Summary
The coagulation of milk (by proteolysis or acidification) is the key operation in cheesemaking. The enzymatic (rennet-induced) coagulation of milk can be divided into two phases: (1) hydrolysis of the micelle-stabilizing protein, κ-casein, (2) aggregation and gelation of the rennet-altered micelles, with the development of a particulate gel.
The mechanism of the primary (enzymatic) phase has been described in molecular terms and the effects of various environmental factors thereon quantified. Aggregation of the rennet-altered micelles occurs when the zeta potential of the micelles, due mainly to the surface layer of κ-casein, has been reduced to a critical level. The effects of various compositional and environmental factors on the aggregation of the altered micelles have been described. Gelation is usually regarded as a continuation of the secondary (aggregation) phase, but requires a different approach and instrumentation for its study. It is the most complex and, at present, the least well understood aspect of the enzymatic coagulation of milk. Several instruments are available with which to study the rheological properties of the gel and there is particular interest in developing methods that can be used to study/quantify gelation in the cheese vat.
An overview of the rennet-induced coagulation of milk will be presented in this chapter.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
As discussed in Chap. 2, the milk for most cheese varieties is coagulated through the action of selected proteinases, called rennets . The rennet-induced coagulation of milk is a two-stage process (Fig. 7.1). The primary phase involves the specific enzymatic modification of the casein micelles to produce para-casein micelles which aggregate in the presence of Ca2+ at a temperature >~ 20 °C; aggregation of the rennet-altered micelles is referred to as the secondary phase of coagulation. The primary phase of rennet action is well characterized but the secondary phase is less clear. The subject has been reviewed by Fox (1984), Fox and Mulvihill (1990), Dalgleish (1992, 1993), Green and Grandison (1993), Fox et al. (1996), Fox and McSweeney (1997), Hyslop (2003), Horne and Banks (2004) and Lucey (2011).
7.2 Primary Phase of Rennet Coagulation
As discussed in Chap. 4, the caseins exist as micelles stabilized by a surface layer of κ-casein. Following its isolation in 1956, it was shown that κ-casein is the micelle-stabilizing protein and that its stabilizing properties are destroyed on renneting. Shortly afterwards, it was shown that κ-casein is the only protein hydrolyzed during the rennet-induced coagulation of milk and that it is hydrolyzed specifically at the Phe105-Met106 bond (Fig. 7.2). The N-terminal part of the molecule, κ-CN f1-105, referred to as para-κ-casein, remains attached to the casein micelle while the C-terminal part, referred to as the caseinomacropeptide (CMP) or glycomacropeptide (GMP), because it contains the carbohydrate moieties of κ-casein, is lost into the surrounding aqueous medium. It has been recognised since the end of the nineteenth century that small peptides are produced on renneting. As discussed in Chap. 4, there are nine forms of κ-casein which differ in sugar content; hence, 9 CMPs are produced. All the CMPs are soluble in 2 % TCA but only the glycosylated forms are soluble at higher concentrations of TCA. Thus, TCA-soluble N, or more specifically TCA-soluble sugars, e.g., N-acetyl neuramic acid, can be used to monitor the primary phase of rennet coagulation (Fig. 7.3).
The unique sensitivity of the Phe-Met bond of κ-casein has aroused interest. The dipeptide, H.Phe-Met.OH, is not hydrolyzed, nor are tri- or tetra-peptides containing a Phe-Met bond. However, this bond is hydrolyzed in the pentapeptide, H.Ser-Leu-Phe-Met-Ala-OMe, and reversing the positions of serine and leucine, to give the correct sequence of κ-casein, increases the susceptibility of the Phe-Met bond to chymosin. Both the length of the peptide and the sequence around the Phe-Met bond are important determinants of enzyme-substrate interaction. Serine104 appears to be particularly important and its replacement by Ala, or even L-Ser, in the above pentapeptide renders the Phe-Met bond very resistant to hydrolysis by chymosin . Extension of the pentapeptide, H.Ser.Phe.Met.Ala.Ile.OH (i.e., κ-CN f104-108), from the N- and/or C-terminal to reproduce the sequence of κ-casein around the chymosin-susceptible bond increases the efficiency with which the Phe-Met bond is hydrolyzed by chymosin (Table 7.1). The sequence κ-CN f98-111 includes all the residues necessary to render the Phe-Met bond as susceptible to hydrolysis by chymosin at pH 4.7 as it is in intact κ-casein; it is hydrolyzed ~66,000 times faster than the parent pentapeptide (κ-CN f104-108), with a kcat/Km of ~2 M−1 s−1, which is similar to that for intact κ-casein (Visser 1981; Visser et al. 1987). κ-Casein and the peptide κ-CN f98-111 are also readily hydrolyzed at pH 6.6 but smaller peptides are not.
The Phe and Met residues in the chymosin-sensitive bond of κ-casein are not intrinsically essential for chymosin action. There are numerous Phe and a substantial number of Met residues in all milk proteins. In porcine and human κ-caseins, the chymosin-sensitive bond is Phe-Ile, while in rat and mouse κ-caseins, it is Phe-Leu; yet, these proteins are readily hydrolyzed by calf chymosin, although more slowly than bovine κ-casein. In contrast, porcine milk is coagulated more effectively than bovine milk by porcine chymosin, indicating that unidentified subtle structural features influence chymosin action. Camel milk , the κ-casein in which contains a Phe-Ile bond, is not coagulated by calf chymosin but bovine milk is coagulated faster by camel chymosin than by calf chymosin. Peptides in which Phe was replaced by Phe (NO2) or cyclohexylamine are also hydrolyzed by chymosin although less effectively than those with a Phe-Met bond; oxidation of Met106 reduces kcat/Km ca. tenfold but substitution of Nle for Met increases it ca. threefold.
A genetically engineered mutant of κ-casein, in which Met106 was substituted by Phe106, i.e., the chymosin-sensitive bond was changed from Phe105-Met106 to Phe105-Phe106, was hydrolyzed 1.8 times faster by chymosin than natural κ-casein. These findings suggest that the sequence around the Phe-Met bond, rather than the residues in the bond itself, contains the important determinants of hydrolysis by chymosin. The particularly important residues are Ser104, the hydrophobic residues Leu103 and Ile108, at least one of the three histidines (residues 98, 100 or 102, as indicated by the inhibitory effect of photooxidation) and Lys111. Studies on chemically or enzymatically modified peptide analogues of κ-CN f98-112 indicated the relative importance of residues in the sequences of 98–102 and 111–112. It has been suggested that the sequence Leu103 to Ile108 of κ-casein, which probably exists as an extended β-structure, fits into the active site cleft of acid proteinases. The hydrophobic residues , Leu103, Phe105, Met106, and Ile108, are directed towards hydrophobic pockets along the active site cleft while the hydroxyl group of Ser104 forms part of a hydrogen bond with some counterpart in the enzyme. It has been proposed that the sequences 98–102 and 109–111 form β-turns around the edges of the active site cleft of the enzyme; this conformation is stabilized by Pro residues at positions 99, 101, 109 and 110. The three His residues at positions 98, 100, 102, and Lys111 are probably involved in electrostatic bonding between enzyme and substrate; none appears to have a predominant role. Lys112 appears not to be important in enzyme-substrate binding as long as Lys111 is present.
The significance of electrostatic interactions in chymosin-substrate complex formation is indicated by the effect of added NaCl on the rennet coagulation time (RCT) of milk: addition of NaCl up to 3 mM reduces RCT but higher concentrations have an inhibitory effect; it is claimed that the effect of NaCl is on the primary, enzymatic phase rather than on the aggregation of rennet-altered micelles. Increasing ionic strength (0.01–0.11) reduces the rate of hydrolysis of κ-CN fHis98-Lys111/112 in a model system; the effect becomes more marked as the reaction pH is increased but is independent of ion type.
As well as serving to elucidate the importance of certain residues in the hydrolysis of κ-casein by chymosin, small peptides that mimic or are identical to the sequence of κ-casein around the Phe-Met bond are very useful substrates for determining the activity of rennets in absolute units, i.e., independent of variations in the non-enzymatic phase of coagulation of different milks. Standard methods for such quantification have been developed and chromogenic peptide substrates are available commercially, e.g., the chromophoric heptapeptides Leu,Ser,Phe(NO2)Nle.Ala.Leu.OMe (Dunn et al. 1986) or Pro.Thr.Glu.Phe(NO2).Phe.Arg.Leu (Hurley et al. 1999). Since the specific activity of different rennets on these peptides varies, methods for quantifying the proportions of acid proteinases in commercial rennets have been proposed.
7.3 Rennet
Several proteinases will coagulate milk under suitable conditions but most are too proteolytic relative to their milk clotting activity (MCA) ; consequently, they hydrolyse the caseins in the coagulum too quickly, causing a reduced cheese yield (MCA is the inverse of RCT; i.e., MCA = 1/RCT). Excessive proteolysis or incorrect specificity may also lead to defects in the flavour, especially bitterness, and texture of the cheese. Although plant proteinases appear to have been used as rennets since prehistoric times, gastric proteinases from calves, kids or lambs have been used traditionally as rennets, with very few exceptions.
Animal rennets are prepared by extracting the dried (usually) or salted gastric tissue (referred to as vells) with 10 % NaCl and activating and standardizing the extract. Standard calf rennet contains ca. 60–70 RU/ml and is preserved by making the extract to 20 % NaCl and adding sodium benzoate or sodium propionate. A rennet unit (RU) is the amount of rennet activity that will coagulate 10 ml of milk (usually low-heat skim milk powder reconstituted in 0.01 % CaCl2 and perhaps adjusted to pH 6.5) in 100 s. Chymosin (an aspartyl acid proteinase, i.e., a proteinase with two aspartic acid residues at the active site and with a pH optimum of 2–4) represents >90 % of the MCA of good quality veal rennet, the remaining activity being due to pepsin. As the animal ages, especially when fed solid food, the secretion of chymosin declines while that of pepsin increases.
Like many other animal proteinases, chymosin is secreted as its zymogen, prochymosin, which is autocatalytically activated on acidification to pH 2–4 by removal of a 44-residue peptide from the N-terminal of the zymogen (see Foltmann 1993).
Chymosin, an acid proteinase, is well characterized at the molecular level (see Foltmann 1993; Chitpinityol and Crabbe 1998; Crabbe 2004). The enzyme, which was crystallized in the 1940s (Hankinson 1943; Berridge 1945), is a single-chain polypeptide containing about 323 amino acid residues with a molecular mass of 35,600 Da. Its primary structure has been established and a considerable amount of information is available on its secondary and tertiary structures (Fig. 7.4). The molecule exists as two domains separated by the active site cleft in which the two catalytically-active aspartyl residues (Asp32 and Asp215)) are located (see Plowman and Creamer 1995).
Calf rennet contains three chymosin isoenzymes, principally A and B, with lesser amounts of C. Chymosins A and B which are produced from the corresponding zymogens, prochymosins A and B, differ by only one amino acid residue at position 254 (Asp in A, Gly in B). Initially chymosin C was considered to be a degradation product of chymosin A which lacks three residues, Asp244-Phe246, but now it appears to be the product of a different allele. The specific activity of chymosin A, B and C is ~120, 100 and 25 RU/mg, respectively. Chymosins A and B have an optimum pH at 4.2 and 3.7, respectively. The properties of different rennets are discussed in Sect. 7.9.
7.4 Factors that Affect the Hydrolysis of κ-Casein and the Primary Phase of Rennet Coagulation
The hydrolysis of κ-casein is influenced by many factors, some of which are discussed below. While many factors influence both the primary and secondary stages, the effects on each will be discussed separately.
-
pH The pH optimum for chymosin and bovine pepsin on small synthetic peptides is ca. 4.7 but is 5.3–5.5 on κ-CN fHis98-Lys111/112. Chymosin hydrolyses insulin, acid-denatured haemoglobin and Na-caseinate optimally at pH 4.0, 3.5 and 3.5, respectively. The pH optimum for the first stage of rennet action in milk is ~6.0 at 4 or 30 °C.
-
Ionic strength The influence of ionic strength on the primary phase of rennet coagulation was discussed in Sect. 7.2.
-
Temperature The optimum temperature for the coagulation of milk by calf rennet at pH 6.6 is 45–48 °C; presumably, the optimum for the hydrolysis of κ-casein is about this value. The effect of temperature depends on the type of rennet (Fig. 7.5). The temperature coefficient (Q10) for the hydrolysis of κ-casein in solutions of Na-caseinate is ca. 1.8, the activation energy, Ea, is ~40,000 J mol−1, and activation entropy, ∆S, is ~−90 J K−1 mol−1; generally similar values have been reported for the hydrolysis of isolated κ-casein by chymosin.
-
Heat Treatment of Milk Heat treatment of milk at a temperature >72 °C adversely affects its rennet coagulability; if the heat treatment is very severe (>90 °C for 10 min), the milk fails to coagulate on renneting. Although changes in salts equilibria are contributory factors, the principal causative factor is intermolecular disulphide bond formation between κ-casein and β-lactoglobulin and/or α-lactalbumin. Both the primary and especially the secondary phases of rennet action are inhibited in heated milk, as reflected by the marked decreases in curd firming rate and in the strength of the resulting gel. The adverse affects of heating can be reversed by acidification to pH values in the region 6.6 to 6.0, before or after heating, or by addition of CaCl2 (which causes a reduction in pH); the secondary, rather than the primary, phase of rennet action probably benefits from these treatments.
7.5 Secondary (Non-enzymatic) Phase of Coagulation and Gel Assembly
Hydrolysis of κ-casein by chymosin or similar enzymes during the primary phase of rennet action releases the highly charged, hydrophilic C-terminal segment of κ-casein (glycomacropeptide), as a result of which the zeta potential of the casein micelles is reduced from −10/−20 to −5/−7 mV and the protruding peptides (hairs) are removed from their surfaces, thus destroying the principal micelle-stabilizing factors (electrostatic and steric) and their colloidal stability. When ~85 % of the total κ-casein has been hydrolyzed, the stability of the micelles is reduced to such an extent that when they collide, they remain in contact and eventually build into a three-dimensional network, referred to as a coagulum or gel (Fig. 7.6). Gel formation is accompanied by sharp increases in viscosity and elastic shear modulus, G′, which is a measure of gel firmness (Fig. 7.7 ; see Sect. 7.8.2). Reducing the pH or increasing the temperature from the normal values (~6.6 and ~31 °C, respectively) permits coagulation at a lower degree of κ-casein hydrolysis. Although the precise reactions involved in aggregation are not known, the kinetics of aggregation have been described.
Schematic representation of the rennet coagulation of milk. (a) Casein micelles with intact κ-casein layer being attacked by chymosin; (b) micelles denuded of κ-casein; (c) extensively denuded micelles in the process of aggregation; (d) release of macropeptides (filled diamond ) and changes in relative viscosity ( ) during the course of rennet coagulation
) during the course of rennet coagulation
Changes in viscoelasticity and microstructure during rennet-induced gelation of milk, showing increases in elastic shear modulus (G′, filled triangle), loss modulus (G″, open triangle), and reduction in phase angle (open square), and aggregation of rennet-hydrolysed casein micelles into a network of para-casein micelles (as indicated by the increased white area in the confocal laser scanning micrographs). Modified from Fox and Guinee 2013
The assembly of rennet-altered micelles into a gel has been studied using various forms of viscometry, electron microscopy and light scattering. Viscosity measurements show that the viscosity of renneted milk remains constant or decreases slightly during a period equivalent to ~60 % of the visually observed RCT (Figs. 7.6 and 7.7). It has been suggested that the initial decrease in viscosity is due to a decrease in the voluminosity of the casein micelles following release of the macropeptides which form a ‘hairy layer’ ~12 nm thick (de Kruif and Holt 2003; Fig. 7.6a–d). The decrease in micelle size has been confirmed by quasi-elastic light scattering.
The gelation process , generally referred to as the secondary phase of rennet coagulation, involves initially the formation of chains and clumps of micelles, leading eventually to the formation of a network of partly-fused micelles. During the first 60 % of the visually observed RCT, the micelles exist as individual particles; the primary enzymatic reaction is ~85 % complete at 60 % of the visual RCT. Between 60 and 80 % of the RCT, the rennet-altered micelles begin to aggregate steadily with no sudden change in the type of extent of aggregation. Small, chain-like aggregates, rather than clumps, form initially (Fig. 7.8). At 100 % of the RCT, most of the micelles have aggregated into short chains, which then begin to aggregate with the formation of a network. Aggregation of the rennet-altered micelles can be described by the von Smoluchowski theory for diffusion-controlled aggregation of hydrophobic colloids when allowance is made for the need to produce, by enzymatic hydrolysis, a sufficient concentration of particles capable of aggregating, i.e., casein micelles in which >97 % of the κ-casein has been hydrolysed. The diffusion of the particles is rate-limiting and is determined by the random fruitful collision of particles (rennet-altered micelles). The rate of aggregation is not consistent with a branching process model since the micellar functionality is 1.8, whereas an average functionality greater than 2 is required for network formation.
Schematic representation of the various stages involved in the formation of cheese curd
from milk, starting from the initial mixture of casein micelles and added enzyme (rennet  ) in the milk (A), and proceeding through rennet-induced hydrolysis of κ-casein (B–C), aggregation of para-casein micelles (C) and formation of a para-casein gel network (D), which is dehydrated and concentrated into cheese curd (E) (from Fox and Guinee 2013)
) in the milk (A), and proceeding through rennet-induced hydrolysis of κ-casein (B–C), aggregation of para-casein micelles (C) and formation of a para-casein gel network (D), which is dehydrated and concentrated into cheese curd (E) (from Fox and Guinee 2013)
According to Dalgleish (1980), the overall rennet coagulation of milk can be described by combining three factors :
-
proteolysis of κ-casein, which may be described by Michaelis-Menten kinetics;
-
requirement that ~97 % of the κ-casein on a micelle be hydrolysed before it can participate in aggregation;
-
aggregation of para-casein micelles via a von Smoluchowski process.
The overall clotting time, tc, is the sum of the enzymatic phase and the aggregation phase :
where: Km and Vmax are the Michaelis-Menten parameters , αc is the extent of κ-casein hydrolysis, So is the initial concentration of κ-casein, ks is the rate constant for aggregation, Co concentration of aggregating material, Mcrit = weight average molecular weight at tc (~10 micellar units), Mo = weight average molecular weight at t = 0.
Darling and van Hooydonk (1981) proposed an alternative model for rennet coagulation, again by combining Michaelis-Menten enzyme kinetics with von Smoluchowski aggregation kinetics. The stability factor in von Smoluchowski’s theory is considered as a variable determined by the concentration of unhydrolysed surface κ-casein. The coagulation time, tc, is given by:
where, V = velocity of enzymatic hydrolysis of κ-casein, So = initial concentration of κ-casein, Cm = a constant relating the stability of the casein micelle to κ-casein concentration, Wo = initial stability factor for casein micelles, no = initial concentration of casein micelles and nc = concentration of casein aggregates at the observed clotting time, tc. It is claimed that this theoretical model explains the experimentally observed influence of protein concentration, enzyme concentration and temperature on RCT, and the occurrence of a lag phase equal to 60 % of RCT.
With milk of normal concentration, ~90 % of the micelles are incorporated into the curd at 100 % of the visual RCT but only ~50 % are incorporated in a fourfold concentrate when the same level of rennet is used. The micelles that are ‘free’ at or after the RCT may react differently from those free prior to RCT, i.e., before visual coagulation, all micelles are freely dispersed in the serum and can aggregate randomly but once a gel matrix has started to form, free micelles may react either with the gel matrix or with other free micelles. Therefore, a gel assembly may be regarded as a two-stage process and the properties of the final gel may be affected considerably by the amount of casein ‘free’ at the RCT. Since this is particularly high in concentrated milks, it may explain the coarser structure of curd made from concentrated milk.
Based on viscometric data , Tuszynski (1971) suggested that gel assembly is a two-stage process: flocculation and gelation. Turbidity experiments also suggest a two-stage gel assembly process (Surkov et al. 1982):
where E is enzyme, S is substrate, P is the reaction product, P* is para-casein micelles with transformed quaternary structure and Pn is the gelled micelle aggregate. The first two steps are the Michaelis-Menten model for the primary, enzymatic, phase and are essentially as proposed by Payens et al. (1977):
where P1 is para-κ-casein and M is a macropeptide. Payens et al. (1977) suggest that the second, non-enzymatic phase may be represented by:
where i is any number of aggregating particles, P1.
Surkov et al. (1982) suggested that the enzyme-altered micelles (para-casein micelles, P) undergo a co-operative transition in quaternary structure to yield clot-forming particles (P*) with a rate constant, kc; the activation energy, Ea, was 191 kJ mole−1 and the Q10 °C was 1.6. These values are very similar to those reported by Tuszynski (1971).
The sites involved in the aggregation process are not known. Following reduction of the micellar zeta potential by proteolysis of κ-casein, linkage of particles is facilitated. Inter-particle linkage could be via calcium bridges and/or hydrophobic interactions (which the marked temperature dependence of the secondary phase indicate). Changes in the surface hydrophobicity of casein micelles during renneting have been demonstrated through changes in the binding of the fluorescent marker, 8-anilino naphthalene-1-sulphonate (Peri et al. 1990; Iametti et al. 1993). The hydrophobic amino terminal segment (residues 14–24) of αs1-casein appears to be important in the establishment of a rennet-induced gel structure. It has been suggested that the matrix of young cheese curd consists of a network of αs1-casein molecules linked together via hydrophobic patches which extends throughout the cheese structure. The softening of the texture during the early stages of ripening is considered to be due to breaking of the network on hydrolysis of the Phe23-Phe24 bond of αs1-casein. Modification of histidyl, lysyl and arginyl residues in κ-casein inhibits the secondary phase of rennet coagulation, suggesting that a positively-charged cluster on para-κ-casein interacts electrostatically with unidentified negative sites. In native micelles, this positive site may be masked or covered by the macropeptide segment of κ-casein but becomes exposed and reactive when this peptide is released (Hill 1970).
Normally, the rate of an enzymatic reaction increases linearly with enzyme concentration, within certain limits. In the case of rennet coagulation, RCT is inversely related to enzyme concentration, as expressed in the formula:
This equation, which assumes that visually observed coagulation is dependent only on the enzymatic process, has been modified to take account of the duration of the secondary, non-enzymatic phase (Foltmann 1959)
where, x is time required for the coagulation of the enzymatically-altered casein micelles and (tc-x) is the time required for the enzymatic stage. Rearrangement of this equation gives a more convenient form,
which is valid within a certain range of rennet concentrations and under certain conditions of temperature and pH. A very good linear relationship exists between clotting time and the reciprocal of enzyme concentration (Fig. 7.9).
The coagulation equations developed by Dalgleish (1980) and by Darling and van Hooydonk (1981) might be regarded as greatly refined versions of these simpler equations and reduce to them on first approximations. Rennet clotting time , tc, has also been expressed (Payens and Wiersma 1980) by the equation:
where ks, the diffusion-controlled flocculation rate constant according to von Smoluchowski’s theory (non-enzymatic phase), is proportional to the concentration of reactive (coagulable) particles (proteolysed micelles) and hence to enzyme concentration; Vmax = maximum velocity in Michaelis-Menten kinetics (enzymatic phase) and is proportional to enzyme concentration.
7.6 Factors that Affect the Non-enzymatic Phase of Rennet Coagulation
The coagulation of renneted micelles is very temperature-dependent (Q10 ~ 16) and bovine milk does not coagulate <~18 °C unless Ca2+ concentration is increased. The marked difference between the temperature dependence of the enzymatic and non-enzymatic phases of rennet coagulation has been exploited in studies on the effects of various factors on the rennet coagulation of milk, in attempts to develop a system for the continuous coagulation of milk for cheese or rennet casein manufacture and in the application of immobilized rennets. The very high temperature dependence of rennet coagulation suggests that hydrophobic interactions are important.
Coagulation of rennet-altered micelles depends on a critical concentration of Ca2+ which may act by cross-linking rennet-altered micelles, possibly via serine phosphate residues, or simply by charge neutralization. Colloidal calcium phosphate is also essential for coagulation but can be replaced by increased [Ca2+]. Partial enzymatic dephosphorylation of casein, which reduces micellar charge, reduces coagulability; interaction of casein micelles with various cationic species predisposes them to coagulation by rennet and may even coagulate unrenneted micelles (Green and Marshall 1977; Marshall and Green 1980). Chemical modification of histidine, lysine or arginine residues inhibits coagulation (Hill 1970), presumably by reducing micellar positive charge.
The apparent importance of micellar charge in the coagulation of rennet-altered micelles suggests that pH should have a major influence on the secondary phase of coagulation. Reduction in pH in the range 6.6 to 6.0 is accompanied by increases in the rates of the enzymic and coagulation reactions, reductions in gelation time (time for gel onset) and the degree of κ-casein hydrolysis necessary for the onset of gelation (e.g., from ~97 to ~80 % of total κ-casein), and increases in curd firming rate and firmness after a given renneting time. Although it is claimed that pH has essentially no effect on the coagulation process, the rate of firming of the resultant gel is significantly increased on reducing the pH (Fig. 7.10).
The rate of firming of renneted milk gels is influenced by the type of rennet, especially under unfavourable conditions, e.g., high pH or low [Ca2+]. Perhaps such differences reflect the effect of pH on rennet activity or perhaps some general proteolysis by rennet substitutes.
Heat treatment of milk under conditions that denature β-lactoglobulin and promote its interaction with κ-casein via sulphydryl-disulphide interaction adversely affect all aspects of rennet coagulation but especially the build-up of a gel network (Fig. 7.11) (van Hooydonk et al. 1987). Presumably, the attachment of denatured β-lactoglobulin to the surface of the casein micelles (as is evident from electron micrographs of casein micelles) prevents their aggregation in a form capable of building up a gel network.
Release of casein macropeptides (filled square, open square) and changes in the viscosity (filled triangle, open triangle) and curd firmness (filled circle, open circle) in skim milk which was unheated (closed symbols) or heated at 95 °C for 15 s (open symbols). Arrows indicate the time at which cutting is initiated during cheese manufacture (redrawn from data of van Hooydonk et al. 1987)
7.7 Measurement of Rennet Coagulation Properties
The rennet gelation of milk under quiescent conditions involves the conversion of milk from a colloidal dispersion of stable micelles to a network (gel) of aggregated para-casein micelles, which forms a continuous phase, entrapping moisture and fat globules in its pores; the gel becomes more elastic and firm with time (i.e., on ageing). The transformation is accompanied by a number of physico-chemical changes, e.g., hydrolysis of κ-casein with a concomitant increase in the concentration of the glycomacropeptide; aggregation of the sensitized para-casein micelles; increases in viscosity and elasticity; a decrease in the ratio of viscous:elastic character of the milk. Such changes may also alter some of the physical properties of the milk, e.g., light reflectance and thermal conductivity.
Numerous methods , the principles of which are based on detection of one or more of the above changes, have been developed to measure the rennet coagulation characteristics of milk or the activity of rennets. Owing to the commercial importance of gel formation from milk, as a means of recovering milk fat and casein in the form of cheese curd, most methods measure gel formation (also referred to as curd formation or rennet coagulability), i.e., combined first and second stages, but some specifically monitor the hydrolysis of κ-casein. Various terms or descriptors, some of which are used interchangeably, are used to describe the rennet coagulation of milk; these may be defined simply as follows:
-
aggregation : the joining of particles (e.g., para-casein micelles) by various types of electrostatic or hydrophobic bonds; the aggregates are visible by electron microscopy.
-
coagulation or flocculation : the collision and joining of aggregates, especially under non-quiescent conditions, to form flocs, visible to the naked eye.
-
gelation : the aggregation of particles (e.g., micelles or aggregates of micelles) to form particulate strands, in which the particles undergo limited touching, and eventually form a gel network.
-
elasticity : the ability of the gel to recover, instantaneously, its original shape and dimensions after removal of an applied stress; viscoelastic materials, such as a rennet-induced milk gel, are elastic at relatively small strains (e.g., 0.025; ≪ fracture strain); in this region of strain, known as the linear viscoelastic stress–strain region, the strain is directly proportional to the applied stress and the material (e.g., section of a gel strand which bears the applied stress and is strained) recovers its original dimensions immediately on removal of the stress.
-
viscosity : the physical property of a gel given by the ratio between stress and strain rate.
-
Gel (curd) firmness, gel strength or gel tension : the stress required to cause a given strain or deformation. (Curd tension is a term frequently used to express the firmness of formed gels).
Some of the methods used to measure gel-forming characteristics include:
-
measurement of flocculation time under non-quiescent conditions, e.g., rennet coagulation test,
-
dynamic measurement of the viscous drag created by gelling milk on a pendulum suspended in the renneted milk, by determining the tilt of the pendulum from its ‘zero’-position, e.g., using instruments such as the Thrombelastrograph, Formagraph, Gelometer or Lattodinamografo.
-
dynamic measurement of the ability of gelling milk to transmit a pressure, e.g., using the hydraulically-operated oscillating diaphragm apparatus,
-
measurement of the apparent viscosity of gelled milk after a given time at a fixed shear rate (e.g., using various types of rotational viscometers), or alternatively measuring the firmness of the gel using various types of penetrometer,
-
dynamic measurement of parameters such as viscosity, elastic shear modulus (G′), loss modulus (G″) and phase angle (δ), by applying a low-amplitude oscillating strain or stress to the milk sample, e.g., using a controlled strain or controlled stress rheometer,
-
dynamic measurement of some physical properties of the gelling milk in the cheese vat using special probes, e.g., thermal conductivity (using a hot wire probe), reflectance of near infrared light (NIR diffuse reflectance probe).
Some of the more commonly used laboratory and on-line methods for monitoring the rennet-induced gelation of milk are described below.
7.7.1 Measurement of Primary Phase of Rennet Coagulation
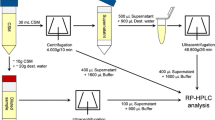
The primary phase of rennet action may be monitored by measuring the formation of either product, i.e., para-κ-casein or the CMP. Para-κ-casein may be measured by SDS-polyacrylamide gel electrophoresis (PAGE), which is slow and cumbersome or by ion-exchange high performance liquid chromatography (HPLC). The CMP is soluble in TCA (2–12 % depending on its carbohydrate content; Fig. 7.3) and may be quantified by the Kjeldahl method or more specifically by determining the concentration of N-acetyl neuraminic acid (Fig. 7.3) or by RP-HPLC. The activity of rennets can be determined easily using chromogenic peptide substrates, a number of which are available, e.g., the hexapeptide Pro.Thr.Glu.Phe(NO2).Phe.Arg.Leu (Hurley et al. 1999). The latter methods are generally used as research tools to study rennet characteristics and/or the kinetics of the primary phase of rennet coagulation.
7.7.2 Methods for Assessing Coagulation, Gel Formation and/or Curd Tension
Various methods have been used to measure the coagulation and gel-forming properties of milk (O’Callaghan et al. 2002; Castillo et al. 2006). Some of these are discussed briefly.
-
Measurement of rennet coagulation time (RCT)
The simplest laboratory method for measuring the overall rennet coagulation process is to monitor the time elapsed between the addition of a measured amount of diluted rennet to a sample of milk in a temperature-controlled water bath at, e.g., 30 °C, and the onset of visual coagulation. If the coagulating activity of a rennet preparation is to be determined, a “reference” milk, e.g., low-heat milk powder reconstituted in 0.01 % CaCl2, and perhaps adjusted to a certain pH, e.g., 6.5, should be used. A standard method has been published (IDF 1992) and a reference milk powder may be obtained from Institut National de la Recherche Agronomique, Poligny, France. If the coagulability of a particular milk is to be determined, the pH may or may not be adjusted to a standard value (e.g., 6.55) to reflect that which is typical at setting (rennet addition) during cheese manufacture. A better method [ISO 11851/IDF 157:2007], called the REMCAT [Relative Milk Clotting Activity Test] method, has been introduced for determination of the total milk clotting activity of calf rennet by comparison with a standard rennet supplied by Chr Hansen, Copenhagen, at pH 6.5. Activity is expressed International Milk Clotting Units [IMCU].
The coagulation point may be determined by placing the milk sample in a bottle or tube which is rotated in a water bath (Fig. 7.12); the fluid milk forms a film on the inside of the rotating bottle/tube but flocs of protein form in the film on coagulation. The rennet coagulation time (RCT) provides a very good index of the gelation potential of milk; a low RCT usually indicates potentially good gel formation and high gel strength after a given renneting time. The method is simple and enables the accurate measurement of several samples simultaneously. This principle has been used to accumulate much of the extensive information reported in the scientific literature on the effects of various processing parameters on the rennet coagulability of milk. However, in contrast to cheese manufacture, where milk is renneted under quiescent conditions to ensure gel formation, this method determines the time for coagulation (i.e., aggregation and flocculation) of the para-casein under agitation.
Fig. 7.12 Apparatus for measuring the rennet coagulation time of milk (from Sommer and Matsen 1935)
-
Nondynamic penetrometer, viscosity and gel firmness tests
Measuring the apparent viscosity, or firmness, of the coagulum after a fixed renneting time, using one of various types of viscometer or penetrometer, respectively, may be used to assess the coagulability of milk. However, this approach permits measurement at only a single point in time, which is a serious limitation in kinetic studies and also requires meticulous test conditions, since curd strength increases with time after renneting.
-
Dynamic gel firmness tests
Various instruments, involving different principles, have been developed to monitor changes continuously throughout the gelation process and are discussed below.
7.7.2.1 Lattodinamografo
The most popular of the dynamic measuring instruments, although with limited use, is the Lattodinamografo (e.g., Foss Italia S.p.A., Padova, Italy). The apparatus consists of:
-
an electrically heated metal block,
-
a sample rack with cavities (usually 10) into which sample cuvettes fit,
-
a set of pendulums,
-
the displacement of pendulum is measured by a transducer, and captured electronically. Samples of milk to be analysed are placed in the cuvettes and tempered to the desired temperature (typically 31 °C) in the heating block. Rennet is then added, the cuvettes replaced in the instrument so that a loop-shaped pendulum is suspended in each sample. The metal block is moved back and forth, creating a “drag” on, and displacing, the pendulum in the milk. The displacement is measured by a transducer and captured electronically on a personal computer, and plotted dynamically during measurement. While the milk is fluid, the viscosity is low and the drag on the pendulum is slight and it scarcely moves from its vertically suspended ‘zero-time’ position; hence, a single straight line is visualised on the computer screen. As the milk coagulates, its viscosity increases and the pendulum is dragged from its zero-time position, resulting in bifurcation of the trace. The rate at, and extent to, which the arms of the trace diverge are indicators of the gel-forming forming characteristics of the milk. A typical trace, shown in Fig. 7.13, may be used to calculate the following parameters:
Fig. 7.13 Typical trace from the Lattodimamografo apparatus for measurement of the rennet gelation properties of milk: *Point of rennet addition, r is rennet coagulation time, k20 is the time required from coagulation for the bifurcated signal (related to the oscillation of the pendulum) to reach a width of 20 mm, and a30 is the extent of bifurcation 30 min after rennet addition
-
rennet coagulation time (r) in min, i.e., the time from rennet addition to the onset of gelation (i.e., point where the trace begins to fork);
-
k20 in min, is the time from the onset of gelation until a firmness corresponding to a trace width of 20 mm is obtained; the rate of curd firming may be calculated from 1/k20.
-
at in mm, the curd firmness at time, t, (e.g., 30 min, a30 or 60 min, a60) after rennet addition, is the trace width at time t.
Good gel-forming properties are characterized by a relatively rapid coagulation time (low r value), high curd firming rate (low k20 value) and good gel firmness or strength after a given renneting time (high a30 value). Typical values for these parameters for a pasteurized mid-lactation milk (3.3 %, w/w, protein), renneted under normal conditions (rennet dosage, ~16 RU/L; pH 6.55, temperature, 31 °C) are: r, 5.5 min; k20, 11 min and a30, 48.5 mm.
While the latter parameters have no precise rheological significance, the Lattodinamografo offers many advantages over the RCT test:
-
the method simulates gel formation during cheesmaking;
-
the results of the assay are less subjective, being independent of operator judgement;
-
the test parameters provide more information on the changes in curd strength over time.
7.7.2.2 Hydraulically Oscillating Diaphragm
A hydraulically-operated oscillating diaphragm apparatus was developed by Vanderheiden (1976). In this apparatus, a sample of milk is placed between two diaphragms (Fig. 7.14) and rennet is added. One diaphram (the transmitting diaphram) is made to vibrate through the cyclical application of hydraulic pressure. When the milk is liquid, the effect of the vibration is dissipated rapidly and does not affect the receiving diaphragm. When a gel is formed, the vibrations emitted by the transmitting diaphragm reach the receiving diaphragm, causing it to vibrate. These vibrations are detected and quantified by a suitable sensing device. An output generally similar to that of the Lattodinamograffo is obtained, from which the coagulation time and a measure of gel strength is obtained. A variation of this apparatus was evaluated by Kowalchyk and Olson (1978), but has not been commercialised (Castillo et al. 2006).
7.7.2.3 Low-Amplitude Stress or Strain Rheometry
Since the 1980s, several controlled strain (e.g., Bohlin VOR, Bohlin Rheologi, Sweden; Rotovisco RV 100/CV 100, Haake Bucchler Instruments, USA; Physica MCR 501, Anton Paar GmbH, A-8054 Graz, Austria) or controlled stress rheometers (e.g., Bohlin CS, Bohlin Rheologi, Sweden; Cari-med CSL2, TA Instruments, New Castle, DE 19720, USA; Rheometric Scientific SR5, Rheometric Scientific Inc, USA) are being used increasingly as a research tool for the continuous measurement of the viscoelastic properties of renneted milks as a function of time from rennet addition.
Dynamic measurements are performed by applying a low-amplitude oscillating shear stress (σ) or shear strain (γ), depending on the type of rheometer, to the milk sample, via oscillations of the outer cylinder. The value of σ or γ are maintained sufficiently low so as to stay within the linear viscoelastic limits of the sample (i.e., region where σ and γ are directly proportional); hence, the term low-amplitude stress or strain oscillation, where amplitude refers to the maximum displacement of any point on the oscillating cup (and hence in the milk sample or on the inner bob) from its mean, or ‘zero’, position. Under these conditions, the gel strands of the gelling milk are strained to a fixed displacement (within their elastic limit) and recover instantaneously when the stress is removed. The stress required to achieve a fixed strain increases as the gel strands become more elastic and rigid; hence, measurement of stress energy provides a measure of the gel strength.
When using a controlled strain rheometer, the sample of renneted milk is subjected to an harmonic, low-amplitude shear strain, γ, of angular frequency ω:
where: γ0 = shear strain amplitude, ω = angular frequency (i.e., 2πf; f = frequency of oscillation), and cos ωt is a term of the simple harmonic function. The shear strain results in an oscillating shear stress, σ, on the milk, of the same angular frequency but which is out of phase by the angle δ:
where: σ0 = stress amplitude; δ = the phase angle between shear stress and shear strain oscillations, the magnitude of which depends on the viscoelasticity (ratio of viscous to elastic properties) of the gelling system (Fig. 7.7). The following rheological parameters are computed continually from the measurement of stress energy over time:
-
Storage or elastic shear modulus, G′, which represents elastically stored stress energy and thus gel elasticity or firmness, is given by the equation:
$$ \mathrm{G}^{\prime } = \left({\sigma}_0/{\gamma}_0\right)\ \cos \delta, $$where σ0 = stress amplitude, γ0 = shear strain amplitude, and δ = the phase angle between shear stress and shear strain oscillations.
-
Viscous or loss modulus, G″, which represents energy dissipated in flow, is given by the equation:
$$ {\mathrm{G}}^{{\prime\prime} } = \left({\sigma}_0/{\gamma}_0\right)\ \sin \delta $$ -
The phase angle, δ, the angle between the stress and strain, ranges from 0° for an ideal elastic solid to 90° for a Newtonian liquid, and between these for viscoelastic materials. Typical changes in the above rheological parameters with time after rennet addition are presented in Fig. 7.7. The onset of gelation is marked by sharp increases in G′ and G″ and a decrease in δ, which decreases abruptly from ~80° in milk to ~10° and marks the transition from a viscoelastic material which is largely viscous, i.e., milk, to a gel which is largely elastic in character.
G″ and δ are useful parameters for monitoring the viscoelastic changes in the gel during ageing, but are not directly related to gel strength. In contrast, G′, is a direct measure of curd firmness and is thus of significance in cheese manufacture. Various objective rennet coagulation parameters, which are pertinent in cheesemaking, may be derived from the G′-time curve, on modelling, as described below (Guinee et al. 1996):
-
Gel time, defined as the time at which G′ reaches a threshold value, Gg, arbitrarily set at 0.2 Pa
-
The firmness after a fixed renneting time, e.g. 30 or 60 min, G′30 or G′60
-
Maximum curd firming rate, defined as the maximum slope, Smax, of the G′-t graph
-
Set-to-cut time (SCT, i.e., time between rennet addition and gel cutting) at a suitable firmness, e.g., 40Pa, SCT40Pa
7.7.2.4 On-Line Sensors for Predicting Gel Firmness and Cutting Time During Cheese Manufacture
Following the onset of gelation, there is a progressive increase in firmness and the gel eventually attains an optimum firmness (e.g., 40 Pa, after 40–50 min, depending on milk composition and renneting conditions) which, for a given vat design, allows it to withstand the mechanical action of the cutting knives without shattering. Curd shattering results from fracturing of individual curd particles (e.g., by the cheese knife, by impact with other curd particles and/or the knife and walls of the vat), especially if the gel is too soft or too rigid at cutting. Shattering results in an excessively large curd particle surface area (through which fat is lost into the cheese whey) and forms very small curd particles (i.e., curd fines, <1 mm) which are also lost in the whey. Thus, cutting at the optimum firmness and rate of curd firming are crucial in obtaining the correct particle size, minimizing the losses of fat and fines in the whey and maximizing cheese yield (Fig. 7.15).
The firmness of the gel after a given set-to-cut time is influenced by many factors, such as the concentrations of fat and casein in the milk, the stage of lactation and diet of cow, milk pH, starter activity (which influences pH at setting and cutting) and rennet-to-casein ratio. Variation in the firmness at cutting can lead to variations in cheese composition (especially moisture), yield and quality. Hence, standardizing and optimizing the firmness at cutting is essential for ensuring a high cheese yield and good quality cheese.
Formerly, the time at which the gel was cut was usually determined by the cheese maker, who subjectively assessed firmness by various means, e.g., making a small cut with a knife and observing “cleanness” of the cut and the clarity of exuding whey. However, in large modern factories, conditions are not conducive to testing gel firmness in cheese vats from different milk silos because of the large scale of operation ( >2–3 × 106 L/day) and the use of pre-programmed vats with limited operator access. Hence, most of the cheesemaking operations are performed on the basis of a pre-set time schedule rather than on the basis of objective criteria, such as gel firmness at cutting, pH at whey drainage, etc. The criteria on which gel cut times are established include ongoing composition of the cheese, and/or losses of fat or curd fines in the whey.
The above methods do not enable cutting at constant gel firmness in every vat. The limitation of the current methods for assessing gel firmness in the cheese factory has led to the development of in-vat gel firmness sensors, which dynamically monitor milk coagulation and, when incorporated into an integrated system, activate the curd knives to cut the gel when it has attained the desired firmness (strength). The mechanisms used to date in designing in-vat sensors to monitor the development of curd firmness over time include monitoring related changes in:
-
Convective heat transfer from a probe (a ‘hot wire’) to the surrounding milk, as in hot wire probe sensors (Hori 1985; Bellon et al. 1988; Lefevre and Richardson 1990).
-
Turbidity (McMahon et al. 1984).
-
Diffuse reflectance of visible (e.g., λ, 660 nm), NIR (e.g., λ, 820 nm) or IR light (e.g., λ, 950 nm), as in various fibre optic probes, the CoAguLite fibre optic sensor and Omron E3XA (Payne et al. 1993).
-
Transmission NIR probes, e.g., TxPro, Gelograph NT (O’Callaghan et al. 1999).
-
absorption and attenuation of ultrasound waves, or pulses, of different frequencies (e.g., >0.5 MHz) passed through the milk (Gunasekaran and Ay 1994; Benguigui et al. 1994).
The subject has been comprehensively reviewed by O’Callaghan et al. (2002) and O’Callaghan (2011). The hot wire and NIR reflectance probes are manufactured commercially by Stoelting Inc. (Kiel, WI) and Reflectronics, Inc (Lexington, KY) as Optiset and CoAguLite, respectively.
7.7.2.5 The Hot Wire Probe
The principle of measurement is based on changes in heat transfer from a hot wire to the milk (http://www.reflectronics.com/products.html). A constant current is passed through the wire, generating heat, which is dissipated readily, by convection currents near the wire, while the milk is liquid. As the milk coagulates, its viscosity increases and generated heat is no longer readily dissipated; the temperature of the wire increases, causing an increase in its resistance. The resistance and temperature of the wire are dynamically measured by monitoring changes in voltage across the wire, giving a continuous output signal. Equations have been developed to relate the output from the hot wire to the rheological properties of the gel.
7.7.2.6 NIR Diffuse Reflectance Fibre Optic Probe
The principle of measurement is based on changes in the light scattering properties of milk (http://www.trademarkia.com/optiset-74011453.html). Light scattered by both the fat globules and casein micelles is detected by the optical fibres and transmitted to a photodetector. As the milk coagulates, more light is reflected (due to the aggregation of the para-casein micelles) and transmitted to the photodetector, the output signal from which is directly proportional to the amount of light received. The output signal is related to the rheological properties of the gel, which is then related to a cut time at a given firmness, as determined by laboratory instruments.
7.8 Factors that Affect Rennet Coagulation
In addition to the actual coagulation time, the strength of the resulting gel (curd tension, CT) is equally, and perhaps more, important, especially from the point of view of cheese yield. The gel assembly process is quite slow (Fig. 7.7) and in the case of most cheese varieties a period roughly equal to the RCT is allowed from the onset of visual coagulation for the gel to become sufficiently firm prior to cutting. If the gel is too soft or too rigid when cut, fat and casein losses may be high (Bynum and Olson 1982; Guinee et al. 1994). In general, there is an inverse relationship between RCT and CT and therefore any factor that reduces RCT increases CT and vice versa. The effect of various compositional and environmental factors on the primary and secondary phases of rennet coagulation are summarized in Table 7.2.
7.8.1 Concentration of Milk Protein
The coagulation time of milk decreases markedly with protein (and thus casein) content, in the range 2.0–3.0 % (w/w), when rennet is added on a volume basis (Figs. 7.16 and 7.17). Further increases in milk protein level (i.e., >3.0 %, w/w) result in a slight increase in gelation time, an effect attributable to the decreasing rennet-to-casein ratio, which necessitates an increase in the time required to generate sufficient hydrolysis of κ-casein to induce aggregation of para-casein micelles. At a constant rennet:casein ratio, the RCT decreases with increasing casein concentration, e.g., as obtained by ultrafiltration, and vice versa. From a practical viewpoint, a minimum protein level of 2.5–3.0 % (w/w) is necessary for gel formation in cheese manufacture, i.e., within 40–60 min. The maximum gel firming rate (Smax) and gel firmness (G′) increase more than proportionally with protein level (Fig. 7.17), with a power law dependence of the latter parameters and protein concentration, i.e., Smax ∝ Pn1 and G′ ∝ Pn2, where n1 and n2 > 1.0, typically ~2.0 (Guinee et al. 1996, 1997). Hence, small variations in protein content, as can occur throughout the cheesemaking season, exert a relatively large effect on its rennet coagulation properties. The positive effects of the higher milk protein content on the rennet coagulation properties probably ensue from the higher level of gel-forming protein which increases the proximity of casein micelles and thus augments the rate of casein aggregation.
Effect of milk protein level [3.0 (filled triangle), 3.5 (open square), 4.5 (filled square), 6.9 (open circle) or 8.2 (filled circle) g/kg] on the elastic modulus of rennet-coagulated milk. Milks with protein levels of 3.5–8.2 % were prepared by ultrafiltration of milk with 3.0 % protein. Coagulation parameters that may be derived from the curve are: gelation time, point at which G′ begins to increase; curd firming rate, slope of G′/time curve in the linear region; and curd firmness, the value of G′ at a given time from rennet addition (redrawn from Guinee et al. 1994)
Effect of increasing level of protein (filled circle) in skim milk, or fat (open circle) in milks containing 3.3 % protein, on rennet coagulation properties: maximum curd firming rate (a), curd firmness at 40 min after rennet addition (b) and set-to-curd time at a firmness of 20 Pa (c) (redrawn from Guinee et al. 1997)
One of the economic attractions in using of UF-concentrated milk in cheesemaking is the savings that accrue from using less rennet. Cheese made from UF-concentrated milk ripens more slowly than normal, due partly to slower proteolysis, for which there may be a number of causes, including the lower ratio of rennet:casein, elevated levels of plasmin inhibitors, a higher degree of casein aggregation.
7.8.2 Concentration of Milk Fat
Increasing fat content in the range 0.1–10 %, w/w, while maintaining the protein level constant (e.g., at 3.3 %, w/w), enhances the rennet coagulation properties, as reflected by decreases in coagulation time and set-to-cut time and higher values for Smax and G′ (Fig. 7.17). However, the positive effects are much smaller than those obtained on increasing protein content in the same range. Indeed, in a milk in which the level of fat plus protein is maintained constant, increasing the fat content results in significant decreases in Smax and G′. In commercial cheese manufacture where standardization of milk protein to a fixed level (e.g., by ultrafiltration of skim milk) is not normally practised, Smax and G′ increase progressively on adding cream to give a fat content of ~4 %, w/w, and decrease thereafter. The latter effect is due to dilution of the protein, which eventually offsets the benefits of increasing fat content. The effect of increasing the fat level in milk, where the level of gel-forming protein is constant, is probably due to the concomitant increase in viscosity.
7.8.3 Pasteurization Temperature
Pre-heating milk up to ~65 °C has a beneficial effect on rennet coagulation, owing to heat-induced precipitation of calcium phosphate and a concomitant decrease in pH. These changes occur also at higher temperatures but their beneficial effects on rennet coagulation are offset and eventually overridden by the combined effects of:
-
whey protein denaturation and the interaction of denatured β-lactoglobulin with micellar κ-casein, and
-
the deposition of heat-induced, insoluble calcium phosphate and the reduction on subsequent cooling of the concentration of native micellar calcium phosphate, which is important for cross-linking para-casein micelles, and hence aggregation, during gel formation.
The complexation of denatured whey protein with κ-casein adversely affects both the enzymatic and non-enzymatic phases of rennet-indeced coagulation, but especially the latter. Increasing the extent of whey protein denaturation (as a % of total) to a level >15 % by high heat treatment of milk (e.g., >80 °C × 15 s), impairs the rennet coagulation characteristics to such an extent that the milk is unsuitable for cheese manufacture (Fig. 7.18). Very severely heated milk, e.g., 90 °C × 10 min (80–90 % total whey protein denatured), is not coagulable by rennet.
Effect of pasteurization temperature, for 15 s, of milk on the level of whey protein denaturation (a), maximum firming rate (b), curd firmness 60 min after rennet addition (c) and set-to-curd time at a firmness of 20 Pa (d) (redrawn from Guinee et al. 1997)
If heated milk is cooled, the RCT increases further (Fig. 7.19), a phenomenon referred to as ‘rennet hysteresis’. The effect can be explained as follows: the adverse influence of the interaction of β-lg with κ-CN on rennet-induced coagulation is offset to some extent by the beneficial effect of heat-precipitated calcium phosphate and reduced pH. However, heat-induced changes in calcium phosphate are at least partially reversible on cooling and hence the full adverse effects of the protein interaction become fully apparent on cooling. In practice, milk should be pasteurized immediately before cheesemaking and should not be cold-stored before use.
7.8.4 Cooling and Cold Storage of Milk
Cooling and cold storage of milk (raw or heated) have adverse effects on the cheesemaking properties of milk. Apart from the growth of psychrotrophs, two undesirable changes occur:
-
Some indigenous colloidal calcium phosphate dissolves, with a concomitant increase in pH, and
-
Some proteins, especially β-casein, dissociate from the micelles.
These changes are reversed by HTST pasteurization or by heating at a lower temperature, e.g., 31 °C, for a longer period.
7.8.5 Milk Homogenization
Homogenization of milk is practised in the manufacture of some cheese varieties where lipolysis is important for flavour development, e.g., blue cheese, the objective being to increase the accessibility of the fat to fungal lipases, and thus to increase the formation of fatty acids and their derivatives (e.g., methyl ketones). Homogenization is an essential part of the manufacturing process for cheeses from recombined milks. Homogenization reduces fat globule size and increases the surface area of the fat by a factor of 5–6. Simultaneously, the fat globules become coated with a protein layer consisting of casein micelles, micelle subunits and whey proteins. Hence, the newly-formed fat globules behave as pseudo-protein particles with the ability to become part of the gel network. Numerous studies have been undertaken to evaluate the effect of homogenization under different conditions of temperature, pressure and/or milk fat level. While there are some discrepancies between the results of these studies, the main trends indicate that homogenization lowers the gelation time slightly, has no effect on curd firming rate and causes a slight increase in G′. However, the higher moisture content of cheese made from homogenized milk, compared to that from unhomogenized milk, suggests that homogenization may alter the rate of casein aggregation during the later stages of cheese manufacture (i.e., after cutting).
7.8.6 Renneting (Set) Temperature
The principal effect of set temperature is on the secondary, non-enzymatic phase of coagulation, which does not occur at temperatures <~18 °C. Above this temperature, the coagulation time decreases to a broad minimum at 40–45 °C and then increases again as the enzyme becomes denatured (Fig. 7.20). In cheesemaking, rennet coagulation normally occurs at a temperature well below the optimum temperature, e.g., 31 °C for many varieties. The lower temperature is necessary to optimize the growth of mesophilic starter bacteria, which have an optimum growth temperature of ~27–28 °C and will not grow at, or perhaps not even survive, >40 °C. In addition, the structure of the coagulum is improved at the lower temperature which is therefore used even for cheeses made using thermophilic cultures.
Effect of various factors on the rennet coagulation time of milk (from Fox and McSweeney 1998)
As shown by changes in absorbance at 600 nm, sedimentability at 5000 ×g of 1 h, viscosity, or inelastic light scattering, micelles do aggregate on renneting at 10 °C, apparently due to hydrolysis of β-casein released from the micelles at low temperatures (Bansal et al. 2007). Aggregation of renneted micelles is promoted by adding CaCl2 or reducing the pH (Bansal et al. 2008).
7.8.7 pH
Due to the effect of pH on the activity of the enzyme, the rennet coagulation time increases with increasing pH, especially >pH 6.4 (Fig. 7.20). The sensitivity to pH depends on the rennet used; porcine pepsin is particularly sensitive while the microbial rennets are relatively insensitive. Owing to the dependence of the rennet coagulation of milk on pH, factors that affect the pH of milk (amount and form of starter added, addition of CaCl2, ripening of milk, pH adjustment by addition of acid or acidogen, pH of the milk itself as effected by mastitis or stage of lactation) affect rennet coagulability. Gel firmness increases markedly with decreasing pH to a maximum at pH 5.9–6.0; the decrease in CT at lower pH values may be due in part to solubilization of colloidal calcium phosphate as the pH is reduced. The pH of milk increases markedly on mastitic infection and may exceed 7.0, i.e., it approaches the pH of blood (~7.4). Mastitic milk has a longer RCT and lower CT than milk from healthy cows, due to a combination of factors, e.g., high pH, low casein content, and the high somatic cell count (e.g., >1 × 106 cells/ml) and associated proteolytic activity (which causes extensive hydrolysis of αs- and β-caseins). The pH of first colostrum may be as low as 6.0 but increases to the normal value (6.7) within about 1 week and then remains relatively constant for most of the lactation, before increasing substantially (to pH 7 or even higher) at the end of lactation.
7.8.8 Added CaCl2
The addition of CaCl2 to milk, which is common practice, promotes rennet coagulation via three beneficial changes:
-
increase in [Ca2+],
-
an increase in the concentration of colloidal calcium phosphate, and
-
a decrease in pH (the addition of CaCl2 to 0.02 % (0.2 g/L), i.e., 1.8 mM Ca, reduces the pH by ~0.05–0.1 units, depending on protein level).
Hence, the addition of CaCl2 (to 0.2 g/L, i.e., ~1.8 mM Ca) enhances the rennet coagulation properties as reflected by a reduction in RCT and increases in curd firming rates and curd firmness (Fig. 7.20). However, on addition of >0.2 g CaCl2/L, the curd firming rate and curd firmness plateau and decrease again at levels greater ≥1.0 g/L (i.e., ≥9 mM Ca). The decrease in CT at the higher Ca levels may be due to the interaction of the excess Ca2+ with the negatively charged carboxyl groups on para-casein, which increases the positive charge on the casein, making it less prone to aggregation. As expected, the addition of calcium chelators (e.g., EDTA, sodium phosphates) reduces gel firmness. Addition of NaCl increases gel firmness up to 0.35 M but markedly decreases it at higher concentrations, possibly via displacement of micellar Ca by Na+.
7.8.9 Rennet Concentration
Obviously, the rate of the enzymatic phase of rennet-induced coagulation is directly related to the amount of rennet used; there is an inverse relationship between enzyme concentration and milk clotting activity (MCA) (Fig. 7.20). However, the results of studies differ in relation to the effect of rennet level on the curd firming rate and curd firmness, with some studies showing increases in the latter parameters and others no effect or slight decreases, depending on the stage of lactation.
In cheesemaking, the amount of rennet added is sufficient to coagulate the milk in 30–40 min [200–220 ml of standard calf rennet (~60 RU/ml) per 1000 l of milk]. This level of rennet is traditional and is presumably based on experience. However, the amount of rennet retained in the curd is proportional to the amount of rennet added to the milk (at least for calf rennet) and this has a major effect on the rate of proteolysis during ripening. The retention of gastric rennets (e.g., calf chymosin or bovine pepsin) increases with decreases in pH at gel cutting and at whey drainage. On the other hand, the retention of R. miehei (i.e., Rennilase) and R. pusillus (i.e., Emporase) proteinases is not influenced by pH at cutting or at whey drainage.
Gel strength is strongly influenced by the type of rennet used: calf chymosin gives a more rapid increase in gel strength than microbial rennets although the substrate on which the rennets were standardized for clotting activity is of some significance. The fact that rennets standardized to equal clotting activity cause different rates of curd firming and the response thereof to compositional factors, e.g., [Ca2+], suggests possible differences in the extent and/or specificity of proteolysis during the enzymatic phase of rennet coagulation. As far as is known, the primary phase of coagulation by all the principal coagulants involves cleavage of the Phe105-Met106 bond except C. parasitica, which hydrolyses Ser104-Phe105; other bonds are also hydrolysed by microbial rennets (Tam and Whitaker 1972; Vanderpoorten and Weckx 1972; see Chap. 12). The amount of rennet used seems to be optional but the influence of increasing concomitantly both the level of rennet used and starter cell numbers does not seem to have been investigated as a possible means of accelerating cheese ripening.
7.8.10 Other Factors
The rennet coagulation properties of milk may be influenced by the stage of lactation and diet , which cause changes in milk composition (i.e., casein, fat, mineral level, pH), degree of casein hydrolysis (e.g., as influenced by plasmin and other proteinases) and the health of the cow. These effects tend to be more marked in countries such as Ireland, New Zealand and Australia, where milk is largely from spring-calving herds, fed predominantly on pasture. Late lactation milk, especially when the lactose level is <4.1 % (w/w), is frequently associated with a long coagulation time and low gel firmness. These defects may be alleviated by drying-off cows at a milk yield >8 L/day, improving the quality of the feed, blending late lactation milk with early lactation milk, and standardizing the cheesemaking process, e.g., pH at set or rennet-to-casein ratio.
7.9 Rennet Substitutes
Owing to increasing world production of cheese (~2–3 % p.a. over the past 30 years), concomitant with a reduced supply of calf vells (due to a decrease in calf numbers and a tendency to slaughter calves at an older age), the supply of calf rennet has been inadequate for many years. This has led to an increase in the price of veal rennet and to a search for rennet substitutes. Despite the availability of numerous potentially useful milk coagulants, only six rennet substitutes (all aspartyl proteinases) have been found to be acceptable for cheese production: bovine, porcine and chicken pepsins and the acid proteinases from Rhizomucor miehei , R. pusillus and Cryphonectria parasitica . (Rhizomucor and Cryphonectria were previously known as Mucor and Endothia, respectively).
In addition to fulfilling the criteria laid down by legislative agencies regarding purity, safety and the absence of antibiotics (IDF 1990), rennet substitutes must possess the following characteristics (Guinee and Wilkinson 1992):
-
A high ratio of MCA-to-proteolytic activity , as for example with calf rennet, prevents excessive non-specific proteolysis during manufacture and hence protects against a weak gel structure, high losses of protein and fat in the whey and reduced yields of cheese solids. Moreover, it avoids excessive proteolysis during maturation and thus ensures the correct balance of peptides and hence desirable flavour, body and functional characteristics in the ripened cheese, and its suitability for certain applications (e.g., processed cheese products, cheese powder). Excessive proteolysis, especially of β-casein, is associated with the development of a bitter flavour.
-
A MCA which is not very pH-dependent in the region 6.5–6.9 (a sharp decrease in MCA with increasing pH may lead to slow gelation and a low gel strength at cutting, especially if the milk pH at setting is high, e.g., 6.7–6.8, as may occur in late lactation) or when the casein concentration is low (e.g., <2.4 %, w/w). These conditions are conducive to low recovery of fat and a reduced cheese yield and can occur in large factories where the duration of milk ripening is short (especially with the use of direct-to-vat starters) and the production steps (including cutting) are generally carried out according to a fixed time schedule. The addition of CaCl2 or acidulants (e.g., gluconic acid-δ-lactone) may overcome the latter problems.
-
Thermostability comparable to that of calf rennet at the pH values and temperatures used during cheesemaking. This can markedly influence the level of residual rennet in high cook cheeses such as Emmental, Pecorino Romano, Provolone and low-moisture Mozzarella and hence the level of proteolysis, texture and functionality of the cheese during maturation (see Chaps. 12 and 19).
-
Low thermostability of rennets during whey processing is desirable; otherwise, the rennet in the whey (~90 % of that added to the cheesemilk) may lead to coagulation of formulated milks on reconstitution, which normally include whey (e.g., infant formulae, calf milk replacer).
-
Must give finished cheese with the desired flavour, body and texture characteristics. Chicken pepsin is the least suitable of the commercial rennet substitutes and was used widely only in Israel where it has now been replaced by fermentation chymosin . Owing to its high ratio of proteolytic activity-to-MCA, chicken pepsin promotes extensive degradation of both αs1- and β-caseins, leading to the development of flavour (e.g., bitterness) and textural (soft body and greasiness) defects during maturation. The activity of porcine pepsin is very sensitive to pH >6.6 and it may be denatured extensively during cheesemaking and consequently proteolysis during cheese ripening may be impaired. A 50:50 mixture of porcine pepsin and calf rennet gave generally acceptable results but porcine pepsin has been withdrawn from most markets. Bovine pepsin is probably the most satisfactory rennet substitute; good quality veal rennet contains ~10 % bovine pepsin and many commercial “calf rennets ” contain ~50 %. Its proteolytic specificity is similar to that of calf chymosin and it gives generally satisfactory results with respect to cheese yield and quality.
Although the proteolytic specificity of the three commonly used fungal rennets is considerably different from that of calf chymosin, they have given generally satisfactory results with most cheese varieties. However, the proteolytic activity of all the rennet substitutes is higher than that of calf chymosin, resulting in higher levels of protein in the cheese whey and lower cheese yields (Fig. 7.21). Prior to the introduction of genetically engineered chymosin, microbial rennets were used widely in the United States but not in most European countries, Australia or New Zealand. The extensive literature on rennet substitutes has been reviewed (see Guinee and Wilkinson 1992; Grag and Johri 1994; Fox and McSweeney 1997 and Jacob et al. 2011).
Yield of Cheddar cheese made using different types of rennet: FPC, fermentation chymosin (Chymax plus); CR, calf rennet (Standard 190), RM Rhizormucor miehei (Fromase 220 TL), CP Cryphonectria parasitica (Suparen 600), RP Rhizormucor pusillus (Emporase). Cheesemaking was performed in quadruplicate under carefully-standardized conditions (rennet-to-casein ratio, pH at different stages of manufacture and firmness of gel at cut). Guinee (unpublished results)
Like chymosin, all commercially successful rennet substitutes are acid (aspartyl) proteinases. The molecular and catalytic properties of the principal rennet substitutes are generally similar to those of chymosin (see Foltmann 1993; Chitpinityol and Crabbe 1998; Crabbe 2004). Acid proteinases have a relatively narrow specificity, with a preference for peptide bonds to which a bulky hydrophobic residue supplies the carboxyl group; this narrow specificity is significant for the success of these enzymes in cheese manufacture. The fact that the pH of cheese is far removed from their optima (ca. 2 for porcine pepsin) is probably also significant. However, not all acid proteinases are suitable as rennets because they are too active even under the prevailing relatively unfavourable conditions in milk and cheese. The specificity of porcine and bovine pepsins on αs1- and β-caseins is quite similar to that of chymosin but the specificity of the fungal rennet substitutes is quite different (see Chap. 12). Like chymosin, the Phe105-Met106 bond of κ-casein is also preferentially hydrolyzed by pepsins and the acid proteinases of Rhizomucor miehei and R. pusillus but the acid proteinase of Cryphonectria parasitica preferentially cleaves the Ser104-Phe105. However, unlike chymosin, the Rhizomucor and Cryphonectria parasitica proteinases also cleave several other bonds in κ-casein.
The MCA of commercial rennets (calf rennet, R. miehei, R. pusillus and C. parasitica) increases with temperature in the range 28–36 °C. The MCA of porcine pepsin, calf rennet and bovine pepsin at pH 6.6 increases with temperature up to 44, 45 and 52 °C, respectively. The fungal enzymes lose activity at 47, 57 and 57 °C, respectively. The MCA of the pepsins, especially porcine pepsin, is more pH-dependent than that of chymosin, while that of the fungal rennets is less sensitive in the pH region 6.2–6.8 (Fig. 7.22) . The coagulation of milk by C. parasitica proteinase is also less sensitive than calf rennet to added Ca2+ but coagulation by Rhizomucor proteinases is more sensitive. For a given MCA, the rate of gel firming depends on the rennet used; this aspect of milk coagulation should be independent of rennet type and may indicate non-specific proteolysis by the fungal enzymes.
Effect of pH on the rennet coagulation time (RCT) of milk using: (a) calf chymosin (open square), bovine pepsin (filled square), ovine pepsin (open circle) or porcine pepsin (filled circle); (b) calf rennet (open square), Rhizomucor miehei (filled diamond), R. pusillus (open diamond), Cryphonectria parasitica (filled diamond) or Bacillus polymyxa (open triangle) proteinases [redrawn from the data of Fox 1969 (a) and Phelan 1973) (b)]
Rennets are also produced commercially from lamb, kid and buffalo calf stomachs. Frequently, lamb rennet is commercialized as rennet paste rather than rennet extract. The extract contains a lipase, pregastric esterase, in addition to chymosin and is used for cheeses with a high level of free fatty acids, e.g., Pecorino Romano. Lamb, kid and buffalo chymosin are similar to calf chymosin (Mohanty et al. 2003; Jacob et al. 2011).
The thermal stability of rennets which differs considerably (Fig. 7.23) is important when the whey is to be used in food processing; the early fungal rennets were considerably more thermo-stable than chymosin or pepsins but the present products have been modified (by oxidation of methionine residues in the molecule) and have thermal stability similar to that of chymosin. The thermal stability of C. parasitica proteinase is less than that of chymosin at pH 6.6. The thermal stability of all rennets increases markedly with decreasing pH (Fig. 7.23) (Thunell et al. 1979).
Effect of heating time at 68.3 °C on the residual activity of various coagulants in whey at pH 5.2: calf rennet (open square), bovine pepsin (filled square), porcine pepsin (filled triangle), Rhizomucor miehei (filled circle), R. pusillus (open circle) or Cryphonectria parasitica (open triangle) (redrawn from Thunell et al. 1979)
Although they are relatively cheap, rennets represent the largest single industrial application of enzymes, with a world market of ca. 40 × 106 l of standard rennet per annum (worth ~€300 × 106). Therefore, rennets have attracted the attention of industrial enzymologists and biotechnologists. The gene for prochymosin has been cloned in E. coli, Saccharomyces cerevisiae, Kluyveromyces marxianus var. lactis, Aspergillus nidulans, A. niger and Tricoderma reesei (see Pitts et al. 1992; Foltmann 1993; Crabbe 2003; Jacob et al. 2011, for references). The enzymatic properties of the recombinant enzymes are indistinguishable from those of calf chymosin although they contain only one of the isoenzymes, A or B. The cheesemaking properties of recombinant/fermentation-produced chymosins have been assessed on many cheese varieties, always with very satisfactory results (Fox and Stepaniak 1993; Jacob et al. 2011). Recombinant chymosins have been approved for commercial use in many, but not all, countries. Two fermentation-produced chymosins are now marketed commercially: Maxiren, secreted by K. marxianus var. lactis and produced by Gist Brocades (the Netherlands), ChyMax® (secreted by A. niger, Hansen, Denmark) and Chymax (secreted by E. coli, Hansen). The gene for Maxiren was isolated from calf abomasum while that used for and ChyMax® was synthesized. Fermentation chymosins have taken market share from both calf rennet and especially fungal rennets and now represent 70–80 % of the total rennet market (see Jacob et al. 2011).
An interesting recent development is the cloning of camel (Camelus dromedaries) chymosin in A. niger Camel milk is not coagulated by calf chymosin but bovine milk is coagulated readily by camel chymosin which has been characterized by Kappler et al. 2006). It has 70 % higher clotting activity per mole on bovine milk than calf chymosin but only 20 % of the general proteolytic activity, i.e., has a sevenfold higher ratio of milk clotting activity to general proteolytic activity than calf chymosin. This finding was confirmed in Cheddar cheese , in which for equal milk clotting activity, camel chymosin produced Cheddar cheese with a much lower level of proteolysis but good flavour (Bansal et al. 2009); the higher MCA of camel chymosin has been explained by Møller et al. (2012a). The specificity of camel chymosin on bovine αs- and β-caseins is generally similar to that of Møller et al. (2012b). Camel chymosin, as CHYMAX M, is being used commercially for cheese manufacture.
The fermentation-produced chymosins currently available are identical, or nearly so, to calf chymosin but there are several published studies on engineered chymosins (see Fox and McSweeney 1997). At present, attention is focussed on elucidating the relationship between enzyme structure and function but this work may lead to rennets with improved MCA or modified general proteolytic activity, i.e., on αs1- and/or β-casein. The natural function of chymosin is to coagulate milk in the stomach of the neonate; it was not intended for cheesemaking and it is probable that the wild-type enzyme may not be the most efficient or effective proteinase to catalyse proteolysis in cheese during ripening. Therefore, it may be possible to modify chymosin so as to accelerate its action on specific bonds of casein during ripening and/or to reduce its activity on others, hydrolysis of which may have undesirable consequences, e.g., bitterness. To date, the pH optimum, thermal stability, kcat and KM on synthetic peptides have been modified through genetic engineering. We are not aware of any cheesemaking studies using engineered chymosins and approval has not been obtained for their use.
The gene for R. miehei proteinase has been cloned in and expressed by A. oryzae (Novo Nordisk A/S, Denmark). It is claimed that this new rennet (Marzyme GM) is free of other proteinase/peptidase activities present in fungal rennets and which may reduce cheese yield. Excellent cheesemaking results with Marzyme GM have been reported. Cloning of the gene for R. miehei proteinase has created the possibility for site-directed mutagenesis of the enzyme.
7.10 Immobilized Rennets
Most (>90 % for Cheddar) of the rennet added to cheesemilk is lost in the whey, representing an economic loss and creating potential problems for whey processors; both problems could be solved through the use of immobilized rennets. A further incentive for immobilizing rennets is the possibility of producing cheese curd continuously (using a cold-renneting technique, i.e., renneting at ~10 °C which allows the primary phase, but not the secondary phase, to occur) which should facilitate process control. The feasibility of continuous coagulation using cold-renneting principles has been demonstrated but the technique has not been commercially successful to date. However, as discussed in Chap. 12, the chymosin (or rennet substitute) retained in cheese curd plays a major role in cheese ripening; consequently if an immobilized rennet was used to coagulate milk, it would be necessary to add some chymosin (or similar proteinase) to the curd and uniform incorporation of this enzyme(s) would be problematic, as has been experienced with the use of exogenous proteinases to accelerate cheese ripening (see Chap. 12).
In modern cheesemaking, most operations are continuous or nearly so; the actual coagulation step is the only major batch operation remaining, although the use of small “batches” of milk, as in the Alpma process for Camembert, makes coagulation, in effect, a continuous process. However, in modern, large Cheddar and Gouda cheese factories very large (20–30,000 L) vats are used.
There is interest in the manufacture of rennet-free curd for studies on the contribution of enzymes from different sources to cheese ripening. A number of approaches have been used to produce rennet-free curd (see Fox et al. 1993) but a completely immobilized, effective rennet would be very useful.
Several investigators have immobilized different rennets on a range of supports and have claimed that these can coagulate milk. However, it appears that in such studies, some enzyme leached from the support and that this solubilized enzyme was responsible for coagulation. An irreversibly immobilized rennet was unable to coagulate milk although it could hydrolyse non-micellar casein; presumably, the κ-casein on the surface of casein micelles is unable to enter the active site cleft of the immobilized enzyme due to steric factors.
Even if immobilized rennets could coagulate milk, they may not be cost-competitive (rennets are relatively cheap) and would be difficult to use in factory situations. The strategy envisaged for their use involves the passage of cold milk (e.g., 10 °C) through a column of immobilized enzyme where the enzymatic phase of renneting would occur without coagulation, owing to the low temperature. The rennet-altered micelles would then be coagulated by heating the milk exiting the column to ~30 °C. Heating would have to be conducted under quiescent conditions to ensure the formation of a good gel and to minimize losses of fat and protein; quiescent heating may be difficult on a very large industrial scale (e.g., many cheese factories process 2–3 × 106 L milk/day). Hygiene and phage-related problems may present serious problems since cleaning the column by standard regimes would inactivate the enzyme. Plugging of the column and loss of activity have been problematic even on a laboratory scale, and power cuts, which would probably lead to an increase in temperature, would be disastrous as the column reactors would become plugged with cheese curd which would be difficult or impossible to remove. In short, the prospects for the use of immobilized rennets on a commercial scale are not bright and they are not being used but research in the subject continues, e.g., Pessela et al. (2004) and Sales-Gomes and Lima-Costa (2008).
References
Bansal N, Fox PF, McSweeney PLH (2007) Aggregation of rennet-altered casein micelles at low temperatures. J Agric Food Chem 55:3120–3126
Bansal N, Fox PF, McSweeney PLH (2008) Factors that affect the aggregation of rennet-altered casein micelles at low temperatures. Int J Dairy Technol 61:56–61
Bansal N, Drake MA, Piriano P et al (2009) Suitability of camel (Camelus dromedaries) chymosin as a coagulant for Cheddar cheese. Int Dairy J 19:510–517
Bellon JL, Quiblier JP, Durier C et al (1988) Un noveau capteur industrial de mesure du temps de coagulation du lait: le coagulomètre. Technique Latière & Marketing, 1031:29–32. Dairy Science Abstracts 50, 775
Benguigui L, Emerery J, Durand D et al (1994) Ultrasonic study of milk clotting. Lait 74:197–206
Berridge NJ (1945) The purification and crystallization of rennin. Biochem J 39:179–186
Bynum DG, Olson NF (1982) Influence of curd firmness at cutting on Cheddar cheese yield and recovery of milk constituents. J Dairy Sci 65:2291–2290
Castillo M, Lucey JA, Wanga T et al (2006) Effect of temperature and inoculum concentra-tion on gel microstructure, permeability and syneresis kinetics. Cottage cheese-type gels. Int Dairy J 16:153–163
Chitpinityol S, Crabbe MJC (1998) Chymosin and aspartic proteinases. Food Chem 61:395–418
Crabbe MJC (2004) Rennets: general and molecular aspects. In: Fox PF, McSweeney PLH, Cogan TM et al (eds) Cheese: Chemistry, Physics and Microbiology, Vol 1, General Aspects, 3rd edn. Elsevier, Oxford, pp 19–45
Dalgleish DG (1980) Effect of milk concentration on the rennet coagulation time. J Dairy Res 47:231–235
Dalgleish DG (1992) The enzymatic coagulation of milk. In: Fox PF (ed) Advanced Dairy Chemistry 1- Proteins. Elsevier Applied Science Publishers, London, pp 579–619
Dalgleish DG (1993) The enzymatic coagulation of milk. In: Fox PF (ed) Cheese: Chemistry, Physics and Microbiology, vol 1, 2nd edn. Chapman & Hall, London, pp 69–100
Darling DF, van Hooydonk ACM (1981) Derivation of a mathematical model for the mechanism of casein micelle coagulation by rennet. J Dairy Res 48:189–200
De Kruif CG, Holt C (2003) Casein micelle structure, functions and interactions. In: Fox PF, McSweeney PLH (eds) Advanced Dairy Chemistry, Vol 1, Part A, Proteins, 3rd edn. Kluwer Academic – Plenum Publishers, New York, pp 233–276
Dunn BM, Jimenez M, Parten BF et al (1986) A systematic series of synthetic chromophoric substrates for aspartic proteinases. Biochem J 237:899–906
Foltmann B (1959) On the enzymatic and the coagulation stages of the renneting process. Proceedings of 15th International Dairy Congress, London, vol 2, Section 3, pp 655–661
Foltmann B (1987) General and molecular aspects of rennets. In: Fox PF (ed) Cheese: Chemistry, Physics and Microbiology, Vol 1, General Aspects. Elsevier Applied Science, London, pp 33–61
Foltmann B (1993) General and molecular aspects of rennets. In: Fox PF (ed) Cheese: Chemistry, Physics and Microbiology, Vol 1, General Aspects, 2nd edn. Chapman & Hall, London, pp 37–68
Fox PF (1969) Milk-clotting and proteolytic activities of rennet, and of bovine pepsin and porcine pepsin. J Dairy Res 36:427–433
Fox PF (1984) Proteolysis and protein-protein interactions in cheese manufacture. In: Hudson BJF (ed) Developments in Food Proteins - 3. Elsevier Applied Science Publishers, London, pp 69–112
Fox PF, Guinee TP (2013) Cheese science and technology. In: Park YW, Haenlein GFW (eds) Milk and Dairy Products in Human Nutrition. Wiley Blackwell, Oxford, pp 357–389
Fox PF, McSweeney PLH (1997) Rennets: their role in milk coagulation and cheese ripening. In: Law BA (ed) Microbiology and Biochemistry of Cheese and Fermented Milk, 2nd edn. Chapman & Hall, London, pp 1–49
Fox PF, McSweeney PLH (1998) Dairy Chemistry and Biochemistry. Blackie Academic and Professional London
Fox PF, Mulvihill DM (1990) Casein. In: Harris P (ed) Food Gels. Elsevier Applied Science Publishers, London, pp 121–173
Fox PF, Stepaniak L (1993) Enzymes in cheese technology. Int Dairy J 3:509–530
Fox PF, Law J, McSweeney PLH et al (1993) Biochemistry of cheese ripening. In: Fox PF (ed) Cheese: Chemistry, Physics and Microbiology, vol 1, 2nd edn. Chapman & Hall, London, pp 389–438
Fox PF, O’Connor TP, McSweeney PLH et al (1996) Cheese, physical, biochemical and nutritional aspects. Adv Food Sci Nutr Res 39:163–329
Grag SA, Johri BN (1994) Rennet: current trends and future research. Food Rev Intern 10:313–355
Green ML, Grandison AS (1993) Secondary (non-enzymatic) phase of rennet coagulation and postcoagulation phenomena. In: Fox PF (ed) Cheese: Chemistry, Physics and Microbiology, Vol. 1, General Aspects. Elsevier Applied Science New York, pp. 101–140
Green ML, Marshall RJ (1977) Acceleration by ionic materials of the coagulation of casein micelles by rennet. J Dairy Res 44:521–531
Guinee TP, Wilkinson MG (1992) Rennet coagulation and coagulants in cheese manufacture. J Soc Dairy Technol 45:94–104
Guinee TP, Pudja PD, Mulholland EO (1994) Effect of milk protein standardization by ultrafiltration on the manufacture, composition and maturation of Cheddar cheese. J Dairy Res 61:117–131
Guinee TP, O’Callaghan DJ, Mulholland EO et al (1996) Rennet coagulation properties of retentates obtained by ultrafiltration of skim milks heated to different temperatures. Int Dairy J 6:581–596
Guinee TP, Gorry C, O’Callaghan DJ et al (1997) The effects of composition and some processing treatments on the rennet coagulation properties of milk. Int J Dairy Technol 50:99–106
Gunasekaran S, Ay C (1994) Evaluating milk coagulation with ultrasonics. Food Technol 48:774–780
Hankinson CL (1943) The preparation of crystalline rennin. J Dairy Sci 26:53–62
Hill RD (1970) The effect of modification of arginine side chains in casein on the coagulation time of renner-altered casein. J Dairy Res 37:187–192
Hori T (1985) Objective measurement of the process of curd formation during rennet treatment of milks by the hot wire method. J Food Sci 50:911–917
Horne DS, Banks JM (2004) Rennet-induced coagulation of milk. In: Fox PF, McSweeney PLH, Cogan TM et al (eds) Cheese: Chemistry, Physics and Microbiology, Vol 1, General Aspects, 3rd edn. Elsevier, Oxford, pp 47–70
Hurley MJ, O’Driscoll BM, Kelly AL et al (1999) Novel assay for the determination of residual coagulant activity in cheese. Int Dairy J 9:553–568
Hyslop DB ((2003) Enzymatic coagulation of milk. In: Fox PF, McSweeney PLH (eds) Advanced Dairy Chemistry, Vol 1, Part B, Proteins, 3rd edn. Kluwer Academic – Plenum Publishers, New York, pp 839–878
Iametti S, Giangiacomo R, Messina G et al (1993) Influence of processing on the molecular modifications of milk proteins in the course of enzymic coagulation. J Dairy Res 60:151–159
IDF (1990) Use of enzymes in cheesemaking. Bulletin No. 247, International Dairy Federation, Brussels, pp 24–34
IDF (1992) Bovine rennets. Determination of Total Milk-Clotting Activity. Provisional Standard 157. International Dairy Federation, Brussels
Jacob M, Jaros D, Rohm H (2011) Recent developments in milk clotting enzymes. Int J Dairy Technol 64:14–33
Kappler SR, van der Brink HM, Nielsen HR et al (2006) Characterization of recombinant camel chymosin reveals superior properties for the coagulation of bovine and camel milk. Biochem Biophys Res Commun 342:647–654
Kowalchyk AW, Olson NF (1978) Firmness of enzymatically-formed milk gels measured by resistance to oscillatory deformation. J Dairy Sci 61:1375–1379
Lefevre MJ, Richardson GH (1990) Monitoring cheese manufacture using a hot wire probe. J Dairy Sci 73 (Suppl. 1), 74 (Abstr.)
Lucey JA (2011) Rennet-induced coagulation of milk. In: Fuquay JW, Fox PF, McSweeney PLH (eds) Encyclopedia of Dairy Sciences, vol 1, 2nd edn. Academic, Oxford, pp 579–584
Marshall RJ, Green ML (1980) The effect of the chemical structure of additives on the coagulation casein micelle suspension by rennet. J Dairy Res 47:359–369
McMahon DJ, Brown RJ, Ernstrom CA (1984) Enzymic coagulation of milk. J Dairy Sci 67:745–748
Mohanty AK, Mukhopadhoyay UK, Kaushik JK et al (2003) Isolation, purification and characterization of chymosin from riverine buffalo (Bubalos bubalis). J Dairy Res 70:37–43
Møller KK, Rattray FP, Sorensen JC et al (2012a) Comparison of the hydrolysis of bovine k-casein by camel and bovine chymosin: a kinetic and specificity study. J Agric Food Chem 60:5454–5460
Møller KK, Rattray FP, Ardo Y (2012b) Camel and bovine chymosin hydrolysis of bovine αs1- and β-caseins studied by comparative peptide mapping. J Agric Food Chem 60:11421–11432
O’Callaghan DJ (2011) Gel firmness and its measurement. In: Fuquay JW, Fox PF, McSweeney PLH (eds) Encyclopedia of Dairy Sciences, vol 1. Academic, Oxford, pp 585–590
O’Callaghan DJ, O’Donnell CP, Payne FA (1999) Effect of protein content of milk on the storage and loss moduli in renneting milk gels. J Food Process Eng 22:249–261
O’Callaghan DJ, O’Donnell CP, Payne FA (2002) Review of systems for monitoring curd setting during cheesemaking. Int J Dairy Technol 55:65–74
Payens TAJ, Wiersma A (1980) On enzymic clotting processes. V. Rate equations for the case of arbitrary rate of production on the clotting species. Biophys Chem 11:137–146
Payens TAJ, Wiersma A, Brinkhuis J (1977) On enzymic clotting processes. I. Kinetics of enzymetriggered coagulation reactions. Biophys Chem 6:253–261
Payne FA, Hicks CL, Shen PS (1993) Predicting optimal cutting time of coagulating milk using diffuse reflectance. J Dairy Sci 76:48–61
Peri C, Pagliarini E, Imetti S et al (1990) A study of surface hydrophobicity of milk proteins during enzymic coagulation and curd hardening. J Dairy Res 57:101–108
Pessela BCC, Torres R, Fuentes M et al (2004) Immobilization of rennet from Mucor miehei via its sugar chain; its use in milk coagulation. Biomacromolesules 5:2029–2033
Phelan JA (1973) Laboratory and field tests on new milk coagulants. Dairy Industries Int 38:418–423
Pitts JE, Dhanaraj V, Dealwics CG et al (1992) Multidisciplinary cycles for protein engineering. Site-directed mutagenesis and x-ray structural studies on aspartic proteinases. Scand J Clin Lab Invest 52(Suppl 210):39–50
Plowman JE, Creamer LK (1995) Restrained molecular dynamics study of the interaction between bovine k-casein peptide 98–111 and bovine chymosin and porcine pepsin. J Dairy Res 62:451–467
Sales-Gomes M, Lima-Costa ME (2008) Immobilization of endoproteases from crude extract of Cynara cardunculus L. flowers. Food Sci Technol Int 14:271–276
Sommer HH, Matsen H (1935) The relation of mastitis to rennet coagulability and curd strength of milk. J Dairy Sci 18:742–749
Surkov BA, Klimovskii II, Krayushkin VA (1982) Turbidimetric study of kinetics and mechanism of milk clotting by rennet. Milchwissenschaft 37:393–395
Tam JJ, Whitaker JR (1972) Rates and extents of hydrolysis of several caseins by pepsin, rennin, Endothia parasitica protease and Mucor pusillus protease. J Dairy Sci 55:1523–1531
Thunell RK, Duersch JW, Ernstrom CA (1979) Thermal inactivation of residual milk clotting enzymes in whey. J Dairy Sci 62:373–377
Tuszynski WB (1971) A kinetic model of the clotting of casein by rennet. J Dairy Res 38:115–125
van Hooydonk ACM, de Koster PG, Boerrigter IJ (1987) The renneting properties of heated milk. Neth Milk Dairy J 41:3–18
Vanderheiden G (1976) An apparatus for continuously monitoring the structural rigidity of a gel. CSIRO Food Res Quart 36:45–47
Vanderpoorten R, Weckx M (1972) Breakdown of casein by rennet and microbial milk-clotting enzymes. Neth Milk Dairy J 26:47–59
Visser S (1981) Proteolytic enzymes and their action on milk proteins. A review. Neth Milk Dairy J 35:65–88
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer New York
About this chapter
Cite this chapter
Fox, P.F., Guinee, T.P., Cogan, T.M., McSweeney, P.L.H. (2017). Enzymatic Coagulation of Milk. In: Fundamentals of Cheese Science. Springer, Boston, MA. https://doi.org/10.1007/978-1-4899-7681-9_7
Download citation
DOI: https://doi.org/10.1007/978-1-4899-7681-9_7
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4899-7679-6
Online ISBN: 978-1-4899-7681-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)