Abstract
The chemical defense of insects is effective for avoiding predation, but may carry a cost in terms of life history traits. If chemical defenses require the resources and/or nutrients necessary for the larva or post-larval stages to survive, grow, and reproduce, there will be a trade-off between chemical defense and other traits, particularly in habitats where larvae are subjected to frequent predator attacks. The larvae of Osmylus hyalinatus McLachlan (Neuroptera: Osmylidae) are semiaquatic, inhabiting the edges of small streams and ponds, where they encounter multiple predators both on land and in water. Larvae of this species spray a hyaline liquid from an anal opening when disturbed. The liquid is stored in the posterior half of the hindgut. Daily stimulation of larvae to exhaust the stored liquid, thereby simulating repeated predator attacks, resulted in smaller adult body size at emergence than the control, but had little effect on the larval/pupal period, cocoon production (for predator avoidance of prepupae and pupae), reproductive potential, or chemical defense of adults in which prothoracic glands release a substance that smells unpleasant to predators. The lack of such effects is explained in part by adults gaining more resources through feeding than the larval stages, as well as nuptial gifts from males to females. The spraying liquids and silk used to spin cocoons are both discharged from an anal opening; therefore, a trade-off between these two materials is plausible and should be examined in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cost and benefit balances impose limits on an organism’s evolution. Many insects have developed chemical defenses, either through synthesis or acquisition from food, which help them escape predation (Eisner et al. 2005; Walker et al. 2018; Dettner 2019). However, the production and maintenance of defensive chemicals, including fluid, is costly in terms of lifetime energetics or resource budgets, and thus has adverse effects on other energy- and resource-intensive fitness parameters such as growth and reproductive rates (Bowers 1992; Zvereva and Kozlov 2016; Lindstedt et al. 2019; Beran and Petschenka 2022). The synthesis of chemical defenses requires enzymes to catalyze the reactions that produce the required compounds; moreover, when used to defend against an attacking predator, replenishing the compounds imposes a further cost (Bowers 1992).
The extent and duration of the costs associated with chemical defense in relation to developmental and behavior events in the insect’s life cycle was studied by several authors. Chemical defense can increase the larval period (Bayoumy et al. 2020) and reduce the larval growth rate (Camara 1997; Smilanich et al. 2009; Zvereva et al. 2017), larval immunity (Smilanich et al. 2009), larval survival (Higginson et al. 2011; Lindstedt et al. 2018), adult body size (Rowell-Rahier and Pasteels 1986; Grill and Moore 1998; Sato et al. 2009; Higginson et al. 2011; Bayoumy et al. 2020; Pacheco et al. 2021), fecundity (Higginson et al. 2011), and egg fertility (Bayoumy et al. 2020). Concerning the adults, chemical defense can delay egg maturation (Knapp et al. 2020) and reduce adult immunity (Knapp et al. 2020) and fecundity (Lindstedt et al. 2020).
The chemical defense is thus costly not only to one developmental stage, but also to the next stages. However, studies examining these costs have been limited to two species of butterflies (Camara 1997; Smilanich et al. 2009; Higginson et al. 2011), a moth (Lindstedt et al. 2020), several species of ladybird beetles (Grill and Moore 1998; Sato et al. 2009; Lee et al. 2018; Bayoumy et al. 2020; Knapp et al. 2020; Pacheco et al. 2021) and leaf beetles (Rowell-Rahier and Pasteels 1986; Kearsley and Whitham 1992; Zvereva et al. 2017), and a pine sawfly (Lindstedt et al. 2018). Furthermore, most of the defensive chemicals of the studied insects are derived from their host plants since herbivorous insects co-opt plant toxins for their own defense (Bowers 1992; Hartmann 2004). The cost of sequestration is assumed to be lower than that of synthesis, which is one potential explanation for the prevalence of sequestration in insect chemical defenses (Zvereva and Kozlov 2016; Zvereva et al. 2017; Beran and Petschenka 2022). To elucidate the evolutionary background of insect chemical defenses, more information about the costs thereof is needed for a variety of insect groups, particularly those with self-synthesized defensive chemicals, e.g., larvae of Osmylus hyalinatus McLachlan (Neuroptera: Osmylidae).
The predatory larvae of O. hyalinatus spray a hyaline liquid from the anal opening when disturbed by enemies (Iwanami et al. 2021). Although it has not been chemically characterized, the sprayed liquid has a pungent odor. The larvae inhabit the edges of small streams and ponds, where they encounter multiple enemies on land and water; spraying is an effective method for escaping predation by both aquatic and terrestrial predators (Iwanami et al. 2021). Little information is available about the ecology or behavior of Osmylidae. Our preliminary observations of O. hyalinatus indicate that the larvae are semiaquatic and suck fluids (mostly the hemolymph of prey) with a pair of fine stylets (Fig. 1A; Zimmermann et al. 2019). This species has a univoltine life cycle (Hayashi 2018), with hatched larvae growing from late summer to the following spring. In April, the fully grown final- (3rd-) instar larvae make cocoons in spaces between fallen leaves or twigs on the ground (Fig. 1B); the cocoons consist of outer (coarse-mesh) and inner (fine-mesh) layers (Fig. 1C, D). The prepupae pupate in the cocoon after about 7 days. After a further 10 days, the pupae break out of the cocoon with their mandibles and adults emerge (Fig. 1B).
General biology and morphology of Osmylus hyalinatus (Neuroptera: Osmylidae). A A larva sucking on a chironomid larva. B A cocoon (co) with outer and inner layers after adult emergence, revealing a pupal exuvium (pe) and meconial pellet (mp) deposited by the emerged adult. C, D Silk nets of the outer and inner layers of the cocoon, respectively. E, F Internal reproductive organs consisting of paired testes (tes) and accessory glands (ag) of just emerged and mature males, respectively. G, H Ovaries of the just emerged female and the mature female including mature eggs (me), respectively. I, J A large gelatinous spermatophore attached to the mated female just after copulation and a female eating the spermatophore, respectively. K Internal organs of the larva; defensive spray liquids are stored in the posterior part of the hindgut (hg). L Internal organs of the prepupa, containing silk to make cocoons throughout from the Malpighian tubules (Mt) to the posterior part of the hindgut (hg)
Larvae have a dead-end digestive system at the midgut. The hindgut is not involved in food digestion (Walker et al. 2018), and its posterior part is where the liquid, which is sprayed from the anal opening, is stored (Fig. 1K; Iwanami et al. 2021). Larvae cease anal spraying for 1–2 days prior to cocoon construction. Silk material is made in the Malpighian tubules and stored in the posterior part of the hindgut (Fig. 1L; Sutherland et al. 2010). During the imaginal development of pupae, the adult digestive system is constructed, which is open throughout the gut, and the excrement stored until this time is expelled from the anal opening as a meconial pellet (Fig. 1B) after adults emerge.
The emerged adults have immature internal reproductive organs, including testes and accessory glands in males (Fig. 1E), and ovaries in females (Fig. 1G). They likely feed on other arthropods, pollen, nectar, and honeydew (Devetak and Klokočovnik 2016) during the adult stage. Mature males have a paired large accessory gland (Fig. 1F) and attach a large gelatinous spermatophore to the female reproductive opening during mating (Fig. 1I), which is a nuptial gift and is completely eaten by the mated female over several minutes (Fig. 1J), while sperm are transferred to the female sperm storage organ. Mature females have large ovaries (Fig. 1H) and lay individual eggs on streamside substrates. The newly hatched larvae have a semiaquatic lifestyle.
Both sexes of adults have a pair of prothoracic glands (Fig. 2) that are also present in several green lacewing species (Neuroptera: Chrysopidae). In Chrysopidae, the gland opens laterally in pleurites via ducts and may deter potential predators through secretion of chemicals such as skatole and tridecene, whereas Osmylidae has eversible sac glands with a glandular reservoir (gr in Fig. 2) bearing setae that protrude when disturbed (Blum et al. 1973; Güsten and Dettner 1991; Güsten 1996; Aldrich et al. 2009; Dettner 2015; Aldrich and Zhang 2016).
Paired prothoracic glands of adult Osmylus hyalinatus that were undisturbed (A–C) and disturbed (D–F). The glandular reservoir (gr) is an eversible sac. When disturbed, it protrudes outside of its gland opening (go) and numerous small setae appear externally (F). A, D in ventral view. B, E in lateral view
In this study, we examine how this larval defense constrains post-larval development and behavior through comparison of life history traits between larvae that were repeatedly stimulated to spray stored liquids and non-stimulated control larvae. Our examinations focused on three ecological topics specific to O. hyalinatus. First, we examined the trade-off of chemical defenses between larval defensive spraying and the unpalatability of adults with eversible prothoracic glands. Second, the cost associated with reproductive investment by adults was examined, as nuptial gifting may occur via large gelatinous spermatophores attached to the female by the male during mating. This type of nuptial gift is classified as an endogenous gift, which may have substantial manufacturing costs; thus, affordability depends on the donor’s physiological condition (Lewis et al. 2014). Finally, we examined the structure and function of neuropteran cocoons, which have not yet been investigated experimentally. Both the defensive spray and silk used for cocoons seem to be stored in and extruded from the larval hindgut, into which the Malpighian tubules open. If a trade-off exists between these substances, costly defensive spraying may impact prepupal and pupal behavior and survival through effects on cocoon structure.
Materials and methods
Experiment 1 (adult unpalatability)
A total of 28 final- (3rd-) instar larvae of O. hyalinatus were collected from February 18 to March 1, 2021, from substrates such as moss-covered stones and wet fallen tree twigs at the edges of small hill streams in Hidaka, Chichibu, Hanno, Ogose and Moroyama, western Saitama, central Japan. The larvae were maintained individually in small plastic cups (50 mm diameter, 35 mm deep) containing several wet fallen leaves and live mosses, without food at outdoor temperatures (< 15 °C). On March 5, 2021, the larvae were placed in a constant-temperature room (20 ± 1 °C, 14 h light and 10 h dark cycle) to examine the effect of larval defensive spraying on post-larval development and adult unpalatability to potential predators. After measuring the fresh body weight of larvae, they were sorted alternately by weight into two groups, each containing 14 individuals. In the control group, larvae were not stimulated, while in the experimental group stored spray material was exhausted through repeated (ten or more) stimulation of the larval body with forceps every day during the larval stage. One living final-instar larva of the chironomid Propsilocerus akamusi (Tokunaga) (ca. 13 mm body length) was provided every other day. We recorded the day on which larvae ceased feeding on chironomids, ceased spraying when stimulated, and formed cocoons, as well as the day on which adults emerged. Adult fresh body weight was measured on the next day after emergence to avoid accidental death caused by touching the soft bodies and wings of just emerged adults. Empty cocoons were carefully removed from the substrates using forceps and weighed after drying for 1 h at 50 °C in an oven. Larvae failed to make complete cocoons were not weighed.
Unpalatability of the emerged adults to potential predators was tested through feeding of the adults to the lizard Plestiodon finitimus Okamoto et Hikida. Seven (six males and one female) lizards were caught at Hachioji, western Tokyo, central Japan, on March 31, 2021. The lizards were reared individually in plastic containers (180 × 330 mm, 220 mm high) at 25 ± 1 °C (14 h light and 10 h dark cycle) and given two mealworms (larvae of the beetle Tenebrio molitor Linnaeus) during a 3-h basking period every other day. The plastic container included a mixture of red soil and peat moss on the bottom (ca. 10 mm thick), a small unglazed pot functioning as a nest, and a small plastic cup of water. Feeding experiments were conducted with the seven lizards on April 4, 6, 8 and 10. After a 30-min basking period, a mealworm was given to confirm feeding motivation, and an adult, making a shallow cut of wings to prevent flight, was then placed approximately 150 mm from the lizard’s head. Male and female adults at 1–5 days from emergence were randomly selected from the control and experimental larval groups. Reuse was generally avoided, except for three males and two females, after being offered to the lizard. Lizard behavior was video recorded for 120 s using an iPhone 8 smartphone camera. After the experiment, the snout–vent length (SVL) of each lizard was measured and the lizards were released at their capture sites. A video of this prey–predator behavior was posted on the Movie Archives of Animal Behavior (MOMO) website.
Experiment 2 (reproductive investment)
A total of 30 final-instar larvae were collected from small streams in Hanno, western Saitama, central Japan, on February 23, 2022. The larvae were maintained individually in small plastic cups (50 mm in diameter, 35 mm deep) containing several wet fallen leaves and live mosses, without food at outdoor temperatures (< 15 °C). On February 26, after measurement of fresh body weight, 29 larvae (excluding an injured one) were sorted alternately by weight into the control (non-stimulated, N = 15) and experimental (stimulated to spray, N = 14) groups, as in Ex. 1. Larvae were reared individually in 90-mm disposable Petri dishes (15 mm deep) with wet 55-mm filter paper on the bottom at 20 ± 1 °C (14 h light and 10 h dark cycle), to examine the effect of larval defensive spraying on post-larval development and investment in adult reproductive organs. The rearing and measurement methods for larval development were the same as those in Ex. 1. Adults were placed in a freezer at − 20 °C for 5 min on the next day of emergence, and male testis and accessory glands (Fig. 1E), or female ovaries (Fig. 1G), were then dissected out and weighed. Dissection was also conducted the field-caught two mature males (Fig. 1F) and one mature female (Fig. 1H). Investment in male and female reproductive organs was reflected in organ weight, calculated as a percentage of body weight.
Experiment 3 (functions of cocoons)
A total of 27 final-instar larvae were collected from small streams in Hidaka and Ogano, western Saitama, central Japan, on March 23 and 25, 2021. These larvae were reared individually using the same methods as in Ex. 2. In total, 24 larvae formed cocoons, while 3 died before that stage. To examine the functionality of the cocoons against predators, three groups of prepupae were prepared by manipulating their cocoons. The first group included control prepupae with intact two-layered cocoons (N = 6). The second group contained prepupae with single-layered cocoons which were made after artificial removal of the firstly spun outer layer (N = 6), and the last group included prepupae without cocoons (N = 9). Foragers of the ant Formica japonica Motschulsky were used as the experimental predator of terrestrial prepupae inside fallen leaves, or under stones and twigs alongside the stream (Iwanami et al. 2021). One ant colony was cultured at 25 ± 1 °C (14 h light and 10 h dark cycle) after being collected at Hachioji, western Tokyo, central Japan. An ant forager that had been walking outside the colony chamber was placed gently into each Petri dish containing a prepupa. The behaviors of the paired prepupa and ant were observed for 2 h after setup, which occurred around 3 p.m. The Petri dish was left undisturbed and checked again (to determine living/dead status) at around 9 a.m. the following morning. In this experiment, the function of the cocoon in the normal developmental processes of pupation and adult emergence was also examined by observing additional prepupae without cocoons (N = 3).
Statistical analysis
Values are presented as mean ± standard error (SE). Pearson’s correlation coefficient (r) was used to examine the effect of initial larval body weight on larval period, cocoon weight, and adult body weight. However, the effect of initial larval body weight on larval unfed period before prepupation and also on prepupal and pupal period was examined by Spearman’s rank correlation coefficient (rs), because the data were not normally distributed when the data was checked by the Shapiro–Wilk normality test. If no correlation was found, Student’s t test with equal variances, or Welch’s t test with unequal variances, was used to compare the means of the control and experimental groups, but Mann–Whitney U test was used in the data with non-normal distributions for comparisons of the two groups. If a significant correlation was found, analysis of covariance (ANCOVA) was used to control for the effects of initial larval body weight. The percentage data of reproductive investment were arcsine transformed before comparisons between the control and experimental groups. Fisher’s exact test and Holm’s multiple comparison test were used to compare survival rates of prepupae in the ant predation experiment among the three groups of cocoon treatments, as well as survival rates of adults emerged from the control and experimental larvae in the lizard predation experiment. These tests were performed using the IBM SPSS Statistics (ver. 25.0; IBM Corp., Armonk, NY, USA) and G ∗ Power 3.1 (University of Dusseldorf, Dusseldorf, Germany) programs, or the base package of R 3.6.0 (R Core Team 2019).
Results
Post-larval development (Ex. 1, 2)
In Ex. 1, 24 adults emerged excluding two of 14 control larvae and two of 14 experimental larvae that failed to pupate or emerge from the pupae, and in Ex. 2, 29 adults emerged from all 15 control and 14 experimental larvae. The two larvae that failed to pupate in Ex. 1 were excluded from the data analysis, and therefore, a total of 29 control and 26 experimental larvae were used for comparisons of their life history parameters (Table 1). Although individual developments were checked every day, some life history parameters could not be determined completely because two control larvae failed in adult emergence and it was impossible to remove cocoons in some pupae and to determine spraying behavior, feeding behavior and prepupation timing in some larvae.
Larvae in the experimental group sprayed for 9.1 days (N = 25, SE = 0.5) and then ceased spraying for 1.5 days (N = 17, SE = 0.1) prior to cocoon formation. The mean initial larval body weight was slightly heavier in the control group, but this difference has no statistical importance (Table 1). The unfed duration prior to cocoon construction, prepupal and pupal periods, and cocoon weight were not correlated with initial larval body weight, and their mean values did not differ between the two groups (Table 1). The larval period after the initiation of the experiments was negatively correlated with initial larval body weight (Table 1), but the larval period did not differ between the control and experimental groups (ANCOVA; F1, 49 = 2.669, P = 0.11 for slope; F1, 50 = 0.002, P = 0.966 for intercept). The body weight of emerged adults was positively correlated with initial larval body weight (Table 1, Fig. 3), and adult body weight was lower in the experimental than control group (ANCOVA; F1, 49 = 0.000, P = 0.98 for slope; F1, 50 = 9.28, P = 0.004 for intercept). Thus, defensive spraying by larvae reduced adult body weight at emergence.
The fresh body weight relationship of Osmylus hyalinatus between the larvae at the initiation of experiments (x) and the adults of the next day of emergence (y) in the intact control group (N = 27) and experimental group (N = 26) in which larvae were stimulated to spray liquids daily during the larval stage. The solid and broken lines are the regression equations of the control (y = 0.631 x + 27.633) and experimental (y = 0.625 x + 23.999) groups, respectively. For statistical results, see Table 1 and the text
Adult unpalatability (Ex. 1)
The experimental lizards ate all mealworms but only one of 28 adult O. hyalinatus (Table 2). The eaten adult was a male that emerged from one of the experimental larvae. The feeding time (from biting to swallowing the whole body) was 102 s. No difference in predation rate was observed between the two larval treatments, intact control (0/14) and stimulated to spray (1/14) (Fisher’s exact test, P = 1.0). Lizards approached the offered adult and touched it while sniffing frequently, but finally left without biting (for a digital video, see http://www.movspec.mus-nh.city.osaka.jp/ethol/showdetail-e.php?movieid=momo220409oh01b). Thus, chemical defense from the adult thoracic glands appeared to function as a deterrent to lizard predation, even in adults that emerged from the stimulating larvae to spray intensively.
Reproductive investment (Ex. 2)
The male internal reproductive organ size as a proportion of body weight was 4.81% (N = 5, SE = 0.75) in the control group and 5.11% (N = 8, SE = 0.49) in the experimental group; these values did not differ between the groups (t test; t = 0.31, df = 11, P = 0.76). The respective values for females were 1.73% (N = 10, SE = 0.21) and 1.68% (N = 6, SE = 0.11), which also did not differ (t test; t = 0.17, df = 14, P = 0.87). However, the field-caught mature males had the larger reproductive organs, 27.13% (N = 2, Range 22.80–31.46) of body weight, and the mature female had the larger ovaries, 36.47% (N = 1) of body weight. Thus, the emerged males and females are quite immature, and the intensive spraying at the larval stage are unlikely to affect adult reproductive investment. Instead of larval conditions, the feeding rate of adults, also the mating frequency in females to eat externally attached spermatophores, may be more important to determine adult reproduction.
Functions of cocoons (Ex. 3)
Most (5/6) prepupae in the intact cocoons survived until the following morning in the presence of ants (Fig. 4), although one prepupa that was not attacked during the 2-h observation period was dead, with a hole found in its cocoon on the following morning (Fig. 4B). Only two of six prepupae with single-layered cocoons survived, and all nine prepupae without cocoons died (Fig. 4). Thus, the cocoons affected the prepupal survival rate of ant predation (P = 0.002 in Fisher’s exact test and P < 0.05 only between intact and no cocoon treatments in Holm’s multiple comparison test). Behavioral observation suggested that ants did not avoid walking on the surface of the cocoons, and attacked the prepupae by breaking through the net of the cocoon (Fig. 4A, B). The first attack by an ant occurred after 60.0 min (SE = 7.1, N = 4) for prepupae with single-layered cocoons and 5.6 min (SE = 3.1, N = 9) for those with no cocoons (t test; t = 8.36, df = 11, P < 0.0001). The cocoons may delay encounters with, and attacks by, ants.
Survival rate of Osmylus hyalinatus prepupae exposed to the ant Formica japonica overnight in their three treatments: no cocoon, single-layered cocoon obtained by removing the outer layer, and normal two-layered cocoon. Ants attacked several prepupae in both single-layered (A) and normal two-layered cocoons (B) by making a hole in the cocoon
Finally, four of five prepupae that escaped ant attack with intact cocoons pupated, and two adults (one male and one female) emerged. The two prepupae in single-layered cocoons pupated, and both adults (one male and one female) emerged. Aditional three prepupae without cocoons, which were not used in the ant predation experiment, pupated normally and all adults (one male and two females) emerged. These results suggest that the cocoons are not necessary for pupation and adult eclosion.
Discussion
Our results show that defensive spraying by larval O. hyalinatus reduces adult body size at emergence (Fig. 3), but does not affect the larval period from the start of the experiment to prepupation (Table 1). These results suggest that larval spraying of liquids stored in the posterior part of the hindgut is costly and affects their growth rate. The costs of defense are apparently not reflected in delayed development, but instead in smaller adult size, which may indicate an adaptation to pay those costs reflected in reduced adult fitness. Synchronous emergence may drive this cost–benefit relationship, as delayed emergence could lead to a loss of mating opportunities (Higginson et al. 2011). The life cycle of O. hyalinatus is univoltine and adults appear in late spring to early summer (Hayashi 2018). Our rearing results showed that final- (3rd-) instar larvae tend to synchronize their adult emergence, as the larval period is negatively correlated with larval body size; moreover, a constant ca. 17-day prepupal and pupal period was observed (Table 1).
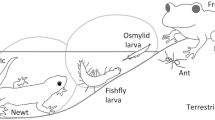
However, decrease in adult body size may suffer their lifetime fitness because the potential fitness of longevity and reproduction depends on it (Kingsolver and Huey 2008; Chown and Gaston 2010). The reason why O. hyalinatus larvae pay costs to reduce adult body size at emergence may be a strong predation pressure in their semiaquatic habitat where both terrestrial and aquatic predators attack them. In our study site, potential predators include insectivorous birds, frogs, fishing spiders and ants on land, and carnivorous fish, amphibians such as larval salamanders and newts, and predatory aquatic insect larvae such as dragonfly, dobsonfly, and fishfly in water. Larval defensive spraying is effective to deter these predators, and promotes survival after being attacked by predators such as frogs and newts (Iwanami et al. 2021). Selection pressure is expected to act more strongly on the survival (all or none) of these larvae than on adults with decreased body size (not all or none, but quantitative change in longevity and fecundity depending on the body size).
We found little effect of larval spraying on adult reproductive investment, i.e., on male and female internal reproductive organ size at emergence (Ex. 2). Unfortunately, we were unable to rear adults to maturity under laboratory conditions due to difficulty in adult feeding procedure. Newly emerged adults were quite immature, and had much smaller internal reproductive organs than field-captured mature adults (Fig. 1E, F for males, Fig. 1G, H for females). The adults feed on living arthropods, pollen, nectar, and honeydew (Devetak and Klokočovnik 2016) to support maturation, mating, and egg laying. Moreover, females feed on the large gelatinous spermatophore donated by the male at copulation (Fig. 1I, J). This may be a nuptial gift from the male to the mated female, being the first observation of such gifts in Neuroptera. Some groups of Megaloptera belonging to the same superorder Neuropterida, together with Neuroptera and Raphidioptera, are also known to transfer a large gelatinous spermatophore at mating (Hayashi 1992; Liu et al. 2015). Female megalopterans that mated multiple times and consumed multiple spermatophores had increased longevity and lifetime fecundity (Hayashi 1998), although spermatophore donation is costly for males in terms of mating frequency (Hayashi 1993) and the development of weapons for male–male competition (Liu et al. 2015). In O. hyalinatus, this additional resource acquisition by adult females may contribute greatly to their longevity and reproductive investment, which may in turn offset the cost of larval spraying reflected in the smaller body size of adults at emergence.
Neuropteran larvae form cocoons at the prepupation stage using silk secretions from Malpighian tubules (Sutherland et al. 2010). This silk is pulled from the anal opening via the hindgut, which is the same pathway used for defensive spray liquids observed in this study (Fig. 1K, L), although the organ that synthesizes defensive liquids remains unknown. Larvae ceased spraying for 1.5 days prior to the beginning of cocoon spinning, suggesting a potential trade-off in the processes of production and storage between spraying liquids and cocoon silk. In larval lacewings, both anal adhesive/defensive secretion and silk spinning are also passed from the hindgut to an anal opening with a similar time lag to our results and which suggested the constraint between these secretion and silk production (Spiegler 1962; LaMunyon 1988; LaMunyon and Adams 1987). Control larvae spun larger cocoons (0.72 mg) than larvae repeatedly stimulated to spray (0.63 mg), although the difference was not statistically significant (Table 1). Our examination of cocoon function suggested that the cocoon is not necessary for larvae to prepupate, pupate, and emerge as adults, because larvae with artificially removed cocoons metamorphosed normally.
Predation experiments using ants, which have been used previously as a model predator (Sugiura 2020), revealed that the cocoons allow prepupae and pupae to escape ant predation (Fig. 4). The cocoons themselves are unlikely to include defensive chemicals against predators similar to the sprayed materials (Fig. 4A, B), but ants are likely unable to recognize prey within two-layered cocoons. In the field, cocoons were found in spaces among fallen leaves, twigs, and stones on the ground, where terrestrial arthropods such as ants, earwigs, centipedes, and spiders may hunt them. The structure and function of Malpighian tubules are complex and still unclear in larvae of Neuroptera (Pacheco et al. 2014; Martins and Ardila-Camacho 2018). The number of the tubules is reported eight in several neuropteran larvae (Spiegler 1962; Martins and Ardila-Camacho 2018). However, the larval spongefly Sisyra nigra (Sisylidae), closely related to Osmylidae, modifies one of eight Malpighian tubules to attach to the anterior part of the hindgut with its distal end (Jandausch et al. 2019). The number of free Malpighian tubules are also seven in O. hyalinatus. Therefore, the possible trade-off between spraying liquids and cocoon silk should be examined in greater detail also based on morphology and histology.
The cost of larval chemical defense, in terms of the efficacy of adult chemical defenses, has not been studied previously in insects. The paired prothoracic glands of adults release an odorous substance when disturbed that deters attacking by lizards, one of the potential predators of larvae (Sugiura 2020). This unpalatability was maintained even in adults that emerged from larvae experimentally induced to exhaust their defensive chemicals (Table 2), which suggests no cost associated with larval spraying in terms of adult chemical defense.
Overall, our results indicate that repeated stimulation of larvae to spray reduces adult body size at emergence, but has little effect on the larval/pupal period, cocoon production, reproductive potential, or chemical defense via adult prothoracic glands, even with intensive exhaustion and replenishment of the spraying liquids. Such chemical defense is reportedly costly in terms of life history traits during the larval period and subsequent developmental stages. In larvae, chemical defense lengthens the larval period (Bayoumy et al. 2020) and reduces the larval growth rate (Camara 1997; Smilanich et al. 2009; Zvereva et al. 2017), larval immunity (Smilanich et al. 2009), larval survival (Higginson et al. 2011; Lindstedt et al. 2018), adult body size (Rowell-Rahier and Pasteels 1986; Grill and Moore 1998; Sato et al. 2009; Higginson et al. 2011; Bayoumy et al. 2020; Pacheco et al. 2021), fecundity (Higginson et al. 2011), and egg fertility (Bayoumy et al. 2020). In adults, chemical defense delays egg maturation (Knapp et al. 2020), and reduces adult immunity (Knapp et al. 2020) and fecundity (Lindstedt et al. 2020). However, estimates of insect chemical defense costs vary among studies. With larval chemical defense, little or no cost is reported in terms of the larval feeding rate (Kearsley and Whitham 1992; Zvereva et al. 2017; Pacheco et al. 2021), larval period (Kearsley and Whitham 1992; Zvereva et al. 2017; Pacheco et al. 2021), prepupal/pupal period (Zvereva et al. 2017; Bayoumy et al. 2020), larval growth rate (Lindstedt et al. 2018), larval immunity (Lindstedt et al. 2018), larval survival (Zvereva et al. 2017), adult body size (Kearsley and Whitham 1992; Smilanich et al. 2009; Zvereva et al. 2017), adult body color (Grill and Moore 1998), or female fecundity (Bayoumy et al. 2020).
Regarding adult chemical defense, no cost in terms of adult body size (Knapp et al. 2020), fecundity (Lee et al. 2018; Knapp et al. 2020), or fertility (Lee et al. 2018; Lindstedt et al. 2020) has been reported. One factor driving differences in the costs of chemical defenses is the source of defensive chemicals, which may be synthesized by the insects themselves or obtained from food (plants) (Zvereva and Kozlov 2016). Zvereva et al. (2017) compared the costs of defensive chemicals between leaf beetles that self-synthesize chemicals and those that acquire chemicals from leaves of food plants; the former group is expected to pay more costs than the latter. However, no differences in costs were detected in terms of larval survival, weight or duration of the developmental period between these two groups. The resource and energy budgets of individuals may be another important factor underling different effects of chemical defense. Adult O. hyalinatus is immature at emergence and acquire additional resources through feeding and nuptial gifts given to females. If adults are already mature at emergence and use only resources acquired during the larval stages for reproduction, the cost of larval chemical defense is expected to be high. This is an interesting topic for future research.
Data availability
The authors make all the data available to readers upon request.
References
Aldrich JR, Zhang QH (2016) Chemical ecology of neuroptera. Ann Rev Entomol 61:197–218
Aldrich JR, Le TC, Zhang QH, Torres J, Winterton SL, Han B, Miller GL, Chauhan KR (2009) Prothoracic gland semiochemicals of green lacewings. J Chemical Ecol 35:1181–1187
Bayoumy MH, Osawa N, Hatt S (2020) Fitness costs of reflex bleeding in the ladybird beetle Harmonia axyridis: the role of parental effects. Insect Sci 27:1346–1359
Beran F, Petschenka G (2022) Sequestration of plant defense compounds by insects: from mechanisms to insect–plant coevolution. Ann Rev Entomol 67:163–180
Blum MS, Wallace JB, Fales HM (1973) Skatole and tridecene: identification and possible role in a chrysopid secretion. Insect Biochem 3:353–357
Bowers MD (1992) The evolution of unpalatability and the cost of chemical defense in insects. In: Roitberg BD, Isman MB (eds) Insect chemical ecology. An evolutionary approach. Chapman & Hall, New York, pp 216–244
Camara MD (1997) Physiological mechanisms underlying the costs of chemical defence in Junonia coenia Hübner (Nymphalidae): a gravimetric and quantitative genetic analysis. Evol Ecol 11:451–469
Chown SL, Gaston KJ (2010) Body size variation in insects: a macroecological perspective. Biol Rev 85:139–169
Dettner K (2015) Toxins, defensive compounds and drugs from insects. In: Hoffmann KH (ed) Insect molecular biology and ecology. CRC, Boca Raton, FL, pp 39–93
Dettner K (2019) Defenses of water insects. In: Del-Claro K, Guillermo R (eds) Aquatic insects. Springer, Cham, pp 191–262
Devetak D, Klokočovnik V (2016) The feeding biology of adult lacewings (Neuroptera): a review. Trends in Entomol 12:30–42
Eisner T, Eisner M, Siegler M (2005) Secret weapons. Defenses of insects, spiders, scorpions, and other many-legged creatures. Harvard University Press, Cambridge
Grill CP, Moore AJ (1998) Effects of a larval antipredator response and larval diet on adult phenotype in an aposematic ladybird beetle. Oecologia 114:274–282
Güsten R (1996) A review of epidermal glands in the order Neuroptera (Insecta). In: Canard M, Aspöck H, Mansell MW (eds) Pure and applied research in neuropterology. Proceedings of the fifth international symposium on neuropterology, Toulouse, France, pp 129–146
Güsten R, Dettner K (1991) The prothoracic gland of the Chrysopidae (Neuropteroidea: Planipennia). In: Zombori L, Peregovits L (eds) Proceedings of the 4th European congress of entomology and the XIII internationale Symposium für die entomofaunistik Mitteleuropas, Hungrian Natural History Museum, Budapest, pp 60–65
Hartmann T (2004) Plant-derived secondary metabolites as defensive chemicals in herbivorous insects: a case study in chemical ecology. Planta 219:1–4
Hayashi F (1992) Large spermatophore production and consumption in dobsonflies Protohermes (Megaloptera, Corydalidae). Kontyû 60:59–66
Hayashi F (1993) Male mating costs in two insect species (Protohermes, Megaloptera) that produce large spermatophores. Anim Behav 45:343–349
Hayashi F (1998) Multiple mating and lifetime reproductive output in female dobsonflies that receive nuptial gifts. Ecol Res 13:283–289
Hayashi F (2018) Neuroptera. In: Kawai T, Tanida K (eds) Aquatic insects of Japan: manual with keys and illustrations, 2nd edn. Tokai University Press, Kanagawa, pp 437–442 ((In Japanese))
Higginson AD, Delf J, Ruxton GD, Speed MP (2011) Growth and reproductive costs of larval defence in the aposematic lepidopteran Pieris brassicae. J Anim Ecol 80:384–392
Iwanami T, Yu P, Hayashi F (2021) Defensive spray by a semiaquatic osmylid larva (Insecta: Neuroptera) for both aquatic and terrestrial predators. J Ethol 39:369–377
Jandausch K, Beutel RG, Bellstedt R (2019) The larval morphology of the spongefly Sisyra nigra (Retzius, 1783) (Neuroptera: Sisyridae). J Morphol 280:1742–1758
Kearsley MJ, Whitham TG (1992) Guns and butter: a no cost defense against predation for Chrysomela confluens. Oecologia 92:556–562
Kingsolver JG, Huey RB (2008) Size, temperature, and fitness: three rules. Evol Ecol Res 10:251–268
Knapp M, Řeřicha M, Židlická D (2020) Physiological costs of chemical defence: repeated reflex bleeding weakens the immune system and postpones reproduction in a ladybird beetle. Sci Rep 10:1–7
LaMunyon C (1988) Hindgut changes preceding pupation and related cocoon structure in Chrysoperla comanche Banks (Neuroptera, Chrysopidae). Psyche 95:203–209
LaMunyon CW, Adams PA (1987) Use and effect of an anal defensive secretion in larval Chrysopidae (Neuroptera). Ann Entomol Soc Am 80:804–808
Lee BW, Ugine TA, Losey JE (2018) An assessment of the physiological costs of autogenous defenses in native and introduced lady beetles. Environ Entomol 47:1030–1038
Lewis SM, Vahed K, Koene JM, Engqvist L, Bussiere LF, Perry JC, Gwynne D, Lehmann GU (2014) Emerging issues in the evolution of animal nuptial gifts. Biol Lett 10:20140336
Lindstedt C, Miettinen A, Freitak D, Ketola T, López-Sepulcre A, Mäntylä E, Pakkanen H (2018) Ecological conditions alter cooperative behaviour and its costs in a chemically defended sawfly. Proceed Roy Soc B 285:20180466
Lindstedt C, Murphy L, Mappes J (2019) Antipredator strategies of pupae: how to avoid predation in an immobile life stage? Philos Trans R Soc B 374:20190069
Lindstedt C, Suisto K, Burdfield-Steel E, Winters AE, Mappes J (2020) Defense against predators incurs high reproductive costs for the aposematic moth Arctia plantaginis. Behav Ecol 31:844–850
Liu X, Hayashi F, Lavine LC, Yang D (2015) Is diversification in male reproductive traits driven by evolutionary trade-offs between weapons and nuptial gifts? Proceed Roy Soc B 282:20150247
Martins CC, Ardila-Camacho A (2018) Order neuroptera. In: Hamada N, Thorp JH, Rogers DC (eds) Keys to neotropical hexapoda, 4th edition: thorp and Covich’s freshwater invertebrates, vol 3. Academic Press, Cambridge, pp 229–236
Pacheco CA, Alevi KCC, Ravazi A, Oliveira MTVDA (2014) Malpighian tubule, an essential organ for insects. Entomol Ornithol Herpetol 3:122
Pacheco P, Borges I, Branco B, Lucas E, Soares AO (2021) Costs and benefits of wax production in the larvae of the ladybeetle Scymnus nubilus. Insects 12:458
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rowell-Rahier M, Pasteels JM (1986) Economics of chemical defense in Chrysomelinae. J Chem Ecol 12:1189–1203
Sato S, Kushibuchi K, Yasuda H (2009) Effect of reflex bleeding of a predatory ladybird beetle, Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae), as a means of avoiding intraguild predation and its cost. Appl Entomol Zool 44:203–206
Smilanich AM, Dyer LA, Chambers JQ, Bowers MD (2009) Immunological cost of chemical defence and the evolution of herbivore diet breadth. Ecol Lett 12:612–621
Spiegler PE (1962) The origin and nature of the adhesive substance in larvae of the genus Chrysopa (Neuroptera: Chrysopidae). Ann Entomol Soc Am 55:69–77
Sugiura S (2020) Predators as drivers of insect defenses. Entomol Sci 23:316–337
Sutherland TD, Young JH, Weisman S, Hayashi CY, Merritt DJ (2010) Insect silk: one name, many materials. Ann Rev Entomol 55:171–188
Walker AA, Robinson SD, Yeates DK, Jin J, Baumann K, Dobson J, Fry BG, King GF (2018) Entomo-venomics: the evolution, biology and biochemistry of insect venoms. Toxicon 154:15–27
Zimmermann D, Randolf S, Aspöck U (2019) From chewing to sucking via phylogeny–from sucking to chewing via ontogeny: mouthparts of Neuroptera. In: Krenn HW (ed) Insect mouthparts, zoological monographs 5. Springer, Cham, pp 361–385
Zvereva EL, Kozlov MV (2016) The costs and effectiveness of chemical defenses in herbivorous insects: a meta-analysis. Ecol Monogr 86:107–124
Zvereva EL, Zverev V, Kruglova OY, Kozlov MV (2017) Strategies of chemical anti-predator defences in leaf beetles: is sequestration of plant toxins less costly than de novo synthesis? Oecologia 183:93–106
Acknowledgements
We thank Yasukazu Okada for offering the worker ants of Formica japonica as the model predator from his cultured colonies and the two anonymous reviewers for their helpful comments on the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the completion of this study; particularly, PY and FH designed the study, TI and KS collected materials, and PY, TI, and FH performed rearing experiments, HY, MT, and FH performed the lizard predation experiment, and PY and FH analyzed all data and wrote the manuscript which was finally checked by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines (A3-3 in Tokyo Metropolitan University) for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yu, P., Iwanami, T., Yazaki, H. et al. Cost of defensive spraying by larval Osmylus hyalinatus (Neuroptera: Osmylidae) for post-larval development. J Ethol 41, 129–139 (2023). https://doi.org/10.1007/s10164-023-00779-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-023-00779-0