of a redox system as functions of the applied scan rate by using the cyclic voltammetry (CV), and the electron transfer rate constant can also be gained from Tafel plots at zero current (open circuit potential) (Harnisch and Freguia 2012).
8.5.3.1 Cyclic Voltammetry (CV)
The most common and direct technique for the determination of the mechanisms of electrode reactions underlying oxidation or reduction reactions can be done by using CV, which requires a three-electrode configuration to obtain accurate results (Zhao et al. 2009).
It is comparatively easy to find using CV whether a chemical system under study is reversible or irreversible. MFC studies employing CV generally use forward and backward voltage sweeps with rates in the range of 1–100 mV s−1. Multiple peaks in the cyclic voltammograms of the bioelectrochemical system can be witnessed due to multistep parallel or consecutive (series) mechanisms, or to the presence of several different redox species (Zhao et al. 2009).
In MFC electrochemical studies, CV experiments have been extensively used to (i) examine the mechanisms of electrode reactions involving both direct and indirect electron transfer between the biofilm and the electrode, (ii) examine the redox potentials of the chemical or biological species involved at the anode or cathode (for a reversible redox couple, the average of the cathodic and anodic peak gives the reversible potential for that couple referenced against the RE being employed), and (iii) evaluate the performance of the catalysts being studied (Zhao et al. 2009).
Even though CV is a simple technique and the results are obtained in a relatively short time, the background experiments (with blank electrolyte) are mandatory for high excellence mechanistic studies.
8.5.3.2 Rotating Ring Disk Electrode (RRDG) and Rotating Disk Electrode (RDE)
In electrochemical experiments where mass transfer is sensibly controlled and crucial for studying the precise kinetic parameters of electron transfer, detailed probing of electrochemical reaction mechanisms including enzyme electrodes can be done by using the hydrodynamic techniques, namely, RDE and RRDE.
These techniques have been used in the evaluation of catalyst or modified electrode performances and for quantifying the number of electrons involved in ORR (Bard et al. 2000). The desired cathode process is the full (4e−) reduction of oxygen to water, but partial (2e−) ORR will occur on carbon-based electrodes and results in the generation of substantial quantities of highly reactive hydrogen peroxide (H2O2), which might affect the microbial metabolism (Zhao et al. 2009).
RRDE studies take in an RDE with an additional ring electrode (separately poised at a controlled potential—hence it needs a biopotentiostat) to identify the products (including peroxides) of the electrochemical reactions occurring on the central disk electrode. Very small rotating disk electrodes can be used to find an expression of linear sweep voltammograms (LSV) in a most precise manner.
However, these techniques cannot always be used to probe the electrochemical manners of biofilms on electrode surfaces as the biofilm can be fragile and likely to be destroyed under the conditions of high-speed rotation. An additional possibility that will permit experiments with the rapid mass transfer is to design experiments, which can study the electrochemistry of a single microbe on some form of electrochemically inactive but electronically conductive electrode surface. The hydrogen oxidation reaction can be studied over electrode tips containing single Pt nanoparticles of defined size (Chen and Kucernak 2004; Zhao et al. 2009).
8.5.3.3 Differential Pulse Voltammetry (DPV)
DPV is also a voltammetry method with an improved sensitivity when compared to CV and LSV methods. A significant limitation of both CV and LSV is the substantial background levels from capacitive to non-Faradaic currents. Enhanced discrimination of Faradaic currents (electron transfer to and from an electrode) can be acquired using DPV, where the potential disconcertion consisting of small pulses will be superimposed upon a staircase waveform. DPV studies can also provide and improve the selectivity for observing different redox processes compared with CV and LSV (Zhao et al. 2009).
8.5.4 Electron Impedance Spectroscopy
Electrochemical impedance spectroscopy technique has a larger potential to examine and study the intricate impedance characteristics of microbial fuel cells (Ramaraja and Ramasamy 2013).
It is one of the most powerful tools for examining chemical and physical processes in solutions, at solid-solid interfaces and at solid-liquid interfaces as it permits the separation of the different voltage loss phenomena (Ramaraja and Ramasamy 2013).
With EIS technique the conductivity of electrode materials and membranes can also be measured easily. Above all, the way of nondestructive measurements makes EIS, a highly fascinating tool for studying the MFC performance without troubling its operation (Ramaraja and Ramasamy 2013).
Even after all the advantages, the knowledge of impedance from EIS alone is not enough, because this technique is only applicable to linear or quasi-linear system, but combining the EIS with the other electrochemical and biochemical techniques could make it better in understanding the performance of the bioelectrochemical systems (Ramaraja and Ramasamy 2013; Kashyap et al. 2014).
EIS of a system can be understood by two common graphical representations:
-
(i)
Nyquist plots: The main limitation of Nyquist plots is that they will not show the frequency denoted by each data point (each point being a depiction of the impedance vector in the complex plane at a particular frequency).
-
(ii)
Bode plots: This shows the frequency information as they are plots of the magnitude and phase angle of the impedance vector (Zhao et al. 2009).
In a microbial fuel cell, the bacterial populations will synthesize an endogenous mediator which may facilitate the electron transfer in the intracellular environment . The microorganisms adhere to the electrode surface and favor electron transfer, whereas the other microbes may secrete soluble mediators for electron transfer shuttles that is electron from bacteria to the electrode and vice versa (Kashyap et al. 2014).
EIS has been magnificently exploited to find the response of such mediators on charge transfer resistance. The charge transfer reaction and the bioelectrochemical reaction provide high impedance and mostly observed from mid- to high-frequency region in the Nyquist plot.
8.6 Challenges and Influencing Factors Defining the Performance of MFCs
There are numerous hurdles remaining to get overcome for MFCs to get adopted widely. One of the main disadvantages in the MFCs operation is the poor power output, which edges the performance of MFCs to drive electronic devices. The power output of MFCs cannot achieve the high-power level as like other sources of renewable energy , such as solar power, tidal power, nuclear power, wind power, and others for industrial applications (Xia et al. 2018).
More than all the material cost of the electrode, PEM and catalyst result in reducing the economic competitive scopes when compared with other sources of energy (Xia et al. 2018).
So far, the performances of the laboratory MFCs are still much lower than the ideal performance due to its operating condition effects. The power generation of an MFC is influenced by various factors including the type of microbes, organic substrate’s concentration, pH, temperature, ionic strength, and reactor configuration (Liu et al. 2005a; Du et al. 2007).
With a given MFC system, the following influencing parameters can be controlled to decline the polarizations in order to improve the performance of an MFC.
8.6.1 Voltage Reversal
The phenomena named voltage reversal and power overshoot are frequently witnessed in microbial fuel cell (MFC) systems, which results in the decline of the MFC’s performance (An et al. 2016).
One in four microbial fuel cell (MFC) units undergoes the issues of power overshoot. Series connection and stacks of the MFC unit to a high-current generating unit showed significant current loss of 57% owing to power overshoot and the resultant voltage reversal (Zhu et al. 2011; An et al. 2016).
The doubling back of power (or current) in a high-overvoltage region (i.e., power overshoot) is related with a drop in the current production rate below a specific working voltage. Researchers have reported that anode malfunctions may lead to power overshoot instigated by the population of the anodic bacterial community, rate of substrate utilization by the anode bacteria, and the maturity of the anodic biofilm (An et al. 2016; Sugnaux et al. 2017).
Peng et al. (2013b) claimed that the nonexistence of anodic abiotic capacitance might be a reason for power overshoot in MFCs.
Based on the literature survey, it was supposed that the power overshoot happened due to the lack of electrons (i.e., oxidation current), and it might be removed by supplying electrons to the anode of the MFC unit that experiences the power overshoot issue (An et al. 2016).
Zhu et al. (2013) stated that enhancement and acclimation of anode bacteria with a higher anode onset potential may possibly lead to increasing the maximum current of MFCs and be effective for reducing power overshoot related to the performance at a low anode onset potential.
The current loss for series connection of the two parallel-connected MFCs was reported by An et al. (2016) as small as 3%, and it clearly shows that series connection of parallel-connected MFCs might be a readily appropriate way for concurrently controlling the power overshoot and voltage reversal in MFC systems.
The challenges due to voltage reversals are resolvable, and optimal power generation is possible by using an MFC stack with shared anolyte was demonstrated by the most convenient techniques like electrical circuit alternation in the serial MFC stack, fast electrostimulation of biofilm growth, higher nutrient concentrations, and anolyte recirculation postponed voltage reversals, while the asymmetry of voltages in the stack endured and the power output persisted well below a balanced state (Sugnaux et al. 2017).
An interesting study demonstrated by Zhu et al. (2011) that stacked MFCs connected with reverse diodes, the voltages of each unit MFC almost equal their open circuit voltages (~0.75 V) and the voltage reversal occurs in the unit MFC with reverse diodes at the cathode end only. It clearly indicates that the imbalanced consumption of electrons in MFCs units, and the potential changes of a specific electrode directly result in voltage reversal.
8.6.2 Effect of Electrode Materials
Electrode modification studies are being actively investigated by several research groups to advance the MFC performances. In order to improve the performance of the MFC, the better performing electrodes should be used, because the anode materials may end up with the various potential losses mainly the activation which occurs during the transfer of electrons from or compound reacting at the electric surfaces and the concentration losses caused by rate of mass transport of a species to and from the electrode surface (Du et al. 2007).
Pt and Pt black electrodes are superior to other electrode materials like graphite plate, graphite felt, and carbon cloth electrodes for both anode and cathode constructions, due to its higher catalytic activity with regard to oxygen than graphite material, but their costs are high. MFCs with Pt or Pt-coated cathodes material may produce high-power densities than with graphite or graphite felt cathodes (Jang et al. 2004; Oh et al. 2004; Moon et al. 2006; Du et al. 2007).
Generally, the electrode in the MFC will have a certain resistance; therefore, the most efficient one should have the very least resistive (Liu et al. 2013a).
As the use of highly efficient electrode materials (i.e. platinum) is not economically feasible for large-scale applications, studies on exploring more cost-effective alternatives are in the priority in MFC research (Wei et al. 2011).
The role of the nanomaterials may pave the way for making the electrode materials more efficient with the characteristics like active electron transfer with high conductivity and mechanical strength. There is no requirement for bacteria adhesion on the electrode as well. The high performance, sustainable, scalable, and cost-effective MFC can be made only with the incorporation of the nanomaterials in the system (Juan and Nixon 2013).
8.6.3 pH Buffer and Electrolyte
The role of the electrolyte’s pH is very crucial in the proper functioning of the MFCs. If there is no pH buffer solution used in a working MFC, there will be a huge pH difference between both the chambers of the MFC reactor. Ideally there will be no shift in the pH, when the reaction rate of protons, electrons and oxygen at the cathode equals the production rate of protons at the anode. But this may lead to cause the transport barrier in the PEM during cross-membrane diffusion of the protons; proton transport through the membrane is slower than its production rate in the anode and, finally, results with the developed high pH level in the anodic chamber (Gil et al. 2003).
8.6.4 Proton Exchange Membrane System
Proton exchange membrane system is able to affect an MFC system’s internal resistance, concentration, and polarization loss; finally they will have the influence over the power output of the MFC. Nafion (DuPont, Wilmington, Delaware) is the most popular and commonly used PEM, because of its highly selective permeability of protons (Juan and Nixon 2013).
Nafion is still the outstanding PEM membrane in the market, despite the attempts by researchers to develop less expensive and more durable substitutes for it (Juan and Nixon 2013). As it is very expensive with another side effect like the cations, transport through the membrane is inevitable during the MFC operation.
In a batch mode accumulative system, the transportation of cation species other than protons through the Nafion dominates the charge balance between the anodic and cathodic chambers because its huge concentrations of Na +, K+, NH4+, Ca2+, and Mg2+ are much higher than the proton concentrations in the anolyte and catholyte (Logan et al. 2006).
Due to this, Nafion and other PEMs used in the MFCs are not necessarily proton-specific membranes but actually cation-specific membranes.
To ensure the optimum power output, the ratio of PEM surface area to system volume is very important. The PEM surface area has a huge impact on maximum power output if the power output is lower than a critical threshold. Eventually, the MFC internal resistance decreases with the increase of PEM surface area over a relatively larger range (Oh and Logan 2006). The phenomenon of the larger surface area in the PEM can be achieved with the usage of nanomaterials with the very high surface area.
8.6.5 Anodic Chamber Operating Conditions
The anodic chamber of the MFC directly relies on the important factors like the substrate/fuel type, pH, concentration, and feed rate. Even with the employed microbial consortium for the purpose of substrate degradation, power generation varies with the different fuels provided (Juan and Nixon 2013).
Various studies have shown that electricity production is mostly dependent on fuel concentration, in both the batch and fed-batch mode of operations. Generally in a wide concentration range of higher fuel concentration, a higher-power output is achieved (Juan and Nixon 2013). The increased inflow rate of feed is unlikely to affect the flora of microbes grown around the electrodes as biofilms.
The possible reason may be that the high feed rate brings in other alternate electron acceptors competing with the anode to lower the power output of the MFC (Juan and Nixon 2013).
8.6.6 Cathodic Chamber Operating Conditions
Oxygen is the most often used electron acceptor in MFCs for the cathodic reduction reaction. The power output of an MFC intensely is influenced by the concentration levels of electron acceptors. Notable, research studies of (Gil et al. 2003; Oh et al. 2004; Pham et al. 2004) had shown that DO was a major limiting factor for the electron acceptors when it remained below the air-saturated level.
Remarkably, a catholyte sparged with pure oxygen resulted with 38 mg/L DO did not further increased the power output, when compared to that of the air-saturated water at 7.9 mg/L DO (Min and Logan 2004; Oh et al. 2004; Pham et al. 2004).
8.7 Synergetic Applications of MFC’s
8.7.1 Powering Implanted Medical Devices
Among one of the strange and newly emerging applications of MFC to power the implanted medical devices with glucose and oxygen which are present in the blood, the implanted MFC may deliver power open-endedly and avoids the need for replacing the batteries with surgery (Kerzenmacher et al. 2008).
Due to the growing interests in MFC for powering the implanted devices, the human white blood cells were used as a source of electrons for an anode. This experiment using white blood cells in phosphate-buffered saline solution with a ferricyanide cathode generated a low current level of 1–3 μAcm2, but it perhaps could not be determined, if electron transported to the anode was through a direct or indirect mediated process (Tekle and Demeke 2015).
Several developments and improvements concerning the increase in the development of MFC for implanted devices are being added up. Scientists forecast that in the future a miniature MFC can be implanted in a human body to power an implantable medical device with the utilization of the nutrients supplied by the human body. This technology is principally favored for sustainable long-term power applications for the implanted devices. Nevertheless, this MFC will be used for this purpose only after studying the potential health and safety issues brought by the microorganisms in the MFC are comprehensively explained (Chia 2002).
8.7.2 Biohydrogen as Secondary Fuel
MFCs can be employed to generate secondary fuel like hydrogen (H2) as an alternate of electricity (Das and Mangwani 2010). Due to the energy-intensive and sustainable behavior, biological hydrogen (biohydrogen) production processes are found to be advantageous over the thermochemical and electrochemical processes (Parkash 2016).
Biohydrogen can be successfully produced by the dark fermentation produce, but the efficiency is low. For the fermentation of carbohydrate-rich wastewater resulted in the yield of less than 15% (Liu et al. 2005a; Huang et al. 2016). Moreover, the methanogenic depletion of hydrogen in the fermentation process ended with the substrates being majorly converted to acetate or butyrate as by-products (Cheng and Hamelers 2008; Parkash 2016).
An MFC can also be modified to generate the hydrogen gas (H2) by removing oxygen at the cathode region and the addition of a small voltage by the bioelectrochemically assisted microbial reactor (BEAMR) process or the biocatalyzed electrolysis process (Liu et al. 2005b; Logan and Regan 2006).
Around 8–9 mol-H2 could be generated in a process that uses glucose as a substrate, where the first-stage fermentation system achieves 2–3 mol-acetate/mol glucose and a second-stage BEAMR process recovers 2.9 mol-H2/mol-acetate. The power needed for the second stage is estimated to be equivalent to 0.5 mol-H2/mol-(Liu et al. 2005b; Logan and Regan 2006).
The production of biohydrogen through the BEAMR process is not restricted to glucose alone. Any biodegradable substrates which produce electricity in an MFC may work in a BEAMR system as well (Heilmann and Logan 2006; Logan and Regan 2006).
Through extensive study, Liu et al. (2005b) had revealed that the MFCs can produce about 8–9 mol H2/mol glucose potentially, when related to the typical 4 mol2/mol glucose achieved in other form of conventional fermentation methods. Consequently, MFCs will provide a renewable hydrogen source that may contribute to the overall hydrogen demand in this current hydrogen economy (Liu et al. 2005b; Tekle and Demeke 2015).
In a recent research work, an exfoliated molybdenum disulfide (MoS2-EF) catalyst was synthesized, and the obtained particle size was 200 ± 50 nm, 50-fold smaller than the pristine MoS2 catalyst . Hydrogen production rates in the same MEC with a Geobacter sulfurreducer act as a biocatalyst at the anode along with Pt, MoS2-EF, and the pristine MoS2 cathodes were 0.106, 0.133, and 0.083 m3d−1 m−3, respectively. This result promoted that MoS2-EF led to highly purified hydrogen and that this catalyst can serve as an electrochemical active and cost-effective alternative to Pt (Rozenfeld et al. 2018).
8.7.3 Wastewater Treatment
In the earlier 1991 (Habermann and Pommer 1991), the MFCs were employed for treating the municipal or community wastewater which contains a huge amount of organic compounds that can be utilized as the fuel for the MFCs.
The power generated by MFCs through the wastewater treatment process potentially halved the electricity needed in a conventional treatment process that consumes a lot of electric power for aerating in the activated sludge reactors along with the removal of 50–90% less solids (Tekle and Demeke 2015).
The alternate methods to treat wastewater are favored even with the need of high operating cost and huge operating energy. The significant ability of MFCs is to treat wastewater with the benefits of low-energy requirement along with the additional energy generation. The first demonstration of consuming domestic wastewater as the substrate for the generation of electricity by MFC was reported by Liu et al. (2005b) and Parkash (2016).
An MFC would be used in a wastewater treatment system as a substitute for the existing energy-demanding bioreactor like an activated sludge system as a net energy-producing system. On the other hand, economical scale-up of an MFC is yet a challenge, and also the costs should be to replace a conventional treatment system with an MFC-based design (Logan and Regan 2006).
The development of the high internal resistance during the scale-up of the MFC and its high materials costs are the greatest challenges in bringing the MFCs for the real-time application in the wastewater treatment system (Logan and Regan 2006).
8.7.4 Benthic/Sediment Microbial Fuel Cells
Sediment or benthic microbial fuel cells (SMFCs) come under the category of the bioelectrochemical systems (BESs), where this type of BESs comprising an anode embedded in the anoxic sediments and a cathode suspended at the surface of the water column to ensure aerobic condition.
Owing to their exceptional characteristics, SMFCs can be explored as new technology for eliminating organic pollutants from sediments (Yan et al. 2014) and for in situ bioremediation of organic-rich sediment (Zhou et al. 2015) and wastewater (Huang et al. 2011a).
SMFC became an exciting application in the microbial fuel cell research area that is able to generate electricity from the organic matter in aquatic sediments (Liu et al. 2005b). The sediment MFC design can be made to power devices placed on the seafloor or underwater environment , where it will be expensive and technically difficult to exchange traditional batteries routinely, and the sediment MFC is also known as benthic unattended generators (BUGs) (Kim et al. 2003).
Due to the abundance of exoelectrogenic bacteria in the sediments, the sediment MFCs are set to generate electricity. Remarkable works suggested that the sediment-anode united with seawater-cathode configuration harvested high energy from the net oxidation of marine sediment’s organic matter (Reimers et al. 2001; Parkash 2016).
The data about the natural environment can be supportive in understanding and modeling ecosystem responses, but the sensors distributed in the natural environment require power for operation. SMFCs can feasibly be used to power such devices, mostly in the river and deep-water environments where it is challenging to routinely access the system to replace the batteries. Sediment fuel cells are being advanced to monitor environmental systems such as creeks, rivers, and the ocean (Logan and Regan 2006).
The developed power densities are low in sediment fuel cells due to the low organic matter concentrations and their high intrinsic internal resistance. SMFCs developed to this date are limited to producing <30 mW/m2. Nevertheless, the low-power density can be equalized with the energy storage systems that release data in gusts to central sensors (Logan and Regan 2006).
8.8 Importance of Nanomaterials for the Sustainable Development of MFC Technology
Microbial fuel cells (MFCs) are the promising technology to solve the futuristic energy related the issues. The performance levels and unit cost of the MFCs significantly rely on the basis of architecture, choice of materials, and overall geometry. In this endeavor for the enhanced performance either with the readily available or bespoke nanomaterials, a number of researches are continuing in hunt of the optimum combination of “high performance/low cost/multi-functional” materials that can create a formula for easy and economical scale-up of the MFCs (Santoro et al. 2017a).
The limited productivity of MFCs compared to other fuel cell technologies and the high cost of their components are the two major obstacles to commercialization. Since the early emergence of nanomaterial, there has been a great concern in its potential applications due to its excellent conductivity, enormous surface area, and good mechanical strength (ElMekawy et al. 2017).
The application of nanomaterials could help to overcome such challenges while integrating with biocatalysts for the construction of MFCs, either as an anode to enhance the electron transfer efficiency or as a cathode to successfully catalyze the oxygen reduction reaction (ORR) (ElMekawy et al. 2017).
8.8.1 The Ultimate Doping Processes for Nanomaterials
There are quite a remarkable efforts that have been taken for modifying the electrocatalysts to ensure optimum performance of the microbial fuel cell; in that list, the doping of heteroatoms of the approximately the same radius, namely, boron, nitrogen, sulfur, and phosphorous, was substituted over the carbon atoms in the sp2 network (Zehtab Yazdi et al. 2015).
The O2 is electrocatalytically reduced on the cathode surface via scavenging the electrons from the anodic region, and thus the oxygen reduction reaction (ORR) efficiency predicts the cell’s overall performance (Liu et al. 2013b).
In general, the ORR takes place majorly through the four-electron pathway on platinum group metals (PGMs) as a catalyst (Bing et al. 2010) or two-electron pathway on non-noble materials like carbon black, Vulcan X, and activated carbon (Liu et al. 2013b).
Among the carbon nanomaterials, the metal-free electrocatalysts, namely, graphene, are at the forefront and also the promising alternative to the costlier Pt catalyst. Moreover, the reduced graphene was reported as a competent ORR catalyst which is proven for its fast electron transfer kinetics and excellent electrocatalytic activity (Tang et al. 2009)
8.8.1.1 Chemical Vapor Deposition (CVD)
The chemical vapor deposition (CVD) technique allows the carbon nanotubes (CNT) development in a diversity of forms, namely, powder, thick and thin films, aligned or entangled, straight or coiled, or even a preferred architecture of nanotubes, at predefined sites over a patterned substrate. This technique also offers better control over the growth parameters in contrast to other synthesis approaches. The parameters like atmosphere, carbon source , catalyst, and growth temperature are essential for the development of CNT growth in CVD technique (Koziol et al. 2010).
8.8.1.2 Thermal Catalytic Chemical Vapor Deposition (TCCVD)
The method of chemical vapor deposition (CVD) relies on the pyrolysis of hydrocarbons or other carbon feedstock through the addition of a nitrogen source like nitrogen, amines, ammonia, and nitriles in the furnace system over the surface of metallic catalyst particles, namely, ferrocene, cobaltocene, nickelocene, and others. The different quality of growth products was synthesized on the basis of varied conditions and parameters (Fig. 8.10). It has been recommended that only a minor concentration of nitrogen (<15%) could be introduced into MWNTs (Terrones et al. 1997).
Schematic diagram of thermal catalytic chemical vapor deposition (TCCVD). (Figure drawn with modifications after Koziol et al. 2010)
Because of the distinctive properties like high oxygen reduction reaction (ORR) efficiency due to their increased catalytic activity, outstanding reliability, and environmental friendliness, the nitrogen-doped carbon materials have attracted substantial attention (Wen et al. 2014).
In the case of nitrogen doping process, there are two exclusive routes available to the synthesis of nitrogen-doped carbon nanotubes (N-CNTs):
-
(i)
Direct delivery of heteroatoms with the carbon source stream, during the development of the nanotubes.
-
(ii)
Replacement of carbon atoms by heating the nitrogen-comprising compound with CNTs. The operating temperature range of TCVD is 500–1200 °C at atmospheric pressure for the synthesis processes (Koziol et al. 2010).
A novel low-price, scalable, artificial technique for the preparation of porous nitrogen-doped carbon nanosheet on grapheme (PNCN) is the carbonization of graphite oxide-polyaniline hybrid (GO-PANI), subsequently followed by KOH activation treatment (Wen et al. 2014). Due to its high concentration of nitrogen and high specific surface area, PNCN exhibited an excellent catalytic activity for ORR. As a result, the maximum power density of 1159.34 mW m−2 obtained with PNCN catalyst was higher than that of Pt/C catalyst (858.49 mW m−2) in an MFC. Therefore, porous nitrogen-doped carbon nanosheet could be a good alternative to Pt catalyst in MFCs.
The technique of plasma-enhanced CVD (PECVD) can be employed to synthesize carbon nanotubes and nanofibers, where the gaseous form of hydrocarbons will be in an ionized state over the transition metal catalyst, namely, nickel, iron, cobalt, etc. (Fig. 8.11). The electrical self-bias fields from plasma can be used to get the carbon nanotube and nanofiber with the optimum aligned growth which is perpendicular to the substrate (Koziol et al. 2010). There are two types of PECVD available, namely, hot filament PECVD which uses thermal energy for plasma generation and microwave PECVD used for the preparation of diamond films.
Schematic diagram of plasma-enhanced chemical vapor deposition (PECVD). (Figure drawn with modifications after (Koziol et al. 2010)
During a study, atmospheric pressure plasma jets (APPJs) were used to treat the carbon cloth, which resulted in increased hydrophilic nature of the carbon cloth, and notably no observable cracks or flaws were found. MFCs constructed with APPJ-treated carbon cloth electrodes had shown maximum power density of 7.56 mW m−2, which was superior to that of MFCs configured with untreated carbon cloth electrodes of the maximum power density of 2.38 mW−2 (Chang et al. 2016)
8.8.1.3 Hydrothermal/Solvothermal Process
The solvothermal process including the hydrothermal process is one of the oldest methods in the green chemistry (Fig. 8.12). This hydrothermal/solvothermal process generally called as a green technique due to its closed system conditions available in the hydrothermal reactors is being extensively used for synthesizing quartz and metal oxides and also for growing the single crystals (Komarneni et al. 2010).
Schematic illustration of the preparation of nitrogen-doped graphene by a solvothermal method in autoclave reactor. (Figure drawn with modifications after Ghanbarlou et al. 2015)
With the knowledge of the theoretical studies, it is predicted that graphene doping with nitrogen can alter its electronic properties and chemical reactivity. A novel process of one-pot direct synthesis of N-doped grapheme is through the reaction of tetrachloromethane with lithium nitride under mild conditions, which renders fabrication in gram scale. This new type of solvothermal process had maintained the temperature below 350 °C for the synthesis of N-doped graphene based on the reaction of tetrachloromethane with lithium nitride (Deng et al. 2011).
An innovative nanoflower-like NG with designed nitrogen types was directly produced by a low-temperature solvothermal process, and then Fe, Co, and Fe-Co nanoparticles are precipitated onto the NG using a reformed polyol technique. The electrocatalytic activity was measured by using cyclic voltammetry, electrochemical impedance spectroscopy, and linear sweep voltammetry. The higher electrocatalytic activity was exhibited by M/NG catalysts than NG catalysts. Further, the high stability of the Co/NG catalyst was proven by using the chronoamperometric techniques (Ghanbarlou et al. 2015).
In a recent endeavor, α -MnO2 nanorods (MN), α-MnO2 nanorods supported on reduced graphene oxide (MN/rGO), and α -MnO2 nanorods supported on nitrogen-doped reduced graphene oxide (MN/NrGO) were synthesized through a facile one-step hydrothermal method (Gautam et al. 2016). The synthesized MN/NrGO and MN/rGO electrocatalysts were coated over the cathode of the air-cathode microbial fuel cell for the experimental studies with a very low loading of 0.5 mg/cm2. The MFCs produced maximum power densities of 135.27 mW m2 and 85.45 mW m2, respectively, which was significantly higher as compared to pure rGO (57.63 mW m2) and MN (45.56 mW m2). From the experimental results, it was shown that MN/NrGO electrocatalysts are potentially efficient and cost-effective cathode catalysts for the practical application in MFCs.
8.8.2 Advancements in Anode Modifications
Microbial fuel cell (MFC) is one the most capable device, which can yield electrical energy through the conversion of the organic load with the help of microbial populations by the electron transfer mechanism (ETM). The need for the anode modification is very crucial in order to create a platform to permit the optimum level of extracellular electrons transfer (EET). Recently, remarkable levels in anode modifications are available by using the metal oxide nanomaterials due to their unique properties like good chemical stability, eco-friendliness, biocompatibility, etc.
Paramount importance is being provided in the selection of anode materials and architectures, which can directly influence the key performance parameters, namely, adhesion of microbial population, oxidation of organic loads, and electron transfer mechanisms (Fig. 8.13 and Table 8.3). There are several exclusive studies done to provide new knowledge in the area of anode surface modification by using the promising nanocomposite materials like nanometal oxides, nanotubes, nanofibers, nanosheets, etc.
8.8.2.1 Anodic Modifications with Metal Oxides Nanocomposite
The advancements and modifications in the surface of the anode play a vital role in enhancing the overall performance of the MFC and also ensuring the optimal biocatalytic activity. The anode surface can be modified to provide a favorable ambience for the microbial population, which results in the improved electron transfer from the bacterial biofilm to the anodic surfaces. The optimal bacterial population’s adhesion over the anode surfaces may result in increased power production with minimal loss. Notably, the performance of TiO2 nanosheet (TiO2-NS)-modified carbon paper anode (TiO2-NSs/CP) in Shewanella loihica PV-4 inoculated MFCs was significantly enhanced by intentional N doping of TiO2-NSs to modify its electronic properties (Yin et al. 2017). TiO2-NSs/CP was synthesized by calcination alone, respectively.
With NH3 atmosphere at various temperatures (T = 400, 500, 600, and 700 °C, T – temperature) N-doped anode nanocomposite (T-NTiO2-NSs/CP) was obtained. Out of other nanocomposite materials, the T600C-NTiO2-NSs/CP electrode had shown the maximal power density output increased by 196% and 50% compared to that from the bare CP and TiO2-NSs/CP electrodes.
Most fascinatingly, the improvement of electrocatalytic activity of the composite modified electrode was reported through the bioelectrochemical sensing platform with a high potential for the microbial-electrode interactions. It was designed based on decorated graphene oxide (GO) sheet with alumina (Al2O3) nanocrystals to get GO-Al2O3 nanocomposite through the self-assembly of GO and Al2O3 (Hassan et al. 2018). The cell viability was evaluated by monitoring the bioelectrochemical response of the living microbial cells (bacteria and yeast) upon stimulation with carbon source by using the modified GO-Al2O3 electrode nanocomposite.
8.8.2.2 Anodic Modifications with Nanotubes Composite
Anode materials like carbon nanotubes are being considered as the essential component of microbial fuel cells (MFCs), with enormous impacts on power generation performance and general cost. Due to the unique electronic and textural attributes, carbon nanotubes (CNTs) have been recognized as an electrode material with promising prospects with protruding properties of the large specific surface area, the capacity to be modified with various target groups and good chemical stability (Filip and Tkac 2014; Mehdinia et al. 2014). In a versatile study, supercapacitor (SC)-activated carbon (AC)-carbon nanotubes (CNTs) (SC-AC-CNTs) were used first to modify the carbon cloth (CC) anodes of microbial fuel cells (MFCs) and related with that of SC-AC and CC (Zhang et al. 2017). This study reveals that the specific surface area is increased from 219.519 to 283.643 m2g−1 after modifications. The anode’s effectiveness was tested in a urine-powered MFC (UMFC) also. The obtained power densities values of the UMFC assembled with SC-AC-CNTs and SC-AC-modified anodes are 899.52 mW m−2 and 555.10 mW m−2, which are 2.9 and 1.8 times higher than that of the blank UMFC, respectively.
A simple and effective study was done to illustrate clearly the activity effect of multiwalled carbon nanotubes (MWCNTs) and their functionality on anodic exoelectrogen in microbial fuel cells (Fan et al. 2017). In addition, the growth of E. coli and anode biofilm over the MWCNT-, MWCNT-COOH-, and MWCNT-NH2-modified anodes was related to a bare carbon cloth anode. The MFCs tested with the MWCNT-COOH-modified anode have attained a maximum power density of 560.40 mW/m2, which was 49% greater than the one gained with pure carbon cloth.
8.8.2.3 Anodic Modifications with Nanoarrays Composite
Modification of the anode surfaces with three-dimension (3D) materials in nanoscale and microscale is also a possible approach to enhance the surface participating in electron transfer and biofilm growth mechanism.
Edifying work was carried to generate brush-like polyaniline (BL-PANI) nanowire arrays over the surface of the Carbon cloth and it was employed as anode material for improving the power output from the MFCs. The pulsed voltage technique was used to fabricate BL-PANI with PANI nanowires of 230 nm in length (Zhang et al. 2017). Due to the BL-PANI modified carbon cloth anode, the power output was enhanced by 58.1% and 36.1% compared to that of plain carbon cloth and PANI-modified carbon cloth with ordinary structure , respectively. The remarkable performance was reported because of the high specific area and capacitive manners caused by superior morphology contributed to higher-power output when compared with normal PANI-modified or plain carbon cloth.
Notably, a facile approach for production of conductive polyaniline (PANI) nanoflower modified carbon cloth electrode was made and employed in the microbial fuel cell for energy production. It was done by simple tuning of aniline monomer’s concentration (Sonawane et al. 2018). By in situ polymerization technique, a uniformly distributed PANI nanoflower that was assembled from PANI nanoflakes which got anchored over the surface of carbon cloth electrode was fabricated. Moreover, the electrode modified with PANI nanoflower was employed as the anode of microbial fuel cells (MFC), which generated 2.6 and 6.5 times higher voltage and power output than these of pristine carbon cloth electrode, respectively.
8.8.3 Advancements in Cathode Modifications with Electro Catalyst for Improved Oxygen Reduction Reaction (ORR)
Abiotic catalysts used in MFCs cathodes can be classified in three main categories on the basis of the presence/absence of platinum and the presence/absence of earth-abundant metals. They are: (i) Platinum-based (PGM-based) with a 4e− transport mechanism identified, (ii) Carbonaceous-based (metal-free) with a 2e− transport mechanism identified and (iii) Platinum-group metal-free (PGM-free) with a more complex electron transfer mechanism (Santoro et al. 2017a).
Numerous methods are used to apply or incorporate the catalyst over the cathode surface. These can be based on: (i) Spraying technique, (ii) Doctor blade technique, (iii) Rolling, (iv) Pressing and (v) Drop casting.
In an MFC reactor, the biological oxidation may occur at the anode chamber with the presence of the exoelectrogenic bacterial populations which may produce free electrons and ions (Table 8.4 and Fig. 8.14). Those electrons will flow to the cathode via an external path where oxygen, a terminal electron acceptor get reduced. Even with the easy availability of Oxygen in the environment as an electron acceptor, the mechanism of oxygen reduction reaction (ORR) is slow and retains high-energy obstruction for its activation, which can be reduced by the usage of a competent catalyst.
8.8.3.1 Cathodic Modifications with Metal Oxides Nanocomposite
There were several studies reported on the development of the noble catalysts to enhance the oxygen reduction reaction (ORR) in the cathode surfaces. Notably, in a study, modified cathodes with titanium dioxide (TiO2) or hybrid graphene (HG) was employed in the dual-chamber MFC and reported with an enhanced catalytic activity of the cathode surface (Mashkour et al. 2017). A significant enhancement in the MFC performance was noted by calculating the power density value of 80 mW/m2 for GP-TiO2 and 220 mW/m2 for GP-HG compared to 30 mW/m2 for GP electrode. Furthermore, lower charge transfer resistance (Rct) was revealed by the modified electrodes compared to the bare electrode.
The highly projectable study was done by using the in situ growth and in situ polymerized embedded polyaniline (PANI) inpetaline NiO (NiO@PANI-CF) for electricity generation and dye degradation (Zhong et al. 2018). Due to the high capacitive property of NiO and the high conductivity of PANI reported with the improved electricity generation capacity on basis of its maximum output power density of 1078.8 mW·m−2 and less charge transfer resistance of 10.4 Ω. Notably, the color and COD removal capability of Reactive Brilliant Red X-3B attained 95.94% and 64.24% at 48 h, respectively.
A novel and simplistic method were proposed for the first time to fabricate the electrocatalyst for oxygen reduction reaction (ORR) namely the surface-oxidized cobalt phosphide (CoP) nanocrystals (NCs), for electricity generation in microbial fuel cell (MFC). This corallite-like CoP NCs were successfully synthesized by a hydrothermal reaction with subsequent phosphating treatment in the N2 atmosphere (Zou et al. 2016). The maximum power density of MFC overstated with 10% CoP reached 1914.4 ± 59.7 mW m−2, which is 108.5% higher than the control. Furthermore, characterizations of material pointed out the important and beneficial for ORR due to the surface oxide layer (CoOx) around the metallic CoP core.
8.8.3.2 Cathodic Modifications with Nanotube Composites
On one side, rigorous studies were attempted to overcome the challenges in making the very efficient electrocatalysts with the high electron transfer’s activity rate between anode and electrolyte solution. The carbon nanotubes (CNTs) were used to modify the carbon electrode surface is confirmed to be the efficient catalytic support for several electrochemical applications, due to its exclusive electrical conductivity, large specific surface area, and structural properties.
To improve the reduction of Orange II azo dye in cathode compartment of microbial fuel cells (MFCs) and power generation, through immobilization of redox mediators (RMs) with self-assembled peptide nanotubes (PNTs) as the carrier, a novel cathode modification was done over the carbon paper (CP) (Xu et al. 2017). MFCs loaded with the riboflavin (RF)-/PNT-altered cathode (PNT/RF/CP) or anthraquinone-2,6-disulfonate (AQDS)-/PNT-altered cathode (PNT/AQDS/CP) resulted in an enhanced decolorization rate to Orange II azo dyes, compared with the control electrode, along with 1.3 and 1.2-folds increased by the reduction kinetic constants. In addition, the MFCs with the PNT/AQDS/CP cathode and PNT/RF/CP cathode produced a very higher-power density of 55.5 mW m−2 and 72.6 mW m−2, respectively, than the unmodified control electrode (15.5 mW m−2).
To emphasize the remarkable work done on the carbon nanotube platinum (CNT/Pt) nanocomposite was fabricated by using the in situ method, used as a novel cathode catalyst in MFCs, and its performance was compared with platinum in MFC system (Halakoo et al. 2015). The results show that CNT coupled with Pt has reported with better performance than Pt in MFCs, due to better interaction with oxygen in the cathode chamber and high catalytic activity of CNT/Pt for oxygen reduction reaction. The catalytic power of the cathode improved when the quantity of Pt is raised to 0.2 mg/cm2and reached 73.01 mW/m2 at 246.67 mA/m2. After that the power density was greater than before slightly and reached to 75.95 (251.58), 77.028 (327.08), and 84.01 mW/m2 (341 mA/m2) with the loading rate of 0.3 mg/cm 2, 0.4 mg/cm2, and 0.5 mg/cm2, respectively.
Interestingly, the performance of sediment microbial fuel cells (SMFCs) was increased by fabricating it with the multiwalled, carbon nanotube (MWNT)-modified graphite felt (GF) cathode (Zhu et al. 2015). They proposed three types of MWNT-modified GF cathodes prepared by different electrophoretic deposition (EPD) times of the duration of 10, 30, and 60 min. Notably, the highest-power density of SMFC with MWNT-GF cathode at 60 min EPD was 215 ± 9.9 mW m2, which was nearly 1.6 times higher than the SMFC with an unmodified control GF cathode .
8.8.3.3 Cathodic Modifications with Graphene Nanocomposites
A novel and environmental-friendly study was proposed to develop a ternary composite containing reduced graphene oxide (rGO), poly(3,4-ethylene dioxythiophene) (PEDOT), and iron oxide (Fe3O4) nanorods. Further, the significant influence of the energy generation through air-cathode microbial fuel cells (ACMFC) was due to the application of competent oxygen reduction reaction (ORR) catalyst (Gnana kumar et al. 2016). Among the prepared composites, rGO/PEDOT/CC showed the ACMFC power and current densities of 2337 mW/m2and 7410 mA/m2, respectively, with the greater sturdiness of more than 600 h and this behavior is mainly due to its protracted surface area, extraordinary electrical conductivity, and large oxygen adsorption sites.
A versatile work was reported on the viability of graphene-based nanomaterials (RGOHI-AcOH vs. RGO/Ni nanoparticle composite) as a unique cathode catalyst in single-chamber air-cathode MFCs (Valipour et al. 2016). The prepared RGOHI-AcOH nanocomposite cathode has shown higher-power densities (>37%) than those with the RGO/Ni nanoparticle cathodes. Furthermore, MFCs with the highest-power density of 1683 ± 23 mW/m2 (CE ¼72 ± 3%), which covers 77% of that estimated for Pt/C (2201 ± 45 mW/m2 (CE¼81 ± 4%), were obtained from the double-loading RGOHI-AcOH cathodes.
Recently, three-dimensional graphene nanosheets (3D-GNS) were synthesized and employed as catalysts over the cathode surface in microbial fuel cells, operating under the neutral conditions (Santoro et al. 2017b). Further, the overall performances in MFCs exhibited that 3D-GNS had the utmost performances with power densities of 2.059 ± 0.003 Wm−2, 1.855 ± 0.007 Wm−2, and 1.503 ± 0.005 Wm−2 for loading of 10, 6, and 2 mg/cm−2, respectively.
8.8.4 Recent Trends in the Development of Nanocomposite Electrolyte Membranes
Proton exchange membrane (PEM), as well as other structural materials like electrode and electro catalysts, plays a crucial role in enduring the development of MFCs, especially for the real-time practical implementations (Santoro et al. 2017a).
Most of MFC designs need the separation of the anode and the cathode compartments by a cation exchange membrane (CEM). Exemptions are naturally separated systems such as sediment MFCs (Reimers et al. 2001).
The most commonly used CEM is Nafion (DuPont Co., USA), which is available from numerous suppliers, and other options to Nafion, such as Ultrex CMI-7000 (Membranes International Incorp., Glen Rock, NJ), also are well suited for MFC applications (Rabaey et al. 2004), but all the other types of membranes are significantly more cost-effective than Nafion (Logan et al. 2006).
Initially, various examples focused on the utilization of cation exchange membranes (CEM) (e.g., Nafion) that came from the existing hydrogen proton exchange membrane fuel cell technology (PEMFC) and membrane-based water treatment systems (Santoro et al. 2017a).
In the present endeavor, the market for ion exchange membranes is continually growing, and more systematic studies are necessary to develop the membranes with the nanocomposite electrolyte membranes for the enhanced performance and long-term stability in the MFCs (Logan et al. 2006). Several varieties of the nanocomposite electrolyte membranes can be fabricated on basis of the appropriate carrier polymers and the functional nanocomposites (Table 8.5).
8.8.4.1 Sulfonated Polymer Nanocomposite Electrolyte Membranes
The nanocomposite electrolyte membranes fabricated from the sulfonated groups of metal oxides not only create a path for proton transport but also act as means of transportation due to its negative charge. Both metal oxide (-OH group metal oxide) and water molecule can only create a path for proton transport but cannot act as vehicle due to their neutral charge (Ayyaru and Dharmalingam 2015).
A most promising study was carried with solution cast sulfonated TiO2 (S-TiO2)/polystyrene ethylene butylene polystyrene) SPSEBS nanocomposite membranes and the performance was analyzed by using single-chamber microbial fuel cell (SCMFC) (Ayyaru and Dharmalingam 2015). The results exposed that the incorporation of sulfonated TiO2 composite enhanced the proton conductivity of the SPSEBS membrane efficiently and showed the maximum peak power density of 1345 ± 17 mWm−2 for SPSEBS-S-TiO27.5% when compared to 695 ± 7 mWm−2and 835 ± 8 mWm−2obtained for SPSEBS and SPSEBS-TiO2 membranes, respectively. Furthermore, the prepared SPSEBS-S-TiO27.5% membrane was also compared to previously reported work with Nafion®(300 ± 10 mWm−2) in MFCs. Interestingly, the composite membrane supplied more than fourfold higher-power density. In addition, the oxygen mass transfer coefficient (KO) of nanocomposite membranes reduced due to the usage of the sulfonated TiO2 which in turn improved the columbic efficiency (CE).
The highly projectable study was reported by using nanocomposites QPSU, QPSU/GO, and QPSU/FGO which was fabricated by solvent-casting method (Elangovan and Dharmalingam 2016). The agar plate test confirmed the better antibacterial property of the QPSU/FGO-1.0% membrane among all five different composites and also reported with an extreme power density of 1036.15 mW m−2 and a current density of 2880.70 mAm−2. Additionally, the outperformed composite membrane was compared with the commercially available anion exchange membranes (AEMs) like AMI-7001, and it reveals that the membrane QPSU/FGO-1.0 wt% had the least resistance with enhanced the anti-biofouling efficiency even for long-term use.
In a recent study, nanocomposite membranes based on polyethersulfone (PES) with different amounts of Fe3O4 nanoparticles were synthesized and utilized as proton exchange membranes in a microbial fuel cell (MFC). The maximum power (9.59 ± 1.18 mW m−2) and current density (38.38 ± 4.73 mA m−2) generation were achieved by using a composite with 20 wt% of nanoparticles . The obtained results of mechanical characterization pointed out that increasing the nanoparticles content can compromise the mechanical properties of membranes leading to an extensive brittle behavior, while the tensile strength was found to be appropriate for durable operation of MFC (Di Palma et al. 2018).
This particular study demonstrated the evaluation of synthesized non-noble bimetallic [1:1 nickel (Ni): cobalt (Co)] nanocatalyst supported on sulfonated polyaniline (SPAni) in MFC. Furthermore, in MFC, a maximum power density of ~659.79 mW/m2 was witnessed with prospective Ni-Co/SPAni catalyst also compared to the corresponding Pt/C catalyst (~483.48 mW/m2). The results strongly direct the potential application of a conducting polymer such as SPAni as supporting matrix in bimetallic Ni-Co catalyst system that could also alternatively function as an efficient cathode catalyst over the traditionally used costly Pt/C catalyst in MFCs operation (Papiya et al. 2018).
8.8.4.2 Non-sulfonated Polymer Nanocomposite Electrolyte Membranes
Other than the sulfonated polymer, presently the non-sulfonated polymer nanocomposite electrolyte membrane has got remarkable consideration as well. The low-cost non-sulfonated polymer like PVA with the inorganic composite had shown an outstanding dimensional and thermal stabilities, good mechanical stability, controllable physical properties, and good hydrophilic and electrochemical properties , all of which are necessary for ion exchange membranes (Anis et al. 2008).
Noteworthy, a study was presented with the development and application of a proton exchange polymer membrane separator containing of graphene oxide (GO), poly(vinyl alcohol) (PVA), and silicotungstic acid (STA), and it was employed in a single-chamber microbial fuel cell (SMFC) (Khilari et al. 2013). Interestingly, an extreme power density of 1.9 W/m3 was attained when acetate wastewater was treated in a MFC equipped with a PVA-STA-GO non-sulfonated polymer nanocomposite.
Remarkable work was reported with the fabricated 12-mm-thin nanocomposite fuel cell membranes, where poly(vinylidene fluoride -co-hexafluoropropylene) (PVDF-HFP) nanofibers are directly electrospunned onto the gas diffusion electrodes (Breitwieser et al. 2017). At the temperature of 120 °C and 35% relative humidity, stoichiometric 1.5/2.5 H2/airflow and atmospheric pressure, the power density of the DMD fuel cell (0.19 W cm−2), resulted with 1.7 times higher than that of the reference fuel cell (0.11 W cm−2) with Nafion HP membrane and identical catalyst. Additionally, a 100 h accelerated stress test exposed a voltage decline of below 0.8 mV h−1, which is in the range of literature values notably for the thicker reinforced membranes.
8.8.4.3 Natural Polymer Nanocomposite Electrolyte Membranes
Time being we are in the growing need of finding the solution to replace the costlier and not environmental-friendly polymer electrolyte membranes like Nafion® without compromising the maximum power density generation.
An adaptable work reported the influence of multiwalled carbon nanotube (MWCNT) in water absorption, oxygen crossover, and performance of chitosan (CHIT) in single-chamber microbial fuel cell (SCMFC) (Narayanaswamy Venkatesan and Dharmalingam 2013). They also reported the maximum power density yield of CHIT–MWCNT composite membrane, and it was greater compared to that of CHIT membrane with 38.16 mW/m2 and 46.94 mW/m2 for CHIT and CHIT–MWCNT, respectively.
A promising study was reported by using a novel quaternized poly(vinyl alcohol)/chitosan/molybdenum disulfide (QPVA/CS/MoS2) anion exchange membranes (AEMs) prepared with MoS2 nanosheets (0, 0.1, 0.2, 0.5 and 1 wt%) into QPVA/CS mixture solution. The membrane was cast by the solution casting method and employed in the direct methanol alkaline fuel cell (DMAFC) (Jiang et al. 2018). Notably, with the casted nanocomposite electrolyte membranes of 1.0 wt% MoS2, the QPVA/CS/MoS2 was reported with the lowest methanol permeability of 0.210 × 10−7 cm2·s−1.
For the first time, chitosan nano-ZnO composite film was prepared by a simple one-pot method. The potential applicability of composite films as an efficient antimicrobial packaging material was evaluated, and it exhibited enhanced antimicrobial efficacy as compared with pure chitosan film, and it is linearly related to the amount of ZnO particles in the matrix (Mujeeb Rahman et al. 2018).
8.9 Conclusions
In general, the MFCs exploiting the naturally occurring microbes decomposing the organic materials through the exoelectrogenic pathways are used to both clean water and produce electrical energy by oxidizing the organic materials from the wastewater. Therefore, it has created tremendous attraction to the multidisciplinary researchers. Even though it has many barriers in the power production, it has viable application with usage of cost-effective materials as a possible future technology in the field of wastewater treatment sector. With the aid of future leading nanotechnology, a great platform can be obtained in terms of maximum power production by minimizing the obstacle in the MFCs operation in various components like anodic and cathodic electrode modification and membrane development. Moreover, with the support of nanotechnology in MFCs, the surface area to volume ratio can be enlarged, for providing an easier and more direct access between microbes and electrodes for a more an efficient transfer of electrons at the anode modification .
Furthermore, modification of cathode with nanomaterials could reduce the potential in the air cathode and creates the opportunities to search for the alternative non-noble metal catalyst. A recent advance in the hybrid bio-supercapacitor with the electrodes modified with the nanomaterials has shown to improve the efficiency of the capacitance behavior and makes the process viable. Moreover, few issues such as biocompatibility, environmental-friendly synthesis, and minimum nanotoxicology effect to the environment could take into account the implementation of nanotechnology in MFCs application.
As afore-discussed elaborately, MFCs can be used potentially for different applications. When used in wastewater treatment, a large surface area is needed for biofilm to build upon the anode. An advanced nanomaterial is needed in creating a less expensive and high-performance MFCs. It is impractical to expect the power density output from an MFC to match the conventional chemical fuel cell like a hydrogen-powered fuel cell. The fuel in an MFC is more often the wastewater treatment in the anodic chamber that has a limited releasable energy and inherent naturally low catalytic rate of the microbes and even at their fastest-growing microbes are relatively slow transformers.
Even with all the abovementioned challenges, the coulombic efficiency of more than 90% has been reported with the MFCs incorporated with nanomaterials. Currently, basic knowledge has been gained through a century of extensive research in the field of MFCs; there is still a lot to be learned about the extensive applications of the nanomaterials for developing MFC to serve the wide-range and large-scale applications. Through all the above-discussed research, MFC can be strongly considered as the promising technology with sustainable materials for the development of the low-cost and futuristic high-performance microbial fuel cells.
References
Aelterman P, Rabaey K, Verstraete W (2006) Continuous electricity generation at high voltages and currents using stacked microbial fuel cells. Environ Sci Technol 40:3388–3394. https://doi.org/10.1021/es0525511
An J, Kim T, Chang IS (2016) Concurrent control of power overshoot and voltage reversal with series connection of parallel-connected microbial fuel cells. Energ Technol 4:729–736. https://doi.org/10.1002/ente.201500466
Anis A, Banthia AK, Bandyopadhyay S (2008) Synthesis & characterization of PVA/STA composite polymer electrolyte membranes for fuel cell application. J Mater Eng Perform 17:772–779. https://doi.org/10.1007/s11665-008-9200-1
Ayyaru S, Dharmalingam S (2015) A study of influence on nanocomposite membrane of sulfonated TiO2and sulfonated polystyrene-ethylene-butylene-polystyrene for microbial fuel cell application. Energy 88:202–208. https://doi.org/10.1016/j.energy.2015.05.015
Babanova S, Carpenter K, Phadke S et al (2017) The effect of membrane type on the performance of microbial electrosynthesis cells for methane production. J Electrochem Soc 164:H3015–H3023. https://doi.org/10.1149/2.0051703jes
Bajracharya S, Srikanth S, Mohanakrishna G et al (2017) Biotransformation of carbon dioxide in bioelectrochemical systems: state of the art and future prospects. J Power Sources 356:256–273. https://doi.org/10.1016/j.jpowsour.2017.04.024
Bard AJ, Faulkner LR, Swain E, Robey C (2000) Electrochemical methods fundamentals and applications
Bhunia P, Dutta K (2018) Biochemistry and electrochemistry at the electrodes of microbial fuel cells. Elsevier B.V
Bing Y, Liu H, Zhang L, Ghosh D, Zhang J (2010) Nanostructured Pt-alloy electrocatalysts for PEM fuel cell oxygen reduction reaction. Chem Soc Rev 39(6):2184
Bond DR, Lovley DR (2003) Electricity production by Geobacter sulfurreducens attached to electrodes electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol 69:1548–1555. https://doi.org/10.1128/AEM.69.3.1548
Bond DR, Lovley DR (2005) Evidence for involvement of an Electron shuttle in electricity generation by Geothrix fermentans evidence for involvement of an Electron shuttle in electricity generation by Geothrix fermentans. Appl Environ Microbiol 71:2186–2189. https://doi.org/10.1128/AEM.71.4.2186
Breitwieser M, Klose C, Klingele M et al (2017) Simple fabrication of 12 μm thin nanocomposite fuel cell membranes by direct electrospinning and printing. J Power Sources 337:137–144. https://doi.org/10.1016/j.jpowsour.2016.10.094
Bullen RA, Arnot TC, Lakeman JB, Walsh FC (2006) Biofuel cells and their development. Biosens Bioelectron 21:2015–2045. https://doi.org/10.1016/j.bios.2006.01.030
Busalmen JP, Esteve-Nuñez A, Feliu JM (2008) Whole cell electrochemistry of electricity-producing microorganisms evidence an adaptation for optimal exocellular electron transport. Environ Sci Technol 42:2445–2450. https://doi.org/10.1021/es702569y
Cao X, Huang X, Liang P et al (2009) A new method for water desalination using microbial desalination cells. Environ Sci Technol 43:7148–7152. https://doi.org/10.1021/es901950j
Chae KJ, Choi MJ, Lee JW et al (2009) Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour Technol 100:3518–3525. https://doi.org/10.1016/j.biortech.2009.02.065
Chang IS, Moon H, Bretschger O et al (2006) Electrochemically active bacteria (EAB) and mediator-less microbial fuel cells. J Microbiol Biotechnol 16:163–177
Chang SH, Liou JS, Liu JL et al (2016) Feasibility study of surface-modified carbon cloth electrodes using atmospheric pressure plasma jets for microbial fuel cells. J Power Sources 336:99–106. https://doi.org/10.1016/j.jpowsour.2016.10.058
Chen S, Kucernak A (2004) Electrocatalysis under conditions of high mass transport: investigation of hydrogen oxidation on single submicron Pt particles supported on carbon. J Phys Chem B 108:13984–13994. https://doi.org/10.1021/jp048641u
Cheng S, Hamelers HVM (2008) Critical review microbial electrolysis cells for high yield hydrogen gas production from organic matter. 42
Cheng S, Liu H, Logan BE (2006) Increased power generation in a continuous flow MFC with advective flow through the porous anode and reduced electrode spacing. Environ Sci Technol 40:2426–2432. https://doi.org/10.1021/es051652w
Chia MA (2002) Miniatured microbial fuel cell. Technical digest of solid state sensors and actuators workshop, Hilton Head Island, pp 59–60
Choi Y, Jung E, Kim S, Jung S (2003) Membrane fluidity sensoring microbial fuel cell. Bioelectrochemistry 59(1–2):121–127
Coates JD, Wrighton KC (2009) Microbial fuel cells: plug-in and power-on microbiology. Microbe Mag 4:281–287. https://doi.org/10.1128/microbe.4.281.1
Das S, Mangwani N (2010) Recent developments in microbial fuel cells: a review. J Sci Ind Res (India) 69:727–731
Davis F, Higson SPJ (2007) Biofuel cells-recent advances and applications. Biosens Bioelectron 22:1224–1235. https://doi.org/10.1016/j.bios.2006.04.029
De Juan A, Nixon B (2013) Technical evaluation of the microbial fuel cell technology in wastewater applications declaration: 1–18. https://doi.org/10.13140/2.1.4481.0569
Deng D, Pan X, Yu L et al (2011) Toward N-doped graphene via solvothermal synthesis. Chem Mater 23:1188–1193. https://doi.org/10.1021/cm102666r
Di Palma L, Bavasso I, Sarasini F et al (2018) Synthesis, characterization and performance evaluation of Fe3O4/PES nano composite membranes for microbial fuel cell. Eur Polym J 99:222–229. https://doi.org/10.1016/j.eurpolymj.2017.12.037
Du Z, Li H, Gu T (2007) A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. Biotechnol Adv 25:464–482. https://doi.org/10.1016/j.biotechadv.2007.05.004
Elangovan M, Dharmalingam S (2016) A facile modification of a polysulphone based anti biofouling anion exchange membrane for microbial fuel cell application. RSC Adv 6:20571–20581. https://doi.org/10.1039/c5ra21576e
ElMekawy A, Hegab HM, Mohanakrishna G et al (2016) Technological advances in CO2conversion electro-biorefinery: a step toward commercialization. Bioresour Technol 215:357–370. https://doi.org/10.1016/j.biortech.2016.03.023
ElMekawy A, Hegab HM, Losic D et al (2017) Applications of graphene in microbial fuel cells: the gap between promise and reality. Renew Sust Energ Rev 72:1389–1403. https://doi.org/10.1016/j.rser.2016.10.044
Escapa A, Mateos R, Martínez EJ, Blanes J (2016) Microbial electrolysis cells: an emerging technology for wastewater treatment and energy recovery. From laboratory to pilot plant and beyond. Renew Sust Energ Rev 55:942–956. https://doi.org/10.1016/j.rser.2015.11.029
Fan Y, Xu S, Schaller R et al (2011) Nanoparticle decorated anodes for enhanced current generation in microbial electrochemical cells. Biosens Bioelectron 26:1908–1912. https://doi.org/10.1016/j.bios.2010.05.006
Fan M, Zhang W, Sun J et al (2017) Different modified multi-walled carbon nanotube–based anodes to improve the performance of microbial fuel cells. Int J Hydrog Energy 42:22786–22795. https://doi.org/10.1016/j.ijhydene.2017.07.151
Feng Y, Wang X, Logan BE, Lee H (2008) Brewery wastewater treatment using air-cathode microbial fuel cells. Appl Microbiol Biotechnol 78:873–880. https://doi.org/10.1007/s00253-008-1360-2
Filip J, Tkac J (2014) Is graphene worth using in biofuel cells? Electrochim Acta 136:340–354. https://doi.org/10.1016/j.electacta.2014.05.119
Gautam RK, Bhattacharjee H, Venkata Mohan S, Verma A (2016) Nitrogen doped graphene supported α-MnO2 nanorods for efficient ORR in a microbial fuel cell. RSC Adv 6:110091–110101. https://doi.org/10.1039/C6RA23392A
Ghanbarlou H, Rowshanzamir S, Kazeminasab B, Parnian MJ (2015) Non-precious metal nanoparticles supported on nitrogen-doped graphene as a promising catalyst for oxygen reduction reaction: synthesis, characterization and electrocatalytic performance. J Power Sources 273:981–989. https://doi.org/10.1016/j.jpowsour.2014.10.001
Gil GC, Chang IS, Kim BH et al (2003) Operational parameters affecting the performance of a mediator-less microbial fuel cell. Biosens Bioelectron 18:327–334. https://doi.org/10.1016/S0956-5663(02)00110-0
Gnana kumar G, Joseph Kirubaharan C, Yoo DJ, Kim AR (2016) Graphene/poly(3,4-ethylenedioxythiophene)/Fe3O4nanocomposite – an efficient oxygen reduction catalyst for the continuous electricity production from wastewater treatment microbial fuel cells. Int J Hydrog Energy 41:13208–13219. https://doi.org/10.1016/j.ijhydene.2016.05.099
Habermann W, Pommer EH (1991) Biological fuel cells with sulphide storage capacity. Appl Microbiol Biotechnol 35(1)
Halakoo E, Khademi A, Ghasemi M et al (2015) Production of sustainable energy by carbon nanotube/platinum catalyst in microbial fuel cell. Procedia CIRP 26:473–476. https://doi.org/10.1016/j.procir.2014.07.034
Harnisch F, Freguia S (2012) A basic tutorial on cyclic voltammetry for the investigation of electroactive microbial biofilms. Chem – Asian J 7:466–475. https://doi.org/10.1002/asia.201100740
Hassan M, Wei H, Qiu H, Su Y, Jaafry SWH, Zhan L, Xie B (2018) Power generation and pollutants removal from landfill leachate in microbial fuel cell: variation and influence of anodic microbiomes. Bioresour Technol 247:434–442
He Z, Angenent LT (2006) Application of bacterial biocathodes in microbial fuel cells. Electroanalysis 18:2009–2015. https://doi.org/10.1002/elan.200603628
Heilmann J, Logan BE (2006) Production of electricity from proteins using a microbial fuel cell. Water Environ Res 78:531–537. https://doi.org/10.2175/106143005X73046
Huang L, Chai X, Chen G, Logan BE (2011a) Effect of set potential on hexavalent chromium reduction and electricity generation from biocathode microbial fuel cells. Environ Sci Technol 45:5025–5031. https://doi.org/10.1021/es103875d
Huang YX, Liu XW, Xie JF et al (2011b) Graphene oxide nanoribbons greatly enhance extracellular electron transfer in bio-electrochemical systems. Chem Commun 47:5795–5797. https://doi.org/10.1039/c1cc10159e
Huang Z, Jiang D, Lu L, Ren ZJ (2016) Ambient CO2capture and storage in bioelectrochemically mediated wastewater treatment. Bioresour Technol 215:380–385. https://doi.org/10.1016/j.biortech.2016.03.084
Jang JK, Pham TH, Chang IS et al (2004) Construction and operation of a novel mediator- and membrane-less microbial fuel cell. Process Biochem 39:1007–1012. https://doi.org/10.1016/S0032-9592(03)00203-6
Ji J, Jia Y, Wu W et al (2011) A layer-by-layer self-assembled Fe2O3nanorod-based composite multilayer film on ITO anode in microbial fuel cell. Colloids Surf A Physicochem Eng Asp 390:56–61. https://doi.org/10.1016/j.colsurfa.2011.08.056
Jiang X, Sun Y, Zhang H, Hou L (2018) Preparation and characterization of quaternized poly(vinyl alcohol)/chitosan/MoS2composite anion exchange membranes with high selectivity. Carbohydr Polym 180:96–103. https://doi.org/10.1016/j.carbpol.2017.10.023
Kadier A, Simayi Y, Abdeshahian P et al (2016) A comprehensive review of microbial electrolysis cells (MEC) reactor designs and configurations for sustainable hydrogen gas production. Alex Eng J 55:427–443. https://doi.org/10.1016/j.aej.2015.10.008
Kamaraj SK, Romano SM, Moreno VC et al (2015) Use of novel reinforced cation exchange membranes for microbial fuel cells. Electrochim Acta 176:555–566. https://doi.org/10.1016/j.electacta.2015.07.042
Kano K et al (1999) Bifidobacterium longum. Biochim Biophys Acta 1428:241–250
Karube I, Matsunaga T, Tsuru S, Suzuki S (1976) Continuous hydrogen production by immobilized whole cells of Clostridium butyricum. Biochim Biophys Acta – Gen Subj 444:338–343. https://doi.org/10.1016/0304-4165(76)90376-7
Kashyap D, Dwivedi PK, Pandey JK et al (2014) Application of electrochemical impedance spectroscopy in bio-fuel cell characterization: a review. Int J Hydrog Energy 39:20159–20170. https://doi.org/10.1016/j.ijhydene.2014.10.003
Kerzenmacher S, Ducrée J, Zengerle R, von Stetten F (2008) Energy harvesting by implantable abiotically catalyzed glucose fuel cells. J Power Sources 182:1–17. https://doi.org/10.1016/j.jpowsour.2008.03.031
Khilari S, Pandit S, Ghangrekar MM et al (2013) Graphene oxide-impregnated PVA-STA composite polymer electrolyte membrane separator for power generation in a single-chambered microbial fuel cell. Ind Eng Chem Res 52:11597–11606. https://doi.org/10.1021/ie4016045
Kim BH, Chang IS, Gil GC et al (2003) Novel BOD (Biochemical Oxygen Demand) sensor using mediator-less microbial fuel cell. Biotechnol Lett 25:541–545. https://doi.org/10.1023/A:1022891231369
Kokabian B, Gude VG, Smith R, Brooks JP (2018) Evaluation of anammox biocathode in microbial desalination and wastewater treatment. Chem Eng J 342:410–419. https://doi.org/10.1016/j.cej.2018.02.088
Komarneni S, Noh YD, Kim JY, et al (2010) ChemInform abstract: solvothermal/hydrothermal synthesis of metal oxides and metal powders with and without microwaves. ChemInform 41:no-no. https://doi.org/10.1002/chin.201043010
Koziol K et al (2010) Synthesis of carbon nanostructures by CVD method. Carbon oxide nanostructures. Adv Struct Mater 5:23–49. https://doi.org/10.1007/8611_2010_12
Labelle E, Bond DR (2009) Cyclic voltammetry for the study of microbial electron transfer at electrodes. Bioelectrochemical Syst Extracell Electron Transf Biotechnol Appl 137–152
Liu H, Logan BE (2004) Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ Sci Technol 38:4040–4046. https://doi.org/10.1021/es0499344
Liu H, Ramnarayanan R, Logan BE (2004) Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ Sci Technol 38:2281–2285. https://doi.org/10.1021/es034923g
Liu H, Cheng SA, Logan BE (2005a) Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ Sci Technol 39:5488–5493. https://doi.org/10.1021/es050316c
Liu H, Grot S, Logan BE (2005b) Electrochemically assisted microbial production of hydrogen from acetate. Environ Sci Technol 39:4317–4320. https://doi.org/10.1021/es050244p
Liu H, Hu H, Chignell J, Fan Y (2010) Microbial electrolysis: novel technology for hydrogen production from biomass. Biofuels 1:129–142. https://doi.org/10.4155/bfs.09.9
Liu J, Qiao Y, Guo CX et al (2012) Graphene/carbon cloth anode for high-performance mediatorless microbial fuel cells. Bioresour Technol 114:275–280. https://doi.org/10.1016/j.biortech.2012.02.116
Liu L, Tsyganova O, Lee DJ et al (2013a) Double-chamber microbial fuel cells started up under room and low temperatures. Int J Hydrog Energy 38:15574–15579. https://doi.org/10.1016/j.ijhydene.2013.02.090
Liu Y, Liu H, Wang C et al (2013b) Sustainable energy recovery in wastewater treatment by microbial fuel cells: stable power generation with nitrogen-doped graphene cathode. Environ Sci 47:13889–13895
Liu XW, Chen JJ, Huang YX et al (2014) Experimental and theoretical demonstrations for the mechanism behind enhanced microbial electron transfer by CNT network. Sci Rep 4:1–7. https://doi.org/10.1038/srep03732
Logan BE, Regan JM (2006) Microbial fuel cells—challenges and applications. Environ Sci Technol 40:5172–5180. https://doi.org/10.1021/es0627592
Logan BE, Murano C, Scott K et al (2005) Electricity generation from cysteine in a microbial fuel cell. Water Res 39:942–952. https://doi.org/10.1016/j.watres.2004.11.019
Logan BE, Hamelers B, Rozendal R et al (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192. https://doi.org/10.1021/es0605016
Lovley DR (2006a) Bug juice: harvesting electricity with microorganisms. Nat Rev Microbiol 4:497–508. https://doi.org/10.1038/nrmicro1442
Lovley DR (2006b) Microbial fuel cells: novel microbial physiologies and engineering approaches. Curr Opin Biotechnol 17:327–332. https://doi.org/10.1016/j.copbio.2006.04.006
Lu L, Ren ZJ (2016) Microbial electrolysis cells for waste biorefinery: a state of the art review. Bioresour Technol 215:254–264. https://doi.org/10.1016/j.biortech.2016.03.034
Mahadevan A, Gunawardena D A, Fernando S (2014) Biochemical and electrochemical perspectives of the anode of a microbial fuel cell. Technol Appl Microb Fuel Cells 13–32. https://doi.org/10.5772/57200
Malvankar NS, Vargas M, Nevin KP et al (2011) Tunable metallic-like conductivity in microbial nanowire networks. Nat Nanotechnol 6:573–579. https://doi.org/10.1038/nnano.2011.119
Mashkour M, Rahimnejad M, Pourali SM et al (2017) Catalytic performance of nano-hybrid graphene and titanium dioxide modified cathodes fabricated with facile and green technique in microbial fuel cell. Prog Nat Sci Mater Int 27:647–651. https://doi.org/10.1016/j.pnsc.2017.11.003
Mehdinia A, Ziaei E, Jabbari A (2014) Multi-walled carbon nanotube/SnO2 nanocomposite: a novel anode material for microbial fuel cells. Electrochim Acta 130:512–518. https://doi.org/10.1016/j.electacta.2014.03.011
Menicucci J, Beyenal H, Marsili E, Veluchamy GD, Lewandowski Z (2006) Procedure for determining maximum sustainable power generated by microbial fuel cells. Environ Sci Technol 40(3):1062–1068
Min B, Logan BE (2004) Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ Sci Technol 38:5809–5814. https://doi.org/10.1021/es0491026
Min B, Cheng S, Logan BE (2005) Electricity generation using membrane and salt bridge microbial fuel cells. Water Res 39(9):1675–1686
Moon H, Chang IS, Kim BH (2006) Continuous electricity production from artificial wastewater using a mediator-less microbial fuel cell. Bioresour Technol 97:621–627. https://doi.org/10.1016/j.biortech.2005.03.027
Mujeeb Rahman P, Abdul Mujeeb VM, Muraleedharan K, Thomas SK (2018) Chitosan/nano ZnO composite films: enhanced mechanical, antimicrobial and dielectric properties. Arab J Chem 11:120–127. https://doi.org/10.1016/j.arabjc.2016.09.008
Myers JM, Myers CR (2001) Role for outer membrane cytochromes OmcA and OmcB of Shewanella putrefaciens MR-1 in reduction of manganese dioxide. Appl Environ Microbiol 67:260–269. https://doi.org/10.1128/AEM.67.1.260-269.2001
Narayanaswamy Venkatesan P, Dharmalingam S (2013) Characterization and performance study on chitosan-functionalized multi walled carbon nano tube as separator in microbial fuel cell. J Memb Sci 435:92–98. https://doi.org/10.1016/j.memsci.2013.01.064
Nevin KP, Woodard TL, Franks AE et al (2010) Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. MBio 1:e00103-10. https://doi.org/10.1128/mBio.00103-10
Niessen J, Harnisch F, Rosenbaum M, Schroder U, Scholz F (2006) Heat treated soil as convenient and versatile source of bacterial communities for microbial electricity generation. Electrochem Commun 8(5):869–873
Oh S-E, Logan BE (2006) Proton exchange membrane and electrode surface areas as factors that affect power generation in microbial fuel cells. Appl Microbiol Biotechnol 70(2):162–169
Oh S, Min B, Logan BE (2004) Cathode performance as a factor in electricity generation in microbial fuel cells. Environ Sci Technol 38:4900–4904. https://doi.org/10.1021/es049422p
Pant D, Van Bogaert G, Diels L, Vanbroekhoven K (2010) A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour Technol 101:1533–1543. https://doi.org/10.1016/j.biortech.2009.10.017
Papiya F, Pattanayak P, Kumar P et al (2018) Development of highly efficient bimetallic nanocomposite cathode catalyst, composed of Ni:Co supported sulfonated polyaniline for application in microbial fuel cells. Electrochim Acta 282:931–945. https://doi.org/10.1016/j.electacta.2018.07.024
Park DH, Zeikus JG (2000) Electricity generation in microbial fuel cells using neutral red as an electronophore electricity generation in microbial fuel cells using neutral red as an electronophore. Appl Environ Microbiol 66:1292–1297. https://doi.org/10.1128/AEM.66.4.1292-1297.2000.Updated
Park DH, Zeikus JG (2003) Improved fuel cell and electrode designs for producing electricity from microbial degradation. Biotechnol Bioeng 81:348–355. https://doi.org/10.1002/bit.10501
Park DH, Laivenieks M, Guettler MV et al (1999) Microbial utilization of electrically reduced neutral red as the sole electron donor for growth and metabolite production. Appl Environ Microbiol 65:2912–2917
Parkash A (2016) Microbial fuel cells: a source of bioenergy. J Microb Biochem Technol 8:247–255. https://doi.org/10.4172/1948-5948.1000293
Peng X, Yu H, Wang X et al (2013a) Enhanced anode performance of microbial fuel cells by adding nanosemiconductor goethite. J Power Sources 223:94–99. https://doi.org/10.1016/j.jpowsour.2012.09.057
Peng X, Yu H, Yu H, Wang X (2013b) Lack of anodic capacitance causes power overshoot in microbial fuel cells. Bioresour Technol 138:353–358. https://doi.org/10.1016/j.biortech.2013.03.187
Pham CA, Jung SJ, Phung NT, Lee J, Chang IS, Kim BH, Yi H, Chun J (2003) A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to, isolated from a microbial fuel cell. FEMS Microbiol Lett 223(1):129–134
Pham TH, Jang JK, Chang IS, Kim BH (2004) Improvement of cathode reaction of a mediatorless microbial fuel cell. J Microbiol Biotechnol 14:324–329
Piccolino M (2008) Visual images in Luigi Galvani’s path to animal electricity. J Hist Neurosci 17:335–348. https://doi.org/10.1080/09647040701420198
Potter MC (1911) Electrical effects accompanying the decomposition of organic compounds. Proc R Soc B Biol Sci 84:260–276. https://doi.org/10.1098/rspb.1911.0073
Rabaey K, Rozendal RA (2010) Microbial electrosynthesis – revisiting the electrical route for microbial production. Nat Rev Microbiol 8:706–716. https://doi.org/10.1038/nrmicro2422
Rabaey K, Boon N, Siciliano SD et al (2004) Biofuel cells select for microbial consortia that self-mediate electron transfer biofuel cells select for microbial consortia that self-mediate electron transfer. Appl Environ Microbiol 70:5373–5382. https://doi.org/10.1128/AEM.70.9.5373
Rabaey K, Boon N, Höfte M, Verstraete W (2005a) Microbial phenazine production enhances electron transfer in biofuel cells. Environ Sci Technol 39:3401–3408. https://doi.org/10.1021/es048563o
Rabaey K, Clauwaert P, Aelterman P, Verstraete W (2005b) Tubular microbial fuel cells for efficient electricity generation. Environ Sci Technol 39:8077–8082. https://doi.org/10.1021/es050986i
Rahimnejad M, Adhami A, Darvari S et al (2015) Microbial fuel cell as new technol ogy for bioelectricity generation: a review. Alex Eng J 54:745–756. https://doi.org/10.1016/j.aej.2015.03.031
Ramaraja P, Ramasamy NS (2013) Electrochemical impedance spectroscopy for microbial fuel cell characterization. J Microb Biochem Technol. https://doi.org/10.4172/1948-5948.S6-004
Reguera G, McCarthy KD, Mehta T et al (2005) Extracellular electron transfer via microbial nanowires. Nature 435:1098–1101. https://doi.org/10.1038/nature03661
Reguera G, Nevin KP, Nicoll JS et al (2006) Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl Environ Microbiol 72:7345–7348. https://doi.org/10.1128/AEM.01444-06
Reimers CE, Tender LM, Fertig S, Wang W (2001) Harvesting energy from the marine sediment−water Interface. Environ Sci Technol 35:192–195. https://doi.org/10.1021/es001223s
Ringeisen BR, Henderson E, Peter KW, Pietron J, Ray R, Little B, Biffinger JC, Jones-Meehan JM (2006) High power density from a miniature microbial fuel cell using DSP10. Environ Sci Technol 40(8):2629–2634
Rizzi F, Annunziata E, Liberati G, Frey M (2014) Technological trajectories in the automotive industry: are hydrogen technologies still a possibility? J Clean Prod 66:328–336. https://doi.org/10.1016/j.jclepro.2013.11.069
Roy S, Schievano A, Pant D (2015) Electro-stimulated microbial factory for value added product synthesis. Bioresour Technol 213:129–139. https://doi.org/10.1016/j.biortech.2016.03.052
Rozenfeld S, Teller H, Schechter M et al (2018) Exfoliated molybdenum di-sulfide (MoS2) electrode for hydrogen production in microbial electrolysis cell. Bioelectrochemistry 123:201–210. https://doi.org/10.1016/j.bioelechem.2018.05.007
Santoro C, Arbizzani C, Erable B, Ieropoulos I (2017a) Microbial fuel cells: from fundamentals to applications. Rev J Power Sources 356:225–244. https://doi.org/10.1016/j.jpowsour.2017.03.109
Santoro C, Kodali M, Kabir S et al (2017b) Three-dimensional graphene nanosheets as cathode catalysts in standard and supercapacitive microbial fuel cell. J Power Sources 356:371–380. https://doi.org/10.1016/j.jpowsour.2017.03.135
Schröder U (2007) Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys Chem Chem Phys 9:2619–2629. https://doi.org/10.1039/b703627m
Schröder U, Nießen J, Scholz F (2003) A generation of microbial fuel cells with current outputs boosted by more than one order of magnitude. Angew Chem Int Ed 42(25):2880–2883
Sekoai PT, Gueguim Kana EB (2014) Semi-pilot scale production of hydrogen from organic fraction of solid municipal waste and electricity generation from process effluents. Biomass Bioenergy 60:156–163. https://doi.org/10.1016/j.biombioe.2013.11.008
Sonawane JM, Al-Saadi S, Singh Raman RK et al (2018) Exploring the use of polyaniline-modified stainless steel plates as low-cost, high-performance anodes for microbial fuel cells. Electrochim Acta 268:484–493. https://doi.org/10.1016/j.electacta.2018.01.163
Srikanth S, Maesen M, Dominguez-Benetton X et al (2014) Enzymatic electrosynthesis of formate through CO2sequestration/reduction in a bioelectrochemical system (BES). Bioresour Technol 165:350–354. https://doi.org/10.1016/j.biortech.2014.01.129
Srinophakun P, Thanapimmetha A, Plangsri S et al (2017) Application of modified chitosan membrane for microbial fuel cell: roles of proton carrier site and positive charge. J Clean Prod 142:1274–1282. https://doi.org/10.1016/j.jclepro.2016.06.153
Straub KL, Straub KL, Schink B, Schink B (2004) Ferrihydrite-dependent growth of. Society 70:5744–5749. https://doi.org/10.1128/AEM.70.10.5744
Sugnaux M, Savy C, Cachelin CP et al (2017) Simulation and resolution of voltage reversal in microbial fuel cell stack. Bioresour Technol 238:519–527. https://doi.org/10.1016/j.biortech.2017.04.072
Tang L, Wang Y, Li Y et al (2009) Preparation, structure, and electrochemical properties of reduced graphene sheet films. Adv Funct Mater 19:2782–2789. https://doi.org/10.1002/adfm.200900377
Tekle Y, Demeke A (2015) Review on microbial fuel cell. Basic Res J Microbiol 1:1–32
Terrones M, Grobert N, Olivares J, Zhang JP, Terrones H, Kordatos K, Hsu WK, Hare JP, Townsend PD, Prassides K, Cheetham AK, Kroto HW, Walton DRM (1997) Controlled production of aligned-nanotube bundles. Nature 388(6637):52–55
Thygesen A, Poulsen FW, Min B, Angelidaki I, Thomsen AB (2009) The effect of different substrates and humic acid on power generation in microbial fuel cell operation. Bioresour Technol 100(3):1186–1191
Trapero JR, Horcajada L, Linares JJ, Lobato J (2017) Is microbial fuel cell technology ready? An economic answer towards industrial commercialization. Appl Energy 185:698–707. https://doi.org/10.1016/j.apenergy.2016.10.109
Turick CE, Tisa LS, Caccavo F (2002) Melanin production and use as a soluble electron shuttle for Fe (III) oxide reduction and as a terminal electron acceptor by Shewanella algae BrY melanin production and use as a soluble electron shuttle for Fe (III) oxide reduction and as a terminal E. Appl Environ Microbiol 68:2436–2444. https://doi.org/10.1128/AEM.68.5.2436
Valipour A, Ayyaru S, Ahn Y (2016) Application of graphene-based nanomaterials as novel cathode catalysts for improving power generation in single chamber microbial fuel cells. J Power Sources 327:548–556. https://doi.org/10.1016/j.jpowsour.2016.07.099
Varanasi JL, Nayak AK, Sohn Y et al (2016) Improvement of power generation of microbial fuel cell by integrating tungsten oxide electrocatalyst with pure or mixed culture biocatalysts. Electrochim Acta 199:154–163. https://doi.org/10.1016/j.electacta.2016.03.152
Vega CA, Fernández I (1987) Mediating effect of ferric chelate compounds in microbial fuel cells with Lactobacillus plantarum, Streptococcus lactis, and Erwinia dissolvens. Bioelectrochem Bioenerg 17(2):217–222
Villano M, Monaco G, Aulenta F, Majone M (2011) Electrochemically assisted methane production in a biofilm reactor. J Power Sources 196:9467–9472. https://doi.org/10.1016/j.jpowsour.2011.07.016
Wang Y, Li B, Zeng L et al (2013) Polyaniline/mesoporous tungsten trioxide composite as anode electrocatalyst for high-performance microbial fuel cells. Biosens Bioelectron 41:582–588. https://doi.org/10.1016/j.bios.2012.09.054
Watson VJ, Hatzell M, Logan BE (2015) Hydrogen production from continuous flow, microbial reverse-electrodialysis electrolysis cells treating fermentation wastewater. Bioresour Technol 195:51–56. https://doi.org/10.1016/j.biortech.2015.05.088
Wei J, Liang P, Huang X (2011) Recent progress in electrodes for microbial fuel cells. Bioresour Technol 102:9335–9344. https://doi.org/10.1016/j.biortech.2011.07.019
Wen Q, Wang S, Yan J et al (2014) Porous nitrogen-doped carbon nanosheet on graphene as metal-free catalyst for oxygen reduction reaction in air-cathode microbial fuel cells. Bioelectrochemistry 95:23–28. https://doi.org/10.1016/j.bioelechem.2013.10.007
Werner CM, Logan BE, Saikaly PE, Amy GL (2013) Wastewater treatment, energy recovery and desalination using a forward osmosis membrane in an air-cathode microbial osmotic fuel cell. J Memb Sci 428:116–122. https://doi.org/10.1016/j.memsci.2012.10.031
Winter CJ (2005) Into the hydrogen energy economy – milestones. Int J Hydrog Energy 30:681–685. https://doi.org/10.1016/j.ijhydene.2004.12.011
Xafenias N, Mapelli V (2014) Performance and bacterial enrichment of bioelectrochemical systems during methane and acetate production. Int J Hydrog Energy 39:21864–21875. https://doi.org/10.1016/j.ijhydene.2014.05.038
Xia C, Zhang D, Pedrycz W et al (2018) Models for microbial fuel cells: a critical review. J Power Sources 373:119–131. https://doi.org/10.1016/j.jpowsour.2017.11.001
Xiao L, Damien J, Luo J et al (2012) Crumpled graphene particles for microbial fuel cell electrodes. J Power Sources 208:187–192. https://doi.org/10.1016/j.jpowsour.2012.02.036
Xie X, Hu L, Pasta M et al (2011) Three-dimensional carbon nanotube-textile anode for high-performance microbial fuel cells. Nano Lett 11:291–296. https://doi.org/10.1021/nl103905t
Xie X, Zhao W, Lee HR et al (2014) Enhancing the nanomaterial bio-interface by addition of mesoscale secondary features: crinkling of carbon nanotube films to create subcellular ridges. ACS Nano 8:11958–11965. https://doi.org/10.1021/nn504898p
Xu H, Quan X, Xiao Z, Chen L (2017) Cathode modification with peptide nanotubes (PNTs) incorporating redox mediators for azo dyes decolorization enhancement in microbial fuel cells. Int J Hydrog Energy 42:8207–8215. https://doi.org/10.1016/j.ijhydene.2017.01.025
Yan Z, Jiang H, Li X, Shi Y (2014) Accelerated removal of pyrene and benzo[a]pyrene in freshwater sediments with amendment of cyanobacteria-derived organic matter. J Hazard Mater 272:66–74. https://doi.org/10.1016/j.jhazmat.2014.02.042
Yin T, Su L, Li H et al (2017) Nitrogen doping of TiO2nanosheets greatly enhances bioelectricity generation of S. loihica PV-4. Electrochim Acta 258:1072–1080. https://doi.org/10.1016/j.electacta.2017.11.160
Zehtab Yazdi A, Fei H, Ye R et al (2015) Boron/nitrogen co-doped helically unzipped multiwalled carbon nanotubes as efficient electrocatalyst for oxygen reduction. ACS Appl Mater Interfaces 7:7786–7794. https://doi.org/10.1021/acsami.5b01067
Zhang Y, Mo G, Li X et al (2011) A graphene modified anode to improve the performance of microbial fuel cells. J Power Sources 196:5402–5407. https://doi.org/10.1016/j.jpowsour.2011.02.067
Zhang W, Xie B, Yang L et al (2017) Brush-like polyaniline nanoarray modified anode for improvement of power output in microbial fuel cell. Bioresour Technol 233:291–295. https://doi.org/10.1016/j.biortech.2017.02.124
Zhao F, Slade RCT, Varcoe JR (2009) Techniques for the study and development of microbial fuel cells: an electrochemical perspective. Chem Soc Rev 38:1926–1939. https://doi.org/10.1039/b819866g
Zhong D, Liao X, Liu Y et al (2018) Enhanced electricity generation performance and dye wastewater degradation of microbial fuel cell by using a petaline NiO@ polyaniline-carbon felt anode. Bioresour Technol 258:125–134. https://doi.org/10.1016/j.biortech.2018.01.117
Zhou YL, Jiang HL, Cai HY (2015) To prevent the occurrence of black water agglomerate through delaying decomposition of cyanobacterial bloom biomass by sediment microbial fuel cell. J Hazard Mater 287:7–15. https://doi.org/10.1016/j.jhazmat.2015.01.036
Zhu NW, Chen X, Tu LX et al (2011) Voltage reversal during stacking microbial fuel cells with or without diodes. Adv Mater Res 396–398:188–193. https://doi.org/10.4028/www.scientific.net/AMR.396-398.188
Zhu X, Tokash JC, Hong Y, Logan BE (2013) Controlling the occurrence of power overshoot by adapting microbial fuel cells to high anode potentials. Bioelectrochemistry 90:30–35. https://doi.org/10.1016/j.bioelechem.2012.10.004
Zhu D, Wang D-B, Song T et al (2015) Effect of carbon nanotube modified cathode by electrophoretic deposition method on the performance of sediment microbial fuel cells. Biotechnol Lett 37:101–107. https://doi.org/10.1007/s10529-014-1671-6
Zou L, Qiao Y, Wu XS, Li CM (2016) Tailoring hierarchically porous graphene architecture by carbon nanotube to accelerate extracellular electron transfer of anodic biofilm in microbial fuel cells. J Power Sources 328:143–150. https://doi.org/10.1016/j.jpowsour.2016.08.009
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kuppurangam, G., Selvaraj, G., Ramasamy, T., Venkatasamy, V., Kamaraj, SK. (2019). An Overview of Current Trends in Emergence of Nanomaterials for Sustainable Microbial Fuel Cells. In: Rajendran, S., Naushad, M., Raju, K., Boukherroub, R. (eds) Emerging Nanostructured Materials for Energy and Environmental Science. Environmental Chemistry for a Sustainable World, vol 23. Springer, Cham. https://doi.org/10.1007/978-3-030-04474-9_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-04474-9_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-04473-2
Online ISBN: 978-3-030-04474-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)