Abstract
The acid coagulation of milk is the basis for a wide diversity of cultured dairy products. Acidification directly impacts the stability of casein micelles, reducing their charge, dissolving some of the insoluble calcium phosphate crosslinks and modifying internal bonding between proteins. The formation of aggregates and ultimately gels occurs at some critical point when electrostatic repulsion is reduced and is not sufficient to overcome attractive forces, like hydrophobic interactions. Acid-induced milk gels increase in stiffness with time due to on-going bond formation between casein particles within the network. In gels made from heated milk, an increase in the loss tangent parameter is observed for a short period after gelation; this phenomenon is due to the loss of insoluble calcium phosphate crosslinks within the casein particles that are already forming the gel matrix. The texture and physical properties of acid-induced gels are dependent on the specific conditions used for gel formation including: the rate of acidification, temperature, extent of whey protein denaturation, protein content, and presence of polysaccharide stabilizers. On-going studies are still investigating the exact physicochemical mechanisms involved in the acid coagulation of milk including trying to gain a better understanding of how exopolysaccharides modify yoghurt properties.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Fermented milks and yoghurts are one of the oldest and most popular foodstuffs that are produced throughout the world. Worldwide production of fermented milk products (such as yoghurt) probably exceeds 25 million tonnes (IDF, 2012). In 2011, per capita consumption of yoghurt in the US was 6.2 kg (IDFA, 2013) but this is still only a fraction of the consumption levels in many parts of the world.
Historically, the souring of milk was unpredictable and the exact processes involved were not known until well into the twentieth century. For example, Revis and Payne (1907) in an article entitled “The Acid Coagulation of Milk” investigated why during milk souring the initial increase in bacterial numbers was not directly proportional to the increase in acidity, as well as the rate of calcium solubilisation from the caseins as a function of acid development. It was just over one hundred years ago that Metchnikoff first proposed that consumption of fermented milk could help to prolong the life of man, igniting interest in health benefits. Over the past 100 years much has been discovered about the microbiology/biochemistry of the various cultures used for these fermentations, the therapeutic benefits of certain probiotic bacteria, as well as the formation and physicochemical properties of acid coagulated products. The formation and physical properties of acidified milk gels have been reviewed (Lucey and Singh, 1997, 2003; Horne, 1999).
A wide variety of fermented milk products are produced; some of the main types are briefly described here. Yoghurt is formed by the slow fermentation of lactose to lactic acid by the thermophilic starter bacteria, Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus. In set-style yoghurt, gels are formed (undisturbed) in the retail pot. Stirred-type yoghurt is made by breaking a set gel before mixing with fruit or other inclusions and filling into retail containers. Concentrated yoghurt (called Greek or Greek-style yoghurt in the US) is made by removing some acid whey after fermentation using straining with cloth bags, mechanical separation or membrane filtration. The microbiology/biochemistry of the starter cultures used, as well as the manufacturing technologies involved in yoghurt and fermented milks, have been extensively reviewed (e.g., Chandan, 2006; Tamime, 2006; Tamime and Robinson, 2007).
The effects of compositional and processing parameters on the textural properties of acid milk gels and fresh acid cheeses have been reviewed (Lucey and Singh, 1997, 2003; Lucey, 2011). In fresh acid cheeses, the coagulation of milk, cream or whey is achieved via a combination of acid and heat. The production of fresh acid cheeses generally involves pre-treatments (which may include heat treatment and/or homogenisation of the milk), slow acidification (usually with mesophilic starter cultures) and gelation, whey separation and/or curd treatment (Guinee et al., 1993). A small amount of rennet may be used in the production of Quarg, Cottage cheese and Fromage frais to promote greater syneresis. Compositional and processing parameters, such as fat content of the milk, milk heat treatment, method of whey separation, heat treatment of the curd and addition of hydrocolloids to the curd, are varied to produce different types of fresh cheese. The use of rennet in acid gels has a dramatic influence on the rheological properties of these “combined” gels (Lucey et al., 2000; Tranchant et al., 2001). Acid casein is produced by the acidification of milk by starter (lactic) or mineral acids (e.g., HCl), followed by heating, whey separation, washing with water, mechanical dewatering and drying of the resultant precipitate (Chap. 2). A common factor in all of these different acidified milk products is that the initial step involves the formation of an acid-induced gel, which is then further processed. This chapter focuses primarily on the formation of acid milk gels and their physical, rheological and microstructural properties.
12.2 Acidification of Milk
12.2.1 Method of Acidification of Milk
Milk can be acidified by bacterial cultures, which ferment lactose to lactic acid, the direct addition of mineral acids, such as hydrochloric acid (HCl), the use of glucono-δ-lactone (GDL) which hydrolyses to gluconic acid, injection of carbon dioxide, or by a combination of these methods. Many studies on the formation of acid gels have involved the use of GDL (Lucey and Singh, 1997). An extensive study on the formation and rheological properties of acid gels formed by cold acidification of milk with HCl and subsequent heating to the gelation temperature has been reported (Roefs et al., 1990; Roefs and van Vliet, 1990). The cold acidification method allowed the acidification process to be completed separately from gelation, which only occurred during the warming step. The rate of acidification is different between milk acidified with GDL and bacterial cultures; GDL is hydrolysed rapidly to gluconic acid (especially at high temperatures) whereas after the addition of starter bacteria, the pH changes little initially, but then decreases gradually with time. The final pH attained in GDL-induced gels is a function of the amount of GDL added to the milk whereas starter bacteria can continue to produce acid until a very low pH (e.g., <4.1) is attained when the bacteria are inhibited by the low pH; in practice, bacterially acidified gels are cooled when sufficient acidity has been attained (Tamime and Robinson, 2007) or the product will become too acidic.
In the manufacture of casein, skim milk is mixed with mineral acid at room temperature and the decrease in pH is very rapid. The rate of pH change during fermentation or addition of acid is controlled by the buffering properties of milk (Lucey et al., 1993). The rheological and physical properties of gels made with GDL differ from those of fermentation-produced gels (Lucey et al., 1998d), probably due to the different rates of acidification during the critical stage of aggregation of the casein particles, different degrees of particle/cluster rearrangements, as well as concomitant physico-chemical changes in casein micelles.
Preacidification of milk (addition of acid prior to addition of culture) has been explored as a means of accelerating the fermentation process and facilitating a more continuous manufacturing process (Driessen et al., 1977; Peng et al., 2009a). Yoghurt gels made with lower preacidification pH and longer fermentation time had lower storage modulus and yield stress values, higher whey separation and permeability and formed coarse networks (Peng et al., 2009a). Preacidification removed colloidal calcium phosphate (CCP) crosslinks within casein micelles resulting in weaker gels. Fermentation time significantly affects the rate of solubilization of CCP. Long fermentation times (slow acidification) allows more time for the solubilization of CCP (higher soluble Ca content in gels), whereas short fermentation times (fast acidification) allows less time for this process to occur (lower soluble Ca content in gels) (Peng et al., 2009a).
12.2.2 Effect of Acidification on the Properties of Casein Micelles
Caseins constitute approximately 80 % of the protein in bovine milk, present as four types (αs1-, αs2-, β-, and κ-casein) in combination with appreciable quantities of CCP in the form of aggregates called casein micelles. The structure and formation of casein micelles has been intensively investigated in the past with many different models proposed; but now most researchers are using the dual-binding model (Horne, 1998) as the most realistic approach. The dual binding model emphasizes that the assembly of the micelle occurs due to the combined impact of both hydrophobic and electrostatic interactions. The hydrophobic interactions occur between hydrophobic segments on the casein molecules. Phosphoserine clusters on casein molecules provide the location for the formation of CCP nanoclusters, but they also help to prevent excessive growth of these nanoclusters by capping these growing structures, limiting their growth. Recently, the molecular weight of CCP was experimentally estimated to be around 7500 g/mol (Choi et al., 2011), which was within the range (4900–9800 g/mol) predicted theoretically by Horne et al. (2007).
Originally, it was assumed that micelles were held together only by bridges of CCP (Schmidt, 1982) but it is now clear that a number of factors are responsible for maintaining the integrity of the micelle. The formation of CCP is an effective means of “burying” a considerable amount of Ca and phosphate within casein micelles (which is important for milk to achieve its primary role of nutrition of the young calf) but CCP can be removed without completely disrupting the micelle if milk is acidified at temperatures >20 °C (Dalgleish and Law, 1988; Lucey et al., 1997a); casein dissociation is limited at >20 °C. The CCP-depleted casein particles dissociate from the micelle if the pH is adjusted back to 6.6, which indicates that electrostatic interactions are important for micellar stability (Lucey et al., 1997a). Hydrophobic and hydrogen bonding are important for micelle integrity since the addition of urea disrupts the micelle structure (McGann and Fox, 1974); calcium ions (Ca2+) also play a role in the integrity of the micelle (Holt et al., 1986).
During acidification of milk, many of the physicochemical properties of the casein micelles undergo considerable change, especially in the pH range 5.5–5.0, including increased voluminosity and solvation of the caseins (Roefs et al., 1985; Walstra, 1990). The decrease in pH also results in a reduction in the charge on the κ-casein hairs and they eventually collapse on the micellar surface (de Kruif, 1999). During acidification there is an initial (small) decrease in average micellar mass and radius and also a redistribution of mass (rearrangements) within the micelles (Moitzi et al., 2011). These complex changes occurring during acidification are dependent on environmental conditions including concentration/dilution of the system, rate of acidification, and temperature (Moitzi et al., 2011).
As the pH of unheated milk is reduced, CCP is dissolved (Pyne and McGann, 1960) and the caseins are liberated into the serum phase (Roefs et al., 1985; Dalgleish and Law, 1988). The extent of liberation of caseins depends on the temperature at acidification; at 30 °C, a decrease in pH causes virtually no liberation; at 4 °C about 40 % of the caseins are liberated into the serum at pH ~5.5 (Dalgleish and Law, 1988). The temperature of acidification has little (direct) effect on the solubilization of CCP. Apparently, little change in the average hydrodynamic diameter of casein micelles occurs during acidification of (unheated) milk to pH ~5.0 (Roefs et al., 1985). Aggregation of casein occurs as the isoelectric point (pH 4.6) is approached.
Milk used for yoghurt manufacture is subjected to an extensive heat treatment. Heat treatment of milk at a temperature above 70 °C causes denaturation of whey proteins, some of which associate with casein micelles, involving κ-casein, via hydrophobic interactions and intermolecular disulphide bonds (Haque and Kinsella, 1988; Singh, 1995). Moderate heating (<90 °C) does not appear to affect the size of casein micelles although these treatments cause the whey proteins to denature and bind to micellar κ-casein; more extensive heat treatment (>100 °C) causes some degree of micellar aggregation and an increase in particle size (Dalgleish et al., 1987). Heat treatment of milk markedly effects the formation and properties of acid gels, as described in detail in Sect. 12.3.1.2.
The effect of heat treatment on the solubilization of CCP and the release of caseins during acidification of milk has been studied (Law, 1996; Singh et al., 1996). Heat treatment (in the range 70–90 °C) prior to acidification has little effect on the extent of solubilization of calcium and Pi from the micelles (Law, 1996; Singh et al., 1996).
From this discussion, it appears that during acidification of unheated or heated milk to pH values ~5.1 at temperatures above 22 °C, most of the CCP in the micelles is solubilized, the charge on individual caseins is altered and the ionic strength of the solution increases. As a result, the forces responsible for the integrity of these “micelle-like” CCP-depleted casein particles are considerably different from those in native micelles even if their average hydrodynamic diameter appears largely unchanged. The balance between the (intermolecular) attractive and repulsive forces, which is important for gelation properties, is also modified (Horne, 1999).
12.3 Formation and Properties of Acid-Induced Gels
12.3.1 Mechanisms Involved in the Formation of Acid-Induced Gels
12.3.1.1 Theoretical Models
Acid milk gels (like yoghurt) are examples of particle gels (Horne, 1999). At least three theoretical models, namely fractal, adhesive hard spheres and percolation models have been used to model the formation of acidified milk gels.
Fractal aggregation theory has been applied to the formation of various casein gels (Bremer et al., 1989, 1990, 1993; Vertier et al., 1997; Chardot et al., 2002) and is described in this section. In particle gels, a fractal scaling regime may occur only over small length scales, which are of the order of the aggregating clusters. At longer length scales, the microstructure appears homogeneous. Fractal behaviour is not expected in gels made from high volume fraction systems (Dickinson, 1997; Horne, 1999). Fractal aggregation assumes that (hard) spherical particles of radius a can move by Brownian motion and that they can aggregate when they encounter each other. The aggregates formed then also aggregate with each other. If no further changes occur among the particles in an aggregate, once they are incorporated, this cluster-cluster aggregation process leads to aggregates obeying the scaling relation:
where N p is the number of particles in an aggregate of radius R, N 0 is the total number of primary particles that could form such a floc, D is a constant called the fractal dimensionality (D < 3) and a eff is the radius of the effective building blocks forming the fractal cluster.
For casein gels (both rennet- and acid-induced), D ≈ 2.3 has been observed generally (Bremer et al., 1989, 1993), although the rheological properties of these gels differ markedly. This simple fractal approach, although it has successfully described semi-quantitative features in irreversibly aggregating systems, appears to have some deficiencies, including the lack of any allowance for aggregate rearrangement (before, during and after gelation) and interpenetration, and the assumption that all aggregates have the same size at the gel point (Dickinson, 1997). Horne (1999) concluded that although casein gels can exhibit some scaling behaviour, the fractal approach does not provide insights into the dynamics of gel development.
The aggregation of casein particles during the acidification of milk has also been modelled using the adhesive hard sphere theory (de Kruif et al., 1995; de Kruif and Roefs, 1996; de Kruif, 1997, 1999). In this model, it is proposed that the glycomacropeptide (GMP) part of κ-casein sterically stabilizes casein micelles and the GMP is considered as a polyelectrolyte brush which collapses on the surface of the micelle as the pH of the system approaches the pK a of the charged groups on the brush (reduced charge density).
Horne (1999, 2003) pointed out that the adhesive hard sphere model could be applied only to situations where there is weak attraction between particles (i.e., onset of instability) and it has no inherent time scale. Acidification of milk causes significant changes in the internal structure and integrity of casein micelles which greatly impacts milk gelation properties (Choi et al., 2007; Ozcan Yilsay et al., 2007). These results indicate that simple hard sphere models are inadequate for the characterization of milk gel networks.
Horne (1999) reviewed the suitability of percolation models for acid milk gels. In this theory, it is assumed that percolation clusters form bonds between close neighbours on a lattice. Bond formation is assumed to be random and as the number of bonds between neighbours increases, the clusters increase in size. In computer simulations, these functions can be calculated (de Kruif et al., 1995). Above a certain threshold, a large cluster occurs which extends through the lattice. Analogies between percolation and gelation can be drawn with particles establishing an increasing number of links as they aggregate, until, at a certain threshold, a cluster is created which spans the system. At this stage, only a fraction of bonds have been joined and, even more importantly, not all individual fractions have been incorporated into the system-spanning cluster and this mechanism would explain the continued increase in G′ after gelation (Horne, 1999); however, the author suggests that the percolation model may be suitable only at the gel point and suggests that it is difficult to use this theory to model the mechanical properties of acid gels.
12.3.1.2 Possible Physico-Chemical Mechanisms Involved in the Formation of Gels from Unheated and Heated Milks
Native casein micelles (in milk at normal pH) are stabilized by a negative charge and steric repulsion (Walstra, 1990; Mulvihill and Grufferty, 1995). On acidification, casein particles aggregate as a result of (mainly) charge neutralization, leading to the formation of chains and clusters that are linked together to form a three-dimensional network (Mulvihill and Grufferty, 1995). Acid casein gels can be formed from sodium caseinate and these gels can have generally similar properties to acid gels made from milk (Lucey et al., 1997c, d), which suggests that CCP is not essential for acid gelation. Hydrophobic interactions are unlikely to play a direct role in the strength of acid gels as the elastic or storage modulus (G′) of acid gels increases with decreasing assay temperature (Roefs and van Vliet, 1990; Lucey et al., 1997b, c). Cooling gels to a low temperature results in an increase in the G′ probably due to swelling of casein particles and an increase in the contact area between particles (Lucey et al., 1997c). Roefs and van Vliet (1990) reported that increasing the concentration of NaCl added to cold-acidified skim milk samples resulted in a decrease in the dynamic moduli of gels formed on subsequent heating, confirming that electrostatic interactions are important for particle interactions. With increasing ionic strength, charged groups on casein would be screened, thereby weakening interactions between particles (Roefs and van Vliet, 1990; Lucey et al., 1997c); calcium binding by caseins also decreases with increasing ionic strength (Dalgleish and Parker, 1980).
Lucey et al. (1997b) suggested that gels made from unheated milks undergo extensive particle rearrangements during the gel formation, resulting in the formation of dense clusters of aggregated casein particles, which aggregate to form a gel. From these dense clusters, it would be expected that many particles would hardly contribute to cross linking of the network; thus, unheated milk gels would probably have a low G′ value, which has indeed been reported (Lucey et al., 1997b).
Acid gels made from heated milk have a higher pH at gelation (Heertje et al., 1985; Horne and Davidson, 1993) and produce considerably firmer gels than unheated milk (Lucey et al., 1997b, 1998c). High heat treatment of milk causes denaturation of whey proteins and subsequently a proportion of denatured whey protein associates with the casein micelles, involving κ-casein. These whey proteins appear as appendages or filaments on the casein micellar surface in electron micrographs (Davies et al., 1978; Kalab et al., 1983; Mottar et al., 1989). When heated milk is acidified, the denatured whey proteins associated with the casein micelles become susceptible to aggregation, as the repulsive charge on the proteins is reduced. The isoelectric pH of the major whey protein, β-lactoglobulin, is ~5.3 which is higher than that of the caseins (Kinsella and Whitehead, 1989). This would explain the high pH of gelation of heated milk. Denatured whey proteins associated with casein micelles (“bound”) act as bridging material by interacting with other denatured whey proteins associated with micelles (Lucey et al., 1998c). This increases the number and strength of bonds between protein particles. Altering the pH of heating impacts the proportion of denatured whey protein associated with the micelle or in the form of soluble complexes (“soluble”). There has been considerable interest in understanding the impact of different proportions of “bound” and “soluble” denatured whey proteins on acid gels (Lucey et al., 1998c; Donato et al., 2007; Guyomarc’h et al., 2009).
The different physico-chemical mechanisms involved in the formation of acid gels made from heated milk result in the formation of gels with different microstructure from those formed from unheated milk. There is more branching or interconnectivity of the gel network in heated milk gels than in unheated milk gels (Lucey et al., 1998e). The presence of denatured whey proteins on the surface of casein particles may hinder the close approach of other casein particles and lessen the likelihood that dense clusters of casein particles could be formed. Cross-linking of aggregating particles may occur via the denatured whey proteins associated with the casein micelle surface (Lucey et al., 1998c) instead of between charged residues or hydrophobic groups on casein molecules. Thus, “bound” denatured whey proteins play a vital role in strengthening acid gels made from heated milk.
12.3.2 Physical Properties of Acid-Induced Gels
12.3.2.1 Rheological Properties of Acid Milk Gels
In many studies, the textural properties of acid gels have been measured empirically as firmness or viscosity (e.g., Parnell-Clunies et al., 1986; Dannenberg and Kessler, 1988b). Empirical methods do not help in understanding the interactions or mechanisms involved in the formation of acid gels and are more suited for purposes of quality control. The relevance of viscosity measurements in a set gel is unclear, although it is important in stirred-style yoghurts. Fundamental small and large deformation studies of acid gels are much more powerful and useful tools in understanding the formation and physico-chemical properties of acid gels.
Most rheological parameters characterizing casein gels depend on the number and strength of bonds between the casein particles, on the structure of the latter and the spatial distribution of the strands making up these particles (Roefs et al., 1990; Lucey and Singh, 1997).
Dynamic non-destructive testing, which involves an oscillatory applied strain or stress, can provide very useful information on the gel formation process (Lopes da Silva and Rao, 1999). Some of the main parameters that are usually determined from these tests include the G′, which is a measure of the energy stored per oscillation cycle, the viscous or loss modulus (G″), which is a measure of the energy dissipated as heat per cycle, and the loss tangent (tan δ), which is the ratio of the viscous to elastic properties (Lopes da Silva and Rao, 1999). These parameters are defined as follows:
where τ 0 is the amplitude of the shear stress, γ 0 is the amplitude of the strain and δ is the phase angle.
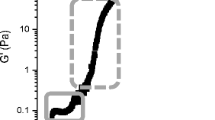
The rheological properties of yoghurt gels made from unheated and heated milk at 40 °C are shown in Fig. 12.1. Unheated milk forms a weak gel and the pH at the onset of gelation is generally ~4.9–4.8. After gelation, G′ initially increases rapidly but starts to plateau (<30 Pa) during ageing of the gel, tan δ decreased to <0.4 soon after gelation and decreased to ~0.25 during ageing of the gel. Roefs (1986) demonstrated that for acid gels made by cold acidification and quiescent heating, G′ continues to increase for a period of up to several days, due presumably to slow ongoing fusion of casein particles.
There have been a number of reports on the effects of heat treatment on the rheological properties of acid gels determined by dynamic low amplitude (strain) oscillation (van Vliet and Keetels, 1995; Lucey et al., 1997b, 1998c). van Vliet and Keetels (1995) reported that acid skim milk gels made from reconstituted low-heat skim milk powder (SMP) had much lower dynamic moduli than gels made from high-heat SMP. Lucey et al., (1997b) reported that heating milk at a temperature ≥78 °C greatly increased the G′ of GDL-induced acid milk gels compared to unheated milk. Increased cross-linking or bridging, by denatured whey proteins, within gels made from heated milk may be responsible for the increased rigidity and G′ of the network (Lucey et al., 1997b, 1998c).
An unusual rheological phenomenon is observed soon after the formation of an acid gel from heated milk; tan δ decreases initially but then increases to a maximum value before decreasing again (Fig. 12.1) (Biliaderis et al., 1992; Rönnegård and Dejmek, 1993; van Marle and Zoon, 1995; Lucey et al., 1998c). A high tan δ indicates an increased susceptibility of bonds and strands in the gel to break or relax, thus facilitating more rearrangements of the gel (van Vliet et al., 1991). The solubilization of CCP also results in a change in the light scattering properties of the milk system, as can be observed from the second peak in first derivative (R′) profile of yoghurt gels (Castillo et al., 2006) (Fig. 12.2). The maximum in tan δ is a consequence of a partial loosening of the weak initial gel network due to the solubilization of CCP (Fig. 12.3), while at lower pH values there would be increased protein-protein attractions between casein particles as the net charge decreases on approaching of the isoelectric point (Lucey et al., 1998c). The maximum in tan δ does not occur in acid gels made from unheated milk or in milk heated in the presence of a sulfhydryl blocking agent because gelation in these systems occurs at low pH values (<5.2), which is below the pH range where most of the dramatic changes in the physicochemical properties of casein micelles (e.g., solubilization of CCP) occur (Lucey et al., 1998c).
Comparison of light scattering and rheological properties during yoghurt gelation. Dashed line light is R′, the first derivative of the light backscatter ratio (R), which is calculated by dividing the sensor output voltage by the initial/starting sample voltage. Method basically as described by Castillo et al., (2006) using a CoAguLab instrument (Reflectronics Inc., Lexington, KY, USA). Storage modulus (G′) (filled circle) and loss tangent (unfilled circle) values (Pachekrepapol and Lucey, unpublished results)
Horne (1999) reported that the rheological properties of acidified milk gels exhibit a form of scaling behaviour. In this procedure, the complex shear modulus (G*) for individual milk samples are replotted as a function of reduced time, defined as the reaction time, t, divided by the gelation time for that profile. Then, each of these individual reduced time plots is normalized against the value of its own shear modulus at three times the gelation time. For acid gels made from unheated or heated (90 °C for 10 min) milk, there are two distinct “master curves”. Horne (1999) suggested that since heated milk and unheated milk had different “master curves” this implies that there are fundamental differences in the kinetics and dynamics of the gel formation process in these two types of gels. It would appear that the rate at which bonds form between protein particles and the mechanism by which clusters grow into a network are altered by heat treatment.
The effects of fat on the fundamental rheological properties of acid milk gels have been reported (van Vliet and Dentener-Kikkert, 1982; van Vliet, 1988; Lucey et al., 1998b; Cho et al., 1999). The nature of the fat globule membrane determines the types of interaction that can occur between fat globules and the protein matrix. Fat globules act as an inert filler if the native fat globule membrane is intact since this membrane does not interact with casein particles (van Vliet and Dentener-Kikkert, 1982; van Vliet, 1988). The G′ value of acid milk gels decreases with an increasing volume fraction of fat, which has an intact native fat globule membrane (van Vliet and Dentener-Kikkert, 1982; van Vliet, 1988). In homogenized or recombined milk, the native membrane is replaced largely with casein and some whey protein so that the surfaces of fat particles can interact with the protein matrix (largely casein but some denatured whey proteins when the gel is made from heated milks) of acid milk gels (van Vliet and Dentener-Kikkert, 1982; van Vliet, 1988). In acid gels made from recombined milk, G′ increases with an increasing volume fraction of fat (van Vliet and Dentener-Kikkert, 1982; van Vliet, 1988; Lucey et al., 1998b). Cho et al., (1999) showed that the G′ of acid gels made from either heated or unheated milk was influenced by the nature of fat globule membrane; gels containing fat globules stabilized by sodium caseinate or denatured whey proteins had very high G′ values compared with those stabilised with SMP (containing casein micelles and whey proteins) or native (undenatured) whey proteins.
In experiments where the time-scale of the applied deformation was varied (frequency sweeps), log G′ versus log angular frequency gave linear lines with a slope of ~0.15 for various types of acid casein gels (Roefs and van Vliet, 1990; Lucey et al., 1997b). This suggests that similar structural components are present in all types of acid casein gels.
Large deformation studies provide information on properties that may be related to the consistency of the gel during consumption or shearing (which is used in the production of stirred-style yoghurt). There is little information on the large scale deformation properties of yoghurts (Rönnegård and Dejmek, 1993). Mixing and stirring of set gels prior to rheological testing means that many reported (e.g., Dannenberg and Kessler, 1988b) yield properties are not those of the original ‘set’ gel. Fundamental large deformation rheological properties of acid casein gels have been reported (Bremer et al., 1990; van Vliet et al., 1991; van Vliet and Keetels, 1995; Lucey et al., 1997b, c). Gross fracture of acid casein gels made with GDL was observed at a strain of 0.5–0.6 (Roefs, 1986; van Vliet et al., 1991; van Marle and Zoon, 1995). Lucey et al., (1997c), using a low constant shear rate technique for acid casein gels made in situ, found that the apparent shear stress at fracture increased with decreasing gelation temperature. The apparent shear stress at fracture of acid casein gels increased with ageing while the strain at fracture decreased (Lucey et al., 1997c). Lucey et al., (1997b) reported that heat treatment of milk prior to acidification resulted in a large reduction in the strain at fracture, from ~1.5 for gels made from unheated milk to 0.5–0.8 for gels made from milks heated at temperatures ≥80 °C.
12.3.2.2 Microstructure of Acid Gels
Electron microscopy (EM) studies on acid gels, such as yoghurt, have shown that these gels consist of a coarse particulate network of casein particles linked together in clusters, chains and strands (Kalab et al., 1983). The network has pores or void spaces in which the aqueous phase is confined. The diameter of these pores varies considerably, with larger pores in gels made at a high gelation temperature or from milk with a low protein content. There have been several EM studies on the microstructure of gels formed by acidification of heated milk (Davies et al., 1978; Parnell-Clunies et al., 1987; Mottar et al., 1989). Harwalkar and Kalab (1980) proposed, based on the examination of electron micrographs, that yoghurt gels made from unheated milk had larger protein clusters than gels made from heated milk, which they described as highly branched.
Unless great care is taken, many of the preparation steps required in EM, including dehydration, fixation, embedding, sectioning and staining, can disrupt the native structure of milk products and lead to the creation of artefacts. Confocal scanning laser microscopy (CSLM) is a technique which enables samples to be observed with minimal preparation due to its unique optical sectioning capabilities and high spatial resolution (Brooker, 1995) and is very suitable for observing the overall microstructure of milk gels (Hassan et al., 1995; Lucey et al., 1997d, 1998b, e). Confocal images are very amenable to image analysis since the images are already in a digital form and, for example, they can be used to calculate the D of acid casein gels (Bremer et al., 1993).
Many of the preparation steps used in EM of whole milk yoghurt can result in partial extraction of fat globules (Allan-Wojtas and Kalab, 1984). Barrantes et al. (1996) reported that in yoghurt made from recombined milk, the fat globules were not noticeable using scanning EM but could be observed using transmission EM. The microstructure of acid gels with different fat contents was investigated using CSLM by Lucey et al., (1998b). In acid-induced gels made from recombined milk, fat globules appeared to be dispersed throughout the gel and the microstructure was quite different from that of skim milk gels (Lucey et al., 1998b). No large pores were visible in acid gels made from recombined milk; probably fat globules obscured the finer details of pores and strands. Fluorescence micrographs of a yoghurt gel made from heated milk are shown in Fig. 12.4 (at two different magnifications). The effects of heat treatment on the microstructure of GDL-induced milk gels have been reported (Lucey et al., 1998e).
12.3.2.3 Permeability of Acid Milk Gels
Permeability measurements give information about inhomogeneities at the level of the gel network (van Dijk and Walstra, 1986; Roefs et al., 1990; Lucey et al., 1998e) and the permeability coefficient of acid gels can be calculated as follows:
where B is the permeability coefficient (m2), h∞ is the height of the whey in the reference tube (m), ht1 is the height of the whey in the gel tube at t1 (m), ht2 is the height of the whey in the gel tube at t2, η is the viscosity of the whey, H is the height of the gel (m), ρ is the density of the whey and g is acceleration due to gravity. In acid gels made at 30 °C with GDL, the value of B is usually in the range 1–2 × 10−13 m2 (Roefs et al. 1990; Lucey et al., 1998e).
In rennet-induced milk gels, B increases with time, which has been taken as evidence of “microsyneresis” or breakage of strands in the network resulting in the formation of larger pores (Walstra, 1993). Studies on the permeability of acid-induced gels have shown that the value of B does not change with time (Roefs et al., 1990; Lucey et al., 1997d).
12.3.2.4 Appearance
Set-style yoghurt gels should have a smooth, semi-solid consistency with no surface whey (Lucey and Singh, 1997). The appearance of a set gel should be smooth with no cracks or ‘blemishes’. Acid gels made from severely heated milks with GDL had a “rough” surface with visible cracks and some whey separation (Lucey et al., 1998a, e). It was suggested that spontaneous rearrangement of the network just after gel formation might be responsible for these defects. Gels made from severely heated milk had a low strain at fracture compared to gels made from unheated milk and this may make heated gels more susceptible to localized fracturing of strands in the network (Lucey, 2001). The “transition” in the rheological properties (as indicated by the maximum in loss tangent) may increase the susceptibility of protein-protein bonds to relax and if these bonds have a relatively short lifetime, this may lead to yielding or breaking of strands (van Vliet et al., 1991).
12.3.2.5 Whey Separation
Whey separation refers to the appearance of liquid (whey) on the surface of a milk gel and is a common defect in fermented milk products. Syneresis is defined as shrinkage of a gel and this occurs concomitantly with expulsion of whey. It is useful to define spontaneous syneresis as the contraction of a gel without the application of any external force (e.g., centrifugation) and this is related to instability of the gel network (i.e., due to large scale rearrangements) (Walstra, 1993). In practice, yoghurt manufacturers try to prevent whey separation by adding stabilizers (e.g., pectin or gelatin) or whey protein concentrate (WPC).
A simple method for quantifying spontaneous whey separation in acid gels is often used (Lucey et al., 1998a). Older studies had determined the quantity of whey expelled from yoghurt as a result of high speed centrifugation or drainage through a screen (Harwalkar and Kalab, 1983, 1986; Dannenberg and Kessler, 1988a). The drainage of whey from a broken gel distributed over a screen measures whey separation when a very large surface area is available and is more relevant to products such as Cottage cheese or casein, than to set gels like yoghurt (Lucey et al., 1998a). High speed centrifugation measures the water holding capacity of the gels under relatively high forces. Therefore, both of these methods for measuring whey expulsion are not relevant to the spontaneous separation of whey defect from set-style gels. The approach of Lucey et al. (1998a) was to make gels in containers and determine the amount of surface whey that was expelled during gelation. Whey separation (in volumetric flasks) was increased significantly by heat treatment and gelation temperature (Lucey et al., 1998a); coincidentally a high heat treatment and high gelation temperature are commonly used in the manufacture of yoghurt.
A high incubation temperature is one of the main causes of whey separation in acid gels like yoghurt (Lucey and Singh, 1997). Acid-induced milk gels formed by slow acidification of milk at a low temperature and quiescent heating exhibit little wheying-off or spontaneous syneresis (Roefs, 1986).
It has been shown (van Dijk and Walstra, 1986) that the one dimensional syneresis of milk gels is related to the flow of liquid (whey) through the network and is governed by the equation of Darcy:
where v is the superficial flow velocity of the syneresing liquid, B is the permeability coefficient, η is the viscosity of the liquid, p is the pressure acting on the liquid and x the distance over which the liquid must flow. In milk gels, an endogenous syneresis pressure can occur if there is a tendency of the casein network to rearrange after its formation (Lucey et al., 1997d). It has been shown that endogenous syneresis pressure is mostly small in acid sodium caseinate gels and this results in lesser tendency for shrinkage of these gels compared to rennet-induced gels (Lucey et al., 1997d). One-dimensional syneresis of acid milk gels made with GDL increased at high gelation temperatures and high pH values (van Vliet et al., 1997).
Differences in the ease of water loss from different casein gels have been related to the susceptibility of the network to rearrangements just after gel formation (van Vliet and Walstra, 1994). Parameters that affect the time-scale for rearrangements of bonds in a gel include the dynamic moduli, which indicates the strength and number of bonds in the network, the yield stress and shear deformation at yielding, which determine the susceptibility of the strands to breakage, and tan δ, with higher values favouring the relaxation of bonds (van Vliet et al., 1991; Lucey et al., 1997b, d). In freshly made gels, the number of bonds between each junction is not yet very high, as indicated by the low dynamic moduli and tan δ is higher than in aged gels; these factors might explain why wheying–off occurs sometimes in young but not as much in aged gels. The “maximum in tan δ” which has been observed in acid gels made from heated milk (Fig. 12.1) would indicate an even greater likelihood of relaxation of bonds during the initial period after gel formation.
Acid-induced milk gels that were first cooled to a low temperature (e.g., 5 °C) before wetting their surface actually increased slightly in height, possibly due to the absorption of water by casein particles, which swell at low temperatures (Lucey et al., 1997d). Surface whey that was expelled during gelation is sometimes reabsorbed by the gel on cooling and storage at low temperature (Lucey and Singh, 1997).
12.3.2.6 Textural Defects
A wide range of cultured milk products is now on the market and each has very different textures or consistencies. Many processing parameters influence the properties of yoghurt gels (Table 12.1). The textural properties of acid milk gels can be assessed by a range of fundamental and empirical (instrumental) methods such as dynamic low amplitude oscillation, large amplitude oscillatory shear, creep or stress relaxation, penetration, rotational viscometry and flow through an orifice such as a Posthumus funnel. An excessively firm texture can be caused by factors such as a very high total solids content of the mix or an excessive amount of added stabilizers (this is exploited in the manufacture of “custard-style” yoghurt). A weak body can be caused by factors such as a low solids (fat) content of the mix, insufficient heat treatment of the milk, low acidity (high pH) and a high gelation temperature (>40 °C). Textural defects described as ‘lumpiness’ or ‘granular’ are objectionable as consumers usually expect a smooth, fine-bodied product (Tamime and Robinson, 2007; Clark et al., 2009) Lumpiness usually refers to the presence of large protein aggregates in yoghurt that can often range in size from 1 to 5 mm (Lucey and Singh, 1997). Factors such as excessive production of acid at a high incubation temperature, poorly rehydrated powders, and the use of rennet have been associated with these defects (Lucey and Singh, 1997). Some of these defects may be caused by conditions that favour the formation of dense protein clusters during gelation. Excessive heat treatment of milk and the addition of a high level of WPC have also been associated with other textural defects.
12.3.3 Addition of Dairy Proteins
In many acid gel systems additional dairy proteins are added to improve textural attributes, reduce whey separation, or for nutritional fortification. There are many options for adjusting the dairy protein levels of cultured products including the addition of skim milk powder, WPC, membrane concentrates, and milk protein concentrates.
There has been considerable interest in replacing skim milk powder with whey powders. The decision on possible substitution depends on factors such as their relative prices and the type of whey product. Bland whey products should be used to avoid off-flavours. Whey proteins have been used to substitute for up to 35–40 % of skim milk protein in yoghurt formulations. High concentration can result in defects and quality depends on processing conditions (i.e., heat treatment and incubation temperature). High protein whey products contain little lactose and have high gelling protein content. In yoghurt samples in which ≥20 % of milk solids-non-fat (SNF) were replaced by WPC, a ‘grainy’ texture was observed (Greig and Van Kan, 1984). Substituting WPC for SMP to elevate the total solids content of yoghurt mixes increased the ‘lumpy’ or ‘granular’ defect (Guirguis et al., 1988), while replacement of casein by WPC resulted in a yoghurt with a ‘less smooth and clumpy’ appearance (Jelen et al., 1987). Addition of WPC to milk, followed by heat treatment, resulted in acid gels becoming more brittle (Lucey et al., 1999). With the addition of high levels (4 %) of WPC, there is an increased risk of coagulation during heat treatment (Jelen et al., 1987). Possible benefits of using whey powders include: cost reduction compared with skim milk powder, improved texture, reduction in wheying-off, possible replacement of non-dairy ingredients (“cleaner label”), as well as the addition of nutritionally beneficial whey proteins, minerals and other bioactive compounds.
Peng et al., (2009b) prepared yoghurt bases from reconstituted SMP with 2.5 % protein and fortified with additional 1 % protein (wt/wt) from four different milk protein sources: SMP, milk protein isolate (MPI), micellar casein (MC) and sodium caseinate. Heat-treated, yoghurt mixes were fermented at 40 °C with a commercial yoghurt culture until pH 4.6. They reported that yoghurt firmness increased in the order: skim milk powder = micellar casein < milk protein isolate < sodium caseinate. Various other studies have also suggested that the use of sodium caseinate to fortify milk was the most effective means of increasing yoghurt firmness and reducing syneresis compared to other types of milk powders (Modler et al., 1983; Tamime et al., 1984; Sodini et al., 2004).
12.4 Interactions Between Acidified Casein Systems and Polysaccharides
12.4.1 Possible Interactions Between Caseins and Polysaccharides
In many dairy systems polysaccharides are added to increase/control viscosity or to stabilize the system, e.g., from sedimentation. Many different types of polysaccharides are used including pectins, starches, gelatin and gums.
In stirred-type yoghurt, stabilizers are added to control textural defects and prevent whey separation but stabilizers are not normally added to plain, set-style yoghurt (Lucey and Singh, 1997) although pectin is sometimes added in the US. Generally, usage levels for stabilizers in stirred yoghurt are <0.5 %. The most commonly added stabilizers in cream cheeses are xanthan gum and galactomannans (locust bean gum and guar gum), which act synergistically to increase viscosity or form a weak gel.
The nature of the interaction between milk proteins and polysaccharides is dependent on many factors including the specific types of intermolecular forces between the two biopolymers, concentrations of the two biopolymers and environmental factors (pH, ionic strength and calcium content) (Dickinson, 1998; Syrbe et al., 1998).
Some possible interactions between caseins and polysaccharides are shown in Fig. 12.5. In the presence of a nonadsorbing biopolymer there can be an attractive force between casein particles due to an osmotic effect associated with the exclusion of polysaccharides from the narrow region around each casein particle (Fig. 12.5a). The polysaccharides are “depleted” in the region around, and between, casein particles if they are too large to enter the depletion region. Flocculation of casein particles causes the depletion layers to overlap, thereby increasing the volume of solvent available for the polymer. Some polysaccharides are able to complex with proteins at specific pH values, for example high methoxy pectin can adsorb on caseins at low pH and provide electrostatic repulsion for acidified beverages (Fig. 12.5b). At neutral pH an electrostatic complex can be formed between oppositely charged biopolymers (Fig. 12.5c), e.g., κ-casein and κ-carrrageenan. Phase separation (Fig. 12.5d) results from incompatibility between the protein and polysaccharide molecules. Addition of high methoxy pectin to milk (at neutral pH values) can cause phase separation. Some polysaccharides have the ability to form weak gels (or systems with high zero shear viscosity) and thereby stabilize the sedimentation of casein particles (e.g., xanthan is able to form weak gels, especially in the presence of galactomannans) (Fig. 12.5e). Other biopolymers can form stiff gel networks, e.g., gelatin. Bridging flocculation occurs where there is a mixture of an adsorbing biopolymer and protein molecules and if there is insufficient polymer to cover all the particle surface completely and some polymer becomes attached simultaneously to more than one particle; this is called bridging flocculation (Fig. 12.5f). Bridging flocculation has been reported as possible between caseins and biopolymers like pectins or carrageenans (De Kruif and Holt, 2003). With a change in biopolymer concentration, there can be change in the nature of the interaction with caseins, e.g., the system can change from a depletion type interaction to a biopolymer gel network with an increase in the biopolymer concentration.
Low methoxy pectins are widely used as gelling/thickening agents in yoghurts. Pectin is an anionic carboxylated polysaccharide and in yoghurt its gelation occurs due to the release of calcium ions from micelles during acidification, which then initiates interactions between blocks of galacturonic acid on the pectin molecules (Matia-Merino and Singh, 2007).
12.4.2 Impact of Exopolysaccharides in Acid-Induced Gels
Some yoghurt starter cultures produce exopolysaccharides (EPS) during the fermentation process and are considered to help increase the viscosity of yoghurt and reduce whey separation. They can be viewed as a naturally produced thickener. This EPS can be produced as a capsular layer around the bacterial cell or excreted into the medium to produce an effect sometimes called “ropy” or “stringy” (Hassan, 2008); popular examples of ropy yoghurt are Viili and Långfil from Scandinavia. Capsular EPS has little impact on yoghurt gelation or texture. Ropy EPS can be either charged or uncharged. Some bacterial strains produce EPS which are considered to help increase the viscosity of yoghurt and reduce whey separation. The reported EPS concentrations produced in yoghurt fermentations is relatively small <150 mg/L. It is not clear how EPS modify yoghurt texture but various mechanisms have been suggested including: EPS in the serum phase increasing product viscosity, EPS forming bridges with the casein matrix and incompatibility between the EPS and casein modifying gel structure. EPS-producing starter cultures produce a number of specific types of EPS that differ in chemical structure/sugar composition, molar mass, concentration, charge, location and structural characteristics. It is possible that charged EPS may associate electrostatically with the caseins, depending on the pH of the milk, whereas uncharged EPS may influence gelation via a depletion flocculation type mechanism (Girard and Schaffer-Lequart, 2007). The exact period during fermentation (before, during or after gelation) when EPS is produced may play a role in determining the impact of EPS on yoghurt gels.
12.5 Concluding Remarks
Although acidified milk gels have been made for thousands of years only the technological and microbiological aspects of these products were studied in any detail until relatively recently. Considerable progress has been made during the past 20 years on the formation and rheological properties of acidified milk gels. Recent developments were reviewed but it is believed that further work is needed in the following areas:
-
The mechanism by which EPS impact the formation of acid gels, as well as, their influence on the detailed textural and sensory attributes of yoghurt. More knowledge is needed about precise physicochemical properties (molar mass, charge, branching, etc) of EPS and their importance for texture modifications of yoghurt.
-
Studies that probe possible linkages between fundamental rheological properties and empirical tests and their correlation with sensory perception of texture and taste.
-
Developing or modifying existing theoretical models for the formation of acid milk gels; such theories should be able to describe the kinetics of gelation and the dynamics of the rheological properties, help explain the different gelation behaviour of heated milk, be consistent with rearrangements of the aggregating particles before, during and after gelation and explain the continued increase in G′ after visual gelation.
-
Concentrated yoghurts are becoming more popular but little is known about the impact of processing on their textural or physical properties, e.g., impact of heating, shearing or pumping of the fermented product. Different approaches to concentration (fortification, mechanical separation or membrane filtration) are used industrially and they produce different products but little is known about the reasons for these differences.
References
Allan-Wojtas P, Kalab M (1984) Milk gel structure. XIV. Fixation of fat globules in whole milk yoghurt for electron microscopy. Milchwissenschaft 39:323–327
Barrantes E, Tamime AY, Sword AM, Muir DD, Kalab M (1996) The manufacture of set-style natural yoghurt containing different oils—2: rheological properties and microstructure. Int Dairy J 6:827–837
Biliaderis CG, Khan MM, Blank G (1992) Rheological and sensory properties of yogurt from skim milk and ultrafiltered retentates. Int Dairy J 2:311–323
Bremer LGB, van Vliet T, Walstra P (1989) Theoretical and experimental study of the fractal nature of the structure of casein gels. J Chem Soc Faraday Trans 1(85):3359–3372
Bremer LGB, Bijsterbosch BH, Schrijvers R, van Vliet T, Walstra P (1990) On the fractal nature of the structure of acid casein gels. Colloids Surf 51:159–170
Bremer LGB, Bijsterbosch BH, Walstra P, van Vliet T (1993) Formation, properties and fractal structure of particle gels. Adv Colloid Interf Sci 46:117–128
Brooker BE (1995) Imaging food systems by Confocal Laser Scanning Microscopy. In: Dickinson E (ed) New physico-chemical techniques for the characterization of complex food systems. Blackie Academic & Professional, Glasgow, pp 53–68
Castillo M, Lucey JA, Payne FA (2006) The effect of temperature and inoculum concentration on rheological and light scatter properties of milk coagulated by a combination of bacterial fermentation and chymosin. Cottage cheese-type gels. Int Dairy J 16:131–146
Chandan RC (2006) Manufacturing yogurt and fermented milks. Blackwell Publishing, Oxford
Chardot V, Banon S, Misiuwianiec M, Hardy J (2002) Growth kinetics and fractal dimensions of casein particles during acidification. J Dairy Sci 85:8–14
Cho YH, Lucey JA, Singh H (1999) Rheological properties of acid milk gels as affected by the nature of the fat globule surface material and heat treatment of milk. Int Dairy J 9:537–545
Choi J, Horne DS, Lucey JA (2007) Effect of insoluble calcium concentration on rennet coagulation properties of milk. J Dairy Sci 90:2612–2623
Choi J, Horne DS, Lucey JA (2011) Determination of molecular weight of a purified fraction of colloidal calcium phosphate derived from the casein micelles of bovine milk. J Dairy Sci 94:3250–3261
Clark S, Costello M, Drake MA, Bodyfelt F (2009) The sensory evaluation of dairy products, 2nd edn. Springer, New York
Dalgleish DG, Law AJR (1988) pH-induced dissociation of casein micelles. 1. Analysis of liberated caseins. J Dairy Res 55:529–538
Dalgleish DG, Parker TG (1980) Binding of calcium ions to bovine αS1-casein and precipitability of protein-calcium ion complexes. J Dairy Res 47:113–122
Dalgleish DG, Pouliot Y, Paquin PA (1987) Studies on the heat stability of milk. II. Association and dissociation of particles and the effects of added urea. J Dairy Res 54:39–49
Dannenberg F, Kessler H-G (1988a) Effect of denaturation of β-lactoglobulin on texture properties of set-style nonfat yoghurt. 1. Syneresis. Milchwissenschaft 43:632–635
Dannenberg F, Kessler H-G (1988b) Effect of denaturation of β-lactoglobulin on texture properties of set-style nonfat yoghurt. 2. Firmness and flow properties. Milchwissenschaft 43:700–704
Davies FL, Shankar PA, Brooker BE, Hobbs DG (1978) A heat-induced change in the ultrastructure of milk and its effect on gel formation in yoghurt. J Dairy Res 45:53–58
de Kruif CG (1997) Skim milk acidification. J Colloid Interface Sci 185:19–27
de Kruif CG (1999) Casein micelle interactions. Int Dairy J 9:183–188
De Kruif CG, Holt C (2003) Casein micelle structure, functions and interactions. In: Fox PF, McSweeney PLH (eds) Advanced dairy chemistry, vol 1, 3rd edn, Proteins. Kluwer Academic/Plenum, New York, pp 233–276
de Kruif CG, Roefs SPFM (1996) Skim milk acidification at low temperatures: a model for the stability of casein micelles. Neth Milk Dairy J 50:113–120
de Kruif KG, Hoffman MAM, van Marle ME, van Mil PJJM, Roefs SPFM, Verheul M, Zoon N (1995) Gelation of proteins from milk. Faraday Discuss 101:185–200
Dickinson E (1997) Aggregation processes, particle interactions, and colloidal structure. In: Dickinson E, Bergenståhl B (eds) Food colloids: proteins, lipids and polysaccharides. Royal Society of Chemistry, Cambridge, pp 107–126
Dickinson E (1998) Stability and rheological implications of electrostatic milk protein-polysaccharide interactions. Trends Food Sci Technol 9:347–354
Donato L, Alexander M, Dalgleish DG (2007) Acid gelation in heated and unheated milks: interactions between serum protein complexes and the surfaces of casein micelles. J Agric Food Chem 55:4160–4168
Driessen FM, Ubbels J, Stadhouders J (1977) Continuous manufacture of yogurt. 1. Optimal conditions and kinetics of the prefermentation process. Biotechnol Bioeng 19:821–839
Girard M, Schaffer-Lequart C (2007) Gelation and resistance to shearing of fermented milk: role of exopolysaccharides. Int Dairy J 17:666–673
Greig RIW, Van Kan J (1984) Effect of whey protein concentrate on fermentation of yogurt. Dairy Ind Int 49(10):28–29
Guinee TP, Pudja PD, Farkye NY (1993) Fresh acid-curd cheese varieties. In: Fox PF (ed) Cheese: chemistry, physics and microbiology, vol 1, 2nd edn, General aspects. Chapman & Hall, London, pp 363–419
Guirguis N, Hickey MW, Freeman R (1988) Some factors affecting nodulation in yoghurt. Aust J Dairy Technol 43:45–47
Guyomarc’h F, Jemin M, Le Tilly V, Madec M-N, Famelart M-H (2009) Role of the heat-induced whey protein/κ-casein complexes in the formation of acid milk gels: a kinetic study using rheology and confocal microscopy. J Agric Food Chem 57:5910–5917
Haque Z, Kinsella JE (1988) Interaction between κ-casein and β-lactoglobulin: predominance of hydrophobic interactions in the initial stages of complex formation. J Dairy Res 55:67–80
Harwalkar VR, Kalab M (1980) Milk gel structure. XI. Electron microscopy of glucono-δ-lactone-induced skim milk gels. J Texture Stud 11:35–49
Harwalkar VR, Kalab M (1983) Susceptibility of yoghurt to syneresis. Comparison of centrifugation and drainage methods. Milchwissenschaft 38:517–522
Harwalkar VR, Kalab M (1986) Relationship between microstructure and susceptibility to syneresis in yoghurt made from reconstituted nonfat dry milk. Food Microstruct 5:287–294
Hassan AN (2008) Possibilities and challenges of exopolysaccharide-producing lactic cultures in dairy foods. J Dairy Sci 91:1282–1298
Hassan AN, Frank JF, Farmer MA, Schmidt KA, Shalabi SI (1995) Formation of yogurt microstructure and three-dimensional visualization as determined by confocal scanning laser microscopy. J Dairy Sci 78:2629–2636
Heertje I, Visser J, Smits P (1985) Structure formation in acid milk gels. Food Microstruct 4:267–277
Holt C, Davies DT, Law AJR (1986) Effects of colloidal calcium phosphate content and free calcium ion concentration in milk serum on the dissociation of bovine casein micelles. J Dairy Res 53:557–572
Horne DS (1998) Casein interactions: casting light on the black boxes, the structure in dairy products. Int Dairy J 8:171–177
Horne DS (1999) Formation and structure of acidified milk gels. Int Dairy J 9:261–268
Horne DS (2003) Casein micelles as hard spheres: limitations of the model in acidified gel formation. Colloids Surf A Physicochem Eng Asp 213:255–263
Horne DS, Davidson CM (1993) Influence of heat treatment on gel formation in acidified milks. International Dairy Federation, Brussels, pp 267–276, Special Issue no. 9303
Horne DS, Lucey JA, Choi JW (2007) Casein interactions: does the chemistry really matter? In: Dickinson E, Leser ME (eds) Food colloids: self-assembly and material science. Royal Society of Chemistry, Cambridge, pp 155–166
IDF (2012) The world dairy situation 2012. Bulletin 458. International Dairy Federation, Brussels
IDFA (2013) Dairy facts 2012 edition. International Dairy Foods Association, Washington, DC
Jelen P, Buchheim W, Peters K-H (1987) Heat stability and use of milk with modified casein: whey protein content in yogurt and cultured milk products. Milchwissenschaft 42:418–421
Kalab M, Allan-Wojtas P, Phipps-Todd BE (1983) Development of microstructure in set-style nonfat yoghurt. Food Microstruct 2:51–66
Kinsella JE, Whitehead DM (1989) Proteins in whey: chemical, physical, and functional properties. Adv Food Nutr Res 33:343–438
Law AJR (1996) Effects of heat treatment and acidification on the dissociation of bovine casein micelles. J Dairy Res 63:35–48
Lopes da Silva J, Rao MA (1999) Rheological behavior of food gel systems. In: Rao MA (ed) Rheology of fluid and semisolid foods. Aspen, Gaithersburg, MD, pp 319–368
Lucey JA (2001) The relationship between rheological parameters and whey separation in milk gels. Food Hydrocoll 15:603–608
Lucey JA (2011) Acid and acid/heat coagulated cheese. In: Fuquay JW, Fox PF, McSweeney PLH (eds) Encyclopedia of dairy sciences, 2nd edn. Elsevier, London, pp 678–705
Lucey JA, Singh H (1997) Formation and physical properties of acid milk gels: a review. Food Res Int 30:529–542
Lucey JA, Singh H (2003) Acid coagulation of milk. In: Fox PF, McSweeney PLH (eds) Advanced dairy chemistry, vol 1, 3rd edn, Proteins. Kluwer Academic/Plenum Publishers, New York, pp 1001–1025
Lucey JA, Hauth B, Gorry C, Fox PF (1993) Acid-base buffering of milk. Milchwissenschaft 48:268–272
Lucey JA, Dick C, Singh H, Munro PA (1997a) Dissociation of colloidal calcium-phosphate depleted casein particles as influenced by pH and concentration of calcium and phosphate. Milchwissenschaft 52:603–606
Lucey JA, Teo CT, Munro PA, Singh H (1997b) Rheological properties at small (dynamic) and large (yield) deformations of acid gels made from heated milk. J Dairy Res 64:591–600
Lucey JA, van Vliet T, Grolle K, Geurts T, Walstra P (1997c) Properties of acid casein gels made by acidification with glucono-δ-lactone. 1. Rheological properties. Int Dairy J 7:381–388
Lucey JA, van Vliet T, Grolle K, Geurts T, Walstra P (1997d) Properties of acid casein gels made by acidification with glucono-δ-lactone. 2. Syneresis, permeability and microstructural properties. Int Dairy J 7:389–397
Lucey JA, Munro PA, Singh H (1998a) Whey separation in acid skim milk gels made with glucono-δ-lactone: effects of heat treatment and gelation temperature. J Texture Stud 29:413–426
Lucey JA, Munro PA, Singh H (1998b) Rheological properties and microstructure of acid milk gels as affected by fat content and heat treatment. J Food Sci 63:660–664
Lucey JA, Tamehana M, Singh H, Munro PA (1998c) Effect of interactions between denatured whey proteins and casein micelles on the formation and rheological properties of acid skim milk gels. J Dairy Res 65:555–567
Lucey JA, Tamehana M, Singh H, Munro PA (1998d) A comparison of the formation, rheological properties and microstructure of acid skim milk gels made with a bacterial culture or glucono-δ-lactone. Food Res Int 31:147–155
Lucey JA, Teo CT, Munro PA, Singh H (1998e) Microstructure, permeability and appearance of acid gels made from heated skim milk. Food Hydrocoll 12:159–165
Lucey JA, Munro PA, Singh H (1999) Effects of heat treatment and whey protein addition on the rheological properties and structure of acid skim milk gels. Int Dairy J 9:275–279
Lucey JA, Tamehana M, Singh H, Munro PA (2000) Rheological properties of milk gels formed by a combination of rennet and glucono-δ-lactone. J Dairy Res 67:415–427
Matia-Merino L, Singh H (2007) Acid-induced gelation of milk protein concentrates with added pectin: effect of casein micelle dissociation. Food Hydrocoll 21:765–775
McGann TCA, Fox PF (1974) Physico-chemical properties of casein micelles reformed from urea-treated milk. J Dairy Res 41:45–53
Modler HW, Larmond ME, Lin CS, Froehlich D, Emmons DB (1983) Physical and sensory properties of yogurt stabilized with milk proteins. J Dairy Sci 66:422–429
Moitzi C, Menzel A, Schurtenberger P, Stradner A (2011) The pH induced sol-gel transition in skim milk revisited. A detailed study using time-resolved light and X-ray scattering experiments. Langmuir 27:2195–2203
Mottar J, Bassier A, Joniau M, Baert J (1989) Effect of heat-induced association of whey proteins and casein micelles on yogurt texture. J Dairy Sci 72:2247–2256
Mulvihill DM, Grufferty MB (1995) Effect of thermal processing on the coagulability of milk by acid. In: Fox PF (ed) Heat-induced changes in milk, 2nd edn. International Dairy Federation, Brussels, pp 188–205, Special Issue No. 9501
Ozcan Yilsay T, Lee W, Horne DS, Lucey JA (2007) Effect of trisodium citrate on rheological, physical properties and microstructure of yogurt. J Dairy Sci 90:1644–1652
Parnell-Clunies EM, Kakuda Y, deMan JM (1986) Influence of heat-treatment of milk on the flow properties of yoghurt. J Food Sci 51:1459–1462
Parnell-Clunies E, Kakuda Y, Smith AK (1987) Microstructure of yogurt as affected by heat treatment of milk. Milchwissenschaft 42:413–417
Peng Y, Horne DS, Lucey JA (2009a) The impact of preacidification of milk and fermentation time on the properties of yogurt. J Dairy Sci 92:2977–2990
Peng Y, Serra M, Horne DS, Lucey JA (2009b) Effect of fortification with various types of milk proteins on the rheological properties and permeability of nonfat set yogurt. J Food Sci 74:666–673
Pyne GT, McGann TCA (1960) The colloidal calcium phosphate of milk. II. Influence of citrate. J Dairy Res 27:9–17
Revis C, Payne GA (1907) The acid cogulation of milk. J Hyg 7:216–231
Roefs SPFM (1986) Structure of acid casein gels. A study of gels formed after acidification in the cold. Ph.D. Thesis, Wageningen Agricultural University, The Netherlands
Roefs SPFM, van Vliet T (1990) Structure of acid casein gels. 2. Dynamic measurements and type of interaction forces. Colloids Surf 50:161–175
Roefs SPFM, Walstra P, Dalgleish DG, Horne DS (1985) Preliminary note on the change in casein micelles caused by acidification. Neth Milk Dairy J 39:119–122
Roefs SPFM, de Groot-Mostert AEA, van Vliet T (1990) Structure of acid casein gels. 1. Formation and model of gel network. Colloids Surf 50:141–159
Rönnegård E, Dejmek P (1993) Development and breakdown of structure in yoghurt studied by oscillatory rheological measurements. Lait 73:371–379
Schmidt DG (1982) Association of casein and casein micelle structure. In: Fox PF (ed) Developments in dairy chemistry, vol 1, Proteins. Applied Science Publishers, London, pp 61–86
Singh H (1995) Heat-induced changes in casein, including interactions with whey proteins. In: Fox PF (ed) Heat-induced changes in milk, 2nd edn. International Dairy Federation, Brussels, pp 86–104
Singh H, Roberts MS, Munro PA, Teo CT (1996) Acid-induced dissociation of casein micelles in milk: effects of heat treatment. J Dairy Sci 79:1340–1346
Sodini I, Remeuf F, Haddad S, Corrieu G (2004) The relative effect of milk base, starter, and process on yogurt texture: a review. Crit Rev Food Sci Nutr 44:113–137
Syrbe A, Bauer WJ, Klostermeyer H (1998) Polymer science concepts in dairy systems-an overview of milk protein and food hydrocolloid interaction. Int Dairy J 8:179–193
Tamime AY (2006) Fermented milks. Blackwell Science, Oxford
Tamime AY, Robinson RK (2007) Tamime and Robinson’s yoghurt: science and technology, 3rd edn. CRC Press, Boca Raton, FL
Tamime AY, Kalab M, Davies G (1984) Microstructure of set-style yogurt manufactured from cow’s milk fortified by various methods. Food Microstruct 3:83–92
Tranchant CC, Dalgleish DG, Hill AR (2001) Different coagulation behaviour of bacteriologically acidified and renneted milk: the importance of fine-tuning acid production and rennet action. Int Dairy J 11:483–494
van Dijk HJM, Walstra P (1986) Syneresis of curd. 2. One-dimensional syneresis of rennet curd in constant conditions. Neth Milk Dairy J 40:3–30
van Marle ME, Zoon P (1995) Permeability and rheological properties of microbially and chemically acidified skim-milk gels. Neth Milk Dairy J 49:47–65
van Vliet T (1988) Rheological properties of filled gels. Influence of filler matrix interaction. Colloid Polym Sci 266:518–524
van Vliet T, Dentener-Kikkert A (1982) Influence of the composition of the milk fat globule membrane on the rheological properties of acid milk gels. Neth Milk Dairy J 36:261–265
van Vliet T, Keetels CJAM (1995) Effect of preheating of milk on the structure of acidified milk gels. Neth Milk Dairy J 49:27–35
van Vliet T, Walstra P (1994) Water in casein gels; how to get it out or keep it in. J Food Eng 22:75–88
van Vliet T, van Dijk HJM, Zoon P, Walstra P (1991) Relation between syneresis and rheological properties of particle gels. Colloid Polym Sci 269:620–627
van Vliet T, Lucey JA, Grolle K, Walstra P (1997) Rearrangements in acid-induced casein gels during and after gel formation. In: Dickinson E, Bergenståhl B (eds) Food colloids: proteins, lipids and polysaccharides. Royal Society of Chemistry, Cambridge, pp 335–345
Vétier N, Desobry-Banon S, Ould Eleya MM, Hardy J (1997) Effect of temperature and acidification rate on the fractal dimension of acidified casein aggregates. J Dairy Sci 80:3161–3166
Walstra P (1990) On the stability of casein micelles. J Dairy Sci 73:1965–1979
Walstra P (1993) The syneresis of curd. In: Fox PF (ed) Cheese: chemistry, physics and microbiology, vol 1, 2nd edn, General aspects. Chapman & Hall, London, pp 141–191
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Lucey, J.A. (2016). Acid Coagulation of Milk. In: McSweeney, P., O'Mahony, J. (eds) Advanced Dairy Chemistry. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2800-2_12
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2800-2_12
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2799-9
Online ISBN: 978-1-4939-2800-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)