Abstract
Vascular calcification (VC) is seen ubiquitously in aging blood vessels and prematurely in disease states like renal failure. It is thought to be driven by a number of systemic and local factors that lead to extra-osseous deposition of mineral in the vascular wall and valves as a common endpoint. The response of resident vascular smooth muscle cell to these dystrophic signals appears to be important in this process. Whilst in vivo models allow the observation of global changes in a pro-calcific environment, identifying the specific cells and mechanisms involved has been largely garnered from in vitro experiments, which provide added benefits in terms of reproducibility, cost, and convenience. Here we describe a 7–21 day cell culture model of calcification developed using immortalized murine vascular smooth muscle cells (MOVAS-1). This model provides a method by which vascular smooth muscle cell involvement and manipulation within a mineralizing domain can be studied.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
Vascular calcification (VC) is a feature of aging and a number of diseases including chronic kidney disease (CKD ), where it is an important predictor of adverse outcomes and mortality in patients [1]. The process is characterized by the deposition of insoluble mineral in cardiovascular tissues, especially the arterial vasculature, both in intimal plaque and the tunica media [2, 3].
The underlying molecular mechanisms of VC are thought to resemble those of physiological skeletal bone formation. Whilst in health, mineralization is tightly regulated, in disease, vascular calcification is thought to be driven by mineral imbalance, bone morphogenetic proteins, some pro-atherosclerotic lipids as well as loss of negative regulators of calcification [4, 5].
Our current understanding suggests that vascular calcification is not purely due to passive precipitation of calcium phosphate but may be cell driven to some extent [6]. Several cell types have been implicated in extra-osseous calcification [7], but the vascular smooth muscle cell (VSMC) appears to play a pivotal role. In response to pro-calcific triggers, many of which remain poorly understood, the VSMC develops phenotypic changes characteristic of osteoblast or chondrocyte-like cells. Thus, a better understanding of these VSMC pro-osteoblastic traits and their regulatory mechanisms may be beneficial in the prevention of calcification. Given that research in animal models indicates that VC is multifaceted and complex, the study of contributing pathways in simpler settings provides many advantages.
In vitro models of VC enable the study of mechanisms influencing this process in isolation. While cultivation of primary cell cultures is a useful method for studying VSMC behavior in vitro, the need to reduce inter-assay variation, convenience, and cost has driven research towards the use of cell lines for kinetic and mechanistic investigation.
One such model employs the SV40 large T antigen-immortalized murine VSMC line MOVAS-1. First described by Afroze et al. the cell line has been used in the study of vascular circadian rhythms [8], cell cycling [9] and lipid loading [10].
A variety of methods can be used to induce procalcific and osteochondrocytic behavior in these cells, as they have an inherent propensity to calcify. Here we describe several in vitro techniques for studying calcification in MOVAS-1. Whilst more sensitive techniques can be employed to detect changes in genotype that occur early in culture, the formation of calcium containing nodules is not observed prior to 14 days of cultivation. After this time, staining techniques can be used to demonstrate presence of nodules in the cell monolayer. This model enables the study of mechanisms driving calcification as well as potential agonists and antagonists of the process in a controlled and reproducible environment.
2 Materials
All aqueous solutions are prepared in double deionized water (ddH2O) unless otherwise stated.
2.1 Cell Culture
2.1.1 Cell Culture
-
1.
MOVAS-1 (ATCC, Manassas, VA, USA).
-
2.
Tissue culture flasks (75 or 150 cm2).
-
3.
6-well tissue culture plates (34.5 mm diameter).
-
4.
Sterile 50 mL plastic tubes.
-
5.
Sterile glass or plastic 10 mL pipettes.
-
6.
Pipetting aid suitable for use in cell culture.
-
7.
DMEM + 10 % fetal calf serum (FCS) (see Note 1 ).
-
8.
Sterile Tris buffered saline (TBS): 50 mM Tris–HCl, 150 mM NaCl, pH 7.4.
-
9.
Sterile trypsin–EDTA (0.25 % trypsin in 0.02 % EDTA).
-
10.
Laminar flow hood.
-
11.
Phase-contrast microscope suitable for viewing cells in culture flasks.
-
12.
Trypan blue solution (0.4 % w/v in ddH2O).
-
13.
Hemocytometer or automated cell counter.
2.2 Additives to Promote Calcification
2.2.1 Calcium and Phosphate Salt Solutions
-
1.
20 mM calcium chloride (CaCl2) in TBS, pH 7.4.
-
2.
14 mM sodium phosphate (NaHPO4) in TBS, pH 7.4.
-
3.
Sterile TBS.
-
4.
0.22 μM syringe microfilter.
2.2.2 Calciprotein Particles (CPP)
-
1.
50 mL sterile tubes.
-
2.
Sterile TBS.
-
3.
FCS or human serum (as a source of fetuin-A and other plasma proteins).
-
4.
20 mM CaCl2 in TBS, pH 7.4 (Ca).
-
5.
14 mM NaHPO4 in TBS pH 7.4 (Pi).
-
6.
High-speed refrigerated centrifuge.
2.2.3 β-Glycerophosphate
-
1.
β-glycerophosphate (Cell culture grade, Sigma-Aldrich), in ddH2O at a concentration of 10–25 mM.
-
2.
50 μg/mL ascorbic acid solution in ddH2O.
-
3.
Aliquot solutions and store at −20 °C prior to use in cell culture.
2.2.4 Naked Apatite Crystals
-
1.
Synthetic hydroxyapatite nanocrystals, <200 nm 10 % w/v in ddH2O (Sigma-Aldrich).
-
2.
Spin filter columns, 300 kDa molecular weight cutoff (Sartorius AG, Dandenong South, Victoria, Australia).
-
3.
Sterile TBS.
-
4.
High-speed refrigerated centrifuge.
2.3 Histological Methods for Detection of Calcification
2.3.1 Alizarin Red Staining
-
1.
10 % Neutral buffered formalin.
-
2.
Transfer pipettes.
-
3.
TBS.
-
4.
2 % Alizarin Red (w/v) in ddH2O, pH 4, adjust using 0.5 % ammonium hydroxide and filter to remove particulates. Stable when stored in dark for 1 month.
2.3.2 von Kossa Staining of Nodules
-
1.
4 % paraformaldehyde (w/v) in TBS.
-
2.
1 % silver nitrate (w/v) in ddH2O.
-
3.
5 % sodium thiosulfate (w/v) in ddH2O.
-
4.
Harris hematoxylin (see Note 2 ).
-
5.
Ultraviolet light source.
-
6.
Aqueous mounting medium.
2.4 Quantifying Mineralization
2.4.1 Calcium and Phosphate Assays
-
1.
0.6 M hydrochloric acid in ddH2O.
-
2.
TBS (cold).
-
3.
Cell scrapers.
-
4.
96-well flat bottom plates.
-
5.
RIPA® Buffer (cold) supplemented with protease inhibitor cocktail (Sigma).
-
6.
Calcium colorimetric assay (Sigma-Aldrich).
-
7.
Phosphate Quantichrom® colorimetric kit (BioAssay Systems, Hayward, CA, USA).
-
8.
Micro BCA® colorimetric protein kit (Thermo Scientific, Waltham, MA, USA).
-
9.
Microplate reader.
3 Methods
3.1 MOVAS-1 Cell Culture and Characterization
3.1.1 Culturing and Maintaining Cells
-
1.
Immortalized murine vascular smooth muscle cell line (MOVAS-1) are maintained in 75–150 cm2 sterile vented cell culture flasks in DMEM + 10 % FCS supplemented with glutamine and antibiotics (see Note 1 ).
-
2.
Cells are incubated at 37 °C in a humidified atmosphere of 95 % air–5 % CO2.
-
3.
Media is replaced with 10–15 mL fresh media on alternate days (Fig. 1; see Notes 1 and 3 ).
3.1.2 Seeding Cells for Experiments
-
1.
Using a sterile pipette, remove media from flask.
-
2.
Wash MOVAS-1 monolayer twice with 5 mL cold, sterile TBS.
-
3.
Add 5 mL Trypsin/EDTA solution per 75 cm2 flask.
-
4.
Replace flask lid and return flask to incubator for approximately 5 min to allow dislodging of monolayer from plastic surface. Monitor progress after 2.5 min. Gentle tapping on the outside of the flask may aid cells lifting.
-
5.
Stop trypsin action by adding 5 mL media.
-
6.
Mix by gentle aspiration.
-
7.
Remove cell solution and place into a clean, sterile 50 mL tube.
-
8.
Sediment cells by centrifugation for 5 min at 800 × g at 4 °C.
-
9.
Remove media and replace with 1–5 mL fresh media.
-
10.
Resuspend pellet by gentle aspiration.
-
11.
Perform cell count.
-
12.
Seed cells at desired density in 6-well plates (see Notes 3 and 4 ). Add 2 mL media to each well.
-
13.
Grow to confluence in media changing media on alternate days (see Note 5 ).
-
14.
Replace with DMEM + 10 % FCS containing treatments (see Note 6 ).
-
15.
Change media every 2–3 days for the duration of the experiment.
3.2 Calcifying Growth Conditions
3.2.1 Spontaneous Calcification
MOVAS-1 are known to calcify following long-term culture (approximately 30 days) in serum containing media. This process can be accelerated by addition of procalcific reagents to the growth media.
3.2.2 Calcium Phosphate Induced Calcification
Calcification can be induced by incubating cells in the presence of moderate doses of either calcium or phosphate or both in serum-containing media.
Under sterile conditions:
-
1.
Double filter CaCl2 and NaHPO4 solutions separately through sterile 0.22 μM microfilters.
-
2.
Prepare desired treatment doses of Ca and Pi using 1 M stock solutions diluted in media.
-
3.
A range of doses should be used, including a combination of Ca and Pi. For example, 2, 3.6 and 4.8 mM Ca; 1.5, 3 and 5 mM Pi; 2 mM Ca and 2 mM Pi; 2.8 mM Ca and 3 mM Pi.
-
4.
Culture cells for 7–21 days using the method detailed (see Subheading 3.1.1) for nodule formation.
3.2.3 Calciprotein Particle (CPP) Preparation
-
1.
Under sterile conditions combine the following with end-over-end mixing after each addition:
-
5.0 mL sterile FCS (see Note 7 ).
-
10.0 mL 20 mM CaCl2 in TBS, pH 7.4.
-
10.0 mL 14 mM NaHPO4 in TBS, pH 7.4.
-
15.0 mL sterile TBS.
in a sterile 50 mL tube.
-
-
2.
Incubate with continual slow mixing on rotator for 12 h at room temperature.
-
3.
Aliquot into sterile 2 mL tubes.
-
4.
Pellet particles by centrifugation at 24,000 × g for 2 h at 4 °C.
-
5.
Remove supernatant and wash pellet twice with ice-cold TBS.
-
6.
Resuspend the pellet in 200 μL warmed (37 °C) TBS (see Note 8 ).
-
7.
Pool supernatant mixture into a single tube.
-
8.
Spin at 1,000 × g for 10 min at room temperature to pellet large aggregates and collect supernatant (CPP stock).
-
9.
Test concentration of CPP mix using calcium assay and adjust to 1 mg/mL with sterile TBS.
-
10.
Aliquot CPP suspension into sterile 1.5 mL tubes and store at 4 °C (1–2 days) or at –80 °C for long-term storage.
-
11.
Aliquots should be thawed and diluted in media immediately prior to incubation with cells (see Note 9 ).
3.2.4 β-Glycerophosphate
β-glycerophosphate serves as a free phosphate ion donor (cleavable by alkaline phosphatase) to cells in culture. Ascorbic acid is thought to enhance both the collagen-producing and proliferative capabilities of the cells.
-
1.
Add β-glycerophosphate solution and ascorbic acid solution in media to confluent cells (see Note 10 ).
-
2.
Change media every 2–3 days, for 21 days.
3.2.5 Naked Apatite Particles
Hydroxyapatite particles formed by the premixing of calcium and phosphate salt solutions are known to induce calcification.
-
1.
Filter hydroxyapatite particles through a Vivaspin® column by filling column to its maximum volume.
-
2.
Centrifuge at 10,000 × g for 20 min at room temperature.
-
3.
Discard the filtrate and resuspend the retentate with a maximum volume of TBS.
-
4.
Centrifuge at 10,000 × g for 20 min at room temperature.
-
5.
Discard the filtrate and recover apatite in TBS solution from the concentrator pocket of the column.
-
6.
Test the concentration of apatite crystals by performing a calcium assay (see Subheading 3.4.2).
-
7.
Add to media and mix well (see Note 11 ). Treat confluent cell monolayers.
3.3 Qualitative Methods for Assessing Calcification
3.3.1 Alizarin Red Staining for Calcium Deposition
Alizarin Red is used to stain calcium deposits. The dye forms a complex with calcium during the chelation process and appears as a red salt in stained sections and cell monolayers.
-
1.
Aspirate media from cell monolayers.
-
2.
Gently wash monolayers twice with Dulbecco’s PBS (Ca2+/Mg2+ free) twice.
-
3.
Aspirate wash and fix cells with enough neutral buffered formalin (10 %) to completely cover the cell monolayer at room temperature for at least 30 min.
-
4.
Remove fixative and wash with ddH2O.
-
5.
Carefully aspirate and cover cell monolayer with Alizarin Red staining solution. Incubate at room temperature for 45 min (see Note 12 ).
-
6.
Remove excess stain and wash gently four times with ddH2O.
-
7.
Add PBS to each well.
-
8.
View under a light microscope. Calcium deposits stain red (Fig. 2a).
3.3.2 von Kossa Staining of Calcium-Containing Nodules
The von Kossa staining protocol is used to identify calcium phosphate deposits localized to cells or within tissue sections. Silver nitrate solution is used to deposit silver in areas of concentrated calcium phosphate. This is based on a reaction between silver nitrate and phosphate ions. The resulting silver phosphate salt is then degraded to silver under UV light (or strong white light).
-
1.
Remove media.
-
2.
Rinse monolayers twice with ice-cold TBS.
-
3.
Fix cells in 4 % paraformaldehyde in TBS for 5 min at room temperature.
-
4.
Remove fixative and air-dry (approximately 30 min).
-
5.
Incubate cell monolayers in 1 % sodium nitrate for 30–90 min at room temperature under ultraviolet light (see Note 13 ).
-
6.
Remove sodium nitrate and wash cells with two changes of ddH2O.
-
7.
Remove unreacted silver particles by adding 5 % sodium thiosulfate for 5 min at room temperature.
-
8.
Counterstain using Harris hematoxylin for 30 s (see Note 2 ).
-
9.
Remove and rinse twice with tap water.
-
10.
Mount by placing a small amount of aqueous mounting media on a coverslip. Invert.
-
11.
Place over cells in well.
-
12.
View under a light microscope. Calcium-containing nodules appear as black deposits (Fig. 2b).
3.4 Quantitative Methods of Assessing Calcification
3.4.1 Harvesting Samples for Estimation of Calcium Concentration
-
1.
Wash cell monolayers with TBS.
-
2.
Aspirate wash and dissolve mineral within cell monolayers by incubating with 0.6 M hydrochloric acid (6–24 h) at 4 °C (see Note 14 ).
-
3.
Collect extracts and centrifuge at 10,000 × g for 20 min at 4 °C to pellet debris.
-
4.
Collect supernatants in clean 1.5 mL tubes and store at −20 °C until analysis.
3.4.2 Estimating Calcium Concentration by Colorimetric Assay
This assay measures the chromogenic complex formed by calcium ions binding with o-cresophthalein.
-
1.
Prepare calcium standards by diluting 10 μL of calcium standard solution (500 mM) in 990 μL of ddH2O to prepare the first standard of 5 mM. Mix by aspiration.
-
2.
From this, add 0, 2, 4, 6, 8, and 10 μL into the wells of a 96-well plate. Bring the volume to 50 μL with ddH2O. Your standards will have the concentrations 0 (blank), 0.4, 0.8, 1.2, 1.6, and 2.0 μg/mL Ca (see Note 15 ).
-
3.
Aliquot 50 μL each sample into 96-well plate. Prepare in duplicate.
-
4.
Add 90 μL of chromogenic reagent to each well. Mix gently.
-
5.
Add 60 μL of Calcium Assay reagent to each well. Mix gently.
-
6.
Protect plate from light by wrapping whole plate in foil. Incubate the reaction for 5–10 min at room temperature.
-
7.
Read absorbance of wells at 575 nM (see Note 16 ).
-
8.
Plot concentration (x axis) versus average absorbance (y axis) to obtain a standard curve.
-
9.
Read average sample absorbances from the standard curve to obtain concentrations in μg/mL (see Note 17 ).
-
10.
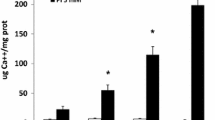
Calcium concentrations should be normalized to total protein. Neutralize and solubilize calcium extracts with 10 % TBS supplemented with 1 % SDS. Determine total protein concentration using micro BCA assay. Exemplar data showing dose-dependent CPP-induced calcium deposition on MOVAS-1 cell monolayers after 7 day treatment compared to basal media or media supplemented with equivalent doses of free calcium (CaCl2) are shown in Fig. 3.
3.4.3 Estimation of Phosphate Concentration
This commercial phosphate kit is based on the reaction of malachite green and molybdate with ionic phosphate.
-
1.
Wash cell monolayers twice using ice-cold TBS.
-
2.
Lyse cells in 200 μL ice-cold RIPA® buffer.
-
3.
Scrape cells from the plastic using a cell scraper.
-
4.
Collect the cell suspension in sterile 1.5 mL tubes.
-
5.
Incubate on ice for 45 min. Gently vortex suspensions periodically.
-
6.
Centrifuge at 10,000 × g for 20 min at 4 °C to pellet debris.
-
7.
Place supernatants in clean 1.5 mL tubes (can be stored at −80 °C prior to analysis).
-
8.
Prepare standards by performing serial dilutions of phosphate (Pi, 30 μM) standard in ddH2O. The working linear range of the assay is 0.3–50 μM, so standards and samples should be kept within this range. Dilute with ddH2O as necessary.
-
9.
In a 96-well plate, add 50 μL standards and samples into each well. Each standard and sample should be tested in duplicate. However, triplicate is optimal.
-
10.
Add 100 μL working reagent (provided in kit) to each well. Tap plate gently to mix.
-
11.
Seal and incubate plate at room temperature for 30 min.
-
12.
Read absorbance at 620 nM (600–660 nM).
-
13.
Generate a standard curve by plotting concentration (x axis) versus average absorbance (y axis) of the standards.
-
14.
Read the average sample absorption from the standard curve. Alternatively, curve plotting software may be used to devise an equation to estimate phosphate concentration within samples.
-
15.
Phosphate concentrations should be normalized to total protein using the micro BCA assay.
4 Notes
-
1.
DMEM + 10 % FCS is prepared by combining 10 mL HEPES Buffer (1 M), 4 mL glutamine (200 mM), 8 mL penicillin–streptomycin (5000 U/mL), 40 mL fetal calf serum (FCS), and 348 mL 1× DMEM and passing through a 0.22 μM filter. Store at 4 °C in a sterile bottle for up to 2 weeks. Warm to 37 °C immediately prior to use.
-
2.
Harris hematoxylin is used to add definition to the cell monolayer for the purpose of orientation under the microscope. Other stains may be used, e.g., Nuclear Fast Red.
-
3.
Cells should be maintained at 1 × 104 to 1 × 105 cells/cm2. The doubling time of MOVAS-1 cultures is approximately 15 h. This should be factored in when choosing initial seeding density and timing of your experiment.
-
4.
Cell counts can be performed either using a hemocytometer or automated cell counter. A Bio-Rad TC20® Automated Cell counter is used routinely in our lab.
-
5.
Media can be changed every 2–3 days without compromising experiments.
-
6.
DMEM + 10 % FCS is used as the base media into which treatments are placed immediately prior to addition to cells.
-
7.
Alternatively cell culture-grade human serum (Sigma) may be used in the preparation of CPP.
-
8.
The pellet does not resuspend easily. Mix vigorously by aspiration. Warm TBS is used to aid resuspension.
-
9.
Sonicate particulate suspension and media well by aspiration immediately prior to treating cells. Prepare fresh on the day of use and discard unused treatment media. A detailed methodology for CPP preparation is provided elsewhere by Smith [11].
-
10.
Current literature indicates calcification can be induced in MOVAS-1 and vascular smooth muscle cell s within the concentration range provided.
-
11.
For best results, sonicate hydroxyapatite in media for 1 min prior to addition to cell cultures.
-
12.
The pH of Alizarin Red solution affects the overall staining quality. We recommend using 0.5 % ammonium hydroxide to adjust pH when preparing 2 % aqueous (w/v) solution. Store at room temperature. Discard unused portion after 1 month.
-
13.
Light from a 60 W (or higher) bulb can be used instead of UV light. However, slides may need a longer incubation time. If the silver is removed during the wash step following incubation, the light source is not strong enough.
-
14.
When demineralizing monolayers, agitation such as use of a plate mixer is recommended.
-
15.
The linear range of this assay is 0.4–2.0 μg/mL. Samples may need to be diluted to achieve readings within the working range of the assay. A new standard curve needs to be set up with each assay run.
-
16.
Read samples within 30 min as the calcium chromophore is prone to fading over time.
-
17.
Alternatively, curve-fitting software may be employed to generate equation and concentrations can be calculated using this method.
References
Blacher J, Guerin AP, Pannier B et al (2001) Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 38:938–942
Schwarz U, Buzello M, Ritz E et al (2000) Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol Dial Transplant 15:218–223
Schlieper G, Aretz A, Verberckmoes SC et al (2010) Ultrastructural analysis of vascular calcifications in uremia. J Am Soc Nephrol 21(4):689–696
Sage AP, Tintut Y, Demer LL (2010) Regulatory mechanisms in atherosclerotic calcification. Nat Rev Cardiol 7:528–536
Jia G, Stormont RM, Gangahar DM et al (2012) Role of matrix Gla protein in angiotensin II-induced exacerbation of vascular calcification. Am J Physiol Heart Circ Physiol 303:H523–H532
Wu M, Rementer C, Giachelli CM (2013) Vascular calcification: an update on mechanisms and challenges in treatment. Calcif Tissue Int 93:365–373
Johnson RC, Leopold JA, Loscalzo J (2006) Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res 99:1044–1059
Chalmers JA, Martino TA, Tata N et al (2008) Vascular circadian rhythms in a mouse vascular smooth muscle cell line (MOVAS-1). Am J Physiol Regul Integr Comp Physiol 295:R1529–R1538
Afroze T, Yang LL, Wang C et al (2003) Calcineurin-independent regulation of plasma membrane Ca2+ ATPase-4 in the vascular smooth muscle cell cycle. Am J Physiol Cell Physiol 285:C88–C95
Rivera J, Walduck AK, Thomas RR et al (2013) Accumulation of serum lipids by vascular smooth muscle cells involves a macropinocytosis-like uptake pathway and is associated with the downregulation of the ATP-binding cassette transporter A1. Naunyn Schmiedebergs Arch Pharmacol 386:1081–1093
Smith ER (2015) Isolation, characterization of calciprotein particles in biological fluids. Methods Mol Biol (in press)
Acknowledgements
Development of these methods was supported by a Jacquot Research Establishment Award to SGH. The authors are grateful to A/Prof. Grant Drummond for supplying MOVAS-1.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Kelynack, K.J., Holt, S.G. (2016). An In Vitro Murine Model of Vascular Smooth Muscle Cell Mineralization. In: Hewitson, T., Smith, E., Holt, S. (eds) Kidney Research. Methods in Molecular Biology, vol 1397. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3353-2_14
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3353-2_14
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3351-8
Online ISBN: 978-1-4939-3353-2
eBook Packages: Springer Protocols