Abstract
The presence of calcification in the aortic valve is responsible for important prognostic information for the natural history of this disease [1, 2]. Although calcification in aortic valves has been described in the literature for over 100 years, little is known about the synthesis of bone matrix proteins in the aortic valve. Studies evaluating aortic valve calcification have focused on the expression of osteopontin (OP) expression in the mineralization zones of heavily calcified aortic valves obtained at autopsy and surgery [3, 4]. Furthermore, studies in cardiovascular calcification demonstrate parallel histologic findings in the valve and the vasculature in regards to the cellular abnormalities involved in the calcification [5].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Aortic Valve
- Cyclic Stretch
- Myofibroblast Differentiation
- Aortic Valve Calcification
- Osteoblast Bone Formation
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The presence of calcification in the aortic valve is responsible for important prognostic information regarding the natural history of this disease [1, 2]. Although calcification in aortic valves has been described in the literature for over 100 years, little is known about the synthesis of bone matrix proteins in the aortic valve. Studies evaluating aortic valve calcification have focused on the expression of osteopontin (OP) expression in the mineralization zones of heavily calcified aortic valves obtained at autopsy and surgery [3, 4]. Furthermore, studies in cardiovascular calcification demonstrate parallel histologic findings in the valve and the vasculature in regards to the cellular abnormalities involved in the calcification [5].
Vascular biologists [6], have demonstrated that lipids are important in the differentiation of vascular smooth muscle cells to calcifying cells. The field of valvular biology has also demonstrated that a similar phenotypic switch occurs in myofibroblasts isolated from the aortic valve [7–12]. Several lines of evidence indicate that osteoblasts, chondrocytes, and adipocytes are all derived from a common progenitor cell called an undifferentiated mesenchymal cell [13, 14]. The mineralized valve has been characterized as an osteoblast-like bone phenotype [11, 15].
Bone is the major component of the skeleton and is formed by two distinct process, intramembranous and endochondral. Intramembranous bone arises directly from mesenchymal cells condensing at ossification centers and transforming directly into osteoblasts. This form of ossification gives rise to the flat bones of the skull, parts of the clavicle, and the periosteal surface of long bones. Endochondral ossification differs from the intramembranous component in that is formed in the presence of a cartilaginous blastema. It is a complex, multistep process requiring the sequential formation and degradation of cartilaginous structures that serve as templates for the development of axial and appendicular bones. This formation of calcified bone on a cartilage scaffold occurs not only during skeletogenesis, but is an integral part of post-natal growth and fracture repair [16].
Valve Myofibroblast Differentiation to Bone: Cell Proliferation to Mineralization to Bone
Bone is a mineralized connective tissue, comprising an exquisite assembly of functionally distinct cell populations that are required to support the structural integrity and remodeling of the skeleton. The bone forming OB cells are derived from mesenchymal precursor cells in the bone marrow stroma and periosteum, and have the capacity for extensive proliferation [17]. In cell culture, osteoblasts are morphologically indistinguishable from fibroblasts [18, 19]. Figure 2.1, Panel a, demonstrates the first stage of OB differentiation, which is the transition of the undifferentiated mesenchymal cells to OB progenitors [20]. Figure 2.1 Panel b, demonstrates the second stage is the maturation of osteoblast progenitors into functional OB cells, which produce collagen, osteocalcin, osteopontin, bone sialoprotein and high levels of alkaline phosphatase activity. Figure 2.1, Panel c, demonstrates the final stage of OB mineralization and binding of hydroxyapatite to the newly synthesized matrix in the tissues. The regulatory mechanism of osteoblast differentiation from OB progenitor cells into terminally differentiated cells that produce bone matrix proteins has been extensively studied and requires the actions of specific paracrine/hormonal factors [21]. Genes which code for the bone extracellular matrix proteins in OB cells include alkaline phosphatase (AP), osteopontin (OP), osteocalcin (OC), bone sialoprotein (BSP) and matrix Gla protein (MGP) [22]. Interestingly, Giachelli et al. have shown that OP may have an inhibitory role in mineralization in the vascular smooth muscle cells [23, 24]. This data supports a potential regulatory mechanism that these matrix proteins may play in the development of biomineralization. To date, many of these markers have been shown to be critical in the extracellular mineralization and bone formation developing in normal OB differentiation [19]. Recent descriptive studies from patient specimens have demonstrated the critical features of aortic valve calcification, including osteoblast expression, cell proliferation and atherosclerosis [3, 11, 12, 15]. These studies define the biochemical and histological characterization of these valve lesions. Furthermore, these studies have also shown that specific bone cell phenotypes are present in calcifying valve tissue from human specimens [25, 26]. Early studies in vascular smooth muscle cells demonstrate the ability of calcifying vascular cells to have the multipotential ability to differentiate to calcifying phenotypes [13]. The first description of a possible bone protein in the valve was the discovery of the expression of the bone matrix protein osteopontin in the diseased calcific aortic valves [3, 4]. This concept was confirmed once the molecular phenotype was published demonstrating the RNA osteogenic regulation in the control versus the calcified valves from surgery explants [11]. Figure 2.2 demonstrates the first evidence at the RNA level for the activation of the osteoblast gene program in calcified human aortic valves from surgical valve replacement as compared to valve removed at the time of heart transplantation [11]. Increased gene expression of osteopontin, bone sialoprotein, and Cbfa1 (the osteoblast specific transcription factor) were all increased in the calcified aortic valves as compared to the control valves from heart transplantation. This data has provided the first molecular evidence that a parallel osteoblast gene program is important in the mineralizing phenotype found in calcified human aortic valves. The gene expression and the histomorphometric data that endochondral bone formation provide the foundation for an ossification phenotype. Osteoblast bone formation is a complex process involving multiple growth and differentiation cellular mechanisms. The presence of osteoblast bone formation in the aortic valve has provided the foundation for the hypothesis that the cells residing in the aortic valve have the potentiality to trans-differentiate into a bone forming cell, which over time mineralizes and expresses and ossification phenotype. There have been a number of studies that have identified the signaling pathways critical in the development of calcific aortic stenosis. A number of these signaling factors are similar to those found in vascular atherosclerosis and bone formation. Matrix MetalloProteinases (MMP) [27], Interleukin1 [28], Transforming Growth Factor-beta(TGF-beta), purine nucleotides [29, 30], RANK [31], osteoprotegrin(OPG) [31], and TNF alpha [32], have all been identified as signaling pathways important in the development of this disease process.
Demonstrates the three phases for developing a porcine valvular fibroblast model system to follow the osteoblast (OB) differentiation and mineralization process, important in the phenotypic switch. (a) Stage one, cell proliferation stage. (b) Stage two, extracellular matrix synthesis. (c) Stage three, mineralization phase
RNA level for the activation of the osteoblast gene program in calcified human aortic valves from surgical valve replacement as compared to valve removed at the time of heart transplantation [11]
Cardiac Valve Cell Types: Valvular Interstitial Cells
VICs are abundant in all layers of the heart valves and are crucial to function. VICs synthesize VECM and express matrix degrading enzymes (including matrix metalloproteinases [MMPs] and their inhibitors [TIMPs]) that mediate and regulate remodeling of collagen and other matrix components. VICs comprise a diverse, dynamic, and highly plastic population of resident cells [33]. They modulate function among phenotypes in response to changes in stimulation by the mechanical environment or by certain chemicals, during valvular homeostasis, adaptation, and pathology. Adult heart valve VICs in-situ have characteristics of resting fibroblasts; they are quiescent, without synthetic or destructive activity for ECM. VICs are activated during intrauterine valvular maturation, by abrupt changes in the mechanical stress state of valves and in disease states, and VICs continuously repair a low level of injury to the VECM that occurs during physiological functional remodeling of AV tissue [34]. Table 2.1 demonstrates the phenotypic transitions of the VIC cells, which are critical for normal development, homeostasis, and function of the aortic valve, and likely mediate the development of valve calcification [33]. Once activated, VICs can differentiate into a variety of other cell types [35], including myofibroblasts and osteoblasts, although valve osteoblasts may respond to cellular signals differently than skeletal osteoblasts.
Establish an In Vitro Model of Aortic Valve Calcification
This cell culture model is currently being utilized in other laboratories [7, 36, 37]. We established this model in order to investigate the molecular mechanisms of calcification in the aortic valve. The myofibroblast cell is the suspected target for differentiation into OB like cells in the aortic valve. Therefore, determining the signaling pathways in this cell will help to define the changes found in the ex vivo calcified aortic valves and define future medical therapies for this disease. In vitro studies have been instrumental to determine the timing and phenotypic characterization of calcifying nodules using an in vitro cell culture model of porcine aortic valve cells. Chapter 1, describes the cell isolation technique for myofibroblast cells from porcine aortic valves.
Osteogenic Transcription Factor Expression in Calcified Aortic Valves
The evidence for osteoblastogeneis in aortic valve myofibroblast is dependent on defining the regulatory elements controlling osteoblast (OB) differentiation as in Fig. 2.2, which demonstrates the bone transcription factors upregulated in the calcified valves. The next step to study the osteogenic regulation of bone formation in the valve, Fig. 2.3, demonstrates the in vitro evidence for osteogensis in the myofibroblast cell.
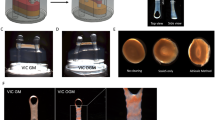
Panel (a) Matrix gene expression in valve myofibroblast cells, Panel (b) Alkaline phosphatase assay in myofibroblast cells treated with dexamethasone and TGF beta, and Panel (c), is the Control experiment with the non-calcifying cells, (Negative Von Kossa stain), Panel (d), is the Calcifying cells treated with the osteogenic media (Positive Von kossa stain) after 6 weeks of treatment in vitro
Myofibroblast cells in culture were differentiated to osteoblast-like cells using osteogenic media. Figure 2.3 demonstrates the three phases for developing a porcine valvular fibroblast model system to follow the osteoblast (OB) differentiation and mineralization process, and determine the cell type responsible for the phenotypic switch as shown in the model for Fig. 2.1. Figure 2.3, Panel a, demonstrates the mRNA expression of the bone markers, osteopontin and type-I collagen in porcine valve fibroblast cells cultured for 2–20 days with dexamethasone and TGFbeta. The results indicate that the growth of these cells in culture for longer periods and under specified certain conditions induces expression of two important OB markers. Figure 2.3, Panel b, demonstrates the increase in myofibroblast synthesis of alkaline phosphatase after treatment with TGFbeta for 32 days. Figure 2.3, Panel d, Von Kossa staining of the valvular fibroblasts reveals nodule formation after treatment with dexamethasone and TGFbeta for 20 days whereas the control myofibroblast cells, Fig. 2.3, Panel c, received only 0.5 % media and no dex and no TGFbeta demonstrates no nodule formation. These results demonstrating that dexamethasone and TGFbeta are important in myofibroblast differentiation are also important agents in OB differentiation [38–40]. This data supports the feasibility of using an in vitro system to investigate and characterize potential signaling pathways. Also, the preliminary data supports the hypothesis that myofibroblasts can differentiate into an OB-like cell. An in vitro model system will be invaluable to determine the cellular mechanisms involved in the cellular differentiation/transformation and induction of the calcification process as well as determining the mechanism by which these cells undergo a phenotype switch from a valve myofibroblast cell to an OB-like cell.
The Role of the Stem Cell Niche: Myofibroblast Cell Differentiating to Osteogenic Bone
The next assay is to test the myofibroblast cell’s ability to differentiate to mineralized bone via upregulation of the Lrp5 receptor. The concept of the cell-cell communication was the foundation for these sets of experiments to test the role of conditioned media released from the endothelial cell to initiate the myofibroblast differentiation. There are two necessary corollaries for this experiment: (1) cell-cell communication, (2) oxidative stress gradient in the presence of elevated LDL [41].
Myofibroblasts were treated with three different conditions to determine the microenvironment necessary to activate the Lrp5 pathway of bone formation. The initial set of conditions includes treatment of the myofibroblasts with osteogenic differentiating media. This osteogenic differentiation media provides the mineralizing microenvironment necessary for the calcification of bone mineralization [42]. The cells were treated with osteogenic media in Fig. 2.4, Panel a. Over time the myofibroblast cells stain positive for Alcian blue, indicating the transformation to a chondrocyte phenotype. After 6 weeks, the cells begin to mineralize and form bone as implicated with the positive stain for Alizarin read as shown in Fig. 2.4, Panel b. The microCT indicates mineralization in this in vitro model with upregulation of Runx2 and Lrp5 the key regulators of Wnt regulation of bone formation, Fig. 2.4, Panels c and d.
Osteogenic mineralization assays in a stem cell niche. Panel (a) The myofibroblast cells stain for Alcian blue, indicating a cartilaginous phenotype treated with osteogenic media. Panel (b) The myofibroblast cells stain positive for Alizarin red, indicating an osteoblast phenotype treated with osteogenic media. Panel (c) RTPCR for Cbfa1 and Lrp5, in the myofibroblast cells treated with osteogenic media. Panel (d) MicroCT of the calcifying cells indicating mineralization present in the cells. Panel (e) RTPCR for Lrp5, Cbfa1 and osteopontin in the myofibroblast cells treated with directly with lipids with and without Atorvastatin. Panel (f) RTPCR for Lrp5, Cbfa1 and osteopontin in the myofibroblast cells treated with conditioned media from Endothelial cells with lipids with and without Atorvastatin. Panel (g) RTPCR for Lrp5, Cbfa1 and osteopontin in the myofibroblast cells treated with cyclic stretch and with lipids with and without Atorvastatin. Panel (h) RTPCR for Lrp5, Cbfa1 and osteopontin in the myofibroblast cells treated with cyclic stretch and conditioned media from Endothelial cells with lipids with and without Atorvastatin
Next, the cells were treated with LDL, with and without Atorvastatin directly shown in Fig. 2.4, Panel e. When the cells were treated with lipids directly, there was no gene expression of the Lrp5 receptor and low level expression of Runx2 and osteopontin. Figure 2.4, Panel f, demonstrates the gene expression in myofibroblast cells treated with conditioned media from aortic valve endothelial cells. The conditioned media was produced in the presence of LDL, with and without Atorvastatin. The AEC conditioned media is required to upregulate the Lrp5 gene in this model of endothelial/mesenchmyal cross talk. The conditioned media from the endothelial cells treated with lipids induced the Lrp5 gene expression and mildly attenuated with Atorvastatin therapy. Figure 2.4, Panels g and h, demonstrates the final set of conditions, the response to mechanical force by measuring the Lrp5 expression in the myofibroblast cells with cyclic stress. The Lrp5 is expressed after the application of the cyclic stretch, and is further increased with the application of the cyclic stretch and the Conditioned media treated cells. This data, in combination with previously published data [10, 43, 44], indicates that LDL and Pressure are both necessary for the upregulation of the Lrp5/Wnt3a pathway [45, 46].
Summary
The model proposed in the study as described in Fig. 2.2 provides the cellular architecture for the development of this disease process. The stem cell niche is a unique model for the development of an oxidative stress communication within the aortic valve endothelium. As shown in Fig. 2.2, oxidative stress contributes to the release of Wnt3a into the subendothelial space to activate Lrp5/Frizzled receptor complex on the extracellular membrane of the myofibroblast. This trimeric complex then induces glycogen synthase kinase to be phosphorylated. This phosphorylation event causes β-catenin translocation to the nucleus. β-catenin acts as a coactivator of osteoblast specific transcription factor Runx2 to induce mesenchymal osteoblastogenesis in the aortic valve myofibroblast cell.
References
Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O’Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Circulation. 2011;124(16):1783–91.
Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–7.
O'Brien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE, Otto CM. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995;92:2163–8 [comment].
Mohler 3rd ER, Adam LP, McClelland P, Graham L, Hathaway DR. Detection of osteopontin in calcified human aortic valves. Arterioscler Thromb Vasc Biol. 1997;17:547–52.
Vattikuti R, Towler DA. Osteogenic regulation of vascular calcification: an early perspective. Am J Physiol Endocrinol Metab. 2004;286:E686–96.
Parhami F, Basseri B, Hwang J, Tintut Y, Demer LL. High-density lipoprotein regulates calcification of vascular cells. Circ Res. 2002;91:570–6.
Mohler 3rd ER, Chawla MK, Chang AW, Vyavahare N, Levy RJ, Graham L, Gannon FH. Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis. 1999;8:254–60.
Rajamannan NM. Calcific aortic valve disease: cellular origins of valve calcification. Arterioscler Thromb Vasc Biol. 2011;31:2777–8.
Rajamannan NM. Role of oxidative stress in calcific aortic valve disease: from bench to bedside. In: Morales-Gonzalez J.A.Oxidative Stress and Chronic Degenerative Diseases – A Role for Antioxidants, ] InTech Publisher, 2013; Chapter 11, pp. 265–87.
Rajamannan NM, Subramaniam M, Caira F, Stock SR, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the lrp5 receptor pathway. Circulation. 2005;112:I229–34.
Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–4.
Rajamannan NM, Subramaniam M, Springett M, Sebo TC, Niekrasz M, McConnell JP, Singh RJ, Stone NJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation. 2002;105:2260–5.
Tintut Y, Alfonso Z, Saini T, Radcliff K, Watson K, Bostrom K, Demer LL. Multilineage potential of cells from the artery wall. Circulation. 2003;108:2505–10.
Rawadi G, Vayssiere B, Dunn F, Baron R, Roman-Roman S. Bmp-2 controls alkaline phosphatase expression and osteoblast mineralization by a wnt autocrine loop. J Bone Miner Res. 2003;18:1842–53.
Mohler 3rd ER, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–8.
Bonner F, Farach-Carson MC. Bone formation. New York: Springer; Topics in Bone Biology. 2003. Vol 1. p. 1–15.
Bostrom K, Tintut Y, Kao SC, Stanford WP, Demer LL. Hoxb7 overexpression promotes differentiation of c3h10t1/2 cells to smooth muscle cells. J Cell Biochem. 2000;78:210–21.
Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–54 [see comment].
Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science. 2000;289:1501–4.
Waters KM, Rickard DJ, Riggs BL, Khosla S, Katzenellenbogen JA, Katzenellenbogen BS, Moore J, Spelsberg TC. Estrogen regulation of human osteoblast function is determined by the stage of differentiation and the estrogen receptor isoform. J Cell Biochem. 2001;83:448–62.
Aubin JE, Liu F, Malaval L, Gupta AK. Osteoblast and chondroblast differentiation. Bone. 1995;17:77S–83.
Young MF, Kerr JM, Ibaraki K, Heegaard AM, Robey PG. Structure, expression, and regulation of the major noncollagenous matrix proteins of bone. Clin Orthop Relat Res. 1992;281:275–94.
Speer MY, McKee MD, Guldberg RE, Liaw L, Yang HY, Tung E, Karsenty G, Giachelli CM. Inactivation of the osteopontin gene enhances vascular calcification of matrix gla protein-deficient mice: evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. J Exp Med. 2002;196:1047–55.
Steitz SA, Speer MY, McKee MD, Liaw L, Almeida M, Yang H, Giachelli CM. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol. 2002;161:2035–46.
Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47:1707–12.
Jian B, Jones PL, Li Q, Mohler 3rd ER, Schoen FJ, Levy RJ. Matrix metalloproteinase-2 is associated with tenascin-c in calcific aortic stenosis. Am J Pathol. 2001;159:321–7.
Kaden JJ, Vocke DC, Fischer CS, Grobholz R, Brueckmann M, Vahl CF, Hagl S, Haase KK, Dempfle CE, Borggrefe M. Expression and activity of matrix metalloproteinase-2 in calcific aortic stenosis. Z Kardiol. 2004;93:124–30.
Kaden JJ, Dempfle CE, Grobholz R, Tran HT, Kilic R, Sarikoc A, Brueckmann M, Vahl C, Hagl S, Haase KK, Borggrefe M. Interleukin-1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis. 2003;170:205–11.
Osman L, Chester AH, Amrani M, Yacoub MH, Smolenski RT. A novel role of extracellular nucleotides in valve calcification: a potential target for atorvastatin. Circulation. 2006;114:I566–72.
Osman L, Amrani M, Isley C, Yacoub MH, Smolenski RT. Stimulatory effects of atorvastatin on extracellular nucleotide degradation in human endothelial cells. Nucleosides Nucleotides Nucleic Acids. 2006;25:1125–8.
Kaden JJ, Bickelhaupt S, Grobholz R, Haase KK, Sarikoc A, Kilic R, Brueckmann M, Lang S, Zahn I, Vahl C, Hagl S, Dempfle CE, Borggrefe M. Receptor activator of nuclear factor kappab ligand and osteoprotegerin regulate aortic valve calcification. J Mol Cell Cardiol. 2004;36:57–66.
Kaden JJ, Kilic R, Sarikoc A, Hagl S, Lang S, Hoffmann U, Brueckmann M, Borggrefe M. Tumor necrosis factor alpha promotes an osteoblast-like phenotype in human aortic valve myofibroblasts: a potential regulatory mechanism of valvular calcification. Int J Mol Med. 2005;16:869–72.
Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. 2007;171:1407–18.
Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113:1344–52.
Chen JH, Yip CY, Sone ED, Simmons CA. Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential. Am J Pathol. 2009;174:1109–19.
Tintut Y, Parhami F, Bostrom K, Jackson SM, Demer LL. Camp stimulates osteoblast-like differentiation of calcifying vascular cells. Potential signaling pathway for vascular calcification. J Biol Chem. 1998;273:7547–53.
Johnson CM, Hanson MN, Helgeson SC. Porcine cardiac valvular subendothelial cells in culture: cell isolation and growth characteristics. J Mol Cell Cardiol. 1987;19:1185–93.
Selvamurugan N, Kwok S, Alliston T, Reiss M, Partridge NC. Transforming growth factor-beta 1 regulation of collagenase-3 expression in osteoblastic cells by cross-talk between the smad and mapk signaling pathways and their components, smad2 and runx2. J Biol Chem. 2004;279:19327–34.
Roelen BA, Dijke P. Controlling mesenchymal stem cell differentiation by tgfbeta family members. J Orthop Sci. 2003;8:740–8.
Chang W, Parra M, Ji C, Liu Y, Eickelberg O, McCarthy TL, Centrella M. Transcriptional and post-transcriptional regulation of transforming growth factor beta type ii receptor expression in osteoblasts. Gene. 2002;299:65–77.
Rajamannan NM. Oxidative-mechanical stress signals stem cell niche mediated lrp5 osteogenesis in enos(-/-) null mice. J Cell Biochem. 2012;113:1623–34.
Stringa E, Filanti C, Giunciuglio D, Albini A, Manduca P. Osteoblastic cells from rat long bone. I. Characterization of their differentiation in culture. Bone. 1995;16:663–70.
Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine wnt signals. J Clin Invest. 2005;115:1210–20.
Kirton JP, Crofts NJ, George SJ, Brennan K, Canfield AE. Wnt/beta-catenin signaling stimulates chondrogenic and inhibits adipogenic differentiation of pericytes: potential relevance to vascular disease? Circ Res. 2007;101:581–9.
Rajamannan NM. The role of lrp5/6 in cardiac valve disease: Ldl-density-pressure theory. J Cell Biochem. 2011;112(9):2222–9.
Rajamannan NM. The role of lrp5/6 in cardiac valve disease: experimental hypercholesterolemia in the apoe-/-/lrp5-/- mice. J Cell Biochem. 2011;112:2987–91.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Rajamannan, N.M., Cicek, M., Hawse, J.R., Spelsberg, T.C., Subramaniam, M. (2014). In Vitro Cell Culture Model of Calcification: Molecular Regulation of Myofibroblast Differentiation to an Osteoblast Phenotype. In: Rajamannan, N. (eds) Molecular Biology of Valvular Heart Disease. Springer, London. https://doi.org/10.1007/978-1-4471-6350-3_2
Download citation
DOI: https://doi.org/10.1007/978-1-4471-6350-3_2
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-6349-7
Online ISBN: 978-1-4471-6350-3
eBook Packages: MedicineMedicine (R0)