Abstract
Diffuse low-grade gliomas are rare tumors. Therapeutic strategies have dramatically changed in recent years, thanks to observational data, insight of some authors, retrospective studies, and, incidentally, results of few phase III and II trials. Surgery has become the cornerstone of the treatment. Radiotherapy, because of its potential delayed neurotoxicity and the equivalent results in terms of survival whatever the timing of the treatment (early or late), is increasingly offered to patients with unresectable tumors (or tumor that cannot be reoperated) and in case of progression after chemotherapy. Chemotherapy, subject of this chapter, has shown clinical benefits regarding tumor progression for nonsurgical patients, before or after radiotherapy: initial chemosensitivity almost constant, improvement of epilepsy and thus of cognition, and preservation of quality of life (despite a possible transient alteration). Its articulation with surgery has been more recently discussed by allowing, thanks to tumor shrinkage, subtotal or total resection (whose impact on anaplastic transformation and survival has been demonstrated), in addition to potential effects on cerebral plasticity. It remains to show the direct or indirect impact on survival, to refine its risk-benefit ratio, especially in the context of prolonged treatment with temozolomide, and to develop further research from a neurological (impact on plasticity) and oncological (involved molecular pathways, identifying new therapeutic targets) points of view.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Diffuse low-grade glioma (DLGG) is a rare disease whose therapeutic concepts have profoundly been challenged these recent years [1].
Thus, surgery, marginally considered until the 1990s, saw his place reinforced, thanks to the development of neuroscience researches through the cortical and subcortical stimulations, awake surgery, and functional imaging progress [2, 3].
Potential neurotoxicity of long-term conventional radiotherapy was stressed. Therefore, this treatment was little used in the initial stages of the disease. Recent technical advances allow nevertheless considering a more focused therapeutic volume. It should so lead to new assessments with a clear evaluation of the ratio between expected benefits/potential risks [4].
Finally, chemotherapy, despite many theoretical limitations (intrinsic chemoresistance, difficult access to the tumoral site, low number of available molecules), gradually developed, first in case of progression after conventional treatments deliverance and then more precociously in the disease history and in close coordination with surgery.
We will develop this point below by considering the conceptual and historical bases while highlighting the unresolved issues.
Current Practices
Conceptual Bases of Chemotherapy
The place of the chemotherapy for DLGG remains difficult to encircle. Many theoretical arguments can be opposed to the principle of prescription: subnormal blood–brain or blood-tumor barrier (low penetration of drugs), spontaneous chemoresistance of gliomas, and very limited number of potentially active molecules.
Nevertheless, there are situations like tumor progression in case of patients with unresectable tumor previously treated by radiotherapy (RT) where chemotherapy (CT), in absence of therapeutic alternative, could be discussed or considered before anaplastic transformation.
The literature on the subject remains relatively poor.
For a long time, only the paper by Eyre et al. from the South Western Oncology Group (SWOG) served as a reference and discouraged all therapeutic inclinations. In fact, the SWOG conducted the first randomized trial concerning CT for DLGG. It has compared RT alone versus RT plus lomustine-based CT after subtotal/partial surgery or biopsy. No benefit was shown and the trial was prematurely terminated [5]. To date, it is difficult to consider this relatively old trial because of the associated methodological bias regarding the selected population (more specifically from a pathological and radiological point of view).
Cairncross and Macdonald have, the first, evoked the possibility of a real objective response for diffuse low-grade tumors within a series of aggressive oligodendrogliomas [6, 7]. Six years later, Mason was able to note 9/9 responders under procarbazine + cecenu + vincristine association (PCV) [8], while Soffietti [9] reported 13/13 stabilized or responding patients also under PCV.
Since then, a little over 30 articles have been published. There are mostly retrospective series that included most often a small number of patients (see below). We will come back to some of these articles in the following chapters.
Available Data and Chronology
Table 25.1 adapted from Ducray [10] summarizes the literature on the subject. As we can appreciate, series are very heterogeneous in term of disease entities, time of illness, treatment modalities, and evaluation of the response.
We can nevertheless confirm that, in recent years, regardless the growing role of surgery, we have seen a real interest in chemotherapy (especially temozolomide) in the management of these tumors [11].
This led to the creation of a dedicated European Task Force and to the establishment of recommendations recently published (and being updated). These recommendations clearly propose chemotherapy in specific situations to which we will return in the course of this chapter: “Chemotherapy can be useful both at recurrence after radiotherapy and as initial treatment after surgery to delay the risk of late neurotoxicity from large-field radiotherapy” [12].
We should nonetheless note that all except eight published series have fewer than 50 patients. This low number of inclusions reflects the relative scarcity of the pathology but also the difficulties to include such patients in therapeutic trials probably because of the specificity of this particular tumoral entity (too heterogeneous for normative constraints of clinical trials) and the conceptual differences between major involved groups.
Types of Chemotherapy
Two main modalities of chemotherapy were used for DLGG: procarbazine + cecenu + vincristine (PCV) association and temozolomide (TMZ), according to different patterns.
There are little variations in the reported dosages concerning the PCV combination used first by Gutin in 1975 [13] and Levin in 1980 and 1985 [14, 15]. Classically, cecenu is administered on day (D) 1 (110 mg/m2), procarbazine (60 mg/m2) from D8 to D21 and vincristine (1.4 mg/m2 – max 2 mg) at D8 and D29. A cycle is administered every 6–8 weeks. Intensified protocols have also been described but not used in DLGG [16].
Temozolomide (TMZ) is, to date, the most widely used treatment. The conventional scheme proposes a daily dose of 150 mg/m2 for 5 days during the first course. If it is well tolerated, the dose is increased to 200 mg/m2 per day for 5 days from the second course. Cycles last for 28 days. Other plans, including intensified protocols, have been proposed. Lashkari et al. attempted to assess the impact of these different TMZ regimens on the treatment of DLGG. They performed a systematic review of the literature and identified all the studies published in PubMed, Embase, and Cochrane databases that met the inclusion criteria. Eighteen studies and 736 patients were analyzed. Although there is possibly an indication that metronomic regimens of TMZ result in better progression-free survival (PFS) and response rate when compared with the conventional standard 5 day regimen, insufficient available data and study heterogeneity preclude any safe conclusions. Authors offer as conclusion that “well-designed randomized controlled clinical trials are needed to establish the efficacy of metronomic regimens of TMZ in LGGs” [17].
To date, we can consider, mainly because of the good immediate tolerance and the respect for the quality of life (cf. infra), that temozolomide used with conventional doses remains the reference treatment.
Results
Chemotherapy, Volumes, and Growth Rate
The response assessment after chemotherapy for DLGG remains a difficult and nonconsensual issue.
For many years, MacDonald criteria, created to evaluate WHO grade III and IV gliomas [18] and based on 2D enhanced tumor measurements on computed tomography or magnetic resonance imaging (in conjunction with clinical and steroid dosage evaluations), were used for DLGG after adaptation (especially by considering the two largest diameters on T2-weighted or FLAIR slides and not on injected images and by abandoning the reference to steroids). This procedure does not allow to objectively monitor the evolution of a tumor under treatment and underestimates the number of responders. This was clearly the case for many initially reported studies [19–21].
New recommendations were proposed [22]. These latter do not appear optimal by considering that published studies that have compared calculations based on single, multidimensional, and true volumetric measurements and the strength of their correlations with the outcome (PFS, OS) are absent and thus that evidence-based data for the preferred measurement system are not available. We disagree with this opinion (see the dedicated chapter), because we consider that the volumetric evaluation is absolutely necessary for monitoring DLGG patients receiving chemotherapy. Otherwise, the risk is to dramatically underestimate responses and thus to be in an absolute inability to properly monitor the treatment duration.
The papers by Hoang-Xuan et al. [23] and Ricard et al. [24] were the firsts most important considering the impact of chemotherapy on DLGG. In the second one’s, authors were, indeed, among the first to report a longitudinal real volumetric assessment in a population of 107 patients treated exclusively with temozolomide. The method of the three diameters was used to obtain volumes and mean tumoral diameters (MTD) [25]. During the treatment, they found that more than 60 % of patients achieved a minor or partial response. At the onset of TMZ treatment, the MTD decreased in 92 % of patients, demonstrating an early initial chemosensitivity (38 of 39 patients who had a pre-, per-, and postevaluation of the MTD slope experienced a breakdown of the MTD growth curves after chemotherapy onset). After the initial phase of MTD decrease and despite continuous administration of TMZ, the tumors of some patients started to resume growth again, whereas others continued to decrease. Tumor regrowth occurred in 16.6 % of 1p19q codeleted tumors and in 60.6 % in non-codeleted tumors (p < 0.0004). Tumors overexpressing p53 had also a much greater rate of relapse (70.5 % vs. 25 %). The evolution of the MTD was also tested after discontinuation of TMZ. The greater part of the population remains stable or sometimes continues to decrease despite the interruption of treatment. Nevertheless, a majority of tumors starts to grow again: 59 % rate of MTD regrowth after a median follow-up of 200 days after TMZ discontinuation (range 60–630 days).
Our group has also published a retrospective study concerning chemotherapy followed by surgical resection for DLGG. The impact of chemotherapy on the tumor volume was estimated using Volume Viewer® software (General Electric GE Healthcare, Milwaukee, WI, USA). For exams in which only printed images were accessible, a three-diameter technique was used. We also demonstrated that chemotherapy induced a tumor shrinkage (median volume decrease 38.9 %) in 10/10 cases (ipsilateral in six patients and in the contralateral hemisphere in four patients) [26].
Chemotherapy and Epilepsy
Seizures are the most common initial symptom in patients with DLGG. Their occurrence strongly depends on the tumor location including insular and central topography [27]. Some authors have also suggested a link between IDH 1/2 mutation (frequent in DLGG) and the onset of metabolic changes capable of promoting seizures [28].
For a long time, chemotherapy and irradiation were considered having just some minor beneficial effects on the patients’ seizure disorder using the argument that overall 60–70 % of patients may experience recurrent epilepsy during long-term follow-up [29].
The progressive development of this therapeutic modality, its conceptual changes (prolongation of treatment time), and more precise analysis of the impact of such therapy on seizures have radically changed the view of many authors. Thus, it is now considered (despite the usual difficulties with seizure quantification in retrospective studies) that (1) the negative course of seizure frequency was mostly correlated to tumor progression, (2) surgery had almost always a favorable effect on epilepsy, and (3) chemotherapy had a mostly favorable effect with acceptable tolerance [2, 30, 31].
The improvement in seizure frequency during treatment with temozolomide seems, moreover, independent of antiepileptic drug adjustment [32].
An extensive experience with insular DLGG (topography considered as the most epileptogenic) was also reported by our group. We confirmed the interest of a surgical removal and supported the role of chemotherapy from an epileptological point of view [33].
We need to address in this chapter, regarding the relationship between chemotherapy and DLGG, the special place of antiepileptic treatments. Recommendations in this area are identical to the recommendations for all brain tumors. Most authors recommend first-line noninducing drugs such as lamotrigine, levetiractam, or lacosamide [34, 35]. The place of valproate remains debated. A clear efficiency is reported [35]. Combined antiproliferative activity through its inhibitory properties of histone deacetylase could improve survival as it was evoked for glioblastomas [36]. Nevertheless there are potential side effects (weight gain, thrombocytopenia) and enzyme inhibition may increase the hematologic toxicity of chemotherapy.
Chemotherapy and Cognition
Cognitive functioning is correlated with quality of life, itself linked with return to work [37]. This point is absolutely crucial in general neuro-oncology and, still more, in the management of patients with DLGG. Approximately one quarter of patients with DLGG reported serious neurocognitive symptoms [38]. Neurocognitive deficits are far more frequent than previously thought and can be caused by the tumor itself, tumor-related epilepsy, treatments, and psychological distress [12]. For some authors, the role of radiotherapy and chemotherapy in the treatment of DLGG remains controversial regarding their effect on survival and the development of neurotoxicity. Forty DLGG patients participated in the study of Correa et al. 16 patients had RT +/− chemotherapy and 24 patients had no treatment. In this series, RT +/− chemotherapy, disease duration, and antiepileptic treatment contributed to mild cognitive difficulties [39]. The same team published a new paper with 25 DLGG patients who underwent neuropsychological evaluations at study entry, 6 and 12 months subsequently. Nine patients had RT +/− chemotherapy prior to enrollment and 16 had no treatment [40]. Longitudinal follow-up showed that both disease duration and treatment with RT +/− chemotherapy contributed to a mild decrement in nonverbal recall and in some aspects of executive functions and quality of life. In these two articles, the widespread use of combined strategies (radiotherapy + chemotherapy) makes difficult to analyze the specific contribution of chemotherapy in the cognition modulation. Our group [26] reported a retrospective work with a neuropsychological assessment (NPA) of ten patients who underwent a strategy with a first chemotherapy followed by functional surgery. Nine patients were right-handed and one left-handed. No one presented with premorbid intelligence deterioration. Three patients did not show any neuropsychological deficit. Seven patients failed at three or less out of the 18 cognitive tests that were applied. The three others failed at least four tests. The main cognitive domains where deficits were observed concern episodic memory, especially verbal modality (five patients), and executive functions (five patients). Interestingly, the patients who did not continue to work were not the same who presented the most severe cognitive impairment. Our conclusion was that this combined strategy is highly likely to preserve cognitive function.
Chemotherapy and Quality of Life
As already mentioned, quality of life is correlated with cognitive functioning with itself linked with return to work [37]. Works on these three fundamental aspects of DLGG patient’s evaluation are very rare. We know, generally, that female sex, epilepsy burden, and number of objectively assessed neurocognitive deficits were associated significantly with both generic and condition-specific HRQOL [38]. The major impact of PCV on HRQOL is on nausea/vomiting, loss of appetite, and drowsiness during and shortly after treatment. There are no long-term effects of PCV chemotherapy, since patients recover a “normal” state when they move away from the treatment period [41].
Liu et al. described the quality of life (QOL) of DLGG patients at baseline prior to chemotherapy and through 12 cycles of temozolomide. The Functional Assessment of Cancer Therapy-Brain (FACT-Br) was obtained at baseline (prior to chemotherapy) and at 2-month intervals under chemotherapy. Patients at baseline had higher reported social well-being scores (mean difference = 5.0; p < 0.01) but had lower reported emotional well-being scores (mean difference = 2.2; p < 0.01) compared with a normal population. Patients with right hemisphere tumors reported higher physical well-being scores (p = 0.01): 44 % could not drive, 26 % did not feel independent, and 26 % were afraid of having a seizure. Difficulty with work was noted in 24 %. Mean change scores at each chemotherapy cycle compared with baseline for all QOL subscales showed either no significant change or were significantly positive (p < 0.01). Authors concluded that DLGG patients on therapy were able to maintain their QOL in all realms. Patients’ QOL may be further improved by addressing their emotional well-being and their loss of independence in terms of driving or working [42].
In our work concerning patients treated with presurgical chemotherapy [26], the Karnofsky Performance Scale (KPS) scores ranged from 80 to 100 (median 90) and were globally stable during the whole follow-up period. The main domain that presented with significant impairment in the QOL assessment was role functioning (feeling of independence and socio-professional life) with a median score of 66.7 % (range 50–100). The global QOL score was preserved after chemotherapy and surgery for most patients with a median value of 66.7 % (range 33.3–83.3). Cognitive, emotional, physical, and social well-being scores were also relatively preserved (medians 83.3, 79.2, 100, and 100 %, respectively). Among the general symptoms, the main complaints were fatigue (median score 33.3 %, range 11.1–100) and pain (median score = 16.6 %, range 0–66.7) due to different associated diseases like osteoarthritis and arteriopathy. Sleeping troubles (mean score = 20 ± 30.6 %), financial impact (mean score = 23.3 ± 39.6 %), and digestive troubles (mean score = 20 ± 30.6 %) seemed to have a moderate influence on the QOL. No patient reached the cut-off of 15 in the inventory for signs or symptoms of depression (BDI) with a mean score of 8.7 ± 3.6. However, seven subjects showed a tendency for “mild depression,” characterized by a score between 8 and 14.
We can therefore consider that TMZ alone or combined with surgery is able to maintain or even to improve the quality of life and that PCV alters transiently the QOL, with a return to the “normal” situation when we once move away from the treatment period.
Chemotherapy and Survival
To date, there is no direct evidence for DLGG patients that confirms the impact of chemotherapy on patients’ survival. We know, however, that presumed eloquent location of DLGGs is an important but modifiable risk factor predicting disease progression and death [43] and that the risk of malignant transformation and subsequent survival may be predicted by pretreatment and also by treatment-related factors [44].
We are thus entitled to imagine that indirectly, this treatment modality may have an impact on patient survival.
In a retrospective selected series (personal unpublished data), 17 patients, considered at diagnosis or recurrence as “nonoperable” because of a functional area infiltration or a too large contralateral extension, underwent temozolomide-based chemotherapy inducing tumor volume decrease immediately followed by a radical surgery. The median follow-up since initial radiological diagnosis was 5.9 years (range 1.4–11). Median time to malignant transformation was 99.6 months. We demonstrated that age, volume at diagnosis, 1p19q, IDH, and MGMT promoter status had no impact on time to malignant transformation. Chemotherapy reduced tumor volume (median −33.43 %, range – 61.6 % to −5.1 %) and significantly decreased the imaging tumor growth whatever 1p19q, IDH, and MGMT status. We confirmed that a tumor volume decrease of more than 20 % was significantly correlated with a lower postoperative residual tumor (median = 3.4 cc, p = 0.003), a greater extent of resection (p = 0.03), and a better prognosis (p = 0.05). Postoperative tumor volume less than 10 cc was significantly associated with better outcome (p = 0.042). We thus concluded that, regardless of the molecular status, neoadjuvant chemotherapy could optimize surgical resection of DLGGs and could have an impact on their natural history (Blonski et al. submitted).
Tolerance
Hematological Toxicity
The “PCV” association possesses a cumulative acute hematologic toxicity making impossible the administration of more than six courses. Previous papers provide evidence that nitrosoureas are leukemogenic in human beings and confirm observations that adjuvant chemotherapy with alkylating agents may increase the risk of leukemia [45]. In the paper of Boice et al. concerning adjuvant treatment of gastrointestinal cancer with semustine (methyl-CCNU), the 6-year cumulative mean risk of acquiring a leukemic disorder after treatment with semustine was 4.0 +/− 2.2 % for an incidence rate of 2.3 cases per 1,000 persons per year [46]. In a meta-analysis of five randomized clinical trials for adult patients with brain tumors, Greene et al. identified 2 of 1,628 individuals who experienced acute nonlymphocytic leukemia after carmustine chemotherapy [47]. The risk of developing this complication was 24.6 times higher than expected [45]. Baehring et al. identified well-documented case reports and small case series of patients who developed therapy-induced myelodysplasia (t-MDS) and therapy-induced acute myeloid leukemia (t-AML) during or after treatment with alkylating chemotherapy for primary brain neoplasms. Moreover, they performed a comprehensive review of the literature on the subject and they noted that the overall incidence of primary MDS was estimated at 3–20 cases per 100,000 population with 10–15 % of all MDS cases arising in patients exposed to chemo- or radiation therapy administered for other tumors [48]. It seems that t-MDS/t-AML risk among patients with brain tumors may be lower than in patients with other primary neoplasms [49]. Nevertheless, this observation may be linked to the often-reserved prognosis of the central nervous system tumors, not allowing the late hematological complications emergence. Perry et al. reported two cases of AML following therapy for malignant glioma and found 26 other examples of therapy related leukemia in adult and pediatric brain tumor patients (including 12 patients with malignant glioma). The median interval from treatment to diagnosis of AML was 31 months. Nine adult malignant glioma patients received all nitrosoureas and some of them as the sole chemotherapy. Authors concluded that “if regimens such as PCV continue to prove valuable in neurooncology the risk of leukemia will require integration into the clinical decision process” and recommended a search for “more effective therapy with minimal mutagenicity remains critical” [50].
The risk of late hematological complications with TMZ seems low compared with other alkylating agents like nitrosoureas mentioned above. An Australian team reported the cases of three patients treated with TMZ for a progressive glioma. These patients have continued the treatment respectively for 5, 7, and 8 years! No serious side effects were reported. Thus, it was often considered that most individuals receiving exceptionally large doses of alkylating agents over an extended period did not develop T-MDS/AML. This is true for patients receiving TMZ [51]. In contrast, Natelson et al. published a case report concerning a patient who had received temozolomide as a single agent for treatment of malignant glioma and who developed t-MDS. After a literature review, authors suggested that the cumulative dose threshold (CDT) for temozolomide that could predispose to t-MDS and which may potentially lead to acute myeloid leukemia would be around 18,000–20,000 mg/m2 [52]. The authors acknowledge, however, that the objective assessment of the real risk appears much difficult for tumors with a worse prognosis such as gliomas than for tumors associated with a long survival like Hodgkin’s lymphoma, testicular cancer, or breast cancer. They concluded that all alkylating agents, including TMZ, should be considered potentially leukemogenic when administered long term. Nevertheless, the risk of direct (progression or recurrence, malignant evolution) or indirect tumor complications (permanent deficit, seizures) or short latency adverse reactions to treatment (myelosuppression, opportunistic infection, encephalopathy due to radiation therapy) remains, at this day, much higher than the t-MDS/t-AML risk [48].
We nevertheless have to be careful with our prescription and to demonstrate in well-structured databases that prolonged use of alkylating chemotherapy until tumor progression or unacceptable toxicity is superior to treatment with a defined and limited number of cycles.
Chemotherapy and Gonadotoxicity
Data concerning chemotherapy, DLGG, and gonadotoxicity are almost nonexistent. Alkylating chemotherapy containing procarbazine (and/or cyclophosphamide) causes prolonged azoospermia in 90–100 % of men and premature ovarian failure in 5–25 % of women under the age of 30 [53]. We are also entitled to fear a marked gonadal toxicity of vincristine [54]. Thus, we can assume, although with no specific published data, that the PCV association is clearly gonadotoxic. We so recommend (1) to warn patients of this possibility, (2) to propose systematically a fertility preservation (easier in men than in women), and (3) to avoid this association in patients wishing to preserve essentially their reproduction capabilities.
Concerning temozolomide, a retrospective study was recently published. It concerns 24 female patients treated for a glioma. Fifteen patients had no fertility preservation and the remaining nine had a cryopreservation of embryos with or without an oocyte cryopreservation. Four patients are or have been pregnant (delivery, spontaneous miscarriage, pregnancy in the group of preserving fertility, and a current pregnancy in the group where no fertility preservation has been achieved). The conclusion of the authors is that temozolomide is not totally gonadotoxic [55]. Paternities have also been reported after temozolomide [56]. We could apply the two previous recommendations (information, fertility preservation) when a TMZ-based chemotherapy is needful in the course of a DLGG and when the patient wishes to preserve its reproduction capabilities while integrating the concept of a likely lower toxicity compared with that seen with nitrosoureas.
Other Toxicities
The peripheral neurological risk of vincristine cannot be neglected. There is currently no way to prevent it [57]. The risk of lung fibrosis with cecenu is also a parameter to be integrated during the establishment of such a combined therapy with cecenu [58]. Otherwise, patients under the PCV association complain frequently about an intense asthenia and/or about a loss of weight [41].
Temozolomide-induced hepatitis can be particularly severe, especially the cholestatic form [59].
Open Questions
How to Evaluate the Benefit of Chemotherapy
For more objective assessment of the impact of chemotherapy, it is conventional in neuro-oncology to use parameters such as overall survival and progression-free survival.
Overall survival is sensitive to all instituted treatments including “salvage” therapies. In this type of disease, treatments are often multiple and repeated. That makes difficult to analyze the specific impact of a given treatment (chemotherapy in our case) on survival. Progression-free survival could be interesting parameters to use if and only if (1) there is longitudinal and rigorous volumetric assessment and (2) this morphological parameters are associated with quality of life data [60]. The same remark can be made for the classical time to malignant transformation.
It was recently pointed out that clinical trials for DLGG “need to consider other measures of patient’s benefit such as cognition, symptom burden, and seizure activity, to establish whether improved survival is reflected in prolonged wellbeing” [22] should move in this direction also emphasized by Klein and colleagues “the multidimensional scales used to study changes in HRQOL studies in brain tumor patients provide a more comprehensive view of what is important to the patient concerning living with their disease and receiving treatment” [61].
How to Monitor the Treatment (Response Assessment)
To date, most radiologists and physicians analyze the images and decide the direction of treatment for gliomas and especially DLGG via a side-by-side comparison of images. This procedure can be considered as very imperfect and even dangerous. It was indeed clearly demonstrated that automated change detection and image subtraction are superior to side-by-side image comparison for brain tumors in general [62] and more obviously for DLGG [63].
In the same manner, the majority of dedicated centers simply monitored patients with conventional MRI without volumetric assessment and a fortiori without multiparametric examinations able to assess tumor cellularity, hypoxia, disruption of normal tissue architecture, changes in vascular density, and vessel permeability [64]. However, today, these parameters seem absolutely essential [65].
Links Between Chemotherapy and Clinico-radiological Factors
There are several factors clearly related to the prognosis of DLGG. These factors formed the “EORTC scoring system” [66] or the “UCSF LGG prognostic scoring system” [67] by combining different parameters: (1) location of tumor in presumed eloquent cortex (UCSF), (2) tumor crossing the midline (EORTC), (3) presence of neurologic deficit (EORTC), (4) Karnofsky Performance Scale score ≤80 (UCSF), (5) age > 50 years (UCSF)/≥40 years (EORTC), (6) maximum diameter (≥6 cm for EORTC/>4 cm for UCSF), and (7) histology (astrocytoma histology subtype for EORTC). Patients that combine two or more factors are classified in the high-risk group for the EORTC scoring system. For UCSF, the stratification of patients is based on score-generated groups (0–4) with statistically different OS and PFS estimates (p < 0.0001, logrank test). It has more recently been shown by a multivariate analysis constructed on the basis of two European Organisation for Research and Treatment of Cancer radiation trials for low-grade gliomas that tumor size and MMSE score were significant predictors of OS whereas tumor size, astrocytoma histology, and MMSE score were significant predictors of “PFS” [68]. It is so far difficult if not impossible to determine whether these factors are only prognostic factors or predictors of treatment response, including chemotherapy response.
Dynamic susceptibility-weighted contrast-enhanced perfusion imaging can identify progression and can also predict treatment failure during follow-up of DLGG with, for some authors, the best diagnostic performance [69].
Concerning spectroscopy, Murphy et al. reported in 2004 that there was interest to evaluate the reduction in the tumor choline/water signal in parallel with tumor volume change and that this marker could reflect the therapeutic effect of temozolomide [70]. In addition and very interestingly, Guillevin et al. demonstrated that the mean relative decrease of metabolic ratio – Δ(Cho/Cr)(n)/(Cho/Cr)(o)– 3 months after the start of a TMZ-based chemotherapy was predictive of tumor response over the 14 months of follow-up. The (1) H-MRS profile changes more widely and rapidly than tumor volume and represents an early noninvasive predictive factor of outcome under temozolomide-based chemotherapy [65].
Links Between Chemotherapy and Pathological Phenotype
The diagnostic criteria, in particular for oligoastrocytoma but also for “simple” astrocytomas or oligodendrogliomas, are highly subjective [71]. Most authors now propose to go beyond the pathological (morphological) classification by including other criteria, notably molecular [72, 73], to refine the prognostic significance of the diagnosis according to the WHO classification. Due to these important limitations of the morphological analysis of DLGG, it seems difficult to build clinical trials for chemotherapy or to decide, in the daily practice, the indication of chemotherapy on the sole basis of histology despite the fact that oligodendroglioma differentiation seems to respond better to chemotherapy than astrocytomas [10, 44].
Links Between Chemotherapy and Molecular Biology
Before talking about chemotherapy, the prognostic role of molecular markers after surgery alone could be discussed. Thus, even if the concept of “progression-free survival” (PFS) after partial surgery in the context of a DLGG is highly questionable, Hartmann et al. considered that no molecular marker was prognostic for this endpoint after surgery alone using multivariate adjustment for histology, age, and extent of resection [74] and all, on their side, screened 360 WHO grade II gliomas for mutations in the IDH1, IDH2, and TP53 genes and for 1p/19q loss and correlated these factors with clinical outcome. TP53 mutation was considered as a significant prognostic marker for shorter survival (p = 0.0005) and 1p/19q loss for longer survival (p = 0.0002), while IDH1/2 mutations had no prognostic value (p = 0.8737). Their conclusion was that “molecular classification on the basis of IDH1/2 mutation, TP53 mutation, and 1p/19q loss has power similar to histological classification and avoids the ambiguity inherent to the diagnosis of oligoastrocytoma” [71].
Data regarding chemotherapy are partly contradictory. Iwadate et al. have treated 36 consecutive low-grade oligodendroglioma patients (postoperative residual tumors or recurrence after total resection) by a modified PCV-based chemotherapy-preceding strategy and without radiotherapy. In this study 1p and 19q status were analyzed by fluorescence in situ hybridization. 1p/19q codeletion was observed in 72 % of cases. There was no significant association between 1p/19q codeletion and chemotherapy response rate. No significant difference has been found as well in terms of survival: median PFS of 121 months for 1p/19q-deleted tumors and 101 months for nondeleted tumors (logrank test: p = 0.894). Recurrent tumors were also well controlled by chemotherapy irrespective of 1p/19q status [75]. Following the work of the Hoang-Xuan et al. [23] and in contrast, Kaloshi et al. reported a retrospective single center observational study with 149 consecutive patients. The median number of TMZ cycles delivered was 14 (range 2–30). Seventy-seven patients (53 %) experienced an objective response (15 % of partial response, 38 % of minor response, 37 % of stable disease, and 10 % of progression). The median time to maximum tumor response was 12 months (3–30 months). The median “PFS” was 28 months (95 % CI, 23.4–32.6). Material for genotyping was available for 86 patients. Combined 1p/19q LOH was present in 42 % of the cases. Codeletion was significantly associated with (1) a higher response rate (p < 0.02), (2) a longer objective response to chemotherapy (p < 0.017), (3) a longer PFS (p < 4.105), and (4) a longer overall survival (p < 0.04) [76]. The same team, through Houiller et al., reported a series of 271 patients with a DLGG in which 84 patients were treated up front. IDH (1 or 2) mutations were found in 132/189 patients (70 %). IDH mutation and 1p19q codeletion were associated with a prolonged overall survival in multivariate analysis (p = 0.003 and p = 0.004). 1p19q codeletion, MGMT promoter methylation, and IDH mutation (p = 0.01) were also correlated with a higher rate of response to temozolomide. Inside the untreated subgroup, 1p19q codeletion was associated with prolonged “progression-free survival” (this concept is highly questionable in an untreated population) in univariate analysis, whereas IDH mutation was not [77]. Our understanding of the problem may also be informed by the work of Ochsenbein et al. Twenty-two patients with histologically verified DLGG (WHO grade II) were treated with temozolomide (TMZ) for tumor progression. LOH 1p and/or 19q correlated with longer time to progression but not with radiological response to TMZ. The volumetric response to chemotherapy analyzed by MRI and time to progression correlated with the level of MGMT promoter methylation [78]. Data on tumors considered as pure astrocytomas are likewise difficult to interpret. In the study of Taal et al. concerning temozolomide-based chemotherapy, MGMT promoter methylation and IDH1 mutations were not correlated with “PFS,” but the interval between the first symptom and the start of the TMZ was significantly (p = 0.02) longer in the patients with a methylated MGMT promoter and with IDH mutations (p = 0.01) [79].
The reported results appear thus contradictory. Although, at a population level, there is a quite pronounced correlation between 1p19q deletion (and a smaller correlation between MGMT promoter methylation or IDH1 mutation) and response to chemotherapy, it appears today absolutely impossible to consider the indication of chemotherapy on this sole argument at an individual level. We have previously shown [24] that 1p19q codeletion was primarily a marker of the duration of response and not a marker of response. In the case of a presurgical chemotherapy continued under a strict volumetric monitoring until obtaining a plateau, depriving patients of such a strategy (which can potentially change the natural history of the disease by allowing to move towards a possible subtotal resection not originally envisaged) seems to us a significant error.
When to Treat
To date only four large randomized trials in patients with low-grade glioma have been published. They allow concluding that early radiotherapy does not improve overall survival and supports alternative approaches like chemotherapy without providing evidence on the timing of chemotherapy [12]. We have previously emphasized the heterogeneity of the various reported series concerning chemotherapy. Often, within the same study, patients could be included before or after the anaplastic transformation of the tumor. To be very convenient, prescriptions at the “low-grade” stage can theoretically be proposed (1) in case of progression after surgery (regardless the quality of debulking) and radiotherapy, (2) for nonoperable progressive tumor and before radiotherapy in order to delay radiation and so radiation induced cognitive impairment, (3) in case of progression after a first-line surgery if reoperation cannot be immediately considered and before either surgery if the volume reduction obtained with chemotherapy allows it, and (4) up front in order to allow, in case of volume reduction, a surgical procedure before radiotherapy.
Only one randomized trial has been achieved. It compared primary temozolomide versus radiotherapy in progressive low-grade gliomas (EORTC/NCIC 22033/26033) regardless of the initial surgical status. The results are not yet published except data concerning dummy run and conformity indices [80].
Chemotherapy After Surgery and Radiation Therapy
Historically, the first prescriptions of chemotherapy [8, 9] were made after standard surgery and radiation therapy, which were unambiguously considered as the reference treatments while chemotherapy was considered as less or not effective. Clinical and radiological responses were clearly observed [8, 9, 21, 81, 82]. Can we nevertheless draw from these publications that chemotherapy is able, at this time of prescription, to modify enough the natural history by delaying, for a given time, the evolution of the disease including the anaplastic evolution? Is the impact of post radiation chemotherapy, considering the duration of response more or less important than before the radiotherapy? More or less important before malignant transformation than after? Does it allow the patient to maintain longer a high quality of life in comparison with earlier (pre radiotherapy) or later (after anaplastic evolution) prescription of chemotherapy?
It is difficult if not impossible to answer these questions. This point will, indeed, depend on many parameters: tumor volume, tumor heterogeneity, time of the disease where radiation therapy was performed, time from the end of radiotherapy, type of chemotherapy or duration of this one…. It is also very difficult to assess the type of response evaluation (both clinically and radiologically) in the various published papers. The majority of reported series does not relate real reproductible parameters. The limited retrospective data (which constitute the majority of available data) on all of these parameters does not help to get a clear vision of the real impact of chemotherapy at this time of the disease.
Nevertheless, a phase III prospective study was conducted by the RTOG group (9,802 trial). This is the only phase III trial that raised the question of the role of adjuvant chemotherapy in DLGG. Inclusion criteria of this trial were related to high-risk patients with a residual tumor after surgery and an age over 40. Patients were stratified by age, histology, Karnofsky performance status, and presence or absence of preoperative contrast enhancement (suggesting that an unspecified proportion of patient had anaplastic transformation!). They were randomized to radiotherapy (RT) alone (54 Gy/30 fractions) versus RT followed by six cycles of standard dose PCV. Finally, results have so far not been published except through an abstract presented at the ASCO meeting in 2008 [83]. We often find in the literature statements such as “adjuvant use of PCV-chemotherapy in high-risk patients failed to improve progression-free and overall survival in comparison with radiotherapy” [84]. Accurate analysis of data in the abstract contradicts significantly this assertion. 251 cases were indeed included in the study between 1998 and 2002. The median follow-up was 5.9 years. The median “PFS” time were not reached for the RT + PCV group and at 7.5 years for the RT group. The 5 years OS were 72 % for the RT + PCV group and 63 % for the RT group (p = 0.06, logrank p = 0.005). Beyond 2 years, the OS and PFS curves separated significantly favoring PCV + RT patients. For 2 years survivors (n = 211), the probability of OS for an additional 3 years was 84 % with RT + PCV versus 72 % with RT (p = 0.03) with comparable data for “PFS” (74 % with RT + PCV versus 52 % with RT alone, p = 0.002). Finally, The hazard ratio for RT + PCV versus RT was 0.52 for death (p = 0.02) and 0.45 for progression (p = 0.0004). The conclusion is rather oddly worded. It is indeed initially specified that PFS but not OS were improved for adult WHO grade II glioma patients receiving RT + PCV versus RT alone and then that beyond 2 years, the addition of PCV to RT conferred both a significant OS and PFS advantage, and reduced the risk of death by 48 % and progression by 55 %, suggesting a delayed benefit for chemotherapy. Nonetheless, after analyzing the abstract, we would like to conclude to a positive impact of chemotherapy as for “PFS” and for OS. This point, clearly in favor of an early chemotherapy after radiotherapy, seems to have been insufficiently reported. The absence of the final publication must be an explanation to consider.
We must specify that another phase III study was conducted by the ECOG group as “E3F05 trial” [11]. In the reference arm, patients undergo 3D conformal or intensity-modulated radiotherapy once daily, 5 days a week for 5½ weeks (28 fractions). In the experimental arm, patients undergo radiotherapy as in the previous arm and receive concurrent oral temozolomide once daily for 5½ weeks. Beginning 28 days after completion of chemoradiotherapy, patients receive also oral temozolomide alone once daily on days 1–5. Treatment with temozolomide repeats every 28 days for 12 courses in the absence of disease progression or unacceptable toxicity. The primary objective is to determine whether the addition of temozolomide to fractionated radiotherapy improves the “progression-free survival” of patients with symptomatic or progressive DLGG and to determine whether the addition of temozolomide to fractionated radiotherapy improves the median overall survival (OS) of these patients. The design of this trial may fear an excess of delayed neurotoxicity.
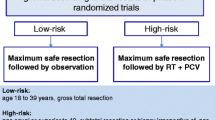
Finally, a randomized phase II tries to analyze the place of temozolomide as an adjuvant treatment (RTOG 04-24). High-risk LGGs (at least 3 risk factors: age ≥ 40, tumor diameter ≥6 cm, tumor crossing midline, astrocytoma subtype, preoperative neurologic deficit) are treated with RT + temozolomide. Comparison will be the historical EORTC patient population. The study is closed, but the results are not yet reported [85].
Chemotherapy Before Radiotherapy
Chemotherapy for Nonsurgical Tumor
Some locations (e.g., primary motor area), multifocal tumors, or “gliomatosis-like” aspects remain forever nonsurgical. These tumors are evolving as much as the other gliomas, clinically and radiologically. A primary chemotherapy course has to be discussed because, especially in the case of multifocal tumors or gliomatosis, volumes to be theoretically irradiated remain at risk of high cognitive toxicity. Data from the literature remain, again, rare and affect mainly the gliomatosis. We know that chemotherapy (temozolomide, “PCV”) can be effective in terms of symptoms and volumes [86]. The main question concerns the duration of temozolomide treatment for long responders. Can we continue the treatment for a very long time (even several years) when chemotherapy is (1) well tolerated and (2) able to produce a volume reduction and stability or do we fear the risk of late complications related to it (myelodysplasia, induced leukemia) [87]? There is to date no formal response. The question must be clearly asked by analyzing the risk/benefit ratio with on one side a tumor often with pejorative prognostic factors and on the other side a low risk at a medium term of complications.
Articulation Between Chemotherapy as First-Line Treatment and Surgery
Our group reported the first case of a complete surgery made possible thanks to an initial chemotherapy. This patient was initially diagnosed because of seizures. He benefited from a first partial conventional resection. The tumor continued logically to grow with an invasion of the contralateral hemisphere via the corpus callosum. A temozolomide-based chemotherapy was then prescribed and allowed a regression of the contralateral extension. Post chemotherapy surgery was performed with intraoperative functional mapping and allowed a complete resection without sequelae [88]. The patient now continues to enjoy a normal life with 10 years of follow-up since the first surgery (without radiotherapy). Spena et al. reported the same strategy for a patient and concluded that this new therapeutic approach of chemotherapy followed by surgery can offer safer and more radical surgical resection while improving the quality of life of the patient [89].
We then published a series of ten patients who benefited from a presurgical chemotherapy. In all cases we observed a tumor shrinkage that made possible the resection of these initially inoperable tumors. All the patients were secondarily evaluated from a cognitive and quality of life points of view. We demonstrated that the combined treatment was (1) feasible, (2) efficient, and (3) well tolerated with few cognitive deficits (mostly related to the tumor location) and with a good quality of life [26]. Martino et al., also within our group, reported a series of 19 patients who benefited from two operations separated by at least 1 year. Nine of these patients received chemotherapy before the second operation that allowed a subtotal or total resection in 14/19 cases. After the second operation, 16/19 patients improved or stabilized their clinical situation, while, in 14/17 cases, seizures were reduced or disappeared. Therefore, the authors concluded that chemotherapy did not prevent or even favored a second operation [90].
We described in chapter II.E our submitted series of selected 17 initially nonoperable patients who underwent temozolomide-based chemotherapy. Here, we just would like to point out that chemotherapy was able to reduce the growth slope in all 17 cases whatever the molecular status and that the quality of surgery was directly related to the magnitude of response to chemotherapy. TMZ appears thus as a way to optimize surgery and an additional way to potentially modify the natural history of this disease.
How Long to Treat (Efficacy Versus Potential Toxicity)
Temozolomide
Duration According to Volumic Evolution
The article that can argue, at best, this question about the duration of chemotherapy is that of Ricard and colleagues [24] we talked about earlier. In this paper, the great majority (92 %) of patients experienced initial decrease of the mean tumoral diameter after initiation of temozolomide. Ricard et al. found a clear correlation between 1p19q codeletion or absence of p53 overexpression and the duration of the response. Under chemotherapy, the volume is better controlled in codeleted patients (while recognizing that the maximum duration of temozolomide was 24 months). Otherwise, a majority of the patients resumed their progressive growth within a year after discontinuation of the chemotherapy. This observation of Ricard et al. raises the question of the validity of an arbitrary interruption of treatment in patients whose mean tumoral diameter (MTD) is still decreasing when the a priori fixed number of chemotherapy courses has been reached. Should treatment be pursued as long as the MTD continues to decrease (or stabilize), knowing that this option should be balanced with the potential long-term toxicity of prolonged treatment? Alternatively, should we abbreviate chemotherapy to four to six cycles to prevent formation of drug resistance? In this case, can we be certain that the tumor will remain sensitive to the treatment resumed after several months of interruption? Outside volume aspects, must we incorporate other parameters in the longitudinal follow-up of patients? We know that H-MRS imaging 3D volumetric maps of choline (Cho) over creatine (Cr) is more accurate in DLGG for the detection of glioma progression in comparison with conventional magnetic resonance imaging (MRI) and clinical symptoms [65, 91] and that the (1)H-MRS profile represents an early predictive factor of outcome over 14 months of follow-up under temozolomide [65]. It appears so obvious that, in the future, we will focus on a multimodal monitoring.
Duration According Tolerance
We know that prolonged administration of adjuvant temozolomide is safe and can be favorable for patients with anaplastic gliomas [92]. Other authors have nevertheless a more pessimistic view highlighting the fact that 15–20 % of patients (in a high-grade glioma population) treated with TMZ develop clinically significant toxicity, which can leave further treatment unsafe [23]. This point was developed in chapter II.F.
PCV
Peyre et al. [93] reported a series of 21 patients with a DLGG. All the patients presented a decrease of the mean tumoral diameter (MTD) during the chemotherapy. After discontinuation of the PCV, all but one patient presented an ongoing decrease of the MTD. The mean duration of the MTD decrease after PCV onset was 3.4 years (0.8–7.7). The mean duration of the MTD decrease after the end of PCV was 2.7 years (0–7). According to adapted McDonald’s criteria, the rates of partial and minor responses were 43 % at the end of PCV but 80 % at the time of maximal mean tumoral diameter decrease, which occurred after a median period of 3.4 years after PCV onset [94]. This prolonged impact of treatment is to be balanced against the increased toxicity of the association mainly during its administration [41] and also sometimes after, via lung and hematological long-term complications (cf. supra).
Retreatment with Chemotherapy
Taking again temozolomide for a patient initially responder with this drug and after an interruption for a given period can possibly be discussed even if the data are very rare [95]. The alternative way may be based, in this configuration (TMZ pretreatment) and in the absence of other possible therapeutic modality, on the prescription of a nitrosourea. The results reported by Kaloshi et al. appear nevertheless disappointing. The authors have indeed described a series of 30 patients treated with a nitrosourea-based chemotherapy for low-grade gliomas failing initial treatment with temozolomide. Response rate was 10 % (three minor responses achieved in nonenhancing tumors). Tolerance was considered as acceptable. Median PFS was 6.5 months. Median OS from start of salvage treatment was 23.4 months. Chromosomes 1p/19q codeletion was not predictive for objective response to salvage treatment but correlated with a better PFS (p = 0.02). The conclusion of the authors was that salvage NU chemotherapy provides disappointing results in TMZ-pretreated DLGG. They recommend in priority conventional radiotherapy, especially in DLGG that display contrast enhancement at progression [96].
Platinum salts [97] or CPT11 [98] seems also to have a modest effect. Therefore, the development of new drugs is highly desirable.
Conclusions and New Horizons
A DLGG is a tumor that, in the absence of treatment, shows a continuous growth resulting, usually within a few years and through a crescendo evolution of tumor aggressivity, in life threatening. During the initial period, contrary to a classical belief, this tumor entity alters so much quality of life through, most often, the existence of cognitive disorders and crescendo socially debilitating epilepsy. The idea of an initial “simple” monitoring (“wait and see” attitude) should definitely be abandoned in favor of an active therapeutic strategy implying the preservation of the core neurological sensory-motor and visual functions, but also the cognition and language, and thus the quality of life. In recent years, we have very clearly seen an improvement of the surgical component of care. It has been shown that the extent of surgery had a major impact on the delay before anaplastic transformation and survival itself. Under the development of functional surgery (cortical and subcortical stimulations, intraoperative awakening), percentage of patients undergoing a subtotal or total excision has increased considerably. At the same time, morbidity was significantly reduced with a mortality tending to zero. Meanwhile, indications of early radiotherapy were reduced because of inducing late treatment risks, mainly related to cognitive disorders. In this chapter, we saw that chemotherapy could also take place in the armamentarium. Currently, two strategies remain advisable: monochemotherapy by temozolomide or PCV-type association (vincristine + procarbazine + cecenu). The first (temozolomide) offers the advantage of a better tolerance to short and medium terms (hematologic, gonadal, and general parameters). Serious complications (myelodysplasia, leukemia) remain exceptional. The limit is clearly based on tumor regrowth in the months following discontinuation of therapy, inciting to discuss an extension of the treatment duration. The second (PCV) is somewhat more toxic when administered. Serious hematological complications also exist. Nevertheless, it provides tumor control over long periods up to several months or years after treatment discontinuation.
In all cases, we now know that chemotherapy (1) allows a volume decrease in the vast majority of cases and (2) improves the neurological symptoms, in particular epilepsy and cognition. We have also shown that this treatment could make “the bed of surgery” allowing the realization of subtotal or total excision, of which we know the impact on survival and quality of life. We are thus entitled to imagine, although we have not formally demonstrated it, that chemotherapy could improve directly or indirectly the survival of patients, while preserving or even improving the quality of life. Much work remains, however, to perform. Two major prescription frames seem to be well considered: (1) tumors (at diagnosis or progression) for which surgery could be discussed in case of a more or less volume reduction eventually associated with a redistribution of functional areas able to afford a subtotal or total resection and (2) tumors that will remain, with regard to their topography and or extension (gliomatosis-like, multifocality), forever inoperable. In both cases, there are, to date, many unanswered questions regarding (1) timing of the prescription (after demonstrating a lesional scalability with two successive MRI at 3 or 4 months of interval? later in the disease, after a period of “wait and see” with the risk of being confronted with a bulkier tumor having accumulated a greater number of genetic abnormalities potentially promoting a chemoresistance?), (2) decision-making criteria for prescription assistance (pathological, molecular, or radiological predictors of chemosensitivity or chemoresistance criteria? parameters to exclude patients who are at risk for serious side effects like myelodysplasia or induced leukemia?), (3) specific strategies (conventional temozolomide? intensified temozolomide? PCV? alternative treatment in case of progression after PCV and temozolomide for a tumor remaining a real WHO grade II glioma? If so which one?), and (4) duration (when to operate after a chemotherapy has been established? from the time when the tumor seems, based on probabilistic maps, able to benefit from subtotal resection? when the tumor stabilizes from a volumic point of view, which implies the necessity of close volumetric monitoring? in the event of a definitive inoperable tumor, how long will temozolomide be continued if the choice fells on this molecule? in case of well hematological and general tolerance, until when will the tumor is at least stabilized? for defined periods and if so, how long? after discontinuation, by repeating the treatment at a new documented growth, can we be certain of the chemosensitivity persistence?).
Furthermore, it is obviously important to continue basic research, both from neurological and oncological points of view. Neurologically, as well as for surgery, we must, again and again, better assess the impact of our treatment strategies, for each topography and each patient on cognitive or language impairments and on the capabilities of the brain to redistribute itself, with chemotherapy alone or in combination with parallel strategies of speech therapy and cognitive rehabilitation. Oncologically, a better understanding of energy processes or cellular and molecular mechanisms will allow the development of specific therapeutic targets able to extend the rather limited armamentarium at our disposal. Despite the relatively low number of DLGGs, this adventure remains exciting by itself, but also in that it will be able to open up original concepts declinable for a number of other entities, starting with WHO grade III and IV gliomas.
References
Whittle IR. The dilemma of low grade glioma. J Neurol Neurosurg Psychiatry. 2004;75 Suppl 2:ii31–6.
Duffau H. The challenge to remove diffuse low-grade gliomas while preserving brain functions. Acta Neurochir (Wien). 2012;154:569–74.
Sanai N, Chang S, Berger MS. Low-grade gliomas in adults. J Neurosurg. 2011;115:948–65.
Douw L, Klein M, Fagel SS, van den Heuvel J, Taphoorn MJ, Aaronson NK, Postma TJ, Vandertop WP, Mooij JJ, Boerman RH, Beute GN, Sluimer JD, Slotman BJ, Reijneveld JC, Heimans JJ. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8:810–8.
Eyre HJ, Crowley JJ, Townsend JJ, Eltringham JR, Morantz RA, Schulman SF, Quagliana JM, Al-Sarraf M. A randomized trial of radiotherapy versus radiotherapy plus CCNU for incompletely resected low-grade gliomas: a southwest Oncology Group Study. J Neurosurg. 1993;78:909–14.
Cairncross JG, Macdonald DR. Oligodendroglioma: a new chemosensitive tumor. J Clin Oncol. 1990;8:2090–1.
Macdonald DR, Gaspar LE, Cairncross JG. Successful chemotherapy for newly diagnosed aggressive oligodendroglioma. Ann Neurol. 1990;27:573–4.
Mason WP, Krol GS, DeAngelis LM. Low grade oligodendroglioma responds to chemotherapy. Neurology. 1996;46:203–7.
Soffietti R, Ruda R, Bradac GB, Schiffer D. PCV chemotherapy for recurrent oligodendrogliomas and oligoastrocytomas. Neurosurgery. 1998;43:1066–73.
Ducray F. Chemotherapy for diffuse low-grade gliomas in adults. Rev Neurol (Paris). 2011;167:673–9.
Pouratian N, Schiff D. Management of low-grade glioma. Curr Neurol Neurosci Rep. 2010;10:224–31.
Soffietti R, Baumert BG, Bello L, von Deimling A, Duffau H, Frénay M, Grisold W, Grant R, Graus F, Hoang-Xuan K, Klein M, Melin B, Rees J, Siegal T, Smits A, Stupp R, Wick W, European Federation of Neurological Societies. Guidelines on management of low-grade gliomas: report of an EFNS-EANO Task Force. Eur J Neurol. 2010;17:1124–33.
Gutin PH, Wilson CB, Kumar AR, Boldrey EB, Levin V, Powell M, Enot KJ. Phase II study of procarbazine, CCNU, and vincristine combination chemotherapy in the treatment of malignant brain tumors. Cancer. 1975;35:1398–404.
Levin VA, Edwards MS, Wright DC, Seager ML, Schimberg TP, Townsend JJ, Wilson CB. Modified procarbazine, CCNU, and vincristine (PCV 3) combination chemotherapy in the treatment of malignant brain tumors. Cancer Treat Rep. 1980;64:237–44.
Levin VA, Wara WM, Davis RL, Vestnys P, Resser KJ, Yatsko K, Nutik S, Gutin PH, Wilson CB. Phase III comparison of BCNU and the combination of procarbazine, CCNU, and vincristine administered after radiotherapy with hydroxyurea for malignant gliomas. J Neurosurg. 1985;63:218–23.
Mohile NA, Forsyth P, Stewart D, Raizer JJ, Paleologos N, Kewalramani T, Louis DN, Cairncross JG, Abrey LE. A phase II study of intensified chemotherapy alone as initial treatment for newly diagnosed anaplastic oligodendroglioma: an interim analysis. J Neurooncol. 2008;89:187–93.
Lashkari HP, Saso S, Moreno L, Athanasiou T, Zacharoulis S. Using different schedules of Temozolomide to treat low grade gliomas: systematic review of their efficacy and toxicity. J Neurooncol. 2011;105:135–47.
Macdonald DR, Cascino TL, Schold Jr SC, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–80.
Brada M, Viviers L, Abson C, Hines F, Britton J, Ashley S, Sardell S, Traish D, Gonsalves A, Wilkins P, Westbury C. Phase II study of primary temozolomide chemotherapy in patients with WHO II grade gliomas. Ann Oncol. 2003;14:1715–21.
Buckner JC, Gesme Jr D, O’Fallon JR, Hammack JE, Stafford S, Brown PD, Hawkins R, Scheithauer BW, Erickson BJ, Levitt R, Shaw EG, Jenkins R. Phase II trial of procarbazine, lomustine and vincristine as initial therapy for patients with low-grade oligodendroglioma or oligoastrocytoma: efficacy and associations with chromosomal abnormalities. J Clin Oncol. 2003;21:251–5.
Pace A, Vidiri A, Galie E, Carosi M, Telera S, Cianciulli AM, Canalini P, Giannarelli D, Jandolo B, Carapella CM. Temozolomide chemotherapy for progressive low-grade glioma :clinical benefits and radiological response. Ann Oncol. 2003;14:1722–6.
van den Bent MJ, Wefel JS, Schiff D, Taphoorn MJ, Jaeckle K, Junck L, Armstrong T, Choucair A, Waldman AD, Gorlia T, Chamberlain M, Baumert BG, Vogelbaum MA, Macdonald DR, Reardon DA, Wen PY, Chang SM, Jacobs AH. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12:583–93.
Hoang-Xuan K, Capelle L, Kujas M, Taillibert S, Duffau H, Lejeune J, Polivka M, Crinière E, Marie Y, Mokhtari K, Carpentier AF, Laigle F, Simon JM, Cornu P, Broët P, Sanson M, Delattre JY. Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol. 2004;22(15):3133–8.
Ricard D, Kaloshi G, Amiel-Benouaich A, Lejeune J, Marie Y, Mandonnet E, Kujas M, Mokhtari K, Taillibert S, Laigle-Donadey F, Carpentier AF, Omuro A, Capelle L, Duffau H, Cornu P, Guillevin R, Sanson M, Hoang-Xuan K, Delattre JY. Dynamic history of low-grade gliomas before and after temozolomide treatment. Ann Neurol. 2007;61:484–90.
Mandonnet E, Delattre JY, Tanguy ML, Swanson KR, Carpentier AF, Duffau H, Cornu P, Van Effenterre R, Alvord Jr EC, Capelle L. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol. 2003;53:524–8.
Blonski M, Taillandier L, Herbet G, Maldonado IL, Beauchesne P, Fabbro M, Campello C, Gozé C, Rigau V, Moritz-Gasser S, Kerr C, Rudà R, Soffietti R, Bauchet L, Duffau H. Combination of neoadjuvant chemotherapy followed by surgical resection as a new strategy for WHO grade II gliomas: a study of cognitive status and quality of life. J Neurooncol. 2012;106:353–66.
Duffau H, Capelle L, Lopes M, Bitar A, Sichez JP, van Effenterre R. Medically intractable epilepsy from insular low-grade gliomas: improvement after an extended lesionectomy. Acta Neurochir (Wien). 2002;144:563–72.
Stockhammer F, Misch M, Helms HJ, Lengler U, Prall F, von Deimling A, Hartmann C. IDH1/2 mutations in WHO grade II astrocytomas associated with localization and seizure as the initial symptom. Seizure. 2012;21:194–7.
Kurzwelly D, Herrlinger U, Simon M. Seizures in patients with low-grade gliomas – incidence, pathogenesis, surgical management, and pharmacotherapy. Adv Tech Stand Neurosurg. 2010;35:81–111.
Hu A, Xu Z, Kim RY, Nguyen A, Lee JW, Kesari S. Seizure control: a secondary benefit of chemotherapeutic temozolomide in brain cancer patients. Epilepsy Res. 2011;95:270–2.
Ochsenbein AF, Schubert AD, Vassella E, Mariani L. Quantitative analysis of O6-methylguanine DNA methyltransferase (MGMT) promoter methylation in patients with low-grade gliomas. J Neurooncol. 2011;103:343–51.
Sherman JH, Moldovan K, Yeoh HK, Starke RM, Pouratian N, Shaffrey ME, Schiff D. Impact of temozolomide chemotherapy on seizure frequency in patients with low-grade gliomas. J Neurosurg. 2011;114:1617–21.
Taillandier L, Duffau H. Epilepsy and insular grade II gliomas: an interdisciplinary point of view from a retrospective monocentric series of 46 cases. Neurosurg Focus. 2009;27(2):E8.
Maschio M, Dinapoli L, Mingoia M, Sperati F, Pace A, Pompili A, Carapella CM, Vidiri A, Muti P. Lacosamide as add-on in brain tumor-related epilepsy: preliminary report on efficacy and tolerability. J Neurol. 2011;258:2100–4.
Vecht CJ, Wilms EB. Seizures in low- and high-grade gliomas: current management and future outlook. Expert Rev Anticancer Ther. 2010;10:663–9.
Weller M, Gorlia T, Cairncross JG, van den Bent MJ, Mason W, Belanger K, Brandes AA, Bogdahn U, Macdonald DR, Forsyth P, Rossetti AO, Lacombe D, Mirimanoff RO, Vecht CJ, Stupp R. Prolonged survival with valproic acid use in the EORTC/NCIC temozolomide trial for glioblastoma. Neurology. 2011;77:1156–64.
Moritz-Gasser S, Herbet G, Maldonado IL, Duffau H. Lexical access speed is significantly correlated with the return to professional activities after awake surgery for low-grade gliomas. J Neurooncol. 2012;107:633–41.
Aaronson NK, Taphoorn MJ, Heimans JJ, Postma TJ, Gundy CM, Beute GN, Slotman BJ, Klein M. Compromised health-related quality of life in patients with low-grade glioma. J Clin Oncol. 2011;29:4430–5.
Correa DD, DeAngelis LM, Shi W, Thaler HT, Lin M, Abrey LE. Cognitive functions in low-grade gliomas: disease and treatment effects. J Neurooncol. 2007;81:175–84.
Correa DD, Shi W, Thaler HT, Cheung AM, DeAngelis LM, Abrey LE. Longitudinal cognitive follow-up in low grade gliomas. J Neurooncol. 2008;86:321–7.
Taphoorn MJ, van den Bent MJ, Mauer ME, Coens C, Delattre JY, Brandes AA, Sillevis Smitt PA, Bernsen HJ, Frénay M, Tijssen CC, Lacombe D, Allgeier A, Bottomley A, European Organisation for Research and Treatment of Cancer. Health-related quality of life in patients treated for anaplastic oligodendroglioma with adjuvant chemotherapy: results of a European Organisation for Research and Treatment of Cancer randomized clinical trial. J Clin Oncol. 2007;25:5723–30.
Liu R, Solheim K, Polley MY, Lamborn KR, Page M, Fedoroff A, Rabbitt J, Butowski N, Prados M, Chang SM. Quality of life in low-grade glioma patients receiving temozolomide. Neuro Oncol. 2009;11:59–68.
Chang EF, Clark A, Smith JS, Polley MY, Chang SM, Barbaro NM, Parsa AT, McDermott MW, Berger MS. Functional mapping-guided resection of low-grade gliomas in eloquent areas of the brain: improvement of long-term survival. J Neurosurg. 2011;114:566–73.
Prabhu VC, Khaldi A, Barton KP, Melian E, Schneck MJ, Primeau MJ, Lee JM. Management of diffuse low-grade cerebral gliomas. Neurol Clin. 2010;28:1037–59.
Greene MH, Boice Jr JD, Greer BE, Blessing JA, Dembo AJ. Acute nonlymphocytic leukemia after therapy with alkylating agents for ovarian cancer: a study of five randomized clinical trials. N Engl J Med. 1982;307:1416–21.
Boice Jr JD, Greene MH, Killen Jr JY, Ellenberg SS, Keehn RJ, McFadden E, Chen TT, Fraumeni Jr JF. Leukemia and preleukemia after adjuvant treatment of gastrointestinal cancer with semustine (methyl-CCNU). N Engl J Med. 1983;309:1079–84.
Greene MH, Boice Jr JD, Strike TA. Carmustine as a cause of acute nonlymphocytic leukemia. N Engl J Med. 1985;313:579.
Baehring JM, Marks PW. Treatment-related myelodysplasia in patients with primary brain tumors. Neuro Oncol. 2012;14:529–40.
Duffner PK, Krischer JP, Horowitz ME, et al. Second malignancies in young children with primary brain tumors following treatment with pro- longed postoperative chemotherapy and delayed irradiation: a Pediatric Oncology Group study. Ann Neurol. 1998;44(3):313–6.
Perry JR, Brown MT, Gockerman JP. Acute leukemia following treatment of malignant glioma. J Neurooncol. 1998;40:39–46.
Khasraw M, Bell D, Wheeler H. Long-term use of temozolomide: could you use temozolomide safely for life in gliomas? J Clin Neurosci. 2009;16:854–5.
Natelson EA, Pyatt D. Temozolomide-induced myelodysplasia. Adv Hematol Vol. 2010;Article ID 760402:5.
Harel S, Fermé C, Poirot C. Management of fertility in patients treated for Hodgkin’s lymphoma. Haematologica. 2011;96:1692–9.
Dobrzyńska MM, Czajka U, Słowikowska MG. Reproductive effects after exposure of male mice to vincristine and to a combination of X-rays and vincristine. Reprod Fertil Dev. 2005;17:759–67.
Sitbon Sitruk L, Sanson M, Prades M, Lefebvre G, Schubert B, Poirot C. Unknown gonadotoxicity chemotherapy and preservation of fertility: example of Temozolomide. Gynecol Obstet Fertil. 2010;38:660–2.
Palmieri C, Brock C, Newlands ES. Maintenance of fertility following treatment with temozolomide for a high grade astrocytoma. J Neurooncol. 2005;73:185.
Sioka C, Kyritsis AP. Central and peripheral nervous system toxicity of common chemotherapeutic agents. Cancer Chemother Pharmacol. 2009;63:761–7.
Mertens AC, Yasui Y, Liu Y, Stovall M, Hutchinson R, Ginsberg J, Sklar C, Robison LL, Childhood Cancer Survivor Study. Pulmonary complications in survivors of childhood and adolescent cancer. A report from the Childhood Cancer Survivor Study. Cancer. 2002;95:2431–41.
Sarganas G, Orzechowski HD, Klimpel A, Thomae M, Kauffmann W, Herbst H, Bronder E, Garbe E. Severe sustained cholestatic hepatitis following temozolomide in a patient with glioblastoma multiforme: case study and review of data from the FDA adverse event reporting system. Neuro Oncol. 2012;14:541–6.
Mandonnet E, Duffau H, Bauchet L. A new tool for grade II glioma studies: plotting cumulative time with quality of life versus time to malignant transformation. J Neurooncol. 2012;106:213–5.
Klein M. Health-related quality of life aspects in patients with low-grade glioma. Adv Tech Stand Neurosurg. 2010;35:213–35.
Erickson BJ, Wood CP, Kaufmann TJ, Patriarche JW, Mandrekar J. Optimal presentation modes for detecting brain tumor progression. AJNR Am J Neuroradiol. 2011;32:1652–57.
Mandonnet E, Pallud J, Clatz O, Taillandier L, Konukoglu E, Duffau H, Capelle L. Computational modeling of the WHO grade II glioma dynamics: principles and applications to management paradigm. Neurosurg Rev. 2008;31:263–9.
Nelson SJ. Assessment of therapeutic response and treatment planning for brain tumors using metabolic and physiological MRI. NMR Biomed. 2011;24:734–49.
Guillevin R, Menuel C, Taillibert S, Capelle L, Costalat R, Abud L, Habas C, De Marco G, Hoang-Xuan K, Chiras J, Vallée JN. Predicting the outcome of grade II glioma treated with temozolomide using proton magnetic resonance spectroscopy. Br J Cancer. 2011;104:1854–61.
Pignatti F, van den Bent M, Curran D, Debruyne C, Sylvester R, Therasse P, Afra D, Cornu P, Bolla M, Vecht C, Karim AB, European Organization for Research and Treatment of Cancer Brain Tumor Cooperative Group; European Organization for Research and Treatment of Cancer Radiotherapy Cooperative Group. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20:2076–84.
Chang EF, Clark A, Jensen RL, Bernstein M, Guha A, Carrabba G, Mukhopadhyay D, Kim W, Liau LM, Chang SM, Smith JS, Berger MS, McDermott MW. Multiinstitutional validation of the University of California at San Francisco Low-Grade Glioma Prognostic Scoring System. Clinical article. J Neurosurg. 2009;111:203–10.
Daniels TB, Brown PD, Felten SJ, Wu W, Buckner JC, Arusell RM, Curran WJ, Abrams RA, Schiff D, Shaw EG. Validation of EORTC prognostic factors for adults with low-grade glioma: a report using intergroup 86-72-51. Int J Radiat Oncol Biol Phys. 2011;81:218–24.
Vöglein J, Tüttenberg J, Weimer M, Gerigk L, Kauczor HU, Essig M, Weber MA. Treatment monitoring in gliomas: comparison of dynamic susceptibility-weighted contrast-enhanced and spectroscopic MRI techniques for identifying treatment failure. Invest Radiol. 2011;46:390–400.
Murphy PS, Viviers L, Abson C, Rowland IJ, Brada M, Leach MO, Dzik-Jurasz AS. Monitoring temozolomide treatment of low-grade glioma with proton magnetic resonance spectroscopy. Br J Cancer. 2004;90:781–6.
Kim YH, Nobusawa S, Mittelbronn M, Paulus W, Brokinkel B, Keyvani K, Sure U, Wrede K, Nakazato Y, Tanaka Y, Vital A, Mariani L, Stawski R, Watanabe T, De Girolami U, Kleihues P, Ohgaki H. Molecular classification of low-grade diffuse gliomas. Am J Pathol. 2010;177:2708–14.
Figarella-Branger D, Maues de Paula A, Colin C, Bouvier C. Histomolecular classification of adult diffuse gliomas: the diagnostic value of immunohistochemical markers. Rev Neurol (Paris). 2011;167:683–90.
Pollo B. Neuropathological diagnosis of brain tumours. Neurol Sci. 2011;32 Suppl 2:S209–11.
Hartmann C, Hentschel B, Tatagiba M, Schramm J, Schnell O, Seidel C, Stein R, Reifenberger G, Pietsch T, von Deimling A, Loeffler M, Weller M, for the German Glioma Network. Molecular markers in low-grade gliomas: predictive or prognostic? Clin Cancer Res. 2011;17:4588–99.
Iwadate Y, Matsutani T, Hasegawa Y, Shinozaki N, Higuchi Y, Saeki N. Favorable long-term outcome of low-grade oligodendrogliomas irrespective of 1p/19q status when treated without radiotherapy. J Neurooncol. 2011;102:443–9.
Kaloshi G, Benouaich-Amiel A, Diakite F, Taillibert S, Lejeune J, Laigle-Donadey F, Renard MA, Iraqi W, Idbaih A, Paris S, Capelle L, Duffau H, Cornu P, Simon JM, Mokhtari K, Polivka M, Omuro A, Carpentier A, Sanson M, Delattre JY, Hoang-Xuan K. Temozolomide for low grade gliomas: predictive impact of 1p/19q on response and outcome. Neurology. 2007;68:1831–6.
Houillier C, Wang X, Kaloshi G, Mokhtari K, Guillevin R, Laffaire J, Paris S, Boisselier B, Idbaih A, Laigle-Donadey F, Hoang-Xuan K, Sanson M, Delattre JY. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75:1560–6.
Taal W, Dubbink HJ, Zonnenberg CB, Zonnenberg BA, Postma TJ, Gijtenbeek JM, Boogerd W, Groenendijk FH, Kros JM, Kouwenhoven MC, van Marion R, van Heuvel I, van der Holt B, Bromberg JE, Sillevis Smitt PA, Dinjens WN, van den Bent MJ, Dutch Society for Neuro-Oncology. First-line temozolomide chemotherapy in progressive low-grade astrocytomas after radiotherapy: molecular characteristics in relation to response. Neuro Oncol. 2011;13:235–41.
Musat E, Roelofs E, Bar-Deroma R, Fenton P, Gulyban A, Collette L, Stupp R, Weber DC, Bernard Davis J, Aird E, Baumert BG. Dummy run and conformity indices in the ongoing EORTC low-grade glioma trial 22033–26033: First evaluation of quality of radiotherapy planning. Radiother Oncol. 2010;95:218–24.
Quinn JA, Reardon DA, Friedman AH, Rich JN, Sampson JH, Provenzale JM, McLendon RE, Gururangan S, Bigner DD, Herndon 2nd JE, Avgeropoulos N, Finlay J, Tourt-Uhlig S, Affronti ML, Evans B, Stafford-Fox V, Zaknoen S, Friedman HS. Phase II trial of temozolomide in patients with progressive low-grade glioma. J Clin Oncol. 2003;21:646–51.
van den Bent MJ. Can chemotherapy replace radiotherapy in low-grade gliomas? Time for randomized studies. Semin Oncol. 2003;30(6 Suppl 19):39–44.
Shaw EG, Wang M, Coons S, Brachman D, Buckner JC, Stelzer K, Barger G, Brown PD, Gilbert MR, Mehta MP. Final report of Radiation Therapy Oncology Group (RTOG) protocol 9802: radiation therapy (RT) versus RT + procarbazine, CCNU, and vincristine (PCV) chemotherapy for adult low-grade glioma (LGG). 2008 ASCO annual meeting. J Clin Oncol 2008; 26(May 20 suppl):abstr 2006
Baumert BG, Stupp R. Is there a place for radiotherapy in low-grade gliomas? Adv Tech Stand Neurosurg. 2010;35:159–82.
Ruiz J, Lesser GJ. Low-grade gliomas. Curr Treat Options Oncol. 2009;10:231–42.
Sanson M, Cartalat-Carel S, Taillibert S, Napolitano M, Djafari L, Cougnard J, Gervais H, Laigle F, Carpentier A, Mokhtari K, Taillandier L, Chinot O, Duffau H, Honnorat J, Hoang-Xuan K, Delattre JY, ANOCEF Group. Initial chemotherapy in gliomatosis cerebri. Neurology. 2004;63:270–5.
Wick W, Platten M, Weller M. New (alternative) temozolomide regimens for the treatment of glioma. Neuro Oncol. 2009;11:69–79.
Duffau H, Taillandier L, Capelle L. Radical surgery after chemotherapy: a new therapeutic strategy to envision in grade II glioma. J Neurooncol. 2006;80:171–6.
Spena G, Garbossa D, Barletta L, Prevost C, Versari P. Preoperative chemotherapy for infiltrative low-grade oligoastrocytoma: a useful strategy to maximize surgical resection – case report. Neurol Med Chir (Tokyo). 2010;50:410–3.
Martino J, Taillandier L, Moritz-Gasser S, Gatignol P, Duffau H. Re-operation is a safe and effective therapeutic strategy in recurrent WHO grade II gliomas within eloquent areas. Acta Neurochir (Wien). 2009;151:427–36.
Imani F, Boada FE, Lieberman FS, Davis DK, Deeb EL, Mountz JM. Comparison of proton magnetic resonance spectroscopy with fluorine-18 2-fluoro-deoxyglucose positron emission tomography for assessment of brain tumor progression. J Neuroimaging. 2012;22:184–90.
Freyschlag CF, Smolczyk DR, Janzen E, Schmieder K, Thomé C, Lohr F, Wenz F, Weiss C, Tuettenberg J, Seiz M. Prolonged administration of temozolomide in adult patients with anaplastic glioma. Anticancer Res. 2011;31:3873–7.
Chamberlain MC. Temozolomide: therapeutic limitations in the treatment of adult high-grade gliomas. Expert Rev Neurother. 2010;10:1537–44.
Peyre M, Cartalat-Carel S, Meyronet D, Ricard D, Jouvet A, Pallud J, Mokhtari K, Guyotat J, Jouanneau E, Sunyach MP, Frappaz D, Honnorat J, Ducray F. Prolonged response without prolonged chemotherapy: a lesson from PCV chemotherapy in low-grade gliomas. Neuro Oncol. 2010;12:1078–82.
Franceschi E, Omuro AM, Lassman AB, Demopoulos A, Nolan C, Abrey LE. Salvage temozolomide for prior temozolomide responders. Cancer. 2005;104:2473–6.
Kaloshi G, SierradelRio M, Ducray F, Psimaras D, Idbaih A, Laigle-Donadey F, Taillibert S, Houillier C, Dehais C, Omuro A, Sanson M, Delattre JY, Hoang-Xuan K. Nitrosourea-based chemotherapy for low grade gliomas failing initial treatment with temozolomide. J Neurooncol. 2010;100:439–41.
Lesser GJ. Chemotherapy of low-grade gliomas. Semin Radiat Oncol. 2001;11:138–44.
Chamberlain MC. Salvage chemotherapy with CPT-11 for recurrent oligodendrogliomas. J Neurooncol. 2002;59:157–63.
Ius T, Angelini E, Thiebaut de Schotten M, Mandonnet E, Duffau H. Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: towards a “minimal common brain”. Neuroimage. 2011;56:992–1000.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag London
About this chapter
Cite this chapter
Taillandier, L. (2013). Chemotherapy for Diffuse Low-Grade Gliomas. In: Duffau, H. (eds) Diffuse Low-Grade Gliomas in Adults. Springer, London. https://doi.org/10.1007/978-1-4471-2213-5_25
Download citation
DOI: https://doi.org/10.1007/978-1-4471-2213-5_25
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-2212-8
Online ISBN: 978-1-4471-2213-5
eBook Packages: MedicineMedicine (R0)