Abstract
The catalytic microorganisms oxidise the organic matter to produce electrical energy in microbial fuel cells (MFCs). The microorganisms that can shuttle the electrons exogenously to the electrode surface without utilising artificial mediators are referred as exoelectrogens. The microorganisms produce specific proteins or genes for their inevitable performance towards electricity generation in MFCs. Multiple studies have confirmed the expression of certain genes for outer membrane multiheme cytochromes (e.g. OmcZ), redox-active compounds (e.g. pyocyanin), conductive pili, and their potential roles in the exoelectrogenic activity of various microorganisms, particularly in the members of Geobacteraceae and Shewanellaceae family. This chapter explores the various mechanisms of microorganisms that are advantageous for the technology: biofilm formation, metabolism, electron transfer mechanisms from inside the microorganisms to the electrodes and vice versa.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
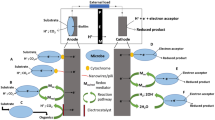
Microbial fuel cells (MFCs) are fascinating biological fuel cells that typically contain two compartments, i.e. the anode and the cathode, and use biological catalysts (mostly bacteria) to produce electric energy from organic matter present naturally in the environment or in waste (Wang and Ren 2013). General principle of a microbial fuel cell is presented in Fig. 9.1. The microorganisms that act as biocatalysts oxidise organic and inorganic substrate to carbon dioxide and generate electrons at the anode. It requires transferring these electrons from inside the cells to the anode (surface) in anoxic conditions to produce electric current (Logan and Rabaey 2012). The bacteria can transfer these electrons to the anode by producing electron shuttles (e.g. flavins, phenazines, etc.) or by using electron mediators generally found in extracellular environment (e.g. humic substances) (Brutinel and Gralnick 2012, Kotloski and Gralnick 2013). Alternatively, the electrons can be transferred via electrically conductive proteinaceous filaments, referred as ‘microbial nanowires’ produced by the bacteria (Malvankar and Lovley 2012). The electron shuttles further may be reduced by outer surface redox-active molecules, such as c-type cytochromes (Inoue et al. 2010a; Voordeckers et al. 2010; Orellana et al. 2013). In earlier MFC studies, chemical mediators (e.g. neutral red) were added to the system to carry the electrons from inside the cell to the electrode for electricity production (Park and Zeikus 1999; Bond et al. 2002). Electrons from the anode surface are passed through a resistor or another type of electrical device to the cathode surface and protons through a proton exchange membrane (PEM) or cation-selective membrane (commonly used Nafion, Ultrix and Salt Bridge) where they combine with oxygen to form water (Huang et al. 2012).
With the instantaneous increase in the global energy demand every year, overconsumption and dwindling of nonrenewable sources of energy, microbial electricity production may become a pivotal form of bioenergy because MFCs offer effective opportunities of extracting current from a wide range of biodegradable organic matter and renewable biomass from simple molecules such as carbohydrates and proteins to complex mixtures of organic matter present in animal, human and food processing wastewaters. The versatility and availability of different microorganisms to use wide range of organic matter makes MFC an exemplary and quirky technology for renewable bioelectricity production. The MFC technology is not a new technology, but it is only recently MFCs are in the limelight of research for bioelectricity production (Rabaey et al. 2003, 2004; Schroder et al. 2003; Liu et al. 2004). MFC is a promising technology for harvesting energy and can be advantageously combined with various applications, such as bioremediation, sensors and powering electronic monitoring devices (Patil et al. 2012; Ren et al. 2012).
The diverse microbes (mostly bacteria) from different phylogenetic groups have been reported to generate electricity in MFCs without using a mediator. Five classes of Proteobacteria, Firmicutes and Acidobacteria phyla have shown electrical current generation, but also, some microalgae, yeast and fungi have been reported in MFCs, being used as substrate or assist the anode or the cathode. The nomenclature of such microorganisms is not standardised yet; however, some terms have been given for microorganisms that can transfer electrons exogenously to the anode without using any artificial mediator. These terms include exoelectrogens, electrogenic microorganisms, electrochemically active bacteria, anodophiles, anode-respiring bacteria and electricigens. Moreover, microorganisms can also be termed according to their functions in the MFC, e.g. sulphate-reducing bacteria and iron-reducing bacteria can be referred as sulphate reducers and iron reducers, respectively. The microorganisms that donate electrons to the electrode (anode) in MFCs can be referred to as electrode reducers, while those that accept electrons from the electrodes are referred to as electrode oxidisers. The prevalent bacterial species known to produce electricity in MFCs include dissimilatory iron-reducing Geobacter spp. (Bond and Lovley 2003), Shewanella spp. (Gorby et al. 2006) Rhodoferax ferrireducens (Chaudhuri and Lovley 2003), Aeromonas hydrophila (Pham et al. 2003), Pseudomonas aeruginosa (Jayapriya and Ramamurthy 2012), Clostridium butyricum (Park et al. 2001) and Enterococcus gallinarum (Chisti 2007). Alternatively, microalgae have been used as a substrate or biocathode in MFC (Wang et al. 2012). In yeast, besides Saccharomyces cerevisiae, Hansenula anomala also showed current production successfully in MFC (Prasad et al. 2007). However, use of yeasts for electricity generation using MFCs in general does not seem to have been considered further very deeply. An oxygenic phototrophic cyanobacterium Synechocystis sp. which produces nanowires has been discovered to generate electricity in MFC (El-Naggar et al. 2010). The microorganisms can contribute effectively for power generation those able to oxidise organic compounds completely and transfer the electrons with accelerated rates to the anode. Biofilms on the anode have been demonstrated to increase the current density due to the direct electron transfer between the microbes and the surface of the anode. Earlier studies have shown that biofilms of mixed cultures have more capability to produce higher current density than the biofilms of pure cultures (Dumas et al. 2008). For example, a bacterium Brevibacillus sp. produced little power as a pure culture in MFC but produced comparatively high power when a Pseudomonas sp. was added in MFC (Pham et al. 2008). The bacteria capable of dissimilatory metal reduction can effectively produce electricity in a mediatorless MFC. Such bacteria transfer electrons either by excreting electron shuttles or by direct contact via outer membrane cytochromes. Later, another mechanism for electron transfer was revealed providing the evidence that bacteria synthesise appendages known as microbial nanowires that are capable of transferring electrical current. In a study, a bacterium Pelotomaculum thermopropionicum was found connected to the methanogen Methanothermobacter thermautotrophics by an electrically conductive appendage, promoting the interspecies electron transfer (Gorby et al. 2006). Multiple studies suggest that quorum-sensing chemicals (e.g. fatty acyl-homoserine lactones) play an important role in the communication between the bacteria of different species within the biofilm (Schaefer et al. 2008). Pseudomonas aeruginosa produces pyocyanin that acts as an electron shuttle and signalling molecule to upregulate the transcription of quorum-sensing genes (Dietrich et al. 2006).
This chapter describes the different microbial mechanisms that are advantageous to the MFC technology, including formation of biofilm by the microorganisms, different mechanisms of electron transfer from microorganisms to electrode and vice versa, followed by the description of high current-producing microorganisms used for electricity production.
9.2 Biofilm Formation and Its Regulation
Bacteria prefer to live in polymeric matrix (contains proteins, lipids, carbohydrate, etc.) produced by the bacteria attached to a surface, which is known as a biofilm. In MFCs, it is highly significant to produce electroactive biofilms to generate electricity more efficiently. Biofilm formation is regulated via different pathways depending on the microbe used in the MFC, the substrates, electrode material and the operating conditions of the MFC. The physiological and morphological properties of electrode surface also influence biofilm formation. Some particular studies demonstrated that microorganisms favour to adhere on hydrophobic surfaces in rival to hydrophilic materials (Patil et al. 2012). The earlier studies suggest that the bacteria unable to form biofilms on the electrode can’t generate substantial current densities in MFCs. However, the bacteria able to form thick biofilms on the anode generate higher current densities in rival to bacteria adept to form thin biofilms. For example, confocal microscopy revealed that Thermincola ferriacetica, Gram-positive bacteria which form thick biofilms (~38 μm), generated a sustained current density 7–8 Am−2 (Prathap et al. 2013), while Thermincola potens, which form monolayer biofilms, produced comparatively lower current densities (Wrighton et al. 2011).
The process of biofilm formation is triggered by the transport of microbes to a surface, followed by their attachment to the surface (in MFC, on anode or cathode), formation of microcolonies and biofilm maturation (Sauer et al. 2002). The bacterial cells produce some adhesins, and carbohydrates (polysaccharides), nucleic acids and proteins interconnect and encase the bacteria in the form of a biofilm (Pamp and Nielsen, 2007). The most distinguished feature of electroactive biofilms is their ability to respire terminal electrons from metabolism onto electrode surfaces or soluble electron acceptors (Bond et al. 2002). It has been demonstrated that outer membrane c-type cytochromes are crucial for biofilm formation in Geobacter spp. and Shewanella spp. (Bond and Lovley 2003; Gorby et al. 2006). While, type IV pili protein composed of PilA monomers are chiefly responsible for Geobacter spp. and Aeromonas spp. conductive biofilm formation (Pham et al. 2003; Malvankar and Lovley 2012). G. sulfurreducens deficient in omcZ and pilA genes inhibited biofilm formation and consequently, the current production, suggesting the role of c-type cytochromes and the protein pilin in biofilm formation (Inoue et al. 2010a). While in Shewanella spp. other redox-active components such as flavins mediate the exocellular electron transport through biofilm (Kotloski and Gralnick 2013). In P. aeruginosa biofilm formation, bacteria transfer to the surface with the movement of flagella. Cellular aggregation and microcolony formation is driven by type IV pili, and the subsequent formation of mushroom-shaped biofilm occurs via a maturation process that requires cell-to-cell signalling (Stoodley et al. 2002; Merritt et al. 2010; Malone et al. 2012). The mechanism known as quorum sensing (QS) allows bacterial population to communicate and coordinate group behaviour. QS regulates the expression of biofilm-related genes and is pivotal for structural development of biofilm in P. aeruginosa and other microorganisms as well (Diggle et al. 2003; Holm et al. 2006).
The biofilms of mixed culture generate high-power densities than pure culture. For example, a mixed culture-inoculated MFC produced ca. 20 % more power in rival to pure culture in the similar MFC (Ishii et al. 2008). However, the role of non-exoelectrogens (the microorganisms when used as pure cultures were not able to generate electric current) in power generation is not known. In monolayer biofilms, bacterial cells remain in the close proximity with anodic surface and transfer the electrons directly to the anode either via c-type cytochromes or electron shuttles. While in thick multilayer biofilms, it has been found that biofilms produce pili that mediate the electron transfer from the distant cells to the anode surface (Reguera et al. 2006). The role of pili and its role in electron transfer are discussed in the later section of the chapter. The use of microorganisms at the cathode to catalyse oxygen reduction has increased the interest in cathodic biofilm studies. In rival to anodic biofilms, it has been observed that power generation slowly decreases with increase in thickness of cathodic biofilms (Behera et al. 2010).
9.3 Microbial Metabolism and Bioelectrogenesis
Many microorganisms have been experimented in MFCs for electricity generation, bioremediation and other manifold applications. Besides, several nutrients (acetate, glucose, starch, sucrose, ethanol, lactate and xylose, etc.) and wastewaters (beer brewery wastewater, chocolate industry wastewater, swine wastewater, paper recycling wastewater and protein-rich wastewater, etc.) from various sources have been used as substrate for microbial growth in MFC technology (Liu et al. 2004). Despite the availability of wide range of substrates and microorganisms, only restricted and specific microorganisms are known to produce electricity in MFCs. Exoelectrogens from various categories such as Gram-positive bacteria, Gram-negative bacteria, yeast, cyanobacteria, algae and even fungi have already been utilised in different kinds of MFCs. Those organisms are substantially efficient for electricity generation that can completely oxidise complex organic substrates into their respective components in the anodic chamber. But, a particular exoelectrogen can oxidise specific substrates or a specific type of substrate for its growth and energy production. Moreover, depending on the type of substrate, every exoelectrogen has different pathways and genes, enzymes or proteins for its degradation or oxidation. Therefore, selection of a suitable bacterial consortia and preferred substrate determine the output of MFC. For example, a MFC fed with aerobic-anaerobic sludge inoculum and glucose, when operated for 3 months, increased the bacterial substrate to electricity conversion rates sevenfolds (Rabaey and Verstraete 2005).
In MFC, organic substrates containing carbohydrates, lipids and proteins serve as electron donors for redox reactions at the anode to produce energy. These complex organic molecules further undergo through glycolysis and other respective processes to yield acetyl Co-A, which then participate in citric acid cycle. Three equivalents of reduced NADH are generated from three nicotinamide adenine dinucleotide (NAD+), one flavin adenosine dinucleotide (FAD) reduces to FADH2, and CO2 is released as by-product in single turn of citric acid cycle. These metabolic pathways (glycolysis and Krebs cycle) occur in cytoplasm in both prokaryotes (bacteria) and eukaryotes (yeast). NADH and FADH2 act as electron carriers, which then transfer their electrons to electron transport chain (ETC) to produce energy carrier molecule, adenosine triphosphate (ATP). In bacteria, respiratory reaction occurs in the cell membrane (constituting outer cell membrane, inner cell membrane and periplasm), the machinery containing all the proteins or enzymes required for the electron transfers (the basis of MFC). While in yeast, ETC resides on the inner mitochondrial membrane. The ETC typically contains four intermediary proteins, NADH dehydrogenase, ubiquinone, coenzyme Q and cytochromes (however, these intermediary proteins may vary with species). The electrons are passed through these proteins to the final electron acceptor, and the protons (reduced) are pumped out of the cell, in the anode which is then transferred to the cathode through PEM. Prior to the prominence that bacteria can facilitate electron transfer, chemical mediators were utilised to catalyse electron transfer from inside the bacterial cell to the anode surface. These mediators react with ETC components and get reduced, release out of the cell and transfer their electrons to the anode.
Moreover, metabolism of the bacteria can switch from oxidative phosphorylation (metabolism) to fermentative metabolism depending on the anode potential. At low anode potential, in the presence of electron acceptors (sulphate, nitrate, etc.), bacteria adapt to oxidative metabolism, and the electrons are deposited on electron acceptors. But, when electron acceptors are not present, bacteria prefer the fermentation metabolism. During the fermentation process, e.g. of glucose, one-third of electrons can be used for electricity generation, while the rest of electrons reside in the fermentation products, which can be further oxidised by anaerobic bacteria such as Geobacter sp. in MFC for current generation (Logan 2004; Rabaey et al. 2005; Reguera et al. 2005). Beyond electricity generation, many bacteria (Clostridium sp., Enterococcus sp.) have been inoculated anaerobically in MFCs to produce fermentation products (Logan 2009). Like Geobacter sp. is the most efficient exoelectrogen known in MFC, Clostridium sp. is the most efficient hydrogen producer in MFC (Singh et al. 2013; Singh and Wahid 2015). No doubt, biofilms of mixed consortia studied so far in MFC have showed higher-power densities than pure cultures, and it can be conceived due to the networks of metabolisms between the bacteria in biofilms, but it needs a complete elucidation and experimental corroboration over the matter. The potential of anode plays an important role to determine the bacterial metabolism. Negative anode potential influences the bacteria to deliver the electrons through more reduced complexes (Logan 2009). As a result, the bacteria extract less energy and greater is the energy recovery in MFC and thus the power output. Evidently, microbial community of sulphate-reducing bacteria at negative anode potentials produced higher-power density, 45 mA m−2 at −0.6 V than 15 mA m−2 at −0.2 V (Chou et al. 2014). Also, setting the cathode potential has shown to improve the performance of MFC. A study demonstrated that MFC for Cr (VI) reduction with set cathodic potentials at -300 V increased the maximum power density from 4.1 W/m3 (control, no set potential) to 6.4 W/m3, and the start-up time was reduced to 19 days from 26 days as compared to control (Huang et al. 2011).
9.4 Mechanisms of Electron Transfers
In MFC, electron transfer chiefly occurs in two directions: at the anode, from microorganisms to electrode, and at the cathode, from electrode to microorganisms when biocathodes are used to catalyse oxygen reduction.
9.4.1 Electron Transfer from Microorganisms to Electrode
Microorganisms can transfer electrons to an electrode directly by three mechanisms (see Fig. 9.2) known till date: (1) short-range electron transfer via redox-active proteins such as cytochromes present on the outer surface of bacterial cell membrane; (2) electron transport via microbial-secreted soluble electron shuttles, for example, flavins and pyocyanin; and (3) long-range electron transfer through conductive pili.
9.4.1.1 Direct Electron Transfer via Cytochromes
Geobacter sulfurreducens has been studied most extensively to comprehend the mechanisms for direct electron transfer. G. sulfurreducens contains the enzymes for the central metabolism to anaerobically oxidise carbon (effectively acetate) completely to carbon dioxide and water and can transfer electrons to different electron acceptors (Kiely et al. 2011). The genetic studies of G. sulfurreducens genome unveiled the presence of many c-type cytochromes containing heme groups in their motifs, exposing on the outer surface of cell (Leang et al. 2010; Inoue et al. 2010a). The abundance of cytochromes is an advantageous characteristic for the organism that ameliorates electron transport across cell/electrode interface. The other compounds or proteins that help in electron transport include quinones, iron-sulphur proteins and b-type cytochromes. The electron transport proteins are present in the periplasm or on outer membrane of G. sulfurreducens. Besides, many studies including gene deletions demonstrated that c-type cytochromes transfer electrons to diverse extracellular electron acceptors in vitro as well as in vivo (Leang et al. 2003; Inoue et al. 2010a; Voordeckers et al. 2010). The immunogold labelling of G. sulfurreducens biofilms validated the accumulation of profuse OmcZ at biofilm and anode interface (Inoue et al. 2010a), while OmcZ mutant strain halted the electron transfer between biofilm and the anode. Hence, all the results confirmed the vital role of OmcZ in direct electron transfers. Nevin et al. compared the gene expression in cells of G. sulfurreducens biofilms growing on different electron acceptors, between cells grown on graphite and graphite with fumarate. The microarray studies revealed the genes omcB, omcT, omcE, omcS and omcZ encode c-type cytochromes. OmcZ and OmcE cytochromes were most abundant in current harvesting cells, while OmcS was least abundant. Further, the cells deficient in omcZ inhibited the current production and biofilm formation, showing the importance of the cytochrome in the electron transfer. The cells deficient in other genes didn’t show any impact on current generation as well as on biofilm formation (Leang et al. 2003; Inoue et al. 2010a; Smith et al. 2013). Multiple evidences suggest that OmcZ is the most important cytochrome in high current-producing biofilms and is an octaheme hydrophobic protein which occurs in two forms, one large (OmcZL) and one short (OmcZS, the predominant form) (Inoue et al. 2010a, b). It has been suggested that in G. sulfurreducens biofilms, OmcZ mediates the electron transfer through the biofilm, while OmcB mediates the electron transfer across the biofilm/electrode interface. The cytochromes OmcS and OmcE also play a secondary role in electron transfer through the biofilm (Richter et al. 2009). A study demonstrated that OmcF mutant strain of G. sulfurreducens showed low current density (Kim et al. 2008). Further, the results suggested that the OmcF is either directly or indirectly involved in electron transfer process, and hence OmcF is a pivotal role in electricity production (Kim et al. 2008).
In G. sulfurreducens proteins other than the outer membrane c-type cytochromes, the outer-membrane multicopper protein OmpB and OmpC are also required for Fe (III) oxide reduction (Mehta et al. 2006; Holmes et al. 2008). However, it’s not clear how these multicopper proteins affect the electricity production in MFCs, therefore, will be a highly interesting topic for future research. Desulfovibrio alaskensis G20, a sulphate-reducing bacteria studied to identify the components involved in electron flow, revealed a new model for electron transfer and showed that type I tetraheme cytochrome c 3 (TpIc 3) and the transmembrane complexes (QrcA) also play a key role to transfer the electrons across the cell membrane for sulphate reduction (Keller et al. 2014).
Gram-positive species of the genus Thermincola potens has also been studied to elucidate the direct electron transfer mechanism. Surface-enhanced Raman spectroscopy evinced the expression of profuse multiheme c-type cytochromes (MHCs) on the cell wall or cell surface during T. potens growth on hydrous ferric oxides or AQDS, an analogue of the redox-active components of humic substances. The results unveiled unique evidence for cell wall-associated cytochromes and involvement of MHC in transporting the electrons across the cell envelope of a Gram-positive bacterium (Wrighton et al. 2011). A better understanding of genes or proteins involved in direct electron transfer along with genetic manipulation can amend increases in current production and efficiency of MFCs.
9.4.1.2 Electron Transfer via Electron Shuttles Secreted by Microorganisms
Some microorganisms have been identified that can mediate the electron transfer to soluble or insoluble electron acceptors or electrodes by secreting soluble electron shuttles, for example, Shewanella oneidensis, Pseudomonas aeruginosa and Geothrix fermentans, etc. G. fermentans releases a soluble electron shuttle which promotes reduction of Fe (III) oxides (Bond and Lovley 2005). G. fermentans secreted two different soluble redox-active electron shuttles to reduce Fe (III); first was riboflavin at redox potential of −0.2 V and the other, still unknown at redox potential of 0.3 V (Mehta and Bond 2012). P. aeruginosa produces pyocyanin and phenazine-1-carboxamide that are very important for electron transfers. A mutant strain of P. aeruginosa, deficient in the synthesis of pyocyanin and phenazine-1-carboxamide, achieved only 5 % power output as compared to wild type’s strains (Baron et al. 2009). Further, the study demonstrated that pyocyanin promotes substantial electron transfer, not only used by P. aeruginosa but also by other bacterial species (Baron et al. 2009; Shen et al. 2014). Moreover, overexpression of phzM (methyltransferase-encoding gene) in P. aeruginosa-phzM-inoculated MFC increased the pyocyanin production by 1.6-folds and consequently the exocellular electron transfer efficiency and power output (Yong et al. 2014).
Shewanella species produces flavin mononucleotide and riboflavin as the extracellular electron shuttles to reduce of Fe (III) oxides coupled with anoxic growth of the species (Von et al. 2008; Chaudhuri and Lovley 2003). Fluorescence emission spectra showed an increase in concentration of quinone derivatives, and riboflavin in the cell-free supernatant of Shewanella loihica PV-4 strain grown on graphite electrode, responsible for direct electron transfer and mediated electron transfer, produced maximum anodic current density of 90 μAcm−2 (Jain et al. 2012; Marsili et al. 2008). Kotloski and Gralnick (2013) identified a flavin adenine dinucleotide transporter in S. oneidensis, responsible for the export of flavin electron shuttles to further the electron transfer to insoluble substrates. In S. oneidensis MR-1, decaheme c-type cytochromes MtrC and OmcA present on the outer surface of the cell; part of multiprotein complex helps in hopping the electrons cell membrane (Baron et al. 2009). The cytochrome OmcA also plays an important role in the attachment of bacteria to the electrode surface during biofilm formation (Coursolle et al. 2010). Electron transfer complex MtrCAB responsible for direct and mediated exocellular electron transport in S. oneidensis (Baron et al. 2009) was introduced in E. coli with a more tunable induction system. The strains showed limited control of MtrCAB expression and impaired cell growth, and the results demonstrated that maximum current densities was produced not by the strains that expressed more MtrC and MtrA but by the strains with improved cell growth and fewer morphological changes (Goldbeck et al. 2013).
Lactococcus lactis produces variegated membrane-associated quinones which mediate electron transfer to extracellular electron acceptors such as Fe (III) and Cu (II) (Fuller et al. 2014). The bacterium transfers electrons to the anode via soluble redox mediators. The study suggested that one of these two mediators was 2-amino-3-dicarboxy-1,4-naphthoquinone (Freguia et al. 2009). Klebsiella pneumoniae strain L17 also studied in MFC produces a recycle electron shuttle 2, 6-di-tert-butyl-p-benzoquinon to transfer electrons to the anode (Lifang et al. 2010; Torres et al. 2010).
9.4.1.3 Electron Transfer via Microbial Nanowires
Long-range electron transfer is mediated by dense network of conductive pili produced by the microorganism, responsible for the conductive biofilms of high current production. Though, diverse microorganisms are known to produce pili, only Shewanella sp. (Leung et al. 2013; Pirbadian and El-Naggar 2012) and Geobacter sp. (Malvankar et al. 2012; Snider et al. 2012; Bonanni et al. 2013) are competent to produce conductive pili that account for electricity production.
The role of conductive pili in long-range electron transfer in biofilms was demonstrated earlier in Geobacter sulfurreducens, and the study revealed that these electronic networks contributed for more than tenfold increase in electricity production (Reguera et al. 2006). G. sulfurreducens pili are type IV pili composed by the monomers of PilA protein (Craig et al. 2004). Type IV pili are small structural proteins of molecular weight ca. 7–20 kDa, 10–20 μm long and 3–5 μm broad with a conserved N-terminal domain forming α-helix with a transmembrane domain and a protein-protein interaction domain (Craig et al. 2004). Moreover, C terminus of PilA contains a conserved sequence of aromatic amino acids (Trp, Phe, Tyr, His and Met) responsible for overlapping of pi-pi orbitals in the pili structure and consequently for metal-like conductivity and lacks in nonconductive biofilms (Vargas et al. 2013). The function of PilA is directly regulated by PilR which functions as an RpoN-dependent enhancer-binding protein. Further, the study revealed that a strain deficient in pilR gene showed waned insoluble Fe (III) reduction as well as soluble Fe (III) reduction (Juárez et al. 2009). The hypothesis that cytochromes are associated with G. sulfurreducens pili and serve a key role in electron transfer along with pili was ruled out with the publication of Malvankar et al. (2011); the study unveiled that conductivity of G. sulfurreducens nanowires don’t attribute to cytochromes because the spacing between cytochrome to cytochrome was ca. 200 times greater than required for electron hopping. It was further clarified by Liu et al., who demonstrated a G. sulfurreducens strain PA, that pilA gene was replaced with pilA gene of Pseudomonas aeruginosa PAO1, expressed the pili subunits and c-type cytochrome OmcS similar to control strain, but showed waned current production and Fe (III) oxides reduction. Further, the results suggested that c-type cytochrome OmcS on pili don’t confer for the conductivity of pili (Liu et al. 2014a, b; Smith et al. 2014). Fanghua et al. revealed that magnetite can facilitate microbial extracellular electron transfer. The study demonstrated that magnetite compensated for the extracellular electron transfers for OmcS-deficient strain in Fe (III) oxide reduction (Liu et al. 2014a).
Conducting probe atomic force microscopy technique and gene deletion studies of MtrC and OmcA suggested that S. oneidensis MR-1 nanowires are conductive in nature (El-Naggar et al. 2010). Electronic transport characteristics of S. oneidensis MR-1 nanowires was further studied and exhibited p-type, tunable electronic behaviour with a field-effect mobility (Leung et al. 2013). In an alternative study, deletion of the structural pilin genes (mshA-D) which encode for extracellular Msh (mannose-sensitive hemagglutinin) structural proteins in S. oneidensis MR-1 produced 20 % less current compared to control strain, indicating extracellular electron transfer ability of intracellular- and membrane-bound Msh biogenesis complex in S. oneidensis MR-1 (Fitzgerald et al. 2012). A multistep hopping mechanism has been proposed for extracellular charge transfer in S. oneidensis MR-1 biofilms, suggesting that redox components are associated with each other at less than 1 nm distance, forming a chain along extracellular appendages, responsible for electron hopping or electron tunnelling (Polizzi et al. 2012). However, the actual organisation of cytochromes on S. oneidensis MR-1 nanowires and their exact role in electron transfer mechanism is yet to be clarified.
The pilus-associated c-type cytochrome OmcS and pili have also been associated with electron transfer via direct interspecies electron transfer (DIET). Gorby et al. provided the first evidence that nanowire production is not limited to dissimilatory metal-reducing bacteria; further the study demonstrated that an oxygenic phototrophic cyanobacterium Synechocystis and thermophilic, fermentative bacterium Pelotomaculum thermopropionicum produced electrically conductive nanowires that established connections with the methanogen Methanothermobacter thermautotrophicus for efficient electron transfer and energy distribution (Gorby et al. 2006). The mechanism DIET has also been seen within aggregates of G. metallireducens and Methanosaeta harundinacea in anaerobic digesters (Rotaru et al. 2014). Granular-activated carbon (GAC) has been hypothesised to stimulate DIET between bacteria and methanogens (Liu et al. 2012). GAC simulates the role of pili and associated c-type cytochrome involved DIET (Liu et al. 2012). The molecular mechanism of DIET and its contribution towards energy production is not understood well and, therefore, demands a deep investigation into the matter.
9.4.2 Electron Transfer from Electrodes to Microorganisms
Many microorganisms have already been used as biocathodes in the technology, but only limited information is available on electron transport mechanisms from electrode to microbes. Though, it’s clear that microorganisms use different mechanisms to accept electrons from the cathode (see Fig. 9.3) than to donate electrons to the anode, Gregory et al. provided the first evidence that Geobacter species can accept electrons directly from an electrode (Gregory et al. 2004). Alternatively, Shewanella oneidensis MR-1 in the aerated cathode produced riboflavin, an electron shuttle mediator to transfer electrons to Cr (VI) (Xafenias et al. 2013). Acinetobacter calcoaceticus and Shewanella putrefaciens as pure cultures excrete redox compound similar to pyrroloquinoline quinone (PQQ) that further use outer membrane-bound redox compounds for extracellular electron transfer (Freguia et al. 2010). An acidophile microorganism, Acidithiobacillus ferrooxidans, used as biocathode demonstrated that the redox species, an outer membrane-bound cytochrome c (Cyc2), is associated to microbial-catalysed O2 reduction (Carbajosa et al. 2010). Transmission electron microscopy of immunogold-labelled Leptospirillum group II bacterium-dominated biofilm (acidophilic microbial communities) revealed that Cyt579 (structurally, 70 % α-helical) is localised in periplasmic space (Jeans et al. 2008) and helps in accepting the electrons derived from Fe (II) oxidation (Jeans et al. 2008). Similarly, another unusual membrane protein, Cyt572 (structurally, β-helical), isolated from acidophilic microbial communities showed the ability for Fe (II) oxidation (Jeans et al. 2008), but it’s still elusive that the protein participates in electron transfer mechanisms. Recently, cyclic voltammetry scanned an unidentified redox-active molecule secreted from P. aeruginosa, involved in the electron transfer from the electrode to targeted azo bonds, leading to decolorisation of azo dye (Wang et al. 2014). A biocathodic microbial community predominated by Proteobacteria, Bacteroidetes and Firmicutes during dechlorination of pentachlorophenol (PCP) in MFC transferred the electrons directly, as cyclic voltammetry characterisation of the medium didn’t confirm any redox mediator secreted by the bacteria (Liu et al. 2013). Besides, many Gram-negative and Gram-positive bacteria utilised as biocathodes such as Dechlorospirillum anomalous, Acinetobacter calcoaceticus, Staphylococcus carnosus, Streptococcus mutans, Enterococcus faecalis, Shigella flexneri, Kingella denitrificans and Lactobacillus farciminis have shown the ability to transfer electrons directly or accept the electrons indirectly from different electrodes through redox-active compounds for manifold applications of the technology (Thrash et al. 2007; Aulenta et al. 2010; Cournet et al. 2010). Unfortunately, the molecular mechanism of accepting electrons from the electrodes in any microorganism is yet not understood well and can be taken as a future aspect.
9.5 High-Power-Producing Microorganisms in MFC
The power density produced by a particular microorganism such as Geobacter sp. can’t be compared to other microorganism, e.g. Shewanella sp., unless the MFC structure, operating conditions, nutrients and chemical solutions used for the study will be indistinguishable. Till date, many microorganisms used in different MFCs produced electrical energy in unalike conditions. Although this chapter describes only the prevalent microorganisms studied (in anode and cathode) that produced efficient power densities in MFC technology, also, some novel microorganisms were discovered recently. The microorganisms with known and unknown natural electron mediators are given in Tables 9.1 and 9.2, respectively.
9.5.1 Microorganisms in Anode
The most studied and efficient exoelectrogens in MFC technology belong to Geobacteraceae family of bacteria. G. sulfurreducens, δ-proteobacteria, can reduce acetate with ca.100 % electron recovery to generate electricity. The organism has successfully produced the current density of 3147 mA/m2 in a MFC with gold electrodes, acetate as the electron donor and fumarate as the electron acceptor (Richter et al. 2008). However, G. metallireducens (pure culture) could produce only 40 mWm−2 power output in MFC using wastewater as inoculum (Min et al. 2005). Shewanella spp., γ-proteobacteria, can reduce iron and manganese and can use them as electron acceptors. Shewanella oneidensis DSP10 in a miniature MFC using lactate as the anolyte and ferricyanide as catholyte produced power density of 3000 mW/m2 which is quite appreciable (Ringeisen et al. 2006). Recently, S. putrefaciens in a single-chamber microbial fuel cell (sMFC) produced maximum power density of 4.92 W/m3using CaCl2 as anolyte (Pandit et al. 2014). Rhodopseudomonas palustris, α-proteobacteria and a photosynthetic purple non-sulphur bacterium, can utilise volatile acids, yeast extract and thiosulphate and produce power density of 2720 mW/m2 higher than mixed cultures in indistinguishable MFCs (Xing et al. 2008). A thermophilic, Gram-positive, metal-reducing bacterium, Thermincola ferriacetica, is able to generate current from acetate and exhibited maximum current density 12 Am−2 (Prathap et al. 2013). Pseudomonas aeruginosa, γ-proteobacteria, in MFC produced power density of 4310 mWm−2 using glucose as electron donor and graphite electrodes as the electron acceptor (Rabaey et al. 2004). A sulphate-reducing bacterium, Desulfovibrio desulfuricans, in MFC with surface-treated graphite felt electrodes generated maximum current density of 233 mA/m2 which was ca. 50 % higher than with untreated electrodes (Kang et al. 2014). E. coli, Gram-negative bacteria in MFC successfully achieved power density of 1300 mW/m2 at 3390 mA/m2 current density (Qiao et al. 2008).
Saccharomyces cerevisiae in sMFC (open-air cathode) fed with synthetic wastewater using noncatalysed graphite as electrodes without the use of artificial mediators generated maximum current density 282.83 mA/m2 (Raghavulu et al. 2011). Other yeast Hansenula anomala using Pt electrode and ferricyanide as catholyte produced power density of 2.9 W/m3 (Prasad et al. 2007). Moreover, Candida melibiosica in a MFC of modified carbon felt electrode with surface nickel nanostructures produced significant power output of 720 mW/m2 (Hubenova and Mitov 2010). Some microorganisms not used commonly in MFC and a few novel exoelectrogens discovered recently have also shown the ability to produce electricity.
Analysis of 16S rRNA gene sequences has unveiled a new exoelectrogen; Geobacter anodireducens showed 98 % similarity to Geobacter sulfurreducens but cannot reduce fumarate as the electron acceptor (Sun et al. 2014a, b). Another novel strain, Ochrobactrum sp. 575 isolated recently from the anodic chamber of a xylose MFC, produced maximum power density of 2625 mW/m3. Further, the results suggested that xylose digestion in Ochrobactrum sp. 575 was different to other electroactive bacterial strains, which depends on the succinate oxidation respiratory chain instead of traditional NADH oxidation respiratory chain (Li et al. 2014). Klebsiella pneumonia, Gram-negative, nonmotile, lactose-fermenting bacteria in a cubic air-chamber MFC, generated 199.2 mA/m2 current density and maximum voltage output of 426.2 mV (Lifang et al. 2010). A Gram-positive bacterium Lysinibacillus sphaericus in MFC using graphite felt as electrode generated a maximum current density of ca. 270 mA/m2 and power density of 85 mW/m2 (Nandy et al. 2013). Further, Citrobacter sp. SX-1 can utilise diverse simple substrates like acetate, glucose, sucrose, glycerol and lactose in MFCs but produced the highest current density of 205 mA/m2from citrate (Xu and Liu 2011; Kimura et al. 2014; Zhang et al. 2013). Besides bacteria and yeast, microalgae have been also used in MFC technology either as bioanode or a substrate assisting the anode for the prevalent application. Scenedesmus, green algae in powder form as substrate, was used in anode and Chlorella vulgaris as a biocathode in MFC produced maximum power density of 1926 mW/m2 (Cui et al. 2014). In another study, Arthrospira maxima was used as a substrate as well as a carbon source for the metabolism and growth of R. palustris in a micro-MFC and exhibited volumetric power density of 10.4 mW/m3, the highest in rival to other substrates used in the study (Inglesby et al. 2012). Furthermore, blue-green algae (cyanobacteria) in a sMFC produced maximum power density of 114 mW/m2 at 0.55 mA/m2 current density (Yuan et al. 2011). In microalgae-assisted MFCs, algae degradation produces intermediate compounds like acetate and lactate which can be further used by exoelectrogens such as G. sulfurreducens for bioelectricity production.
9.5.2 Microorganisms in Cathode
Geobacter spp. highly efficient as bioanodes in MFC also evidenced to be prelusive biocathodes to accept the electrons from cathodic electrodes (Gregory et al. 2004). The study revealed that G. metallireducens reduced nitrate to nitrite and G. sulfurreducens reduced fumarate to succinate with the electrode as the sole electron donor (Gregory et al. 2004). Furthermore, G. sulfurreducens reduced fumarate in a reactor with stainless steel electrodes producing the current density of 20.5 Am−2 (Dumas et al. 2008). Shewanella oneidensis MR-1 as biocatalyst in the air-cathode MFC and lactate as electron donor showed increase in Cr (VI) reduction rate with maximum current density of 32.5 mA/m2 (Xafenias et al. 2013). The study demonstrated the expression of riboflavin in the electron transport. In an alternative investigation, Shewanella putrefaciens and Acinetobacter calcoaceticus showed the ability to reduce the oxygen to water with increased rate by utilising outer membrane-bound cytochromes and self-excreted PQQ respectively (Freguia et al. 2010). An acidophile microorganism, Acidithiobacillus ferrooxidans, fed as a biocathode in MFC, up to 5 Am−2 of current densities, were obtained for O2 reduction at low pH (Yuan et al. 2011). In a study, Enterobacter and Pseudomonas spp. demonstrated for the catalysis of acetate oxidation actually resulted to catalyse the electrochemical reduction of oxygen-producing maximum current density of 145 mA−2 (Parot et al. 2009). Cyclic voltammetry unveiled that Micrococcus luteus and other Gram-positive (Staphylococcus spp., Lactobacillus farciminis) and Gram-negative bacteria (Pseudomonas fluorescens, Escherichia coli, Acinetobacter sp.) are able to catalyse the electrochemical reduction of oxygen on the carbon electrode (Cournet et al. 2010). Seawater-formed aerobic biofilms coated on stainless steel electrodes have shown significant ability to catalyse oxygen reduction and achieved current densities up to 460 mA/m2 at different set potentials (Bergel et al. 2005).
An acetate-fed MFC utilising Chlorella vulgaris as a biocathode produced maximum power density of 1926 mW/m2. CO2 produced at the anode was used by C. vulgaris as a carbon source for its growth. Further, the study demonstrated that C. vulgaris could not grow in acetate-fed MFC without anodic CO2 supply (González et al. 2013; Cui et al. 2014). The immobilisation of C. vulgaris into the cathode chamber turned the MFC highly efficient, consequently producing the power density 2485.35 mWm−3 at a current density of 7.9 Am−3, while the MFC with suspended C. vulgaris achieved 1324.68 mWm−3 power density (Zhou et al. 2012). A strain of white-rot fungus, Coriolus versicolor (secretes laccase to reduce oxygen at the cathode), inoculated in the cathode chamber of a MFC to catalyse the cathodic reaction generated the maximum power density 320 mWm−3 (Wu et al. 2012).
9.6 Future Directions
It is unfortunate for the MFC technology that the studied applications of the technology are still confined to the four walls of the laboratory. In other words, the technology is not commercialised yet. The electron transfer mechanisms from exoelectrogens to electrodes are well understood only in Geobacter spp. and Shewanella spp.; hence, the investigations about electron transfer mechanisms in other microorganisms are also intended. Further, the efficiency of the exocellular electron transfer rates can be increased by genetic manipulations. The microorganisms exhibiting conductive pili are proposed to be discovered, though such microorganisms can generate higher-power densities. The electron transfer mechanisms from electrodes to microorganisms are still not known. The microorganisms that can accept the electrons from the electrode will have a great significance in cathode compartment. The outer membrane multicopper proteins, OmpB and OmpC, showed their key role in Fe (III) oxide reduction, but a deep investigation is needed to explore their functions in electron transfer mechanisms.
9.7 Conclusions
In MFCs, the microorganisms act as the power houses of the MFC, and those that can form conductive biofilms is of great importance in MFCs. The bacteria produce specific proteins such as c-type cytochromes, pili and QS that play important roles in the formation of a conductive biofilm. Moreover, line of multiple studies suggests that c-type cytochromes, OmcZ and OmcB, are crucially required in electron transfer mechanisms. So far, only Geobacter spp. and Shewanella spp. are able to perform the long-range electron transport through pili. The other exoelectrogens like Pseudomonas spp. secretes pyocyanin, and Shewanella spp. prefers to use flavins to transfer electrons to the electrodes. The latter exoelectrogen has shown to accept electrons from the electrodes using riboflavin as electron mediator. Further, some unusual cytochromes, Cyt579 and Cyt572, have been reported to mediate the electron transfers from the electrode to bacterial cells. The use of biocathodes has made the technology more economic. Moreover, MFC has become the only technology towards renewable energy production and other manifold applications.
References
Aulenta F, Reale P, Canosa A, Rossetti S, Panero S, Majone M (2010) Characterization of an electro-active biocathode capable of dechlorinating trichloroethene and cis-dichloroethene to ethane. Biosens Bioelectron 25:1796–1802. doi:10.1016/j.bios.2009.12.033
Baron DB, LaBelle E, Coursolle D, Gralnick JA, Bond DR (2009) Electrochemical measurements of electron transfer kinetics by Shewanella oneidensis MR-1. J Biol Chem 284:28865–28873. doi:10.1074/jbc.M109.043455
Behera M, Jana PS, Ghangrekar JMM (2010) Performance evaluation of low cost microbial fuel cell fabricated using earthen pot with biotic and abiotic cathode. Bioresour Technol 101:1183–1189. doi:10.1016/j.biortech.2009.07.089
Bergel A, Féron D, Mollica A (2005) Catalysis of oxygen reduction in PEM fuel cell by seawater biofilm. Electrochem Commun 7:900–904. doi:10.1016/j.elecom.2005.06.006
Bonanni S, Massazza D, Busalmen JP (2013) Stepping stones in the electron transport from cells to electrodes in Geobacter sulfurreducens biofilms. Phys Chem Chem Phys 15:10300–10306. doi:10.1039/c3cp50411e
Bond DR, Lovley DR (2003) Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol 69:1548–1555. doi:10.1128/AEM.69.3.1548- 1555.2003
Bond DR, Lovley DR (2005) Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans. Appl Environ Microbiol 71:2186–2189. doi:10.1128/AEM.71.4.2186-2189.2005
Bond DR, Holmes DE, Tender LM, Lovley DR (2002) Electrode reducing microorganisms that harvest energy from marine sediments. Science 295:483–485. doi:10.1126/science.1066771
Brutinel E, Gralnick J (2012) Shuttling happens: soluble flavin mediators of extracellular electron transfer in Shewanella. Appl Microbiol Biotechnol 93:41–48. doi:10.1007/s00253-011-3653-0
Carbajosa S, Malki M, Caillard R, Lopez MF, Palomares FJ, Martín-Gago J, De Lacey AL (2010) Electrochemical growth of Acidithiobacillus ferrooxidans on a graphite electrode for obtaining a biocathode for direct electrocatalytic reduction of oxygen. Biosens Bioelectron 26:877–880. doi:10.1016/j.bios.2010.07.037
Chaudhuri SK, Lovley DR (2003) Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat Biotechnol 21:1229–1232, doi.org/10.1038/nbt867
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306. doi:10.1016/j.biotechadv.2007.02.001
Chou TY, Whiteley CG, Lee DJ (2014) Anodic potential on dual-chambered microbial fuel cell with sulphate reducing bacteria biofilm. Int J Hydrog Energy 39:19225–19231. doi:10.1016/j.ijhydene.2014.03.236
Cournet A, Délia ML, Bergel A, Roques C, Bergé M (2010) Electrochemical reduction of oxygen catalyzed by a wide range of bacteria including Gram-positive. Electrochem Commun 12:505–508. doi:10.1016/j.elecom.2010.01.026
Coursolle D, Baron DB, Bond DR, Gralnick JA (2010) The Mtr respiratory pathway is essential for reducing flavins and electrodes in Shewanella oneidensis. J Bacteriol 192:467–474. doi:10.1128/JB.00925-09
Craig L, Piquie ME, Tainer JA (2004) Type IV pilus structure and bacterial pathogenicity. Nat Rev Microbiol 2:363–378. doi:10.1038/nrmicro885
Cui Y, Rashid N, Hu N, Rehman MSU, Han JI (2014) Electricity generation and microalgae cultivation in microbial fuel cell using microalgae-enriched anode and bio-cathode. Energy Conv Manag 79:674–680. doi:10.1016/j.enconman.2013.12.032
Dietrich LE, Prince-Whelan A, Petersen A, Whiteley M, Newman DK (2006) The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol Microbiol 61:1308–1321. doi:10.1111/j.1365-2958.2006.05306.x
Diggle SP, Winzer K, Chhabra SR, Worrall KE, Camara M, Williams P (2003) The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol Microbiol 50:29–43. doi:10.1046/j.1365-2958.2003.03672.x
Dumas C, Basseguy R, Bergel A (2008) Electrochemical activity of Geobacter sulfurreducens biofilms on stainless steel anodes. Electrochim Acta 53:5235–5241. doi:10.1016/j.electacta.2008.02.056
El-Naggar MY, Wanger G, Leung KM, Yuzvinsky TD, Southam G, Yang J, Lau WM, Nealson KH, Gorby YA (2010) Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. Proc Natl Acad Sci U S A 107:18127–18131. doi:10.1073/pnas.1004880107
Fitzgerald L, Petersen ER, Ray RI, Little BJ, Cooper CJ, Howard EC, Biffinger JC (2012) Shewanella oneidensis MR-1 Msh pilin proteins are involved in extracellular electron transfer in microbial fuel cells. Proc Biochem 47:170–174. doi:10.1016/j.procbio.2011.10.029
Freguia S, Masuda M, Tsujimura S, Kano K (2009) Lactococcus lactis catalyses electricity generation at microbial fuel cell anodes via excretion of a soluble quinone. Bioelectrochem 76:14–18. doi:10.1016/j.bioelechem.2009.04.001
Freguia S, Tsujimura S, Kano K (2010) Electron transfer pathways in microbial oxygen biocathodes. Electrochim Acta 55:813–818. doi:10.1016/j.electacta.2009.09.027
Fuller SJ, McMillan DGG, Renz MB, Schmidt M, Burke IT, Stewart ID (2014) Extracellular electron transport-mediated Fe(III) reduction by a community of alkaliphilic bacteria that use flavins as electron shuttles. Appl Environ Microbiol 80:128–137. doi:10.1128/AEM.02282-13
Goldbeck CP, Jensen HM, TerAvest M, Beedle N, Appling Y, Hepler M, Ajo-Franklin CM (2013) Tuning promoter strengths for improved synthesis and function of electron conduits in Escherichia coli. ACS Synth Biol 2:150–159. doi:10.1021/sb300119v
González del Campo A, Cañizares P, Rodrigo M, Fernández FJ, Lobato J (2013) Microbial fuel cell with an algae-assisted cathode: a preliminary assessment. J Power Sources 242:638–645. doi:10.1016/j.jpowsour.2013.05.110
Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, Beveridge TJ, Chang IS, Kim BH, Kim KS, Culley DE, Reed SB, Romine MF, Saffarini DA, Hill EA, Shi L, Elias DA, Kennedy DW, Pinchuk G, Watanabe K, Ishii S, Logan B, Nealson KH, Fredrickson JK (2006) Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci U S A 103:11358–11363. doi:10.1073/pnas.0604517103
Gregory KB, Bond DR, Lovley DR (2004) Graphite electrodes as electron donors for anaerobic respiration. Environ Microbiol 6:596–604. doi:10.1111/j.1462-2920.2004.00593.x
Holm M, Barken KB, Yang L, Klausen M, Webb JS, Kjelleberg S, Molin S, Givskov M, Tolker-Nielsen T (2006) A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol Microbiol 59:1114–1128. doi:10.1111/j.1365-2958.2005.05008.x
Holmes DE, Mester T, O’Neil RA, Perpetua LA, Larrahondo MJ, Glaven R, Sharma ML, Ward JE, Nevin KP, Lovley DR (2008) Genes for two multicopper proteins required for Fe(III) oxide reduction in Geobacter sulfurreducens have different expression patterns both in the subsurface and on energy-harvesting electrodes. Microbiology 145:1422–1435. doi:10.1099/mic.0.2007/014365-0
Huang L, Chai X, Chen G, Logan BE (2011) Effect of set potential on hexavalent chromium reduction and electricity generation from biocathode microbial fuel cells. Environ Sci Technol 45:5025–5031. doi:10.1021/es103875d
Huang L, Chai X, Quan X, Logan BE, Chen G (2012) Reductive dechlorination and mineralization of pentachlorophenol in biocathode microbial fuel cells. Bioresour Technol 111:167–174. doi:10.1016/j.biortech.2012.01.171
Hubenova Y, Mitov M (2010) Potential application of Candida melibiosica in biofuel cells. Bioelectrochem 78:57–61. doi:10.1016/j.bioelechem.2009.07.005
Inglesby AE, Beatty DA, Fisher AC (2012) Rhodopseudomonas palustris purple bacteria fed Arthrospira maxima cyanobacteria: demonstration of application in microbial fuel cells. RSC Adv 2:4829–4838. doi:10.1039/C2RA20264F
Inoue K, Leang C, Franks AE, Woodard TL, Nevin KP, Lovley DR (2010a) Specific localization of the c-type cytochrome OmcZ at the anode surface in current-producing biofilms of Geobacter sulfurreducens. Environ Microbiol Rep 3:211–217. doi:10.1111/j.1758-2229.2010.00210.x
Inoue K, Qian X, Morgado L, Kim BC, Mester T, Izallalen M, Lovley DR (2010b) Purification and characterization of OmcZ, an outer-surface, octaheme c-type cytochrome essential for optimal current production by Geobacter sulfurreducens. Appl Environ Microbiol 76:3999–4007. doi:10.1128/AEM.00027-10
Ishii S, Watanabe K, Yabuki S, Logan BE, Sekiguchi Y (2008) Comparison of electrode reducing rates of Geobacter sulfurreducens and an enriched electricity-generating mixed consortium in a microbial fuel cell. Appl Environ Microbiol 74:7348–7355. doi:10.1128/AEM.01639-08
Jain A, Zhang X, Pastorella G, Connolly JO, Barry N, Woolley R, Marsili E (2012) Electron transfer mechanism in Shewanella loihica PV-4 biofilms formed at graphite electrode. Bioelectrochem 87:28–32. doi:10.1016/j.bioelechem.2011.12.012
Jayapriya J, Ramamurthy V (2012) Use of non-native phenazines to improve the performance of Pseudomonas aeruginosa MTCC 2474 catalysed fuel cells. Bioresour Technol 124:23–28. doi:10.1016/j.biortech.2012.08.034
Jeans C, Singer SW, Chan CS, Verberkmoes NC, Shah M, Hettich RL, Banfield JF, Thelen MP (2008) Cytochrome 572 is a conspicuous membrane protein with iron oxidation activity purified directly from a natural acidophilic microbial community. ISME J 2:542–550. doi:10.1038/ismej.2008.17
Juarez K, Kim BC, Nevin K, Olvera L, Reguera G, Lovley DR, Methé BA (2009) PilR, a transcriptional regulator for pilin and other genes required for Fe(III) reduction in Geobacter sulfurreducens. J Mol Microbial Biotechnol 16:3–4. doi:10.1159/000115849
Kang CS, Eaktasang N, Kwon DY, Kim HS (2014) Enhanced current production by Desulfovibrio desulfuricans biofilm in a mediator-less microbial fuel cell. Bioresour Technol 165:27–30. doi:10.1016/j.biortech.2014.03.148
Keller KL, Rapp-Giles BJ, Semkiw ES, Porat I, Brown SD, Wall JD (2014) New model for electron flow for sulfate reduction in Desulfovibrio alaskensis G20. Appl Environ Microbiol 80:855–868. doi:10.1128/AEM.02963-13
Kiely PD, Regan JM, Logan BE (2011) The electric picnic: synergistic requirements for exoelectrogenic microbial communities. Curr Opin Biotechnol 22:378–385. doi:10.1016/j.copbio.2011.03.003
Kim BC, Postier BL, Didonato RJ, Chaudhuri SK, Nevin KP, Lovley DR (2008) Insights into genes involved in electricity generation in Geobacter sulfurreducens via whole genome microarray analysis of the OmcF-deficient mutant. Bioelectrochem 73:70–75. doi:10.1016/j.bioelechem.2008.04.023
Kimura ZI, Chung KM, Itoh H, Hiraishi A, Okabe S (2014) Raoultella electrica sp. isolated from anodic biofilms of a glucose-fed microbial fuel cell. Int J Syst Evol Microbiol 64:1384–1388. doi:10.1099/ijs.0.058826-0
Kotloski NJ, Jeffrey AG (2013) Flavin electron shuttles dominate extracellular electron transfer by Shewanella oneidensis. MBio 4.1: e00553–12
Leang C, Coppi MV, Lovley DR (2003) OmcB, a c-type polyheme cytochrome, involved in Fe(III) reduction in Geobacter sulfurreducens. J Bacteriol 185:2096–2103. doi:10.1128/JB.185.7.2096-2103.2003
Leang C, Qian X, Mester T, Lovley DR (2010) Alignment of the c-type cytochrome OmcS along pili of Geobacter sulfurreducens. Appl Environ Microbiol 76:4080–4084. doi:10.1128/AEM.00023-10
Leung KM, Wanger G, El-Naggar MY, Gorby Y, Southam G, Lau WM, Yang J (2013) Shewanella oneidensis MR-1 bacterial nanowires exhibit p-type, tunable electronic behaviour. Nano Lett 13:2407–2411. doi:10.1021/nl400237p
Li X, Zhong G, Qiao Y, Huang J, Weihua H, Wang X, Li C (2014) A high performance xylose microbial fuel cell enabled by Ochrobactrum sp. 575 cells. RSC Adv 4:39839–39843. doi:10.1039/C4RA05077K
Lifang D, Frang L, Shungui Z, Yin HD, Jinren NI (2010) A study of electron-shuttle mechanism in Klebsiella pneumoniae based microbial fuel cells. Environ Sci Technol 55:99–104. doi:10.1007/s11434-009-0563-y
Liu H, Ramnarayanan R, Logan BE (2004) Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ Sci Technol 38:2281–2285. doi:10.1021/es034923g
Liu F, Rotaru A-E, Shrestha PM, Malvankar NS, Nevin KP, Lovley DR (2012) Promoting direct interspecies electron transfer with activated carbon. Energy Environ Sci 5:8982–8989. doi:10.1039/C2EE22459C
Liu D, Lei L, Yang B, Yu Q, Li Z (2013) Direct electron transfer from electrode to electrochemically active bacteria in a bioelectrochemical dechlorination system. Bioresour Technol 148:9–14. doi:10.1016/j.biortech.2013.08.108
Liu F, Rotaru AE, Shrestha PM, Malvankar NS, Nevin KP, Lovley DR (2014a) Magnetite compensates for the lack of a pilin-associated c-type cytochrome in extracellular electron exchange. Environ Microbiol. doi:10.1111/1462-2920.12485
Liu X, Tremblay P-L, Malvankar NS, Nevin KP, Lovley DR, Vargas M (2014b) A Geobacter sulfurreducens strain expressing Pseudomonas aeruginosa type IV pili localizes OmcS on pili but is deficient in Fe(III) oxide reduction and current production. Appl Environ Microbiol 80:1219–1224. doi:10.1128/AEM.02938-13
Logan BE (2004) Extracting hydrogen electricity from renewable resources. Environ Sci Technol 38:160–167. doi:10.1021/es040468s
Logan BE (2009) Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol 7:375–381. doi:10.1038/nrmicro2113
Logan BE, Rabaey K (2012) Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 337:686–690. doi:10.1126/science.1217412
Malone JG, Jaeger T, Manfredi P, Dotsch A, Blanka A, Bos R, Cornelis GR, Haussler S, Jenal U (2012) The YfiBNR signal transduction mechanism reveals novel targets for the evolution of persistent Pseudomonas aeruginosa in cystic fibrosis airways. PLoS Pathog 8:e1002760. doi:10.1371/journal.ppat.1002760
Malvankar NS, Lovley DR (2012) Microbial nanowires: a new paradigm for biological electron transfer and bioelectronics. Chem Sus Chem 5:1039–1046. doi:10.1002/cssc.201100733
Malvankar NS, Vargas M, Nevin KP, Franks AE, Leang C, Kim B-C, Inoue K, Mester T, Covalla SF, Johnson JP, Rotello VM, Tuominen MT, Lovely DR (2011) Tunable metallic-like conductivity in microbial nanowire networks. Nat Nanotechnol 6:573–579. doi:10.1038/nnano.2011.119
Malvankar NS, Tuominen MT, Lovley DR (2012) Lack of cytochrome involvement in long-range electron transport through conductive biofilms and nanowires of Geobacter sulfurreducens. Energy Environ Sci 5:8651–8659. doi:10.1039/C2EE22330A
Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR (2008) Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci U S A 105:3968–3973. doi:10.1073/pnas.0710525105
Mehta MG, Bond DR (2012) Geothrix fermentans secretes two different redox-active compounds to utilize electron acceptors across a wide range of redox potentials. Appl Environ Microbiol 78:6987–6995. doi:10.1128/AEM.01460-12
Mehta T, Childers SE, Glaven R, Lovley DR, Mester T (2006) A putative multicopper protein secreted by an atypical type II secretion system involved in the reduction of insoluble electron acceptors in Geobacter sulfurreducens. Microbiology 152:2257–2264. doi:10.1099/mic.0.28864-0
Merritt JH, Ha DG, Cowles KN, Lu W, Morales DK, Rabinowitz J, Gitai Z, O’Toolea GA (2010) Specific control of Pseudomonas aeruginosa surface-associated behaviors by two c-di-GMP diguanylate cyclases. mBio1: e00183-10. doi:10.1128/mBio.00183-10
Min B, Cheng S, Logan BE (2005) Electricity generation using membrane and salt bridge microbial fuel cells. Water Res 39:1675–1686. doi:10.1016/j.watres.2005.02.002
Nandy A, Kumar V, Kundu PP (2013) Utilization of proteinaceous materials for power generation in a mediatorless microbial fuel cell by a new electrogenic bacteria Lysinibacillus sphaericus VA5. Enzyme Microb Technol 53:339–344. doi:10.1016/j.enzmictec.2013.07.006
Orellana R, Leavitt JJ, Comolli LR, Csencsits R, Janot N, Flanagan KA, Gray AS, Leang C, Izallalen M, Mester T (2013) U(VI) reduction by a diversity of outer surface c-type cytochromes of Geobacter sulfurreducens. Appl Environ Microbiol 79:6369–6374. doi:10.1128/AEM.02551-13
Pamp SJ, Tolker-Nielsen T (2007) Multiple roles of biosurfactants in structural biofilm development by Pseudomonas aeruginosa. J Bacteriol 189:2531–2539. doi:10.1128/JB.01515-06
Pandit S, Khilari S, Roy S, Pradhan D, Das D (2014) Improvement of power generation using Shewanella putrefaciens mediated bioanode in a single chambered microbial fuel cell: Effect of different anodic operating conditions. Bioresour Technol 166:451–457. doi:10.1016/j.biortech.2014.05.075
Park HS, Kim BH, Kim HS, Kim HJ, Kim GT, Kim M, Chang IS, Park YK, Chang HI (2001) A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell. Anaerobe 7:297–306. doi:10.1006/anae.2001.0399
Park DH, Zeikus JG (1999) Utilization of electrically reduced neutral Red by Actinobacillus succinogenes: physiological function of neutral Red in membrane driven fumarate reduction and energy conservation. J Bacteriol 181:2403–2410
Parot S, Nercessian O, Delia ML, Achouak W, Bergel A (2009) Electrochemical checking of aerobic isolates from electrochemically active biofilms formed in compost. J Appl Microbiol 106:1350–1359. doi:10.1111/j.1365-2672.2008.04103.x
Patil S, Hagerhall C, Gorton L (2012) Electron transfer mechanisms between microorganisms and electrodes in bioelectrochemical systems. Bioanal Rev 4:159–192. doi:10.1007/s12566-012-0033-x
Pham CA, Jung SJ, Phung NT, Lee J, Chang IS, Kim BH, Yi H, Chun J (2003) A novel electrochemically active and Fe(III)- reducing bacterium phylogenetically related to Aeromonas hydrophila, isolated from a microbial fuel cell. FEMS Microbiol Lett 223:129–134. doi:10.1016/S0378-1097(03)00354-9
Pham TH, Boon N, Aelterman P, Clauwaert P, Schamphelaire LD, Vanhaecke L, Maeyer KD, Hofte M, Verstraete W, Rabaey K (2008) Metabolites produced by Pseudomonas sp. enables a Gram positive bacterium to achieve extracellular electron transfer. Appl Microbiol Biotechnol 77:1119–1129. doi:10.1007/s00253-007-1248-6
Pirbadian S, El-Naggar MY (2012) Multistep hopping and extracellular charge transfer in microbial redox chains. Phys Chem Chem Phys 14:13802–13808. doi:10.1039/C2CP41185G
Polizzi NF, Skourtis SS, Beratan DN (2012) Physical constraints on charge transport through bacterial nanowires. Faraday Discuss 155:43–61. doi:10.1039/C1FD00098E
Prasad D, Arun S, Murugesan M, Padmanaban S, Satyanarayanan RS, Berchmans S, Yegnaraman V (2007) Direct electron transfer with yeast cells and construction of a mediatorless microbial fuel cell. Biosens Bioelectron 22:2604–2610. doi:10.1016/j.bios.2006.10.028
Prathap P, Cesar IT, Tyson B, Sudeep CP, Bradley GL, Bruce ER (2013) Kinetic, electrochemical, and microscopic characterization of the thermophilic, anode-respiring bacterium Thermincola ferriacetica. Environ Sci Technol 47:4934–4940. doi:10.1021/es400321c
Qiao Y, Li CM, Bao SJ, Lu Z, Hong Y (2008) Direct electrochemistry and electrocatalytic mechanism of evolved Escherichia coli cells in microbial fuel cells. Chem Commun 11:1290–1292. doi:10.1039/B719955D
Rabaey K, Verstraete W (2005) Microbial fuels: novel biotechnology for energy generation. Trends Biotechnol 23:291–298. doi:10.1016/j.tibtech.2005.04.008
Rabaey K, Lissens G, Siciliano SD, Verstraete W (2003) A microbial fuel cell capable of converting glucose to electricity at high rate and efficiency. Biotechnol Lett 25:1531–1535. doi:10.1023/A:1025484009367
Rabaey K, Boon N, Siciliano SD, Verhaege M, Verstraete W (2004) Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl Environ Microbiol 70:5373–5382. doi:10.1128/AEM.70.9.5373-5382.2004
Rabaey K, Hofte M, Vesrtraete W, Boon N (2005) Microbial phenazine production enhances electron transfer in biofuel cells. Environ Sci Technol 39:3401–3408. doi:10.1021/es048563o
Raghavulu SV, Goud RK, Sarma PN, Mohan SV (2011) Saccharomyces cerevisiae as anodic biocatalyst for power generation in biofuel cell: influence of redox condition and substrate load. Bioresour Technol 102:2751–2757. doi:10.1016/j.biortech.2010.11.048
Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR (2005) Extracellular electron transfer via microbial nanowires. Nature 435:1098–1101. doi:10.1038/nature03661
Reguera G, Nevin KP, Nicoll JS, Covalla SF, Woodard TL, Lovley DR (2006) Biofilm and Nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl Environ Microbiol 72:7345–7348. doi:10.1128/AEM.01444-06
Ren H, Lee HS, Chae J (2012) Miniaturizing microbial fuel cells for potential portable power sources: promises and challenges. Microfluid Nanofluid 13:353–381. doi:10.1007/s10404-012-0986-7
Richter H, McCarthy K, Nevin KP, Johnson JP, Rotello VM, Lovley DR (2008) Electricity generation by Geobacter sulfurreducens attached to gold electrodes. Langmuir 24:4376–4379. doi:10.1021/la703469y
Richter H, Nevin KP, Jia H, Lowy DA, Lovley DR, Tender LM (2009) Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type IV pili, and protons in extracellular electron transfer. Energy Environ Sci 2:506–516. doi:10.1039/B816647A
Ringeisen BR, Henderson E, Wu PK, Pietron J, Ray R, Little B, Jones-Meehan JM (2006) High power density from a miniature microbial fuel cell using Shewanella oneidensis DSP10. Environ Sci Technol 40:2629–2634. doi:10.1021/es052254w
Rotaru AE, Shrestha PM, Liu F, Shrestha M, Shrestha D, Embree M, Zengler KP, Wardman C, Nevin KP, Lovley DR (2014) A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ Sci 7:408–415. doi:10.1039/C3EE42189A
Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG (2002) Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J Bacteriol 184:1140–1154. doi:10.1128/jb.184.4.1140-1154.2002
Schaefer AL, Greenberg EP, Oliver CM, Oda Y, Huang JJ, Banin G, Peres CM, Schimdt S, Juhaszova K, Sufrin JR, Harwood CS (2008) A new class of homoserine lactone quorum-sensing signals. Nature 454:595–599. doi:10.1038/nature07088
Schroder U, Nieben J, Scholz F (2003) A generation of microbial fuel cells with current outputs boosted by more than one order of magnitude. Angew Chem Int Ed Engl 42:2880–2883. doi:10.1002/anie.200350918
Shen HB, Yong XY, Chen YL, Liao ZH, Si RW, Zhou J, Zheng T (2014) Enhanced bioelectricity generation by improving pyocyanin production and membrane permeability through sophorolipid addition in Pseudomonas aeruginosa-inoculated microbial fuel cells. Bioresour Technol 167:490–494. doi:10.1016/j.biortech.2014.05.093
Singh L, Wahid ZA (2015) Enhancement of hydrogen production from palm oil mill effluent via cell immobilisation technique. Int J Energy Res 39:215–222. doi:10.1002/er.3231
Singh L, Siddiqui MF, Ahmad A, Rahim MH, Sakinah M, Wahid ZA (2013) Biohydrogen production from palm oil mill effluent using immobilized mixed culture. J Ind Eng Chem 19:659–664. doi:10.1016/j.jiec.2012.10.001
Smith J, Lovley DR, Tremblay PL (2013) Outer cell surface components essential for Fe (III) oxide reduction by Geobacter metallireducens. Appl Environ Microbiol 79:901–907. doi:10.1128/AEM.02954-12
Smith JA, Tremblay PL, Shrestha PM, Snoeyenbos-West OL, Franks AE, Nevin KP, Lovley DR (2014) Going wireless: Fe(III) Oxide reduction without Pili by Geobacter sulfurreducens Strain JS-1. Appl Environ Microbiol 80:4331–4340. doi:10.1128/AEM.01122-14
Snider RM, Strycharz-Glaven SM, Tsoi SD, Erickson JS, Tender LM (2012) Long-range electron transport in Geobacter sulfurreducens biofilms is redox gradient-driven. Proc Natl Acad Sci U S A 109:15467–15472. doi:10.1073/pnas.1209829109
Stoodley P, Sauer K, Davies DG, Costerton JW (2002) Biofilms as complex differentiated communities. Annu Rev Microbiol 56:187–209. doi:10.1146/annurev.micro.56.012302.160705
Sun D, Call D, Wang A, Cheng S, Logan BE (2014a) Geobacter sp. SD-1 with enhanced electrochemical activity in high-salt concentration solutions. Environ Microbiol Rep. doi:10.1111/1758-2229.12193
Sun D, Wang A, Cheng S, Yates MD, Logan BE (2014b) Geobacter anodireducens sp. nov., a novel exoelectrogenic microbe in Bioelectrochemical systems. Int J Syst Evol Microbiol. doi:10.1099/ijs.0.061598-0
Thrash JC, Van Trump IV, Weber KA, Miller E, Achenbach LA, Coates JD (2007) Electrochemical stimulation of microbial perchlorate reduction. Environ Sci Technol 41:1740–1746. doi:10.1021/es062772m
Torres CI, Marcus AK, Lee H-S, Parameswaran P, Krajmalnik-Brown R, Rittmann BE (2010) A kinetic perspective on extracellular electron transfer by anode-respiring bacteria. FEMS Microbiol Rev 34:3–17. doi:10.1111/j.1574-6976.2009.00191.x
Vargas M, Malvankar NS, Tremblay PL, Leang C, Smith J, Patel P, Synoeyenbos-West O, Nevin KP, Lovley DR (2013) Aromatic amino acids required for pili conductivity and long-range extracellular electron transport in Geobacter sulfurreducens. mBio 4:e00105-13. doi:10.1128/mBio.00105-13
Von CH, Ogawa J, Shimizu S, Lloyd JR (2008) Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl Environ Microbiol 74:615–623. doi:10.1128/AEM.01387-07
Voordeckers JW, Kim BC, Izallalen M, Lovley DR (2010) Role of Geobacter sulfurreducens outer surface c-type cytochromes in reduction of soil humic acid and anthraquinone-2, 6- disulfonate. Appl Environ Microbiol 76:2371–2375. doi:10.1128/AEM.02250-09
Wang H, Ren ZJ (2013) A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol Adv 31:1796–1807. doi:10.1016/j.biotechadv.2013.10.001
Wang H, Lu L, Cui F, Liu D, Zhao Z, Xu Y (2012) Simultaneous bioelectrochemical degradation of algae sludge and energy recovery in microbial fuel cells. RSC Adv 2:7228–7234. doi:10.1039/C2RA20631E
Wang Y, Wang A, Zhou A, Liu W, Huang L, Xu M, Tao H (2014) Electrode as sole electrons donor for enhancing decolorization of azo dye by an isolated WYZ-2. Bioresour Technol 152:530–533. doi:10.1016/j.biortech.2013.11.001
Wrighton KC, Thrash JC, Melnyk RA, Bigi JP, Byrne-Bailey KG, Remis JP, Schichnes D, Auer M, Chang CJ, Coates JD (2011) Evidence for direct electron transfer by a Gram-positive bacterium isolated from a microbial fuel cell. Appl Environ Microbiol 77:7633–7639. doi:10.1128/AEM.05365-11
Wu C, Liu XW, Li WW, Sheng GP, Zang GL, Cheng YY, Yu HQ (2012) A white-rot fungus is used as a biocathode to improve electricity production of a microbial fuel cell. Appl Energy 98:594–596. doi:10.1016/j.apenergy.2012.02.058
Xafenias N, Zhang Y, Banks CJ (2013) Enhanced performance of hexavalent chromium reducing cathodes in the presence of Shewanella oneidensis MR-1 and lactate. Environ Sci Technol 47:4512–4520. doi:10.1021/es304606u
Xing D, Zuo Y, Cheng S, Regan JM, Logan BE (2008) Electricity generation by Rhodopseudomonas palustris DX-1. Environ Sci Technol 42:4146–4151. doi:10.1021/es800312v
Xin L, Guo-Zhen Z, Yan Q, Jing H, Weihua H, Xing-Guo W, Changming L (2014) A high performance xylose microbial fuel cell enabled by Ochrobactrum sp.575 cells. RSC Adv 4:39839–39843. doi:10.1039/C4RA05077K
Xu S, Liu H (2011) New exoelectrogen Citrobacter sp. SX-1 isolated from a microbial fuel cell. J Appl Microbiol 111:1108–1115. doi:10.1111/j.1365-2672.2011.05129.x
Yong XY, Shi DY, Chen YL, Feng J, Xu L, Zhou J, Zheng T (2014) Enhancement of bioelectricity generation by manipulation of the electron shuttles synthesis pathway in microbial fuel cells. Bioresour Technol 152:220–224. doi:10.1016/j.biortech.2013.10.086
Yuan Y, Chen Q, Zhou S, Zhuang L, Hu P (2011) Bioelectricity generation and microcystins removal in a blue–green algae powered microbial fuel cell. J Hazard Mater 187:591–595. doi:10.1016/j.jhazmat.2011.01.042
Zhang J, Yang G, Zhou S, Wang Y, Yuan Y, Zhuang L (2013) Fontibacter ferrireducens sp., an Fe(III)-reducing bacterium isolated from a microbial fuel cell. Int J Syst Evol Microbiol 63:925–929. doi:10.1099/ijs.0.040998-0
Zhou M, He H, Jin T, Wang H (2012) Power generation enhancement in novel microbial carbon capture cells with immobilized Chlorella vulgaris. J Power Sources 214:216–219. doi:10.1016/j.jpowsour.2012.04.043
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer India
About this chapter
Cite this chapter
Kumar, R., Singh, L., Wahid, Z.A. (2015). Role of Microorganisms in Microbial Fuel Cells for Bioelectricity Production. In: Kalia, V. (eds) Microbial Factories. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2598-0_9
Download citation
DOI: https://doi.org/10.1007/978-81-322-2598-0_9
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2597-3
Online ISBN: 978-81-322-2598-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)