Abstract
The surface properties of metals and alloys become important when these materials are used especially for tribological applications. Some basic concepts involved during wear of metals and alloys are briefly discussed in this chapter. Delamination theory of adhesive wear which is dominating wear mechanism for most metals and alloys is discussed. Most of the tribological joints are exposed to environmental oxygen when used in atmospheric conditions. Oxidation becomes problematic for such and high-temperature sliding applications when oxygen source is readily available at the interface. The debris formation mechanism and oxidation during sliding are included in this chapter. Information on oxidation and tribological behavior of 60NiTi is reviewed as it is a potential alloy for tribo-element applications. A brief description on phase transformation and high-temperature tribology of metallic materials is also included. The wear of materials at the interface depends on the interfacial strength of the sliding materials. In high-temperature oxidative wear, wear performance can be determined by the type of oxides formed on the sliding surfaces.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Tribology has contributed to human culture as it evolved alongside of it; however, its formal study was not conducted for centuries. Sir Leonardo da Vinci’s early work provided directions to the scientific study of tribology. Tribology is described in various sources such as “The science of mechanisms of friction, lubrication, and wear of interacting surfaces that are in relative motion” [1]. In another source, it is described as “a branch of mechanical engineering that deals with the design, friction, wear, and lubrication of interacting surfaces (as bodily joints) in relative motion” [2]. It is also described as “the branch of engineering that deals with the interaction of surfaces in relative motion (as in bearings or gears): their design and friction and wear and lubrication” [3]. In general, the purpose of tribological study is to design surfaces to reduce or regulate the friction. Despite extensive research efforts that have been made for decades, even today, the phenomenon of friction is not completely understood. It is empirically established that frictional force is directly proportional to applied load and the constant of proportionality is known as the coefficient of friction and represented as
where “μ” is the coefficient of friction, “F” is the frictional force, and “P” is the applied load. Experimental methods always face some difficulty understanding friction due to surface features such as asperities, surface roughness, and the scale of measurement such as micrometer length scale or nanometer length scale. Recent development in metrological techniques such as the atomic force microscope [4, 5], the surface force apparatus [6], and the quartz crystal balance [7, 8] enabled us to study surface forces at atomic length scales which are responsible for friction at that level.
2 Wear of Metallic Materials
Wear is defined as a process of material removal from the solid surface by means of mechanical, thermal, or chemical action. Writing with pencil on paper is an example of useful wear, whereas wear of machines is not useful wear. Wear takes place on the surface by one or combination of mechanisms like adhesion, sliding (delamination), fretting, abrasion, erosion, fatigue, or corrosion. Most of these types of wear involve the adhesion which is responsible for the surface damage. Among metals and alloys, adhesive wear is the most common. During sliding, metal from one sliding surface is transferred to another surface due to adhesive forces. This transfer stays on the surface or detaches from the surface in the form of debris [9].
2.1 Adhesive Wear Mechanism
The surface damage during relative motion of two surfaces could be due to the bombardment of one surface on the other, or due to the reaction between two surfaces, or with the surroundings. When two surfaces slide together, shear takes place at the junction. The shear strength of the interface and the type of metals involved in sliding determine the possibility of fragmentation. Different possibilities for shear to occur are [10]:
-
When the interface is slightly weaker than both the mating surfaces, shear occurs at the interface.
-
When the interface is stronger than one of the mating surface and weaker than the other, shear occurs within the softer metal, and the detached fragments adhere to the harder sliding metal.
-
When the interface is stronger than that of the mating surface but slightly harder than the other, severe shear occurs on the softer metal and a significant amount of metal transfers to the harder metal. The shear of the harder metals also occurs occasionally.
-
When the interface is significantly stronger than both the sliding metals, shear occurs at some distance from the interface. In such a situation, transfer of metals occurs from both the surfaces and the surface damage encountered in this situation increases.
The schematic of all these possibilities is shown in Fig. 6.1 [10].
Wear of metals due to shear [10]
The delamination theory also explains adhesive wear [11, 12]. Adhesive wear occurs when cracks formed under the surface propagate under continuous loading conditions. These cracks upon reaching the surface lead to surface damage. During continuous sliding the dislocations pile up at a finite distance from the surface. As the sliding goes on, voids are formed at the pileup region. The rate of void formation is more if the sliding surface is an alloy with a hard second phase for dislocations to pile against. When there are large secondary-phase particles in the metal, voids are formed by a plastic flow of matrix around hard particles. Voids cluster together and result in a crack, which then propagate parallel to the surface and ultimately fragment the surface. The separated particle in this fashion is a platelike structure. The void formation at a distance from the surface is illustrated in Fig. 6.2 [11].
Formation of debris by shear deformation [11]
Wear volume during adhesive wear can be expressed quantitatively [13, 14], i.e., the amount of wear is dependent on load, sliding distance, and hardness of the surface. The wear volume is given by
where “c” is a nondimensional constant, “L” is the load, “x” is the sliding distance, and “p” is the hardness of the surface.
3 Surface Reaction During Sliding: Tribo-oxidation
Frictional heat or external heat can affect the sliding wear of metallic materials significantly. The temperature rise facilitates the oxidation of the sliding surfaces. This can reduce the wear rate due to the transformation of metallic debris to oxide debris [15]. In metals such as Cu, Ni, Fe, Co, W, Mo, and Zn, surface oxide films form and grow as crystalline layers. However, in the case of Al, Si, Cr, Ge, Nb, and Ta, oxidation initially takes place forming amorphous films, and then these films transfer into crystalline depending upon temperature and time [16–20]. In aluminum alloys, an amorphous alumina oxide film can be thermodynamically more stable than the corresponding crystalline γ-Al2O3 film up to a certain critical oxide-film thickness. This amorphous film can also be more stable even for higher oxidation temperatures [17].
3.1 Case Study: Aluminum–Copper Sliding
During sliding of Al and Cu in air, copper oxidizes to form a crystalline copper oxide layer, whereas aluminum forms amorphous and converts to crystalline aluminum oxide if sufficient time is permitted. Crystalline alumina can present on the surface from as-received sample or can form during the beginning of the sliding. The oxidation potential of Cu is −0.34 EoV (Cu → Cu2+ + 2e−) and that of Al is 1.66 EoV (Al → Al3+ + 3e−) [21]. Copper oxidizes at a lower rate than aluminum. Initially, both oxide layers wear out. As wear continues, pure metals underneath those oxide layers expose to the atmosphere. As soon as the exposure of pure metals to the atmosphere, further oxidation commences. For Al, the initial oxide layer formed on the surface is amorphous alumina. Without sufficient time to crystallize, the amorphous alumina will be removed during sliding as amorphous debris. The TEM analysis of such debris formed during sliding of Cu–Al system showed the amorphous phase (Fig. 6.3) [22].
TEM micrograph of Al–Cu debris [22]
This phenomenon of the tribo-oxidation wear is represented schematically in Fig. 6.4. There are three steps in this process: pre-oxidation, removal of the initial oxide layer, and removal of the newly formed oxide layer. Pre-oxidation is the natural oxidation of the surface of mating metals.
3.2 Oxidation and Tribological Properties of 60NiTi [23]
Oxidation and tribological behavior of two Ni–Ti alloys was recently reviewed [23]. Ni–Ti alloy with 55 wt% of Ni (50 at.% of Ni) has shape memory effect, superelasticity, and very good corrosion resistance and is recognized as 55NiTi. Another Ni–Ti alloy with 60 wt% of Ni (55 at.% of Ni) shows shape memory effect but has higher brittleness and is recognized as 60NiTi. 60NiTi is a promising material for tribological applications such as tribo-elements, bearings, gears, or tools. NASA’s Engineering and Safety Center (NESC) is promoting 60NiTi as it showed shockproof, corrosion-resistant properties that can be used for aerospace bearing applications [24].
55NiTi has been studied for its oxidation behavior [25, 26]. Oxidation behavior of 60NiTi can be comparable to nitinol. At room temperature and 450 °C with low oxygen pressure 10−4 Torr, Ni did not oxidize but Ti was oxidized to TiO2 [25]. High-temperature oxidation (500 and 600 °C) of this alloy producing Ni-free zone with protective oxide coating of titanium oxide means Ni did not oxidize [26]. 60NiTi has high Ni content which might provide additional resistance to oxidation. NiTi alloys can be utilized for room and moderate temperature application as it showed good oxidation resistance up to 600 °C.
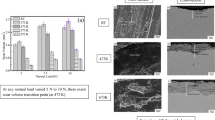
Study was conducted for tribological feasibility of 60NiTi for bearing applications in space using Spiral Orbit Tribometer (SOT) [27–31]. Powder metallurgical procedures were used to prepare the 60NiTi ball. In SOT the bearing ball slid between two parallel rotating disks. 60NiTi bearing ball was tested against 440C plates under boundary lubricated conditions using Pennzane 2001A oil. Under these conditions 60NiTi friction behavior (lower friction than 440C) was comparable to the high-performance 440C bearing balls which showed potential for these applications. No galling but mild abrasive wear on the tested was observed when the surface of 60NiTi ball was operated well beyond the lubricant life of the oil [27]. Tribological performance is compared with several other bearing materials in Fig. 6.5 which is adopted from the NASA/TM—2011-217105. The superiority of 60NiTi is evident from these results.
Coefficient of friction of different bearing ball against 440C plate under Pennzane 2001A oil lubrication [29]
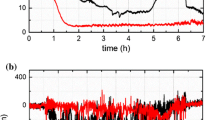
In our study of a typical dry sliding of thermally sprayed nitinol coating against 52100 bearing ball [32], it was observed that the friction was suddenly reduced after sometime during the test. This might be the indication of phase transformation of this shape memory alloy. The coefficient of friction was found to be very high (Fig. 6.6a) [32]. This behavior could limit nitinol’s applications as tribo-element.
(a) Friction coefficient with time plot for nitinol in dry test and (b) an atomic force microscopic image of worn nitinol surface [32]
The topological study of the worn surface of nitinol depicts grain boundary failure. The hard Ni3Ti phases along the grain boundary [33] might have influenced this failure. The debris generated was very fine due the brittle fracture of these particles (Fig. 6.6b) [32].
4 Interface Phenomenon During Sliding of Metallic Materials: Debris Formation
The material behavior and the surface changes at the interface during the sliding of two different surfaces are a very complex process so as the debris formation. Materials used in tribological applications are mostly elastoplastic in nature. Due to continuous load and frictional forces on the surface, these materials deform plastically. The sliding also increases the interfacial temperature and is sudden at the asperities. It can reach the melting point of the mating surfaces. This temperature is termed as flash temperature [10, 34, 35]. When two metallic surfaces slide, deformation at the interface occurs. The severity of the deformation depends on the lubricating condition such as lubricated or unduplicated. The deformation is avoided only in ideal hydrodynamic conditions [36]. This results in plastic strains and the strain gradients near the severely deformed interfaces. The deformation is very well observed most of the time by visual observation in a form of wear scar. The strain decreases exponentially under the sliding surface. Therefore, at the sliding interface, the shear strain is very high and it diminishes rapidly under the sliding surface [36].
Hardness of the materials deformed or fragmented at the interface can be harder or softer compared to the interfaces. If “h” be the hardness of the layer material and “H” be the hardness of the highly deformed materials, the topology of the deformed surfaces or debris can be predicted. Debris flakes are generated when h/H ≤ 1 and wear scar will be rough, whereas irregular debris formed when h/H ≥ 1 and wear scar will be smooth [36, 37].
There are different possibilities for the debris particle to follow once it separates from the parent materials. These possibilities are explained in Fig. 6.7a. The detached particle can adhere to the other surface by adhesive interaction which is represented by “a.” It can react with the surrounding atmosphere and get oxidized depending upon its reactivity with oxygen; this is represented by “b.” It can also roll in between two sliding surfaces as a third body which is represented by “c.” If the third-body rolling debris particle is hard, it will damage the softer surface and form groove on it which is represented by “d.” The deformation of the mating surface that can occur during sliding is represented by “e.” The debris formed during this process has a very complex structure. Figure 6.7b shows the schematic of the debris particle.
5 Phase Transformation During Sliding
Structural as well as chemical changes occur between the interfaces of two sliding bodies. Stable binary phases can form when alloying elements of binary alloys satisfy the criteria [38] given by the Hume–Rothery rules, i.e., (a) the ratio of atomic radii of constituent should not exceed 15 %, (b) the crystal structure of both the metals should be the same, (c) their electronegativity should be close, and (d) two metal ions must have the same valance. Most of the binary alloy system follows these rules.
On the other hand, the atomic radii of two constituent metals are important parameter that defines the amorphous structure [39, 40]. The difference between radii should be more than 15 %. Therefore, most of the binary alloys, which follow the Hume–Rothery rules, cannot be solidified as amorphous structures. These conventionally solidified alloys (in general, which follow the Hume–Rothery rules) show the long-range crystal structures. If this crystal structure is broken below the crystallization temperature, the alloys retain their metastable structure. Mechanical milling and mechanical alloying can produce such structures [41–47]. In these processes, the phase change occurs by interdiffusion and accumulation of lattice defects such as vacancies. During milling, amorphization can take place if the stored energy in the material exceeds the energy of the amorphous phase [48]. Mechanical deformation assists in interdiffusion to occur and subsequently to form the homogeneous amorphous alloy [49]. The experimental conditions in mechanical milling are in many ways analogous to the sliding of two surfaces. Sliding of two metals and alloys provides all the necessary experimental parameters and driving forces for amorphization to occur such as plastic deformation, temperature rise, and the accumulation of the lattice defects. Temperature rise which is the driving force for the diffusion to occur speeds up the solid reaction and phase changes by interdiffusion between sliding metals; this mechanism is explained for the formation of an amorphous alloy from pure crystalline metals [50, 51]. Another example is the rail and wheel sliding. During the skid along the rail, the wheel material close to the contact surface undergoes excessive mechanical and thermal events. The frictional heat generated raises the temperature up to approximately 800–1,000 °C; this causes phase transformations in the wheel steel [52].
Material polishing produces a thin film on the surface that is different from its parent material. In the tribology community, this layer is known as the “Beilby Layer” [53]. This layer is amorphous and formed due to the rapid solidification of locally melted asperities [10, 54]. It is also possible that during sliding the nanocrystalline materials could develop [55, 56]. These experimental observations of nanocrystalline and amorphous material formation during sliding were also validated with Molecular Dynamics (MD) simulations [57–60]. Sliding of annealed nanocrystalline Zr–Ti–Cu–Ni–Be bulk metallic glass (BMG) in vacuum and in air produced a work-softened layer where the plastic deformation was highest and formed the amorphous layer. The debris formed during this process was re-amorphized [59].
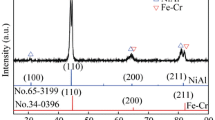
Let’s consider another case of Cr and Fe; both crystals have body center cubic (BCC) structures. The bond lengths of Cr and Fe are 0.24980 and 0.24823 nm, respectively [61]. Fe–Cr is a typical binary alloy which, according to Hume–Rothery rules [53], forms a substitutional solid solution. Although this alloy system is not readily glass forming (RGF), the middle composition range formed an amorphous state by thermal evaporation at room temperature using very low deposition rates [62]. During sliding, the rise in temperature at interface will be the driving force for the interdiffusion of Fe and Cr. This diffusion can occur on one of the mating surfaces, on both surfaces, or on the debris after they detach from their parent surfaces. Plastic deformation which occurred due to the continuously applied load during sliding is another influencing factor for amorphization to occur.
6 Elevated Temperature Tribology of Metallic Materials
Elevated temperature tribology of metallic materials is even complicated than their tribological behavior at room temperature. These complications are due to more favorable thermodynamics conditions occurring particularly for oxidation of sliding partners. Excessive oxidation at elevated temperature results in loss of surface metals when the oxide formed on the surface is not adhesive to the surface and result in spalling. To improve the adhesiveness of the oxide, the oxide formed on the surface is believed to be ductile. For example, the wear resistance of the Inconel 617 and Stellite 6 alloys at 750 °C is improved due to adhesive oxides growing slowly on Inconel 617 and Stellite 6 alloys. These oxides sustain the wear action and reduce spalling [63]. Some of the key points that contribute to high-temperature tribology are mentioned here [64]:
-
Change in mechanical properties at elevated temperature coupled with the oxidation affects the friction behavior at the interface which will have varying effects at different temperatures.
-
Tribo-chemistry at elevated temperature that is affected by other phenomenon such as diffusion between sliding surfaces and phase transformations.
-
The performance of these sliding materials which is governed by mechanical properties of reaction products formed at elevated temperature, the surface layers formed, and the actual response of the subsurface materials to the applied forces (i.e., deformation and fracture).
-
Geometry change at the sliding interface (scar formation) with possible wear phenomenon that took place at the interface.
7 Conclusions
The delamination theory can explain the wear and possibility of debris formation. Formation and type of oxide (amorphous or crystalline) on the surface of the sliding metals and alloys can influence the nature of debris formed. Whereas their adhesiveness to the surface on which they are formed influence further wear process, debris formed could be a complex composition than any sliding counterparts. Same as in milling, during sliding of metals and alloys, amorphization can take place if the stored energy in the material exceeds the energy of the amorphous phase.
Exercise
-
1.
Describe adhesive wear mechanism.
-
2.
Discuss the delamination theory of adhesive wear.
-
3.
Discuss debris formation during sliding of metallic materials.
-
4.
Describe the oxidation and tribological properties of 60NiTi.
-
5.
What factors do affect wear of metallic materials at high temperature?
References
In The American Heritage® Dictionary of the English Language, Houghton Mifflin Company
In Merriam-Webster Medical Dictionary© 2002, Merriam-Webster, Inc
In WordNet® 2.0 © 2003, Princeton University
Binnig G et al (1982) Surface studies by scanning tunneling microscopy. Phys Rev Lett 49(1):57–61
Binnig G, Quate CF, Gerber C (1986) Atomic force microscope. Phys Rev Lett 56(9):930–933
Israelachvili JN (1989) Techniques for direct measurements of forces between surfaces in liquids at the atomic scale. Chemtracts Anal Phys Chem 1:1–12
Krim J, Widom A (1988) Damping of a crystal oscillator by an adsorbed monolayer and its relation to interfacial viscosity. Phys Rev B 38(17):12184–12189
Krim J, Solina DH, Chiarello R (1991) Nanotribology of a Kr monolayer: a quartz-crystal microbalance study of atomic-scale friction. Phys Rev Lett 66(2):181–184
Rabinowicz E (1995) Friction and lubrication of materials. Wiley, New York
Bowden FP, Tabor D (1964) The friction and lubrication of solids, vol I and II. Clarendon Press, Oxford
Suh NP (1973) The delamination theory of wear. Wear 25(1):111–124
Suh NP (1986) Tribophysics. Printice-Hall, Englewood Cliffs, NJ
Holm R (1946) Electric contacts. Almquist and Wiksells, Stockholm
Archard J (1953) Contact and rubbing of flat surfaces. J Appl Phys 24(8):981–988
Stott FH (1998) The role of oxidation in the wear of alloys. Tribol Int 31(1–3):61–71
Fehlner FP (1986) Low temperature oxidation, the role of vitreous oxides. OSTI ID: 5328041. Retrieved from http://www.osti.gov/scitech/servlets/purl/5328041
Jeurgens L et al (2000) Thermodynamic stability of amorphous oxide films on metals: application to aluminum oxide films on aluminum substrates. Phys Rev B 62(7):4707
Doherty P, Davis R (1963) Direct observation of the oxidation of Aluminum single crystal surfaces. J Appl Phys 34(3):619–628
Eldridge J et al (1988) Thermal oxidation of single-crystal aluminum at 550 °C. Oxidation Metals 30(5):301–328
Snijders P, Jeurgens L, Sloof W (2002) Structure of thin aluminium-oxide films determined from valence band spectra measured using XPS. Surf Sci 496(1):97–109
Francis E (1999) Standard oxidation potentials. ©1998 [cited 2012 Oct 30, 2012]; Accessed http://dl.clackamas.cc.or.us/ch105-09/standard.htm
Ingole SP (2005) Nanotribological characterization of dynamic surfaces. University of Alaska Fairbanks, Fairbanks, AK, USA
Ingole S (2013) 60NiTi alloy for tribological and biomedical surface engineering applications. JOM 65(6):792–798. doi:10.1007/s11837-013-0610-7
Industry updates (2011) J Fail Anal Prev 11(6):645–653. doi:10.1007/s11668-011-9515-3
Chan CM, Trigwell S, Duerig T (2004) Oxidation of an NiTi alloy. Surf Interface Anal 15(6):349–354
Firstov G et al (2002) Surface oxidation of NiTi shape memory alloy. Biomaterials 23(24):4863–4871
DellaCorte C et al (2009) Intermetallic Nickel–Titanium alloys for oil-lubricated bearing applications. NASA, Cleveland, OH
DellaCorte C, Glennon GN (2012) Ball bearings comprising nickel-titanium and methods of manufacture thereof, in Google Patents, The United States of America as represented by the National Aeronautics and Space Administration, Abbott Ball Company, USA
DellaCorte C et al (2011) Resilient and corrosion-proof rolling element bearings made from superelastic Ni-Ti alloys for aerospace mechanism applications. August 2011, NASA/TM—2011-217105, http://www.ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20110016524_2011017534.pdf
Pepper SV et al (2009) NITINOL 60 as a material for spacecraft triboelements. 2009. Proc. ‘13th European Space Mechanisms and Tribology Symposium – ESMATS 2009’, Vienna, Austria, 23–25 September 2009 (ESA SP-670, July 2009), http://www.esmats.eu/esmatspapers/pastpapers/pdfs/2009/pepper.pdf
Pepper SV, DellaCorte C, Glennon G (2010) Lubrication of Nitinol 60, June 2010, NASA/TM-2010: 215331-1-8, http://www.grc.nasa.gov/WWW/StructuresMaterials/TribMech/highlights/documents/additional/TM-2010-216331.pdf
Ingole S, Liang H, Mohanty P (2005) Tribology characteristics of thermal sprayed NiTi coatings. In presented at 4th ASM international surface engineering congress and 19th international conference on surface modification technologies. ASM International, Saint Paul
Stanford MK, Thomas F, DellaCorte C (2012) Processing issues for preliminary melts of the intermetallic compound 60-NITINOL. Nov 01, 2012, NASA/TM-2012-216044; E-18479; GRC-E-DAA-TN4035, Doc ID: 20130000580, http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20130000580_2012018813.pdf
Jaeger J (1942) Moving sources of heat and the temperature of sliding contacts. in. J Proc Roy Soc NSW 76:203–224
Cook N, Bhushan B (1973) Sliding surface interface temperatures(solid–solid interface temperature rise during sliding from model with surface topography statistics, frictional conditions, surface hardness and thermal parameters). ASME Trans Ser F J Lubr Technol 95:59–64
Rigney D et al (1986) Low energy dislocation structures caused by sliding and by particle impact. Mater Sci Eng 81:409–425
Don J, Sun TC, Rigney DA (1983) Friction and wear of Cu–Be and dispersion-hardened copper systems. Wear 91(2):191–199
Hume-Rothery W (1931) The metallic state. Oxford University Press, London
Davies H, Luborsky F (1983) Amorphous metallic alloys. Butterworths, London, pp 8–25
Giessen BC (1982) In: Proceedings of 4th international conference on rapidly quenched metals, Japan Institute of Metals, Sendai
Xia S et al (1999) Formation of disordered structures in Cr–Fe alloy by mechanical milling. J Phys Condens Matter 5(17):2729
Gaffet E et al (1988) Ball milling amorphization mechanism of Ni⋅Zr alloys. J Less Common Metals 145:251–260
Hellstern E, Schultz L (1988) Formation and properties of mechanically alloyed amorphous Fe⋅Zr. Mater Sci Eng 97:39–42
Koch C et al (1983) Preparation of “amorphous” Ni 6 0 Nb 4 0 by mechanical alloying. Appl Phys Lett 43(11):1017–1019
Thompson J, Politis C (1987) Formation of amorphous Ti–Pd alloys by mechanical alloying methods. EPL (Europhys Lett) 3(2):199
Dolgin B et al (1986) Mechanical alloying of Ni, CO, and Fe with Ti. Formation of an amorphous phase. J Non-Crystalline Solids 87(3):281–289
Politis C, Johnson W (1986) Preparation of amorphous Ti 1−x Cu x (0.10≪x ≤ 0.87) by mechanical alloying. J Appl Phys 60(3):1147–1151
Schwarz RB, Koch CC (1986) Formation of amorphous alloys by the mechanical alloying of crystalline powders of pure metals and powders of intermetallics. Appl Phys Lett 49(3):146–148
Atzmon M et al (1984) Formation and growth of amorphous phases by solid-state reaction in elemental composites prepared by cold working. Appl Phys Lett 45(10):1052–1053
Schwarz R, Johnson W (1983) Formation of an amorphous alloy by solid-state reaction of the pure polycrystalline metals. Phys Rev Lett 51(5):415–418
Johnson WL (1986) Thermodynamic and kinetic aspects of the crystal to glass transformation in metallic materials. Prog Mater Sci 30(2):81–134
Ahlström J, Karlsson B (2002) Modelling of heat conduction and phase transformations during sliding of railway wheels. Wear 253(1–2):291–300
Beilby SG (1921) Aggregation and flow of solids. Macmillon, London
Bowden F, Hughes T (1937) Physical properties of surfaces. IV. Polishing, surface flow and the formation of the Beilby layer. Proc Roy Soc Lon Ser A Math Phys Sci 160(903):575–587
Rigney D, Hammerberg J (1999) Mechanical mixing and the development of nanocrystalline material during the sliding of metals. Proc TMS Fall Meet 465–474
Ganapathi S et al (1990) A comparative study of the nanocrystalline material produced by sliding wear and inert gas condensation. In MRS proceedings. Cambridge University Press, Cambridge
Rigney D et al (2003) Examples of structural evolution during sliding and shear of ductile materials. Scripta Materialia 49(10):977–983
Kim HJ, Karthikeyan S, Rigney D (2009) A simulation study of the mixing, atomic flow and velocity profiles of crystalline materials during sliding. Wear 267(5–8):1130–1136
Fu XY, Rigney D, Falk M (2003) Sliding and deformation of metallic glass: experiments and MD simulations. J Non-Crystalline Solids 317(1):206–214
Heilmann P et al (1983) Sliding wear and transfer. Wear 91(2):171–190
Weast R, Selby S, Hodgman C (1965/1966) Handbook of chemistry and physics, 46th edn. The Chemical Rubber Co, Cleveland, OH
Xia SK, Saitovitch EB (1994) Formation of an amorphous phase in Cr(1−x)FeX films obtained by thermal evaporation. Phys Rev B 49(5):927
Birol Y (2010) High temperature sliding wear behaviour of Inconel 617 and Stellite 6 alloys. Wear 269(9–10):664–671
Blau PJ (2010) Elevated-temperature tribology of metallic materials. Tribol Int 43(7):1203–1208
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Ingole, S.P. (2013). Tribology of Metals and Alloys. In: Menezes, P., Nosonovsky, M., Ingole, S., Kailas, S., Lovell, M. (eds) Tribology for Scientists and Engineers. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-1945-7_6
Download citation
DOI: https://doi.org/10.1007/978-1-4614-1945-7_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-1944-0
Online ISBN: 978-1-4614-1945-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)