Abstract
This chapter compiles active and passive defensive mechanisms of aquatic and semiaquatic developmental stages of all insect orders against various predators. Mainly escape reactions, mechanical defense, defensive stridulation, and especially chemical defenses are described, illustrated, and tabulated. Apart from the large aquatic groups of ephemeropteran, Odonata or Trichoptera larvae especially aquatic bugs and water beetles are considered by even including small groups from Collembola up to Mecoptera.
Differences between defensive mechanisms and strategies in aquatic and terrestrial insects are described. Aquatic insects especially rely on escape, mechanical defenses, defensive stridulation, and chemical defenses. Exocrine glands are mainly restricted to large taxa with both terrestrial and aquatic representatives (adephagan beetles, Heteroptera) and not invented in aquatic groups. Chemically aquatic insects especially evolved biosynthesis of aromatic and few aliphatic compounds against microorganisms. In contrast mainly steroids are targeted against cold-blooded vertebrates such as fishes and amphibians. As compared with terrestrial insects, aquatic representatives lack many mechanisms of defense such as reflex bleeding, incorporation of toxic compounds from plants, freshwater animals, or microorganisms. Exocrine secretions of water insects are usually externalized by secretion grooming in order to receive a clean body surface, to achieve an optimal breathing, and to modify the wettability of the body surface. Generally there exists a considerable lack of knowledge concerning bionomy and especially defenses of aquatic insects.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Adults and developmental stages of aquatic insects are eaten by many predators (e.g., carnivorous fishes, predatory insects, various invertebrates), suffer from parasitoids, and have problems with insect pathogenic microorganisms (Protozoa, Bacteria) and fungi. In extreme cases water insects may suffer from snake attacks (Peddle and Larson 1999) and reversally prey on vertebrates (McCormick and Polis 1982). Therefore water insects which are found in various sometimes unique habitats (Heckman 2018) have to defend against these natural enemies and evolved various strategies of defense, sometimes even multiple defenses against these target organisms.
There exist many important general books dealing with defense mechanisms of animals or insects: Curio (1976), Edmunds (1974), Evans and Schmidt (1990), and Ruxton et al. (2004). In 1990 Witz studied antipredator mechanisms of hundreds of arthropods from terrestrial and aquatic habitats and showed that active or secondary defenses are most important. These defenses are energetically expensive mechanisms, which increase the probability of surviving attacks of predators. They include for example chemical defenses (46%), fighting (11%), escape reactions (8%), postural defenses (4%), armors (3%), or defensive stridulation (2%). In contrast Witz (1990) showed that passive or primary defenses such as cryptic appearance (9%) or group size (4%) are of minor importance. These defenses seem relatively inexpensive and reduce the probability of an encounter between predator and prey.
The main aim of this review is to compile such active and passive defenses in all aquatic insect orders and aquatic or semiaquatic developmental stages (especially larvae) of water insects. Also aquatic representatives of usually terrestrial orders are considered, because now many data from these and other taxa are available due to publications on biodiversity and many valuable books, for example “Thorp and Covich’s Freshwater Invertebrates ” (Thorp and Rogers 2015, 2016; Hamada et al. 2018). With regard to insect species numbers in different biogeographical regions (Stork 2018) especially the incredible species diversity in the neotropics (1,620,000 insect species) as compared with the Palaearctic (524,165 insect species; albeit terrestrial taxa) were helpful for this compilation, because the various strategies of defense increase towards tropical areas.
Since theoretical data on predator-prey interactions are abundant (e.g., Resh and Rosenberg 1984; Kerfoot and Sih 1987; Williams and Feltmate 1992; Lampert and Sommer 2007; Lancaster and Downes 2013) these interesting aspects were not considered. The same applies to chemical signals such as pheromones or kairomones which were compiled by Chivers and Smith (1998), Burks and Lodge (2002), Sotka et al. (2009), Ferrari et al. (2010), and Brönmark and Hansson (2012), although our knowledge on chemistry of these interesting signal molecules is very poor. For this review it is highly interesting to consider and to compare with valuable papers dealing with defense of water insects such as from Peckarsky (1984), Scrimshaw and Kerfoot (1987), Crespo (2011), or Kicklighter (2012). Also chemical defenses of lower freshwater animals (apart from insects) may be of interest (Dettner 2010) because these animals might be fed by aquatic insects (Bay 1974).
Unfortunately data on bionomy of eggs, egg deposition (Hinton 1981; Hilker and Meiners 2002), and especially pupation in most holometabolous taxa are scarce or unknown especially with respect to aquatic and semiaquatic taxa. Therefore these data are nearly unavailable for this compilation. Moreover it seems interesting for forthcoming evaluations to consider the significance of attachment devices (Gorb 2007) and the role of adhesives (Betz and Kölsch 2004; Betz 2010) as defense mechanisms of aquatic insects. Apart from the dytiscid beetles (Dettner 2014) the same lack of knowledge concerns interactions between symbiontic and parasitic internal but also external microorganisms (bacteria, fungi), protozoa (peritrichic Ciliata, Laboulbeniales), and in/external parasites (mites, trematodes) and their water insect hosts.
Depending on the number of aquatic taxa within each insect order, the data on defenses of aquatic insects are presented on the level of the order or alternatively on the family level. The percentual value of aquatic or semiaquatic species as compared with the total species number per taxon is indicated after each insect order. Bold numbers in the text and legends indicate numbers of compounds (see Tables 9.1, 9.2, and 9.3).
I hope that further studies of biology and bionomy of water insects may help to complete our fragmentary knowledge concerning defense mechanisms of these interesting animals, especially if tropical taxa are additionally taken into consideration.
A lot of phylogenetic trees indicate that the elder arrangement of entognathous taxa as insects (e.g., Dettner and Peters 2010) has to be corrected. Rather now ectognathous taxa represent Insecta whereas Entognatha + Ectognatha are named Hexapoda (e.g., Beutel et al. 2014). Nevertheless semiaquatic Collembola as representatives of Entognatha are treated in this chapter.
COLLEMBOLA (springtails; about 45% hydrophilous): With worldwide approximately 9000 species (Stork 2018: 8140; according to Cipola et al. 2018 probably 50,000 species) this is the largest group of apterygote and endognathous insects or hexapods. Due to their jumping ability with furca and retinaculum (springtails) and detachable hairs and scales this is a well-defended taxon. Unusually for insects springtails continue to molt (up to 52 stages) after reaching sexual maturity. However there is a gap of knowledge with respect to collembolan biology which might be correlated to their small body size and the difficulty to determine all species. With respect to their habitats about 45% of all Collembola species are hydrophilous, represent riparian species, are associated with wet habitats as shores of ponds and lakes, or are frequent in marine littoral zones (Thorp and O’Neill 2015). Cipola et al. (2018) divided Collembola into primary or water-dependent and secondary (epigean hydrophilous) aquatic-associated species. However no species has developed a permanent underwater lifestyle, wherefore no special adaptations for swimming or diving have evolved. Instead most species have hydrophobic hairs, a type of wax and/or a specific surface geometry like microtubercles. Even under high pressure, with the addition of surfactants or other organic fluids Collembola are not immersed due to their unwettable cuticle. Only representatives of genera Spinactaletes, Arlesminthurus, and Pseudobourletiella are capable of being submerged (Cipola et al. 2018). Interestingly young springtails after molting lose their nonwetting properties for a short time. If they stand on the water surface this is due to their nonwetting claws. If forced to submerge larvae will sink, have cutaneous respiration, and can also be invaded by microorganisms (Chang 1966; Thorp and O’Neill 2015). These underwater larvae will not molt. If gravid females are disturbed expelled eggs float on the water surface and later sink down. The underwater development continued and most eggs hatched (Chang 1966). An interesting movement in order to escape predators was observed in Anurida maritima (Fig. 9.1/4) on coastal pools and Podura aquatica (Fig. 9.1/3) at the surface of ponds. They lay on the water surface and pull up with a central wetting tube or ventral tube (collophore) at the same time as nonwetting head and abdominal tip are pushed down. When they release the wetting tube from the surface film, the animal is propelled into the air (Fig. 9.1/4, Bush et al. 2008). This posture by forming a meniscus also enables them to attract neighbors over a small distance.
Defenses of Collembola (1–5), Ephemeroptera (6–8). 1. Onychiurus spec. depletes defensive droplet from pseudocell (scale: 0.5 mm). 2. Pseudocell of Tetrodontophora bielanensis (scale: 10 um). 3. Podura aquatica on water surface. 4. Springtail Anurida maritima escapes predators by using its ventral tube. 5. Unusual hydrophobous hydrocarbon poduran from P. aquatica. 6. High-intensity scorpion posture of Ephemerella subvaria. 7. First larval instar of Baetisca rogersi. 8. Last larval instar of Baetisca spec. Sources: 1, 2, 3: Dettner and Peters 2010. 4. Modified after Lancaster and Downes 2013. 5. After Schulz et al. 1997. 6. Modified after Peckarsky 1987. 7. Modified after Pescador and Peters (1974). 8. Modified after Pescador et al. (2009)
Some semiaquatic species live and feed on water surfaces. Especially Podura aquatica (Fig. 9.1/3, Poduridae), Isotomurus palustris (Isotomidae ), Sminthurides aquaticus (Sminthuridae ) and S. malmgreni live on open water surfaces. Other more specialized species such as representatives of Arrhopalites (Sminthuridae) live on surface films of cave waters or other species prefer surfaces of aquatic plants such as Lemna or Nasturtium). Interestingly Collembola which are mainly found on water surfaces are characterized by distinctly paddle-like broadened mucrones, the claws of the abdominal springing organ (Podura aquatica; Proisotoma crassicauda, P. borealis Isotomidae; Archisotoma Isotomidae; Sminthurides; Schulze 1924). These mucrones prevent the breakthrough of the surface tension during a jump (Hopkin 1997).
Chemical defense is obvious in very different Collembola taxa and not only significant for those species which are eyeless or have lost their jumping ability (Dettner 2015). Defensive compounds were especially found in representatives of Onychiuridae (Fig. 9.1/1 Onychiurus spec. depletes secretion from a pseudocellus), Hypogastruridae, Neanuridae, or Tullbergiidae. These allomones are usually externalized by integumental pores, so-called pseudocells , (Fig. 9.1/2 pseudocellus from Tetrodontophora spec.), but there exist also springtails exhibiting warning colorations or exposing club-like defensive glands. Among the semiaquatic families there exist chemical data from Poduridae and Isotomidae . From Podura aquatica body surfaces a new hydrocarbon named poduran, with an unusual tricyclo(6.2.0.0)decane system was identified (Fig. 9.1/5; Schulz et al. 1997). Finally representatives of Isotomidae such as Folsomia fimetaria contain unknown hemolymph toxins which negatively influence the reproduction of the main predatory species, the spider Erigone atra (Marcussen et al. 1999).
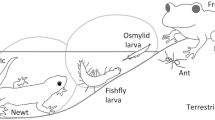
EPHEMEROPTERA (mayflies; 100% aquatic): Mayflies represent the oldest order of winged insects. About 3500 species worldwide (Salles et al. 2018; Stork 2018: 3240) are characterized by aquatic eggs, phytophagous larvae (nymphs), and 2 terrestrial winged stages (subimago, imago) (Sartori and Brittain 2015a, b). The unique subimago is covered by hydrofugous hairs which allows this stage to escape the water surface tension (Sartori and Brittain 2015a, b). There are 9–45 larval stages which live 3 weeks to 2 years, whereas adults live from 1 h to few days (Morse 2017). Mayfly larvae and subimagines which may be very diverse with respect to morphology represent a major part of the macroinvertebrate biomass and are therefore eaten by a wide range of aquatic invertebrates (Plecoptera, Trichoptera, Sialidae, Odonata, aquatic bugs and water beetles, leeches, triclads, crayfish) and vertebrates (fishes, specialized birds; Grant 2001). 42 fish families analyzed fed on aquatic insects and in Nearctic plecopteran genera 50% of the gut contents consisted of mayfly larvae (Bauernfeind and Soldan 2012). As compared with larvae of stoneflies which often show a greater degree of chitinization, larvae of Ephemeroptera are more soft and often have large lamellar gills (Brittain 1990). It is astonishing that there exist no chemical or acoustical defense mechanisms of mayfly developmental stages (neither stridulation, Aiken 1985, nor chemical defense, Dettner 2015) against predators, parasitoids, or pathogenic organisms. Instead depending on the presence of predators mayflies have evolved behavioral defensive mechanisms such as drifting, swimming, crawling away, hiding, scorpion posturings, or timing of activities (mass emergence, mating swarms) in order to reduce the chance of predation (Peckarsky 1996). As shown by Crespo (2011) for mayfly larvae chemical signals are highly important. Synchronous emergence could saturate potential predators whereas dispersed emergence eventually lowers the possibility of predator-prey encounters (Sartori and Brittain 2015a, b). After contact with predacious stonefly larvae Ephemerella infrequens (Ephemerellidae) exhibited a scorpion posture (Fig. 9.1/6). This reaction was usually evoked by touch of body parts; sometimes the reaction was shown when predators approached from upstream (Peckarsky 1987). In contrast Baetis bicaudatus (Baetidae) showed a “tail curl” posture in flexing cerci (which represent sensory structures, Crespo 2011) and posterior abdominal segments against predators. Peckarsky (1980, 1987) reports that Ephemerella deflects stoneflies because the posture may increase the apparent size of the mayflies which are then rejected by tactile predators. On the other hand Baetis detects predator’s wave disturbances and enables the prey to avoid predator encounters. In other species defence reactions such as crawling evasions and tactile (chemotactile) stimuli were observed as being responsible for these behaviors (Peckarsky 1980). Moreover chemical interactions between predator and mayfly-larvae-prey were supposed, because larvae of Baetis actively left the vicinity of stonefly larvae (see Bauernfeind and Soldan 2012). Elder larvae of Baetiscidae (Fig. 9.1/8) as compared with freshly hatched stages (Fig. 9.1/7) have a unique and bizarre appearance due to the thoracic notum and the presence of large spines (two lateral, two dorsal projections) and various lobes and humps (“armored mayflies”; Pescador et al. 2009). In addition these larvae exhibit cuticles which are harder than most mayfly larvae. The thoracic notum of Baetiscidae is fused, covers various abdominal segments (5), and encloses the gills underneath. According to Pescador and Peters (1974) the mesonotal shield helps to protect the gills and the lateral spines act as balancers and maintain the dorsoventral position of the larvae. Here I interpret the spines and the hardened cuticle as a typical mechanical defense against larger predators. Moreover it is noteworthy that Baetisca larvae exhibited thanatosis after mechanical molestation and show a coloration closely resembling their habitat (Pescador and Peters 1974). Both cryptic colorations (other larval genera such as Baetis and Rhithrogena) and aposematic color patterns (Ecdyonurus, Heptagenia, Ephemerella) are observed (Bauernfeind and Soldan 2012). However the presence of toxic natural compounds in mayflies has not been observed at all. Apart from thanatosis or scorpion postures common other defensive reactions observed in mayfly larvae are escape into the substrate (Bauernfeind and Soldan 2012). There are no data available on how mayflies defend against parasitic or commensalic organisms. However mayflies parasitized by mermithid nematodes showed increased defensive activities against predaceous stoneflies (Benton & Pritchard 1990).
9.2 ODONATA (damselflies, dragonflies; 99–100% aquatic)
Worldwide there exist about 6000 species (Stork 2018: 5899; another 1000 to 1500 species are expected to exist per Pessaq et al. 2018) of Odonata which comprise the three suborders Zygoptera (damselflies), Anisoptera (dragonflies), and Anisozygoptera (damsel dragons) (Suhling et al. 2015; Morse 2017; St. Quentin and Beier 1968). Eggs and larvae are aquatic and are found in various types of freshwater habitats including water-filled bromeliads. Few species are semiaquatic, whereas adults are terrestrial flying near water. Depending on species larvae need few weeks to 5 years to complete their development comprising 10–15 instars. Aquatic larvae and aerial adults of Odonata are predators with extrudable mouthparts of larvae and developed sight and flight in adults. Odonata represent one of the best known insect orders concerning taxonomy, zoogeography, or studies on sexual selection and conflicts by sperm displacement (Cordoba-Aguilar 2008; Dijkstra et al. 2014).
In addition predators of Odonata larvae, their prey, and their various defense mechanisms against opponents are well known in spite of the fact that there was not realized any chemical defense mechanism in larvae or adults of this insect order (Corbet 1999; Suhling et al. 2015). The risk of fish predation is reduced by various behavioral and morphological features of the odonate larvae. As was demonstrated by Wohlfahrt et al. (2005) these traits may be fixed and occur both in the presence and absence of predators. On the other hand on response to predators these traits may be flexible. Main predators of Odonata larvae are fishes which are significant in shaping odonate community structures as was demonstrated in North American Enallagma species (Johnson 1991). Larvae of certain species reduce their movements and their foraging activity when fishes are present, whereas other species escape predators by swimming away (Johnson 1991). As was shown by Crespo (2011), larvae of Odonata can sense infochemicals from various predators and even learn to associate them with predator presence. Invertebrate predators include other Odonata larvae including conspecifics (cannibalism), dytiscid beetles (larvae and adults), crayfish, belostomatid bugs, and several water birds (see Suhling et al. 2015). Other larvae exhibit nocturnal circadian rhythms in order to reduce predation pressure (Pierce 1988). It was also reported from zygopteran larvae that avoidance responses after contacts with predators may change during ontogeny (Corbet 1999). Many Odonata larvae show cryptic colorations which correspond to the microhabitat background, being greenish, yellowish, darkened, or with dark spots on pale ground (mixture or mixtures of sediments). In young instars of Anax transverse dark bands are observed followed by pale stripes. As in adults also in larvae there are abilities to change coloration. Larvae of other species may bear many setae on their body surface; adhering to fine detritus may result in an excellent camouflage. Many Odonata larvae hide in dense vegetation (Thompson 1987) or burrow in suitable substrates (Suhling and Müller 1996) in order to reduce predation pressure.
Larvae of Epiophlebia (Anisozygoptera) which represent living fossils are unusual in producing sound and sometimes exhibiting thanatosis when molested. The production of sounds after molestation was also reported in Uropetala carvei (Petaluridae; see Corbet 1999). Larvae with 14 instars need maximally 8 years (longest record for Odonata) for their development. Elder larvae are terrestrial for several months and breathe via thoracic stigma. In laboratory experiments it could be shown that larval stridulation was produced in response to mechanical disturbance (Aiken 1985; Asahina 1950). In order to stridulate lateral stridulatory files on abdominal segments 3–7 (Fig. 9.2/1) are pressed against metafemur serrations. Sounds are produced when the abdomen is twisted and telescoped. Stridulatory files are first evident in the sixth instar before eclosion (Aiken 1985). Epiophlebia larvae which appear like larvae from the Anisoptera are unable to use the anisopteran jet propulsion mode of escape but walk. In elder anisopteran larvae a kind of jet propulsion as a rapid escape mechanism was described (Corbet 1999) which is elicited by stimulation of paraproct nerves. These larvae may suddenly deplete their water-filled hindgut. In elder larvae this is the only way of locomotion, because leg muscles degenerate before larvae leave the water. When zygopteran larvae are irritated they may lose their caudal appendages (Stoks 1999), which possess breaking joints (Corbet 1999). Thus influx of water or loss of hemolymph is prevented. Autotomized body parts regenerate during succeeding moltings. These abdominal appendages are sometimes broadened in order to increase swimming speed (McPeek 2000). In other genera such as Aeshna, Enallagma, or Ischnura also whole legs are autotomized (Corbet 1999). In many cases larvae of Odonata are immobilized and show thanatosis (Fig. 9.2/6, 7) when they are molested (Wildermuth 2000). In Somatochlora larvae either extended their legs laterally, held them obliquely upwards (Fig. 9.2/6), or folded them close to their bodies. Among 18 investigated anisopteran species larvae of Brachytron pratense and three Somatochlora species revealed an obligatory reaction to tactile stimulation (Wildermuth 2000). Corbet (1999) reports reflex immobilization from 11 families of Odonata (Aeshnidae, Coenagrionidae, Cordulegastridae, Corduliidae, Epiophlebiidae, Gomphidae, Hemiphlebiidae, Libellulidae, Neopetaliidae, Petaluridae, and Pseudolestidae). Within ten species duration of immobilization ranged from more than 20 s to about 889 s; the maximum recorded time was more than 30 min. Many zygopteran larvae show distinct “unnatural” postures mainly of their caudal appendages (Xanthocnemis, Corbet 1999). Various anisoptera larvae have extremely flexible abdomina. Due to spines which are arranged laterally and dorsally various predators and especially fishes are warded off (Johansson and Mikolajewski 2008). Spines of anisopteran larvae occur especially on the last third of the abdomina and form a spiky pyramid in order to “sting” potential predators. Remarkable examples for abdominal “weapons” are known from representatives of the anisopteran genera Aeshnosoma (Fig. 9.2/2), Melanocacus, Orionothemis (Fig. 9.2/4, 5), or Paracordulia and even from zygopteran species such as Heteragrion consors (Fig. 9.2/3; Pessacq et al. 2018). Sometimes large anisopteran larvae as Tanypteryx pryeri or Anax junius use their mandibles in order to bite aggressors (Corbet 1999).
Defenses of Odonata (1–7), Plecoptera (8). 1. Last-stage larva of Epiophlebia superstes and stridulatory files on tergites of abdominal segments 5/6 (left). 2. Apex half of abdomen larva of Aeschnosoma sp. (dorsal view). 3. Apex of abdomen with caudal appendages of female larva of Heteragrion consors (Megapodagrionidae, dorsal view). 4. Left lateral view of abdomen of last-stage larva of Orionothemis felixorioni. 5. Half last-stage larva (dorsal view) of Orionothemis felixorioni. 6. Thanatosis of Somatochlora flavomaculata after molestation (side view). 7. Somatochlora flavomaculata last instar larva (dorsal view), normal sitting position (left), thanatosis after molestation (right). 8. Plecopteran larva of family Taeniopterygidae after molestation in curling defence position. Sources: 1. Modified after Aiken 1985. 2. Modified after Neiss et al. 2018. 3. Modified after Anjos-Santos et al. 2018. 4, 5. Modified after Fleck et al. 2009. 6, 7. Modified after Wildermuth 2000. 8. Modified after Zwick 1980
Recently there were published several results in order to test hypotheses about parasite (water mites, gregarines) mediated selection with odonate hosts (Forbes and Robb 2008; Honkavaara et al. 2009). Probably mite infections may compromise a male’s resistance against further infections by parasites which is achieved by hemolymph encapsulation in Coenagrion armatum. In addition the pseudopeptide antibiotic amicoumacin was isolated from Bacillus species which were isolated from Coenagrion guts. However it is not known if the bacteria produce this compounds in the Zygoptera larva (see Dettner 2015).
PLECOPTERA (stoneflies; 100% aquatic): As basal order of the Neoptera stoneflies have worldwide about 4000 species (Stork 2018: 3743) and are characterized by terrestrial adults and aquatic eggs and larvae (Avelino-Capistrano et al. 2018). Plecoptera are usually associated with cool and cold waters and possess 10–22 larval instars (Morse 2017). Stoneflies and especially their larvae have a lot of enemies and parasites ranging from salmonid fishes, birds, and predatory arthropods to gregarines, insect-pathogenic fungi, and water mites (Zwick 1980). Communication between males and females is often achieved by tapping on substrate (“drumming”; DeWalt et al. 2015). Crespo (2011) demonstrated in stonefly larvae both mechanosensory and chemical cues, which means nonvisual modalities were involved in detection of prey. In various especially young adult species (Pteronarcys proteus, Peltoperla maria, Benfield 1974; Perla burmeisteriana, Acroneuria arenosa, Zwick 1980) and one larval species (P. dorsata; Moore and Williams 1990) reflex bleeding behavior was recorded after molestations. Beaty (2015) reported that preserved larvae of Pteronarcyidae showed white exudates originating from thoracic nota and coxae. Also in various Plecoptera adults reflex bleeding was observed; however no active compound could be isolated (Stocks 2004). Zwick (1980) suggested that plecopteran hemolymph acts as an adhesive. Zwick (1980) reported that hemolymph of Pteronarcys can be sprayed away up to 25 cm by reflex bleeding, a behavior which is accompanied by an audible popping sound. Apparently hemolymph of stoneflies is not malodorous and represents no deterrent (Zwick 1980). The basis for this autohemorrhage was presumed to be increased hemolymph pressure (Stocks 2004). Moore and Williams (1990) were the first who demonstrated that the larvae of Pteronarcys dorsata show different responses which depends on the predators. Reflex bleeding and retreat were observed after contact with benthic crayfish predators. In contrast freezing and thanatosis were observed after contacts with benthic and pelagic fish predators. Beaty (2015) reported that Pteronarcys larvae exhibit thanatosis and turn into a ball. Further predator-prey studies were performed by Peckarsky (1980). Finally it is well known that larvae of Taeniopterygidae (e.g., Brachyptera) exhibit a characteristic of curled thanatosis position (Fig. 9.2/8; Zwick 1980). In various plecopteran taxa coiling movements were observed in order to escape from carnivorous insect larvae such as Odonata or Dytiscidae (Zwick 1980). Few larvae are also characterized by spines which might be located on thorax (Kempnyia, Gripopteryx) or abdomen (Neopentura) and one species may be covered by detritus (Pelurgoperla personata) (Avelino-Capistrano et al. 2018).
ORTHOPTERA (bush crickets, crickets, grasshoppers, locusts; 0.3% aquatic): Among the over 28,000 orthopteran species (Stork 2018: 23,855) about 80 species with adaptations to aquatic life are aquatic whereas additional 110 species are water dependant (associated with aquatic habitats; Cover and Bogan 2015; Heckman 2018). Adaptations of aquatic Orthoptera are paddle-shaped hind tibiae (adapted for swimming), use of a plastron when submerged (Acrididae: Leptysminae), and diving in order to feed on submerged plants and to escape predators (Gutjahr and Braga 2018). Following families include Nearctic aquatic species: grasshoppers (Acrididae), pygmy grasshoppers (Tetrigidae), pygmy mole grasshoppers (Tridactylidae), katydids (Tettigoniidae), crickets (Gryllidae), and mole crickets (Gryllotalpidae). Also from Asia aquatic representatives are registered from the subfamilies Oxyinae (Acrididae), Tropidopolinae, and Hemiacridinae. Interestingly one aquatic and South American acridid species Cornops aquaticum feeds on water hyacinths and is being bred for possible release as a way to control this weed (Cover and Bogan 2015). Other South American species from the genera Paulinia and Marellia exclusively consume aquatic plants and are used in biocontrol of aquatic weeds in Africa and Australia (Cover and Bogan 2015). Unfortunately biology and ecology of these aquatic species are mainly unknown. However the semi-aquatic grasshopper Paulinia acuminata and its food plant Salvinia auricularia exhibit mimicry and ultrastructural analogy in order to achieve water-repellent surfaces (Barthlott et al. 1994). Generally among Orthoptera a lot of primary and secondary defensive mechanisms such as crypsis, aposematism, jumping, biting, flight, stridulation, or autotomy are realized (Dettner 2015). Employed chemical defenses are regurgitation, defecation, glandular defensive secretions, and internal toxins (Dettner 2015).
Within Tetrigoidea (about 1000 species), members of Scelimini are fully aquatic and can swim effectively (Rentz and Su 2003). Usually Tetrigidae are common near water, to which they readily take to escape from predators. They swim below the surface, and cling to plants until the danger has passed. Honma et al. (2006) studied the death-feigning posture in Criotettix japonicus against the frog Rana nigromaculata, which represents a sit-and-wait, gape-limited predator. The characteristic posture of this pygmy grasshopper enlarges its body size because pronotum, hind legs, and lateral spines project in different directions. Therefore the predator is unable to swallow the prey. Therefore it was shown that thanatosis does not generally means to mimic the posture of dead animals.
Concerning aquatic Orthoptera only few defensive mechanisms have been described. Members of Tridactylidae prefer damp habitats, where they construct nests out of mud and debris. Many species are active swimmers. Larvae and both sexes of adult Tridactylidae possess paired sternal glands with openings and opening muscles associated with the second abdominal sternum. When disturbed Tridactylus japonicus discharges a characteristic scent. Chemically the defensive secretions are unknown but they effectively repel Tetramorium ants and Pardosa spiders (Moriya and Ichinose 1988; Moriya 1989). In addition within mesotibiae of Tridactylus variegatus further exocrine glands were identified (Messner 1969). Probably the mucus secretion serves to strengthen the wall of the nest tunnels of the pygmy mole grasshopper. Stridulation in which the tegmina are pulled against the hind wings occurs in some species. Members of Pauliniidae (Acridoidea) represent small- to medium-sized aquatic grasshoppers from South America. They can skate on water surface, they dive, and they can swim. Egg deposition takes place below water on water plants. Another species Paulinia acuminata was introduced into Africa for control of Salvinia (Rentz and Su 2003). There were reported further defensive mechanisms of selected orthopterans (Heckman 2018). When attacked by insectivorous fishes various Cornops species (Acrididae) fly away. Other species such as Southamerican cricket Argizala spec. (Gryllidae) or Marilia spec. dive beneath water surface and conceal among roots of water plants. Few species such as Paraneonotus spec. (Stenopelmatidae) dive as long as 12 m and remain motionless until the danger has departed.
BLATTODEA (roaches, termites; 0.8% aquatic): Among the more than 6400 described cockroach species (Stork 2018: 7314) including termites, most representatives of the aquatic and amphibious Blattodea belong to subfamily Epilamprinae (Blaberidae ; Cover and Bogan 2015). Many species inhabit small ecosystems as phytotelmata in neotropics and Indomalaya and about 60 cockroach species were reported from leaf bases of bromeliads. Especially representatives of the genera Epilampra, Phlebonotus, Poeciloderrhis, Opisthoplatia, Rhabdoblatta, and Rhicnoda have been reported as aquatic/amphibious in spite of the fact that little is known on both the biology of these phytotelmata species and those taxa which are found along streams and rivers. When disturbed the rapidly swimming species Epilampra maya from Central America enters the aquatic habitat and remain submerged up to 15 min (Crowell 1946). Females of Phlebonotus pallens from India protect their larvae when they are crawling along the stream bottom (Bell et al. 2007). Larvae and apterous females of the Indian species Rhicnoda natatrix are active swimmers and hide under submerged stones (Nesemann et al. 2010).
Reports on defensive mechanisms especially in aquatic and amphibious cockroaches are not available. Generally Blattodea show a diverse array of defensive mechanisms. Sometimes they are camouflaged and resemble dead or green leafs; otherwise they mimic toxic insects (Dettner 2015). In addition they show evasive behavior; they stridulate; they fight, fly, or run away; and they dive into and quickly swim in water. Finally they cause allergic symptoms in humans and many species have exocrine glands where a lot of allomones are produced (Brossut 1993; Schal et al. 1982; Dettner 2015).
HETEROPTERA (true bugs; 13% aquatic): Heteroptera represent an insect order (about 40,500 sp.; Stork (2018): Hemiptera: 103,590) within Hemiptera. Numbers of special heteropteran taxa were from Lytle (2015) and Moreira et al. (2018); careful morphological characterizations of complex exocrine glands are compiled by Slater (1982); chemical data concerning complex glands were given by Aldrich (1988), Blum (1981), Dazzini-Valcurone and Pavan (1978), Weatherston and Percy (1978), and Millar (2005). Data on maxillary glands are based on Puchkova (1965). Moreira et al. (2018) report that representatives of Gerromorpha, Nepomorpha, and Leptopodomorpha (the three infraorders have more than 4800 species) are primarily associated with freshwater and sometimes with saltwater. Biological data on Heteroptera are especially based on Wesenberg-Lund (1943), Andersen and Weir (2004), Scholtz and Holm (1986), Schuh and Slater (1995), and Scholtz and Holm (1986).
Gerromorpha (semiaquatic bugs): According to this compilation there exist 2846 Gerromorpha species (Moreira et al. (2018): 2100 sp.). Members of this specialized group use the surface water film and glide on it rapidly in search for prey. Several data on Gerromorpha were compiled by Andersen (1982). In accordance with Moreira et al. (2018) only eight families (not superfamilies, and Madeoveliidae) are considered. Antipredator adaptations in Gerromorpha include cryptic coloration, countershading, and defensive behavior (Andersen 1996). The metasternal secretions of Gerromorpha are chemically unknown but may have repellent functions against predators (Andersen 1996). Nearly nothing is known on the composition of salivary glands (Walker et al. 2016). Schmidt (1982) reports that representatives of Gerromorpha (e.g., Veliidae, Gerridae) possess lethal and painful venoms. Aldrich (1988) speculates that the widespread occurrence of communication via waves may explain the frequent loss of exocrine glands especially in these semiaquatic bugs.
Mesoveliidae (water treaders; 49 sp.): Metasternal scent glands are present as a single median reservoir which is at least partially lined with glandular cells. Larvae have a somewhat reduced dorsal scent gland opening between terga III and IV.
Hydrometridae (water measurers; 129 sp.): Representatives of Hydrometra exhibit thanatosis and have an extreme slender body. Combined with rhythmical, vertical movements probably this may have the function of obscuring the outline of the insect (Andersen 1996). Hydrometridae possess a rapidly lethal venom within their salivary glands (Schmidt 1982). The metasternal scent glands have glandular cells opening into the collecting ducts and there is a reservoir with a dorsal accessory gland. Larvae lack dorsal abdominal scent glands.
Hebridae (velvet shore bugs; 233 sp.): The metasternal scent gland is a single simple median pouch with a narrow slit opening and without differentiated evaporative area. Larvae have a somewhat reduced dorsal scent gland opening between abdominal terga III and IV.
Paraphrynoveliidae (two sp.): The abdominal scent gland is situated between terga III and IV. No data are available with respect to the metasternal glands.
Macroveliidae (Macroveliid shore bugs; 3 sp.): The metasternal scent glands open into collecting ducts and contain a reservoir with a dorsal accessory gland and an opening of the median elevation on the posterior part. There are no fully developed lateral scent gland channels. There is a dorsal abdominal scent gland opening on the tubercle on tergum IV.
Veliidae (riffle bugs; 903 sp.): Representatives of this family can hop or jump or remain motionless (Scrimshaw and Kerfoot 1987). The escape movements called expansion skating might be the most elaborate defense mechanism of Heteroptera at all. It is observed in the genera Velia and Microvelia (Andersen 1996) and is comparable to the staphylinid beetles of Steninae (Betz et al. 2018). The detergent saliva which is ejected through the rostrum lowers the surface tension of the water. The metasternal scent glands on the sternum open into collecting ducts and have a dorsal accessory gland. The ostiolar channels on the metapleuron are usually elongated and prolonged on metapleura, ending in elongated evaporative areas with hair tufts in front of the hind coxae. Henrikson and Stenson (1993) concluded that aggregations of the dark, highly contrasting neustonic Velia caprai in exposed areas advertise their bad taste, which represents a case of aposematism. Species of Velia caprai were rejected by Salmo trutta and Lepomis macrochirus (Scrimshaw and Kerfoot 1987).
Madeoveliidae (one sp.): These predacious bugs live among wet rocks. Lateral metathoracic scent gland channels are present.
Gerridae (water striders; 713 sp.): Within genus Gerris death feigning and thanatosis are observed (Andersen 1996). Water striders can also hop and jump and often have countershading to reduce shadows (Scrimshaw and Kerfoot 1987). In order to defend optimally against bottom-striking predators it was found that long midlegs were especially important. The genetic basis of this interaction between a Hox protein Ubx and a new target gene (gilt) was studied by Armisen et al. (2015). The metasternal scent gland apparatus consists of two lateral glands and a median reservoir discharging through the median ventral aperture. Obviously the secretions evaporate due to many setae (Scrimshaw and Kerfoot 1987). Lateral secretory channels are “retained” in Brachymetra and Cylindrostethus. Usually there are no dorsal abdominal scent glands present in adults or larvae. Bioassays showed that some adult gerrids were rejected by bluegills (Lepomis macrochirus; Scrimshaw and Kerfoot 1987). Interestingly diapausing representatives of Gerris develop a diapause secretion that means there is a coat of densely packed microparticles on their body surfaces (Fig. 9.3/8) before leaving the water surface (Hauser 1985). It was speculated that these particles either represent fungicides or prevent freezing.
Defenses of Heteroptera (1–8), Neuroptera (9). 1 and 2: Probable operating of opening and closing mechanism of defensive glands in Ilyocoris cimicoides. 1. Opening muscles (opm) are relaxed and secretion flows from the secretory lobes (sl) into the reservoir (res). 2. Opening muscles are contracted and secretion flows from the reservoir into the vestibule and through efferent channels to the ostioles (arrows). 3. Dorsal view of adult I. cimicoides showing the position of metathoracic gland reservoir (res) and secretory lobes (sl). 4. Secretion grooming of Plea minutissima; Fig. 9.3 (continued) emission of secretion is indicated by arrow. 5. Secretion grooming of Sigara arguta on the water surface prior to flying away. 6. Glands of Ranatra linearis: Paired maxillary glands (max) and bipartite paired labial glands (only one gland system figured) consisting of an accessory gland (ag) and a main gland (mg). Reservoirs of maxillary glands open at the base of proboscis (paired arrows); emission of secretion of labial glands occurs at the apex of proboscis (arrow). 7. Foreleg of male Anisops stali with stridulation organ (arrow). 8. Diapause secretion (stippled) according to REM figure of Gerris costae 5 weeks after imaginal molt. 9. Adult larva of Nevrorthus fallax; side view (left), after molestation, side view (right). Gland constituents: 1. (E)-4-oxo-hex-2-enal; 2. (E)2-hexenal, 3. (E)-2-octenal, 4. hydrogen peroxide, 5. deoxycorticosterone, 6. 4-hydroxy benzaldehyde. Sources: 1–3. Modified according to Staddon and Thorne (1973). 4. Modified according to Kovac et al. 1991. 5. Modified according to Kovac and Maschwitz (1991). 6. Modified according to Pawlowsky (Pawlowsky 1927) and A. Böttcher (Böttcher and Dettner unpubl.). 7–9. Modified according to Andersen and Weir (7. Andersen and Weir 2004, 8. Hauser 1985, and 9. Zwick 1967).
Hermatobatidae (coral treaters, 12 sp.): In this family the abdomen is extremely short and has a single scent gland opening on the fourth mediotergite.
Leptopodomorpha (shore bugs): There exist 382 sp. worldwide. Chemical data from metathoracic glands are not available. Maxillary glands are present in representatives of Leptopodomorpha (Puchkova 1965). According to Moreira et al. (2018) Saldidae and Aepophilidae represent sister groups to Omaniidae and Leptopodidae.
Saldidae (shore bugs; 382 sp.) and Aepophilidae (marine bugs; 1 sp.): When disturbed saldids run fast, leap into the air, and land some distance away after which they scurry into shelter. The metathoracic scent gland has one median reservoir and one ostiole located on the sternum of the metathorax. Pregenital eversible glands are present at the posterolateral corners of the seventh sternum (not in Aepophilidae). Schmidt (1982) reports that Saldidae probably produce venomous saliva.
Omaniidae (intertidal dwarf bugs; five sp.): Omaniidae are predacious and intertidal bugs. They are able to jump readily. The metathoracic scent gland has four reservoirs of a paired gland system with a single median ostiole. Chemical data are not available.
Leptopodidae (spiny-legged bugs; 32 sp.): Leptopodidae fly swiftly from rock to rock when disturbed. The metathoracic scent gland has two reservoirs and two ostioles. Other exocrine glands (including pregenital glands) are missing.
Nepomorpha ( aquatic bugs ): According to Slater (1982) the group has 2404 sp. worlwide. Both aquatic and terrestrial nepomorphs inject venom to immobilize and liquefy their prey. Apart from Nepidae, Aphelocheiridae, and few Belostomatidae, Gelastocoridae, and Notonectidae within all remaining species there exists a metasternal gland (see Staddon and Thorne 1979). Absence of this type of gland is interpreted as a secondary condition. Several representatives of Nepomorpha are able to spread secretion from the metathoracic gland actively over their bodies (Andersen and Weir 2004). Therefore these species crawl out of the water (Naucoridae, Notonectidae, Pleidae) or lie on the water surface (Corixidae) in order to show secretion grooming. Usually these antimicrobic secretions serve to clean the hair pile which holds the air store (Andersen and Weir 2004). Therefore secretion grooming is primarily found in species with a physical gill. In addition also cephalic glands are present. The classification of Nepomorpha was based on Moreira et al. (2018).
Nepoidea : According to Moreira et al. (2018) Nepoidea (Belostomatidae + Nepidae) represent a basal sister group to other Nepomorpha. The Nepoidea species are ambush predators, which are hidden in submerged vegetation to wait on prey. They possess no abdominal scent glands.
Nepidae (water scorpions) 268 sp.: Nepa rubra and also the Australian genus Laccotrephes are well camouflaged due to their brown color and their mud body cover (Andersen and Weir 2004). Its dorsoventral compression is similar to dead leaf. On molestations Nepa and other genera show thanatosis; in some cases with its raptatorial forelegs water scorpions cling 1 h motionless to vegetation. Bites of both Nepa and Ranatra are painful for humans (Pawlowsky 1927). The poisonous secretion is produced by paired salivary or labial glands, which consist of a main gland and an accessory gland (Fig. 9.3/6). The accessory gland duct transports secretion from the accessory gland into the bipartid main gland. From there the lateral duct leads to the salivary pump and proboscis (Pawlowsky 1927). By transcriptomic studies the salivary glands of Ranatra chinensis revealed many transcripts encoding proteins with homology to proteases, acid phosphatases, apyrases, dipeptidylpeptidases IV, hyaluronidases, and prophenoloxidases. No metathoracic scent glands are present within Nepidae; however it is of interest that Nepidae preen only the eyes and the respiratory siphon (Andersen and Weir 2004). This indicates that they might use other secretions as from dermal or salivary glands or anal fluids (see Weiss 2006). Larvae lack dorsal abdominal scent glands. The secretion of the cephalic or maxillary glands (Fig. 9.3/6) is insecticidal and produces a burning sensation on human skin (Walker et al. 2016). Stridulations and vibrations were reported for the forelegs of Ranatra with a femoral plectrum and a coxal pars stridens (Aiken 1985).
Belostomatidae (giant water bugs) 169 sp.: They are general predators and feed on aquatic invertebrates and vertebrates (amphibians, fishes, turtles, birds), which are captured with their raptorial forelegs and quickly immobilized with the poisonous secretion from the salivary glands (de Carlo et al. 1973). The bites which are very painful for humans also represent effective defense mechanisms (Haddad et al. 2010). The belostomatid saliva reveals proteins from 5 to 55 kDa (Walker et al. 2016). Enzymic assays revealed activities of phospholipase A2, hyaluronidase, protease, amylase, esterase, a-glucosidase, glucosaminidase, invertase, lipase, nuclease, phosphatase, and phosphohydrolase (Walker et al. 2016). The saliva of the larger Lethocerus uhleri (Lethocerinae) which only feed on small vertebrates contains three proteolytic enzymes and no amylase, while the smaller Belostoma lutarium (Belostomatidae), which feeds on small invertebrates and snails, produces two proteolytic enzymes and amylase (Swart et al. 2006). The authors suggest that the presence of amylase allows to exploit the plant material already ingested by their prey. There are also reports of rich lipidic contents from the venom of Belostoma anurum (Walker et al. 2016). Walker et al. (2018) presented a transcriptomic and proteomic study on Lethocerus distinctifemur and found 132 venom proteins including putative enzymes, cytolytic toxins, and antimicrobial peptides. 73% of proteins were homologous to assassin bugs (Reduviidae), and 21% are not known from other sources (Walker et al. 2018). Metasternal scent glands of Lethocerinae and Horvathiniinae contain a median undivided lip valve, dorsoventral valve-opener muscles, and accessory glands (glands absent in Belostomatinae; Aldrich 1988). Especially in males these glands are well developed and may be detected due to their odor. (E)-2-hexenyl acetate was identified as main constituent in Lethocerus indicus males, with the corresponding butyrate as minor compound (Aldrich 1988). The functions of these esters remain unknown and might fulfill a secondary, probably sexual role (Scrimshaw and Kerfoot (1987). In Asia where especially Lethocerus is eaten by humans the scent gland odor and taste can be perceived (Schuh and Slater 1995). Belostomatidae larvae lack dorsal abdominal scent glands (Aldrich 1988). As defense reaction most Belostomatidae are additionally capable of ejecting a foul-smelling inky black anal liquid. Finally many families have paired so-called cephalic or maxillary glands (Fig. 9.3/6) which are saclike and open at the base of the beak (de Carlo 1961). The gland reservoirs are lined by a cuticle and bear ventrally glandular cells. In Abedus herberti the reservoirs are depleted after a molestation (Eisner et al. 2005). The viscous white fluid contains the four pregnanes desoxycorticosterone (Fig. 9.3/5), pregnenolone, progesterone, and 3a-hydroxy-pregn-5-en-20-one (Lokensgard et al. 1993). The first three pregnanes were also identified from prothoracic defensive glands of dytiscid beetles. It was evaluated that one A. herberti specimen contained about 0.1 mg desoxycorticosterone (in dytiscid beetles 0.03–0.4 mg/beetle). In the genus Abedus a soft chirping was described, which is produced by expulsion of air through the ventral spiracles (Aiken 1985).
Corixoidea: Corixidae (water boatmen; 662 sp.): Corixids are unable to use their rostrum and salivary secretion for defense. The opening of the large metathoracic scent glands is located laterally of the mesocoxae and contains a median, an undivided flap valve, and a ventral valve opener muscles. Obviously the pale yellow oil-like secretion with an aldehyde odor is depleted due to the elasticity of the gland reservoir. Larvae have dorsal abdominal scent gland openings between terga III–IV, IV–V, and V–VI. Corixids have defensive secretions composed of (E)-4-oxo-hex-2-enal (95% of adult secretion; Fig. 9.3/1) and of (E)-4-oxo-oct-2-enal (92% in larval secretions; Staddon et al. 1979). When attacked both adult corixids and larvae may eject secretions under water. It was found that fish guts only contain low numbers of corixids (Scrimshaw and Kerfoot 1987). The majority of corixids (Diaprepocorinae, Cymatiainae, Corixinae) rub their metathoracic gland secretions over their body parts covered with hydrofuge hairs. Within 2–3 s secretion grooming was observed while floating on the water surface (Fig. 9.3/5; Kovac and Maschwitz 1991). Secretion grooming of Diaprepocoris takes place on land. The behavior is released when light intensity and water temperature are raised. Due to the antibacterial properties of the aldehyde secretion, the contamination of hydrofuge hairs by microorganisms is minimized and thus loss of air store is reduced. It is peculiar that in all instars of Micronecta and earliest corixid instars secretion grooming is lacking: They have no hydrofuge hairs and obtain oxygen by cutaneous diffusion. Probably their strongly smelling secretions are directed against aquatic or terrestrial predators (Kovac and Maschwitz 1991).
Stridulation in Corixidae was found to be a component of the reproductive behavior and was observed in Micronectinae and Corixinae and probably occurs in Diaprepocorinae and Cymatiinae (Aiken 1985). The position and function of stridulatory organs vary considerably within Corixidae (Aiken 1985). In few cases (e.g., Cenocorixa) stridulation was shown to maintain individual distances between males. Corixids are able to leap out of the water into the air and take flight. Maxillary glands are present (Puchkova 1965).
Ochteroidea : Ochteridae + Gelastocoridae: Schmidt (1982) reports that Ochteridae and Gelastocoridae produce saliva which is probably venomous (Ochteridae) or paralytic and painful (Gelastocoridae).
Gelastocoridae (toad bugs; 116 sp.): Many representatives of toad bugs are cryptic and take on the color of the substrate; moreover they are characterized by their jumping ability. They usually occur near water in wet mud and vegetable debris where they usually blend well with their background. The metathoracic scent glands of Gelastocorinae contain a pair of stop valves and a dorsoventral valve with a polygonal cuticular microsculpture (scent glands are absent in Nerthrinae). The glands contain 4-oxo-2-hexenal (63%), octenal (25%; Fig. 9.3/3), 4-oxo-octenal (9%), and hexanal (3%; see Scrimshaw and Kerfoot 1987). Larvae lack dorsal abdominal scent glands. Maxillary glands are present (Puchkova 1965).
Ochteridae (velvety shore bugs; 75 sp.): Some Ochteridae species have larvae that scoop sand over the head and then push it over the dorsal surface with their front legs, thus making them closely resemble sand grains. Metathoracic scent glands are present. Adults and larvae lack dorsal abdominal scent glands. Maxillary glands are present (Puchkova 1965).
Naucoroidea : Naucoridae (creeping water bugs; 398 sp.): Naucorid bugs such as Pelocoris and Ilyocoris (= Naucoris) cimicoides should be handled with care as they can inflict a painful bite (Pawlowsky 1927) due to the venom of their salivary glands (Schmidt 1982). Their bite is characterized by Wesenberg-Lund (1943) as “worse than a bee sting.” The metathoracic scent gland system of Ilyocoris cimicoides consists of a pair of glands, a reservoir, a closing apparatus, and an efferent apparatus (Fig. 9.3/1–3; Staddon and Thorne 1973). The complex gland system possesses a medium undivided lip valve and dorsoventral valve opener muscles (Fig. 9.3/1, 2); accessory glands are missing. In Ilyocoris cimicoides the aromatic compounds 4-hydroxy-benzaldehyde (Fig. 9.3/6) and 4-hydroxy benzoic acid methylester were identified (see Scrimshaw and Kerfoot 1987) along with minor constituents. From time to time Ilyocoris cimicoides leave the water in order to distribute metathoracic gland secretion over the ventral hairs (Kovac and Maschwitz 1990a). This grooming behavior prevents the contamination of these hairs with microorganisms, which would result in wetting of this region and loss of the air bubble. There is one pair of larval scent glands (gland divided) present between abdominal terga III and IV. Maxillary glands are present (Puchkova 1965). A sound production was reported from Ilyocoris cimicoides males; however both stridulatory structures and significance of sound production are discussed controversially (Aiken 1985). Horta et al. (2010) described a possible case of mimicry involving the naucorid Limnocoris porphyrus and an anuran tadpole of the hylid frog Scinax machadoi. Because both can sting or might be toxic it is not clear whether it is Mullerian mimicry or Bates mimicry.
9.3 Aphelocheiroidea : Potamocoridae + Aphelocheiridae
Potamocoridae (eight sp.): Tropical species which are related to Aphelocheiridae (Lytle 2015).
Aphelocheiridae (benthic water bugs; 78 sp.): There are reports that Aphelocheirus may occasionally sting or bite, which can be painful to man (Andersen and Weir 2004). No metathoracic scent glands are present in this family (Staddon and Thorne 1979) and it is of interest that Aphelocheirus does not show grooming behavior (Andersen and Weir 2004). Larvae possess divided dorsal abdominal scent gland openings between terga III and IV. Maxillary glands are present (Puchkova 1965).
Notonectoidea : Notonectidae (backswimmers; 422 sp.): Backswimmers swim on their backs, and often have reverse countershading. When disturbed they swim rapidly down. They are also able to leap into the air from the water and take flight. When handled they can inject painful venomous saliva (German name “Wasserbiene,” water bee; Pawlowsky 1927; Schmidt 1982). In Notonectinae the metasternal scent glands have paired stop valves, dorsoventral valve opener muscles, and a polygonal cuticular microsculpture. The glands are absent in representatives of Anisopinae, e.g., Anisops and Buenoa. Interestingly Anisops does not show any grooming behavior (Andersen and Weir 2004). Apart from Anisopinae in other notonectids paired accessory glands are associated with the reservoir which has a midventral opening. The odorless, brownish secretion consists of 4-hydroxybenzaldehyde (Fig. 9.3/6) and methyl-4-hydroxy benzoate, compounds which were also identified from pygidial glands of water beetles. It is remarkable that these antimicrobics also are deterrents (at 6 × 10−7 moles) against cichlids as Tilapia (Scrimshaw and Kerfoot 1987). Larvae lack dorsal abdominal scent gland openings. Maxillary glands are present (Puchkova 1965). It is remarkable that only members of genera Buenoa, Anisops, and Walambianisops can stridulate, which definitely lack thoracic defensive glands. In contrast other genera of Notonectinae possess no stridulatory apparatus (Aiken 1985; Andersen and Weir 2004). The stridulatory repertoire of Buenoa is very complex (tibial comb/rostral prong; femur/coxal peg; femoral setae/rostral flange) but obviously and exclusively used in courtship and mating. The same type of chirping behavior as in Buenoa is also typical for males of Anisops stridulating with their forelegs (Fig. 9.3/7). It seems important to denote that Notonecta maculata as predators release hydrocarbons such as n-heneicosane and n-tricosane which repel oviposition by mosquitoes such as Culiseta longiareolata (Silberbush et al. 2010). It would be interesting to know if these hydrocarbons, which might be valuable in eliminating mosquitoes, are produced in complex glands.
9.4 Pleoidea: Helotrephidae + Pleidae
Pleidae (pygmy backswimmers; 44 sp.): Like Notonectidae, representatives of Pleidae swim in the inverted position. The predacious pygmy backswimmers have a saclike metathoracic scent gland with a single midventral opening (Aldrich 1988). Paired accessory glands are associated with the scent gland. The colorless secretion of Plea leachi was reported to contain a 10–15% solution of hydrogen peroxide (Fig. 9.3/4) together with traces of an unknown carbonyl compound (Maschwitz 1971). From time to time Plea minutissima leaves the water in order to distribute this secretion by its legs (secretion grooming) over its hydrofuge ventral pubescence (Fig. 9.3/4; Kovac 1993). This efficient disinfectant probably destroys epzoic microorganisms and guarantees respiration via an intact air sheath when submerged. Increase of light intensity and higher water temperatures promote secretion grooming and probably flight behavior (Kovac 1993). If adults of Plea are dissected very often foams are apparent, which is obviously due to a catalytical decomposition of hydrogen peroxide into water and oxygen. Scrimshaw and Kerfoot (1987) also report that the Plea secretion is an effective deterrent against small fishes as Gambusia. Pleidae larvae have dorsal abdominal scent gland openings between terga III and IV. Stridulation is known to occur in both sexes of Plea minutissima by contacts of a mesothoracic file with a prothoracic projection (Aiken 1985). Maxillary glands are present (Puchkova 1965). According to Schmidt (1982) the saliva of Pleidae is probably venomous.
Helotrephidae (164 sp.): As Notonectidae they swim with the venter up. Larvae of the tropical Helotrephidae have a single median unpaired dorsal scent gland opening located between terga II and IV. In Helotrephinae stridulatory structures were identified. Especially a serrated lateral margin of hemielytron contacts a ridge dorsally on hind femur (Schuh and Slater 1995). Finally it must be noted that the first aquatic plant-sucking insects are two homopteran larvae of spittlebugs of genus Mahanarva from Costa Rica. They live submerged in water filled flowers of various Heliconia species (Thompson 1997).
MEGALOPTERA (alderflies, dobsonflies, fish flies; 100% aquatic): Worldwide there exist around 380 megalopteran species (Stork 2018: 354; Dijkstra et al. 2014; Ardila-Camacho and Contreras-Ramos 2018) which contain the families Sialidae (alderflies) and Corydalidae (dobsonflies and fish flies). All representatives have long-living aquatic larvae (up to 5 years; 10–12 stages) and short-lived terrestrial pupae and adults (Cover and Bogan 2015). The larvae feed on small invertebrates and vertebrates (e.g., mayfly and caddisfly larvae; small tadpoles) and also cannibalize smaller conspecifics. Few publications dealing with larval bionomy show that there exist defensive mechanisms. In the genus Neohermes (Corydalidae) an avoidance reaction was observed, when conspecific larvae had contacts (Smith 1970). When disturbed last-stage Neohermes californicus larvae (and prepupae) vomit an extremely foul-smelling, dark, greenish liquid (Smith 1970). Also adults of Corydalus can bite when they are disturbed (Parfin 1952). Recently defensive reactions were described for Neoneuromus ignobilis (Corydalidae; Cao et al. 2012). When disturbed due to the high flexibility of their bodies the larvae curl up into a ball or immediately open the palates. Cao et al. (2012) also observed the depletion of milky and water-insoluble substances from the end of the larval abdomen, a behavior which was compared with depletion of defensive secretions by whirligig beetles (Gyrinidae) or secretion grooming in aquatic Plea bugs. Corydalus females protect their egg masses with a hardening substance which appears like bird feces (Ardila-Camacho and Contreras-Ramos 2018).
NEUROPTERA (net-winged insects; 4% aquatic): There exist more than 6000 neuropteran species worldwide (Stork 2018: 5868; probably 10,000). Among the 17 known familes only 2 are aquatic (Sisyridae: 60 sp.; Nevrorthidae: 19 sp.) and 1 semiaquatic (Osmylidae: 160 sp.). Neuropteran larvae are characterized by specialized sucking jaws which are formed by mandibles and distal parts of maxillae (Beutel et al. 2014). This food uptake apparatus exhibits a sucking channel and a poison channel; the paired poison and salivary glands are associated with this specialized food apparatus (Beutel et al. 2010; Beutel et al. 2014). Aquatic larvae of Sisyridae (spongillaflies) have piercing mouthparts and are specialized predators or commensales of freshwater sponges (with symbiontic Zoochlorellae) and limnic bryozoans (Heckman 2018). Their stylets curve outwards distally. For constructing their pupal cocoons by using their spinning glands, full-grown third-stage larvae (lengths 2.7–8.5 mm) leave the water (Cover and Bogan 2015). Because other larval Neuroptera such as lacewings (Chrysopidae) use their pointed jaws in piercing and sucking prey and even are capable of piercing human skins where they may cause injuries (Southcott 1991), the same situation is suggested for Sisyridae. Larvae of Nevrothidae and even their pupal stages are aquatic and larvae represent predators of benthic invertebrates. Nevrorthidae represent the sister group of all other Neuroptera, suggesting an aquatic ancestor for all Neuroptera (Cover and Bogan 2015). The slender and agile predatory larvae are usually found in mountain brooks. As described by Zwick (1967) larvae of Nevrothus fallax showed an unusual behavior after molestation. The larvae rapidly moved foreward and backward and then suddenly lifted the head by an extremely quick tossing movement in order to form a right angle between head with prominent sucking jaws and rest of the body (Fig. 9.3/9). This peculiar behavior is interpreted as defensive behavior. Several representatives of the Osmylidae larvae are associated with freshwater habitats and occur in riparian areas. Many species consume trichopteran eggs, and hunt on Tipulidae and Chironomidae larvae (Cover and Bogan 2015). Because chironomid prey larvae die immediately after encountering osmylid larva, it can be suggested that the larval saliva is toxic (Wesenberg-Lund 1943; Schmidt 1982). Adults of Osmylidae are characterized by defensive glands and disagreable odors are perceivable after molestation (Dettner 2015).
COLEOPTERA (beetles; 3% aquatic): Most data are from Short (2018), Jäch and Balke (2008), Arnett and Thomas (2001), Beutel and Leschen (2016), and Leschen et al. (2010). According to Jäch and Balke (2008) about 3% of the known Coleoptera species described are regarded “aquatic.” In considering undescribed species it can be assumed that 18,000 water beetle species might exist among 386,500 species worldwide (Stork 2018). The following beetle families are not arranged phylogenetically but according to Jäch and Balke (2008).
9.5 Predominantly Aquatic Families (Jäch and Balke 2008)
Myxophaga : Defensive mechanisms of the four families are only known from Lepiceridae (Navarrete-Heredia 2005). Hydroscaphidae (skiff beetles and their larvae are hygropetric; 13 sp.). Skiff beetles live on algae covered by a thin water film.
Lepiceridae (two sp.): Adults are found at the edge of rivers and prefer moist environments; larvae are probably aquatic. Recently collecterd Lepicerus adults were capable to retract body appendages and show thanatosis. In addition they cover their body surface with subtrate particles, are strongly sclerotized and colored similar to substrate where they are living (Navarrete-Heredia et al. 2005). Sphaeriusidae (minute bog beetles; 19 sp.): Adults of many species are found along banks of streams, in stagnant waters, or in Sphagnum mosses. Ecological data are scarce. Torridincolidae (torrent beetles; Short 2018: 60 sp.): All torrent beetles have aquatic developmental stages and prefer hygropetric habitats. Even pupae are aquatic with plastron-bearing gills (Lawrence 1991).
Adephaga : All aquatic Adephaga (= Hydradephaga) possess paired abdominal pygidial glands (Fig. 9.4). Secretions of all Hydradephaga hitherto tested topically on the cuticles of each representative show an increase in wettability for water of treated cuticles (Fig. 9.6).
Site of defensive glands (prothoracic glands and pygidial glands) of Hydradephaga (1–10; according to Dettner 1985) and Geadephaga (11–12) in Nebrioporus depressus (1). 2. Acilius sulcatus, 3. Colymbetes fuscus, 4. Liopterus haemorrhoidalis, 5. Laccophilus minutus, 6. Hygrobia hermanni, 7. Gyrinus substriatus, 8. Noterus clavicornis, 9. Haliplus heydeni, 10. Amphizoa lecontei, 11. Trachypachus gibbsi, 12. Omophron dentatum. 13–14: Prothoracic gland reservoirs of Ilybius fenestratus (13) and Acilius canaliculatus (14). Abbreviation: Pro prothoracic glands; Pyg pygidial glands; res reservoir; gc glandular cells; sch secretory channel; tu tubule; tr trachea. 1–10: Modified according to Dettner 1985. 11–12: Modified according to Forsyth (1972). 13–14: Originals in Dettner 2014
Amphizoidae (trout stream beetles): Larvae and adults (five species worldwide; Dettner 2016b) prefer fast-flowing rivers but are poorly adapted to aquatic environment. They share plesiomorphic features with Carabidae. Eggs are deposited submers, and mature larvae leave the water for pupation. Freshly emerged adults are often covered with mud. Larvae live gregariously, show thanatosis, and roll their bodies into balls when disturbed (Yu et al. 1993). Adults possess pygidial glands with a tripartite secretory lobe (Fig. 9.4/10; prothoracic glands absent) which are filled with a yellow fluid, which is depleted over the hind body (as in Carabidae) upon disturbance (Edwards 1953). The gland reservoir is covered by muscles; as in Hygrobia (Fig. 9.4/6) the gland tissue is lengthened (Fig. 9.4/10; Forsyth 1970). The secretion contains the strongly smelling dimethyl disulfide (Fig. 9.9/Formula 3), and antimicrobics and fungicides like methyl-3-indole carboxylate (Fig. 9.9/Formula 1) and methyl-4-hydroxycarboxylate (Fig. 9.5/Formula 6; Dettner 1990). The yellow coloration of the secretion, which according to Darlington (1929) leaves a cigarette-like stain on the fingers, is due to 4′,5-dihydroxybenzalisocumaranone (= marginalin; Fig. 9.5/Formula 15) which was first discovered from pygidial glands of Dytiscus marginalis (Dettner 2014).
Aspidytidae (cliff water beetles; two sp.): Larvae and adults of this monophyletic family (Vasilikopoulos et al. 2019) prefer hygropetric habitats and are probably predacious (Beutel et al. 2016). When disturbed adults walk rapidly. Pygidial glands must be present; until now however they are not recorded from this family , which is also true for the prothoracic glands.
Dytiscidae (predacious diving beetles; according to Yee 2014: 4303 sp.): Jäch and Balke (2008) estimated up to 4800 sp. of diving beetles and predacious diving beetles, which means that this water beetle family which occurs on all continents where it is found in all aquatic habitats has the first position with respect to global water beetle diversity (Balke and Hendrich 2016; Yee 2014; Miller and Bergsten 2016). Larvae and adults of nearly all species are carnivorous and aquatic. Habitats range from ponds and bogs to rivers, phytotelmata, hygropetric areas, and even subterranean waters (Yee 2014; Miller and Bergsten 2016). A lot of predacious diving beetles leave the water for secretion grooming or before flight, another fraction of species stay on land during winter or summer, and few species of the genera Geodessus and Typhlodessus are reported to be terrestrial. Thus they have reduced swimming hairs and are unable to swim (Brancucci 1985).
Dytiscidae have a lot of predators ranging from vertebrates such as fishes or amphibians to invertebrates as Odonata, Heteroptera, or other predacious water beetles. Therefore we find a lot of defensive mechanisms (Dettner 2014; Miller and Bergsten 2016). Various mechanical defenses can be observed. Larger species such as Cybistrinae or Dytiscinae can kick with their hind legs which are supplied with large spurs. In addition both larvae and adults of these larger dytiscids bite with their mandibles which is painful for humans and may result in bleeding. In addition various Laccophilinae show a characteristic jumping behavior. In contrast many dytiscid beetles may quickly escape or in contrast can hide, whereas certain larvae of Cybistrinae exhibit thanatosis when molested (Miller and Bergsten 2016). As in other water insects coloration in Dytiscidae can be variable ranging from crypsis to aposematism and resulting from structural, secretion, and pigmentary colors (Dettner 2014). Colorful taxa which are attractively marked often are found in clear water with mineral substrates (Larson 1996; Young 1960) whereas dark melanistically or green-colored species (Laccophilus with yellow carotenoids and blue bile pigments; Dettner 2014) often prefer habitats with dark substrates or dense vegetation (Balke et al. 1997).
Several species of Dytiscidae can produce sounds and have organs of possible stridulatory function (Larson and Pritchard 1974; Franciscolo 1979). Although detailed bioacoustical studies are missing, the significance of most stridulations seems to be courtship behavior or intraspecific communication. It is suggested that stridulations also represent alarm and stress signals and might represent defense signals (Smith 1973).
Chemical defense through allomones is widespread in Dytiscidae (Dettner 2014). Both adults and larvae possess huge rectal ampullae that means diverticuli of hindgut. These ampullae primarily represent hydrostatic organs which are normally filled with feces and water. If adults of larger Dytiscidae (e.g., Dytiscinae) are handled at first they deplete an unpleasant smelling fluid from their rectal ampulla containing also hydrogen sulfide and ammonia. During severe molestations the two complex gland secretions from pygidial and prothoracic glands are suddenly or gradually depleted. Pygidial glands are present in all Dytiscidae and contain an ovoid reservoir, covered by muscles (Fig. 9.4). The lengthened, sometimes branched, secretory lobes (Fig. 9.4/1–5) are connected to the reservoir by a shorter or longer central collecting canal (Forsyth 1968). There exist various types of glandular cells with tubules. In several hydroporine species (Hyphydrus, Stictotarsus) an accessory gland and an integumental gland were described (see Dettner 2014).
Pygidial gland secretion very often is like a paste and dytiscid beetles distribute the material (about 10–15% of one gland reservoir) on body surface by using their legs. This behavior was described by Dettner (1985) and Kovac and Maschwitz (1990b). The secretion represents one of the richest sources for aromatic compounds within arthropods (Figs. 9.5 and 9.11). 14 aromatic compounds were identified (Table 9.1), 7 aliphatic gland constituents, the unusual yellow pigment marginalin, the tryptophane metabolite 3-indoleacetic acid, and tiglic acid which is probably also derived from amino acids (Schildknecht et al. 1962; Dettner 2014). Now chemical data are known from 91 dytiscid species comprising 29 genera. Several aromatics can be used as chemotaxonomic markers (phenylacetic acid for Hydroporinae and Copelatinae and responsible for the strong sweetish odor of Hydrodessus fragrans or Hydrotarsus; 4-hydroxybenzaldehyde for Colymbetinae/Dytiscinae) (Dettner 2014). It is remarkable that 4-hydroxybenzaldehyde and 4-hydoxybenzoic acid methylester from pygidial glands of Dytiscidae are also present in metathoracic glands of water bugs Notonecta glauca and Ilyocoris cimicoides (see Naucoridae, Notonectidae).
Concerning the functions of pygidial glands (Fig. 9.5) both aromatics (e.g., 3, 6, 11) and aliphatics (e.g.,17, 19, 20, 22) represent excellent preservatives which maintain a clean body surface that means protection from contamination of body surface by bacteria, protozoa, or fungi. Even in the terrestrial water beetle Geodessus besucheti phenylacetic acid represents the main constituent of the pygidial glands as compared with its aquatic bidessine relatives (Dettner 2014; Brancucci 1985). In addition it was suggested that each of the following pygidial gland constituents, the yellow pigment marginalin (Fig. 9.5/Formula 15) acts as fixative), the glycoproteins (form coherent films), and 3-hydroxy acids (17, 20, 22; polyesters) fix and entangle epizoic microorganisms which are subsequently stripped off (Schildknecht and Bühner 1968; Barbier 1990). The significance of the plant auxin 3-indoleacetic acid (Fig. 9.5/16) in Hydroporinae is unknown. The pygidial gland secretion of Acilius sulcatus especially with compounds 5 and 6 was shown to act against ciliates. This seems important because many aquatic arthropods and especially dytiscid beetles are contaminated with many epitrichic ciliates. The effect of pygidial gland secretion on the wettability of dytiscid integument by water is highly significant. As compared with the untreated elytral control, contact angles of standard water droplets on elytra previously treated with pygidial gland secretion of various Dytiscidae species were significantly lowered (Fig. 9.6). This increase of wettability was also observed with solutions of single pygidial gland constituents (Dettner 2014). When water beetles leave the water their integument becomes hydrophobic and they have enormous difficulties to break through the water surface and reenter into the water (Brancucci 1975). In addition especially after flights the weight of the beetles is reduced due to the filled tracheal system and the empty rectal ampulla. Therefore especially small Dytiscidae such as Hydroporinae are overcompensated and are pressed out of the water (Fig. 9.7/Hydroporinae 1). In this situation the beetles start rubbing and show secretion grooming in distributing pygidial gland secretion over their bodies (Fig. 9.7/Hydroporinae 9–11). After few minutes on or below the water surface the beetles fill their rectal ampulla with water and become more and more hydrophilous and as a consequence receive a stable position within the water (Fig. 9.7/Hydroporinae 12, 13; Dettner 1985). These findings amply demonstrate that exocrine glands and their secretions have usually a multifunctional significance.
Contact angles of water droplets, placed on elytral surfaces of different Hydradephaga species. Vertical bars: measurements per specimen: above value = untreated control elytra; below = elytra treated with pygidial gland secretion (according to Dettner 1985)
Diving behavior (left), subsequent cleaning behavior (center), and sloping position (right side) of Hydroporinae (Dytiscidae). Diving behavior of Haliplidae (below). According to Dettner (1985)
Seasonal fluctuations of pygidial gland titers in Acilius (Newhart and Mumma 1979) and Agabus (Classen and Dettner 1983) do not reflect different degrees of utilization of the secretion but are due to the different age structures of population titers. Young specimens namely after pupation have low amounts of aromatics and other compositions as compared with elder specimens (Dettner 1985).
Prothoracic exocrine glands are present in all dytiscid beetles (Fig. 9.4/1, 13, 14). The homology of the prothoracic gland of Hygrobiidae (Fig. 9.9/10) with the gland of Dytiscidae is questionable. Prothoracic exocrine glands represent paired saclike reservoirs which open anterolaterally of prothorax. As exocrine glands they are not homologues with endocrine prothoracic glands which are present in all insects. Forsyth (1968) describes that tubule-carrying gland cells cover these paired reservoirs which have no muscles. The usually odorous prothoracic secretion often is fluid and can be exuded as a milk after a strong molestation. Several Dytiscidae such as Ilybius fenestratus (Fig. 9.4/13), Hyphydrus ovatus, or Laccophilus minutus possess large prothoracic gland reservoirs extending from prothorax into the abdomen (Dettner 2014).
The chemistry of the prothoracic glands was especially investigated by Schildknecht (1970). An impressive array of vertebrate steroidal hormones was identified and a lot of new beetle steroids with C18, C19, and C21 skeletons (Fig. 9.8). Together with Chrysomelidae, Silphidae, Lampyridae, and Belostomatidae, Dytiscidae represent those insects, with an extraordinary biosynthetical capacity to produce these essential compounds from cholesterol precursors from their food. It was shown that labeled steroid precursors are incorporated into defensive steroids of Acilius sulcatus (Chapman et al. 1977). Obviously microorganisms from the crop of these beetles play an important role in transforming these steroids into prothoracic gland compounds (for details see Dettner 2014). Altogether 63 steroids are recorded (e.g., Compounds 25, 28, 37, 57, 67, 73, Fig. 9.8, Table 9.2). Among the nonsteroidal constituents of the prothoracic glands there is methylisobutanoate (Fig. 9.8/87), benzoic acid (Fig. 9.5/3), pentadecanoic and octadecanoic acids, and monoglycerides with saturated and unsaturated side chains (Fig. 9.8/103, 107; Table 9.2). In addition the yellow alkaloid methyl-8-hydroxquinoline carboxylate was found (Fig. 9.8/100) in Ilybius fenestratus, which is derived from tryptophane, and the same species together with Dytiscus marginalis also contains methyl esters of leucine, valine, and isoleucine. In Ilybius fenestratus and Platambus maculatus Schildknecht and his group (Schildknecht 1977) identified 13 sesquiterpenes (including for example platambin, Fig. 9.8/101, and alpha muurolene, Fig. 9.8/89) which are defensive compounds and additionally responsible for the characteristic odor of the prothoracic secretion of these two and other species. Colymbetes fuscus does not produce steroids but instead contains the nucleoproteid colymbetin (Table 9.2), which can lower blood pressure in small mammals.
Prothoracic gland constituents 24–108 (for names see Table 9.2) from predacious diving beetles (Dytiscidae ) (according to Dettner 2014). 25: C18 steroid of estratriene structure; 28: C19 steroid of androstene structure; 37: C21 steroid of pregn-4-ene structure; 57: C21 steroid of pregna-4,6-diene structure; 67: C21 steroid of pregn-5-ene structure; 73: C21 steroid of pregnane structure; 89 and 101: sesquiterpenes alpha-muurolene (89) and platambin (101). Alkaloid (100), ester (87), and monoglycerides (103, 107)
Biological activities of prothoracic steroids which are often present in huge amounts in the secretion are primarily directed against fishes and amphibians (newts and frogs). If the beetles are ingested, the secretion and especially the steroids are absorbed via the gill membranes, partly via skin. Many of these compounds from prothoracic glands of dytiscid beetles but also constituents from pygidial glands of whirligig beetles (Gyrinidae, which lack prothoracic glands) are toxic and exhibit especially anesthetic activities, which was investigated by Miller and Mumma (1976a, b) with minnows (Fig. 9.10; Pimephales promelas) or by Gerhart et al. (1991) with bluegill sunfishes (Lepomis macrochirus). In many experiments they evaluated survival time of test fishes which was correlated with the steroid concentration and the type of steroids used in the experiments (Fig. 9.10). The activity of steroids was highly related to the degree of oxygenation (see Dettner 2014). It was suggested that the aforementioned alkaloid (Fig. 9.8/100) and the sesquiterpene platambin (Fig. 9.8/101) are directed against small mammals, because Ilybius fenestratus often stays on land (Schildknecht 1970, 1977). The amino acids and their methyl esters exhibit various effects on fishes and they can be both feeding stimulants and deterrents (Dettner 2014). According to Kasumyan and Doving (2003) this applies especially to the found valine, isoleucine, and leucine. In bioassays with minnows (Phoxinus phoxinus) it was found that two polyunsaturated monoglycerides showed a deterrent effect and may act as emulsifiers for prothoracic steroids (Schaaf and Dettner 2000).
As with pygidial gland secretion also prothoracic defensive fluid is not exclusively directed against predatory vertebrates. When peritrichic ciliates isolated from the water beetle Agabus sturmii were treated with prothoracic gland secretion there was a drastic negative effect that means stop of cilia movement after a short time (Dettner 2014).
When last-stage dytiscid larvae leave the water in order to construct a firmly closed pupal chamber it was noticed that within the opened chamber an aromatic lactonic odor was perceivable (Blunck 1923). It is suggested that peristigmatic glands of pupae produce these antisepticals; the same phenomenon was described in Carabidae pupal chambers (Giglio et al. 2009).
Within Dytiscidae larvae (e.g., Hydroporinae, Laccophilinae, Colymbetinae, Dytiscinae) there exist glandular organs of epipharynx. From outside many spines are found which are confined to those areas where the tips of mandibles touch the ventral surface of head (De Marzo and Nilsson 1986). Glandular cells without tubules either form a single epithelium behind spinulose areas or represent a large gland with secretory ducts (Cybister). The role of these secretions is discussed controversially as detergents for cleaning mandibular tips (“Organes de toilette,” Bertrand 1928), poisonous secretions, or attractants for prey.
Gyrinidae (whirligig beetles; 850 sp.; Beutel and Roughley 2016; Jäch and Balke 2008 estimated 1000 sp.; with respect to global diversity among water beetles this family has position 6); Phylogenetically there exist three subfamilies (Spanglerogyrinae, Heterogyrinae, Gyrininae) with Orectochilini, Gyrinini, and Enhydrini based on molecular and morphological data from both adults (Miller and Bergsten 2012) and larvae (Beutel and Roughley 1993). Adults by active movements whirl on the water surface (loops, zigzags), but they can also dive and fly after leaving the water. Gyrinids are often found in aggregations with sometimes 50,000 individuals, which is interpreted as a kind of defense strategy (Yee and Kehl 2015; Vulinec and Miller 1989). Vulinec and Miller (1989) showed that adult whirligig beetles are adapted to aquatic and terrestrial life by divided compound eyes and by hind leg strokes of about 50 to 60 s with speeds of 53–144 cm/s (Yee and Kehl 2015). Larvae are strictly aquatic, need a lot of oxygen, and possess tracheal gills. Preys are digested extraintestinally. All developmental stages are carnivorous, and pupation takes place on land. If there is a danger, the heavily sclerotized adults drastically increase their movements, can immediately dive, and may move under the water surface. There they swim and must quickly sting on solid objects. Apart from these unusual neustonic adaptations whirligig beetles possess paired abdominal pygidial glands (Fig. 9.4/7; Fig. 9.9/2) with odoriferous, volatile, and fluid secretions. The paired glands of Gyrinus caspius were described by Forsyth (1968), and the glands of Enhydrus sulcatus were figured by Barth (Fig. 9.9/2; 1960). The gland reservoirs are covered by muscles, and the secretory tissue evacuates secretion into efferent ducts of reservoirs close to or far away from the reservoir opening. Enhydrus is unusual as its gland reservoir hosts a second type of secretory tissue, apart from the secretory lobe (Fig. 9.9/2), an analogue situation resembling the anal glands of primitive Steninae staphylinid beetles (Fig. 9.13; Betz et al. 2018). It would be interesting to know if phylogenetic data from pygidial gland morphology and chemistry are in accordance with the abovementioned phylogenies of whirligig beetles. There exist various investigations with respect to the defensive secretions which contain the norsesquiterpenes gyrinidal, isogyrinidal, gyrinidon, and gyrinidion (Fig. 9.9/Formula 4) exhibiting strong repellencies against fishes (Fig. 9.10), newts (Notophthalmus), birds, and small mammals (Schildknecht et al. 1972; Miller et al. 1975; Scrimshaw and Kerfoot 1987).
Morphological defenses (5), stridulation (8, 9), coloration (7), pygidial glands (1–4), prothoracic gland (10) of Gyrinidae (2, 5), Hygrobiidae (6–10), Haliplidae (1,3,4). Pygidial glands of 1. Haliplus (Neohaliplus) lineatocollis (Haliplidae). 2. Enhydrus sulcatus (Gyrinidae). 3. Brychius elevatus (Gyrinidae). 4. Peltodytes caesus (Haliplidae). 5. Morphological defence Porrorhynchus marginatus (Gyrinidae). 6. Secretion grooming of Hygrobia hermanni. 7. Coloration of last instar larva of H. hermanni. 8. Stridulatory file on interior elytral apex of H. hermanni. 9. Oscillogram (above) and Fig. 9.9 (continued) spectrogram (below) of stridulation noise of H. hermanni, 10. Half of prothorax of H. hermanni with prothoracic gland, covered by muscles. Abbreviation: cl closure; ed efferent duct; op opening of reservoir; ogl gland; m muscles; res reservoir; sf stridulatory file; sl secretory lobe; st secretory tissue. Pygidial gland constituents: Amphizoa: 1. Methylindol-3-carboxylate, 2. methyl homogentisate, 3. dimethyl disulfide; Gyrinidae, 4. gyrinidione, 5. 3-methyl-1-butanal, 6. 3-methyl-1-butanoic acid, 7. 3-methyl-1-butanol, 8. 2-methyl-1-propanol, 9. octanal, 10. 6-methyl-5-hepten-2-on, Hygrobia: 11. 2-hydroxyhexanoic acid, 12. S-methyl-2-mercaptobutanoi acid, 13. lactide between 11 and 12. 1, 3, 4: Dettner and Böhner 2009, 8–10: Dettner 1997, modified according to Barth 1960 (2), Miller and Bergsten 2012 (5), Kovac and Maschwitz 1990a, b (6), Klausnitzer 1996 (7)
Structure-activity of steroid deoxycorticosterone (1), gyrinidal (2) and gyrinidone (3) from pygidial rsp. prothoracic glands of whirligig beetles (2, 3) and predacious water beetles (1). Log x log plots of the survival time of fathead minnows (Pimephales promelas) in 50 mL solutions of norsesquiterpenes and steroid (ug/ml). Modified according to Miller et al. (1975)
In all cases when the beetles were attacked all whirligig beetles were rejected and the individuals survived without damage. Eisner and Aneshansley (2000) demonstrated that the oral tolerance of the bass Micropterus salmoides varied, dependent on gyrinidal dosage and satiation of the fish. Most of these experiments were performed by offering topically treated mealworms to the bass in an aquarium. Moreover the bass showed an intensive oral flushing behavior during which beetle sesquiterpenes were absorbed (about 80%) by the gills of the bass. Depending on gyrinid species there are also stinking volatiles in the pygidial gland secretions (Ivarsson et al. 1996) such as 3-methyl-1-butanal (Fig. 9.9/Formula 5), 3-methyl-1-butanol (Fig. 9.9/Formula 7), 2-methyl-1-propanol (Fig. 9.9/Formula 8), 3-methyl butyric acid (Fig. 9.9/Formula 6), and octanal (Fig. 9.9/Formula 9). In few Gyrinus species traces of 6-methyl-5-hepten-2-one (Fig. 9.9/Formula 10) and phenylacetaldehyde were recorded (Table 9.3; Fig. 9.11). Remarkably Macrogyrus oblongus did not contain the aforementioned four norsesquiterpenes, but instead other unidentified terpenes. Finally pygidial glands of Orectochilus villosus contained C17, C18, C21, and C22 alkanes; however the chemistry of the strong violet odor as described by Lucht (1974) remains unknown (Table 9.3). In addition the pygidial gland secretions are applied to the body surface by using their legs in order to keep it free from contaminating bacteria, protozoa, or fungi. In gyrinid beetles this secretion grooming was described by Kovac and Maschwitz (1990b). In addition pygidial gland secretion of Gyrinus substriatus increased wettability of the beetle integument (Fig. 9.6). As compared with the untreated elytral control, contact angles of standard water droplets on elytra previously treated with pygidial gland secretion were significantly lowered.
Aromatic pygidial gland constituents in aquatic and terrestrial adephagan beetles (Dettner and Böhner 2009). Fat boxes: Aromatics are present in nearly all members of appropriate families. Interrupted boxes: Compounds only present in these taxa, especially large rectangular box of Dytiscidae. Thin boxes: Compounds only in minor amounts in single species per taxon. Phylogeny according to Beutel et al. 2006. Terrestrial families: Cicindelidae, Carabidae, Rhysodidae, Trachypachidae
As demonstrated by fossil Permian Gyrinidae this family became adapted to swimming on the water surface long before the mesozoic invasion of the aquatic environment by Dytiscoidea took place (Yan et al. 2018). In the primitive Spanglerogyrinae the dorsal and ventral eyes are only narrowly separated as compared with the more derived Gyrininae. Molested Spanglerogyrus albiventris shows a peculiar jumping behavior (Steiner and Anderson 1981) which is also typical for some other gyrinid beetles. It is remarkable that Gyrinus larvae have a nonwettable integument and therefore are captured at the water surface (Bott 1928). Gyrinus larvae also gather debris which is mixed with mucopolysaccharides from midgut cells (Rupprecht 1971) in order to construct a pupal chamber on the top of emergent plants.
Various studies demonstrated the role of the aggregations of gyrinid beetles. It was found that they aggregate in the daytime into single- and multispecies groups (rafting of 100 to 1000 individuals). With increasing temperature the beetles dispersed from these rafts to shallow edges of the waters (Fitzgerald 1987; Heinrich and Vogt 1980). Naive fishes ate single beetles; however they learned to avoid these beetles, indicating that rafting places represent safe places for gyrinids (Heinrich and Vogt 1980). Henrikson and Stenson (1993) finally demonstrated that water prepared with gyrinid elicited evasive behavior in the beetles. They concluded that aggregations of the dark, highly contrasting beetles in exposed areas advertise their bad taste, which represents a case of aposematism. Probably, the spreading activity of the Gyrinidae secretion on the water surface has no relevance for living beetles (Vulinec 1987), since this activity is typically found in defensive secretions of many arthropods. It is important to denote that most adult Gyrinidae have a hard integument. Very often the upper body sideboard is brightly colored and elytra exhibit large and small spines at elytral apices as found in Porrorhynchus marginatus (Fig. 9.9/5; Gustafson and Miller 2016), which might be interpreted as defense against fishes.
Haliplidae (crawling water beetles; 240 sp.): Haliplidae hold the 11th position with respect to global water beetle diversity according to Jäch and Balke (2008). Larvae and adults are strictly aquatic and prefer stagnant or slow-running waters often with filamentous algae and characeans (van Vondel 2016). Adults are characterized by metacoxal plates and move their hind legs alternately. The three herbivorous larval stages have tracheal gills, move very slowly, and are often hidden within water plants. Haliplid larvae have a slender body shape and show a hard integument. Elder larvae are often camouflaged and covered by diatoms rendering a black body coloration; however they are fed by fishes, frogs, or carnivorous insect larvae (Hickman 1931). According to Falkenström (1926) pupae of Haliplus and in contrast to Dytiscidae pupae exhibit thanatosis. Mousseau and Roughley (2007) discuss that characteristic elytral maculations in Haliplidae and especially in Brychius can have deceptive character or may represent a warning coloration. Adult haliplids are consumed by fishes, frogs, ducks, or carnivorous insects (larvae of Dytiscidae and Odonata, adult dytiscid beetles, Hickman 1931).
The paired pygidial glands of adults of Haliplus ruficollis were described by Forsyth (1968), and the glands of other species from the genera Peltodytes, Brychius, and Haliplus were figured by Dettner and Böhner (2009). The gland reservoirs are covered by muscles, which either are confined to the reservoir in the primitive genus Peltodytes (Fig. 9.9/4) or extend to the reservoir opening in other genera (Fig. 9.9/1). The more primitive Peltodytes representatives exhibit compact glandular tissues (Fig. 9.9/4) as in Carabidae (Fig. 9.4/12) and show longer collecting channels which unite with the gland reservoir far away from the gland opening. In Brychius and Haliplus the glandular tissue is lengthened; however Brychius and Haliplus (subgenus Neohaliplus) exhibit short collecting channels and have reservoirs which gradually narrow towards opening (Fig. 9.9/1, 3, 4). Phylogenetic data from pygidial gland morphology are in accordance with phylogenies based on adult characters (Beutel and Ruhnau 1990). Phenylacetic acid (Fig. 9.5/11) which is also found in hydroporine Dytiscidae (and Copelatus) and Noteridae was recorded in all haliplid species investigated (Table 9.3). In all genera also further antimicrobials from selected Dytiscidae (Hydrovatus, Hygrotus, Laccophilus) such as 3-hydroxy octanoic, 3-hydroxy decanoic acid (Fig. 9.5/17, 22), benzoic acid (Fig. 9.5/3; Dytiscidae: Colymbetinae, Dytiscinae), or 4-hydroxyphenylacetic acid (Fig. 9.5/12; Dytiscidae: Hydroporinae; Noteridae) were recorded (Dettner and Böhner 2009). Exclusively few Haliplidae species secrete 3-hydroxyphenylacetic acid and phenyllactic acid (Fig. 9.11). All aliphatic and aromatic recorded gland constituents from Haliplidae are bactericidals, fungicidals, and disinfectants and are distributed on the beetle surfaces by secretion grooming (Dettner 1985; Kovac and Maschwitz 1990b). On land and on water surface the beetles distributed secretions with their legs on their body surface in order to dive in a characteristic way typical for Haliplidae (Fig. 9.7/Haliplidae 1–3). In addition phenylacetic acid and other components increase wettability of the haliplid body surface when the beetles hang on the water surface and show their characteristic cleaning behavior in order to dive (Fig. 9.6/Haliplidae; Dettner 1985). Whether the 3-hydroxyesters are transformed to polyesters on the body surface in order to enclose, kill, and eliminate epizoic microorganisms has to be investigated (Dettner and Böhner 2009). Stridulation was reported from three Haliplus species (Beier 1929; Seeger 1971; Aiken 1985). Sclerotized teeth at the underside of the elytra represent the pars stridens, whereas the plectrum is found in the pleural fold of abdominal segments 2–4. Stridulation behavior which is achieved by stretching of abdomen was described before and during mating and probably is not a defense mechanism.
Hygrobiidae (squeak beetles; six sp.): It is a relict family (also named Paelobiidae) with species in China, Australia, and Europe. Larvae and adults live in mud, silt, and detritus of ponds (Dettner 1997b; Dettner 2016c). Eggs are deposited on submerged plants. There exist three larval instars with tubular gills and characteristic strong but variable brownish pigmentation (Fig. 9.9/7). They pupate in a pupal chamber on land. Adults possess pygidial glands consisting of a gland reservoir (covered by muscles) with efferent duct and bifurcated secretory lobe (Fig. 9.4/6; Forsyth 1970) filled with colorless to white fluid which is depleted on land and distributed on the body surface of adult beetles (Kovac and Maschwitz 1990b). Hygrobia produces a series of unusual 2-hydroxyacids such as 2-hydroxyhexanoic acid (Fig. 9.9/Formula 11) and S-methyl-2-hydroxy mercaptobutanoic acid (Fig. 9.9/Formula 12), which may form diverse lactides (Fig. 9.9/Formula 13). Traces of benzoic acid and 4-hydroxybenzaldehyde, which also represent main gland constituents in Dytiscidae, were also recorded (Fig. 9.11; see Dettner 1985, 1987). Hygrobia hermanni also showed a typical behavior which was described as secretion grooming (Fig. 9.9/6, Kovac and Maschwitz 1990b). In addition pygidial gland secretion of H. hermanni increased wettability of the beetle integument (Fig. 9.6). As compared with the untreated elytral control, contact angles of standard water droplets on elytra previously treated with pygidial gland secretion were significantly lowered (Fig. 9.6). As in Dytiscidae the sister group of Hygrobiidae (Fig. 9.11) Hygrobia hermanni is characterized by paired prothoracic glands which are covered by secretory cells and a muscle cover (Fig. 9.9/10; Forsyth 1970). In contrast to Dytiscidae the prothoracic glands of Hygrobia are situated posterolaterally within the prothorax wherefore homology seems questionable. Recent molecular data indicate that there is a sistergroup relationship of Hygrobiidae to a clade comprising Amphizoidae, Aspidytidae and Dytiscidae (Vasilikopoulos et al. 2019). The separate evolution of a prothoracic gland in Hygrobiidae therefore seems possible. Since there are no chemical data available, significance of the secretion remains unknown. When molested both sexes of the beetles (“squeak beetles”) produce audible stridulations (Fig. 9.9/9) by rubbing abdominal apex on a file at the underside of an elytron (Fig. 9.9/8; Beutel 1986; Aiken 1985; Wichard et al. 1995). Probably Hygrobia also stridulates during contacts with conspecifics and in courtship.
Meruidae (comb-clawed cascade beetles; one sp.): The single small-sized species (adult body length 0.9 mm) was described from Venezuela and lives in the margins of cascades and mountain streams (Balke et al. 2018). Its larva has several characteristics of Noterus larvae. In order to submerge, adults turnover with the head directed downward and kick with their alternating leg motion until finding an underwater foothold. Similar behaviors are known from Haliplidae or small Dytiscidae. Pygidial glands were identified morphologically, and prothoracic glands are absent (Beutel et al. 2006).
Noteridae (burrowing water beetles; 280 sp., according to Jäch and Balke (2008)): Noteridae hold the 9th position with respect to global water beetle diversity. Noteridae are especially found burrowing in muddy substrate and in root mats in ponds and marshes, there exist few subterranean species. Adults and larvae are mainly carnivorous, and larvae (only few species have been described) have a siphon at abdominal tip in order to expire oxygen from aquatic plant aerenchyma. Air-filled pupae of some species are attached to the aerenchyma of water plants. Prothoracic glands are absent, and typical paired pygidial glands are present. They consist of a reservoir covered with muscles and a bipartite secretory lobe with two types of glandular cells (Fig. 9.4/8; Forsyth 1968). As in Dytiscidae main gland constituents are antimicrobics as phenylacetic (sweet odor; Fig. 9.5/11; Fig. 9.11), 4-hydroxybenzoic (Fig. 9.5/5), benzoic (Fig. 9.5/3), and 4-hydroxyphenylacetic acids (Fig. 9.5/12). Also 3-indole-acetic acid (Fig. 9.5/16) of unknown function is present. Noteridae also show secretion grooming behavior outside the water in order to distribute pygidial gland secretions on their bodies (Kovac and Maschwitz 1990b). In addition pygidial gland secretion of Noterus clavicornis increased wettability of the beetle integument (Fig. 9.6; Dettner 1985, 1997a, 2016a). As compared with the untreated elytral control, contact angles of standard water droplets on elytra previously treated with pygidial gland secretion were significantly lowered.
9.5.1 Polyphaga: Hydrophiloidea
Hydrophilidae (water scavenger beetles or silver water beetles; 2932 sp.; Short: 2950 sp.; according to Jäch and Balke 2008: 3rd position with respect to global water beetle diversity): Hydrophilids and their larvae are mainly found in stagnant and also running waters and hygropetric habitats; however their ecologic habits are extremely broad. Adults prefer plants and decaying organic matters as food whereas larvae are usually predacious. There are no complex glands present. Nevertheless Kovac and Maschwitz (2000) observed terrestrial and submerged grooming in various Hydrophilidae (Hydrophilus, Laccobius, Berosus, Enochrus, Spercheus: Spercheidae) apart from the terrestrial Helophorus nubilus (see Helophoridae). It is remarkable that Hydrochus, Hydrobius, and Berosus use anal droplets for grooming behavior.
Stridulation is widely distributed in adult Hydrophilidae and was obviously evolved several times (Aiken 1985). In many cases hydrophilid beetles stridulate when they are irritated, and sometimes sonograms indicate that sounds are important during courtship (Scheloske 1975). In several cases stridulatory structures in hydrophilid beetles are controversially discussed (Hansen 1991; Scheloske 1975).
Within Berosini (Berosus) and Oocyclini (Laccobius; Scheloske 1975; Fig. 9.12/2) both sexes stridulate. In both genera laterosternite 3, which is concealed under the elytra, exhibits a ribbed area representing a stridulatory organ together with protuberances on the underside of elytra (Hansen 1991). Within Hydrophilini stridulatory organs are present in genera Hydrophilus, Hydrobius, Limnoxenus, and Tropisternus (Wilson et al. 2015) and seem to be reduced in Helochares and Enochrus (Hansen 1991). In Tropisternus this behavior was named disturbance stridulation and acoustic aposematism which deters predators such as wolf spiders (Masters 1979). In Chaetarthriini, Sperchopsini, and Sphaeridiinae stridulatory structures are missing (Hansen 1991).
Defensive structures of Hydrophilidae , Hydraenidae , and Lampyridae and defensive components. 1. Posterior metasternal process of Hydrophilus aterrimus (Hydrophilidae). 2. Stridulation organs of Laccobius minutus (Hydrophilidae) with protuberances on underside of elytron and pleural area (pars stridens) of third abdominal segment. 3. Dorsal side of Limnebius piceus (Hydraenidae) with areas groomed by protibia and protarsus (a), protibia (b), mesotibia (c), Fig. 9.12 (continued) and metatibia and metatarsus (d). 4. Ventral aspect of head and prothorax of Ochthebius quadricollis (Hydraenidae) with part of exocrine secretion delivery system (e: end apparatus of gland cells, su: sulcus for collecting secretion, ssd: setae for secretion delivery). 5. Venter of left side of prothorax of Hydraena riparia with part of secretion delivery system (e: end apparatus of gland cells; hh hydrofuge hypomeron; su sulcus; wh wet hypomeron). 6 and 7. Macerated larvae of Luciola leii (Lampyridae) with everted, fork-like reservoirs of defensive glands (arrows in enlarged right figure). 8. Georyssus spec. (Georyssidae) with camouflage (covered by sand/mud; left), clean surface (right). 9. Aposematically colored Ancyronyx spec. (Elmidae). Gland constituents of lampyrid larva L. leii: 1. Terpinolene, 2. gamma-terpinene. Originals (1, 6, 7). Modified according to Scheloske (2), Perkins 1997 (3–5), Merritt and Cummins 1984 (8), 1996 (9).
The posterior metasternal process of Hydrophilus-species (Fig. 9.12/1) probably represents a defensive structure. In addition this process serves for keeping the ventral air bubble. Predator avoidance in Hydrophilidae also may include crypsis. For example Derallus larvae bear setiferous projections with a lot of algae and detritus. When molested larvae of Hydrophilus may immediately defecate like an explosion and suddenly lift their head backwards (Pavlovsky 1922).
Helophoridae (192 sp.): Helophoridae hold the 12th position with respect to global water beetle diversity according to Jäch and Balke (2008). Due to sticky dermal glands, adults are often dirty or covered by sand and must be cleaned before determination of these brownish, metallic, or black specimens. Adult Helophoridae are found especially in shallow water, and their larvae are found among vegetation an in soil near water (Angus 1992). The laterosternite 3 of adult Helophorus has no ribbed area (which would represent a stridulatory organ; Hansen 1991) and no stridulation has been recorded (Oliva 1992).
Epimetopidae (56 sp.; Short 2018: 66 sp.): The beetles seem to be riparian and live within sand substrates. Adults may be covered by soil particles, and they possess no abdominal stridulatory organ (Hansen 1991).
Hydrochidae (181 sp.): Hydrochidae hold the 11th position with respect to global water beetle diversity according to Jäch and Balke (2008). Hydrochid beetles are found in stagnant to slowly flowing water. The dark or metallic adults are often covered by a secretion and crust of vegetable matter (Oliva 1992). Adults feign death when disturbed and possess no abdominal stridulatory organ (Hansen 1991).
Spercheidae (filter-feeding water scavenger beetles; 20 sp., Yee and Kehl 2015): Representatives of these filter-feeding beetle family are found in stagnant waters and in mud. When molested on land or within water Spercheus adults of both sexes are able to stridulate in a different manner. Obviously stridulation is primarily a defensive reaction but can also be important in sexual communication (Von Frankenberg 1937). Laterosternites 3, which are concealed under the elytra, exhibit transversely ribbed areas which are moved against the ridges under each elytra in order to stridulate. Remarkably Spercheus adults also exhibit thanatosis (longer lasting) together with the defensive stridulation (Von Frankenberg 1937).
9.5.2 Polyphaga; Staphylinoidea
Hydraenidae (minute moss beetles; about 1300 sp.; Short 2018: 1600 described species; according to Jäch and Balke 2008 there was an estimation of 2500 sp. which means that Hydraenidae occupy the second position among water beetles with respect to global water beetle diversity): Many species of these true water beetles (few of them are terrestrial) possess a microplastron that means a ventrally situated thin layer of air, which is held by hydrofuge setae (Jäch et al. 2016). An exocrine secretion delivery system with various glands especially located at head and prothorax, e.g., in Ochthebius or Hydraena species and various cuticular structures such as grooves, ridges, and setae (Fig. 9.12/4, 5), plays an enormous role in secretion grooming (Jäch et al. 2016; Kovac and Maschwitz 2000; Perkins 1997). Perkins (1997) presented a detailed morphological investigation on these secretion delivery systems, the wetted and hydrofuge integumental body areas, and studied grooming behavior of selected species such as Limnebius (Fig. 9.12/3); however due to the small body size of these hydraenid beetles no chemical data are available. Integument of terrestrial and coastal species is covered with encrustations. Adult hydraenids cannot swim but walk on underside of surface film. Stridulation is quite common and two main stridulatory areas are known: (a) abdomen/elytra (all species) and (b) head/pronotum (only few species). Since stridulatory organs are sexually dimorphic, a role in courtship and mating was suggested (Aiken 1985). The similar grooming patterns of Hydraenidae within water and on land were described by Kovac and Maschwitz (2000).
Scirtidae (marsh beetles; Yee and Kehl 2015: 1000 sp.; over 1800 sp.): Scirtidae hold the 11th position with respect to global water beetle diversity according to Jäch and Balke (2008). They prefer various habitats such as streams, pools, phytotelmata, and groundwater. Marsh beetle larvae are usually aquatic, and ecology and bionomy of the family are poorly studied. If molested larvae are very sensitive and immediately stop feeding and may even escape (Klausnitzer 1996).
Elmidae (riffle beetles; 1500 sp.; Yee and Kehl: 1300 sp.): Elmidae hold the 11th position with respect to global water beetle diversity according to Jäch and Balke (2008). Aquatic Elmidae are herbivores or detrivores and are usually found in running water with high oxygen content, and seldom lakes and ponds. Adults possess an efficient plastron that means a thin air film coats the body surface. Interestingly secretion grooming by brushing legs over the ventral air layer is important but can be only observed under water (Kovac and Maschwitz 2000). White (1989) offered elmid specimens to a turtle and predatory fishes and showed that aposematically colored (red to yellow markings on elytra and thorax) beetles were almost always rejected (Fig. 9.12/9). Larvae do not have aposematic colorations and were never rejected or ignored by fishes. It was further proven that elmid adults have a chemical defense restricted to the elytra; until now active principles have not been elucidated. Elliott (2008) showed that lanceolate larvae of Elmis, Esolus, or Oulimnius rolled up like an armadillo when disturbed. Both larvae and adults usually have hard integuments.
Dryopidae (long-toed water beetles; 280 sp.; according to Jäch and Balke 2008/Yee and Kehl 2015: 300 sp.): Dryopidae hold the 8th position with respect to global water beetle diversity according to Jäch and Balke (2008). Adults are found in aquatic or other habitats, and larvae are terrestrial or under water surface. Beetles and larvae are slowly moving and generally lethargic insects. Adults are purely aquatic and are characterized by a plastron. For Dryops secretion grooming by using their legs was reported (Kovac and Maschwitz 2000). Ecology of Dryopidae is only purely known. Adults and larvae do not have aposematic colorations and are accepted by predators (Scrimshaw and Kerfoot 1987). According to Lawrence (1991) Dryopidae pupae have unpaired gin traps, which means sclerotized and often armed portions of two adjacent sclerites (Lawrence 1991).
Psephenidae (water pennies; 287 sp.; Jäch and Balke 2008: 272 sp.): Psephenidae hold the 11th position with respect to global water beetle diversity according to Jäch and Balke (2008): The flattened larvae with their powerful claws are usually in running waters. They can hug the rocks as tenaciously as limpets, so they are safe from predators. Their coloration varies and often they match with their background substrate (Lawrence 1991). Aquatic psephenid pupae are characterized by plastron-bearing gills; in addition they have gin traps (Lawrence 1991), and adults are terrestrial. The nocturnal feeding behavoir of the larvae was reported to be a defense mechanism against predators. In addition the larvae can form a ball when being swept away (Beutel and Leschen 2016).
Cneoglossidae (eight sp.; Yee and Kehl 2015: ten sp.): Beetles are found in submerged bushwood in small streams. Larvae are aquatic, and adults are terrestrial. The life history of this small family is unknown.
Eulichadidae (forest stream beetles; 42 sp.): Larvae are aquatic; the beetles are in sand or litter under rocks in streams.
Lutrochidae (travertine beetles; Jäch and Balke 2008/Yee and Kehl 2015: 15 sp.): Travertine beetles occur submerged in decaying wood. Their life history is unknown and their taxonomy has to be revised. Adults which inhabit calcareous water streams may be encrusted with this material (Yee and Kehl 2015).
9.6 Not Predominantly Aquatic Families According to Jäch and Balke (2008)
Nitidulidae (sap beetles; according to Jäch and Balke 2008 about 3000 sp.): One species Amphicrossus japonicus is aquatic and can be found in in water-filled stems of bamboo.
Carabidae (ground beetles; 37,600 sp.; Beutel and Leschen 2016): Among these Geadephaga there are a lot of species which are riparian, found near freshwater habitats, or even stay underwater for feeding or concealing (Carabus clathratus, Oodes, Nebria, Bembidion, Chlaenius, Brachygnathus). Representatives of the genus Omophron are found within wet sand and even resemble dytiscid water beetles. However phylogenetically there is no relation between Omophron and Hydradephaga. All Geadephaga share the same pygidial glands with Hydradephaga. However the gland morphology of Carabus or Omophron (Fig. 9.4/12) is not similar with Hydradephaga, because secretory tissues are compact and not lengthened. In addition they produce other pygidial gland constituents (e.g., Omophron: 2-methylpropanoic acid, 3-methylbutyric acid; Carabus: 2-methylpropenoic acid, (E)-2-methyl-but-2-enoic acid; also in water beetle Ilybius; Dazzini Valcurone and Pavan 1980). In Omophron stridulation was investigated and was named disturbance stridulation and acoustic aposematism which deters predators such as wolf spiders (Masters 1979).
Staphylinidae (rove beetles; 55,244 sp. Beutel and Leschen 2016): Rove beetles from many subfamilies (e.g., Aleochara, Omalium, Paederus, Philonthus, Thinobius) are semiaquatic and can be found on beaches, along rivers, ponds, bogs, and nearly every kind of freshwater. Due to their short elytra and their unprotected abdomina most staphylinid species have defensive glands or toxic hemolymph in order to defend against predators (Dettner 1993). Especially two taxa have special relationships with water. Many species of Bledius (Oxytelinae) live subsocially within wet sand of shores (freshwater; sea) where they feed on algae. Females feed and defend their larvae and use their quinoid abdominal gland secretions against aggressors (Dettner 1993; Thayer 2016). In many cases the formulation of these mixtures of defensive secretions is optimized against special target organisms (Steidle and Dettner 1993, 1995). Another rove beetle group, representatives of Steninae (worldwide with 3300 sp.), is typical for the water surface of all kind of freshwaters (Betz et al. 2018). The beetles are predators of aquatic springtails and other small arthropods and are found in every wet place. Many species walk on the water surface and depending on species show a unique skimming behavior after a molestation. They secrete terpenes and alkaloids such as stenusin or cicindeloin from their anal glands (Fig. 9.13; Dettner 1987, 1993; Betz et al. 2018). Comparable to Enhydrus glands (Gyrinidae), Stenus abdominal glands have two types of secretory tissues (gland 1 and gland 2) which are morphologically separated and synthesize different compounds. The large gland 1 delivers alkaloids; in contrast the small gland produces terpenes. All alkaloids are biosynthetically derived from amino acids from food (e.g., springtails, aphids). Together with molecular data, the Steninae alkaloids are excellent chemotaxonomic markers (Betz et al. 2018).
Anal glands and secretions of Stenus rove beetles (Staphylinidae). Paired anal glands with large reservoirs (gland tissue g1 and gland reservoir r1; blue) and small gland reservoirs (r2) and gland tissue (g2; red). Alkaloids from the large gland are figured above (blue box), terpenes from the small gland are figured below (red box). Circle demonstrates quantitative amounts of alkaloids stenusin and norstenusine from large gland as compared with remaining terpenes from smaller glands of Stenus comma (according to Betz et al. 2018)
Both special alkaloids (formerly named “spreading alkaloids”) and selected terpenes may have considerable spreading activity. However the spreading ability and velocity of a Stenus species on the water surface vary. These differences are due to gland composition and the ability of the beetle species to deplete their outer gland reservoir on the water surface (Fig. 9.13; Lang et al. 2012). These compounds have multiple functions as antimicrobics, fungicides, and deterrents against predatory arthropods and fishes (Schierling et al. 2013). In addition the rove beetles exhibit grooming behavior or deplete parts of their anal glands, when molested. Spreading abilities are unusual phenomena in nature; however it is also observed in semiaquatic Veliidae (Heteroptera) which in contrast spray saliva on the water surface . As mentioned by Bush et al. (2008) even pine needles floating on the water surface are propelled across the surface since the resin at its base decreases the surface tension.
Leiodidae (round fungus beetles): Platypsyllus castoris lives on beavers, and Silphopsyllus desmanae lives on water moles. These two species represent parasitic water beetles (Jäch and Balke 2008).
Scarabaeidae (scarab beetles; 27,000 sp.): Among this large cosmopolitan family there exists the aquatic rice beetle Dyscinetus morator. This species is often found underwater, especially while escaping disturbances (Jäch and Balke 2008).
Ptilodactylidae (toe-winged beetles; Yee and Kehl 2015: approximately 500 sp.): They are found in aquatic, especially riparian, habitats. Larvae feed on submerged decaying wood. As defense ptilodactylid pupae have abdominal gin traps (Beutel and Leschen 2016). Not all species are aquatic. Ecological information is mostly lacking.
Lampyridae (lightning bugs/fireflies; approx. 2000 sp. according to Jäch and Balke (2008) and Yee and Kehl (2015)): Larvae of few species (Luciola, Pyractomena, Pristolycus) are secondarily aquatic (sometimes with tracheal gills) and are found in running water (lotic systems) or ditches (lentic systems). As terrestrial larvae which feed on land snails, the aquatic larvae feed on aquatic molluscs such as mussels and snails. Elder larvae may be good swimmers in order to catch prey. Since the snail preys in Japan sometimes represent hosts for human parasites these larvae are important for biocontrol (Yee and Kehl 2015). Aquatic and semiaquatic lampyrid larvae leave the water before pupation in order to construct terrestrial mud cells (Branham 2010). All lampyrid larvae aposematically produce light via paired photic organs which are located ventrally at the eighth abdominal segment (Branham 2010). Fu et al. (2007, 2009) reported that the glow intensity of three aquatic Luciola species is weak. Okada (1928) and Fu et al. (2007, 2009) described defensive glands in larvae of the genus Luciola. After molestation from meso- and metathorax and every abdominal segment (8) paired forked organs are everted from slit-like crevices which are situated above the gills (Fig. 9.12/6, 7). The anterior branch of these glandular sacs is longer than the posterior one. Fu et al. (2007) described the odor of Luciola leii secretion (present in all six instars) as pine oil-like, whereas Okada (1928) reported an odor in a Japanese Luciola-species resembling resin and peppermint. Until now terpinolene (Fig. 9.12/Formula 1) and gamma-terpinene (Fig. 9.12/Formula 2) could be identified in the defensive secretion of L. leii (Fu et al. 2007). When aquatic larvae are under attack they may run away, and exhibit thanatosis, glowing and with release of defensive secretion. Preliminary bioassays showed that L. leii larvae were refused by various predatory fishes or dragonfly larvae (Fu et al. 2007). However one has to take into consideration that most lampyrid beetles and their larvae synthesize toxic cardiac glycosides, the lucibufagins (Eisner et al. 2005).
Chrysomelidae (leaf beetles; about 46,000 species in many subfamilies are known): From several subfamilies such as Alticinae, Chrysomelinae, Galerucinae, or Hispinae there are known members living on emergent aquatic plants or even within phytotelmata. Adults of several species can live underwater or at the water surface (Jäch and Balke 2008). Representatives of subfamily Donaciinae with genera such as Donacia, Macroplea, or Plateumaris are especially adapted to freshwater. Their larvae and pupae are generally submerged, but also adults, which may carry an extensive plastron, are often found underwater. Larvae have caudal spines in order to penetrate tissues of aquatic plants to take up air. Cocoons which are spun from larval silk glands are fixed underwater and are water- and airtight. Donaciinae usually feed on monocotyledonous host plants or Nymphaeaceae; adults also prefer pollen. Defense mechanisms in Donaciinae are not known; however the abovementioned subfamilies of Chrysomelidae with semiaquatic species represent interesting taxa to investigate, since they exhibit various mechanisms of mechanical (Hispinae) and chemical defenses including sequestration of plant toxins (e.g., Burse and Boland 2015; Dettner 1987, 2015; Hilker and Meiners 2002; Opitz and Müller 2009; Pasteels et al. 1984, 1990).
Nanophyidae (about 300 species Jäch and Balke 2008): Several species of Nanophyes represent phytophilous water beetles feeding on Alternanthera and Ludwigia.
Erirhinidae (about 300 species, Jäch and Balke 2008) with some phytophilous water beetles.
Curculionidae (weevils; largest family of animals; more than 60,000 species, Jäch and Balke 2008): Few species from various subfamilies have invaded aquatic environments. Most species belong to Bagoinae but also those from Cleoninae, Notarinae, Stenopelminae, Tanysphyrinae, or Ceutorhynchinae aquatic representatives are known. Many species (larvae and adults) are mono- and oligophageous (e.g., Alisma, Azolla, Equisetum, Butomus, Eichhornia, Glyceria, Lemna, Myriophyllum, Pistia, Potamogeton, Ranunculus, Ricciocarpus, Salvalina, Stratiotes) and few species are used as biological control agents of introduced nuisance water plants (Merritt and Cummings 1996).
Most aquatic weevils swim and dive underwater and use plastron respiration. Larvae are mining but usually slowly crawl on host plants and pupate within air-filled cocoons located on roots of plants (Klausnitzer 1996). Adults of most species are mechanically defended by hard integuments; sometimes as in Dicranthus elegans they have additionally long and hard elytral spines. In several genera such as Helodytes, Eubrychius, or Hydronomus terrestrial and aquatic grooming pattern was described (Kovac and Maschwitz 2000). For grooming dark and later clear fecal droplets were repeatedly released from the abdominal tips.
Trachypachidae (false ground beetles; six sp.): The enigmatic Trachypachidae (Adephaga) have many morphological similarities with Dytiscoidea (e.g., Arndt and Beutel 1995); however they are not found in wet habitats and therefore not covered by Jäch and Balke (2008). In contrast they are xerophilous (Beutel and Arndt 2016) and molecular data place them phylogenetically within Geadephaga (Maddison et al. 2008). Although biology of this family is nearly unknown, the pygidial glands resemble those of carabids (Fig. 9.4/11, 12). The defensive chemicals of two Trachypachus species (Attygalle et al. 2004) with methacrylic acid and tiglic acid also resemble those secretions of terrestrial carabids. In contrast, trachypachids secrete octanoic acid, unknown sulfur compounds, or an aromatic (Fig. 9.11) which is typical for Hydradephaga (Dettner 1985; Dettner and Böhner 2009). Therefore further research is necessary in order to evaluate the chemotaxonomic value of these micrometabolites.
9.7 Shore Beetles According to Jäch and Balke (2009)
Georissidae (minute mud-loving beetles; 80 sp.): These beetles are usually riparian. By using buccal secretions adults are often covered by sand or mud (Fig. 9.12/8) and it is difficult to observe the melanized or metallic surfaces. Bameul (1989) described the camouflage of G. crenulatus which even prevents flight (Shepard 2003). In spring when georissid beetles are very active they exhibit a short thanatosis before moving away. Adults have no stridulatory organs (Hansen 1991).
Limnichidae (minute mars-loving beetles; 387 sp., Beutel and Leschen 2016; according to Yee and Kehl 2015: 335 sp.): These beetles feed on algae, are semiaquatic, and can be found on sandy beaches or coralline formations. Adults of Thaumastodinae (jumping shore beetles) can jump. Beetles of this family are accepted by fishes (Scrimshaw and Kerfoot 1987).
Heteroceridae (variegated mud-loving beetles; 300 sp.; Yee and Kehl: over 250 sp.): Adults and larvae live in tunnels in wet sand at water edges, in riparian areas. Adult heterocerid larvae can use secretion from dermal or salivary glands in order to stabilize their pupal chamber. After molestations beetles break through the mud and fly away (Klausnitzer 1996).
HYMENOPTERA (sawflies, wood wasps, bees, wasps, ants; 0.1% aquatic): Among worldwide 132,000 hymenoptera species (Stork 2018: 116,861) only about 150 from 11 families represent mostly internal parasitoids of aquatic stages (eggs, larvae) of various water insects and hygrophilic spiders (Cover and Bogan 2015; Morse 2017; Wesenberg-Lund 1943). In contrast selected Agriotypinae and Pompilidae (e.g., Anoplius) represent external parasitoids of Trichoptera pupae (Goera, Silo) of, respectively, Dolomedes or Pardosa spiders (Morse 2017). Interestingly by abdominal silk glands Agriotypus larvae produce a helical outgrowth from the trichopteran pupa which serves as a breathing device (Wesenberg-Lund 1943). Wesenberg-Lund (1943) and Heckman (2018) summarized typical aquatic hymenopterans from Chalcididae (e.g., Pleurotropis, Trichogramma, Prestwichia; hosts: cocoons and eggs of Gyrinidae, Dytiscidae, Sialis, Odonata, water bugs), Proctotrupidae (Limnodytes, Litus, Anteris, Anagrus, Anaphes = Polynema; hosts: eggs of water beetles, and water bugs, Odonata), Ichneumonidae (Hemiteles, Apsilops, Atractodes, Trichocryptus; hosts: cocoons of Gyrinidae, larvae, and pupae of various Diptera, Trichoptera, Lepidoptera), Braconidae (e.g., Dacnusa, Chaenusa, Liposcia, Gyrocampa Hydroplitis; hosts: larvae of Hydrellia, Hydrocampa), and Agriotypinae (see above). Several biological aspects of aquatic parasitoid species were discussed by Heckman (2018).
As in other insects, parasitic wasps are attacked by various predators ranging from vertebrates to invertebrates such as spiders and other insects. Because natural histories of aquatic hymenopteran species are mainly unknown, here only selected defensive mechanisms within those families are presented where aquatic representatives were reported. As known from various hymenopteran parasitoids they may be brightly colored, which indicates their unpalatability (Quicke 1997). Other larger species such as ichneumonids and braconids defend against vertebrates and invertebrates by stinging (Quicke 1997), which sometimes might be really painful. Certainly only females are effective, since males lack ovipositors for venom injection. Certain parasitoids in addition produce distinct, sometimes unpleasant, odors which originate from exocrine glands. In female Alloxysta species mandibular glands produce deterrents such as 6-methyl-5-hepten-2-one, actinidin, and various iridoids (Völkl et al. 1994) which are directed against predators. Other species produce also perillene-isomers or iridoids such as nepetalactone and iridomyrmecin (Hübner et al. 2002). Many ichneumonids but also braconids and certain Oppiinae are odoriferous which may also originate from mandibular glands (own observations, Quicke 1997) and are probably directed against wasps. Unfortunately only few data exist on exocrinology of hymenopteran parasitoid wasps; however some pheromones, kairomones, and venom proteins are compiled by Keeling et al. (2004), Dettner (2015), and Asgari and Rivers (2010). Other parasitic Hymenoptera show thanatosis (death feigning). Interestingly some braconids are polymorphic for this behavior and in Habrobracon the gene controlling thanatotic behavior is inherited as a single dominant allele (Grosch 1988; Quicke 1997). Parasitic wasps may act as models in mimicry systems or may be mimics themselves (for theory see Dettner and Liepert 1994). In addition especially ichneumonids also might construct cryptic or mimetic cocoons (Quicke 1997). Finally larger braconids may be protected from predators by heavily sclerotized thoracic spines (Quicke 1997). In larger ichneumonids there occur even poison claws and urticating hairs (Hanson and Gauld 1995).
DIPTERA (true flies; about 50% aquatic): Worldwide there exist about 154,000 species of Diptera (Stork 2018: 155,477), most of which have aquatic or semiaquatic eggs and larvae. There exist various reviews where aquatic and semiaquatic dipteran larvae are compiled (e.g., Italian and European species: Rivosecchi 1984; Faasch 2015; neotropical species: Fusari et al. 2018; world: Hennig 1968, Morse 2017). Especially the following aquatic and subaquatic families should be denoted: Ceratopogonidae, Chironomidae, Deuterophlebiidae, Simuliidae, Thaumaleidae, Canacidae, Culicidae, Dixidae, Corethrellidae, Chaoboridae, Blephariceridae, Nymphomyiidae, Psychodidae, Stratiomyidae, Dryomyzidae, Syrphidae, Periscelididae, and Ephydridae. In addition further families may also be of importance: Cylindrotomidae, Tipulidae, Limoniidae, Tanyderidae, Ptychopteridae, Scatopsidae, Athericidae, Rhagionidae, Tabanidae, Pelecorhynchidae, Empididae, Dolichopodidae, Lonchopteridae, Phoridae, Muscidae, Scathophagidae, Sarcophagidae, and Sciomyzidae. Since bionomics of many Diptera are unknown (Oosterbroek 2006), especially at the larval stage (Hennig 1968), the knowledge on defenses of dipteran larvae is deficient. As demonstrated by Crespo (2011) Chaoborus larvae (Chaoboridae) can sense fish kairomones which affect their vertical migration. Also chironomids can assess different concentrations of infochemicals. For the predatory Toxorhynchites larvae it was shown that not chemical cues but vibrations (detected by thoracic and abdominal setae) were necessary to detect prey organisms (Crespo 2011). As a whole Crespo (2011) stressed that studies on predator avoidance have not received the same attention in Diptera as in other aquatic insects.
One fascinating aspect of mosquito perceiving of kairomones of larval predators was presented by Crespo (2011). Recent molecular data on olfactory binding proteins in larvae and adult mosquitoes showed that several of them are present in both immature and mature stages. Therefore it seems conceivable that adult mosquitoes may utilize information which is relevant during their larval stages. That female Culiseta mosquitoes only oviposit in those waters where no Notonecta kairomones (such as n-heneicosane), the predators of the Culiseta-larvae, are present was discussed in chapter Notonectidae.
Apart from flight reactions (often achieved by peristaltic movements) which might be abundant in dipteran larvae, secondary defenses such as chemical defense or thanatosis obviously are rare (Dettner 2015). However primary defense mechanisms such as larval shelters ( Chironomidae, Simuliidae), crypsis, translucence, or stable and elastic cuticles may be more abundant in spite of the fact that larval body colors are not abundant (Dettner 2015). Tergites and sternites of fly larvae usually are reduced in order to obtain optimal mobility.
Larval integuments of Diptera may sometimes possess micro- and macrotrichia, spines, and dermal glands but no complex glands. Often larvae show fleshy projections, papillae, pseudopods, or suctorial discs. Even pupae of aquatic Diptera often have abdominal spines. That the abovementioned structural characters such as hairs and/or body sizes of the larvae can protect chironomid larvae from different kind of predators is amply illustrated by the study of Hershey and Dodson (1987). Large larvae of Cricotopus bicinctus were less susceptible to predation by Hydra and small Ischnura larvae . In contrast long hairs protected Cricotopus sylvestris from Hydra but not from Ischnura.
In the following examples of defense in aquatic or semiaquatic dipteran larvae are given.
Ceratopogonidae (biting midges): According to Ronderos et al. (2018) there exist worldwide 6268 sp. In Ceratopogonidae it was proved that female canthariphilous species such as Atrichopogon trifasciatus are able to transfer toxic cantharidin into their eggs and larvae (Dettner 1997). Larvae of Forcipomyia possess secretory setae dorsally on their bodies (Fig. 9.14/2), where various antibacterial and hygroscopic compounds such as fatty acids (Fig. 9.14/1, 2, 4), glycerol (Fig. 9.14/6), and pyroglutamic acid (Fig. 9.14/5) are produced (Urbanek et al. 2012). Atrichopogon larvae and pupae of various genera bear abdominal spines which may represent a kind of mechanical defense (Ronderos et al. 2018).
Morphological defense , defensive glands , and stridulatory organ of dipteran (1–4) and trichopteran larvae (5, 6). 1. Tabanus spec. larva (Tabanidae; right) and head capsule with pierced mandible (left). 2. Fore-end of Forcipomyia larva (Ceratopogonidae) with secretory setae (black; left side). Enlargement of secretory setae (right) showing depletion of secretion and its hydroscopic effects. 3. Larva of Stratiomys spec. (Stratiomyidae) with hard integument by calcareous crystals (enlargement). 4. Pupa of Ephydra bivittata (Ephydridae) with gin trap between segments 6 and 8. 5. Head and thorax of Apataniana hellenica (Limnephilidae) with everted prothoracic glands (arrows). 6. Stridulatory organ of Hydropsyche pellucidula (Hydropsychidae) with file on head underside and scraper on profemur (arrows). Defensive secretions: 1. Caprylic acid (Apatania, Forcipomyia), 2. capric acid (Apatania, Forcipomyia), 3. 3-dodecenoic acid (Apatania), 4. palmitic acid (Forcipomyia), 5. pyroglutamic acid (Forcipomyia), 6. glycerol (Forcipomyia).
Modified according to Jacobs and Renner 1974 (1, 3), Urbanek et al. 2011 (2), Rivosecchi 1984 (4), Wichard et al. 1995 (5), Aiken (1985)
Lonchopteridae (spur-winged flies): 58 species worldwide (Fusari et al. 2018): Lonchopterid larvae live on shores of water. They are often dark colored and have broad dorsal and smaller lateral sclerotized plates which may have a protective value.
Tabanidae (horse and deer flies): According to Fusari et al. (2018) there exist worldwide about 4500 species. Larvae are predators and often have a thick integument (Fig. 9.14/1). From each segment 4–10 of the often colored larvae there exists a thick ring, each supplied with various parapodia and sometimes with additional spines. Larvae have a retractile head capsule with pierced mandibles, where toxins from the salivary gland are applied (Fig. 9.14/1). Bites of some Tabanus larvae are known to be as painful as bee stings. Injections of tabanid venoms into insects produce moveless animals after one or two spasms (Schmidt 1982). In the field even tadpoles are killed immediately.
Stratiomyidae (soldier flies): According to Pitaluga de Godoi and Pujol-Luz (2018) there exist worlwide 2800 species (928 species are aquatic, Morse 2017). The integument of armored, dorsoventrally flattened larvae is leatherlike, with a lot of calcareous crystals which are produced within malpighian tubules (Fig. 9.14/3). The hard and flexible integument protects the larvae from predators but also represents a protective device against hymenopteroid parasitoids (Wesenberg-Lund 1943).
Syrphidae (syrphid flies; worldwide about 6000 sp.; 1341 aquatic species according to Morse 2017): Certain larvae of usually saprophagous aquatic Eristalinae possess a long anal segment (“rat-tailed larvae”) which acts as a protecting sheath for the breathing tube. Since Eristalis tenax and various other syrphid species are able to survive in extremely polluted aquatic habitats various antibacterial peptides could be identified within the hemolymph of these larvae (Altincicek and Vilcincskas 2007). In contrast cuticles of syrphid larvae with both short and long breathing tubes have impervious cuticles which prevent entering of many toxic substances from the water (Heckman 2018). Similar microbiological results and unusual products were achieved by analysis of Helaeomyia petrolei larvae (Ephydridae, shore flies; 1251 species Morse 2017) from extreme habitats (Kadavy et al. 2000). Pupal representatives of this family such as Ephydra bivittata also possess gin traps as defense against parasitoids (Fig. 9.14/4). Predatory syrphid larvae of Ocyptamus live in tanks of bromeliads and subdue their prey with venoms (Miranda and Rotheray 2018).
TRICHOPTERA (caddisflies; more than 99% aquatic): Few defensive mechanisms are known from Trichoptera , the sister group of Lepidoptera. They have worldwide about 15,000 species (Stork 2018: 14,391) and are found in nearly all freshwater habitats (Heckman 2018; Pes et al. 2018). Nearly all species have long-living aquatic larvae with about five larval stages. In 1984 Otto showed that trout in daylight preferably attacked caddis-larvae which possessed head capsules with contrasting color patterns compared to uniformly colored specimens. However this preference was reversed under reduced illuminations (Otto 1984). Many Trichoptera larvae produce transportable case buildings from silk glands as primary defensive behavior against both vertebrate and invertebrate predators (Morse 2003). A wide range of plant and mineral materials are used and are orientated longitudinally or horizontally. Such caddisfly cases may be classified as tube cases (most Integripalpia: Limnephilidae, Goeridae, Phryganeidae, Brachycentridae, Lepidostomatidae, Beraeidae, Sericostomatidae, Odontoceridae, Molannidae, Leptoceridae), saddle or tortoise cases (Glossosomatidae), or purse cases (Hydroptilidae). Some cases are only made from silk, and may contain shells of freshwater molluscs, freshwater sponges, or pieces of corals (marine species). In contrast about half the caddisfly species do not build cases, but spin silken retreats which are fixed on the substrates and serve for capturing food (e.g., Philopotamidae, Psychomyiidae, Ecnomidae, Polycentropodidae, Hydropsychidae, Arctopsychidae). There are also free-living larvae moving on substrates (e.g., Rhyacophilidae, Hydrobiosidae). Efficiency of different trichopteran larvae with various types of cases was tested against predatory Anax dragonfly larvae (Ferry et al. 2013). It was astonishing that the presence of any case, regardless of the material used, offers survival benefits against dragonfly larvae. In contrast caddisflies removed from their case were attacked and captured. Larval cases of Limnephilus larvae surviving exposure to dragonfly larvae and predacious minnows were significantly stronger (greater mineral fraction), longer, and wider than cases of victims (Nislow and Molles Jr 1993). Finally Boyero et al. (2006) showed that Potamophylax larvae can discriminate predators such as dragonfly larvae, salamander larvae, and trouts by chemical cues and alter their choice of case type according to risks of predation. As mentioned by Crespo (2011) long-range identification of food was not dependent on intact antennae of trichopteran larvae but may be achieved by sensilla in maxillary and galeal palps. He also stressed that predator avoidance responses had not been studied so far in trichopteran larvae.
However one exceptional example is described below. When disturbed aquatic larvae of the genera Apatania and Apataniana (Limnephilidae) release a defensive fluid (1–2 μg secretion per specimen) from an eversible prothoracic gland (Fig. 9.14/5; Wichard et al. 1995), which shows paralyzing effects against small invertebrates (other trichopteran larvae such as Rhyacophila). When Rhyacophila penetrates the Apatania case, at first the larvae withdraw, and then release droplets of the secretion. In contrast the secretion is ineffective against larger targets such as plecopteran larvae or fishes (Wagner et al. 1990). The secretion contains about 40 C7- to C14-carboxylic acids (Fig. 9.14/1–3) with the main constituents 5-octenoic-, octanoic-, decanoic-, 3-dodecenoic- (Fig. 9.14/3), 3,5-dodecadienoic-, and 3,5,7,11-tetradecatetrenoic acids (Wagner et al. 1990). These eversible larval limnephilid defensive glands resemble the osmeteria which are found in various caterpillars such as Notodontidae or Papilionidae. It is noteworthy that a single irritation does not result in a complete loss of defensive secretion. Another defensive behavior was observed in larvae of Hydropsychidae. In order to defend their retreats against intruders they communicate and produce stridulatory signals in rubbing the scraper of the forefemur against a file on the underside of the head (Fig. 9.14/6; Aiken 1985). Also Plectrocnemia larvae will defend their retreats and fight with open mandibles against intruders (Solem and Gullefors 1996).
LEPIDOPTERA (moths, butterflies; 0.5% aquatic): According to Graca and Solis (2018) there exist 157,000 lepidopteran species worldwide (Stork 2018: 157,338). Most of the species are terrestrial; however several families contain few or various semiaquatic or aquatic species at least caterpillars. Females of aquatic species usually deposit eggs in underwater vegetation. In few species females cover their egg masses with thick secretion. In Acentria ephemerella there exist even females which have no wings and are living completely underwater (Reichholf 1970; Vallenduuk and Cuppen 2004; Wesenberg-Lund 1943). Newly hatched larvae may attach silken threads to the leaf as anchor. As a kind of protection from enemies especially early larval instars of several species feed as miners or boreres within stems or leaves of aquatic or semiaquatic plants. By using their salivary glands later instars or larvae from other species may construct cases from their food plants like trichopteran larvae and therefore to some extent might be protected. Oxygen intake by larvae may be achieved through tracheal gills, plastron-like devices, and use of bubbles in interstitial places or in cases made with the host plant. Depending on species and larval stages hydrophobicity and wettability of larval integuments may vary considerably. Pupation of aquatic lepidopteran larvae takes place in or out of water inside silken cocoons.
Examples of aquatic lepidopteran families, some genera involved, and food plant families are given below: Cosmopterigidae (1792 sp.): Cosmopterix atte (Cyperaceae); Momphidae (114 sp.): Monpha (Onagraceae); Tortricidae (10,387 sp.): Bactra (Cyperaceae); Clepsis more than 60 species associated with (Cyperaceae) Choristoneura and Archips (Typhaceae); Pyralidae (5921 sp.): Acola (Amaranthaceae); and Crambidae (9655 sp.) within subfamily Acentropinae according to Cover and Bogan (2015) most of the 730 species worldwide are aquatic: Acentria, Argyractis, Cataclysta, Donacaula, Elophila, Hygraula, Neargyractis, Niphograpta, Nomophila, Nymphula, Ostrinia, Oxyelophia, Parapoynx, Petrophila, Samea, Sameodes, Schoenobius, Thalia, Usingeriessa, and Xubida. Their food plant families are Alismataceae, Amaranthaceae, Araceae, Callitrichaceae, Cannaceae, Ceratophyllaceae, Characeae, Cyperaceae, Gramineae, Haloragaceae, Hydrocharitaceae, Lemnaceae, Marantaceae, Nelumbonaceae, Nymphaeaceae, Poaceae, Polygonaceae , Pontederiaceae, Potamogetonaceae, Salviniaceae, Sparganiaceae, Typhaceae, and Zosteraceae.
Sphingidae (1463 sp.): Eumorpha (Onagraceae), Erebidae (24,600 sp. including Arctiidae and Lymantriidae): Paracles, and Noctuidae : Bellura (Cambombaceae, Gramineae, Hydrocharitaceae, Lentibulariaceae, Nymphaceae, Pontederiaceae, Typhaceae).
As a whole there are not known defensive mechanisms of aquatic Lepidoptera. Generally Pyralidae larvae are known to regurgitate as a response to disturbance (Dettner 2015). A lot of terrestrial caterpillars feed on toxic plants and are able to tolerate and to enrich these toxins as defense mechanisms (Nishida 2002; Opitz and Müller 2009). In comparing the abovementioned aquatic lepidopteran families and their food plants (plant family level) with defensive mechanisms and toxin sequestration in terrestrial Lepidoptera there was no correspondence at all.
MECOPTERA (scorpionflies, hangingflies; 1% aquatic): There is one relict family (with 8 species) in Australia and South America among 9 families worldwide (600 species; Stork 2018: 757) which is characterized by long, slender aquatic larvae, which resemble Corydalidae or Trichoptera larvae. These representatives of Nannochoristidae have four instars and live in stream benthos and prey upon larvae of Chironomidae and other dipterans (Machado 2018; Cover and Bogan 2015; Pilgrim 1972). Adults of many Mecoptera fall to the ground and show thanatosis when disturbed (Dettner 2015). Very often they emit a malodorous digestive fluid from the mouth when they are irritated. Until now larval defense in Mecoptera is unknown.
9.8 Conclusions
After surveying defense mechanisms of aquatic and semiaquatic water insects and after comparing the results with antipredatory data of Witz (1990) there are some interesting results. Generally there is a high significance of thanatosis, escape, withdrawal, and especially stabile elastic or hard exoskeleton. Comparable to all arthropods (Witz 1990) defensive stridulation is more abundant in water insects and especially distributed in adults of different water beetles or Heteroptera, but also uniquely in larvae of Odonata and Trichoptera. Following result is astonishing: Exclusively notonectid bugs which have secondarily lost their complex thoracic defensive gland exhibit stridulatory organs. Such stridulatory organs are not found in those notonectids with intact thoracic glands. Generally stridulation in water insects seems primarily defensive stridulation, before it is used intraspecifically for alarm or communication.
The presence of hard exoskeletons and spines as observed in larvae of Odonata or Ephemeroptera should not only represent defenses against fishes but also might be due to kairomonal effects which seem to be abundant in freshwater ecosystems (Brönmark and Hansson 2012). Many phenomena concerning such mechanisms of defense have to be investigated in the future and especially in tropical water insects (especially Indo-Malayan or Afrotropical regions, see Stork 2018). Moreover behavior and bionomy of aquatic dipteran larvae are mostly unknown.
Exocrine glands and chemical defense in aquatic insects are not so widespread and abundant as in terrestrial arthropods (Witz 1990) or in marine systems. The only widespread complex gland in adephagan beetles is present within all Adephaga . In addition the thoracic gland of water bugs is primarily present in all Heteroptera. The same applies to the diversity and biosynthetic variations of the constituents. It is remarkable that the ubiquitous defensive system in terrestrial habitats, the quinones, is absolutely absent in water insects (Blum 1981; Dettner and Peters 2010). The extensive use of aromatic compounds and antimicrobics in freshwater in contrast to terrestrial systems is amply demonstrated by comparing adephagan land and water beetles. Since insects do not possess the metabolic capacity to generate the steroid skeleton de novo, steroids are seldom, because precursors must be taken from outside. Therefore it is remarkable that steroids are abundant in prothoracic glands of larger Dytiscidae and the maxillary glands of belostomatid bugs. Three of four steroids from the maxillary gland of belostomatids are also present in prothoracic beetle glands and this convergent evolution might indicate that these qualitatively identical compounds in extremely high concentrations are targeted against larger predators such as fishes or amphibians. Typical defensive mechanisms of terrestrial taxa such as reflex bleeding of toxic hemolymph or sequestration of toxic plant compounds and use for own defense are absolutely missing in water insects. The only case of reflex bleeding in Ephemeroptera seems to be a mechanical defense and has nothing to do with toxic hemolymph. Moreover there are no toxic spines or urticating hairs in aquatic insects. Apart from tabanid larvae and several predatory bugs also venoms and stings in freshwater insects evolved only few times as compared with terrestrial arthropods.
Highly astonishing is the scarcity of defensive mechanisms in aquatic larval stages . Chemical defense by exocrine glands in larval stages is only present in aquatic lampyrids and one trichopteran group. In contrast larvae of Odonata, Plecoptera, Ephemeroptera, and most of Trichoptera lack any defensive chemicals. Also defensive stridulation is rare within larvae; however larval mechanical defense seems widespread. Many larvae of Heteroptera possess abdominal defensive glands; however there exist no chemical or behavioral data. In contrast to Heteroptera larvae the larval dytiscid cephalic gland seems not to be defensive. What are the reasons for missing larval defenses in aquatic freshwater habitats? Larvae are often short-lived (not in Odonata, etc.); therefore biosynthesis of allomones or development of stridulation structures might be too expensive.
As compared with marine or terrestrial systems, in freshwater arthropods we cannot observe any uptake of toxic compounds from plants in order to generate and optimize own chemical defense and increase of fitness. Obviously there exist no trichopteran or aquatic lepidopteran larvae, which would incorporate toxic plant structures in their case or alternatively would incorporate these compounds into their body. In terrestrial systems incorporation of toxic plant constituents is widely distributed and overwhelming at least in lepidopteran larvae (Nishida 2002; Opitz and Müller 2009). In addition there was no record that toxic invertebrates from freshwater such as aposematically colored and toxic water mites or flatworms (Dettner 2010) were fed by carnivorous water insects in order to incorporate toxic chemicals. Finally urticating hairs or allergic reactions which are widespread in terrestrial arthropods are not known in aquatic insects apart from allergic reactions to haemoglobin of certain Chironomidae.
There is another chapter with only few data in aquatic entomology . The significance of microorganisms as producers of behavioral modifying compounds such as allomones or their role as symbionts or parasites of their insect hosts is mainly unknown. In Dytiscidae a rich aerobic, heterotrophic bacterial flora was identified inside the foregut which shows elements of an autochthonous bacterial flora in the foregut (Schaaf and Dettner 1997). Later it was shown that actinomycete species within the crops of beetles play an important role in transformation of food steroid precursors into prothoracic defensive steroids (Dettner 2014). Furthermore the role and significance of intracellular microorganisms in water insects have to be investigated. However their phylogenetic relationships seem interesting: Rickettsia species from Dytiscidae were shown to be related to rickettsial isolates from aquatic Limoniidae (Diptera), Lutzomyia apache (Diptera: Psychodidae), or Hemiclepsis or Torix leeches (Küchler et al. 2009).
As was demonstrated by various publications of Kovac and Maschwitz (1990b, 1991, 2000) secretion grooming is an abundant behavior especially in adult freshwater insects. This behavior prevents contamination of the hydrofuge respiratory regions by bacteria or peritrichic ciliates , which would otherwise lead to loss of the air bubble, wetting of these areas, and finally drowning of the insect. Usually the antimicrobial secretions of complex glands such as pygidial glands of water beetles or thoracic glands of aquatic bugs increase wettability of the hydrophobous integuments of aquatic insects (Dettner 1985). In order to decrease this wettability there must be certain dermal glands which can rapidly produce (maybe through oil-secreting) waterproofing surface areas (Holdgate 1955; Beament 1976). There is considerable knowledge on physicochemical properties of integuments of water-walking arthropods (Bush et al. 2008) in contrast to true water insects which may also show amphibious habits. Certainly the qualitative and quantitative distribution, chemistry and functional role of these dermal glands must be investigated in selected water insects especially with respect to the passage from terrestrial into aquatic environment and vice versa (a), a stable and balanced position within the water (b), and an optimal function of breathing (c). In order to fulfill these various demands as change of terrestrial/aquatic habitats, breathing, care of body, and predator defenses it is conceivable that most exocrine secretions of water insects have a multifunctional significance.
References
Aiken RB (1985) Sound production by aquatic insects. Biol Rev 60:163–211
Aldrich JR (1988) Chemical ecology of the Heteroptera. Annu Rev Entomol 33:211–238
Altincicek B, Vilcincskas A (2007) Analysis of the immune-inducible transcriptome from microbial stress resistant, rat-tailed maggots of the drone fly Eristalis tenax. BMC Genomics 8:326
Andersen NM (1982) The semiaquatic bugs (Hemiptera, Gerromorpha). Phylogeny, adaptations, biogeography and classification. Entomonograph 3:1–455
Andersen NM (1996) Heteroptera: Gerromorpha. Semiaquatic bugs. In: Nilsson A (ed) Aquatic insects of North Europe. Apollo Books, Stenstrup, pp 77–90
Andersen NM, Weir TA (2004) Australian water bugs. Apollo Books, Stenstrup
Angus R (1992) Insecta Coleoptera Hydrophilidae Helophorinae. In: Schwoerbel J, Zwick P (eds) Süßwasserfauna von Mitteleuropa 20/10-2. Fischer, Stuttgart
Anjos-Santos D, Neiss UG, Pessacq P (2018) Superfamily Calopterygidae. In: Hamada N, Thorp JH, Rogers DC (eds) Keys to neotropical hexapoda, Thorp and Covich’s freshwater invertebrates, vol 3. Academic Press, London, pp 449–468
Ardila-Camacho A, Contreras-Ramos A (2018) Order Megaloptera. In: Hamada N, Thorp JH, Rogers DC (eds) Keys to neotropical hexapoda, Thorp and Covich’s freshwater invertebrates, vol III. Academic Press, London, pp 217–227
Armisen D, Refki PN, Crumiere AJJ, Viala S, Toubiana W, Khila A (2015) Predator strike shapes antipredator phenotype through new genetic interactions in water striders. Nat Commun 6:8153
Arndt E, Beutel RG (1995) Larval morphology of Systolosoma solier and Trachypachus motschulsky (Coleoptera: Trachypachidae) with phylogenetic considerations. Ent Scand 26:439–446
Arnett RH, Thomas MC (2001) American Beetles, vol 1. CRC, Boca Raton, FL
Asahina S (1950) On the life history of Epiophlebia superstes (Odonata, Anisozygoptera). Proceedings of the VIIIth Congress of Entomology, Stockholm, pp 337–341
Asgari S, Rivers DB (2010) Venom proteins from endoparsitoid wasps and their role in host-parasite interactions. Annu Rev Entomol 56:313–335
Attygalle AB, Wu X, Ruzicka J, Rao S, Garcia S, Herath K, Meinwald J, Maddison DR, Will KW (2004) Defensive chemicals of two species of Trachypachus motschulski. J Chem Ecol 30:577–588
Avelino-Capistrano F, Pessacq P, Barbosa LS (2018) Order Plecoptera. In: Hamada N, Thorp JH, Rogers DC (eds) Keys to neotropical hexapoda, Thorp and Covich’s freshwater invertebrates, vol 3. Academic Press, London, pp 119–141
Balke M, Hendrich L (2016) 7.6 Dytiscidae Leach, 1815. In: Beutel RG, Kristensen NP (eds) Handbook of zoology, Arthropoda: Insecta. Coleoptera, Beetles, vol 1, 2nd edn. De Gruyter, Berlin, pp 118–140
Balke M, Larson DJ, Hendrich L (1997) A review of the New Guinea species of Laccophilus Leach 1815 with notes on regional melanism (Coleoptera Dytiscidae). Trop Zool 10:295–320
Balke M, Alarie Y, Beutel RG (2018) 7.8 Meruidae Spangler & Steiner, 2005. In: Beutel RG, Kristensen NP (eds) Handbook of zoology, Arthropoda: Insecta. Coleoptera, Beetles, vol 1, 2nd edn. De Gruyter, Berlin, pp 149–158
Bameul F (1989) Description du comportement de camouflage d’un Coléoptère: le déguisement actif de Georissus crenulatus (Coleoptera Georissidae), et proposition d’une nouvelle classification des déguisements chez les invertébrés. Compt Rend Acad Sciences Paris 309(série III):351–356
Barbier M (1990) Marginalin, a substance from the pygidial glands of Dytiscus marginalis (Coleoptera): molecular associations with polyamines in vitro. Z Naturfoschg 45b:1455–1456
Barth R (1960) Ueber die Pygidialdrüse von Enhydrus sulcatus (Wied., 1821) (Coleoptera, Gyrinidae). Mem Inst Oswaldo Cruz 58(2):135–147
Bauernfeind E, Soldan T (2012) The mayflies of Europe (Ephemeroptera). Brill Academic Publishers, Leiden
Bay EC (1974) Predator-prey relationships among aquatic insects. Annu Rev Entomol 19:441–453
Barthlott W, Riede K, Wolter M (1994) Mimicry and ultrastructural analogy between the semi-aquatic grasshopper Paulinia acuminata (Orthoptera: Pauliniidae) and ist foodplant, the water-fern Salvinia auriculata (Filicatae: Salviniaceae). Amazoniana 12:47-58
Beament JWL (1976) The ecology of cuticle. In: Hepburn HR (ed) The insect integument. Elsevier, Amsterdam, pp 359–374
Beaty SR (2015) The Plecoptera of North Carolina: a biologist’s handbook for the identification of stonefly nymphs with standard taxonomic effort levels. Version 4.0. North Carolina Department of Environmental Quality, Division of water resources, Biological Assessment Branch, Raleigh, NC
Beier M (1929) Zur Kenntnis der Lebensweise von Haliplus wehnckei Gerh. Z f Morph Ökol Tiere 14:191–233
Bell WJ, Roth LM, Nalepa CA (2007) Cockroaches: ecology, behavior, and natural history. Hopkins University Press, Baltimore, MD
Benfield EF (1974) Autohemorrhage in two stoneflies (Plecoptera) and its effectiveness as a defense mechanism. Ann Entomol Soc Am 67:739–742
Benton MJ, Pritchard G (1990) Mayfly locomotory Responses to endoparasitic infection and predator presence: the effects on predator Encounter rate. Freshw Biol 23:363-371
Bertrand H (1928) Les Larves et Nymphes des Dytiscides, Hygrobiides, Haliplides. Encyclopédie Entomologique X, Lechevalier, Paris
Betz O (2010) Adhesive exocrine glands in insects: morphology, ultrastructure, and adhesive secretion. In: Byern JV, Grunwald I (eds) Biological adhesive systems. Springer, Wien, pp 111–152
Betz O, Kölsch G (2004) The role of adhesion in prey capture and predator defence in arthropods. Arthropod Struct Dev 33:3–30
Betz O, Koerner L, Dettner K (2018) The Biology of Steninae. In: Betz O, Irmler U, Klimaszewski J (eds) Biology of Rove Beetles (Staphylinidae). Springer, Cham, pp 229–283
Beutel R (1986) Skelet und Muskulatur des Kopfes und Thorax von Hygrobia tarda (Herbst). Ein Beitrag zur Klärung der phylogenetischen Beziehungen der Hydradephaga (Insect: Coleoptera). Stuttg Beitr Naturk Ser A 7 Nr 388:1–54
Beutel RG, Arndt E (2016) Trachypachidae C. G. Thomson, 1857. In: Beutel RG, Kristensen NP (eds) Handbook of zoology, Arthropoda: Insecta. Coleoptera, Beetles, vol 1, 2nd edn. De Gruyter, Berlin, pp 159–162
Beutel RG, Leschen AB (eds) (2016) Handbook of zoology, Arthropoda: Insecta. Coleoptera, Beetles, vol 1, 2nd edn. De Gruyter, Berlin
Beutel RG, Roughley RE (1993) Phylogenetic analysis of Gyrinidae based on characters of the larval head (Coleoptera: Adephaga). Entomo Scand 24:459–468
Beutel RG, Roughley RE (2016) 7.1 Gyrinidae Latreille, 1810. In: Beutel RG, Kristensen NP (eds) Handbook of zoology, Arthropoda: Insecta. Coleoptera, Beetles, vol 1, 2nd edn. De Gruyter, Berlin, pp 80–89
Beutel RG, Ruhnau S (1990) Phylogenetic analysis of the genera of Haliplidae (Coleoptera) based on characters of adults. Aquat Insects 12:1–17
Beutel RG, Balke M, Steiner ES Jr (2006) The systematic position of Meruidae (Coleoptera, Adephaga) and the phylogeny of the smaller aquatic adephagan beetle families. Cladistics 22:102–131
Beutel RG, Friedrich F, Aspöck U (2010) The larval head of Nevrothidae and the phylogeny of Neuroptera (Insecta). Zool J Linnean Soc 158:533–562
Beutel RG, Friedrich F, Ge SQ, Yang XK (2014) Insect morphology and phylogeny. De Gruyiter, Berlin
Beutel RG, Balke M, Ribera I (2016) 3.1. Aspidytidae Ribera, beutel, Balke and Vogler, 2002. In: Beutel RG, Kristensen NP (eds) Handbook of zoology, Arthropoda: Insecta. Coleoptera, Beetles, vol 1, 2nd edn. De Gruyter, Berlin, pp 21–28
Blum MS (1981) Chemical defenses of Arthropods. Academic Press, New York
Blunck H (1923) Die Entwicklung des Dytiscus marginalis L. vom Ei bis zur Imago. 2.Teil. Die Metamorphose (B Das Larven und das Puppenleben). Z Wiss Zool Abt A 121:172–392
Bott HR (1928) Beiträge zur Kenntnis von Gyrinus natator substriatus Steph. Z Morph Ökol Tiere 10:207–306
Boyero L, Rincon PA, Bosch J (2006) Case selection by a limnephilid caddisfly (Potamophylax latipennis (Curtis)) in response to different predators. Behav Ecol Sociobiol 59:364–372
Brancucci M (1975) Die Dytisciden und die Oberflächenspannung des Wassers (Coleoptera). Deutsche Entomol Z NF 24:423–424
Brancucci M (1985) A Review of the biology and structure of Geodessus besucheti Brancucci (Coleoptera, Dytiscidae). Proc Acad Nat Sci Phil 137:29–32
Branham MA (2010) 4.15 Lampyridae. In: Kristenasen NP, Beutel RG (eds) Handbook of zoology, Coleoptera, Beetles, vol 2, 2nd edn. De Gruyter, Berlin, pp 141–149
Brittain JE (1990) Life history strategies in Ephemeroptera and Plecoptera. In: Campbell IC (ed) Mayflies and stoneflies. Kluwer, Amsterdam, pp 1–12
Brönmark C, Hansson LA (2012) Chemical ecology in aquatic systems. Oxford University Press, Oxford
Brossut R (1993) Allomonal secretions in cockroaches. J Chem Ecol 9:143–158
Burks RL, Lodge DM (2002) Cued in: advances and opportunities in freshwater chemical ecology. J Chem Ecol 28:1901–1917
Burse A, Boland W (2015) RNAi based functional analysis of biosynthetic enzymes and transport proteins involved in the chemical defense of juvenile leaf beetles. In: Hoffmann KH (ed) Insect molecular biology and ecology. CRC, Boca Raton, FL, pp 350–375
Bush JWM, Hu DL, Prakash M (2008) The integument of water-walking arthropods: form and function. Adv Insect Physiol 34:117–192
Cao C-Q, Liu ZW, Chen SZ, Tong C (2012) Swimming behavior of the aquatic larva of Neoneuromus ignobilis (Megaloptera: Corydalidae: Corydalinae). Acta Entomol Sin 55:133–138
Chang SI (1966) Some physiological observations on two aquatic Collembola. Trans Am Microsc Soc 85:359–371
Chapman JC, Lockley WJS, Rees HH, Goodwin TW (1977) Stereochemistry of olefine bond formation in defensive steroids of Acilius sulcatus (Dytiscidae). Eur J Biochem 81:293–298
Chivers DP, Smith RJF (1998) Chemical alarm signalling in aquatic predator-prey systems: a review and prospectus. Ecoscience 5:338–352
Cipola NG, Silva DD, Bellini BC (2018) Class Collembola. In: Hamada N, Thorp JH, Rogers DC (eds) Keys to neotropical hexapoda, Thorp and Covich’s freshwater invertebrates, vol 3. Academic Press, London, pp 11–55
Classen R, Dettner K (1983) Pygidial defensive titer and population structure of Agabus bipustulatus L. and Agabus paludosus F (Coleoptera, Dytiscidae). J Chem Ecol 9:201–209
Corbet PS (1999) Dragonflies: behaviour and ecology of odonata. Harley Books, Colchester
Cordoba-Aguilar A (2008) Dragonflies and damselflies – model organisms for ecological and evolutionary research. Oxford University Press, Oxford
Cover MR, Bogan MT (2015) Minor Insect orders. In: Ecology and general biology: Thorp and Covich’s freshwater invertebrates, vol I, 4th edn. Academis Press/Elsevier, Amsterdam, pp 1059–1072
Crespo FG (2011) A review of chemosensation and related behavior in aquatic insects. J Insect Sci 11:62; insectscience.org/11.62
Crowell HH (1946) Notes on an amphibious cockroach from the republic of Panama. Entomol News 75:171–172
Curio E (1976) The ethology of predation. Springer, Berlin
Darlington PJ Jr (1929) Notes on the habits of Amphizoa. Psyche 36:383–385
Dazzini Valcurone M, Pavan M (1980) Glandole pigidiali e secrezione difensive dei Carabidae (Insecta Coleoptera). Pubbl Ist Entomol Univ Pavia 12:1–36
Dazzini-Valcurone M, Pavan M (1978) Scent glands and defensive secretions of Rhynchota. Pubbl Ist Entomol Univ Pavia 5:1–46
De Carlo JM (1961) Anatomia e histologia de las glandulas maxilares o cefalicas de Belostoma elegans (Mayr). Actas Trabajos Congr Sudamericano LaPlata Zool 5:127–141
De Carlo JM, Pellerano GN, Maggese MC (1973) Anatomia microscopica del tracto digestivo y glandulas salivales de Lethocerus mazzai (Hemiptera, Belostomatidae). Physis Buenos Aires Sect C 32(85):295–314
De Marzo L, Nilsson AN (1986) Morphological variation and fine structure of some head structures of Dytiscidae (Coleoptera). Entomol Basil 11:29–42
Dettner K (1985) Ecological and phylogenetic significance of defensive compounds from pygidial glands of hydradephaga (Coleoptera). Proc Acad Nat Sci Phil 137:156–171
Dettner K (1987) Chemosystematics and evolution of beetle chemical defenses. Annu Rev Entomol 32:17–48
Dettner K (1990) Chemische Abwehr bei der ursprünglichen Käferfamilie der Amphizoidae- ein beitrag zur Evolution der Pygidialdrüse der Hydradephaga. Mitt D Ges Allg Angew Entomol 7:519–526
Dettner K (1993) Defensive secretions and exocrine glands in free-living staphylinid beetles-Theit bearing on phylogeny (Coleoptera: Staphylinidae). Biochem Syst Ecol 21:143–162
Dettner K (1997) Inter- and intraspecific transfer of toxic Insect compound cantharidin. Ecol Stud 130:115–145
Dettner K (1997a) Insecta: Coleoptera: Noteridae. In: Schwoerbel J, Zwick P (eds) Süßwasserfauna von Mitteleuropa 20/2,3,4. Fischer, Stuttgart, pp 97–126
Dettner K (1997b) Insecta: Coleoptera: Hygrobiidae. In: Schwoerbel J, Zwick P (eds) Süßwasserfauna von Mitteleuropa 20/2,3,4. Fischer, Stuttgart, pp 127–147
Dettner K (2010) Chemical defense and toxins of lower terrestrial and freshwater animals. In: Mori K (ed) Comprehensive natural products II Chemistry and Biology, Chemical Ecology, vol 4. Elsevier, Amsterdam, pp 387–410
Dettner K (2014) Chapter 6 Chemical Ecology and Biochemistry of Dytiscidae. In: Ecology, systematics, and the natural history of predacious diving beetles (Coleoptera: Dytiscidae). Springer, Dordrecht, pp 235–306
Dettner K (2015) Toxins, defensive compounds and drugs from Insects. In: Hoffmann KH (ed) Insect molecular biology and ecology. CRC, Boca Raton, FL, pp 39–93
Dettner K (2016a) 7.3 Noteridae Thomson, 1857. In: Beutel RG, Kristensen NP (eds) Handbook of zoology, Arthropoda: Insecta. Coleoptera, Beetles, vol 1, 2nd edn. De Gruyter, Berlin, pp 98–107
Dettner K (2016b) 7.4 Amphizoidae LeConte, 1853. In: Beutel RG, Kristensen NP (eds) Handbook of zoology, Arthropoda: Insecta. Coleoptera, Beetles, vol 1, 2nd edn. De Gruyter, Berlin, pp 107–112
Dettner K (2016c) 7.5 Hygrobiidae Régimbart, 1879, 112-118. In: Beutel RG, Kristensen NP (eds) Handbook of zoology, Arthropoda: Insecta. Coleoptera, Beetles, vol 1, 2nd edn. De Gruyter, Berlin
Dettner K, Böhner M (2009) Dier Pygidialdrüse der Wassertreter (Coleoptera: Haliplidae): Morphologie, Chemie, Funktion und phylogenetische Bedeutung. Contr Nat Hist 12:437–460
Dettner K, Liepert C (1994) Chemical mimicry and camouflage. Annu Rev Entomol 39:129–154
Dettner K, Peters W (2010) Lehrbuch der Entomologie Teil 1 & 2. Spektrum, Heidelberg
DeWalt RE, Kondratieff B, Sandberg JB (2015) Order Plecoptera. In: Ecology and general biology: Thorp and Covich’s freshwater invertebrates, vol 1, 4th edn. Academic Press/Elsevier, Amsterdam, pp 933–949
Dijkstra KDB, Monaghan MT, Pauls SU (2014) Freshwater biodiversity and aquatic insect diversification. Annu Rev Entomol 59:143–163
Edmunds M (1974) Defence in animals. Longman, Harlow
Edwards JG (1953) The real source of Amphizoa-secretions. Coleopt Bull 7:4
Eisner T, Aneshansley DJ (2000) Chemical defense: aquatic beetle (Dineutes hornii) vs. fish (Micropterus salmoides). PNAS 97:11313–11318
Eisner T, Eisner M, Siegler M (2005) Secret weapons. Belknap Press, Cambridge
Elliott JM (2008) The ecology of riffle beetles (Coleoptera: Elmidae). Fr Rev 1:189–203
Evans DL, Schmidt JO (1990) Insect defenses. Adaptive mechanisms and strategies of prey and predators. State University of New York Press, Albany
Faasch H (2015) Identification guide to aquatic and semiaquatic Diptera larvae. DGL Arbeitshilfe 1-2015. DGL, Hardegesen
Falkenström G (1926) Beiträge zur Kenntnis der Biologie der Haliplidae und der Metamorphose von Haliplus immaculatus Gerh. Entomol Tidskr Arg 47(1):1–28
Ferrari MCO, Wisenden BD, Chivers DP (2010) Chemical ecology of predator-prey interactions in aquatic ecosystems: a review and prospectus. Can J Zool 88:698–724
Ferry EE, Hopkins GR, Stokes AN, Mohammadi S, Brodie ED Jr, Gall BG (2013) Do all portable cases constructed by caddisfly larvae function in defense ? J Insect Sci 13:5
Fitzgerald VJ (1987) Social behavior of adult whirligig beetles (Dineutes nigrior and D. discolor Coleoptera: Gyrinidae). Am Midl Nat 118:439–447
Fleck G, Hamada N, Carvalho AL (2009) A remarkable new genus and species of dragonfly (Odonata: Anisoptera: Libellulidae) from Brazil and notes on its bionomics and phylogenetic affinities. Ann Soc Entomol Fr (ns) 45:275–284
Forbes MR, Robb T (2008) Testing hypotheses about parasite-mediated selection using odonate hosts. In: Cordoba-Aguilar A (ed) Dragonflies and damselflies – model organisms for ecological and evolutionary research. Oxford University Press, Oxford, pp 175–188
Forsyth DJ (1968) The structure of the defence glands in the Dytiscidae, Noteridae, Haliplidae and Gyrinidae (Coleoptera). Trans R Entomol Soc Lond 120:159–181
Forsyth DJ (1970) The structure of the defence glands of Cicindelidae, Amphizoidae, and Hygrobiidae (Insecta: Coleoptera). J Zool Lond 160:51–59
Forsyth DJ (1972) The structure of the pygidial defence glands of Carabidae (Coleoptera). Transact Zool Soc Lond 32:249–309
Franciscolo ME (1979) Coleoptera Haliplidae, Hygrobiidae, Gyrinidae, Dytiscidae, Fauna d’Italia, vol 14. Calderini, Bologna
Fu X, Vencl FC, Nobuyoshi O, Meyer-Rochow VB, Lei C, Zhang Z (2007) Structure and function of the eversible glands of the aquatic firefly Luciola lei (Coleoptera: Lampyridae). Chemoecology 17:117–124
Fu X, Meyer-Rochow VB, Tylwer J, Suzuki H, de Cock R (2009) Structure and function of the eversible organs of several genera of larval firefly (Coleoptera: Lampyridae). Chemoecology 19:155–168
Fusari LM, Dantas GPS, Pinho LC (2018) Order Diptera. In: Hamada N, Thorp JH, Rogers DC (eds) Keys to neotropical hexapoda, Thorp and Covich’s freshwater invertebrates, vol 3. Academic Press, London, pp 607–623
Gerhart DJ, Bondura ME, Commito JA (1991) Inhibition of sunfish feeding by defensive steroids from aquatic beetles: structure-activity relationships. J Chem Ecol 17:1363–1370
Giglio A, Brandmayr P, Dalpozzo R, Sindona G, Tagarelli A, Talarico F, Brandmayr TZ, Ferrero EA (2009) The defensive secretion of Carabus lefebrei Dejean 1826 pupa (Coleoptera, Carabidae): gland ultrastructure and chemical identification. Microsc Res Tech 72:352–361
Gorb SN (2007) Smooth attachment devices in insects: functional morphology and biomechanics. Adv Insect Physiol 34:81–115
Graca MB, Solis MA (2018) Order Lepidoptera. In: Hamada N, Thorp JH, Rogers DC (eds) Keys to neotropical hexapoda, Thorp and Covich’s freshwater invertebrates, vol 3. Academic Press, London, pp 325–338
Grant PM (2001) Mayflies as food. In: Dominguez E (ed) Trends in research in Ephemeroptera and Plecoptera. Kluwer, Amsterdam, pp 107–124
Grosch DS (1988) Genetic research on braconid wasps. Adv Genet 35:109–184
Gustafson GT, Miller KB (2016) Revision of the Southeast Asian whirligig beetles genus Porrorhynchus Laporte, 1835 (Coleoptera: Gyrinidae: Gyrininae: Dineutini). Colept Bull 70:675–714
Gutjahr ALN, Braga CES (2018) Order Orthoptera. In: Hamada N, Thorp JH, Rogers DC (eds) Keys to neotropical hexapoda, Thorp and Covich’s freshwater invertebrates, vol 3. Academic Press, London, pp 143–168
Haddad V, Schwartz EF, Schwartz CA (2010) Bites caused by giant water bugs belonging to Belastomatidae family (Hemiptera, Heteroptera) in humans: a report of seven cases. Wilderness Environ Med 21:130–133
Hamada N, Thorp JH, Rogers DC (2018) Keys to neotropical Hexapoda, Thorp and Covich’s freshwater invertebrates, vol 3. Academic Press, London
Hansen M (1991) The hydrophiloid beetles. Biolog Skrifter 40:1–368
Hanson P, Gauld LD (eds) (1995) The hymenoptera of Costa Rica. Oxford University Press, Oxford
Hauser R (1985) Ein Diapausesekret bei Wasserläufern (Hemiptera, Gerridae). Mitt Schweiz Entomol Ges 85:511–525
Heckman CW (2018) Ecological strategies of aquatic insects. CRC, Baton Rouge
Heinrich B, Vogt FD (1980) Aggregation and foraging behavior of whirligig beetles (Gyrinidae). Behav Ecol Sociobiol 7:179–186
Hennig W (1968) Die Larvenformen der Dipteren. Akademieverlag, Berlin
Henrikson BI, Stenson JAE (1993) Alarm substance in Gyrinus aeratus (Coleoptera, Gyrinidae). Oecologia 93:191–194
Hershey AE, Dodson SI (1987) Predator avoidance by Cricotopus cyclomorphosis and the importance of being big and hairy. Ecology 68:913–920
Hickman JR (1931) Contribution to the biology of the Haliplidae (Coleoptera). Ann Entomol Soc Am 24:129–142
Hilker M, Meiners T (2002) Chemoecology of Insect eggs and egg deposition. Blackwell, Berlin
Hinton HE (1981) Biology of insect eggs, vol 1-3. Pergamon Press, Oxford
Holdgate MW (1955) The wetting of insect cuticles by water. J Exper Biol 32:591–617
Honkavaara J, Rantala MJ, Suhonen J (2009) Mating status, immune defence, and multi-parasite burden in the damselfly Coenagrion armatum. Entomol Exp Appl 132:165–171
Honma A, Oku S, Nishida T (2006) Adaptive significance of death feigning posture as a specialized inducible defense against gape-limited predators. Proc Biol Sci 273:1631–1636
Hopkin SP (1997) Biology of the Springtails (Insecta: Collembola). Oxford University Press, Oxford
Horta MAP, de Melo AL, Bertoluci J (2010) A possible case of mimicry involving a heteropteran insect and an anuran tadpole. Herpetol Bull 114:1-7
Hübner G, Völkl W, Francke W, Dettner K (2002) Mandibular gland secretions in alloxystine wasps (Hymenoptera, Cynipoidea, Charipidae): do ecological or phylogenetic constraints influence occurrence or composition ? Biochem Syst Ecol 30:505–523
Ivarsson P, Henrikson BI, Stenson JAE (1996) Volatile substances in the pygidial secretion of gyrinid beetles (Coleoptera: Gyrinidae). Chemoecology 7:191–193
Jäch MA, Balke M (2008) Global diversity of water beetles (Coleoptera) in freshwater. Hydrobiologia 595:419–442
Jäch MA, Beutel RG, Delgado JA, Diaz JA (2016) 14.1 Hydraenidae Mulsant, 1844. In: Beutel RG, Kristensen NP (eds) Handbook of zoology, Arthropoda: Insecta. Coleoptera, Beetles, vol 1, 2nd edn. De Gruyter, Berlin, pp 316–345
Jacobs W, Renner M (1974) Taschenlexikon zur Biologie der Insekten. Fischer, Stuttgart
Johansson F, Mikolajewski DJ (2008) Evolution of morphological defenses. In: Cordoba-Aguilar A (ed) Dragonflies and damselflies- Model organisms for ecological and evolutionary Research. Oxford University. Press, Oxford, pp 70–74
Johnson MD (1991) Behavioural ecology of larval dragonflies and damselflies. Trends Ecol Evol 6:8–13
Kadavy DR, Hornby JM, Haverkost T, Nickerson KW (2000) Natural antibiotic resistance of bacteria isolated from larvae of the oil fly Helaeomyia petrolei. Appl Environ Microbiol 66:4615–4619
Kasumyan AO, Doving KB (2003) Taste preferences in fishes. Fish Fish 4:289–347
Keeling CI, Plettner E, Slessor KN (2004) Hymenopteran Semiochemicals. In: Schulz S (ed) Topics in current chemistry, vol 1. Springer, Berlin, pp 133–177
Kerfoot WC, Sih A (1987) Predation-direct and indirect impacts on aquatic communities. University Press of New England, Hanover
Kicklighter C (2012) Chemical defences against predators. Oxford University Press, Oxford, pp 236–249
Klausnitzer B (1996) Käfer im und am Wasser. Westarp, Magdeburg & Spektrum, Heidelberg
Kovac D (1993) A quantitative analysis of secretion-grooming behaviour in the water bug Plea minutissima Leach (Heteroptera, Pleidae): Control by abiotic factors. Ethology 93:41–61
Kovac D, Maschwitz U (1990a) Sekretputzen bei Ilyocoris cimicoides (Heteroptera, Naucoridae). Nachr Entomol Ver Apollo Frankfurt NF 11:155–164
Kovac D, Maschwitz U (1990b) Secretion-grooming in aquatic beetles (Hydradephaga): a chemical protection against contamination of the hydrofuge respiratory region. Chemoecology 1:131–138
Kovac D, Maschwitz U (1991) The function of the metathoracic scent gland in corixid bugs (Hemiptera, Corixidae): Secretion-grooming on the water surface. J Nat Hist 25:331–340
Kovac D, Maschwitz U (2000) Protection of hydrofuge respiratory structures against detrimental microbic growth by terrestrial grooming water beetles (Coleoptera: Hydrophilidae, Hydraenidae, Dryopidae, Elmidae, Curculionidae). Entomol Gener 24:277–292
Kovac D, Maschwitz U, Hirschel K (1991) Plea minutissima (Pleidae)-Sekretputzen Secretion-grooming. Film E 3101. Publ Wiss Film Biol 21:13–20
Küchler SM, Kehl S, Dettner K (2009) Characterization and localization of Rickettsia sp. in water beetles of genus Deronectes (Coleoptera; Dytiscidae). FEMS Microbiol Ecol 68:201–211
Lampert W, Sommer U (2007) Limnoecology, 2nd edn. Oxford University Press, Oxford
Lancaster J, Downes BJ (2013) Aquatic entomology. Oxford University Press, Oxford
Lang C, Seifert K, Dettner K (2012) Skimming behaviour and spreading potential of Stenus species and Dianous coerulescens (Coleoptera: Staphylinidae). Naturwissenschaften 99:937–947
Larson DJ (1996) Color patterns of dytiscine water beetles (Coleoptera: Dytiscidae, Dytiscinae) of arroyos, billabongs and wadis. Coleopt Bull 50:231–235
Larson DJ, Pritchard G (1974) Organs of possible stridulatory function in water-beetles (Coleoptera: Dytiscidae). Coleopt Bull 28:53–63
Lawrence JW (1991) Order Coleoptera. In: Stehr FW (ed) Immature insects, vol 2. Kendall & Hunt, Dubuque, pp 144–185
Leschen RAB, Beutel RG, Lawrence JF (eds) (2010) Handbook of zoology, Arthropoda: Insecta. Coleoptera, Beetles, vol 2. De Gruyter, Berlin
Lokensgard J, Smith RL, Eisner T, Meinwald J (1993) Pregnanes from defensive glands of a belostomatid bug. Experientia 49:175–176
Lucht W (1974) Studien über die Biologie und Ethologie des Orectochilus villosus Müll. (Col., Gyrinidae). Entomol Blätter 70:12–34
Lytle DA (2015) Order Hemiptera. In: Ecology and general biology: Thorp and Covich’s freshwater invertebrates, vol 1, 4th edn. Academic Press/Elsevier, Amsterdam, pp 951–963
Machado RJP (2018) Order Mecoptera. In: Hamada N, Thorp JH, Rogers DC (eds) Keys to neotropical hexapoda, Thorp and Covich’s freshwater invertebrates, vol 3. Academic Press, London, pp 349–351
Maddison DR, Moore W, Baker MD, Ellis TM, Ober KA, Cannone JJ, Gutell RR (2008) Monophyly of terrestrial adephagan beetles as indicated by three nuclear genes I (Coleoptera: Carabidae and Trachypachidae). Zool Scr 38:43–62
Marcussen BM, Axelsen JA, Toft S (1999) The value of two Collembola species as food for a linyphiid spider. Entomol Exp Appl 92:29–36
Maschwitz U (1971) Wasserstoffperoxid als Antiseptikum bei einer Wasserwanze. Naturwissenschaften 68:572
Masters WM (1979) Insect disturbance stridulation: its defensive role. Behav Ecol Sociobiol 5:187–200
McCormick S, Polis GA (1982) Arthropods that prey on vertebrates. Biol Rev 57:29–58
McPeek MA (2000) Predisposed to adapt? Clade-level differences in characters affecting swimming performance in damselflies. Evolution 54:2072–2080
Merritt R, Cummins KW (1984) An introduction to the aquatic insects of North America, 1. edn. Kandall/Hunt, Dubuque
Merritt R, Cummins KW (1996) An introduction to the aquatic insects of North America, 3. edn. Kandall/Hunt, Dubuque
Messner B (1969) Zur Morphologie und Histologie einer neuen Drüse in den Mitteltibien von Tridactylus variegatus Taltr. 1809 (Orthoptera). Wiss. Z. Ernst-Moritz-Arndt-Universität Greifswald XVIII. Math Naturwiss 18:93–98
Millar JG (2005) Pheromones of true bugs. Top Curr Chem 240:37–84
Miller KB, Bergsten J (2012) Phylogeny and classification of whirligig beetles (Coleoptera: Gyrinidae): relaxed-clock model outperforms parsimony and time-free Bayesian analyses. Syst Entomol 37:706–746
Miller KB, Bergsten J (2016) Diving beetles of the world. Johns Hopkins University Press, Baltimore, MD
Miller JR, Mumma RO (1976a) Physiological activity of water beetle defensive agents. I Toxicity and anesthetic activity of steroids and norsesquiterpenes administered in solution in the minnow Pimephales promelas Raf. J Chem Ecol 2:115–130
Miller JR, Mumma RO (1976b) Physiological activity of water beetle defensive agents. II Absorption of selected anesthetic steroids and norsesquiterpenes across gill membranes of the minnow Pimephales promelas Raf. J Chem Ecol 2:131–146
Miller JR, Hendry LB, Mumma RO (1975) Norsesquiterpenes as defensive toxins of whirligig beetles (Coleoptera: Gyrinidae). J Chem Ecol 1:59–82
Miranda GFG, Rotheray G (2018) Family Syrphidae. In: Hamada N, Thorp JH, Rogers DC (eds) Keys to neotropical hexapoda, Thorp and Covich’s freshwater invertebrates, vol 3. Academic Press, London, pp 779–783
Moore KA, Williams DD (1990) Novel strategies in the complex defense repertoire of a stonefly (Pteronarcys dorsata) nymph. Oikos 57:49–56
Moreira FFF, Rodrigues HDD, Sites RW, Cordeiro IRS, Magalhaes OM (2018) Order Hemiptera. In: Hamada N, Thorp JH, Rogers DC (eds) Keys to neotropical hexapoda, Thorp and Covich’s freshwater invertebrates, vol 3. Academic Press, London, pp 175–216
Moriya N (1989) Morphology and histology of the scent glands of the pigmy mole cricket, Tridactylus japonicus de Haan (Orthoptera: Tridactylidae). Appl Entomol Zool 24:161–168
Moriya N, Ichinose T (1988) Function of the scent from the pigmy mole crickets, Tridactylus japonicus de Haan (Orthoptera: Tridactylidae). Appl Entomol Zool 23:321–328
Morse JC (2003) Trichoptera (Caddisflies). In: Resh VC, Cardé RT (eds) Encyclopedia of insects. Academic Press, Amsterdam, pp 1154–1151
Morse JC (2017) Biodiversity of aquatic insects. In: Foottit RG, Adler PH (eds) Insect biodiversity, vol 1. Wiley-Blackwell, Oxford, pp 205–227
Mousseau T, Roughley RE (2007) Taxonomy, classification, reconstructed phylogeny and biogeography of Nearctic species of Brychius Thomson (Coleoptera: Haliplidae). Coleopt Bull 61:351–397
Navarrete-Heredia JL, Cortés-Aguilar J, Beutel RG (2005) New findings on the enigmatic beetle family lepiceridae (Coleoptera: Myxophaga). Entomol Abh 62:193–201
Neiss UG, Fleck G, Pessacq P, Tennessen KJ (2018) Odonata: Superfamily Libelluloidea. In: Hamada N, Thorp JH, Rogers DC (eds) Keys to neotropical hexapoda, Thorp and Covich’s freshwater invertebrates, vol 3. Academic Press, London, pp 399–447
Nesemann H, Shah RDT, Shah DN, Sharma S (2010) First records of Rhicnoda natatrix and Ricnoda rugosa (Blattodea: Blaberidae) from Nepal and India (Maharashtra) with notes on habitat quality. J Threat Taxa 2:648–652
Newhart AT, Mumma RO (1979) Defensive secretions of three species of Acilius (Coleoptera, Dytiscidae) and their seasonal variations as determined by high-pressure liquid chromatography. J Chem Ecol 5:643–652
Nishida R (2002) Sequestration of defensive substances from plants by Lepidoptera. Annu Rev Entomol 47:57–92
Nislow KH, Molles MC Jr (1993) The influence of larval case design on vulnerability of Limnephilus frijole (Trichoptera) to predation. Freshw Biol 29:411–417
Okada YK (1928) Two Japanese aquatic glowworms. Trans R Entomol Soc Lond 76:101–108
Oliva A (1992) Cuticular microstructure in some genera of Hydrophilidae (Coleoptera) and their phylogenetic significance. Bull Inst R Sci Nat Belg Entomol 62:33–56
Oosterbroek P (2006) The European families of the Diptera. KNNV Publishing, Utrecht
Opitz SEW, Müller C (2009) Plant chemistry and insect sequestration. Chemoecology 19:117–154
Otto, C. (1984) Coloração de cabeça adaptativa em larvas de caddis produtoras de casos. Freshw Biol 14:317–321.
Parfin S (1952) Notes on the Bionomics of Corydalus cornutus (Linné), Chauliodes rastricorn is Rambur. C. pectinicornis (Linné) and Neohermes spec. The American Midl. Naturalist 47:426-434.
Pasteels JM, Rowell-Rahier M, Braekman JC, Daloze D (1984) Chemical defences in leaf beetles and their larvae: the ecological, evolutionary and taxonomic significance. Biochem Syst Ecol 12:395–406
Pasteels JM, Duffey S, Rowell-Rahier M (1990) Toxins in chrysomelid beetles: Possible evolutionary sequence from de novo synthesis to derivation from food-plant chemicals. J Chem Ecol 16:211–222
Pavlovsky EN (1922) On the biology and structure of the larvae of Hydrophilus caraboides L. J Cell Sci S2-66:627-655
Pawlowsky EN (1927) Gifttiere und ihre Giftigkeit. Gustav Fischer, Jena
Peckarsky B (1980) Predator-prey interactions between stoneflies and mayflies: Behavioral observations. Ecology 61:932–943
Peckarsky BL (1984) Predator-Prey interactions among aquatic insects. In: Resh VH, Rosenberg DM (eds) The ecology of aquatic insects. Praeger, New York, pp 196–253
Peckarsky B (1987) Mayfly cerci as defense against stonefly predation: deflection and detection. Oikos 48:161–170
Peckarsky B (1996) Alternative predator avoidance syndromes of stream-dwelling mayfly larvae. Ecology 77:1888–1905
Peddle SM, Larson DJ (1999) Cuticular evidence of traumatic experiences of water beetles (Coleoptera: Dytiscidae, Hydrophilidae). Coleopt Bull 53:42–51
Perkins PD (1997) Life on the effective bubble: Exocrine secretion delivery systems (ESDS) and the evolution and classification of beetles in the family Hydraenidae (Insecta, Coleoptera). Ann Carnegie Museum 66:89–207
Pes AM, Holzenthal RW, Sganga JV, Santos APM, Barcelos Silva P, Camargos LM (2018) Order Trichoptera. In: Hamada N, Thorp JH, Rogers DC (eds) Keys to neotropical hexapoda, Thorp and Covich’s freshwater invertebrates, vol 3. Academic Press, London, pp 237–324
Pescador ML, Peters WL (1974) The life history and ecology of Baetisca rogersi Berner (Ephemeroptera: Baetiscidae). Bull Fla State Mus Biol Sci 17:151
Pescador ML, Richard BA, Hubbard MD, Staniczek AH (2009) Evolution of Baetiscidae (Ephemeroptera): current state of knowledge of the family. Aquat Insects 31(Suppl 1):137–147
Pessacq P, Muzon J, Neiss UG (2018) Order Odonata. In: Hamada N, Thorp JH, Rogers DC (eds) Keys to neotropical Hexapoda, Thorp and Covich’s freshwater invertebrates, vol 3. Academic Press, London, pp 355–494
Pierce CL (1988) Predator avoidance, microhabitat shift, and risk-sensitive foraging in larval dragonflies. Oecologia 77:81–90
Pilgrim RL (1972) The aquatic larva and the pupa of Choristella philpotti Tillyard 1917 (Mecoptera: Nannochoristidae). Pac Insects 14:151–168
Pitaluga di Godoi FS, Pujol-Luz JR (2018) Family Stratiomyidae. In: Hamada N, Thorp JH, Rogers DC (eds) Keys to neotropical hexapoda, Thorp and Covich’s freshwater invertebrates, vol 3. Academic Press, London, pp 717–777
Puchkova LV (1965) Maxillary glands of Heteroptera, their function and origin. Zool Zh Akad Nauk SSSR 44:1801–1808
Quicke DLJ (1997) Parasitic wasps. Chapman & Hall, London
Reichholf J (1970) Untersuchungen zur Biologie des Wasserschmetterlings Nymphula nymphaeata L. (Lepidoptera, Pyralidae). Int Rev ges Hydrobiol 55:687-728
Rentz DCF, Su YN (2003) Orthoptera. In: Resh VH, Cardé RT (eds) Encyclopedia of insects. Academic Press, Amsterdam, pp 827–839
Resh VH, Rosenberg DM (1984) The ecology of aquatic insects. Praeger, New York
Rivosecchi L (1984) Ditteri (Diptera) in Guide per il riconoscimento delle specie animali delle aque interne Italiane. 177 pp, Consiglio Nazionale delle Ricerche AQ/1/206
Ronderos MM, Diaz F, Marino PI, Fereira-Keppler RL (2018) Family Ceratopogonidae. In: Hamada N, Thorp JH, Rogers DC (eds) Keys to neotropical hexapoda, Thorp and Covich’s freshwater invertebrates, vol 3. Academic Press, London, pp 625–659
Rupprecht R (1971) Der Kokonbau der Taumelkäferlarve (Gyrinus substriatus Stephens). MZ Naturwiss Arch 10:195–202
Ruxton GD, Sherratt TN, Speed MP (2004) Avoiding attack. The evolutionary ecology of crypsis, warning signals, and mimicry. Oxford University Press, Oxford
Salles FF, Dominguez E, Molineri C, Boldrini R, Nieto C, Dias LG (2018) Order Ephemeroptera. In: Hamada N, Thorp JH, Rogers DC (eds) Keys to neotropical hexapoda, Thorp and Covich’s freshwater invertebrates, vol 3. Academic Press, London, pp 61–117
Sartori M, Brittain JE (2015a) Order Ephemeroptera. In: Ecology and general biology: Thorp and Covich’s freshwater invertebrates, vol 1, 4th edn. Academic Press/Elsevier, Amsterdam, pp 873–891
Sartori M, Brittain JE (2015b) Order Ephemeroptera 873–891, in: Ecology and General Biology: Thorp and Covich’s Freshwater Invertebrates. Vol I, 4th edition. Academic Press/Elsevier, Amsterdam. In defensive glands of the water beetle Agabus affinis. Lipids 35:543–550
Schaaf O, Dettner K (1997) Microbial diversity of aerobic heterotrophic bacteria inside the foregut of two tyrphophilous water beetle species (Coleoptera: Dytiscidae). Microbiol Res 152:57–64
Schaaf O, Dettner K (2000) Polyunsaturated monoglycerides and a pregnadiene in defensive glands of water beetles Agabus affinis. Lipids 35:543–550
Schal C, Frase J, Bell WJ (1982) Disturbance stridulation and chemical defence in nymphs of the tropical cockroach Megaloblatta blaberoides. J Insect Physiol 28:541–552
Scheloske HW (1975) Fortpflanzungsverhalten und Lauterzeugung bei Laccobius minutus (L.) (Coleoptera, Hydrophilidae). Verh Dtsch Zool Ges 1974:329–334
Schierling A, Seifert K, Sinterhauf SR, Rieß JB, Rupprecht JC, Dettner K (2013) The multifunctional pygidial gland secretion of the Steninae (Coleoptera: Staphylinidae): ecological significance and evolution. Chemoecology 23:45–57
Schildknecht H (1970) Die Wehrchemie von Land- und Wasserkäfern. Angew Chemie 82:17–25
Schildknecht H (1977) Protective substances of arthropods and plants. Pontif Acad Sci Scr Varia 41:1–49
Schildknecht H, Bühner R (1968) Über ein Glykoproteid in den Pygidialwehrblasen des Gelbrandkäfers. Z Naturforsch 23b:1209–1213
Schildknecht H, Holoubek K, Wolkenstörfer M (1962) Über einen Inhaltsstoff in den Pygidialblasen vom Gelbrandkäfer. Z Naturforsch 17b:81–83
Schildknecht H, Neumaier H, Tauscher B (1972) Gyrinal, die Pygidialdrüsensubstanz der Taumelkäfer (Coleoptera: Carabidae). Liebigs Ann Chem 756:155–161
Schmidt JO (1982) Biochemistry of Insect venoms. Ann Rev Ent 27:339–368
Scholtz CH, Holm E (1986) Insects of Southern Africa. Butterworths, Durban
Schuh RT, Slater JA (1995) True bugs of the world. Comstock Publishing Associates/Cornell University Press, Ithaca
Schulz S, Messer C, Dettner K (1997) Poduran, an unusual tetraterpen from the springtail Podura aquatica. Tetrahedron Lett 38:2077–2080
Schulze P (1924) Biologie der Tiere Deutschlands. Apterygota Teil 25.2, 25–56. Borntraeger, Berlin
Scrimshaw S, Kerfoot WC (1987) 16. Chemical defenses of freshwater organisms: beetles and bugs. In: Kerfoot WC, Sih A (eds) Predation. University Press New England, Hanover, pp 240–262
Seeger W (1971) Morphologie, Bionomie und Ethologie von Halipliden, unter besonderer berücksichtigung funktionsmorphologischer Gesichtspunkte (Haliplidae: Coleoptera). Arch Hydrobiol 68:400–435
Shal C, Gautier JY, Bell WJ (1984) Behavioural ecology of cockroaches. Biol Rev 59:209–254
Shepard WD (2003) The biology of Georissus californicus LeConte (Coleoptera, Hydrophilidae, Georissinae). Spec Bull Jpn Soc Coleopt 6:121–128
Short AEZ (2018) Systematics of aquatic beetles (Coleoptera): current state and future directions. Syst Entomol 43:1–18
Silberbush A, Markman S, Lewinsohn E, Bar E, Cohen JE, Blaustein L (2010) Predator-released hydrocarbons repel oviposition by a mosquito. Ecol Lett 13:1129–1138. https://doi.org/10.1111/j.1461-0248.2010.01501.x
Slater JA (1982) Hemiptera. In: Parker S (ed) Synopsis and classification of living organisms, vol 2. McGraw-Hill, New York, pp 417–447
Smith EL (1970) Biology and structure of the Dobsonfly, Neohermes californicus (Walker). Pan Pac Entomol 46:142–150
Smith RL (1973) Aspects of the biology of three species of the genus Rhantus (Coleoptera: Dytiscidae) with special reference to the acoustical behavior of two. Can Entomol 105:909–919
Solem JO, Gullefors B (1996) Trichoptera, caddisflies. In: Nilsson A (ed) Aquatic Insects of North Europe. Apollo Books, Stenstrup, pp 223–255
Sotka EE, Forbey J, Horn M, Poore AGB, Raubenheimer D, Whalen KE (2009) The emerging role of pharmacology in understanding consumer-prey interactions in marine and freshwater systems. Integr Comp Biol 49:291–313
Southcott RV (1991) Injuries from larval Neuroptera. Med J Aust 154:329–332
St. Quentin D, Beier M (1968) V. Überordnung Libelluloidea und 6. Ordnung Odonata (Libellen). 39 pp, Handbuch der Zoologie IV, 2-2/6
Staddon BW, Thorne MJ (1973) The structure of the metathoracic scent gland system of the water bug Ilyocoris cimicoides (L.) (Heteroptera: Naucoridae). Trans R Entomol Soc Lond 124:343–363
Staddon BW, Thorne MJ (1979) The metathoracic gland system in Hydrocorisae (Heteroptera: Nepomorpha). Syst Entomol 4:239–250
Staddon BW, Everton IJ, Games DE (1979) Organ specificity and scent constitution in Corixidae (Heteroptera: Hydrocorisae). Comp Biochem Physiol 62B:259–262
Steidle JLM, Dettner K (1993) Quantitative composition of the defensive secretion of Bledius species (Coleoptera: Staphylinidae: Oxytelinae) is adapted to naturally occurring predators. Chemoecology 4:63–71
Steidle JLM, Dettner K (1995) Abdominal gland secretion of Bledius rove beetles as an effective defence against predators. Entomol Exp Appl 76:211–216
Steiner WE, Anderson JJ (1981) Notes on the Natural history of Spanglerogyrus albiventris Folkerts, with a new Distribution record (Coleoptera: Gyrinidae). Pan Pac Entomol 57:124–132
Stocks I (2004) Reflex bleeding (autohemorrhage). In: Capinera JP (ed) Encyclopedia of entomology. Springer, Berlin, pp 3132–3238
Stoks R (1999) Effect of lamellae on survival and foraging success of the damsel fly Lestes sponsa (Odonata: Lestidae). Oecologia 117:443–448
Stork NE (2018) How many species of insects and other terrestrial arthropods are there on earth? Annu Rev Entomol 63:31–45
Suhling F, Müller O (1996) Die Flußjungfern Europas (Gomphidae). Westarp, Magdeburg & Spektrum, Heidelberg
Suhling F, Sahlen G, Gorb S, Kalkman VJ, Dijkstra K-D B, van Tol J (2015) Order Odonata. In: Ecology and general biology: Thorp and Covich’s freshwater invertebrates, vol 1, 4th edn. Academic Press/Elsevier, Amsterdam, pp 893–932
Swart CC, Deaton LE, Felgenhauer BE (2006) The salivary gland and salivary enzymes of the giant water bugs (Heteroptera; Belostomatidae). Comp Biochem Physiol Part A 145:114–122
Thayer MK (2016) Staphylinidae Latreille, 1802. In: Kristenasen NP, Beutel RG (eds) Handbook of zoology, Coleoptera, Beetles, vol 2, 2nd edn. De Gruyter, Berlin, pp 394–442
Thompson DJ (1987) Regulation of damselfly populations: the effects of weed on larval mortality due to predation. Frehw Biol 17:367–371
Thompson V (1997) Spittlebug nymphs (Homoptera: Cercopidae) in Heliconia Flowers (Zingiberales: Heliconiaceae): Preadaptation and Evolution of the first aquatic Homoptera. Rev Biol Trop 45:905-912
Thorp JH, O’Neill BJ (2015) Hexapoda-Introduction to Insects and Collembola. In: Ecology and general biology: Thorp and Covich’s freshwater invertebrates, vol 1, 4th edn. Academic Press/Elsevier, Amsterdam, pp 849–871
Thorp JH, Rogers DD (2015) Ecology and general biology: Thorp and Covich’s freshwater invertebrates, vol 1, 4th edn. Academic Press/Elsevier, Amsterdam
Thorp JH, Rogers DD (2016) Keys to Nearctic fauna: Thorp and Covich’s freshwater invertebrates, vol 2, 4th edn. Academic Press/Elsevier, Amsterdam
Urbanek A, Richert M, Gilka W, Szadziewski R (2011) Morphology and histology of secretory setae in terrestrial larvae of biting midges of the genus Forcipomyia (Diptera: Ceratopogonidae). Arthropod Struct Dev 40:485–494
Urbanek A, Szadziewski R, Stepnowski P, Boros-Majewska J, Dawgul M, Kamysz W, Sosnowska D, Golebiowski M (2012) Composition and antimicrobial activity of fatty acids detected in the hygroscopic secretion collected from the secretory setae of larvae of the biting midge Forcipomyia nigra (Diptera: Ceratopogonidae). J Insect Physiol 58:1265–1276
Vasilikopoulos A, Balke M, Beutel RG, Donath A, Podsiadlowski L, Pflug JM, Waterhouse RM, Meusemann K, Peters RS, Escalona HE, Mayer C, Liu S, Hendrich L, Alarie Y, Bilton DT, Jia F, Zhou X, Maddison DR, Niehuis O, Misof B (2019) Phylogenomics of the superfamily Dytiscoidea (Coleoptera: Adephaga) with an Evaluation of the phylogenetic conflict and systematic error. Mol Phyl and Evol 135:270-285
Vallenduuk HJ, Cuppen HMJ (2004) The aquatic living caterpillars (Lepidoptera: Pyraloidea: Crambidae) of Central Europe. A key to the larvae and autecology. Lauterbornia 49:1–17
Van Vondel BJ (2016) 7.2 Haliplidae Aubé, 1836. In: Beutel RG, Kristensen NP (eds) Handbook of zoology, Arthropoda: Insecta. Coleoptera, Beetles, vol 1, 2nd edn. De Gruyter, Berlin, pp 89–98
Völkl W, Hübner G, Dettner K (1994) Interactions between Alloxysta brevis (Hymenoptera, Cynipoidea, Alloxystidae) and honeydew collecting ants: how an aphid hyperparasitoid overcomes ant aggression by chemical defense. J Chem Ecol 20:2621–2635
Von Frankenberg G (1937) Das Zirporgan des Wasserkäfers Spercheus. Aus der Heimat 50:47–50
Vulinec K (1987) Swimming in whirligig beetles (Coleoptera: Gyrinidae): a possible role of the pygidial gland secretion. Coleopt Bull 41:151–153
Vulinec K, Miller MC (1989) Aggregation and predator avoidance in whirligig beetles (Coleoptera: Gyrinidae). J N Y Entomol Soc 97:483–447
Wagner R, Aurich M, Reder E, Veith HJ (1990) Defensive secretions from the larvae of Apatania fimbriata (Pictet) (Trichoptera: Limnephilidae). Chemoecology 1:96–104
Walker AA, Weirauch C, Fry BG, King GF (2016) Venoms of Heteropteran insects: a treasure Trove of diverse pharmacological toolkits. Toxins 8:1–32
Walker AA, Vargas MJH, Corzo G, Frau BG, King GG (2018) Giant fish-killing water bug reveals ancient and dynamic venom evolution in Heteroptera. Cell Mol Life Sci 75:3215–3229
Weatherston J, Percy JE (1978) Venoms of rhynchota (Hemiptera). In: Bettini S (ed) Arthropod venoms. Springer, Berlin, pp 489–509
Weiss MR (2006) Defecation behavior and ecology of insects. Annu Rev Entomol 51:635–661
Wesenberg-Lund C (1943) Biologie der Süßwasserinsekten. Nordisk Vorlag, Kopenhagen & Springer Berlin
White DS (1989) Defense mechanisms in riffle beetles (Coleoptera: Dryopoidea). Ann Entomol Soc Am 82:237–241
Wichard W, Arens W, Eisenbeis G (1995) Atlas zur Biologie der Wasserinsekten. Gustav Fischer, Stuttgart
Wildermuth H (2000) Totstellreflex bei Großlibellenlarven (Odonata). Libellula 19:17–39
Williams DD, Feltmate BW (1992) Aquatic insects. CAB International, Wallingford
Wilson N, Flinn MB, West B, Hereford J (2015) Identification of sound-producing hydrophilid beetles (Coleoptera: Hydrophilidae) in underwater recordings using digital signal processing. Coleopt Bull 69:305–315
Witz BW (1990) Antipredator mechanisms in arthropods: a twenty year literature survey. Florida Entomol 73:71–99
Wohlfahrt B, Mikolajewski DJ, Joop G, Suhling F (2005) Are behavioural traits in prey sensitive to the risk imposed by predatory fish? Freshw Biol 51:76–84
Yan EV, Beutel RG, Lawrence JF (2018) Whirling in the late Permian: ancestral Gyrinidae show early radiation of beetles before Permian-Triassic mass extinction. BMC Evol Biol 18:33 (1-10)
Yee DA (2014) Ecology, systematics and the natural history of predacious diving beetles (Coleoptera: Dytiscidae). Springer, Dordrecht
Yee DA, Kehl S (2015) Order Coleoptera. In: Ecology and general biology: Thorp and Covich’s freshwater invertebrates, vol 1, 4th edn. Academic Press/Elsevier, Amsterdam, pp 1003–1042
Young FN (1960) The colors of desert water beetles - environmental effect or protective coloration? Ann Entomol Soc Am 53:422–425
Yu P, Xie W, Fengqin L (1993) Bionomics and morphology of the larvae of Amphizoa sinica Yu & Stork (Coleoptera: Amphizoidae). Scient treatises Syst Evol Zool 2:107–114
Zwick P (1967) Beschreibung der aquatischen Larve von Neurorthus fallax (Rambur) und Errichtung der neuen Planipennierfamilie Neurorthidae fam. nov. Gewässer Abwasser 44:65–86
Zwick P (1980) Handbuch der Zoologie. IV. Band Arthropoda; 2. Hälfte Insecta, 2. Teil: 7. Plecoptera (Steinfliegen). De Gruyter, Berlin
Heckman CW (2018) Ecological strategies of aquatic insects. CRC, Boca Raton
Heckman CW (2018) Ecological strategies of aquatic insects. CRC, Boca Raton
Acknowledgments
In order to prepare this manuscript the help of the following collaborators and colleagues is highly acknowledged: A. Böttcher (Bayreuth), B. Dettner (Bayreuth), E. Helldörfer (Bayreuth), H. Luthardt (Bayreuth), S. Wagner (Bayreuth), V. Lavanya (Springer), Prof. Dr. Kleber del Claro (Fed. Univ. Uberlandia, Brazil) and Prof. Dr. R. Guillermo Ferreira (Sao Carlos, Brazil). During my Ernst Bresslau guest professorship at the Zoological Institute (chemical ecology in marine and freshwater systems) of University of Cologne I was highly supported by Prof. Dr. E. von Elert and PD Dr. P.Fink.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dettner, K. (2019). Defenses of Water Insects. In: Del-Claro, K., Guillermo, R. (eds) Aquatic Insects. Springer, Cham. https://doi.org/10.1007/978-3-030-16327-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-16327-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16326-6
Online ISBN: 978-3-030-16327-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)