Abstract
Despite the accumulating evidences of high chemosensitivity especially in anaplastic oligodendrogliomas with loss of chromosomes 1p and 19q, the optimal management strategy for low-grade tumors using the 1p/19q information remains controversial. We have treated all low-grade oligodendrogliomas by a chemotherapy-preceding strategy without radiotherapy, and here we analyzed the survival outcomes of 36 consecutive patients in relation to 1p/19q status. The treatment protocol was as follows: (1) simple observation after gross total resection, and (2) modified PCV chemotherapy for postoperative residual tumors or recurrence after total resection. The 1p and 19q status were analyzed by fluorescence in situ hybridization. The median follow-up period was 7.5 years and no patient was lost during the follow-up periods. 1p/19q co-deletion was observed in 72% of the patients, and there was no significant association between 1p/19q co-deletion and chemotherapy response rate. The 5- and 10-year progression-free survival (PFS) rate was 75.1 and 46.9%, respectively, and the median PFS was 121 months for 1p/19q-deleted tumors and 101 months for non-deleted tumors (log-rank test: P = 0.894). Extent of surgery did not affect PFS (P = 0.685). In contrast, the elder patients (>50) had significantly shorter PFS (P = 0.0458). Recurrent tumors were well controlled by chemotherapy irrespective of 1p/19q status, and 35 out of 36 patients survived without receiving radiotherapy. The 5- and 10-year overall survival rates were 100 and 93.8%, respectively. Two of the patients in their sixties (29%) suffered from severe cognitive dysfunctions and marked brain atrophy following chemotherapy alone. These results show that low-grade oligodendrogliomas could be successfully treated by surgical resection and nitrosourea-based chemotherapy alone without radiotherapy irrespective of 1p/19q status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The therapeutic strategy for adult low-grade gliomas, especially as regards the choice and timing of radiotherapy, is still controversial [1–4]. Although radiotherapy is undoubtedly beneficial for a subset of patients with low-grade gliomas, the natural history of gliomas when not irradiated after surgery is largely unknown. Only four prospective randomized trials have been conducted regarding the efficacy of radiotherapy for low-grade gliomas, and none of them could demonstrate any significant benefits on overall survival (OS). The EORTC 22845 randomized trial suggested that immediate postoperative radiotherapy for any residual tumors has advantages in terms of progression-free survival (PFS) but not in terms of OS [1, 3]. On the other hand, radiation-induced toxicities such as delayed cognitive dysfunction and leukoencephalopathy are important factors to determine treatment strategy [5, 6].
Stratification or personalization of the treatment strategy based on some markers is expected. Among gliomas, deletions of chromosomes 1p and 19q are shown to be associated with tumors including oligodendroglial components [7]. The co-deletion has also been associated with responsiveness of anaplastic oligodendroglial tumors to radiotherapy and chemotherapy as well as with prolonged survival of the patients [8, 9]. However, this predictive and prognostic relevance of the 1p/19q co-deletion are more controversial for low-grade tumors [10–17]. To elucidate this issue, a long follow-up period is necessary because the patients with low-grade oligodendroglial tumors usually have more favorable outcome than anaplastic tumors.
In addition to the controversy in the application of radiotherapy, it has been reported that low-grade oligodendroglial tumors respond well to chemotherapy [18–21]. Therefore, we have treated all patients without using radiotherapy and have applied a nitrosourea-based chemotherapy (PAV, a modified PCV) when postoperative progressing tumors were verified [19]. The aim of this study was to elucidate the long-term outcome of low-grade oligodendrogliomas treated with chemotherapy-preceding strategy without radiotherapy in relation to 1p/19q co-deletion.
Methods
Patients and treatment
Since 1995, we have prospectively treated all patients having low-grade oligodendrogliomas by a radiotherapy-deferring and chemotherapy-preceding strategy using a standard nitrosourea-based chemotherapy (PAV, a modified PCV). The classic oligodendroglioma histological features were defined by areas composed of uniform and round nuclei surrounded by perinuclear halos and in an even tissue distribution [15]. The treatment protocol was: (1) simple observation after complete resection of tumors, and (2) PAV for postoperative residual tumors or recurrence after total resection. In this chemotherapy, lomustine (CCNU) was replaced with nimustine (ACNU; [1-(4-amino-2-methyl-5-pyrimidinyl)-methyl-(2-chloroethyl)-3-nitrosourea hydrochloride] which is a water- and lipid-soluble nitrosourea derivative. The chemotherapy protocol was ACNU 75 mg/m2 on day 1, vincristine 1 mg/m2 on days 8 and 29, and procarbazine 100 mg/day on days 8–21; this cycle was administered four times a year for 2 years [19]. Patients were required to provide written informed consent before receiving the chemotherapy.
Data collection
All patients histologically confirmed to have oligodendroglioma or oligoastrocytoma were enrolled in this study. Age, sex, tumor location, tumor size, pathological diagnosis, and extents of resection were recorded. None of the patients was excluded from analysis because of early recurrence within 1 year after surgery. Magnetic resonance imaging (MRI) studies were performed preoperatively, postoperatively within 2 weeks, and after every course of chemotherapy. Tumor volume was estimated as the product of the three largest perpendicular diameters of all measurable lesions on fluid-attenuated inversion recovery (FLAIR) with reference to pre- and postgadolinium T1-weighted MRI. Regarding the extent of surgery, gross total resection was defined as a disappearance of the tumor on MRI, and subtotal resection as a ≥70% reduction of the tumor size. Responses to chemotherapy were determined in the patients with postoperative residual tumors using the modified Macdonald criteria [22], in which complete response (CR) was defined as disappearance of all measurable disease, and partial response (PR) was defined as ≥50% decrease in the measured tumor size compared with baseline. Progressive disease (PD) was defined as ≥25% increase of the tumor size and stable disease (SD) was applied to all other situations. Toxicity was graded according to the National Cancer Institute’s Common Toxicity Criteria version 3.0.
The histological diagnosis was confirmed by a neuropathologist other than the initial diagnostician. Chromosome 1p- and 19q-deletion analyses were done using a standard fluorescence in situ hybridization (FISH) of fixed cytogenetic preparation from fresh tumor tissues [23]. FISH probes for 1p were the target region of 1p36 with a control region of 1q25, and those for 19q were the control region of 19p13 with the target region of 19q13. The total number of signals was counted, and the ratio of 1p:1q or 19q:19p of <0.75 was diagnosed as loss.
Statistical analysis
Progression-free survival was calculated from the date of diagnosis until the first sign of radiological progression, death, or last follow-up. OS was calculated from the date of diagnosis until the date of death or last follow-up. The Kaplan–Meier method was used to estimate survival rates and the log-rank test was applied to compare the survival differences using StatView software (SAS Institute, Cary, NC, USA). A Fisher exact test was performed to determine the association between 1p/19q co-deletion and chemotherapy response rate. Cox’s proportional hazard regression model was used to perform multivariate analysis for the possible prognostic variables including age, extent of resection, 1p19q status (SPSS, Chicago, IL, USA).
Results
Thirty-six consecutive patients with histologically proven low-grade oligodendrogliomas were treated between 1995 and 2008 (Table 1). Thirty-three patients had oligodendrogliomas and three had oligoastrocytomas. There were 24 men and 12 women with a mean age of 43 years (range 22–68 years). The patients were followed up with for a median period of 7.5 years and no patient was lost during the follow-up period. Fifteen patients (42%) underwent gross total resection, 10 patients (28%) underwent subtotal tumor resection, and the other 11 (30%) underwent partial resection. Twenty-six patients were treated with chemotherapy. Tumor recurrence occurred in 15 patients (42%); 5 patients after total resection (5/15: 33%), 4 after subtotal resection (4/10: 40%), and 6 after partial resection (6/11: 55%).
The 5- and 10-year PFS rates were 75.1 and 46.9%, respectively, and the median PFS was 101 months (Fig. 1a). There was no significant difference of PFS between the patients who were observed after total resection and those with incomplete resection followed by the chemotherapy (median PFS, 121 vs 93 months, respectively, P = 0.685) (Fig. 1b). In contrast, the elder patients (>50) had significantly shorter PFS (P = 0.0458) (Fig. 1c). There was no difference in clinical course including PFS between the patients with oligodendroglioma and oligoastrocytoma. A salvage second surgery was performed in seven cases, and malignant transformation was not observed in the present non-irradiated series. There was a patient whose tumor had 1p/19q loss but finally could not be controlled by chemotherapy. This patient refused radiotherapy and died at 81 months after surgery. Therefore, no patient in the present study received radiotherapy, and 35 out of 36 patients survived without receiving radiotherapy at the follow-up period of 10 years; 5- and 10-year OS rates were 100 and 93.8%, respectively (Fig. 2).
1p/19q co-deletion was observed in 23 of 32 cases (72%) analyzed with FISH. Isolated loss of 1p or 19q was not observed in the present series. Median PFS rate for the patients with 1p/19q co-deleted tumors was 121 months and that for non-deleted tumors was 101 months. There was no significant difference in PFS between the patients with 1p/19q co-deleted tumors and those without co-deletion (P = 0.894) (Fig. 3). The multivariate analysis showed that neither of age, extent of resection, nor 1p/19q status was significantly associated with the length of PFS (Table 2).
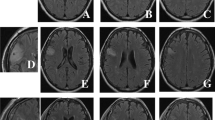
Two patients in their sixties manifested marked brain atrophy without tumor recurrence 4–5 years after the initiation of chemotherapy (Fig. 4). These patients required intensive nursing care and observation because of their developing cognitive deficits. The MRI finding of tight high-convexity, which is typical for normal pressure hydrocephalus, was not observed and the CSF tap test was negative in the patients. A grade 3 or 4 leukopenia mandating a treatment delay occurred in two patients (9%).
The representative MRI pictures (upper panel; gadolinium-enhanced T1 weighted images, lower; T2-weighted images) of a patient aged 68 years. A left parietal tumor at initial diagnosis (a) was completely resected after surgery (b), and the tumor recurred 2.5 years after the surgery (c). Although chemotherapy with ACNU, vincristine and procarbazine achieved a complete response, the patient’s neurological condition gradually worsened. Marked enlargement of the cortical sulci and lateral ventricles was observed without tumor recurrence at 7 years from diagnosis (d)
Discussion
The present study showed that, when treated with a radiotherapy-deferring and chemotherapy-preceding strategy using modified PCV chemotherapy, the 10-year OS rates of low-grade oligodendrogliomas were over 90% irrespective of 1p/19q status. This outcome compares favorably with those of previous reports including immediate postoperative radiotherapy without chemotherapy; the median survival times were within 5.3–14.9 years [24–27, 31], and 5- and 10-year OS rates were 52–95% [24–31] and 24–85% [24, 25, 27–29, 31], respectively. The median times to tumor progression were within 5.6–13.2 years [26, 31], and the 5-year PFS rate was reported as 67% [30]. In addition, it was reported that neither PFS and OS were significantly improved by radiotherapy in retrospective studies employing chemotherapy [32–34]. As a new therapeutic strategy for low-grade oligodendrogliomas, the effectiveness of PCV chemotherapy has been reported [18–21, 35–38], and some authors have concluded that radiotherapy could be postponed until malignant transformation occurs [3, 18–21, 32, 35]. The present result is in accordance with these studies. In contrast, although PCV chemotherapy for low-grade oligodendrogliomas achieved stabilization or shrinkage of tumors, its efficacy was not curative in many cases, as shown by the increased recurrence rate at 10-year follow-up. A longer observation period in a larger cohort would be necessary to clarify the validity of the radiotherapy-deferring and chemotherapy-preceding strategy against low-grade oligodendrogliomas.
Since a subset of low-grade gliomas progresses to malignant tumors, some stratification or personalization in the treatment planning are expected. In addition to the diagnostic relevance for oligodendroglial tumors, the prognostic role of 1p/19q loss is well defined for anaplastic oligodendrogliomas [8, 9]. For grade III tumors, 1p/19q loss may characterize a less malignant variant of the tumor, and the gene products lost as a consequence of 1p/19q loss may be mediators of resistance to genotoxic therapies [14]. In contrast, the prognostic relevance is less defined for low-grade oligodendroglial tumors [10–17]. Our result showed that the outcome of patients with histologically typical low-grade oligodendrogliomas was generally favorable irrespective of 1p/19q status when treated without radiotherapy. Although 1p/19q loss is one of the major genetic alterations in oligodendroglial tumors [7, 23], other important genetic alterations would exist as an early event. The previous contradictory results may be partly due to heterogeneity in histology and treatments [10–17]. Radiotherapy would negatively modify the survival results of the patients having tumors without 1p/19q deletions [23]. However, the small sample size may have contributed to this result, and a future prospective study including more patients with 1p/19q information is needed.
Recently, temozolomide (TMZ) has been frequently used as the initial treatment for oligodendrogliomas with high response rates almost equivalent to those of PCV chemotherapy [10, 16]. Both of these chemotherapy regimens would be effective for low-grade oligodendrogliomas. TMZ is advantageous due to its safety profile especially with regard to hematologic toxicity. Standard 42-day PCV chemotherapy induces significant hematologic toxicity, requiring a dose reduction and/or a cycle delay [19, 36]. Therefore, we applied a prolonged-cycle interval schedule to avoid the cumulative hematologic toxicity of PAV, and the incidence rate of grade 3 or 4 leukopenia was acceptably low.
In the present study, the other adverse effect following the modified PAV chemotherapy was found after long-term observations. Two patients in their sixties underwent marked brain atrophy following chemotherapy without receiving radiotherapy. Other causes of brain atrophy due to aging, such as normal pressure hydrocephalus or multiple cerebral infarctions, could not be completely excluded. However, the MRI findings of tight high-convexity and the CSF tap test were both negative, and they had not had the risk factors for cerebral infarction. Although neurotoxicities of an intensive PCV regimen have been reported [38, 39], this is the first report of a potential neurotoxicity following a standard nitrosourea-based chemotherapy alone. This adverse effect could not be detected within short-term follow-up periods. Careful application of chemotherapy for patients older than 60 years is recommended. In contrast, the recurrence rate was higher in older patients than in younger patients, which highlighted the importance of surgical resection. Attempting the greatest possible surgical resection without neurological deteriorations followed by simple observation may be the best way to treat low-grade oligodendrogliomas, especially for the elder patients.
References
Karim AB, Afra D, Cornu P, Bleehan N, Schraub S, De Witte O, Darcel F, Stenning S, Pierart M, Van Glabbeke M (2002) Randomized trial on the efficacy of radiotherapy for cerebral low-grade gliomas in the adult: European Organization for Research and Treatment of Cancer Study 22845 with Medical Research Council Study BRO4: an interim analysis. Int J Radiat Oncol Biol Phys 52:316–324
Shaw E, Arusell R, Scheithauer B et al (2002) Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Group study. J Clin Oncol 20:2267–2276
van den Bent MJ, Afra D, de Witte O, Ben Hassel M, Schraub S, Hoang-Xuan K, Malmström PO, Collette L, Piérart M, Mirimanoff R, Karim AB, EORTC Radiotherapy and Brain Tumor Groups and the UK Medical Research Council (2005) Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet 366:985–990
Papagikos MA, Shaw EG, Stieber VW (2005) Lessons from randomized clinical trials in adult low grade glioma. Lancet Oncol 6:240–244
Behin A, Hoang-Xuan K, Carpentier AF, Delattre J-Y (2003) Primary brain tumors in adults. Lancet 361:323–331
Wessels PH, Weber WE, Raven G, Ramaekers FC, Hopman AH, Twijnstra A (2003) Supratentorial grade II astrocytoma: biological features and clinical course. Lancet Neurol 2:395–403
Bello MJ, Vaquero J, de Campos JM, Kusak ME, Sarasa JL, Saez-Castresana J, Pestana A, Rey JA (1994) Molecular analysis of chromosome 1 abnormalities in human gliomas reveals frequent loss of 1p in oligodendroglial tumors. Int J Cancer 57:172–175
Caincross G, Berkey B, Shaw E et al (2006) Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol 24:2707–2714
van den Bent MJ, Carpentier AF, Brandes AA et al (2006) Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organization for Research and Treatment of Cancer phase III trial. J Clin Oncol 24:2715–2722
Hoang-Xuan K, Capelle L, Kujas M et al (2004) Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol 22:3133–3138
Kanner AA, Staugaitis SM, Castilla EA et al (2006) The impact of genotype on outcome in oligodendroglioma: validation of the loss of chromosome arm 1p as an important factor in clinical decision making. J Neurosurg 104:542–550
Mariani L, Deiana G, Vassella E, Fathi AR, Murtin C, Arnold M, Vajtai I, Weis J, Siegenthaler P, Schobesberger M, Reinert MM (2006) Loss of heterozygosity 1p36 and 19q13 is a prognostic factor for overall survival in patients with diffuse WHO grade 2 gliomas treated without chemotherapy. J Clin Oncol 24:4758–4763
Jaeckle KA, Ballman KV, Rao RD, Jenkins RB, Buckner JC (2006) Current strategies in treatment of oligodendroglioma: evolution of molecular signature of response. J Clin Oncol 24:1246–1252
Weller M, Berger H, Hartmann C et al (2007) Combined 1p/19q loss in oligodendroglial tumors: predictive or prognostic biomarker? Clin Cancer Res 13:6933–6937
Giannini C, Burger PC, Berkey BA, Cairncross JG, Jenkins RB, Mehta M, Curran WJ, Aldape K (2008) Anaplastic oligodendroglial tumors: refining the correlation among histopathology, 1p 19q deletion and clinical outcome in Intergroup Radiation Therapy Oncology Group Trial 9402. Brain Pathol 18:360–369
Kaloshi G, Benouaich-Amiel A, Diakite F et al (2007) Temozolomide for low-grade gliomas. Predictive impact of 1p/19q loss on response and outcome. Neurology 68:1831–1836
Capelle L, Oei P, Teoh H, Hamilton D, Palmer D, Low I, Campbell G (2009) Retrospective review of prognostic factors, including 1p19q deletion, in low-grade oligodendrogliomas and a review of recent published works. J Med Imaging Radiat Oncol 53:305–309
Mason WP, Krol GS, DeAngelis LM (1996) Low-grade oligodendrogliomas respond to chemotherapy. Neurology 46:203–207
Higuchi Y, Iwadate Y, Yamaura A (2004) Treatment of low-grade oligodendroglial tumors without radiotherapy. Neurology 63:2384–2386
Sunyach MP, Jouvet A, Perol D, Jouanneau E, Guyotat J, Gignoux L, Carrie C, Frappaz D (2007) Role of exclusive chemotherapy as first line treatment in oligodendroglioma. J Neurooncol 85:319–328
Bromberg JEC, van den Bent MJ (2009) Oligodendrogliomas: molecular biology and treatment. Oncologist 14:155–163
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Jenkins RB, Blair H, Ballman KV et al (2006) A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res 66:9852–9861
Shaw EG, Scheithauser BW, O’Fallon JR, Tazellar HD, Davis DH (1992) Oligodendrogliomas: the Mayo Clinic experience. J Neurosurg 76:428–434
Celli P, Nofrone I, Palma L, Cantore G, Fortuna A (1994) Cerebral oligodendroglioma: prognostic factors and life history. Neurosurgery 35:1018–1034
Leighton C, Fisher B, Bauman G, Depiero S, Stitt L, MacDonald D, Cairncross G (1997) Supratentorial low-grade glioma in adults: an analysis of prognostic factors and timing of radiation. J Clin Oncol 15:1294–1301
Leonardi MA, Lumenta CB (2001) Oligodendrogliomas in CT/MR-era. Acta Neurochir 143:1195–1203
Okamoto Y, Di Patre OL, Birkhard C et al (2004) Population-based study on incidence, survival rates, and genetic alterations of low-grade diffuse astrocytomas and oligodendrogliomas. Acta Neuropathol 108:49–56
Lebrun C, Fontaine D, Ramaioli A, Vandenbos F, Chanalet S, Lonjon M, Michiels JF, Bourg V, Paquis P, Chatel M, Frenay M, Nice Brain Tumor Study Group (2004) Long-term outcome of oligodendrogliomas. Neurology 62:1783–1787
Yeh SA, Lee TC, Chen HJ, Lui CC, Sun LM, Wang CJ, Huang EY (2002) Treatment outcome and prognostic factors of patients with supratentorial low-grade oligodendroglioma. Int J Radiat Oncol Biol Phys 54:1405–1409
Kang H-C, Kim IH, Eom K-Y, Kim JH, Jung H-W (2009) The role of radiotherapy in the treatment of newly diagnosed supratentorial low-grade oligodendrogliomas: comparative analysis with immediate radiotherapy versus surgery alone. Cancer Res Treat 41:132–137
Olson JD, Riedel E, DeAngelis LM (2000) Long-term outcome of low-grade oligodendroglioma and mixed glioma. Neurology 54:1442–1448
Ozyigit G, Onal C, Gurkaynak M, Soylemezoklu F, Zorlu F (2005) Postoperative radiotherapy and chemotherapy in the management of oligodendroglioma: single institutional review of 88 patients. J Neurooncol 75:189–193
El-Hatter H, Souhami L, Roberge D, Del Maestro R, Leblanc R, Eldebawy E, Muanza T, Melancon D, Kavan P, Guiot MC (2009) Low-grade oligodendroglioma: an indolent but incurable disease? J Neurosurg 111:265–271
Stege EM, Kros JM, de Bruin HG, Enting RH, van Heuvel I, Looijenga LH, van der Rijt CD, Smitt PA, van den Bent MJ (2005) Successful treatment of low-grade oligodendroglial tumors with a chemotherapy regimen of procarbazine, lomustine, and vincristine. Cancer 103:802–809
Ty AU, See SJ, Rao JP, Khoo JB, Wong MC (2006) Oligodendroglial tumor chemotherapy using “decreased-dose-intensity” PCV: a Singapore experience. Neurology 66:247–249
Lebrun C, Fontaine D, Bourg V, Ramaioli A, Chanalet S, Vandenbos F, Lonjon M, Fauchon F, Paquis P, Frenay M (2007) Treatment of newly diagnosed symptomatic pure low-grade oligodendrogliomas with PCV chemotherapy. Eur J Neurol 14:391–398
Buckner JC, Geame D, O’Fallon JR et al (2003) Phase II trial of procarbazine, lomustine, and vincristine as initial therapy for patients with low grade oligodendroglioma and oligoastrocytoma: efficacy and associations with chromosomal abnormalities. J Clin Oncol 21:251–255
Postma TJ, van Groeningen CJ, Witjes RJ, Weerts JG, Kralendonk JH, Heimans JJ (1998) Neurotoxicity of combination chemotherapy with procarbazine, CCNU and vincristine (PCV) for recurrent glioma. J Neurooncol 38:69–75
Conflict of interest
There are no financial disclosures from the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iwadate, Y., Matsutani, T., Hasegawa, Y. et al. Favorable long-term outcome of low-grade oligodendrogliomas irrespective of 1p/19q status when treated without radiotherapy. J Neurooncol 102, 443–449 (2011). https://doi.org/10.1007/s11060-010-0340-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0340-4